Abstract

This paper describes a conceptual design of hierarchical composite hydrogels. The hydrogel materials comprise MoS2 flakes and interpenetrating polymer networks, and further exhibit controlled release and tunable strength that are caused by the synergistic combination of select components. In terms of design, MoS2 flakes initiate radical polymerization of chosen monomers and simultaneously provide physical cross-linking points, both of which afford a primary composite network. Then, the sequential formation of additional networks results in functional, hierarchical, composite hydrogels. Therefore, we were able to demonstrate double-network hydrogels as a stimuli-responsive vector for programmed release of cargo molecules in response to heat or light or to form triple-network hydrogels showing tunable mechanical strength owing to intermolecular interaction between charged monomers and MoS2 flakes. The design concept would be expanded by incorporating other chalcogenides or functional monomers, which advance the properties and functionalities of materials and broadens the versatility of nanocomposite hydrogels.
Introduction
Polymer nanocomposites contain diverse nanomaterials in viscoelastic polymer matrices, exhibiting advanced performance far exceeding the performance of conventional polymers. In particular, the inclusion of a considerable amount of water results in the formation of nanocomposite hydrogels that exhibit unique physicochemical properties and are adaptable for numerous applications.1−3 The composite hydrogels have been known to exhibit designed functionality while maintaining general properties of hydrogels since the pioneering work by Haraguchi et al.,4,5 and thus, have provided synthetic platforms for functional systems. Therefore, composite hydrogels have been widely used in agricultural as well as biomedical fields, and currently, they give rise to high-profile materials for sustainable applications.6,7
From the synthetic perspective of the composite hydrogels, multidimensional colloidal nanomaterials are required to be uniformly dispersed—on a macroscopic scale—in the continuous phase of hydrophilic polymers including biopolymers (e.g., polysaccharides and polynucleotides), synthetic polymers (e.g., poly(ethylene glycol) (PEG), poly(acrylamide) (PAAm), and poly(vinyl alcohol) (PVA)) and polyelectrolytes. Then, exogenous components are embedded during gelation in situ or introduced after gelation8 and also can provide a focal point to induce noncovalent or covalent cross-linking of the polymer chains,9 which limits the mobility of the chains and forms networks that hold water. As a result, the hybrid materials not only maintain their bulk structures as composite hydrogels but also exhibit enhanced mechanical properties or synergistic functions, which are sophisticatedly designed by a rational combination for the composites.10−13
A variety of nanocomponents are extensively prepared from metals, clays, or carbon allotropes, and transition metal dichalcogenides (TMDs) have been also used for composite hydrogels.14 Among the chalcogenides, molybdenum disulfide (MoS2) has received great attention due to tunable band gap, weak out-of-plane interaction, and synthetic flexibility.15−17 When forming composite hydrogels, the sulfur-rich flakes are typically incorporated during gelation and play a pivotal role in providing cross-linking points; this not only reinforces hydrogel networks but also communicates their optical and electronic properties to the networks. Therefore, the resultant materials could be obtained as soft yet durable monoliths that exhibit specific functions, for example, catalytic activity, heavy metal removal, or light-sensitive behavior.18−24 However, fledgling efforts to explore the potential of composite hydrogels containing MoS2 have been made so far, when considering (i) many polymeric materials that can form hydrogels, (ii) diverse structures of hydrogels such as topological hydrogels or supramolecular hydrogels, or (iii) existing additive processing techniques. Moreover, a suitable combination of the point aforementioned would achieve highly sought-after applications in energy and catalysis fields, which causes growing interest in MoS2-based materials.
In this conceptual study, we newly demonstrate the design of MoS2-containing hierarchical composite hydrogels. For this purpose, we incorporated MoS2 flakes into interpenetrating network hydrogels. This class of hydrogels consists of individual networks that provide specific functions25 or exhibit remarkable mechanical properties as notably researched by Gong et al.26 Additionally, orthogonal control of each network in the integrated system induces desired macroscopic responses of materials, circumventing a devastating collapse or degeneration of a whole structure.27−30 Herein, we create a primary hydrogel network due to the multifunctional role of MoS2 and further develop sequential hierarchical structures. Therefore, the resulting functional materials not only showed intrinsic durability but also enabled controlled responses that arise from the cooperative combination of select polymer networks and the MoS2 flakes.
Results and Discussion
Figure 1 illustrates the synthesis procedure for functional, hierarchical, and composite hydrogels. First, we dispersed MoS2 (2H phase; diameter, <2 μm) by sonication under aqueous conditions in the presence of N,N-dimethylacrylamide (DMA) and then used the exfoliated nanoflakes to form single-network composite hydrogels (SNCHs) following the previous method.31 The MoS2 flakes induce polymerization of the monomer after redox radical initiation with potassium persulfate (KPS) and provide physical cross-linking points for growing polymer chains, which leads to DMA-based SNCHs without using a chemical cross-linker. Owing to the amphiphilicity of DMA and the nature of cross-linking in the network, the resulting SNCHs can well absorb various solvents and cohere with other monomers, which then allows the formation of double-network composite hydrogels (DNCHs). The amalgamation of additional networks results in integrated functions and alters the material properties of the composite hydrogels. For example, while being associated with the embedded MoS2, we were able to form double- or triple-network structures and also achieved the controlled release of cargo molecules as manipulated by light or heat for the proof of concept. Likewise, further inclusion of other monomers or additives would improve this conceptual design for the development of advanced composite hydrogels.
Figure 1.

Schematic description of the synthesis of MoS2-containing hierarchical composite hydrogels. Single-network composite hydrogels (SNCHs) are formed from N,N-dimethylacrylamide (DMA) initiated by the redox reaction of MoS2 flakes and persulfates in the absence of a chemical cross-linker. Sequential introduction of the second network causes double-network composite hydrogels (DNCHs).
