Abstract
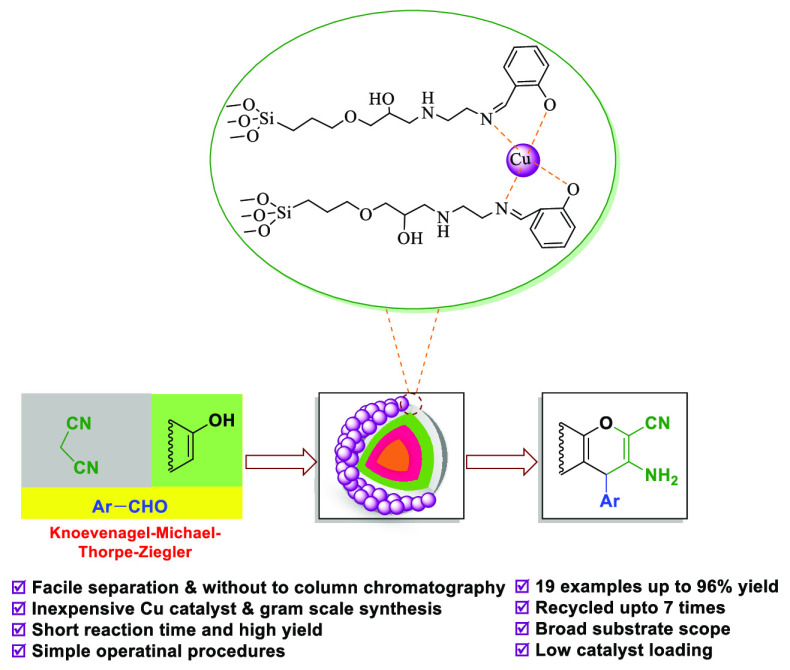
An ecofriendly inorganic–organic hybrid and novel Schiff base complex of copper coated on epoxy-modified Fe3O4@SiO2 MNPs was successfully designed and prepared from readily available chemicals. In this method, a Schiff base complex as a linker is utilized to protect copper nanoparticles to the core–shell Fe3O4 exterior without agglomeration. The resulted Schiff base complex of copper coated on epoxy-modified Fe3O4@SiO2 MNPs was characterized and confirmed via different analyses such as FT-IR, TGA, XRD, VSM, FE-SEM, TEM, ICP, EDX, and BET. The novel catalyst was examined for the synthesis of various chromene-annulated heterocycles through the one-pot three component reaction of aromatic aldehydes, various phenols (2-hydroxynaphthalene-1,4-dione/resorcinol/β-naphthol), and malononitrile in ethanol at reflux conditions. This method includes important aspects like no usage of column chromatography, very short reaction times, simplicity of product isolation using ethanol, excellent yields, simple procedures, and magnetic recoverability of the catalyst. All in all, our method makes a novel and significant advancement in the synthesis of various chromene-annulated heterocycles.
Introduction
Nowadays, the application of the fundamentals of green chemistry including application of green solvent, decreasing energy utilization and by-product, application of non-toxic substances, and usage of catalyst has attracted considerable attention. In the meantime, among the principles of green chemistry, the application of hybrid organic and inorganic materials as heterogeneous catalysts is most important.1
In recent years, core–shell magnetic nanoparticles of Fe3O4 or γ-Fe2O3 are widely studied in various areas such as enzyme and protein separations,2 environmental remediation,3 MRI contrast agent,4 magneto thermal therapy,5,6 drug delivery,7,8 bio separation,9 data storage,10 and biomolecular sensing.11 Moreover, MNPs due to having a variety of notable advantages such as low cost, high surface area, superparamagnetism properties, high stability, convenient and cost-effective synthesis, separability, and reusability have become an increasing importance in organic synthesis.12
In the last decades, the synthesis and application of Schiff base complexes along with various ligand-coated core–shell magnetic nanoparticles in various sciences such as pharmaceutical and industrial fields have gained significant attention due to Schiff base complexes having a variety of advantages such as chemical inertness, high surface-to-volume ratios, environmentally friendly nature, proper thermal stability, nontoxicity, effectuality, easy separability and reusability, and also merit to design for various uses.13−16
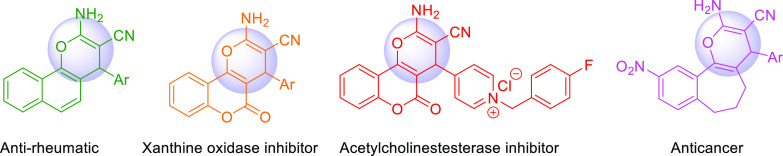
4H-Chromene and their derivatives like 4H-pyran derivatives and 4H-pyran-annulated heterocyclic moieties are one of the primary classes of natural compounds, which include six-membered-ring heterocycles with oxygen. The synthesis of 4H-chromene and their derivatives have considerably drawn scientists’ attention throughout the globe owing to their diverse biological and pharmaceutical activities like antifungal and antimicrobial,17 antitumor,18 anti-inflammatory,19 xanthine oxidase inhibiting,20 anti-HIV,21 antiallergenic,22,23 antiproliferative and anticancer,22,24 and anti-rheumatic25 (Scheme 1). Also, they have been used for the disease of Alzheimer, Huntington, and Parkinson.26
Scheme 1. Examples of Chromene-Based Bioactive Compounds.
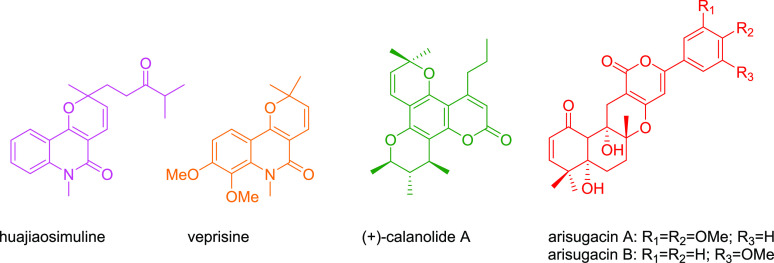
In addition, these compounds have attracted the attention of scientists especially medicinal and and organic chemists to design, synthesize, and develop a lot of alkaloid compounds, for instance, huajiaosimuline and veprisine,27,28 (+)-calanolide A,29 and arisugacin,30 which are examples of main pyran-annulated pharmacophoric scaffolds (Scheme 2).
Scheme 2. Selected Biologically Active Compounds Containing the Pyran-Annulated Motif.
2-Amino-3-cyano-4H-chromene derivatives have been synthesized through a multicomponent reaction involving malononitrile,31 aldehyde, and enolizable C–H acids like dimedone, Kojic acid, barbituric acid, α- and β-naphthol, 2-hydroxy-1,4-naphthoquinone-4-hydroxy coumarin, and resorcinol in a one-pot reaction. It seems that the reaction was carried out to proceed through a Knoevenagel–Carba–Michael–Thorpe–Ziegler-type cascade method.32 Recently, various heterogeneous or homogeneous catalysts such as ionic liquids,33 diammonium hydrogen phosphate (DAHP),34 α-Fe2O3 nanoparticles,35 H6P2W18O62·18H2O,36 cetrimonium bromide,37 Mg–La mixed metal oxides,38 silica-grafted ionic liquid,39 TiCl4,40 InCl3,41 triethylbenzylammonium chloride,42 Preyssler heteropoly acid,43 and γ-alumina.44 However, each of these approaches may have its own merits and some typical shortcomings like high cost, long reaction times, high temperature, usage of toxic solvent, low product yields, and difficulty of removal and recovery. Hence, the introduction of novel, eco-friendly, and simple heterogeneous catalysts is still valuable.
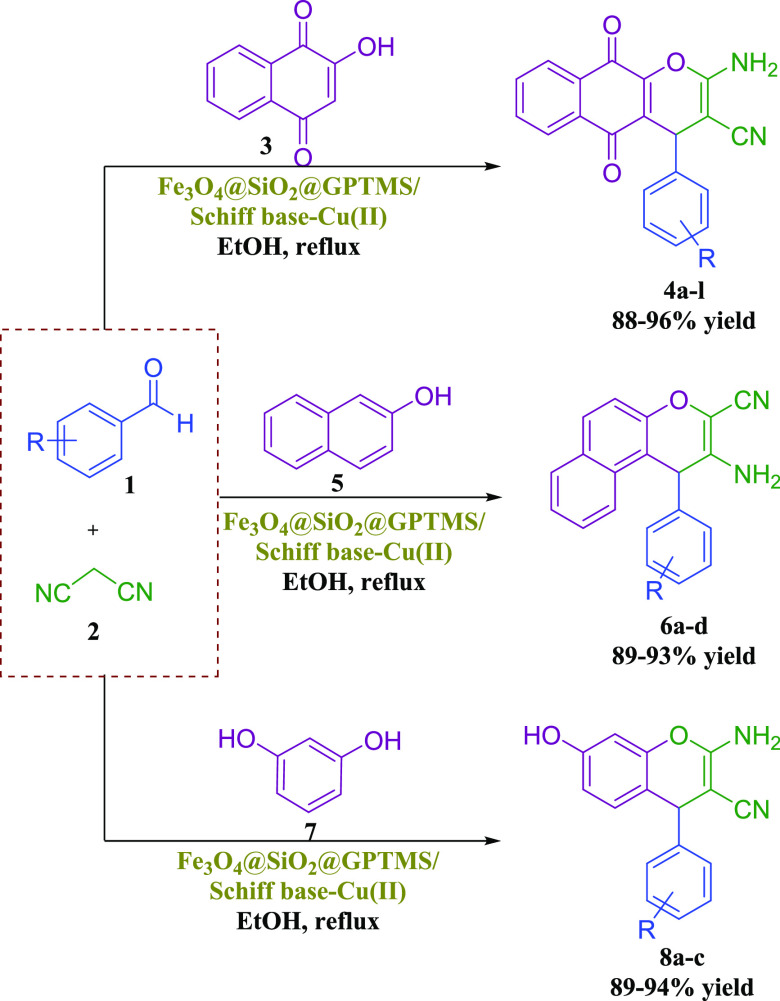
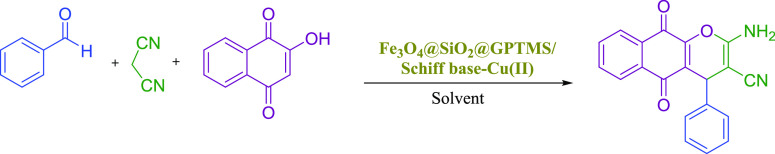
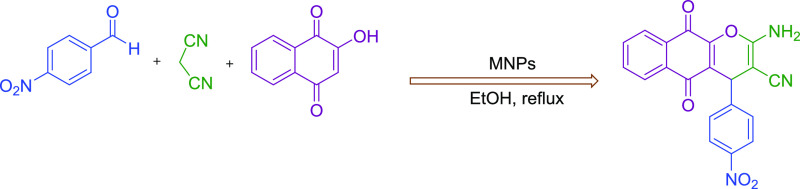
Therefore, based on the above-mentioned catalytic reaction conditions, herein, we now reported for the first time the synthesis, characterizations, and employment of novel Schiff base complexes of copper-coated core–shell MNPs (Fe3O4@SiO2@GPTMS/Schiff base-Cu(II)) as a magnetically recoverable heterogeneous nanocatalyst for the preparation of 2-amino-4H-chromene derivatives through a one-pot three component reaction of aromatic aldehydes, various phenols (2-hydroxynaphthalene-1,4-dione/resorcinol/β-naphthol), and malononitrile in ethanol at reflux conditions (Scheme 3).
Scheme 3. Synthesis of 2-Amino-4H-chromene Derivatives Catalyzed by the Schiff Base Complex of Copper Coated on Epoxy-Modified Fe3O4@SiO2 MNPs.
