Abstract
Background:
Caloric restriction (CR) is the only consistently reproducible non-genetic means of minimizing age-related diseases and increasing maximum lifespan in short-lived animals but few human studies exist.
Objective:
Since elderly Okinawans exhibit several phenotypic features of CR including low BMI, low prevalence of chronic diseases, and exceptional longevity, we hypothesized that this phenotype may be reflected in candidate biomarkers of human aging.
Methods:
We retrospectively estimated adult energy balance across the life course for septuagenarian birth cohorts (born ca 1915–1925) from Okinawa and the U.S. based on archived data. We then compared plasma DHEA, estrogen and testosterone in a sample of community dwelling members from these birth cohorts.
Results:
Elderly Okinawans had much lower caloric intake than Americans and appeared mildly calorically restricted (10–15%) at younger ages relative to their estimated energy requirements. Okinawans also had significantly higher plasma DHEA, testosterone and estrogen levels as septuagenarians versus non-CR Americans of similar chronological age.
Conclusion:
These cross-sectional data are consistent with the caloric restriction hypothesis in humans and support further longitudinal investigation into biomarkers of human aging and their potential modification by caloric restriction.
Keywords: Caloric restriction, Okinawa, longevity, human, biomarker, DHEA
INTRODUCTION
Caloric restriction (CR) is the only consistent non-genetic method of markedly reducing age-related diseases and lengthening maximum lifespan in short-lived animals [1–5]. Ongoing studies of CR in long-lived non-human primates have found that CR lowers body weight and fat mass, decreases blood pressure, lowers glucose, induces beneficial changes in blood cholesterol, higher cortisol levels, higher DHEA levels, and other alterations of phenotype and gene expression suggesting longer lifespan [6–8].
Recent short-term studies on humans are consistent with the biological effects in animals, where CR lowers blood pressure, induces leaner BMI, improved lipid profiles, lower blood glucose and increased insulin sensitivity [9–12]. But an effect for CR on human lifespan is difficult to assess since the length of human lifespan renders such interventional studies nearly impossible. Gauging the effects of CR on aging have been difficult since valid biological markers that can measure the rate of human aging don’t exist and therefore controversy exists whether short term studies are a valid model for gauging CR’s potential effects on the aging process [13,14].
The role of CR in human aging is an important unanswered question in aging [11,15]. A few epidemiological studies support CR-related health effects [16] but only one long-term study (>10 years) has reported the effects of low caloric intake on human mortality. A 36-year epidemiological study of the Honolulu Heart Program cohort found a weak trend for lower all-cause mortality in never smoking men who ate fewer calories [17]. This trend persisted until caloric intake dropped to less than 50% of the group mean, consistent with the animal literature.
Of significant epidemiological interest is that pre-World War 2 birth cohorts of elderly Okinawans (currently aged 65-plus years) allegedly ate fewer calories than other Japanese and have several characteristics of the CR phenotype [18,19]. This phenotype includes the smallest body size, the longest life expectancy and the lowest age-specific mortality risk in Japan. Age-related diseases such as coronary heart disease and hormone-related cancers are also the lowest in Japan. Okinawa also has the highest prevalence of exceptionally aged individuals in Japan, the world’s longest-lived country [20,21]. Thus, elderly Okinawans may be a very important population for assessing the long-term effects of caloric restriction in humans.
Previous research has implicated diet as a contributor to the healthy phenotype seen in elderly Okinawans [18,19]. However, long term analyses of caloric intake and energy balance that are supported by biomarker evidence for CR-related health effects are rare.
We hypothesized that if elderly Okinawans were truly a calorically restricted population they would exhibit phenotypic evidence of slower physiological aging, relative to non-CR human populations, and that this would measurable by biomarker assay. Therefore, to test the CR hypothesis in elderly Okinawans, we asked two questions. Were Okinawan septuagenarians calorically restricted? And, is there biomarker evidence of slower physiological aging in this cohort? Thus, to test the first question, we estimated caloric intake and energy expenditure and collected anthropometric data from the birth cohort representing Okinawan septuagenarians at four time points: young adulthood, late adulthood, middle age, older age. DHEA appears to be a promising candidate biomarker of aging in non-human primates [22], so to answer question two, we measured plasma DHEA and its downstream products testosterone and estrogen in Okinawan septuagenarians. Subsequently, these data were compared to previously published data from non-CR Americans of similar chronological age. Population differences in energy balance, anthropometry and age-related biomarkers should be large between Okinawans and Americans, assuming the former truly underwent CR.
