Abstract

Whole genome sequencing is emerging as a promising tool for the untargeted detection of a broad range of microbial species for diagnosis and analysis. However, it is logistically challenging to perform the multistep process from sample preparation to DNA amplification to sequencing and analysis within a short turnaround time. To address this challenge, we developed a digital microfluidic device for rapid whole genome amplification of low-abundance bacterial DNA and compared results with conventional in-tube DNA amplification. In this work, we chose Corynebacterium glutamicum DNA as a bacterial target for method development and optimization, as it is not a common contaminant. Sequencing was performed in a hand-held Oxford Nanopore Technologies MinION sequencer. Our results show that using an in-tube amplification approach, at least 1 pg starting DNA is needed to reach the amount required for successful sequencing within 2 h. While using a digital microfluidic device, it is possible to amplify as low as 10 fg of C. glutamicum DNA (equivalent to the amount of DNA within a single bacterial cell) within 2 h and to identify the target bacterium within 30 min of MinION sequencing—100× lower than the detection limit of an in-tube amplification approach. We demonstrate the detection of C. glutamicum DNA in a mock community DNA sample and characterize the limit of bacterial detection in the presence of human cells. This approach can be used to identify microbes with minute amounts of genetic material in samples depleted of human cells within 3 h.
Introduction
Bacterial diagnosis often relies on detecting the DNA of various microorganisms ranging from bacterial pathogens in clinical and environmental samples1,2 to harmful foodborne microbial species.3,4 Standard bacterial detection includes microbial culture, followed by identification and antimicrobial susceptibility testing of isolated microorganisms. This is a time-consuming and laborious process that can last for days or weeks. Targeted polymerase chain reaction (qPCR) is a faster alternative but requires a priori suspicion of a known bacterial strain. 16S ribosomal RNA amplification followed by sequencing is becoming a popular option for untargeted detection of a broad range of microbial species, but it is limited to the straightforward bacterial classification without the potential to generate additional genomic information.5 Droplet-based digital PCR has gained attention as a new methodology for the accurate amplification of single copies of DNA, however, it requires additional laboratory instruments including droplet generators, thermocyclers, and droplet readers.6,7
Over recent years, whole genome sequencing (WGS) is emerging as a promising alternative for microbial diagnosis and analysis.8−10 However, it is often logistically challenging to perform the multistep process, which includes DNA extraction, whole genome amplification (WGA), next-generation sequencing (NGS), and bioinformatics analysis within a short turnaround time, which is essential in urgent cases such as medical emergencies. Oxford Nanopore Technologies (ONT) offers a paradigm-shifting MinION sequencer that enables rapid, real-time, and long-read nucleic acid sequencing in a palm-sized device powered by a laptop computer.11,12 This device offers benefits for rapid untargeted bacterial detection with increased rapidity and minimal experimental complexity compared with NGS sequencing strategies. Among ONT sequencing options, we chose rapid sequencing for this work, as this sequencing strategy is designed for users with limited access to laboratory equipment for sample preparation with a quick turnaround time.12 However, ONT recommends a few hundred nanograms of DNA input for rapid sequencing, which is magnitudes higher than the typical amount of bacterial DNA (as low as femtograms) present in many samples.13,14 To enable rapid and sensitive bacterial detection using the MinION sequencer, it is essential to amplify femtograms of the bacterial DNA with high purity in a rapid manner.
Microfluidic platforms are becoming popular for WGA due to their ability to handle microscale reactions in a controlled manner with minimal contamination.13−17 Multiple displacement amplification18 is an ideal option for microfluidic-based WGA.19−21 It is based on φ29 DNA polymerase and random primers to replicate template DNA with high fidelity and lower error rates following relatively simple procedures in an isothermal setting, which is compatible with most microfluidic systems.22−24 However, these microfluidic platforms often rely on complex and specialized setups for precise pressure control systems and highly skilled personnel for device operation.
Digital microfluidics (DMF) is starting to gain attention as an alternative for biomedical applications due to its ability to accurately handle microscale droplets in a programmed manner, offering the feasibility to integrate multiple steps in a single device and automate the processes.25−27 Briefly, these devices are composed of electrode arrays that support droplet transport, splitting, and mixing based on electrowetting principles28 and can be operated by a hand-held control system through a software.29 Therefore, droplets can be programmed to mix in a constant manner alternately turning on and off the electrodes to agitate the droplets during an experiment, thus enhancing the reaction efficiency and shortening the reaction time with minimal user intervention. These unique features make DMF devices ideal for amplifying low biomass bacterial DNA to reach the quantity required for MinION sequencing within a short time, enabling the detection of bacterial presence in a rapid manner.
In this work, we present a proof-of-concept demonstration of amplifying as low as 10 fg of bacterial DNA in a DMF device for rapid MinION sequencing to identify the bacterial species within 3 h. We also show the feasibility of detecting Corynebacterium glutamicum, Porphyromonas somerae, and Escherichia coli bacteriophage DNA in a mock bacterial community DNA sample. To pave the way for bacterial detection in complex samples such as biospecimens, we characterize the limit of bacterial detection in the presence of human cells using this approach. This work lays the foundation for rapidly identifying low abundance bacterial species in urgent settings.
Materials and Methods
Cell Culture and DNA Extraction
C. glutamicum ATCC 13032 was cultured in LB broth (Research Product International) at 37 °C in a shaker incubator (Thermo Fisher Scientific). P. somerae ATCC BAA1230 was cultured in chopped meat carbohydrate broth (DB) at 37 °C in an anaerobic chamber (Coy). Both cultures were harvested during the log phase (∼107/mL) and pelleted at 10,000 g for 3 min at 4 °C followed by supernatant removal. Human KLE ATCC 1622 (endometrial adenocarcinoma) cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM/F-12, Thermo Fisher Scientific) containing 10% fetal bovine serum at 37 °C with 5% CO2 in an incubator (Thermo Fisher Scientific). C. glutamicum was diluted to 103 cells/mL. DNA was extracted using the DNeasy Powersoil Kit (Qiagen) following manufacturer’s instructions. Extracted C. glutamicum and P. somerae DNA was quantified by High Sensitivity Qubit Assay and then initially diluted to 10 ng/μL, followed by a series of 10× dilution in nuclease-free water (Ambion) to obtain samples that contain 100 pg/μL, 10 pg/μL, 1 pg/μL, 100 fg/μL, and 10 fg/μL of the extracted DNA. E. coli lambda bacteriophage DNA was included in the ONT Rapid Sequencing Kit (ONT, SQK-RAD004). DNA mixture samples were produced by diluting and mixing extracted DNA from C. glutamicum, P. somerae, and E. coli lambda bacteriophage DNA.
