Abstract
The signal intensity (SI) in gradient-echo, echo-planar magnetic resonance images (repetition time/echo time = 1,000/40) of anterior tibialis muscle in active [estimated energy expenditure 42.4 ± 3.7 (SD), n = 8] vs. sedentary (32.3 ± 0.6 kcal ·kg−1 ·day−1, n = 8) young adult (18–34 yr old) human subjects was measured after single, 1-s-duration maximum voluntary ankle dorsiflexion contractions. There was no difference between groups in anterior tibial muscle cross-sectional area or peak force. In both groups there was a transient increase in anterior tibialis muscle SI, which peaked 5–7 s after the end of each contraction. The magnitude of the SI transient was over threefold greater [5.5 ± 1.0 (SE) vs. 1.5 ± 0.4%] and persisted twice as long (half-recovery time 5.4 ± 0.4 vs. 2.7 ± 0.3 s) in the active subjects. In the same subjects, blood flow in popliteal, anterior tibial, and posterior tibial arteries was measured by cardiac-gated CINE magnetic resonance angiography before and after 2 min of dynamic, repetitive ankle dorsiflexion exercise. There was no difference between groups in resting or postexercise flow in anterior tibial artery, although popliteal and posterior tibial artery flow after exercise tended to be greater in the active group. The results indicate that transient hyperemia and oxygenation in muscle after single contractions are enhanced by chronic physical activity to a greater extent than peak muscle blood flow.
Keywords: magnetic resonance imaging angiography, hyperemia
The beneficial effects of physical activity on health and longevity are well known (1), and, to a significant extent, these benefits may arise from the effects of activity on vascular function (13). Documented vascular changes associated with aerobic exercise training include both macrovascular adaptations [e.g., increased arterial compliance (12), increased flow-mediated arterial dilation (4)] and microvascular adaptations [e.g., increased vascular density, enhanced arteriolar endothelium-mediated dilation (6, 14)]. Assessment of the macrovascular adaptations to exercise training can be accomplished by ultrasound or magnetic resonance imaging (MRI) of major conduit vessels such as the brachial and popliteal arteries. However, direct assessment of microvascular structure and function in human subjects in response to exercise or other interventions has been limited to superficial measurements, for example in the skin (8, 16), or to measurements on biopsy samples (10). Thus the microvascular adaptations in human muscle during disuse or after training have been largely inferred from gross measurements of muscle blood flow.
Magnetic resonance (MR) images of muscle and other tissues are sensitive to microvascular density, perfusion, and oxygenation, as well as to the cellular properties of the surrounding parenchymal tissue. For example, one version of the brain “functional” MRI methods now widely used to map neural activity is not actually a measure of neural activity per se, but is instead an indirect measure of the local microvascular response to neural activity. This brain functional imaging method relies on the fact that the magnetic susceptibility and T2 relaxation rate are less in oxygenated blood than in deoxygenated blood, a phenomenon referred to as the blood oxygen level-dependent (BOLD) effect (17, 23). In activated brain regions, the local increase in blood flow exceeds the local increase in oxygen consumption, so blood oxygen saturation increases, apparent T2 relaxation rate decreases, and the MR signal intensity increases in T2-weighted images. Thus application of this method to measure neural activity implicitly assumes that vascular density and reactivity are the same in different regions of the brain and in different individuals.
A previous report (20) showed that a transient BOLD effect analogous to that in the brain can be measured by MRI in skeletal muscles of healthy subjects after single, 1-s-duration maximal isometric contractions. The time course of the BOLD transients is similar to the time course of transient hyperemia measured in major conduit arteries after single contractions of human (2, 3, 30) and dog muscles (7, 21). As in the brain, the transients are caused at least in part by increased blood oxygen saturation (20), presumably because the transient hyperemia delivers oxygen in excess of that required to restore the small PCr depletion [~2 mM/s (24)] during a brief contraction. However, unlike in the brain, one would expect both the microvascular density and reactivity in skeletal muscle to vary with subject physical activity level. In fact, in the previous study (20), the magnitude of the postcontractile BOLD transients did vary widely between different subjects, although subject activity level was not recorded in that study. Therefore, the main purpose of this study was to examine the magnitude and time course of muscle postcontractile BOLD transients in the anterior tibialis muscle of healthy sedentary vs. chronically active human subjects. In addition, anterior tibial blood flow after repetitive, dynamic ankle dorsiflexion exercise was measured in the same subjects by conventional phase-contrast CINE MR angiography (19). The results indicate that chronic activity enhances the magnitude of the postcontractile BOLD response by over threefold but that it has no effect on the peak muscle blood flow after isolated, repetitive exercise of the same muscle. Thus postcontractile BOLD transients may be a more sensitive index of peripheral muscle vascular conditioning than conventional measurements of peak muscle blood flow.
METHODS
Subjects.
Sixteen healthy subjects (18–34 yr of age, 3 female) were recruited from the university community. Before participation, the subjects gave informed written consent, in accordance with the University’s Committee on Research Involving Human Subjects. All subjects were apparently healthy and were not taking any medications known to affect blood flow. Subjects were asked to refrain from strenuous physical activity on the day of the testing. To limit the potential effects of food or caffeine on blood flow subjects were asked to not eat for 2 h before nor consume any caffeinated drinks 3 h before their scheduled visit.
Physical activity.
Subjects were selected for either the active or sedentary group on the basis of their self-reported physical activity levels. Subjects reporting participation in running or other aerobic training greater than 30 min/day and at least 5 days/wk were assigned to the active group; subjects in the sedentary group reported no regular participation in physical activity. Physical activity levels were confirmed using a 7-day physical activity diary (28) and the 7-day physical activity recall questionnaire as described by Sallis et al. (25).
