Abstract
In this study, the removal of methylene blue dye (MB) from aqueous solution was examined using a novel green adsorbent to overcome the obstacles encountered in chemical methods. Ricinus communis (RC) aqueous seeds extract was herein used as a reducing and capping agent to synthesize a novel nano-zero-valent iron (RC-nZVI) for the adsorption of harmful MB. Structural and morphological characterization of the synthesized RC-nZVI were performed using several techniques, e.g., steady-state absorption, scanning electron microscopy (SEM), transmission electron microscopy (TEM), Fourier transform infrared (FT-IR), energy-dispersive X-ray spectroscopy (EDS), X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), and zeta potential. The maximum efficiency of the removal was 96.8% at pH 6 and 25 °C. According to the kinetics study results, the adsorption process obeys the pseudo-first-order model. The experimental equilibrium data were fitted to the Freundlich isotherm model, the maximum adsorption capacity reached was 61.37 mg·g–1, and the equilibrium parameters were determined. The synthesized RC-nZVI possesses good reusability and can be considered as a potential economic and environmentally friendly adsorbent.
1. Introduction
There is no doubt that water is a vital resource for all creatures, and its treatment is a must nowadays due to the augmentation of water pollution with various pollutants. Nitroaromatic compounds, chlorinated compounds, dyes, heavy metals, and anthropogenic chemicals are the most harmful categories of pollutants that need to be removed from the aquatic environment.1,2 Different manufacturing applications that use dye compounds, such as food, textiles, cosmetics, and paper industries, discharge thousands of tons of dye effluents annually.3,4 Organic pollutants, notably aromatic compounds with a benzene ring structure, have a more stable structure, are difficult to decompose, and are highly toxic, resulting in serious pollution of the environment.5−7 Among the most harmful pollutants, methylene blue (MB) is one of the most commonly used cationic dye; however, its discharge can cause kidney, brain, liver, and central nervous system deterioration.8,9 Owing to its complex chemical structure, MB is nonbiodegradable and can adversely affect aquatic ecosystems by preventing the penetration of sunlight into water and streams.10−12 In addition, MB can inhibit microorganisms’ catalytic activity, contributing to their death, which can cause severe health problems, such as vomiting, nausea, diarrhea, and stomach upset.13
Recently, the removal of these organic pollutants has been a major concern, due to their deleterious effects. Diverse physical, chemical, biological, and electrochemical processes have been used to treat these pollutants from wastewater.14−16 Catalytic degradation is one of the main routes for the removal of these dyes.17,18 Advanced oxidation processes, another effective way for the removal of dyes, is mainly concerned with the formation of hydroxyl radicals (•OH) that readily oxidize these dyes into H2O, CO2, and other nontoxic small molecules.19,20 Among the used techniques, adsorption has been recognized as a favorable one due to its high efficiency and relatively low cost of energy and capital expenditure.16,21−24
Green synthesis of nanomaterials represents a key solution to overcome the unfavorable impacts of their production via chemical routes.25,26 Green synthesis of nanoparticles should be widely used in real life and moved beyond the laboratory owing to its unique features, e.g., environmentally friendly, nontoxic, and safe. Among the green-synthesized nanoparticles, nano-zero-valent iron (nZVI) has attracted great attention in industrial and environmental applications27 due to its unique properties, e.g., small size, large specific surface area, high interfacial reactivity, and high magnetic nature.28,29 The unique structural and electronic properties of the synthesized nZVI make it a promising material for removing the toxic pollutants from contaminated water, such as heavy metals,30,31 antibiotics,32 chloroorganics,33 bromides,34 nitrates,34 dye pollutants,29,35 and cesium.36
Compared to the ease of fabrication, low cost, and environmentally friendly nature of the green-synthesized nZVI, the traditional chemical methods used for the synthesis of nZVI are still facing many problems such as hazardous byproducts generated by precursors and solvents, the contamination resulting from use of harmful chemical compounds, as well as low production rates and high energy consumption.37−39 In addition, the strong magnetic forces in nZVI usually lead to aggregation of particles, which has been the main impediment to its practical application.40 The surface modification of nZVI with dispersants has been extensively studied, which proved to be a viable approach to promoting the stability and fluidity of nZVI in the ambient medium.41,42 Nonbiodegradable dispersants, however, are generally considered to have significant toxicity and could cause secondary contamination if used on a large scale. Therefore, alternative methods of low cost and green synthesis are urgently required. The reduction of ferric or ferrous iron using bioactive substances derived from plant extracts has received extensive attention as one of the nZVI synthesis alternatives.43 Plant extracts can be used as a highly effective and environmentally sustainable alternative for the synthesis of nanoparticles, instead of physiochemical methods, which have less energy efficiency (require high temperature and pressure). Besides, the abundance and easy availability of plants make them a significant candidate for biogenic synthesis.44,45 Various plant extracts have been reported in the green synthesis of nZVI, including the leaf extract of Mangifera indica, pomegranate peel extract, Thymus vulgaris, Urtica dioica, and Rosa damascene.46−48Ricinus communis belongs to the family Euphorbiaceae, which is a monotypic genus, and is found throughout the country and is widely cultivated in the tropics and warm regions for its seeds, which yield the well-known castor oil. Experimental and clinical studies demonstrate that R. communis has a wider spectrum of pharmacological and therapeutic effects.49
To the best of our knowledge, green synthesis of nZVI using castor oil (R. communis) is rare in the literature. Taking the advantages of green synthesis and the unique properties of nZVI into consideration, we report herein the green synthesis of nZVI using R. communis seeds extract as a reducing and stabilizing agent, and characterization of the synthesized nZVI using various spectroscopic and analytical techniques, and evaluate the potentiality of the synthesized nZVI for the removal of cationic methylene blue dye (MB).
2. Results and Discussion
2.1. Phytochemical Screening of R. communis
The phytocomponents of plant extract act as a reducing as well as stabilizing agent. However, the basic mechanism of phytosynthesis of MNPs has not been well understood, and various putative mechanisms have been proposed for their synthesis.50−52 It is a tedious process to identify any specific bioreducing and stabilizing mediators responsible for the synthesis and stabilization of MNPs due to the complex nature of phytochemicals in plant extracts. To date, phytocomponents including polyphenols (flavonoids, phenolic acid, and terpenoids), or organic acids, and proteins are considered as possible bioreducing and stabilizing agents for the synthesis of MNPs.53,54 So, in the present study, a preliminary phytochemical screening of some bioactive compounds of R. communis was carried out using suitable chemical reagents to indicate the presence or absence of the chemical constituents that are usually involved in the phytosynthesis of Fe0. The test results indicated the presence of high contents of tannins, alkaloids, fats and fixed oils, and phenols, a moderate content of saponins and carbohydrates, and low contents of anthraquinone glycosides as presented in Table 1. This variety in the phytochemical compounds provides strong reducing properties that mediate the synthesis of nZVI through the reduction of Fe3+ ions.
Table 1. Qualitative Phytochemical Screening for the Aqueous Extract of RC Seedsa.
| no | test name | phytochemical compound | result |
|---|---|---|---|
| 1 | Ferric chloride test | tannins | +++ |
| 2 | Dragendorff’s test | alkaloids | +++ |
| 3 | Foam test | saponins | ++ |
| 5 | Alkaline reagent test | flavonoids | +++ |
| 6 | Molisch’s test | carbohydrates | ++ |
| 7 | Potassium ferricyanide test | phenols | +++ |
| 8 | Copper sulfate test | fats and fixed oils | +++ |
| 9 | Hydroxyanthraquinone test | anthraquinone glycosides | + |
(+++) = high amount [appears immediately after addition of reagent]; (++) = moderate amount [appears after 5 min of reagent addition]; (+) = low amount [appears after 10 min of reagent addition]; and (−) = absence of active compound [not detected after 20 min].
2.2. Characterization of RC-nZVI Nanoparticles
2.2.1. UV–vis Spectral Analysis and Effect of Incubation Time
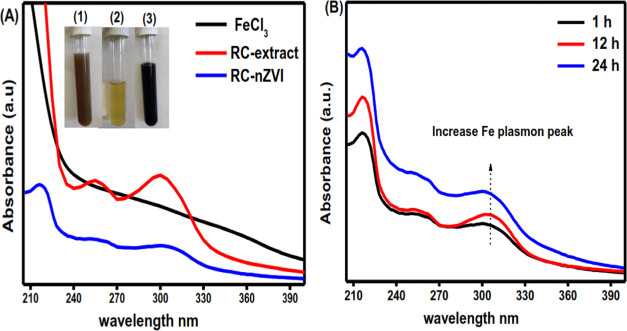
The reduction of Fe3+ to nZVI was confirmed by the visible color change in the reaction mixture, serving as a preliminary indication of the formation of nZVI.55 In the current study, an immediate change in the solution color from reddish brown to black at room temperature was detected after the addition of the plant seeds extract, indicating the rapid synthesis of RC-nZVI (Figure 1A). It was reported that the formation of the dark color of the synthesized nZVI is basically due to the complexation of Fe3+ with the phenolic group in the plant extract as a metal–ligand interaction between Fe0 and the polyphenolic compound via the C=O bond.56−58 The pH of the plant extract was found to be 5.2; however, after adding it to the Fe3+ ions solution, the pH dropped to 2.25, which could be due to the generation of H+ during the reduction of Fe3+ by polyphenols or other reducing agents present in plant extracts, as shown in the reaction below.59
where n is the number of hydroxyl groups oxidized by Fe3+, and Ar stands for the polyphenol group.
Figure 1.
(A) UV–visible absorption spectra of an aqueous solution of Fe3+ ions, RC seed extract, and synthesized RC-nZVI. (B) UV–visible absorption spectra of the synthesized RC-nZVI at different incubation times.
UV–vis absorption spectra for the Fe3+ ions aqueous solution, RC seed extract, and the synthesized RC-nZVI are represented in Figure 1A. The absorption spectrum of RC-nZVI exhibited a maximum in the range of 216–300 nm, which is identical to the characteristic absorption peak for the reported metallic iron.60 These results are in good agreement with a previously published work by Pattanayak and Nayak,61 where nZVI was synthesized using Azadirachta indica with an absorption peak for nZVI in the wavelength range 216–268 nm. Furthermore, Somchaidee and Tedsree62 recorded the absorption peaks for the phytosynthesized nZVI using guava (Psidium guajava L) leaf extract at 200–300 nm. In addition, Eslami et al.63 reported the absorption bands of the aqueous extract of Myrtus communis leaves and the development of the nZVI at 260 and 210 nm, which are considered the signature bands for nZVI.
