Abstract

Macrocyclic lactones have redolent characteristics of muscones that originate from the rectal musk organs of the musk deer. These lactones are the primary raw material in the flavor and fragrance industry and are also found within the cyclic frameworks of various bioactive molecules. Due to great demand, many efforts have been made for their synthesis; however, strategies generating a large number of macrocyclic analogues from renewable resources have not been fully realized and are urgently required. Here, we outline a sustainable, straightforward, and eco-friendly approach to synthesize high-valued macrocyclic lactones utilizing olive oil under greener reaction conditions. The outlined method allows us to turn biomass into valuable 12- to 29-membered lactones and dilactones.
1. Introduction
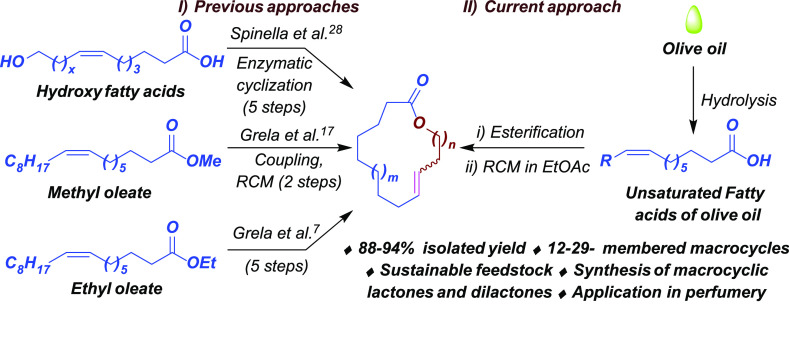
Plant-based feedstocks have become an intriguing raw material for various industries in environment-friendly and economically sustainable processes.1−3 These feedstocks mainly include vegetable oils, which predominantly contain fatty acids. Olefinic bonds of (mono or poly) unsaturated fatty acids offer the inherent possibility for versatile chemical transformations, for example, oxidation,4 epoxidation,5,6 metathesis,7,8 hydroalkylation,9 and polymerization.10,11 They are renewable feedstocks for constructing high value-added products such as macrocycles via greener approaches.12,13 Also, there is a considerable demand for macrocyclic molecules in the fragrances and flavor industry, particularly for macrocyclic lactones, that is, musk lactones.7 Traditionally, musk lactone is obtained from the musk deer’s rectal musk gland and is one of the oldest ingredients in perfumery.7 In traditional Chinese medicine, musk is a sedative and stimulant to treat various sicknesses and is used in more than 300 medicines. Musk’s excellent olfactory properties and use in pharmaceutical formulations put the species in the endangered category due to overexploitation.14,15 Alternatively, these molecules can be obtained from plant sources; however, their low natural abundance makes them unsustainable.16
Olefin metathesis, a catalytic reaction, became a powerful tool for producing natural products, polymers, and fine chemicals from biomass valorization.17−21 This reaction frequently utilizes Ru- and Mo-based catalysts owing to their high activity, efficiency, and stability.22,23 In olefin metathesis, ring-closing metathesis (RCM) is often used to develop the cyclic framework, gaining importance in synthesizing varieties of macrocyclic cores.7,8,24 In 1996, Fürstner and Langemann reported conformationally unbiased macrocyclization reactions for the synthesis of macrolactones having musky odor using RCM for the first time.25 Thereafter, the same group utilized the RCM strategy to synthesize a range of olfactory macrocycles from terminal olefins.26 Matsuda et al. synthesized musk macrolides from β-butyrolactone utilizing Grignard reaction, esterification, and RCM reaction.27 Recently, in 2018, the Grela group employed the RCM strategy to synthesize musk-smelling macrolactones from the methyl/ethyl oleate (Figure 1I).7,17 The Spinella group described a non-RCM-based protocol to synthesize macrocyclic lactones from hydroxy fatty acids using Candida antarctica lipase B enzyme (Figure 1I).28
Figure 1.
(I) Previous approaches and (II) current approach for synthesizing macrocyclic lactones.
In the quest for a bioresource for sustainable chemistry to prepare high value-added macrocycles, we for the first time report olive oil utilization to synthesize high-valued macrocyclic lactones. The olive oil is obtained from the seeds of the plant Olea europaea that is cultivated worldwide.29 It produces seeds containing 20–30% oil30 with a massive quantity of unsaturated fatty acids (70–90%), mainly oleic acid (∼65–80%).31 The presence of enormous unsaturation in olive oil makes it possible to utilize for amalgamation into various value-added products.
2. Results and Discussion
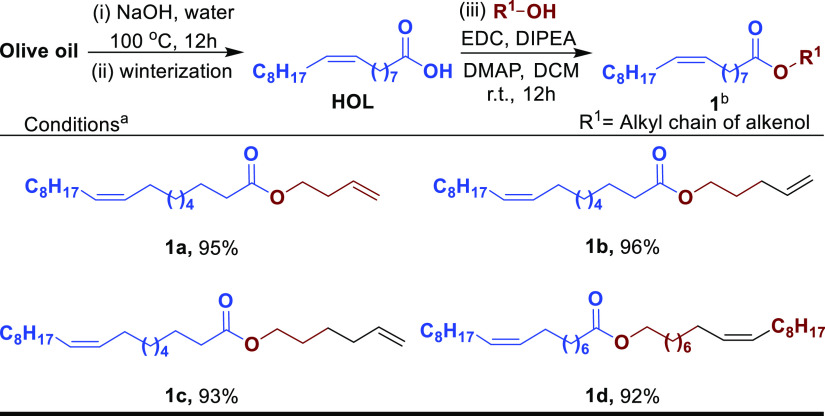
To begin our studies, we have commercially purchased olive oil from TCI Chemicals (India). Olive oil was subjected to hydrolysis in the presence of aq sodium hydroxide solution to obtain hydrolyzed oil. Next, to remove saturated fatty acids, the hydrolyzed oil was subjected to winterization, that is, it was slowly cooled to 15–18 °C and stood for 2 h, resulting in saturated fatty acids to crystallize and unsaturated fatty acids to remain in the liquid state. The liquid part was collected by decantation to furnish an oleic acid-rich hydrolyzed olive oil fraction (HOL) that contains 91% oleic acid (Supporting Information, Figure S2). Further, oleic acid-rich HOL was condensed with various unsaturated alcohols to obtain corresponding esters (Scheme 1, 1a–1d). Initially, homoallyl alcohol was reacted with HOL using N-ethylcarbodiimide hydrochloride (EDC·HCl) in dichloromethane (DCM) to furnish the desired ester 1a in excellent yield. To introduce further diversity, alcohols with varying alkyl chain lengths such as pent-4-en-1-ol and hex-5-en-1-ol were condensed with HOL to furnish corresponding esters (1b–1c) in 96 and 93% yields, respectively. Accordingly, an internal double bond containing alkenol, that is, oleyl alcohol, was smoothly condensed with HOL to furnish the corresponding ester 1d in excellent yield.
