Abstract
Mesenchymal stem cells (MSCs) as undifferentiated cells are specially considered in cell-based cancer therapy due to unique features such as multi-potency, pluripotency, and self-renewal. A multitude of cytokines secreted from MSCs are known to give such multifunctional attributes, but details of their role are yet to be unknown. In the present study, MSCs were cultured, characterized and co-cultured with Molt-4 cells as acute lymphoblastic leukemia cell line in a trans-well plate. Then, cultured Molt-4 alone and Molt-4 co-cultured with MSCs (10:1) were collected on day 7 and subjected to real time-PCR and Western blotting for gene and protein expression assessment, respectively. Ki-67/caspase-3 as well as telomere length were investigated by flow cytometry and real time-PCR, respectively. The results showed that MSCs caused significant decrease in telomere length as well as hTERT gene expression of Molt-4 cells. Also, gene and protein expression of BAD and P53 were significantly increased. Furthermore, the flow cytometry analysis indicated the decrease and increase of the Ki-67 and caspaspase-3 expression, respectively. It was concluded that MSCs co-cultured with Molt-4 cells could be involved in the promotion of Molt-4 cell apoptosis via caspase-3, BAD, and P53 expression. In addition, the decrease of telomere length is another effect of MSCs on Molt-4 leukemic cells.
Key Words: Mesenchymal stem cells, telomere length, hTERT, BAD, P53, caspase-3, apoptotic pathway
Mesenchymal stem cells (MSCs) are multipotent undifferentiated cells which have a potential to differentiate into different cell types such as adipocytes, osteocytes, neuron like cells etc. (1). Other features of MSCs including self-renewal, plasticity, and non-immunogenic characteristics have led to consider them for cell-based therapy (2). The role of MSCs in some diseases such as cancer, blood disorders, heart failure, genetic and neurodegenerative diseases have been considered in more recent studies (3, 4). Hematologic malignancies have received more attention for cell transplantation with MSCs. The promoting and inhibiting effects of MSCs on cancer cells progression is one of the challenges associated with cell therapy (5, 6). Most studies confirmed the inhibitory effects of MSCs on tumor growth, however, only a few have shown stimulatory effects (7). The reason for this contradiction may be related to different types of tumor cells, the heterogeneity of MSCs population, and the number of injected cells (8). Because of these contradictions, further studies are suggested for investigating the precise bidirectional interaction between tumor cells and MSCs. In one study, Zhang and Zhang (2009) indicated that factors such as cytokines which are released from MSCs could inhibit the proliferation rate of chronic myeloid leukemia mononuclear cells (CML-MNCs) in patients (9). In contrast to the study mentioned above, Paino et al. (2018) demonstrated that SAOS2 osteosarcoma and MCF7 breast cancer cell lines are able to maintain adipose tissue-derived MSCs (ADSCs) in a stemnes state, and if these cells persist following surgery, they will most likely induce resident MSCs to boost tumor angiogenesis and proliferation (10). In more detail, the inhibitory/promoting effects of MSCs on tumor cells are driven by cytokines and other factors released from them. In this regard, Fonseka et al. (2012) pointed that interleukins (IL)-6 and IL-8 secreted from MSCs could inhibit the growth, and arrest the cell cycle of K562 cells as
CML cell line (11).
Regarding the effect of MSCs on tumor cells, some signaling pathways such as mitogen-activated protein kinases (MAPK), AKT, glycogen synthase kinase (GSK)-3α/β, and extracellular signal-regulated kinase (ERK) 1/2 have been investigated. The aim of the present study was to investigate the effect of MSCs on telomere length (TL) and caspase-3 expression as an apoptotic marker of the Molt-4 cell line through BAD and P53 protein expression. For this purpose, Molt-4 cells cultured alone and co-cultured with MSCs (10:1) were collected on day 7, and subjected to TL assessment, human telomerase reverse transcriptase (hTERT) gene expression, and caspase-3 expression analyses. The protein expression of signaling pathways components involved in this process, including BCL2 associated agonist of cell death (BAD) and P53, were also evaluated.
Materials and Methods
Cell culture
MSCs and Molt-4 cell line were purchased from Royan and Pasteur Institute (Tehran, Iran), respectively. Dulbecco’s Modified Eagle Medium (DMEM) with low glucose (Gibco Co. UK) and Roswell Park Memorial Institute (RPMI) (Gibco Co. UK) supplemented with 10% fetal bovine serum (FBS) (Gibco Co. UK) were used for MSCs and Molt-4 cell line culture, respectively (12).
