Abstract
Background: Essential oils represent a major class of natural products which are known for their antimicrobial activity. This study aimed to determine the composition of four Piper essential oils by gas chromatography mass spectrometry, attenuated total reflection infrared, and chemometric analysis. Results: Monoterpene was the most predominant class in Piper nigrum and white pepper (87.6 and 80%, respectively) with the dominance of α-pinene, β-pinene, 3-carene, limonene, and β-caryophyllene. Sesquiterpenes represented 50, 19.6, and 12.3% of the essential oils of Piper longum, white pepper, and P. nigrum, respectively. Unlike other species, Piper cubeba oil was found to be rich in aromatics (59%), with eugenol (10.7%) and methyl eugenol (47.4%) representing the major components along with β-myrcene (21.2%) and 1,8-cineole (6.4%). Only P. longum essential oil comprised about 18.2% of alkanes and 13.6% of alkenes. Application of chemometric analysis utilizing GC/MS and ATR-IR data displayed the same segregation pattern where both principal component analysis and hierarchal cluster analysis revealed that white pepper was most closely related to P. nigrum while being completely discriminated from other Piper species. The Piper oils showed promising inhibitory effects on Helicobacter pylori. P. longum oil recorded the most efficient anti-Helicobacter activity [minimum inhibitory concentration (MIC) value of 1.95 μg/ml, which is the same as the MIC of clarithromycin], followed by the oil of white pepper (MIC = 3.90 μg/ml), while P. cubeba and P. nigrum produced the lowest activity (MIC value of 7.81 μg/ml). Conclusion:Piper essential oils can be used as nutritional supplements or therapeutic drugs to protect against H. pylori infection.
Introduction
The genus Piper (Piperaceae) comprises 2000 species which are native to tropical and subtropical regions mainly in Asia, South America, and Africa. The characteristic spicy aroma of Piper leaves and fruits has made it popular for global use as a food spice since ancient times.1,2 Black pepper (Piper nigrum) has been known as the king of spices.3 White pepper is a processed product of P. nigrum collected when the fruit is reddish in color; the outer black skin layer is peeled off and then sun dried.4,5 Centuries ago, different Piper species were used in traditional folk medicine for many applications. P. nigrum L. was used for its rubefacient, stimulant, anti-inflammatory, and anti-infection activities, as well as for the treatment of gastric ailments.2,4 In addition, white pepper was used in the treatment of infections and stomachache. Piper longum L. (long pepper) is widely used in Ayurvedic and Unani folk medicines for the treatment of respiratory tract disorders, including cold and bronchitis, and as a cough remedy.2,4 Furthermore, Piper cubeba L.F. (tailed pepper) was traditionally used for the alleviation of various infections and gastric disorders.2
Essential oils are well known for their complex chemical composition, and to guarantee their consistency, a fingerprint should be processed. The available targeted techniques to recognize major chemical constituents could be insufficient for understanding the functional food pharmacological actions. A reliable functional fingerprint could be achieved by using different analytical chromatographic tools including gas chromatography (GC) and high-performance liquid chromatography along with mass spectrometry (MS) and infrared (IR) which could be coupled to chemometry.6,7
Essential oils represent a major class of natural products which are known for their antimicrobial activity and for being used as a food preservative. Essential oils obtained from different Piper species have been investigated in previous literature, which revealed the presence of monoterpene hydrocarbons (e.g., α-pinene, β-pinene, β-myrcene, and limonene), oxygenated monoterpenoids (e.g., 1,8-cineole, linalool, terpinen-4-ol, and borneol), sesquiterpene hydrocarbons (e.g. β-caryophyllene, humulene, and α-cubebene), oxygenated sesquiterpenoids (e.g., (E)-nerolidol, caryophyllene oxide, and bisabolol), and some phenylpropanoids.1
Helicobacter pylori is a Gram negative bacteria localized in colonies within the gastric mucosa and affects more than half of the world’s population as per WHO.8 The prevalence may reach even 80–90% in developing countries.9,10H. pylori is implicated as the most common factor in the pathogenesis of gastritis, peptic ulcer, and gastric adenocarcinoma,11 and the WHO has highlighted H. pylori as a class I gastric carcinogen.3
Management of H. pylori infection is based on conventional triple therapy which comprises the combination of a proton pump inhibitor and two antibiotics. However, antibiotic resistance and the development of severe side effects from this therapy are the major causes of treatment failure. The acquired resistance to various antibiotics is also a worldwide concern.8 Eradication rates have been reduced to 80% in the last few years due to increasing antibiotic resistance.9 Considering this major problem, the use of natural products in the therapeutic management of H. pylori represents a new and beneficial strategy over conventional drugs due to the lower side effects of natural products along with their potential new mechanisms of actions which can overcome the multiple drug resistance of this organism.8,12
A previous study revealed that piperine alkaloid from P. nigrum inhibited the growth of H. pylori through suppression of its adhesion to gastric cells as well as its motility.3 However, the anti-H. pylori effect of Piper essential oils has not been illustrated before. The present study aims at testing the inhibitory activity of the essential oils obtained from different Piper species against H. pylori, along with comparison of the metabolic profiling of these oils using GC/MS and ATR-IR coupled to chemometrics.
Materials and Methods
Plant Material and Extraction of Essential Oils
The dried entire fruits of P. nigrum (black and white pepper), P. cubeba, and P. longum were brought in July 2018 from Ragb Al-Attar commercial stores of herbs and spices (Cairo, Egypt). All Piper species have been generously identified by Mohamed El-Gebaly, Professor of Plant Taxonomy, Faculty of Science, Ain Shams University. Voucher specimens of P. nigrum (PHG-P-PN-342), P. cubeba (PHG-P-PC-343) and P. longum (PHG-P-PL-344) were kept at Pharmacognosy department, Faculty of Pharmacy, Ain Shams University.
The dried entire fruits of different Piper species (200 g) were subjected to hydrodistillation (500 ml) for 4 h using a Clevenger-type apparatus. The water-to-plant material ratio was 500 mL: 200 g, and the rate of distillation was 10 mL/min at 100 °C. The yields were 2.35 mL/200 g for P. nigrum, 1.25 mL/200 g for white pepper, 2.50 mL/200 g for P. cubeba, and 0.8 mL/200 g for P. longum; the yield is expressed as volume mL/200 gm weight. The oil yield in percentage (oil g/100 g plant material) wase calculated as follows: 0.905% (P. nigrum), 0.44% (white pepper), 1.01% (P. cubeba), and 0.285% (P. longum).
The oils were collected, desiccated, and then stored at −4 °C in sealed vials protected from light for further analysis.