The effect of incubation time on the green synthesis process is another important factor that greatly affects the characteristics of nZVI. On this account, the UV–vis spectra of the synthesized nZVI were monitored at different time intervals of 1, 12, and 24 h. The UV–vis spectra of the synthesized nZVI using RC seed extract showed an increase in intensity with time as shown in Figure 1B. This may arise from the excitation of longitudinal plasmon vibrations in MNPs in the solution, reflecting the continued reduction of the synthesized nZVI.64
2.2.2. Electron Microscopy Analysis
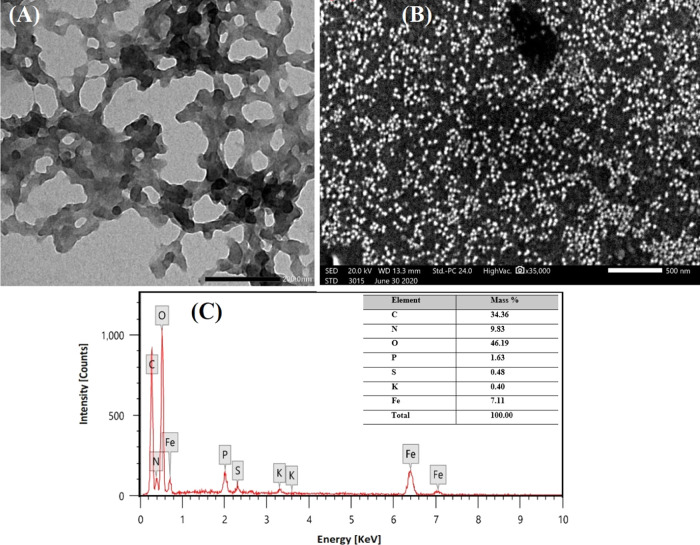
Transmission electron microscopy (TEM) and scanning electron microscopy (SEM) were carried out to understand the particle size, shape, dispersity, and topology of the synthesized RC-nZVI. From TEM images (Figure 2A), it can be seen that RC-nZVI exhibited a spherical shape along with some irregular shapes and has a tendency to form chain-like aggregates with an average particle size of 20 nm. The nanoscale particle size in the chain provides its high specific surface area and reactivity with contaminants.65 Furthermore, the surface of the synthesized RC-nZVI is covered by a transparent layer, as demonstrated by the TEM images, which serves as a capping and stabilizing agent to prevent RC-nZVI from agglomerating and plays a crucial role in improving its dispersion and stability as a basic constituent of green-synthesized nZVI.57,66,67 In the current investigation, SEM images added more support to those of TEM and confirmed that the synthesized RC-nZVI was mostly granular with a spherical shape, as shown in Figure 2B. In addition, the EDX analysis was performed to investigate the elemental composition of the synthesized RC-nZVI. As observed from Figure 2C, Fe, C, and O peaks confirm the formation of RC-nZVI. Moreover, the Fe, C, and O contents were found to be 7.11, 34.36, and 46.16%, respectively. These findings were in line with other works that reported the green synthesis of nZVI using tea and Eucalyptus leaf plant extracts.68,69
Figure 2.
SEM images (A, B), TEM images (C, D), and EDX pattern of RC-nZVI (E).
2.2.3. Dynamic Light Scattering (DLS) Analysis and Size Distribution
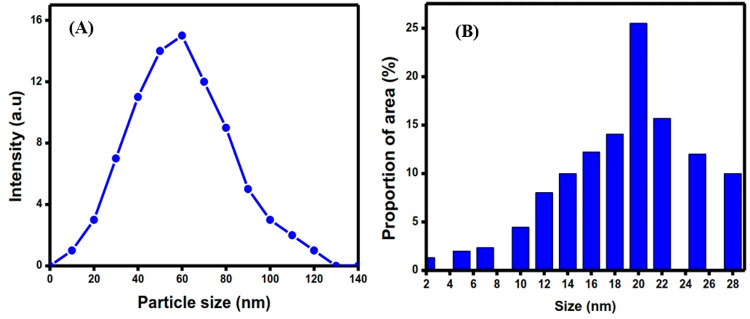
Dynamic light scattering (DLS) has been utilized to investigate the aggregation phenomena of FeNPs in solution.70 The final colloidal state and its evolution in terms of aggregation, reaction, and core–shell formation are all influenced by the metal colloid’s synthesis. In this study, the average size of RC-nZVI was determined through DLS (Figure 3A). Results indicated that the size distribution of RC-nZVI in aqueous solution lies between 40 and 50 nm. However, the variation of size distribution and mean size of RC-nZVI obtained from the TEM photomicrograph (Figure 3C) indicated that the size distribution of the particle lies between 10 and 30 nm and the highest frequency of the particle size is 20 nm.
Figure 3.
(A) DLS of the size distribution and (B) TEM micrograph of the particle size distribution.
2.2.4. ζ-Potential (ZP)
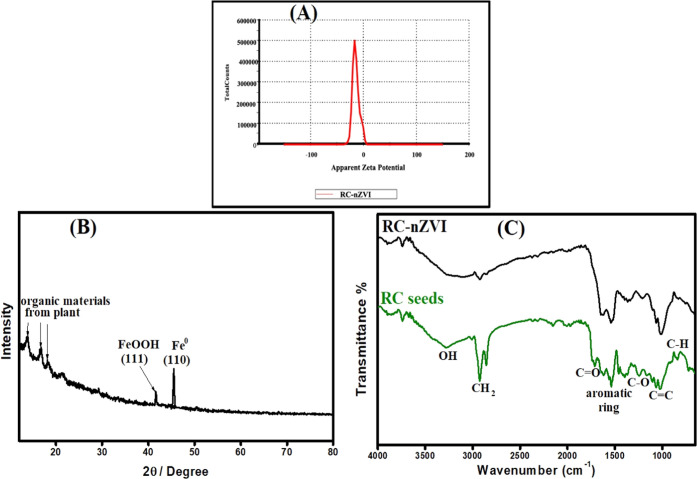
The ability to measure the ζ-potential of nanoparticles in dilute concentrations is a useful tool in a variety of fields. This parameter, for example, could be used in the nanotoxicological community to predict the environmental impact of commercial nanoparticles as well as their effects on human health. As a result, the ζ-potential can be used as a base for analyzing specific regulatory end points in relation to nanoparticle safety.71 Additionally, the ζ-potential (ZP) was used for determining the degree of electrostatic attraction or repulsion between particles in a liquid suspension. On this basis, ZP is one of the main tools that measure the stability of nanoparticles in an aqueous solution. In the absence of steric stabilization, particles with ζ-potentials greater than +30 mV and less than −30 mV are considered stable for colloidal dispersion.72 The recorded ζ-potential for the synthesized RC-nZVI is −14.9 mV, as shown in Figure 4A and Table 2. Accordingly, the synthesized RC-nZVI acquires a stable dispersal potential in the solution than the uncoated nanoparticles (−2.43 mV).73 These negative charges on its surface suggest that RC-nZVI is a potential adsorbent for cationic dyes.
Figure 4.
ζ-potential (A) and X-ray diffraction (XRD) (B) of RC-nZVI and Fourier transform infrared (FT-IR) spectra for both RC seeds and RC-nZVI (C).
Table 2. ζ-Potential Parameters and Deviation Values.
| mean (mV) | area (%) | st dev (mV) | ||
|---|---|---|---|---|
| ζ-potential (mV) | –14.9 | peak 1: −14.9 | 100.0 | 4.41 |
| ζ-deviation (mV) | 4.31 | peak 2: 0.00 | 0.0 | 0.00 |
| conductivity (mS cm–1) | 0.340 | peak 3: 0.00 | 0.0 | 0.00 |
| result quality | good |
2.2.5. XRD Pattern
The XRD pattern of RC-nZVI (Figure 4B) showed that the pattern of the synthesized RC-nZVI acquired a diffraction peak with high intensities at 2θ = 41.7° corresponding to the (111) plane of α-FeOOH. This is due to the shell layer of zero-valent iron nanoparticles as reported by Li et al.74 They explained that nZVI is composed of the core (metallic iron) and the shell, i.e., the oxide layer of Fe(II) and Fe(III), which is formed due to the rapid metallic iron oxidation in the environment. However, the strong peak at 44.5° corresponding to the (110) plane represents Fe0 (110). This characteristic peak at 2θ = 44.5° is attributed to the cubic phase structure of nZVI according to JCPDS card, No. 06–0696.75 These results confirm the successful formation of nZVI. The presence of only one peak for the nZVI in the XRD pattern may be due to the high background caused by the X-ray florescence of Fe. When performing XRD of materials containing elements such as Fe and Mn, the secondary X-ray emitted by the elements causes high background levels that ruin the pattern. Increasing the scanning time slightly improves the quality of the diffraction pattern. Moreover, the three different peaks at 2θ = 13.81, 16.86, and 18.49° are attributed to the organic matter on the surface of the RC-nZVI.14 The presence of these diffraction peaks confirms the fundamental role of the RC seed extract in the bioreduction and stabilization of nZVI.41
2.2.6. FT-IR Analysis
FT-IR spectra provide information about the surface structures and functional characteristic groups responsible for the reduction and stabilization of the green-synthesized RC-nZVI. The FT-IR spectra for the RC seed extract and RC-nZVI are shown in Figure 4C. For the RC seed extract, the peak at 3285 cm–1 is assigned to the characteristic O–H stretching vibrations in H2O molecules, as well as the O–H groups of polyphenols in the RC seeds.43,76 The peak at 2919 cm–1 is corresponding to the C–H and CH2 vibrations of aliphatic hydrocarbons.77,78 The peak at 1735 cm–1 is attributed to the C=O stretching vibration of the carbonyl group derived from the RC seeds.68 The absorption peak at 1534 cm–1 is associated to the aromatic ring stretching vibration in the phenolic compound. The peak located at 1252 cm–1 is assigned to C–O, C–O–H, and symmetric and asymmetric C–O–C groups.79,80 Furthermore, the FT-IR spectrum of RC-nZVI shows the main characteristic peaks well matching that of the RC seeds, indicating that the nZVI surface is covered with active ingredients of RC seeds.
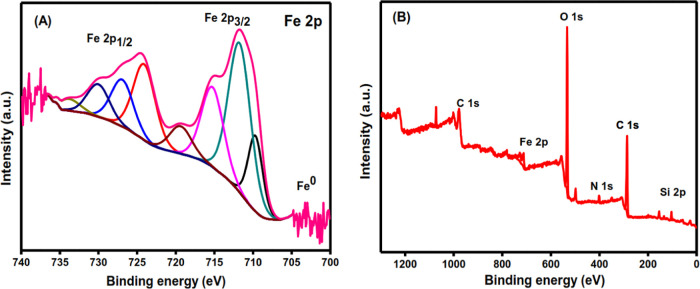
2.2.7. XPS Analysis
XPS is the most sensitive and widely used technique for determining the exact elemental ratio and bonding nature of the elements in NP materials. It is a surface-sensitive technique that can be used in depth-profiling studies to determine the overall composition and variation in composition with depth.81 The wide XPS spectrum of RC-nZVI showed three peaks at 709.69, 724, and 726 eV (Figure 5A), representing the binding energies of Fe (2p3/2) and shake-up satellite (2p3/2 and 2p1/2) peaks, which confirm the existence of Fe(II) and Fe(III). The small peak at 704 eV is related to the 2p3/2 peak of nZVI.82 This peak is expected to be small in XPS since it is a surface analysis and the nZVI surface is largely made up of oxides. The co-existence of a small fraction of Fe0 compared to that of iron oxides confirms the core–shell structure of nZVI.83 The main composition of RC-nZVI is carbon, oxygen, nitrogen, and silicon, as shown in Figure 5B, which was previously confirmed by the EDX analysis (Figure 2C).
