Abstract
Temperature stress (TS) is a significant issue in poultry production, which has implications for animal health and welfare, productivity, and industry profitability. Temperature stress, including both hot (heat stress) and cold conditions (cold stress), is associated with increased incidence of meat quality defects such as pale, soft, and exudative (PSE) and dark, firm, and dry (DFD) meat costing poultry industries millions of dollars annually. A meta-analysis was conducted to determine the effect of ambient TS on meat quality parameters of poultry. Forty-eight publications which met specific criteria for inclusion were identified through a systematic literature review. Temperature stress was defined by extracting 2 descriptors for each treatment mean from the chosen studies: (1) temperature imposed for the experimental treatments (°C) and duration of temperature exposure. Treatment duration was categorized for analysis into acute (≤24 h) or chronic (>24 h) treatments. Meat quality parameters considered were color (L*-a*-b* scheme), pH (initial and ultimate), drip loss, cooking loss, and shear force. Linear mixed model analysis, including study as a random effect, was used to determine the effect of treatment temperature and duration on meat quality. Model evaluation was conducted by performing a k-fold cross-validation to estimate test error, and via assessment of the root mean square prediction error (RMSPE), and concordance correlation coefficient (CCC). Across both acute and chronic durations, treatment temperature was found to have a significant effect on all studied meat quality parameters. As treatment temperature increased, meat demonstrated characteristics of PSE meat and, as temperature decreased, meat demonstrated characteristics of DFD meat. The interaction between treatment temperature and duration was significant for most traits, however, the relative impact of treatment duration on the studied traits was inconsistent. Acute TS had a larger effect than chronic TS on ultimate pH, and chronic stress had a more considerable impact on color traits (L* and a*). This meta-analysis quantifies the effect of ambient TS on poultry meat quality. However, quantitative effects were generally small, and therefore may or may not be of practical significance from a processing perspective.
Key words: color, cooking loss, drip loss, pH, shear force
INTRODUCTION
When ambient temperature deviates from an animal's thermoneutral zone, it forces the animal to employ additional heat-saving or heat-dissipating measures (Silva, 2006). Exposure to temperature stress (TS) is an important issue in poultry production since poultry are inefficient at regulating body temperature. In particular, poultry are at high risk in hot environments because they do not possess functional sweat glands, have a high core body temperature, and a rapid metabolism (Jahejo et al., 2016). However, both heat and cold can negatively impact poultry growth and production performance (Zhang et al., 2011; Lara and Rostagno, 2013; Zhao et al., 2013; Habibian et al., 2014; Tawfeek et al., 2014).
Heat and cold stress have also been implicated in the development of meat quality defects in poultry, such as pale, soft, and exudative (PSE), and dark, firm, and dry (DFD) meat (Barbut et al., 2005). In susceptible birds, heat stress can accelerate the postmortem (PM) degradation of muscle glycogen to lactic acid, which causes muscle pH to decrease rapidly after slaughter (Owens et al., 2009; Barbut, 2015). This rapid drop in pH, combined with the warm muscle, denatures the muscle proteins, causing the meat to have a low water holding capacity, pale color, and exude moisture (Pietrzak et al., 1997; King and Whyte, 2006; Carvalho et al., 2014). The rise in consumer demand for deboned smaller cuts and further processed products has increased focus on the presentation of skinless portions. This means meat color and water-holding capacity (WHC), which are highly related to consumer acceptance, are more important (Min and Ahn, 2012; Barbut, 2015). As such, PSE meat has been estimated to cost both the US turkey and broiler industry $200 million annually (Lubritz, 2007; Owens et al., 2009).
While heat stress is more likely to cause PSE meat, cold stress is implicated more often in DFD meat development. Several studies have found correlations between cold exposure during rearing and transportation and the incidence of DFD meat in poultry (Froning et al., 1978; Babji et al., 1982; Holm and Fletcher, 1997; Bianchi et al., 2006). The increased metabolic demand to maintain core body temperature during cold conditions results in the use of muscle glycogen as an energy source (Lee et al., 1976; Haman et al., 2002,2005; Dadgar et al., 2011, 2012a). The depletion of muscle glycogen, prior to slaughter, decreases the PM potential lactate formation in the meat and results in higher pH of the muscle (Berri et al., 2005; Dadgar et al., 2011). Eventually, this results in a darker product that is unappealing from a consumer perspective and as such, can result in economic loss to the industry.
Poultry production is year-round, occurring during many types of inclement weather, so TS can be a concern during all phases of production. Typically, studies use chronic exposure (>24 h) to simulate the long-term or seasonal effects of TS during production, whereas acute exposure (<24 h) is typically used to simulate short-term or daily fluctuations in ambient temperature soon before slaughter (i.e., during transit). Both chronic and acute TS affect poultry meat quality parameters; however, acute stress, as opposed to chronic, is typically implicated in the development of PSE meat since the exposure to extreme temperatures soon before slaughter has a more substantial effect on meat quality (Barbut et al., 2008; Gonzalez-Rivas et al., 2020). However, this effect may be related to genetic susceptibility in certain birds, meaning that not all animals are prone to the malignant hyperthermia associated with heat stress-induced PSE meat (Barbut, 2015). Interestingly, studies of swine and ruminants report that chronic stress can lead to the development of DFD meat in these species (Gregory, 2010; Adzitey and Nurul, 2011). In poultry, the relationship between acute and chronic exposure is not clear. Studies of TS in poultry use widely different stress durations leading to a substantial source of variation in the results. Few studies include both short-term and long-term stress durations within the same experiment, so it is difficult to elucidate which treatment has a larger effect on meat quality.
There have been several reviews detailing the effect of TS on meat quality in poultry, ruminants, and swine (Ali et al., 2008; Xing et al., 2019; Gonzalez-Rivas et al., 2020) as well as describing meat quality defects in poultry (Lesiow and Kijowski, 2003; Barbut, 2009; Owens et al., 2009; Petracci et al., 2009; Mir et al., 2017). However, to our knowledge, a quantitative meta-analysis focusing on the effect of TS on poultry meat quality has not yet been conducted. Meta-analyses are useful for accounting for variability between studies and can overcome the limitations of small sample sizes in individual studies. Meta-analysis can also overcome the subjective nature of qualitative reviews and help interpret conflicting results in the literature.
The objective of the present study was therefore to synthesize the results of the numerous empirical studies in this area and quantify the effect of TS on various parameters of poultry meat quality. An additional objective was to investigate whether the studied meat quality traits are affected differently by acute and chronic exposure to TS.
MATERIALS AND METHODS
Dataset Development
A systematic literature search was conducted in February 2020 using the Web of Science database and hand-searching the Poultry Science journal. No temporal or language restrictions were applied to the searches. Keyword combinations were used to identify papers that included poultry, stress, and meat quality which formed the initial dataset for this study (N = 1389 studies). Exclusion criteria were then applied to this dataset to determine the studies that will be included in the meta-analysis (Figure 1).
Figure 1.
Literature funnel (Preferred reporting items for systematic reviews and meta-analyses (PRISMA) diagram adapted from (Moher et al., 2009).
Papers were excluded from further analysis if they did not include poultry, stress, or meat quality in the title, abstract, or keywords or were not primary research articles (i.e., reviews or conference abstracts). Poultry species considered for this meta-analysis were broiler chickens and turkeys.
Full-length papers were reviewed to determine if they included the effect of TS (i.e., heat and cold stress) on typical meat quality parameters. All studies included in the analysis were required to have TS as an experimental treatment. This means that the objective of the studies included needed to focus on the impact of temperature stress on meat quality outcomes, and comparable to a control (nonstress conditions). Meat quality parameters of interest included color traits of lightness (L*), redness (a*), yellowness (b*), pH traits (initial, ultimate), drip loss (%), cooking loss (%), and shear force (N). Color was recorded in all selected studies based on the Commission Internationale de l'Eclairage dimensions (CIE, 1976). Meat color was recorded between 0.25 and 48 h PM in the final database, with the mean recording time of 24 h PM. Initial pH was defined as the first pH measurement taken after slaughter. In the final database, initial pH measurements were taken from 0 to 240 min PM. The average measurement time was approximately 45 min PM. Ultimate pH was defined as the pH measurement recorded at 24 h PM. Of the initial 1,389 search results, 48 studies met the criteria for inclusion in the meta-analysis and are summarized in Table 1.
Table 1.
Summary of studies used to assess the effect of TS on meat quality of poultry.
| Reference | Country | Species | Temp. type1 | Stress type2 | Control temperature3 | Treatment temperature3 |
|---|---|---|---|---|---|---|
| Aksit et al. (2006) | Turkey | broiler | CON, HS | Chronic* | 22 | 31 |
| Babji et al. (1982) | USA | turkey | CON, HS, CS | Acute | 21 | 5 & 38 |
| Bautista et al. (2016) | Mexico | broiler | CON, HS | Acute | 24 | 40 |
| Bianchi et al. (2006) | Italy | broiler | CON, HS, CS | Acute | 15 | 12 & 18 |
| Brossi et al. (2018) | Brazil | broiler | CON, HS | Acute | 22 | 35 |
| Cheng et al. (2018) | China | broiler | CON, HS | Chronic | 20 | 32.5 |
| Chiang et al. (2008) | USA | turkey | CON, HS | Chronic | 23 | 35 |
| Cramer et al. (2018) | USA | broiler | CON, HS | Chronic | 22 | 32 |
| Dadgar et al. (2011) | Canada | broiler | CON, CS | Acute | 21 | -8.5 |
| Dadgar et al. (2012b) | Canada | broiler | CON, CS | Acute | 22 | -8.25 |
| Dai et al. (2009) | China | broiler | CON, HS | Chronic | 23 | 28 |
| Debut et al. (2003) | France | broiler | CON, HS | Acute | 21 | 35 |
| Feng et al. (2008) | China | broiler | CON, HS | Chronic | 22 | 31 |
| Fernandes et al. (2016) | Brazil | broiler | HS | Acute | NA | 33 |
| Ferreira et al. (2015) | Brazil | broiler | CON, HS, CS | Chronic | 27.5 | 23 & 32 |
| Froning et al. (1978) | USA | turkey | HS, CS | Acute | NA | 4 & 42 |
| Goo et al. (2019) | South Korea | broiler | CON, HS | Chronic | 20 | 27.8 |
| Gu et al. (2008) | China | broiler | CON, HS, CS | Chronic | 22 | 15 & 33 |
| Hadad et al. (2014) | Israel | broiler | CON, HS | Chronic | 26 | 32 |
| Hashizawa et al. (2013) | Japan | broiler | CON, HS | Chronic | 24 | 30 |
| Henrikson et al. (2018) | Canada | turkey | CON, CS | Acute | 20 | -18 |
| Holm and Fletcher (1997) | Sweden | broiler | CON, HS, CS | Acute | 18 | 7 & 29 |
| Kanani et al. (2017) | Iran | broiler | CON, HS | Chronic | 22 | 32 |
| Liu et al. (2019) | China | broiler | CON, HS | Chronic | 26 | 32 |
| Lu et al. (2007) | China | broiler | CON, HS | Chronic | 21 | 34 |
| Lu et al. (2017) | China | broiler | CON, HS | Chronic | 22 | 32 |
| Mazur-Kuśnirek et al. (2019) | Poland | broiler | CON, HS | Chronic | 20.5 | 34 |
| N'dri et al. (2007) | France | broiler | CON, HS | Chronic | 20 | 30 |
| Owens et al. (2000b) | USA | turkey | CON, HS | Chronic | 17 | 34 |
| Petracci et al. (2001) | Italy | broiler | CON, HS, CS | Acute | 29.5 | 24 & 34 |
| Sandercock et al. (2001) | UK | broiler | CON, HS | Acute | 21 | 32 |
| Schneider et al. (2012) | Canada | broiler | CON, HS, CS | Acute | 21 | 7 & 30 |
| Shao et al. (2019) | China | broiler | CON, HS | Chronic | 23 | 35 |
| Sifa et al. (2018) | China | broiler | CON, HS | Acute | 23 | 34 |
| Skomorucha et al. (2010) | Poland | broiler | CON, HS | Chronic | 21 | 35 |
| Tang et al. (2013) | China | broiler | CON, HS | Acute | 22 | 37 |
| Tavaniello et al. (2020) | Italy | broiler | HS | Chronic | NA | 30 |
| Toplu et al. (2014) | Turkey | broiler | CON, HS | Chronic* | 24 | 35 |
| Vermette et al. (2017) | Canada | turkey | CON, HS | Acute | 20 | 35 |
| Wan et al. (2018) | China | broiler | CON, HS | Chronic | 22 | 34 |
| Wang et al. (2017) | China | broiler | CON, HS | Acute | 25 | 36 |
| Wen et al. (2019) | China | broiler | CON, HS | Chronic | 22 | 34 |
| Zahoor et al. (2016) | Pakistan | broiler | CON, CS | Chronic | 21 | 17 |
| Zeferino et al. (2016) | Brazil | broiler | CON, HS | Chronic | 24 | 32 |
| Zhang et al. (2012) | China | broiler | CON, HS | Chronic* | 23 | 35 |
| Zhang et al. (2017) | China | broiler | CON, HS | Chronic | 22 | 33 |
| Zhang et al. (2019) | China | broiler | CON, HS | Acute | 25 | 38 |
| Zhao et al. (2019) | China | broiler | CON, HS | Chronic | 22 | 34 |
CON, control treatment; CS, cold stress treatment; HS, heat stress treatment.