DMF Device Microfabrication
The DMF device layout was designed in AutoCAD (Autodesk) and microfabricated following previously described methods.27,30 Briefly, the device was microfabricated on a 2″ × 3″ glass slide precoated with a 200 nm chromium layer, with an AZ1500 photoresist layer (Telic Company) as a bottom plate, and an indium-tin oxide (ITO)-coated glass slide (Delta Technologies) was used as a top plate. Electrodes were photolithographically patterned on the bottom plate in a mask aligner (KLOE), developed in 1:4 MF 351 developer (Microposit) and etched in CR-Chrome Etch (KMG Electronic Chemicals). Each actuation square electrode was 2.2 × 2.2 mm in size. The bottom plate was coated with 5 μm Parylene C (Speciality Coating Systems). 1 mm through-holes were drilled (MicroLux) on the top plate as the inlet and outlet ports of the device. The patterned side of the bottom plate and the ITO side of the top plate were spin-coated (1000 rpm, 60 s) with 2.25% type M fluoropolymer CYTOP solution (Bellex International Corporation) and incubated on a hotplate at 180 °C for 15 min. A 76.2 μm thick copper tape and a 102 μm thick electrically conductive adhesive transfer tape (3 M) were used as spacers between top and bottom plates. All sides of the device were sealed using epoxy glue and incubated for an hour at room temperature.
DMF Experimental Setup
The overall workflow of rapid bacterial detection in this work is illustrated in Figure 1. Briefly, the DMF device was operated by a Dropbot system (Sci-bot Inc.)29 that consisted of electrical circuitry compacted into a portable metal case (5.7″ × 4″ × 3″). The Dropbot system was connected to a laptop through a USB cable to control the DMF device via MicroDrop software. A 2″ × 3″ DMF device was inserted into the Dropbot system, and the parameters (e.g., voltage, frequency, and timing) were set to perform droplet transport, splitting, and mixing in a programmed manner.28 To better illustrate the functions of the DMF device, we manipulated droplets of dyes of different colors in the device for easy visualization (Supporting Information Video S2).
Figure 1.
Overview of the workflow of bacterial WGA in a DMF device for WGS in a MinION sequencer. The process is completed within 3 h.
The structure of the DMF device is illustrated in Figure 2. Overall, the device was composed of an electrode-patterned glass substrate at the bottom and an ITO-coated glass substrate on the top. The two substrates were connected through double-sided electrically conductive tape and copper tape as the spacer layer (Figure 2a). The device was prefilled with OS-30 silicone oil (Dow Corning) prior to loading aqueous samples and was operated at 80 VRMS at 1 k Hz, while the ITO-coated glass was connected to electrical ground. Fluids were transferred into the device by pipetting through the drilled inlets on the top plate and onto the electrically activated reservoir electrodes (Figure 2b). To avoid cross-contamination, different samples were introduced onto different reservoir electrodes. Each square electrode in the matrix can hold a ∼1 μL droplet. The final product was transported to an unused reservoir electrode and transferred out of the device by pipetting. All supplies and reagents were filtered (0.2 μm), autoclaved, or UV-sterilized, except for DNA polymerase and library preparation reagents. All samples and reagents used in the DMF device except library preparation reagents contained a final volume of 0.1% v/v poloxamine detergent Tetronic 90R4 (Octochem) to enhance droplet movement and minimize biofouling.31,32
Figure 2.
Overview of the structure of the DMF device. (a) Close-up of a DMF device inserted into the Dropbot system, with colored fluids on reservoir electrodes. (b) Cross-section diagram of a DMF device and fluid manipulation between the top and bottom substrates.
Bacterial WGA and Library Preparation in DMF Device
A REPLI-g Single Cell Kit (Qiagen), which contains DNA denaturing buffer (D2), neutralization buffer, and DNA polymerase, was used for bacterial WGA. Briefly, a 1 μL droplet of bacterial DNA and a 1 μL droplet of D2 buffer were mixed in the device and incubated at room temperature for 3 min. A 1 μL droplet of neutralization buffer was added to the sample and incubated for 10 min to terminate DNA denaturation. 9 μL of the DNA polymerase was introduced into the device to mix with the sample. The sample was incubated at room temperature for 2 h, while the electrodes were programmed to turn on and off alternately in a constant manner to agitate the droplets to enhance mixing and thus amplification efficiency. For larger droplets of >3 μL, the constant mixing was achieved by programmed splitting of the droplet into two smaller droplets, moving these droplets and merging them again in a repetitive manner. To terminate amplification, the DMF device was taken out of the Dropbot system, incubated at 65 °C on a hotplate for 3 min, and then placed on ice for 1 min before sliding it back into the Dropbot system. The same procedure was used for amplifying DNA from C. glutamicum of different concentrations as well as DNA mixture samples.
A Rapid Sequencing Kit (ONT, SQK-RAD004) was used for library preparation in the DMF device and sequencing using a MinION. 3 μL of the fragmentation reagent was introduced into the device and mixed with the sample. The device was then incubated on a hotplate at 30 °C for 1 min followed by 80 °C for 1 min and was then briefly placed on ice. 1 μL of the rapid adapter was added to the sample in the device and incubated at room temperature for 5 min. As the aqueous fluid was within ambient silicone oil, evaporation was not observed. The sample was moved to an unused reservoir electrode and transferred out of the device into a 0.2 mL microcentrifuge tube. DNA was quantified using a Qubit assay.