MRI measurements.
All MR images were acquired using a standard clinical extremity coil on a 1.5-T GE Horizon system (GE Medical Systems, Milwaukee, WI). Subjects lay supine in the imager with both legs extended. The subject’s right foot was secured to the plate of a custom-built foot device using a nylon strap with Velcro closures. The footplate angle was either fixed at 120°, for isometric exercise, or could be moved through a 30° range of motion from 120° to 90°, for dynamic exercise. The force system consisted of a load cell (Interface, model SSM-EV-250, Scottsdale, AZ) mounted to the underside of the footplate. Force during the isometric and dynamic exercise was digitized (DATAQ Instruments, model DI-195B, Akron, OH), sampled at 60 Hz, and recorded on a personal computer. Force during both the isometric and dynamic exercise was measured as the peak force during each contraction.
T1-weighted [3-plane, TR 100 ms, TE 1.6 ms, 24-cm field of view (FOV), 5-mm slice thickness, 11 slices per plane, 256 × 128 acquisition matrix, 1 number of excitation (NEX)] and T2-weighted (axial fast spin echo, TR 1,500 ms, TE 24 ms, echo train length 4, 256 × 160 acquisition matrix, 16-cm FOV, 1-cm slice, 1 NEX) images were acquired to locate the largest cross-sectional area of the anterior tibialis muscle. One-shot gradient-recalled echo planar images (TR 1,000 ms, TE 40 ms, 90° pulse, 18-cm FOV, 1-cm slice thickness, 62.5-kHz bandwidth, 64 × 64 acquisition matrix) were acquired from a single axial slice transecting the largest cross-sectional area of the tibialis anterior muscle. Echo planar images were acquired continuously for 4 min, during which time subjects performed a single, 1-s-duration maximal isometric ankle dorsiflexion every 30 s (total of 7 contractions). The time course of signal intensity (SI) changes in the anterior tibialis muscle and in posterior muscles were computed from manually demarked regions of interest (ROI) drawn with care taken to exclude any vessels resolved in the corresponding anatomical images. The peak change in SI above baseline, time to peak, and half-recovery time were calculated for the postcontractile transients as described previously (20).
After the above single-contraction protocol, a set of two-dimensional (2D) gradient-recalled, echo time of flight (TOF) flow images was acquired to identify suitable axial/oblique planes for flow measurements. The 2D TOF sequence consisted of 92 adjacent axial images (TR 18 ms, TE 4.5 ms, 45° pulse, 16-cm FOV, 1.5-mm slice thickness , 256 × 128 acquisition matrix, 1 NEX) centered on a region 5 cm below the fibular head. The axial images were used to construct a three-dimensional (3D) representation of the vessels within that region. Based on the 3D image, a pair of parallel slices was chosen, one transecting the popliteal artery 1–2 cm above the popliteal bifurcation and the second transecting both the anterior and posterior tibial arteries 1–3 cm below their bifurcation from the popliteal artery. The two parallel slices were prescribed as near as possible to be perpendicular to the axis of the two tibial arteries.
Flow velocity images (TR 18 ms, TE 6 ms, 30° pulse, 1-cm slice thickness, 14-cm FOV, 256 × 128 acquisition matrix, 1 NEX) of the selected slices were acquired in retrospectively ECG-gated CINE mode as described previously (19). This method depends on the measurement of the extra phase (ΔΘ) acquired by spins moving along the direction of a bipolar flow-encoding gradient. The extra phase depends directly on velocity V (cm/s) according to ΔΘ = γATV, where γ (rad/G) is the gyromatic ratio, A is the time integral of gradient lobes (G·s/cm), and T is the time between the two gradient lobes. In this study, the flow-encoding gradient was applied perpendicular to the prescribed slices (i.e., parallel to the vessels), with maximum velocity encoding (VENC; corresponding to ±180° phase shifts) set at 160 cm/s. Retrospective gating of the data acquired over 128 heartbeats (total acquisition time 2–4 min, depending on the subject’s heart rate) yielded 32 cardiac-gated flow-velocity and magnitude images per slice. Flow (ml/min) was calculated from the individual velocity images by integrating velocity (cm/s) across the area (cm2) of each vessel as described previously (19). Mean flow in each vessel was then calculated from the mean across all 32 images in each set.
Vessel flow was measured immediately before and twice during the recovery period after the subjects performed 2 min of dynamic ankle dorsiflexion exercise at 50% duty cycle, 0.5 Hz contraction rate. For each contraction, the subjects moved the footplate through a 30° range of motion (120° to 90°) against a resistance applied by rubber tubing. When the footplate was at 90° relative to the perpendicular, the resistance applied by the tubing was approximately equal to 40% of the subjects’ isometric maximal. This exercise protocol has been shown to activate the anterior tibialis muscle without significant activation of plantar flexor muscles (5).
Magnitude images reconstructed from the velocity-encoded data sets were used to estimate indexes of arterial compliance and flow-mediated dilation. Apparent arterial compliance was estimated as % change in anterior tibial artery cross-sectional area [100*(systolic vessel area − diastolic vessel area)/diastolic vessel area] from the first set of postexercise velocity-encoded images. Flow-mediated dilation was calculated as the relative change in anterior tibial artery area during diastole from resting to first postexercise scans [100*(postexercise vessel area − resting vessel area)/(resting vessel area)].
Near-infrared spectroscopic measurements.
