Abstract
Background:
Previous research has demonstrated that women instructed in fertility awareness methods can identify the Peak Day of cervical mucus discharge for each menstrual cycle, and the Peak Day has high agreement with other indicators of the day of ovulation. However, previous studies enrolled experienced users of fertility awareness methods or were not fully blinded.
Objective:
To assess the agreement between cervical mucus Peak Day identified by fertile women without prior experience on assessing cervical mucus discharge with the estimated day of ovulation (1 day after urine luteinising hormone surge).
Methods:
This study is a secondary analysis of data from a randomised trial of the Creighton Model FertilityCare™ System (CrM), conducted 2003–2006, for women trying to conceive. Women who had no prior experience tracking cervical mucus recorded vulvar observations daily using a standardised assessment of mucus characteristics for up to seven menstrual cycles. Four approaches were used to identify the Peak Day. The referent day was defined as one day after the first identified day of luteinising hormone (LH) surge in the urine, assessed blindly. The percentage of agreement between the Peak Day and the referent day of ovulation was calculated.
Results:
Fifty-seven women with 187 complete cycles were included. A Peak Day was identified in 117 (63%) cycles by women, 185 (99%) cycles by experts, and 187 (100%) by computer algorithm. The woman-picked Peak Day was the same as the referent day in 25% of 117 cycles, within ±1 day in 58% of cycles, ±2 days in 84%, ±3 days in 87%, and ±4 days in 92%. The ±1 day and ± 4 days’ agreement was 50% and 90% for the expert-picked and 47% and 87% for the computer-picked Peak Day, respectively.
Conclusions:
Women’s daily tracking of cervical mucus is a low-cost alternative for identifying the estimated day of ovulation.
Keywords: biomonitoring, cervical mucus, creighton model fertilitycare system, fertility, ovulation
1 |. BACKGROUND
Ovulation is a central event in a woman’s reproductive or menstrual cycle. However, the timing of ovulation varies considerably, and for some women ovulation occurs irregularly or not at all.1,2 This contributes to subfertility (including delayed time to pregnancy or the inability to conceive a wanted pregnancy), which occurs in at least one in seven (14.3%) and one in four (25%) couples in western and developing countries.3–5 For couples experiencing subfertility, identifying the precise time of ovulation is helpful for the timing of sexual intercourse to increase their chance of conception.6,7 Irregular or absent ovulation is also a marker for general health and a variety of possible diseases in women.8,9
The use of biomarkers for identifying ovulation is of interest to couples, fertility support services, and researchers interested in periconceptional exposures and their relation to fertility and pregnancy outcomes.10–13 Candidate biomarkers to identify ovulation for clinical or research purposes include calendar-based calculations derived from menstrual cycle length, basal body temperature, measurements of luteinising hormone (LH) and other hormones in the urine, and observations of cervical mucus; each of these biomarkers has advantages and disadvantages.7
In the processes that lead to ovulation, the ovary is triggered into releasing an egg from the mature follicle by increasing LH. Measured in urine, the LH surge (meaning the initial rise of LH several folds over the baseline level) precedes the event of ovulation. Prior studies, identifying ovulation sonographically, have shown the LH surge to occur within 1 day before or after the day of ovulation in 74% of cycles, and within 2 days in about 90%.14,15 Thus, urine LH is generally considered an accurate and appropriate marker for ovulation when ultrasound is not available.16–18
Woman’s observations of their own cervical mucus (cervical fluid) constitute another biomarker for ovulation, which has minimal cost, can be used many settings, and has been used to develop and validate protocols for identifying ovulation.11,19–22 Based on cervical mucus observations, the estimated day of ovulation, or “Peak Day”, is defined as the “last day” when women observe cervical mucus present which is clear, stretchy, or has a slippery or lubricative sensation at the vulva, with some slight variations of definition for different investigators.19,23 Studies in many different settings have confirmed that the cervical mucus Peak Day identified by women falls within plus or minus 3 days of ovulation in at least 95% of cycles, as determined by serum or urine LH, serum progesterone, or ultrasound.19,20,22,24−26However, these studies enrolled women who had established experience monitoring their cervical mucus, rather than new users. Also, in most studies, the women were using multiple indicators in addition to the cervical mucus, most commonly basal body temperature.
A highly standardised protocol to teach women to observe and interpret the vaginal discharge from the cervix is found in the Creighton Model FertilityCare™ System (CrM).23,27 Trained CrM instructors teach women and couples to observe and systematically record cervical mucus fertility signs and help women with individualised assessments for special circumstances, such as infertility.
In previous research, the Peak Day determined by the users of the CrM fell within ±3 days of ovulation in at least 97% of cycles.22,26However, these studies also enrolled at least some experienced CrM users and included other biomarkers that were not blinded to participants (basal body temperature or urine LH).
The objectives of this study were as follows: (a) to assess how consistently new CrM users without prior experience in monitoring cervical mucus can identify their estimated day of ovulation or Peak Day; (b) to assess the agreement of four methods of Peak Day selection: woman-picked Peak Day, expert-picked Peak Day, computer-picked Peak Day, and computer-picked Best Quality Day, with the reference standard being urinary LH surge plus one day measured with a blinded monitor.
