Abstract
FreeStyle Libre has been approved for use in patients undergoing hemodialysis (HD) in Japan, unlike Europe and the United States; however, evidence regarding its accuracy in such patients is sparse. Forty‐one participants with type 2 diabetes undergoing HD were recruited. The overall mean absolute relative difference and mean absolute difference were 23.4% and 33.9 mg/dL, respectively. Sensor glucose levels and capillary glucose levels were significantly correlated (r = 0.858, P < .01), although the sensor glucose levels were significantly lower than the capillary glucose levels. The accuracy of FreeStyle Libre in patients undergoing HD became deteriorated with the days of usage. The percentage of sensor results in Zones A and B in the consensus error grid analysis and in the Clarke error grid analysis were 99.7% and 99.0%, respectively. Its insufficient accuracy necessitates adjunct usage of FreeStyle Libre with self‐monitoring of blood glucose in patients undergoing HD.
Keywords: accuracy, continuous glucose monitoring, diabetic nephropathy, FreeStyle Libre, hemodialysis, type 2 diabetes
1. INTRODUCTION
Diabetic nephropathy is the most common cause of hemodialysis (HD) initiation. As the majority of oral antidiabetic drugs are contraindicated for patients with type 2 diabetes (T2D) undergoing HD, insulin therapy is a main treatment option for these patients. As patients undergoing HD are unable to generate glucose in the kidney, they are particularly susceptible to hypoglycemia [1, 2]. In addition, as the glucose concentration in dialysis fluid affects the blood glucose levels of patients undergoing HD, they show unique glycemic excursion, with some presenting with hypoglycemia during and hyperglycemia after HD [3, 4, 5, 6]. Therefore, monitoring the blood glucose levels during and after HD is important for ensuring the safety of HD in such patients.
Self‐monitoring of blood glucose (SMBG) has been the standard modality for determining the blood glucose levels and adjusting the insulin dose appropriately. However, multiple daily repetitions of SMBG impose a large psychological and social burden on the patient due to the pain of fingertip puncture and the inconvenience of the procedure. In addition, it is difficult to determine the detailed blood glucose profile, for example, glycemic variability, as SMBG only detects the blood glucose levels at the time of measurement.
To overcome the limitations associated with SMBG, the technology of continuous glucose monitoring (CGM) was developed to measure the subcutaneous interstitial glucose levels and approximate blood glucose levels. Two main types of CGM are available: real‐time CGM, which displays the approximate blood glucose levels at all times, and retrospective CGM, which is used to analyze the past glucose profile. Real‐time CGM has been used mainly in patients with type 1 diabetes, and evidence of its utility is accumulating. However, it is not widely used in patients with T2D, as it is more expensive than SMBG and evidence of its utility for T2D patients is not accumulated enough at present.
Intermittent‐scanning CGM (isCGM) is a variant of CGM that displays the approximate blood glucose levels only when the sensor is scanned by the reader. At present, FreeStyle Libre (Abbott Diabetes Care, Alameda, CA, USA), is the only isCGM device available in Japan, and its manufacturer named it as “flash glucose monitoring” (FGM) as its commercial nomenclature. Unlike real‐time CGM, FreeStyle Libre has no alarm function to alert for hypoglycemia or hyperglycemia. Notably, it is less expensive than other real‐time CGM devices available in Japan. FreeStyle Libre is factory‐calibrated and does neither require nor accept SMBG measurements for calibration during usage [7]. Interestingly, FreeStyle Libre is approved only for adjunctive use to SMBG in Japan, in contrast to Europe and the United States [8].
In Japan, FreeStyle Libre was not approved for patients undergoing HD when it was initially approved in January 2017 [8], but later such approval was granted in August 2018 [9]. Of note, the usage of FreeStyle Libre in patients undergoing HD is not approved in Europe or the United States [10, 11].
The accuracy of FreeStyle Libre in patients with diabetes not undergoing HD has been widely investigated, and the mean absolute relative difference (MARD) was shown to range between 11.4% and 13.7% in such patients [12, 13, 14, 15]. At present, evidence regarding the accuracy of FreeStyle Libre in patients undergoing HD is sparse. A small study involving seven patients with diabetes mellitus undergoing HD reported the MARD of FreeStyle Libre to be 17.3%, although this study did not include detailed analyses with the day‐to‐day comparison of the accuracy [16]. The accuracy of FreeStyle Libre may be affected by HD, as HD causes rapid and marked changes in the fluid volume of the body, including subcutaneous interstitial tissue fluid.
Given the above, we conducted the precise evaluation of the accuracy of FreeStyle Libre in patients with T2D undergoing HD.
