Abstract
Background
Melioidosis-related mycotic aneurysm (MA) is rare but a potentially life-threatening disease with high morbidity and mortality rate.
Case presentation
We report a case series of mycotic aneurysm caused by Burkholderia pseudomallei and the subsequent outcomes. Here, we illustrate their clinical characteristics, laboratory results, radiological findings, mode of therapies and clinical outcomes.
Conclusion
Melioidosis-associated MA may manifest in an atypical presentation. Its outcome is often lethal if antimicrobial therapy and surgical intervention are not offered promptly.
Abbreviation: BA, Blood Agar; CTA, Aortographic computed tomography; EVAR, Endovascular repair; MA, Mycotic aneurysm; MALDI-TOF MS, Matrix-assisted laser desorption/ionisation mass spectrometry; MAC, MacConkey Agar; RRT, renal replacement therapy; OS, Open surgery; TEVAR, Thoracic endovascular aortic repair; TMP/SMX, Trimethoprim/Sulfamethoxazole; WCC, White blood cells, in cells/μL
Keywords: Melioidosis, Mycotic aneurysm, Outcome
Background
Melioidosis is a tropical infectious disease caused by Burkholderia pseudomallei, prevalent in South East Asia and Northern Australia [1]. The incidence rate of melioidosis in Sabah is 2.6 per 100,000 populations per year [1]. The mortality rate ranges from 37.9% to 61% with optimal therapy and indeed approaches 100% in septicaemic forms if untreated. It was reported that up to 75% of patients with melioidosis had type 2 diabetic mellitus [1].
In contrast to a true aneurysm defined as a segmental dilation of the arterial wall, a pseudoaneurysm results from a mechanical tear in the vessel wall with subsequent perivascular haematoma formation. The haematogenous seeding of damaged arteriosclerotic vessels leads to the development of MA. MA caused by B.pseudomallei was first described in Taiwan, in 1994 [2]. Anunnatsiri et al. reported B. pseudomallei as the common causative microorganism of MA in Thailand (17 out of 40 cases, 42.5%) [3]. Our literature review showed the incidence rate of melioidosis-related MAs varied in Asia-Pacific regions, for instances, 0.4% (2/540) in Australia [4], 5.0%(8/159) in China [5], 7.5% (5/67) in Malaysia [6]. With its low prevalence, a high index of suspicion is required to diagnose the melioidosis-related MAs.
Here, we present three cases with melioidosis-related MA in Queen Elizabeth Hospital II, Sabah, Malaysia.
Case presentation
Case 1
A 55-year-old lady with underlying type II diabetes mellitus, presented with abdominal discomfort and fever for a week. On examination, she appeared septic with a pulsatile mass in her left abdomen. The blood investigations showed anaemia (Hb 7.5 g/L) with leucocytosis (WCC 15 × 103 cells/L) and elevated CRP (191 mg/L). She proceeded with the aortographic computer tomography (CTA) (Fig. 1A, B). The blood culture processed in the laboratory demonstrated the grey, translucent colonies on BA and light pink colonies on MAC . The detected microorganism was an aerobic, motile, oxidase-positive, indole-negative, Gram-negative rod-shaped bacillus. As B. pseudomallei was suspected, the subsequent laboratory works were immediately handled in Class II Biosafety Cabinet. The identification of B.pseudomallei was confirmed by using the matrix-assisted laser desorption/ionisation mass spectrometry (MALDI-TOF MS). The diagnosis was confirmed to be melioidosis-related mycotic aneurysm. She was commenced with intravenous (IV) ceftazidime. She underwent the left external iliac artery stenting and Endovascular Aortic Repair (EVAR) with bilateral renal chimney graft. A postoperative CTA was performed (Fig. 1C). During the intensive-phased therapy, she received six weeks of intravenous ceftazidime, followed by life-long doxycycline for the eradication-phased treatment as she was intolerant to trimethoprim/sulfamethoxazole (TMP/SMX). She was discharged well after three-month hospitalisation. Unfortunately, two months after discharge, she presented again with relapsed melioidosis with dissemination (bacteraemia, gallbladder empyema and pneumonia). The clinical and radiological examinations did not reveal any new endoleaks. She succumbed to death within two days of the hospitalisation from the overwhelming sepsis.