Scheme 1. Synthesis of Olive Oil-Derived Esters.
Reaction conditions: (i) olive oil (1.0 g, 1.0 equiv), NaOH (1.1 equiv), water, 100 °C, 12 h. (ii) Winterization and decantation, 76% after two steps. (iii) HOL (1.0 g, 1.0 equiv), alkenol (1.1 equiv), EDC·HCl (1.5 equiv), dimethylaminopyridine (DMAP) (0.22 equiv), diisopropylethylamine (DIPEA) (3.0 equiv), DCM, r.t., 12 h.
Isolated yield.
In the quest for sustainable methods for active pharmaceutical ingredients and key starting materials, we recently reported a method for synthesizing azelaic acid utilizing renewable oils over the vanadia catalyst.32,33 Initially, we synthesized azelaic acid from the oleic acid-rich HOL. To continue our efforts, the HOL-derived azelaic acid was coupled with different alkenols to furnish substrates for macrocyclization (Scheme 2, 2a–2d). First, allyl alcohol was condensed with azelaic acid to provide the corresponding diester 2a in a 96% yield. Similarly, a terminal double bond containing but-3-en-1-ol and dec-9-en-1-ol was condensed with azelaic acid to deliver the corresponding diesters (2b–2c) in excellent yield. Finally, an internal double bond containing oleyl alcohol was coupled with azelaic acid to obtain dioleyl nonanedioate 2d in 85% yield.
Scheme 2. Synthesis of Azelaic Acid-Derived Esters.
Reaction conditions: (i) HOL (1.0 g, 1.0 equiv), VO@TiO2 (30 wt %), TBHP (20 vol), 80 °C, 12 h, 65%. (ii) Azelaic acid (376 mg, 1.0 equiv), alkenol (2.2 equiv), EDC·HCl (3.0 equiv), DMAP (0.44 equiv), DIPEA (6.0 equiv), DCM, r.t., 12 h.
Isolated yield.
After successfully synthesizing a diverse range of olefins (1a–1d and 2a–2d), our subsequent attention turned toward identifying suitable reaction conditions for macrocyclization using the RCM protocol. Benzoquinone-based additives such as 1,4-benzoquinone (BQ) and 1,1,4,4-tetrafluorobenzoquinone (TFBQ) were also utilized to prevent oligomerization to a great extent by preventing undesirable reactions.7,34 Grubbs second generation (GB-2) and Hoveyda–Grubbs second generation (HG-2) catalysts were selected for RCM due to their high reactivity and thermal stability over other catalysts. For the RCM, chlorinated and aromatic solvents such as DCM, dichloroethane, toluene, benzene, and xylene were frequently employed. However, previously, we had performed the RCM reaction in ethyl acetate (EtOAc), a nonchlorinated, environmentally benign, and economical solvent due to health and environmental issues associated with these solvents.24
In this context, to obtain the best reaction conditions for metathesis, various parameters such as the catalyst, reaction temperature, mode of catalyst addition, and additives were optimized, selecting compound 1a as a model substrate. Initially, a reaction in EtOAc at 70 °C without any catalyst resulted in no reaction, whereas reaction with the GB-2 catalyst resulted in reactant consumption, but no desired product was obtained (Table 1, entries 1 and 2). Next, reaction with portion-wise catalyst addition gave the desired product with poor selectivity (Table 1, entry 3). Further, reaction with the BQ additive resulted in a relatively improved yield for the product (Table 1, entry 4). Reaction with the TFBQ additive instead of BQ gave an excellent yield for the desired product (Table 1, entry 5). Interestingly, a decrease in the temperature from 70 to 50 °C resulted in no change in the product yield (Table 1, entry 6). However, reactions with high olefinic concentrations (5 and 10 mM) furnished poor selectivity for the desired product (Table 1, entries 7 and 8). A reaction without the TFBQ additive resulted in a drastic decrease in the product yield (Table 1, entry 9). Reactions in DCM instead of EtOAc (Table 1, entry 10) at reduced temperature (Table 1, entries 11 and 12) resulted in poor yields for the product. A reaction with low catalyst loading leads to low conversion and poor yield even after 12 h (Table 1, entries 13 and 17). When the reactions were performed with the HG-2 catalyst, a decreased yield under various conditions was obtained, and multiple spots were observed using thin-layer chromatography (TLC) (Table 1, entries 14–18).
Table 1. Optimization of Reaction Conditions for RCMa.
| entry | cat. (mol %) | additive (10 mol %) | solvent | temp (°C) | timeb (h) | yieldc (%) |
|---|---|---|---|---|---|---|
| 1 | EtOAc | 70 | 24 | |||
| 2 | GB-2d (5) | EtOAc | 70 | 24 | ||
| 3 | GB-2 (5) | EtOAc | 70 | 1 + 24 | 10 | |
| 4 | GB-2 (5) | BQ | EtOAc | 70 | 1 + 2 | 40 |
| 5 | GB-2 (5) | TFBQ | EtOAc | 70 | 1 + 2 | 90 |
| 6 | GB-2(5) | TFBQ | EtOAc | 50 | 1 + 2 | 90 |
| 7e | GB-2 (5) | TFBQ | EtOAc | 50 | 1 + 2 | 10 |
| 8f | GB-2 (5) | TFBQ | EtOAc | 50 | 1 + 2 | 40 |
| 9 | GB-2 (5) | EtOAc | 50 | 1 + 24 | 15 | |
| 10 | GB-2 (5) | TFBQ | DCM | 50 | 1 + 2 | 85 |
| 11 | GB-2 (5) | TFBQ | DCM | 25 | 1 + 24 | 40 |
| 12 | GB-2 (5) | TFBQ | EtOAc | 25 | 1 + 24 | 45 |
| 13 | GB-2 (3) | TFBQ | EtOAc | 50 | 1 + 12 | 40 |
| 14 | HG-2 (5) | TFBQ | EtOAc | 50 | 1 + 2 | 60 |
| 15 | HG-2 (5) | TFBQ | DCM | 50 | 1 + 2 | 40 |
| 16 | HG-2 (5) | BQ | EtOAc | 50 | 1 + 2 | 30 |
| 17 | HG-2 (3) | TFBQ | EtOAc | 50 | 1 + 12 | 30 |
| 18 | HG-2 (5) | TFBQ | EtOAc | 70 | 1 + 2 | 75 |
Reaction conditions: 1a (50 mg, 1.0 equiv), GB-2 (5 mol %), TFBQ (10 mol %), EtOAc (1 mM), 50 °C, (1 + 2) h.
