Abstract
Rhythmic rest-activity cycles are controlled by an endogenous clock. In Drosophila, this clock resides in ∼150 neurons organized in clusters whose hierarchy changes in response to environmental conditions. The concerted activity of the circadian network is necessary for the adaptive responses to synchronizing environmental stimuli. Thus far, work was devoted to unravel the logic of the coordination of different clusters focusing on neurotransmitters and neuropeptides. We further explored communication in the adult male brain through ligands belonging to the bone morphogenetic protein (BMP) pathway. Herein we show that the lateral ventral neurons (LNvs) express the small morphogen decapentaplegic (DPP). DPP expression in the large LNvs triggered a period lengthening phenotype, the downregulation of which caused reduced rhythmicity and affected anticipation at dawn and dusk, underscoring DPP per se conveys time-of-day relevant information. Surprisingly, DPP expression in the large LNvs impaired circadian remodeling of the small LNv axonal terminals, likely through local modulation of the guanine nucleotide exchange factor Trio. These findings open the provocative possibility that the BMP pathway is recruited to strengthen/reduce the connectivity among specific clusters along the day and thus modulate the contribution of the clusters to the circadian network.
SIGNIFICANCE STATEMENT The circadian clock relies on the communication between groups of so-called clock neurons to coordinate physiology and behavior to the optimal times across the day, predicting and adapting to a changing environment. The circadian network relies on neurotransmitters and neuropeptides to fine-tune connectivity among clock neurons and thus give rise to a coherent output. Herein we show that decapentaplegic, a ligand belonging to the BMP retrograde signaling pathway required for coordinated growth during development, is recruited by a group of circadian neurons in the adult brain to trigger structural remodeling of terminals on a daily basis.
Keywords: circadian remodeling, DPP, LNvs, PDF, structural plasticity, Trio
Introduction
A balanced communication between relevant neuronal clusters is essential to achieve a correct modulation of physiology and behavior. In the adult brain, groups of neurons containing a molecular clock coordinate different behaviors (e.g., locomotor activity, sleep, feeding) and physiology with time of day and are collectively known as circadian neurons (Welsh et al., 2010). Although in mammals this function is fulfilled by ∼10,000 neurons located in the suprachiasmatic nucleus (Evans and Gorman, 2016), ∼150 clock neurons support the circadian pacemaker in the adult fly brain (Shafer et al., 2006); other clocks can be found scattered throughout the body and are termed peripheral clocks (Dibner et al., 2010). The molecular clock is conserved across animals; in Drosophila this clock relies on the activity of transcription factors CLOCK (CLK) and CYCLE (CYC), which drive circadian oscillations by promoting rhythmic transcription of hundreds of genes, including period (per), timeless (tim), and clockwork orange (cwo), which in turn repress CLK/CYC-mediated transcription (Abruzzi et al., 2011; Ozkaya and Rosato, 2012; Abruzzi et al., 2017). Clock neurons are anatomically clustered in distinct groups: small and large ventrolateral (s-LNvs, l-LNvs, and the fifth s-LNv), the dorsolateral (LNds), the lateral posterior (LPNs) and three subgroups of dorsal neurons (DNs1-3) (Shafer et al., 2006). The LNvs are the only ones that express a neuropeptide called pigment dispersing factor (PDF), which plays a major role in the synchronization of the circadian network. PDF is essential for normal circadian activity patterns in light/dark (LD) cycles and for persistent circadian rhythms under free running conditions in constant darkness (DD; Renn et al., 1999; Peng et al., 2003; Yoshii et al., 2009).
The coordinated operation of the circadian network is necessary for the adaptive responses to synchronizing environmental stimuli; despite the key role of PDF within the circadian ensemble (Liang et al., 2017), clock neurons use a heterogeneous set of neuropeptides, that is, short neuropeptide F, ion transport peptide, and CCHamide1 (Hermann et al., 2012; He et al., 2013; Hermann-Luibl et al., 2014; Yao and Shafer, 2014; Fujiwara et al., 2018), and neurotransmitters (Guo et al., 2016; Frenkel et al., 2017; Duhart et al., 2020) for communication, contributing to the rhythmic profile of locomotor activity. Behavioral missexpression screens also retrieved unexpected candidates mediating communication between circadian clusters in the adult brain; one such example are ligands belonging to the bone morphogenetic protein (BMP) pathway (Beckwith et al., 2013). Through genetic manipulation of the intracellular components of BMP it was shown that sustained activation of this pathway within the LNvs generates a long period phenotype because of the decrease in CLK levels, providing a venue to integrate signals from different circadian clusters (Beckwith et al., 2013). The BMP pathway is a highly conserved retrograde signaling pathway that influences synaptic connectivity, ultimately controlling gene transcription (McCabe et al., 2003; Ball et al., 2010); it plays fundamental roles in tissue patterning and homeostasis (Hamaratoglu et al., 2014). Several ligands belonging to the BMP pathway are expressed during development in the Drosophila embryo and larvae: Maverick (MAV), Myoglianin (MYO), Glassbottom boat (GBB), Activin β (ACTβ), Dawless (DAW), Screw (SCW) and decapentaplegic (DPP), and were found to be relevant for rhythmic activity patterns in the adult fly (Beckwith et al., 2013). BMP ligands transmit biological information by binding to type I and type II receptors, which form heterotetrametric complexes in the presence of dimerized ligands; these complexes transduce the information to the nucleus through phosphorylation and hence, activation, of a group of proteins called SMADs; in Drosophila there is only one orthologue, Mothers against DPP (MAD). In the nucleus, phosphorylated MAD (p-MAD) regulates gene expression alone or by association with different coregulators (Raftery and Sutherland, 1999; Moustakas and Heldin, 2009). The most compelling evidence that this pathway could be recruited in the adult brain, and in particular by the circadian network, is the effect of the activation of this pathway over CLK abundance (Beckwith et al., 2013); additional evidence points to miR-124, which targets, among other genes, several BMP components (Garaulet et al., 2016; Zhang et al., 2016).
Herein we show that DPP mediates rapid changes in connectivity among circadian clusters in the adult brain promoting the remodeling of the sLNv terminals at the dorsal protocerebrum. Interestingly, the arousal promoting large LNvs contribute to this process; within the sLNvs, activation of the BMP pathway could result in the expression of the Rho-type guanine exchange factor TRIO and thus promote the activation of small GTPases such as Rac1 to modulate actin cytoskeletal dynamics and hence the complexity of the terminals (Ball et al., 2010). Moreover, acute DPP released from the large LNvs reduces the complexity of the sLNv dorsal terminals and affects circadian behavior, confirming a link between these two processes (Petsakou et al., 2015). These findings suggest that cluster-specific ligand release contributes to the refinement of the communication strength between pairs of circadian clusters to adjust the hierarchy of the clusters and support an adaptive behavioral response.
Materials and Methods
Fly rearing and strains
Flies were grown and maintained at 25°C in vials containing standard cornmeal medium under 12:12 h LD cycles. Adult-specific induction was achieved through the TARGET system (McGuire et al., 2004). Adult-specific thermosensitive Gal4 expression was induced transferring flies, 4–5 d, raised at 18–20°C during development to 30°C for the number of days indicated in the corresponding figure legend. The reporter lines dpp::GFP and mad::GFP were obtained from the Vienna Stock Center (stock #318414 and #318395, respectively). The sLNvs-Gal4 (Herrero et al., 2020) and R10H10-Gal4 (Sekiguchi et al., 2020) driver lines were provided by Taishi Yoshii. Stocks C929-Gal4 (Taghert et al., 2001), pdf-Gal4 (stock #6900), tuIb-Gal80ts (stock #7017), UAS-dpp (stock #1486), UAS-dppRNAi (stock #33618), UAS-GFP (stock #4776), UAS-trio (stock #9513) were obtained from the Bloomington Stock Center. The dClk856-Gal4 line was shared by Nicholas Glossop (Gummadova et al., 2009), and pdf-RFP and UAS-dppGFP (Dahal et al., 2017) were provided by Justin Blau and Emily Bates, respectively.
Adult locomotor activity
For locomotor activity experiments male flies 45 days old and raised at 22°C were entrained for 4 d in 12:12 LD cycles at 30°C and then transferred to DD at 30°C. Males were placed in glass tubes containing standard food and monitored for activity with infrared detectors and an automated data collection system (TriKinetics). Activity was monitored for 17 d (4 d in LD and 10–13 d in DD). Period and rhythmicity (power significance) in DD were estimated using ClockLab software (Actimetrics) as previously described (Depetris-Chauvin et al., 2011).
Average activity
Average activity plots were made using the plug-in ActogramJ (Schmid et al., 2011). Individual actograms were normalized by a reference actogram, and all of them were scaled so that overall activity matched that of the reference one. Next, actograms were transformed into average activity plots, summarizing the activity during LD and dividing for the number of days. A Gaussian normalization was applied, and all activity plots for individuals were average per genotype.
Anticipation indexes
The anticipation indexes were calculated as reported (Delventhal et al., 2019). For each fly an index for the anticipation of the morning peak [morning anticipation index (MAI)] and for the evening peak [evening anticipation index (EAI)] was calculated. Indexes per fly were calculated as the ratio between the mean activity exhibited during the last 1.5 h and the last 3 h before lights on and off for the MAI and EAI, respectively. We purposely excluded from the analysis the 30 min bin including the startle effect. In the graph each dot represents the index for the morning (or evening) anticipation of a single fly.
Immunohistochemistry and image acquisition
For consistency between behavioral and immunohistochemistry experiments only male flies were used in these experiments. Adult flies and fly heads were fixed with 4% p-formaldehyde, pH 7.5, for 40–60 min at room temperature. Brains were dissected and rinsed five times in PBS with 0.1% Triton X-100 (PT) for 30 min. Samples were blocked in 7% normal goat serum (in PT) for 1 h and incubated with primary antibodies at 4°C for 2 d. The primary antibodies used were chicken anti-GFP (1:250; Aves Labs), chicken anti-RFP (1:250; Clontech Laboratories), homemade rat anti-PDF (1:250; Depetris-Chauvin et al., 2011), and rabbit anti-pMAD (1:100; Epitomics). Samples were washed 4× 15 min in PT and incubated with secondary antibodies at 1:250 for 2 h at room temperature. Secondary antibodies were washed 4× 15 min in PT and mounted in Vectashield antifade mounting medium (Vector Laboratories). The secondary antibodies used were Cy2-conjugated donkey anti-chicken, Alexa Fluor 647-conjugated AffiniPure donkey anti-rat and Cy3-conjugated AffiniPure donkey anti-rabbit and anti-chicken (Jackson ImmunoResearch). Images were taken on a Zeiss LSM 710 confocal microscope.
