Abstract
Currently available human immunodeficiency virus type 1 (HIV-1) RNA quantification assays can detect most viruses of the group M subtypes, but a substantial number are missed or not quantified reliably. Viruses of HIV-1 group O cannot be detected by any commercially available assay. We developed and evaluated a quantitative assay based on nucleic acid sequence-based amplification (NASBA) technology, with primers and probes located in the conserved long terminal repeat (LTR) region of the HIV-1 genome. In 68 of 72 serum samples from individuals infected with HIV-1 subtypes A to H of group M, viruses could be detected and quantified. In serum samples from two patients infected with HIV-1 group O viruses, these viruses as well could be detected and quantified. In contrast, the currently used gag-based assay underestimated the presence of subtype A viruses and could not detect subtype G and group O viruses. The discrepancy between the results of the two assays may be explained by the number of mismatches found within and among the probe and primer regions of the subtype isolates. These data indicate that LTR-based assays, including the NASBA format chosen here, are better suited to monitoring HIV-1 therapy than are gag-based assays in an era in which multiple HIV-1 subtypes and groups are spreading worldwide.
The human immunodeficiency virus type 1 (HIV-1) RNA level in plasma or serum has become one of the most important markers for monitoring HIV-1-infected patients. Other than HIV-1 DNA, it is the only evidence for mother-to-child transmission, since maternal antibodies present in infant serum hamper antibody-screening assays. The HIV-1 RNA level is the most valuable marker for predicting disease progression in nontreated patients (10, 13, 21, 22, 34) and is highly useful for evaluating the effectiveness of antiretroviral drug therapy (14, 26, 43). The decision to start antiretroviral drug therapy is currently made on the basis of the viral RNA level (31). A patient with an HIV-1 RNA level of less than 10,000 copies per ml generally will not progress to AIDS for at least 5 years (22, 34). Highly active antiretroviral therapy, consisting of treatment with a combination of three drugs, results in a decline of the viral RNA level of approximately 99% (28). The therapy’s goal, for optimal delay of disease, is to decrease the viral RNA level until it cannot be detected by RNA quantification assays.
Although HIV-1 subtype B has been the predominant cause of AIDS in Europe and the United States, other HIV-1 subtypes, particularly subtypes A and C, are now taking over. As these different clades of HIV-1 spread rapidly around the world, there is an increased need for assays that can reliably quantify the level of RNA of all known subtypes, i.e., group M subtypes A to H and group O viruses, in plasma, serum, or culture supernatants.
Commercially available RNA quantification assays are based either on the amplification of a fragment of the gag gene of the HIV-1 genome (e.g., NucliSens HIV-1 QT assay [Organon Teknika, Boxtel, The Netherlands] or Amplicor version 1.5 HIV Monitor test [Roche Diagnostics, Basel, Switzerland]) (15, 23, 24, 41, 42) or on the direct detection of HIV-1 RNA by hybridization with labeled probes (e.g., Quantiplex HIV 3.0 assay; Chiron Diagnostics, Emeryville, Calif.) (27, 35, 36, 46). The NucliSens and Amplicor assays were developed with reagents derived from HIV-1 subtype B, but they can detect most group M viruses. The Quantiplex assay uses 45 target probes designed to hybridize with all known HIV-1 group M viruses and is thus more likely than the other two assays to detect and quantify genetically divergent HIV-1 subtypes (7, 12, 25). It has been reported that there is no difference in general performance, for instance, with regard to sensitivity, accuracy, and reproducibility, among the three assays (7, 11, 30, 32, 37, 38) even though some viruses not detected by one assay have been detected by another assay (1, 8, 25). None of the assays can detect HIV-1 group O viruses (12, 20, 29). The sensitivity of both the Quantiplex and the NucliSens assays, if an ultrasensitive protocol is applied, is currently 50 copies of RNA per ml of plasma or serum (5, 44), with an input of 1,000 or 200 μl, respectively. The Amplicor assay has variable sensitivity, with a detection limit of generally ranging from 30 to 60 RNA copies per ml of plasma when an ultrasensitive protocol is applied with an input of 500 μl.
We developed and evaluated a new, broad-clade HIV-1 RNA quantification assay based on nucleic acid sequence-based amplification (NASBA) technology. The evaluation was performed with plasma or serum samples that together contained all group M subtypes and group O viruses. We show that the number of mismatches in sequences of primers and probes was the major determinant of accuracy in the detection and quantification of HIV-1 RNA.
MATERIALS AND METHODS
Three-calibrator gag-based NASBA and one-calibrator long terminal repeat (LTR)-based NASBA.
The three-calibrator gag-based NASBA is a commercially available assay (NucliSens HIV-1 QT assay; Organon Teknika). The assay was performed by following the instructions of the manufacturer.
Four regions having highly conserved sequences, which were found in the 5′ end of the genomic RNA (LTR region) after screening of the known HIV-1 genomes (17), were used to develop an LTR-based NASBA. The assay was based on standard NASBA technology (42) but used one internal calibrator (Q) molecule instead of three, as in the gag-based assay (15, 41, 42). A fragment of approximately 135 bases of antisense RNA was amplified and detected with two primers and two probes. Calibrator molecules were added to a 200-μl plasma or serum sample, and RNA was isolated by a silica-based method (4). Five microliter volumes of the 50 μl of isolated viral RNA and calibrator RNA were used in the NASBA reaction with a 5′ sense primer (5′ CTCAATAAAGCTTGCCTTGA) (HIVHXB2CG [GenBank accession no. K03455] nucleotides [nt] 508 to 523) and a 3′ antisense primer elongated with a T7 sequence (in lowercase italics) (5′ aattctaatacgactcactatagggagagGGGCGCCACTGCTAGAGA) (nt 643 to 628) were used to amplify the LTR fragment. After 1.5 h of incubation at 41°C, 5 μl of the reaction mixture was diluted 31 times in detection diluent (Organon Teknika). From the diluted sample, 5 μl was added either to a mixture of ruthenium tag-labeled wild-type detection (5′ AATGTGTGCCCGTCTGTT) (nt 555 to 572) and biotin-labeled capture (5′ TCTGGTAACTAGAGATCCCTC) (nt 580 to 600) probes or to a mixture of Q detection and identical capture probes. The detection probes were labeled so they could be detected by electrochemiluminescence (3). The number of wild-type RNA copies per milliliter of serum was calculated by the ratio between the wild-type signal and the Q signal. Serum samples in which no viral RNA could be detected were reanalyzed by following an ultrasensitive protocol (44).