Addition of the catalyst in six equal portions in intervals of 10 min that took 1 h for complete catalyst addition.
Isolated yield.
One portion catalyst addition.
Olefin concentration in solvent = 10 mM.
Olefin concentration in solvent = 5 mM. Reactions were performed at 1 mM concentration of olefin.
After optimization, the best reaction conditions require portion-wise addition of 5 mol % of GB-2 and 10 mol % of TFBQ and maintaining a concentration of 1 mM concerning olefins in EtOAc at 50 °C for 2 h after complete addition of the catalyst. Having optimized reaction conditions in hand, we explored the substrate scope for the developed protocol. For this purpose, various synthesized olefinic (di)esters (1a–1d and 2a–2d) were tested for the RCM reaction.
The macrocyclization of olive oil-derived olefinic esters (1a–1d) was performed (Scheme 3I). First, homoallyl ester 1a was subjected to RCM, which resulted in industrially valuable 13-membered macrocyclic lactone 3a (yuju lactone)7 in 90% yield. Similarly, internal-terminal double bonds containing compounds 1b and 1c were cyclized smoothly to furnish respective macrocyclic lactones 3b (14-membered) and 3c (15-membered) in excellent yields. Further, terminal–terminal double bonds containing compound 1d was cyclized under the developed protocol to deliver the 19-membered macrocyclic lactone 3d in 94% yield. The result shows that long-chain alkenol-derived esters furnished the corresponding macrolactones in higher yield than the esters derived from small-chain alkenols.
Scheme 3. (I) Synthesis of Macrolactones from Alkyl Oleate. (II) Synthesis of Macrolactones from Dialkyl Azelate.
Reaction conditions: 1 or 2 (100 mg, 1.0 equiv), GB-2 (5 mol %), TFBQ (10 mol %), EtOAc (1 mM), 50 °C, (1 + 2) h.
Isolated yield.
Yield from 2c & 2d each.
Next, cyclization of diesters obtained from HOL-derived azelaic acid (2a–2d) was attempted (Scheme 3II). For this purpose, diallyl azelate (2a) containing terminal double bonds under RCM conditions yielded the desired 15-membered dilactone 4a in 88% yield. Similarly, 17- and 29-membered dilactones (4b–4c) were synthesized from their respective precursors (2b–2c) under the developed protocol in excellent yields. Interestingly, a cyclization reaction of dioleyl azelate (2d) containing the internal–internal double bond resulted in 29-membered dilactone 4c in excellent yield.
3. Conclusions
In summary, the work successfully demonstrates the first-time utilization of olive oil to synthesize high-valued macrocyclic lactones and dilactones. The hydrolyzed olive oil was successfully coupled with various unsaturated alcohols, including terminal and internal double bond containing alcohols, to produce eight olefinic (di)esters. These synthesized olefinic (di)esters were cyclized using the Grubbs catalyst under greener reaction conditions to furnish a range of macrocyclic products with diverse ring sizes varying from 12- to 29-membered. Utilizing natural resources made this strategy an efficient and proficient approach to deliver high-valued macrocycles, which has the potential for industrial application.
4. Experimental Section
4.1. General Information
All commercially available starting materials and reagents were purchased from Sigma-Aldrich, TCI chemicals, and other sources and used without further purification unless otherwise noted. All solvents for routine synthesis, isolation, and chromatography were of reagent grade and used without further purification. All glassware were dried in a hot air oven at 120 °C for 12 h before use. All reactions were performed under air. Reaction monitoring was conducted by TLC using aluminum plates precoated with silica gel 60 F254 (0.25 mm thickness) purchased from Merck. TLC was visualized by staining with iodine and charring with p-anisaldehyde or potassium permanganate. Silica gel (60–120, 100–200, 230–400 mesh, Spectrochem make) was used for column chromatography/flash chromatography (CombiFlash Rf Teledyne ISCO) eluting with a gradient of n-hexane and diethyl ether/EtOAc. All evaporations were carried out below 50 °C temperature and under reduced pressure using a Büchi rotary evaporator. Yields refer to chromatographically and spectroscopically pure compounds unless otherwise stated. NMR spectra were recorded on Bruker Avance 600 and 300 MHz instruments using trimethylsilane as the internal standard and deuterated chloroform as the solvent. The chemical shift values are on the δ scale, and the coupling constants (J) are in Hz. Mass spectra (ESI-MS/ESI-HRMS) were recorded either on a Water Q-TOF mass spectrometer or an Agilent 6560 ion mobility Q-TOF LC/MS spectrometer. Gas chromatography–mass spectrometry (GC–MS) analysis was carried out on a Shimadzu QP 2010 GC–MS system with an AOC 5000 autoinjector equipped with a Zebron ZB-5MS capillary column (30 m × 0.25 mm, 0.25 mm film thickness); carrier gas: helium (flow: 1.0 mL/min); split ratio 1:50; ionization energy: 70 eV; ion source temperature: 250 °C; injector temperature: 240 °C. Oven temperature program: initially at 70 °C for 3 min, increased at 4 °C/min to 220 °C, and then held isothermal (5 min) at 220 °C. Full spectral data for all novel compounds are given; all previously characterized compounds gave spectra consistent with the literature.
4.2. Hydrolysis of Olive Oil
The olive oil was subjected to hydrolysis under basic conditions to obtain free fatty acids. For this purpose, in a 250 mL round-bottom flask was added 5 g of olive oil and 800 mg of NaOH in 30 mL of water. The resultant reaction mixture was refluxed for 24 h, and water (30 mL) was added in intervals of 3 h (3 × 10 mL) to ensure complete hydrolysis. After completion (TLC monitoring), the reaction mixture was cooled to room temperature, 1 N HCl solution (30 mL) was added dropwise, and the product was extracted with EtOAc (3 × 50 mL). Next, pooled organic layers were washed with brine solution and dried over anhy. Na2SO4, and the solvent was removed under vacuum to furnish the crude material. The obtained hydrolyzed crude was subjected to winterization, for which it was slowly cooled at 15–18 °C and stood for 2 h, resulting in saturated fatty acids to crystallize and unsaturated fatty acids to remain in the liquid state due to the high freezing point of saturated fatty acids over unsaturated fatty acids. The liquid layer was collected by decantation to furnish oleic acid-rich fraction (HOL, 3 g, 76%).351H NMR (300 MHz, CDCl3): δ 5.34–5.33 (m, 2H), 2.36–2.31 (m, 2H), 2.01–1.99 (m, 4H), 1.65–1.60 (m, 2H), 1.30–1.25 (m, 20H), 0.87–0.85 (m, 3H). 13C{1H} NMR (75 MHz, CDCl3): δ 180.1, 130.0, 129.7, 34.0, 31.8, 29.7, 29.6, 29.5, 29.3, 29.1, 29.0, 27.2, 27.1, 24.6, 22.6, 14.0.