Results and Discussion
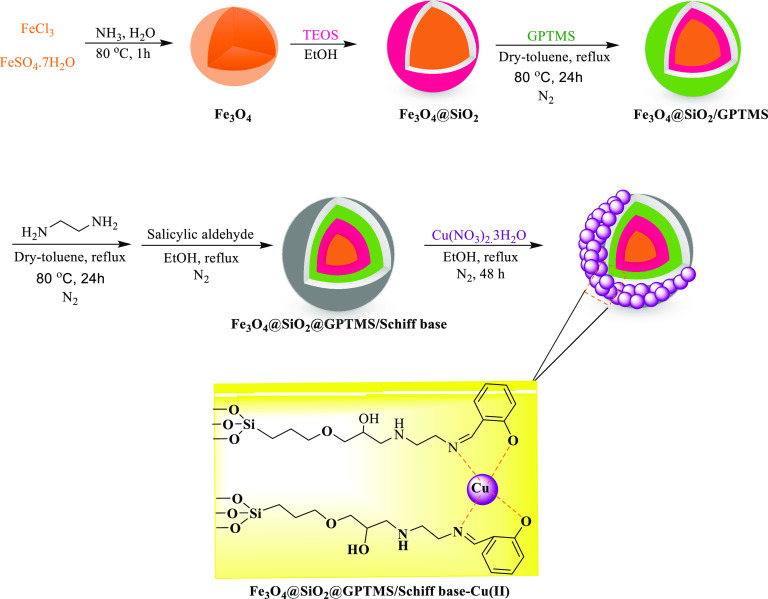
The Schiff base complex of copper coated on epoxy-modified Fe3O4@SiO2 MNPs as a simple and environmentally friendly nanocatalyst was prepared by the immobilization of Schiff-base complex on core–shell magnetic nanoparticles followed by treatment with copper salt (Scheme 4). Then, the structure of the catalyst was characterized and confirmed using FT-IR, XRD, TGA, VSM, FE-SEM, TEM, ICP, EDX, and BET analysis.
Scheme 4. Preparation of the Schiff Base Complex of Copper Coated on Epoxy-Modified Fe3O4@SiO2 MNPs.
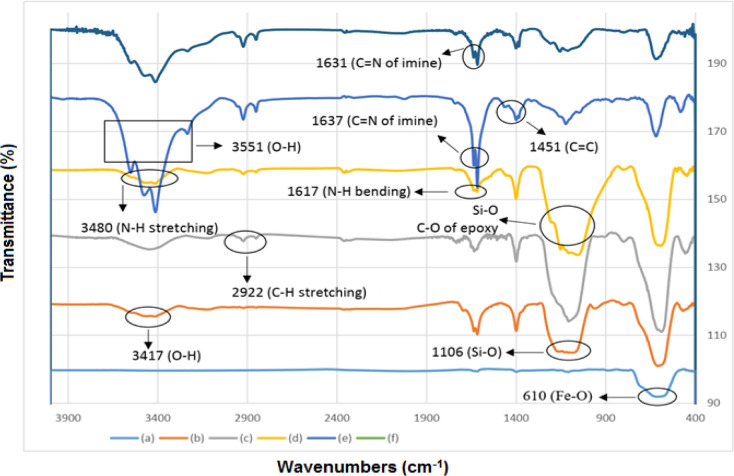
As demonstrated in Figure 1, with a comparative style of each layer with the previous one, the FT-IR spectra of the Schiff base complex of copper coated on epoxy-modified Fe3O4@SiO2 MNPs was investigated step by step. As can be seen in Figure 1a–f, a very strong peak at 610 cm–1 is related to vibrations of Fe–O bonds of Fe3O4 and functionalized types of them. The FT-IR spectra of Fe3O4@SiO2 show that the wide peak at 1106 cm–1 confirms the existence of Si–O groups within the structure of the catalyst. After coating (3-glycidyloxypropyl)trimethoxysilane (GPTMS) with Fe3O4@SiO2 (Figure 1c), a new band appeared at 2922 cm–1 that can be given to the alkyl CH2 stretching vibration. Moreover, characteristic absorption bands can be assigned to the epoxy group at 1108 cm–1 that overlapped with the strong absorption of the bare silica.45,46 As expected, after the reaction between Fe3O4@SiO2@GPTMS and diamine (Figure 1d), new absorption bands appear at 3480 and 1617 cm–1, analogous to the nitrogen–hydrogen stretching frequency and bending vibration of nitrogen–hydrogen, respectively. The reaction of Fe3O4@SiO2@GPTMS/EDA with 2-hydroxybenzaldehyde produces Fe3O4@SiO2@GPTMS/Schiff base (Figure 1e). As can be seen in Figure 1e, new absorption bands appear at 3551, 1637, and 1451 cm–1, analogous to the O–H stretching, stretching vibration of C=N, and stretching vibration of C=C, respectively. Moreover, after the reaction of Fe3O4@SiO2@GPTMS/Schiff base with Cu(NO3)2·3H2O, it produces a Schiff base complex of copper coated on epoxy-modified Fe3O4@SiO2 MNPs (Figure 1f). It can be seen in Figure 2f that the wavenumber of C=N in the Schiff base complex of copper coated on epoxy-modified Fe3O4@SiO2 MNPs shifts to lower frequency (1631 cm–1), which shows the coordination of metal–ligand bonds.47−49
Figure 1.
FT-IR spectra of (a) Fe3O4, (b) Fe3O4@SiO2, (c) Fe3O4@SiO2-GPTMS, (d) Fe3O4@SiO2-GPTMS@EDA, (e) Fe3O4@SiO2-GPTMS/Schiff base, and (f) Fe3O4@SiO2-GPTMS/Schiff base-Cu(II).
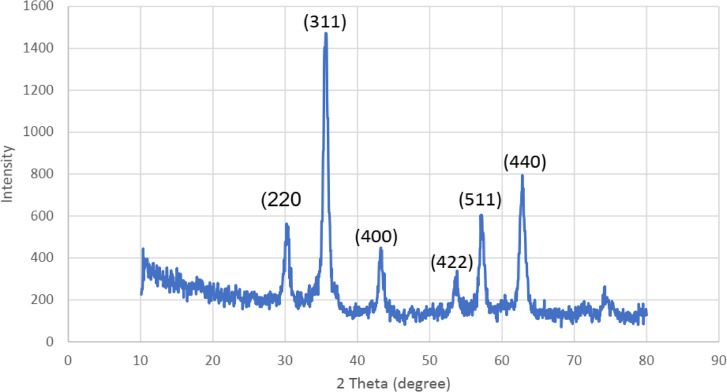
Figure 2.
XRD patterns of the Schiff base complex of copper coated on epoxy-modified Fe3O4@SiO2 MNPs.
The crystalline structure of the Schiff base complex of copper coated on epoxy-modified Fe3O4@SiO2 MNPs were measured with the XRD analysis at room temperature (Figure 2). As illustrated in Figure 2, the XRD pattern exhibited reflection peaks at 2θ = 30.17, 35.63, 43.64, 54.04, 57.32, and 63.08°, that are specified to the (220), (311), (400), (422), (511), and (440) crystallographic faces in well in line with the standard XRD pattern of cubic Fe3O4 (JCPDS 88-0866).50 These results suggested the acceptable purity of the catalyst.
Figure 3a illustrates the magnetic properties of (a) Fe3O4, (b) Fe3O4@SiO2, (c) Fe3O4@SiO2@GPTMS, (d) Fe3O4@SiO2@GPTMS/EDA, (e) Fe3O4@SiO2@GPTMS/Schiff base, and (f) Fe3O4@SiO2@GPTMS/Schiff base-Cu(II) that were studied using a vibrating sample magnetometer (VSM) at room temperature. As a comparison, we decided to compare the saturation magnetization of Fe3O4 and the Schiff base complex of copper coated on epoxy-modified Fe3O4@SiO2 MNPs with each other. The maximum saturation magnetization value of Fe3O4 was found to be about 58.5 emu/g, and the saturation magnetization value of the Schiff base complex of copper coated on epoxy-modified Fe3O4@SiO2 MNPs was found to be about 27.7 emu/g; this decrease is due to the successful coating of five layers on the surface of Fe3O4 MNP. As depicted in Figure 3a, all products had good magnetic properties, and this property is an important advantage of our catalyst for separation. Also, Figure 3b illustrates the separation of the Schiff base complex of copper coated on epoxy-modified Fe3O4@SiO2 MNPs from the reaction mixture through an external magnet.
Figure 3.
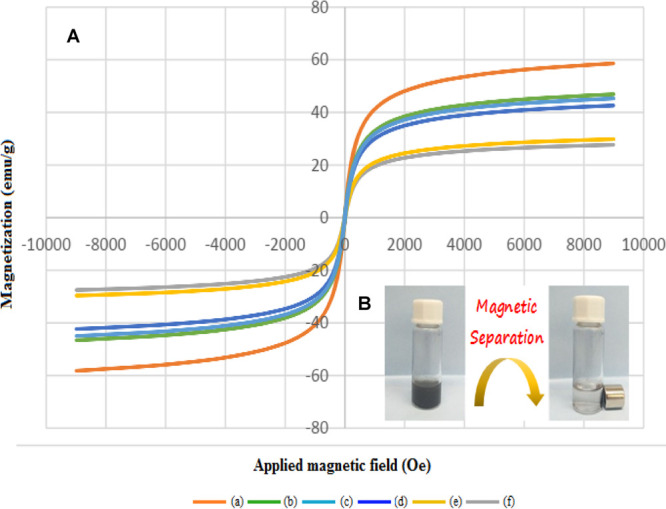
Magnetization curves for (a) Fe3O4, (b) Fe3O4@SiO2, (c) Fe3O4@SiO2@GPTMS, (d) Fe3O4@SiO2@GPTMS/EDA, (e) Fe3O4@SiO2@GPTMS/Schiff base, and (f) Fe3O4@SiO2@GPTMS/Schiff base-Cu(II).
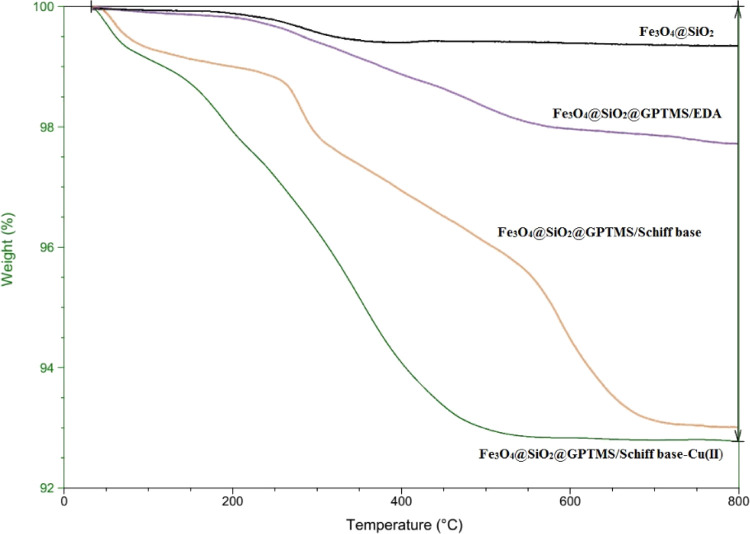
The thermo gravimetric analysis (TGA) of the Schiff base complex of copper coated on epoxy-modified Fe3O4@SiO2 MNPs, Fe3O4@SiO2@GPTMS/Schiff base, Fe3O4@SiO2@GPTMS/EDA, and Fe3O4@SiO2 were investigated in the range of 25–800 °C (Figure 4). The TGA curve of Fe3O4@SiO2@GPTMS/Schiff base-Cu(II) show that the first weight loss of 1% pertains to the removal of moisture contents at 110 °C. The second weight loss of 2% is related to the removal of the organic groups on the surface of Fe3O4 around 200 °C. Also, the third weight loss of 7% between 400 and 500 °C is related to the removal of the organic compounds. It should be mentioned that the complete decomposition of the Schiff base complex of copper coated on epoxy-modified Fe3O4@SiO2 MNPs appeared at 500 °C.
