Abstract
Cholecystokinin A receptor (CCKAR) is a key receptor mediating satiety. Previous studies found that decreased expression of CCKAR attenuated satiety, and thus contributed to the high-growth of broiler chickens. The objective of this study is to map sequence variants associated with the growth of chickens in the CCKAR. The CCKAR and upstream 1.4 kb genomic sequences were resequenced to find out all sequence variants using 35 Lueyang black-boned chickens (LBC). Haplotypes were reconstructed using the PHASE program. Linkage disequilibrium between variants was analyzed using the Haploview software. Associations of 33 tag SNPs that captured 89% of all variants with body weight of LBC (n = 675) at 16 (BW16), 20 (BW20) weeks of age and the onset (BWOEP) of egg production were tested using linear mixed models. A total of 126 SNPs were found and formed 41 haplotypes in 35 resequenced samples. Average length of haplotype blocks is 129 bp, indicating that LBC maintains low linkage disequilibrium at the CCKAR locus. Eleven of 33 tag SNPs were significantly associated with BW16, but not with BW20 and BWOEP. These significantly associated variants were most (8/11) distributed in a 2 kb region (chr4:73206169-73208244) around the Exon3. They together with 33 captured variants potentially disrupted binding sites of 471 transcription factors. Twelve variants can disrupt appetite (FOXO1) or lipid metabolism-related TF (AR and C/EBP) motifs. This study recognized chr4:73206169-73208244 as a key region harboring functional variants affecting the growth of chickens.
Key words: Lueyang black-boned chicken, CCKAR, satiety, body weight
INTRODUCTION
Cholecystokinin (CCK) is a peptide hormone that is mainly synthesized in small intestinal endocrine cells and nervous fibers (Martinez et al., 1993). CCK induces satiety and thus is a key signaling molecule modulating food intake and energy balance of animals. Cholecystokinin A receptor (CCKAR) and cholecystokinin B receptor (CCKBR) are 2 key receptors mediating CCK actions. CCKBR binds CCK and gastrin with almost equal affinities. Its activation in the stomach stimulates gastric acid secretion, and in the brain is associated with anxiety and pain perception (Guilloteau et al., 2006). In contrast, CCKAR that exhibits a 500-fold higher affinity for CCK than for gastrin is believed to be the primary receptor mediating satiety (Guilloteau et al., 2006). The absence of CCKAR attenuated the satiety signal, which contributed to increasing food intake and obesity in human and rat (Miller et al., 1995; Moran and Bi, 2006). This link between abundant of CCK receptor and appetite was also found in chicken. Decreased expression of CCKAR facilitated the growth of chicken by changing satiety set point (Dunn et al., 2013). Sequence variants that affect CCKAR expression were associated with body weight of chickens, suggesting that cis-regulatory variants in the CCKAR form molecular basis controlling the growth of chickens (Rikimaru et al., 2013). Previous studies reported some sequence variants that were significantly associated with diverse growth traits of chickens (Dunn et al., 2013; Rikimaru et al., 2013; Yi et al., 2018). A fine mapping remains necessary to narrow down the region containing functional variants at the gene level. This study resequenced the CCKAR locus to find out all sequence variants, and mapped functional variants using 33 tag SNPs.
MATERIALS AND METHODS
Genetic Features of Lueyang Black-Boned Chickens and Measurement of Body Weight
Lueyang black-boned chicken (LBC) is an indigenous breed from Lueyang city of Shaanxi province, China. The breed has a dermal hyperpigmentation phenotype like the Silkie chicken. But its adult BW is 2 folds as large as the Silkie chicken. As LBC was not subjected to selection for production traits, it shows large variations in egg production and growth traits, implying that some QTL could not possibly be fixed in this population (Dang et al., 2020). In addition, our previous studies found that this breed sustained low genomic linkage disequilibrium (Wang et al., 2016). These genetic features make it being a suitable population for fine mapping of some functional variants. In this study, 675 pullets that were produced by mating 47 cocks with 296 hens were used in the association analysis. These pullets were simultaneously hatched and raised in the same hen house by Lueyang LongJia Agro-Tech Ltd. Co. (Lueyang city, Shaanxi, China) from March 2018 to January 2019. Body weights at the 16 (BW16), 20 (BW20) weeks of age and the onset of egg production (BWOEP) were measured for each bird after fasting for 12 h. Animal care, slaughter and experimental procedures were approved by Institutional Animal Care and Institutional Ethic Committee of Northwest A&F University (DK-2021020).