2 |. METHODS
2.1 |. Study population
This study is a secondary analysis of data from a randomised trial of the CrM for women trying to conceive.28 Sixty-three women, aged 18–36, who had prior proven fertility and desired to conceive were recruited into the arm of the trial which received CrM instruction and began charting with the CrM. Women who were breastfeeding or pregnant at the time of the study were ineligible. No participants had prior experience with CrM or any other system for assessing vaginal or cervical mucus. Women were asked to avoid genital intercourse and pregnancy during the first cycle, while they were being taught to track their cervical mucus patterns. The main reason for study completion was pregnancy (54 women).
2.2 |. Cervical mucus Peak Day
Women recorded their vaginal bleeding and vulvar mucus observations daily on the CrM fertility charts (daily diaries). We used three methods to identify the Peak Day from the women’s self-observed and recorded fertility charts: woman-picked, expert-picked, and computer-picked. For the woman-picked method, women identified their CrM ovulation day according to the CrM instructions, which are based on colour, stretch, and sensation of the discharge. In general, the “last day” of clear, stretchy, or lubricative cervical mucus is defined as the Peak Day.23 There are some additional definitions for special situations, such as, when stretchy mucus is observed for the entire cycle. Based on the CrM instructions, women identified on their fertility charts the day they identified as Peak Day; we abstracted this information as the woman-picked Peak Day.
For the expert-picked method, two independent and blinded CrM trained expert reviewers carefully reviewed all cycles on the CrM fertility charts and selected a Peak Day for each cycle based on the women’s record. The expert reviewers were blinded to the urine LH surge (described below) as well as ovulatory status of the cycle. When the two experts disagreed, a third expert reviewer reviewed and made the final decision (JBS; 18% of cycles). The Peak Day decided by this method was defined as the expert-picked Peak Day.
For the computer-picked methods, we also used the daily diary data recorded by the women. A complication for computer methods is the common presence of premenstrual fluid that has similar characteristics to cervical mucus. Human reviewers know to pay close attention to the middle of the cycle when determining an estimated day of ovulation, but computers need a specific rule to follow, and we elected to have the computer algorithms exclude the last seven days of the cycle. We used two computer algorithms to select the Peak Day: one that identifies the Peak Day conventionally as the last day of mucus that was clear, stretchy, or lubricative; and a Best Quality Day. The Best Quality Day algorithm identifies the last day during a menstrual cycle when a woman observed the highest number of the three fertile-type cervical mucus characteristics. For example, if on cycle day 15 the mucus observed was clear, stretchy, and lubricative, and on day 16 it was stretchy but not clear or lubricative, and then from day 17 on there were none of these characteristics, the conventional algorithm would pick day 16 as the Peak Day, while the Best Quality algorithm would pick day 15. We have applied these same computer algorithms to analyse fertility charts in a previous study that also included basal body temperature; in that study the algorithms agreed well with expert reviewers.11
2.3 |. Day of luteinising hormone surge
The women in this study also performed daily morning urine tests using a blinded research version of the ClearBlue® Easy Fertility Monitor (CBFM) (Clearblue™, Swiss Precision Diagnostics GmbH) which recorded the urinary levels of conjugated estrone and LH. The blinded device did not give participants any feedback from the tests, but always requested daily morning tests from day 2 to day 31 of each menstrual cycle. Daily results of estrone and LH values were stored on a data card and transferred to the study database, and then, LH values were used to determine the day of LH surge. The day after the LH surge was used as the estimated ovulation day as an objective reference standard in this study. In most cases, we identified the day of LH surge by the same algorithm in the monitor for the first day of peak fertility, which is the first rapid rise in LH following a rise in oestrogen. In a few cycles, the monitor did not identify an LH surge, but we identified one by direct review of the monitor data, during a review that was blinded to participants and the daily diary data for cervical mucus. The high sensitivity and accuracy of commercially available urinary LH monitors, and the ClearBlue monitor in particular, has been established by previous studies in comparison with serum hormones and serial follicular ultrasound.29–31
We compared the woman-picked Peak Day, expert-picked Peak Day, computer algorithm–picked Peak Day, and computer algorithm–picked Best Quality Day with the referent day, which was one day after the urine LH surge recorded by the fertility monitor. We conducted sensitivity analyses excluding cycles that resulted in conception.
2.4 |. Covariate assessment
At study enrolment, women completed a baseline questionnaire capturing demographic, life style, and reproductive history factors. For each cycle, intention to conceive (intend, do not intend, and unsure) and cycle length were captured prospectively via the CrM charts.