Figure 4.
TGA diagram of the Schiff base complex of copper coated on epoxy-modified Fe3O4@SiO2 MNPs, Fe3O4@SiO2@GPTMS/Schiff base, Fe3O4@SiO2@GPTMS/EDA, and Fe3O4@SiO2.
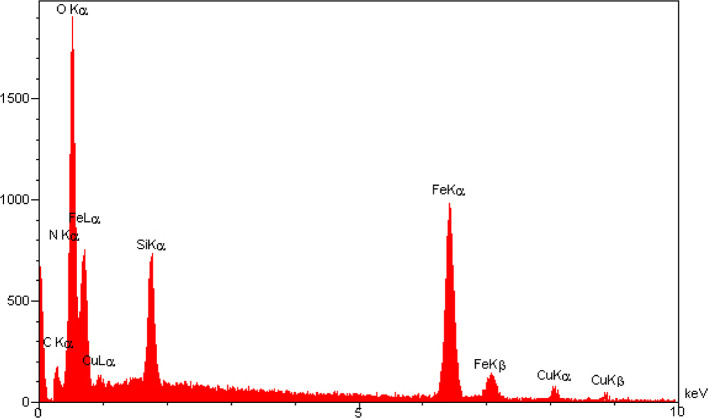
The energy dispersive X-ray (EDS) analysis of the Schiff base complex of copper coated on epoxy-modified Fe3O4@SiO2 MNPs was investigated to show the adsorption of Cu on the surface of Fe3O4 and other organic contents in the structure of the nanocatalyst (Figure 5). The EDS image show that the peaks related to with iron, oxygen, nitrogen, carbon, silicium, and copper can be observed. In addition, it was found that the coating of copper on the surface of Fe3O4@SiO2@GPTMS/Schiff base was successful.
Figure 5.
EDX image of the Schiff base complex of copper coated on epoxy-modified Fe3O4@SiO2 MNPs.
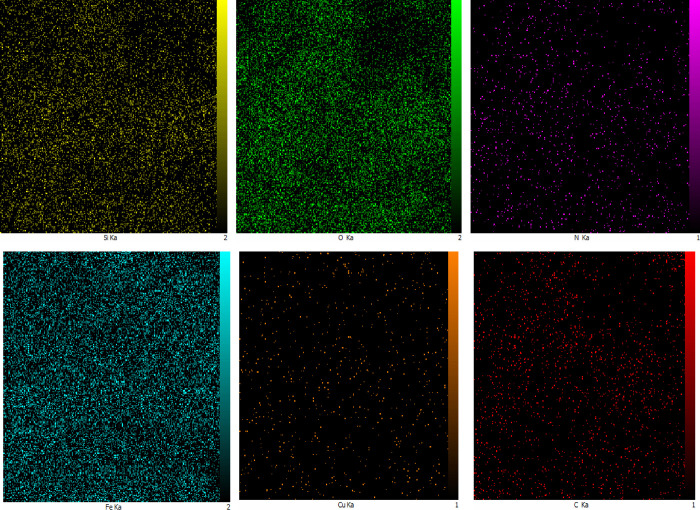
For the distribution of a variety of chemical elements in the nanocatalyst matrix, the wavelength-dispersive X-ray analysis (WDX) of the Schiff base complex of copper coated on the epoxy-modified Fe3O4@SiO2 MNP catalyst is indicated in Figure 6. As shown in Figure 6, the WDX analysis shows that Cu is well distributed on the surface of the nanocatalyst.
Figure 6.
X-ray map analysis of the Schiff base complex of copper coated on epoxy-modified Fe3O4@SiO2 MNPs.
To investigate the exact molar ratio of Cu in the Schiff base complex of copper coated on epoxy-modified Fe3O4@SiO2 MNPs, ICP-OES analysis was applied and the exact amount of Cu in the catalyst has been calculated. According to the ICP analysis, the exact amount of Cu in the Schiff base complex of copper coated on epoxy-modified Fe3O4@SiO2 MNPs was 2.79 wt %.
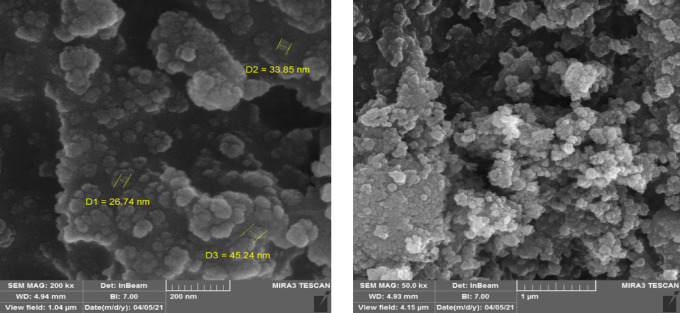
To show the surface morphology, particle size, and particle shape of the synthesized Schiff base complex of copper coated on epoxy-modified Fe3O4@SiO2 MNPs, the field-emission scanning electron microscopy image (FE-SEM) technique was conducted in various magnifications (Figure 7). As shown in the Figure 7, the prepared Schiff base complex of copper coated on epoxy-modified Fe3O4@SiO2 MNPs was formed with a spherical morphology and an average size of 26–45 nm.
Figure 7.
FE-SEM analysis of the Schiff base complex of copper coated on epoxy-modified Fe3O4@SiO2 MNPs.
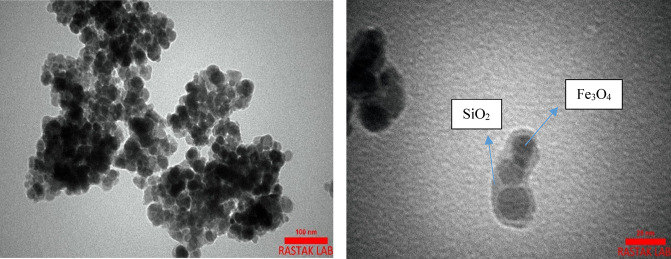
Figure 8 illustrate TEM images of the Schiff base complex of copper coated on epoxy-modified Fe3O4@SiO2 MNPs. As illustrated in Figure 8, the TEM image confirmed that the amorphous silica (bright area) coated with successful on the Fe3O4 magnetic nanoparticles (dark core). Furthermore, the average size of catalyst is approximately 26 to 45 nm, and the TEM revealed that nanoparticles with almost spherical.
Figure 8.
TEM analysis of the Schiff base complex of copper coated on epoxy-modified Fe3O4@SiO2 MNPs.
Brunauere–Emmette–Teller (BET) analysis is an efficient technique to the measure of the surface area and pore volume of the adsorbent Schiff base complex of copper coated on epoxy-modified Fe3O4@SiO2 MNPs. Figure 9a,b indicates its BET plot and nitrogen adsorption–desorption isotherms at a temperature of 77 K. As illustrated Figure 9, the BET surface area, mean pore diameter, and total pore volume of the Schiff base complex of copper coated on epoxy-modified Fe3O4@SiO2 MNPs were found to be 40.081 m2/g, 26.94 nm, and 0.3104 cm3/g, respectively. Also, according to IUPAC classification, our nanocatalyst is classified as a type IV isotherm, and the adsorbent is mesoporous.51
Figure 9.
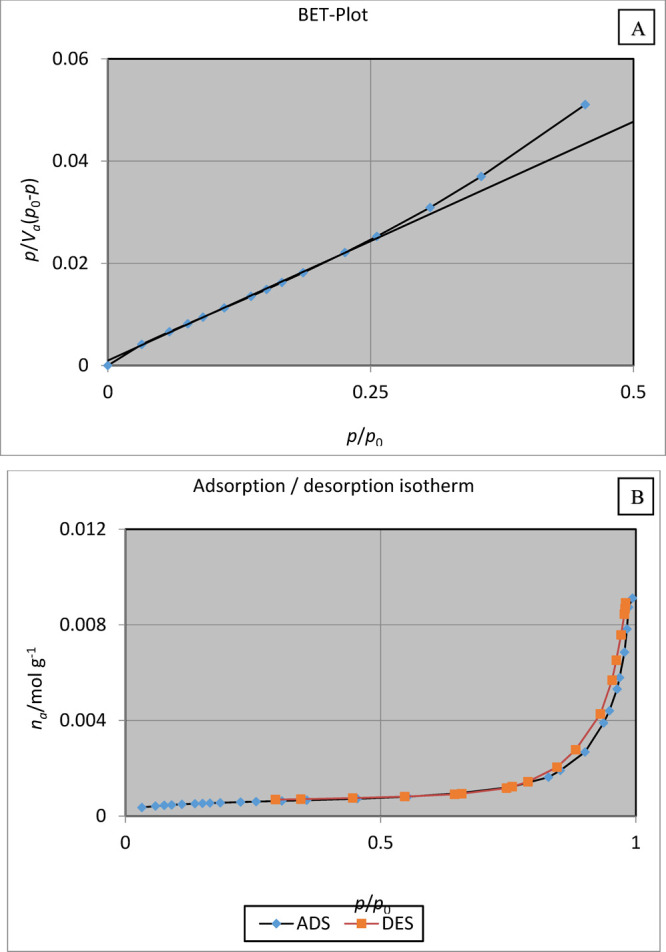
(a) BET plot of nitrogen adsorption. (b) BET plot of the nitrogen adsorption–desorption isotherms of the Schiff base complex of copper coated on epoxy-modified Fe3O4@SiO2 MNPs.
Catalytic Activity
Considering the Schiff base complex of copper coated on epoxy-modified Fe3O4@SiO2 MNPs, as a magnetically recoverable and environmentally benign catalyst, after its complete identification, we investigated its catalytic activity to synthesize a large number of 2-amino-4H-chromenes derivatives through the multicomponent reaction of benzaldehydes 1, malononitrile 2, and 2-hydroxynaphthalene-1,4-dione 3, as a model reaction and then the impacts of different experimental parameters such as temperatures, solvents, and catalyst loading were investigated. For this study, the effect of the catalyst loading, temperature, and various solvents were investigated, and the summary of the discovered optimal conditions is presented in Table 1. The effect of various solvents such as EtOH, H2O, H2O:EtOH (1:1), CH3OH, CH2Cl2, CH3CN, toluene, and DMF, the catalyst amount (0.001 to 0.15 g), and temperature were investigated. It can be concluded that 0.05 g of Fe3O4@SiO2@GPTMS/Schiff base-Cu(II) is the needed amount of catalyst to create a 96% yield of 4a in 10 min under reflux conditions (Table 1, entry 5). The reaction was done in the absence of Fe3O4@SiO2@GPTMS/Schiff base-Cu(II), and no conversion of product 4a was found after 120 min at room temperature (Table 1, entry 1). In addition, the higher amount of the catalyst up to 0.1 and 0.15 g did not present ther reaction time and better yields of 4a (Table 1, entries 6–8). Both CH3CN and toluene in the presence of 0.05 g of the Schiff base complex of copper coated on epoxy-modified Fe3O4@SiO2 MNPs led to 40 and 45% of the 4a, respectively (Table 1, entries 13 and 14). Moreover, the desired product 4a was obtained in 10% and in trace amounts in the absence of Fe3O4@SiO2@GPTMS/Schiff base-Cu(II) in EtOH under reflux conditions and room temperature, respectively (Table 1, entries 16 and 17).