Analysis of Essential Oils by GC/MS
A gas chromatograph [Shimadzu GCMS-QP 2010 (Kyoto, Japan)] coupled to a mass spectrometer (SSQ 7000 quadrupole; Thermo-Finnigan, Bremen, Germany) was used for identification and quantification of essential oil components. A capillary column (Rtx-5MS-Restek, USA) was used with the following specifications: 30 m length, 0.25 mm internal diameter, and 0.25 μm thickness. The oven initial temperature was set at 45 °C for 2 min and then linearly programmed to 300 °C at 5 °C per min for 5 min. The temperature of the injector and detector was maintained at 250 °C and 280 °C, respectively. A carrier gas (He) was used at a flow rate of 1.41 mL/min. Automated sample injection at (1 μL, 1% v/v) with a split ratio of 1:15 was employed. The operating parameters of the mass spectrometer were 70 eV for the EI mode, 280 °C for the interface temperature, and 200 °C for the ion source temperature, and the scan range was adjusted from 35 to 500 amu. The volatile constituents were identified depending on their Kovats indices (KIs) and their mass spectra. These data were compared with NIST-11, Wiley library database, and previous published data.13 KIs were calculated relative to n-alkane (C8–C28) series injected under the same conditions. Each peak area was calculated relative to the entire chromatogram area (100%). Each sample was measured in three replicates.
Analysis of Essential Oils by ATR-IR Spectroscopy
The mid-IR spectra were measured in the range 375–4000 cm–1 with an attenuated total reflection Fourier transform infrared (ATR/FT-IR) spectrometer (Alpha II, Bruker Optics GmbH, Ettlingen, Germany) utilizing a single reflection configuration (spectral resolution = 4 cm–1 and 32 scans). A zero filling factor of 2 along with a Blackman–Harris 3-term apodization function was employed. The essential oils (5–10 μL) were placed on the surface of the ATR crystal. Each sample was measured in three replicates.
Chemometric Analysis
Chemometric analysis was applied to both GC/MS and ATR-IR phytochemical profiling. Principal component analysis (PCA) comprises the first step in data interpretation, recognition of sample grouping, trends, and strong outliers.14,15 Furthermore, application of hierarchal cluster analysis (HCA) was carried out for clustering of various Piper essential oils. For group building, the clustering pattern was determined by employing the complete linkage method.14,15 For PCA and HCA, Unscrambler X 10.4 software (Computer Aided Modeling, AS, Norway) was utilized.
Evaluation of Anti-Helicobacter Activity
A reference strain (RCMB 031124, ATCC 43504) of Helicobacter pylori was used for testing the inhibitory effect of Piper essential oils. The strain was obtained from the Regional Center for Mycology and Biotechnology (RCMB), Al-Azhar University, Cairo, Egypt. The minimum inhibitory concentrations (MICs) of Piper oils were measured by the well-dilution method with some modifications,16,17 where a 96-well plate was filled with brain heart infusion growth medium in 10% fetal bovine serum (40 μL) and an inoculum (10 μL) of H. pylori (106 cfu/mL). Aliquots (50 μL) of two-fold serial dilutions of each essential oil or the reference antibiotic clarithromycin in DMSO (final concentration ranges were 125 to 0.24 μg/mL) were then added. Sterile growth and control wells were prepared. The sterile wells contained DMSO and media only, while the growth wells contained media and H. pylori without antimicrobials. Incubation of all plates was carried out at 37 °C for 3 days under microaerophilic conditions (85% N2, 10% CO2, and 5% O2). Standard MTT assay was used to determine the bacterial growth.16 40 μL of MTT was added per well and incubated for half an hour. The change to purple color indicated bacterial survival and was recorded using a microplate reader where the absorbance was measured at 550 nm. The percent of inhibition (%) was calculated as follows: [(absorbance of control – absorbance of tested oil)/(absorbance of control)] × 100. All tests were performed in triplicate, and the means (±SD) were calculated.
Results and Discussion
Chemical Composition of Piper Essential Oils
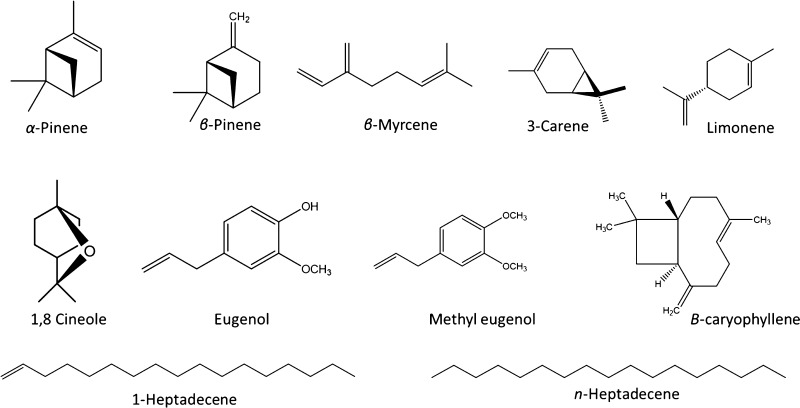
GC/MS analysis of the essential oils of Piper revealed major essential oil classes with variant percentiles (Figure 1). Monoterpene was the most predominant class in P. nigrum and white pepper (87.6 and 80%, respectively) followed by P. cubeba (38.3%) and then P. longum (10.5%). Almost 50% of P. longum volatiles belongs to sesquiterpene class, exemplified by β-caryophyllene (11.9%), α-caryophyllene (6.3%), and caryophyllene oxide (4.7%), whereas sesquiterpenes represented 19.6 and 12.3% of the essential oil of white pepper and P. nigrum, respectively. Unlike the other species, P. cubeba oil was found to be rich in aromatic compounds (59%). It is worth to mention that only P. longum essential oil comprises about 18.2% of alkanes and 13.6% of alkenes. Individual volatile components of P. nigrum and white pepper revealed the predominance of α-pinene (6.6 and 7.3%, respectively), β-pinene (15.9 and 16.2%, respectively), 3-carene (17.6 and 18.0%, respectively), limonene (35.6 and 26.0%, respectively), and β-caryophyllene (9.5, 14.4%). The identified components are illustrated in Table 1. As for the P. longum essential oil, GC/MS analysis recorded the presence of β-caryophyllene (11.8%), n-heptadecene (11.0%), and n-heptadecane (11.9%) as major compounds, whereas β-myrcene (21.2%), 1,8-cineole (6.4%), eugenol (10.7%), and methyl eugenol (47.4%) represented the major components of P. cubeba essential oil. The identification was achieved through the comparison of Kovats retention index along with mass spectrometric data (molecular ion peaks and fragmentation patterns) with those reported in NIST Mass Spectral Library and other published data for reference compounds under the same analytical conditions.13,18 GC/MS analysis provided a useful tool to develop characteristic chromatographic fingerprints for the authentication of the essential oils of Piper. The identified compounds were in accordance with those reported for different Piper species.19,20 The chemical structures of the marker compounds are illustrated in (Figure 2).
Figure 1.
GC/MS chromatograms of Piper essential oils: (a): white pepper, (b) P. nigrum, (c) P. longum, and (d) P. cubeba.