Acute = stress treatments applied for ≤24 h, Chronic = stress treatments applied for >24 h.
Average control or treatment temp. in°C, NA = control temp. not specified.
Study contains cyclic stress treatments which have been reclassified as chronic.
Additional parameters extracted included species (broiler or turkey), experimental treatment temperature, experimental treatment duration, slaughter age, and muscle (breast vs. thigh). Typically, each study has multiple experimental treatments (e.g., a control treatment and a heat stress treatment) which were extracted separately from the study with the corresponding treatment means for the meat quality traits. Each treatment represented a separate row of data in the developed database. To collapse variation in study design for analysis, the duration of TS was categorized for each treatment mean as either control (no TS applied), acute (≤24 h) or chronic (>24 h). Treatment means for cyclic TS (stress applied for <24 h for more than 2 consecutive days) were reclassified as chronic stress.
Descriptive statistics (mean, median, min, max, and standard deviation) for the dependent and independent variables in the final database (before model development) are shown in Table 2.
Table 2.
Descriptive statistics for X variables and meat quality traits included in the meta-analysis.
| Variable | Mean | SD | Min | Max | Median | N |
|---|---|---|---|---|---|---|
| Lightness (L*) | 51.19 | 5.947 | 36.600 | 71.100 | 51.270 | 195 |
| Yellowness (b*) | 7.02 | 5.029 | −3.800 | 18.480 | 6.320 | 167 |
| Redness (a*) | 6.82 | 5.499 | 0.002 | 27.400 | 4.800 | 169 |
| Initial pH | 6.18 | 0.284 | 5.570 | 6.840 | 6.170 | 129 |
| Ultimate pH | 6.28 | 4.292 | 5.300 | 7.070 | 5.920 | 161 |
| Cooking loss (%) | 21.78 | 9.657 | 3.700 | 49.400 | 49.400 | 108 |
| Drip loss (%) | 1.96 | 1.442 | 0.320 | 5.720 | 1.560 | 77 |
| Shear force (N) | 31.12 | 16.551 | 11.470 | 77.960 | 27.670 | 90 |
Model Development
Prior to full model development, a correlation analysis was performed on the dependent variables using PROC CORR in SAS to identify key potential drivers of meat quality outcomes, as well as redundant and collinear variables within the database (Table 3). Due to the high number of variable correlations, r > 0.4 was used as an arbitrary cut-off value for discussion. All correlations, regardless of correlation coefficient, are reported in Table 3.
Table 3.
Pearson correlation coefficients between meat quality outcome variables in the database.
| Trait | L* | a* | b* | pH initial | pH ultimate | Drip loss | Cooking loss | Shear force | Temperature3 | Age4 |
|---|---|---|---|---|---|---|---|---|---|---|
| Lightness (L*) | 1 | |||||||||
| Redness (a*) | −0.2101 | 1 | ||||||||
| Yellowness (b*) | 0.2541 | 0.0297 | 1 | |||||||
| Initial pH | 0.0288 | −0.0271 | −0.3251 | 1 | ||||||
| Ultimate pH | −0.4822 | 0.0264 | −0.2011 | 0.2921 | 1 | |||||
| Drip loss (%) | 0.4521 | −0.111 | 0.2921 | 0.173 | −0.4391 | 1 | ||||
| Cooking loss (%) | 0.2411 | −0.2851 | −0.105 | −0.5592 | −0.3972 | 0.6062 | 1 | |||
| Shear force (N) | −0.107 | −0.0343 | −0.0343 | 0.0330 | −0.0824 | 0.4401 | 0.2991 | 1 | ||
| Temperature | 0.3852 | 0.0589 | 0.4592 | −0.3412 | −0.4942 | 0.4151 | 0.4412 | 0.159 | 1 | |
| Age | 0.123 | 0.154 | −0.2751 | −0.2761 | −0.2021 | −0.2861 | 0.2411 | 0.4842 | 0.0842 | 1 |
P < 0.05.
P < 0.0001.
Average treatment temperature (°C).
Age in days when birds were slaughtered.
To model the effect of TS on meat quality, a linear mixed model analysis was performed with study modeled as a random effect (St-Pierre, 2001) and treatment temperature as the main fixed effect. The general model used was:
| (1) |
where i = 1, … , 48 studies, j = 1, … , ni treatment means, = treatment temperature in Celcius, = slope term for temperature, =treatment duration (control, acute, or chronic), = interaction between temperature and duration, = additional variables examined (species, slaughter age, muscle type, measurements time), and = residual error of the model. The fixed effect component of the model was expanded as additional variables were considered. The Y variables considered for analysis were L*, a*, b*, initial pH, ultimate pH, drip loss, cooking loss, and shear force.
Models were developed and tested using PROC MIXED in SAS (SAS version 9.4, SAS/STAT, SAS Institute Inc, Cary, NC). Fixed effects were tested against a P-value of 0.05 for inclusion in the model. A backward stepping modeling approach was taken by first creating a ‘full’ model (Equation 1), with temperature, species, slaughter age, muscle type, measurement time, and stress duration as x-variables (depending on the trait), and then sequentially removing the least significant term at each step and evaluating the change in the significance in the remaining variables. At each step, the residuals were assessed for normality, and the Akiake Information Criterion (AICc) value was evaluated for model improvement/worsening. Since color and pH measurements were not always taken at the same time PM in the different studies, the ‘measurement time’ variable was included in the analysis for these traits to account for some of the resulting variation.
To account for the heterogeneity in sample size and error between treatment means across experiments, the WEIGHT statement in PROC MIXED was used to weight the observations by the inverse of their variance. To do so, the inverse of the squared standard error of the mean (SEM) of each observation was determined. This value was then divided by the average squared SEM inverse of all observations, which centers the value around 1, and is the metric used to weight observations (St-Pierre, 2001).
To identify individual observations with a significant influence on the parameter estimates (outliers), the Cook's distance statistic was computed for each observation for each of the trait models in PROC MIXED. Cook's distance represents the change in fitted response values of the regression after the removal of an observation (Cook, 1977). Influential observations with a Cook's distance >4/n, where n represents the total number of treatment mean observations, were removed from the data set in a step-wise sequential manner.
Model Evaluation
After the final models were developed, a k-fold cross-validation approach was taken to determine the best model for each meat quality trait based on the calculated test error. The developmental dataset was divided into 5 folds, and each model was refitted on 4of the 5 folds in turn, keeping one fold for evaluation. Test error (CVK, Equation 2), root mean square prediction error (the root of the MSPE, Equation 3), and concordance correlation coefficient (CCC, Equations 7) were then calculated for the refitted model on each of the remaining folds (not used for model development), and can be summarized as:
| (2) |
where K represents each fold (1-5), = number of observations in the kth fold, n = the total number of observations, and = mean squared error of the kth fold.
| (3) |
Where n = total number of treatment mean observations, YPred = predicted value, and YObs = observed value. Root mean square prediction error (RMSPE) was calculated by taking the square root of the MSPE and is expressed as a percentage of the observed mean. RMSPE provides an estimate of the overall prediction error for the equation.
The RMSPE was decomposed into error due to bias (ECT, Equation 4), error due to deviation of the regression slope from unity (ER, Equation 5), and error due to random disturbance (ED, Equation 6) (Bibby and Toutenburg, 1977).
| (4) |
| (5) |
| (6) |
Where = observed mean, = predicted mean, and = observed and predicted standard deviations, and R = Pearson correlation coefficient.
The CCC, or reproducibility index, simultaneously assess a model's precision and accuracy (Lin, 1989; Tedeschi, 2006). The CCC ranges from −1 to 1, with −1 indicating that the observed and predicted values are perfectly unrelated, 0 indicating no relationship, and 1 indicating they are perfectly related (Lin, 1989). The CCC was calculated as:
| (7) |
where R is the Pearson correlation coefficient which indicates the equation's precision, and Cb is a bias correction factor which indicates the equation's accuracy (Equation 8). Cb ranges from 0 to 1 where 1 indicates there is no deviation of the regression line from the line of unity (Tedeschi, 2006). Cb is calculated as:
| (8) |
where:
| (9) |
| (10) |
and where v is an indicator of change in standard deviation, or scale shift, between predicted and observed values. The parameter µ is an indicator of location shift whereby a positive µ value indicates equation under-prediction and a negative µ value indicates over-prediction.
The model which yielded the lowest test error (CVK) for each trait was chosen as the best developed model. Models reported are then those developed on the full dataset. The MSPE and CCC represent the average ± SEM values across the 5 k-folds.
To test for slope and mean bias, an analysis of the residuals was performed using PROC REG. The conditional residuals were regressed on the predicted values to obtain slope/intercept parameter estimates which were tested for significance against zero. A visual assessment of predicted values vs. observed and predicted values vs. residuals was also performed for color traits, pH traits, and cooking loss, drip loss, and shear force traits using PROC SGPLOT.
Subanalysis: Heat vs. Cold Stress
For the general equations, heat and cold stress studies were grouped together, however, a subanalysis was also conducted to compare the effect of heat stress versus cold stress on meat quality attributes in poultry. This attribute of the TS variable was not included in the main model development section due to the imbalanced nature of the database with respect to the number of heat stress (N = 131) and cold stress (N = 36) treatment means. Within the subanalysis, treatment means from each study were classified as either control (standard commercial temperature, no TS applied), heat stress, or cold stress. Stress type was included as a categorical fixed effect in the best prediction model for each meat quality trait in place of the temperature variable. Other significant fixed effects included in the best prediction model for each trait (i.e., muscle type or slaughter age) were kept in the model. Models were run in PROC MIXED of SAS to derive least-squared means for the heat stress and cold stress treatments for each meat quality trait. P-values from pairwise comparisons were adjusted using the Tukey HSD method to account for the effect of multiple comparisons.