Table 1. Optimization of the Catalyst Amount, Solvent, and Temperature for the Synthesis of 4aa.
| entry | catalyst (g) | solvent | temperature (°C) | time (min) | yield (%)b | conv (%)c |
|---|---|---|---|---|---|---|
| 1 | no catalyst | r.t. | 120 | trace | 0 | |
| 2 | 0.005 | EtOH | reflux | 60 | 45 | 70 |
| 3 | 0.01 | EtOH | reflux | 30 | 65 | 81 |
| 4 | 0.03 | EtOH | reflux | 30 | 77 | 95 |
| 5 | 0.05 | EtOH | reflux | 10 | 96 | 100 |
| 6 | 0.07 | EtOH | reflux | 20 | 80 | 100 |
| 7 | 0.1 | EtOH | reflux | 20 | 75 | 100 |
| 8 | 0.15 | EtOH | reflux | 30 | 71 | 99 |
| 9 | 0.05 | H2O | reflux | 30 | 55 | 100 |
| 10 | 0.05 | H2O:EtOH (1:1) | reflux | 45 | 73 | 100 |
| 11 | 0.05 | CH3OH | reflux | 60 | 70 | 97 |
| 12 | 0.05 | CH2Cl2 | reflux | 110 | 55 | 87 |
| 13 | 0.05 | CH3CN | reflux | 110 | 40 | 85 |
| 14 | 0.05 | toluene | reflux | 120 | 45 | 66 |
| 15 | 0.05 | DMF | reflux | 100 | 53 | 75 |
| 16 | no catalyst | EtOH | reflux | 120 | 10 | 15 |
| 17 | no catalyst | EtOH | r.t. | 120 | trace | 0 |
Reaction conditions: benzaldehyde (1 mmol), phenol (1 mmol), malononitrile (1 mmol), various solvents (2 mL).
Isolated yield.
Conversions were calculated from the 1H NMR spectrum of crude products.
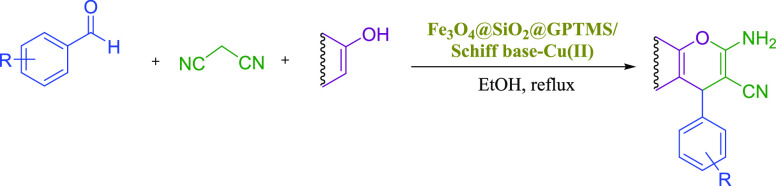
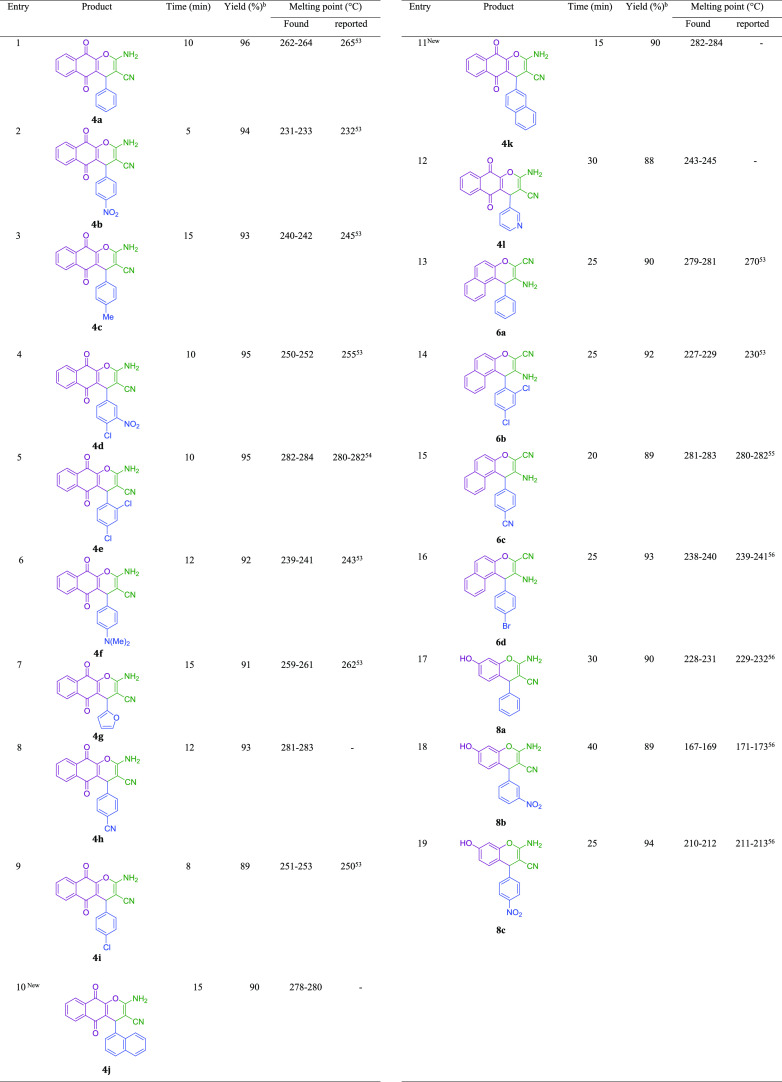
Subsequently, after getting the optimized conditions, derivatives of 4a-l were synthesized through the reaction of electron-withdrawing or electron-donating substituents on the aromatic aldehydes, 2-hydroxynaphthalene-1,4-dione, and malononitrile in the presence of a magnetic nanocatalyst (0.05 g) in ethanol under reflux conditions (Table 2). Table 2 illustrates the corresponding products 4a-l are obtained in 88–96% after 5–30 min. In another study, 0.05 g of the magnetic nanocatalyst successfully employed for the preparation of 3-amino-4-alkyl-1H-benzo[f] chromene-2-carbonitrile 6a-d. Derivatives of 6a-d were synthesized through the reaction of various aromatic aldehydes (benzaldehyde, 2,4-dichlorobenzaldehyde, 4-cyanobenzaldehyde, and 4-bromobenzaldehyde), β-naphthol, and malononitrile and are summarized in Table 2. Table 2 illustrates that the corresponding products 6a-d are obtained in 89–93% after 20–25 min. Encouraged by these results, 0.05 g of the magnetic nanocatalyst successfully employed for the preparation of another major category of chromene-annulated heterocycles, namely, 2-amino-7-hydroxy-4-alkyl-4H-chromene-3-carbonitrile 8a-c. Derivatives of 8a-c were synthesized through the reaction of various aromatic aldehydes (benzaldehyde, 3-nitrobenzaldehyde, and 4-nitrobenzaldehyde), recorcinol, and malononitrile and are summarized in Table 2. Table 2 illustrates that the corresponding products 8a-c are obtained in 89–94% after 25–40 min.
Table 2. Preparation of 2-Amino-4H-chromene Derivatives Using the Schiff Base Complex of Copper Coated on Epoxy-Modified Fe3O4@SiO2 MNPsa.
Reaction conditions: various aldehyde (1 mmol), phenol (1 mmol), malononitrile (1 mmol), phenol (1 mmol), catalyst (0.05 g), and EtOH (2 mL) reflux conditions.
Isolated yield.
Proposed Mechanism
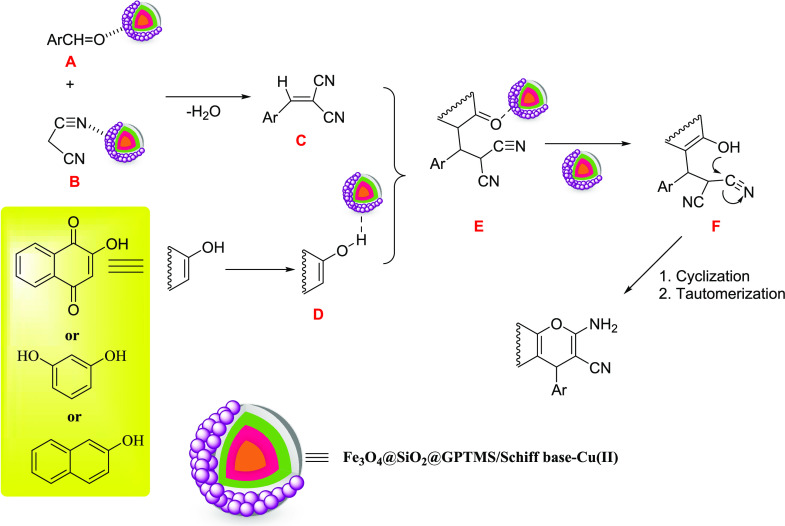
A plausible mechanism to prepare 2-amino-4H-chromene derivatives via one-pot three-component condensation of aldehydes, malononitrile, and various phenols (2-hydroxynaphthalene-1,4-dione/resorcinol/β-naphthol) in the presence of the Schiff base complex of copper coated on epoxy-modified Fe3O4@SiO2 MNPs is shown in Scheme 5. The first step involved performing Knoevenagel product C through the Knoevenagel condensation reaction between various aldehydes A and malononitrile B in the presence of the Schiff base complex of copper coated on epoxy-modified Fe3O4@SiO2 MNPs as a Lewis acid magnetic nanocatalyst. In the second step, the Michael addition of 2-hydroxynaphthalene-1,4-dionein D with Knoevenagel product C gave intermediate E. In the last step, by enolization of intermediate E, intermediate F was produced, which under intramolecular nucleophilic cyclization gives the 2-amino-4H-chromene derivatives. Then, the Schiff base complex of copper coated on epoxy-modified Fe3O4@SiO2 MNPs was removed by a magnetic field to the reaction cycle to reuse.
Scheme 5. A Suggested Mechanism to Synthenize 2-Amino-4H-chromene Derivatives Catalyzed by the Schiff Base Complex of Copper Coated on Epoxy-Modified Fe3O4@SiO2 MNPs.
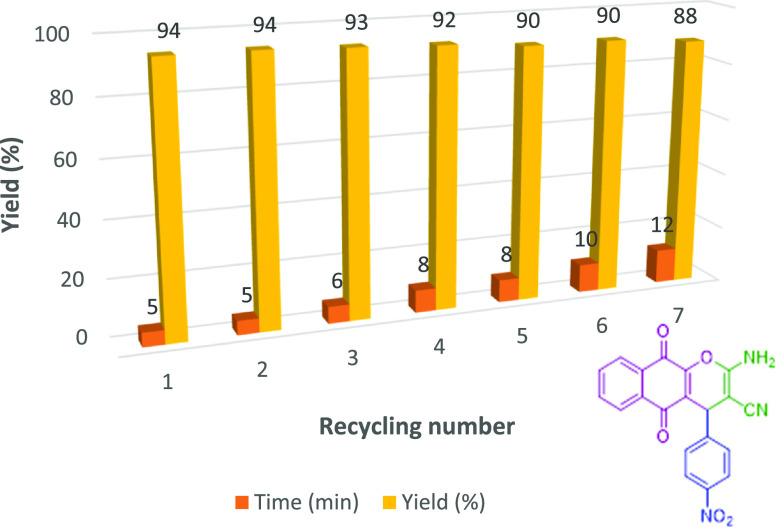
Reusability of the Schiff Base Complex of Copper Coated on Epoxy-Modified Fe3O4@SiO2 MNPs
One of the important factors for the design of eco-friendly catalytic systems in both industrial and synthetic pathways is recyclability and reusability. In our method, the recyclability of the Schiff base complex of copper coated on epoxy-modified Fe3O4@SiO2 MNPs was evaluated with the reaction of 4-nitrobenzaldehydes, 2-hydroxynaphthalene-1,4-dione, and malononitrile as the model reaction (Figure 10). After the reaction model was completed, the reaction mixture was cooled to room temperature and then the nanocatalyst can be isolated through an exterior magnet followed by washing with distilled water and EtOH, drying in air, and reusing directly for the subsequent reaction cycle.
Figure 10.
Recyclability of the catalyst.