On a different experimental day, the subjects repeated the above single contraction protocol (i.e., 1-s-duration maximum isometric contractions at 30-s intervals) while relative hemoglobin saturation was recorded using either a NIM Runman CW2000 dual-wavelength spectrometer (Nim, Philadelphia, PA) or an INVOS Cerebral Oximeter (Somanetics, Troy, MI). After the contraction protocol, saturation data were acquired during 4–8 min of cuff ischemia (thigh cuff pressure greater than 220 Torr) and the subsequent hyperemia after release of the cuff. The magnitude of the postcontraction saturation transients is reported as percentage of the maximum change observed after release of the cuff, as described previously (18, 20).
Statistics.
Comparisons between groups for descriptive characteristics, mean peak BOLD magnitude, time to peak, half-relaxation, apparent arterial stiffness, and flow-mediated dilation were made using a Student’s t-test. A Folded Form F-statistic was used to test the assumption of equal variances. If the assumption of equal variances was violated, the Satterthwaite approximation for degrees of freedom was used. Time series data from the BOLD and flow portions of the study were analyzed using a two-factor (group × time) ANOVA, with repeated measures on time point. Data were analyzed using SAS statistical software package version 9.1.2 (Cary, NC), using the MIXED procedure and the REPEATED statement. The appropriate covariance matrix for the repeated measure was selected based on the structure of the time series data and the fit statistics. Data are presented as means ± SD or SE where appropriate.
RESULTS
Physical characteristics of the subjects are shown in Table 1. There were no differences between groups in age, height, weight, or maximum cross-sectional area of the anterior tibialis muscle. The active group was significantly more active than the inactive, sedentary group as estimated by the physical activity questionnaire [42.4 ± 3.7 vs. 32.3 ± 0.6 (SD) kcal·kg−1 ·day−1, respectively, P < 0.00].
Table 1.
Subject physical characteristics
| Active (n = 8, 2 female) |
Sedentary (n = 8, 1 female) |
|
|---|---|---|
| Age, yr | 24.9±4.8 | 24.6±2.9 |
| Height, cm | 178.1±7.7 | 171.3±10.3 |
| Body wt, kg | 66.8±8.8 | 72.6±14.0 |
| Anterior tibialis cross section, cm2 | 10.8±1.1 | 12.3±2.1 |
Values are means ± SD.
Figure 1 shows representative axial anatomical and corresponding echo-planar images from an active and a sedentary subject. The green marks on the echo-planar images enclose the ROI drawn for analysis of the time course of SI changes during the brief isometric contraction protocol. Figure 2 shows the time course of SI changes (as percent change from baseline) in the anterior tibialis muscle ROI (top row) and in the posterior muscle ROI (next row) for the same two subjects. The spikes in both anterior and posterior muscle SI coincident with the contractions (force, third panels) are saturation artifacts caused by muscle length changes and distortion during the contractions. As reported previously (20), these motion artifacts are followed by delayed, transient increases in SI in the anterior, but not in the posterior, muscle. Figure 2, bottom, shows the mean transient response in anterior muscle, obtained by averaging the responses after each contraction, and clearly illustrates the larger response observed in the active compared with the sedentary subject.
Fig. 1.
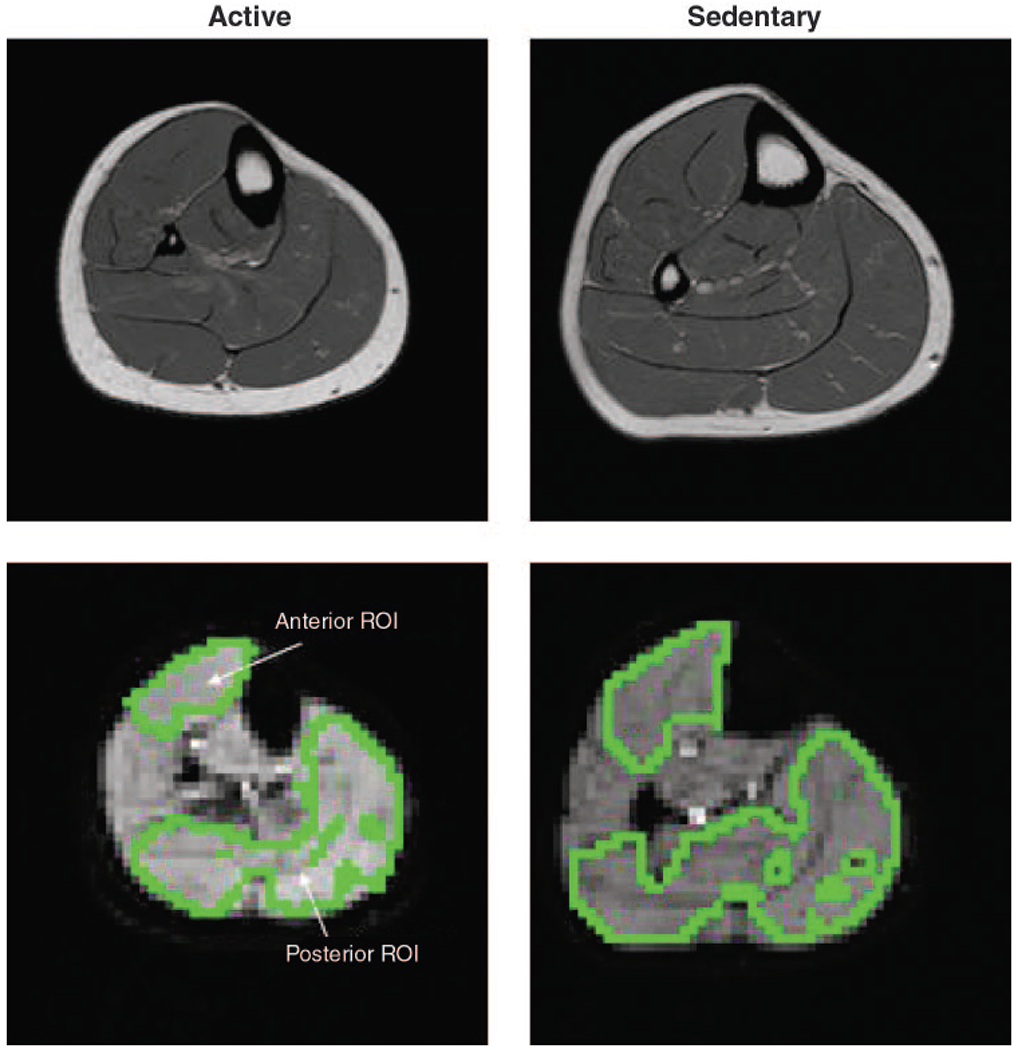
Representative anatomical (top: fast spin echo, repetition time/echo time (TR/TE) = 1,500/24) and echo-planar (bottom: TR/TE = 1,000/40) images from an active (left) and sedentary (right) subject. Voxels marked in green on the echo-planar images surround the regions of interest (ROI) from which the time courses of signal intensity (SI) change were obtained.
