Abstract
Developing an appropriate method to broaden the color of long persistent luminescence materials has important scientific significance and practical value but remains a great challenge. Herein, we have developed a unique strategy to fine-tune the persistent luminescence using the inclusion complex of rhodamine 6G with (2-hydroxypropyl)-β-cyclodextrin as efficient light conversion materials. The emitting color of the novel persistent luminescence material could be tuned from green to orange by changing the concentration of the light conversion agent. Furthermore, afterglow decay measurements showed that the initial afterglow brightness is 9.65 cd/m2, and the initial afterglow brightness gradually decreased as the cyclodextrin inclusion compound coating increased. This design concept introduces a new perspective for broadening the luminescence color of afterglow phosphors, which may open up new opportunities for persistent luminescence materials toward many emerging applications.
1. Introduction
Persistent luminescence, also known as long-lasting phosphorescence or afterglow, is a special optical phenomenon characterized by the ability of some materials to emit light even after the cessation of external light stimulations.1 In 1996, Matsuzawa et al. reported for the first time an ultralong green phosphor SrAl2O4:Eu2+,Dy3+(SAOED) with 30 h afterglow lifetime.2 This pioneering work has prompted the rapid development of persistent luminescence materials in the past few decades. Long persistent luminescence (LPL) materials have a wide range of applications,3−6 such as low light illumination and decorative indicators due to their unique optical properties.7 Although, blue and green luminescent materials have excellent performance,8−11 other colors developed in the laboratory are still in the research stage due to their poor performance. Based on the requirement for trichromatic materials, developing an appropriate method to broaden the luminescence color of the LPL has important scientific significance and practical value but remains a challenge.
In recent years, researchers have conducted in-depth research on the color properties of LPL.12−15 For example, Gong et al. realized the light color conversion by spinning the CsPbX3 perovskite quantum dots with strong absorption and high fluorescence quantum yield as an efficient light conversion layer on the surface of a CaAl2O4:Eu2+,Nd3+ (CAO) afterglow phosphor.16 Since the preparation method is spin coating, the application in many scenarios is restricted. Zhu et al. prepared a new luminous material SrAl2O4:Eu2+,Dy3+/light conversion by combining light conversion agent with SrAl2O4:Eu2+,Dy3+, whereas the composite material emits faint red light in the darkness after it excited.17 Chen et al. selected TEOS as silicon coating regent to form the SiO2 layer and encapsulated the SAOED phosphor particles and a coumarin-type fluorescent pigment via the sol–gel process.18 The blue-green light of SAOED is converted into red light due to the energy transfer from SAOED to light conversion agent, but the afterglow duration and luminous brightness are seriously reduced due to the influence of the coating layer.
To enhance the luminous intensity, supramolecular assemblies based on macro-cycles, including ionic crown ethers,19,20 ionic macrocyclic arenes,21,22 cyclodextrins,23−25 cucurbiturils,26,27 etc., have been a research hotspot topic in recent years. Because fluorescent molecules are sensitive to their steric environments, the preparation of highly fluorescent photofunctional materials by accommodating fluorophore guests in coordinating hosts and macrocyclic receptors, so-called spatial confinement, has emerged as a highly promising strategy.28−32 Cyclodextrins (CDs) are naturally occurring water-soluble toroidally shaped polysaccharides with a hydrophobic central cavity and hydrophilic exterior.33,34 The highly hydrophobic central cavity of CDs enables it to form inclusion complexes with a variety of substrates.35 (2-Hydroxypropyl)-β-cyclodextrin (HP-β-CD) is derived from β-CD. Compared to β-CD, HP-β-CD has much higher water solubility and low toxicity.36,37 In this work, the HP-β-CD as a carrier was employed to form an inclusion compound with rhodamine 6G (Rh6G). Thus, the fluorophores were spatially and electronically confined to avoid photoelectric energy coupling or quenching, thereby achieving enhancement of fluorescence intensity. Then, the SAOED long-lasting phosphor, as the phosphorescent light source, was prepared. The inclusion complex of Rh6G with HP-β-CD with high fluorescence intensity and a large molar absorption coefficient was used as the light conversion agent. Finally, the cyclodextrin inclusion compounds were coated on the surface of SAOED at a certain mass ratio through a silane coupling agent. Meanwhile, there are a large number of hydroxyl groups on the outside of the cyclodextrin,33 which bond to aluminum, strontium, oxygen, and other atoms on the surface of the phosphorescent light source through hydrogen bonding and electrostatic interaction. Consequently, it shortens the distance between the light conversion agent and the surface of the light source and reduces the energy loss in the photon transfer process.38 At the same time the surface coating effect of the SAOED is improved. The light conversion layer absorbed continuously emitted photons from SAOED and then emitted light through the down-conversion mechanism,16,39 as shown in Figure 1.
Figure 1.
Illustration of the synthesis process of the SrAl2O4:Eu2+,Dy3+/light conversion composite material.
2. Results and Discussion
Fourier-transform infrared (FT-IR) spectra of HP-β-CD, Rh6G, and the cyclodextrin inclusion compounds are displayed in Figure 2. The peaks of the hydroxyl stretching resonances of HP-β-CD shifted from 3328 to 3297 cm–1 and the intensity of the bands also reduced due to the strong hydrogen bond interaction between hydroxyl groups of HP-β-CD and the carbonyl and the ester group of Rh6G molecules. The specific peaks at 2975 and 2868 cm–1, which correspond to the methyl stretching resonances of Rh6G, shifted to 2968 and 2874 cm–1 because the methyl groups were present (included) in the nanocavity of HP-β-CD and the dipolar interaction of the methyl groups proton with the cavity proton. The hydrogen bond interaction between the NH bond of Rh6G and the glycosidic bonds inside the HP-β-CD cavity may restrict the rotation of the NH bond, so the NH stretching resonances at 3631 cm–1 disappeared. The Ar–CO–O–(C=O) and the ester group (COC) stretching resonance peak intensity was significantly weak, indicating the formation of strong hydrogen bond interaction between HP-β-CD and Rh6G, after the HP-β-CD formed the inclusion compounds with Rh6G.
Figure 2.

FT-IR spectra of HP-β-CD, Rh6G, and the inclusion complex of Rh6G with HP-β-CD.