Characterization of MSCs
MSCs were characterized with cell surface markers investigation and multi-lineage differentiation potential to adipogenic and osteogenic cells as previously reported by Farahzadi et al. (2016) (13). For this purpose, MSCs from passage 4 were trypsinized, collected, and stained with specific antibodies against CD34, CD56, CD44, and CD90 (Santa Cruz Biotech- nology, CA, USA) for 30 min at 4 ℃. At the end of staining time, cells were washed using washing buffer (PBS supplemented with 3-5% FBS) and cell surface markers expression was quantified using FACS instrument. In the following, MSCs were seeded in the presence of adipogenic and osteogenic differentiation medium for 21 days. The components of differentiation medium were previously mentioned by Farahzadi et al. (2016) (13). Ethical consent was approved by Tabriz University of Medical Sciences, Tabriz, Iran (Ethic Code No: IR.TBZMED.VCR.REC. 1398. 215).
Co-culture of MSCs and Molt-4 cell line
MSCs of passage 4-6 were seeded at the density of 2×105 cells/well into trans-well insert with 0.4 µm microporous membrane (SPL Life Sciences Co, South Korea, Cat: 37306). After 12-16 h of culture, 1×106 Molt-4 cells/well was added to lower wells of trans-well plate. In this way two groups of cells were formed; control group (culture of Molt-4 alone) and experimental group (co-cultured Mol-4 and MSCs in a ratio of 10:1). At the end of co-culture at day 7, cultured Molt-4 cells alone and co-cultured Molt-4 cells with MSCs were subjected to genes, proteins, and caspase-3 expression assessment.
Real-time PCR
At the end of co-culture period, Molt-4 cells in both control and experimental groups were collected and mRNA expression of target genes hTERT, BAD, and P53 were examined by real-time PCR. In more detail, total RNA was extracted using RNA extraction kit (Yekta Tajhiz Azma, Tehran, Iran). 2 μg RNA was used for the first strand cDNA synthesis according to manufacturer’s instructions. Corbett Rotor-Gene 6000 HRM was used for performing PCR reactions in a total volume of 20 μl containing forward and reverse primers (1 µM), SYBER Green master mix (Yekta Tajhiz Azma, Tehran, Iran) (2X), cDNA and H2O (14). The annealing temperature was 61 ˚C (BAD), 54 ˚C (P53), and 59 ˚C (hTERT and β-actin) for cDNA amplification. The final data was analyzed as Ct values in relation to β-actin Ct values by the 2-ΔΔCT method (15, 16). Primers are presented in Table 1.
Table 1.
Primer sequences used for real time-PCR
| No. | Gene | Primer pair sequence (5'-3') | Product length (bp) |
|---|---|---|---|
| NM_032989.3 | BAD | ACTTCCTCGCCCGAAGAGC CTTCCCCTGCCCAAGTTCC |
105 |
| NM_001126118.1 | P53 | TCAGTCTACCTCCCGCCATAA AGTGGGGAACAAGAAGTGGAG |
86 |
| NM_001193376.3 | hTERT | CAGCAAGTTTGGAAGAACCC GACATCCCTGCGTTCTTGG |
98 |
| NM_001101.4 | β-actin | AAACTGGAACGGTGAAGGTG TATAGAGAAGTGGGGTGGCT |
174 |
Western blotting
Protein expression of BAD and P53 was investigated by Western blotting. For this purpose, cells from control and experimental groups were collected and total cell proteins were extracted. Next, protein samples were electrophoresed on 12% polyacrylamide gels and transferred to a PVDF membrane. In the following, the membrane was incubated with primary antibodies against BAD and P53, and it was then incubated with corresponding secondary antibodies. The protein bands were detected with X-ray film and GAPDH was used as the internal control to normalize (17, 18).
Absolute telomere length (aTL) measurement
aTL was measured using real-time PCR as previously reported by Fathi et al. (2020) (19). Briefly, DNA was isolated from both control and experimental groups at the end of co-culture period and 20 ng/µl DNA was suitable for aTL measurement. Data obtained from real-time PCR for aTL was analyzed as kb/reaction and genome copies/reaction for telomere and single copy gene. The primers used for aTL measurment are listed in Table 2 (20).