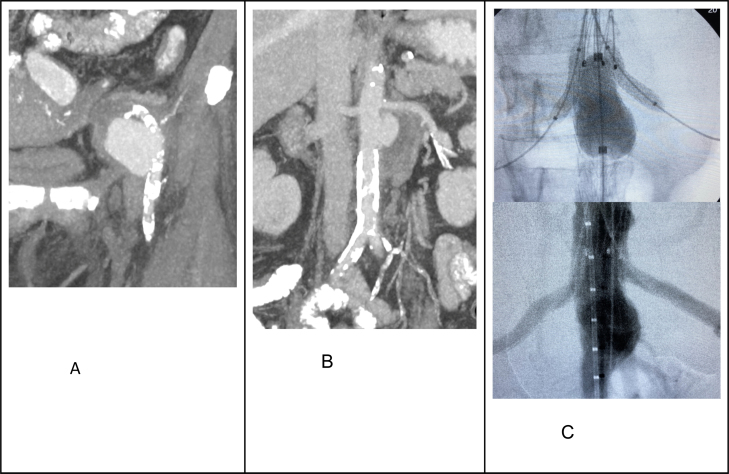
Fig. 1.
Showed the radiological images for the first case. Fig. A and B illustrated the CTA of a ruptured MA at the left external iliac artery and a leaking MA at the juxta-renal aorta. Fig. C showed the aortic stenting with bilateral renal chimney and a completion of angiogram showing an exclusion of the aneurysm.
Case 2
A 57-year-old man with a background history of type II diabetes mellitus, chronic renal disease (CKD) stage I-II and ischaemic heart disease, presented with left abdominal pain and constitutional symptoms for two months. He had a past history of disseminated melioidosis involving the lungs, liver and scalp. After eight weeks of intensive-phased therapy, he was discharged well with lifelong TMP/SMX for eradication. On examination, a pulsatile mass was felt in the periumbilical area. His blood test reviewed elevated septic markers (WCC 27 × 103 cells/μL and CRP 250 mg/L). The CTA was performed (Fig. 2A, B). The result of blood culture later depicted a growth of B. pseudomallei. Intravenous meropenem 2 gm twice a day (renal dose adjusted) was administered for eight weeks. This was followed by a completion of the endovascular repair of the remaining abdominal and thoracic aorta with a 4-vessels fenestration custom-made device (Cook Medical). He was discharged well with doxycycline. The TMP/SMX was not chosen in the background of worsening renal impairment requiring RRT in the near future. A surveillance CT scan was performed after a month of the procedure (Fig. 2). However, a month later, he presented with fever and chills and blood culture grew B. pseudomallei again. He was treated with another month of intravenous meropenem. He responded well with subsequent negative culture and discharged well with regular monitoring of renal function while on TMP/SMX.
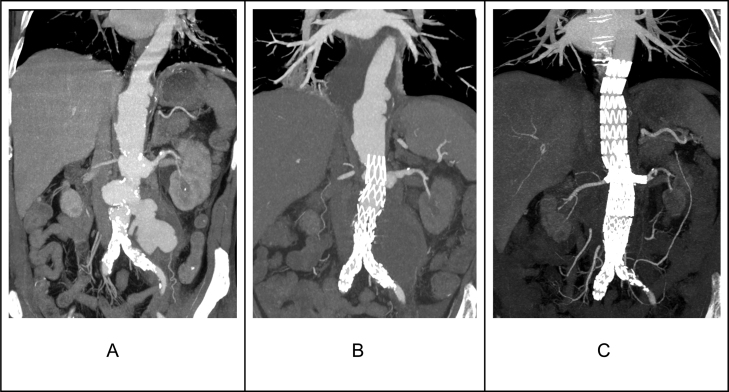
Fig. 2.
Demonstrated the CTA images for the second case. Fig. A revealed multiple MAs involving the thoracic and abdominal aorta. The MA involving the abdominal aorta had ruptured with a contained leak. Fig. B illustrates CTA performed after the initial EVAR to exclude an abdominal MA with a contained leak. Fig. C revealed a total exclusion of the thoracic and abdominal MAs with good perfusion to the targeted visceral vessels.
Case 3
A 57-year-old man presented with fever and constitutional symptoms for two weeks. Clinical examination was unremarkable. The routine blood test showed leucocytosis (WCC 15 × 103 cells/μL) and elevated CRP (250 mg/L). The chest x-ray (Fig. 3A) was suspicious of aortic abnormality in the thorax. Thus, he proceeded with the CTA (Fig. 3B). He underwent an urgent procedure of aortic arch debranching with carotid-carotid bypass, left carotid-left axillary bypass with ligation of proximal left common carotid artery and embolisation of the left subclavian artery proximal to the left vertebral artery with Amplatzer vascular plug (Abbott Vascular), followed by the thoracic endovascular aortic repair (TEVAR) with Zone 0 landing and chimney of the brachiocephalic trunk. The postoperative CTA was performed (Fig. 3C). His blood culture later grew B. pseudomallei. IV ceftazidime 2 gm 6 hourly was administered for six weeks. Due to the intolerance to TMP/SMX, he was discharged well with the advice of continuing lifelong doxycycline.