Table 1. GC/MS Analysis of four Piper Essential Oilsa.
| Rt (min) | component | class | KI obs. | KI lit. | P. longum (avg ± SD) | white pepper (avg ± SD) | P. cubeba (avg ± SD) | P. nigrum (avg ± SD) | molecular formula | identification | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 7.34 | tricyclene | monoterpene hydrocarbon | 917 | 914 | 1.71 ± 0.47 | 0.03 ± 0.05 | _ | _ | C10H16 | MS, KI |
| 2 | 7.59 | a-pinene | monoterpene hydrocarbon | 925 | 925 | _ | 7.32 ± 0.68 | 0.40 ± 0.02 | 6.61 ± 3.10 | C10H16 | MS, KI |
| 3 | 8.02 | camphene | monoterpene hydrocarbon | 941 | 941 | _ | 0.21 ± 0.01 | _ | 0.1 ± 0.17 | C10H16 | MS, KI |
| 4 | 8.88 | β-pinene | monoterpene hydrocarbon | 972 | 970 | 4.66 ± 0.58 | 16.18 ± 0.19 | 0.27 ± 0.02 | 15.87 ± 3.48 | C10H16 | MS, KI |
| 5 | 9.42 | β-myrcene | monoterpene hydrocarbon | 992 | 990 | _ | 3.15 ± 0.15 | 21.19 ± 1.21 | 3.02 ± 0.32 | C10H16 | MS, KI |
| 6 | 9.75 | a-phellandrene | monoterpene hydrocarbon | 1003 | 1003 | _ | 3.85 ± 0.23 | 0.12 ± 0.01 | 3.88 ± 0.09 | C10H16 | MS, KI |
| 7 | 9.96 | 3-carene | monoterpene hydrocarbon | 1010 | 1010 | _ | 18.02 ± 1.31 | _ | 17.57 ± 1.12 | C10H16 | MS, KI |
| 8 | 10.13 | a-terpinene | monoterpene hydrocarbon | 1015 | 1015 | _ | 0.12 ± 0.00 | 0.43 ± 0.01 | 0.15 ± 0.01 | C10H16 | MS, KI |
| 9 | 10.38 | p-cymene | monoterpene hydrocarbon | 1024 | 1022 | _ | 1.78 ± 0.02 | 0.24 ± 0.01 | 1.54 ± 0.17 | C10H14 | MS, KI |
| 10 | 10.52 | limonene | monoterpene hydrocarbon | 1028 | 1029 | 3.98 ± 0.14 | 26.03 ± 1.57 | 1.80 ± 0.03 | 35.6 ± 3.60 | C10H16 | MS, KI |
| 11 | 10.6 | 1,8-cineole | oxygenated monoterpene | 1031 | 1030 | _ | _ | 6.41 ± 0.06 | _ | C10H18O | MS, KI |
| 12 | 10.81 | cis-β-ocimene | monoterpene hydrocarbon | 1037 | 1037 | _ | _ | 0.14 ± 0.01 | _ | C10H16 | MS, KI |
| 13 | 11.13 | trans-β-ocimene | monoterpene hydrocarbon | 1048 | 1048 | _ | _ | 1.74 ± 0.01 | _ | C10H16 | MS, KI |
| 14 | 11.46 | γ-terpinene | monoterpene hydrocarbon | 1058 | 1058 | _ | 0.14 ± 0.01 | 0.59 ± 0.01 | 0.13 ± 0.12 | C10H16 | MS, KI |
| 15 | 12.39 | α-terpinolene | monoterpene hydrocarbon | 1088 | 1088 | _ | 0.87 ± 0.09 | 0.64 ± 0.02 | 1.07 ± 0.17 | C10H16 | MS, KI |
| 16 | 12.75 | β-linalool | oxygenated monoterpene | 1100 | 1100 | _ | 0.35 ± 0.05 | 0.63 ± 0.01 | 1.8 ± 0.30 | C10H18O | MS, KI |
| 17 | 14.66 | trans-3-caren-2-ol | oxygenated monoterpene | 1161 | 1160 | _ | 0.19 ± 0.12 | _ | _ | C10H16O | MS, KI |
| 18 | 15.19 | terpinen-4-ol | oxygenated monoterpene | 1178 | 1177 | _ | 0.34 ± 0.06 | 1.15 ± 0.02 | _ | C10H18O | MS, KI |
| 19 | 15.43 | p-cymen-8-ol | aromatic | 1186 | 1185 | _ | 0.13 ± 0.11 | _ | _ | C10H14O | MS, KI |
| 20 | 15.62 | α-terpineol | oxygenated monoterpene | 1192 | 1191 | _ | 1.02 ± 0.23 | 2.41 ± 0.03 | 0.23 ± 0.04 | C10H18O | MS, KI |
| 21 | 15.83 | estragole | aromatic | 1199 | 1197 | _ | 0.04 ± 0.08 | 0.95 ± 0.01 | _ | C10H12O | MS, KI |
| 22 | 15.94 | cis-p-mentha-1(7),8-dien-2-ol | oxygenated monoterpene | 1202 | 1200 | _ | 0.23 ± 0.06 | _ | _ | C10H16O | MS, KI |
| 23 | 16.16 | verbenone | oxygenated monoterpene | 1210 | 1209 | _ | 0.04 ± 0.06 | _ | _ | C10H14O | KI, MS |
| 24 | 16.3 | linalyl formate | oxygenated monoterpene | 1215 | 1215 | _ | 0.04 ± 0.06 | _ | _ | C11H18O2 | MS, KI |
| 25 | 17.52 | piperitone | oxygenated monoterpene | 1258 | 1253 | _ | _ | 0.14 ± 0.01 | _ | C10H16O | MS, KI |
| 26 | 19.87 | δ-eiemene | sesquiterpene hydrocarbon | 1340 | 1337 | _ | 0.36 ± 0.05 | _ | _ | C15H24 | MS, KI |
| 27 | 20.21 | α-cubebene | oxygenated monoterpene | 1352 | 1350 | 0.09 ± 0.17 | 0.07 ± 0.06 | _ | _ | C15H24 | MS, KI |
| 28 | 20.57 | eugenol | aromatic | 1364 | 1359 | _ | 0.06 ± 0.10 | 10.66 ± 0.22 | _ | C10H12O2 | MS, KI |
| 29 | 20.98 | α-copaene | sesquiterpene hydrocarbon | 1379 | 1378 | 0.23 ± 0.20 | 0.41 ± 0.05 | _ | 1.12 ± 0.13 | C15H24 | MS, KI |
| 30 | 21.42 | β-elemene | sesquiterpene hydrocarbon | 1394 | 1392 | 0.97 ± 0.11 | 0.16 ± 0.02 | 0.22 ± 0.00 | _ | C15H24 | MS, KI |
| 31 | 21.96 | methyl eugenol | aromatic | 1415 | 1410 | _ | _ | 47.42 ± 0.83 | _ | C11H14O2 | MS, KI |
| 32 | 22.0 | trans-α-bergamotene | sesquiterpene hydrocarbon | 1416 | 1415 | 0.14 ± 0.12 | _ | _ | _ | C15H24 | MS, KI |
| 33 | 22.24 | β-caryophyllene | sesquiterpene hydrocarbon | 1426 | 1423 | 11.85 ± 1.96 | 14.42 ± 0.87 | 2.13 ± 0.05 | 9.48 ± 1.20 | C15H24 | MS, KI |
| 34 | 22.6 | trans-β-bergamotene | sesquiterpene hydrocarbon | 1437 | 1436 | 0.28 ± 0.07 | _ | _ | _ | C15H24 | MS, KI |
| 35 | 23.14 | α-humulene | sesquiterpene hydrocarbon | 1461 | 1460 | 6.25 ± 0.48 | 0.79 ± 0.12 | 0.32 ± 0.00 | 0.52 ± 0.07 | C15H24 | MS, KI |
| 36 | 23.6 | n- dodecanol | long chain alcohol | 1478 | 1473 | 0.76 ± 0.18 | _ | _ | _ | C12H26O | MS, KI |