Table 2.
Oligomers and characterizations used for absolute telomere length measurement
| Oligomer name |
Oligomer sequence
(5'-3') |
Molecular
weight (MW) |
Other calculations | Purification method | ||
|---|---|---|---|---|---|---|
| To draw standard curve | Telomere standard | (TTAGGG)14 | 26667.2. | Weight (g) | 2.6667×104/6.02×1023= 0.44×10-19 | HPLC |
| Number of molecules of oligomer in TEL standard A | 60×10-12/0.44× 10-19 = 1.36×109 | |||||
| Amount of telomere sequence in TEL standard A (kbp) | 1.36 ×109×84 = 1.18×108 | |||||
| 36B4 standard | 5'CAGCAAGTGGGAAGGTGTAATCCGTCTCCACAGACAAGGCCAGGACTCGTTTGTACCCGTTGATGATAGAATGGG 3' | 23268.1 | Weight (g) | 2.32681×104/6.02×1023= 0.38×10-19 | ||
|
Number of copies
of 36B4 |
200×10-12/0.44× 10-19= 5.26×109 | |||||
| To calculate telomere length | Telo | Fwd: CGGTTTGTTTGGGTTTGGGTTTGGGTTTGGGTTTGGGTT Rev: GGCTTGCCTTACCCTTACCCTTACCCTTACCCTTACCCT |
||||
| 36B4 | Fwd: CAGCAAGTGGGAAGGTGTAATCC Rev: CCCATTCTATCATCAACGGGTACAA |
|||||
Apoptosis investigation by Ki-67/caspase-3 assay
Ki-67/aaspase-3 assay was performed in co-cultured and non-co-cultured groups. In this regard, 20 ×104 cells/well were washed with washing buffer and incubated with 0.2% Triton X-100 for 15 min. Next, the cells were stained with 5 μ Ki-67 antibody solution (BD Biosciences, USA, Cat: 556027) for 30 min and analyzed by flow cytometry.
In addition, for the caspase-3 assay, cells from both groups were fixed and then permeabilized using fixation and permeabilization buffers (supplemented in kit), respectively. Washed cells were immediately stained using PE conjugated anti caspase (BD Biosciences, USA, Cat: 556027) and analyzed by flow cytometry.
Statistical analysis
The results were analyzed using t-test. The statistical significance was determined at P <0.05 by Graph Pad Prism version 6.01.
Results
Characterization of MSCs with flow-cytometry and multi-lineage differentiation
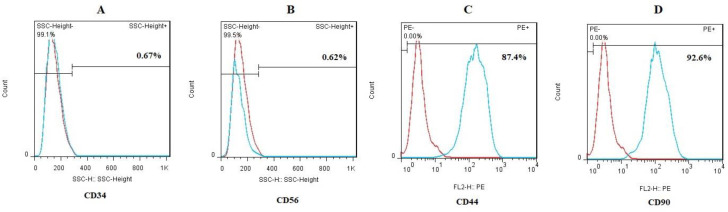
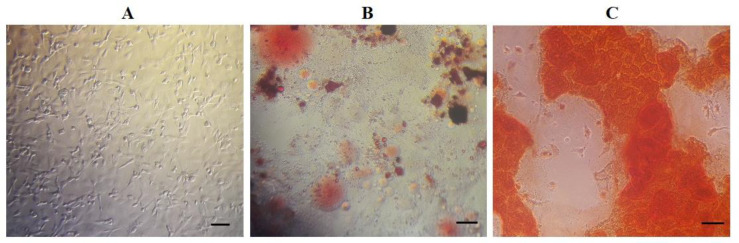
Flow cytometry analyzes (Figures 1A-D) show that MSCs expressed CD44 (87.4%) and CD90 (92.6%) as mesenchymal surface markers. However, hematopoietic surface markers CD34 (0.67%) and CD56 (0.62%) were not expressed in MSCs. In addition to flow-cytometry, adipogenic and osteogenic differentiation was investigated for multi-lineage differentiation potency of MSCs. As shown in Figure 2, lipid droplets as well as calcium deposits were stained with Oil-red O and Alizarin-red, respectively (13).
Fig. 1.