Figure 5.
(A) XPS spectra for Fe 2p, and (B) wide spectrum of RC-nZVI.
2.3. Study of MB Removal
2.3.1. Adsorption Kinetics Studies
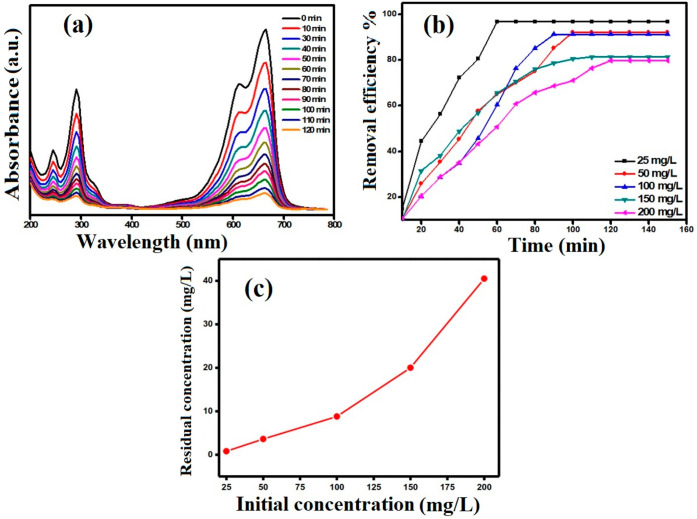
Figure 6A represents the UV–vis spectrum for the decrease in absorbance of MB (200 mg·L–1) with contact time in the presence of RC-nZVI (30 mg). The impact of variation of MB concentration on the adsorption was analyzed by taking different concentrations (25–200 mg·L–1) with a fixed quantity of RC-nZVI (30 mg) while the pH was adjusted at 6. It was observed that the percentage removal of MB decreases with the increase in initial concentration of the MB dye. The removal efficiency decreases from 96.8 to 79.75% as the initial concentration increases from 25 to 200 mg·L–1. The increase in loading capacity of the adsorbent concerning dye ions is probably due to a high driving force for mass transfer. The more concentrated the solution, the better the adsorption capacities. As seen from Figure 6B, the adsorption capacities of RC-nZVI increase from 8.1 to 53.16 mg·g–1. The concentrations of MB before and after adsorption onto RC-nZVI are shown in Figure 6C.
Figure 6.
(a) UV–vis absorption spectra [Co = 200 mg·L–1, m = 30 mg, pH = 6, V= 10 mL], (b) qt versus contact time, and (c) initial versus residual concentration for the removal of MB onto RC-nZVI.
Adsorption kinetics study is a useful method to evaluate the dye adsorption performance, which is necessary to evaluate the dye adsorption efficiency. Hence, different kinetic models were applied on the experimental data of the adsorption of MB onto RC-nZVI, including pseudo-first-order, pseudo-second-order, and Elovich models. Table 3 lists the linear forms of these kinetic equations.
Table 3. Kinetic Models and Adsorption Isotherms Used in the Present Studya.
| model | linear form | |
|---|---|---|
| kinetic models | pseudo-first-order | 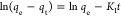 |
| pseudo-second-order | 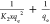 |
|
| Elovich | 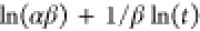 |
|
| goodness of adsorption (Chi-square) | 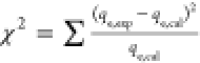 |
|
| adsorption isotherm | Langmuir isotherm | 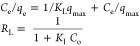 |
| Freundlich isotherm | 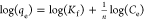 |
|
| Temkin | 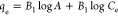 |
qe, qt, and qm are the amount of adsorbed dye at equilibrium, the amount of dye adsorbed at time t, and the maximum adsorption capacity (mg·g–1), respectively. K1 and K2 are the rate constant of pseudo-first-order (min–1) and rate constant of pseudo-second-order (g·mg–1·min–1), respectively. β is the desorption constant (g·mg–1) and α is the initial adsorption rate (mg·g–1·min–1). Ce is the equilibrium concentration (mg·L–1), KL is the adsorption equilibrium constant (L·mg–1), RL is the separation factor, B1 is the Temkin constant, and A is the equilibrium bond constant.
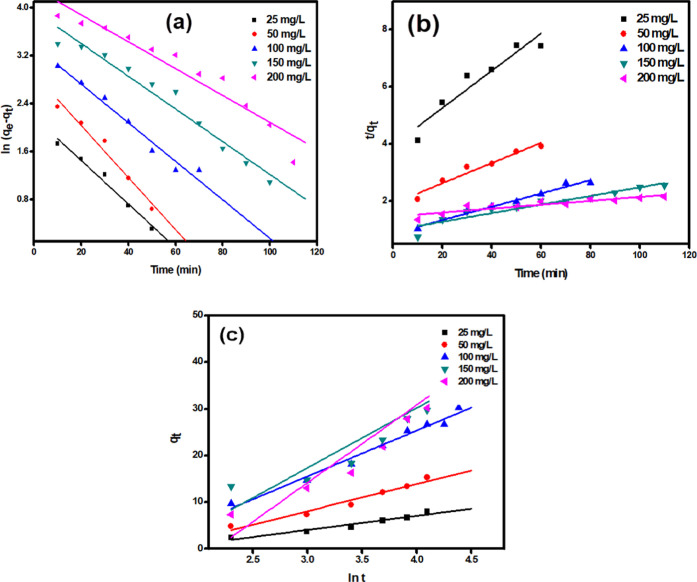
The results obtained from these models indicated that the adsorption of MB onto RC-nZVI follows the pseudo-first-order kinetic model, where the R2 value obtained from this kinetic model (R2 > 0.97) were found to be higher than those of the other two models (Table 4 and Figure 7), thus confirming that this kinetic model better describes the MB adsorption onto RC-nZVI. In addition, the calculated values of (qe,cal) were found to be in good agreement with the experimental ones (qe,exp). Furthermore, K1 values decrease with increase in the initial concentration of MB dye, revealing that the adsorption of MB by RC-nZVI is a physical adsorption reaction. The removal of MB by biowaste granules also fitted to pseudo-first-order in a study performed by Jaiyeola et al.84 and another study performed by Sahu et al.85 They found that the adsorption of MB onto nZVI follows the pseudo-first-order kinetic model. The Elovich model effectively describes the interaction between a solid and liquid interface, and takes into account that the real solid surface is energetically heterogeneous and that the desorption process and interactions between adsorbed species have little effect on the adsorption kinetics.86 As it can be seen from Table 4, the value of α is higher than that of β for MB, which indicates a low desorption rate and a higher adsorption rate, implying that MB has an effective tendency to be adsorbed onto RC-nZVI.87
Table 4. Kinetic Parameters for Adsorption of the MB Dye onto RC-nZVI.
| concentration (mg·L–1) |
|||||
|---|---|---|---|---|---|
| parameter | 25 | 50 | 100 | 150 | 200 |
| qe,exp (mg·g–1) | 8.10 | 15.46 | 30.40 | 43.33 | 53.16 |
| pseudo-first-order | |||||
| qe, cal (mg·g–1) | 8.78 | 18.14 | 31.16 | 39.10 | 55.76 |
| K1 (min–1) | 0.046 | 0.033 | 0.035 | 0.027 | 0.019 |
| R2 | 0.988 | 0.975 | 0.971 | 0.961 | 0.961 |
| (χ2) | 0.944 | ||||
| pseudo-second-order | |||||
| qe cal (mg·g–1) | 15.34 | 28.18 | 43.22 | 66.46 | 155.4 |
| K2 (mg·g–1) | 0.0038 | 0.0021 | 0.0012 | 0.0014 | 0.0002 |
| R2 | 0.907 | 0.949 | 0.970 | 0.910 | 0.831 |
| (χ2) | 34.529 | ||||
| Elovich | |||||
| β (g·mg–1) | 0.328 | 0.171 | 0.102 | 0.103 | 0.050 |
| α (mg·g·min–1) | 0.575 | 1.612 | 2.393 | 2.92 | 5.779 |
| R2 | 0.944 | 0.961 | 0.970 | 0.920 | 0.918 |
Figure 7.
Adsorption kinetic models: (a) pseudo-first-order, (b) pseudo-second-order, and (c) Elovich model.
The chi-square (χ2) test is used to assess the difference between the experimental and model data. If the model’s data match the experimental data, χ2 will be a small value, but if they do not, χ2 will be a high value.88 According to the results obtained from the model, the χ2 values for the pseudo-first-order were 0.944 and 34.529. Thus, the lower χ2 value for the pseudo-first-order suggests that MB dye adsorption onto RC-nZVI follows pseudo-first-order; the value of pseudo-second-order suggests poor fitting to the data for MB adsorption onto RC-nZVI.