Structural plasticity analysis and PDF immunoreactivity
Images were taken with a 63× objective and an optical zoom of 1.4×. The RFP signal was adjusted to threshold levels generating a selection that delimits the area of sLNv axonal terminals. This selection was then applied to the PDF channel, and mean intensity was measured. For the analysis of PDF immunoreactivity, all pictures were taken using the same confocal settings, and quantification was performed using the ImageJ package (https://rsbweb.nih.gov/ij/). Structural plasticity was analyzed by Scholl analysis using the ImageJ plug-in already described (Stanko et al., 2015).
Data analysis and statistics
Statistical analyses were performed and graphs created with RStudio (version 0.98.501) and GraphPath (Prism 5). Normality and homogeneity of variances were examined using Shapiro–Wilk's test and Bartlett's test, respectively; in case data did not adjust to these parameters nonparametric analysis was used, that is, a Mann–Whitney test for comparing between groups and a Kruskal–Wallis test instead of a two-way ANOVA. In the graphs describing the analysis of structural plasticity and PDF levels, only experimental groups that share no letter indicate statistically significant differences, with p < 0.05. Effects on structural plasticity and PDF levels were analyzed by a Kruskal–Wallis test, followed by Dunn's post hoc test. The number of flies or brains in each experiment is indicated as n.
Results
The BMP pathway is active in the adult brain
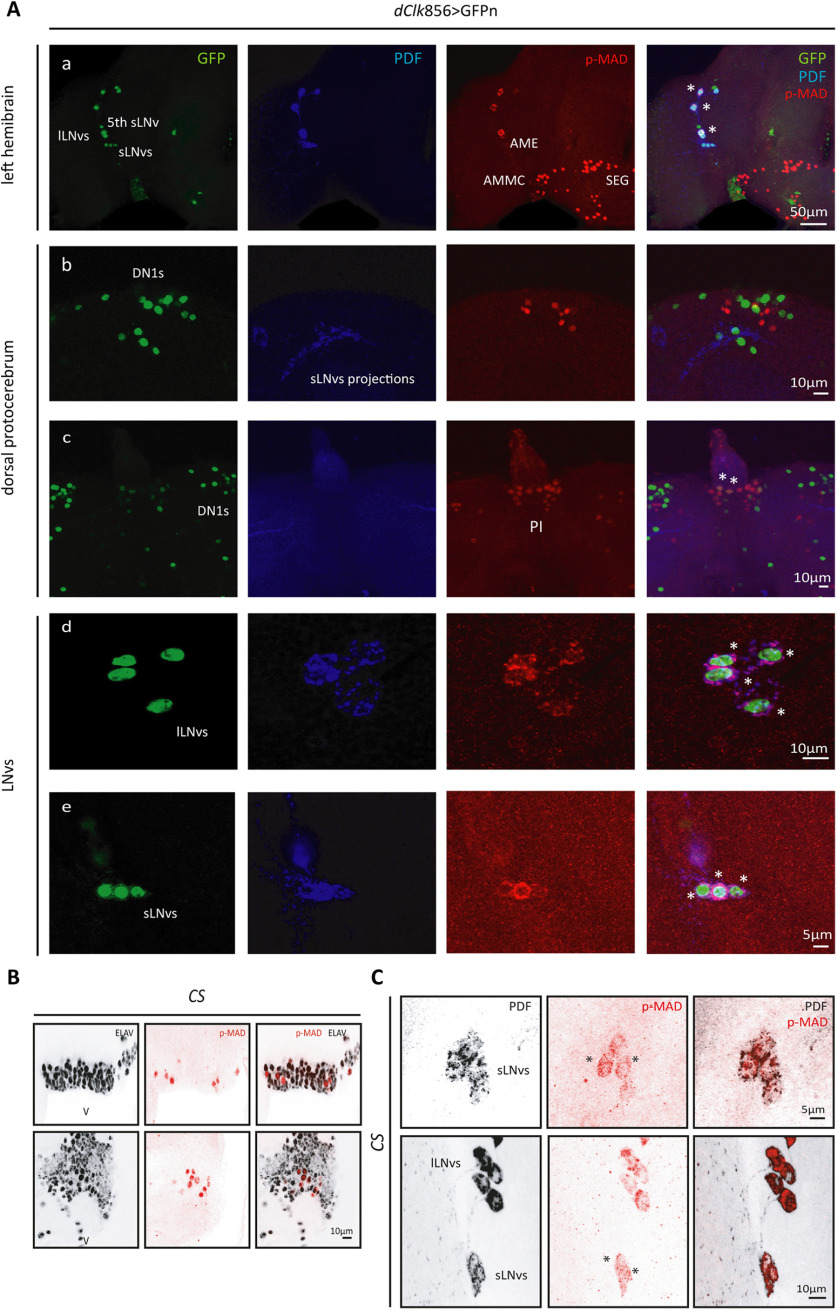
To examine the possibility that the BMP pathway is active under physiological conditions (i.e., in the absence of a genetic manipulation that upregulates or downregulates the activity of this signaling pathway intracellularly) we took advantage of the GAL4/upstream activating sequence (GAL4/UAS) system to drive expression of nuclear GFP (GFPn) in all circadian clusters; the p-MAD antibody was used to recognize those where the pathway is active. Previous work in zebrafish revealed that the transcription factor MAD is regulated by the circadian clock with a peak in mRNA levels at the beginning of the light phase (Sloin et al., 2018), thus we wondered whether the pathway could be active at that time in the fruit fly.
As shown in Figure 1A, we analyzed p-MAD immunoreactivity at Zeitgeber time (ZT)02 (2 h after lights on), which is also the peak of the circadian-relevant clock protein PERIOD (PER; Shafer et al., 2002). At the beginning of the day, the pathway is active (indicated by the nuclear localization of p-MAD immunoreactivity) in several brain areas including the pars intercerebralis (PI), subesophageal ganglion, the antenna-mechanosensory and motor center, and also the antennal lobes; but, to our surprise, among clock neurons only the LNvs showed this proxy of pathway activation during the early day. Nuclear p-MAD immunoreactivity was also detected in ventral areas of the brain, and the signal colocalized with the neuronal marker ELAV, suggesting the pathway is mostly active in neurons at ZT02 (Fig. 1B). Interestingly, in the LNvs, p-MAD immunoreactivity localizes to the cytoplasm rather than the nucleus as it has been described in most biological settings (Ramel and Hill, 2012), and it is observed in noncircadian adult neurons (Fig. 1A). A cytoplasmic p-MAD signal could be a result of a recent pathway activation where the p-MAD and MEDEA complex had not yet entered the nucleus, or alternatively, is actively retained in the cytoplasm through phosphorylation on regulatory sites (Aleman et al., 2014) or shuttled from the nucleus to modulate activity (Hill, 2009), potentially in a time-dependent fashion. To shed light on either possibility, we analyzed an earlier time point (ZT1.5). Interestingly, p-MAD was detected in the nucleus of the LNvs at that time confirming that the pathway is active in the early morning (Fig. 1C). Surprisingly, we were not able to detect p-MAD at other times throughout the day (data not shown), likely a reflection of the transient intrinsic nature of p-MAD (Berke et al., 2013) and the tight post-translational regulation it is subjected to (Urrutia et al., 2016).
Figure 1.
The BMP pathway is active in the LNvs. Representative confocal images of different regions of the adult brain. A, Left hemibrain displaying the dorsal ventral part of the brain and LNvs (a). Dorsal protocerebrum, showing the DN1 cluster (b) and the PI (c). Detail of the lLNvs (d) and sLNvs (e). Nuclear GFPn staining (green) was used to identify the different circadian clusters driven by dClk856-G4, and PDF (blue) labels the LNvs; p-MAD (red) was used as a reporter of the activity of the BMP pathway. Asterisks denote colocalization of GFP and PDF (only in the LNvs) with p-MAD. B, Representative confocal images from ventral areas of the brain in wild-type (CS) flies. BMP pathway activation is reported by the detection of p-MAD (red) colocalized with the pan-neuronal marker ELAV (gray) at ZT02. C, Detection of nuclear p-MAD in the sLNvs at ZT1.5. LNvs were identified by PDF staining (gray). Asterisks highlight nuclear p-MAD signal.
Previous work from our laboratory showed that some BMP ligands are relevant for the control of circadian locomotor activity (Beckwith et al., 2013); to identify the source of specific ligands in the adult brain, we analyzed the expression pattern of each morphogen belonging to this pathway (DPP, GBB, Actβ, MYO, MAV, DAW and SCW; data not shown). DPP was the only one with restricted expression to the LNvs, making it the focus of our attention; in fact, chronic downregulation leads to a significant deconsolidation of the locomotor activity patterns (Beckwith et al., 2013).