Resequencing of CCKAR and SNP Calling
The CCKAR gene and upstream 1.4 kb genomic sequence (chr4:73201947-73210290) of 35 chickens were resequenced by the Sanger sequencing. The resequenced samples were randomly selected from 675 pullets. Blood samples were collected using wing venipuncture. Genomic DNA was extracted using FlexGen Blood DNA Kit (CWBIO, Beijing, China) according to the manufacture's instruction. Seven fragments were amplified using 5’- CTACCAAATCAATGCCTGTC-3’ and 5’- TACATCTATCTCATTTAGCG-3’ for fragment1, 5’- AGCAGCTCAAGTTTACAAGT-3’ and 5’- TCAGATCACATAACTCCAAT-3’ for fragment2, 5’- GGCAGGAAAGAGTTCAGTAT-3’ and 5’-CAAAGTTCTCAAGGGCGTAA-3’ for fragment3, 5’-CGCCCTTGAGAACTTTGATG-3’ and 5’-GCAAATGGCACTGTACCGCT-3’ for fragment4, 5’-AGCACGGAGACCAAGACCCA-3’ and 5’-CAGAAGGACAGGAGCGGTTG-3’ for fragment5, 5’-CAACCCTCCAGTAGGTGCCA-3’ and 5’-TCACAGGCGGCTTCTGCTTA-3’ for fragment6, and 5’-TCCCATCTCCTTTATCCACC-3’ and 5’-AGCTCACATCTGCCCTTTAC-3’ for fragment7. PCR was performed using Dye-added 2 × Es Taq MasterMix (CWBIO, Beijing, China) in the Bio-Rad T100 Thermal Cycler (Bio-Rad Laboratories, Inc., Hercules, CA). Thermal cycle program was 95°C 3 min, 33 cycles of 95°C 30s, 60°C 30 s and 72°C 60 s followed by final elongation at 72°C for 5 min. PCR products were purified using Universal DNA purification kit (TIANGEN, Beijing, China). Purified PCR products were sequenced in 2 directions by GENERAL BIOL Co (Chuzhou, China). Sequence polymorphisms were found by multiple sequence alignment using ChromasPro 1.33.
Linkage Disequlibrium Analysis and Haplotype Reconstruction
SNPs with Hardy-Weinberg equilibrium P-value >0.001 and minor allele freq >0.05 were included in the linkage disequilibrium (LD) study. LD was analyzed using the Haploview 4.2 software. Haplotype block was defined using the confidence interval algorithm. Twenty-six tag SNPs were picked out in pairwise mode with r2 ≥0.7 and LOD ≥3.0 using the Tagger program in the Haploview 4.2 software. Additional 7 tag SNPs that belong to non-synonymous mutations or were reported to be associated with body weight of chickens were forced into the tag list. CCKAR haplotypes were reconstructed using the PHASE 2.1.1 program with the default parameters.
Genotyping Tag SNPs
Thirty-three tag SNPs were genotyped in 675 chickens using Sequenom MassARRAY platform. Multiplex PCR was performed in 384-well plates using PCR mix consisting 10 ng of gDNA, 0.5 μM PCR primer mix, 8 mM MgCl2, 2.5 mM dNTP, and 1 U of hot-start enzyme (Qiagen, Hilden, Germany), 0.625 μL of 10 × PCR buffer and 1.75 μL of ddH2O per reaction. Non-incorporated dNTPs were dephosphorylated by adding 2 μL mixture consisting of 0.3 μL (1.7 U/μL) shrimp alkaline phosphatase (NEB, Ipswich, MA), 0.17 μL SAP buffer (10 ×) and 1.53 μL H2O. The dephosphorylated products were then subjected to the single base extension reaction according to the iPLEX Assay protocol (Sequenom, San Diego,CA). The resulting products were dispensed onto a 384-element SpectroCHIP Bioassay with a nanodispenser (Sequenom). Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry MassARRAY was performed on a MassARRAY Analyzer Compact. MassARRAY results were analyzed with the SpectroTYPER 4.0 (Sequenom).