RESULTS
Preliminary analysis in PROC CORR of the continuous X and Y variables are presented in Table 3. For the color traits, correlations where R > 0.4 included the correlation between L* and ultimate pH (R = −0.482, P < 0.0001), and L* and drip loss (R = 0.452, P < 0.05). For the pH traits, correlations where R > 0.4 included the correlation between initial pH and cooking loss (R = −0.559, P < 0.0001), as well as between ultimate pH and drip loss (R = −0.439, P < 0.05). For drip loss, cooking loss, and shear force traits, correlations where R > 0.4 included drip loss and cooking loss (R = 0.606, P < 0.0001), as well as drip loss and shear force (R = 0.440, P < 0.05). Temperature was significantly correlated with all studied traits, except for a* and shear force (P < 0.05). Slaughter age was significantly correlated with b*, initial pH, ultimate pH, drip loss, cooking loss, and shear force (P < 0.05).
Models developed using the mixed model approach are presented in Table 4, Table 5, Table 6, and model evaluation statistics in Table 7. In Table 4, Table 5, Table 6, one model may be documented across multiple rows if it contained categorical x continuous variable interactions (e.g., a separate equation was developed for turkeys vs. broilers, TS type, or muscle type).
Table 4.
Estimated parameters of linear mixed models for meat lightness (L*), redness (a*), and yellowness (b*) obtained for broilers or turkeys undergoing TS.1
| Trait | Model2 | Equation | Species | Muscle | Intercept | Stress3 | Temperature4 | Species × Age5 | Time6 |
|---|---|---|---|---|---|---|---|---|---|
| L* | 1 | L1 | Broiler | - | 56.19 ± 2.703 | Control | 0.03 ± 0.015 | −0.16 ± 0.065 | 0.06 ± 0.026 |
| L* | 1 | L2 | Broiler | - | 56.19 ± 2.703 | Acute | 0.02 ± 0.015 | −0.16 ± 0.065 | 0.06 ± 0.026 |
| L* | 1 | L3 | Broiler | - | 56.19 ± 2.703 | Chronic | 0.07 ± 0.021 | −0.16 ± 0.065 | 0.06 ± 0.026 |
| L* | 1 | L4 | Turkey | - | 56.19 ± 2.703 | Control | 0.03 ± 0.015 | −0.07 ± 0.013 | 0.06 ± 0.026 |
| L* | 1 | L5 | Turkey | - | 56.19 ± 2.703 | Acute | 0.02 ± 0.015 | −0.07 ± 0.013 | 0.06 ± 0.026 |
| L* | 1 | L6 | Turkey | - | 56.19 ± 2.703 | Chronic | 0.07 ± 0.021 | −0.07 ± 0.013 | 0.06 ± 0.026 |
| a* | 2 | R1 | - | Breast | 5.27 ± 0.405 | - | -0.02 ± 0.007 | - | - |
| a* | 2 | R2 | - | Thigh | 7.06 ± 0.606 | - | -0.02 ± 0.007 | - | - |
| b* | 3 | Y1 | - | - | 7.06 ± 0.821 | - | 0.02 ± 0.010 | - | - |
Empty boxes, denoted with (-), indicate that the effect is not included in the best prediction model. Parameter estimates for temperature represent the interaction between stress and temperature when stress type is specified. If stress type is not specified (-), then the interaction was not included in the best prediction model.
Equations with the same model ID were parameterized within the same statistical model.
Control = no treatment applied, Acute = TS for ≤24 h, Chronic = TS for >24 h.
Average treatment temperature (°C).
Interaction between Species and Age in days when birds were slaughtered.
Time in hour PM that the color measurement was taken.
Table 5.
Estimated parameters of linear mixed models for meat initial pH (pHi) and ultimate pH (pHu) obtained for broilers or turkeys undergoing TS.1
| Trait | Model2 | Equation | Species | Muscle | Intercept | Stress3 | Temperature4 | Species × age5 | Time6 |
|---|---|---|---|---|---|---|---|---|---|
| pHi | 4 | PI1 | Broiler | - | 6.939 ± 0.1822 | - | −0.002 ± 0.0011 | −0.013 ± 0.0043 | −0.002 ± 0.0001 |
| pHi | 4 | PI2 | Turkey | - | 6.939 ± 0.1822 | - | −0.002 ± 0.0011 | −0.005 ± 0.0014 | −0.002 ± 0.0001 |
| pHu | 5 | PU1 | - | Breast | 5.906 ± 0.0091 | - | −0.002 ± 0.0004 | - | - |
| pHu | 5 | PU2 | - | Thigh | 6.119 ± 0.0304 | - | −0.002 ± 0.0004 | - | - |
Empty boxes, denoted with (-), indicate that the effect is not included in the best prediction model. Parameter estimates for temperature represent the interaction between stress and temperature when stress type is specified. If stress type is not specified (-), then the interaction was not included in the best prediction model.
Equations with the same model ID were parameterized within the same statistical model.
Control = no treatment applied, Acute = TS for ≤24 h, Chronic=TS for >24 h.
Average treatment temperature (°C).
Interaction between Species and Age in days when birds were slaughtered.
Time in minute PM that the pH measurement was taken.
Table 6.
Estimated parameters of linear mixed models for meat drip loss (%), cooking loss (%), and shear force (N) for broilers or turkeys undergoing TS.1
| Trait | Model2 | Equation | Species | Muscle | Intercept | Stress3 | Temperature4 | Species × age5 | Time6 |
|---|---|---|---|---|---|---|---|---|---|
| Drip loss | 6 | D1 | - | - | 2.129 ± 0.2715 | Control | 0.003 ± 0.0027 | - | - |
| Drip loss | 6 | D2 | - | - | 2.129 ± 0.2715 | Acute | 0.004 ± 0.0022 | - | - |
| Drip loss | 6 | D3 | - | - | 2.129 ± 0.2715 | Chronic | 0.010 ± 0.0022 | - | - |
| Cooking loss | 7 | C1 | - | Breast | 21.664 ± 1.3298 | - | 0.045 ± 0.0176 | - | - |
| Cooking loss | 7 | C2 | - | Thigh | 24.831 ± 1.7958 | - | 0.045 ± 0.0176 | - | - |
| Shear force | 8 | S1 | - | Breast | 25.917 ± 2.4483 | - | 0.136 ± 0.0533 | - | - |
| Shear force | 8 | S2 | - | Thigh | 30.175 ± 3.4533 | - | 0.136 ± 0.0533 | - | - |
Empty boxes, denoted with (-), indicate that the effect is not included in the best prediction model. Parameter estimates for temperature represent the interaction between stress and temperature when stress type is specified. If stress type is not specified (-), then the interaction was not included in the best prediction model.
Equations with the same model ID were parameterized within the same statistical model.
Control = no treatment applied, Acute = TS for ≤24 h, Chronic = TS for >24 h.
Average treatment temperature.
Interaction between Species and Age in days when birds were slaughtered.
Time in hour PM that the measurement was taken.
Table 7.
Evaluation statistics for the best prediction equation determined by meta-analysis.
| Lightness (L*) | Redness (a*) | Yellowness (b*) | Initial pH | Ultimate pH | Drip loss (%) | Cooking loss (%) | Shear force (N) | |
|---|---|---|---|---|---|---|---|---|
| Model ID | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Observed | ||||||||
| Mean | 51.12 | 6.25 | 7.07 | 6.17 | 5.92 | 1.56 | 21.78 | 28.98 |
| SD | 5.190 | 5.212 | 4.916 | 0.281 | 0.203 | 1.145 | 9.783 | 15.301 |
| Predicted | ||||||||
| Mean | 51.30 | 5.63 | 7.08 | 6.19 | 5.89 | 1.53 | 21.87 | 28.67 |
| SD | 4.606 | 4.008 | 4.824 | 0.247 | 0.105 | 1.082 | 9.440 | 14.548 |
| RMSE (%)1 | 2.47 ± 0.173 | 2.30 ± 0.242 | 0.81 ± 0.131 | 0.09 ± 0.011 | 0.14 ± 0.006 | 0.20 ± 0.025 | 1.21 ± 0.1827 | 3.90 ± 0.375 |
| ECT (%)2 | 1.4 | 7.4 | 0.01 | 3.1 | 3.3 | 2.2 | 0.5 | 0.7 |
| ER (%)3 | 5.1 | 11.4 | 0.01 | 6.7 | 14.9 | 5.4 | 5.03 | 0.4 |
| ED (%)4 | 93.4 | 81.2 | 99.9 | 90.2 | 81.8 | 92.3 | 94.5 | 98.9 |
| CCC5 | 0.92 ± 0.007 | 0.86 ± 0.014 | 0.97 ± 0.005 | 0.92 ± 0.007 | 0.76 ± 0.005 | 0.98 ± 0.008 | 0.99 ± 0.002 | 0.94 ± 0.006 |
| R6 | 0.956 | 0.918 | 0.983 | 0.957 | 0.783 | 0.986 | 0.993 | 0.967 |
| Cb7 | 0.962 | 0.936 | 0.985 | 0.963 | 0.806 | 0.994 | 0.992 | 0.971 |
| CVK8 | 1.900 | 1.299 | 0.750 | 0.00484 | 0.00850 | 0.0128 | 1.455 | 13.195 |
| Plots | ||||||||
| Intercept9 | 7.93 ± 1.065* | 1.21 ± 0.201* | 0.26 ± 0.128* | 1.01 ± 0.156* | 3.50 ± 0.158* | 0.08 ± 0.039* | 1.00 ± 0.276* | 2.01 ± 0.926* |
| Slope10 | 0.08 ± 0.026* | 0.19 ± 0.042* | 0.002 ± 0.015 | 0.09 ± 0.033* | 0.51 ± 0.100* | 0.04 ± 0.023 | 0.03 ± 0.012±* | 0.02 ± 0.031* |
Root mean square prediction error expressed as a percentage of the observed mean ± SD for all 5 k-folds.
Error due to bias expressed as a percentage of MSPE.
Error due to regression slope deviation expressed as a percentage of MSPE.
Error due to disturbance expressed as a percentage of MSPE.
Mean concordance correlation coefficient. ± SD for all 5 K-folds.
Pearson correlation coefficient.
Bias correction factor.
Test error based on the K-fold cross validation approach.
Intercept of predicted versus observed regression. Values are presented as estimate ± SE and * indicates a significant difference from zero.
Slope of residual versus predicted regression. Values are presented as estimate ± SE and * indicates a significant difference from zero.