Ultrasensitive protocol for NASBA.
An ultrasensitive protocol for NASBA (UltraSens protocol) (44), which improved the sensitivity of both the gag-based and the LTR-based NASBAs to 50 copies/ml, had already been developed. Briefly, the eluted nucleic acids remaining from the isolation procedure described in the previous section were taken off of the silica beads. The noneluted nucleic acids, still attached to the silica beads, were eluted again in 70 μl of elution buffer (Organon Teknika) and pooled with the previously eluted nucleic acids. The nucleic acids were precipitated with Pellet Paint (Novagen, Madison, Wis.) and ethanol. After the pellet was washed, the nucleic acids were again amplified and detected by following the standard NASBA amplification and electrochemiluminescence detection procedure.
Samples.
Seventy-two serum samples taken from individuals suspected or known to be infected with a non-B HIV-1 subtype were selected from the collection at the outpatient clinic of the Academic Medical Center, Amsterdam, The Netherlands. Most of these individuals were non-European and non-U.S. immigrants to The Netherlands, who probably were infected in their home country, or were individuals known or suspected to be infected with an HIV-1 strain of non-European and non-U.S. origin. They had been identified by a thorough epidemiological investigation that is part of the routine evaluation of every newly diagnosed HIV case at the outpatient clinic of the Academic Medical Center. In addition, four serum samples from two individuals infected with an HIV-1 group O virus (ANT70 and partner [9]), taken before and during antiretroviral therapy, were the kind gift of G. van der Groen and W. Janssens (Institute for Tropical Medicine, Antwerp, Belgium). Sequence analysis was performed on the gag sequences of all samples to identify the viral subtypes.
To obtain supernatants from the viral cultures of subtypes A to G, infectious virus stocks were collected and prepared by the World Health Organization (WHO) Network for HIV Isolation and Characterization (18). Expanded virus stocks were produced (45) by the inoculation of 4.0 × 106 phytohemagglutinin-stimulated donor peripheral blood mononuclear cells with a supernatant from cultures of the primary isolate. After being incubated and washed, cells were resuspended in culture medium and incubated at 37°C. Cell-free supernatant was harvested after 10 to 11 days.
PCR.
Nucleic acids were isolated from 200 μl of serum by a silica-based method (4). After washing and elution from the silica with 100 μl of sterile water, 10 μl of the eluate was used in a reverse transcription reaction with avian myeloblastosis virus reverse transcriptase. For amplification of the gag gene, we used the antisense primer 3′ SK39 (5′ GCATTCTGGACATAAGACAAGGACCAAA) (nt 1658 to 1631). For amplification of the 5′ LTR, we used 3′ L-GagUniM2 (5′ GCACCCATCTCTCTCCTTCTAGCCTCCGC) (nt 797 to 759). After incubation of the eluate for 45 min at 41°C, a PCR mixture containing the sense primers 5′ Gag-1 (GCGAGAGCGTCAGTATTAAGC) (nt 796 to 816) for the gag gene and 5′ L-R1-M2 (5′ GGTCTCTCTTGTTRGACCAGATYTGAGCC) (nt 455 to 484) for the 5′ LTR, PCR buffer, deoxynucleoside triphosphates, 2.5 mM MgCl2 for the gag gene and 5 mM MgCl2 for the 5′ LTR, and 2 U of Taq polymerase was added. After incubation for 5 min at 95°C, the reaction mixture was subjected to 35 cycles of amplification (1 min at 95°C, 1 min at 55°C, and 2 min at 72°C). Nested PCRs with 25 cycles of amplification each were performed before direct sequencing. The nested gag gene product was obtained after amplification with the primers 5′ Gag-2-SP6 (sense; 5′ atttaggtgacactatagGGGAAAAATTCGGTTAAGGCC) (nt 836 to 857) and 3′ Gag AE3-T7 (antisense; 5′ taatacgactcactatagggTAGGACCCTAATTTATTTTATCA) (nt 1610 to 1588; SP6 and T7 sites are in lowercase italics). The product of the nested 5′-LTR PCR was obtained after amplification with the primers 5′ L-T7-R2M2 (sense; 5′ taatacgactcactatagggGAGCCTGGGAGCTCTCTGGCTA) (nt 479 to 500) and 3′ L-GagM-SP6 (antisense; 5′ atttaggtgacactatagAGCAAGCCGAGTCCTGCGTC) (nt 707 to 688). The conditions for these PCRs were similar to those described for the first PCR but with a concentration of 4 mM instead of 5 mM MgCl2 for the nested 5′-LTR PCR. The presence of amplified PCR products was verified with 1% agarose gels stained with ethidium bromide.
DNA sequencing.
Both strands of the nested-PCR fragments were directly sequenced with the SP6 and T7 primer sequences. Sequencing was performed with Taq dye primers (Applied Biosystems, Foster City, Calif.) and the Thermo Sequenase fluorescence-labeled primer cycle-sequencing kit (Amersham International, Little Chalfont, England). The sequence products were analyzed on an automatic DNA sequencer (model 373A stretch; Applied Biosystems).