Association Analysis
This study fit a univariate linear mixed model (LMM) to test association of tag SNPs with body weight in the following form: y = Wα+xβ+u+ε; u∼MVNn(0, λτ−1K), ε∼MVNn(0, τ−1In) where y is an n-vector of BW; W is an n × c matrix of covariates consisting of a column of 1s and c-1 column of covariates; α is a c-vector of coefficents; x is an n-vector of marker genotypes; β is the effect size of the marker; u is an n-vector of random effects; ε is an n-vector of errors; τ−1 is the variance of the residual errors; λ is the ratio of random effect and residual error variances; K is a n × n relatedness matrix and In is a n × n identity matrix. MVNn denotes the n-dimensional multivariate normal distribution. The W is a 675-vector of 1s in the LMM for BW16 and BW20, and a 675 × 2 matrix consisting of a column of 1 s and a column of ages at the onset of egg production in the LMM for BWOEP. Missing genotypes were imputed based on LD information in 35 resequenced and 675 MassARRAY genotyped samples using the BIMBAM software prior to association analysis. Missing BW data was predicted using outputs from Bayesian sparse linear mixed model. Relatedness matrix was calculated using the kinship2 library in R program based on the pedigree of 675 chickens. LMMs were fit using the GEMMA software (Zhou and Stephens, 2012). GEMMA performed Wald test to get the effect size estimate for each tag SNPs and the corresponding p value. P-values were adjusted using the Benjamini and Hochberg approach to overcome the multiple testing problems. A Benjamini and Hochberg adjusted P -value (q-value) <0.05 is considered significant (Benjamini and Hochberg, 1995).
RESULTS AND DISCUSSION
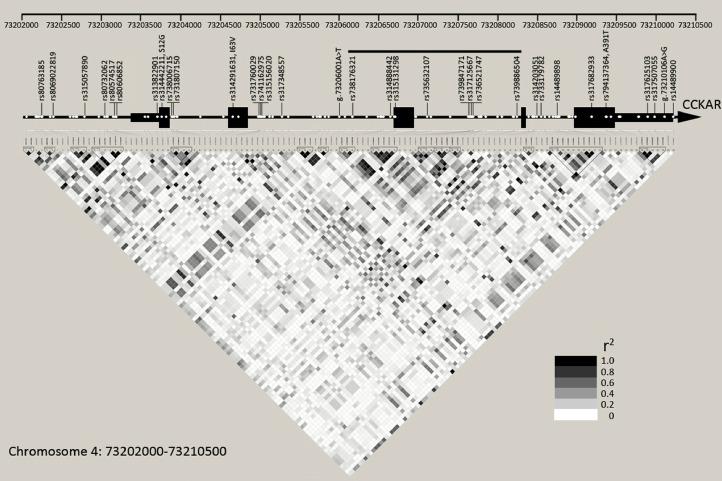
A total of 126 SNP were found in the CCKAR and upstream 1.4 kb region, and formed 41 haplotype in the 35 resequenced samples (Figure 1). There were no structural variants in the region. Twenty-four SNP were located in exons, and the others were distributed in promoter and introns (Figure 1). These variants sustained low LD as only 15 short haplotype blocks with an average length of 129 bp were present in the region (Figure 1). Low LD enables us to map functional variants within the CCKAR locus.
Figure 1.
Linkage disequilibrium structure and distribution of sequence variants in the CCKAR. This diagram shows the linkage disequilibrium structure of genomic region at Chromosome 4: 73202000-73210500. This region covers the whole CCKAR gene and upstream 1.4 kb region from transcription start site. The white points in the gene structure diagram represent 124 variants. The other two variants were excluded from linkage disequilibrium analysis due to minor allele frequencies less than 0.05. Those shown on the gene structure diagram are 33 tag SNPs. The bold black line indicates the region where most (8/11) of significantly associated tag SNPs were distributed. Haplotype blocks are outlined in black triangles on the linkage disequilibrium structure graph.