| 37 | 23.7 | γ-muurolene | sesquiterpene hydrocarbon | 1481 | 1477 | 0.26 ± 0.23 | _ | _ | _ | C15H24 | MS, KI |
| 38 | 23.8 | γ-himachalene | sesquiterpene hydrocarbon | 1486 | 1483 | 5.04 ± 1.29 | _ | _ | _ | C15H24 | MS, KI |
| 39 | 23.98 | β-selinene | sesquiterpene hydrocarbon | 1493 | 1490 | 1.91 ± 0.06 | _ | _ | 0.25 ± 0.03 | C15H24 | MS, KI |
| 40 | 24.0 | n- pentadecane | alkane | 1500 | 1500 | 5.31 ± 0.25 | _ | _ | _ | C15H32 | MS, KI |
| 41 | 24.1 | isodaucene | sesquiterpene hydrocarbon | 1505 | 1503 | 1.41 ± 0.10 | _ | _ | _ | C15H24 | MS, KI |
| 42 | 24.3 | α-farnesene | sesquiterpene hydrocarbon | 1505 | 1504 | 0.99 ± 0.03 | _ | _ | _ | C15H24 | MS, KI |
| 43 | 24.4 | β-bisabolene | sesquiterpene hydrocarbon | 1511 | 1509 | 3.66 ± 0.29 | _ | _ | _ | C15H24 | MS, KI |
| 44 | 24.7 | epi-α-selinene | sesquiterpene hydrocarbon | 1520 | 1517 | 1.45 ± 0.22 | _ | _ | _ | C15H24 | MS, KI |
| 45 | 24.89 | δ-cadinene | sesquiterpene hydrocarbon | 1529 | 1528 | 0.86 ± 0.27 | 0.26 ± 0.05 | _ | 0.39 ± 0.05 | C15H24 | MS, KI |
| 46 | 24.9 | (E)-γ-bisabolene | sesquiterpene hydrocarbon | 1535 | 1530 | 0.24 ± 0.09 | _ | _ | _ | C15H24 | MS, KI |
| 47 | 25.2 | γ-cuprenene | sesquiterpene hydrocarbon | 1544 | 1539 | 1.11 ± 0.17 | _ | _ | _ | C15H24 | MS, KI |
| 48 | 25.7 | E-nerolidol | oxygenated sespuiterpene | 1564 | 1561 | 0.89 ± 0.18 | _ | _ | _ | C15H26O | MS, KI |
| 49 | 26.2 | copaen-4-α-ol | oxygenated sespuiterpene | 1584 | 1584 | 0.28 ± 0.07 | _ | _ | _ | C15H24O | MS, KI |
| 50 | 26.47 | caryophyllene oxide | oxygenated sespuiterpene | 1591 | 1590 | 4.65 ± 1.06 | 1.78 ± 0.26 | _ | 0.57 ± 0.05 | C15H24O | MS, KI |
| 51 | 27.12 | humulene-1,2-epoxide | oxygenated sespuiterpene | 1618 | 1615 | 2.59 ± 0.08 | 0.03 ± 0.05 | _ | _ | C15H24O | MS, KI |
| 52 | 27.55 | muurola-4,10(14)-dien-1β-ol | oxygenated sespuiterpene | 1637 | 1635 | 0.71 ± 0.11 | 0.39 ± 0.06 | _ | _ | C15H24O | MS, KI |
| 53 | 27.65 | alloaromadendrene oxide | oxygenated sespuiterpene | 1640 | 1639 | 0.87 ± 0.34 | _ | _ | _ | C15H24O | MS, KI |
| 54 | 27.70 | caryophylla-4(12),8(13)-dien-5α-ol | oxygenated sespuiterpene | 1642 | 1640 | _ | 0.22 ± 0.03 | _ | _ | C15H24O | MS, KI |
| 55 | 27.77 | caryophylla-4(12),8(13)-dien-5β-ol | oxygenated sespuiterpene | 1646 | 1641 | 0.85 ± 0.10 | 0.72 ± 0.12 | _ | _ | C15H24O | MS, KI |
| 56 | 28.1 | cedr-8(15)-en-9-α-ol | oxygenated sespuiterpene | 1651 | 1651 | 0.22 ± 0.19 | _ | _ | _ | C15H24O | MS, KI |
| 57 | 28.41 | n-tetradecanol | long chain alcohol | 1676 | 1676 | 5.84 ± 0.92 | _ | _ | _ | C14H30O | MS, KI |
| 58 | 28.48 | germacra-4(15),5,10(14)-trien-1α-ol | oxygenated sespuiterpene | 1680 | 1680 | 0.94 ± 0.21 | _ | _ | _ | C15H24O | MS, KI |
| 59 | 28.56 | occidentalol acetate | oxygenated derivative | 1683 | 1682 | _ | 0.22 ± 0.04 | _ | _ | C17H26O2 | MS, KI |
| 60 | 28.64 | 1-heptadecene | alkene | 1684 | 1682 | 11.03 ± 1.77 | _ | _ | _ | C17H34 | MS, KI |
| 61 | 28.94 | n-heptadecane | alkane | 1703 | 1700 | 11.93 ± 1.83 | _ | _ | _ | C17H36 | MS, KI |
| 62 | 29.10 | (Z,Z)-2,6-farnesol | oxygenated sespuiterpene | 1705 | 1705 | 0.32 ± 0.29 | _ | _ | _ | C15H26O | MS, KI |
| 63 | 32.86 | N-hexadecanol | long chain alcohol | 1872 | 1871 | 1.3 ± 0.30 | _ | _ | _ | C16H34O | MS, KI |
| 64 | 33.06 | 1-nonadecene | alkene | 1881 | 1880 | 2.53 ± 0.68 | _ | _ | _ | C19H38 | MS, KI |
| 65 | 33.23 | N-nonadecane | alkane | 1892 | 1900 | 0.93 ± 0.16 | _ | _ | _ | C19H40 | MS, KI |
| 66 | 39.14 | incensole acetate | oxygenated derivative | 2200 | 2189 | _ | 0.03 ± 0.05 | _ | _ | C21H34O3 | MS, KI |
| total monoterpenes | 10.45 | 79.96 | 38.31 | 87.59 | |||||||
| total sesquiterpenes | 49.0 | 19.55 | 2.67 | 12.33 | |||||||
| total alkenes | 13.56 | 0 | 0 | 0 | |||||||
| total alkanes | 18.16 | 0 | 0 | 0 | |||||||
| total aromatics | 0 | 0.23 | 59.03 | 0 | |||||||
| total long chain alcohols | 7.9 | 0 | 0 | 0 | |||||||
| oxygenated derivatives | 0 | 0.25 | 0 | 0 | |||||||
| total | 99.07 | 99.99 | 100.01 | 99.92 |
Results are expressed as means ± SD of triplicate values. Compounds are listed according to their elution order on a RTX-5 GC column, KI observed, Kovats index determined experimentally on a RTX-5 column relative to a series of C8–C28 n-alkanes. KI lit., Kovat indices reported from previous literature; MS, identification was based on mass spectral data; KI, identification was based on comparison with published Kovats retention indices in NIST Mass Spectral Library (Wiley Registry of Mass Spectral Data, 8th edition) and literature. (_): absent. Bold values are the major constituents in the volatile oil. Percentiles are presented as an average of three runs.