2.5 |. Statistical analysis
We calculated descriptive statistics for the participating women including age at enrolment (years, continuous), race/ethnicity (White/non-Hispanic, White/Hispanic, and non-White/non-Hispanic), pregnancy history (1 or 2+), education (below or above college), family income (<$40 000 or ≥ $40 000 per year), BMI (<18.5, 18.5–24.9, 25–29.9, and ≥30 kg/m2), any prior use of oral contraceptives (yes/no), consumed alcohol in the prior month (yes/no), and any prior history of smoking (yes/no). For each cycle, we report intent to conceive (intend, do not intend, and unsure) and cycle length (short, <26 days; normal, 26–35 days; and long, ≥35 days). For each cycle, we calculated the number (%) classified correctly for the Peak Day determined by each of the approaches with referent day (1 day after urine LH surge) as follows: exactly the same day, within ±1 day, 2 days, 3 days, and 4 days. We assessed agreement between the four approaches and the reference day (1 day after urine LH surge) via the Pearson correlation coefficient (within ± 4 days and for all days within the cycle) as well as the weighted Kappa coefficient (95% confidence interval [CI]) for exact agreement (day ≤12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, ≥23), using Fleiss-Cohen weights. Finally, in order to better understand demographic, reproductive, and life style factors related to agreement (within ±1 day and exact), we calculated unadjusted and adjusted prevalence ratio (PR) and 95% CIs using modified Poisson regression with robust error variance taking into account multiple cycles per woman.32
2.6 |. Sensitivity analysis
Multiple sensitivity analyses were conducted to determine the robustness of our findings. To assess whether the identification of the Peak Day improved over time, we calculated per cent agreement between the various algorithms compared to reference day stratified by cycle number (1st, 2nd, and 3rd-7th cycles). Additionally, to assess whether the Peak Day was more likely to occur before or after ovulation, we calculated agreement for all methods separating out the days prior (within −1, −2, −3, and −4) and days after (within + 1, +2, +3, and + 4) estimated day of ovulation. All analyses were performed with SAS 9.4 (SAS Institute Inc).
2.7 |. Ethics approval
The trial was registered at clinicaltrials.gov, NCT00161395.28 The study was approved by the University of Utah Institutional Review Board. We obtained written informed consent from all participants.
3 |. RESULTS
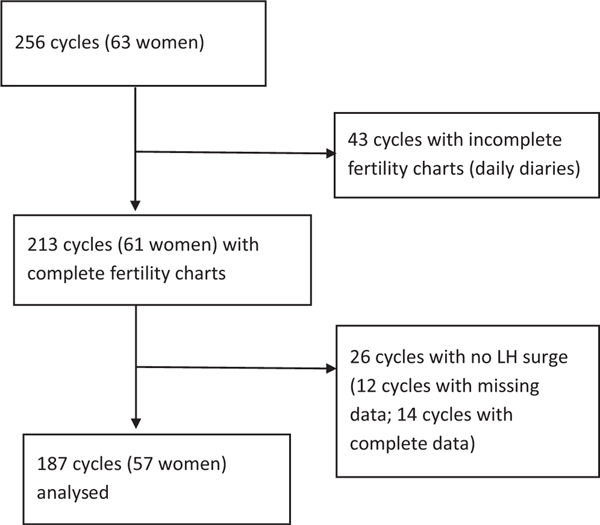
Of the total consented population (63 women over 256 menstrual cycles), 43 cycles were excluded for incomplete fertility charting that prevented selection of a Peak Day; 26 cycles were excluded because no urine LH surge could be identified, of which 12 cycles had missing daily urine testing data, and 14 cycles had complete daily urine testing data but still had no LH surge seen. Thus, the final dataset included 57 women contributing a total of 187 cycles for analysis (Figure 1).
FIGURE 1.
Selection of cycles for analysis
The 57 women with included cycles were predominantly White non-Hispanic (95%), college graduate or above (61%) and reporting a family income of $40 000 or more (63%) (Table 1). Very few (13%) had a prior history of smoking (ever) or of alcohol consumption (9%) in the past month and the majority were of normal weight BMI (18.5–24.9 kg/m2) and had previously used oral contraceptives (93%). The majority of cycles had an intention of trying to conceive (57%) and were of normal length (78%). Among the 187 included cycles, the mean cycle length was 30.7 (SD = 7.9), median cycle length 29.0, mode cycle length 27, and range 23 to 91. The mean day of LH surge plus one was day 19, median day 17, mode day 15, and range day 5 to day 64.
TABLE 1.
Characteristics of participants
| Totala | |
|---|---|
| Baseline characteristics (n = 57 women) | |
| Age (mean ± SD) | 28.7 ± 3.0 |
| Race/Ethnicity, n (%) | |
| White non-Hispanic | 53 (95) |
| White Hispanic | 2 (4) |
| Non-White non-Hispanic | 1 (2) |
| Pregnancy history, n (%) | |
| 1 | 25 (45) |
| ≥2 | 31 (55) |
| Education, n (%) | |
| Below college graduate | 22 (39) |
| College graduate or higher | 34 (61) |
| Family income per year, n (%) | |
| <$40 000 | 20 (37) |
| ≥$40 000 | 34 (63) |
| Body Mass Index (kg/m2) n (%) | |
| <18.5 | 4 (7) |
| 18.5–24.9 | 36 (64) |
| 25–29.9 | 9 (16) |
| ≥30 | 7 (13) |
| Any prior use of oral contraceptives, n (%) | 52 (93) |
| Consumed alcohol in the past month, n (%) | 5 (9) |
| Any prior history of smoking, n (%) | 7 (13) |
| Cycle characteristics (187 cycles) | |
| Intent to conceive, n (%) | |
| Trying to get pregnant | 106 (57) |
| Avoiding pregnancyb | 65 (35) |
| Unsure | 15 (8) |
| Cycle length, n (%) | |
| Short (<26 d) | 17 (9) |
| Normal (26 to 35 d) | 146 (78) |
| Long (≥35 d) | 24 (13) |
Missing values include n = 1 for age, race/ethnicity, pregnancy history, education, BMI, prior use of oral contraceptives, alcohol and smoking, cycle intent; and n = 3 for income.
Assessed at beginning of each cycle. Note that women were instructed to avoid pregnancy and genital intercourse in the first cycle of the study, but not all followed the instruction.