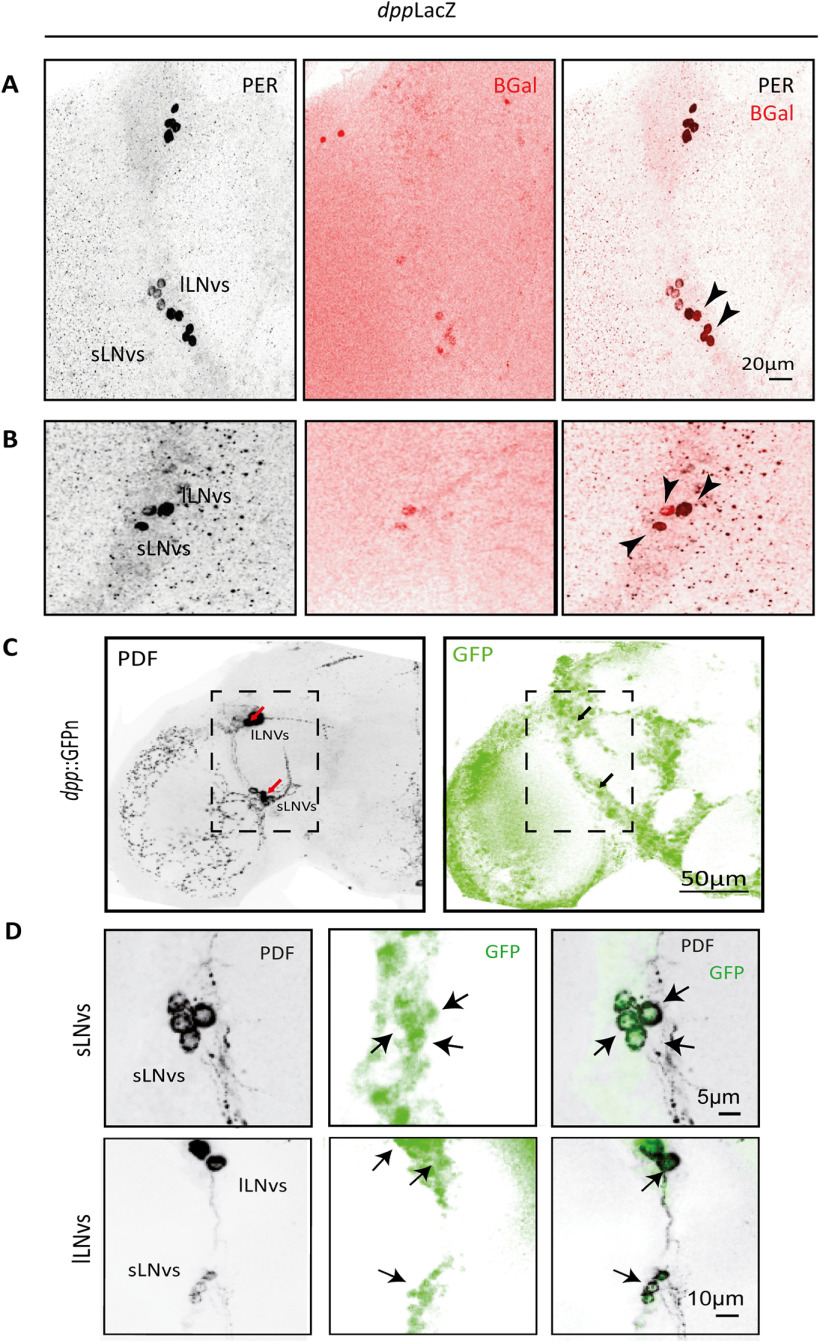
Taking advantage of the reporter line dppLacz, we analyzed the expression pattern of DPP in the adult brain. Both lLNvs and sLNvs express DPP (Fig. 2A,B), which acts via type I serine threonine receptors TKV (Thickveins) and SAX (Saxophone), the same receptors that when overexpressed activate the pathway in the sLNvs (Beckwith et al., 2013). To confirm this localization we analyzed the expression of DPP through the reporter line DPP::GFP. We detected DPP in all four PDF+ small and large LNvs (Fig. 2C,D); both reporter lines also showed DPP expression in noncircadian clusters, which was taken into account in later experiments. Given the restricted expression within the circadian network, we chose to explore DPP as the putative signal used by the LNvs to communicate circadian-relevant information.
Figure 2.
DPP is expressed in the LNv cluster. A, B, Representative confocal images of two hemibrains showing the detection of DPP with the reporter line dppLacZ. The sLNvs and lLNvs were identified by PER immunoreactivity (in gray) and DPP through β-Galactosidase staining (β-Gal, in red). Black arrowheads indicate localization of the two signals in the same groups of cells. C, D, Representative confocal images of a different reporter line, dpp::GFPn; the PDF signal (left, gray) and GFP signal (right, green) are shown. D, Higher magnification of the sLNv and lLNv cluster from a different brain. Arrows highlight DPP reporter accumulation in the LNvs.
Reduced DPP levels in the lLNvs affect anticipation to dawn and dusk as well as rhythmicity under free-running conditions
To unveil the relevance of the communication between the sLNvs and lLNvs through the BMP pathway, particularly through DPP, we took advantage of the Target System, a version of the GAL4/UAS enabling temporal control of expression only in adult stages; thus, we downregulated DPP either in the small or large LNvs postdevelopmentally. To drive expression in the sLNvs we took advantage of a recently described sLNv-specific driver (Herrero et al., 2020). In the case of the lLNvs, we resorted to the R10H10G4 Gal4 line, whose circadian expression is restricted to the 4 lLNvs (Sekiguchi et al., 2020). The latter is also expressed in a few somas at the dorsal protocerebrum as well as in a few PI neurons (Fig. 3A). The dpp knock-down was achieved through RNAi expression separately in the sLNvs and in the lLNvs (Fig. 3B–D).
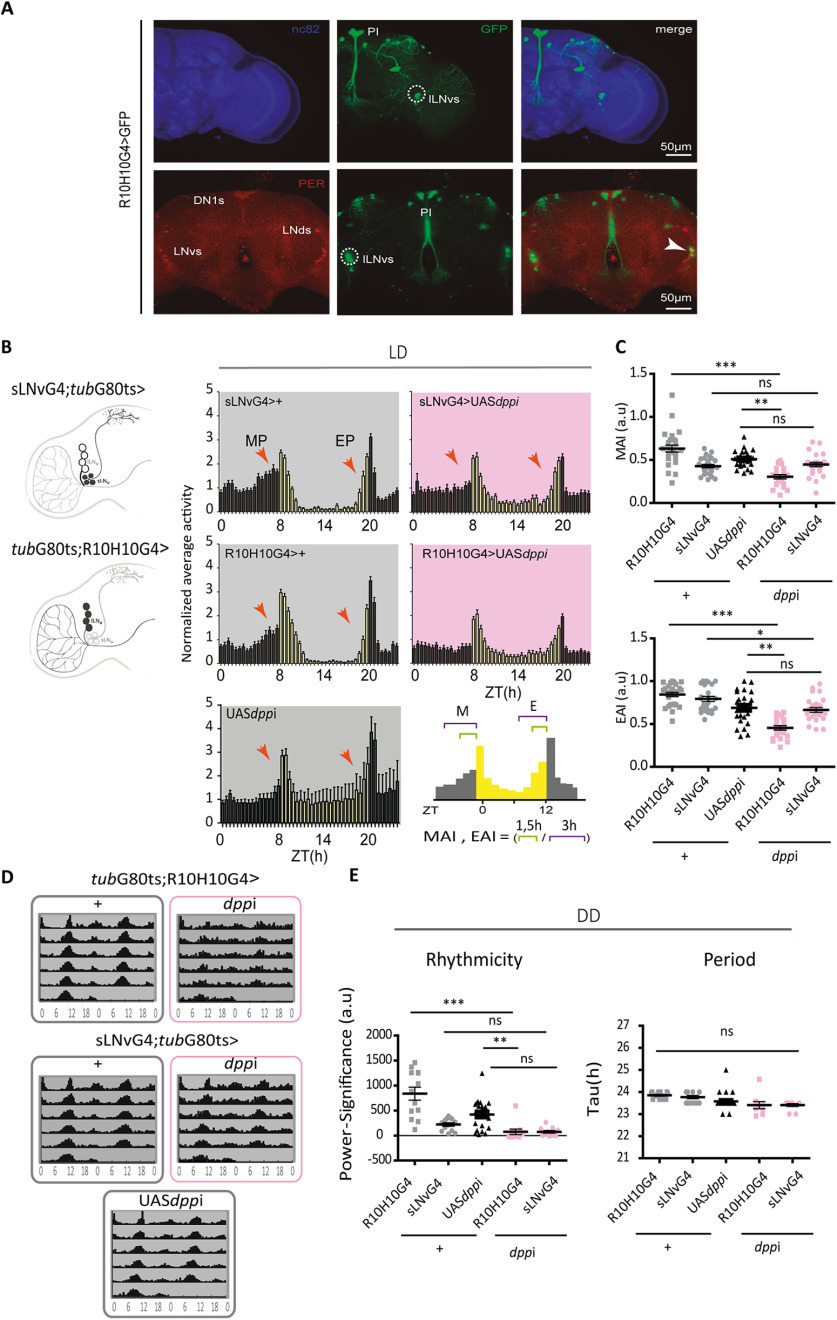
Figure 3.
DPP knock-down in the lLNvs affects anticipations in LD and rhythmicity in DD. A, Expression pattern of the R10H10G4 driver line. Confocal images of an adult brain; R10H10G4+ neurons are labeled with GFP (middle, green); the circadian clusters immunostained against PER are shown in red, neuropils are labeled with nc82 (blue). Dashed circles highlight the lLNvs, and the white arrowhead shows the colocalization of GFP and PER signals in the cluster. B, Schematic diagram of a hemibrain with the affected clusters, the sLNvs (top) or the lLNvs (bottom), highlighted in black. Average activity plots of normalized activity for control flies sLNvsG4;tubG80ts>+ or tubG80ts;R10H10G4>+ and UASdppi (gray and black), and flies with DPP knock-down in the sLNvs (sLNvsG4;tubG80ts>dppi) or in the lLNvs (tubG80ts;R10H10G4>dppi, in pink). Plots represent the normalized average activity during 2 consecutive days in LD (LD2 and LD3) at 30°C. Bars represent the mean of at least 15 flies, and error bars indicate SEM. Red arrowheads mark the morning and evening peaks (MP and EP, respectively). Right, The schematic diagram (bottom) shows how anticipation indexes (included in C) were calculated. C, Anticipation indexes for controls (gray and black) and dpp downregulation (pink) in either cluster. Each dot corresponds to the index calculated for a single fly. Statistical analysis: Kruskal–Wallis test; for the MAI, χ2 = 45.56, p < 0.001, df = 4; for the EAI, χ2= 55.99, p < 0.0001, df = 4. D, Adult-specific DPP downregulation in the LNvs reduces the consolidation of circadian locomotor activity. Average (population) actograms (left) of control flies slNvsG4;tubG80ts>+ or tubG80ts;R10H10G4>+ and UAS dppi/+ (gray outline), flies with downregulated DPP sLNvsG4;tubG80ts>dppi and tubG80ts;R10H10G4>dppi (pink outline) kept at 30°C. E, Rhythmicity (defined as power significance) and period quantitation (tau) in control (gray and black) and dpp downregulated flies (pink). Statistical analysis: power significance (an indication of the degree of rhythmicity), Kruskal–Wallis test, χ2 = 35.73, p < 0.0001, df = 4; Period, χ2 = 28.99, p < 0.0001, df = 4. Dots represent independent flies, the mean and SEM are shown. In Dunn's multiple comparisons test, statistically significant differences *p < 0.05, ***p < 0.0001. ns, not significant.
Adult-specific dpp downregulation in the LNvs affected locomotor activity patterns under LD conditions, particularly the steady increase in activity that precedes dawn and dusk, so-called morning and evening anticipation, respectively. Normalized average activity profiles during two consecutive days in LD at 30°C were compared between controls and dpp knock-down flies (Fig. 3B,C). In the case of the sLNvs, we did not observe significant differences in the anticipatory activity at dawn or dusk, and the increase of the activity in the middle of the day appears to be characteristic of the parental line UASdppi. On the other hand, lack of DPP in the lLNvs drastically impaired morning and evening anticipation without significantly affecting overall activity (Fig. 3B,C). These observations confirm that the BMP pathway is recruited for the temporal organization of daily activity; moreover, these results lend support to the notion that the lLNvs are also relevant in the control of rhythmic behavior.