The sequences were aligned manually. Phylogenetic analysis of the gag gene sequences of all serum samples was performed by the neighbor-joining method of the TREECON program (39). The distance matrix was generated by Kimura’s two-parameter estimation (16).
Statistical analysis.
Statistical analysis was performed by using the Pearson product moment correlation procedure as well as the paired t test as implemented in the SigmaStat version 1.0 software package (Jandel Corporation, San Rafael, Calif.).
RESULTS
Comparison of the quantitative performance of the gag- and LTR-based NASBAs on HIV-1 subtype B RNA.
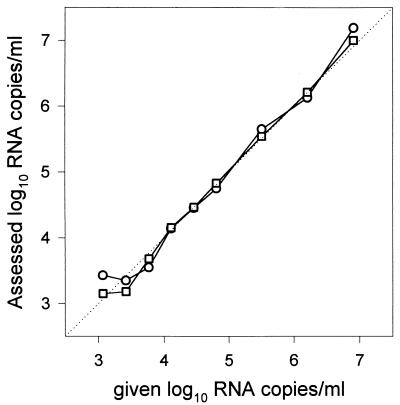
The gag-based NASBA, based on subtype B sequences, has already shown its ability to detect and quantify HIV-1 subtype B genomic RNA (13, 38, 40–42, 47). We tested whether the new LTR-based NASBA could equal its performance. The two assays were compared with a panel consisting of dilution series of a well-characterized subtype B standard (HXB3) in 0.2 ml of human plasma (19). Analysis of the dilution series yielded similar results for both assays (Fig. 1). The quantification of both assays was linear and accurate over a range of 103 to 107 copies of genomic HIV RNA per ml when a sample volume of 0.2 ml was applied. The precision and accuracy of the LTR-based NASBA were within 0.2 and 0.1 log10, respectively, for up to 250 copies of genomic HIV RNA per input volume (0.2 ml). This result was determined with a group of 47 human plasma samples mixed with known amounts of HXB3 (19) (data not shown). The analytical sensitivity of the assay, in which amplification occurred in 50% of the reactions, was approximately 10 genomic RNA copies per reaction. An input volume of 0.2 ml led to a sensitivity of 500 copies of genomic HIV RNA per ml, as only 1/10 of the sample was used in a reaction. The sensitivity was improved to 50 copies of genomic RNA per ml when the UltraSens protocol was applied.
FIG. 1.
Assessed viral RNA levels versus given viral RNA levels in serial dilutions of HIV-1 HXB3 (19) in plasma as determined with the LTR-based (○) and the gag-based (□) NASBAs. The results shown are the means of at least three independently performed experiments.
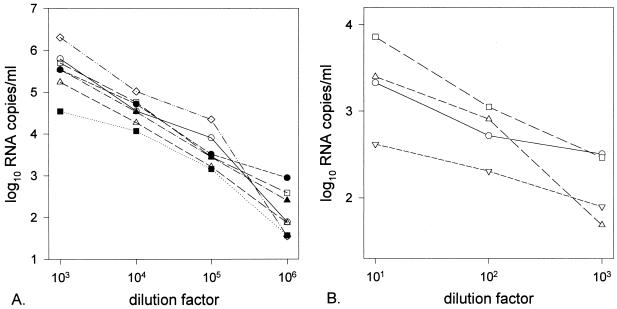
Linear quantification capacities of the LTR-based NASBA on various HIV-1 subtypes.
The linear quantification abilities of the LTR-based assay were determined by serial dilutions of a viral culture supernatant for each subtype (A to G) of the HIV-1 M group and for four viruses of the HIV-1 O group. The results of representative dilution series for the HIV-1 M and O groups are plotted in Fig. 2A and 3B, respectively. For subtypes A to G of the HIV-1 M group, a linear decrease of the assessed viral RNA levels as the level of dilution increased was observed, indicating that all subtypes were quantified similarly. The variation in initial RNA levels was determined by differences in RNA input. One representative experiment of three that were performed for each subtype was plotted (Fig. 2). The slope of the linear decrease of the viral RNA levels was not as steep for the group O viruses. This finding suggests that the efficiency of quantification was less for the group O viruses than for the group M viruses and was probably due to a greater number of mismatches in the capture probe.
FIG. 2.
Assessed viral RNA levels versus dilution factor of supernatant from cultures of HIV-1 group M subtypes A (○), B (□), C (▵), D (●), E (■), F (▴), and G (◊) (A) and from four different HIV-1 group O viruses (B).
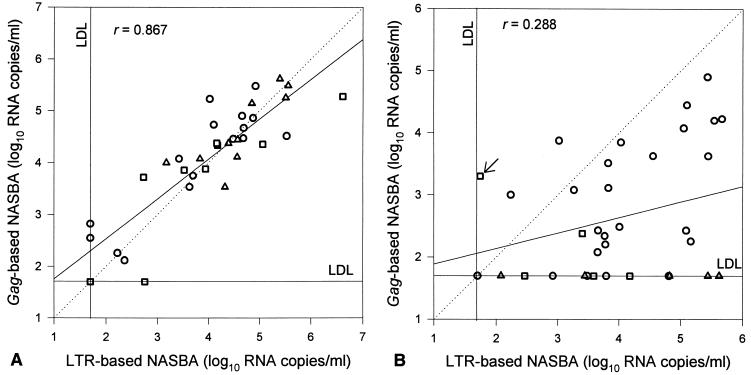
FIG. 3.
Scatter diagrams of log10 RNA levels as assessed by the LTR-based NASBA versus the log10 RNA levels as assessed by the gag-based NASBA for HIV-1 subtypes B (○), C (□), and D (▵) (A) and subtypes A (○), E (□), and G (▵) (B). The arrow in panel B indicates a sample positive for subtype E in both assays. LDL, lower detection limit.