The content of MoS2 flakes significantly affects the mechanical strength of the first network. Figure 2a shows changes in the strength of the DMA-based SNCHs. As more MoS2 was embedded, the resultant DMA networks became sturdier up to 0.8 wt %. The average Young’s modulus at the concentration was measured to be 9.6 ± 0.2 kPa, which is similar to that of the tissues.32 The modulus sharply decreased when exceeding 0.8 wt % MoS2. It is considered that undesired restacking of the inorganic nanoplatelets would occur during polymerization, which lowered the overall strength of networks.33 Thus, we prepared an elastic yet sturdy material at 0.8 wt % MoS2 and investigated swelling behavior, as shown in Figure 2b. The resultant SNCH absorbed water and N,N-dimethylformamide (DMF) as well and exhibited swelling degrees of 30 and 20%, respectively, after 200 min immersion, which is essential for the sufficient inclusion of additional monomers for establishing hierarchical structures.30 For comparison, we prepared a control network using acrylamide (AM) at the same concentration of MoS2 but obtained a stiff, brittle monolith. The AM network absorbed only small amounts of water, exhibiting a swelling degree of 15%, and could not be wet with DMF (Figure S1a). These results support that a monomer structure not only determines the appropriate contents of MoS2 for hydrogels but also alters macroscopic properties of hydrogels and further encourages us to explore the potential ability of SNCHs to design hierarchical networks.
Figure 2.
(a) Change in the elastic moduli of SNCHs as the MoS2 content varies, measured in a compressive mode. (b) Swelling properties of SNCH (MoS2, 0.8 wt %) when immersed in water (blue) or DMF (gray). (c) Compressive stress–strain curve of SNCH (gray) and that after inclusion of the NIPAM (N-isopropylacrylamide) network (i.e., DNCH; sky blue). The inset depicts the preparation of DNCH from SNCH. (d) Swelling properties of DNCH with the NIPAM network when measured in water (blue) or DMF (gray). The inset shows the LCST behavior of DNCH (solid) and a sole NIPAM network (open). (e, f) Morphological studies on the composite hydrogels. (e) SEM images of dried networks from SNCH (top) and DNCH (bottom) after lyophilization. (f) XPS spectra for Mo 3d core level peaks (2H phase, orange; 1T phase, pink) obtained from SNCH (bottom) and DNCH (top).
We have demonstrated sequential inclusion of the second network after immersion in a DMF solution of N-isopropylacrylamide (NIPAM) (2 M) for 48 h, followed by photopolymerization in the presence of trace amounts of N,N′-methylenebisacrylamide (MBAm) and a photoinitiator. In fact, it is difficult to form MoS2-containing NIPAM-based composite hydrogels under aqueous conditions due to the coil-to-globule transition of poly(NIPAM) that appears above the lower critical solution temperature (LCST; around 32 °C). We presume that the instant local heating generated during gelation was sufficient to induce the volumetric collapse in water, which hampers the formation of uniform networks. However, the amphiphilicity of SNCH allowed the formation of the NIPAM network from DMF solution followed by UV irradiation. Subsequent solvent exchange with water for 48 h at 25 °C resulted in DNCH from the SNCH precursor, as shown in the inset of Figure 2c.
The change in the mechanical strength of materials was investigated in Figure 2c. The elastic modulus of DNCH increased by a factor of ∼1.4 as compared to the modulus of SNCH (i.e., 12.8 ± 1.5 kPa), indicating the interpenetration of the poly(NIPAM) network. The resulting hydrogel was found to swell less in water than the initial network, attributed to the increased cross-linking density, which is 260% larger than that of SNCH (37.42 mol L–1 in SNCH and 96.33 mol L–1 in DNCH at 25 °C). Moreover, the dangling isopropyl groups in the NIPAM units more enhanced the wettability of DNCH toward DMF than water, so the hydrogel rapidly absorbed the same amount of DMF as water (Figure 2d). We further estimated the absorption rates of the materials when immersed in DMF and water following a pseudo-second-order kinetic model.34 The rate constants of SNCH were found to be 0.119 ± 0.019 and 0.074 ± 0.011 g g–1 min–1 in DMF and water, respectively. Meanwhile, DNCH showed a twice higher absorption rate in DMF than in water (0.104 ± 0.002 and 0.052 ± 0.004 g g–1 min–1, each), which is similar to that of SNCH measured in DMF. Linear regression for the estimation is presented in Figure S1b,c. DNCH exhibited thermally reversible LCST behavior, as designed. Thus, when temperature increased, the shrinkage of the network was induced, which was translated into the change in transmittance of DNCH (the inset of Figure 2d). We found a sharp decrease around 38 °C; as a result, the DNCH turned opaque. The LCST of DNCH is higher than the control NIPAM-based hydrogel prepared without MoS2 (32 °C, open circles in the inset), which confirms a bit delayed transition of the second NIPAM network that can be held up by the first DMA network in DNCH.29,30
The formation of the interpenetrating NIPAM network was analyzed by Fourier transform infrared spectroscopy (FTIR) (Figure S2). The control NIPAM hydrogel containing amide bonds exhibited carbonyl stretching at 1640 cm–1 and N–H bending at 1526 cm–1, which were not found in SNCH. In contrast, DNCH showed a merged spectrum, corroborating the presence of both polymer networks. Further, thermogravimetric analyses showed that the dried networks of both materials are thermally stable until 400 °C. The char yield of the network of SNCH was measured as low as 8%, which is slightly larger than that of DNCH or the control NIPAM hydrogel attributed to the combustible NIPAM network (thermograms, Figure S3).
The change in the global morphology of hydrogels was observed using a scanning electron microscope (SEM) (Figure 2e). The dried network of SNCH after lyophilization formed large pores with diameters in the range of 20–85 μm, which was found to decrease to 15–22 μm with a more uniform size distribution after the inclusion of the NIPAM network in DNCH. We confirmed that pore diameters of the materials are quite similar as found in MoS2-containing hydrogels previously reported,19,20,22 and they are also considered large enough (i.e., >10 μm) for rapid solvent exchange or molecular uptake.35 For comparison, the control NIPAM hydrogel exhibited pore diameters ranging from 30 to 50 μm, as shown in Figure S4.
We also investigated the microscopic morphology of the embedded MoS2 flakes using X-ray photoelectron spectroscopy (XPS). The flake we used predominantly comprises a semiconducting 2H phase together with negligible amounts of a metallic 1T phase; thus, we observed Mo 3d peaks at binding energies of 233.3 (3d3/2) and 230.3 eV (3d5/2) for the 2H phase with tiny peaks at 231.9 (Mo 3d3/2) and 228.4 eV (Mo 3d5/2) for the 1T phase (Figure S5). After redox-initiated radical polymerization, an increase in the 1T phase was found (Figure 2f). We conjecture that radical generation followed by polymerization would induce atomic plane gliding in the embedded MoS2 and result in the phase transition to the metallic phase.31,36,37 Therefore, we found 19% 1T phase development in SNCH, which further increased to 30% in DNCH (both bottom and top plots in Figure 2f), probably due to interior distortion imposed by the second NIPAM network.