Color Traits (L*, a*, b*)
Lightness (L*)
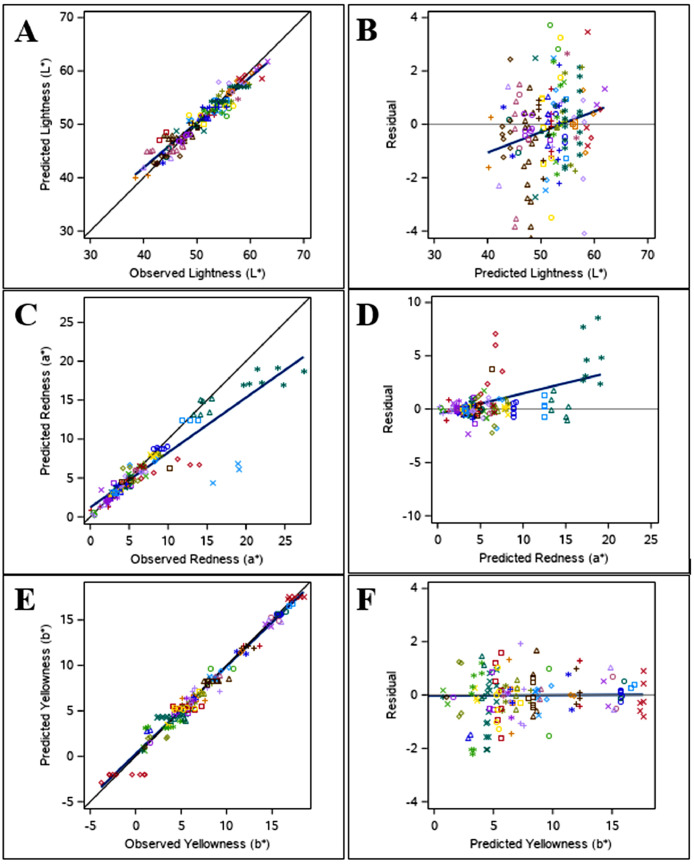
The best prediction model included the fixed effects of slaughter age within species (P < 0.0001), the interaction between stress duration (control vs. acute vs. chronic) and temperature (P < 0.0001), as well as PM measurement time (P < 0.05) (Model 1, Tables 4 and 7). Model evaluation (Table 7) shows a low RMSPE (2.47%) with the majority of error coming from random sources (ED, 93.4%), a high CCC (0.92), and a CVk of 1.9%, indicating good overall fit of the data and homogenous residual error distribution (Figure 2). The model developed indicates that the effect of temperature on L* differed between stress durations. With each degree increase in temperature under chronic stress conditions (>24 h), the L* value increases by 0.07 ± 0.021 (L3 and L6, Table 4), compared to a slope of 0.02 ± 0.015 under acute stress conditions (L2 and L5, Table 4) and a slope of 0.03 ± 0.015 under control conditions. In general, it was found that as slaughter age increases, L* is lower (darker meat) compared to younger broilers (L1–3, Table 4) and turkeys (L4–6, Table 4). There was also a significant influence of PM color measurement time on L* (Model 1, Table 4), with increased L* values over time indicating lighter meat as measurements were taken later.
Figure 2.
Predicted vs. observed and predicted vs. residual plots for the best prediction models for lightness (L*) (A and B), redness (a*) (C and D), and yellowness (b*) (E and F). Symbols that share the same shape and color are treatment means from the same study.
Redness (a*)
The best prediction model included the fixed effects of muscle type (breast vs. thigh; P < 0.0001) and temperature (P < 0.05) (Model 2, Tables 4 and 7). Model evaluation (Table 7) shows a low RMSPE (2.30%) with the majority of error coming from random sources (ED, 81.2%), a high CCC (0.86), and a CVk of 1.3%, indicating good overall fit of the data and homogeneous residual error distribution (Figure 2). In general, thigh meat is more red (higher a*, R2, Table 4) than breast meat (R1, Table 4). It was found that as treatment temperature increases, a* decreases by 0.02 ± 0.007 (Model 2, Table 4). Although the duration of TS was not included in the best prediction model, it was still shown to have a significant effect on a* (P < 0.05) with the largest effect observed under chronic stress conditions. Species was also found to have a significant effect on a* (P < 0.05), however, this effect was not included in the best prediction model for this trait. In general, though, broiler meat tends to have higher a* values than turkey meat.
Yellowness (b*)
The best prediction model included only the fixed effect of temperature (P < 0.05) (Model 3, Tables 4 and 7). Model evaluation (Table 7) shows a low RMSPE (0.81%) with the majority of error coming from random sources (ED, 99.9%), a high CCC (0.97), and CVk of 0.8%, indicating good overall fit of the data with a homogenous residual error distribution and no detected slope bias (Figure 2). An increase in treatment temperature was associated with a higher b* value (Model 3, Table 4). There was a significant effect of species on b* (P < 0.05), however, this was not included in the best prediction model for this trait. In general, broiler meat tended to have higher b* values than turkey meat. Temperature stress duration, muscle, and slaughter age did not significantly affect b* (P > 0.05).
pH Traits (Initial, Ultimate)
Initial pH
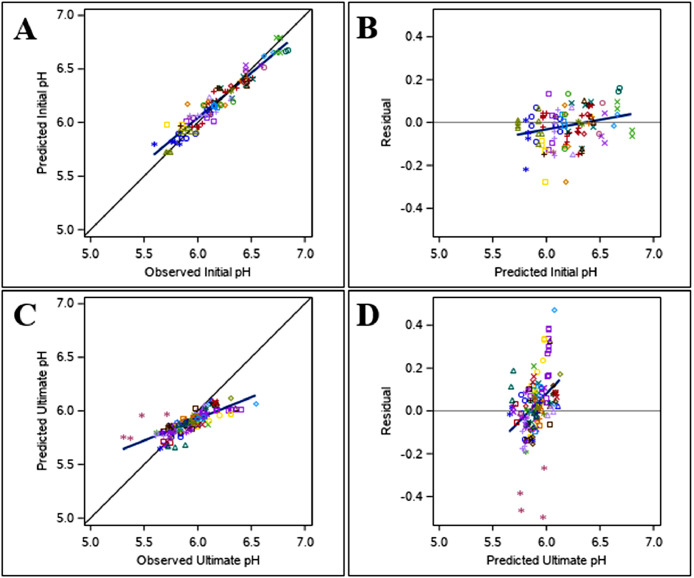
The best prediction model included the fixed effects of slaughter age within species (P < 0.05), the effect of treatment temperature (P < 0.05), and the effect of PM measurement time (P < 0.0001) (Model 4, Tables 5 and 7). Model evaluation (Table 7) shows a low RMSPE (0.09%) with the majority of error coming from random sources (ED, 90.2%), a high CCC (0.92), and a CVk of 0.005%, indicating good overall fit of the data and homogenous residual error distribution (Figure 3). A lower initial pH was observed when treatment temperature was higher (Model 4, Table 5). A significant effect of stress duration (acute vs. chronic) or muscle type (breast vs. thigh) was not found for initial pH (P > 0.05). In general, as slaughter age increased within broilers (PI1, Table 5) or turkeys (PI2, Table 5), the initial meat pH was lower. Additionally, the initial pH decreased by 0.002 ± 0.0001 units/min when the measurement time is delayed (Model 4, Table 5).
Figure 3.
Predicted vs. observed and predicted vs. residual plots for the best prediction models for initial pH (A and B) and ultimate pH (C and D). Symbols that share the same shape and color are treatment means from the same study.
Ultimate pH
The best prediction model included the fixed effect of muscle type (breast vs. thigh; P < 0.0001) and treatment temperature (P < 0.0001) (Model 5, Tables 5 and 7). Model evaluation (Table 7) shows a low RMSPE (0.14%) with the majority of error coming from random sources (ED, 81.8%), a moderate CCC (0.76), and a CVk of 0.009%, indicating good overall fit of the data and homogeneous residual error distribution (Figure 3). In general, breast meat (PU1, Table 6) had a lower ultimate pH than thigh meat (PU2, Table 6). In terms of TS, it was found that as treatment temperature increased, ultimate pH decreased (Model 5, Table 5). The duration of TS was found to have a significant effect on ultimate pH (P < 0.05) but was not included in the best prediction model for this trait. Increasing the temperature under acute stress conditions lowered ultimate pH at a faster rate compared to chronic stress conditions. Species and slaughter age were not found to significantly affect ultimate pH (P > 0.05).
Drip Loss, Cooking Loss, and Shear Force
Drip Loss
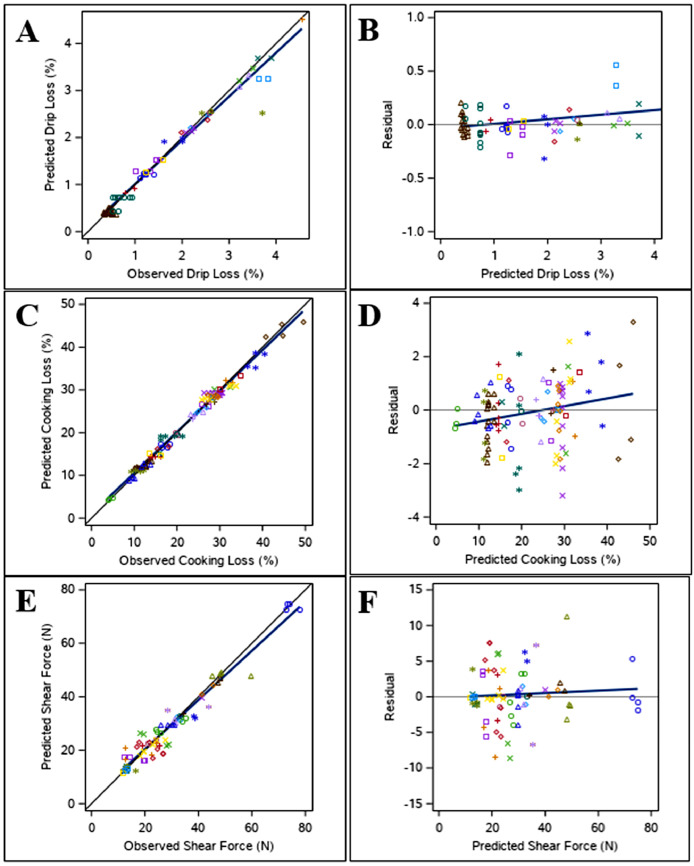
The best prediction model included the interaction between stress duration and treatment temperature (P < 0.0001) (Model 6, Tables 6 and 7). Model evaluation (Table 7) shows a low RMSPE (0.20%) with the majority of error coming from random sources (ED, 92.3%), a high CCC (0.98), and a CVk of 0.01%, indicating good overall fit of the data with homogenous residual error distribution and no detected slope bias (Figure 4). As temperature increased, meat drip loss increased under all conditions. A higher increase was observed under chronic stress conditions (D3, Table 6), followed by acute stress conditions (D2, Table 6), whereas the smallest increase was observed in the control group (D1, Table 6). Species, slaughter age, and muscle were not found to have significant effects on meat drip loss (P > 0.05).
Figure 4.
Predicted vs. observed and predicted vs. residual plots for the best prediction models for drip loss (%) (A and B), cooking loss (%) (C and D), and shear force (N) (E and F). Symbols that share the same shape and color are treatment means from the same study.
Cooking Loss
The best prediction model included the fixed effects of muscle (P < 0.0001) and treatment temperature (P < 0.05) (Model 7, Tables 6 and 7). Model evaluation (Table 7) shows a low RMSPE (1.21%) with the majority of error coming from random sources (ED, 94.5%), a high CCC (0.99), and a CVk of 1.5%, indicating good overall fit of the data and homogenous residual error distribution (Figure 4). In general, breast meat (C1, Table 6) tended to have a lower percent cooking loss than thigh meat (C2, Table 6). As treatment temperature increased by one degree, percent cooking loss increased by 0.045 ± 0.0176% (Model 7, Table 6). Temperature stress duration and slaughter age were found to have significant effects on cooking loss (P < 0.05) but were not included in the best prediction model for this trait. The effect of increasing temperature on cooking loss was larger under acute stress conditions (0.032 ± 0.0258%) compared to chronic stress (0.025 ± 0.0221%). Regarding slaughter age, birds that were older at slaughter had meat with increased cooking loss (0.033 ± 0.0111%).
Shear Force
The best prediction model included the fixed effects of muscle (P < 0.0001) and treatment temperature (P < 0.05) (Model 8, Tables 6 and 7). Model evaluation (Table 7) shows a low RMSPE (3.90%) with the majority of error coming from random sources (ED, 98.9%), a high CCC (0.94), and a CVk of 13.2, indicating good overall fit of the data and homogenous residual error distribution (Figure 4). Thigh meat had a higher shear force than breast meat (S2 vs. S1, Table 6). Like drip loss and cooking loss, higher shear force was observed with increasing treatment temperature (Model 8, Table 6). Temperature stress duration (acute vs. chronic) and species (broiler vs. turkey) were found to have a significant effect on shear force (P < 0.05) but were not included in the best prediction model based on this database. Slaughter age did not have a significant effect on meat shear force (P > 0.05).