RNA quantification in serum samples from 72 individuals infected with HIV-1 group M viruses of subtypes A to H and from two individuals (four samples) infected with HIV-1 group O viruses.
Viral subtypes of the viruses were determined based on phylogenetic analysis of the sequences of the gag genes. The serum samples with a viral RNA level below the detection limit of either the LTR- or the gag-based assay were reexamined by using the UltraSens protocol, which has a lower detection limit (50 copies/ml). The gag-based NASBA was unable to detect viral RNA in samples containing subtype G or HIV-1 group O viruses, whereas the LTR-based assay could detect the RNA of all tested subtypes or groups. For subtype A viruses, RNA levels were significantly lower (P < 0.0001) with assessment by the gag-based NASBA than they were with assessment by the LTR-based assay. For all samples, the RNA levels for subtype E viruses as determined by the gag-based assay were lower than as determined by the LTR-based assay, but not significantly (P = 0.26).
To facilitate analysis, the serum samples were divided into two groups. The first group contained subtypes B, C, and D (Fig. 3A), whereas the second group contained subtypes A, E, and G (Fig. 3B). Only one serum sample each was available for subtypes F and H, and for group O, only four serum samples from two patients were available. Results for these six serum samples were therefore not plotted in the diagrams. The correlation coefficient (r) for the gag-based and LTR-based NASBA results for all sera together, including those not plotted, was 0.52 (n = 76; P < 0.0001). For the group containing the subtype B, C, and D viruses, a strong correlation could be found between the two assays (r = 0.87; P < 0.0001), but this correlation was not as strong for the group with subtype A, E, and G viruses (r = 0.29; P = 0.089). In total, 16 viruses of various subtypes of the M group (and the four O-group viruses) could not be detected by the gag-based NASBA. Of these, five were subtype A, one was subtype C, one was subtype D, two were subtype E, six were subtype G, and the remaining one was the only subtype H in our serum sample set; therefore, 13 of these 16 serum samples (81%) were in the group containing A, E, and G subtypes.
The LTR-based NASBA was unable to detect viral RNA in four serum samples. Two of these, one subtype A and one subtype D, were negative for viral RNA by both assays. The other two serum samples contained subtype B viruses, which could be detected by the gag-based NASBA only after the UltraSens protocol was applied. This result could indicate that for a limited number of subtype B isolates the detection limit of the gag-based NASBA is lower than that of the LTR-based NASBA. Finally, one serum sample (Fig. 3B) contained a subtype E virus and was positive by both assays; however, it was positive in the LTR-based NASBA only with the UltraSens protocol.
Analysis of mismatches in primer and probe regions.
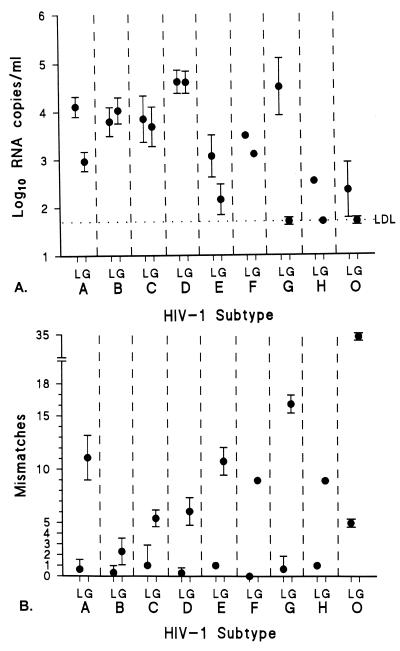
To explain the discrepancies in assessed viral RNA levels between the two assays, we sequenced the relevant LTR and gag regions and analyzed the number of mismatches for the primers and the probes. For analysis, insertions and deletions present only in the noncoding LTR region were counted as one mismatch each. For all samples, the number of mismatches for the primers and probes counted for the gag-based NASBA (range, 0 to 17; n = 71; mean, 7.85; median, 7) was significantly higher (P < 0.0001) than that counted for the LTR-based NASBA (range, 0 to 5; n = 64; mean, 0.61; median, 0). The means and standard deviations of the viral RNA levels (log10 copies per milliliter) for all samples per subtype, as assessed by the LTR-based and the gag-based NASBAs, are plotted in Fig. 4A. The means and standard deviations of the mismatches per subtype for the primers and probes of the LTR- and gag-based NASBAs are plotted in Fig. 4B. We found a significant inverse correlation between the number of mismatches and viral RNA levels detected for the gag-based NASBA (r = −0.78; P = 0.023) which was not present for the LTR-based NASBA. This finding implied that complete or partial assay failure was due to primer and probe mismatches. The mismatches resulted in assessments of significantly lower or absent viral RNA levels for subtype A and G and group O viruses by the gag-based assay. The difference in viral RNA level in the previously described subtype E serum sample, as estimated by the two assays, was not explainable by the number of mismatches for primers and probes for each assay.
FIG. 4.
(A) Mean log10 RNA level with standard deviation (error bars) for each HIV-1 subtype as assessed by the LTR-based NASBA (L) and the gag-based NASBA (G). LDL, lower detection limit. (B) Mean number of mismatches with standard deviation (error bars) for each subtype for the primers and probes of the LTR-based assay (L) and the gag-based assay (G).