Taking advantage of material properties in DNCH, we were able to exploit the material as a stimuli-responsive polymeric carrier. It was deemed that the large, interconnected pores of DNCH could facilitate internal mass transport when loading cargo molecules (i.e., rhodamine 6G); then, the model dye could be physically and reversibly captured in the hydrogel network owing to noncovalent interactions such as multitopic hydrogen bonds or electrostatic forces. Further, the material enables controlled release of the loaded molecules due to (i) the embedded MoS2 flakes that absorb near-infrared (NIR) light38,39 and (ii) the NIPAM network that shows thermoresponsive phase transition (Figure 3a). For this purpose, we immersed DNCH (0.67 g; 3 cm × 1 cm × 0.3 cm) in a solution of rhodamine 6G in DI water (0.03 mM) for 24 h at 25 °C, and obtained the loading amount of 0.2 mg g–1 in DNCH. Subsequently, we estimated the release pattern of the loaded DNCH when immersed in DI water (3 mL) under various conditions.
Figure 3.
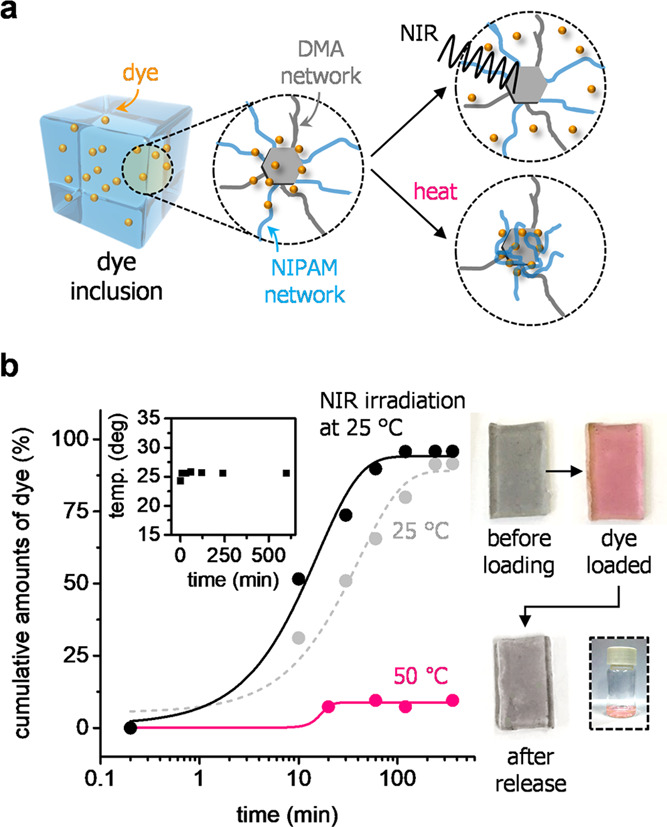
(a, b) Controlled release of DNCHs. (a) Inclusion of dye (rhodamine 6G; orange) in DNCH and controlled release under near-infrared (NIR) light irradiation (up) and when heated (down) are depicted. (b) Change in the release pattern of the dye molecules when irradiated at 850 nm (black) and heated at 50 °C (pink). The release pattern at 25 °C without irradiation is shown for comparison (gray). Photographs of DNCHs before and after loading the dye, the gel after release, and the unloaded dye molecules in the vial are shown in the inset.
The loaded dye was gradually released at 25 °C without any stimulus as time elapsed. We found that most of the dye molecules (∼90%) were unloaded from DNCH when soaked in DI water for 360 min (gray, Figure 3b). The release behavior was promoted adequately when being irradiated at 850 nm; thus, 95% of dye was released within 120 min (black, Figure 3b). We deem that the NIR absorption capability of the MoS2 flakes induced local heating and/or thermal vibration,40−45 which stimulates the release of the dye as compared with the case without irradiation. Meanwhile, the overall temperature of the DNCH sample rarely varied. Consequently, we found minor changes in temperature during irradiation, as shown in the inset of Figure 3b. The low MoS2 content in the interpenetrating structure would generate local heating, yet be insufficient for inducing the volumetric phase transition of the NIPAM network. On the other hand, global heating of the entire networks caused significant changes in the release pattern of DNCH; the volumetric collapse of the NIPAM network dramatically retarded the release of dye at 50 °C, and limited amounts of dye molecules (<10%) barely escaped from the entire material over 360 min (pink, Figure 3b). The obtained absorption spectra for dye molecules during the release tests are shown in Figure S6.
The controlled release behavior was further estimated using the Higuchi kinetic model expressed as follows
where Q, KH, and t indicate the cumulative percentage of the dye, Higuchi rate constant, and time, respectively.46 The obtained linear slopes reveal the Fickian release of dye molecules from DNCH, as shown in Figure S8. The rate constant of DNCH at 25 °C was found to be 8.00 min–0.5, which could be 1.58 times increased under NIR irradiation or 6.35 times reduced when heated over the LSCT temperature (KH: 12.63 and 1.26 min–0.5 respectively). The representative photographs for the DNCH before loading, after loading, and after release are shown on the right of Figure 3b. The sample was slightly gray when prepared owing to the MoS2 flakes being embedded but became slightly pink after loading the dye. After soaking in water, we were able to observe a gray sample and a pink aqueous solution, which supports the capability of DNCHs to manage the programmed release of cargo molecules. In addition, we found that other common dyes such as methylene blue or methyl orange could be loaded with loading capacities of 0.9 and 2.0 mg g–1 and then released spontaneously at 25 °C in DI water (Figure S7), demonstrating the feasibility and versatility of the materials.