2. PATIENTS AND METHODS
2.1. Study participants
This single‐arm prospective observational study was conducted at 11 research sites (Tokai University School of Medicine Hospital, Kanagawa, Japan; Nihon University School of Medicine Hospital, Tokyo, Japan; Kitasato University School of Medicine Hospital, Kanagawa, Japan; Osaka City University School of Medicine Hospital, Osaka, Japan; Hamamatsu University School of Medicine Hospital, Shizuoka, Japan; Tokyo Medical University Hachioji Medical Center, Tokyo, Japan; Yokohama City University School of Medicine Hospital, Kanagawa, Japan; Seichi Clinic, Kanagawa, Japan; Kurata Hospital, Kanagawa, Japan; Sohbudai Nieren Clinic, Kanagawa, Japan; Hamana Clinic, Shizuoka, Japan).
This study protocol was approved by the Institutional Review Board for Clinical Research, Tokai University School of Medicine (18R‐287). All study methods were performed in accordance with the relevant guidelines and the Ethical Guidelines for Medical and Health Research Involving Human Subjects in Japan and under the Code of Ethics of the Helsinki Declaration, 1964, and written informed consent was obtained from all participants.
Forty‐one participants with T2D undergoing HD were recruited. T2D was defined by the Japan Diabetes Society criteria [17]. All participants underwent HD three times a week on inpatient or outpatient days for 2 to 6 h at a time. All patients used insulin, GLP‐1 receptor agonists or both. Participants were confirmed to be free from any of the following exclusion criteria: blindness, the history of severe hypoglycemia within the past year, usage of other implantable medical devices (e.g., pacemakers), usage of insulin pumps, the presence of shunts in both upper extremities, and pregnancy.
2.2. Study design
All participants were fitted with the FreeStyle Libre sensor, and baseline characteristic were collected at visit 1. FreeStyle Libre sensor was attached at the upper arm contralateral of the HD shunt between the start of HD and 2 h later. It was worn on Monday or Wednesday for patients on a Monday/Wednesday/Friday HD schedule and on Tuesday or Thursday for patients on a Tuesday/Thursday/Saturday HD schedule to allow staff to deal with any problems associated with FreeStyle Libre sensor. To measure capillary blood glucose levels as reference, all participants used FS Precision Blood Glucose Measurement Electrode test strips (Abbott Diabetes Care) in combination with FreeStyle Libre reader. FS Precision Blood Glucose Measurement Electrode test strips use glucose dehydrogenase, nicotinamide adenine dinucleotide, and phenanthroline quinone [18, 19]. The first FreeStyle Libre scanning and finger‐prick blood glucose test were performed at the end of HD. We instructed patients to perform sensor scanning at least six times a day and measure capillary blood glucose levels within 3 min of scanning. On the dialysis day, sensor scanning and measuring of capillary blood glucose levels were performed within 30 min before shunt puncture and within 30 min after removing the needle.
Data from FreeStyle Libre reader were downloaded using FreeStyle Libre Software program, Version 1.0 (Abbott Diabetes Care), and saved to log files as text data. Paired measurements of sensor glucose levels and capillary blood glucose levels were compared, and the maximum difference of 3 min between the two different measurements was used to pair the data [20].
Four sensor lots were used through this study (lot number: 181009Q, 190119Q, 190201P, 191004Q). We investigated the relationship between sensor glucose levels and capillary blood glucose levels over 14 days. After the final sensor scanning and measuring of capillary blood glucose levels, FreeStyle Libre reader and sensor were retrieved from the study participants, and the log files were sent to the data center in anonymized form.
Blood samples were drawn before HD at day 1 to measure glycated albumin levels and at both before and after HD at day 1 to measure hemoglobin (Hb) concentration, hematocrit (Ht) and serum albumin levels. Glycated albumin was measured by using Lucica glycated albumin‐L assay kit (Asahi Kasei, Tokyo, Japan).
2.3. Study outcomes
To evaluate the accuracy of FreeStyle Libre, MARD, and mean absolute difference (MAD) were calculated [21]. As these values are affected by the capillary blood glucose levels, MARD and MAD in the ranges of ≥100 and < 100 mg/dL were also calculated [20].