Fig. 2.
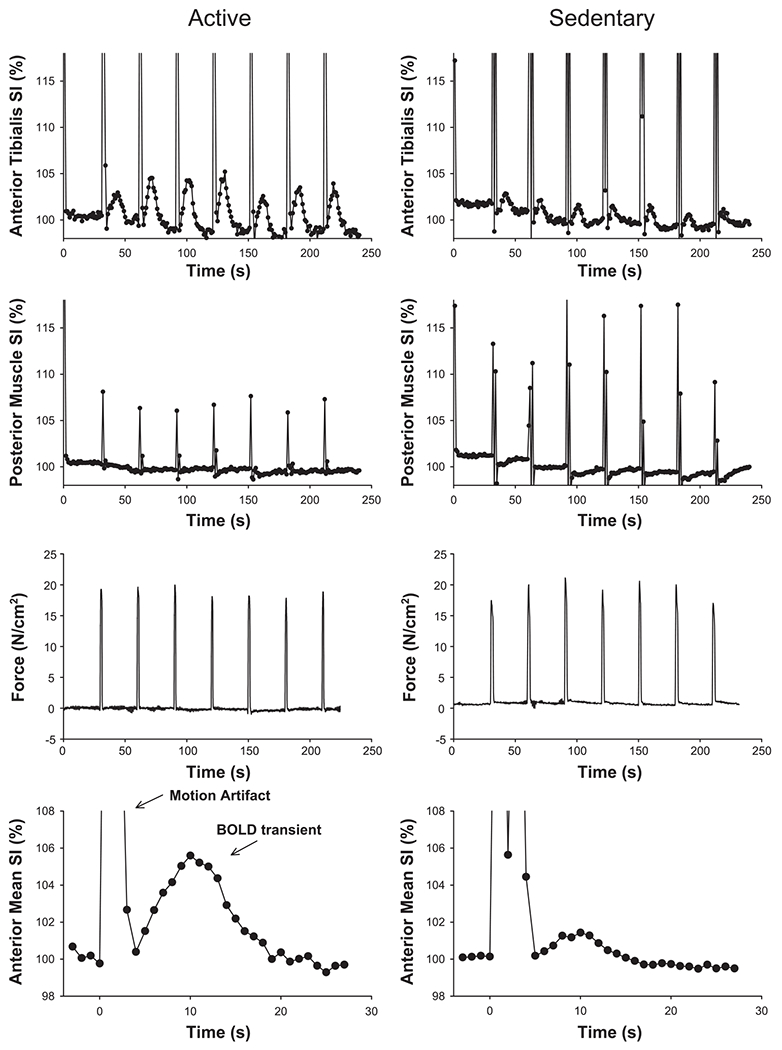
Representative time course of SI changes in anterior tibialis (top) and posterior muscle (2nd panel) during single-contraction protocol. Active (left) and sedentary (right) subjects are the same as in Fig. 1. Spikes coincident with the contractions (force, 3rd panel) are due to changes in signal saturation when the muscles move in the imaged slice during contraction and relaxation (20). Bottom shows the response for each subject averaged over the 7 contractions.
On average, the peak magnitude of the SI transients was over threefold larger in the active compared with the sedentary group (Table 2). The time to peak magnitude (measured in seconds after the end of the motion artifact) was similar in the two groups. However, the half-recovery time was significantly longer in the trained compared with the sedentary group (Table 2). Neither the mean peak magnitude nor peak muscle force changed significantly over the course of the seven contractions in either group, and there was no significant relationship between peak transient magnitude vs. muscle cross-sectional area (r2 = 0.12, P = 0.20, n = 16) or force of contraction (r2 = −0.033, P = 0.48). Near-infrared spectroscopy hemoglobin saturation measurements during the single-contraction protocol were unsuccessful in five subjects due to movement of the probes or other instrument instabilities. Among the remaining subjects, the change in relative hemoglobin saturation after single contractions tended to be greater in active [14.1 ± 2.6% (SE), n = 5] compared with sedentary subjects (7.6 ± 1.4%, n = 6), but this trend was not statistically significant (P = 0.07).
Table 2.
Force and postcontractile BOLD transient responses in anterior tibial muscle after 1-s maximal isometric ankle dorsiflexions
| Active | Sedentary | |
|---|---|---|
| Peak BOLD, %change in SI | 5.5±1.0 | 1.5±0.4* |
| Time to peak, s | 6.7±0.5 | 5.8±0.3 |
| Half relaxation, s | 5.4±0.4 | 2.7±0.3* |
| MVC, N/cm2 | 22.7±1.5 | 19.4±2.0 |
| End force, % initial | 95.1±4.0 | 98.1±3.0 |
Values are means ± SE.