Figure 2.
Chemical structures of the major volatiles in Piper species.
ATR-IR Vibrational Spectroscopic Analysis of Essential Oils
The IR fingerprint region between 1800 and 400 cm–1 demonstrated distinctive spectral data for component identification. Comprehensive spectral analysis of the oils was investigated based on the vibrational spectra of their individual components. The IR spectra showed distinctive key bands which were useful for the discrimination between different Piper species. As shown in Figure 3, the spectra of both white pepper and P. nigrum were closely similar to each other revealing the presence of common major bands. IR spectra of both white pepper and P. nigrum essential oils were dominated by bands pertaining to their main components: limonene (1640 and 885 cm–1), 3-carene (1686, 822, and 721 cm–1), β-pinene (1641 and 873 cm–1), β-caryophyllene (1633, 1452, 1380, and 888 cm–1), and α-pinene (1644, 885 and 787 cm–1). Most of these components showed intense bands in the ATR-IR spectra attributed to (C=C) stretching vibrations at 1640–1680 cm–1, along with the wagging vibrations of CH and CH2 groups (885 and 787 cm–1 for α-pinene and at 885 cm–1 for limonene). These bands are in accordance with the typical bands for these components in the literature.21−23 Regarding P. longum essential oil, the ATR-IR spectrum demonstrated characteristic bands for limonene (1640 and 885 cm–1), β-pinene (1641 and 873 cm–1), β-caryophyllene (1452, 1378, and 885 cm–1), and caryophyllene oxide (1452 and 885 cm–1), whereas bands for β-myrcene (1638, 1590, 989, and 890 cm–1), 1,8-cineole (1374, 1214, 1079, 983, and 848 cm–1), eugenol, and methyl eugenol (1637, 1605, 1590, 1512, 1256, 1028, 995, 910, 848, and 803 cm–1) represented the major components of P. cubeba essential oil. Characteristic bands of 1,8-cineole in the ATR-IR spectrum were attributed to typical C–O–C stretching vibrations of the symmetrical (1079 cm–1) and asymmetrical (1214 cm–1) types along with symmetrical deformation modes pertaining to CH3 at 1374 cm–1, along with wagging vibrations of CH and CH2 groups recognized at 983 cm–1.21,24 Eugenol and methyl eugenol showed a typical signal at 803 cm–1 assigned to CH ring deformation (out-of-plane bending). Spectral CH out-of-plane bending of eugenol and methyl eugenol is seen at 995 cm–1.24,25 These key absorption bands are in accordance with those reported in previous literature concerning essential oils from Piper species.26
Figure 3.
ATR-IR spectra of essential oils of (a) white pepper, (b) P. nigrum, (c) P. longum, and (d) P. cubeba.
Chemometric Analysis
The differences among GC/MS spectra of different Piper species cannot be observed by naked eyes. Consequently, the metabolic profiling elicited by the GC/MS interpretation of Piper essential oils was subjected to PCA together with HCA to illustrate the quantitative and qualitative variations of chemical constituents (66 components, Table 1), as well as the inter-relationships between these oils.
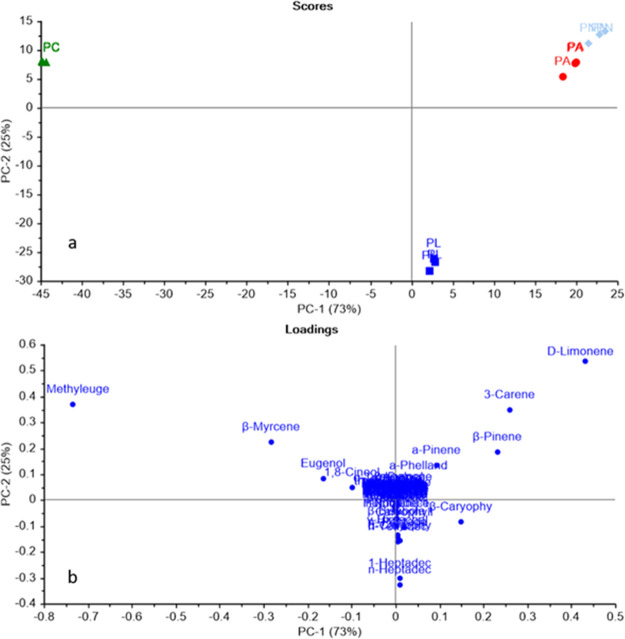
PCA score plot explained 98% of the variance of the data as shown in (Figure 4a) (the first PC represented 73% of the total variance, while the second PC accounted for 25% of variance), where the plot discriminated the four essential oils into three main groups. As observed, P. nigrum and white pepper were completely segregated from other species in a separate quadrant. PCA score plot revealed the closeness of P. longum to both P. nigrum and white pepper as all of them were located on the positive axis with respect to PC1 on the left side, whereas P. cubeba was in a very far distance from all other species on the negative axis of PC1. The loading plot (Figure 4b) showed that limonene, 3-carene, and β-pinene were the main metabolites discriminating P. nigrum and white pepper from other species. As for P. longum essential oil, 1-heptadecene and n-heptadecane distinguished it from other tested oils, whereas methyl eugenol represented the main metabolite discriminating P. cubeba oil. To confirm the results obtained by PCA, HCA was implemented in the unsupervised pattern recognition mode. The HCA dendrogram revealed the discrimination of four oils into three main clutters. As illustrated in (Figure 5), P. nigrum and white pepper were grouped in one main cluster (cluster I); however, they were completely discriminated from each other into two subclusters. HCA dendrograms revealed that P. longum (cluster II) was closely related to P. nigrum and white pepper endorsing the results of PCA. The data obtained from ATR-IR spectroscopy was subjected to chemometric analysis in an attempt to prove the resemblance of GC data with spectral interpretation. As shown in (Figure 6), PCA score plot exhibited the same segregation pattern as that obtained from GC data. Based on these results, it is obvious that the plot discriminated different Piper essential oils into three distinctive groups, with white pepper and P. nigrum in the same quadrant. By applying HCA (Figure 7), it is in accordance with the results of PCA.