Gram Scale Reaction
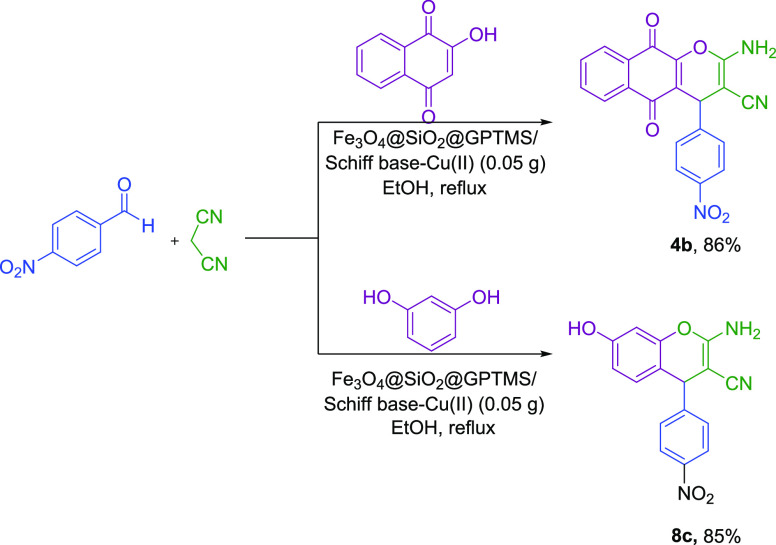
According to the excellent results obtained, the application of the current method has also been investigated in the gram scale (Scheme 6). For this purpose, the reaction of 4-nitrobenzaldehydes (10 mmol, 1.51 g), 2-hydroxynaphthalene-1,4-dione (10 mmol, 1.74 g), and malononitrile (10 mmol, 0.66 g) and also the reaction of 4-nitrobenzaldehydes (10 mmol, 1.51 g), recorcinol (10 mmol, 1.10 g), and malononitrile (10 mmol, 0.66 g) under optimized conditions have been investigated as model reactions. Scheme 6 illustrates that high yields of 86 and 85% were obtained for 4b and 8c, respectively.
Scheme 6. Comparison of Compounds 4b and 8c at Various Scales.
Hot Filtration Test
A hot filtration experiment was conducted to study the heterogeneous nature and stability of the Schiff base complex of copper coated on epoxy-modified Fe3O4@SiO2 MNPs. The hot filtration test aimed at the reaction of 4-nitrobenzaldehydes, 2-hydroxynaphthalene-1,4-dione, and malononitrile in the presence of a catalyst under optimized conditions. After half of the reaction time, 58% of the reaction was obtained. Subsequently, we repeated the above reaction under the same reaction and in half-time of the reaction, the Schiff base complex of copper coated on epoxy-modified Fe3O4@SiO2 MNPs was seperated from the reaction mixture and allowed to continue reaction without a nanocatalyst for a further time. It exhibits that only 58% of 2-amino-4-(4-nitrophenyl)-5,10-dioxo-5,10-dihydro-4H-benzo[g]chromene-3-carbonitrile was obtained. These results clearly show that leaching of Cu has not occurred.
More importantly, the catalytic activity of this Schiff base complex of copper coated on epoxy-modified Fe3O4@SiO2 MNPs was scrutinized in comparison with the catalytic activities of the Fe3O4@SiO2@GPTMS/Schiff base, Fe3O4@SiO2@GPTMS, Fe3O4, Schiff base alone, and the Schiff base complex. Hence, the reaction of 4-nitrobenzaldehydes (1 mmol), 2-hydroxynaphthalene-1,4-dione (1 mmol), and malononitrile in ethanol under reflux conditions has been investigated as the model reaction (Table 3). As shown in Table 3, the results clearly demonstrated that the Fe3O4@SiO2@GPTMS/Schiff base-Cu(II) MNPs in the model reaction gives strong results, and the corresponding product is formed in 96% yield after 10 min. On the other hand, the Fe3O4@SiO2@GPTMS/Schiff base, Fe3O4@SiO2@GPTMS, Fe3O4, Schiff base, and Schiff base complex in the model reaction gives poor results, and the corresponding product is formed in low yields. These results clearly indicated that the enhanced activity is due to the presence of the Cu coated on Fe3O4@SiO2@GPTMS/Schiff base. Also, the Schiff base alone was investigated for the model reaction. Three hours after the reaction, the desired product was not obtained at all (Table 3, entry 5). Moreover, when the Schiff base complex was replaced with Schiff base, the desired product was obtained in 53% yields after 90 min (Table 3, entry 6). Eventually, Fe3O4 (Table 3, entry 4) and Fe3O4@SiO2@GPTMS (Table 3, entry 3) gave the desired product 45 and 35%, respectively. It seems that the Fe3O4 can catalyze the reaction through the presence of OH-free groups on the surface. However, this effect is low in Fe3O4@SiO2@GPTMS than in Fe3O4 because the coating of various groups on the surface of the Fe3O4 led to the unavailability of the OH groups.
Table 3. Comparative Catalytic Activity of the Schiff Base Complex of Copper Coated on Epoxy-Modified Fe3O4@SiO2 MNPs under Optimized Conditions.
| entry | catalyst | time (min) | yield (%)a |
|---|---|---|---|
| 1 | Fe3O4@SiO2@GPTMS/Schiff base-Cu(II) | 25 | 94 |
| 2 | Fe3O4@SiO2@GPTMS/Schiff base | 360 | 33 |
| 3 | Fe3O4@SiO2@GPTMS | 240 | 35 |
| 4 | Fe3O4 | 60 | 45 |
| 5 | Schiff base alone | 180 | no reaction |
| 6 | Schiff base complex | 90 | 53 |
Isolated yield.
Conclusions
A novel Schiff base complex of copper coated on epoxy-modified Fe3O4@SiO2 MNPs was successfully synthesized from readily-available chemicals. The magnetic nanoparticles exhibited high activity in the synthesis of various chromene-annulated heterocycles via a variety of aromatic aldehydes, various phenols (2-hydroxynaphthalene-1,4-dione/resorcinol/β-naphthol), and malononitrile in EtOH at under reflux conditions. One of the factors of the high activity of this nanocatalyst may be the small size of the nanocatalyst between 26 and 45 nm, which leads to the dispersion and diffusion of the nanocatalyst in the reaction mixture. Simplicity of product isolation using ethanol, simple procedures, excellent yields, short reaction time, and no usage of column chromatography are notable advantages of these methods. More importantly, the nanocatalyst can be rapidly taken out from the reaction mixture with the help of an external magnet and after washing with ethanol, drying, and directly reusing in seven sequential runs without any loss in activity.
Experimental Section
Chemicals and Instrumentation
The materials used here including FeSO4·7H2O, FeCl3, aqueous ammonia (25%), acetone, ethanol, tetraethyl orthosilicate, GPTMS, various aromatic aldehydes, toluene (anhydrous), various phenoles, ethylenediamine, malononitrile, salicylaldehyde, and Cu(NO3)2·3H2O were prepared from the Merck or Fluka (Switzerland) Company. 1H NMR (250 MHz) and 13C NMR (62.5 MHz) spectra using dimethyl sulfoxide (DMSO-d6) were acquired on a Bruker DRX-250 AVANCE spectrometer. FT-IR analysis was fulfiled by a Perkin–Elmer 597 spectrophotometer. SEM analysis was recorded by a TE-SCAN, Brno Czech Republic. TEM analysis was recorded on a Zeiss EM10C operating at 80 kV TEM. The VSM analysis was utilized to specify the magnetic trait of the Schiff base complex of copper coated on epoxy-modified Fe3O4@SiO2 MNPs (VSM, Taban, Tehran, Iran). Also, XRD analysis (X’Pert-PRO advanced difractometer operated at 40 kV and 40 mA at r.t.) helped to investigate the crystalline structure of the catalyst. The EDAX spectrum was utilized for the elemental analysis of the Schiff base complex of copper coated on epoxy-modified Fe3O4@SiO2 MNPs.52−55
Catalyst Preparation
The Schiff base complex of copper was successfully coated on epoxy-modified Fe3O4@SiO2 MNPs according to the following steps.
Preparation of Fe3O4
To prepare Fe3O4, we used the co-precipitation method. First, FeSO4.7H2O (0.9 g) and FeCl3 (0.97 g) with 120 mL of distilled water were mixed under vigorous stirring at 80 °C, then 120 mL aqueous ammonia solution 1.5 M with a dropping funnel was added dropwise to it, and it immediately turned black. Second, the resulting black mixture was stirred under N2 gas for 30 min. Next, the reaction mixture was kept for 2.5 h at 25 °C, and the black Fe3O4 was isolated using an exterior super magnet and washed four times with distilled water (4 × 50 mL) and once with acetone (1 × 50 mL) and dried in an oven at 40 °C for 24 h.56
Preparation of Fe3O4@SiO2
Fe3O4 (1 g) in a mixture of ethanol:distilled water (80:20; 100 mL) was dispersed by sonification for 30 min. In sequence, 2 mL of tetraethyl orthosilicate (TEOS) and 2 mL of NH4OH 25% was added and the resulting solution was kept under N2 for 12 h. Finally, the resulting products was collected by an external magnetic field and washed five times with distilled water (5 × 50 mL) and once with ethanol (1 × 50 mL). Eventually, the Fe3O4@SiO2 was synthesized and dried in an oven at 40 °C for 24 h.57
Preparation of Fe3O4@SiO2@GPTMS
Tural and colleagues have reported the Fe3O4@SiO2@GPTMS from GPTMS functionalized with Fe3O4@SiO2 with a facile strategy.45 For this, 1 g of Fe3O4@SiO2 was dispersed in 100 mL of anhydrous toluene for 20 min. In sequence, GPTMS (10 mmol) was added gradually to the Fe3O4@SiO2 solution and was refluxed under nitrogen at 80 °C for 8 h. Finally, the Fe3O4@SiO2@GPTMS was filtered by an external magnet field and washed twice with benzene (2 × 50 mL) and then dried overnight at room temperature.
Preparation of Fe3O4@SiO2@GPTMS/EDA
The Fe3O4@SiO2@GPTMS/EDA compound was prepared easily. A total of 1 g of Fe3O4@SiO2@GPTMS was dispersed in 100 mL of anhydrous toluene for 20 min. Then, ethylenediamine (12 mmol) was added to the Fe3O4@SiO2@GPTMS solution under N2 at reflux conditions for 24 h. Then, the Fe3O4@SiO2@GPTMS/EDA was filtered by employing an external magnet, washed with benzene (2 × 50 mL), and then dried overnight at room temperature.
Preparation of Fe3O4@SiO2@GPTMS/Sciff Base
First, Fe3O4@SiO2@GPTMS/EDA was dispersed in 50 mL anhydrous toluene for 15 min. Then, the solution of salicylaldehyde (10 mmol) in anhydrous toluene (10 mL) was added dropwise into solution of Fe3O4@SiO2@GPTMS/EDA (1 g) under N2 at reflux conditions for 24 h. Then, the precipitate (Fe3O4@SiO2@GPTMS/Sciff base) was collected from the solution employing an external magnet, washed with benzene and ethanol several times, and dried overnight at room temperature.
Preparation of the Schiff Base Complex of Copper Coated on Epoxy-Modified Fe3O4@SiO2 MNPs
To synthesize the Schiff base complex of copper coated on epoxy-modified Fe3O4@SiO2 MNPs, first, Fe3O4@SiO2@GPTMS/Sciff base (1 g) was dispersed in absolute ethanol (30 mL). Afterward, the solution of Cu(NO3)2·3H2O (2.5 mmol) in absolute ethanol (20 mL) was added quickly to the solution of Fe3O4@SiO2@GPTMS/Sciff base under vigorous stirring under reflux conditions for 24 h. Finally, the mixture was gathered employing an exterior magnet, washed several times with water and ethanol, and dried at room temperature to give the Schiff base complex of copper coated on epoxy-modified Fe3O4@SiO2 MNPs.