Thirty-three SNP that captured 89% of 126 SNP at r2 ≥0.7 were selected as tag SNPs to map functional variants (Figure 1). Eleven of 33 tag SNPs were significantly associated with BW16, but not with BW20 and BWOEP (Table 1). Most (8/11) of significantly associated variants were distributed in a 2 kb region (chr4:73206169-73208244) around the Exon3 (Table 1). Rs314291631 is a missense mutation in the Exon2. A previous study found that this variant was significantly associated with feed intake of Tianlu Black chicken (Yi et al., 2018). But rs314291631 and another two missense mutations were not associated with BW of LBC, implying that the changes of protein function are not a key factor affecting the growth of LBC (Figure 1; Table 1).
Table 1.
Association test of 33 tag SNPs in the CCKAR with body weight of Lueyang black-boned chickens.
| dbSNP No. | Description1 | Position | BW163 |
BW203 |
BWOEP3 |
|||
|---|---|---|---|---|---|---|---|---|
| Effect size (kg) | q value2 | Effect size (kg) | q value | Effect size (kg) | q value | |||
| rs80763185 | g.73202243T>A | Promoter | 0.06 ± 0.06 | 0.45 | 0.12 ± 0.08 | 0.32 | 0.03 ± 0.05 | 0.72 |
| rs8069022819 | g.73202391C>T | 0.07 ± 0.10 | 0.57 | 0.16 ± 0.13 | 0.39 | 0.02 ± 0.07 | 0.84 | |
| rs315057890 | g.73202789G>A | 0.02 ± 0.05 | 0.77 | −0.03 ± 0.06 | 0.65 | 0.02 ± 0.03 | 0.74 | |
| rs80732062 | g.73203040C>A | 0.18 ± 0.18 | 0.47 | 0.28 ± 0.25 | 0.46 | 0.05 ± 0.14 | 0.81 | |
| rs80574517 | g.73203169T>C | 0.01 ± 0.04 | 0.87 | −0.04 ± 0.06 | 0.60 | 0.01 ± 0.03 | 0.81 | |
| rs80606852 | g.73203188G>A | 0 ± 0.01 | 0.87 | −0.01 ± 0.02 | 0.60 | 0 ± 0.01 | 0.81 | |
| rs313822901 | g.73203705A>C | 5’UTR | 0.05 ± 0.11 | 0.75 | −0.03 ± 0.15 | 0.89 | 0.09 ± 0.08 | 0.52 |
| rs314442211 | g.73203766A>G | Exon1 | 0.04 ± 0.05 | 0.52 | 0 ± 0.06 | 0.99 | 0.02 ± 0.03 | 0.71 |
| rs738006715 | g.73203887T>G | Intron1 | 0 ± 0.03 | 0.95 | −0.03 ± 0.04 | 0.59 | 0 ± 0.02 | 0.90 |
| rs731807150 | g.73203909T>C | 0.12 ± 0.10 | 0.39 | 0.09 ± 0.14 | 0.64 | 0.05 ± 0.08 | 0.66 | |
| rs314291631 | g.73204661G>A | Exon2 | 0.14 ± 0.06 | 0.06 | 0.18 ± 0.08 | 0.11 | 0.06 ± 0.05 | 0.37 |
| rs731760029 | g.73204992G>A | Intron2 | 0.37 ± 0.20 | 0.13 | 0.15 ± 0.28 | 0.65 | 0.18 ± 0.15 | 0.47 |
| rs741162975 | g.73205010C>T | 0.22 ± 0.14 | 0.21 | 0.07 ± 0.20 | 0.76 | 0.1 ± 0.11 | 0.54 | |