We further employed DNCH as a reaction template for the formation of the third network. Herein, we used 2-acrylamido-2-methyl-1-propanesulfonic acid (AMPS) as the third monomer, which afforded triple-network composite hydrogels (TNCHs) in the same manner as used for DNCH. AMPS is highly soluble in water due to a polar sulfonate group and has been widely used for coating layers, membranes, or smart hydrogels.47,48 We immersed DNCH in 2 M AMPS in water and then polymerized it to form TNCHs, as described in Figure 4a. The resultant material exhibited an open-cell morphology when lyophilized by the SEM measurement (Figure 4b). Furthermore, we investigated the microstructure of the embedded MoS2 and observed that 25% of the metallic phase remained as observed by the XPS measurement (Figure 4c). The swelling behavior of TNCH was measured in DMF and water (Figure 4d). The material sparsely absorbed DMF but absorbed twice larger amounts of water more slowly when compared with DNCH. The absorption rate constant measured 0.001 ± 0.001 g g–1 min–1 as shown in Figure S9. We presume that the inclusion of the negatively charged, AMPS network could increase the overall hydrophilicity of TNCH; concomitantly, the increased concentration of polymer networks in the hydrogel could reduce the absorption rate of water. The incorporated NIPAM network also caused the LCST behavior of TNCH (Figure 4e). Thus, we could confirm the reversible change in transmittance around a temperature of 26 °C, which is lower than that of DNCH. We presume that interchain interaction in the NIPAM network could be more enhanced after the addition of the hydrophilic AMPS network, leading to a more favorable phase transition of TNCH as temperature increased. Meanwhile, the inclusion of the AMPS network remarkably enhanced the mechanical strength of TNCH; the elastic modulus of TNCH was obtained as 70.3 ± 13.4 kPa, which is 5.5 times higher than that of DNCH (gray, Figure 4f), and similar to those for gelatins or synthetic rubbers.32 Furthermore, owing to the NIPAM network, we were able to observe an increase in the modulus of TNCH above the LCST (i.e., 82.6 ± 1.7 kPa), almost 5 times higher than that of DNCH above the LCST (i.e., 17.8 ± 0.5 kPa), which corroborate the thermal control of mechanical strengths of the composite hydrogels (pink, Figure 4f). AMPS is quite efficient to form a MoS2-containing double-network hydrogel. For instance, when preparing a control double-network hydrogel with AMPS instead of NIPAM, we were able to obtain a modulus of 55.3 ± 12.6 kPa. This value is slightly lower than TNCH but much higher than that of the DNCH with NIPAM. On the other hand, we incorporated methacroylcholine chloride (MC) that contains a quaternary ammonium group for the formation of another control double-network hydrogel. The monomer is also hydrophilic but caused a weak hydrogel material from SNCH, and it exhibited a low elastic modulus of 4.8 ± 0.1 kPa. We reason that the positively charged MC molecules are not considered to be uniformly dispersed in SNCH in contrast to the cases of AMPS or neutral NIPAM monomers, given that the pristine MoS2 flakes showed a ζ potential of −22.2 mV (Figure S10). Internal ionic interactions would induce potential coalescence of propagating polymer chains around the MoS2 flakes and hinder the formation of the interpenetrating structure.49−52 We further tested a 1:1 mixture of AMPS and MC but found a weak double-network hydrogel with a low modulus of 4.0 ± 1.4 kPa, expectedly (gray patterned, Figure 4f). Moreover, the addition of the NIPAM network into the control double network from AMPS resulted in another control network that has a similar structure to TNCH conceivably but it exhibited only a modulus of 22.6 ± 4.0 MPa which would suffer from the discordant formation of the third network (orange patterned, Figure 4f). These control samples only caused negligible effects; nonetheless, the implication here is that a judicious combination of monomers and their addition sequence can manipulate structural hierarchy and alter properties of the MoS2-based polyelectrolyte hydrogels. Initial regions of the measured compressive data for TNCH and other control networks are shown in Figure S11.
Figure 4.
Formation of triple-network composite hydrogels (TNCHs). (a) Illustration of the inclusion of the third monomer (violet) in DNCH and the resultant TNCH. (b) SEM image of TNCH using 2-acrylamido-2-methyl-1-propanesulfonic acid (AMPS) after lyophilization. The sample was measured at a magnification of 500×. (c) XPS spectra for Mo 3d core level peaks obtained from TNCH using AMPS. After deconvolution, the 2H phase was mainly observed (orange) together with tiny amounts of 1T phase (pink). (d) Swelling properties of TNCH when soaked in water (blue) or DMF (gray). (e) LCST behavior of TNCH (purple). The data points from DNCH are shown for comparison (sky blue). (f) (left) Change in the elastic modulus of hierarchical hydrogels as embedded with NIPAM and AMPS in sequence (gray). The moduli of DNCH and TNCH when measured above LCST (pink). (right) The moduli of control hydrogels are shown for comparison. *Control double-network hydrogels prepared with AMPS, methacroylcholine chloride (MC), or a 1:1 mixture of both, instead of NIPAM (gray, patterned). **Control triple-network hydrogel prepared via a reverse sequence (orange, patterned). Chemical structures of AMPS and MC are shown on the right.
Conclusions
In summary, we demonstrated the hierarchical design and structural engineering of MoS2-containing composite hydrogels. The inorganic flakes played a pivotal role in the facile formation of the hydrogel networks since they not only initiated redox polymerization but also induced cross-linking. Subsequently, the resultant single-network composite hydrogels are amphiphilic, swellable, and tangible, which provides a reaction template to form functional, hierarchical, composite hydrogels. For the proof of concept, we achieved the design of double-network hydrogels that shows the programmed release of dye molecules taking advantage of the MoS2 flakes and the second NIPAM network. Thus, the release pattern could be promoted under NIR irradiation or suppressed by heating. The addition of the AMPS network caused triple-network hydrogels that show high mechanical strength in conjunction with the MoS2 flakes. In this study, we merged the common polymer networks and formed the resulting hierarchical structures, which would be further developed for hydrogel-based sensory systems that are responsive to heat or light. Also, the embedment of other designed monomers or additives in the future would pave the way for beneficial applications in the field of biomimetics, energy, or catalysis, in combination with MoS2.39,53−56
Experimental Section
MoS2-Containing Single-Network Hydrogels (SNCHs)
The composite hydrogels were prepared following the previous method with minor modifications.30 To a solution of DMA (0.5 g, 5.04 mmol, 1.0 equiv) in water (2 mL) was added the predesigned amounts of MoS2 flakes (1–8 mg; 0.02–1.6 wt % with respect to DMA). The mixture was sonicated for 30 min to disperse and exfoliate the MoS2 nanosheets and degassed by bubbling with nitrogen gas. Then, a solution of potassium persulfate (15.0 mg, 0.06 mmol, 0.01 equiv) in water (0.5 mL) was added in one portion to the mixture. The reaction mixture was then stored at 25 °C for 48 h to afford free-standing, three-dimensional, composite hydrogels. The control acrylamide-based hydrogels were prepared following a similar method as described for SNCHs, except for using AM instead of DMA. The quantities of reagents used were as follows: AM (0.5 g, 7.0 mmol, 1.0 equiv), MoS2 (4 mg, 0.08 wt %), and potassium persulfate (15.0 mg, 0.06 mmol, 0.01 equiv).