Additionally, 100 mg of olive oil and HOL was subjected to NaOH-catalyzed FAME synthesis separately and characterized by GC–MS. According to the GC–MS analysis, olive oil contains 76% of oleic acid (Supporting Information, Figure S1), whereas HOL fraction mainly contains oleic acid (91%) (Supporting Information, Figure S2).
4.3. Synthesis of Azelaic Acid
The azelaic acid synthesis from oleic acid-rich fraction (HOL) was achieved by the vanadia-catalyzed oxidative cleavage method.32 For the oxidative cleavage of oleic acid-rich HOL, a screw-capped glass vial (120 mL capacity) was charged with HOL (1 gm), tert-butyl hydroperoxide (TBHP, 20 mL), and the vanadium catalyst (C-20, 30 wt %). The above reaction mixture was heated to 80 °C under stirring for 12 h. After completing the reaction (TLC monitoring), the catalyst was separated by filtration or by centrifugation methods. The resulting filtrate was subjected to evaporation of the solvent under reduced pressure to furnish a residue. The residue was washed with n-hexane (3 × 30 mL) to remove impurities and dried to obtain a powder which was then recrystallized in ethanol to furnish azelaic acid (436 mg, 65%) as a white powder. 1H NMR (600 MHz, DMSO-d6): δ 11.98 (s, 2H), 2.18 (t, J = 7.3 Hz, 4H), 1.48–1.45 (m, 4H), 1.24 (m, 6H). 13C{1H} NMR (151 MHz, DMSO-d6): δ 174.9, 34.0, 28.9, 28.8, 24.9. The data correspond to the literature.36
4.4. General Procedure for the Synthesis of Olive Oil-Derived Olefinic Ester (1a–1d)
To a stirred solution of oleic acid-rich HOL (1.0 g, 1.0 equiv) in DCM (10 vol) at 0 °C was added EDC·HCl (1.7 equiv) and DMAP (0.22 equiv). After 10 min at 0 °C, alcohol (1.1 equiv) and DIPEA (4.0 equiv) were added, and the reaction mixture was stirred at room temperature for 12 h. After completion (TLC monitoring), the reaction mixture was quenched with water (30 vol) and extracted with DCM (3 × 50 vol). The combined organic fractions were dried over anhydrous Na2SO4, the solvent was removed under reduced pressure, and the residue was purified by silica gel column chromatography to afford the desired product.37
4.4.1. But-3-en-1-yl Oleate (1a)
Colorless oil, (1.13 g, 3.36 mmol, yield 95%). 1H NMR (300 MHz, CDCl3): δ 5.77–5.72 (m, 1H), 5.31–5.29 (m, 2H), 5.07 (d, J = 17.1 Hz, 1H), 5.03 (d, J = 10.2 Hz, 1H), 4.09 (t, J = 6.7 Hz, 2H), 2.34 (q, J = 6.7 Hz, 2H), 2.25 (t, J = 7.5 Hz, 2H), 1.99–1.96 (m, 4H), 1.59–1.57 (m, 2H), 1.27–1.22 (m, 20H), 0.85 (t, J = 6.9 Hz, 3H). 13C{1H} NMR (151 MHz, CDCl3): δ 173.6, 134.0, 129.9, 129.6, 117.0, 63.1, 34.2, 33.1, 31.9, 29.7, 29.6, 29.5, 29.3, 29.1, 29.1, 29.0, 29.0, 27.1, 27.1, 24.9, 24.9, 22.6, 14.0. ESI-HRMS m/z: calcd for C22H40O2 [M + H]+, 337.3101; found, 337.3105.
4.4.2. Pent-4-en-1-yl Oleate (1b)
Colorless oil, (1.19 g, 3.398 mmol, yield 96%). 1H NMR (300 MHz, CDCl3): δ 5.86–5.76 (m, 1H), 5.39–5.28 (m, 2H), 5.03 (d, J = 17.1 Hz, 1H), 4.98 (d, J = 10.0 Hz, 1H), 4.07 (t, J = 6.6 Hz, 2H), 2.28 (t, J = 7.5 Hz, 2H), 2.16–1.99 (m, 6H), 1.76–1.67 (m (5), J = 7.0 Hz, 2H), 1.61 (t, J = 6.4 Hz, 2H), 1.29–1.25 (m, 20H), 0.87 (t, J = 6.3 Hz, 3H). 13C{1H} NMR (151 MHz, CDCl3): δ 173.9, 137.4, 129.9, 129.7, 115.2, 63.6, 34.3, 31.9, 30.0, 29.7, 29.6, 29.5, 29.3, 29.1, 29.1, 29.1, 27.8, 27.2, 27.1, 25.0, 22.6, 14.1. ESI-HRMS m/z: calcd for C23H42O2 [M + H]+, 351.3258; found, 351.3260.
4.4.3. Hex-5-en-1-yl Oleate (1c)
Colorless oil, (1.198 g, 3.398 mmol, yield 93%). 1H NMR (300 MHz, CDCl3): δ 5.86–5.73 (m, 1H), 5.40–5.29 (m, 2H), 5.05–4.95 (m, 2H), 4.07 (t, J = 6.5 Hz, 2H), 2.29 (t, J = 7.5 Hz, 2H), 2.12–2.01 (m, 6H), 1.67–1.60 (m, 4H), 1.51–1.41 (m, 2H), 1.31–1.26 (m, 24H), 0.88 (t, J = 6.4 Hz, 3H). 13C{1H} NMR (151 MHz, CDCl3): δ 173.7, 134.0, 129.9, 129.7, 117.1, 63.2, 34.2, 33.1, 31.9, 29.7, 29.6, 29.5, 29.3, 29.1, 29.1, 29.0, 27.2, 27.1, 24.9, 22.6, 14.0. ESI-HRMS m/z: calcd for C24H44O2 [M + H]+, 365.3414; found, 365.3400.
4.4.4. (Z)-Octadec-9-en-1-yl Oleate (1d)
Colorless oil (1.73 g, 3.26 mmol, yield 92%). 1H NMR (300 MHz, CDCl3): δ 5.40–5.28 (m, 4H), 4.04 (t, J = 6.6 Hz, 2H), 2.27 (t, J = 7.4 Hz, 2H), 2.16 (s, 4H), 2.03–1.94 (m, 7H), 1.65–1.55 (m, 4H), 1.36–1.21 (m, 43H), 0.89–0.84 (m, 6H). 13C{1H} NMR (75 MHz, CDCl3): δ 173.9, 129.9, 129.7, 64.3, 34.3, 32.5, 31.8, 30.8, 29.6, 29.5, 29.3, 29.1, 28.6, 27.1, 25.9, 25.0, 22.6, 14.0. ESI-HRMS m/z: calcd for C36H68O2 [M + H]+, 533.5292; found, 533.5281.