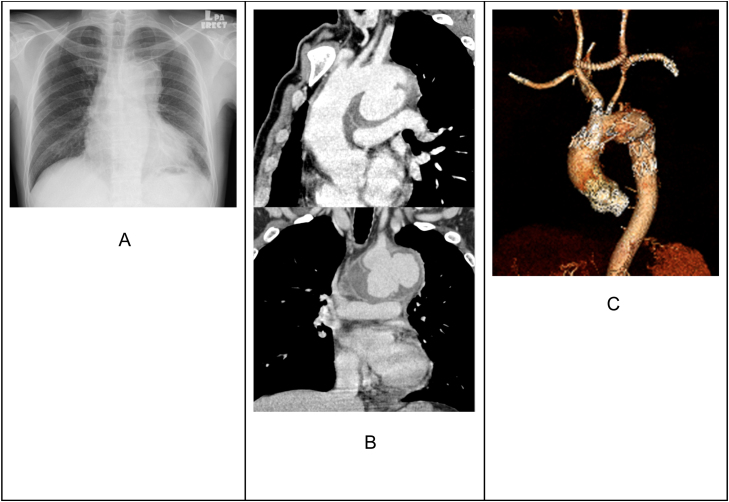
Fig. 3.
Belonged to the third case. Fig. A depicted a widened mediastinum. Fig. B showed a lobulated saccular MA arising from the aortic arch, surrounded by an enhancing walled mural thrombus. In Fig. C, CTA revealed the MA exclusion without an endoleak and the perfusion maintenance to the supra-aortic vessels.
Discussion and conclusion
We presented three cases of melioidosis-related MAs, proven by the positive culture and radiological findings. Two of 3 cases had diabetes mellitus and the relapse of melioidosis. We reported the high incidence of mortality and morbidity and prolonged hospitalization in these cases.
The clinical presentations, in our cases, are atypical and diverse. Paul et al. and colleagues described that pyrexia was the common clinical feature, followed by abdominal pain [6]. Obtaining B.pseudomallei from blood or tissue culture is recognised as the gold standard for melioidosis diagnosis. Classically, B.pseudomallei is able to grow on the BA and MAC, with the appearance of grey-translucent colonies. Rapid identification can be identified via MALDI-TOF MS. The implementation of biosafety practice is mandatory in dealing with any specimens suspected of containing B.pseudomallei to reduce laboratory exposure. The multidetector CTA is utilized to evaluate MAs. Alternatively the contrast-enhanced magnetic resonance (MR) angiography can be performed but it is less favourable in our local settings due to the limited resources. The typical radiological findings are described as the multi-lobular or saccular aneurysms with the periaortic soft tissue mass, commonly involving the aorta, renal or infrarenal and external iliac arteries[7]. The confirmed blood culture and radiological abnormality of arteries are consistent with a diagnosis of mycotic aneurysm caused by melioidosis.
A combination of surgery and long-termed antimicrobial therapy is the definitive treatment in melioidosis-related MA. Its goal is to achieve a complete eradication of infection and establish a smooth arterial flow. The surgical procedures vary, depending on the MA’s anatomical sites, severity of infection, and availability of facilities. Nowadays, the aortic stenting (EVAR or TEVAR) is considered as the gold standard of MA’s surgical treatment, compared to OS. The perioperative outcomes of aortic stenting following ruptured MAs are presumably better than OS [7], [8]. Intravenous ceftazidime, alternatively carbapenem for 6 weeks is recommended as the first-line therapy, followed by oral (TMP-SMX) or doxycycline. Compared with the TMP-SMX-based eradication therapy, non-TMP-SMX-based regimen (namely Doxycycline or Amoxicillin/Clavulanic acid) is inferior and highly associated with a higher rate of relapse [9]. Thus, TMP-SMX is preferable as the mainstay therapy during eradication phase, unless contraindicated.
Despite correct diagnosis and management, the morbidity and mortality remain high. Paul et al. reported a mortality rate of 20% [6]. Experts suggested that with the presence of risk factors (including endemic areas, diabetes mellitus, occupational exposure to contaminated water and soil), an early introduction of empirical antimicrobials is mandatory in any suspected cases of MAs [10]. Prompt diagnosis and management can prevent any fatal complications, including the rupture or embolization of MAs. A delayed diagnosis may result in a poorer outcome.