To further explore the impact of DPP signaling in the synchronization of the circadian network, we analyzed the activity patterns under free running conditions. Adult-specific DPP downregulation in the lLNvs, but not in the sLNvs, correlated with deconsolidation of locomotor rhythmicity with no effect on the circadian period (Fig. 3D). Interestingly, DPP knock-down in the lLNv cluster reinforces the possibility that the large LNvs contribute either directly or indirectly to the synchronization of the circadian network.
Increased DPP release from the lLNvs impairs evening anticipation
We previously showed that activation of BMP signaling through chronic expression of intracellular components (i.e., constitutively active receptors or the nuclear factor schnurri) reduces CLK levels and as a result lengthens the period of the activity patterns (Beckwith et al., 2013). We reasoned that expression of an extracellular component would lead to a more physiological activation, particularly if combined with the target system to drive adult-specific (and short term) expression. Given that DPP expression in the lLNvs is necessary for the temporal organization of locomotor behavior, we examined the consequences of DPP release from this cluster.
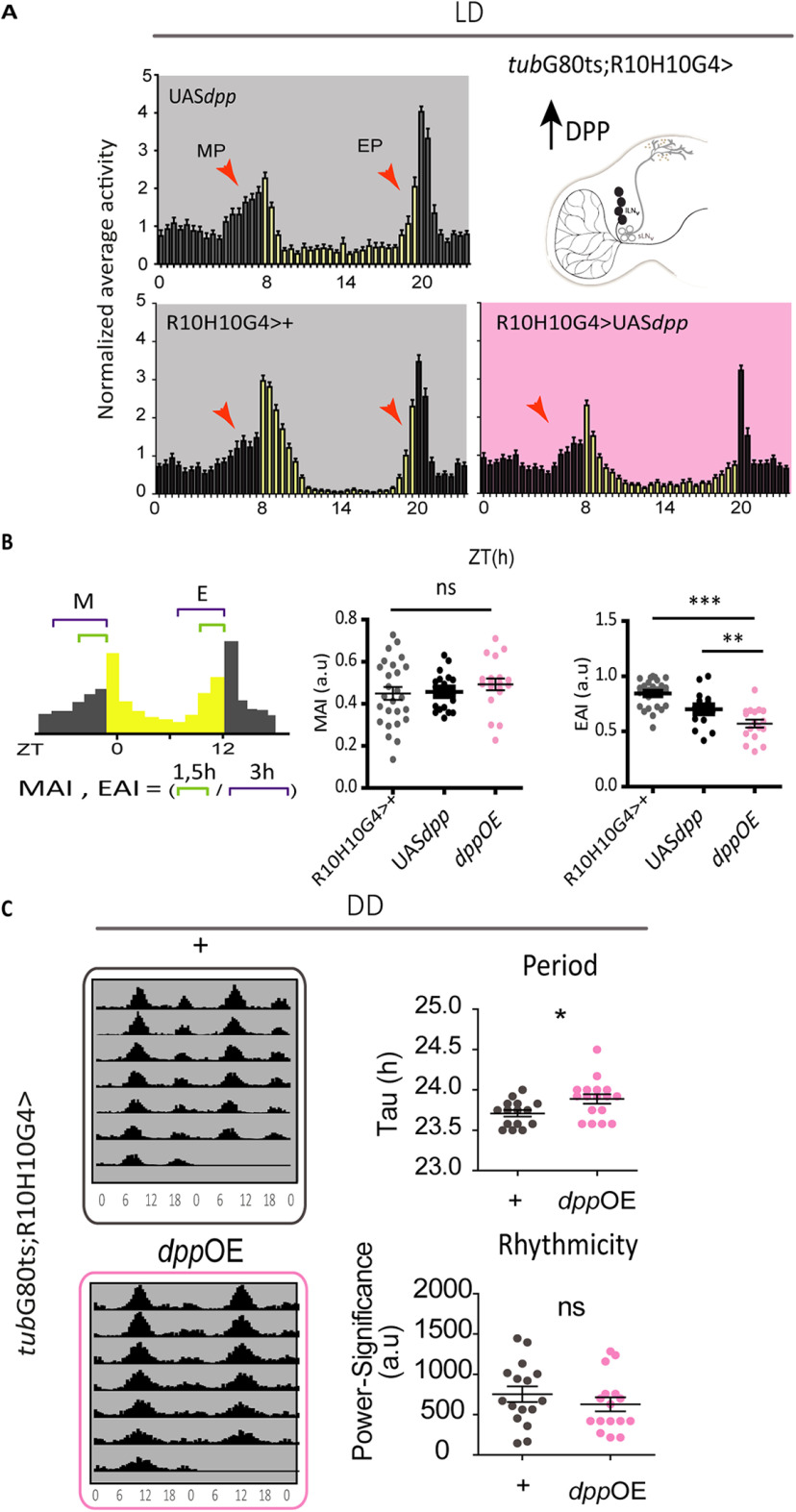
Adult-specific DPP expression in the lLNvs triggers a clear reduction in the anticipatory activity at dusk (Fig. 4A,B), particularly evident right before lights off (i.e., in the last hour). We did not observe any effect in the anticipation of dawn (Fig. 4A,B), suggesting that the anticipatory behavior to lights on and lights off is sensitive to different levels of this ligand.
Figure 4.
Increased DPP release from the lLNvs affects anticipation at dusk as well as circadian period. A, Top, Schematic hemibrain displaying the affected cluster right). Average normalized activity plots of controls (light gray background) and DPP overexpression (pink) for two consecutive days in LD at 30°C; red arrowheads indicate the morning and evening peaks (MP and EP, respectively). Bars represent the mean of at least 15 flies and the error bars represent SEM. B, Left, Graph shows how the MP and EP indexes were calculated. Analysis of the MAI and EAI in controls (gray and black) and DPP overexpression (OE; pink). Dots represent independent flies, mean and SEM are shown. Statistically significant differences, *p < 0.005, ***p < 0.0001. MAI, one-way ANOVA, F(2,63) = 0.65, p = 0.522; Tukey's post hoc multiple comparisons test. EAI, one-way ANOVA, F(2,59) = 19.92, p < 0.0001, Tukey's post hoc multiple comparisons test. C, lLNv-specific DPP overexpression affects free-running period. Representative actograms of control flies (gray outline) and flies overexpressing DPP (pink outline) kept at 30°C. Dot plots show the analysis of circadian parameters in individual flies (period on the left and rhythmicity on the right) in control (gray) and DPP OE flies (pink). Statistical analysis: Period, Mann–Whitney U = 65.50, n1 = 15, n2 = 17, p = 0.0185 two tailed; Rhythmicity, Mann–Whitney U = 106, n1 = 15, n2 = 17, p = 0.4172 two tailed. ns, not significant.
We next explored the effect of DPP expression under constant conditions. Surprisingly, DPP overexpression in the lLNvs subtly lengthens the free running period. No effects on the consolidation of the activity patterns were observed under either condition (Fig. 4D).
DPP release from the lLNvs impairs the circadian remodeling of the sLNv projections
The sLNv projections undergo structural remodeling across the day; these terminals exhibit more elaborated processes at dawn, which undergo changes in the degree of fasciculation and even retraction and pruning as the night proceeds; structural changes are coupled with oscillations in PDF immunoreactivity at these terminals, with higher levels during the day and lower levels at nighttime (Gorostiza et al., 2014; Herrero et al., 2020). In fact, it has been reported that changes in the structure of the sLNvs projections affect locomotor activity patterns (Petsakou et al., 2015).
During larval development and growth, BMP activation at the neuromuscular junctions (NMJs) involves the translocation of p-MAD to the nucleus of the motoneuron, which switches on transcription of different target genes to coordinate actin cytoskeleton remodeling and hence the increase in synaptic contacts (McCabe et al., 2003; Rawson et al., 2003; Berke et al., 2013). To explore the possibility that pathway activation in the sLNvs is related to changes in structural remodeling, that is, in the communication within clock neurons, we examined whether DPP released from the lLNvs could affect, as it happens in the NMJ, the morphology of the sLNvs projections.
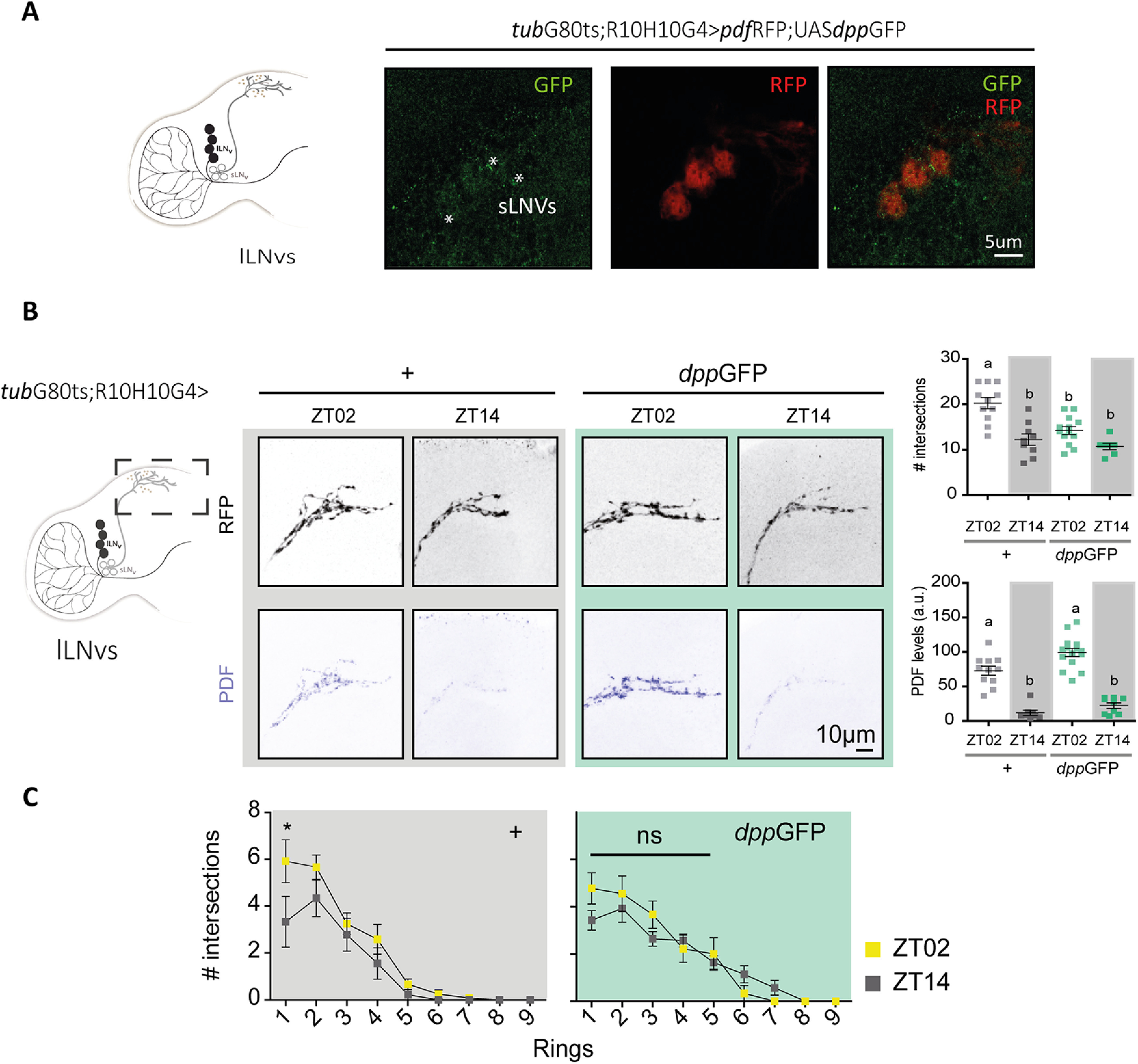
Short-term (24 h) DPP expression in the lLNvs correlates with p-MAD accumulation in the LNv somas (Fig. 5A). Next, we tested the effect of such acute DPP release from the lLNvs on the sLNv structural plasticity. Strikingly, short-term DPP expression affected the morphology of the sLNv projections, which display little complexity across the day (Fig. 5B). PDF immunoreactivity at these terminals still changes between day and night, albeit daytime levels are higher than those of the corresponding controls (Fig. 5B). Similar results were obtained through a widely used lLNv-restricted driver (C929G4; Taghert et al., 2001)) to control the specificity of the effect (Fig. 5D). As this Gal4 line drives expression in a domain that extends beyond the lLNvs, we tested whether DPP released from the remaining (noncircadian) clusters could trigger any circadian phenotype; however, no effect was observed on DPP expression in the C929G4; pdfG80 background, confirming the relevance of the lLNvs in the modulation of specific sLNv outputs (Fig. 5F).