4.5. General Procedure for the Synthesis of Olive Oil-Derived Olefinic (Di)esters (2a–2d)
To a stirred solution of oleic acid-rich fraction (HOL)-derived azelaic acid (376 mg, 1.0 equiv) in DCM (10 vol) at 0 °C was added EDC·HCl (3.0 equiv) and DMAP (0.44 equiv). After 10 min at 0 °C, alcohol (2.2 equiv) and DIPEA (6.0 equiv) were added, and the reaction mixture was stirred at room temperature for 12 h. After completion (TLC monitoring), the reaction mixture was quenched with water (30 vol) and extracted with DCM (3 × 50 vol). The combined organic fractions were dried over anhydrous Na2SO4, the solvent was removed under reduced pressure, and the residue was purified by silica gel column chromatography to afford the desired product.37
4.5.1. Diallyl Nonanedioate (2a)
Colorless oil (514.6 mg, 1.92 mmol, yield 96%). 1H NMR (600 MHz, CDCl3): δ 5.92–5.87 (m, 2H), 5.29 (dd, J = 17.1, 1.4 Hz, 2H), 5.21 (dd, J = 10.4, 1.2 Hz, 2H), 4.55 (d, J = 5.7 Hz, 4H), 2.30 (t, J = 7.5 Hz, 4H), 1.61 (t, J = 7.1 Hz, 4H), 1.30 (m, 6H). 13C{1H} NMR (151 MHz, CDCl3): δ 173.3, 132.3, 118.0, 64.9, 34.1, 28.8, 28.8, 24.8. ESI-HRMS m/z: calcd for C15H24O4 [M + H]+, 269.1747; found, 269.1730.
4.5.2. Di(but-3-en-1-yl) Nonanedioate (2b)
Colorless oil (568.3 mg, 1.92 mmol, yield 96%). 1H NMR (300 MHz, CDCl3): δ 5.81–5.74 (m, 2H), 5.10 (qd, J = 17.6, 1.5 Hz, 2H), 5.06 (dd, J = 10.2, 1.0 Hz, 2H), 4.11 (t, J = 6.7 Hz, 4H), 2.39–2.35 (m, 4H), 2.30–2.27 (m, 4H), 1.65–1.59 (m, 4H), 1.34–1.30 (m, 6H). 13C{1H} NMR (75 MHz, CDCl3): δ 173.7, 173.5, 134.0, 117.1, 102.2, 63.2, 34.2, 34.2, 34.0, 33.1, 29.6, 28.9, 28.8, 28.7, 28.5, 24.8, 24.7, 24.5. ESI-HRMS m/z: calcd for C17H28O4 [M + H]+, 297.2060; found, 297.2066.
4.5.3. Di(dec-9-en-1-yl) Nonanedioate (2c)
Colorless oil (863 mg, 1.86 mmol, yield 93%). 1H NMR (600 MHz, CDCl3): δ 5.81–5.76 (m, 2H), 4.98 (t, J = 17.1 Hz, 2H), 4.91 (t, J = 10.1 Hz, 2H), 4.04 (t, J = 6.6 Hz, 4H), 2.29–2.26 (m, 4H), 2.02 (q, J = 7.0 Hz, 4H), 1.61–1.59 (m, 8H), 1.38–1.35 (m, 4H), 1.30–1.28 (m, 22H). 13C{1H} NMR (151 MHz, CDCl3): δ 173.9, 139.1, 114.1, 64.4, 34.3, 33.8, 29.3, 29.2, 29.0, 28.9, 28.9, 28.8, 28.6, 25.9, 24.9, 24.6. ESI-HRMS m/z: calcd for C29H52O4 [M + H]+, 465.3938; found, 465.3946.
4.5.4. Di((Z)-octadec-9-en-1-yl) Nonanedioate (2d)
Colorless oil (1.17 g, 1.70 mmol, yield 85%). 1H NMR (600 MHz, CDCl3): δ 5.40–5.31 (m, 4H), 4.06 (t, J = 6.7 Hz, 4H), 2.29 (t, J = 7.5 Hz, 4H), 2.05–2.00 (m, 6H), 1.64–1.60 (m, 8H), 1.36–1.27 (m, 52H), 0.89 (t, J = 6.9 Hz, 6H). 13C{1H} NMR (151 MHz, CDCl3): δ 173.8, 130.4, 130.2, 129.9, 129.7, 64.3, 34.3, 32.5, 32.5, 31.9, 29.7, 29.6, 29.6, 29.6, 29.5, 29.4, 29.4, 29.3, 29.3, 29.2, 29.2, 29.1, 29.0, 28.9, 28.9, 28.6, 27.2, 27.1, 25.9, 24.9, 22.6, 21.4, 14.0. ESI-HRMS m/z: calcd for C45H84O4 [M + H]+, 689.6442; found, 689.6420.
4.6. General Procedure for Macrocyclization of Synthesized Olefinic (Di)ester (1a–1d & 2a–2d) Utilizing RCM
To a round-bottom flask, olefinic (di)ester (100 mg, 1.0 equiv) and TFBQ (10 mol %) were dissolved in EtOAc (1 mM) to make conc. [wrt olefinic (di)ester] = 1 mM. The reaction mixture was heated to 50 °C with continuous stirring. To a glass vial charged with the GB-2 catalyst (5 mol %) was added EtOAc (1 mg of catalyst dissolved in 1 mL EtOAc). The catalyst solution was then added to the reaction mixture in 10 min intervals (five to six portions for complete addition) via a syringe. Further, after complete addition of the catalyst, the reaction mixture was stirred for additional 2 h at 50 °C with constant stirring. After completion (TLC monitoring), the reaction mixture was cooled to room temperature, and the catalyst was quenched with ethyl vinyl ether (4 mL of ethyl vinyl ether per 5 mg of catalyst). The reaction mixture was stirred for 15 min to ensure complete quenching of the catalyst to prevent side reactions. It is worth mentioning that the presence of an active catalyst after completion of the reaction often leads to the side reaction/oligomerization via ADMET/ROMP while removing the solvent by evaporation. Next, the solvents were evaporated under reduced pressure, and the obtained crude was purified by column chromatography (eluting with diethyl ether/n-hexane or EtOAc/n-hexane) to deliver the desired macrocycles.