Indicates statistically significant difference between groups, Student’s t-test (P<0.05).
BOLD, blood oxygen level-dependent, MVC, maximal voluntary contraction; SI, signal intensity.
Figure 3 shows representative magnitude images from a subject before and after the dynamic, repetitive exercise, illustrating the location of the flow measurements in popliteal, anterior tibial, and posterior tibial arteries. Figure 4 shows representative cardiac-gated arterial flow waveforms derived from the preexercise and first postexercise sets of flow images in the same subject. There was no significant difference between groups in cross-sectional area or mean preexercise flow in any of the three arteries examined. Peak force decreased to a similar extent in the two groups by the end of the repetitive exercise [11.1 ± 3.7 (SE) vs. 15.9 ± 5.1% decrease in active vs. sedentary, respectively]. As expected, the increase in flow after this fatiguing, repetitive exercise was most dramatic in the anterior tibial artery, which supplies the exercised muscle (Fig. 5). However, there was no significant difference between groups in postexercise flow in any of the arteries, either at the two measured time points or when flow immediately after the exercise was estimated by extrapolation, assuming exponential flow recovery (plotted at time zero in Fig. 5). There were trends toward greater flow in the posterior tibial (ANOVA group × time interaction, P = 0.15) and popliteal (P = 0.08) arteries in the active compared with the sedentary group, but these trends did not reach statistical significance. Finally, there was no difference between groups in the index of anterior tibial artery compliance [%change in arterial cross-sectional area from diastole to systole, 53.4 ± 16.4 vs. 58.0 ± 23.3% (SE) in active and sedentary, respectively] or flow-mediated dilation (%change in diastolic cross-sectional area after exercise, 66.8 ± 16.2 vs. 59.7 ± 14.6%).
Fig. 3.
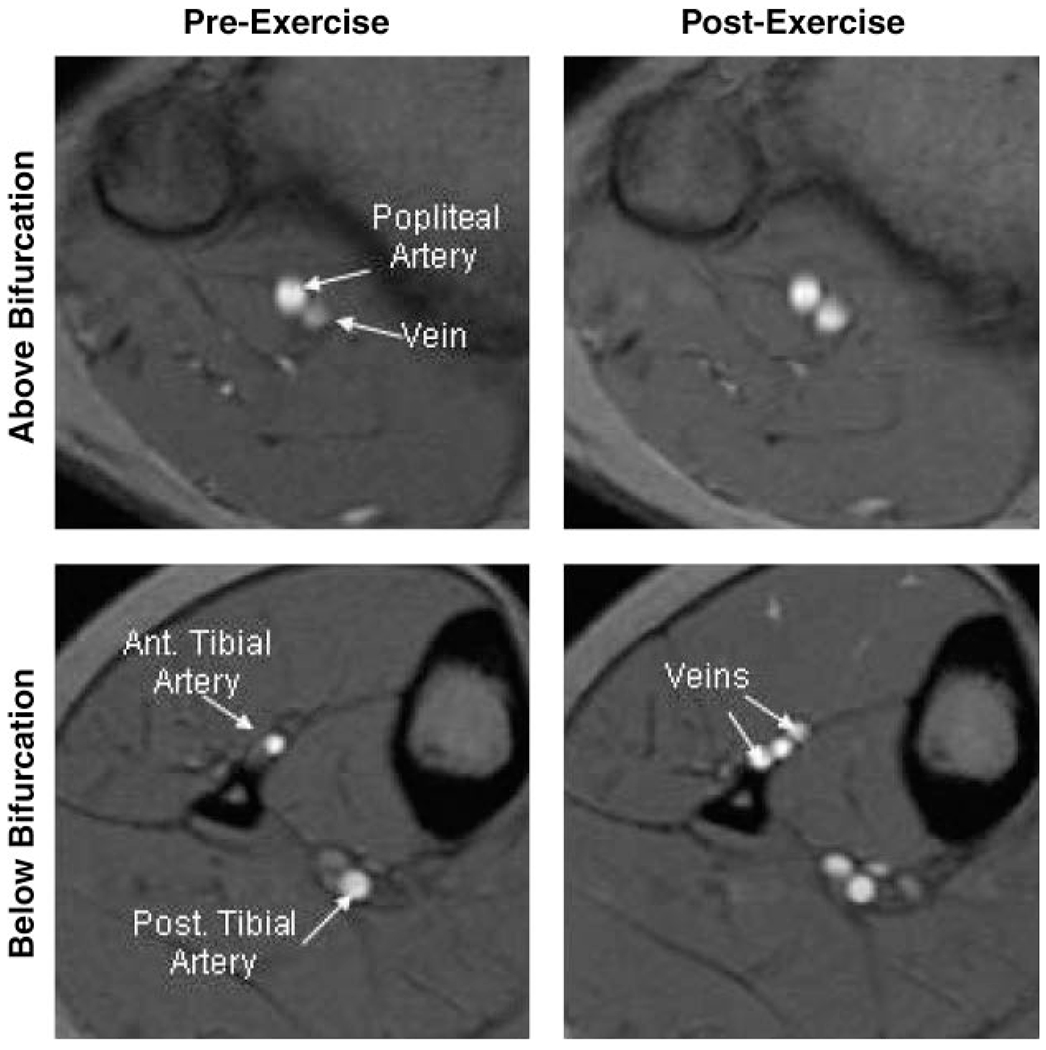
Example magnitude images from the cardiac-gated phase-contrast flow study, illustrating location at which flow measurements were made in popliteal (top) and tibial (bottom) arteries before (left) and after (right) repetitive exercise. These images are from peak systole in the same active subject shown in Figs. 1 and 2. Ant, anterior; Post, posterior.