Melioidosis-related MA is associated with an atypical presentation and its outcomes are lethal if both medical and surgical therapies are delayed. Awareness among clinicians about melioidosis is of paramount importance.
Ethical approval
This study was registered with the National Medical Research Register, Ministry of Health Malaysia.
Consent
Written informed consents were obtained from the guardians for publication of this case series.
Funding
Self-funding.
CRediT authorship contribution statement
All authors equally contributed to the writings and patient managements. Both authors prepared, read and approved the final manuscript.
Competing interests
None to declare.
Acknowledgements
The authors would like to thank the Director General of Health Malaysia for the permission to publish this paper. We would like to express special gratitude to the Department of Pathology and Department of Radiology in Queen Elizabeth Hospital II, Sabah, Malaysia.
Contributor Information
Tan Kok Tong, Email: tankoktong85@gmail.com.
Giri Shan, Email: gsrajahram@gmail.com.
Feona Joseph Sibangun, Email: feonajoseph@gmail.com.
Benjamin Leong Dak Keung, Email: bleongdk@yahoo.com.
References
- 1.Maria Suleiman D.F.K., Polin Ponolin, Gindono Jasni. Public Health Division, Sabah State Health Department; Sabah Malaysia: 2014. Guideline for Clinical and Public Health Management of Melioidosis in Sabah. [Google Scholar]
- 2.Lee S.S., Liu Y.C., Wang J.H., Wann S.R. Mycotic aneurysm due to Burkholderia pseudomallei. Clin Infect Dis. 1998;26(4):1013–1014. doi: 10.1086/517640. [DOI] [PubMed] [Google Scholar]
- 3.Anunnatsiri S., Chetchotisakd P., Kularbkaew C. Mycotic aneurysm in Northeast Thailand: the importance of Burkholderia pseudomallei as a causative pathogen. Clin Infect Dis. 2008;47(11):1436–1439. doi: 10.1086/592975. [DOI] [PubMed] [Google Scholar]
- 4.Currie B.J., Ward L., Cheng A.C. The epidemiology and clinical spectrum of melioidosis: 540 cases from the 20 year Darwin prospective study. PLoS Negl Trop Dis. 2010;4(11):900. doi: 10.1371/journal.pntd.0000900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu H., Wang X., Zhou X., Wu Z., Wang Y., Pan M. Mycotic aneurysm secondary to melioidosis in China: a series of eight cases and a review of literature. PLOS Negl Trop Dis. 2020;14(8) doi: 10.1371/journal.pntd.0008525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kingsley P.V., Leader M., Nagodawithana N.S., Tipre M., Sathiakumar N. Melioidosis in Malaysia: a review of case reports. PLOS Negl Trop Dis. 2016;10(12) doi: 10.1371/journal.pntd.0005182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sörelius K., Wanhainen A., Furebring M., Björck M., Gillgren P., Mani K. Nationwide study of the treatment of mycotic abdominal aortic aneurysms comparing open and endovascular repair. Circulation. 2016;134(23):1822–1832. doi: 10.1161/CIRCULATIONAHA.116.024021. [DOI] [PubMed] [Google Scholar]
- 8.Li Y., Li Z., Wang S., Chang G., Wu R., Hu Z. Endovascular versus open surgery repair of ruptured abdominal aortic aneurysms in hemodynamically unstable patients: literature review and meta-analysis. Ann Vasc Surg. 2016;32:135–144. doi: 10.1016/j.avsg.2015.09.025. [DOI] [PubMed] [Google Scholar]
- 9.Chetchotisakd P., Chierakul W., Chaowagul W., Anunnatsiri S., Phimda K., Mootsikapun P. Trimethoprim-sulfamethoxazole versus trimethoprim-sulfamethoxazole plus doxycycline as oral eradicative treatment for melioidosis (MERTH): a multicentre, double-blind, non-inferiority, randomised controlled trial. Lancet. 2014;383(9919):807–814. doi: 10.1016/S0140-6736(13)61951-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sidrim J.J., Rocha M.F., Bandeira T.J., Cordeiro R.A., Carvalho B.M., Grangeiro T.B. Mycotic aneurysm caused by Burkholderia pseudomallei: report of a Brazilian strain genetically related to Thai strains. Clin Microbiol Infect. 2011;17(5):719–721. doi: 10.1111/j.1469-0691.2010.03405.x. [DOI] [PubMed] [Google Scholar]