General Process to Synthesize 2-Amino-4H-Chromene Derivatives Using the Schiff Base Complex of Copper Coated on Epoxy-Modified Fe3O4@SiO2 MNPs
In round-bottom flask, a variety of aldehydes (1 mmol), various phenols (1 mmol), malononitrile (1 mmol), nanocatalyst (0.05 g), and ethanol (5 mL) were reacted under reflux conditions (Table 2). The reaction completion process was investigated using thin layer chromatography. After the completion of the reaction, the reaction mixture was cooled to 25 °C. Subsequently, the magnetic catalyst was recovered from the reaction mixture by an exterior super magnet, washed with ethanol, and dried in an oven for 24 h. The solid product came through simple filtration and washed with ethanol thoroughly for purification and dried at room temperature. Various chromene-annulated heterocycles were specified using FT-IR, melting point, 1H NMR, and 13C NMR techniques.
Compound 4a
Orange solid, FT-IR (KBr, cm–1): 3403, 3325, 3192, 2936, 2199, 1687, 1671, 1450, 1070, 719. 1H NMR (250 MHz, DMSO-d6) δ: 7.19–8.01 (m, 11H, Ar–H, NH2), 4.57 (s, 1H, C–H). 13C NMR (62.5 MHz, DMSO-d6) δ: 36.9, 57.9, 119.7, 122.4, 126.2, 126.5, 127.5, 128.1, 129, 131, 131.4, 134.5, 134.9, 144, 149.3, 158.8, 177.2, 182.9.
Compound 4b
Orange solid; FT-IR (KBr, cm–1): 3401, 3331, 3196, 3072, 2922, 2204, 1493, 1411, 1301, 1246, 1183, 859, 780 cm–1. 1H NMR (250 MHz, DMSO-d6) δ: 7.46–8.04 (m, 8H, Ar–H), 8.12–8.15 (s, 2H), 4.77 (s, 1H). 13C NMR (62.5 MHz, DMSO-d6) δ: 36.8, 56.7, 119.4, 121, 124.2, 126.2, 126.5, 129.5, 131.1, 131.3, 134.6, 134.9, 146.9, 149.8, 151.4, 158.8, 177.1, 182.9.
Compound 4c
Orange solid, FT-IR (KBr, cm–1): 3408, 3218, 2199, 1662, 1635, 1405, 1243, 1073, 734. 1H NMR (250 MHz, DMSO-d6) δ: 7.06–8.01 (m, 10H, Ar–H, NH2) 2.47 (s, 3H, Me), 4.54 (s, 1H, C–H). 13C NMR (62.5 MHz, DMSO-d6) δ: 21, 36.5, 58, 119.8, 122.6, 126.2, 126.5, 128, 129, 131, 131.4, 134.5, 134.9, 136.7, 141, 149.2, 158.7, 183.
Compound 4d
Red-brown solid, FT-IR (KBr, cm–1): 3409, 3329, 3191, 2193, 1674, 1637, 1529, 1475, 1366, 948. 1H NMR (250 MHz, DMSO-d6) δ: 7.46–8.05 (m, 9H, Ar–H, NH2), 4.79 (s, 1H, C–H). 13C NMR (62.5 MHz, DMSO-d6) δ: 36.2, 56.7, 119.3, 120.4, 123.6, 124.9, 126.2, 126.4, 131.1, 131.4, 131.9, 133.6, 134.6, 134.9, 145.3, 148.3, 150.0, 158.7, 177.2, 183.0.
Compound 4e
Red-brown solid, FT-IR (KBr, cm–1): 3467, 3340, 3194, 2922, 2202, 1733, 1670, 1469, 1400, 1365, 1248, 717 cm–1. 1H NMR (250 MHz, DMSO-d6) δ: 7.29–8.04 (m, 10H, Ar–H, NH2), 5.10 (s, 1H, C–H). 13C NMR (62.5 MHz, DMSO-d6) δ: 33.5, 56.1, 119.1, 121.1, 126.2, 126.5, 128.4, 129.1, 131, 131.3, 132.3, 132.7, 133.3, 134.6, 135, 140.6, 150, 158.8, 177.2, 182.9.
Compound 4f
Dark-orange solid, FT-IR (KBr, cm–1): 3398, 3318, 3006, 2883, 2199, 1686, 1634, 1443, 1245, 923, 772. 1H NMR (250 MHz, DMSO-d6) δ: 8.0–8.02 (m, 1H, Ar–H), 7.83–7.84 (m, 3H, Ar–H), 7.22 (s, 2H, NH2), 7.04–7.07 (d, 2H,3J = 8.5 Hz, Ar–H), 6.59–6.30 (d, 2H,3J = 8.5 Hz, Ar–H), 4.46 (s, 1H, C–H), 2.47–2.81 (s, 6H, 2 Me). 13C NMR (62.5 MHz, DMSO-d6) δ: 35.8, 58.3, 112.8, 119.9, 123.1, 126.2, 126.4, 128.7, 130.9, 131.5, 134.5, 134.9, 148.6, 149.9, 158.7, 177.4, 183.0.
Compound 4g
Orange solid, FT-IR (KBr, cm–1): 3413, 3329, 3118, 2921, 2862, 2197, 1689, 1416, 1210, 742. 1H NMR (250 MHz, DMSO-d6) δ: 7.82–8.01 (m, 5H, Ar–H), 7.40–7.50 (m, 3H, Ar–H), 6.26–6.33 (s, 2H, NH2), 4.73 (s, 1H, C–H). 13C NMR (62.5 MHz, DMSO-d6) δ: 30.3, 54.9, 106.8, 111.1, 119.6, 120.2, 126.3, 126.6, 130.9, 131.3, 134.6, 135.1, 142.8, 149.6, 154.9, 159.6, 177.2, 182.6.
Compound 4h
Brown solid, FT-IR (KBr, cm–1): 3438, 3334, 3191, 3059, 2232, 2196, 1667, 1597, 1403, 1241, 1073, 716, 615. 1H NMR (250 MHz, DMSO-d6) δ: 7.98 (m, 1H, Ar–H), 7.50–7.75 (m, 6H, Ar–H), 7.40 (m, 3H, Ar–H and NH2), 4.67 (s, 1H). 13C NMR (62.5 MHz, DMSO-d6) δ: 37.0, 56.9, 110.3, 119.1, 119.4, 121.1, 126.2, 126.4, 129.2, 131.0, 131.3, 132.9, 134.6, 134.9, 149.3, 149.8, 158.8, 177.1, 182.9.
Compound 4j
Brick red solid, FT-IR (KBr, cm–1): 3444, 3060, 2931, 2194, 1671, 1457, 1246,724. 1H NMR (250 MHz, DMSO-d6) δ: 8.50 (d, 1H, Ar–H) 7.21–8.06 (m, 13H, Ar–H, NH2), 5.58 (s, 1H, C–H). 13C NMR (62.5 MHz, DMSO-d6) δ: 31.2, 58.5, 119.7, 123.3, 124.0, 126.3, 126.7, 127.8, 128.8, 131.0, 131.3, 133.7, 134.6, 134.9, 141.6, 149.8, 158.7, 177.3, 183.0.
Compound 4k
Orange solid, FT-IR (KBr, cm–1): 3378, 2976, 2215, 1707, 1663, 1475, 1214, 762. 1H NMR (250 MHz, DMSO-d6) δ: 7.35–8.05 (m, 14H, Ar–H, NH2), 4.77 (s, 1H, C–H). 13C NMR (62.5 MHz, DMSO-d6) δ: 37.2, 57.8, 119.8, 122.1, 126.5, 127.9, 128.1, 128.7, 131.0, 131.4, 132.6, 133.3, 134.5, 134.9, 141.5, 149.4, 158.7, 177.3, 183.0.
Compound 4l
Brown solid, FT-IR (KBr, cm–1): 3413, 3349, 3062, 2197, 1688, 1673, 1457, 1247, 960, 726. 1H NMR (250 MHz, DMSO-d6) δ: 8.57 (s, 1H, Ar–H), 7.62–8.52 (m, 7H, Ar–H), 7.41 (s, 2H, NH2), 4.68 (s, 1H, C–H). 13C NMR (62.5 MHz, DMSO-d6) δ: 34.7, 57.0, 119.5, 121.1, 124.3, 126.2, 126.4, 131.1, 131.3, 134.6, 134.9, 136.2, 139.7, 148.3, 148.8, 149.1, 149.7, 158.8, 177.1, 183.0.
Compound 6a
White solid, FT-IR (KBr, cm–1): 3432, 3339, 2182, 1651, 1452, 1245, 1027, 753. 1H NMR (250 MHz, DMSO-d6) δ: 7.86 (m, 3H, Ar–H), 7.16–7.36 (m, 8H, Ar–H), 6.94 (s, 2H, NH2), 5.24 (s, 1H, C–H). 13C NMR (62.5 MHz, DMSO-d6) δ: 38.4, 58.31, 116.1, 117.2, 120.9, 124.0, 125.3, 127.0, 127.4, 128.8, 129.1, 129.9, 130.5, 131.2, 146.1, 147.2, 160.1.
Compound 6c
White solid, FT-IR (KBr, cm–1): 3445, 3304, 3166, 2872, 2226, 2184, 1652, 1586, 1468, 1405, 1238, 1083, 807, 780, 620, 558. 1H NMR (250 MHz, DMSO-d6) δ: 5.41 (s, 1H), 7.12 (s, 2H, NH2), 7.34 (m, 5H, Ar–H), 7.63–7.71 (m, 3H, Ar–H), 7.85 (m, 2H, Ar–H). 13C NMR (62.5 MHz, DMSO-d6) δ: 38.3, 57.2, 109.9, 114.8, 117.2, 119.0, 123.7, 125.4, 127.7, 128.4, 128.9, 130.3, 131.2, 133.1, 147.3, 151.3, 160.3.
Compound 8a
Brown solid, FT-IR (KBr, cm–1): 3505, 3429, 2192, 1651, 1619, 1450, 1148; 1H NMR (250 MHz, DMSO-d6) δ: 9.68 (s, 1H, OH), 7.14–7.25 (m, 5H, Ar–H), 6.75–6.83 (m, 3H, Ar–H), 6.38–6.45 (s, 2H, NH2), 3.35 (s, 1H, C–H). 13C NMR (62.5 MHz, DMSO-d6) δ: 56.6, 102.6, 112.8, 114.1, 121.1, 127.0, 127.8, 129.0, 130.4, 146.8, 149.2, 157.5, 160.6.
Compound 8b
Dark brown solid, FT-IR (KBr, cm–1): 3442, 3328, 3195, 3079, 2979, 2193, 1644, 1579, 1471, 1350, 1155, 857, 734. 1H NMR (250 MHz, DMSO-d6) δ: 9.77 (s, 1H, OH), 8.03 (t, 1H, J = 7.8 HZ, Ar–H), 6.99 (s, 2H, NH2), 4.87 (s, 1H, CH), 7.59 (d, 2H, J = 9.25, Ar–H), 6.81 (d, 2H, J = 8 Hz, Ar–H), 6.44–6.49 (m, 2H, Ar–H). 13C NMR (62.5 MHz, DMSO-d6) δ: 18.9, 55.8, 56.5, 102.8, 112.9, 113.1, 120.7, 122.2, 130.3, 130.7, 134.7, 148.3, 148.9, 149.3, 157.9, 160.9.
Acknowledgments
The present study, derived from the Ph.D. thesis by Sobhan Rezayati, was supported by the Iran National Science Foundation INSF and University of Zanjan.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c03672.
Copies of FT-IR, 1H NMR (250 MHz, DMSO-d6), and 13C NMR (62.5 MHz, DMSO-d6) spectra of synthesized compounds (PDF)
The authors declare no competing financial interest.