Bacterial WGS in MinION Sequencer
A FLO-MIN106D flow cell was primed, and the sample was prepared for loading according to manufacturer’s instruction. Briefly, 11 μL of DNA library, 4.5 μL of nuclease-free water, 34 μL of sequencing buffer, and 25.5 μL of loading beads were added to a microcentrifuge tube in a sequential manner and mixed. The final 75 μL sample was immediately loaded into the flow cell sample port in a drop-wise manner to avoid bead aggregation. The sample port, priming port, and MinION lid were then closed. The MinION sequencer was controlled by MinKNOW software, which performed data acquisition, real-time DNA quality analysis, and basecalling. Reads that passed quality filters after basecalling were stored in time-stamped fast5 and fastq files, with 4000 reads in each of the latter.
Bioinformatics Post-Processing
For Oxford Nanopore reads, adapter sequences were removed from the raw reads using Porechop v0.2.4.33 The fastq files were then concatenated based on their time stamps, and reads were mapped using Minimap2 v2.1734 to a reference genome of C. glutamicum ATCC 13032 in NCBI genome database (RefSeq assembly accession GCF_000011325.1). Mapped reads were coordinate-sorted using samtools v1.8.35 All processed sequences were visualized in Integrative Genomics Viewer (v.2.8.0).36 For profiling of taxonomy, reads with adapter removed were processed using BBDuk entropy filtering from the BBMap tool set v38.6937 to mask low complexity regions of the reads. Then, reads were profiled using Centrifuge v1.0.4,38 using a database based on NCBI RefSeq complete genomes from Bacteria, Archaea, and Viruses (as of October 2018), as well as human genome hg38 and mouse genome GRCm.38 Taxonomy calls were reported only if their Centrifuge score was larger than 150. The taxonomy pipeline was implemented using Nextflow v19.10.0.39 As a clarification, we used Centrifuge to test the presence of amplified DNA from the target organism and to survey putative environmental contaminants in the sample. The database used RefSeq genomes marked as “complete” (as opposed to contigs or scaffolds only) from Bacteria, Archaea, and Viruses. The per-read score in Centrifuge is roughly the sum of the square of the k-mer lengths of the matching segments in a read. A threshold of 150 can be interpreted as a matching segment of length 27 bp.
Results and Discussion
In-Tube WGA and Sequencing Quality Control
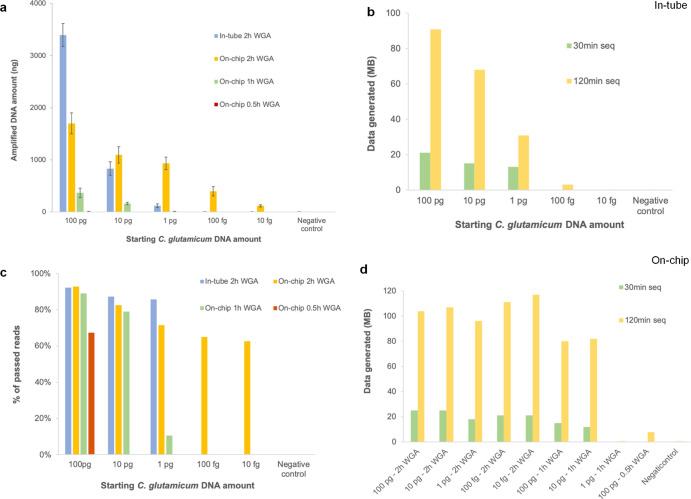
We performed a set of C. glutamicum DNA WGA experiments in 0.2 mL microcentrifuge tubes and DMF devices followed by MinION sequencing as a comparative study (Figure 3). Each WGA experiment was repeated thrice, with one replicate randomly selected from each sample set for sequencing. For in-tube experiments, 2 h of WGA showed success for samples with 100 pg, 10 pg, and 1 pg starting DNA, generating an average of 3.3 μg, 832 ng, and 120 ng DNA (Figure 3a). After a 30 min sequencing run, >20 Mb data were generated and ready for processing (Figure 3b). Samples with higher amounts of starting DNA led to larger amounts of DNA available for sequencing after WGA, and thus, more data generated. The amount of DNA obtained between these samples was different (p < 0.005). During sequencing, DNA sequences with a quality score (Qscore) < 7.0 were filtered and discarded; over 85% of reads passed quality checks with a medium Qscore of 9.6 (Figure 3c). Note that the Qscore threshold was the default setting of the basecaller of the MinION sequencing platform. Due to constant advancements in sequencing technology and bioinformatic tools, the basecalling accuracy has been continuously improved for the same raw signal. In this work, the per-read nucleotide identity to the reference C. glutamicum genome rarely dropped below 89%.
Figure 3.
Comparison between the amount of C. glutamicum DNA amplified in-tube and on-chip. Each amplification experiment was performed thrice. One of the replicates was sequenced. (a) DNA obtained after amplification in-tube and on-chip. (b) Sequencing data generated for samples amplified in-tube for 2 h. (c) Percent of sequencing reads that passed quality control (Qscore > 7) for samples amplified in-tube and on-chip. (d) Sequencing data generated for samples amplified on-chip for 2, 1, and 0.5 h.
However, for samples with 100 fg and 10 fg starting DNA, only ∼4 ng of DNA was generated after 2 h of WGA with no data generated with 30 min of sequencing. The amount of DNA obtained after amplification was not significantly different between samples or compared with the negative control (p = 0.3–0.35). Extending the sequencing time to 120 min led to only ∼0.2 Mb data output. These results show that DNA can be sufficiently amplified in-tube for rapid MinION sequencing if the sample contains at least 1 pg starting DNA; however, in-tube WGA is not suitable for processing samples with femtograms of starting DNA for rapid sequencing due to the low post-amplification DNA amount.
To test the possibility of amplifying femtograms of DNA in-tube for an extended time, we amplified 10 and 100 fg DNA for up to 4 h with three replicates each. For samples with 10 fg starting DNA, only one replicate had a measurement of ∼9 ng DNA after amplification, while the other two replicates were below the detection limit of the Qubit assay (High Sensitivity). For samples with 100 fg starting DNA, an average of ∼11 ng amplified DNA was obtained but all failed sequencing. These tests showed the advantage of using the DMF device for amplifying minute amounts of DNA in a relatively rapid manner.