Figure 5.
Short-term DPP expression in the lLNvs affects structural plasticity. A, Schematic diagram of the experimental design (left). AE, After eclosion. Confocal images showing activation of the BMP pathway in the sLNv reported through p-MAD nuclear staining (green) on short-term (24 h) DPP expression in the lLNvs (right). RFP (red) and PDF (blue) localize the LNv clusters. B, C, Representative confocal images of control (gray) and DPP overexpression (OE, pink) at ZT02 and ZT14. Two different lLNv drivers were used, R10H10G4 (B) and C929G4 (C). Males, 4 d old, were used. Graphs show the quantitation of the arbor complexity (top, number of intersections defined by Sholl analysis) and PDF levels (bottom) at ZT02 and ZT14 in controls (gray) and DPP OE (in pink). Different letters indicate significant differences (that is, treatments sharing any letter are not statistically different). Statistical analysis: #intersections (B), Kruskal–Wallis test, χ2 = 17.18, p = 0.0006, df = 3; PDF levels, Kruskal–Wallis test, χ2 = 51.27 p <0.0001, df = 3; Dunn's post hoc multiple comparisons test; #intersections (C), two-way ANOVA, F(3,27) = 30.05, p < 0.0001; PDF levels, two-way ANOVA, F(3,29) = 33.45, p < 0.0001; Bonferroni post hoc test. Squares represent individual brains that were normalized within at least two independent experiments (all genotypes were considered for the normalization). D, E, DPP released from noncircadian neurons does not affect structural plasticity. D, Confocal images of C929G4 driver line (top) alone and combined with pdfG80 (bottom) to exclude the expression from the LNv cluster; additional noncircadian neurons are shown as GFP+ somas (green) and the LNvs as PDF immunostaining (blue). E, Quantitation of the complexity of the sLNv projections and PDF levels at ZT02 and ZT14 in controls and DPP OE in noncircadian neurons. Statistical analysis: #intersection, Kruskal–Wallis test, χ2 = 17.29, p = 0.0006, df = 3; PDF levels, Kruskal–Wallis test, χ2 =24.79, p <0.0001, df = 3. Dunn's post hoc multiple comparisons test. Different letters highlight statistically significant differences.
The lLNv communicate to the sLNv through DPP signaling
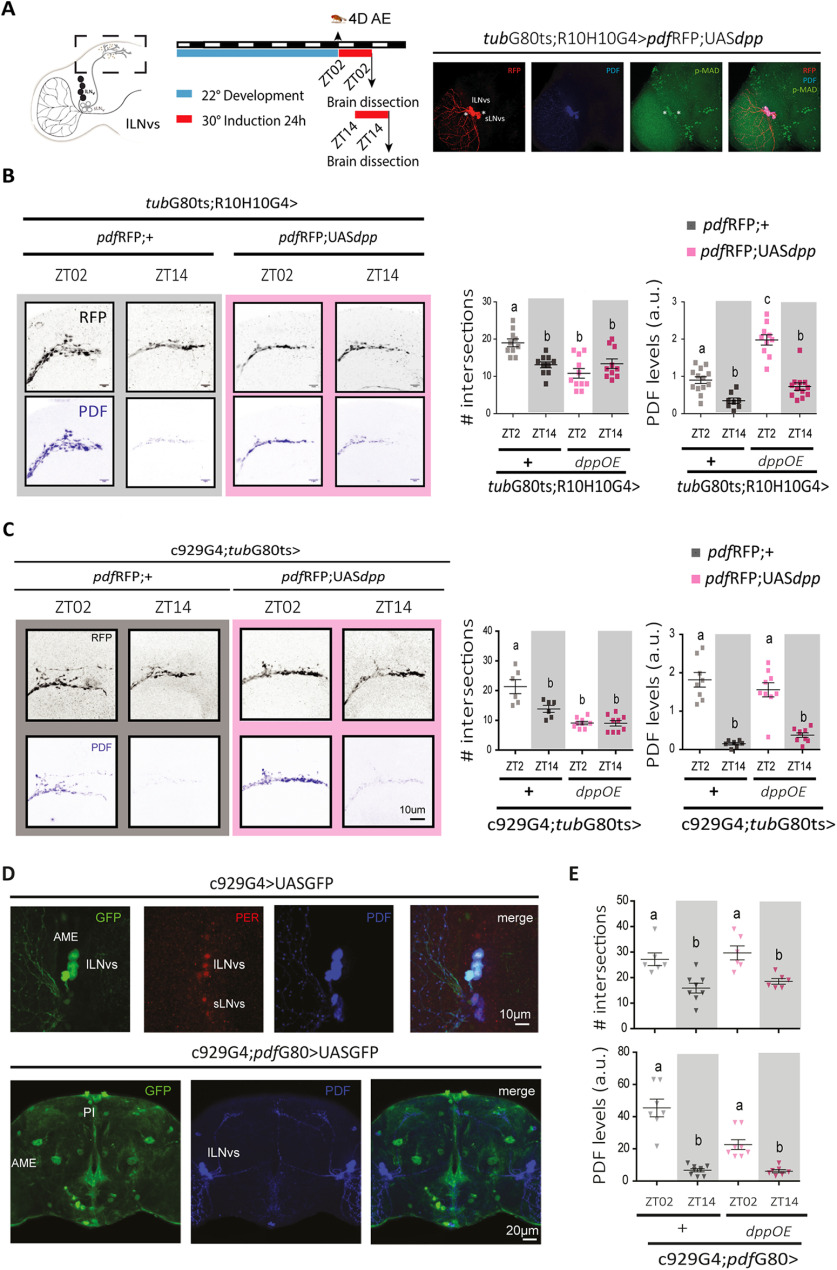
The effects observed on DPP release from the lLNv cluster could be accounted for the accumulation of the ligand in the accessory medulla (AME) where the sLNv somas are located. To test this hypothesis we took advantage of the UASGFP::dpp line to express DPPGFP, enabling ligand detection through the fluorescent reporter (Roy et al., 2014; George and Bates, 2019). As expected, DPPGFP was detected in the AME, within the lLNvs somas and in close proximity to the sLNvs (Fig. 6A), suggesting that DPP released from the lLNvs could bind to receptors in the sLNv somas and result in the activation of the BMP intracellular signaling cascade in this cluster. DPPGFP expression triggers similar phenotypes than DPP alone, with low complexity projections during the day when DPP is released from the lLNvs (Fig. 6B,C), confirming it is biologically functional. No significant changes to PDF levels were observed (Fig. 5).
Figure 6.
Structural remodeling of sLNv projections is affected by DPP release in the AME. A, Schematic diagram of a hemibrain, highlighting the lLNv cluster (left). Representative confocal images of a single focal plane onto the sLNv somas on DPP overexpression from the lLNvs using the dpp::GFP reporter (green) driven by the R10H10G4 driver; LNv somas and projections are labeled with RFP (in red); * indicates colocalization of dpp::GFP. B, Quantitation of the complexity of the sLNv projections and PDF immunoreactivity. Dots represent individual brains; intensity was normalized in at least two independent experiments. Statistical analysis: #intersections, Kruskal–Wallis test, χ2 = 16.22, p = 0.0010, df = 3; PDF levels at ZT02 and ZT14 between control (gray) and DPP OE (green); Kruskal–Wallis test, χ2 = 51.27 p < 0.0001, df = 3; Dunn's post hoc multiple comparisons test. C, Number of intersections per ring (each 10 um) between ZT02 (yellow squares) and ZT14 (gray squares) in control (gray background) and with DPP OE (green background). Statistical analysis included a two-way ANOVA; different letters and * indicate statistically significant differences, p < 0.05.
Conserved BMP effectors mediate structural remodeling
The BMP signaling pathway is recruited for the regulation of synapse assembly, maintenance, and function (Aberle et al., 2002; Marqués et al., 2002; Berke et al., 2013). At the NMJ, the BMP pathway modulates the strength of connectivity between synaptic partners and synapse growth through transcriptional regulation of the Rho guanine nucleotide exchange factor (GEF) Trio (Ball et al., 2010). Trio was shown to mediate Rac and/or Rho activation depending on the biological context, in turn modulating actin cytoskeletal dynamics and hence synaptic remodeling. Additionally, single-cell RNAseq analysis indicated trio cycles in the sLNvs, at least under LD conditions (Ma et al., 2021). To explore the possibility that Trio could mediate the cell-autonomous structural changes triggered by DPP, adult-specific Trio expression was assayed in the sLNvs. Interestingly, acute Trio expression gave rise to poorly elaborated terminals throughout the day, with a complexity reminiscent of the one observed at the beginning of the night in control lines, thus impairing circadian remodeling (Fig. 7A,B). These results parallel those observed on DPP released from the lLNvs, namely reduced complexity projections across the day (Figs. 5, 6), although a causal connection between the two phenomena awaits confirmation.