Separation of Heat and Cold Stress
Based on the results of the meta-analysis, a subanalysis was conducted to examine and consider heat stress vs. cold stress. Overall, they were found to differently affect the quality of poultry meat (Table 8). Treatment temperature for the cold stress and heat stress studies ranged from −18 to 24°C and 18 to 42°C, respectively. Meat lightness (L*) was significantly different between heat-stressed and cold-stressed birds (P = 0.0001). The average meat L* for heat-stressed birds was 52.14 ± 0.750, whereas the average L* for cold-stressed birds was 50.55 ± 0.810. Meat redness (a*) was also found to be significantly different between the temperature treatments (5.49 ± 0.557 (heat) vs. 6.19 ± 0.574 (cold), P = 0.0003). However, there was no significant difference found in meat yellowness (b*) between the groups (P > 0.05).
Table 8.
Results of heat stress (HS) vs. cold stress (CS) subanalysis: LSMeans ± SD for the meat quality traits of birds exposed to either heat stress (HS) or cold stress (CS) as reported in the studies included in the meta-analysis database.
| Trait | HS | N | CS | N | P-value |
|---|---|---|---|---|---|
| Lightness (L*) | 52.14 ± 0.750 | 86 | 50.55 ± 0.810 | 36 | 0.0001 |
| Redness (a*) | 5.49 ± 0.557 | 67 | 6.19 ± 0.574 | 36 | 0.0003 |
| Yellowness (b*) | 7.63 ± 0.784 | 66 | 7.58 ± 0.796 | 36 | 0.9723 |
| Initial pH | 6.19 ± 0.058 | 76 | 6.23 ± 0.071 | 8 | 0.6834 |
| Ultimate pH | 5.95 ± 0.023 | 65 | 6.02 ± 0.026 | 28 | 0.0010 |
| Drip loss (%) | 2.39 ± 0.252 | 38 | 2.14 ± 0.256 | 13 | 0.0003 |
| Cooking loss (%) | 24.57 ± 1.737 | 49 | 24.28 ± 1.783 | 22 | 0.8397 |
| Shear force (N) | 32.35 ± 3.072 | 43 | 32.67 ± 3.698 | 14 | 0.9902 |
No significant difference was found in the initial pH between heat-stressed and cold-stressed birds (P > 0.05), although it was found that the initial pH of meat from heat-stressed birds was significantly lower than that of birds raised at control temperatures (data not shown, P = 0.0018). However, a significant difference was found in meat ultimate pH between heat-stressed and cold-stressed birds (P = 0.001). Birds from cold-stress treatments had a significantly higher ultimate pH (6.015 ± 0.0263) than birds from heat-stress treatments (5.95 ± 0.0226).
A significant difference in mean drip loss was found between heat-stressed and cold-stressed birds (P = 0.0003). Birds in heat-stress treatments had a higher average drip loss (2.39 ± 0.252%) compared to birds in cold-stress treatments (2.14 ± 0.256). However, no similar relationship was found for cooking loss between the groups (P > 0.05). Additionally, there was no significant difference in shear force values (P > 0.05), however, there was a trend for heat-stressed birds to have a significantly higher shear force than birds raised at control temperatures (P = 0.08).
DISCUSSION
Temperature and Meat Quality
Across a large database of published literature, ambient temperature had an effect on all studied meat quality traits. As treatment temperature increased (heat stress), meat tended to be lighter (L*), less red (a*), and more yellow (b*), as well as having a lower initial and ultimate pH, and increased drip loss, cooking loss, and shear force. These results are in line with knowledge on the development of PSE-like meat under heat stress conditions and DFD-like meat under cold stress conditions but quantifies the effect across the body of literature available.
An increase in temperature has previously been shown to increase glycogen breakdown and subsequent acidification and degradation of muscle protein (Pietrzak et al., 1997; King and Whyte, 2006; Owens et al., 2009; Carvalho et al., 2014), while lower temperatures have been shown to counter this effect by causing depletion of glycogen stores prior to slaughter (Lee et al., 1976; Haman et al., 2002,2005; Dadgar et al., 2011,2012b). The fast acidification of PSE meat and degradation of muscle protein and pigments can explain the lower initial and ultimate pH as well as the color fading (higher L*, higher b*, lower a*), and decreased WHC (increased drip and cooking loss) at higher temperatures.
Overall, the prediction equations developed fit the data well based on the model evaluation measures. The RMSPE% was low and for all equations most of the error could be attributed to random disturbance. The CCC values ranged between 0.76 and 0.99 which indicate that the observed and predicted values are closely related. The model test error (CVK) was less than 2% for most models which indicate that the developed models are robust. The model for shear force had the highest CVK (13.2%) which can likely be attributed to the smaller sample size for this trait (N = 90). The plots of predicted vs. observed values indicate good overall fit for most models, except for ultimate pH, which is reflected in the lowest calculated CCC (0.76). A significant slope bias (P < 0.05) was detected for all models except for b* and drip loss. After examination of the predicted vs. residual plots, this bias is potentially due to outlier studies and the fact that the random effect of study is not considered in the regression (PROC REG) analysis used to compare overall predicted vs. residual plots. To be as comprehensive as possible, we did not apply a temporal restriction to our search strategy, however, it may be advisable for future meta-analyses to consider applying a restriction (e.g., last 10 yr) to potentially avoid studies which are outliers because of changes in techniques or genetic selection.
Indicator Traits for Meat Quality Defects
To classify poultry meat quality defects, traits such as meat color and pH are used due to their relative ease of measurement and close relationship with each other, as well as to other relevant traits such as WHC and texture (Barbut, 1998; Owens et al., 2000a; Garcia et al., 2010; Dadgar et al., 2012b). The results of this meta-analysis support the use of both color and pH as indicators for meat quality parameters. In terms of color, we found that L*, and to a lesser extent a* and b*, were significantly correlated with meat quality traits such as pH, drip loss, and cooking loss (Table 3). Furthermore, we found a significant correlation between L* and a* and b* (Table 3). This could explain why a* and b* are not often used to classify PSE meat, as some say that they are largely redundant, and differences in L* tend to be larger. In any case, measuring L* alone has been shown to be reliable at identifying PSE meat (Barbut, 1993; Petracci et al., 2004) and the relationship between a* or b* and other indicator traits (e.g., ultimate pH) are not always as well established in the literature. This could suggest that recording a single value for L* (which today can be done inline at high speed) might be more efficient than additionally assessing a* and b* values. In terms of pH to classify PSE meat in poultry, ultimate pH appears to be a more valid indicator of meat quality defects than initial pH (Garcia et al., 2010; Eadmusik et al., 2011). This could be supported by the results of this meta-analysis, as ultimate pH was correlated with more of the relevant meat quality parameters (i.e., L*, drip loss, and cooking loss) compared to initial pH which was only correlated to cooking loss (Table 3).
Implications for Consumer Acceptance
Our analysis showed that birds exposed to higher temperatures are more likely to produce meat with PSE-like characteristics, whereas birds exposed to colder temperatures are more likely to produce meat with DFD-like characteristics. These characteristics are unappealing to consumers and could have negative implications for the profitability of the industry. We were able to show that ambient TS can significantly affect meat color. Although there may be no classifiable defect, meat color is highly related to consumer acceptance, and is considered one of the most important characteristics at the point of purchase (West et al., 2001; Banović et al., 2009). Meat color is particularly relevant today, as most cut-up poultry is sold in trays covered with clear plastic film (Min and Ahn, 2012; Font-i-Furnols and Guerrero, 2014). Western consumers have been shown to react negatively when chicken meat has a yellow color, and were more likely to act favorably when the yellow color was disguised (Kennedy et al., 2005). Our analyses showed that increasing temperature was more likely to result in paler and more yellow meat. Additionally, with increasing temperature, drip loss, cooking loss, and shear force all showed higher values. Drip and cooking losses are important indicators of WHC. Poor WHC detracts from the product's appearance, reduces the weight of fresh meat, and can impact the juiciness of the meat once it is cooked. Meat that loses a significant amount of moisture while cooking may result in a cooked product that is dry, less tender and is less preferred by consumers (Warriss, 2000).
Separation of Heat vs. Cold Stress
The results of the subanalysis are similar to the regression equations developed for TS and indicate that hot and cold conditions may differently affect some poultry meat quality traits. Birds undergoing heat stress treatments tended to have meat that was lighter and less red, with a lower ultimate pH and higher drip loss compared to cold stress. This is similar to the findings of many published studies and reviews which attribute PSE meat to heat stress conditions and DFD meat to cold stress conditions (Bianchi et al., 2006; Barbut et al., 2008; Gonzalez-Rivas et al., 2020). However, these results should be interpreted with some caution given the relative imbalance between heat and cold studies included, as well as the unequal distribution of studies assessing cold stress between species (NBroiler=9, NTurkey=3). It is possible that with more cold stress studies considered, the results of this analysis could change.
Acute vs. Chronic Stress
Acute TS is typically designed to mimic the effect of temperature during short-term or daily fluctuations soon before slaughter (i.e., during transportation or lairage), whereas chronic TS simulates the effect of temperature during long-term or seasonal changes that can occur during production. The interaction between treatment temperature and duration of TS (acute vs. chronic) was only included in the best prediction model for 2 traits (L* and drip loss), but was also found to be significant (P < 0.05) for all other traits except for yellowness and initial pH.
Based on our analysis, there was no clear consensus as to whether acute or chronic TS has a larger effect on meat quality traits in poultry. Acute stress had a larger effect on ultimate pH, cooking loss, and shear force, compared to chronic stress which had a larger effect on color (L* and a*) and drip loss. From this, we could suggest that acute exposure to extreme temperatures soon before slaughter is more likely to have larger impact on pH traits, whereas chronic exposure to TS is more likely to have a larger impact on meat color.
It is possible that the inconsistent effect magnitude between acute and chronic stress on the studied meat quality traits is due to the varying range of temperatures within the acute and chronic categories used here. The ranges in treatment temperatures for the acute and chronic categories (within this meta-analysis) were −18 to 42°C and 15 to 36°C, respectively, with medians of 34°C and 34°C, respectively. Overall, more extreme temperatures are represented within the acute category, whereas more moderate temperatures are in the control and chronic categories. To be as comprehensive as possible, studies were not excluded from the meta-analysis based on their treatment temperature. Future studies may discern the effects of acute and chronic temperature exposure when temperature ranges are more similar. Additionally, the range of TS exposure time varied considerably within the acute and chronic categories. Within acute stress (≤24 h), the actual duration of exposure in the various studies ranged from 20 min to 24 h before slaughter. Within chronic stress (>24 h), the actual duration of exposure in the various studies ranged from 3 to 70 d. It is possible that the large range of exposure times within these categories contributed to the differing relative effect magnitude of acute and chronic stress on meat quality traits, though they could be representative of what occurs in practice. Recategorization of the treatment duration or using treatment duration as a continuous variable may help clarify this relationship in future studies.