The primary outcome of this study was the MARD over 14 days. The secondary outcomes were as follows:
MAD over 14 days
MAD and MARD in the capillary blood glucose range ≥ 100 mg/dL
MAD and MARD in the capillary blood glucose range < 100 mg/dL
MARD and MAD for each day in capillary blood glucose ranges of all, ≥100 mg/dL and < 100 mg/dL
MARD and MAD before dialysis (within 30 min of shunt puncture) in capillary blood glucose ranges of all, ≥100 mg/dL and < 100 mg/dL
MARD and MAD after dialysis (within 30 min of the removing a needle) in capillary blood glucose range of all, ≥100 mg/dL and < 100 mg/dL
Consensus error grid analysis over 14 days
Clarke error grid analysis over 14 days
Histogram of MARD per FreeStyle Libre sensor
The correlations of MARD with the variables and laboratory data (age, sex, diabetes duration, height, dry weight [DW], body mass index [BMI], delta body weight [Delta BW], water removing quantity, Albumin [Alb], GA, Hemoglobin [Hb] [before HD, after HD] and Hematocrit [Ht] [before HD, after HD])
Adverse events (severe adverse events [SAEs] and adverse events [AEs])
2.4. Statistical analyses
Statistical analyses were performed using Student's t‐test or analysis of variance (ANOVA), followed by Tukey's test for multiple comparisons. The relationship between two continuous variables was expressed by Pearson's correlation coefficient. The consensus error grid for type 1 diabetes used for regulatory purposes and the Clarke error grid were analyzed to assess the clinical significance of difference [22, 23, 24]. P values of <.05 were considered to indicate statistical significance. Analyses were carried out using R version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria).
3. RESULTS
3.1. Patient characteristics
Forty‐one participants were recruited. The baseline characteristics are described in Table 1. Among them, 36 participants wore FreeStyle Libre sensor for 14 days, and five participants experienced the early termination of usage due to the detachment of the sensor at days 3, 6, 8, 10, and 13 (n = 1 each).
TABLE 1.
Baseline characteristics of the participants
| Age, years | 67.4 (11.5) |
|---|---|
| Male, % | 68.3 |
| Diabetes duration, years | 23.4 (9.8) |
| Dry weight, kg | 58.9 (13.9) |
| BMI, kg/m2 | 22.6 (3.9) |
| GA, % | 20.5 (3.6) |
| Hb, g/dL | 10.9 (0.9) |
Note: Data are presented as mean (standard deviation) or percentage.
Abbreviations: BMI, body mass index; GA, glycoalbumin; Hb, hemoglobin.
3.2. MARD and MAD
The overall MARD was 23.4% (Table 2). The overall MAD was 33.9 mg/dL. The MARD after HD was significantly greater than that before HD for all glucose ranges and at blood glucose levels of ≥100 mg/dL.
TABLE 2.
The MARD and MAD
| Variables | All (2885 points) | BG <100 (447 points) | BG ≥100 (2438 points) |
|---|---|---|---|
| MARD, % | |||
| 14 days | 23.4 (12.9) | 25.1 (13.3) | 23.1 (12.8) |
| Before HD | 21.7 (12.2) | 22.3 (16.2) | 21.6 (11.9) |
| After HD | 25.8 (11.9)* | 24.7 (13.3) | 26.3 (11.3)* |
| MAD, mg/dL | |||
| 14 days | 33.9 (22.8) | 21.8 (11.8) | 36.2 (23.7) |
| Before HD | 33.8 (21.3) | 19.9 (15.2) | 34.8 (21.3) |
| After HD | 30.5 (17.1) | 20.9 (11.7) | 35.1 (17.4) |
Note: Data are presented as mean (standard deviation).
Abbreviations: BG, blood glucose; HD, hemodialysis; MARD, mean absolute relative difference; MAD, mean absolute difference.
P < .05 (vs. before dialysis).
3.3. The comparison of sensor and capillary glucose levels
The sensor and capillary glucose levels were significantly correlated (r = 0.858, P < .01). However, the overall sensor glucose levels were significantly lower than the overall capillary glucose levels (116.0 ± 48.0 mg/dL vs. 147.2 ± 51.1 mg/dL, P < .01) and at days 1 to 14 (Table 3).
TABLE 3.
The comparison between the sensor and capillary blood glucose levels by day
| Day | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sensor glucose, mg/dL | 135.4 (59.0) | 134.7 (52.3) | 123.8 (48.3) | 129.3 (43.5) | 125.8 (51.1) | 121.0 (51.4) | 123.3 (51.3) | 110.7 (44.9) | 111.2 (42.5) | 102.2 (39.8) | 104.7 (37.0) | 97.1 (36.7) | 98.3 (39.0) | 95.4 (38.9) |
| Capillary blood glucose, mg/dL | 149.1 (55.7) | 149.3 (48.8) | 144.7 (49.3) | 152.3 (47.2) | 151.5 (55.6) | 148.1 (57.2) | 152.6 (58.3) | 143.5 (51.8) |
144.8 (46.8) |
141.6 (49.5) | 146.9 (46.8) | 143.1 (46.1) | 145.4 (48.6) | 146.3 (49.9) |
| P value | <.01* | <.01* | <.01* | <.01* | <.01* | <.01* | <.01* | <.01* | <.01* | <.01* | <.01* | <.01* | <.01* | <.01* |
Note: Data are presented as the mean (standard deviation) or number.