The UV–vis absorption spectra of HP-β-CD, Rh6G, and the cyclodextrin inclusion compounds were recorded, as shown in Figure 3. It can be observed in Figure 3c that the HP-β-CD shows no absorption in the range of 300–1200 nm and exhibits slight absorption in the range of 220–300nm.40 Rh6G had seven absorption peaks in the scanned range. When the inclusion complex was formed, there were several changes in the UV–vis absorption spectra of the cyclodextrin inclusion compounds compared with that of Rh6G. The absorption peak of Rh6G at 236 nm shifted to 248 nm. The absorption peaks of Rh6G at 280 and 351 nm were slightly affected by the formation of the complex. The absorption peak of Rh6G at 527, 489, and 425 nm increased greatly. The absorption peak of Rh6G at 594 nm increased and blue-shifted to 568 nm. These changes are mainly caused by the host–guest interaction of the HP-β-CD/Rh6G complex, because HP-β-CD showed no absorption in the scanned range. Therefore, the absorption peaks were weakened or shifted, confirming the formation of the cyclodextrin inclusion compounds.41,42
Figure 3.

UV–vis absorption spectra of HP-β-CD (a), Rh6G (b), and the inclusion complex of Rh6G with HP-β-CD (c).
1H NMR spectra provide one of the most direct evidence for the formation of the inclusion complex.431H NMR spectra of HP-β-CD, Rh6G, and the inclusion complex of Rh6G with HP-β-CD are shown in Figure S1. 1H NMR spectra of the inclusion complex showed the proton peaks of both HP-β-CD and Rh6G. Since the interactions between the host and guest molecules are through noncovalent bonding such as van der Waals forces and hydrogen bonds instead of chemical bonds, the chemical shifts (Δδ) in the complex were small.44 Several 1H chemical shifts of Rh6G were changed and Δδ of the protons H-3 was 0.08 ppm (Figure S1). Furthermore, Table 1 lists the chemical shifts (δ) of HP-β-CD before and after forming a complex with Rh6G and the variation of 1H chemical shifts (Δδ). The 1H chemical shifts of HP-β-CD were consistent with the former report.40 Almost all of the 1H chemical shifts of HP-β-CD were changed. The variation of 1H chemical shift confirmed that the inclusion complex was formed.
Table 1. Variation of 1H Chemical Shift (δ/ppm) of HP-β-CD before and after Forming Complex with Rh6G (DMSO, 300 K).
| HP-β-CD | H-1 | H-2 | H-3 | H-4 | H-5 | H-6 | OCH2 | CH3 | OH |
|---|---|---|---|---|---|---|---|---|---|
| δfree | 4.833 | 3.441 | 3.751 | 3.412 | 3.478 | 3.615 | 3.327 | 1.025 | 5.880 |
| δcomplex | 4.834 | 3.438 | 3.748 | 3.394 | 3.467 | 3.614 | 3.314 | 1.024 | 5.885 |
| Δδ | 0.001 | –0.003 | 0.003 | –0.018 | –0.011 | –0.001 | –0.013 | –0.001 | 0.005 |
Thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) curves of HP-β-CD, Rh6G, and the inclusion complex of Rh6G with HP-β-CD are illustrated in Figure 4a–c, respectively. The HP-β-CD exhibited a steep one-step mass loss between 303 and 403 °C (Figure 4a). The decomposition started at 303 °C and ended at 403 °C, which is consistent with the former paper.41 Meanwhile, DSC shows the fusion and degradation of HP-β-CD at 354 occurs in one step. The thermal decomposition process for Rh6G can be divided into two stages, as shown in Figure 4b. The first stage begins at 233 °C and terminates at 265 °C with a mass loss 10.6%. Meanwhile, the second step starts from 320 to 510°C with a mass loss 52.6%. DSC shows four endothermic peaks at 240, 305, 465, and 506 °C. DSC shows the melting of Rh6G at 241, 465, and 506 °C with simultaneous decomposition. In Figure 4c, the TG curve of the inclusion complex of Rh6G with HP-β-CD was also one step. The inclusion complex began to decompose at about 245 °C and this ended at about 603 °C, which is different from HP-β-CD. DSC analysis shows two endothermic peaks at 276 and 294 °C. The decomposition peak of Rh6G was at 276 °C and the decomposition peak of HP-β-CD was at 294 °C. Due to the Van der Waals forces and hydrogen bonds between HP-β-CD and Rh6G molecules, the initial decomposition temperature of Rh6G increased by about 35 °C, and the initial decomposition temperature of HP-β-CD decreased by 60 °C, partly proving the formation of the inclusion complex.
Figure 4.
TGA and DSC curves of HP-β-CD (a), Rh6G (b), and the inclusion complex of Rh6G with HP-β-CD (c).
The morphologies of HP-β-CD, Rh6G, and the inclusion complex of Rh6G with HP-β-CD powder are shown in Figure 5a–c, respectively. The scanning electron microscopy (SEM) micrographs of the inclusion complex of Rh6G with HP-β-CD showed a smooth morphology (Figure 5c), while the HP-β-CD (Figure 3a) and Rh6G (Figure 5b) had a rough surface. The SEM micrographs of the SAOED phosphor particles present irregular shapes with an average diameter of 15 μm, and show sharp edges and clean surfaces (Figure 5d) compared with the surface of SAOED/light conversion composite material (Figure 5e). The surface of SAOED/light conversion composite material presents a completely different and relatively coarse morphology (Figure 5e), which indicates that the cyclodextrin inclusion compound particles adhered to SAOED. The surface details of the SAOED particles showed much small debris formed after grinding, while the surface details of the SAOED/light conversion composite material present small agglomerates mainly composed of the cyclodextrin inclusion compounds (Figure 5f).
Figure 5.
(a) SEM micrograph of HP-β-CD. (b) SEM micrograph of Rh6G. (c) SEM micrograph of the inclusion complex of Rh6G with HP-β-CD. (d) SEM micrograph of SAOED. (e) SEM micrograph of the SAOED/light conversion composite material. (f) SEM micrograph of the surface of the SAOED/light conversion composite material.
Figure S2 shows the energy-dispersive spectrometry (EDS) analysis and X-ray dot mapping of the SAOED/light conversion composite material. The results of EDS analysis confirm the presence of strontium (Sr), aluminum (Al), oxygen (O), carbon (C), europium (Eu), dysprosium (Dy), chlorine (Cl), and nitrogen (N) elements in the samples. X-ray dot mapping analysis indicates that C, Cl, and N elements are uniformly distributed in the surface of the SrAl2O4:Eu2+,Dy3+ host, which indicates that the cyclodextrin inclusion compound particles homogeneously adhered to SrAl2O4:Eu2+,Dy3+.