Table 2 shows the timing of the Peak Day selected by the four different approaches compared with the referent day (urine LH surge plus 1). Overall, 117 cycles (63%) had a women-picked Peak Day recorded among these new Creighton Model users. In the cycles where the women did not record a Peak Day, experts could still review their fertility charts and selected an expert-picked Peak Day. There were a total of 185 and 187 cycles for expert-picked and computer-picked approaches, respectively. Two cycles in which the computer algorithm identified a peak were not considered to have a Peak Day by expert review.
TABLE 2.
The agreement of four approaches for mucus Peak Day with the reference ovulation day (LH surge plus 1 d) (57 women, 187 cycles)
| Reference Standard for ovulation | Woman-picked Peak Day | Expert-picked Peak Day | Computer algorithm-picked Peak Day | Computer algorithm-picked Best Quality Day |
|---|---|---|---|---|
|
|
|
|
|
|
| (117 Cycles) | (185 cycles) | (187 cycles) | (187 cycles) | |
|
|
|
|
|
|
| n (%) | n (%) | n (%) | n (%) | |
| Exact | 29 (25) | 44 (24) | 40 (21) | 29 (16) |
| Within ± 1 d | 68 (58) | 93 (50) | 87 (47) | 71 (38) |
| Within ± 2 d | 98 (84) | 140 (76) | 132 (71) | 109 (58) |
| Within ± 3 d | 102 (87) | 155 (84) | 150 (80) | 129 (69) |
| Within ± 4 d | 108 (92) | 166 (90) | 163 (87) | 139 (74) |
| ρ for within ± 4 d | 0.86 | 0.96 | 0.96 | 0.83 |
| ρ for all days within cycle | 0.69 | 0.83 | 0.82 | 0.80 |
| Weighted κa (95% CI) | 0.71 (0.59, 0.83) | 0.74 (0.66, 0.83) | 0.72 (0.63, 0.81) | 0.58 (0.47, 0.70) |
Abbreviations: κ, Kappa statistic; ρ = Pearson’s correlation coefficient.
Weighted Kappa coefficient (95% Confidence intervals (CIs)) for exact agreement (day ≤12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, ≥23), using Fleiss-Cohen weights.
The woman-picked Peak Day had the best agreement with the urine LH referent day: 84% agreement within ±2 days; 92% agreement within ±4 days. The expert-picked and conventional computer-picked Peak Days had slightly less agreement while also covering more cycles; they performed very similarly with each other: 76% and 71% agreement, respectively, within ±2 days; and 90% and 87% agreement, respectively, within ±4 days. The computer-picked Best Quality Day had lower agreement: 58% agreement within ±2 days; 74% agreement within ±4 days. The Pearson correlation coefficients for all days within the cycle were all 0.80 or greater, except for the women-picked Peak Day, with a coefficient of 0.69. Compared with the urine LH surge plus 1 day, 25% of the women-picked Peak Day and 24% of the expert-picked Peak Day agreed perfectly. Substantial agreement was found for the woman-picked Peak Day (weighted Κ = 0.71 [95% CI: 0.59, 0.83]), the expert-picked Peak Day (weighted Κ 0.74, 95% CI: 0.66, 0.83), and the computer algorithm-picked Peak Day (weighted Κ 0.72, 95% CI: 0.63, 0.81) compared with the referent LH surge plus one day. Moderate agreement was found for computer algorithm-picked Best Quality Day and LH surge plus one day (weighted Κ 0.58, 95% CI: 0.47, 0.70). Age, having had only 1 prior pregnancy vs 2+, lower income and education, and prior history of smoking were somewhat negatively associated with agreement; while BMI was positively associated with agreement (Table 3).
TABLE 3.
Factors affecting agreement between approaches and the reference ovulation day (LH surge plus 1 d) (57 women, 187 cycles)
| Woman-picked Peak Day | Expert-picked Peak Day | Computer algorithm-picked Peak Day | Computer algorithm-picked Best Quality Day | |
|---|---|---|---|---|
|
|
|
|
|
|
| (117 cycles) | (185 cycles) | (187 cycles) | (187 cycles) | |
|
|
|
|
|
|
| Prevalence ratio (95% confidence interval)a | ||||
| Plus or minus 1 d in agreement | ||||
| Age (continuous) | 0.96 (0.91, 1.01) | 0.97 (0.92, 1.02) | 1.00 (0.95, 1.06) | 0.99 (0.91, 1.08) |
| Body Mass Index, (continuous) | 1.03 (1.01, 1.04) | 1.02 (1.00, 1.04) | 1.02 (1.00, 1.04) | 1.01 (0.99, 1.04) |
| Pregnancy history (1 vs 2+) | 0.74 (0.53, 1.04) | 0.79 (0.57, 1.09) | 0.85 (0.60, 1.19) | 0.63 (0.4, 1.00) |
| Family income, (< vs ≥ $ 40,000 per year) | 0.87 (0.69, 1.1) | 0.94 (0.72, 1.22) | 0.80 (0.59, 1.10) | 1.24 (0.86, 1.79) |
| Any prior history of smoking, (yes vs no) | 0.67 (0.4, 1.12) | 0.83 (0.54, 1.29) | 0.53 (0.26, 1.10) | 0.52 (0.21, 1.31) |