4.6.1. Synthesis of Macrocyclic Lactones (3a–3d) from Olive Oil-Derived Olefinic Esters (1a–1d)
4.6.1.1. (10E/Z)-Oxacyclotridec-10-en-2-one (3a)
Colorless oil with musky odor (52.4 mg, 0.27 mmol, 90%). Rf = 0.50 (1:19, diethyl ether/n-hexane). 1H NMR (300 MHz, CDCl3): δ 5.58–5.27 (m, 2H), 4.23–4.07 (m, 2H), 2.43–2.23 (m, 4H), 2.09–1.97 (m, 2H), 1.70–1.58 (m, 2H), 1.50–1.38 (m, 2H), 1.34–1.23 (m, 6H). 13C{1H} NMR (75 MHz, CDCl3): δ 173.9, 134.6, 132.2, 131.7, 130.6, 129.2, 127.0, 126.4, 126.1, 64.1, 63.5, 62.8, 35.5, 35.3, 34.6, 33.9, 32.5, 31.9, 30.7, 29.6, 28.1, 27.5, 27.3, 27.2, 26.9, 26.5, 25.9, 25.8, 25.5, 24.5, 24.3, 24.1, 23.4. ESI-HRMS m/z: calcd for C12H20O2 [M + H]+, 197.1536; found, 197.1510. The data correspond to the literature.38
4.6.1.2. (10E/Z)-Oxacyclotetradec-10-en-2-one (3b)
Colorless oil with musky odor (55.1 mg, 0.26 mmol, 92%). Rf = 0.50 (1:19, diethyl ether/n-hexane). 1H NMR (300 MHz, CDCl3): δ 5.46–5.39 (m, 1H), 5.31–5.27 (m, 1H), 4.11–4.09 (m, 2H), 2.39–2.31 (m, 2H), 2.23–2.20 (m, 2H), 2.04–2.00 (m, 2H), 1.70–1.62 (m, 4H), 1.36–1.25 (m, 8H). 13C{1H} NMR (75 MHz, CDCl3): δ 173.8, 131.0, 130.5, 130.6, 130.3, 128.3, 64.7, 62.7, 62.6, 33.4, 32.9, 31.3, 30.9, 28.9, 28.1, 27.0, 26.8, 26.6, 25.9, 25.1, 24.9, 24.4, 24.0, 23.6. ESI-HRMS m/z: calcd for C13H22O2 [M + H]+, 211.1693; found, 211.1676. The data correspond to the literature.38
4.6.1.3. (10E/Z)-Oxacyclopentadec-10-en-2-one (3c)
Colorless oil with musky odor (55.3 mg, 0.25 mmol, 90%). Rf = 0.50 (1:19, diethyl ether/n-hexane). 1H NMR (300 MHz, CDCl3): δ 5.47–5.29 (m, 2H), 5.15–5.08 (m, 2H), 2.71–2.27 (m, 2H), 2.06–1.94 (m, 4H), 1.67–1.59 (m, 4H), 1.41–1.25 (m, 10H). 13C{1H} NMR (75 MHz, CDCl3): δ 173.9, 135.9, 130.7, 130.6, 130.0, 129.5, 129.3, 129.2, 125.0, 63.8, 35.2, 34.3, 32.2, 31.9, 31.7, 31.5, 29.3, 29.2, 29.1, 28.7, 28.4, 27.9, 27.8, 27.3, 27.2, 27.0, 26.9, 26.3, 25.7, 25.3, 25.0, 23.4. ESI-HRMS m/z: calcd for C14H24O2 [M + H]+, 225.1849; found, 225.1842. The data correspond to the literature.39
4.6.1.4. (10E/Z)-Oxacyclononadec-10-en-2-one (3d)
Colorless oil with musky odor (48 mg, 0.17 mmol, 94%). Rf = 0.50 (1:19, diethyl ether/n-hexane). 1H NMR (600 MHz, CDCl3): δ 5.40–5.22 (m, 2H), 4.09 (t, J = 5.0 Hz, 2H), 2.30 (t, J = 6.9 Hz, 2H), 2.07–1.96 (m, 4H), 1.65–1.56 (m, 4H), 1.40–1.20 (m, 18H). 13C{1H} NMR (151 MHz, CDCl3): δ 174.0, 130.7, 64.5, 34.9, 32.1, 29.6, 29.5, 29.2, 29.1, 28.9, 28.8, 28.0, 27.7, 26.3, 25.2. ESI-HRMS m/z: calcd for C18H32O2 [M + H]+, 281.2475; found, 281.2462. The data correspond to the literature.7
4.6.2. Synthesis of Macrocyclic Dilactones (4a–4c) from Olive Oil-Derived Azelaic Acid Alkenyl Diester (2a–2d)
4.6.2.1. (3E/Z)-1,6-Dioxacyclopentadec-3-ene-7,15-dione (4a)
Colorless oil with musky odor (78.7 mg, 0.33 mmol, 88%). Rf = 0.50 (1:19, diethyl ether/n-hexane). 1H NMR (300 MHz, CDCl3): δ 5.88–5.88 (m, 2H), 4.61–4.57 (m, 4H), 2.35–2.29 (m, 4H), 1.63–1.57 (m, 4H), 1.30–1.27 (m, 6H). 13C{1H} NMR (75 MHz, CDCl3): δ 173.3, 128.9, 62.5, 34.6, 28.3, 27.6, 24.9. ESI-HRMS m/z: calcd for C13H20O4 [M + H]+, 241.1434; found, 241.1430.
4.6.2.2. (4E/Z)-1,8-Dioxacycloheptadec-4-ene-9,17-dione (4b)
Colorless oil with musky odor (87.3 mg, 0.27 mmol, 95%). Rf = 0.48 (1:19, diethyl ether/n-hexane). 1H NMR (600 MHz, CDCl3): δ 5.56–5.49 (m, 2H), 4.19–4.14 (m, 4H), 2.41–2.28 (m, 8H), 1.65–1.59 (m, 4H), 1.34–1.28 (m, 6H) (1H NMR shows the presence of geometrical isomers in the ratio of Z/E = 1:4). 13C{1H} NMR (151 MHz, CDCl3): δ 173.9, 128.9, 128.4, 63.5, 34.4, 34.2, 31.9, 27.9, 27.7, 27.5, 27.3, 24.7, 24.6. ESI-HRMS m/z: calcd for C15H24O4 [M + H]+, 269.1747; found, 269.1740.
4.6.2.3. (20E/Z)-1,11-Dioxacyclononacos-20-ene-2,10-dione (4c)
From compound 2c. Colorless oil with musky odor (84.7 mg, 0.19 mmol, 90%). Rf = 0.50 (1:19, diethyl ether/n-hexane). From compound 2d. Colorless oil with musky odor (84.7 mg, 0.19 mmol, 90%). Rf = 0.50 (1:19, diethyl ether/n-hexane). 1H NMR (300 MHz, CDCl3): δ 5.41–5.35 (m, 2H), 4.07 (d, J = 6.7 Hz, 4H), 2.30 (d, J = 7.5 Hz, 4H), 2.03–1.96 (m, 4H), 1.64–1.60 (m, 10H), 1.31–1.27 (m, 24H). 13C{1H} NMR (75 MHz, CDCl3): δ 173.9, 130.3, 64.4, 34.3, 32.5, 31.9, 29.7, 29.6, 29.6, 29.3, 29.2, 29.1, 28.9, 28.6, 25.9, 24.9, 22.6. ESI-HRMS m/z: calcd for C27H48O4 [M+2H]2+, 438.3698; found, 438.3695.