Double-Network Composite Hydrogels (DNCHs)
The SNCHs (0.48 g; size: 1 cm × 1 cm × 1 cm) were immersed in a solution of NIPAM (6.8 g, 0.06 mol, 1.0 equiv) and N,N′-methylenebisacrylamide (0.19 g, 1.0 mmol, 0.02 equiv) in DMF (30 mL) in the presence of a photoinitiator (Irgacure 2959; 0.4 g, 0.002 mol, 0.03 equiv). After soaking for 48 h at 25 °C, the wet materials were taken out and polymerized under UV irradiation for 5 min at 25 °C to afford the desired double-network composite hydrogels. The control NIPAM-based hydrogels were prepared using a similar method as described for DNCHs except for SNCHs.
Triple-Network Composite Hydrogels (TNCHs)
The SNCHs (0.48 g; size: 1 cm × 1 cm × 1 cm) were immersed in a solution of NIPAM (6.8 g, 0.06 mol, 1.0 equiv) and N,N′-methylenebisacrylamide (0.19 g, 1.0 mmol, 0.02 equiv) in DMF (30 mL) in the presence of a photoinitiator (Irgacure 2959; 0.4 g, 0.002 mol, 0.03 equiv). After soaking for 48 h at 25 °C, the wet materials were taken out and polymerized under UV irradiation for 5 min at 25 °C to afford the desired double-network composite hydrogels.
The Control Double-Network Hydrogels
The control samples were prepared following a similar method as described for TNCHs, except for using SNCHs (0.48 g; size: 1 cm × 1 cm × 1 cm) instead of DNCHs and using solutions of other acrylic monomers. The quantities of reagents for the control sample with AMPS were as follows: AMPS (6.8 g, 32.8 mmol, 1.0 equiv), N,N′-methylenebisacrylamide (0.15 g, 1.0 mmol, 0.03 equiv), and Irgacure 2959 (0.15 g, 0.67 mmol, 0.02 equiv). The quantities of reagents for the control sample with methacroylcholine chloride (MC) were as follows: MC (6.8 g, 32.7 mmol, 1.0 equiv), N,N′-methylenebisacrylamide (0.15 g, 1.0 mmol, 0.03 equiv), and Irgacure 2959 (0.15 g, 0.67 mmol, 0.02 equiv). The quantities of reagents for the control sample with the mixture of both AMPS and MC were as follows: AMPS (6.4 g, 30.9 mmol, 1.0 equiv), MC (6.4 g, 30.8 mmol, 1.0 equiv), N,N′-methylenebisacrylamide (0.15 g, 1.0 mmol, 0.03 equiv), and Irgacure 2959 (0.15 g, 0.67 mmol, 0.02 equiv).
Calculating Swelling Degree and Cross-Linking Density
The hydrogels were immersed in water or DMF at 25 °C and measured the change in their weights. The degree of swelling was calculated by eq 1; the cross-linking density was estimated by eq 2 modified from the Flory–Rehner equation neglecting chain ends57 as follows
| 1 |
where X is the weight of the wet analyte sample and X0 is the weight of the sample dried under vacuum using a lyophilizer for 48 h
| 2 |
where Vsol indicates the molar volume of water, vp is the equilibrium polymer volume fraction determined from the inverse of the degree of swelling, and χ is the solvent–polymer interaction parameter and is equal to 0.46.58
Estimation of Absorption Rate Constants
Absorption rate constants of materials were estimated by a pseudo-second-order kinetic model34 as expressed in eq 3 as follows
| 3 |
where qe and qt indicate the absorbed amounts of water (g g–1) at equilibrium and at each time interval (t, min), respectively, and k2 (g g–1 min–1) is the rate constant.
Dye Loading and Release Tests
DNCHs (0.67 g; size: 3 cm × 1 cm × 0.3 cm) were immersed in a solution of rhodamine 6G (0.03 mM) for 24 h at 25 °C. The uptake amounts were estimated from the change in UV–vis absorption spectra of the dye solution. The release of dye was performed in DI water (3 mL) under NIR irradiation or after being heated to 50 °C and monitored by UV–vis spectrometry.
Acknowledgments
This work was partly supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2019R1F1A1055458) and by the Korea Institute for Advancement of Technology (KIAT) grant funded by the Korea Government (MOTIE) (P0012770).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c03690.