| Education, (< vs ≥ college graduate) | 0.80 (0.59, 1.08) | 0.87 (0.60, 1.27) | 0.84 (0.54, 1.31) | 0.96 (0.62, 1.48) |
| Consumed alcohol in the past month, (yes vs no) | 0.80 (0.32, 1.98) | 1.09 (0.51, 2.31) | 1.06 (0.55, 2.06) | 1.01 (0.41, 2.53) |
| Race/ethnicity (other vs white/non-Hispanic) Cycles Length | 0.72 (0.22, 2.35) | 0.99 (0.74, 1.33) | 1.03 (0.72, 1.48) | 1.63 (0.89, 3.00) |
| Long vs Normal (≥35 vs 26 to 35 d) | 1.42 (0.90, 2.24) | 1.04 (0.62, 1.75) | 0.92 (0.51, 1.67) | 1.36 (0.81, 2.29) |
| Short vs Normal (<26 vs 26 to 35 d) | 1.16 (0.59, 2.31) | 0.78 (0.44, 1.39) | 0.83 (0.45, 1.54) | 0.28 (0.11, 0.74) |
| Intend to Conceive (do not intend or unsure vs intend) | 0.85 (0.59, 1.20) | 1.02 (0.77, 1.35) | 1.06 (0.79, 1.43) | 0.65 (0.44, 0.96) |
| Exact agreement | ||||
| Age (continuous) | 0.82 (0.66, 1.01) | 0.88 (0.77, 1.00) | 0.92 (0.81, 1.05) | 0.84 (0.68, 1.03) |
| Body Mass Index, (continuous) | 1.04 (1.00, 1.08) | 1.04 (1.01, 1.08) | 1.04 (1.01, 1.08) | 0.97 (0.92, 1.03) |
| Pregnancy history (1 vs 2+) | 0.56 (0.24, 1.30) | 0.46 (0.24, 0.89) | 0.54 (0.27, 1.09) | 0.47 (0.17, 1.29) |
| Family income, (< vs ≥ $40 000 per year) | 0.42 (0.14, 1.22) | 0.62 (0.31, 1.21) | 0.52 (0.26, 1.05) | 0.43 (0.19, 0.99) |
| Any prior history of smoking, (yes vs no) | 0.55 (0.10, 2.98) | 0.86 (0.31, 2.37) | 0.72 (0.28, 1.89) | 1.45 (0.27, 7.85) |
| Education, (< vs ≥college graduate) | 0.60 (0.28, 1.29) | 0.46 (0.27, 0.77) | 0.64 (0.36, 1.13) | 0.75 (0.36, 1.58) |
| Consumed alcohol in the past month, (yes vs no) | 0.94 (0.18, 5.03) | 0.57 (0.12, 2.66) | 0.55 (0.12, 2.59) | 0.85 (0.13, 5.65) |
| Race/ethnicity (other vs white/non-Hispanic) Cycles Length | —b | 0.65 (0.33, 1.31) | 0.80 (0.44, 1.45) | 1.54 (0.53, 4.44) |
| Long vs Normal (≥35 vs 26 to 35 d) | 0.65 (0.15, 2.87) | 0.80 (0.38, 1.66) | 1.01 (0.50, 2.07) | 0.82 (0.18, 3.72) |
| Short vs Normal (<26 vs 26 to 35 d) | 0.70 (0.13, 3.76) | 0.40 (0.09, 1.70) | 0.35 (0.08, 1.59) | 0.25 (0.03, 2.39) |
| Intend to Conceive (do not intend or unsure vs intend) | 1.20 (0.59, 2.45) | 1.45 (0.79, 2.65) | 1.12 (0.58, 2.16) | 0.60 (0.3, 1.22) |
Multivariable Poisson regression model accounting for multiple cycles per woman.
Removed since model would not converge due to too few observations.
The Peak Day (whether identified by the woman, expert, or computer) is slightly more likely to occur before the referent day for ovulation than after it. For example, agreement between woman-picked, expert-picked, and computer-picked Peak Day and gold-standard LH referent was 46%, 40%, and 35%, respectively, in the interval one day before and through the day of estimated ovulation versus 37%, 34%, and 33% in the interval of estimated ovulation to one day afterwards (Table S1). However, this discrepancy diminished the further out from estimated day of ovulation for all approaches. We did not find any evidence of a trend for increasing accuracy with successive cycles in the study (Table S2).
4 |. COMMENT
4.1 |. Principal findings
We found that the Peak Day of cervical mucus had high agreement with the reference ovulation day determined by LH surge plus one day. The agreement was highest for the woman-picked Peak Day, but women only recorded a Peak Day in about two thirds of cycles (117/187). The expert-picked Peak Day (also blinded to urine LH) also had high agreement and identified a mucus peak in all of the cycles that were selected based on complete daily diary data and the presence of a urine LH surge. In addition, the expert-picked Peak Day and computer-picked Peak Day were very similar, with levels of agreement within ±4 days of the urinary LH surge 90% and 87% of cycles, respectively. That the woman-picked day had higher agreement than the expert-picked or computer-picked might reflect a lower likelihood of women to identify a Peak Day in cycles where the pattern was less clear. However, it is unclear to what extent the lower number of cycles with a Peak Day recorded by women may be related to incomplete documentation by the women, versus uncertainty of the women to interpret their chart. We also cannot rule out the possibility that in some cases, women might have been using another indicator (such as basal body temperature) that they did not report to us.