Fig. 4.
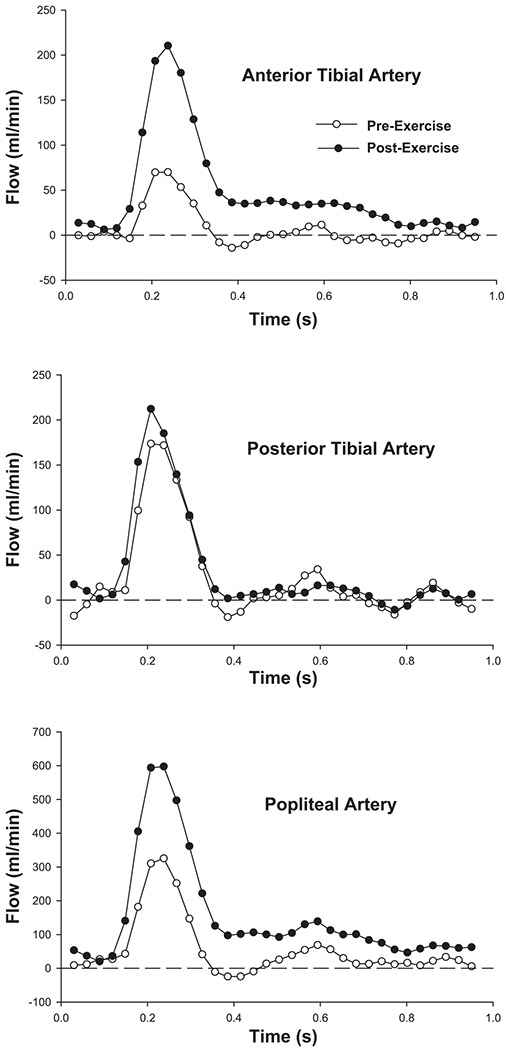
Example cardiac-gated flow waveforms from anterior tibial (top), posterior tibial (middle), and popliteal (bottom) arteries before (◯) and after (●) repetitive ankle dorsiflexion exercise. Results from the same subject as Fig. 3.
Fig. 5.
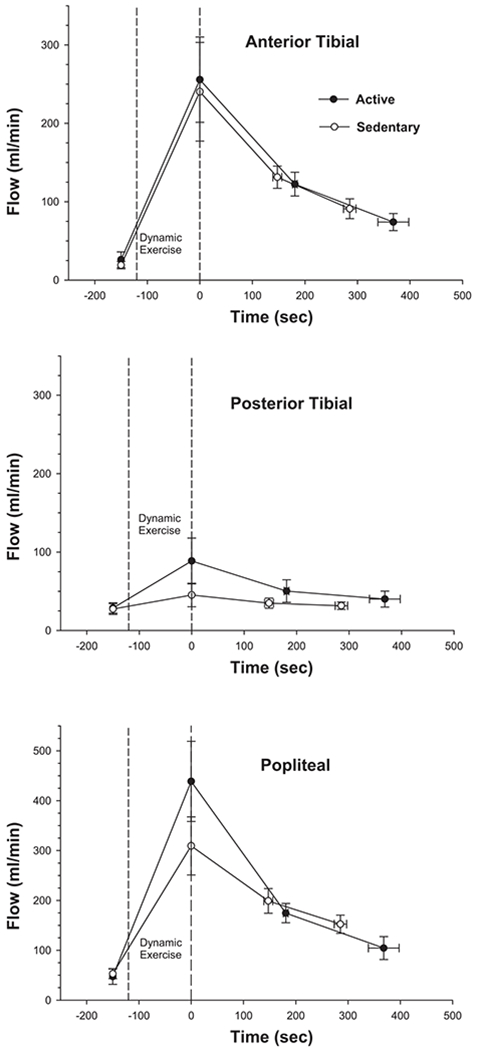
Mean flow (±SE) in anterior tibial (top), posterior tibial (middle), and popliteal (bottom) arteries in active (●) and sedentary (◯) subjects before and after 2-min dynamic, repetitive ankle dorsiflexion exercise. Measurements after exercise are on average slower in the active compared with the sedentary group due to the slower heart rate in the active group [52.5 ± 2.5 (SE) vs. 69.5 ± 4.2 beats/min]. Flow immediately after exercise (plotted at time zero) was computed for each individual measurement assuming exponential flow recovery to the preexercise flow level.
DISCUSSION
The main result of this study is that the transient increase in MRI-measured SI in anterior tibialis muscle after single, 1-s contractions is over threefold greater in chronically active compared with sedentary subjects (Fig. 2, Table 2). In contrast, there was no significant difference between groups in anterior tibial artery flow, or in any other macrovascular measurement, in response to a 2-min dynamic dorsiflexion exercise. Thus it appears that the MRI-measured response after single, brief contractions is a more sensitive index of skeletal muscle vascular conditioning than conventional measurements of vessel flow after repetitive exercise.
The physical mechanism for the MRI-measured BOLD transients after single contractions is not fully understood. The time course of the transients is similar to the time course of increased hemoglobin saturation measured by near-infrared spectroscopy (20), as well as to the time course of conduit artery hyperemia after single contractions of human forearm muscles (2). Previous model calculations suggested that the MRI transients arise predominantly from an intravascular BOLD effect (20), i.e., from the increasing contribution of well oxygenated blood to the total muscle signal when blood flow transiently exceeds muscle oxygen consumption. Therefore, assuming that the oxygen cost of muscle contractions is not substantially different in active compared with sedentary subjects (11), our results suggest that the hyperemia after single, brief contractions is substantially larger in active subjects. The trend toward increased hemoglobin saturation after contractions in the active subjects is consistent with this mechanism. Surprisingly, to our knowledge no previous study has examined the effect of training status on the magnitude of muscle hyperemia after single contractions. However, there is evidence that the immediate increase in muscle blood flow at the onset of repetitive exercise occurs more quickly after training (27).