Figure 4.
PCA score (a) and loading (b) plots of three replicates of different Piper species based on GC/MS analysis (PL: P. longum, PA: white pepper, PC: P. cubeba, and PN: P. nigrum).
Figure 5.
HCA dendrogram of three replicates of different Piper species based on GC/MS analysis (PL: P. longum, PA: white pepper, PC: P. cubeba, and PN: P. nigrum).
Figure 6.
PCA score plot of three replicates of different Piper species based on ATR-IR spectral analysis (PL: P. longum, PA: white pepper, PC: P. cubeba, and PN: P. nigrum).
Figure 7.
HCA dendrogram of three replicates of different Piper species based on ATR-IR spectral analysis (PL: P. longum, PA: white pepper, PC: P. cubeba, and PN: P. nigrum).
Anti-Helicobacter pylori Activity
In the present study, the four volatile oils of Piper showed promising inhibitory activities against H. pylori. P. longum oil recorded the most efficient anti-Helicobacter activity with an MIC value of 1.95 μg/mL, which is the same as the MIC of clarithromycin (Table 2). This was followed by the oil of white pepper (MIC = 3.90 μg/mL), while P. cubeba and P. nigrum produced the highest MIC value of 7.81 μg/mL. This variation in MIC values could be related to the individual components in each volatile oil. Experimental evidence hase demonstrated that crude essential oils have better antibacterial effects than their single terpene components due to synergistic interactions between the individual essential oil components.27 Therefore, crude essential oils are usually used as antimicrobial agents in many medical and food applications rather than their individual components.28 However, previous articles have illustrated that the presence of certain components in the essential oils contribute to the anti-Helicobacter activity of these oils. α-Pinene, β-pinene, limonene, linalool, cineol, α-terpineol, and eugenol have been shown to be among the most active terpenes against H. pylori.8,29,30 The antibacterial mechanisms include the possibility of these terpenoids to perforate the membrane bacterial cells, affecting the permeability characters of the bacterial cell and leading to cell leakage.31 The presence of these components as dominant ones in the tested essential oils of Piper may explain their anti-Helicobacter activity. Furthermore, the essential oil of mastic gum against clarithromycin-and metronidazole-resistant strain H. pylori infection was attributed mainly to its α-terpineol and methyl eugenol contents. The presence of linalool, limonene, α-pinene, and β-caryophyllene contributed also to the anti-H. pylori activity of mastic gum.11 Similarly, the essential oil of Citrus lemon showed a marked inhibitory effect on H. pylori infection which was attributed to the presence of limonene.8 A recent study has demonstrated that monoterpenes exhibit anti-Helicobacter activity through different mechanisms, for example, decreasing the acid secretions while increasing the mucus.32 Both limonene and β-pinene recorded a marked antimicrobial activity against H. pylori.33 These two monoterpenes were shown to reduce the bacterial resistance to antibiotics. Meanwhile, α-pinene reduced the gastric injury induced by oxidative stress, the reduction of gastric prostaglandins and elevation of histamine resulting from H. pylori infection.31 The major β-myrcene content in P. cubeba (21.2%) could explain its protective action against H. pylori injurious effect on gastric mucosa.9 Furthermore, the high content of aromatic compounds in P. cubeba oil (59%), exemplified by methyl eugenol (47.4%) and eugenol (10.6%), could also contribute to its high antimicrobial activity. In previous studies, eugenol inhibited the viability of 30 strains of H. pylori (MIC = 2 μg/mL) without development of resistance.10,34 The mechanistic role of eugenol on the bacterial cell could be referred to the free hydroxyl group of the aromatic ring.35,36 Methyl eugenol (47.4%), a major constituent of P. cubeba, also exhibited antimicrobial activity as mentioned in previous studies.37,38 All these components may partly explain the anti-Helicobacter activity of Piper essential oils. Notably, this is the first study to demonstrate the anti-Helicobacter activity of these four essential oils of Piper.
Table 2. Anti-Helicobacter pylori Effect of Piper Essential Oilsa.
| inhibition
% |
|||||
|---|---|---|---|---|---|
| sample conc. (μg/mL) | P. longum | white pepper | P. cubeba | P. nigrum | clarithromycin |
| 125 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| 62.5 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| 31.25 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| 15.63 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| 7.81 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| 3.9 | 100 ± 0 | 100 ± 0 | 89.41 ± 0.52 | 82.32 ± 0.83 | 100 ± 0 |
| 1.95 | 100 ± 0 | 84.41 ± 1.96 | 72.32 ± 1.30 | 50.37 ± 0.82 | 100 ± 0 |
| 0.98 | 91.17 ± 0.55 | 61.60 ± 0.73 | 56.73 ± 0.59 | 22.31 ± 0.88 | 91.98 ± 0.42 |
| 0.48 | 73.44 ± 1.45 | 29.97 ± 0.87 | 29.10 ± 0.18 | 9.84 ± 0.51 | 86.28 ± 1.27 |
| 0.24 | 50.74 ± 0.87 | 16.82 ± 1.04 | 12.99 ± 0.85 | 0 | 81.83 ± 0.43 |
| 0 | 0 | 0 | 0 | 0 | 0 |
| MIC (μg/mL) | 1.95 | 3.9 | 7.81 | 7.81 | 1.95 |
The test was done using the standard MTT assay to determine the bacterial growth. For MIC, different concentrations of the tested oils or reference antibiotic clarithromycin were prepared in DMSO (final concentration ranges in the wells were 125 to 0.24 μg/mL). Results are expressed as means ± SD of triplicate values.
Experimental evidence has indicated that P. nigrum, P. cubeba, and P. longum ethanol extracts exhibited potential anti-H. pylori effect, with MIC ranging from 62 to 125 μg/mL.39 Previous studies have indicated that piperine alkaloid inhibited H. pylori growth completely at concentrations of 125 and 250 μM, while the MIC was 125 μM. This may highlight the significance of our results which demonstrated that the essential oils of Piper, especially from P. longum (MIC = 1.95 μg/mL), may have a better inhibitory activity against H. pylori than piperine alkaloid or Piper extracts. Being common ingredients in regular diet, the availability of Piper oils suggests their potential therapeutic use to clear H. pylori infection in asymptomatic patients or as a complementary therapy to conventional drugs to overcome bacterial resistance. Further in vivo and clinical trials are warranted to determine the efficacy of Piper oils against H. pylori infection in human gastric tissues.