| rs315156020 | g.73205026C>T | 0.08 ± 0.04 | 0.12 | 0.05 ± 0.05 | 0.53 | 0.04 ± 0.03 | 0.37 | |
| rs317348557 | g.73205278G>A | 0.37 ± 0.13 | 0.03 | 0.44 ± 0.18 | 0.11 | 0.16 ± 0.10 | 0.34 | |
| Novel SNP | g.73206001T>A | 0.18 ± 0.10 | 0.13 | 0.25 ± 0.13 | 0.15 | 0.08 ± 0.07 | 0.48 | |
| rs738176321 | g.73206169C>T | 0.09 ± 0.03 | 0.03 | 0.10 ± 0.04 | 0.11 | 0.04 ± 0.02 | 0.34 | |
| rs314888442 | g.73206650G>A | 0.04 ± 0.02 | 0.03 | 0.04 ± 0.02 | 0.11 | 0.02 ± 0.01 | 0.34 | |
| rs315131298 | g.73206714C>T | Exon3 | 0.03 ± 0.01 | 0.03 | 0.04 ± 0.02 | 0.11 | 0.02 ± 0.01 | 0.34 |
| rs735632107 | g.73207113A>G | Intron3 | 0.21 ± 0.08 | 0.03 | 0.22 ± 0.11 | 0.12 | 0.10 ± 0.06 | 0.34 |
| rs739847171 | g.73207633A>G | 0.20 ± 0.08 | 0.03 | 0.23 ± 0.11 | 0.11 | 0.10 ± 0.06 | 0.34 | |
| rs317125667 | g.73207665T>C | 0.06 ± 0.02 | 0.03 | 0.07 ± 0.03 | 0.11 | 0.03 ± 0.02 | 0.34 | |
| rs736521747 | g.73207685T>C | 0.09 ± 0.03 | 0.03 | 0.10 ± 0.05 | 0.11 | 0.05 ± 0.03 | 0.34 | |
| rs739886504 | g.73208244G>C | Exon4 | 0.34 ± 0.14 | 0.04 | 0.44 ± 0.19 | 0.11 | 0.17 ± 0.10 | 0.34 |
| rs314203051 | g.73208495C>T | Intron4 | 0.08 ± 0.04 | 0.12 | 0.09 ± 0.05 | 0.21 | 0.04 ± 0.03 | 0.37 |
| rs733179782 | g.73208549T>C | 0.14 ± 0.06 | 0.07 | 0.14 ± 0.09 | 0.24 | 0.05 ± 0.05 | 0.47 | |
| rs14489898 | g.73208752A>T | 0.07 ± 0.06 | 0.40 | 0.07 ± 0.08 | 0.53 | 0.06 ± 0.04 | 0.37 | |
| rs317682933 | g.73209189T>C | Exon5 | 0.02 ± 0.03 | 0.48 | 0.04 ± 0.04 | 0.46 | 0.02 ± 0.02 | 0.61 |
| rs794137364 | g.73209364G>A | 0.13 ± 0.14 | 0.48 | 0.27 ± 0.19 | 0.31 | 0.08 ± 0.10 | 0.61 | |
| rs317625103 | g.73209893T>C | 3’UTR | 0.18 ± 0.06 | 0.03 | 0.18 ± 0.09 | 0.11 | 0.10 ± 0.05 | 0.34 |
| rs317507055 | g.73209978A>G | 0.09 ± 0.11 | 0.51 | 0.11 ± 0.15 | 0.60 | 0.01 ± 0.08 | 0.90 | |
| Novel SNP | g.73210106A>G | 0.36 ± 0.14 | 0.03 | 0.47 ± 0.19 | 0.11 | 0.19 ± 0.11 | 0.34 | |
| rs14489900 | g.73210214A>C | 0.18 ± 0.10 | 0.16 | 0.13 ± 0.14 | 0.53 | 0.08 ± 0.08 | 0.52 | |
Alleles in front are effect alleles. Physical positions of SNP at the chromosome 4 are given according to the chicken reference genome Galgal 6.0 in the UCSC database.
Q-values represent Benjamini and Hochberg adjusted P-values. Those shown in bold indicate significant association between tag SNPs and body weight.
BW16, BW20, and BWOEP represent body weight at 16, 20 weeks of age and the onset of egg production.