2.3.2. Adsorption Isotherm Models
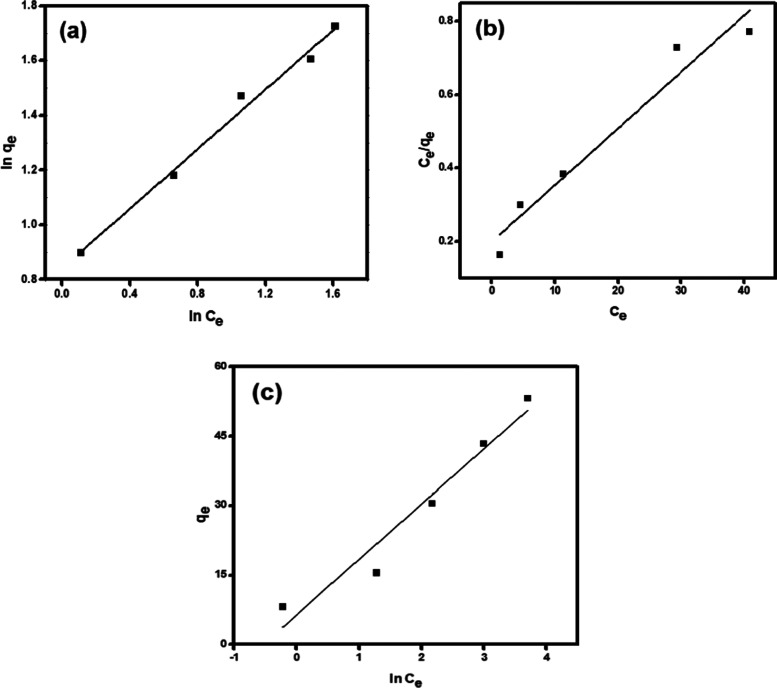
Adsorption isotherms play an essential part in describing the interaction between the adsorbate and the adsorbent at equilibrium. Therefore, the adsorption isotherm study is an important step in the designing of the adsorption system.89 In this study, the equilibrium data for adsorption of MB onto RC-nZVI were analyzed by different isotherm models, including Langmuir, Freundlich, and Temkin models. This information can provide the relationship between the adsorbate and absorbent, in which the surface and adsorbate might adhere via chemisorption or physisorption.90 The linear forms of these models are summarized in Table 3. It is clear from the isotherm plots (Figure 8) and the derived parameters (Table 5) that the adsorption of MB dye onto RC-nZVI is fitted to the Freundlich model (R2 = 0.990). Therefore, the MB molecules were adsorbed on the film surface by way of multilayer adsorption. The derived n value is greater than 1 (1.838), indicating moderate adsorption intensity of MB onto RC-nZVI. Moreover, the maximum adsorption capacity (qmax) calculated from Langmuir was 61.73 mg·g–1. Moreover, the dimensionless constant separation factor (RL) used at different initial concentrations of the MB dye lies between 0 and 1, which signifies that the adsorption of RC-nZVI is favorable. In the literature, a similar isotherm model fitting has been obtained for the adsorption isotherms of various pollutants onto different adsorbents.13,91,92 The linear plot for the Temkin adsorption isotherm (Figure 8c) suggests that the adsorption of MB onto RC-nZVI is physical adsorption, since the value of b is less than 82 KJ mol–1.93
Figure 8.
Adsorption models: Langmuir (a), Freundlich (b), and Temkin (c) for adsorption of the MB dye onto RC-nZVI.
Table 5. Adsorption isotherm parameters for the adsorption of MB dye onto RC-nZVI.
| isotherm model | parameter | value |
|---|---|---|
| Langmuir | qm (mg·g–1) | 61.73 |
| KL (L·mg–1) | 0.092 | |
| RL | 0.259 | |
| R2 | 0.950 | |
| Freundlich | KF (L·mg–1) | 2.321 |
| n | 1.838 | |
| R2 | 0.990 | |
| Temkin | B (J·mol–1) | 207.55 |
| B (KJ·mol–1) | 0.207 | |
| A | 1.708 | |
| R2 | 0.950 |
2.3.3. Comparison with the Reported Green-Synthesized nZVI
To evaluate the potentiality of the synthesized RC-nZVI as an adsorbent, its adsorption capacity in the removal process of MB was compared with those of the reported green-synthesized nZVI adsorbents. It is obvious from Table 6 that RC-nZVI has a good adsorption capacity towards MB and hence can be applied as a green and clean adsorbent for the treatment of cationic dyes from wastewater.
Table 6. Comparison of the Maximum Adsorption Capacity (qm) of RC-nZVI with Those of Other Reported nZVI Adsorbentsa.
| adsorbent | pollutant | qm (mg·g–1) | ref |
|---|---|---|---|
| nZVI (using sweet lime pulp) | MB | 14.76 | (85) |
| TP-nZVI | MB | 72.60 | (68) |
| nZVI (liquid-phase reduction) | MB | 5.53 | (94) |
| nZVI (using Platanus occidentalis) | MB | 126.60 | (95) |
| nZVI | Amoxicillin | 40.28 | (96) |
| nZVI | Pb2+ | 50.31 | (97) |
| nZVI (using Korla fragrant) | Cr(VI) | 46.62 | (98) |
| RC-nZVI | MB | 64.94 | this study |
TP = Tea polyphenols.
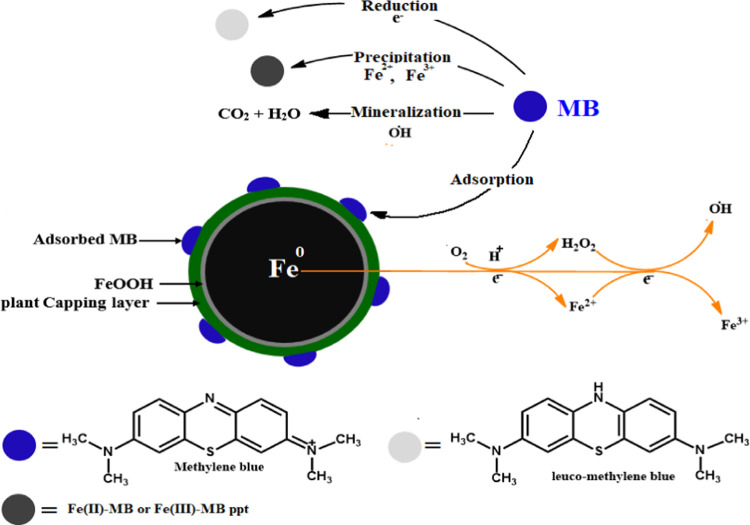
2.3.4. Mechanism of MB Removal onto RC-nZVI
Figure 9 shows the proposed mechanism for the removal of MB onto RC-nZVI. MB removal by RC-nZVI can proceed via different pathways, including the following:
Figure 9.
Proposed mechanism for the removal of MB onto RC-nZVI.
(i) Adsorption of MB particles onto RC-nZVI via electrostatic interaction between the cationic MB dye molecules and the negatively charged RC-nZVI (ZP of −14.9 mV), as clarified from ZP measurements.
(ii) Degradation that takes place firstly via MB adsorption on RC-nZVI, followed by reductive cleavage of MB molecules to mineralization products.99 The electrons produced from the oxidation of Fe0 are utilized in the reduction of MB dye, causing decolorization (eq 1 and 24).100
| 1 |
| 2 |
Moreover, Fe0 forms H2O2 in the presence of O2 and H+ (eq 3), which produces reactive •OH radicals (eq 4).101•OH radicals are strong oxidants of organic dyes, which is responsible for decolorization (eq 5).102
| 3 |
| 4 |
| 5 |
(iii) Formation of Fe2+/Fe3+ ion–MB complexes as a result of MB precipitation due to the combination with Fe2+, and Fe3+ that is produced from the further oxidation of Fe2+ according to eq 3.103
| 6 |
(iv) Oxidation of nZVI to Fe2+, producing free electrons that easily reduce MB into colorless leuco-methylene blue35,104 (eq 7).
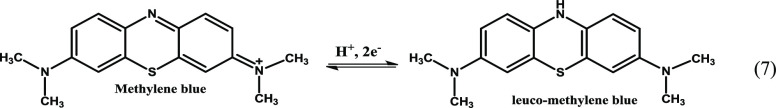 |
7 |
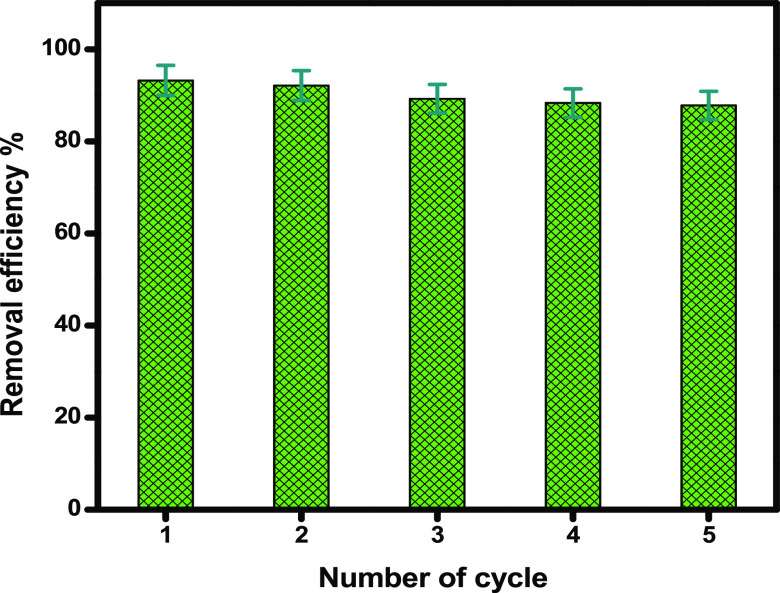
2.3.5. Reusability of nZVI
Reusability is a major factor for any adsorbent to be applicable in large-scale applications. In this contest, the synthesized RC-nZVI was tested for reuse for 5 cycles. After complete adsorption of the MB dye, RC-nZVI was easily separated by centrifugation, washed with ethanol (99%) as a desorption medium, dried in an air oven at 60 °C for 3 h, and then tested for the next adsorption run. Figure 9 shows the reusability of RC-nZVI in the removal of MB; the synthesized RC-nZVI can retain its adsorption ability (>87%) even after 5 consecutive cycles, revealing that RC-nZVI represents a green and environmentally friendly and low-cost adsorbent (Figure 10).
Figure 10.
Reusability of RC-nZVI in MB dye adsorption.
3. Conclusions
In this work, a novel and green synthesis of nZVI was achieved by a facile and one-step method using R. communis seed extract. R. communis acts as a reducing and capping agent for nanoparticles. The biomolecules in the extract, which were determined by the phytochemical screening, reduce the aggregation of nZVI and enhance its reactivity. The phytochemical screening of the plant extract also indicated the presence of flavonoids, tannins, saponins, and phenols, which provide a synergistic effect for the reduction and stabilization of RC-nZVI. The MB removal efficiency reached a maximum of 96.8%. The regeneration efficiency was decreased to 87% after five cycles, indicating good stability and activity.
4. Materials and Methods
4.1. Materials
All reagents used in this study were of analytical grade and used without further purification. All aqueous solutions were prepared using double-distilled water. Ferric chloride hexahydrate (97%, FeCl3·6H2O) was purchased from Sigma-Aldrich. Ethanol (99.9%, C2H5OH), sodium hydroxide (97%, NaOH), and hydrochloric acid (37%, HCl) were obtained from Merck. Methylene blue (MB) dye (C16H18N3SCl, 319.85 g mol–1) was purchased from MP Bio-medical, LLC.