Immunophenotypic characterization of MSCs; The MSCs were negative for (A) CD34 (0.67%) and (B) CD56 (0.62%), and positive for (C) CD44 (87.4%) and (D) CD90 (92.6%). Also, isotype control is seen with red dots. Flow cytometry data was analyzed by FlowJo software (version 6.2)
Fig. 2.
Morphology of MSCs. A) Fibroblast-like morphology of cells was seen (bar = 20 μm); B) Lipid vacuoles were stained by Oil-red O after abiogenesis (bar = 20 μm); C) Mineralized cell aggregates were stained by Alizarin red at the end of osteogenic differentiation (bar = 20 μm).
Investigation of the gene and protein expression of BAD and P53 in Molt-4 cells following co-culture with MSCs
For investigating the effect of cytokines secreted from MSCs on Molt-4 cells as acute lymphoblastic leukemia, mRNA and protein expression was examined by real time-PCR and Western blotting, respectively.
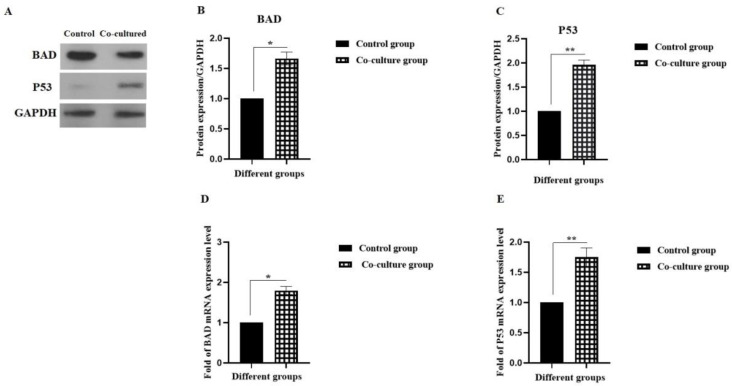
In this regard, BAD and P53 as apoptotic elements were investigated. As shown in Figures 3A-C, the protein expression levels of BAD and P53 was significantly increased by about 1.66 and 1.96 folds in the experimental group in comparison with the control group, respectively (*P <0.05 and **P < 0.01). In addition, the mRNA levels of BAD and P53 were significantly increased by about 1.8 and 1.75 folds in the experimental group in comparison with the control group, respectively (Figs 3D and E) (*P <0.05 and **P < 0.01).
Fig. 3.
Effect of MSCs on gene and protein expression of BAD and P53 in Molt-4 cell line. A-C) The protein expression of BAD and P53 ; D and E) The mRNA expression levels of BAD and P53. *P < 0.05 and **P < 0.01
Investigation of aTL and hTERT gene expression following co-culture with MSCs
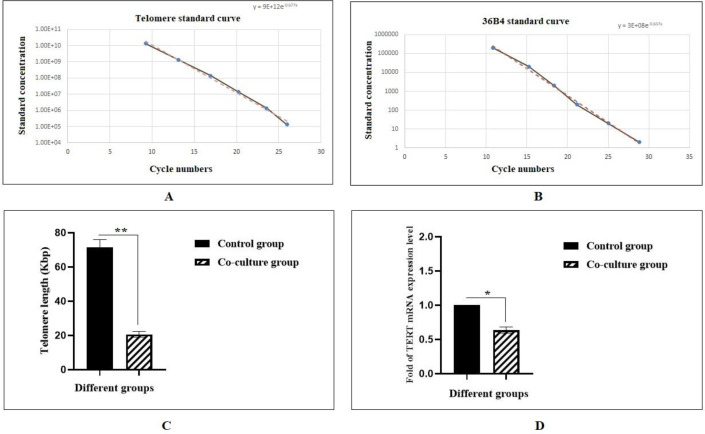
Following the co-culture period, aTL was measured using real-time PCR. As shown in Figs 3 A-C, aTL was significantly decreased (20.63 Kbp) compared to the control group (71.61 Kbp) (**P<0.01). Also, the hTERT gene expression has decreased by about 0.68-fold in the experimental group in comparison with the control group (Figure 4D) (*P <0.05).
Fig. 4.