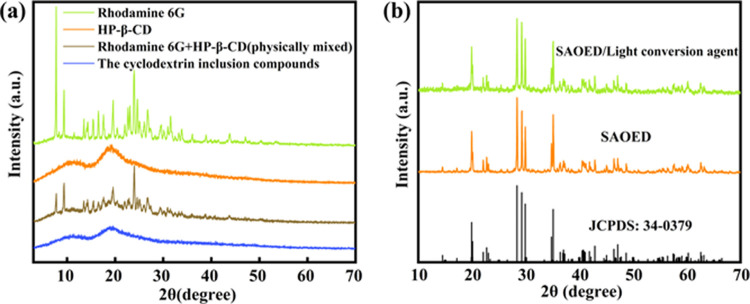
Crystallinity characterization gives out more information about the formation of the inclusion complex. Figure 5a shows the X-ray diffraction (XRD) patterns of guest (Rh6G), host (HP-β-CD), physical mixture, and the inclusion of Rh6G with HP-β-CD. Actually, pure Rh6G is a crystalline material having several sharp high-intensity peaks at different diffraction angles (2θ) of 7.7, 9.4, 19.6, 23.1, 23.9, 24.6, 26.7, 31.5, 33.9, 36.1, and 38.9. The XRD data of the HP-β-CD displayed a broad peak, which confirmed its amorphous structures, consistent with the result in ref (45). The physical mixture (Figure 5a) reflects an overlapping pattern of both Rh6G and HP-β-CD. In the case of the inclusion complex, the pattern is significantly different from those of individual components and the physical mixture. The regular pattern of the solid inclusion complex disappears, which strongly suggests that Rh6G molecules were encapsulated in the HP-β-CD cavity and ulteriorly proves that intermolecular interaction exists between HP-β-CD and Rh6G,46,47 which is consistent with IR spectra.
The XRD patterns of the SAOED and SAOED/light conversion composite material are shown in Figure 6b. Through analysis of data using MDI Jade5.0 software, the characteristic diffraction peaks of the prepared SAOED were identical to those of the monoclinic SrAl2O4 phase (JPCDS No. 34-0379), which means that we successfully prepared the SAOED phosphors. The characteristic XRD peaks of the cyclodextrin inclusion compounds in the XRD patterns of coated samples are not found because the coating light conversion agent is amorphous (Figure 6b). This indicates that the coating of the cyclodextrin inclusion compounds does not affect the phase of SAOED, which ensured that light source luminescence properties and light conversion process is achieved by the cyclodextrin inclusion compounds.
Figure 6.
(a) XRD patterns of Rh6G, HP-β-CD, the physical mixture of Rh6G with HP-β-CD, and the inclusion of Rh6G with HP-β-CD. (b) XRD patterns of the SAOED and SAOED/light conversion composite material.
As shown in Figure 7a, after the formation of the Rh6G/HP-β-CD inclusion complex, the excitation spectrum of the light converter was significantly enhanced, indicating that the inclusion complex was more efficiently excited. Meanwhile, the emission spectrum of the cyclodextrin inclusion compound (Figure 7b) displayed a broad visible band. More importantly, the maximum fluorescence emission intensity of the Rh6G/HP-β-CD inclusion complex is about 10 times stronger than that of Rh6G, which briefly illustrates the formation of the inclusion complex. The fluorophore is mainly deactivated through nonradiative routes at room temperature due to the active intramolecular vibrations and rotations.48,49 As the size of the cavity of the cyclodextrin molecule50 can only accommodate one Rh6G molecule, the nanoconfined space in the cavity of HP-β-CD can effectively prevent the nonradiative relaxation by locking the Rh6G fluorophore and inhibiting their intramolecular motions, resulting in enhanced fluorescence. In addition, the inclusion of Rh6G with HP-β-CD produces both spatial and electronic isolation of the fluorophores. So, it avoids the photoelectric energy coupling and the quenching of the emitted fluorescence to realize the enhancement of the fluorescence intensity of the light conversion agent. An increase in the fluorescence intensity on the formation of an inclusion complex was observed earlier.51−53
Figure 7.
(a) Excitation spectra of the cyclodextrin inclusion compounds and Rh6G ((λEM = 653 nm)). (b) Emission spectra of the cyclodextrin inclusion compounds and Rh6G (λEX = 525 nm). (c) Excitation (λEM = 525 nm) and emission (λEX = 365 nm) spectra of the SAOED phosphor. (d) Emission spectrum (λEX = 365 nm) of the SAOED/light conversion composite material with various contents of the cyclodextrin inclusion compounds (1# = 3 wt %, 2# = 5 wt %, 3# = 7 wt %, 4# = 8 wt %, 5# = 9 wt %, 6# = 10 wt %, 7# = 11 wt %). Multi-peak fitting curve of the prepared 2# (e) and 6# (f) samples.
The spectrum of SAOED/light conversion composite material with various contents of the cyclodextrin inclusion compounds (Figure 7d) exhibits two broad emission bands, compared with that of SAOED (Figure 7c). The peak between 450 to 530 nm (peak I) is assigned to the phosphorescence emission of SAOED and the peak between 530 and 650 nm (peak II) is assigned to the fluorescence emission of the cyclodextrin inclusion compounds. The emission peak at 525 nm is assigned to 4f65d1 to 4f7 transition of Eu2+ ions.39,54 The role of Dy3+ is to form a hole trap level and prolong the afterglow.55,56 With the cyclodextrin inclusion compounds content increasing, the emission peak at around 580 nm shifts to the right gradually, which is because the red light component increases and electronic interaction exists among the cyclodextrin inclusion compounds. It can also be seen from the figure that the intensity of the red light band is much higher than that of the green light band, so the composite phosphor successfully realizes the conversion and energy transfer from green to yellow or orange light. The emission peak of 2# (Figure 7e) and 6# samples (Figure 7f) were well fitted, in which fitting curves 2–1 and 6–1 could be divided into 2–2, 2–3, 2–4 and 6–2, 6–3, 6–4 three-component peaks, respectively. The ratio of peak area is 0.15:0.33:0.52 for fitting peak 2–2, 2–3, 2–4 and 0.08:0.39:0.53 for fitting peak 6–2, 6–3, 6–4, respectively, which is consistent with the concentration ratio of adding the cyclodextrin inclusion compounds. Although the physical meaning of the three-component peak was not clear, it is significant that this method can be used to make a semi-quantitative interpretation.