On-Chip WGA and Sequencing Quality Control
Five starting amounts of C. glutamicum DNA ranging from 100 pg to 10 fg were amplified in a DMF device followed by on-chip library preparation and MinION sequencing. Each on-chip WGA was repeated thrice, with one replicate randomly selected from each sample set for sequencing. After performing WGA in the DMF device for 2 h, samples with 100 pg were amplified to an average of 1.7 μg DNA (Figure 3a), significantly higher (p = 0.01) than samples with 10 pg and 1 pg starting DNA (∼1.1 μg and 933 ng). For samples with 100 fg and 10 fg starting DNA, the average amplified DNA was ∼400 and ∼118 ng, respectively (p < 0.005).
To test the feasibility of obtaining sufficient DNA within a minimal time, we repeated the on-chip WGA experiments with reduced amplification times (Figure 3a). For samples with 100 pg and 10 pg starting DNA, the average DNA after 1 h amplification was 367 and 164 ng, respectively (p = 0.04). For samples with lower starting DNA amounts, amplified DNA was within range of the negative control (p = 0.3) or below the detection limit of the High Sensitivity Qubit assay. Further reducing on-chip amplification time to 0.5 h led to only ∼7 ng DNA for samples with 100 pg starting DNA, while DNA in the rest of the samples was below the Qubit assay detection limit. Therefore, to obtain a minimum amount of DNA needed for downstream rapid sequencing from as low as 10 fg starting DNA, we determined the minimum time for DNA amplification to be 2 h. However, the time for DNA amplification can be increased to achieve a larger amount of amplified DNA, if necessary. Note that for samples with 100 pg starting DNA, the in-tube amplification outperformed on-chip amplification. It can be easier to deplete the DNA polymerase and thus saturate the amplification when amplifying relatively larger amounts of DNA in a smaller volume, and therefore standard in-tube amplification can be a better option for samples with at least 100 pg starting DNA.
For samples at all five starting DNA amounts amplified on-chip for 2 h, the first fastq files were generated within 20-30 min after sequencing started, with each of the fastq files containing ∼20 Mb data (4000 reads) with a medium Qscore of 9.5 and 65–90% sequenced reads passing quality check (Figure 3c,d). These generated fastq files were immediately sent to the taxonomy calling pipeline to identify the bacterial species, which takes approximately 10 min. Extending sequencing to 2 h generated multiple fastq files that were concatenated and processed in the same manner. As a comparison, we also sequenced samples amplified for 1 and 0.5 h on-chip with detectable ranges of DNA (Figure 3c,d). These samples had low amounts of DNA and insufficient high-quality DNA. The percentage of sequencing reads passing quality check was low and required extended sequencing times to obtain minimal data (∼0.5 Mb). Because the aim of this work was to achieve bacterial identification within 30 min of sequencing in a reliable manner, we did not sequence samples amplified on-chip that were below the detection limit of the Qubit assay.
Contamination Profile in MinION Sequencing
Contaminants detected by MinION sequencing are shown in Figure 4. For the 100 pg C. glutamicum DNA amplified on-chip, the target species was reliably detected after 30 min sequencing with ∼3900 C. glutamicum reads and <10 contaminant reads. Extending the sequencing time led to a higher number of C. glutamicum reads, with the number of reads doubling every 30 min. 120 min sequencing generated ∼5× more C. glutamicum reads than 30 min sequencing. The number of reads of the contaminants also increased by 5× but remained low overall (<50 reads) (Figure 4a). Figure 4b shows the contamination profile within 30 min of sequencing samples with different amounts of starting DNA amplified for 2 h on-chip and in-tube. Despite the contaminants present in the samples sequenced, the target species displayed at least 10× higher number of reads.
Figure 4.
Contamination profile of sequenced samples. (a) Contamination profile of samples with different starting amounts of C. glutamicum DNA amplified on-chip and in-tube. The results show the profile after 30 min of sequencing. Sequencing failed for samples with <1 pg starting DNA amplified in-tube. (b) Contamination profile of 100 pg C. glutamicum DNA amplified for 2 h on-chip. Extended sequencing times led to increased numbers of target species reads with marginal increases of contaminant reads.
Possible contaminating sources can include the original culture, reagent and airborne contaminants, human-related contamination, and equipment sources. Homo sapiens is reported as an expected contamination in WGA.40 These contaminations can be introduced at any stage of the entire process, including initial cell cultivation, handling, experimentation, sample transfer, library preparation, and sequencing. Among the contaminant reads, Cutibacterium acnes reads appeared higher than other contaminants; this organism is commonly found on human skin.41,42 Low percent contaminant reads appeared as Corynebacterium. This is likely due to the low quality of some C. glutamicum reads that could not be called beyond the genus level and therefore were not assigned to the target species. To investigate the level of contamination of reagents, we sequenced negative control samples (sterile PBS) amplified in-tube and on-chip for 2 h, and the results are shown in Figures S1 and S2. Note that we sequenced for 2 and 4 h to obtain the data (instead of the 30 min sequencing for other samples). C. glutamicum and E. coli lambda virus DNA was detected in these negative control samples, at low amounts. To investigate if these cross-contaminations stem from the original DNA samples, we performed direct sequencing on the extracted C. glutamicum DNA, P. somerae DNA, and E. coli lambda DNA without amplification. The results showed that there was no cross-contamination of any of the three species in the original samples (Figures S3–S5).
Coverage of MinION Sequenced Reads
Raw reads were mapped to the reference genome of C. glutamicum ATCC 13032 after adapters were removed (Figure 5). As a general trend, samples with higher starting DNA amounts showed more genome coverage after 30 min of sequencing (Table 1). For samples with picograms of starting DNA, those amplified in-tube showed more complete genome coverage than those amplified on-chip. However, for samples with femtograms of starting DNA, not enough DNA was obtained for rapid MinION sequencing with in-tube amplification. Therefore, to obtain sufficient DNA from samples with low DNA amounts in a rapid manner, it is essential to enhance amplification efficiency by implementing constant mixing strategies. For samples amplified on-chip, higher amounts of starting DNA led to increased genome coverage. Approximately 76.7% genome coverage was achieved for the sample with 100 pg starting DNA, however, genome coverage decreased as the amount of starting DNA decreased. Note that three replicates were sequenced for samples with femtograms of starting DNA, as this is the range that DMF-based amplification is advantageous over in-tube amplification. Another observation is that samples with a higher percentage of quality sequencing reads showed better genome coverage. For samples with picograms of starting DNA, in-tube amplification showed a higher percentage of quality reads compared to on-chip amplification. A possible explanation can be that the high operating voltage of the DMF device may cause a certain level of damage to the DNA during the on-chip amplification, especially if the dielectric layer (Parylene C) is spotted with sporadic, nanoscale pinholes; however, this is a speculation that requires further verification. Potential ways to solve this problem can be to deposit an additional layer of Al2O3 using atomic layer deposition technology to create a pinhole-free dielectric layer.43,44
Figure 5.