There are 6 tag SNPs distributed within upstream 1.4 kb region of CCKAR, a key region controlling gene expression (Figure 1). But none of them were associated with BW of LBC (Table 1). Rs313822901 were reported to disrupt YY1 binding, and was significantly associated with BW of Japanese indigenous chickens (Rikimaru et al., 2013). But this variant was not associated with BW of the LBC (Table 1). Rs315131298 was another marker that was reported to have a significant additive effect on BW (Dunn et al., 2013). We confirmed the association not only for rs315131298, but for other 8 variants around the rs315131298 (Table 1). In addition, 2 SNPs located in the 3’ UTR also showed significant association with BW (Table 1).
These significantly associated tag SNPs together with adjacent 33 SNPs captured by them became promising candidates affecting CCKAR expression and the growth of LBC. We predicted effect of the 44 SNPs on TF motifs using the JASPAR database. These variants can disrupt 471 TF motifs. They were widely involved in differentiation of hematopoietic, lymphoid, endothelial, cardiac muscle and bone resorbing cells, regulation of premature reproductive senescence, energy metabolism, tumorigenesis, and development of vascular calcification, metabolic and cardiovascular diseases, etc. Forkhead Box O1 (FOXO1) represents one of promising TFs as it is involved in appetite regulation by integrating leptin and insulin signals in hypothalamic neurons (Peng et al., 2020). Three variants (rs316313304, rs737347140, and rs317125667) have a potential disrupting effect on the FOXO1 motif. Although almost nothing is known about relationship between FOXO1 and CCK system, these FOXO1 motif variants can affect avian appetite by regulating CCKAR expression given CCK system may play a more important role in appetite regulation in birds than that in mammals (Honda et al., 2017). Androgen receptor (AR) and members of the CCAAT/enhancer-binding protein (C/EBP) are another class of TFs that may regulate CCKAR expression. AR and C/EBP members are involved in lipid metabolism by regulating expression of IGF-1, leptin, inulin, PPARγ etc. (Staiger et al., 2009; Rana et al., 2011). Variants in TF itself and motifs were widely associated with obesity and related metabolic diseases in humans and mice (Bennett et al., 2010; Rana et al., 2011; Ren et al., 2014). In this study we found that 9 variants potentially disrupted the AR (rs314054492) and the C/EBP (rs318084470, rs741082602, rs14489892, rs735511990, rs317507055, rs739162066, rs315744114, and rs315844714) motifs. CCK signals play an important role in regulation of energy balance (Richards, 2003). Therefore, it is possible that these AR and C/EBP motif variants modulate the growth of chickens by affecting CCKAR expression.
This study identified a 2 kb region (chr4:73206169-73208244) around the Exon3 as a promising region containing functional variants affecting the growth of chicken.
ACKNOWLEDGMENTS
This study was funded by grants from Key Research and Development Program of Shaanxi Province (2021NY-028) and Egg laying Lueyang Black-boned Chicken Breeding Project (WJYJY-2021-3).
DISCLOSURES
The authors declare that they have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled “Fine mapping of sequence variants associated with body weight of Lueyang black-boned chicken in the cholecystokinin A receptor gene”. The authors have no conflicts of interest.
REFERENCES
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Series. B Stat. Methodol. 1995;57:289–300. [Google Scholar]
- Bennett C.E., Nsengimana J., Bostock J.A., Cymbalista C., Futers T.S., Knight B.L., McCormack L.J., Prasad U.K., Riches K., Rolton D., Scarrott T., Barrett J.H., Carter A.M. CCAAT/enhancer binding protein alpha, beta and delta gene variants: associations with obesity related phenotypes in the Leeds Family Study. Diab. Vasc. Dis. Res. 2010;7:195–203. doi: 10.1177/1479164110366274. [DOI] [PubMed] [Google Scholar]
- Dang L.P., Liu R.F., Zhao W.Y., Zhou W.X., Min Y.N., Wang Z.P. Investigating structural impact of a valine to isoleucine substitution on anti-Müllerian hormone in silico and genetic association of the variant and AMH expression with egg production in chickens. J. Integr. Agric. 2020;19:1635–1643. [Google Scholar]