P < .05.
3.4. Consensus and Clarke error grid analyses
The percentage of sensor results in Zones A and B in the consensus error grid analysis (Figure 1(A)) and in the Clarke error grid analysis (Figure 1(B)) were 99.7% and 99.0%, respectively .
FIGURE 1.
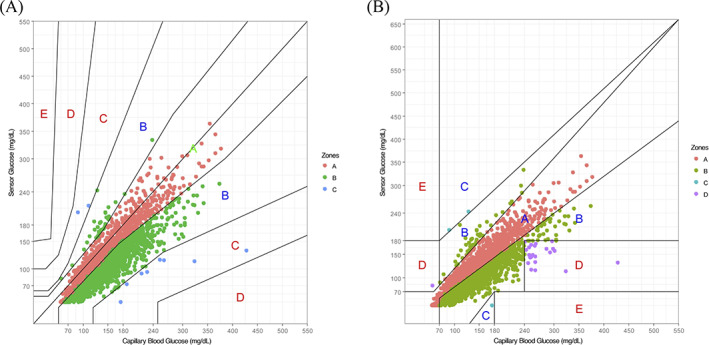
Error grid analyses. (A) Consensus error grid analysis. The ratios of zone A, zone B, zone C, zone D, and zone E were 39.8%, 59.9%, 0.3% 0.0%, and 0.0%, respectively. (B) Clarke error grid analysis. The ratios of zone A, zone B, zone C, zone D, and zone E were 41.8%, 57.2%, 0.1%, 0.8%, and 0.0%, respectively [Color figure can be viewed at wileyonlinelibrary.com]
3.5. Changes in the MARD and MAD by day
The MARD and MAD differed significantly among days (Table 4). The MARD (all ranges and ≥ 100 mg/dL) and MAD values (all ranges and ≥ 100 mg/dL) from day 5 were significantly greater than those at day 1.
TABLE 4.
Changes in the MARD and MAD by day
| Day | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MARD, % |
13.8 (11.1) |
13.9 (9.7) |
17.1 (10.1) |
16.9 (9.7) |
18.9 * (8.6) |
21.0 * (13.1) |
21.6 * (9.3) |
24.5 * (10.0) |
25.3 * (11.6) |
28.7 * (10.9) |
29.7 * (10.7) |
33.5 * (10.3) |
33.3 * (13.1) |
36.1 * (13.1) |
|
MARD, % BG ≥100 |
12.9 (10.7) |
14.2 (9.8) |
16.9 (10.4) |
16.5 (9.6) |
19.0 * (8.6) |
20.0 * (10.9) |
21.0 * (9.2) |
24.3 * (9.8) |
25.0 * (11.2) |
27.8 * (10.9) |
29.8 * (10.6) |
33.2 * (9.8) |
34.0 * (13.2) |
36.5 * (13.8) |
|
MARD, % BG <100 |
18.1 (12.1) |
12.2 (8.8) |
17.8 (8.3) |
20.6 (9.8) |
18.5 (8.5) |
25.7 (20.2) |
24.5 (9.4) |
25.8 (11.3) |
27.0 (13.3) |
32.5 * (10.2) |
29.1 * (11.5) |
35.7 * (12.7) |
29.2 * (11.6) |
34.2 * (9.4) |
| MAD, mg/dL |
19.2 (15.8) |
20.4 (15.8) |
23.7 (15.3) |
25.4 (17.4) |
28.1 * (16.0) |
30.9 * (28.3) |
32.5 * (18.6) |
34.4 * (17.8) |
36.3 * (20.0) |
40.5 * (22.5) |
44.2 * (22.9) |
47.8 * (21.3) |
48.3 * (24.8) |
53.2 * (28.0) |
|
MAD, mg/dL BG ≥100 |
20.0 (16.6) |
21.8 (16.3) |
25.4 (16.0) |
26.4 (18.0) |
29.9 * (16.2) |
32.7 * (29.8) |
34.6 * (19.2) |
36.9 * (18.0) |
38.5 * (20.3) |
43.5 * (23.6) |
47.6 * (22.8) |
50.5 * (21.4) |
52.6 * (24.2) |
58.3 * (28.2) |
|
MAD, mg/dL BG <100 |
15.6 (10.5) |
11.0 (7.6) |
15.4 (7.2) |
17.6 (7.6) |
16.4 (7.2) |
22.6 (18.1) |
21.5 (8.9) |
21.8 (9.6) |
23.3 (11.6) |
28.0 * (9.8) |
25.4 * (11.0) |
31.7 * (12.2) |
24.4 * (10.3) |
30.3 * (8.1) |
Note: Data are presented as mean (standard deviation).