Of importance, not all birds are PSE-susceptible under heat stress conditions. Stress-susceptibility is well documented in pigs, and it has been shown that susceptible pigs are more likely to develop PSE meat than nonsusceptible animals (Offer, 1991). In pigs, a point mutation in the RYR1 gene (ryanodine receptor) has been determined as the genetic cause of malignant hyperthermia resulting in PSE meat (Fujii et al., 1991; Paiao et al., 2013). In chickens and turkeys, RYR1 polymorphisms and variants in RYR1 transcripts have been discovered but were not related to the development of PSE meat, so the genetic cause for this myopathy in poultry remains unknown (Chiang et al., 2004; Droval et al., 2012). Although the cause has not been identified, it is possible that some birds within a given study treatment are susceptible to PSE meat and others are not, which could result in variation in effect magnitude between studies.
Practical Significance
Although TS was demonstrated to have a significant effect on meat quality parameters, it must be discussed whether these effects are practically significant from a meat production perspective. In general, with increasing or decreasing temperature, the parameter estimates for the effect of temperature on meat quality traits were low. To illustrate, reported L* cut-offs for PSE meat can range between 49 and 56 depending on the study (Petracci et al., 2009), or poultry meat having an ultimate pH of <5.8 has also been used in practice (Garcia et al., 2010). If we assume an L* cut-off of 53, in the middle of the range, a broiler slaughtered at 42 d of age would have to be chronically exposed to 30°C or greater, or acutely exposed to 75°C or greater to have an L* value greater than 53, based on (linear) extrapolation of the prediction equations. For ultimate pH, a bird would need to be exposed to temperatures over 50°C for ultimate pH to drop below 5.8. While chronic exposure to 30°C may be plausible, exposure to temperatures greater than 50°C are unlikely in most production systems. It is, however, possible that these temperatures could be reached acutely during transportation or heat waves in poultry operations in hot climates or when the apparent temperature (i.e., combination of high air temperature with low air velocity, high humidity, and high stocking density) is considered. Humidity is especially important to consider for poultry, which are especially poor at dissipating heat and panting is less effective under high humidity conditions (Jahejo et al., 2016; van Dyk et al., 2019). Unfortunately, only 19 of the 48 studies reported relative humidity so we did not include this variable in the analysis, although it undoubtedly influences heat stress. Including aspects of ‘apparent’ temperature should be the focus of future studies or meta-analyses.
Heat stress impairs the performance of poultry by reducing feed intake and increasing the feed conversion ratio, with ultimately slower growth (Lara and Rostagno, 2013; Habibian et al., 2014; Tawfeek et al., 2014). An estimate from 2003 indicates that economic losses from heat stress in the US poultry industry can amount to $128 million annually (St-Pierre et al., 2003). This could indicate that the greatest impact of TS may be its effect on body weight gain/efficiency and not its impact on meat quality, since it appears to take extreme temperature conditions to result in a classifiable defect based on the results of this meta-analysis. However, since body and muscle growth is undoubtedly connected to meat quality (Dransfield and Sosnicki, 1999), these effects are not necessarily independent, especially in the case of chronic exposure over the growing period (i.e., seasonal heat stress). Furthermore, the extrapolation of our prediction equations assumes a continuous linear relationship between temperature and meat quality traits. The relationship may be nonlinear, and so extrapolations of these equations beyond the breadth of the developmental database (temp range: −8 to 42°C) may not be accurate. Regardless, even if the effects of temperature on meat quality do not result in classifiable defects, they may still negatively affect consumer acceptance of the product and are certainly relevant for welfare.
CONCLUSIONS
The results of this meta-analysis demonstrate that TS significantly affects each of the studied meat quality traits. Furthermore, we also show that these effects can be numerically quantified across a large database of published studies. In brief, it was found that as overall treatment temperature increases, poultry meat has a tendency to be lighter (L*), less red (a*), and more yellow (b*), as well as having a lower initial and ultimate pH, and increased drip loss, cooking loss, and shear force. Conversely, birds exposed to colder temperatures are more likely to produce meat that is darker, redder, and less yellow, as well as having a higher initial and ultimate pH, and decreased drip loss, cooking loss, and shear force. This meta-analysis quantifies previously published research describing heat stress induced PSE meat and cold stress induced DFD meat in poultry. Significant effects of temperature were found for all examined traits; however, the effect magnitude is generally small. Future studies should perform a separate analysis of heat stress and cold stress to better examine the effect of duration or analyze the impact of effective temperature (including air velocity, humidity, stocking density, etc.).
Acknowledgments
ACKNOWLEDGMENTS
The authors would like to express their gratitude to all authors whose studies were included in the meta-analysis. We would also like to thank Neila Ben Sassi, Claire Mindus, Valerie Monckton, and Carolina Reyes Rodriguez for their thoughtful suggestions throughout the development of this manuscript.
This project was funded by the Government of Canada through Genome Canada and the Ontario Genomics Institute (OGI-133). This study was part of the project entitled “Application of genomic selection in turkeys for health, welfare, efficiency and production traits” funded by the government of Canada through the Genome Canada Genomic Application Partnership Program and administered by Ontario Genomics (recipients: B.J. Wood (Industry) and C.F. Baes (Academic)). The authors would also like to acknowledge NSERC and Hybrid Turkeys for financial support. The funders had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Authors’ contributions: All authors (EL, JE, NvS, SB, RJV, VO, BW, AH, and CB) made substantial intellectual contributions to the conception of the study, study design, and interpretation of the data. EL conducted data collection and conducted statistical analysis with assistance from JE and NvS. EL drafted the first version of the manuscript. JE, NvS, SB, RJV, VO, BW, AH, and CB provided substantive input and contributions to manuscript revision. All authors (EL, JE, NvS, SB, RJV, VO, BW, AH, and CB) read and approved the final manuscript.
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Availability of data and materials: The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
DISCLOSURES
All authors declare that they have no known competing financial interests or personal relationships that influence the work reported in this paper.
References
- Adzitey F., Nurul H. Pale soft exudative (PSE) and dark firm dry (DFD) meats: causes and measures to reduce these incidences - a mini review. Int. Food Res. J. 2011;18:11–20. [Google Scholar]
- Aksit M., Yalcin S., Ozkan S., Metin K., Ozdemir D. Effects of temperature during rearing and crating on stress parameters and meat quality of broilers. Poult. Sci. 2006;85:1867–1874. doi: 10.1093/ps/85.11.1867. [DOI] [PubMed] [Google Scholar]
- Ali M.S., Kang G.H., Joo S.T. A review: influences of pre-slaughter stress on poultry meat quality. Asian-Australas. J. Anim. Sci. 2008;21:912–916. [Google Scholar]
- Babji A.S., Froning G.W., Ngoka D.A. The effect of preslaughter environmental temperature in the presence of electrolyte treatment on turkey meat quality. Poult. Sci. 1982;61:2385–2389. [Google Scholar]
- Banović M., Grunert K.G., Barreira M.M., Fontes M.A. Beef quality perception at the point of purchase: a study from Portugal. Food Qual. Prefer. 2009;20:335–342. [Google Scholar]
- Barbut S. Colour measurements for evaluating the pale soft exudative (PSE) occurrence in turkey meat. Food Res. Int. 1993;26:39–43. [Google Scholar]
- Barbut S. Estimating the magnitude of the PSE problem in poultry. J. Muscle Foods. 1998;9:35–49. [Google Scholar]
- Barbut S. Pale, soft, and exudative poultry meat—reviewing ways to manage at the processing plant. Poult. Sci. 2009;88:1506–1512. doi: 10.3382/ps.2009-00118. [DOI] [PubMed] [Google Scholar]
- Barbut S. Pages 39–45 in The Science of Poultry and Meat Processing. University of Guelph; Ontario, Canada: 2015. Evaluating water/fat binding and colour. ISBN: 978-0-88955-625-6. [Google Scholar]
- Barbut S., Sosnicki A.A., Lonergan S.M., Knapp T., Ciobanu D.C., Gatcliffe L.J., Huff-Lonergan E., Wilson E.W. Progress in reducing the pale, soft and exudative (PSE) problem in pork and poultry meat. Meat Sci. 2008;79:46–63. doi: 10.1016/j.meatsci.2007.07.031. [DOI] [PubMed] [Google Scholar]
- Barbut S., Zhang L., Marcone M. Effects of pale, normal, and dark chicken breast meat on microstructure, extractable proteins, and cooking of marinated fillets. Poult. Sci. 2005;84:797–802. doi: 10.1093/ps/84.5.797. [DOI] [PubMed] [Google Scholar]
- Bautista Y., Narciso C., Pro A., Hernandez A.S., Becerril C.M., Sosa E., Velasco J. Effect of heat stress and holding time ante-mortem on the physicochemical and quality characteristics of chicken meat. Arch. Med. Vet. 2016;48:89–97. [Google Scholar]
- Berri C., Debut M., Santé-Lhoutellier V., Arnould C., Boutten B., Sellier N., Baéza E., Jehl N., Jégo Y., Duclos M.J., Le Bihan-Duval E. Variations in chicken breast meat quality: implications of struggle and muscle glycogen content at death. Br. Poult. Sci. 2005;46:572–579. doi: 10.1080/00071660500303099. [DOI] [PubMed] [Google Scholar]
- Bianchi M., Petracci M., Cavani C. The influence of genotype, market live weight, transportation, and holding conditions prior to slaughter on broiler breast meat color. Poult. Sci. 2006;85:123–128. doi: 10.1093/ps/85.1.123. [DOI] [PubMed] [Google Scholar]
- Bibby J., Toutenburg T. John Wiley & Sons, Ltd.; Chichester, UK: 1977. Prediction and Improved Estimation in Linear Models. [Google Scholar]
- Brossi C., Montes-Villanueva N., Rios-Mera J.D., Delgado E.F., Menten J.M., Contreras-Castillo C.J. Acute heat stress detrimental effects transpose high mortality rate and affecting broiler breast meat quality. Sci. Agropecu. 2018;9:305–311. [Google Scholar]