4.2. Preparation of Plant Extract
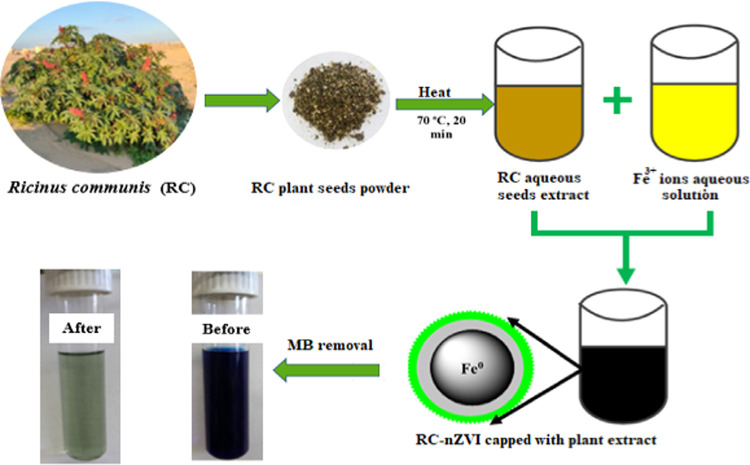
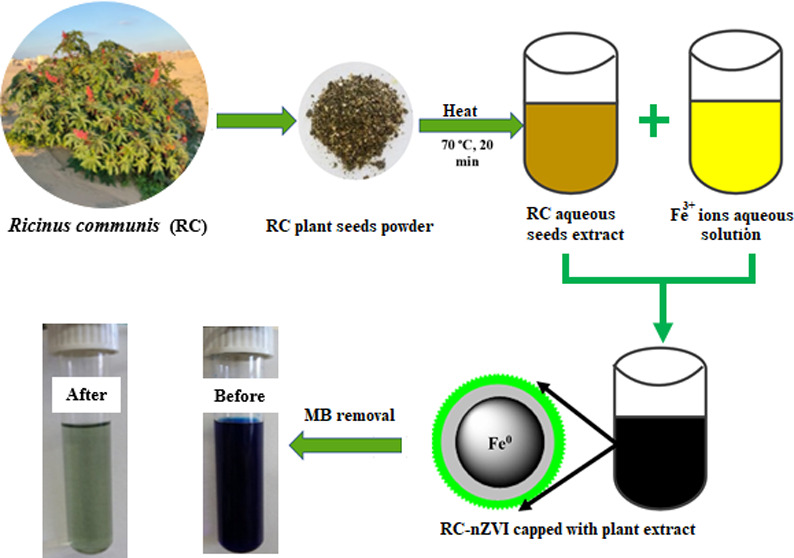
Fresh R. communis specimens were collected from different localities of the black sand area along the Mediterranean Sea Coast, Al Burullus lake, Kafr El-Shaikh, Egypt, with coordinates 31°35′30.2″N 31°05′40.7″E, during November–December 2019. The plant seeds were collected and washed using tap water and distilled water repeatedly to remove any dust and particulate matter on them. The seeds were then left in air at room temperature till they were completely dried. Afterward, specimens were shredded into small pieces using a sterilized knife before grinding by a stainless-steel mill to get a homogeneous fine powder (0.5 μm), and then kept in a dark place at room temperature for further use. A certain amount of plant seed fine powder was soaked in distilled water (5% W/V) and gently stirred at 70 °C for 20 min to get a reddish-brown extract. The extract was collected and centrifuged at 5000 rpm for 10 min, and the supernatant was filtered using Whatman filter paper number 1. Then, the resulting clear solution was stored in a clean, sterilized, and dried Falcon tube in a refrigerator at 4 °C for further use.
4.3. Phytochemical Screening of R. communis
Phytochemical screening of R. communis seeds was conducted as done by Yadav and Agarwala.105Table 1 shows various tests for the presence or absence of tannins, alkaloids, saponins, terpenoids, flavonoids, carbohydrates, fats and fixed oils, phenols, and anthraquinone glycosides.
4.4. Green Synthesis of nZVI
Ten milliliters of plant seed extract was added drop by drop with constant stirring to an aqueous solution of Fe3+ ions (5 mL, 0.05 M) in a clean sterilized flask. The resultant solution was then stirred for another 15 min to enhance the size distribution, followed by centrifugation at 12 000 rpm for 5 min. The obtained black product was washed three times using distilled water and ethanol and dried in an oven at 60 °C for 12 h and nominated as RC-nZVI.
Scheme 1 represents the green synthesis of RC-nZVI using the RC aqueous extract and Fe3+ ions for the adsorptive removal of the MB dye.
Scheme 1. Schematic Diagram and Digital Photographs for the Green Synthesis of nZVI using RC seed extract and Removal of the MB dye (Photographs taken by Ahmed M. Abdelfatah).
4.5. Characterization of RC-nZVI
The optical properties of the green-synthesized RC-nZVI were characterized using a double-beam UV–visible spectrophotometer (T70/T80 series UV/Vis Spectrophotometer, PG Instruments Ltd, U.K.) in the wavelength range 200–800 nm. The morphology and size distribution were determined using transmission electron microscopy (TEM) (JEM-1400 Plus instrument, operated at an accelerating voltage of 80 KV). To assess the possible functional group in the seed extract that is responsible for the reduction and stabilization process, the Fourier transform infrared (FT-IR) technique was utilized; the measurement was conducted for a ground sample with KBr on a JASCO spectrometer over the range 4000–600 cm–1. The elemental analysis was performed using energy-dispersive X-ray spectroscopy (EDS) on a scanning electron microscope, JEOL model JSM-IT100. The surface charge was determined using ζ-potential (Nano-ZS90, Malvern, U.K.). To determine the crystallographic structure of nZVI powder, the XRD spectrum was recorded using an X-ray diffractometer (PXRD-6000 SHIMADZU) with a voltage of 40 KV and a current of 30 mA with Cu Kα radiation (K = 1.54056 Å) in the angle range of 10–80 °C. Furthermore, the binding energies and elemental composition of the RC-nZVI were examined by X-ray photoelectron spectroscopy (XPS, Thermo Scientific ESCALAB 250Xi VG). The particle size of RC-nZVI was determined using a zeta nanosizer (Nano-ZS90, Malvern, U.K.), equipped with a He–Ne laser (λ = 633 nm, max 5 mW) and operated at a scattering angle of 173°.
4.6. Batch Experiment
A stock solution of MB (1000 mg·L–1) was prepared and kept at 25 °C in the dark. A series of diluted MB solutions (25–200 mg·L–1) were prepared by dilution from the stock solution. In all, 0.03 g of RC-nZVI was added to 10 mL of MB dye solution (25 °C and pH 6) and stirred using an orbital shaker (Stuart, orbital shaker/SSL1) at 150 rpm. Sample aliquots were drawn at different time intervals (1–160 min). The residual concentration of MB was determined using a UV–visible spectrophotometer (T70/T80 series UV/Vis Spectrophotometer, PG Instruments Ltd, U.K.) equipped with a quartz cell at λmax = 665 nm. The percentage removal, R%, of the MB and the amount of MB transferred onto the surface of the adsorbent, qt (mg·g–1), were calculated using the following equations.106
| 8 |
where R (%) is the MB removal efficiency (%), and Co and C are the concentrations of MB at time 0 and t, respectively (mg·L–1), and
| 9 |
where q is the quantity of MB adsorbed per unit mass of adsorbent (mg·g–1), Co is the initial concentration of MB, C is the concentration of MB at time t, V is the volume of the solution (L), and m is the mass of RC-nZVI (g).
Acknowledgments
This work was financially supported by SMART WATIR, ERANETMED-3–227 project, Egyptian Academy of Scientific Research and Technology.
The authors declare no competing financial interest.
References
- Li Y.; Du Q.; Liu T.; Peng X.; Wang J.; Sun J.; Wang Y.; Wu S.; Wang Z.; Xia Y.; Xia L. Comparative study of methylene blue dye adsorption onto activated carbon, graphene oxide, and carbon nanotubes. Chem. Eng. Res. Des. 2013, 91, 361–368. 10.1016/j.cherd.2012.07.007. [DOI] [Google Scholar]
- Omer A. M.; Abd El-Monaem E. M.; Abd El-Latif M. M.; El-Subruiti G. M.; Eltaweil A. S. Facile fabrication of novel magnetic ZIF-67 MOF@ aminated chitosan composite beads for the adsorptive removal of Cr (VI) from aqueous solutions. Carbohydr. Polym. 2021, 265, 118084 10.1016/j.carbpol.2021.118084. [DOI] [PubMed] [Google Scholar]
- Hamdy A.; Mostafa M. K.; Nasr M. Regression analysis and artificial intelligence for removal of methylene blue from aqueous solutions using nanoscale zero-valent iron. Int. J. Environ. Sci. Technol. 2019, 16, 357–372. 10.1007/s13762-018-1677-z. [DOI] [Google Scholar]
- Hynes N. R. J.; Kumar J. S.; Kamyab H.; Sujana J. A. J.; Al-Khashman O. A.; Kuslu Y.; Ene A.; Suresh Kumar B. Modern enabling techniques and adsorbents based dye removal with sustainability concerns in textile industrial sector -A comprehensive review. J. Cleaner Prod. 2020, 272, 122636 10.1016/j.jclepro.2020.122636. [DOI] [Google Scholar]
- Du M.; Yi Q.; Ji J.; Zhu Q.; Duan H.; Xing M.; Zhang J. Sustainable activation of peroxymonosulfate by the Mo(IV) in MoS2 for the remediation of aromatic organic pollutants. Chin. Chem. Lett. 2020, 31, 2803–2808. 10.1016/j.cclet.2020.08.002. [DOI] [Google Scholar]
- Yan Q.; Lian C.; Huang K.; Liang L.; Yu H.; Yin P.; Zhang J.; Xing M. Constructing an Acidic Microenvironment by MoS2 in Heterogeneous Fenton Reaction for Pollutant Control. Angew. Chem. 2021, 133, 17292–17300. 10.1002/ange.202105736. [DOI] [PubMed] [Google Scholar]
- Ji J.; Yan Q.; Yin P.; Mine S.; Matsuoka M.; Xing M. Defects on CoS2– x: Tuning Redox Reactions for Sustainable Degradation of Organic Pollutants. Angew. Chem. 2021, 133, 2939–2944. 10.1002/ange.202013015. [DOI] [PubMed] [Google Scholar]
- Li X.; Zhang M.; Liu Y.; Li X.; Liu Y.; Hua R.; He C. Removal of U(VI) in aqueous solution by nanoscale zero-valent iron (nZVI). Water Qual., Exposure Health 2013, 5, 31–40. 10.1007/s12403-013-0084-4. [DOI] [Google Scholar]
- Lellis B.; Fávaro-Polonio C. Z.; Pamphile J. A.; Polonio J. C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innovation 2019, 3, 275–290. 10.1016/j.biori.2019.09.001. [DOI] [Google Scholar]
- Chen D.; Zeng Z.; Zeng Y.; Zhang F.; Wang M. Removal of methylene blue and mechanism on magnetic γ-Fe2O3/SiO2 nanocomposite from aqueous solution. Water Resour. Ind. 2016, 15, 1–13. 10.1016/j.wri.2016.05.003. [DOI] [Google Scholar]
- Hosny M.; Fawzy M.; Abdelfatah A. M.; Fawzy E. E.; Eltaweil A. S. Comparative study on the potentialities of two halophytic species in the green synthesis of gold nanoparticles and their anticancer, antioxidant and catalytic efficiencies. Adv. Powder Technol. 2021, 32, 3220–3233. 10.1016/j.apt.2021.07.008. [DOI] [Google Scholar]