Under 365 nm excitation, the steady-state photoluminescent quantum yield (ηPLQY) of the SAOED/light conversion composite material (1#–7#) are summarized in Table S1, and the measurement details are shown in Figure S3. Because the SAOED/light conversion composite material in this work is a long afterglow material, the absorbed energy is continuously and slowly released instead of being completely released in a short time. Therefore, the photoluminescence quantum yield is about 10%. However, the absorption efficiency (αabs) of the SAOED/light conversion composite material (1#–7#) is about 55%.
Figure 8a shows the CIE chromaticity diagram of SAOED/light conversion composite material with different mass ratios. The emitting color of SAOED/light conversion composite material was a mixed fluorescence color consisting of SAOED and the cyclodextrin inclusion compound, which can be controlled by varying the concentration of the cyclodextrin inclusion compounds. Apparently, the color coordinates of the uncoated SAOED were located in the green area with the coordinate of (x = 0.2933, y = 0.5789). The color coordinates position of the novel material presented an obvious redshift from the green region to the yellow and orange regions as the cyclodextrin inclusion compound coating increased. The color coordinate position of the SAOED/light conversion composite material reached the yellow area with a coordinate of (x = 0.4472, y = 0.4924) when the cyclodextrin inclusion compounds content was added to 5%, and then arrived the orange area with the coordinate of (x = 0.4856, y = 0.4667) when the cyclodextrin inclusion compounds were added at 10%. Thus, by coating the cyclodextrin inclusion compounds, we have successfully realized persistent luminescence tuning.
Figure 8.
(a) CIE 1931 chromaticity diagram for SAOED and SAOED/light conversion composite materials with various contents of the cyclodextrin inclusion compounds (1# = 3 wt %, 2# = 5 wt %, 3# = 7 wt %, 4# = 8 wt %, 5# = 9 wt %, 6# = 10 wt %, 7# = 11 wt %). (b) Afterglow decay curves (0–3600 s) of the SAOED/light conversion composite material with various cyclodextrin inclusion compounds (1# = 3 wt %, 2# = 5 wt %, 3# = 7 wt %, 4# = 8 wt %, 5# = 9 wt %, 6# = 10 wt %, 7# = 11 wt %). (c) Optical images photographed in a darkroom by casting the SAOED/light conversion composite material with various content of the cyclodextrin inclusion compounds onto the flexible silicone logo substrates (afterglow images recorded at a delay time of 5 min after saturated excitation with a 365 nm UV lamp).
Figure 8b shows the afterglow decay curves of the SAOED/light conversion composite material, the corresponding data of which are summarized in Table S2. The afterglow decay process can be divided into two processes: rapid decay and slow decay processes.57,58 The initial stage is the fastest process of the afterglow brightness decay and then the decay rate gradually slows down. These afterglow decay plots show a similar tendency, which verifies the similar radiative energy transfer mechanism.16 As the cyclodextrin inclusion compounds coating increased, the afterglow initial brightness gradually decreases. This is because the SAOED/light conversion composite material has a core–shell structure. The shell part absorbed and reflected a fraction of light, causing the absorbed energy of the core SAOED to reduce. Consequently, the photons emitted by SAOED were significantly reduced.1618 The higher the content of light conversion agent coating, the less light energy can be absorbed by the SAOED core.59 Therefore, the initial afterglow brightness of the SAOED/light conversion composite material decreased gradually with the increase of the content of the light conversion layer. As shown in Figure 8c, by means of casting the SAOED/light conversion composite material samples onto the flexible silicone substrates, we fabricated colorful design patterns.
3. Conclusions
In summary, we developed a facile route for preparing efficient light conversion materials using (2-hydroxypropyl)-β-cyclodextrin (HP-β-CD) to form inclusion compounds with Rhodamine 6G (Rh6G). It was demonstrated that the maximum fluorescence emission intensity of Rh6G/HP-β-CD inclusion compounds is about 10 times stronger than that of Rh6G. Moreover, we have realized persistent luminescence tuning using the as-prepared cyclodextrin inclusion compounds as light conversion materials. Our strategy provides a useful method for fine-tuning the persistent luminescence of afterglow phosphors and can be extended to other energy storage and light conversion materials. This strategy may open up new opportunities for persistent luminescence materials toward many emerging applications.
4. Experimental Section
4.1. Materials and Synthesis
Aluminum oxide (Al2O3, 99.99%), strontium carbonate (SrCO3, 99.99%), europium nitrate hexahydrate (Eu(NO3)3, 99.99%), dysprosium nitrate hexahydrate (Dy(NO3)3, 99.99%), boric acid (H3BO3, AR), (2-hydroxypropyl)-β-cyclodextrin (HP-β-CD, 97%), (3-aminopropyl)triethoxysilane (KH-550, 98%) and ethanol (C2H5OH, AR) were purchased from Aladdin Chemistry Co., Ltd. (Shanghai, China). Rhodamine 6G (Rh6G, AR) was purchased from Macklin Chemistry Co., Ltd. (Shanghai, China). All of the reagents were used as received without any further purification.
4.2. Preparation of the Light Conversion Agent
Cyclodextrin inclusion compounds were prepared by the saturated aqueous solution method according to ref (60). Accurately weighed 2 g of HP-β-CD was added to 500 mL of deionized water, followed by ultrasound for 30 minutes, and then allowed to stand overnight. The HP-β-CD solution was added to rhodamine 6G solution (nHP-β-CD/nRh-6G = 1:1). The mixed solution was continuously stirred for 5 h at 40 °C. Then, the reaction mixture was dried at 105 °C for 10 h and the solid was ground to powder. Finally, the light conversion agent (cyclodextrin inclusion compounds) was obtained.
4.3. Preparation of SAOED Phosphors
The powders of SAOED phosphors were synthesized by the solid-state reactions according to ref (61). The molar ratio of SrCO3, Al2O3, Eu(NO3)3, Dy(NO3)3, and H3BO3 is 1:1:0.02:0.04:0.1. The starting materials were mixed thoroughly in a ball mill for 4 h and subsequently heated to 1350°C under a mild reducing atmosphere of activated carbon for 4 h. After cooling, the sintered products were re-milled and sieved to get the phosphors.