Increased microvascular volume might also contribute to the larger MRI-measured BOLD transients in active subjects, even if the net change in hemoglobin saturation after the contraction were the same as in sedentary subjects. Assuming the same mean change in saturation, but a 50% larger microvascular volume, one would naively predict a 50% larger intravascular BOLD transient. This possibility might be tested by varying muscle Po2 independent of blood flow, for example, by hyperoxia. Interestingly, Noseworthy et al. (22) already reported that the increase in SI of resting muscle during cycles of hyperoxia was greater in the soleus compared with the gastrocnemius muscle of human subjects, a result attributed to greater vascular volume in the soleus. on the other hand, the effects of vascular volume on the BOLD signal are more difficult to predict if extravascular effects are considered, because extravascular BOLD effects depend strongly on the orientation of the vessels in the magnetic field (23). For example, it may be that the greater BOLD response in soleus compared with gastrocnemius (20, 22) is in part due to different muscle fiber and vessel orientations in these muscles (32).
Whatever the precise physical mechanisms of the MRI-observed transients, it is likely that they are related to the postcontraction hyperemia observed in many previous studies of human (2, 3, 30) and dog (7, 21) muscles. Despite over four decades of study, the physiological mechanism for this “immediate” (30) hyperemia in response to a contraction is still unknown. The role of the “muscle pump” in hyperemia after single contractions is still debated (31), although recent studies strongly suggest that local microvascular dilation in response to as yet unidentified signal(s) is more important than the muscle pump (7, 30). Our results agree with this conclusion, inasmuch as muscle force, and therefore presumably the muscle pump, was the same in our active and sedentary subjects, but the postcontractile transients were dramatically larger in the active subjects. Tschakovsky and Hughson (29) showed that hyperemic transients comparable to those observed after single contractions occur in the brachial artery after raising the arm, suggesting that the signal might result from a reflex related to rapid venous emptying. However, a more recent study found no effect of arm position on contraction-induced hyperemia (30). Similarly, neither sympathectomy nor any specific inhibitory drug (e.g., atropine, NG-nitro-L-arginine methyl ester, ketorolac) has been shown to abolish the phenomenon (26, 31). On the other hand, the hypothesis that release of potassium (21) and/or other substances from active muscle cells triggers “immediate” hyperemia has not been clearly disproved.
It may seem surprising that the estimated peak flow in the anterior tibial artery after the repetitive exercise was similar in the active and sedentary subjects in this study, in view of the many previous reports that chronic activity or training increases peak muscle blood flow (9, 14, 15). However, there was a trend toward greater popliteal and posterior artery flows in the active subjects compared with sedentary subjects. The mean difference in peak popliteal flows (Fig. 5; 439 vs. 309 ml/min or 42% higher in the active subjects) is similar to the difference reported in a previous study of peak muscle flow in active vs. sedentary subjects during exercise of a small muscle mass (9). Interestingly, the higher popliteal flow after exercise in the active subjects was in part accounted for by higher flow in the posterior tibial artery, suggesting that there might be greater collateral flow from the posterior to the anterior muscle compartments in the active subjects. Exercise training has been shown to increase collateral blood flow to muscles, at least in rats (33). However, despite the fact that ankle dorsiflexion exercise nominally recruits only the anterior muscle, we cannot rule out the possibility that posterior muscle perfusion was increased to a greater extent in the active subjects. In any case, result illustrates both the unique ability of MR angiography to simultaneously measure flow in multiple vessels and a potential pitfall in the estimation of muscle perfusion from measurements of flow in major conduit vessels.
In summary, this study shows that the flow-related muscle BOLD transients observed after single, brief contractions are dramatically enhanced in physically active compared with sedentary subjects. Measurement of these transients by MRI or by other methods may provide a sensitive index of peripheral microvascular function and its response to therapy in patients with diabetes or other diseases that alter peripheral vascular health.
GRANTS
This study was supported by National Institutes of Health Grant AR-043903.