- Eltaweil A. S.; Abd El-Monaem E. M.; El-Subruiti G. M.; Abd El-Latif M. M.; Omer A. M. Fabrication of UiO-66/MIL-101 (Fe) binary MOF/carboxylated-GO composite for adsorptive removal of methylene blue dye from aqueous solutions. RSC Adv. 2020, 10, 19008–19019. 10.1039/D0RA02424D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal S.; Tyagi I.; Gupta V. K.; Ghasemi N.; Shahivand M.; Ghasemi M. Kinetics, equilibrium studies and thermodynamics of methylene blue adsorption on Ephedra strobilacea saw dust and modified using phosphoric acid and zinc chloride. J. Mol. Liq. 2016, 218, 208–218. 10.1016/j.molliq.2016.02.073. [DOI] [Google Scholar]
- Eltaweil A. S.; Elshishini H. M.; Ghatass Z. F.; Elsubruiti G. M. Ultra-high adsorption capacity and selective removal of Congo red over aminated graphene oxide modified Mn-doped UiO-66 MOF. Powder Technol. 2021, 379, 407–416. 10.1016/j.powtec.2020.10.084. [DOI] [Google Scholar]
- El-Subruiti G.; Eltaweil A.; Sallam S. Synthesis of active MFe2O4/γ-Fe2O3 nanocomposites (metal= Ni or Co) for reduction of nitro-containing pollutants and methyl orange degradation. Nano 2019, 14, 1950125 10.1142/S179329201950125X. [DOI] [Google Scholar]
- Omer A. M.; Khalifa R. E.; Tamer T. M.; Ali A. A.; Ammar Y. A.; Eldin M. S. M. Kinetic and thermodynamic studies for the sorptive removal of crude oil spills using a low-cost chitosan-poly (butyl acrylate) grafted copolymer. Desalin. Water Treat. 2020, 192, 213–225. 10.5004/dwt.2020.25704. [DOI] [Google Scholar]
- El-Monaem E. M. A.; El-Latif M. M. A.; Eltaweil A. S.; El-Subruiti G. M. Cobalt nanoparticles supported on reduced amine-functionalized graphene oxide for catalytic reduction of nitroanilines and organic dyes. Nano 2021, 16, 2150039 10.1142/S1793292021500399. [DOI] [Google Scholar]
- Sallam S.; El-Subruiti G.; Eltaweil A. Facile synthesis of Ag−γ-Fe2O3 superior nanocomposite for catalytic reduction of nitroaromatic compounds and catalytic degradation of methyl orange. Catal. Lett. 2018, 148, 3701–3714. 10.1007/s10562-018-2569-z. [DOI] [Google Scholar]
- Cheng M.; Zeng G.; Huang D.; Lai C.; Xu P.; Zhang C.; Liu Y. Hydroxyl radicals based advanced oxidation processes (AOPs) for remediation of soils contaminated with organic compounds: a review. Chem. Eng. J. 2016, 284, 582–598. 10.1016/j.cej.2015.09.001. [DOI] [Google Scholar]
- Deka P.; Hazarika A.; Deka R. C.; Bharali P. Influence of CuO morphology on the enhanced catalytic degradation of methylene blue and methyl orange. RSC Adv. 2016, 6, 95292–95305. 10.1039/C6RA20173C. [DOI] [Google Scholar]
- Eltaweil A. S.; El-Monaem E. M. A.; Mohy-Eldin M. S.; Omer A. M. Fabrication of attapulgite/magnetic aminated chitosan composite as efficient and reusable adsorbent for Cr (VI) ions. Sci. Rep. 2021, 11, 16598 10.1038/s41598-021-96145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Borady O. M.; Fawzy M.; Hosny M. Antioxidant, anticancer and enhanced photocatalytic potentials of gold nanoparticles biosynthesized by common reed leaf extract. Appl. Nanosci. 2021, 10.1007/s13204-021-01776-w. [DOI] [Google Scholar]
- Mohy Eldin M. S.; Omer A.; Soliman E.; Hassan E. Superabsorbent polyacrylamide grafted carboxymethyl cellulose pH sensitive hydrogel: I. Preparation and characterization. Desalin. Water Treat. 2013, 51, 3196–3206. 10.1080/19443994.2012.751156. [DOI] [Google Scholar]
- El-Sayed E.; Tamer T.; Omer A.; Mohy Eldin M. Development of novel chitosan schiff base derivatives for cationic dye removal: methyl orange model. Desalin. Water Treat. 2016, 57, 22632–22645. 10.1080/19443994.2015.1136694. [DOI] [Google Scholar]
- Elashery S. E.; Attia N. F.; Omar M.; Tayea H. M. Cost-effective and green synthesized electroactive nanocomposite for high selective potentiometric determination of clomipramine hydrochloride. Microchem. J. 2019, 151, 104222 10.1016/j.microc.2019.104222. [DOI] [Google Scholar]
- Attia N. F.; Park J.; Oh H. Facile tool for green synthesis of graphene sheets and their smart free-standing UV protective film. Appl. Surf. Sci. 2018, 458, 425–430. 10.1016/j.apsusc.2018.07.066. [DOI] [Google Scholar]
- Puthukkara P A. R.; Jose T S.; lal S D. Plant mediated synthesis of zero valent iron nanoparticles and its application in water treatment. J. Environ. Chem. Eng. 2021, 9, 104569 10.1016/j.jece.2020.104569. [DOI] [Google Scholar]
- Bao T.; Damtie M. M.; Hosseinzadeh A.; Wei W.; Jin J.; Phong Vo H. N.; Ye J. S.; Liu Y.; Wang X. F.; Yu Z. M.; Chen Z. J.; Wu K.; Frost R. L.; Ni B.-J. Bentonite-supported nano zero-valent iron composite as a green catalyst for bisphenol A degradation: Preparation, performance, and mechanism of action. J. Environ. Manage. 2020, 260, 110105 10.1016/j.jenvman.2020.110105. [DOI] [PubMed] [Google Scholar]
- Xiao C.; Li H.; Zhao Y.; Zhang X.; Wang X. Green synthesis of iron nanoparticle by tea extract (polyphenols) and its selective removal of cationic dyes. J. Environ. Manage. 2020, 275, 111262 10.1016/j.jenvman.2020.111262. [DOI] [PubMed] [Google Scholar]
- Li Z.; Xu S.; Xiao G.; Qian L.; Song Y. Removal of hexavalent chromium from groundwater using sodium alginate dispersed nano zero-valent iron. J. Environ. Manage. 2019, 244, 33–39. 10.1016/j.jenvman.2019.04.130. [DOI] [PubMed] [Google Scholar]
- Lin Z.; Weng X.; Owens G.; Chen Z. Simultaneous removal of Pb(II) and rifampicin from wastewater by iron nanoparticles synthesized by a tea extract. J. Cleaner Prod. 2020, 242, 118476 10.1016/j.jclepro.2019.118476. [DOI] [Google Scholar]
- Pirsaheb M.; Moradi S.; Shahlaei M.; Wang X.; Farhadian N. A new composite of nano zero-valent iron encapsulated in carbon dots for oxidative removal of bio-refractory antibiotics from water. J. Cleaner Prod. 2019, 209, 1523–1532. 10.1016/j.jclepro.2018.11.175. [DOI] [Google Scholar]
- Ai Z.; Gao Z.; Zhang L.; He W.; Yin J. J. Core–Shell Structure Dependent Reactivity of Fe@Fe2O3 Nanowires on Aerobic Degradation of 4-Chlorophenol. Environ. Sci. Technol. 2013, 47, 5344–5352. 10.1021/es4005202. [DOI] [PubMed] [Google Scholar]
- Xu J.-h.; Gao N.-y.; Zhao D.-y.; Yin D.-q.; Zhang H.; Gao Y.-q.; Shi W. Comparative study of nano-iron hydroxide impregnated granular activated carbon (Fe–GAC) for bromate or perchlorate removal. Sep. Purif. Technol. 2015, 147, 9–16. 10.1016/j.seppur.2015.03.052. [DOI] [Google Scholar]
- Eltaweil A.; Mohamed H. A.; Abd El-Monaem E. M.; El-Subruiti G. Mesoporous magnetic biochar composite for enhanced adsorption of malachite green dye: Characterization, adsorption kinetics, thermodynamics and isotherms. Adv. Powder Technol. 2020, 31, 1253–1263. 10.1016/j.apt.2020.01.005. [DOI] [Google Scholar]
- Shubair T.; Eljamal O.; Khalil A. M. E.; Tahara A.; Matsunaga N. Novel application of nanoscale zero valent iron and bimetallic nano-Fe/Cu particles for the treatment of cesium contaminated water. J. Environ. Chem. Eng. 2018, 6, 4253–4264. 10.1016/j.jece.2018.06.015. [DOI] [Google Scholar]
- Dai Y.; Hu Y.; Jiang B.; Zou J.; Tian G.; Fu H. Carbothermal synthesis of ordered mesoporous carbon-supported nano zero-valent iron with enhanced stability and activity for hexavalent chromium reduction. J. Hazard. Mater. 2016, 309, 249–258. 10.1016/j.jhazmat.2015.04.013. [DOI] [PubMed] [Google Scholar]
- Khatami M.; Alijani H. Q.; Fakheri B.; Mobasseri M. M.; Heydarpour M.; Farahani Z. K.; Khan A. U. Super-paramagnetic iron oxide nanoparticles (SPIONs): Greener synthesis using Stevia plant and evaluation of its antioxidant properties. J. Cleaner Prod. 2019, 208, 1171–1177. 10.1016/j.jclepro.2018.10.182. [DOI] [Google Scholar]
- Hosny M.; Fawzy M.; El-Borady O. M.; Mahmoud A. E. D. Comparative study between Phragmites australis root and rhizome extracts for mediating gold nanoparticles synthesis and their medical and environmental applications. Adv. Powder Technol. 2021, 32, 2268–2279. 10.1016/j.apt.2021.05.004. [DOI] [Google Scholar]
- Stefaniuk M.; Oleszczuk P.; Ok Y. S. Review on nano zerovalent iron (nZVI): From synthesis to environmental applications. Chem. Eng. J. 2016, 287, 618–632. 10.1016/j.cej.2015.11.046. [DOI] [Google Scholar]
- Wang T.; Jin X.; Chen Z.; Megharaj M.; Naidu R. Green synthesis of Fe nanoparticles using eucalyptus leaf extracts for treatment of eutrophic wastewater. Sci. Total Environ. 2014, 466-467, 210–213. 10.1016/j.scitotenv.2013.07.022. [DOI] [PubMed] [Google Scholar]
- Zhu M.; Wang X.; Yang J.; Liu H.; Ma J. Study on the physicochemical properties of poly(methylmethacrylate) (PMMA) modified Pd/Fe nanocomposites: Roles of PMMA and PMMA/ethanol. Appl. Surf. Sci. 2013, 282, 851–861. 10.1016/j.apsusc.2013.06.070. [DOI] [Google Scholar]