4.4. Preparation of SAOED/Light Conversion Agent Complexes
Accurately weighed cyclodextrin inclusion compounds and as-prepared SAOED with different weight ratios (0# = 0 wt %, 1# = 3 wt %, 2# = 5 wt %, 3# = 7 wt %, 4# = 8 wt %, 5# = 9 wt %, 6# = 10 wt %, 7# = 11 wt %) were put into a flask, and then added with ethanol. The mixture was continuously stirred for 10 min at 40 °C and then (3-aminopropyl)triethoxysilane was added. The ratio of (3-aminopropyl)triethoxysilane added was 10 wt % of the cyclodextrin inclusion compounds. The mixture was continuously stirred for 30 min at 70 °C, and it was evaporated in a vacuum. After removing the solvent, the samples were dried at 100 °C for 2 h, and the products were milled and sieved to get the desired samples.
4.5. Characterization and Measurements
The samples were tested using a Fourier-transform infrared (FT-IR) spectrometer (Thermo Fisher Nicolet iS 50) in the range of 400–4000 cm–1 with a resolution of 4 cm–1. The 1H NMR spectra were recorded on the Bruker AVANCE III 500 MHz spectrometer. Ultraviolet–visible (UV–vis) absorption spectra were recorded on a Cary 5000 spectrophotometer. Thermogravimetric (TGA) measurements and differential scanning calorimetry (DSC) were carried out on a Mettler Toledo DSC1 at a heat ramp of 10 °C min–1 under nitrogen. The surface morphologies of the SAOED phosphor particles and SAOED/light conversion composite material phosphor were inspected using a field emission scanning electron microscope (FESEM, Thermo Fisher-Apreo S LoVac). EDS analysis and X-ray dot mapping of the SAOED/light conversion composite material were performed using a Bruker energy-dispersive X-ray spectrometer (QUANTAX 200 with XFlash 6/100). X-ray diffraction (XRD) measurements were carried out on the Rigaku Miniflex 600 (Cu Kα radiation, λ = 1.5418 Å) with an angle range of 2θ = 3–70°. The photoluminescence performance (excitation and emission spectrum) spectra, steady-state photoluminescent quantum yield and CIE chromaticity coordinates were measured using the Edinburgh Instruments FLS 980 Fluorescence spectrometer (under 365 nm excitation). Afterglow decay curves were tested using a PR-305 afterglow brightness tester (SENSING Instruments Co., Ltd., China) where the samples were excited by a 150 W xenon arc lamp, and the excitation wavelength was 365 nm. The excitation time is 15 min with excitation illumination of 1000 lx to ensure that our samples fully absorb energy. The detector used was Hamamatsu CR114. The data were recorded 10 s later. All of the measurements were carried out at room temperature.
Acknowledgments
This work is supported by the Fujian Science & Technology Innovation Laboratory for Optoelectronic Information of China (2021ZR122), the project “The Key Technology of Rare Earth Long Glow Materials” and the Joint Luminescent Fibre Lab by Xinsiyuan New Materials Co. Ltd. and Xiamen Institute of Rare Earth Materials.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c03670.
1H NMR spectra of the inclusion complex of Rh6G with HP-β-CD (a), Rh6G (b), and HP-β-CD (c) (Figure S1); EDS spectrum of the SAOED/light conversion composite material and elemental distribution state of the SAOED/light conversion composite material (Figure S2); excitation lines of BaSO4 and 1#, and the emission spectrum of 1# collected using an integrating sphere. The inset shows a magnification of the emission spectrum of 1# (Figure S3); the photoluminescent quantum yield (ηPLQY) and absorption efficiency (αabs) of the SAOED/light conversion composite material (1#–7#) (Table S1); and summary of the afterglow decay curves of SAOED/light conversion composite material (1#–7#, afterglow brightness, mcd/m2) (Table S2) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Siraj N.; El-Zahab B.; Hamdan S.; Karam T. E.; Haber L. H.; Li M.; Fakayode S. O.; Das S.; Valle B.; Strongin R. M.; et al. Fluorescence, Phosphorescence, and Chemiluminescence. Anal. Chem. 2016, 88, 170–202. 10.1021/acs.analchem.5b04109. [DOI] [PubMed] [Google Scholar]
- Matsuzawa T.; Aoki Y.; Takeuchi N.; Murayama Y. A New Long Phosphorescent Phosphor with High Brightness, SrAl2O4: Eu2+, Dy3+. J. Electrochem. Soc. 1996, 143, 2670–2673. 10.1149/1.1837067. [DOI] [Google Scholar]
- Jung K. Y.; Lee H. W.; Jung H. Luminescent Properties of (Sr, Zn) Al2O4: Eu2+, B3+ Particles as a Potential Green Phosphor for UV LEDs. Chem. Mater. 2006, 18, 2249–2255. 10.1021/cm060003w. [DOI] [Google Scholar]
- Liu X.; Chen X.; Yu Y.; Xie W.; Zhao Y.; Luo S.; Mei G.; Lin J. Broad-Band Excited and Tunable Luminescence of CaTbAl3O7: RE3+(RE3+ = Ce3+ and/or Eu3+) Nanocrystalline Phosphors for Near-UV WLEDs. Inorg. Chem. 2020, 59, 12348–12361. 10.1021/acs.inorgchem.0c01440. [DOI] [PubMed] [Google Scholar]
- Lin H.; Wang B.; Xu J.; Zhang R.; Chen H.; Yu Y.; Wang Y. Phosphor-in-Glass for High-Powered Remote-Type White AC-LED. ACS Appl. Mater. Interfaces 2014, 6, 21264–21269. 10.1021/am506251z. [DOI] [PubMed] [Google Scholar]