Absolute telomere length measurement of Molt-4 cells following co-culture with MSCs. A) The standard curve for calculating the length of telomere sequence per reaction tube; B) The standard curve for calculating genome copies using the 36B4 copy number; C) Telomere length (TL) of Molt-4 cells were compared to Molt-4 cells co cultured with MSCs. MSCs decreased significantly the TL of Molt-4 cells upon co-culture (**P < 0.01); D) Relative hTERT gene expression levels of Molt-4 cell line at the end of 7th day co-culture period with MSCs. MSCs decreased significantly the expression of hTERT gene in Molt-4 cell line (*P < 0.05).
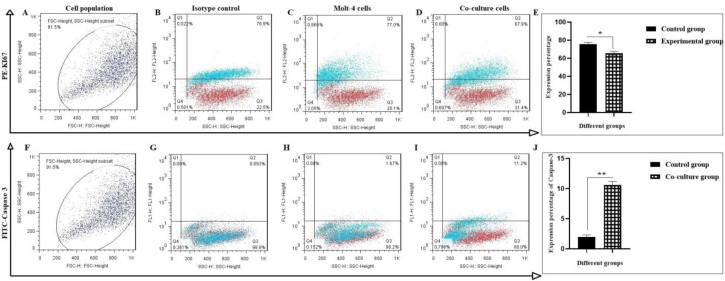
Investigation of cell apoptosis by Ki 67-casapase3 assessment following co-culture with MSCs
The predominant effect of MSCs on inhibition of Molt-4 cell proliferation was detected by Ki-67 expression.
It was shown that co-culture condition attributed to the downregulation of Ki-67 in Molt-4 cells. At the end of co-culture period (7 days), the amount of Ki-67 in Molt-4 cells was 67.9% in comparison with control cells scored that contained 77.0% (Figures 5A-E) (*P < 0.05). Also, Molt-4 cells apoptosis was assessed following their co- culture with MSCs by caspase-3 investigation.
Fig. 5.
Proliferation assay of Molt-4 cells following co-cultured with MSCs. A-E) Harvested cells were evaluated with Ki-67 through flow cytometry; F-J) Flow cytometry analysis of caspase-3 in harvested cells. A and F are selected cell population; B and G are isotype control; C and H are control group; D and I are Molt-4 cells co-cultured with MSCs. Values are mean ± SD from independent experiments (*P < 0.05 and **P < 0.01, n = 3).
Data showed that caspase-3 level in the co-cultured group was increased 6.7-fold (Figurs 5F-J).
Discussion
Hematologic malignancies are among the major causes of mortality throughout the world, and new therapeutic methods attract physicians and investigators. Stem cells present characteristics such as differentiation potential, self-renewal ability, etc. They are classified into two general groups of cells; somatic or adults and embryonic stem cells (21). Adult stem cells are multipotent undifferentiated cells with the multi-lineage differentiation potential. Among these, MSCs have received more attention in cell-based therapy (21). Currently, some diseases including heart failure, chronic wounds, liver disease, sepsis and respiratory diseases, and neurodegenerative diseases, are candidates for cell therapy (22). Recently, anticancer applications of MSCs have been widely considered. Stem cells can function as novel delivery aims by homing to and targeting both primary and metastatic tumor cells (23). There is abundant evidence that different types of MSCs could prevent tumor growth in vitro. Ganta et al., (2009) demonstrated that umbilical cord blood stem cells diminished mammary adenocarcinoma in a rat model (24). Furthermore, it was reported that intra-tumoral injection of ADSCs in a model of pancreatic adenocarcinoma inhibited tumor growth (25). Secchiero et al. (2010) showed that bone marrow (BM)-MSCs could abolish tumor growth in immune deficient mice bearing disseminated non-Hodgkin’s lymphoma xenografts (6). It has further been pointed that the ADSCs inhibited the in vitro proliferation rate of U251 glioma cells (26). Cetintas et al. (2014) indicated that BM-MSCs inhibited significantly the proliferation of ALL cell line CCRF-CEM via changes in the gene and protein expression levels of BAX, caspase 9, and P53 (27). The promoting effects of MSCs on cancerous cells were also been reported as contradictory results (28,29). These contradictory effects can be due to different cancer cells or different MSCs sources. Previous investigations have shown that MSCs affect cancer cells by releasing cytokines, chemokines, and growth factors (30). The results from the current study support the hypothesis that MSCs has inhibitory effects on ALL cell line Molt-4. The decreased and increased protein expression of Ki-67 and caspase3 might be the potential factor for this inhibition, respectively. The accurate role of different factors such as cytokines in leukemic cell survival is not yet well realized. Therefore, studying the cytokine and chemokine array is recommended in future investigations. On the other hand, telomerase expression followed by an increase in TL is critical for cell survival in cancer; thus, its important role as a target for cancer therapeutics is a topic that is widely considered. In most cancers, telomerase is activated to manipulate TL. Accordingly, reduced telomerase activity as well as TL, can be used as therapeutic targets to dominate cancers (31). Keller et al. (2009) reported that TL measured in peripheral blood of patients with CML correlates with disease stage, clinical prognostic scores, and response to treatment (32). In another study, Braig et al. (2014) demonstrated that telomerase-targeting strategy could alleviate the tumor promoting effect of BCR-ABL via inducing senescence in CML-like cells (33). The current results showed a significant relationship between hTERT gene expression and TL in Molt-4 cells co-cultured with MSCs. Since TL is regulated by the expression of the hTERT gene, there might be an association between the TL and hTERT expression. In addition to the mentioned items, many signaling pathways involved in cancer progression and apoptosis have been characterized; among these, the P53, BAD, and caspase3 pathways are important.