Synthetic procedures; experimental details; IR spectra; TGA data; SEM images; XPS spectra; UV–vis absorption spectra; supplementary graphs; ζ potential data; and mechanical data (PDF)
Author Contributions
† K.M.L. and S.J. contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- Bhattacharya S.; Samanta S. K. Soft-Nanocomposites of Nanoparticles and Nanocarbons with Supramolecular and Polymer Gels and Their Applications. Chem. Rev. 2016, 116, 11967–12028. 10.1021/acs.chemrev.6b00221. [DOI] [PubMed] [Google Scholar]
- Thoniyot P.; Tan M. J.; Karim A. A.; Young D. J.; Loh X. J. Nanoparticle–Hydrogel Composites: Concept, Design, and Applications of These Promising, Multi-Functional Materials. Adv. Sci. 2015, 2, 1400010 10.1002/advs.201400010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinson L. C.; Deagen M.; Chen W.; McCusker J.; McGuinness D. L.; Schadler L. S.; Palmeri M.; Ghumman U.; Lin A.; Hu B. Polymer Nanocomposite Data: Curation, Frameworks, Access, and Potential for Discovery and Design. ACS Macro Lett. 2020, 9, 1086–1094. 10.1021/acsmacrolett.0c00264. [DOI] [PubMed] [Google Scholar]
- Haraguchi K.; Takehisa T. Nanocomposite Hydrogels: A Unique Organic–Inorganic Network Structure with Extraordinary Mechanical, Optical, and Swelling/De–swelling Properties. Adv. Mater. 2002, 14, 1120–1124. . [DOI] [Google Scholar]
- Haraguchi K. Nanocomposite Hydrogels. Curr. Opin. Solid State Mater. Sci. 2007, 11, 47–54. 10.1016/j.cossms.2008.05.001. [DOI] [Google Scholar]
- Wahid F.; Zhao X.-J.; Jia S.-R.; Bai H.; Zhong C. Nanocomposite Hydrogels as Multifunctional Systems for Biomedical Applications: Current State and Perspectives. Composites, Part B 2020, 200, 108208 10.1016/j.compositesb.2020.108208. [DOI] [Google Scholar]
- Tseng C.-P.; Silberg J. J.; Bennett G. N.; Verduzco R. 100th Anniversary of Macromolecular Science Viewpoint: Soft Materials for Microbial Bioelectroncis. ACS Macro Lett. 2020, 9, 1590–1603. 10.1021/acsmacrolett.0c00573. [DOI] [PubMed] [Google Scholar]
- Chen T.; Hou K.; Ren Q.; Chen G.; Wei P.; Zhu M. Nanoparticle-Polymer Synergies in Nanocomposite Hydrogels: From Design to Application. Macromol. Rapid Commun. 2018, 39, 1800337 10.1002/marc.201800337. [DOI] [PubMed] [Google Scholar]
- Schexnailder P.; Schmidt G. Nanocomposite Polymer Hydrogels. Colloid. Polym. Sci. 2009, 287, 1–11. 10.1007/s00396-008-1949-0. [DOI] [Google Scholar]
- Zhang H.; Cong Y.; Osi A. R.; Zhou Y.; Huang F.; Zaccaria R. P.; Chen J.; Wang R.; Fu J. Direct 3D Printed Biomimetic Scaffolds Based on Hydrogel Microparticles for Cell Spheroid Growth. Adv. Funct. Mater. 2020, 30, 1910573 10.1002/adfm.201910573. [DOI] [Google Scholar]
- Ji X.; Guo D.; Ma J.; Yin M.; Yu Y.; Liu C.; Zhou Y.; Sun J.; Li Q.; Chen N.; Fan C.; Song H. Epigenetic Remodeling Hydrogel Patches for Multidrug-Resistant Triple-Negative Breast Cancer. Adv. Mater. 2021, 33, 2100949 10.1002/adma.202100949. [DOI] [PubMed] [Google Scholar]
- Pei X.; Zhang H.; Zhou Y.; Zhou L.; Fu J. Stretchable, self-healing and tissue-adhesive zwitterionic hydrogels as strain sensors for wireless monitoring of organ motions. Mater. Horiz. 2020, 7, 1872–1882. 10.1039/D0MH00361A. [DOI] [Google Scholar]
- Wu S.; Shao Z.; Xie H.; Xiang T.; Zhou S. Salt-mediated triple shape-memory ionic conductive polyampholyte hydrogel for wearable flexible electronics. J. Mater. Chem. A 2021, 9, 1048–1061. 10.1039/D0TA08664A. [DOI] [Google Scholar]
- Xu X.; Jerca V. V.; Hoogenboom R. Bioinspired double network hydrogels: from covalent double network hydrogels via hybrid double network hydrogels to physical double network hydrogels. Mater. Horiz. 2021, 8, 1173–1188. 10.1039/D0MH01514H. [DOI] [PubMed] [Google Scholar]
- Nicolosi V.; Chhowalla M.; Kanatzidis M. G.; Strano M. S.; Coleman J. N. Liquid Exfoliation of Layered Materials. Science 2013, 340, 1226419 10.1126/science.1226419. [DOI] [Google Scholar]
- Wang Q. H.; Kalantar-Zadeh K.; Kis A.; Coleman J. N.; Strano M. S. Electronics and Optoelectronics of Two-Dimensional Transition Metal Dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. 10.1038/nnano.2012.193. [DOI] [PubMed] [Google Scholar]
- Xiao L.; Chen X.; Yang X.; Sun J.; Geng J. Recent Advances in Polymer-Based Photothermal Materials for Biological Applications. ACS Appl. Polym. Mater. 2020, 2, 4273–4288. 10.1021/acsapm.0c00711. [DOI] [Google Scholar]
- Jaiswal M. K.; Carrow J. K.; Gentry J. L.; Gupta J.; Altangerel N.; Scully M.; Gaharwar A. K. Vacancy-Driven Gelation Using Defect-Rich Nanoassemblies of 2D Transition Metal Dichalcogenides and Polymeric Binder for Biomedical Applications. Adv. Mater. 2017, 29, 1702037 10.1002/adma.201702037. [DOI] [PubMed] [Google Scholar]
- Zhuang Y. T.; Zhang X.; Wang D. H.; Yu Y. L.; Wang J. H. Three-Dimensional Molybdenum Disulfide/Graphene Hydrogel with Tunable Heterointerfaces for High Selective Hg(II) Scavenging. J. Colloid Interface Sci. 2018, 514, 715–722. 10.1016/j.jcis.2017.12.082. [DOI] [PubMed] [Google Scholar]