- Dirin D. N.; Protesescu L.; Trummer D.; Kochetygov I. V.; Yakunin S.; Krumeich F.; Stadie N. P.; Kovalenko M. V. Harnessing Defect-Tolerance at the Nanoscale: Highly Luminescent Lead Halide Perovskite Nanocrystals in Mesoporous Silica Matrixes. Nano Lett. 2016, 16, 5866–5874. 10.1021/acs.nanolett.6b02688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P.; Xu X.; Zhou D.; Yu X.; Qiu J. Sunlight Activated Long-Lasting Luminescence from Ba5Si8O21: Eu2+, Dy3+ Phosphor. Inorg. Chem. 2015, 54, 1690–1697. 10.1021/ic5026312. [DOI] [PubMed] [Google Scholar]
- Finley E.; Cobb A.; Duke A.; Paterson A.; Brgoch J. Optimizing Blue Persistent Luminescence in (Sr1−δBaδ)2MgSi2O7: Eu2+, Dy3+ via Solid Solution for Use in Point-of-Care Diagnostics. ACS Appl. Mater. Interfaces 2016, 8, 26956–26963. 10.1021/acsami.6b10303. [DOI] [PubMed] [Google Scholar]
- Liao S.; Ji X.; Liu Y.; Zhang J. Highly Efficient and Thermally Stable Blue-Green (Ba0.8Eu0.2O)(Al2O3)4.575×(1+x) Phosphor through Structural Modification. ACS Appl. Mater. Interfaces 2018, 10, 39064–39073. 10.1021/acsami.8b14816. [DOI] [PubMed] [Google Scholar]
- Zu Y.; Xi J.; Li L.; Dai J.; Wang S.; Yun F.; Jiao B.; Dong H.; Hou X.; Wu Z. High-Brightness and Color-Tunable FAPbBr3 Perovskite Nanocrystals 2.0 Enable Ultrapure Green Luminescence for Achieving Recommendation 2020 Displays. ACS Appl. Mater. Interfaces 2020, 12, 2835–2841. 10.1021/acsami.9b18140. [DOI] [PubMed] [Google Scholar]
- Wang L.; Xie R.; Suehiro T.; Takeda T.; Hirosaki N. Down-Conversion Nitride Materials for Solid State Lighting: Recent Advances and Perspectives. Chem. Rev. 2018, 118, 1951–2009. 10.1021/acs.chemrev.7b00284. [DOI] [PubMed] [Google Scholar]
- Terraschke H.; Wickleder C. UV, Blue, Green, Yellow, Red, and Small: Newest Developments on Eu2+ Doped Nanophosphors. Chem. Rev. 2015, 115, 11352–11378. 10.1021/acs.chemrev.5b00223. [DOI] [PubMed] [Google Scholar]
- Qin X.; Liu X.; Huang W.; Bettinelli M.; Liu X. Lanthanide-Activated Phosphors Based on 4f-5d Optical Transitions: Theoretical and Experimental Aspects. Chem. Rev. 2017, 117, 4488–4527. 10.1021/acs.chemrev.6b00691. [DOI] [PubMed] [Google Scholar]
- Ueda J.; Miyano S.; Tanabe S. Formation of Deep Electron Traps by Yb3+ Codoping Leads to Super-Long Persistent Luminescence in Ce3+-Doped Yttrium Aluminum Gallium Garnet Phosphors. ACS Appl. Mater. Interfaces 2018, 10, 20652–20660. 10.1021/acsami.8b02758. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Zhu C. Y.; Yin S. Y.; Wei Z. W.; Zhang J. H.; Fan Y. N.; Jiang J. J.; Pan M.; Su C. Y. A Metal-Organic Supramolecular Box as a Universal Reservoir of UV, WL, and NIR Light for Long-Persistent Luminescence. Angew. Chem., Int. Ed. 2019, 58, 3481–3485. 10.1002/anie.201812708. [DOI] [PubMed] [Google Scholar]
- Gong Z.; Zheng W.; Gao Y.; Huang P.; Tu D.; Li R.; Wei J.; Zhang W.; Zhang Y.; Chen X. Full-Spectrum Persistent Luminescence Tuning Using All-Inorganic Perovskite Quantum Dots. Angew. Chem., Int. Ed. 2019, 58, 6943–6947. 10.1002/anie.201901045. [DOI] [PubMed] [Google Scholar]
- Zhu Y.; Pang Z.; Wang J.; Ge M.; Sun S.; Hu Z.; Zhai J.; Gao J.; Jiang F. Effect of light conversion agent on luminous properties of a new down-converting material SrAl2O4: Eu2+, Dy3+/light conversion agent. J. Rare Earths 2016, 34, 483–488. 10.1016/S1002-0721(16)60053-4. [DOI] [Google Scholar]
- Chen Z.; Zhu Y.; Ge M. Effect of red emitting fluorescent pigment on fluorescent color of SrAl2O4:Eu2+, Dy3+ phosphors. J. Rare Earths 2017, 35, 247–253. 10.1016/S1002-0721(17)60907-4. [DOI] [Google Scholar]
- Merzlyakova E.; Wolf S.; Lebedkin S.; Bayarjargal L.; Neumeier B. L.; Bartenbach D.; Holzer C.; Klopper W.; Winkler B.; Kappes M.; et al. 18-Crown-6 Coordinated Metal Halides with Bright Luminescence and Nonlinear Optical Effects. J. Am. Chem. Soc. 2021, 143, 798–804. 10.1021/jacs.0c09454. [DOI] [PubMed] [Google Scholar]
- Yang Q.; Cai Y. Y.; Zhu Z. Y.; Sun L. X.; Choo Y. S. L.; Zhang Q. G.; Zhu A. M.; Liu Q. L. Multiple Enhancement Effects of Crown Ether in Tröger’s Base Polymers on the Performance of Anion Exchange Membranes. ACS Appl. Mater. Interfaces 2020, 12, 24806–24816. 10.1021/acsami.0c05411. [DOI] [PubMed] [Google Scholar]
- Han X.; Han Y.; Chen C. Pagoda[4]arene andi-Pagoda[4]arene. J. Am. Chem. Soc. 2020, 142, 8262–8269. 10.1021/jacs.0c00624. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Wang H.; Liu P.; Zhu H.; Shi B.; Hong X.; Huang F. Azobenzene-Based Macrocyclic Arenes: Synthesis, Crystal Structures, and Light-Controlled Molecular Encapsulation and Release. Angew. Chem., Int. Ed. 2021, 60, 5766–5770. 10.1002/anie.202015597. [DOI] [PubMed] [Google Scholar]
- Hanayama H.; Yamada J.; Tomotsuka I.; Harano K.; Nakamura E. Rim Binding of Cyclodextrins in Size-Sensitive Guest Recognition. J. Am. Chem. Soc. 2021, 5786–5792. 10.1021/jacs.1c00651. [DOI] [PubMed] [Google Scholar]