Conclusions
In this research, the volatile oils of three Piper species were assessed via GC/MS and ATR-IR. Monoterpene was the most predominant class in P. nigrum and white pepper. By applying chemometric analysis, the same segregation pattern was displayed utilizing GC/MS and ATR-IR data where both PCA and HCA revealed that white pepper was most closely related to P. nigrum. All Piper species were successfully segregated from each other. All Piper oils showed promising inhibitory effects on Helicobacter pylori.
Acknowledgments
The authors would like to thank the research sector of Ain Shams University (2018-R) for providing partial funding for this work.
Glossary
Abbreviations
- ATR-IR
attenuated total reflection-infrared
- DMSO
dimethyl sulfoxide
- GC/MS
gas chromatography–mass spectrometry
- HCA
hierarchal cluster analysis
- He
helium
- H. pylori
Helicobacter pylori
- HPLC
high-performance liquid chromatography
- KI
Kovat’s index
- MIC
minimum inhibitory concentration
- MTT
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]
- NMR
nuclear magnetic resonance
- NIST-11
National Institute of Standards and Technology-11
- PCA
principal component analysis
- RI
refractive (Kovat’s) index
- SD
standard deviation
- WHO
World Health Organization
Author Contributions
§ E.A.-S. and H.A.G. have contributed equally to this paper.
The authors declare no competing financial interest.
Notes
Ethical Approval: This article does not contain any studies with human participants or animals performed by any of the authors.
Notes
Informed Consent: Informed consent is not applicable in this study.
References
- da Silva J.; da Trindade R.; Alves N.; Figueiredo P.; Maia J.; Setzer W. Essential oils from neotropical piper species and their biological activities. Int. J. Mol. Sci. 2017, 18, 2571. 10.3390/ijms18122571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mgbeahuruike E. E.; Yrjönen T.; Vuorela H.; Holm Y. Bioactive compounds from medicinal plants: Focus on Piper species. S. Afr. J. Bot. 2017, 112, 54–69. 10.1016/j.sajb.2017.05.007. [DOI] [Google Scholar]
- Tharmalingam N.; Kim S.-H.; Park M.; Woo H. J.; Kim H. W.; Yang J. Y.; Rhee K.-J.; Kim J. B. Inhibitory effect of piperine on Helicobacter pylori growth and adhesion to gastric adenocarcinoma cells. Infect. Agents Cancer 2014, 9, 43. 10.1186/1750-9378-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaveerach A.; Mokkamul P.; Sudmoon R.; Tanee T. Ethnobotany of the genus Piper (Piperaceae) in Thailand. Ethnobot. Res. Appl. 2006, 4, 223–231. 10.17348/era.4.0.223-231. [DOI] [Google Scholar]
- Abdulazeez M. A.; Sani I.; James B. D.; Abdullahi A. S.. Black Pepper (Piper nigrum L.) Oils. In Essential Oils in Food Preservation, Flavor and Safety; Elsevier, Academic Press, Cambridge, MA, 2016; 277–285. 10.1016/b978-0-12-416641-7.00031-6 [DOI] [Google Scholar]
- Farag M. A.; El-Kersh D. M.; Ehrlich A.; Shokry M.; El-Seedi H.; Frolov A.; Wessjohann L. A. Variation in Ceratonia siliqua pod metabolome in context of its different geographical origin, ripening stage and roasting process. Food Chem 2019, 283, 675. 10.1016/j.foodchem.2018.12.118. [DOI] [PubMed] [Google Scholar]
- Gad H. A.; El-Ahmady S. H.; Abou-Shoer M. I.; Al-Azizi M. M. Application of chemometrics in authentication of herbal medicines: a review. Phytochem. Anal. 2013, 24, 1–24. 10.1002/pca.2378. [DOI] [PubMed] [Google Scholar]
- Bonifácio B.; dos Santos Ramos M.; da Silva P.; Bauab T. Antimicrobial activity of natural products against Helicobacter pylori : a review. Ann. Clin. Microbiol. Antimicrob. 2014, 13, 54. 10.1186/preaccept-1712290149140143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandalari G.; Bisignano C.; Cirmi S.; Navarra M.; Medicine A. Effectiveness of Citrus fruits on Helicobacter pylori. J. Evidence-Based Complementary Altern. Med. 2017, 2017, 8379262. 10.1155/2017/8379262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S. M.; Khan A. A.; Ahmed I.; Musaddiq M.; Ahmed K. S.; Polasa H.; Rao L. V.; Habibullah C. M.; Sechi L. A.; Ahmed N. Antimicrobial activities of Eugenol and Cinnamaldehyde against the human gastric pathogen Helicobacter pylori. Ann. Clin. Microbiol. Antimicrob. 2005, 4, 20. 10.1186/1476-0711-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T.; Okimoto T.; Kuwano M. Chemical Composition of the Essential Oil of Mastic Gum and their Antibacterial Activity Against Drug-Resistant Helicobacter pylori. Nat. Prod. Bioprospect. 2014, 4, 227–231. 10.1007/s13659-014-0033-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sayed E. Unearthing the chemical composition of Taxodium distichum (L.) Rich. leaf essential oil and its antimicrobial activity. Ind. Crops Prod. 2018, 126, 76–82. 10.1016/j.indcrop.2018.10.009. [DOI] [Google Scholar]
- Adams R. P.Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Illinois, USA, 2007. [Google Scholar]
- Brereton R. G.Chemometrics: Applications of Mathematics and Statistics to Laboratory Systems; Ellis Horwood Ltd., 1990. [Google Scholar]
- Brereton R. G.Applied Chemometrics for Scientists; John Wiley & Sons, 2007. [Google Scholar]
- Srikanta B. M.; Harish Nayaka M. A.; Dharmesh S. M. Inhibition of Helicobacter pylori growth and its cytotoxicity by 2-hydroxy 4-methoxy benzaldehyde of Decalepis hamiltonii (Wight & Arn); a new functional attribute. Biochimie 2011, 93, 678–688. 10.1016/j.biochi.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Bonacorsi C.; Raddi M. S. G.; Carlos I. Z.; Sannomiya M.; Vilegas W. Anti-Helicobacter pylori activity and immunostimulatory effect of extracts from Byrsonima crassa Nied. (Malpighiaceae). BMC Complementary Altern. Med. 2009, 9, 2. 10.1186/1472-6882-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIST . The National Institute of Standards and Technology (NIST) Chemistry WebBook. NIST Standard Reference Database Number 69. http://webbook.nist.gov/chemistry/ (accessed January 25, 2021).