- Wei G.; Wei J.; Zhou J.; Chen Y.; Wu D.; Wang Q. MoS2 Nanosheet Initiated Smart Polymeric Hydrogel for NIR-Driven Ag(I) Enrichment. Chem. Eng. J. 2020, 382, 123018 10.1016/j.cej.2019.123018. [DOI] [Google Scholar]
- Liu C.; Zhao X.; Wang S.; Yijie Z.; Ge W.; Li J.; Cao J.; Tao J.; Yang X. Freestanding, Three-Dimensional, and Conductive MoS2 Hydrogel via the Mediation of Surface Charges for High-Rate Supercapacitor. ACS Appl. Energy Mater. 2019, 2, 4458–4463. 10.1021/acsaem.9b00699. [DOI] [Google Scholar]
- Wang X.; Wu P. Aqueous Phase Exfoliation of Two-Dimensional Materials Assisted by Thermoresponsive Polymeric Ionic Liquid and Their Applications in Stimuli-Responsive Hydrogels and Highly Thermally Conductive Films. ACS Appl. Mater. Interfaces 2018, 10, 2504–2514. 10.1021/acsami.7b15712. [DOI] [PubMed] [Google Scholar]
- Hu H.; Zhong X.; Yang S.; Fu H. Tough and stretchable Fe3O4/MoS2/PANI composite hydrogels with conductive and magnetic properties. Composites, Part B 2020, 182, 107623 10.1016/j.compositesb.2019.107623. [DOI] [Google Scholar]
- Xu W.; Wang W.; Chen S.; Zhang R.; Wang Y.; Zhang Q.; Yuwen L.; Yang W. J.; Wang L. Molybdenum Disulfide (MoS2) Nanosheets-Based Hydrogels with Light-Triggered Self-Healing Property for Flexible Sensors. J. Colloid Interface Sci. 2021, 586, 601–612. 10.1016/j.jcis.2020.10.128. [DOI] [PubMed] [Google Scholar]
- Potaufeux J.-E.; Odent J.; Notta-Cuvier D.; Lauro F.; Raquez J.-M. A Comprehensive Review of the Structures and Properties of Ionic Polymeric Materials. Polym. Chem. 2020, 11, 5914–5936. 10.1039/D0PY00770F. [DOI] [Google Scholar]
- Gong J. P. Why Are Double Network Hydrogels So Tough?. Soft Matter 2010, 6, 2583–2590. 10.1039/b924290b. [DOI] [Google Scholar]
- Matsuda T.; Kawakami R.; Namba R.; Nakajima T.; Gong J. P. Mechanoresponsive Self-Growing Hydrogels Inspired by Muscle Training. Science 2019, 363, 504–508. 10.1126/science.aau9533. [DOI] [PubMed] [Google Scholar]
- Behera P. K.; Raut S. K.; Mondal P.; Sarkar S.; Singha N. K. Self-Healable Polyurethane Elastomer Based on Dual Dynamic Covalent Chemistry Using Diels–Alder “Click” and Disulfide Metathesis Reactions. ACS Appl. Polym. Mater. 2021, 3, 847–856. 10.1021/acsapm.0c01179. [DOI] [Google Scholar]
- Jung D.; Lee K. M.; Chang J. Y.; Yun M.; Choi H. -J.; Kim Y. A.; Yoon H.; Kim H. Selective De-Cross-Linking of Transformable, Double-Network Hydrogels: Preparation, Structural Conversion, and Controlled Release. ACS Appl. Mater. Interfaces 2018, 10, 42985–42991. 10.1021/acsami.8b14528. [DOI] [PubMed] [Google Scholar]
- Jung D.; Lee K. M.; Tojo T.; Oh Y.; Yoon H.; Kim H. Dual Cross-Linked Hydrogels That Undergo Structural Transformation via Selective Triggered Depolymerization. Chem. Mater. 2019, 31, 6249–6256. 10.1021/acs.chemmater.9b02365. [DOI] [Google Scholar]
- Lee K. M.; Oh Y.; Yoon H.; Chang M.; Kim H. Multifunctional Role of MoS2 in Preparation of Composite Hydrogels: Radical Initiation and Cross-Linking. ACS Appl. Mater. Interfaces 2020, 12, 8642–8649. 10.1021/acsami.9b19567. [DOI] [PubMed] [Google Scholar]
- Kolahi K. S.; Donjacour A.; Liu X.; Lin W.; Simbulan R. K.; Bloise E.; Maltepe E.; Rinaudo P. Effect of Substrate Stiffness on Early Mouse Embryo Development. PLoS One 2012, 7, e41717 10.1371/journal.pone.0041717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeshwaran S. R.; Jayaganthan R.; Velmurugan R.; Gupta N. K.; Manzhirov A. V. Mechanical and Thermal Properties of MoS2 Reinforced Epoxy Nanocomposites. J. Phys.: Conf. Ser. 2018, 991, 012054 10.1088/1742-6596/991/1/012054. [DOI] [Google Scholar]
- Chae J. A.; Oh Y.; Kim H. J.; Choi G. B.; Lee K. M.; Jung D.; Kim Y. A.; Kim H. Preparation of Compressible Polymer Monoliths that Contain Mesopores Capable of Rapid Oil–Water Separation. Polym. Chem. 2019, 10, 5142–5150. 10.1039/C9PY00967A. [DOI] [Google Scholar]
- Hippauf F.; Nickel W.; Hao G. P.; Schwedtmann K.; Giebeler L.; Oswald S.; Borchardt L.; Doerfler S.; Weigand J. J.; Kaskel S. The Importance of Pore Size and Surface Polarity for Polysulfide Adsorption in Lithium Sulfur Batteries. Adv. Mater. Interfaces 2016, 3, 1600508 10.1002/admi.201600508. [DOI] [Google Scholar]
- Lin Y.-C.; Dumcenco D. O.; Huang Y.-S.; Suenaga K. Atomic Mechanism of the Semiconducting-to-Metallic Phase Transition in Single-Layered MoS2. Nat. Nanotechnol. 2014, 9, 391–396. 10.1038/nnano.2014.64. [DOI] [PubMed] [Google Scholar]
- Huang H. H.; Fan X.; Singh D. J.; Zheng W. T. First Principles Study on 2H–1T′ Transition in MoS2 with Copper. Phys. Chem. Chem. Phys. 2018, 20, 26986–26994. 10.1039/C8CP05445B. [DOI] [PubMed] [Google Scholar]
- Wang H.; Li C.; Fang P.; Zhang Z.; Zhang J. Z. Synthesis, properties, and optoelectronic applications of two-dimensional MoS2 and MoS2-based heterostructures. Chem. Soc. Rev. 2018, 47, 6101–6127. 10.1039/C8CS00314A. [DOI] [PubMed] [Google Scholar]