4.2 |. Strengths of the study
In our study, women determined their ovulation based only on cervical mucus observations and we compared their selections to blinded assessment of urine LH. Hence, our study presents an independent confirmation that the new user can identify the Peak Day based on cervical mucus observations alone, with standardised CrM instruction.
4.3 |. Limitations of the data
The study participants, however, were mainly healthy young women with previously proven fertility; thus, the generalisability to subfertile women was unknown. Future work should explore exactly what type and level of instruction and tracking of cervical mucus is needed to achieve a high level of documentation of mucus Peak Days by women, with a high level of agreement with gold-standard indicators of ovulation, in diverse populations.
4.4 |. Interpretation
We looked for evidence of a learning effect, whereby women might be more accurate in determining a correct Peak Day with longer durations of CrM charting. As seen in Table S2, there is no difference in agreement by cycle number: 22%, 29%, and 24% of women in cycle 1, 2, and ≥3, respectively, were able to identify their exact day of ovulation compared with referent day (LH surge plus 1). In terms of women’s ability to simply choose an estimated day of ovulation, we also found no clear patterns. In cycle 1, 23/33 (70%) of cycles had a woman-picked Peak Day, in cycle 2, 24/50 (48%) of cycles had a woman-picked Peak Day, and in cycle 3 or more 70/104 (67%) of cycles had a woman-picked Peak Day. However, 24 women did not record a complete first cycle, which may indirectly indicate a learning time. In a multi-country of 869 women learning to record cervical mucus patterns without any other referent, 93%, 94%, and 94% recorded an “interpretable ovulatory pattern” (as evaluated by a teacher) in cycles 1, 2, and 3, respectively.33
The referent we used in this study was the urine LH surge plus one day, as assessed by a blinded version of the Clearblue Fertility Monitor. While this referent has been found to have high accuracy for the timing of ovulation, it has some variability in relation to ovulation assessed by follicular ultrasound or other hormonal markers (such as rise in progesterone).18,30 Based on prior studies that have used an ultrasound referent, we believe that the agreement between cervical mucus Peak Day and ovulation assessed by urine LH surge plus one day would be similar to the agreement between cervical mucus Peak Day and ovulation assessed by follicular ultrasound.14
The first published study on the agreement of self-assessment of cervical mucus characteristics to the estimated ovulation day19 compared the cervical mucus Peak Day with serial plasma LH measurements; the 22 participants also measured basal body temperature. This study found that the estimated day of ovulation (the day after the maximum LH level in plasma) occurred an average of 0.9 days after the Peak Day with a range of 2 days before to 3 days after. Subsequent studies have found that the cervical mucus Peak Day identified by women falls within plus or minus 3 days of another indicator of ovulation in at least 95% of cycles, as determined by serum or urine LH, serum progesterone, or ultrasound.11,19,20,22,24−26However, all of these prior studies were based on experienced users of fertility awareness methods, or were not fully blinded; that is, the women had unblinded urine LH test and/or basal body temperature measurements to help them in corroborating their Peak Day. The one exception was a study of 27 Italian women (who were nurses or laboratory technicians).24 Our study builds on the results from these prior studies in showing the agreement of the Peak Day of cervical mucus with a reliable hormonal indicator of ovulation in women maintains in women who are not using any other indicator and who are learning to track their cervical mucus secretions for the first time.
The computer algorithm-picked Peak Day agreed better with the ovulation detected by urinary LH surge than the computer algorithm-picked Best Quality Day (87% and 74% to ±4 days of ovulation, respectively). We tested this algorithm, because of results from our previous study, in which the Best Quality algorithm had higher agreement with the blinded urine LH surge than the Peak Day algorithm.11 While CrM provides users in-person training on how to record their observations,34 the prior study used a brochure to teach a simplified fertility awareness method.11 However, our prior study was much smaller (26 cycles) and women were encouraged to also record basal body temperature (although fewer than half actually did so). In addition, the way women observed the cervical mucus was less standardised in the smaller study. These factors may have contributed to the different comparative performance of the conventional Peak Day versus the Best Quality
Day. Thus, the results of this study are consistent with prior studies, which have used definitions which are more consistent with the “Peak Day” algorithm in this study.11,19,20,22,24–26 However, one possible advantage of the “Best Quality Day” is that it is substantially more likely to occur a few days before the estimated day of ovulation than the computer-picked Peak Day algorithm: 67% vs 49% within the range of 4 days before to the estimated day of ovulation, as seen in Table S1.
The use of cervical mucus and temperature combined is a common practice in users of fertility awareness. In one small study, the combination of mucus and temperature observations identified the timing of ovulation (compared to follicular ultrasound) more precisely than either indicator alone.35 In comparative studies, the mucus Peak Day was more accurate than basal body temperature alone, when compared to a hormonal or ultrasound standard.14,36 Compared with other biomarkers used to determine ovulation, cervical mucus, with or without basal body temperature, is a reliable and minimal cost approach for determining ovulation.37 Currently, mobile phone applications are proliferating that purport to help women identify ovulation; however, few of them use these reliable biomarkers (most are based on calendar calculations only, and most are inaccurate).38,39
5 |. CONCLUSIONS
In summary, more than 80% of the woman-picked, expert-picked, and computer algorithm-picked Peak Days were matched to a hormonal referent for ovulation (urine LH surge plus one day) within ±3 days. Identified by the woman, expert reviewer, or computer algorithm, the mucus Peak Day is a low-cost approach to identify ovulation for achieving pregnancy, or completing time-sensitive assessments of exposure.