Figure 7.
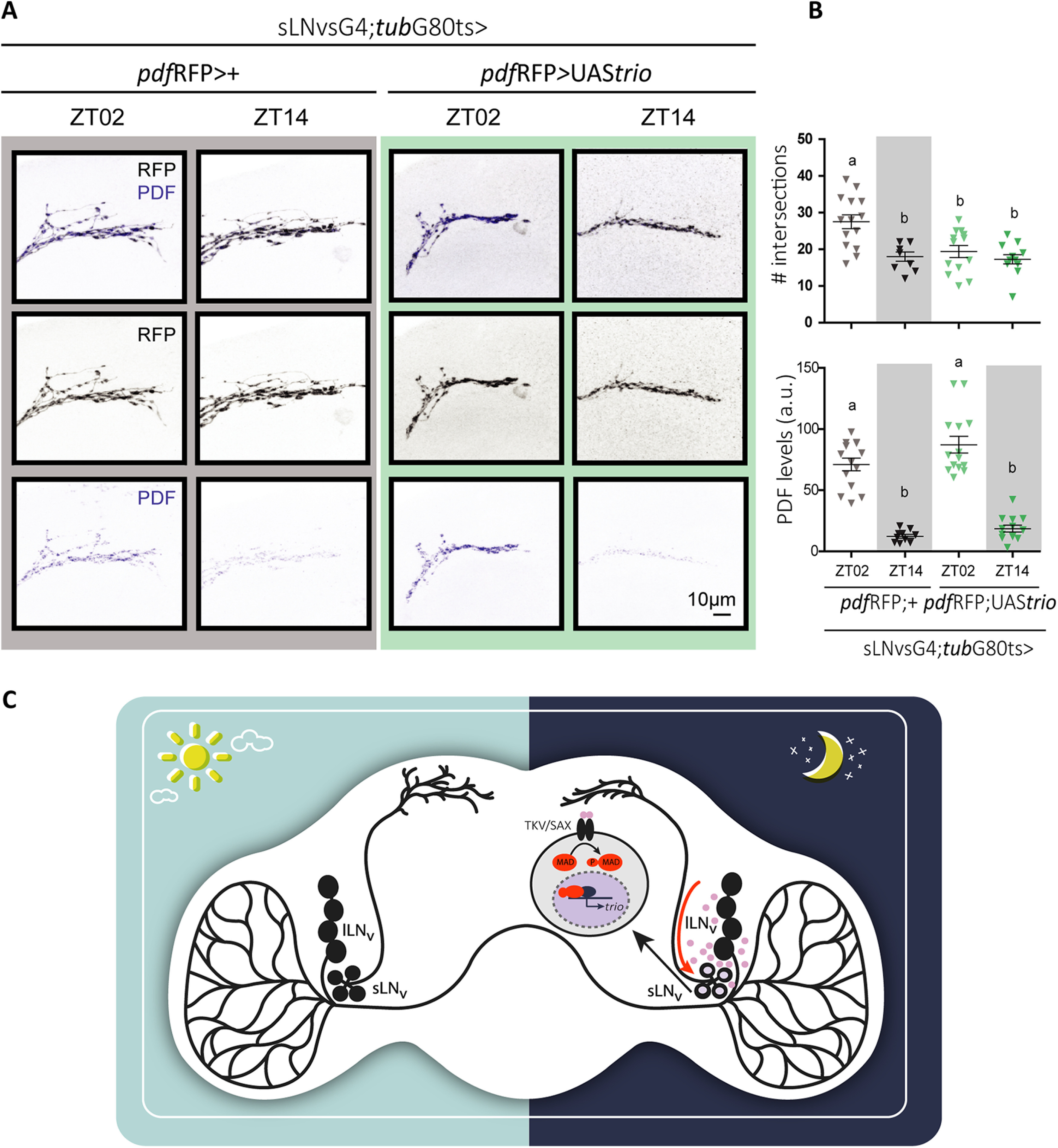
Trio expression in the sLNvs prevents structural remodeling without affecting PDF levels or cycling. A, Representative confocal images of sLNv projections of a control (pdfRFP;+, gray box) and Trio overexpression (pdfRFP;UAStrio, green box) brain. B, Quantitation of the number of intersections (top) and PDF levels (bottom) at ZT02 and ZT14 in control (gray) and DPP OE (green). Statistical analysis: #intersections, Kruskal–Wallis test, χ2 = 15.22, p = 0.016, df = 3; PDF levels, Kruskal–Wallis test, χ2 = 37.89, p < 0.0001, df = 3. Dunn's post hoc multiple comparisons test. Different letters indicate statistically significant differences. C, Working model illustrating the recruitment of the BMP pathway by the circadian network. DPP released by the lLNvs in the accessory medulla activates the BMP pathway in the sLNvs and triggers the pruning of the projections at night through the activation of the RhoGEF Trio.
In sum, our results support the hypothesis that the availability of BMP ligands could fine-tune the connectivity within circadian clusters as it would be anticipated in response to a fluctuating environment (Petsakou et al., 2015).
Discussion
The homeostatic regulation of physiology and behavior is a complex process that requires precise communication and coordination among different neuronal clusters. Circadian locomotor activity, as one of these behaviors, requires coordination of the different neuronal clocks that shape the circadian network to provide coherence and at the same time flexibility to the circuit. A lot of work has been devoted to studying the mechanisms underlying the communication among circadian clusters (Beckwith et al., 2013; Dissel et al., 2014; Gorostiza et al., 2014; Yao and Shafer, 2014; Mezan et al., 2016; Yao et al., 2016; Frenkel et al., 2017; Liang et al., 2017) and the role of the different clusters in the control of specific properties of the activity profile; among others, morning anticipation, evening anticipation, period, and daytime, nighttime sleep (Hamasaka et al., 2010; Zhang et al., 2010a,b; Guo et al., 2014; Beckwith and Ceriani, 2015; Guo et al., 2016, 2018; Fernandez-Chiappe et al., 2020). A few years ago we demonstrated that chronic downregulation of BMP ligands in the LNvs generate decreased rhythmicity, while overexpression of a transcription factor belonging to this pathway affects circadian period (Beckwith et al., 2013). Those initial results revealed this pathway could be recruited for communication among circadian clusters. Herein we show that in addition to the transcriptional effects on dClk levels, short-term pathway activation leads to the remodeling of the LNv synaptic connectivity. Additionally, our work uncovered a yet unexplored communication between the large and small LNvs, and implicates the former in the control of sLNv physiology.
A canonical BMP pathway operates in the LNvs to modulate circadian outputs
BMP ligands are expressed in different tissues throughout development, usually not in concert; we reliably detected at least one ligand expressed in the adult brain, particularly in the circadian LNv neurons. Although most reports focus on the NMJ (Arora et al., 1996), little work has been devoted to characterizing the expression pattern of these small peptides in the adult fly brain. We have shown that communication through these ligands contributes to the synchronization of the circadian network (Beckwith et al., 2013; this article).
BMP pathway activity is tightly regulated; as an example, DAUGHTERS AGAINST DPP (DAD) [an I-SMAD Inhibitory SMADs (I-SMADs)] inhibits the phosphorylation of the transcription factor MAD mediated by type I receptors on ligand binding. An additional MAD phosphorylation site promotes cytoplasmic retention and thus prevents the nuclear function. At the NMJ, SHAGGY (SGG), the Drosophila orthologue of GSK-3, is responsible for this type of regulation (Aleman et al., 2014). Preliminary results suggest that MAD is also phosphorylated by SGG in the LNvs (data not shown) suggesting a potential regulatory step linking the BMP signaling pathway and the clock; in fact, other kinases could also contribute to time-of-day regulation of MAD activity. One interesting candidate is NEMO/NML, which has been shown to modulate normal distribution and accumulation of p-MAD in larval motor neurons (Merino et al., 2009), and it is present in these relevant neurons and directly modulates the speed of the circadian clock (Chiu et al., 2011; Yu et al., 2011).
Strikingly, lLNv-specific DPP knock-down affects the temporal organization of locomotor activity both under light-dark and free-running conditions. The contribution of the lLNvs to the circadian network has been questioned since the report that their molecular clock stops when flies are transferred to constant conditions (Shafer et al., 2002), although they were assigned a key role in sleep control (Shang et al., 2008; Sheeba et al., 2008). However, our current work highlights the relevance of the large to small LNv communication through this pathway and positions the lLNvs as a relevant cluster for circadian locomotor behavior probably through the modulation of sLNv physiology. Whether DPP is expressed or released from the lLNvs in a circadian fashion awaits experimental confirmation. Thus far, high throughput analysis of specific groups of clock neurons have not identified this ligand among the cycling transcripts in the LNvs (Abruzzi et al., 2017; Ma et al., 2021).
BMP activation in the sLNvs modulates structural plasticity
The sLNvs show striking changes in the morphology/connectivity of terminals along the day and recruit different mechanisms likely engaged by the clock at different times to precisely accomplish such structural remodeling. Among the cellular mechanisms underlying this phenomenon, it was shown to depend on a functional clock (Herrero et al., 2017) to respond to changes in membrane excitability (Depetris-Chauvin et al., 2011) through the activity of MEF2, a transcription factor whose expression depends on the circadian clock and neuronal activity; MEF2 also regulates the expression of effector genes responsible for structural changes, that is, Fasciclin II (Sivachenko et al., 2013). Remodeling also depends on MMP1, an extracellular matrix metalloprotease that modulates PDF levels/activity (Depetris-Chauvin et al., 2014). Naturally, circadian structural plasticity ensures actin cytoskeleton remodeling (Petsakou et al., 2015), which depends on the BMP signaling pathway in different cellular contexts. At the NMJ, the BMP pathway modulates the strength of connectivity and synaptic growth through the RhoGEF TRIO that mediates changes in the actin cytoskeleton (Ball et al., 2010; Piccioli and Littleton, 2014). In the sLNvs, activation of the BMP pathway impairs remodeling of the dorsal projections with no significant effect on PDF levels and PDF cycling. Strikingly, short-term DPP release from the lLNvs increased the nuclear p-MAD signal to an extent that is sufficient to generate more fasciculated (hence, less arborized) sLNv projections; this, in turn, correlates with a defective E anticipation at dusk, supporting a link between structural plasticity and the control of locomotor behavior (Petsakou et al., 2015).