DISCUSSION
Because various subtypes of HIV-1 are rapidly spreading around the world, HIV-1 RNA quantification assays that can detect all known subtypes of HIV-1 group M and group O viruses are required. We have developed a new NASBA-based assay that uses the conserved LTR region at the 5′ end of the genomic RNA. We have shown that the LTR-based assay is as good in standard subtype B quantification as the existing gag-based assay (NucliSens HIV-1 QT assay; Organon Teknika). The lower detection limits were similar for both assays. By comparing dilution series of a panel of group M subtype isolates from the WHO collection, we have shown that the LTR-based assay quantifies these viruses as efficiently as it does subtype B viruses. In contrast, the group O viruses were less efficiently quantified than group M viruses (i.e., the viral load was underestimated), probably due to mismatches in the capture probe. Adaptation of the capture probe to a group O matching sequence would most likely resolve this matter. By using serum samples for which the viral subtype was determined by phylogenetic analysis of the gag gene, we have shown that the two assays are similar in their ability to quantify subtypes B to F of HIV-1 group M. However, the LTR-based assay is better suited to quantify subtypes A and G of group M and the group O viruses, as well as the only group M, subtype H, virus in our serum sample set. The set also included some proven recombinant viruses (6), which were also detected and quantified by the LTR-based assay.
The most important improvement in the LTR-based NASBA, compared to the gag-based NASBA, is its decreased number of mismatches for primers and probes. We found a strong inverse correlation (r = −0.78; P = 0.023) between calculated viral RNA levels and the number of mismatches found for the gag-based NASBA. It can therefore be concluded that for subtype A and G viruses, as well as for group O viruses, the RNA levels as assessed by the gag-based NASBA will be underestimated or absent. For these viruses, the RNA levels will be detected with more efficiency and accuracy by the LTR-based NASBA.
It has been reported that the three most widely used commercially available assays, namely, NucliSens HIV-1 RNA, Amplicor HIV-1 Monitor, and Quantiplex HIV-1 RNA, are similar in sensitivity, accuracy, and reproducibility (7, 11, 30, 32, 37, 38) but that the Quantiplex HIV-1 RNA assay is slightly more effective for quantification of isolates of certain subtypes (7). Like the gag-based NucliSens assay, the gag-based Amplicor assay underestimates or cannot detect subtype G viruses (2, 8). Neither the gag-based NASBA, the Amplicor assay, nor the Quantiplex assay was able to detect and quantify group O viruses (12, 20, 29), but the LTR-based NASBA could.
A new group of HIV-1 viruses, the N group, was recently reported (33). This group is genetically different from groups M and O. Using a published genomic sequence (GenBank accession no. AJ006022) of a member (YBF30) of this group and analyzing the number of mismatches, we could speculate whether this virus might be detected with either the gag-based or LTR-based NASBA. Since 22 mismatches were present for the gag-based NASBA primers and probes and this number lies between those for subtype G and group O viruses, which are not detected, it is unlikely that the new group can be detected by the gag-based NASBA. With the LTR-based NASBA, however, only four mismatches were found. This is less than was found for group O viruses, so it seems likely that this assay can detect and quantify the new group N viruses, provided that their LTR sequence resembles that of their representative member, YBF30.
Our LTR-based NASBA would be of use for testing infants born of HIV-1-positive mothers. These infants cannot be diagnosed HIV-1 positive based on the presence of antibodies against HIV-1 antigens, because maternal HIV-1 antibodies are present in the serum of these infants. The presence of HIV-1 DNA or RNA must be detected directly in cellular material or serum from the infant to make the diagnosis. Improving the chance of detection by changing the amplification region from gag to the LTR will probably lead to prompt diagnosis of HIV-1 in infected infants, especially if the infants are infected with a subtype A or G or a group O virus. An additional advantage of the ultrasensitive NASBA format over the Quantiplex and the ultrasensitive Amplicor assay format is the smaller serum volume (200 versus 1,000 versus 500 μl, respectively) necessary to detect HIV-1 RNA with similar sensitivity.
Another application of the LTR-based NASBA could be the monitoring of patients receiving antiviral therapy. Decreased efficiency and accuracy in assessments of viral RNA levels could impact not only the start of therapy but also the judgment of treatment failure or success. Often, the decision to start highly active antiretroviral therapy is made on the basis of the viral RNA levels (19). If assay failure leads to a too-low estimation of viral RNA levels, such therapy could be delayed or omitted, putting the infected individual at an increased risk for developing AIDS (21, 22). If the gag-based NASBA is used to monitor the treatment of individuals infected with group M, subtype A or G, viruses or group O viruses, the viral RNA levels could be determined, too early, to be below the lower detection limit, falsely indicating therapy success. The viral RNA level will rise above the lower detection limit more slowly, causing an unnecessarily later switch to a new drug regimen when drug resistance does begin to appear.
In summary, compared with the gag-based NASBA, our LTR-based NASBA has improved capacities for quantification of the HIV-1 group M, subtype A, virus as well as for the detection and quantification of the subtype G and group O viruses. This assay is a major advancement in HIV diagnostics, affecting decision management for the start and monitoring of therapy and the diagnosis of HIV-1-infected infants.
ACKNOWLEDGMENTS
We thank Guido van der Groen and Wouter Janssens for providing sera from individuals infected with a group O virus; Wim van Est for artwork; Lucy Phillips for editorial review; and Margreet Bakker, Mariel Brok, Remco van den Burg, Marion Cornelissen, and Vladimir V. Lukashov from the Department of Human Retrovirology, Academic Medical Center, University of Amsterdam, and Pierre van Aarle and Paul van de Wiel from Organon Teknika B.V. for excellent technical assistance as well as helpful discussions.
This work was financially supported by Organon Teknika B.V.