- Dosoky N. S.; Satyal P.; Barata L. M.; da Silva J. K. R.; Setzer W. N. Volatiles of black pepper fruits (Piper nigrum L.). Molecules 2019, 24, 4244. 10.3390/molecules24234244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi B.; Zakaria Z. A.; Gyawali R.; Ibrahim S. A.; Rajkovic J.; Shinwari Z. K.; Khan T.; Sharifi-Rad J.; Ozleyen A.; Turkdonmez E.; Valussi M.; Tumer T. B.; Monzote Fidalgo L.; Martorell M.; Setzer W. N. Piper species: A comprehensive review on their phytochemistry, biological activities and applications. Molecules 2019, 24, 1364. 10.3390/molecules24071364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz H.; Özkan G.; Baranska M.; Krüger H.; Özcan M. Characterisation of essential oil plants from Turkey by IR and Raman spectroscopy. Vib. Spectrosc. 2005, 39, 249–256. 10.1016/j.vibspec.2005.04.009. [DOI] [Google Scholar]
- Schulz H.; Baranska M. Identification and quantification of valuable plant substances by IR and Raman spectroscopy. Vib. Spectrosc. 2007, 43, 13–25. 10.1016/j.vibspec.2006.06.001. [DOI] [Google Scholar]
- Baranska M.; Schulz H.; Reitzenstein S.; Uhlemann U.; Strehle M. A.; Krüger H.; Quilitzsch R.; Foley W.; Popp J. Vibrational spectroscopic studies to acquire a quality control method of Eucalyptus essential oils. Biopolymers 2005, 78, 237–248. 10.1002/bip.20284. [DOI] [PubMed] [Google Scholar]
- Farag N. F.; El-Ahmady S. H.; Abdelrahman E. H.; Naumann A.; Schulz H.; Azzam S. M.; El-Kashoury E.-S. A. Characterization of essential oils from Myrtaceae species using ATR-IR vibrational spectroscopy coupled to chemometrics. Ind. Crops Prod. 2018, 124, 870–877. 10.1016/j.indcrop.2018.07.066. [DOI] [Google Scholar]
- Wang L.-H.; Sung W.-C. Rapid evaluation and quantitative analysis of eugenol derivatives in essential oils and cosmetic formulations on human skin using attenuated total reflectance–infrared spectroscopy. J. Spectrosc. 2011, 26, 43. 10.1155/2011/176163. [DOI] [Google Scholar]
- Schulz H.; Baranska M.; Quilitzsch R.; Schütze W.; Lösing G. Characterization of peppercorn, pepper oil, and pepper oleoresin by vibrational spectroscopy methods. J. Agric. Food Chem. 2005, 53, 3358–3363. 10.1021/jf048137m. [DOI] [PubMed] [Google Scholar]
- Nakatsu T.; Lupo A. T.; Chinn J. W.; Kang R. K. L. Biological activity of essential oils and their constituents. Stud. Nat. Prod. Chem. 2000, 21, 571–631. 10.1016/s1572-5995(00)80014-9. [DOI] [Google Scholar]
- Sameh S.; Al-Sayed E.; Labib R. M.; Singab A. N. B. Comparative metabolic profiling of essential oils from Spondias pinnata (Linn. F.) Kurz and characterization of their antibacterial activities. Ind. Crops Prod. 2019, 137, 468–474. 10.1016/j.indcrop.2019.05.060. [DOI] [Google Scholar]
- Bergonzelli G. E.; Donnicola D.; Porta N.; Corthésy-Theulaz I. E. Essential oils as components of a diet-based approach to management of Helicobacter infection. Antimicrob. Agents Chemother. 2003, 47, 3240–3246. 10.1128/aac.47.10.3240-3246.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa-Ruano N.; Becerra-Martínez E.; Cruz-Durán R.; Zarate-Reyes J. A.; Landeta-Cortés G.; Romero-Arenas O. Volatile profiling, insecticidal, antibacterial and antiproliferative properties of the essential oils of Bursera glabrifolia leaves. Chem. Biodiversity 2018, 15, e1800354 10.1002/cbdv.201800354. [DOI] [PubMed] [Google Scholar]
- Khan M. S. A.; Khundmiri S. U. K.; Khundmiri S. R.; Al-Sanea M. M.; Mok P. L. Fruit-derived polysaccharides and terpenoids: Recent update on the gastroprotective effects and mechanisms. Front. Pharmacol. 2018, 9, 569. 10.3389/fphar.2018.00569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Périco L. L.; Emílio-Silva M. T.; Ohara R.; Rodrigues V. P.; Bueno G.; Barbosa-Filho J. M.; Rocha L. R. M. d.; Batista L. M.; Hiruma-Lima C. A. Systematic analysis of monoterpenes: Advances and challenges in the treatment of peptic ulcer diseases. Biomolecules 2020, 10, 265. 10.3390/biom10020265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifácio B. V.; dos Santos Ramos M. A.; Da Silva P. B.; Bauab T. M. Antimicrobial activity of natural products against Helicobacter pylori: a review. Ann. Clin. Microbiol. Antimicrob. 2014, 13, 54. 10.1186/s12941-014-0054-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozioł A.; Stryjewska A.; Librowski T.; Salat K.; Gawel M.; Moniczewski A.; Lochynski S. An overview of the pharmacological properties and potential applications of natural monoterpenes. Mini-Rev. Med. Chem. 2014, 14, 1156–1168. 10.2174/1389557514666141127145820. [DOI] [PubMed] [Google Scholar]
- Marchese A.; Barbieri R.; Coppo E.; Orhan I. E.; Daglia M.; Nabavi S. F.; Izadi M.; Abdollahi M.; Nabavi S. M.; Ajami M. Antimicrobial activity of eugenol and essential oils containing eugenol: A mechanistic viewpoint. Crit. Rev. Microbiol. 2017, 43, 668–689. 10.1080/1040841x.2017.1295225. [DOI] [PubMed] [Google Scholar]
- Kamatou G. P.; Vermaak I.; Viljoen A. M. Eugenol-From the Remote Maluku Islands to the International Market Place: A Review of a Remarkable and Versatile Molecule. Molecules 2012, 17, 6953–6981. 10.3390/molecules17066953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan K. H.; Nishida R. Methyl eugenol: its occurrence, distribution, and role in nature, especially in relation to insect behavior and pollination. J. Insect Sci. 2012, 12, 56. 10.1673/031.012.5601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad A.; Khan A.; Khan L. A.; Manzoor N. In vitro synergy of eugenol and methyleugenol with fluconazole against clinical Candida isolates. J. Med. Microbiol. 2010, 59, 1178–1184. 10.1099/jmm.0.020693-0. [DOI] [PubMed] [Google Scholar]
- Zaidi S. F. H.; Yamada K.; Kadowaki M.; Usmanghani K.; Sugiyama T. Bactericidal activity of medicinal plants, employed for the treatment of gastrointestinal ailments, against Helicobacter pylori. J. Ethnopharmacol. 2009, 121, 286–291. 10.1016/j.jep.2008.11.001. [DOI] [PubMed] [Google Scholar]