Abbreviations: BG, blood glucose; MARD, mean absolute relative difference; MAD, mean absolute difference.
P < .05 (vs. day 1).
3.6. Correlation between the MARD and patient characteristics
The MARD was negatively correlated with the DW and BMI at baseline (Table 5). The MARD was also negatively correlated with the Hb levels and Ht levels after dialysis (Table 5).
TABLE 5.
The correlation of the MARD with variables and laboratory data
| r | P | |
|---|---|---|
| Age, years | 0.276 (−0.034, 0.538) | .080 |
| Male | −0.059 (−0.360, 0.254) | .716 |
| Diabetes duration | 0.043 (−0.322, 0.397) | .820 |
| Height | −0.136 (−0.426, 0.179) | .396 |
| DW | −0.326 (−0.576, −0.020) | .038* |
| BMI | −0.359 (−0.600, −0.058) | .021* |
| Delta BW | −0.079 (−0.397, 0.256) | .646 |
| Water removing quantity | −0.028 (−0.359,0.307) | .865 |
| Alb | −0.281 (−0.542, 0.029) | .075 |
| GA | 0.153 (−0.162, 0.440) | .339 |
| Hb | ||
| Before HD | −0.179 (−0.461, 0.136) | .263 |
| After HD | −0.321 (−0.572, −0.015) | .041* |
| Ht | ||
| Before HD | −0.210 (−0.486, 0.104) | .188 |
| After HD | −0.331 (−0.579, −0.026) | .035* |
Note: Mean (95% confidence interval).
Abbreviations: DW, dry weight; BMI, body mass index; Delta BW, delta body weight; Alb, albumin; GA, glycoalbumin; HD, hemodialysis; Hb, hemoglobin; Ht, hematocrit.
P < .05.
3.7. The MARD per sensor
The distribution of the MARD per sensor was observed in wide range between 10% and 40% with two peaks (Figure 2).
FIGURE 2.
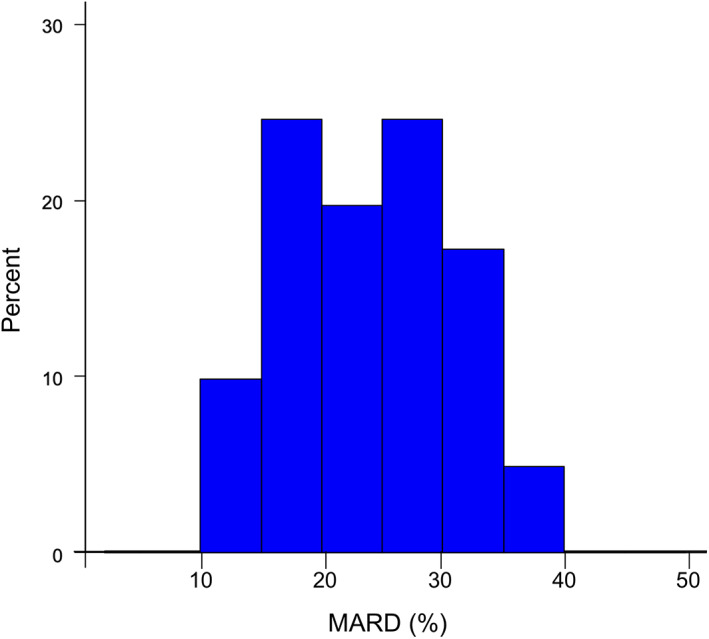
Histogram of the mean absolute relative difference (MARD) per sensor. There were two peaks in the distribution of the MARD per sensor at 15–20% and 25–30% [Color figure can be viewed at wileyonlinelibrary.com]
3.8. Safety
No SAE was observed during the study. Regarding AEs, skin irritation was observed in 7.3% of the participants and no other AE was reported.