REFERENCES
- 1.Alaeus A, Lidman K, Sonnerborg A, Albert J. Subtype-specific problems with quantification of plasma HIV-1 RNA. AIDS. 1997;11:859–865. doi: 10.1097/00002030-199707000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Arnold C, Barlow K L, Kaye S, Loveday C, Balfe P, Clewley J P. HIV type 1 sequence subtype G transmission from mother to infant: failure of variant sequence species to amplify in the Roche Amplicor Test. AIDS Res Hum Retrovir. 1995;11:999–1001. doi: 10.1089/aid.1995.11.999. [DOI] [PubMed] [Google Scholar]
- 3.Blackburn G F, Shah H P, Kenten J H, Leland J, Kamin R A, Link J, Peterman J, Powell M J, Shah A, Talley D B, Tyagi S K, Wilkins E, Wu T-G, Massey R J. Electrochemiluminescence detection for development of immunoassays and DNA probe assays for clinical diagnostics. Clin Chem. 1991;37:1534–1539. [PubMed] [Google Scholar]
- 4.Boom R, Sol C J A, Salimans M M M, Jansen C L, Wertheim-van Dillen P M E, van der Noordaa J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990;28:495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins M L, Irvine B, Tyner D, Fine E, Zayati C, Chang C, Horn T, Ahle D, Detmer J, Shen L P, Kolberg J, Bushnell S, Urdea M S, Ho D D. A branched DNA signal amplification assay for quantification of nucleic acid targets below 100 molecules/ml. Nucleic Acids Res. 1997;25:2979–2984. doi: 10.1093/nar/25.15.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cornelissen, M. Unpublished data.
- 7.Coste J, Montes B, Reynes J, Peeters M, Segarra C, Vendrell J P, Delaporte E, Segondy M. Comparative evaluation of three assays for the quantitation of human immunodeficiency virus type 1 RNA in plasma. J Med Virol. 1996;50:293–302. doi: 10.1002/(SICI)1096-9071(199612)50:4<293::AID-JMV3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 8.Debyser Z, Van Wijngaerden E, Van Laethem K, Beuselinck K, Reynders M, De Clercq E, Desmyter J, Vandamme A M. Failure to quantify viral load with two of the three commercial methods in a pregnant woman harboring an HIV type 1 subtype G strain. AIDS Res Hum Retrovir. 1998;14:453–459. doi: 10.1089/aid.1998.14.453. [DOI] [PubMed] [Google Scholar]
- 9.De Leys R, Vanderborght B, Vanden Haesevelde M, Heyndrickx L, van Geel A, Wauters C, Bernaerts R, Saman E, Nijs P, Willems B, Taelman H, van der Groen G, Piot P, Tersmette T, Huisman J G, Van Heuverswyn H. Isolation and partial characterization of an unusual human immunodeficiency retrovirus from two persons of West-Central African origin. J Virol. 1990;64:1207–1216. doi: 10.1128/jvi.64.3.1207-1216.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Wolf F, Spijkerman I, Schellekens P T, Langendam M, Kuiken C, Bakker M, Roos M, Coutinho R, Miedema F, Goudsmit J. AIDS prognosis based on HIV-1 RNA, CD4+ T-cell count and function: markers with reciprocal predictive value over time after seroconversion. AIDS. 1997;11:1799–1806. doi: 10.1097/00002030-199715000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Dyer J R, Gilliam B L, Eron J J, Jr, Grosso L, Cohen M S, Fiscus S A. Quantitation of human immunodeficiency virus type 1 RNA in cell free seminal plasma: comparison of NASBA with Amplicor reverse transcription-PCR amplification and correlation with quantitative culture. J Virol Methods. 1996;60:161–170. doi: 10.1016/0166-0934(96)02063-0. [DOI] [PubMed] [Google Scholar]
- 12.Gobbers E, Fransen K, Oosterlaken T, Janssens W, Heyndrickx L, Ivens T, Vereecken K, Schoones R, van de Wiel P, van der Groen G. Reactivity and amplification efficiency of the NASBA HIV-1 RNA amplification system with regard to different HIV-1 subtypes. J Virol Methods. 1997;66:293–301. doi: 10.1016/s0166-0934(97)00072-4. [DOI] [PubMed] [Google Scholar]
- 13.Jurriaans S, Van Gemen B, Weverling G J, Van Strijp D, Nara P, Coutinho R, Koot M, Schuitemaker H, Goudsmit J. The natural history of HIV-1 infection: virus load and virus phenotype independent determinants of clinical course? Virology. 1994;204:223–233. doi: 10.1006/viro.1994.1526. [DOI] [PubMed] [Google Scholar]
- 14.Kappes J C, Saag M S, Shaw G M, Hahn B H, Chopra P, Chen S, Emini E A, McFarland R, Yang L C, Piatak M, Jr, Lifson J D. Assessment of antiretroviral therapy by plasma viral load testing: standard and ICD HIV-1 p24 antigen and viral RNA (QC-PCR) assays compared. J Acquired Immune Defic Syndr Hum Retrovirol. 1995;10:139–149. doi: 10.1097/00042560-199510020-00005. [DOI] [PubMed] [Google Scholar]
- 15.Kievits T, Van Gemen B, Van Strijp D, Schukkink R, Dircks M, Adriaanse H, Malek L, Sooknanan R, Lens P. NASBA isothermal enzymatic in vitro nucleic acid amplification optimized for the diagnosis of HIV-1 infection. J Virol Methods. 1991;35:273–286. doi: 10.1016/0166-0934(91)90069-c. [DOI] [PubMed] [Google Scholar]