- Zhu F.; Ma S.; Liu T.; Deng X. Green synthesis of nano zero-valent iron/Cu by green tea to remove hexavalent chromium from groundwater. J. Cleaner Prod. 2018, 174, 184–190. 10.1016/j.jclepro.2017.10.302. [DOI] [Google Scholar]
- Vijayaraghavan K.; Ashokkumar T. Plant-mediated biosynthesis of metallic nanoparticles: A review of literature, factors affecting synthesis, characterization techniques and applications. J. Environ. Chem. Eng. 2017, 5, 4866–4883. 10.1016/j.jece.2017.09.026. [DOI] [Google Scholar]
- Hosny M.; Fawzy M. Instantaneous phytosynthesis of gold nanoparticles via Persicaria salicifolia leaf extract, and their medical applications. Adv. Powder Technol. 2021, 32, 2891–2904. 10.1016/j.apt.2021.06.004. [DOI] [Google Scholar]
- Dhuper S.; Panda D.; Nayak P. Green synthesis and characterization of zero valent iron nanoparticles from the leaf extract of Mangifera indica. Nano Trends: J. Nanotech. App. 2012, 13, 16–22. [Google Scholar]
- Rashtbari Y.; Hazrati S.; Azari A.; Afshin S.; Fazlzadeh M.; Vosoughi M. A novel, eco-friendly and green synthesis of PPAC-ZnO and PPAC-nZVI nanocomposite using pomegranate peel: Cephalexin adsorption experiments, mechanisms, isotherms and kinetics. Adv. Powder Technol. 2020, 31, 1612–1623. 10.1016/j.apt.2020.02.001. [DOI] [Google Scholar]
- Fazlzadeh M.; Rahmani K.; Zarei A.; Abdoallahzadeh H.; Nasiri F.; Khosravi R. A novel green synthesis of zero valent iron nanoparticles (NZVI) using three plant extracts and their efficient application for removal of Cr (VI) from aqueous solutions. Adv. Powder Technol. 2017, 28, 122–130. 10.1016/j.apt.2016.09.003. [DOI] [Google Scholar]
- Jena J.; Gupta A. K. Ricinus communis Linn: a phytopharmacological review. Int. J. Pharm. Pharm. Sci. 2012, 4, 25–29. [Google Scholar]
- Dauthal P.; Mukhopadhyay M. Noble metal nanoparticles: plant-mediated synthesis, mechanistic aspects of synthesis, and applications. Ind. Eng. Chem. Res. 2016, 55, 9557–9577. 10.1021/acs.iecr.6b00861. [DOI] [Google Scholar]
- Ovais M.; Raza A.; Naz S.; Islam N. U.; Khalil A. T.; Ali S.; Khan M. A.; Shinwari Z. K. Current state and prospects of the phytosynthesized colloidal gold nanoparticles and their applications in cancer theranostics. Appl. Microbiol. Biotechnol. 2017, 101, 3551–3565. 10.1007/s00253-017-8250-4. [DOI] [PubMed] [Google Scholar]
- Ovais M.; Ayaz M.; Khalil A. T.; Shah S. A.; Jan M. S.; Raza A.; Shahid M.; Shinwari Z. K. HPLC-DAD finger printing, antioxidant, cholinesterase, and α-glucosidase inhibitory potentials of a novel plant Olax nana. BMC Complementary Altern. Med. 2018, 18, 1 10.1186/s12906-017-2057-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayaz M.; Junaid M.; Ullah F.; Subhan F.; Sadiq A.; Ali G.; Ovais M.; Shahid M.; Ahmad A.; Wadood A.; et al. Anti-Alzheimer’s studies on β-sitosterol isolated from Polygonum hydropiper L. Front. Pharmacol. 2017, 8, 697. 10.3389/fphar.2017.00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovais M.; Khalil A. T.; Raza A.; Islam N. U.; Ayaz M.; Saravanan M.; Ali M.; Ahmad I.; Shahid M.; Shinwari Z. K. Multifunctional theranostic applications of biocompatible green-synthesized colloidal nanoparticles. Appl. Microbiol. Biotechnol. 2018, 102, 4393–4408. 10.1007/s00253-018-8928-2. [DOI] [PubMed] [Google Scholar]
- Rana A.; Kumari N.; Tyagi M.; Jagadevan S. Leaf-extract mediated zero-valent iron for oxidation of Arsenic (III): Preparation, characterization and kinetics. Chem. Eng. J. 2018, 347, 91–100. 10.1016/j.cej.2018.04.075. [DOI] [Google Scholar]
- Sunardi A.; Rahardjo S. Green synthesis and characterization of nano zero valent iron using banana peel extract. J. Environ. Earth Sci. 2017, 7, 80–84. [Google Scholar]
- Saif S.; Tahir A.; Chen Y. Green Synthesis of Iron Nanoparticles and Their Environmental Applications and Implications. Nanomaterials 2016, 6, 209. 10.3390/nano6110209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sravanthi K.; Ayodhya D.; Swamy P. Y. Green synthesis, characterization and catalytic activity of 4-nitrophenol reduction and formation of benzimidazoles using bentonite supported zero valent iron nanoparticles. Mater. Sci. Energy Technol. 2019, 2, 298–307. 10.1016/j.mset.2019.02.003. [DOI] [Google Scholar]
- Mystrioti C.; Xanthopoulou T.; Tsakiridis P.; Papassiopi N.; Xenidis A. Comparative evaluation of five plant extracts and juices for nanoiron synthesis and application for hexavalent chromium reduction. Sci. Total Environ. 2016, 539, 105–113. 10.1016/j.scitotenv.2015.08.091. [DOI] [PubMed] [Google Scholar]
- Bolade O. P.; Williams A. B.; Benson N. U. Green synthesis of iron-based nanomaterials for environmental remediation: A review. Environ. Nanotechnol. Monit. Manage. 2020, 13, 100279 10.1016/j.enmm.2019.100279. [DOI] [Google Scholar]
- Pattanayak M.; Nayak P. L. Green Synthesis and Characterization of Zero Valent Iron Nanoparticles from the Leaf Extract of Azadirachta indica (Neem). World J. Nano Sci. Technol. 2013, 2, 06–09. 10.5829/idosi.wjnst.2013.2.1.21132. [DOI] [Google Scholar]
- Somchaidee P.; Tedsree K. Green synthesis of high dispersion and narrow size distribution of zero-valent iron nanoparticles using guava leaf (Psidium guajava L) extract. Adv. Nat. Sci.: Nanosci. Nanotechnol. 2018, 9, 035006 10.1088/2043-6254/aad5d7. [DOI] [Google Scholar]
- Eslami S.; Ebrahimzadeh M. A.; Biparva P. Green synthesis of safe zero valent iron nanoparticles by Myrtus communis leaf extract as an effective agent for reducing excessive iron in iron-overloaded mice, a thalassemia model. RSC Adv. 2018, 8, 26144–26155. 10.1039/C8RA04451A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darroudi M.; Ahmad M. B.; Zamiri R.; Zak A. K.; Abdullah A. H.; Ibrahim N. A. Time-dependent effect in green synthesis of silver nanoparticles. Int. J. Nanomed. 2011, 6, 677–681. 10.2147/IJN.S17669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D.; Hu L.; Lo I. M. C.; Yu Z. Size Distribution and Phosphate Removal Capacity of Nano Zero-Valent Iron (nZVI): Influence of pH and Ionic Strength. Water 2020, 12, 2939. 10.3390/w12102939. [DOI] [Google Scholar]
- Sharma D.; Kanchi S.; Bisetty K. Biogenic synthesis of nanoparticles: A review. Arabian J. Chem. 2019, 12, 3576–3600. 10.1016/j.arabjc.2015.11.002. [DOI] [Google Scholar]
- Shahwan T.; Sirriah S. A.; Nairat M.; Boyacı E.; Eroğlu A. E.; Scott T. B.; Hallam K. R. Green synthesis of iron nanoparticles and their application as a Fenton-like catalyst for the degradation of aqueous cationic and anionic dyes. Chem. Eng. J. 2011, 172, 258–266. 10.1016/j.cej.2011.05.103. [DOI] [Google Scholar]
- Wang X.; Wang A.; Ma J.; Fu M. Facile green synthesis of functional nanoscale zero-valent iron and studies of its activity toward ultrasound-enhanced decolorization of cationic dyes. Chemosphere 2017, 166, 80–88. 10.1016/j.chemosphere.2016.09.056. [DOI] [PubMed] [Google Scholar]
- Soliemanzadeh A.; Fekri M. Synthesis of clay-supported nanoscale zero-valent iron using green tea extract for the removal of phosphorus from aqueous solutions. Chin. J. Chem. Eng. 2017, 25, 924–930. 10.1016/j.cjche.2016.12.006. [DOI] [Google Scholar]
- Bankar A.; Joshi B.; Kumar A. R.; Zinjarde S. Banana peel extract mediated novel route for the synthesis of silver nanoparticles. Colloids Surf., A 2010, 368, 58–63. 10.1016/j.colsurfa.2010.07.024. [DOI] [PubMed] [Google Scholar]
- Tantra R.; Schulze P.; Quincey P. Effect of nanoparticle concentration on zeta-potential measurement results and reproducibility. Particuology 2010, 8, 279–285. 10.1016/j.partic.2010.01.003. [DOI] [Google Scholar]
- Nayak D.; Ashe S.; Rauta P. R.; Kumari M.; Nayak B. Bark extract mediated green synthesis of silver nanoparticles: evaluation of antimicrobial activity and antiproliferative response against osteosarcoma. Mater. Sci. Eng., C 2016, 58, 44–52. 10.1016/j.msec.2015.08.022. [DOI] [PubMed] [Google Scholar]
- Chen P.-J.; Tan S.-W.; Wu W.-L. Stabilization or oxidation of nanoscale zerovalent iron at environmentally relevant exposure changes bioavailability and toxicity in medaka fish. Environ. Sci. Technol. 2012, 46, 8431–8439. 10.1021/es3006783. [DOI] [PubMed] [Google Scholar]
- Li S.; Wang W.; Liang F.; Zhang W.-x. Heavy metal removal using nanoscale zero-valent iron (nZVI): Theory and application. J. Hazard. Mater. 2017, 322, 163–171. 10.1016/j.jhazmat.2016.01.032. [DOI] [PubMed] [Google Scholar]