Coverage of sequencing reads processed and mapped to the C. glutamicum reference genome. Results shown are from samples amplified for 2 h.
Table 1. Genome coverage of C. glutamicum DNA after 30 min Sequencing in MinIONa.
| 100 pg (%) | 10 pg (%) | 1 pg (%) | 100 fg | 10 fg | |
|---|---|---|---|---|---|
| in-tube | 74.5 | 58.6 | 50.0 | N/A | N/A |
| on-chip | 76.8 | 43.6 | 16.9 | 18.6% (±1.5%) | 10.5% (±5.0%) |
Three replicates were sequenced for samples with femtograms of starting DNA.
Despite the relatively low genome coverage for samples with as low as 10 fg starting DNA (equivalent to the amount of DNA within a single bacterial cell), the genome sequence was unique enough to identify the bacterial strain. In addition, individual reads can be taxonomically assigned, furthering the certainty of the call. However, the accuracy can vary for different microbial species, depending on the extent of their representation in genomic databases. Taken into consideration hands-on time, including flow cell priming, sample loading, and running the sequencing data through the taxonomy pipeline, it is feasible to keep sample-to-answer time within 3 h.
Rapid WGA and Sequencing of Multiple Bacterial Species
We tested the feasibility of this method to detect multiple bacterial species. In these experiments, we used samples with C. glutamicum DNA, P. somerae DNA, and E. coli bacteriophage lambda DNA with starting DNA amounts ranging from 1 pg to 10 fg in randomly generated combinations. Our results show that it is possible to detect all three species if the difference of the amount of starting DNA between these species is no more than 10× (Figure 6a–c). In these cases, the number of reads for all three species is >8× the number of contaminating reads, therefore reliably detecting the presence of these species within the samples. However, in the test where the initial amount of E. coli bacteriophage lambda DNA exceeded C. glutamicum DNA and P. somerae DNA by > 20×, the number of reads of C. glutamicum and P. somerae fell within the range of contaminating reads and thus remained undetected (Figure 6d).
Figure 6.
Sequencing results of on-chip amplified samples with different initial amounts of C. glutamicum, P. somerae, and E. coli bacteriophage lambda DNA. Three amplification tests and sequencing were performed on each sample. (a) 100 fg lambda, 100 fg C. glutamicum, and 50 fg P. somerae DNA. (b)10 fg lambda, 10 fg C. glutamicum, and 50 fg P. somerae DNA. (c) 10 fg lambda, 20 fg C. glutamicum, and 100 fg P. somerae DNA. (d) 1 pg lambda, 50 fg C. glutamicum, and 10 fg P. somerae DNA.
In the long run, these results show promise for rapid detection of bacteria caused by the presence of a single microorganism such as urinary tract infection.45,46 However, a major limitation of this approach is the resolution to detect low-abundance bacterial species within samples with the presence of high-abundance species in polymicrobial arrangements. Potential solutions include the use of high-throughput droplet-based microfluidic platforms to compartmentalize the DNA sample in a large number of picoliters of droplets and amplify DNA in each droplet,47 a concept similar to digital qPCR.
To extract additional critical data from sequencing results, one potential aspect to be improved is the accuracy of sequenced reads. One way of reducing errors in sequencing results is adoption of linear WGA strategies.48,49 In exponential amplification, errors that occur in early cycles of amplification are propagated exponentially. In linear amplification, errors occur at random locations and can be easier to identify and filter out. However, current linear WGA methods rely on complex techniques such as in situ nucleosome depletion and transposon insertion that can be challenging to implement in microfluidic platforms. Besides, different cells may require specific molecular designs and optimization and are thus not ideal for untargeted bacterial detection. We anticipate that new advancements in DNA amplification and sequencing technology could enable adoption of these methods for untargeted bacterial detection. One example of such advancements is the adaptive sampling technology (formerly known as read-until) capable of selective enrichment or depletion of targets during sequencing in Oxford Nanopore sequencers.50
Rapid WGA and Sequencing of Bacterial Cells and Human Cells
A challenge of using a rapid WGA and sequencing for bacterial detection is the effective lysis of low-abundance bacterial cells without compromising DNA quality. To investigate the possibility of performing amplification on cultured bacterial cells, we tested 1 μL of C. glutamicum cells at a concentration of 103 cells/mL in the device, following a protocol for amplifying purified DNA. Amplified products were below the detection limit of high sensitivity Qubit assay. This could be due to the ineffective lysis of bacterial cells. To improve the efficiency of bacterial lysis, we used a custom lysis buffer13 containing 100 mM DTT and 200 U/μL lysozyme and incubated for 15 min at 37 C°. Results showed amplification of C. glutamicum cells at a single cell level, with the number of sequenced reads showing larger variability between amplification tests compared with amplified purified DNA (Figure 7a). This may be due to cell-to-cell variability of C. glutamicum lysis. Nevertheless, the number of C. glutamicum reads was ∼100-fold higher than contaminant reads from which they were easily distinguished.
Figure 7.
Sequencing results of on-chip amplified samples with C. glutamicum and human cells. (a) C. glutamicum cells only. (b) C. glutamicum mix with KLE cells. Three amplification tests and sequencing were performed on each sample. The inset shows a magnified view of the number of C. glutamicum reads and the number of contaminants reads.