4. DISCUSSION
In this study, we evaluated the accuracy of FreeStyle Libre in patients with T2DM undergoing HD by comparing the sensor glucose levels to the capillary blood glucose levels as reference. The overall MARD and MAD values were as great as 23.4% and 33.9 mg/dL, respectively. As described in Table 3, the sensor glucose levels were significantly lower than the capillary blood glucose levels in T2DM patients undergoing HD. Furthermore, the MARD and MAD were significantly greater after day 5 than they had been at day 1. However, both consensus error grid analysis and Clarke error grid analysis showed that the majority of the measurements belonged to zones A (no effect on clinical action, or clinically correct treatment decisions) and B (altered clinical action—little or no effect on clinical outcome, or benign) [22, 23, 24]. The MARD of the individual sensor was distributed widely. The MARD correlated negatively with the DW, BMI, Hb after HD, and Ht after HD, suggesting that a low DW, BMI, Hb after HD, and Ht after HD may predict a greater difference between the sensor glucose levels and the capillary glucose levels used in patients with T2DM undergoing HD.
The MARD and MAD observed in this study were greater than those observed in studies conducted with patients not undergoing HD [12, 13, 14, 15]. Of note, the sensor glucose levels were significantly lower than the capillary blood glucose levels from the first day to the last day of usage, suggesting that the algorithm used in FreeStyle Libre may not be optimized for usage in patients undergoing HD. Unlike some real‐time CGM devices from other manufacturers, FreeStyle Libre cannot be calibrated by capillary glucose levels. Therefore, its adjunct usage with SMBG is strongly recommended when applied to patients undergoing HD. Patients are encouraged to conduct confirmatory SMBG, especially when treatment decisions are being made based on the measurement by FreeStyle Libre.
Nevertheless, using FreeStyle Libre may be beneficial in patients undergoing HD, as it provides extra information as CGM. Information from the downward trend arrow can be used to predict impending hypoglycemia in combination with the information from SMBG, supporting the proactive prevention of hypoglycemia. In addition, information from the trend graph can be used to identify potential hyperglycemia or hypoglycemia between SMBG measurements, especially during the night. Using FreeStyle Libre may also be beneficial in patients with gastroparesis caused by diabetic autonomic neuropathy [25], which is a common complication in diabetic patients undergoing HD. In such patients, glucose excursion can be quite unpredictable; both unexpected hypoglycemia and hyperglycemia occurs due to delayed emptying of the stomach and the mismatch to the action of rapid acting insulin. By using FreeStyle Libre, patients with gastroparesis and their healthcare providers will be able to find and try to mitigate the unfavorable hypoglycemia and hyperglycemia caused by delayed emptying of the stomach. If these patients are able to adjust the timing of the injection of rapid acting insulin or change it to regular insulin, the peak of insulin action and the postprandial glycemia might match better.
Another potential issue with FreeStyle Libre used in patients undergoing HD is that our data suggest the possibility of overestimating the time below range (TBR). As minimizing the TBR is recommended in the guideline [26], the TBR measured by FreeStyle Libre might mislead treatment decisions when used in patients undergoing HD. To address this potential issue, further investigations regarding the accuracy of the TBR in this population will be required.
The present study is strengthened by its inclusion of an assessment of factors associated with the accuracy of FreeStyle Libre in patients undergoing HD. The observation that the MARD correlated negatively with the DW and BMI suggests that a smaller body mass might be associated with a worse accuracy of FreeStyle Libre in patients undergoing HD. In contrast, the observation that the MARD did not correlate with the change in BW or quantity of water removed suggests that these factors might not be determinants of the accuracy of FreeStyle Libre in patients undergoing HD. The observation that the MARD correlated negatively with the Hb after HD and Ht after HD suggests that insufficient water removal after HD might lead to an excess water volume in the interstitial space, thereby affecting the glucose concentration in that location. The two peaks observed in the histogram of the MARD per sensor are consistent with the possibility of the multiple factors that could affect the accuracy of the measurement by FreeStyle Libre sensor, but further investigation is required to clarify the detailed mechanism.
This study is also strengthened by its multicentered, prospective observatory design. However, it is limited by including only type 2 diabetes patients and its lack of body composition data.
5. CONCLUSIONS
The accuracy of FreeStyle Libre in patients undergoing hemodialysis became deteriorated with the days of usage. The mean absolute relative difference after hemodialysis was significantly elevated compared with mean absolute relative difference before hemodialysis. The sensor glucose levels were significantly lower than the capillary glucose levels in these patients. Its insufficient accuracy might potentially lead to the inappropriate diagnosis of hypoglycemia or the missed diagnosis of hyperglycemia, and therefore necessitates adjunct usage of FreeStyle Libre with self‐monitoring of blood glucose in patients undergoing hemodialysis.