Published ASAP on September 14, 2021; Scheme 1, Scheme 4, and abstract graphic revised September 22, 2021.
Supplementary Material
References
- a Yadav L. D. S.; Singh S.; Rai V. K. Catalyst-free, step and pot economic, efficient mercaptoacetylative cyclisation in H2O: synthesis of 3-mercaptocoumarins. Green Chem. 2009, 11, 878–882. 10.1039/b904655k. [DOI] [Google Scholar]; b Rezayati S.; Salehi E.; Hajinasiri R.; Abad S. A. S. Acetic acid functionalized ionic liquid systems: An efficient and recyclable catalyst for the regioselective ring opening of epoxides with NaN3. C. R. Chim. 2017, 20, 554–558. 10.1016/j.crci.2016.07.004. [DOI] [Google Scholar]; c Rezayati S.; Hajinasiri R.; Erfani Z. Microwave-assisted green synthesis of 1,1-diacetates (acylals) using selectfluorTM as an environmental-friendly catalyst under solvent-free conditions. Res. Chem. Intermed. 2016, 42, 2567–2576. 10.1007/s11164-015-2168-1. [DOI] [Google Scholar]; d Hajinasiri R.; Rezayati S. Solvent-free Synthesis of 1,2-Disubstituted Derivatives of 1,2- Dihydroisoquinoline, 1,2-Dihydroquinoline and 1,2-Dihydropyridine. Z. Naturforsch., B 2013, 68, 818–822. 10.5560/znb.2013-3095. [DOI] [Google Scholar]
- Meyer A.; Hansen D. B.; Gomes C. S. G.; Hobley T. J.; Thomas O. R. T.; Franzreb M. Preparation and characterization of surface modified γ-Fe2O3 (maghemite)-silica nanocomposites used for the purification of benzaldehyde lyase. Biotechnol. Prog. 2005, 21, 244–254. 10.1021/bp049656c. [DOI] [PubMed] [Google Scholar]
- Lu A.-H.; Schmidt W.; Matoussevitch N.; Bönnemann H.; Spliethoff B.; Tesche B.; Bill E.; Kiefer W.; Schüth F. Nanoengineering of a Magnetically Separable Hydrogenation Catalyst. Angew. Chem. 2004, 116, 4403–4406. 10.1002/ange.200454222. [DOI] [PubMed] [Google Scholar]
- Weissleder R.; Bogdanov A.; Neuwelt E. A.; Papisov M. Long-circulating iron oxides for MR imaging. Adv. Drug Delivery Rev. 1995, 16, 321–334. 10.1016/0169-409X(95)00033-4. [DOI] [Google Scholar]
- Hiergeist R.; Andrä W.; Buske N.; Hergt R.; Hilger I.; Richter U.; Kaiser W. Application of magnetite ferrofluids for hyperthermia. J. Magn. Magn. Mater. 1999, 201, 420–422. 10.1016/S0304-8853(99)00145-6. [DOI] [Google Scholar]
- Jordan A.; Scholz R.; Wust P.; Fähling H.; Felix R. Magnetic fluid hyperthermia (MFH): Cancer treatment with AC magnetic field induced excitation of biocompatible superparamagnetic nanoparticles. J. Magn. Magn. Mater. 1999, 201, 413–419. 10.1016/S0304-8853(99)00088-8. [DOI] [Google Scholar]
- Pankhurst Q. A.; Connolly J.; Jones S. K.; Dobson J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D: Appl. Phys. 2003, 36, R167–R181. 10.1088/0022-3727/36/13/201. [DOI] [Google Scholar]
- del Campo A.; Sen T.; Lellouche J.-P.; Bruce I. J. Multifunctional magnetite and silica–magnetite nanoparticles: Synthesis, surface activation and applications in life sciences. J. Magn. Magn. Mater. 2005, 293, 33–40. 10.1016/j.jmmm.2005.01.040. [DOI] [Google Scholar]
- Wang D.; He J.; Rosenzweig N.; Rosenzweig Z. Superparamagnetic Fe2O3 Beads–CdSe/ZnS Quantum Dots Core–Shell Nanocomposite Particles for Cell Separation. Nano Lett. 2004, 4, 409–413. 10.1021/nl035010n. [DOI] [Google Scholar]
- Hyeon T. Chemical synthesis of magnetic nanoparticles. Chem. Commun. 2003, 927–934. 10.1039/b207789b. [DOI] [PubMed] [Google Scholar]
- Perez J. M.; Simeone F. J.; Saeki Y.; Josephson L.; Weissleder R. Viral-Induced Self-Assembly of Magnetic Nanoparticles Allows the Detection of Viral Particles in Biological Media. J. Am. Chem. Soc. 2003, 125, 10192–10193. 10.1021/ja036409g. [DOI] [PubMed] [Google Scholar]
- a Abbasi Z.; Rezayati S.; Bagheri M.; Hajinasiri R. Preparation of a novel, efficient, and recyclable magnetic catalyst, γ-Fe2O3@HAp-Ag nanoparticles, and a solvent- and halogen-free protocol for the synthesis of coumarin derivatives. Chin. Chem. Lett. 2017, 28, 75–82. 10.1016/j.cclet.2016.06.022. [DOI] [Google Scholar]; b Sajjadifar S.; Rezayati S.; Arzehgar Z.; Abbaspour S.; Torabi Jafroudi M. Applications of iron and nickel immobilized on hydroxyapatite-core-shell γ- Fe2O3 as a nanomagnetic catalyst for the chemoselective oxidation of sulfides to sulfoxides under solvent-free conditions. J. Chin. Chem. Soc. 2018, 65, 960–969. 10.1002/jccs.201800036. [DOI] [Google Scholar]; c Rezayati S.; Jafroudi M. T.; Rezaee Nezhad E.; Hajinasiri R.; Abbaspour S. Imidazole-functionalized magnetic Fe3O4 nanoparticles: an efficient, green, recyclable catalyst for one-pot Friedländer quinoline synthesis. Res. Chem. Intermed. 2016, 42, 5887–5898. 10.1007/s11164-015-2411-9. [DOI] [Google Scholar]; d Sajjadifar S.; Rezayati S.; Shahriari A.; Abbaspour S. Silver, iron, and nickel immobilized on hydroxyapatite-core-shell γ-Fe2O3 MNPs catalyzed one-pot five-component reactions for the synthesis of tetrahydropyridines by tandem condensation of amines, aldehydes, and methyl acetoacetate. Appl. Organomet. Chem. 2018, 32, e4172 10.1002/aoc.4172. [DOI] [Google Scholar]; e Ezzatzadeh E. Chemoselective oxidation of sulfides to sulfoxides using a novel Zn-DABCO functionalized Fe3O4 MNPs as highly effective nanomagnetic catalyst. Asian J. Nanosci. Mater. 2021, 4, 125–136. 10.26655/AJNANOMAT.2021.2.3. [DOI] [Google Scholar]; f Yadollahzadeh K. Synthesis of 5-arylmethylene-pyrimidine-2,4,6-trione and 2-arylidenemalononitriles derivatives using a new Brønsted acid nano magnetic catalyst. Asian J. Nanosci. Mater. 2021, 4, 81–94. 10.26655/AJNANOMAT.2021.1.7. [DOI] [Google Scholar]
- Ribeiro R. S.; Silva A. M. T.; Figueiredo J. L.; Faria J. L.; Gomes H. T. Catalytic wetperoxide oxidation: a route towards the application of hybrid magnetic car-bon nanocomposites for the degradation of organic pollutants, A review. Appl. Catal., B 2016, 187, 428–460. 10.1016/j.apcatb.2016.01.033. [DOI] [Google Scholar]
- Verma M. L.; Puri M.; Barrow C. J. Recent trends in nanomaterials immobilised enzymes for biofuel production. Crit. Rev. Biotechnol. 2016, 36, 108–119. 10.3109/07388551.2014.928811. [DOI] [PubMed] [Google Scholar]
- Majouga A.; Sokolsky-Papkov M.; Kuznetsov A.; Lebedev D.; Efremova M.; Beloglazkina E.; Rudakovskaya P.; Veselov M.; Zyk N.; Golovin Y.; Klyachko N.; Kabanov A. Enzyme-functionalized gold-coated magnetite nanoparticles as novel hybrid nanomaterials: synthesis, purification and control of enzymefunction by low-frequency magneticfield. Colloids Surf., B 2015, 125, 104–109. 10.1016/j.colsurfb.2014.11.012. [DOI] [PubMed] [Google Scholar]
- Sheoran A.; Kaur L.; Kaur P.; Kumar V.; Tikoo K. B.; Agarwal J.; Bansal S.; Singhal S. Graphene based magnetic nanohybrids as promising catalysts forthe green synthesis of b-amino alcohol derivatives. J. Mol. Struct. 2020, 1204, 127522. 10.1016/j.molstruc.2019.127522. [DOI] [Google Scholar]
- Raj T.; Bhatia R. K.; Kapur A.; Sharma M.; Saxena A. K.; Ishar M. P. S. Cytotoxic activity of 3-(5-phenyl-3H-[1,2,4]dithiazol-3-yl)chromen-4-ones and 4-oxo-4H-chromene-3-carbothioic acid N-phenylamides. Eur. J. Med. Chem. 2010, 45, 790–794. 10.1016/j.ejmech.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Mohr S. J.; Chirigos M. A.; Fuhrman F. S.; Pryor J. W. Pyran Copolymer as an Effective Adjuvant to Chemotherapy against a Murine Leukemia and Solid Tumor. Cancer Res. 1975, 35, 3750–3754. [PubMed] [Google Scholar]
- Moon D.-Q.; Kim K.-C.; Jin C.-Y.; Han M.-H.; Park C.; Lee K.-J.; Park Y.-M.; Choi Y. H.; Kim G.-Y. Inhibitory effects of eicosapentaenoic acid on lipopolysaccharide-induced activation in BV2 microglia. Int. Immunopharmacol. 2007, 7, 222–229. 10.1016/j.intimp.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Kaur R.; Naaz F.; Sharma S.; Mehndiratta S.; Gupta M. K.; Bedi P. M. S.; Nepali K. Screening of a library of 4-aryl/heteroaryl-4H-fused pyrans for xanthine oxidase inhibition: synthesis, biological evaluation and docking studies. Med. Chem. Res. 2015, 24, 3334–3349. 10.1007/s00044-015-1382-0. [DOI] [Google Scholar]
- Rueping M.; Sugiono E.; Merino E. Asymmetric organocatalysis: an efficient enantioselective access to benzopyranes and chromenes. Chem. – Eur. J. 2008, 14, 6329–6332. 10.1002/chem.200800836. [DOI] [PubMed] [Google Scholar]
- Bonsignore L.; Loy G.; Secci D.; Calignano A. Synthesis and pharmacological activity of 2-oxo-(2H) 1-benzopyran-3-carboxamide derivatives. Eur. J. Med. Chem. 1993, 28, 517–520. 10.1016/0223-5234(93)90020-F. [DOI] [Google Scholar]
- Martínez-Grau A.; Marco J. Friedländer reaction on 2-amino-3-cyano-4H-pyrans: Synthesis of derivatives of 4H-pyran [2,3-b] quinoline, new tacrine analogues. Bioorg. Med. Chem. Lett. 1997, 7, 3165–3170. 10.1016/S0960-894X(97)10165-2. [DOI] [Google Scholar]
- Amr A. G. E.; Mohamed A. M.; Mohamed S. F.; Abdel-Hafez N. A.; Hammam A. E. F. G. Anticancer activities of some newly synthesized pyridine, pyrane, and pyrimidine derivatives. Bioorg. Med. Chem. 2006, 14, 5481–5488. 10.1016/j.bmc.2006.04.045. [DOI] [PubMed] [Google Scholar]