Supplementary Material
Synopsis.
Study question
Can fertile women without prior experience in identifying the estimated day of ovulation do so by monitoring cervical mucus (cervical fluid)?
What’s already known
Previous research has demonstrated that women instructed in fertility awareness methods can identify the Peak Day of cervical mucus discharge for each menstrual cycle and that the Peak Day has a high agreement with other indicators of the day of ovulation. However, previous studies enrolled experienced users of fertility awareness methods or were not fully blinded.
What this study adds
Daily tracking of cervical mucus is a low-cost alternative for identifying the estimated day of ovulation among fertile women without prior experience. The estimated day of ovulation can be identified by women, expert review, or a computer algorithm.
ACKNOWLEDGEMENTS
Becky Crockett, Sebrena Banecker, Sara Feltz, and Colette Child assisted in the study visits and collection of the relevant data. Becky Crockett helped with the expert mucus Peak Day reviews. Swiss Precision Diagnostics manufactured the blinded monitors and assisted with extraction of the LH data from the monitors. Jihye Park helped with data cleaning. Shahpar Najmabadi helped with manuscript preparation.
Funding information
Funded in part by 1K23 HD0147901-01A1 from the Eunice Kennedy Shriver National Institute for Child Health and Human Development, and by the Health Studies Fund, Department of Family and Preventive Medicine, University of Utah.
Footnotes
CONFLICT OF INTERESTS
Joseph B. Stanford has served as a scientific consultant for Swiss Precision Diagnostics GmbH, which manufactures the Clearblue™ Fertility Monitor. The other authors declare they have no conflict of interest.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
REFERENCES
- 1.Colombo B, Masarotto G. Daily fecundability: first results from a new data base. Demogr Res. 2000;3:39. [PubMed] [Google Scholar]
- 2.Vollman RF. The menstrual cycle. In: Friedman EA ed. Major Problems in Obstetrics Gynecology. Philadelphia, PA: Saunders WB; 1977:7, 1–193. It is volume 7 of a series of monographs, titled, series editor. [PubMed] [Google Scholar]
- 3.Chandra A, Copen CE, Stephen EH. Infertility service use in the United States: data from the National Survey of Family Growth, 1982–2010. Natl Health Stat Report. 2014;73:1–21. [PubMed] [Google Scholar]
- 4.Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med. 2012;9:e1001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thoma ME, McLain AC, Louis JF, et al. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril. 2013;99(1324–1331):e1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilcox AJ, Weinberg CR, Baird DD. Timing of sexual intercourse in relation to ovulation. Effects on the probability of conception, survival of the pregnancy, and sex of the baby. N Engl J Med. 1995;333:1517–1521. [DOI] [PubMed] [Google Scholar]
- 7.Stanford JB, White GL, Hatasaka H. Timing intercourse to achieve pregnancy: current evidence. Obstet Gynecol. 2002;100:1333–1341. [DOI] [PubMed] [Google Scholar]
- 8.American Academy of Pediatrics Committee on A, American College of O, Gynecologists Committee on Adolescent Health C, Diaz A, Laufer MR, Breech LL. Menstruation in girls and adolescents: using the menstrual cycle as a vital sign. Pediatrics. 2006;118:2245–2250. [DOI] [PubMed] [Google Scholar]
- 9.Pierce SB, Chisholm KM, Lynch ED, et al. Mutations in mitochondrial histidyl tRNA synthetase HARS2 cause ovarian dysgenesis and sensorineural hearing loss of Perrault syndrome. Proc Natl Acad Sci U S A. 2011;108:6543–6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howards PP, Schisterman EF, Wactawski-Wende J, Reschke JE, Frazer AA, Hovey KM. Timing clinic visits to phases of the menstrual cycle by using a fertility monitor: the BioCycle Study. Am J Epidemiol. 2009;169:105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Porucznik CA, Cox KJ, Schliep KC, Stanford JB. Pilot test and validation of the peak day method of prospective determination of ovulation against a handheld urine hormone monitor. BMC Womens Health. 2014;14:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Porucznik CA, Cox KJ, Schliep KC, Wilkins DG, Stanford JB. The Home Observation of Periconceptional Exposures (HOPE) study, a prospective cohort: aims, design, recruitment and compliance. Environ Health. 2016;15:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans-Hoeker E, Pritchard DA, Long DL, Herring AH, Stanford JB, Steiner AZ. Cervical mucus monitoring prevalence and associated fecundability in women trying to conceive. Fertil Steril. 2013;100(1033–1038):e1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ecochard R, Boehringer H, Rabilloud M, Marret H. Chronological aspects of ultrasonic, hormonal, and other indirect indices of ovulation. BJOG. 2001;108:822–829. [DOI] [PubMed] [Google Scholar]
- 15.Miller PB, Soules MR. The usefulness of a urinary LH kit for ovulation prediction during menstrual cycles of normal women. Obstet Gynecol. 1996;87:13–17. [DOI] [PubMed] [Google Scholar]