Also, it was shown that upregulation of P53 and BAD in some cancer cells via downregulation of BCL-2 protein can lead to apoptosis (34, 35). In another study, Chen et al. (2017) pointed that the inhibition of telomerase activity can lead to cellular senescence via a P53-dependent mechanism (36). These results are in line with the results of the current study where overexpression of P53 was associated with decreasing TL and hTERT gene expression. These data suggest that the reduction in TL and hTERT gene expression was governed by the P53, BAD, and caspase3 signaling pathways. Without any ethical concerns, MSCs are easily obtained and cell therapeutic strategy using these cells seems to be a better choice for cancers. However, further researches are needed to use MSCs as clinical application.
Acknowledgment
This project was supported by Stem Cell Research Center, Tabriz University of Medical Sciences, Tabriz, Iran (Pazhoohan ID: 62715).
Conflict of Interests
The authors declare no conflict of interest.
References
- 1.Nuschke A, Rodrigues M, Wells AW, et al. Mesenchymal stem cells/multipotent stromal cells (MSCs) are glycolytic and thus glucose is a limiting factor of in vitro models of MSC starvation. Stem Cell Res Ther. 2016;7:179. doi: 10.1186/s13287-016-0436-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valenti MT, Mori A, Malerba G, et al. Mesenchymal stem cells: A new diagnostic tool? World J Stem Cells. 2015;7:789–92. doi: 10.4252/wjsc.v7.i5.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ebrahimi T, Abasi M, Seifar F, et al. Transplantation of Stem Cells as a Potential Therapeutic Strategy in Neurodegenerative Disorders. Curr Stem Cell Res Ther. 2021;16:133–44. doi: 10.2174/1574888X15666200628141314. [DOI] [PubMed] [Google Scholar]
- 4.Houthuijzen JM, Daenen LG, Roodhart JM, et al. The role of mesenchymal stem cells in anti-cancer drug resistance and tumour progression. Br J Cancer. 2012;106:1901–6. doi: 10.1038/bjc.2012.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin HD, Fong CY, Biswas A, et al. Human Umbilical Cord Wharton's Jelly Stem Cell Conditioned Medium Induces Tumoricidal Effects on Lymphoma Cells Through Hydrogen Peroxide Mediation. J Cell Biochem. 2016;117:2045–55. doi: 10.1002/jcb.25501. [DOI] [PubMed] [Google Scholar]
- 6.Secchiero P, Zorzet S, Tripodo C, et al. Human bone marrow mesenchymal stem cells display anti-cancer activity in SCID mice bearing disseminated non-Hodgkin's lymphoma xenografts. PLoS One. 2010;5:e11140. doi: 10.1371/journal.pone.0011140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okolie O, Irvin DM, Bago JR, et al. Intra-cavity stem cell therapy inhibits tumor progression in a novel murine model of medulloblastoma surgical resection. PLoS One. 2018;13:e0198596. doi: 10.1371/journal.pone.0198596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fathi E, Sanaat Z, Farahzadi R. Mesenchymal stem cells in acute myeloid leukemia: a focus on mechanisms involved and therapeutic concepts. Blood Res. 2019;54:165–74. doi: 10.5045/br.2019.54.3.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang HM, Zhang LS. Influence of human bone marrow mesenchymal stem cells on proliferation of chronic myeloid leukemia cells. Ai Zheng. 2009;28:29–32. [PubMed] [Google Scholar]