- Falaise C.; Moussawi M. A.; Floquet S.; Abramov P. A.; Sokolov M. N.; Haouas M.; Cadot E. Probing Dynamic Library of Metal-Oxo Building Blocks with γ-Cyclodextrin. J. Am. Chem. Soc. 2018, 140, 11198–11201. 10.1021/jacs.8b07525. [DOI] [PubMed] [Google Scholar]
- Jin H.; Yang L.; Ahonen M. J. R.; Schoenfisch M. H. Nitric Oxide-Releasing Cyclodextrins. J. Am. Chem. Soc. 2018, 140, 14178–14184. 10.1021/jacs.8b07661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mako T. L.; Racicot J. M.; Levine M. Supramolecular Luminescent Sensors. Chem. Rev. 2019, 119, 322–477. 10.1021/acs.chemrev.8b00260. [DOI] [PubMed] [Google Scholar]
- Xia Y.; Chen S.; Ni X. White Light Emission from Cucurbituril-Based Host-Guest Interaction in the Solid State: New Function of the Macrocyclic Host. ACS Appl. Mater. Interfaces 2018, 10, 13048–13052. 10.1021/acsami.8b02573. [DOI] [PubMed] [Google Scholar]
- Dube H.; Ams M. R.; Rebek J. Supramolecular Control of Fluorescence through Reversible Encapsulation. J. Am. Chem. Soc. 2010, 132, 9984–9985. 10.1021/ja103912a. [DOI] [PubMed] [Google Scholar]
- Benson C. R.; Kacenauskaite L.; VanDenburgh K. L.; Zhao W.; Qiao B.; Sadhukhan T.; Pink M.; Chen J.; Borgi S.; Chen C.; et al. Plug-and-Play Optical Materials from Fluorescent Dyes and Macrocycles. Chem 2020, 6, 1978–1997. 10.1016/j.chempr.2020.06.029. [DOI] [Google Scholar]
- Frischmann P. D.; Kunz V.; Würthner F. Bright Fluorescence and Host-Guest Sensing with a Nanoscale M4L6 Tetrahedron Accessed by Self-Assembly of Zinc-Imine Chelate Vertices and Perylene Bisimide Edges. Angew. Chem., Int. Ed. 2015, 54, 7285–7289. 10.1002/anie.201501670. [DOI] [PubMed] [Google Scholar]
- Yamashina M.; Sartin M. M.; Sei Y.; Akita M.; Takeuchi S.; Tahara T.; Yoshizawa M. Preparation of Highly Fluorescent Host-Guest Complexes with Tunable Color upon Encapsulation. J. Am. Chem. Soc. 2015, 137, 9266–9269. 10.1021/jacs.5b06195. [DOI] [PubMed] [Google Scholar]
- Fan C.; Wu W.; Chruma J. J.; Zhao J.; Yang C. Enhanced Triplet-Triplet Energy Transfer and Upconversion Fluorescence through Host-Guest Complexation. J. Am. Chem. Soc. 2016, 138, 15405–15412. 10.1021/jacs.6b07946. [DOI] [PubMed] [Google Scholar]
- Saenger W. Cyclodextrin Inclusion Compounds in Research and Industry. Angew. Chem. Int. Ed. 1980, 19, 344–362. 10.1002/anie.198003441. [DOI] [Google Scholar]
- Poór M.; Zand A.; Szente L.; Lemli B.; Kunsági-Máté S. Interaction of α- and β-zearalenols with β-cyclodextrins. Molecules 2017, 22, 1910 10.3390/molecules22111910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Soufi W.; Reija B.; Novo M.; Felekyan S.; Kühnemuth R.; Seidel C. A. M. Fluorescence Correlation Spectroscopy, a Tool to Investigate Supramolecular Dynamics: Inclusion Complexes of Pyronines with Cyclodextrin. J. Am. Chem. Soc. 2005, 127, 8775–8784. 10.1021/ja0508976. [DOI] [PubMed] [Google Scholar]
- Song S.; Chong Y.; Fu H.; Ning X.; Shen H.; Zhang Z. HP-β-CD Functionalized Fe3O4/CNPs-Based Theranostic Nanoplatform for pH/NIR Responsive Drug Release and MR/NIRFL Imaging-Guided Synergetic Chemo/Photothermal Therapy of Tumor. ACS Appl. Mater. Interfaces 2018, 10, 33867–33878. 10.1021/acsami.8b09999. [DOI] [PubMed] [Google Scholar]
- Geng Q.; Xie J.; Wang X.; Cai M.; Ma H.; Ni H. Preparation and Characterization of Butachlor/(2-Hydroxypropyl)-β-cyclodextrin Inclusion Complex: Improve Soil Mobility and Herbicidal Activity and Decrease Fish Toxicity. J. Agric. Food Chem. 2018, 66, 12198–12205. 10.1021/acs.jafc.8b04812. [DOI] [PubMed] [Google Scholar]
- Prochowicz D.; Kornowicz A.; Lewiński J. Interactions of Native Cyclodextrins with Metal Ions and Inorganic Nanoparticles: Fertile Landscape for Chemistry and Materials Science. Chem. Rev. 2017, 117, 13461–13501. 10.1021/acs.chemrev.7b00231. [DOI] [PubMed] [Google Scholar]
- Clabau F.; Rocquefelte X.; Jobic S.; Deniard P.; Whangbo M. H.; Garcia A.; Le Mercier T. Mechanism of Phosphorescence Appropriate for the Long-Lasting Phosphors Eu2+-Doped SrAl2O4 with Codopants Dy3+ and B3+. Chem. Mater. 2005, 17, 3904–3912. 10.1021/cm050763r. [DOI] [Google Scholar]
- Yuan C.; Jin Z.; Xu X. Inclusion complex of astaxanthin with hydroxypropyl-β-cyclodextrin: UV, FTIR, 1H NMR and molecular modeling studies. Carbohydr. Polym. 2012, 89, 492–496. 10.1016/j.carbpol.2012.03.033. [DOI] [PubMed] [Google Scholar]
- Yuan C.; Jin Z.; Xu X.; Zhuang H.; Shen W. Preparation and stability of the inclusion complex of astaxanthin with hydroxypropyl-β-cyclodextrin. Food Chem. 2008, 109, 264–268. 10.1016/j.foodchem.2007.07.051. [DOI] [PubMed] [Google Scholar]
- Bakkialakshmi S.; Menaka T. Fluorescence enhancement of rhodamine 6G by forming inclusion complexes with β-cyclodextrin. J. Mol. Liq. 2011, 158, 117–123. 10.1016/j.molliq.2010.11.004. [DOI] [Google Scholar]