METHODS
Study Population and Procedures
The Okinawa Centenarian Study (OCS) is an ongoing population-based study of hundred-year-olds and other selected elderly aged 65-plus years, residing in the Japanese prefecture of Okinawa, that began in 1976 [23].
At the initial exam a full geriatric assessment is performed, including physical exam and activities of daily living. Other data collected include sociodemographic characteristics, medical history, anthropometric measures, diet, smoking status, alcohol consumption, family pedigree and blood samples. Blood pressure is measured and a 12-lead resting electrocardiogram is recorded.
For the purposes of this study, plasma from healthy community dwelling Okinawan septuagenarians from the OCS was compared to healthy community dwelling American septuagenarians from the Rancho Bernardo Study for the hormones DHEA, testosterone and estrogen. Both septuagenarian groups belonged to similar birth cohorts (circa 1915–1925) and were similarly aged at the time of hormone measurements. Population data on caloric intake, energy expenditure and BMI were compared retrospectively between Okinawa and the U.S. for the same birth cohorts to estimate their energy balance status over the previous several decades.
Study Measurements
Population Dietary Intake and Energy Expenditure
Data on caloric intake were not available for the specific study subjects in whom DHEA was measured. Therefore, we used archived and/or published population data on dietary intake to estimate energy balance status and potential CR status in Okinawan septuagenarians and Americans across the life course. Okinawan population data were derived from Office of the Civil Administrator of the Ryukyu Islands (Okinawa) for the year 1949, when the septuagenarians were aged approximately 30 years [24] . Estimates were also performed at 1960, 1972 and 1998 by the Okinawa Prefectural government when the cohort was in their forties, fifties and seventies, respectively [25,26]. Studies used similar methodology and the Okinawa dietary data were derived from diet surveys of typical dietary habits. These surveys were then used to estimate caloric intake.
USDA food appearance data adjusted for wastage and spoilage were used for the US dietary data [27]. Anthropometric summary data (height and weight) and demographic data (age, occupation) from the Okinawan population and the U.S. population[28] were used as variables to estimate energy expenditure from the Harris-Benedict equation [29]. This equation provides an approximation of resting metabolic rate. To estimate daily total energy expenditure an activity factor for additional calories burned during daily physical activity was added per the guidelines below:
Sedentary = BMR × 1.2 (little or no exercise, desk job)
Lightly active = BMR × 1.375 (light exercise/sports 1–3 days/wk)
Moderately active = BMR × 1.55 (moderate exercise/sports 3–5 days/wk)
Very active = BMR × 1.725 (hard exercise/sports 6–7 days/wk or physical job)
Extremely active = BMR × 1.9 (hard daily exercise/sports & physical job).
Energy balance was calculated in the two populations by subtracting daily total energy expenditure from caloric intake obtained from the diet surveys. Since this method likely has a fairly wide margin of error, body mass index (BMI) was also estimated since this will act as a surrogate of long-term energy balance.
Hormone Measurement in Study Participants
All study participants from Okinawa were unselected men and women aged approximately seventy-five years (n=54 septuagenarians; 29 males, mean age 74.5 ± 0.7 years and 25 females, mean age 74.7 ± 0.6 years) when hormone measurement was done. Septuagenarians were recruited from usual community dwelling subjects who were attending their annual physical exam.
The reference population for hormone levels in Americans was comprised of slightly younger usual community dwelling American sexagenarians and septuagenarians. These data were derived from previously reported worka from the Rancho Bernardo Study (n=991; 534 men, mean age 68.6 ± 9.0 years; 457 women, mean age 72.1± 8.0 years) who had measurements of plasma hormones performed in 1984–1987 [30].
Hormone Measurements
Similar protocols were used for both populations. Common measures included drawing of non-fasting venous blood samples between 8 am and 4 pm, separating and storing at −20 to −80 C for several weeks to months. Plasma was assayed for DHEA, testosterone and estrogen using a solid phase 125I RIA (radio immunoassay) (Okinawa samples: SRL Laboratories, Tokyo). The inter- and intra-assay coefficients of variations were slightly higher in Okinawans at 5.2% and 10.0% in the Okinawan samples and 6.7% versus 6.1% in the U.S. samples, respectively.
Statistical Analysis
Average hormone levels were compared between the Okinawans and the U.S. reference population. Two sample unpaired Student’s t Tests with unequal variances were performed. A statistically significant difference was considered to be a two-tailed p-value of 0.05.
RESULTS
Caloric Intake and Energy Balance in Okinawa vs. the U.S. (Figure 1)
Figure 1. Caloric Intake and Energy Balance in Okinawa vs. the U.S.