Acknowledgments
The authors gratefully acknowledge the Council of Scientific and Industrial Research (CSIR), New Delhi, for the financial support (HCP 0011 and HCP 007) and SRF (R.R.). We are thankful to the Director, CSIR-IHBT, Palampur (H.P.), for providing the necessary infrastructure. CSIR-IHBT communication no. for this publication is 4715.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c03319.
GC–MS chromatogram (olive oil FAME and HOL FAME) and spectrum copies (1H & 13C NMR) for the synthesized compounds (PDF)
Author Contributions
R.R. contributed to the optimization, synthesis of substrate scope, data analysis, and manuscript writing. Shreya contributed to the synthesis of building blocks and substrate scope. R.U. contributed to the substrate scope. S.K.M. conceived and supervised the experiments, contributed to data analysis and editing of the manuscript, and provided overall guidance.
The authors declare no competing financial interest.
Supplementary Material
References
- Chen X.; Song S.; Li H.; Gözaydın G.; Yan N. Expanding the Boundary of Biorefinery: Organonitrogen Chemicals from Biomass. Acc. Chem. Res. 2021, 54, 1711–1722. 10.1021/acs.accounts.0c00842. [DOI] [PubMed] [Google Scholar]
- Dahlquist E.Biomass as Energy Source Resource, System and Application; Dahlquist E., Ed.; Taylor & Francis, 2013. [Google Scholar]
- Twidell J.; Weir T.. Renewable Energy Resources, 3rd ed.; Twidell J., Weir T., Eds.; Routledge, 2015. [Google Scholar]
- Köckritz A.; Martin A. Oxidation of Unsaturated Fatty Acid Derivatives and Vegetable Oils. Eur. J. Lipid Sci. Technol. 2008, 110, 812–824. 10.1002/ejlt.200800042. [DOI] [Google Scholar]
- Wei Y.; Li G.; Lv Q.; Cheng C.; Guo H. Epoxidation of Methyl Oleate and Unsaturated Fatty Acid Methyl Esters Obtained from Vegetable Source over Ti-Containing Silica Catalysts. Ind. Eng. Chem. Res. 2018, 57, 16284–16294. 10.1021/acs.iecr.8b04155. [DOI] [Google Scholar]
- Aguilera A. F.; Tolvanen P.; Heredia S.; Muñoz M. G.; Samson T.; Oger A.; Verove A.; Eränen K.; Leveneur S.; Mikkola J.-P.; Salmi T. Epoxidation of Fatty Acids and Vegetable Oils Assisted by Microwaves Catalyzed by a Cation Exchange Resin. Ind. Eng. Chem. Res. 2018, 57, 3876–3886. 10.1021/acs.iecr.7b05293. [DOI] [Google Scholar]
- Sytniczuk A.; Leszczyńska A.; Kajetanowicz A.; Grela K. Preparation of Musk-Smelling Macrocyclic Lactones from Biomass: Looking for the Optimal Substrate Combination. ChemSusChem 2018, 11, 3157–3166. 10.1002/cssc.201801463. [DOI] [PubMed] [Google Scholar]
- Yapa Mudiyanselage A.; Viamajala S.; Varanasi S.; Yamamoto K. Simple Ring-Closing Metathesis Approach for Synthesis of PA11, 12, and 13 Precursors from Oleic Acid. ACS Sustain. Chem. Eng. 2014, 2, 2831–2836. 10.1021/sc500599u. [DOI] [Google Scholar]
- Green S. A.; Huffman T. R.; McCourt R. O.; Van Der Puyl V.; Shenvi R. A. Hydroalkylation of Olefins to Form Quaternary Carbons. J. Am. Chem. Soc. 2019, 141, 7709–7714. 10.1021/jacs.9b02844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiti B.; De P. RAFT Polymerization of Fatty Acid Containing Monomers: Controlled Synthesis of Polymers from Renewable Resources. RSC Adv. 2013, 3, 24983–24990. 10.1039/c3ra45541f. [DOI] [Google Scholar]
- Wang Z.; Ganewatta M. S.; Tang C. Sustainable Polymers from Biomass: Bridging Chemistry with Materials and Processing. Prog. Polym. Sci. 2020, 101, 101197. 10.1016/j.progpolymsci.2019.101197. [DOI] [Google Scholar]
- Liu H.; Cheng T.; Xian M.; Cao Y.; Fang F.; Zou H. Fatty Acid from the Renewable Sources: A Promising Feedstock for the Production of Biofuels and Biobased Chemicals. Biotechnol. Adv. 2014, 32, 382–389. 10.1016/j.biotechadv.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Biermann U.; Friedt W.; Lang S.; Lühs W.; Machmüller G.; Metzger J. O.; Mark M. R.; Schäfer H. J.; Schneider M. P. New Syntheses with Oils and Fats as Renewable Raw Materials for the Chemical Industry. Angew. Chem., Int. Ed. 2000, 39, 2206–2224. . [DOI] [PubMed] [Google Scholar]
- Yang Q.; Meng X.; Xia L.; Feng Z. Conservation Status and Causes of Decline of Musk Deer (Moschus Spp.) in China. Biol. Conserv. 2003, 109, 333–342. 10.1016/s0006-3207(02)00159-3. [DOI] [Google Scholar]
- Homes V.; Traffic E.. On the Scent: Conserving Musk Deer: The Uses of Musk and Europe’s Role in Its Trade; TRAFFIC Europe, 1999.