AS DPP released from the lLNvs triggers a night-like state in the sLNv projections and this parallels TRIO overexpression in this cluster (of note, a direct link between these two phenomena has yet to be established), we propose that the pathway could be co-opted by the sLNvs at the end of the day to increase trio expression and trigger Rho activation, ultimately affecting actin cytoskeleton (Fig. 7C). This could be a venue through which the lLNvs, also implicated in arousal and sleep, could modulate the network response to different photoperiods; in long days, for example, the activation of the pathway could generate a delay in the evening anticipation.
Given that lack of DPP in the lLNvs affects both morning and evening anticipations, but DPP overexpression mainly affects the evening peak, we propose that communication through this ligand is needed to correctly time anticipations, but there is a signal threshold, and once it is surpassed, additional mechanisms kick in to limit pathway activation, which might be more sensitive at dawn rather than dusk.
Because loss of circadian remodeling was not accompanied by changes in PDF levels or oscillation, we propose that the circadian phenotypes associated with acute and spatially restricted forced DPP signaling are supported by the altered communication between this specific subset of circadian clusters. In sum, our results have shown that short-term activation of the BMP pathway leads to strengthening or decreasing the structural connectivity between key circadian neurons, which, in turn, affects behavioral responses, underscoring a rather direct link between structural plasticity and other circadian outputs.
Footnotes
S.P. was supported by a graduate fellowship from the Argentina National Research Council for Science and Technology and a postgraduate fellowship from the National Agency for the Promotion of Science and Technology of Argentina. This work was supported by grants from the National Agency for the Promotion of Science and Technology of Argentina (PICT2010-1874 and PICT2015-2041 to M.F.C.) and the Japan Society for the Promotion of Science (KAKENHI 15H05600) to T.Y. We thank Esteban Beckwith for reading this manuscript, members of the Ceriani lab for discussion, our technician Andres Liceri for help with fly food and care, the Bloomington and Kyoto stock centers, the Vienna Drosophila Resource Center, Emily Bates for sharing fly stocks, Fundación Williams for a contribution.
The authors declare no competing financial interests.
References
- Aberle H, Haghighi AP, Fetter RD, McCabe BD, Magalhães TR, Goodman CS (2002) wishful thinking encodes a BMP type II receptor that regulates synaptic growth in Drosophila. Neuron 33:545–558. 10.1016/s0896-6273(02)00589-5 [DOI] [PubMed] [Google Scholar]
- Abruzzi KC, Rodriguez J, Menet JS, Desrochers J, Zadina A, Luo W, Tkachev S, Rosbash M (2011) Drosophila CLOCK target gene characterization: implications for circadian tissue-specific gene expression. Genes Dev 25:2374–2386. 10.1101/gad.178079.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abruzzi KC, Zadina A, Luo W, Wiyanto E, Rahman R, Guo F, Shafer O, Rosbash M (2017) RNA-seq analysis of Drosophila clock and non-clock neurons reveals neuron-specific cycling and novel candidate neuropeptides. PLoS Genet 13:e1006613. 10.1371/journal.pgen.1006613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleman A, Rios M, Juarez M, Lee D, Chen A, Eivers E (2014) Mad linker phosphorylations control the intensity and range of the BMP-activity gradient in developing Drosophila tissues. Sci Rep 4:6927. 6927. 10.1038/srep06927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora K, O'Connor MB, Warrior R (1996) BMP signaling in Drosophila embryogenesis. Ann N Y Acad Sci 785:80–97. 10.1111/j.1749-6632.1996.tb56246.x [DOI] [PubMed] [Google Scholar]
- Ball RW, Warren-Paquin M, Tsurudome K, Liao EH, Elazzouzi F, Cavanagh C, An BS, Wang TT, White JH, Haghighi AP (2010) Retrograde BMP signaling controls synaptic growth at the NMJ by regulating trio expression in motor neurons. Neuron 66:536–549. 10.1016/j.neuron.2010.04.011 [DOI] [PubMed] [Google Scholar]
- Beckwith EJ, Ceriani MF (2015) Experimental assessment of the network properties of the Drosophila circadian clock. J Comp Neurol 523:982–996. 10.1002/cne.23728 [DOI] [PubMed] [Google Scholar]
- Beckwith EJ, Gorostiza EA, Berni J, Rezával C, Pérez-Santángelo A, Nadra AD, Ceriani MF (2013) Circadian period integrates network information through activation of the BMP signaling pathway. PLoS Biol 11:e1001733. 10.1371/journal.pbio.1001733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke B, Wittnam J, McNeill E, Van Vactor DL, Keshishian H (2013) Retrograde BMP signaling at the synapse: a permissive signal for synapse maturation and activity-dependent plasticity. J Neurosci 33:17937–17950. 10.1523/JNEUROSCI.6075-11.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu JC, Ko HW, Edery I (2011) NEMO/NLK phosphorylates PERIOD to initiate a time-delay phosphorylation circuit that sets circadian clock speed. Cell 145:357–370. 10.1016/j.cell.2011.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahal GR, Pradhan SJ, Bates EA (2017) Inwardly rectifying potassium channels influence Drosophila wing morphogenesis by regulating Dpp release. Development 144:2771–2783. 10.1242/dev.146647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delventhal R, O'Connor RM, Pantalia MM, Ulgherait M, Kim HX, Basturk MK, Canman JC, Shirasu-Hiza M (2019) Dissection of central clock function in Drosophila through cell-specific CRISPR-mediated clock gene disruption. eLife 8: 10.7554/eLife.48308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depetris-Chauvin A, Berni J, Aranovich EJ, Muraro NI, Beckwith EJ, Ceriani MF (2011) Adult-specific electrical silencing of pacemaker neurons uncouples molecular clock from circadian outputs. Curr Biol 21:1783–1793. 10.1016/j.cub.2011.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depetris-Chauvin A, Fernández-Gamba A, Gorostiza EA, Herrero A, Castaño EM, Ceriani MF (2014) Mmp1 processing of the PDF neuropeptide regulates circadian structural plasticity of pacemaker neurons. PLoS Genet 10:e1004700. 10.1371/journal.pgen.1004700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibner C, Schibler U, Albrecht U (2010) The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol 72:517–549. 10.1146/annurev-physiol-021909-135821 [DOI] [PubMed] [Google Scholar]
- Dissel S, Hansen CN, Özkaya Ö, Hemsley M, Kyriacou CP, Rosato E (2014) The logic of circadian organization in Drosophila. Curr Biol 24:2257–2266. 10.1016/j.cub.2014.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhart JM, Herrero A, de la Cruz G, Ispizua JI, Pírez N, Ceriani MF (2020) Circadian structural plasticity drives remodeling of E cell output. Curr Biol 30:5040–5048.e5. 10.1016/j.cub.2020.09.057 [DOI] [PubMed] [Google Scholar]
- Evans JA, Gorman MR (2016) In synch but not in step: circadian clock circuits regulating plasticity in daily rhythms. Neuroscience 320:259–280. 10.1016/j.neuroscience.2016.01.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Chiappe F, Frenkel L, Colque CC, Ricciuti A, Hahm B, Cerredo K, Muraro NI, Ceriani MF (2020) High frequency neuronal bursting is essential for circadian and sleep behaviors in Drosophila. J Neurosc 41:689–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel L, Muraro NI, Beltrán González AN, Marcora MS, Bernabó G, Hermann-Luibl C, Romero JI, Helfrich-Förster C, Castaño EM, Marino-Busjle C, Calvo DJ, Ceriani MF (2017) Organization of circadian behavior relies on glycinergic transmission. Cell Rep 19:72–85. 10.1016/j.celrep.2017.03.034 [DOI] [PubMed] [Google Scholar]
- Fujiwara Y, Hermann-Luibl C, Katsura M, Sekiguchi M, Ida T, Helfrich-Förster C, Yoshii T (2018) The CCHamide1 neuropeptide expressed in the anterior dorsal neuron 1 conveys a circadian signal to the ventral lateral neurons in Drosophila melanogaster. Front Physiol 9:1276. 10.3389/fphys.2018.01276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garaulet DL, Sun K, Li W, Wen J, Panzarino AM, O'Neil JL, Hiesinger PR, Young MW, Lai EC (2016) miR-124 Regulates diverse aspects of rhythmic behavior in Drosophila. J Neurosci 36:3414–3421. 10.1523/JNEUROSCI.3287-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George LF, Bates EA (2019) Imaging Dpp Release from a Drosophila Wing Disc. J Vis Exp 30:e60528. 10.3791/60528 [DOI] [PubMed] [Google Scholar]
- Gorostiza EA, Depetris-Chauvin A, Frenkel L, Pírez N, Ceriani MF (2014) Circadian pacemaker neurons change synaptic contacts across the day. Curr Biol 24:2161–2167. 10.1016/j.cub.2014.07.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gummadova JO, Coutts GA, Glossop NR (2009) Analysis of the Drosophila clock promoter reveals heterogeneity in expression between subgroups of central oscillator cells and identifies a novel enhancer region. J Biol Rhythms 24:353–367. 10.1177/0748730409343890 [DOI] [PubMed] [Google Scholar]
- Guo F, Cerullo I, Chen X, Rosbash M (2014) PDF neuron firing phase-shifts key circadian activity neurons in Drosophila. eLife 3: 10.7554/eLife.02780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F, Yu J, Jung HJ, Abruzzi KC, Luo W, Griffith LC, Rosbash M (2016) Circadian neuron feedback controls the Drosophila sleep–activity profile. Nature 536:292–297. 10.1038/nature19097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F, Holla M, Díaz MM, Rosbash M (2018) A circadian output circuit controls sleep-wake arousal in Drosophila. Neuron 100:624–635.e4. 10.1016/j.neuron.2018.09.002 [DOI] [PubMed] [Google Scholar]
- Hamaratoglu F, Affolter M, Pyrowolakis G (2014) Dpp/BMP signaling in flies: from molecules to biology. Semin Cell Dev Biol 32:128–136. 10.1016/j.semcdb.2014.04.036 [DOI] [PubMed] [Google Scholar]
- Hamasaka Y, Suzuki T, Hanai S, Ishida N (2010) Evening circadian oscillator as the primary determinant of rhythmic motivation for Drosophila courtship behavior. Genes Cells 15:1240–1248. 10.1111/j.1365-2443.2010.01456.x [DOI] [PubMed] [Google Scholar]