CONFLICT OF INTEREST
The author(s) declare the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Masao Toyoda discloses the following relationships: personal fees from Abbott, Medtronic, Terumo; grants from Abbott, LifeScan, Sanwa Kagaku Kenkyusho, Terumo, and Japan Agency for Medical Research. Takashi Murata discloses the following relationships: grants from Japan Agency for Medical Research and Development and Japan IDDM Network, Non‐Profit Corporation. Moritsugu Kimura discloses the following relationships: personal fees from Abbott, Medtronic. Akinori Hayashi discloses the following relationships: personal fees from Abbott, Medtronic, Roche, Terumo. Daisuke Tsuriya discloses the following relationships: personal fees from Abbott. Takaya Matsushita discloses the following relationships: personal fees from Abbott, Sanwa Kagaku Kenkyusho, Terumo; grants from Abbott, Sanwa Kagaku Kenkyusho. Tadashi Yamakawa discloses the following relationships: personal fees from Abbott, Medtronic, Terumo. Katsuhito Mori discloses the following relationships: personal fees from Sanwa Kagaku Kenkyusho, Medtronic. Akio Kuroda discloses the following relationships: personal fees from Abbott, Medtronic, Terumo, LifeScan. Junnosuke Miura discloses the following relationships: personal fees from Abbott, LifeScan, Medtronic, NIPRO, Terumo. Yushi Hirota discloses the following relationships: personal fees from Abbott, Medtronic, Sanwa Kagaku Kenkyusho, Terumo. Masanori Abe discloses the following relationships: chairs courses endowed by NIPRO and Terumo. Naoki Sakane discloses the following relationships: personal fees from LifeScan. Kiminori Hosoda discloses the following relationships: grant from Japan Agency for Medical Research. The other authors declare no conflict of interest for this article.
ACKNOWLEDGMENTS
The authors thank Ms. Satomi Nakano (Clinical Research Service, Co., Osaka, Japan) for indispensable support to the protocol design.
Toyoda M, Murata T, Saito N, et al. Assessment of the accuracy of an intermittent‐scanning continuous glucose monitoring device in patients with type 2 diabetes mellitus undergoing hemodialysis (AIDT2H) study. Ther Apher Dial. 2021;25:586–594. 10.1111/1744-9987.13618
Funding information Japan Agency for Medical Research and Development, Grant/Award Numbers: 18ek0210104h0001, 19ek0210104h0002, 20ek0210104h0003
The copyright line for this article was changed on 4 October 2021 after original online publication.
REFERENCES
- 1. Cersosimo E, Garlick P, Ferretti J. Renal substrate metabolism and gluconeogenesis during hypoglycemia in humans. Diabetes. 2000;49:1186–93. [DOI] [PubMed] [Google Scholar]
- 2. Ahmad I, Zelnick LR, Batacchi Z, Robinson N, Dighe A, Manski‐Nankervis JAE, et al. Hypoglycemia in people with type 2 diabetes and CKD. Clin J Am Soc Nephrol. 2019;14:844–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kazempour‐Ardebili S, Lecamwasam VL, Dassanyake T, Frankel AH, Tam FWK, Dornhorst A, et al. Assessing glycemic control in maintenance hemodialysis patients with type 2 diabetes. Diabetes Care. 2009;32:1137–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Riveline JP, Teynie J, Belmouaz S, Franc S, Dardari D, Bauwens M, et al. Glycaemic control in type 2 diabetic patients on chronic haemodialysis: use of a continuous glucose monitoring system. Nephrol Dial Transplant. 2009;24:2866–71. [DOI] [PubMed] [Google Scholar]
- 5. Jung HS, Kim HI, Kim MJ, Yoon JW, Ahn HY, Cho YM, et al. Analysis of hemodialysis‐associatedhypoglycemia in patients with type 2 diabetes using a continuous glucose monitoring system. Diabetes Technol Ther. 2010;12:801–7. [DOI] [PubMed] [Google Scholar]
- 6. Hayashi A, Takano K, Masaki T, Yoshino S, Ogawa A, Shichiri M. Distinct biomarker roles for HbA1c and glycated albumin in patients with type 2 diabetes on hemodialysis. J Diabetes Complicat. 2016;30:1494–9. [DOI] [PubMed] [Google Scholar]
- 7. Hoss U, Budiman ES. Factory‐calibrated continuous glucose sensors: the science behind the technology. Diabetes Technol Ther. 2017;19:S44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Murata T, Sakane N, Kato K, Tone A, Toyoda M. The current intermittent‐scanning CGM device situation in Japan: only adjunctive use to SMBG is approved and the latest health insurance coverage details. J Diabetes Sci Technol. 2018;12:729–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abbott Japan, FreeStyle Libre (6th edition, December 2019). https://www.myfreestyle.jp/hcp/products/freestyle-libre/pdf/pdf-spec-02.pdf (in Japanese). Accessed 22 Dec 2020.