To investigate the possibility of rapid detection of bacteria in the presence of human cells, we tested samples of cultured C. glutamicum alone and mixed with human KLE cells. Initially, we mixed the two with a 100:1 ratio for amplification; however, no C. glutamicum sequencing reads were detected. When the ratio of C. glutamicum to KLE cells was increased to 500:1, ∼30 C. glutamicum sequencing reads were detected, ∼100× lower than H. sapiens reads (Figure 7b). To enable the detection of bacterial cells in samples with high complexity (e.g., clinical samples), human cells should ideally be removed prior to amplification and sequencing. Potential strategies to accomplish this, thereby preconcentrating bacterial cells might involve integration of separation strategies such as dielectrophoretic separation,51,52 deterministic lateral displacement,53,54 and/or microfiltration55 into microfluidic platforms. At this stage, we focused on rapid amplification of minute amounts of bacterial DNA with minimal contamination from purified bacteria species. In the future, we will integrate human cell depletion strategies to obtain low-abundance bacterial cells from clinical samples prior to WGA.
Conclusions
Standard bacterial WGS relies on highly specialized instrumentation in laboratory settings and processing time that varies from a few days to a few months. A challenge of using standard WGS for emergent situations is the logistical need for processing and data analysis, which typically require multiple personnel with varied skill sets. In this work, we use novel technologies including a palm-sized DMF device and MinION sequencer to address this challenge to support a 2 h amplification of as low as 10 fg bacterial DNA (equivalent to the DNA amount in a single bacterial cell) with high purity and obtain bacterial species identity within 30 min of sequencing. The approach also showed success in identifying bacterial species within samples with multiple bacteria in a rapid manner. This proof-of-concept work showed feasibility of identifying bacterial species using WGS from samples harboring femtograms of DNA within 3 h with minimal contamination. This approach can be further optimized for other ONT sequencing strategies (e.g., ligation sequencing) to achieve high-throughput sequencing with a low amount of starting DNA to cater to additional experimental needs. Overall, this work paves the way for rapidly identifying low biomass bacterial species in urgent settings. Examples include the rapid detection of bacterial sepsis in blood (1–100 CFU/mL), which can be a life-threatening condition within a short time.56 Further advancements of this platform will focus on the integration of human cell depletion strategies to enable expanded usability for translational applications, as well as the improvement of amplification strategies to increase genome coverage for further analysis. Ultimately, we envision that this portable platform could enable low abundance bacterial DNA identification from host-depleted clinical samples.
Acknowledgments
This work was supported by the Ivan Bowen Family Foundation. In addition, we thank the Microbiome Program and the Center for Individualized Medicine at Mayo Clinic for their support and Dr. Alexander Revzin at Mayo Clinic for granting us the access to his microfabrication facilities. T.M. was supported by the Musculoskeletal Research Training grant (T32 AR56950). This project was supported by CTSA grant number KL2TR002379 from the National Center for Advancing Translational Science (NCATS). This work was also supported, in part, by a career enhancement award from NIH grants P50CA136393. Contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c03683.
Author Present Address
¶ American College of Surgeons, 633 N Saint Clair Street Chicago, IL, USA 60611-3295. The work was conducted when H.N. was at Mayo Clinic
The authors declare no competing financial interest.
Supplementary Material
References
- Deshmukh R. A.; Joshi K.; Bhand S.; Roy U. Recent developments in detection and enumeration of waterborne bacteria: a retrospective minireview. MicrobiologyOpen 2016, 5, 901–922. 10.1002/mbo3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deurenberg R. H.; Bathoorn E.; Chlebowicz M. A.; Couto N.; Ferdous M.; García-Cobos S.; Kooistra-Smid A. M. D.; Raangs E. C.; Rosema S.; Veloo A. C. M.; Zhou K.; Friedrich A. W.; Rossen J. W. A. Application of next generation sequencing in clinical microbiology and infection prevention. J. Biotechnol. 2017, 243, 16–24. 10.1016/j.jbiotec.2016.12.022. [DOI] [PubMed] [Google Scholar]
- Law J. W.-F.; Ab Mutalib N.-S.; Chan K.-G.; Lee L.-H. Rapid methods for the detection of foodborne bacterial pathogens: principles, applications, advantages and limitations. Front. Microbiol. 2015, 5, 770. 10.3389/fmicb.2014.00770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapela M.-J.; Garrido-Maestu A.; Cabado A. G. Detection of foodborne pathogens by qPCR: a practical approach for food industry applications. Cogent Food Agric. 2015, 1, 1013771. 10.1080/23311932.2015.1013771. [DOI] [Google Scholar]
- Rosselli R.; Romoli O.; Vitulo N.; Vezzi A.; Campanaro S.; De Pascale F.; Schiavon R.; Tiarca M.; Poletto F.; Concheri G.; Valle G.; Squartini A. Direct 16S rRNA-seq from bacterial communities: a PCR-independent approach to simultaneously assess microbial diversity and functional activity potential of each taxon. Sci. Rep. 2016, 6, 1–12. 10.1038/srep32165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W.; Zheng J.; Wu C.; Liu S.; Chen Y.; Liu X.; Du J.; Wang J. Breast cancer subtype classification using 4-plex droplet digital pcr. Clin. Chem. 2019, 65, 1051–1059. 10.1373/clinchem.2019.302315. [DOI] [PubMed] [Google Scholar]
- Tozaki T.; Ohnuma A.; Iwai S.; Kikuchi M.; Ishige T.; Kakoi H.; Hirota K.; Kusano K.; Nagata S. Robustness of Digital PCR and Real-Time PCR in Transgene Detection for Gene-Doping Control. Anal. Chem. 2021, 93, 7133–7139. 10.1021/acs.analchem.1c01173. [DOI] [PubMed] [Google Scholar]