- Lopes D.; Strobl H.; Kolodziejczyk P. 14-Methylpentadecano-15-Lactone (Muscolide): A New Macrocyclic Lactone from the Oil of Angelica Archangelica L.. Perspect. Flavor Fragr. Res. 2007, 1, 47–54. 10.1002/9783906390475.ch5. [DOI] [PubMed] [Google Scholar]
- Sytniczuk A.; Dąbrowski M.; Banach Ł.; Urban M.; Czarnocka-Śniadała S.; Milewski M.; Kajetanowicz A.; Grela K. At Long Last: Olefin Metathesis Macrocyclization at High Concentration. J. Am. Chem. Soc. 2018, 140, 8895–8901. 10.1021/jacs.8b04820. [DOI] [PubMed] [Google Scholar]
- Caijo F.; Tripoteau F.; Bellec A.; Crévisy C.; Baslé O.; Mauduit M.; Briel O. Screening of a Selection of Commercially Available Homogeneous Ru-Catalysts in Valuable Olefin Metathesis Transformations. Catal. Sci. Technol. 2013, 3, 429–435. 10.1039/c2cy20524f. [DOI] [Google Scholar]
- Ahmed T. S.; Grubbs R. H. A Highly Efficient Synthesis of Z-Macrocycles Using Stereoretentive, Ruthenium-Based Metathesis Catalysts. Angew. Chem., Int. Ed. 2017, 56, 11213–11216. 10.1002/anie.201704670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Małecki P.; Gajda K.; Gajda R.; Woźniak K.; Trzaskowski B.; Kajetanowicz A.; Grela K. Specialized Ruthenium Olefin Metathesis Catalysts Bearing Bulky Unsymmetrical NHC Ligands: Computations, Synthesis, and Application. ACS Catal. 2019, 9, 587–598. 10.1021/acscatal.8b04783. [DOI] [Google Scholar]
- Smoleń M.; Kośnik W.; Gajda R.; Woźniak K.; Skoczeń A.; Kajetanowicz A.; Grela K. Ruthenium Complexes Bearing Thiophene-Based Unsymmetrical N-Heterocyclic Carbene Ligands as Selective Catalysts for Olefin Metathesis in Toluene and Environmentally Friendly 2-Methyltetrahydrofuran. Chem. Eur. J. 2018, 24, 15372–15379. 10.1002/chem.201803460. [DOI] [PubMed] [Google Scholar]
- Grubbs R. H.; Wenzel A. G.; O’Leary D. J.; Khosrvi E.. Handbook of Metathesis; Wiley-VCH, 2015. [Google Scholar]
- Grela K.Olefin Metathesis Theory and Practice; Grela K., Ed.; John Wiley & Sons, Inc., 2014. [Google Scholar]
- Maurya S. K.; Rana R. An Eco-Compatible Strategy for the Diversity-Oriented Synthesis of Macrocycles Exploiting Carbohydrate-Derived Building Blocks. Beilstein J. Org. Chem. 2017, 13, 1106–1118. 10.3762/bjoc.13.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fürstner A.; Langemann K. Conformationally Unbiased Macrocyclization Reactions by Ring Closing Metathesis. J. Org. Chem. 1996, 61, 3942–3943. 10.1021/jo960733v. [DOI] [PubMed] [Google Scholar]
- Fürstner A.; Langemann K. Macrocycles by Ring-Closing Metathesis. Synthesis 1997, 792–803. 10.1055/s-1997-4472. [DOI] [Google Scholar]
- Matsuda H.; Watanabe S.; Yamamoto K. New Macrocyclic Musk Compounds. Chem. Biodivers. 2004, 1, 1985–1991. 10.1002/cbdv.200490152. [DOI] [PubMed] [Google Scholar]
- Fortunati T.; D’Acunto M.; Caruso T.; Spinella A. Chemoenzymatic Preparation of Musky Macrolactones. Tetrahedron 2015, 71, 2357–2362. 10.1016/j.tet.2015.03.007. [DOI] [Google Scholar]
- Liphschitz N.; Gophna R.; Hartman M.; Biger G. The Beginning of Olive (Olea europaea) Cultivation in the Old World: A Reassessment. J. Archaeol. Sci. 1991, 18, 441–453. 10.1016/0305-4403(91)90037-p. [DOI] [Google Scholar]
- Stefanoudaki E.; Kotsifaki F.; Koutsaftakis A. Classification of Virgin Olive Oils of the Two Major Cretan Cultivars Based on Their Fatty Acid Composition. J. Am. Oil Chem. Soc. 1999, 76, 623–626. 10.1007/s11746-999-0013-7. [DOI] [Google Scholar]
- El Riachy M.; Hamade A.; Ayoub R.; Dandachi F.; Chalak L. Oil Content, Fatty Acid and Phenolic Profiles of Some Olive Varieties Growing in Lebanon. Front. Nutr. 2019, 6, 94. 10.3389/fnut.2019.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay R.; Rana R.; Sood A.; Singh V.; Kumar R.; Srivastava V. C.; Maurya S. K. Heterogeneous Vanadium Catalyzed Oxidative Cleavage of Olefins for Sustainable Synthesis of Carboxylic Acids. Chem. Commun. 2021, 57, 5430–5433. 10.1039/d1cc01742j. [DOI] [PubMed] [Google Scholar]
- Maurya S. K.; Patil P.; Umbarkar S. B.; Gurjar M. K.; Dongare M.; Rudiger S.; Kemnitz E. Vapor Phase Oxidation of 4-Fluorotoluene over Vanadia-Titania Catalyst. J. Mol. Catal. A: Chem. 2005, 234, 51–57. 10.1016/j.molcata.2005.02.020. [DOI] [Google Scholar]
- Hong S. H.; Sanders D. P.; Lee C. W.; Grubbs R. H. Prevention of Undesirable Isomerization during Olefin Metathesis. J. Am. Chem. Soc. 2005, 127, 17160–17161. 10.1021/ja052939w. [DOI] [PubMed] [Google Scholar]
- Gunstone F. D.The Chemistry of Oils and Fats: Sources, Composition, Properties and Uses; Gunstone F. D., Ed.; Blackwell Publishing Ltd Editorial: Oxford, 2004. [Google Scholar]
- Yang J.; Liu J.; Ge Y.; Huang W.; Neumann H.; Jackstell R.; Beller M. Direct and Selective Synthesis of Adipic and Other Dicarboxylic Acids by Palladium-Catalyzed Carbonylation of Allylic Alcohols. Angew. Chem., Int. Ed. 2020, 59, 20394–20398. 10.1002/anie.202008916. [DOI] [PubMed] [Google Scholar]
- Ciardiello J. J.; Galloway W. R. J. D.; O’Connor C. J.; Sore H. F.; Stokes J. E.; Wu Y.; Spring D. R. An Expedient Strategy for the Diversity-Oriented Synthesis of Macrocyclic Compounds with Natural Product-like Characteristics. Tetrahedron 2016, 72, 3567–3578. 10.1016/j.tet.2015.10.061. [DOI] [Google Scholar]
- Hagiwara H.; Nakamura T.; Okunaka N.; Hoshi T.; Suzuki T. Catalytic Performance of Ruthenium-Supported Ionic-Liquid Catalysts in Sustainable Synthesis of Macrocyclic Lactones. Helv. Chim. Acta 2010, 93, 175–182. 10.1002/hlca.200900334. [DOI] [Google Scholar]
- Sytniczuk A.; Forcher G.; Grotjahn D. B.; Grela K. Sequential Alkene Isomerization and Ring-Closing Metathesis in Production of Macrocyclic Musks from Biomass. Chem. Eur. J. 2018, 24, 10403–10408. 10.1002/chem.201800728. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.