- 10.Paino F, La Noce M, Di Nucci D, et al. Human adipose stem cell differentiation is highly affected by cancer cells both in vitro and in vivo: implication for autologous fat grafting. Cell Death Dis. 2017;8:e2568. doi: 10.1038/cddis.2016.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fonseka M, Ramasamy R, Tan BC, et al. Human umbilical cord blood-derived mesenchymal stem cells (hUCB-MSC) inhibit the proliferation of K562 (human erythromyeloblastoid leukaemic cell line) Cell Biol Int. 2012;36:793–801. doi: 10.1042/CBI20110595. [DOI] [PubMed] [Google Scholar]
- 12.Fathi E, Vietor I. Mesenchymal Stem Cells Promote Caspase Expression in Molt-4 Leukemia Cells Via GSK-3alpha/Beta and ERK1/2 Signaling Pathways as a Therapeutic Strategy. Curr Gene Ther. 2021;21:81–8. doi: 10.2174/1566523220666201005111126. [DOI] [PubMed] [Google Scholar]
- 13.Farahzadi R, Mesbah-Namin SA, Zarghami N, et al. L-carnitine Effectively Induces hTERT Gene Expression of Human Adipose Tissue-derived Mesenchymal Stem Cells Obtained from the Aged Subjects. Int J Stem Cells. 2016;9:107–14. doi: 10.15283/ijsc.2016.9.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montazersaheb S, Avci CB, Bagca BG, et al. Targeting TdT gene expression in Molt-4 cells by PNA-octaarginine conjugates. Int J Biol Macromol. 2020;164:4583–90. doi: 10.1016/j.ijbiomac.2020.09.081. [DOI] [PubMed] [Google Scholar]
- 15.Tarhriz V, Wagner KD, Masoumi Z, et al. CDK9 Regulates Apoptosis of Myoblast Cells by Modulation of microRNA-1 Expression. J Cell Biochem. 2018;119:547–54. doi: 10.1002/jcb.26213. [DOI] [PubMed] [Google Scholar]
- 16.Alipour M, Firouzi N, Aghazadeh Z, et al. The osteogenic differentiation of human dental pulp stem cells in alginate-gelatin/Nano-hydroxyapatite microcapsules. BMC Biotechnol. 2021;21 doi: 10.1186/s12896-020-00666-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montazersaheb S, Kazemi M, Nabat E, et al. Downregulation of TdT Expression through Splicing Modulation by Antisense Peptide Nucleic Acid (PNA) Curr Pharm Biotechnol. 2019;20:168–78. doi: 10.2174/1389201020666190206202650. [DOI] [PubMed] [Google Scholar]
- 18.Tarhriz V, Eyvazi S, Musavi M, et al. Transient induction of Cdk9 in the early stage of differentiation is critical for myogenesis. J Cell Biochem. 2019;120:18854–61. doi: 10.1002/jcb.29204. [DOI] [PubMed] [Google Scholar]
- 19.Fathi E, Farahzadi R, Javanmardi S, et al. L-carnitine Extends the Telomere Length of the Cardiac Differentiated CD117(+)- Expressing Stem Cells. Tissue Cell. 2020;67:101429. doi: 10.1016/j.tice.2020.101429. [DOI] [PubMed] [Google Scholar]
- 20.Fathi E, Farahzadi R, Valipour B. Alginate/gelatin encapsulation promotes NK cells differentiation potential of bone marrow resident C-kit(+) hematopoietic stem cells. Int J Biol Macromol. 2021;177:317–27. doi: 10.1016/j.ijbiomac.2021.02.131. [DOI] [PubMed] [Google Scholar]
- 21.Stellavato A, La Noce M, Corsuto L, et al. Hybrid Complexes of High and Low Molecular Weight Hyaluronans Highly Enhance HASCs Differentiation: Implication for Facial Bioremodelling. Cell Physiol Biochem. 2017;44:1078–92. doi: 10.1159/000485414. [DOI] [PubMed] [Google Scholar]
- 22.Fathi E, Farahzadi R, Vietor I, et al. Cardiac differentiation of bone-marrow-resident c-kit(+) stem cells by L-carnitine increases through secretion of VEGF, IL6, IGF-1, and TGF- beta as clinical agents in cardiac regeneration. J Biosci. 2020:45. [PubMed] [Google Scholar]