- Nalwa H. S. A review of molybdenum disulfide (MoS2) based photodetectors: from ultra-broadband, self-powered to flexible devices. RSC Adv. 2020, 10, 30529–30602. 10.1039/D0RA03183F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A.; Kapil N.; Yenuganti M.; Das D. Exfoliated Sheets of MoS2 Trigger Formation of Aqueous Gels with Acute NIR Light Responsiveness. Chem. Commun. 2016, 52, 14043–14046. 10.1039/C6CC05734A. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Du P.; Xu D.; Li Y.; Peng W.; Zhang G.; Zhang F.; Fan X. Near-Infrared Responsive MoS2/Poly(N-isopropylacrylamide) Hydrogels for Remote Light-Controlled Microvalves. Ind. Eng. Chem. Res. 2016, 55, 4526–4531. 10.1021/acs.iecr.6b00432. [DOI] [Google Scholar]
- Carrow J. K.; Singh K. A.; Jaiswal M. K.; Ramirez A.; Lokhande G.; Yeh A. T.; Sarkar T. R.; Singh P.; Gaharwar A. K. Photothermal Modulation of Human Stem Cells Using Light-Responsive 2D Nanomaterials. Proc. Natl. Acad. Sci. U.S.A. 2020, 117, 13329–13338. 10.1073/pnas.1914345117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M.; Zhang K.; Liu Y.; Wang J.; Wang K.; Zhang Y. Multifunctional MoS2 Nanosheets with Au NPs Grown in situ for Synergistic Chemo-Photothermal Therapy. Colloids Surf., B 2019, 184, 110551 10.1016/j.colsurfb.2019.110551. [DOI] [PubMed] [Google Scholar]
- Lee J.; Kim J.; Kim W. J. Photothermally Controllable Cytosolic Drug Delivery Based on Core–Shell MoS2-Porous Silica Nanoplates. Chem. Mater. 2016, 28, 6417–6424. 10.1021/acs.chemmater.6b02944. [DOI] [Google Scholar]
- Shahid R. N.; Scudino S. Strengthening of Al-Fe3Al Composites by the Generation of Harmonic Structures. Sci. Rep. 2018, 8, 6484 10.1038/s41598-018-24824-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes G. A. Nanostructure-Mediated Drug Delivery. Nanomedicine 2005, 1, 22–30. 10.1016/j.nano.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Mahinroosta M.; Farsangi Z. J.; Allahverdi A.; Shakoori Z. Hydrogels as Intelligent Materials: A Brief Review of Synthesis, Properties and Applications. Mater. Today. Chem. 2018, 8, 42–55. 10.1016/j.mtchem.2018.02.004. [DOI] [Google Scholar]
- Liu Z.; Faraj Y.; Ju X. J.; Wang W.; Xie R.; Chu L. Y. Nanocomposite Smart Hydrogels with Improved Responsiveness and Mechanical Properties: A Mini Review. J. Polym. Sci., Part B: Polym. Phys. 2018, 56, 1306–1313. 10.1002/polb.24723. [DOI] [Google Scholar]
- Wang Z.; Cong Y.; Fu J. Stretchable and Tough Conductive Hydrogels for Flexible Pressure and Strain Sensors. J. Mater. Chem. B 2020, 8, 3437–3459. 10.1039/C9TB02570G. [DOI] [PubMed] [Google Scholar]
- Huang S.; Zhao Z.; Feng C.; Mayes E.; Yang J. Nanocellulose Reinforced P(AAm-co-AAc) Hydrogels with Improved Mechanical Properties and Biocompatibility. Composites, Part A 2018, 112, 395–404. 10.1016/j.compositesa.2018.06.028. [DOI] [Google Scholar]
- Frauenlob M.; King D. R.; Guo H.; Ishihara S.; Tsuda M.; Kurokawa T.; Haga H.; Tanaka S.; Gong J. P. Modulation and Characterization of the Double Network Hydrogel Surface-Bulk Transition. Macromolecules 2019, 52, 6704–6713. 10.1021/acs.macromol.9b01399. [DOI] [Google Scholar]
- Liu Y.; Li Z.; Xu J.; Wang B.; Liu F.; Na R.; Guan S.; Liu F. Effects of Amphiphilic Monomers and Their Hydrophilic Spacers on Polyacrylamide Hydrogels. RSC Adv. 2019, 9, 3462–3468. 10.1039/C8RA09644A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.; Kim H.; Chang J. Y. Designing Internal Hierarchical Porous Networks in Polymer Monoliths that Exhibit Rapid Removal and Photocatalytic Degradation of Aromatic Pollutants. Small 2020, 16, 1907555 10.1002/smll.201907555. [DOI] [PubMed] [Google Scholar]
- Le T. H.; Oh Y.; Kim H.; Yoon H. Exfoliation of 2D Materials for Energy and Environmental Applications. Chem.-Eur. J. 2020, 26, 6360–6401. 10.1002/chem.202000223. [DOI] [PubMed] [Google Scholar]
- Lee K. M.; Kim H. One-Step Preparation of Hydrogel Particles that Show Rapid Detection of Hydrogen Peroxide: The Dual Role of New Methylene Blue. Dyes Pigm. 2019, 170, 107546 10.1016/j.dyepig.2019.107546. [DOI] [Google Scholar]
- Park B.; Lee K. M.; Park S.; Yun M.; Choi H.-J.; Kim J.; Lee C.; Kim H.; Kim C. Deep Tissue Photoacoustic Imaging of Nickel(II) Dithiolene-Containing Polymeric Nanoparticles in the Second Near-Infrared Window. Theranostics 2020, 10, 2509–2521. 10.7150/thno.39403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browe D. P.; Wood C.; Sze M. T.; White K. A.; Scott T.; Olabisi R. M.; Freeman J. W. Characterization and Optimization of Actuating Poly(ethylene glycol) diacrylate/acrylic acid Hydrogels as Artificial Muscles. Polymer 2017, 117, 331–341. 10.1016/j.polymer.2017.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. M.; Kim H. J.; Jung D.; Oh Y.; Lee H.; Han C.; Chang J. Y.; Kim H. Rapid Accessible Fabrication and Engineering of Bilayered Hydrogels: Revisiting the Cross-Linking Effect on Superabsorbent Poly(acrylic acid). ACS Omega 2018, 3, 3096–3103. 10.1021/acsomega.8b00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