- He C, Cong X, Zhang R, Wu D, An C, Zhao Z (2013) Regulation of circadian locomotor rhythm by neuropeptide Y-like system in Drosophila melanogaster. Insect Mol Biol 22:376–388. 10.1111/imb.12027 [DOI] [PubMed] [Google Scholar]
- Hermann C, Yoshii T, Dusik V, Helfrich-Förster C (2012) Neuropeptide F immunoreactive clock neurons modify evening locomotor activity and free-running period in Drosophila melanogaster. J Comp Neurol 520:970–987. 10.1002/cne.22742 [DOI] [PubMed] [Google Scholar]
- Hermann-Luibl C, Yoshii T, Senthilan PR, Dircksen H, Helfrich-Förster C (2014) The ion transport peptide is a new functional clock neuropeptide in the fruit fly Drosophila melanogaster. J Neurosci 34:9522–9536. 10.1523/JNEUROSCI.0111-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero A, Duhart JM, Ceriani MF (2017) Neuronal and glial clocks underlying structural remodeling of pacemaker neurons in Drosophila. Front Physiol 8:918. 10.3389/fphys.2017.00918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero A, Yoshii T, Ispizua JI, Colque C, Veenstra JA, Muraro NI, Ceriani MF (2020) Coupling neuropeptide levels to structural plasticity in Drosophila clock neurons. Curr Biol 30:3154–3166.e4. 10.1016/j.cub.2020.06.009 [DOI] [PubMed] [Google Scholar]
- Hill CS (2009) Nucleocytoplasmic shuttling of Smad proteins. Cell Res 19:36–46. 10.1038/cr.2008.325 [DOI] [PubMed] [Google Scholar]
- Liang X, Holy TE, Taghert PH (2017) A series of suppressive signals within the Drosophila circadian neural circuit generates sequential daily outputs. Neuron 94:1173–1189.e4. 10.1016/j.neuron.2017.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D, Przybylski D, Abruzzi KC, Schlichting M, Li Q, Long X, Rosbash M (2021) A transcriptomic taxonomy of Drosophila circadian neurons around the clock. eLife 10: 10.7554/eLife.63056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marqués G, Bao H, Haerry TE, Shimell MJ, Duchek P, Zhang B, O'Connor MB (2002) The Drosophila BMP type II receptor Wishful Thinking regulates neuromuscular synapse morphology and function. Neuron 33:529–543. 10.1016/S0896-6273(02)00595-0 [DOI] [PubMed] [Google Scholar]
- McCabe BD, Marqués G, Haghighi AP, Fetter RD, Crotty ML, Haerry TE, Goodman CS, O'Connor MB (2003) The BMP homolog Gbb provides a retrograde signal that regulates synaptic growth at the Drosophila neuromuscular junction. Neuron 39:241–254. 10.1016/S0896-6273(03)00426-4 [DOI] [PubMed] [Google Scholar]
- McGuire SE, Mao Z, Davis RL (2004) Spatiotemporal gene expression targeting with the TARGET and gene-switch systems in Drosophila. Sci STKE 2004:pl6. [DOI] [PubMed] [Google Scholar]
- Merino C, Penney J, González M, Tsurudome K, Moujahidine M, O'Connor MB, Verheyen EM, Haghighi P (2009) Nemo kinase interacts with Mad to coordinate synaptic growth at the Drosophila neuromuscular junction. J Cell Biol 185:713–725. 10.1083/jcb.200809127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezan S, Feuz JD, Deplancke B, Kadener S (2016) PDF Signaling Is an integral part of the Drosophila circadian molecular oscillator. Cell Rep 17:708–719. 10.1016/j.celrep.2016.09.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustakas A, Heldin CH (2009) The regulation of TGFbeta signal transduction. Development 136:3699–3714. 10.1242/dev.030338 [DOI] [PubMed] [Google Scholar]
- Ozkaya O, Rosato E (2012) The circadian clock of the fly: a neurogenetics journey through time. Adv Genet 77:79–123. 10.1016/B978-0-12-387687-4.00004-0 [DOI] [PubMed] [Google Scholar]
- Peng Y, Stoleru D, Levine JD, Hall JC, Rosbash M (2003) Drosophila free-running rhythms require intercellular communication. PLoS Biol 1:E13. 10.1371/journal.pbio.0000013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petsakou A, Sapsis TP, Blau J (2015) Circadian rhythms in rho1 activity regulate neuronal plasticity and network hierarchy. Cell 162:823–835. 10.1016/j.cell.2015.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccioli ZD, Littleton JT (2014) Retrograde BMP signaling modulates rapid activity-dependent synaptic growth via presynaptic LIM kinase regulation of cofilin. J Neurosci 34:4371–4381. 10.1523/JNEUROSCI.4943-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raftery LA, Sutherland DJ (1999) TGF-beta family signal transduction in Drosophila development: from Mad to Smads. Dev Biol 210:251–268. 10.1006/dbio.1999.9282 [DOI] [PubMed] [Google Scholar]
- Ramel MC, Hill CS (2012) Spatial regulation of BMP activity. FEBS Lett 586:1929–1941. 10.1016/j.febslet.2012.02.035 [DOI] [PubMed] [Google Scholar]
- Rawson JM, Lee M, Kennedy EL, Selleck SB (2003) Drosophila neuromuscular synapse assembly and function require the TGF-beta type I receptor saxophone and the transcription factor Mad. J Neurobiol 55:134–150. 10.1002/neu.10189 [DOI] [PubMed] [Google Scholar]
- Renn SC, Park JH, Rosbash M, Hall JC, Taghert PH (1999) A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell 99:791–802. 10.1016/s0092-8674(00)81676-1 [DOI] [PubMed] [Google Scholar]
- Roy S, Huang H, Liu S, Kornberg TB (2014) Cytoneme-mediated contact-dependent transport of the Drosophila decapentaplegic signaling protein. Science 343:1244624. 1244624. 10.1126/science.1244624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid B, Helfrich-Förster C, Yoshii T (2011) A new ImageJ plug-in “ActogramJ” for chronobiological analyses. J Biol Rhythms 26:464–467. 10.1177/0748730411414264 [DOI] [PubMed] [Google Scholar]
- Sekiguchi M, Inoue K, Yang T, Luo DG, Yoshii T (2020) A catalog of GAL4 drivers for labeling and manipulating circadian clock neurons in Drosophila melanogaster. J Biol Rhythms 35:207–213. 10.1177/0748730419895154 [DOI] [PubMed] [Google Scholar]
- Shafer OT, Rosbash M, Truman JW (2002) Sequential nuclear accumulation of the clock proteins period and timeless in the pacemaker neurons of Drosophila melanogaster. J Neurosci 22:5946–5954. 10.1523/JNEUROSCI.22-14-05946.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer OT, Helfrich-Förster C, Renn SC, Taghert PH (2006) Reevaluation of Drosophila melanogaster's neuronal circadian pacemakers reveals new neuronal classes. J Comp Neurol 498:180–193. 10.1002/cne.21021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y, Griffith LC, Rosbash M (2008) Light-arousal and circadian photoreception circuits intersect at the large PDF cells of the Drosophila brain. Proc Natl Acad Sci U S A 105:19587–19594. 10.1073/pnas.0809577105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheeba V, Fogle KJ, Kaneko M, Rashid S, Chou YT, Sharma VK, Holmes TC (2008) Large ventral lateral neurons modulate arousal and sleep in Drosophila. Curr Biol 18:1537–1545. 10.1016/j.cub.2008.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivachenko A, Li Y, Abruzzi KC, Rosbash M (2013) The transcription factor mef2 links the Drosophila core clock to fas2, neuronal morphology, and circadian behavior. Neuron 79:281–292. 10.1016/j.neuron.2013.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloin HE, Ruggiero G, Rubinstein A, Smadja Storz S, Foulkes NS, Gothilf Y (2018) Interactions between the circadian clock and TGF-β signaling pathway in zebrafish. PloS one 13:e0199777. 10.1371/journal.pone.0199777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanko JP, Easterling MR, Fenton SE (2015) Application of Sholl analysis to quantify changes in growth and development in rat mammary gland whole mounts. Reprod Toxicol 54:129–135. 10.1016/j.reprotox.2014.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taghert PH, Hewes RS, Park JH, O'Brien MA, Han M, Peck ME (2001) Multiple amidated neuropeptides are required for normal circadian locomotor rhythms in Drosophila. J Neurosci 21:6673–6686. 10.1523/JNEUROSCI.21-17-06673.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urrutia H, Aleman A, Eivers E (2016) Drosophila dullard functions as a Mad phosphatase to terminate BMP signaling. Sci Rep 6:32269. 10.1038/srep32269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh DK, Takahashi JS, Kay SA (2010) Suprachiasmatic nucleus: cell autonomy and network properties. Annu Rev Physiol 72:551–577. 10.1146/annurev-physiol-021909-135919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z, Shafer OT (2014) The Drosophila circadian clock is a variably coupled network of multiple peptidergic units. Science 343:1516–1520. 10.1126/science.1251285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z, Bennett AJ, Clem JL, Shafer OT (2016) The Drosophila clock neuron network features diverse coupling modes and requires network-wide coherence for robust circadian rhythms. Cell Rep 17:2873–2881. 10.1016/j.celrep.2016.11.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshii T, Wülbeck C, Sehadova H, Veleri S, Bichler D, Stanewsky R, Helfrich-Förster C (2009) The neuropeptide pigment-dispersing factor adjusts period and phase of Drosophila's clock. J Neurosci 29:2597–2610. 10.1523/JNEUROSCI.5439-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Houl JH, Hardin PE (2011) NEMO kinase contributes to core period determination by slowing the pace of the Drosophila circadian oscillator. Curr Biol 21:756–761. 10.1016/j.cub.2011.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Chung BY, Lear BC, Kilman VL, Liu Y, Mahesh G, Meissner RA, Hardin PE, Allada R (2010a) DN1(p) circadian neurons coordinate acute light and PDF inputs to produce robust daily behavior in Drosophila. Curr Biol 20:591–599. 10.1016/j.cub.2010.02.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu Y, Bilodeau-Wentworth D, Hardin PE, Emery P (2010b) Light and temperature control the contribution of specific DN1 neurons to Drosophila circadian behavior. Curr Biol 20:600–605. 10.1016/j.cub.2010.02.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Ye Y, Long X, Xiao P, Ren X, Yu J (2016) BMP signaling and its paradoxical effects in tumorigenesis and dissemination. Oncotarget 7:78206–78218. 10.18632/oncotarget.12151 [DOI] [PMC free article] [PubMed] [Google Scholar]