- Carvalho R.H., Soares A.L., Honorato D.C.B., Guarnieri P.D., Pedrao M.R., Paiao F.G., Oba A., Ida E.I., Shimokomaki M. The incidence of pale, soft, and exudative (PSE) turkey meat at a Brazilian commercial plant and the functional properties in its meat product. LWT Food Sci. Technol. 2014;59:883–888. [Google Scholar]
- Cheng Y., Du M., Xu Q., Chen Y., Wen C., Zhou Y. Dietary mannan oligosaccharide improves growth performance, muscle oxidative status, and meat quality in broilers under cyclic heat stress. J. Therm. Biol. 2018;75:106–111. doi: 10.1016/j.jtherbio.2018.06.002. [DOI] [PubMed] [Google Scholar]
- Chiang W., Allison C.P., Linz J.E., Strasburg G.M. Identification of two αrYR alleles and characterization of αrYR transcript variants in turkey skeletal muscle. Gene. 2004;330:177–184. doi: 10.1016/j.gene.2004.01.023. [DOI] [PubMed] [Google Scholar]
- Chiang W., Booren A., Strasburg G. The effect of heat stress on thyroid hormone response and meat quality in turkeys of two genetic lines. Meat Sci. 2008;80:615–622. doi: 10.1016/j.meatsci.2008.02.012. [DOI] [PubMed] [Google Scholar]
- CIE . Boreau Central de la CIE; Paris, France: 1976. Colorimetry: Official Recommendations of the International Commission on Illumination. [Google Scholar]
- Cook R.D. Detection of influential observation in linear regression. Technometrics. 1977;19:15–18. [Google Scholar]
- Cramer T.A., Kim H.W., Chao Y., Wang W., Cheng H.W., Kim Y.H.B. Effects of probiotic (Bacillus subtilis) supplementation on meat quality characteristics of breast muscle from broilers exposed to chronic heat stress. Poult. Sci. 2018;97:3358–3368. doi: 10.3382/ps/pey176. [DOI] [PubMed] [Google Scholar]
- Dadgar S., Crowe T.G., Classen H.L., Watts J.M., Shand P.J. Broiler chicken thigh and breast muscle responses to cold stress during simulated transport before slaughter. Poult. Sci. 2012;91:1454–1464. doi: 10.3382/ps.2011-01520. [DOI] [PubMed] [Google Scholar]
- Dadgar S., Lee E.S., Crowe T.G., Classen H.L., Shand P.J. Characteristics of cold-induced dark, firm, dry broiler chicken breast meat. Br. Poult. Sci. 2012;53:351–359. doi: 10.1080/00071668.2012.695335. [DOI] [PubMed] [Google Scholar]
- Dadgar S., Lee E.S., Leer T.L.V, Crowe T.G., Classen H.L., Shand P.J. Effect of acute cold exposure, age, sex, and lairage on broiler breast meat quality. Poult. Sci. 2011;90:444–457. doi: 10.3382/ps.2010-00840. [DOI] [PubMed] [Google Scholar]
- Dai S.F., Wang L.K., Wen A.Y., Wang L.X., Jin G.M. Dietary glutamine supplementation improves growth performance, meat quality and colour stability of broilers under heat stress. Br. Poult. Sci. 2009;50:333–340. doi: 10.1080/00071660902806947. [DOI] [PubMed] [Google Scholar]
- Debut M., Berri C., Baéza E., Sellier N., Arnould C., Guémené D., Jehl N., Boutten B., Jego Y., Beaumont C., Le Bihan-Duval E. Variation of chicken technological meat quality in relation to genotype and preslaughter stress conditions. Poult. Sci. 2003;82:1829–1838. doi: 10.1093/ps/82.12.1829. [DOI] [PubMed] [Google Scholar]
- Dransfield E., Sosnicki A.A. Relationship between muscle growth and poultry meat quality. Poult. Sci. 1999;78:743–746. doi: 10.1093/ps/78.5.743. [DOI] [PubMed] [Google Scholar]
- Droval A.A., Binneck E., Marin S.R.R., Paião F.G., Oba A., Nepomuceno A.L., Shimokomaki M. A new single nucleotide polymorphism in the ryanodine gene of chicken skeletal muscle. Genet. Mol. Res. 2012;11:821–829. doi: 10.4238/2012.April.3.4. [DOI] [PubMed] [Google Scholar]
- van Dyk M., Noakes M.J., McKechnie A.E. Interactions between humidity and evaporative heat dissipation in a passerine bird. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2019;189:299–308. doi: 10.1007/s00360-019-01210-2. [DOI] [PubMed] [Google Scholar]
- Eadmusik S., Molette C., Fernandez X., Rémignon H. Are one early muscle ph and one early temperature measurement sufficient to detect pse breast meat in turkeys? Br. Poult. Sci. 2011;52:177–188. doi: 10.1080/00071668.2011.554798. [DOI] [PubMed] [Google Scholar]
- Feng J., Zhang M., Zheng S., Xie P., Ma A. Effects of high temperature on multiple parameters of broilers in vitro and in vivo. Poult. Sci. 2008;87:2133–2139. doi: 10.3382/ps.2007-00358. [DOI] [PubMed] [Google Scholar]
- Fernandes J.I.M., Santos T.C., Kaneko I.N., Horn D., Leyter J.R., Pasa C.L.B. Effect of thermal embryonic manipulation on the quality of male and female broiler meat submitted to thermal stress pre-Slaughter. Braz. J. Poult. Sci. 2016;18:343–349. [Google Scholar]
- Ferreira I.B., Matos Junior J.B., Sgavioli S., Vicentini T.I., Morita V.S., Boleli I.C. Vitamin C prevents the effects of high rearing temperatures on the quality of broiler thigh meat. Poult. Sci. 2015;94:841–851. doi: 10.3382/ps/pev058. [DOI] [PubMed] [Google Scholar]
- Font-i-Furnols M., Guerrero L. Consumer preference, behavior and perception about meat and meat products: an overview. Meat Sci. 2014;98:361–371. doi: 10.1016/j.meatsci.2014.06.025. [DOI] [PubMed] [Google Scholar]
- Froning G.W., Babji A.S., Mather F.B. The effect of preslaughter temperature, stress, struggle and anesthetization on color and textural characteristics of turkey muscle. Poult. Sci. 1978;57:630–633. [Google Scholar]
- Fujii J., Otsu K., Zorzato F., De Leon S., Khanna V.K., Weiler J.E., O'Brien P.J., Maclennan D.H. Identification of a mutation in porcine ryanodine receptor associated with malignant hyperthermia. Science. 1991;253:448–451. doi: 10.1126/science.1862346. [DOI] [PubMed] [Google Scholar]
- Garcia R.G., de Freitas L.W., Schwingel A.W., Farias R.M., Caldara F.R., Gabriel A.M.A., Graciano J.D., Komiyama C.M., Almeida Paz I.C.L. Incidence and physical properties of PSE chicken meat in a commercial processing plant. Braz. J. Poult. Sci. 2010;12:233–237. [Google Scholar]
- Gonzalez-Rivas P.A., Chauhan S.S., Ha M., Fegan N., Dunshea F.R., Warner R.D. Effects of heat stress on animal physiology, metabolism, and meat quality: a review. Meat Sci. 2020;162 doi: 10.1016/j.meatsci.2019.108025. [DOI] [PubMed] [Google Scholar]
- Goo D., Kim J.H., Park G.H., Reyes J.B.D., Kil D.Y. Effect of heat stress and stocking density on growth performance, breast meat quality, and intestinal barrier function in broiler chickens. Animals. 2019;9:107. doi: 10.3390/ani9030107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory N.G. How climatic changes could affect meat quality. Food Res. Int. 2010;43:1866–1873. [Google Scholar]
- Gu X.H., Li S.S., Lin H. Effects of hot environment and dietary protein level on growth performance and meat quality of broiler chickens. Asian-Australas. J. Anim. Sci. 2008;21:1616–1623. [Google Scholar]
- Habibian M., Ghazi S., Moeini M.M., Abdolmohammadi A. Effects of dietary selenium and vitamin E on immune response and biological blood parameters of broilers reared under thermoneutral or heat stress conditions. Int. J. Biometeorol. 2014;58:741–752. doi: 10.1007/s00484-013-0654-y. [DOI] [PubMed] [Google Scholar]
- Hadad Y., Halevy O., Cahaner A. Featherless and feathered broilers under control versus hot conditions. 1. Breast meat yield and quality. Poult. Sci. 2014;93:1067–1075. doi: 10.3382/ps.2013-03591. [DOI] [PubMed] [Google Scholar]
- Haman F., Péronnet F., Kenny G.P., Massicotte D., Lavoie C., Scott C., Weber J.M. Effect of cold exposure on fuel utilization in humans: plasma glucose, muscle glycogen, and lipids. J. Appl. Physiol. 2002;93:77–84. doi: 10.1152/japplphysiol.00773.2001. [DOI] [PubMed] [Google Scholar]
- Haman F., Péronnet F., Kenny G.P., Massicotte D., Lavoie C., Weber J.M. Partitioning oxidative fuels during cold exposure in humans: muscle glycogen becomes dominant as shivering intensifies. J. Physiol. 2005;566:247–256. doi: 10.1113/jphysiol.2005.086272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashizawa Y., Kubota M., Kadowaki M., Fujimura S. Effect of dietary vitamin E on broiler meat qualities, color, water-holding capacity and shear force value, under heat stress conditions. Anim. Sci. J. 2013;84:732–736. doi: 10.1111/asj.12079. [DOI] [PubMed] [Google Scholar]
- Henrikson Z.A., Vermette C.J., Schwean-Lardner K., Crowe T.G. Effects of cold exposure on physiology, meat quality, and behavior of turkey hens and toms crated at transport density. Poult. Sci. 2018;97:347–357. doi: 10.3382/ps/pex227. [DOI] [PubMed] [Google Scholar]
- Holm C.G.P., Fletcher D.L. Antemortem holding temperatures and broiler breast meat quality. J. Appl. Poult. Res. 1997;6:180–184. [Google Scholar]
- Jahejo A.R., Rajput N., Rajput N.M., Leghari I.H., Kaleri R.R. Effects of heat stress on the performance of Hubbard broiler chicken. Cells Anim. Ther. 2016;2:1–5. [Google Scholar]
- Kanani P.B., Daneshyar M., Aliakbarlu J., Hamian F. Effect of dietary turmeric and cinnamon powders on meat quality and lipid peroxidation of broiler chicken under heat stress condition. Vet. Res. Forum. 2017;8:163–169. [PMC free article] [PubMed] [Google Scholar]
- Kennedy O.B., Stewart-Knox B.J., Mitchell P.C., Thurnham D.I. Flesh colour dominates consumer preference for chicken. Appetite. 2005;44:181–186. doi: 10.1016/j.appet.2004.11.002. [DOI] [PubMed] [Google Scholar]
- King N.J., Whyte R. Does it look cooked? A review of factors that influence cooked meat color. J. Food Sci. 2006;71:31–40. [Google Scholar]
- Lara L.J., Rostagno M.H. Impact of heat stress on poultry production. Animals. 2013;3:356–369. doi: 10.3390/ani3020356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.B., Hargus G.L., Hagberg E.C., Forsythe R.H. Effect of antemortem environmental temperatures on postmortem glycolysis and tenderness in excised broiler breast muscle. J. Food Sci. 1976;41:1466–1469. [Google Scholar]
- Lesiow T., Kijowski J. Impact of PSE and DFD meat on poultry processing: a review. Polish J. Food Nutr. Sci. 2003;12:3–8. [Google Scholar]
- Lin L.I. A concordance correlation coefficient to evaluate reproducibility. Biomatrics. 1989;45:255–268. [PubMed] [Google Scholar]
- Liu W., Yuan Y., Sun C., Balasubramanian B., Zhao Z., An L. Effects of dietary betaine on growth performance, digestive function, carcass traits, and meat quality in indigenous yellow-feathered broilers under long-term heat Stress. Animals. 2019;9:506. doi: 10.3390/ani9080506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z., He X., Ma B., Zhang L., Li J., Jiang Y., Zhou G., Gao F. Chronic heat stress impairs the quality of breast-muscle meat in broilers by affecting redox status and energy-substance metabolism. J. Agric. Food Chem. 2017;65:11251–11258. doi: 10.1021/acs.jafc.7b04428. [DOI] [PubMed] [Google Scholar]
- Lu Q., Wen J., Zhang H. Effect of chronic heat exposure on fat deposition and meat quality in two genetic types of chicken. Poult. Sci. 2007;86:1059–1064. doi: 10.1093/ps/86.6.1059. [DOI] [PubMed] [Google Scholar]
- Lubritz, D. 2007. Breeding for meat quality and high yield products. Accessed July 15, 2020. https://thepoultrysite.com/articles/breeding-for-meat-quality-and-high-yield-products.