- 10.Abbott. The FreeStyle Libre FLASH GLUCOSE MONITORING SYSTEM User's Manual. https://www.diabetescare.abbott/support/manuals/uk.html. Acessed 22 Dec 2020.
- 11.Abbott, FreeStyle Libre Indications and Important Safety Information. https://www.freestyle.abbott/us-en/safety-information.html. Acessed 22 Dec 2020.
- 12. Bailey T, Bode BW, Christiansen MP, Klaff LJ, Alva S. The performance and usability of a factory‐calibrated flash glucose monitoring system. Diabetes Technol Ther. 2015;17:787–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ólafsdóttir AF, Attvall S, Sandgren U, Dahlqvist S, Pivodic A, Skrtic S, et al. A clinical trial of the accuracy and treatment experience of the flash glucose monitor FreeStyle Libre in adults with type 1 diabetes. Diabetes Technol Ther. 2017;19:164–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Staal OM, Hansen HMU, Christiansen SC, Fougner AL, Carlsen SM, Stavdahl O. Differences between flash glucose monitor and Fingerprick measurements. Biosensors (Basel). 2018;8:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boscari F, Galasso S, Facchinetti A, Marescotti MC, Vallone V, Amato AML, et al. The FreeStyle Libre and Dexcom G4 platinum sensors: accuracy comparisons during two weeks of home use and use during experimentally induced glucose excursions. Nutr Metab Cardiovasc Dis. 2018;28:180–6. [DOI] [PubMed] [Google Scholar]
- 16. Odaguchi N, Shimajiri T, Tamaki T, Inamine M, Noomo H, Sunakawa H, et al. Experience of the use of a flash glucose monitoring system by hemodialysis patients. J Jpn Soc Dial Ther. 2019;52:253–60.(in Japanese). [Google Scholar]
- 17. Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus , Seino Y, Nanjo K, Tajima N, Kadowaki T, Kashiwagi A, et al. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Investig. 2010;19(1):212–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abbott, FS Precision Blood Glucose Measurement Electrode (4th edition, December 2019). https://www.pmda.go.jp/PmdaSearch/ivdDetail/ResultDataSetPDF/100159_22600AMX01286000_A_01_03 (in Japanese). Accessed 22 Dec 2020.
- 19.Abbott. Clinical Study "Evaluation of the FreeStyle Precision Neo Blood Glucose Monitoring System, 2015. ". https://www.myfreestyle.com/content/dam/adc/myfreestyle-hcp/provider/pdf/EVALUATION-OF-THE-FS-PRECISION-NEO-BLOOD-GLUCOSE-MONITORING-SYSTEM-HCP-CONSUMER.pdf. Accessed 22 Dec 2020.
- 20. Ida S, Goto H, Ida S, et al. Accuracy of a factory calibrated retrospective CGM device and the comparison to a conventionally calibrated retrospective CGM device: a pilot study. Biomed Sci. 2018;4:32–6. [Google Scholar]
- 21. Murata T, Nirengi S, Kawaguchi Y, Sukino S, Watanabe T, Sakane N. Accuracy of a novel “factory‐calibrated” continuous glucose monitoring device in normal glucose levels: a pilot study. Biomed Sci. 2017;3:109–13. [Google Scholar]
- 22. Parkes JL, Slatin SL, Pardo S, Ginsberg BH. A new consensus error grid to evaluate the clinical significance of inaccuracies in the measurement of blood glucose. Diabetes Care. 2000;23:1143–8. [DOI] [PubMed] [Google Scholar]
- 23. Pfützner A, Klonoff DC, Pardo S, Parkes JL. Technical aspects of the Parkes error grid. J Diabetes Sci Technol. 2013;7:1275–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Clarke WL, Cox D, Gonder‐Frederick LA, Carter W, Pohl SL. Evaluating clinical accuracy of systems for self‐monitoring of blood glucose. Diabetes Care. 1987;10:622–8. [DOI] [PubMed] [Google Scholar]
- 25. Kalantar‐Zadeh K, Derose SF, Nicholas S, Benner D, Sharma K, Kovesdy CP. Burnt‐out diabetes: impact of chronic kidney disease progression on the natural course of diabetes mellitus. J Ren Nutr. 2009;19:33–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Battelino T, Danne T, Bergenstal RM, Amiel SA, Beck R, Biester T, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42:1593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]