- Ahmad M.; Ahmad M.; Usman A. R.; Al-Faraj A. S.; Abduljabbar A. S.; Al-Wabel M. I. Biochar composites with nano zerovalent iron and eggshell powder for nitrate removal from aqueous solution with coexisting chloride ions. Environ. Sci. Pollut. Res. 2018, 25, 25757–25771. 10.1007/s11356-017-0125-9. [DOI] [PubMed] [Google Scholar]
- El Bestawy E.; El-Shatby B. F.; Eltaweil A. S. Integration between bacterial consortium and magnetite (Fe 3 O 4) nanoparticles for the treatment of oily industrial wastewater. World J. Microbiol. Biotechnol. 2020, 36, 141 10.1007/s11274-020-02915-1. [DOI] [PubMed] [Google Scholar]
- Devatha C. P.; Thalla A. K.; Katte S. Y. Green synthesis of iron nanoparticles using different leaf extracts for treatment of domestic waste water. J. Cleaner Prod. 2016, 139, 1425–1435. 10.1016/j.jclepro.2016.09.019. [DOI] [Google Scholar]
- Tamer T. M.; Omer A. M.; Hassan M. A.; Hassan M. E.; Sabet M. M.; Eldin M. M. Development of thermo-sensitive poly N-isopropyl acrylamide grafted chitosan derivatives. J. Appl. Pharm. Sci. 2015, 5, 1–6. 10.7324/JAPS.2015.510.S1. [DOI] [Google Scholar]
- Jafari K.; Heidari M.; Rahmanian O. Wastewater treatment for Amoxicillin removal using magnetic adsorbent synthesized by ultrasound process. Ultrason. Sonochem. 2018, 45, 248–256. 10.1016/j.ultsonch.2018.03.018. [DOI] [PubMed] [Google Scholar]
- Fuente E.; Menéndez J.; Díez M.; Suárez D.; Montes-Morán M. Infrared spectroscopy of carbon materials: a quantum chemical study of model compounds. J. Phys. Chem. B 2003, 107, 6350–6359. 10.1021/jp027482g. [DOI] [Google Scholar]
- Khan I.; Saeed K.; Khan I. Nanoparticles: Properties, applications and toxicities. Arabian J. Chem. 2019, 12, 908–931. 10.1016/j.arabjc.2017.05.011. [DOI] [Google Scholar]
- Romero-González R.; Frenich A. G.. Applications in High Resolution Mass Spectrometry: Food Safety and Pesticide Residue Analysis; Elsevier, 2017. [Google Scholar]
- Zhou X.; Lv B.; Zhou Z.; Li W.; Jing G. Evaluation of highly active nanoscale zero-valent iron coupled with ultrasound for chromium (VI) removal. Chem. Eng. J. 2015, 281, 155–163. 10.1016/j.cej.2015.06.089. [DOI] [Google Scholar]
- Jaiyeola O. O.; Chen H.; Albadarin A. B.; Mangwandi C. Production of bio-waste granules and their evaluation as adsorbent for removal of hexavalent chromium and methylene blue dye. Chem. Eng. Res. Des. 2020, 164, 59–67. 10.1016/j.cherd.2020.09.020. [DOI] [Google Scholar]
- Sahu N.; Rawat S.; Singh J.; Karri R. R.; Lee S.; Choi J.-S.; Koduru J. R. Process optimization and modeling of methylene blue adsorption using zero-valent iron nanoparticles synthesized from sweet lime pulp. Appl. Sci. 2019, 9, 5112. 10.3390/app9235112. [DOI] [Google Scholar]
- Pezoti O.; Cazetta A. L.; Souza I. P. A. F.; Bedin K. C.; Martins A. C.; Silva T. L.; Almeida V. C. Adsorption studies of methylene blue onto ZnCl2-activated carbon produced from buriti shells (Mauritia flexuosa L.). J. Ind. Eng. Chem. 2014, 20, 4401–4407. 10.1016/j.jiec.2014.02.007. [DOI] [Google Scholar]
- Ahamad K. U.; Singh R.; Baruah I.; Choudhury H.; Sharma M. R. Equilibrium and kinetics modeling of fluoride adsorption onto activated alumina, alum and brick powder. Groundwater Sustainable Dev. 2018, 7, 452–458. 10.1016/j.gsd.2018.06.005. [DOI] [Google Scholar]
- Boparai H. K.; Joseph M.; O’Carroll D. M. Kinetics and thermodynamics of cadmium ion removal by adsorption onto nano zerovalent iron particles. J. Hazard. Mater. 2011, 186, 458–465. 10.1016/j.jhazmat.2010.11.029. [DOI] [PubMed] [Google Scholar]
- Eltaweil A. S.; El-Tawil A. M.; Abd El-Monaem E. M.; El-Subruiti G. M. Zero Valent Iron Nanoparticle-Loaded Nanobentonite Intercalated Carboxymethyl Chitosan for Efficient Removal of Both Anionic and Cationic Dyes. ACS Omega 2021, 6, 6348–6360. 10.1021/acsomega.0c06251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somsesta N.; Sricharoenchaikul V.; Aht-Ong D. Adsorption removal of methylene blue onto activated carbon/cellulose biocomposite films: Equilibrium and kinetic studies. Mater. Chem. Phys. 2020, 240, 122221 10.1016/j.matchemphys.2019.122221. [DOI] [Google Scholar]
- Shubair T.; Eljamal O.; Khalil A. M. E.; Matsunaga N. Multilayer system of nanoscale zero valent iron and Nano-Fe/Cu particles for nitrate removal in porous media. Sep. Purif. Technol. 2018, 193, 242–254. 10.1016/j.seppur.2017.10.069. [DOI] [Google Scholar]
- Azzam A. M.; El-Wakeel S. T.; Mostafa B. B.; El-Shahat M. F. Removal of Pb, Cd, Cu and Ni from aqueous solution using nano scale zero valent iron particles. J. Environ. Chem. Eng. 2016, 4, 2196–2206. 10.1016/j.jece.2016.03.048. [DOI] [Google Scholar]
- Erhayem M.; Al-Tohami F.; Mohamed R.; Ahmida K. Isotherm, kinetic and thermodynamic studies for the sorption of mercury (II) onto activated carbon from Rosmarinus officinalis leaves. Am. J. Anal. Chem. 2015, 06, 1–10. 10.4236/ajac.2015.61001. [DOI] [Google Scholar]
- Hamdy A.; Mostafa M. K.; Nasr M. Zero-valent iron nanoparticles for methylene blue removal from aqueous solutions and textile wastewater treatment, with cost estimation. Water Sci. Technol. 2018, 78, 367–378. 10.2166/wst.2018.306. [DOI] [PubMed] [Google Scholar]
- Parlayıcı Ş.; Pehlivan E. Fast decolorization of cationic dyes by nano-scale zero valent iron immobilized in sycamore tree seed pod fibers: kinetics and modelling study. Int. J. Phytorem. 2019, 21, 1130–1144. 10.1080/15226514.2019.1606786. [DOI] [PubMed] [Google Scholar]
- Ali I.; Afshinb S.; Poureshgh Y.; Azari A.; Rashtbari Y.; Feizizadeh A.; Hamzezadeh A.; Fazlzadeh M. Green preparation of activated carbon from pomegranate peel coated with zero-valent iron nanoparticles (nZVI) and isotherm and kinetic studies of amoxicillin removal in water. Environ. Sci. Pollut. Res. Int. 2020, 27, 36732–36743. 10.1007/s11356-020-09310-1. [DOI] [PubMed] [Google Scholar]
- Arancibia-Miranda N.; Baltazar S. E.; García A.; Romero A. H.; Rubio M. A.; Altbir D. Lead removal by nano-scale zero valent iron: Surface analysis and pH effect. Mater. Res. Bull. 2014, 59, 341–348. 10.1016/j.materresbull.2014.07.045. [DOI] [Google Scholar]
- Rong K.; Wang J.; Zhang Z.; Zhang J. Green synthesis of iron nanoparticles using Korla fragrant pear peel extracts for the removal of aqueous Cr(VI). Ecol. Eng. 2020, 149, 105793 10.1016/j.ecoleng.2020.105793. [DOI] [Google Scholar]
- Crane R. A.; Scott T. B. Nanoscale zero-valent iron: future prospects for an emerging water treatment technology. J. Hazard. Mater. 2012, 211–212, 112–125. 10.1016/j.jhazmat.2011.11.073. [DOI] [PubMed] [Google Scholar]
- He Y.; Gao J.-F.; Feng F.-Q.; Liu C.; Peng Y.-Z.; Wang S.-Y. The comparative study on the rapid decolorization of azo, anthraquinone and triphenylmethane dyes by zero-valent iron. Chem. Eng. J. 2012, 179, 8–18. 10.1016/j.cej.2011.05.107. [DOI] [Google Scholar]
- Elkady M.; Shokry H.; El-Sharkawy A.; El-Subruiti G.; Hamad H. New insights into the activity of green supported nanoscale zero-valent iron composites for enhanced acid blue-25 dye synergistic decolorization from aqueous medium. J. Mol. Liq. 2019, 294, 111628 10.1016/j.molliq.2019.111628. [DOI] [Google Scholar]
- Wang K.-S.; Lin C.-L.; Wei M.-C.; Liang H.-H.; Li H.-C.; Chang C.-H.; Fang Y.-T.; Chang S.-H. Effects of dissolved oxygen on dye removal by zero-valent iron. J. Hazard. Mater. 2010, 182, 886–895. 10.1016/j.jhazmat.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Li X.-q.; Elliott D. W.; Zhang W.-x. Zero-valent iron nanoparticles for abatement of environmental pollutants: materials and engineering aspects. Crit. Rev. Solid State Mater. Sci. 2006, 31, 111–122. 10.1080/10408430601057611. [DOI] [Google Scholar]
- Sun X.; Kurokawa T.; Suzuki M.; Takagi M.; Kawase Y. Removal of cationic dye methylene blue by zero-valent iron: effects of pH and dissolved oxygen on removal mechanisms. J. Environ. Sci. Health, Part A 2015, 50, 1057–1071. 10.1080/10934529.2015.1038181. [DOI] [PubMed] [Google Scholar]
- Yadav R.; Agarwala M. Phytochemical analysis of some medicinal plants. J. Phytol. 2011, 3, 10–14. [Google Scholar]
- Ghaedi M.; Heidarpour S.; Kokhdan S. N.; Sahraie R.; Daneshfar A.; Brazesh B. Comparison of silver and palladium nanoparticles loaded on activated carbon for efficient removal of methylene blue: Kinetic and isotherm study of removal process. Powder Technol. 2012, 228, 18–25. 10.1016/j.powtec.2012.04.030. [DOI] [Google Scholar]