- Smith C. W.; Bailey J. M.; Billingham M. E. J.; Chandrasekhar S.; Dell C. P.; Harvey A. K.; Hicks C. A.; Kingston A. E.; Wishart G. N. The anti-rheumatic potential of a series of 2,4-di-substituted-4H-naphtho[1,2-b]pyran-3-carbonitriles. Bioorg. Med. Chem. 1995, 5, 2783–2788. 10.1016/0960-894X(95)00487-E. [DOI] [Google Scholar]
- Andreani L. L.; Lapi E. On some new esters of coumarin-3-carboxylic acid wit balsamic and bronchodilator action. Boll. Chim. Farm. 1960, 99, 583–586. [PubMed] [Google Scholar]
- Schiemann K.; Finsinger D.; Zenke F.; Amendt C.; Knöchel T.; Bruge D.; Buchstaller H. P.; Emde U.; Stähle W.; Anzali S. The discovery and optimization of hexahydro-2H-pyrano[3,2-c]quinolines (HHPQs) as potent and selective inhibitors of the mitotic kinesin-5. Bioorg. Med. Chem. Lett. 2010, 20, 1491–1495. 10.1016/j.bmcl.2010.01.110. [DOI] [PubMed] [Google Scholar]
- Matteelli A.; Carvalho A. C. C.; Dooley K. E.; Kritski A. TMC207: the first compound of a new class of potent anti-tuberculosis drugs. Future Microbiol. 2010, 5, 849–858. 10.2217/fmb.10.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishore N.; Mishra B. B.; Tripathi V.; Tiwari V. K. Alkaloids as potential anti-tubercular agents. Fitoterapia 2009, 80, 149–163. 10.1016/j.fitote.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Mehellou Y.; De Clercq E. Twenty-Six Years of Anti-HIV Drug Discovery: Where Do We Stand and Where Do We Go?. J. Med. Chem. 2009, 53, 521–538. 10.1021/jm900492g. [DOI] [PubMed] [Google Scholar]
- Ballini R.; Bosica G.; Conforti M. L.; Maggi R.; Mazzacani A.; Righi P.; Sartori G. Three-component process for the synthesis of 2-amino-2-chromenes in aqueous media. Tetrahedron 2001, 57, 1395–1398. 10.1016/S0040-4020(00)01121-2. [DOI] [Google Scholar]
- Han Y. F.; Xia M. Multicomponent Synthesis of Cyclic Frameworks on Knoevenagel-Initiated Domino Reactions. Curr. Org. Chem. 2010, 14, 379–413. 10.2174/138527210790231865. [DOI] [Google Scholar]
- Peng Y.; Song G. Amino-functionalized ionic liquid as catalytically active solvent for microwave-assisted synthesis of 4H-pyrans. Catal. Commun. 2007, 8, 111–114. 10.1016/j.catcom.2006.05.031. [DOI] [Google Scholar]
- Abdolmohammadi S.; Balalaie S. Novel and efficient catalysts for the one-pot synthesis of 3,4-dihydropyrano[c]chromene derivatives in aqueous media. Tetrahedron Lett. 2007, 48, 3299–3303. 10.1016/j.tetlet.2007.02.135. [DOI] [Google Scholar]
- Nagabhushana H.; Saundalkar S. S.; Muralidhar L.; Nagabhushana B. M.; Girija C. R.; Nagaraja D.; Pasha M. A.; Jayashankara V. P. α-Fe2O3 nanoparticles: An efficient, inexpensive catalyst for the one-pot preparation of 3,4-dihydropyrano[c]chromenes. Chin. Chem. Lett. 2011, 22, 143–146. 10.1016/j.cclet.2010.09.020. [DOI] [Google Scholar]
- Heravi M. M.; Jani B. A.; Derikvand F.; Bamoharram F. F.; Oskooie H. A. Three component, one-pot synthesis of dihydropyrano[3,2-c]chromene derivatives in the presence of H6P2W18O62.18H2O as a green and recyclable catalyst. Catal. Commun. 2008, 10, 272–275. 10.1016/j.catcom.2008.08.023. [DOI] [Google Scholar]
- Jin T. S.; Liu L. B.; Zhao Y.; Li T. S. Clean, One-Pot Synthesis of 4H-Pyran Derivatives Catalyzed by Hexadecyltrimethyl Ammonium Bromide in Aqueous Media. Synth. Commun. 2005, 35, 1859–1863. 10.1081/SCC-200064898. [DOI] [Google Scholar]
- Babu N. S.; Pasha N.; Rao K. T. V.; Sai P. S. S.; Lingaiah N. A heterogeneous strong basic Mg/La mixed oxide catalyst for efficient synthesis of polyfunctionalized pyrans. Tetrahedron Lett. 2008, 49, 2730–2733. 10.1016/j.tetlet.2008.02.154. [DOI] [Google Scholar]
- Niknam K.; Piran A. Silica-Grafted Ionic Liquids as Recyclable Catalysts for the Synthesis of 3,4-Dihydropyrano[c]chromenes and Pyrano[2,3-c]pyrazoles. Green Sustain. Chem. 2013, 03, 1–8. 10.4236/gsc.2013.32A001. [DOI] [Google Scholar]
- Kumar B. S.; Srinivasulu N.; Udupi R. H.; Rajitha B.; Reddy Y. T.; Reddy P. N.; Kumar P. S. An efficient approach towards three component coupling of one pot reaction for synthesis of functionalized benzopyrans. J. Heterocyclic Chem. 2006, 43, 1691–1693. 10.1002/jhet.5570430641. [DOI] [Google Scholar]
- Pourkazemi A.; Nasouri Z.; Fakhraie F.; Razzaghi A.; Parhami A.; Zare A. Efficient production of 2-amino-4H-chromenes and 14-aryl-14H-dibenzo[a, j]xanthenes catalyzed by N, N-diethyl-N-sulfoethanaminium hydrogen sulfate. Asian J. Nanosci. Mater. 2020, 3, 131–137. 10.26655/AJNANOMAT.2020.2.5. [DOI] [Google Scholar]
- Jin T.-S.; Xiao J.-C.; Wang S.-J.; Li T.-S.; Song X.-R. An Efficient and Convenient Approach to the Synthesis of Benzopyrans by a Three-Component Coupling of One-Pot Reaction. Synlett 2003, 2003, 2001–2004. 10.1055/s-2003-42030. [DOI] [Google Scholar]
- Heravi M. M.; Bakhtiari K.; Zadsirjan V.; Bamoharram F. F.; Heravi O. M. Aqua mediated synthesis of substituted 2-amino-4H-chromenes catalyzed by green and reusable Preyssler heteropolyacid. Bioorg. Med. Chem. Lett. 2007, 17, 4262–4265. 10.1016/j.bmcl.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Chen L.; Li Y. Q.; Huang X. J.; Zheng W. J. N,N-dimethylamino-functionalized basic ionic liquid catalyzed one-pot multicomponent reaction for the synthesis of 4H-benzo[b]pyran derivatives under solvent-free condition. Heteroat. Chem. 2009, 20, 91–94. 10.1002/hc.20516. [DOI] [Google Scholar]
- Tural B.; Tural S.; Ertas E.; Yalınkılıç I.; Demir A. S. Purification and covalent immobilization of benzaldehyde lyase with heterofunctional chelate-epoxy modified magnetic nanoparticles and its carboligation reactivity. J. Mol. Catal. B: Enzym. 2013, 95, 41–47. 10.1016/j.molcatb.2013.05.023. [DOI] [Google Scholar]
- Ulu A.; Ozcan I.; Koytepe S.; Ates B. Design of epoxy-functionalized Fe3O4@MCM-41 core–shell nanoparticles for enzyme immobilization. Int. J. Biol. Macromol. 2018, 115, 1122–1130. 10.1016/j.ijbiomac.2018.04.157. [DOI] [PubMed] [Google Scholar]
- Xie H.; Liu H.; Wang M.; Pan H.; Gao C. L-Tyrosine-Pd complex supported on Fe3O4 magnetic nanoparticles: A new catalyst for C–C coupling and Synthesis of sulfides. Appl. Organomet. Chem. 2020, 34, e5256 10.1002/aoc.5256. [DOI] [Google Scholar]
- Ghorbani-Choghamarani A.; Darvishnejad Z.; Norouzi M. Cu(II)–Schiff base complex-functionalized magnetic Fe3O4 nanoparticles: a heterogeneous catalyst for various oxidation reactions. Appl. Organomet. Chem. 2015, 29, 170–175. 10.1002/aoc.3266. [DOI] [Google Scholar]
- Inaloo I. D.; Majnooni S.; Eslahi H.; Esmaeilpour M. Nickel(II) Nanoparticles Immobilized on EDTA-Modified Fe3O4@SiO2 Nanospheres as Efficient and Recyclable Catalysts for Ligand-Free Suzuki–Miyaura Coupling of Aryl Carbamates and Sulfamates. ACS Omega 2020, 5, 7406–7417. 10.1021/acsomega.9b04450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzaee M.; Bahramian B.; Gholampour P.; Teymouri S.; Khorsand T. Preparation and characterization of Fe3O4@Boehmite core-shell nanoparticles to support molybdenum or vanadium complexes for catalytic epoxidation of alkenes. Appl. Organomet. Chem. 2019, 33, e4792 10.1002/aoc.4792. [DOI] [Google Scholar]
- Chary K. V. R.; Srikanth C. S. Selective Hydrogenation of Nitrobenzene to Aniline over Ru/SBA-15 Catalysts. Catal. Lett. 2009, 128, 164–170. 10.1007/s10562-008-9720-1. [DOI] [Google Scholar]
- Ebrahimiasl H.; Azarifar D. Copper-based Schiff Base Complex Immobilized on Coreshell Fe3O4@SiO2 as a magnetically recyclable and highly efficient nanocatalyst for green synthesis of 2-amino-4Hchromene derivatives. Appl. Organomet. Chem. 2019, 34, e5359 10.1002/aoc.5359. [DOI] [Google Scholar]
- Ebrahimiasl H.; Azarifar D.; Rakhtshah J.; Keypour H.; Mahmoudabadi M. Application of novel and reusable Fe3O4@CoII(macrocyclic Schiff base ligand) for multicomponent reactions of highly substituted thiopyridine and 4H-chromene derivatives. Appl. Organomet. Chem. 2020, 34, e5769 10.1002/aoc.5769. [DOI] [Google Scholar]
- Khan M. N.; Pal S.; Karamthulla S.; Choudhury L. H. Imidazole as organocatalyst for multicomponent reactions: diversity oriented synthesis of functionalized hetero- and carbocycles using in situ-generated benzylidenemalononitrile derivatives. RSC Adv. 2014, 4, 3732–3741. 10.1039/C3RA45252B. [DOI] [Google Scholar]
- Hosseinzadeh-Baghan S.; Mirzaei M.; Eshtiagh-Hosseini H.; Zadsirjan V.; Heravi M. M.; Mague J. T. An inorganic–organic hybrid material based on a Keggintype polyoxometalate@Dysprosium as an effective and green catalyst in the synthesis of 2-amino-4H-chromenes via multicomponent reactions. Appl. Organomet. Chem. 2020, 34, e5793 10.1002/aoc.5793. [DOI] [Google Scholar]
- Yuan D.; Zhang Q.; Dou J. Supported nanosized palladium on superparamagnetic composite microspheres as an efficient catalyst for Heck reaction. Catal. Commun. 2010, 11, 606–610. 10.1016/j.catcom.2010.01.005. [DOI] [Google Scholar]
- Tajbakhsh M.; Farhang M.; Hosseinzadeh R.; Sarrafi Y. Nano Fe3O4 supported biimidazole Cu(i) complex as a retrievable catalyst for the synthesis of imidazo[1,2-a]pyridines in aqueous medium. RSC Adv. 2014, 4, 23116–23124. 10.1039/c4ra03333g. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.