- Dunn I.C., Meddle S.L., Wilson P.W., Wardle C.A., Law A.S., Bishop V.R., Hindar C., Robertson G.W., Burt D.W., Ellison S.J., Morrice D.M., Hocking P.M. Decreased expression of the satiety signal receptor CCKAR is responsible for increased growth and body weight during the domestication of chickens. Am. J. Physiol. Endocrinol. Metab. 2013;304:E909–E921. doi: 10.1152/ajpendo.00580.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilloteau P., Meuth-Metzinger V.Le, Morisset J., Zabielski R. Gastrin, cholecystokinin and gastrointestinal tract functions in mammals. Nutr. Res. Rev. 2006;19:254–283. doi: 10.1017/S0954422407334082. [DOI] [PubMed] [Google Scholar]
- Honda K., Saneyasu T., Kamisoyama H. Gut hormones and regulation of food intake in birds. J. Poult. Sci. 2017;54:103–110. doi: 10.2141/jpsa.0160100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez V., Rodriguez-Membrilla A., Jimenez M., Goñalons E., Vergara P. Immunohistochemical differentiation of gastrin and cholecystokinin in gastrointestinal tract of chickens. Poult. Sci. 1993;72:2328–2336. doi: 10.3382/ps.0722328. [DOI] [PubMed] [Google Scholar]
- Miller L.J., Holicky E.L., Ulrich C.D., Wieben E.D. Abnormal processing of the human cholecystokinin receptor gene in association with gallstones and obesity. Gastroenterology. 1995;109:1375–1380. doi: 10.1016/0016-5085(95)90601-0. [DOI] [PubMed] [Google Scholar]
- Moran T.H., Bi S. Hyperphagia and obesity in OLETF rats lacking CCK-1 receptors. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006;361:1211–1218. doi: 10.1098/rstb.2006.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S.M., Li W., Hou N.N., Huang N. A review of FoxO1-regulated metabolic diseases and related drug discoveries. Cells. 2020;9:184. doi: 10.3390/cells9010184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana K., Fam B.C., Clarke M.V., Pang T.P.S., Zajac J.D., MacLean H.E. Increased adiposity in DNA binding-dependent androgen receptor knockout male mice associated with decreased voluntary activity and not insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2011;301:E767–E778. doi: 10.1152/ajpendo.00584.2010. [DOI] [PubMed] [Google Scholar]
- Ren W., Guo J.J., Jiang F., Lu J., Ding Y., Li A.M., Liang X.B., Jia W.P. CCAAT/Enhancer-binding protein α is a crucial regulator of human fat mass and obesity associated gene transcription and expression. Biomed. Res. Int. 2014;2014 doi: 10.1155/2014/406909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards M.P. Genetic regulation of feed intake and energy balance in poultry. Poult. Sci. 2003;82:907–916. doi: 10.1093/ps/82.6.907. [DOI] [PubMed] [Google Scholar]
- Rikimaru K., Takeda H., Uemoto Y., Komatsu M., Takahashi D., Suzuki K., Takahashi H. Effect of a single-nucleotide polymorphism in the cholecystokinin type A receptor gene on growth traits in the Hinai-dori chicken breed. J. Poult. Sci. 2013;50:206–211. [Google Scholar]
- Staiger J, Lueben M.J., Berrigan D., Malik R., Perkin S.N., Hursting S.D., Johnson P.F. C/EBPb regulates body composition, energy balance-related hormones and tumor growth. Carcinogenesis. 2009;30:832–840. doi: 10.1093/carcin/bgn273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Meng G., Li N., Yu M., Liang X., Min Y., Liu F., Gao Y. The association of very low-density lipoprotein receptor (VLDLR) haplotypes with egg production indicates VLDLR is a candidate gene for modulating egg production. Genet. Mol. Biol. 2016;39:380–391. doi: 10.1590/1678-4685-GMB-2015-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi Z, Li X., Luo W., Xu Z., Ji C., Zhang Y., Nie Q., Zhang D., Zhang X. Feed conversion ratio, residual feed intake and cholecystokinin type A receptor gene polymorphisms are associated with feed intake and average daily gain in a Chinese local chicken population. J. Anim. Sci. Biotechnol. 2018;9:50. doi: 10.1186/s40104-018-0261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Stephens M. Genome-wide efficient mixed-model analysis for association studies. Nat. Genet. 2012;44:821–824. doi: 10.1038/ng.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]