- 23.Zhang CL, Huang T, Wu BL, et al. Stem cells in cancer therapy: opportunities and challenges. Oncotarget. 2017;8:75756–66. doi: 10.18632/oncotarget.20798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ganta C, Chiyo D, Ayuzawa R, et al. Rat umbilical cord stem cells completely abolish rat mammary carcinomas with no evidence of metastasis or recurrence 100 days post-tumor cell inoculation. Cancer Res. 2009;69:1815–20. doi: 10.1158/0008-5472.CAN-08-2750. [DOI] [PubMed] [Google Scholar]
- 25.Cousin B, Ravet E, Poglio S, et al. Adult stromal cells derived from human adipose tissue provoke pancreatic cancer cell death both in vitro and in vivo. PLoS One. 2009;4:e6278. doi: 10.1371/journal.pone.0006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang C, Lei D, Ouyang W, et al. Conditioned media from human adipose tissue-derived mesenchymal stem cells and umbilical cord-derived mesenchymal stem cells efficiently induced the apoptosis and differentiation in human glioma cell lines in vitro. Biomed Res Int. 2014;2014:109389. doi: 10.1155/2014/109389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bozok Cetintas V, Aktug H, Oltulu F, et al. The effects of mesenchymal stem cells on lymphoblastic leukemia cell proliferation. J BUON. 2014;19:1006–17. [PubMed] [Google Scholar]
- 28.Eggenhofer E, Luk F, Dahlke MH, et al. The life and fate of mesenchymal stem cells. Front Immunol. 2014;5:148. doi: 10.3389/fimmu.2014.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galland S, Stamenkovic I. Mesenchymal stromal cells in cancer: a review of their immunomodulatory functions and dual effects on tumor progression. J Pathol. 2020;250:555–72. doi: 10.1002/path.5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reikvam H, Hatfield KJ, Fredly H, et al. The angioregulatory cytokine network in human acute myeloid leukemia - from leukemogenesis via remission induction to stem cell transplantation. Eur Cytokine Netw. 2012;23:140–53. doi: 10.1684/ecn.2012.0322. [DOI] [PubMed] [Google Scholar]
- 31.Mai W, Kawakami K, Shakoori A, et al. Deregulated GSK3{beta} sustains gastrointestinal cancer cells survival by modulating human telomerase reverse transcriptase and telomerase. Clin Cancer Res. 2009;15:6810–9. doi: 10.1158/1078-0432.CCR-09-0973. [DOI] [PubMed] [Google Scholar]
- 32.Keller G, Brassat U, Braig M, et al. Telomeres and telomerase in chronic myeloid leukaemia: impact for pathogenesis, disease progression and targeted therapy. Hematol Oncol. 2009;27:123–9. doi: 10.1002/hon.901. [DOI] [PubMed] [Google Scholar]
- 33.Braig M, Pallmann N, Preukschas M, et al. A 'telomere-associated secretory phenotype' cooperates with BCR-ABL to drive malignant proliferation of leukemic cells. Leukemia. 2014;28:2028–39. doi: 10.1038/leu.2014.95. [DOI] [PubMed] [Google Scholar]
- 34.Basu A, Haldar S. The relationship between BcI2, Bax and p53: consequences for cell cycle progression and cell death. Mol Hum Reprod. 1998;4:1099–109. doi: 10.1093/molehr/4.12.1099. [DOI] [PubMed] [Google Scholar]
- 35.Stickles XB, Marchion DC, Bicaku E, et al. BAD-mediated apoptotic pathway is associated with human cancer development. Int J Mol Med. 2015;35:1081–7. doi: 10.3892/ijmm.2015.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen RJ, Wu PH, Ho CT, et al. P53-dependent downregulation of hTERT protein expression and telomerase activity induces senescence in lung cancer cells as a result of pterostilbene treatment. Cell Death Dis. 2017;8:e2985. doi: 10.1038/cddis.2017.333. [DOI] [PMC free article] [PubMed] [Google Scholar]