- 16.Lynch KE, Mumford SL, Schliep KC, et al. Assessment of anovulation in eumenorrheic women: comparison of ovulation detection algorithms.Fertil Steril. 2014;102(511–518):e512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radin RG, Sjaarda LA, Silver RM, et al. C-Reactive protein in relation to fecundability and anovulation among eumenorrheic women. Fertil Steril. 2018;109(232–239):e231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roos J, Johnson S, Weddell S, et al. Monitoring the menstrual cycle: Comparison of urinary and serum reproductive hormones referenced to true ovulation. Eur J Contracept Reprod Health Care. 2015;20:438–450. [DOI] [PubMed] [Google Scholar]
- 19.Billings EL, Brown JB, Billings JJ, Burger HG. Symptoms and hormonal changes accompanying ovulation. Lancet. 1972;1:282–284. [DOI] [PubMed] [Google Scholar]
- 20.Flynn AM, Lynch SS. Cervical mucus and identification of the fertile phase of the menstrual cycle. Br J Obstet Gynaecol. 1976;83:656–659. [DOI] [PubMed] [Google Scholar]
- 21.Hilgers TW, Stanford JB. Creighton Model NaProEducation Technology for avoiding pregnancy. Use effectiveness. J Reprod Med. 1998;43:495–502. [PubMed] [Google Scholar]
- 22.Hilgers TW, Abraham GE, Cavanagh D. Natural family planning. I. The peak symptom and estimated time of ovulation. Obstet Gynecol. 1978;52:575–582. [PubMed] [Google Scholar]
- 23.Hilgers TW, Prebil AM. The ovulation method–vulvar observations as an index of fertility/infertility. Obstet Gynecol. 1979;53:12–22. [PubMed] [Google Scholar]
- 24.Cortesi S, Rigoni G, Zen F, Sposetti R. Correlation of plasma gonadotrophins and ovarian steroids pattern with symptomatic changes in cervical mucus during the menstrual cycle in normal cycling women. Contraception. 1981;23:629–641. [DOI] [PubMed] [Google Scholar]
- 25.Ecochard R, Duterque O, Leiva R, Bouchard T, Vigil P. Self-identification of the clinical fertile window and the ovulation period. Fertil Steril. 2015;103(1319–1325):e1313. [DOI] [PubMed] [Google Scholar]
- 26.Fehring RJ. Accuracy of the peak day of cervical mucus as a biological marker of fertility. Contraception. 2002;66:231–235. [DOI] [PubMed] [Google Scholar]
- 27.Stanford JB, Smith KR, Dunson DB. Vulvar mucus observations and the probability of pregnancy. Obstet Gynecol. 2003;101: 1285–1293. [DOI] [PubMed] [Google Scholar]
- 28.Stanford JB, Smith KR, Varner MW. Impact of instruction in the Creighton model fertilitycare system on time to pregnancy in couples of proven fecundity: results of a randomised trial. Paediatr Perinat Epidemiol. 2014;28:391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guida M, Tommaselli GA, Palomba S, et al. Efficacy of methods for determining ovulation in a natural family planning program. Fertil Steril. 1999;72:900–904. [DOI] [PubMed] [Google Scholar]
- 30.Behre HM, Kuhlage J, Gassner C, et al. Prediction of ovulation by urinary hormone measurements with the home use ClearPlan Fertility Monitor: comparison with transvaginal ultrasound scans and serum hormone measurements. Hum Reprod. 2000;15:2478–2482. [DOI] [PubMed] [Google Scholar]
- 31.Tanabe K, Susumu N, Hand K, Nishii K, Ishikawa I, Nozawa S. Prediction of the potentially fertile period by urinary hormone measurements using a new home-use monitor: comparison with laboratory hormone analyses. Hum Reprod. 2001;16:1619–1624. [DOI] [PubMed] [Google Scholar]
- 32.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162:199–200. [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization. A prospective multicentre trial of the ovulation method of natural family planning. I. The teaching phase. Fertil Steril. 1981;36:152–158. [PubMed] [Google Scholar]
- 34.Stanford JB, Smith KR. Characteristics of women associated with continuing instruction in the Creighton Model Fertility Care System. Contraception. 2000;61:121–129. [DOI] [PubMed] [Google Scholar]
- 35.Frank-Herrmann P, Gnoth C, Baur S, Strowitzki T, Freundl G. Determination of the fertile window: reproductive competence of women–European cycle databases. Gynecol Endocrinol. 2005;20:305–312. [DOI] [PubMed] [Google Scholar]
- 36.Hilgers TW, Bailey AJ. Natural family planning. II. Basal body temperature and estimated time of ovulation. Obstet Gynecol. 1980;55:333–339. [DOI] [PubMed] [Google Scholar]
- 37.Practice Committee of the American Society for Reproductive Medicine in collaboration with the Society for Reproductive E Infertility, Practice Committee of the American Society for Reproductive Medicine in collaboration with the Society for Reproductive E Infertility. Optimizing natural fertility: a committee opinion. Fertil Steril. 2017;107:52–58.28228319 [Google Scholar]
- 38.Duane M, Contreras A, Jensen ET, White A. The performance of fertility awareness-based method Apps marketed to avoid pregnancy. J Am Board Fam Med. 2016;29:508–511. [DOI] [PubMed] [Google Scholar]
- 39.Freis A, Freundl-Schutt T, Wallwiener LM, et al. Plausibility of menstrual cycle Apps claiming to support conception. Front Public Health. 2018;6:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.