- 16.Kimura M. A simple method for estimating evolutionary rates of base substitution through comparative studies of nucleotide sequences. J Mol Evol. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- 17.Korber B, Foley B, Leitner T, McCutchan F, Hahn B, Mellors J W, Myers G, Kuiken C. Human retroviruses and AIDS 1997. A compilation and analysis of nucleic acids and amino acid sequences. Theoretical Biology and Biophysics Group T-10. N.Mex: Los Alamos; 1997. [Google Scholar]
- 18.Korber B T, Osmanov S, Esparza J, Myers G WHO Network for HIV Isolation and Characterization. The World Health Organization Global Programme on AIDS proposal for standardization of HIV sequence nomenclature. AIDS Res Hum Retrovir. 1994;10:1355–1358. doi: 10.1089/aid.1994.10.1355. [DOI] [PubMed] [Google Scholar]
- 19.Layne S P, Merges M J, Dembo M, Spouge J L, Conley S R, Moore J P, Raina J L, Renz H, Gelderblom H R, Nara P L. Factors underlying spontaneous inactivation and susceptibility to neutralization of human immunodeficiency virus. Virology. 1992;189:695–714. doi: 10.1016/0042-6822(92)90593-e. [DOI] [PubMed] [Google Scholar]
- 20.Loussert-Ajaka I, Descamps D, Simon F, Brun-Vezinet F, Ekwalanga M, Saragosti S. Genetic diversity and HIV detection by polymerase chain reaction. Lancet. 1995;346:912–913. doi: 10.1016/s0140-6736(95)92762-x. [DOI] [PubMed] [Google Scholar]
- 21.Mellors J W, Kingsley L A, Rinaldo C R, Jr, Todd J A, Hoo B S, Kokka R P, Gupta P. Quantitation of HIV-1 RNA in plasma predicts outcome after seroconversion. Ann Intern Med. 1995;122:573–579. doi: 10.7326/0003-4819-122-8-199504150-00003. [DOI] [PubMed] [Google Scholar]
- 22.Mellors J W, Rinaldo C R, Jr, Gupta P, White R M, Todd J A, Kingsley L A. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996;272:1167–1170. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- 23.Mulder J, McKinney N, Christopherson C, Sninsky J, Greenfield L, Kwok S. Rapid and simple PCR assay for quantitation of human immunodeficiency virus type 1 RNA in plasma: application to acute retroviral infection. J Clin Microbiol. 1994;32:292–300. doi: 10.1128/jcm.32.2.292-300.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nkengasong J N, Kalou M, Maurice C, Bile C, Borget M-Y, Koblavi S, Boateng E, Sassan-Morokro M, Anatole-Ehounou E, Ghys P, Greenberg A E, Wiktor S Z. Comparison of NucliSens and Amplicor Monitor assays for quantification of human immunodeficiency virus type 1 (HIV-1) RNA in plasma of persons with HIV-1 subtype A infection in Abidjan, Côte d’Ivoire. J Clin Microbiol. 1998;36:2495–2498. doi: 10.1128/jcm.36.9.2495-2498.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nolte F S, Boysza J, Thurmond C, Clark W S, Lennox J L. Clinical comparison of an enhanced-sensitivity branched-DNA assay and reverse transcription-PCR for quantitation of human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol. 1998;36:716–720. doi: 10.1128/jcm.36.3.716-720.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Brien W A, Hartigan P M, Martin D, Esinhart J, Hill A, Benoit S, Rubin M, Simberkoff M S, Hamilton J D. Changes in plasma HIV-1 RNA and CD4+ lymphocyte counts and the risk of progression to AIDS. Veterans Affairs Cooperative Study Group on AIDS. N Engl J Med. 1996;334:426–431. doi: 10.1056/NEJM199602153340703. [DOI] [PubMed] [Google Scholar]
- 27.Pachl C, Todd J A, Kern D G, Sheridan P J, Fong S J, Stempien M, Hoo B, Besemer D, Yeghiazarian T, Irvine B, Kolberg J, Kokka R, Neuwald P, Urdea M S. Rapid and precise quantification of HIV-1 RNA in plasma using a branched DNA signal amplification assay. J Acquired Immune Defic Syndr Hum Retrovirol. 1995;8:446–454. doi: 10.1097/00042560-199504120-00003. [DOI] [PubMed] [Google Scholar]
- 28.Perelson A S, Essunger P, Cao Y, Vesanen M, Hurley A, Saksela K, Markowitz M, Ho D D. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature. 1997;387:188–191. doi: 10.1038/387188a0. [DOI] [PubMed] [Google Scholar]
- 29.Respess R A, Butcher A, Wang H, Chaowanachan T, Young N, Shaffer N, Mastro T D, Biryahwaho B, Downing R, Tanuri A, Schechter M, Pascu R, Zekeng L, Kaptué L, Gürtler L, Eberle J, Ellenberger D, Fridlund C, Rayfield M, Kwok S. Detection of genetically diverse human immunodeficiency virus type 1 group M and O isolates by PCR. J Clin Microbiol. 1997;35:1284–1286. doi: 10.1128/jcm.35.5.1284-1286.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Revets H, Marissens D, De Wit S, Lacor P, Clumeck N, Lauwers S, Zissis G. Comparative evaluation of NASBA HIV-1 RNA QT, AMPLICOR-HIV Monitor, and QUANTIPLEX HIV RNA assay, three methods for quantification of human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol. 1996;34:1058–1064. doi: 10.1128/jcm.34.5.1058-1064.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saag M S, Holodniy M, Kuritzkes D R, O’Brien W A, Coombs R, Poscher M E, Jacobsen D M, Shaw G M, Richman D D, Volberding P A. HIV viral load markers in clinical practice. Nat Med. 1996;2:625–629. doi: 10.1038/nm0696-625. [DOI] [PubMed] [Google Scholar]