- Mazur-Kuśnirek M., Antoszkiewicz Z., Fijałkowska M., Kotlarczyk S., Lipiński K., Purwin C. The effect of polyphenols and vitamin E on the antioxidant status and meat quality of broiler chickens fed diets naturally contaminated with ochratoxin A. Arch. Anim. Nutr. 2019;73:431–444. doi: 10.1080/1745039X.2019.1639445. http://sfx.scholarsportal.info/guelph/docview/2335123115?accountid=11233 Accessed September 24, 2021. [DOI] [PubMed] [Google Scholar]
- Min B., Ahn D.U. Sensory properties of packaged fresh and processed poultry meat. Advances in meat, poultry and seafood packaging. Woodhead Publishing Ltd. Sawston, UK, 2012:112–153. [Google Scholar]
- Mir N.A., Rafiq A., Kumar F., Singh V., Shukla V. Determinants of broiler chicken meat quality and factors affecting them: a review. J. Food Sci. Technol. 2017;54:2997–3009. doi: 10.1007/s13197-017-2789-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G., Group T.P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. PLOS Med. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- N'dri A.L., Mignon-Grasteau S., Sellier N., Beaumont C., Tixier-Boichard M. Interactions between the naked neck gene, sex, and fluctuating ambient temperature on heat tolerance, growth, body composition, meat quality, and sensory analysis of slow growing meat-type broilers. Livest. Sci. 2007;110:33–45. [Google Scholar]
- Offer G. Modelling of the formation of pale, soft and exudative meat: effects of chilling regime and rate and extent of glycolysis. Meat Sci. 1991;30:157–184. doi: 10.1016/0309-1740(91)90005-B. [DOI] [PubMed] [Google Scholar]
- Owens C.M., Alvarado C.Z., Sams A.R. Research developments in pale, soft, and exudative turkey meat in North America. Poult. Sci. 2009;88:1513–1517. doi: 10.3382/ps.2009-00008. [DOI] [PubMed] [Google Scholar]
- Owens C.M., Hirschler E.M., McKee S.R., Martinez-Dawson R., Sams A.R. The characterization and incidence of pale, soft, exudative turkey meat in a commercial plant. Poult. Sci. 2000;79:553–558. doi: 10.1093/ps/79.4.553. [DOI] [PubMed] [Google Scholar]
- Owens C.M., Mckee S.R., Matthews N.S., Sams A.R. The development of pale, exudative meat in two genetic lines of turkeys subjected to heat stress and its prediction by halothane screening. Poult. Sci. 2000;79:430–435. doi: 10.1093/ps/79.3.430. [DOI] [PubMed] [Google Scholar]
- Paiao F.G., Ferracin L.M., Pedrao M., Kato T., Shimokomaki M. Skeletal muscle calcium channel ryanodine and the development of pale, soft, and exudative meat in poultry. Genet. Mol. Res. 2013;12:3017–3027. doi: 10.4238/2013.August.20.3. [DOI] [PubMed] [Google Scholar]
- Petracci M., Betti M., Bianchi M., Cavani C. Color variation and characterization of broiler breast meat during processing in Italy. Poult. Sci. 2004;83:2086–2092. doi: 10.1093/ps/83.12.2086. [DOI] [PubMed] [Google Scholar]
- Petracci M., Bianchi M., Cavani C. The European perspective on pale, soft, exudative conditions in poultry. Poult. Sci. 2009;88:1518–1523. doi: 10.3382/ps.2008-00508. [DOI] [PubMed] [Google Scholar]
- Petracci M., Fletcher D.L., Northcutt J.K. The effect of holding temperature on live shrink, processing yield, and breast meat quality of broiler chickens. Poult. Sci. 2001;80:670–675. doi: 10.1093/ps/80.5.670. [DOI] [PubMed] [Google Scholar]
- Pietrzak M., Greaser M.L., Sosnicki A.A. Effect of rapid rigor mortis processes on protein functionality in pectoralis major muscle of domestic turkeys. J. Anim. Sci. 1997;75:2106–2116. doi: 10.2527/1997.7582106x. [DOI] [PubMed] [Google Scholar]
- Sandercock D.A., Hunter R.R., Nute G.R., Mitchell M.A., Hocking P.M. Acute heat stress-induced alterations in blood acid-base status and skeletal muscle membrane integrity in broiler chickens at two ages: implications for meat quality. Poult. Sci. 2001;80:418–425. doi: 10.1093/ps/80.4.418. [DOI] [PubMed] [Google Scholar]
- Schneider B.L., Renema R.A., Betti M., Carney V.L., Zuidhof M.J. Effect of holding temperature, shackling, sex, and age on broiler breast meat quality. Poult. Sci. 2012;91:468–477. doi: 10.3382/ps.2010-00952. [DOI] [PubMed] [Google Scholar]
- Shao D., Wang Q., Hu Y., Shi S., Tong H. Effects of cyclic heat stress on the phenotypic response, meat quality and muscle glycolysis of breasts and thighs of yellow-feather broilers. Ital. J. Anim. Sci. 2019;18:301–308. [Google Scholar]
- Sifa D., Bai X., Zhang D., Hu H., Wu X., Wen A., He S., Zhao L. Dietary glutamine improves meat quality, skeletal muscle antioxidant capacity and glutamine metabolism in broilers under acute heat stress. J. Appl. Anim. Res. 2018;46:1412–1417. [Google Scholar]
- Silva J.E. Thermogenic mechanisms and their hormonal regulation. Physiol. Rev. 2006;86:435–464. doi: 10.1152/physrev.00009.2005. [DOI] [PubMed] [Google Scholar]
- Skomorucha I., Muchacka R., Sosnowka-Czajka E. Effect of elevated air temperature on some quality parameters of broiler chicken meat. Ann. Anim. Sci. 2010;10:187–196. [Google Scholar]
- St-Pierre N.R. Invited review. Integrating quantitative findings from multiple studies using mixed model methodology. J. Dairy Sci. 2001;84:741–755. doi: 10.3168/jds.S0022-0302(01)74530-4. [DOI] [PubMed] [Google Scholar]
- St-Pierre N.R., Cobanov B., Schnitkey G. Economic losses from heat stress by US livestock industries. J. Dairy Sci. 2003;86:E52–E77. [Google Scholar]
- Tang S., Yu J., Zhang M., Bao E. Effects of different heat stress periods on various blood and meat quality parameters in young Arbor Acer broiler chickens. Can. J. Anim. Sci. 2013;93:453–460. [Google Scholar]
- Tavaniello S., Slawinska A., Prioriello D., Petrecca V., Bertocchi M., Zampiga M., Salvatori G., Maiorano G. Effect of galactooligosaccharides delivered in ovo on meat quality traits of broiler chickens exposed to heat stress. Poult. Sci. 2020;99:612–619. doi: 10.3382/ps/pez556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawfeek S.S., Hassanin K.M.A., Youssef I.M.I. The effect of dietary supplementation of some antioxidants on performance, oxidative stress. J. Worlds Poult. Res. 2014;4:10–19. [Google Scholar]
- Tedeschi L.O. Assessment of the adequacy of mathematical models. Agric. Syst. 2006;89:225–247. [Google Scholar]
- Toplu H.D.O., Nazligul A., Karaarslan S., Kaya M., Yagin O. Effects of heat conditioning and dietary ascorbic acid supplementation on growth performance, carcass and meat quality characteristics in heat-stressed broilers. Ankara Univ. Vet. Fak. Derg. 2014;61:295–302. [Google Scholar]
- Vermette C.J., Henrikson Z.A., Schwean-Lardner K.V, Crowe T.G. Influence of hot exposure on 12-week-old turkey hen physiology, welfare, and meat quality and 16-week-old turkey tom core body temperature when crated at transport density. Poult. Sci. 2017;96:3836–3843. doi: 10.3382/ps/pex220. [DOI] [PubMed] [Google Scholar]
- Wan X., Ahmad H., Zhang L., Wang Z., Wang T. Dietary enzymatically treated Artemisia annua L. improves meat quality, antioxidant capacity and energy status of breast muscle in heat-stressed broilers. J. Sci. Food Agric. 2018;98:3715–3721. doi: 10.1002/jsfa.8879. [DOI] [PubMed] [Google Scholar]
- Wang R.H., Liang R.R., Lin H., Zhu L.X., Zhang Y.M., Mao Y.W., Dong P.C., Niu L.B., Zhang M.H., Luo X. Effect of acute heat stress and slaughter processing on poultry meat quality and postmortem carbohydrate metabolism. Poult. Sci. 2017;96:738–746. doi: 10.3382/ps/pew329. [DOI] [PubMed] [Google Scholar]
- Warriss P.D. Pages 106–130 in Meat Science: An Introductory Text. CABI Pub; Oxon, UK: 2000. Meat quality. [Google Scholar]
- Wen C., Chen Y., Leng Z., Ding L., Wang T., Zhou Y. Dietary betaine improves meat quality and oxidative status of broilers under heat stress. J. Sci. Food Agric. 2019;99:620–623. doi: 10.1002/jsfa.9223. [DOI] [PubMed] [Google Scholar]
- West G.E., Larue B., Touil C., Scott S.L. The perceived importance of veal meat attributes in consumer choice decisions. Agribusiness. 2001;17:365–382. [Google Scholar]
- Xing T., Gao F., Tume R.K., Zhou G., Xu X. Stress effects on meat quality: a mechanistic perspective. Compr. Rev. Food Sci. Food Saf. 2019;18:380–401. doi: 10.1111/1541-4337.12417. [DOI] [PubMed] [Google Scholar]
- Zahoor I., Mitchell M.A., Hall S., Beard P.M., Gous R.M., De Koning D.J., Hocking P.M. Predicted optimum ambient temperatures for broiler chickens to dissipate metabolic heat do not affect performance or improve breast muscle quality. Br. Poult. Sci. 2016;57:134–141. doi: 10.1080/00071668.2015.1124067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeferino C.P., Komiyama C.M., Pelicia V.C., Fascina V.B., Aoyagi M.M., Coutinho L.L., Sartori J.R., Moura A.S.A.M.T. Carcass and meat quality traits of chickens fed diets concurrently supplemented with vitamins C and E under constant heat stress. Animal. 2016;10:163–171. doi: 10.1017/S1751731115001998. [DOI] [PubMed] [Google Scholar]
- Zhang Z.Y., Jia G.Q., Zuo J.J., Zhang Y., Lei J., Ren L., Feng D.Y. Effects of constant and cyclic heat stress on muscle metabolism and meat quality of broiler breast fillet and thigh meat. Poult. Sci. 2012;91:2931–2937. doi: 10.3382/ps.2012-02255. [DOI] [PubMed] [Google Scholar]
- Zhang Z.W., Lv Z.H., Li J.L., Li S., Xu S.W., Wang X.L. Effects of cold stress on nitric oxide in duodenum of chicks. Poult. Sci. 2011;90:1555–1561. doi: 10.3382/ps.2010-01333. [DOI] [PubMed] [Google Scholar]
- Zhang C., Zhao X., Wang L., Yang L., Chen X., Geng Z. Resveratrol beneficially affects meat quality of heat-stressed broilers which is associated with changes in muscle antioxidant status. Anim. Sci. J. 2017;88:1569–1574. doi: 10.1111/asj.12812. [DOI] [PubMed] [Google Scholar]
- Zhang M., Zhu L., Zhang Y., Mao Y., Zhang M., Dong P., Niu L., Luo X., Liang R. Effect of different short-term high ambient temperature on chicken meat quality and ultra-structure. Asian-Australas. J. Anim. Sci. 2019;32:701–710. doi: 10.5713/ajas.18.0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J.S., Deng W., Liu H.W. Effects of chlorogenic acid-enriched extract from Eucommia ulmoides leaf on performance, meat quality, oxidative stability, and fatty acid profile of meat in heat-stressed broilers. Poult. Sci. 2019;98:3040–3049. doi: 10.3382/ps/pez081. [DOI] [PubMed] [Google Scholar]
- Zhao F.Q., Zhang Z.W., Yao H.D., Wang L.L., Liu T., Yu X.Y., Li S., Xu S.W. Effects of cold stress on mRNA expression of immunoglobulin and cytokine in the small intestine of broilers. Res. Vet. Sci. 2013;95:146–155. doi: 10.1016/j.rvsc.2013.01.021. [DOI] [PubMed] [Google Scholar]