- Fricke W. F.; Rasko D. A. Bacterial genome sequencing in the clinic: bioinformatic challenges and solutions. Nat. Rev. Genet. 2014, 15, 49–55. 10.1038/nrg3624. [DOI] [PubMed] [Google Scholar]
- Didelot X.; Bowden R.; Wilson D. J.; Peto T. E. A.; Crook D. W. Transforming clinical microbiology with bacterial genome sequencing. Nat. Rev. Genet. 2012, 13, 601–612. 10.1038/nrg3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balloux F.; Brønstad Brynildsrud O.; Van Dorp L.; Shaw L. P.; Chen H.; Harris K. A.; Wang H.; Eldholm V. From theory to practice: translating whole-genome sequencing (WGS) into the clinic. Trends Microbiol. 2018, 26, 1035–1048. 10.1016/j.tim.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson J. T.; Workman R. E.; Zuzarte P. C.; David M.; Dursi L. J.; Timp W. Detecting DNA cytosine methylation using nanopore sequencing. Nat. Methods 2017, 14, 407. 10.1038/nmeth.4184. [DOI] [PubMed] [Google Scholar]
- Tyler A. D.; Mataseje L.; Urfano C. J.; Schmidt L.; Antonation K. S.; Mulvey M. R.; Corbett C. R. Evaluation of Oxford Nanopore’s MinION sequencing device for microbial whole genome sequencing applications. Sci. Rep. 2018, 8, 10931–12. 10.1038/s41598-018-29334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.; Schulze-Makuch D.; de Vera J.-P.; Cockell C.; Leya T.; Baqué M.; Walther-Antonio M. The Development of an Effective Bacterial Single-Cell Lysis Method Suitable for Whole Genome Amplification in Microfluidic Platforms. Micromachines 2018, 9, 367. 10.3390/mi9080367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.; Walther-Antonio M. Microfluidics: A new tool for microbial single cell analyses in human microbiome studies. Biomicrofluidics 2017, 11, 061501. 10.1063/1.5002681. [DOI] [Google Scholar]
- Liu Y.; Jeraldo P.; Jang J. S.; Eckloff B.; Jen J.; Walther-Antonio M. Bacterial Single Cell Whole Transcriptome Amplification in Microfluidic Platform Shows Putative Gene Expression Heterogeneity. Anal. Chem. 2019, 91, 8036. 10.1021/acs.analchem.8b04773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry Z. C.; Giovanonni S. J.; Quake S. R.; Blainey P. C. Optofluidic cell selection from complex microbial communities for single-genome analysis. Methods Enzymol 2013, 531, 61. 10.1016/B978-0-12-407863-5.00004-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S.; Arai M.; Katoh H.; Ajioka R.; Baba K. i.; Sato S.; Tomita-Yokotani K.. In Utilization of a cyanobacterium, Nostoc sp. HK-01, under the space environment. 44th International Conference on Environmental Systems; Japan Aerospace Exploration Agency, 2014.
- Dean F. B.; Nelson J. R.; Giesler T. L.; Lasken R. S. Rapid amplification of plasmid and phage DNA using phi29 DNA polymerase and multiply-primed rolling circle amplification. Genome Res. 2001, 11, 1095–1099. 10.1101/gr.180501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zare R. N.; Kim S. Microfluidic platforms for single-cell analysis. Annu. Rev. Biomed. Eng. 2010, 12, 187–201. 10.1146/annurev-bioeng-070909-105238. [DOI] [PubMed] [Google Scholar]
- Binga E. K.; Lasken R. S.; Neufeld J. D. Something from (almost) nothing: the impact of multiple displacement amplification on microbial ecology. ISME J. 2008, 2, 233–241. 10.1038/ismej.2008.10. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Yao J.; Walther-Antonio M. Whole genome amplification of single epithelial cells dissociated from snap-frozen tissue samples in microfluidic platform. Biomicrofluidics 2019, 13, 034109. 10.1063/1.5090235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motley S. T.; Picuri J. M.; Crowder C. D.; Minich J. J.; Hofstadler S. A.; Eshoo M. W. Improved multiple displacement amplification (iMDA) and ultraclean reagents. BMC Genom. 2014, 15, 443. 10.1186/1471-2164-15-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bourcy C. F. A.; De Vlaminck I.; Kanbar J. N.; Wang J.; Gawad C.; Quake S. R. A quantitative comparison of single-cell whole genome amplification methods. PloS One 2014, 9, e105585 10.1371/journal.pone.0105585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M.; Song P.; Zou D.; Hu X.; Zhao S.; Gao S.; Ling F. Comparison of multiple displacement amplification (MDA) and multiple annealing and looping-based amplification cycles (MALBAC) in single-cell sequencing. PloS One 2014, 9, e114520 10.1371/journal.pone.0114520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K.; Ng A. H. C.; Fobel R.; Wheeler A. R. Digital microfluidics. Annu. Rev. Anal. Chem. 2012, 5, 413–440. 10.1146/annurev-anchem-062011-143028. [DOI] [PubMed] [Google Scholar]
- Fair R. B. Digital microfluidics: is a true lab-on-a-chip possible?. Microfluid. Nanofluidics 2007, 3, 245–281. 10.1007/s10404-007-0161-8. [DOI] [Google Scholar]
- Liu Y.; Papautsky I. Heterogeneous Immunoassay Using Channels and Droplets in a Digital Microfluidic Platform. Micromachines 2019, 10, 107. 10.3390/mi10020107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung Kwon Cho S. K.; Hyejin Moon H.; Chang-Jin Kim C.-J. Creating, transporting, cutting, and merging liquid droplets by electrowetting-based actuation for digital microfluidic circuits. J. Microelectromech. Syst. 2003, 12, 70–80. 10.1109/jmems.2002.807467. [DOI] [Google Scholar]
- Fobel R.; Fobel C.; Wheeler A. R. DropBot: An open-source digital microfluidic control system with precise control of electrostatic driving force and instantaneous drop velocity measurement. Appl. Phys. Lett. 2013, 102, 193513. 10.1063/1.4807118. [DOI] [Google Scholar]
- Liu Y.; Banerjee A.; Papautsky I. Precise droplet volume measurement and electrode-based volume metering in digital microfluidics. Microfluid. Nanofluidics 2014, 17, 295–303. 10.1007/s10404-013-1318-2. [DOI] [Google Scholar]