- 32.Schuurman R, Descamps D, Weverling G J, Kaye S, Tijnagel J, Williams I, van Leeuwen R, Tedder R, Boucher C A B, Brun-Vezinet F, Loveday C. Multicenter comparison of three commercial methods for quantification of human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol. 1996;34:3016–3022. doi: 10.1128/jcm.34.12.3016-3022.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simon F, Mauclere P, Roques P, Loussert-Ajaka I, Muller-Trutwin M C, Saragosti S, Georges-Courbot M C, Barre-Sinoussi F, Brun-Vezinet F. Identification of a new human immunodeficiency virus type 1 distinct from group M and group O. Nat Med. 1998;4:1032–1037. doi: 10.1038/2017. [DOI] [PubMed] [Google Scholar]
- 34.Spijkerman I J, Prins M, Goudsmit J, Veugelers P J, Coutinho R A, Miedema F, de Wolf F. Early and late HIV-1 RNA level and its association with other markers and disease progression in long-term AIDS-free homosexual men. AIDS. 1997;11:1383–1388. doi: 10.1097/00002030-199711000-00013. [DOI] [PubMed] [Google Scholar]
- 35.Todd J, Pachl C, White R, Yeghiazarian T, Johnson P, Taylor B, Holodniy M, Kern D, Hamren S, Chernoff D, Urdea M. Performance characteristics for the quantitation of plasma HIV-1 RNA using branched DNA signal amplification technology. J Acquired Immune Defic Syndr Hum Retrovirol. 1995;10(Suppl.):S35–S44. [PubMed] [Google Scholar]
- 36.Urdea M S, Wilber J C, Yeghiazarian T, Todd J A, Kern D G, Fong S J, Besemer D, Hoo B, Sheridan P J, Kokka R, Neuwald P, Pachl C. Direct and quantitative detection of HIV-1 RNA in human plasma with a branched DNA signal amplification assay. AIDS. 1993;7(Suppl.):S11–S14. doi: 10.1097/00002030-199311002-00004. [DOI] [PubMed] [Google Scholar]
- 37.Vandamme A M, Schmit J C, Van Dooren S, Van Laethem K, Gobbers E, Kok W, Goubau P, Witvrouw M, Peetermans W, De Clercq E, Desmyter J. Quantification of HIV-1 RNA in plasma: comparable results with the NASBA HIV-1 RNA QT and the AMPLICOR HIV monitor test. J Acquired Immune Defic Syndr Hum Retrovirol. 1996;13:127–139. doi: 10.1097/00042560-199610010-00003. [DOI] [PubMed] [Google Scholar]
- 38.Vandamme A M, Van Dooren S, Kok W, Goubau P, Fransen K, Kievits T, Schmit J C, De Clercq E, Desmyter J. Detection of HIV-1 RNA in plasma and serum samples using the NASBA amplification system compared to RNA-PCR. J Virol Methods. 1995;52:121–132. doi: 10.1016/0166-0934(94)00151-6. [DOI] [PubMed] [Google Scholar]
- 39.Van de Peer Y, De Wachter R. TREECON for Windows: a software package for the construction and drawing of evolutionary trees for the Microsoft Windows environment. Comput Appl Biosci. 1994;10:569–570. doi: 10.1093/bioinformatics/10.5.569. [DOI] [PubMed] [Google Scholar]
- 40.Van Gemen B, Kievits T, Nara P, Huisman H G, Jurriaans S, Goudsmit J, Lens P. Qualitative and quantitative detection of HIV-1 RNA by nucleic acid sequence-based amplification. AIDS. 1993;7(Suppl.):S107–S110. doi: 10.1097/00002030-199311002-00020. [DOI] [PubMed] [Google Scholar]
- 41.Van Gemen B, Kievits T, Schukkink R, Van Strijp D, Malek L T, Sooknanan R, Huisman H G, Lens P. Quantification of HIV-1 RNA in plasma using NASBA during HIV-1 primary infection. J Virol Methods. 1993;43:177–187. doi: 10.1016/0166-0934(93)90075-3. [DOI] [PubMed] [Google Scholar]
- 42.Van Gemen B, van Beuningen R, Nabbe A, Van Strijp D, Jurriaans S, Lens P, Kievits T. A one-tube quantitative HIV-1 RNA NASBA nucleic acid amplification assay using electrochemiluminescent (ECL) labelled probes. J Virol Methods. 1994;49:157–167. doi: 10.1016/0166-0934(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 43.Wei X, Ghosh S K, Taylor M E, Johnson V A, Emini E A, Deutsch P, Lifson J D, Bonhoeffer S, Nowak M A, Hahn B H, Saag M S, Shaw G M. Viral dynamics in human immunodeficiency virus type 1 infection. Nature. 1995;373:117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- 44.Weverling G J, Lange J M A, Jurriaans S, Prins J M, Lukashov V V, Notermans D W, Roos M, Schuitemaker H, Hoetelmans R M W, Danner S A, Goudsmit J, de Wolf F. Alternative multidrug regimen provides improved suppression of HIV-1 replication over triple therapy. AIDS. 1998;12:F117–F122. doi: 10.1097/00002030-199811000-00003. [DOI] [PubMed] [Google Scholar]
- 45.WHO Network for HIV Isolation and Characterization. HIV type 1 variation in World Health Organization-sponsored vaccine evaluation sites: genetic screening, sequence analysis and preliminary biological characterization of selected viral strains. AIDS Res Hum Retrovir. 1994;11:1327–1343. doi: 10.1089/aid.1994.10.1327. [DOI] [PubMed] [Google Scholar]
- 46.Wilber J C. Branched DNA for quantification of viral load. Immunol Investig. 1997;26:9–13. doi: 10.3109/08820139709048911. [DOI] [PubMed] [Google Scholar]
- 47.Zaaijer H L, Kok W, ten Veen J H, Reesink H W, Foolen H, Winkel I N, Huisman J G, Cuypers H T, Kievits T, Lelie P N. Detection of HIV-1 RNA in plasma by isothermal amplification (NASBA) irrespective of the stage of HIV-1 infection. J Virol Methods. 1995;52:175–181. doi: 10.1016/0166-0934(94)00160-i. [DOI] [PubMed] [Google Scholar]