- Hamdi H.; Abderrahim R.; Meganem F. Spectroscopic studies of inclusion complex of β-cyclodextrin and benzidine diammonium dipicrate. Spectrochim. Acta, Part A 2010, 75, 32–36. 10.1016/j.saa.2009.09.018. [DOI] [PubMed] [Google Scholar]
- Ge X.; He J.; Qi F.; Yang Y.; Huang Z.; Lu R.; Huang L. Inclusion complexation of chloropropham with β-cyclodextrin: Preparation, characterization and molecular modeling. Spectrochim. Acta, Part A 2011, 81, 397–403. 10.1016/j.saa.2011.06.028. [DOI] [PubMed] [Google Scholar]
- Celebioglu A.; Kayaci-Senirmak F.; İpek S.; Durgun E.; Uyar T. Polymer-free nanofibers from vanillin/cyclodextrin inclusion complexes: high thermal stability, enhanced solubility and antioxidant property. Food Funct. 2016, 7, 3141–3153. 10.1039/C6FO00569A. [DOI] [PubMed] [Google Scholar]
- Periasamy R.; Kothainayaki S.; Rajamohan R.; Sivakumar K. Spectral investigation and characterization of host–guest inclusion complex of 4,4′-methylene-bis(2-chloroaniline) with beta-cyclodextrin. Carbohydr. Polym. 2014, 114, 558–566. 10.1016/j.carbpol.2014.08.006. [DOI] [PubMed] [Google Scholar]
- Martínez A.; Ortiz M. C.; Garcia F. J. Cyclodextrin-based multivalent glycodisplays: covalent and supramolecular conjugates to assess carbohydrate-protein interactions. Chem. Soc. Rev. 2013, 42, 4746–4773. 10.1039/c2cs35424a. [DOI] [PubMed] [Google Scholar]
- Wei Z.; Gu Z.; Arvapally R. K.; Chen Y.; McDougald R. N.; Ivy J. F.; Yakovenko A. A.; Feng D.; Omary M. A.; Zhou H. Rigidifying Fluorescent Linkers by Metal–Organic Framework Formation for Fluorescence Blue Shift and Quantum Yield Enhancement. J. Am. Chem. Soc. 2014, 136, 8269–8276. 10.1021/ja5006866. [DOI] [PubMed] [Google Scholar]
- Liu J.; Wang N.; Yu Y.; Yan Y.; Zhang H.; Li J.; Yu J. Carbon dots in zeolites: A new class of thermally activated delayed fluorescence materials with ultralong lifetimes. Sci. Adv. 2017, 3, e1603171 10.1126/sciadv.1603171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szejtli J. Introduction and General Overview of Cyclodextrin Chemistry. Chem. Rev. 1998, 98, 1743–1754. 10.1021/cr970022c. [DOI] [PubMed] [Google Scholar]
- Berberan-Santos M. N.; Choppinet P.; Fedorov A.; Jullien L.; Valeur B. Multichromophoric Cyclodextrins. 8. Dynamics of Homo- and Heterotransfer of Excitation Energy in Inclusion Complexes with Fluorescent Dyes. J. Am. Chem. Soc. 2000, 122, 11876–11886. 10.1021/ja000995l. [DOI] [Google Scholar]
- Takakusa H.; Kikuchi K.; Urano Y.; Higuchi T.; Nagano T. Intramolecular Fluorescence Resonance Energy Transfer System with Coumarin Donor Included in β-Cyclodextrin. Anal. Chem. 2001, 73, 939–942. 10.1021/ac001016a. [DOI] [PubMed] [Google Scholar]
- Martyn T. A.; Moore J. L.; Halterman R. L.; Yip W. T. Cucurbit[7]uril Induces Superior Probe Performance for Single-Molecule Detection. J. Am. Chem. Soc. 2007, 129, 10338–10339. 10.1021/ja073996n. [DOI] [PubMed] [Google Scholar]
- Zeng P.; Wei X.; Yin M.; Chen Y. Investigation of the long afterglow mechanism in SrAl2O4: Eu2+/Dy3+ by optically stimulated luminescence and thermoluminescence. J. Lumin. 2018, 199, 400–406. 10.1016/j.jlumin.2018.03.088. [DOI] [Google Scholar]
- Yu X.; Xu X.; Qiu J. Enhanced long persistence of Sr2SnO4:Sm3+ red phosphor by co-doping with Dy3+. Mater. Res. Bull. 2011, 46, 627–629. 10.1016/j.materresbull.2010.12.028. [DOI] [Google Scholar]
- Wang H.; Liang X.; Liu K.; Zhou Q.; Chen P.; Wang J.; Li J. Synthesis of SrAl2O4:Eu2+ phosphors co-doped with Dy3+, Tb3+, Si4+ and optimization of co-doping amount by response surface method. Opt. Mater. 2016, 53, 94–100. 10.1016/j.optmat.2016.01.030. [DOI] [Google Scholar]
- Kandpal S. K.; Goundie B.; Wright J.; Pollock R. A.; Mason M. D.; Meulenberg R. W. Investigation of the Emission Mechanism in Milled SrAl2O4: Eu, Dy Using Optical and Synchrotron X-ray Spectroscopy. ACS Appl. Mater. Interfaces 2011, 3, 3482–3486. 10.1021/am200710j. [DOI] [PubMed] [Google Scholar]
- Zheng L.; Zhu Y.; Pang Z.; Ge M.. Luminescence Properties of Composite Material Sr2MgSi2O7:Eu2+, Dy3+/Light Conversion Agent with Multilayer Structure. In Journal of Rare Earths; Elsevier, 2020. [Google Scholar]
- Zhu Y.; Ge M. Study on the energy transfer efficiency from SrAl2O4: Eu2+, Dy3+ to light conversion agent of red-emitting phosphor: SrAl2O4: Eu2+, Dy3+ /light conversion agent. Mater. Lett. 2016, 182, 173–176. 10.1016/j.matlet.2016.06.100. [DOI] [Google Scholar]
- Bakkialakshmi S.; Menaka T. A study of the interaction between rhodamine 6g and hydroxy propyl β-cyclodextrin by steady state fluorescence. Spectrochim. Acta, Part A 2011, 81, 8–13. 10.1016/j.saa.2011.04.082. [DOI] [PubMed] [Google Scholar]
- Han S.; Singh K. C.; Cho T.; Lee H.; Jakhar D.; Hulme J. P.; Han C.; Kim J.; Chun I.; Gwak J. Preparation and characterization of long persistence strontium aluminate phosphor. J. Lumin. 2008, 128, 301–305. 10.1016/j.jlumin.2007.07.017. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.