- Rackus D. G.; de Campos R. P. S.; Chan C.; Karcz M. M.; Seale B.; Narahari T.; Dixon C.; Chamberlain M. D.; Wheeler A. R. Pre-concentration by liquid intake by paper (P-CLIP): a new technique for large volumes and digital microfluidics. Lab Chip 2017, 17, 2272–2280. 10.1039/C7LC00440K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leipert J.; Tholey A. Miniaturized sample preparation on a digital microfluidics device for sensitive bottom-up microproteomics of mammalian cells using magnetic beads and mass spectrometry-compatible surfactants. Lab Chip 2019, 19, 3490–3498. 10.1039/C9LC00715F. [DOI] [PubMed] [Google Scholar]
- Wick R. R.; Judd L. M.; Gorrie C. L.; Holt K. E. Completing bacterial genome assemblies with multiplex MinION sequencing. Microb. Genom. 2017, 3, e000132 10.1099/mgen.0.000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. Minimap2: pairwise alignment for nucleotide sequences. Bioinformatics 2018, 34, 3094–3100. 10.1093/bioinformatics/bty191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H.; Handsaker B.; Wysoker A.; Fennell T.; Ruan J.; Homer N.; Marth G.; Abecasis G.; Durbin R. The sequence alignment/map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. T.; Thorvaldsdóttir H.; Winckler W.; Guttman M.; Lander E. S.; Getz G.; Mesirov J. P. Integrative genomics viewer. Nat. Biotechnol. 2011, 29, 24–26. 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell B.; Rood J.; Singer E. BBMerge–accurate paired shotgun read merging via overlap. PloS One 2017, 12, e0185056 10.1371/journal.pone.0185056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.; Song L.; Breitwieser F. P.; Salzberg S. L. Centrifuge: rapid and sensitive classification of metagenomic sequences. Genome Res. 2016, 26, 1721–1729. 10.1101/gr.210641.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Tommaso P.; Chatzou M.; Floden E. W.; Barja P. P.; Palumbo E.; Notredame C. Nextflow enables reproducible computational workflows. Nat. Biotechnol. 2017, 35, 316–319. 10.1038/nbt.3820. [DOI] [PubMed] [Google Scholar]
- Hammond M.; Homa F.; Andersson-Svahn H.; Ettema T. J. G.; Joensson H. N. Picodroplet partitioned whole genome amplification of low biomass samples preserves genomic diversity for metagenomic analysis. Microbiome 2016, 4, 52. 10.1186/s40168-016-0197-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin G. R.; Carlos C.; Chevrette M. G.; Horn H. A.; McDonald B. R.; Stankey R. J.; Fox B. G.; Currie C. R. Evolution and ecology of Actinobacteria and their bioenergy applications. Annu. Rev. Microbiol. 2016, 70, 235–254. 10.1146/annurev-micro-102215-095748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platsidaki E.; Dessinioti C.. Recent advances in understanding Propionibacterium acnes (Cutibacterium acnes) in acne. F1000Research 2018, 7, DOI: 10.12688/f1000research.15659.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj B.; Smith N.; Christy L.; Dhindsa M.; Heikenfeld J.. In Composite dielectrics and surfactants for low voltage electrowetting devices; IEEE, 2008; pp 187–190. [Google Scholar]
- Chang J.-h.; Choi D.-Y.; You X.; Pak J. J.; Han S.. In Low voltage electrowetting on atomic-layer-deposited aluminum oxide; IEEE, 2010; pp 612–615. [Google Scholar]
- Wilson M. L.; Gaido L. Laboratory diagnosis of urinary tract infections in adult patients. Clin. Infect. Dis. 2004, 38, 1150–1158. 10.1086/383029. [DOI] [PubMed] [Google Scholar]
- Puttaswamy S.; Lee B. D.; Sengupta S. Novel electrical method for early detection of viable bacteria in blood cultures. J. Clin. Microbiol. 2011, 49, 2286–2289. 10.1128/JCM.00369-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan F.; Demaree B.; Ahmed N.; Abate A. R. Single-cell genome sequencing at ultra-high-throughput with microfluidic droplet barcoding. Nat. Biotechnol. 2017, 35, 640. 10.1038/nbt.3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.; Xing D.; Tan L.; Li H.; Zhou G.; Huang L.; Xie X. S. Single-cell whole-genome analyses by Linear Amplification via Transposon Insertion (LIANTI). Science 2017, 356, 189–194. 10.1126/science.aak9787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y.; Jiang Y.; Lam K.-W. G.; Berletch J. B.; Disteche C. M.; Noble W. S.; Steemers F. J.; Camerini-Otero R. D.; Adey A. C.; Shendure J. High-throughput single-cell sequencing with linear amplification. Mol. Cell 2019, 76, 676–690. 10.1016/j.molcel.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards H. S.; Krishnakumar R.; Sinha A.; Bird S. W.; Patel K. D.; Bartsch M. S. Real-time selective sequencing with RUBRIC: read until with basecall and reference-informed criteria. Sci. Rep. 2019, 9, 1–11. 10.1038/s41598-019-47857-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L.; Liu X.; Zheng X.; Zhang X.; Yang J.; Tian T.; Liao Y. Dielectrophoretic Separation of Particles Using Microfluidic Chip with Composite Three-Dimensional Electrode. Micromachines 2020, 11, 700. 10.3390/mi11070700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.; Chang H.; Neuzil P. DEP-on-a-chip: Dielectrophoresis applied to microfluidic platforms. Micromachines 2019, 10, 423. 10.3390/mi10060423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstetter A.; Vernekar R.; Austin R. H.; Becker H.; Beech J. P.; Fedosov D. A.; Gompper G.; Kim S.-C.; Smith J. T.; Stolovitzky G.; Tegenfeldt J. O.; Wunsch B. H.; Zeming K. K.; Krüger T.; Inglis D. W. Deterministic lateral displacement: Challenges and perspectives. ACS Nano 2020, 14, 10784–10795. 10.1021/acsnano.0c05186. [DOI] [PubMed] [Google Scholar]
- McGrath J.; Jimenez M.; Bridle H. Deterministic lateral displacement for particle separation: a review. Lab Chip 2014, 14, 4139–4158. 10.1039/C4LC00939H. [DOI] [PubMed] [Google Scholar]
- Wyatt Shields IV C.; Reyes C. D.; López G. P. Microfluidic cell sorting: a review of the advances in the separation of cells from debulking to rare cell isolation. Lab Chip 2015, 15, 1230–1249. 10.1039/C4LC01246A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G. S. Sepsis, severe sepsis and septic shock: changes in incidence, pathogens and outcomes. Expert Rev. Anti Infect. Ther. 2012, 10, 701–706. 10.1586/eri.12.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.