REFERENCES
- 1.Booth FW, Chakravarthy MV, Gordon SE, and Spangenburg EE. Waging war on physical inactivity: using modern molecular ammunition against an ancient enemy. J Appl Physiol 93: 3–30, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Brock RW, Tschakovsky ME, Shoemaker JK, Halliwill JR, Joyner MJ, and Hughson RL. Effects of acetylcholine and nitric oxide on forearm blood flow at rest and after a single muscle contraction. J Appl Physiol 8: 2249–2254, 1998. [DOI] [PubMed] [Google Scholar]
- 3.Corcondilas A, Koroxenidis GT, and Shepherd JT. Effect of a brief contraction of forearm muscles on forearm blood flow. J Appl Physiol 19: 142–146, 1964. [DOI] [PubMed] [Google Scholar]
- 4.Eskurza I, Monahan KD, Robinson JA, and Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human aging. J Physiol 556: 315–324, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisher MJ, Meyer RA, Adams GR, Foley JM, and Potchen EJ. Direct relationship between proton T2 and exercise intensity in skeletal muscle MR images. Invest Radiol 25: 480–485, 1990. [DOI] [PubMed] [Google Scholar]
- 6.Green DJ, Maiorana A, O’Driscoll G, and Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol 561: 1–25, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamann JJ, Buckwalter JB, and Clifford PS. Vasodilation is obligatory for contraction-induced hyperaermia in canine skeletal muscle. J Physiol 55: 1013–1020, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamdy O, Ledbury S, Mullooly C, Jarema C, Porter S, Ovalle K, Moussa A, Caselli A, Caballero AE, Economides PA, Veves A, and Horton ES. Lifestyle modification improves endothelial function in obese subjects with the insulin resistance syndrome. Diabetes Care 26: 2119–2125, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Hepple RT, Babits TL, Plyley MJ, and Goodman JM. Dissociation of peak vascular conductance and Vo2 max among highly trained athletes. J Appl Physiol 87: 1368–1372, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Hermansen L and Wachtlova M. Capillary density of skeletal muscle in well-trained and untrained men. J Appl Physiol 30: 860–863, 1971. [DOI] [PubMed] [Google Scholar]
- 11.Johansen L and Quistorff B. 31P-MRS characterization of sprint and endurance trained athletes. Int J Sports Med 24: 183–189, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Kingwell BA. Large artery stiffness: implications for exercise capacity and cardiovascular risk. Clin Exp Pharmacol Physiol 29: 214–217, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Laughlin MH. Physical activity in prevention and treatment of coronary disease: the battle line is in exercise vascular cell biology. Med Sci Sports Exerc 36: 352–362, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Laughlin MH, Korthuis RJ, Duncker DJ, and Bache RJ. Control of blood flow to cardiac and skeletal muscle during exercise. In: Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems. Bethesda, MD: Am Physiol Soc, 1996, sect. 12, chapt. 16, p. 705–769. [Google Scholar]
- 15.Lawrenson L, Hoff J, and Richardson RS. Aging attenutates vascular and metabolic plasticity but does not limit improvement in Vo2 max. Am J Physiol Heart Circ Physiol 286: H1565–H1572, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Lenasi H and Struel M. Effect of regular physical training on cutaneous microvascular reactivity. Med Sci Sports Exerc 36: 606–612, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Logothetis NK and Wandell BA. Interpreting the BOLD signal. Annu Rev Physiol 66: 735–769, 2004. [DOI] [PubMed] [Google Scholar]
- 18.McCully KK and Hamaoka T. Near-infrared spectroscopy: what can it tell us about oxygen saturation in skeletal muscle? Exerc Sport Sci Rev 28: 123–127, 2000. [PubMed] [Google Scholar]
- 19.Meyer RA, Foley JM, Harkema SJ, Sierra A, and Potchen EJ. Magnetic resonance measurement of blood flow in peripheral vessels after acute exercise. Magn Reson Imaging 11: 1085–1092, 1993. [DOI] [PubMed] [Google Scholar]
- 20.Meyer RA, Towse TF, Reid RW, Jayaraman RC, Wiseman RW, and McCully KK. BOLD MRI mapping of transient hyperemia in skeletal muscle after single contractions. NMR Biomed 17: 392–398, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Mohrman DE and Sparks HV. Role of potassium ions in the vascular response to a brief tetanus. Circ Res 35: 384–390, 1974. [DOI] [PubMed] [Google Scholar]
- 22.Noseworthy MD, Bulte DP, and Alfonsi J. BOLD magnetic resonance imaging of skeletal muscle. Semin Musculoskel Radiol 7: 307–315, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Ogawa S, Menon RS, Tank DW, Kim SG, Merkle H, Ellermann JM, and Ugurbil K. Functional brain mapping by blood oxygenation level-dependent contrast magnetic resonance imaging: a comparison of signal characteristics with a biophysical model. Biophys J 64: 803–812, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russ DW, Elliott MA, Vandenborne K, Walter GA, and Binder-MacLeod SA. Metabolic costs of isometric force generation and maintenance of human skeletal muscle. Am J Physiol Endocrinol Metab 282: E448–E457, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Sallis JF, Haskell WL, Wood PD, Fortmann SP, Rogers T, Blair SN, and Paffenbarger RS Jr. Physical activity assessment methodology in the Five-City Project. Am J Epidemiol 121: 91–106, 1985. [DOI] [PubMed] [Google Scholar]
- 26.Saunders NR, Dinenno FA, Pyke KE, Rogers AM, and Tschakovsky ME. Impact of combined NO and PG blockade on rapid vasodilation in a forearm mild-to-moderate exercise transition in humans. Am J Physiol Heart Circ Physiol 288: H214–H220, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Shoemaker JK, Phillips SM, Green HJ, and Hughson RL. Faster femoral artery blood velocity kinetics at the onset of exercise following short term training. Cardiovasc Res 31: 278−286, 1996. [PubMed] [Google Scholar]
- 28.Timperio A, Salmon J, Rosenberg M, and Bull FC. Do logbooks influence recall of physical activity in validation studies? Med Sci Sports Exerc 36: 1181–1186, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Tschakovsky ME and Hughson RL. Venous emptying mediates a transient vasodilation in the human forearm. Am J Physiol Heart Circ Physiol 278: H1007–H1014, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Tschakovsky ME, Rogers AM, Pyke KE, Saunders NR, Glenn N, Lee SJ, Weissgerber T, and Dwyer EM. Immediate exercise hyperemia in humans is contraction intensity dependent: evidence for rapid vasodilation. J Appl Physiol 96: 639–644, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Tschakovsky ME and Sheriff DD. Immediate exercise hyperemia: contributions of the muscle pump vs. rapid vasodilation. J Appl Physiol 97: 739–747, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Vermathen P, Boesch C, and Kreis R. Mapping fiber orientation in human muscle by proton MR spectroscopic imaging. Magn Reson Med 4: 424–432, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Yang HT, Laughlin MH, and Terjung RL. Prior exercise training increases collateral-dependent blood flow in rats after acute femoral artery occlusion. Am J Physiol Heart Circ Physiol 279: H1890–H1897, 2000. [DOI] [PubMed] [Google Scholar]