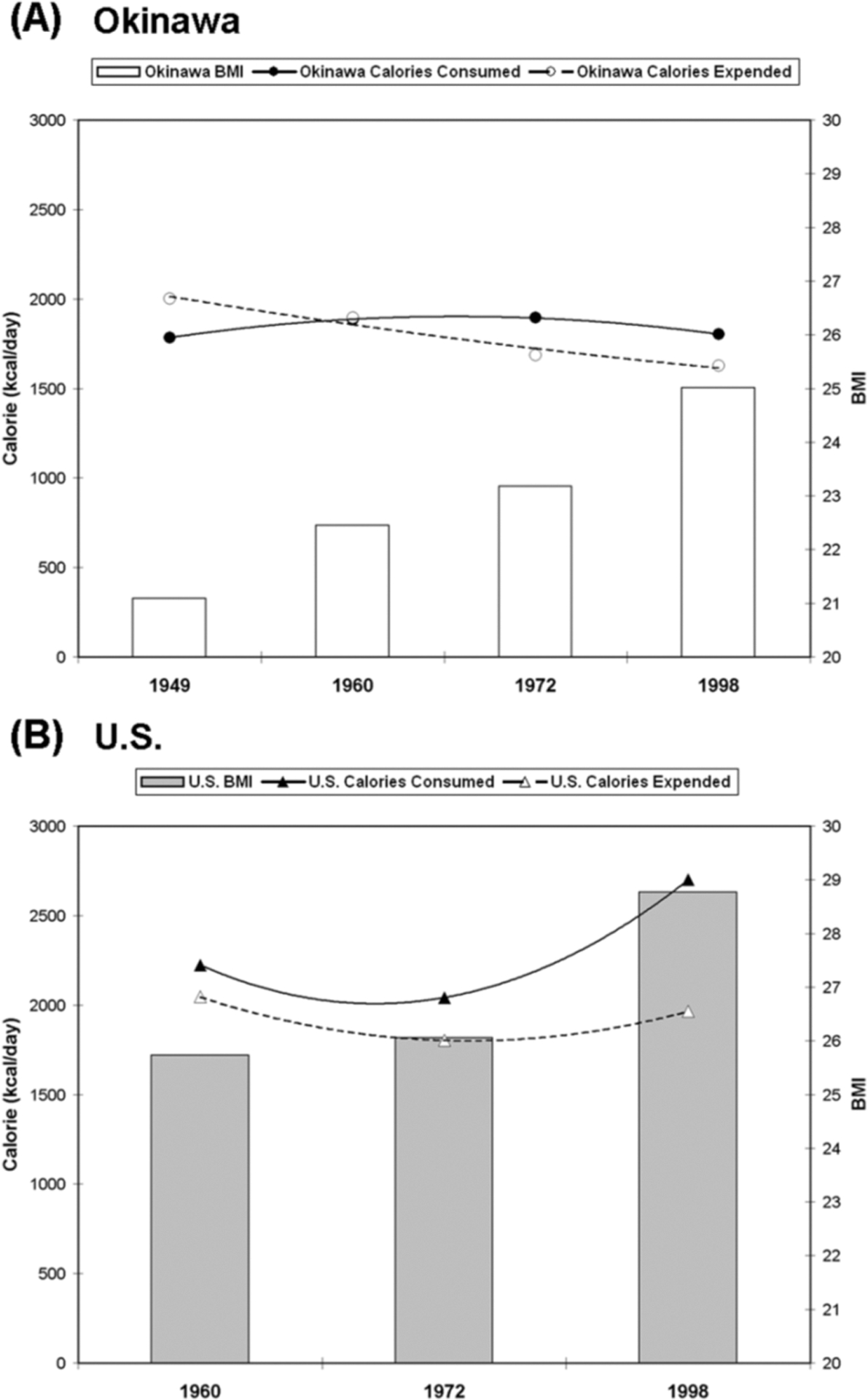
Data for the Okinawan population indicate that consistent positive energy balance did not occur until the 1960s, corresponding to late adulthood/early middle age for the Okinawans (A) but the U.S. population has been in positive energy balance since measurements began circa 1960 (B). Data also indicate markedly higher BMI in Americans vs. Okinawans at all ages and time points.
Data for the Okinawan population indicate that consistent positive energy balance did not occur until the 1960s, corresponding to late adulthood/early middle age for the Okinawans (A). Data indicate that the U.S. population has been in positive energy balance since measurements began circa 1960 (B). These data also indicate markedly higher BMI in Americans vs. Okinawans at all ages and time points.
DHEA Levels (Figure 2)
Figure 2. DHEA Levels.
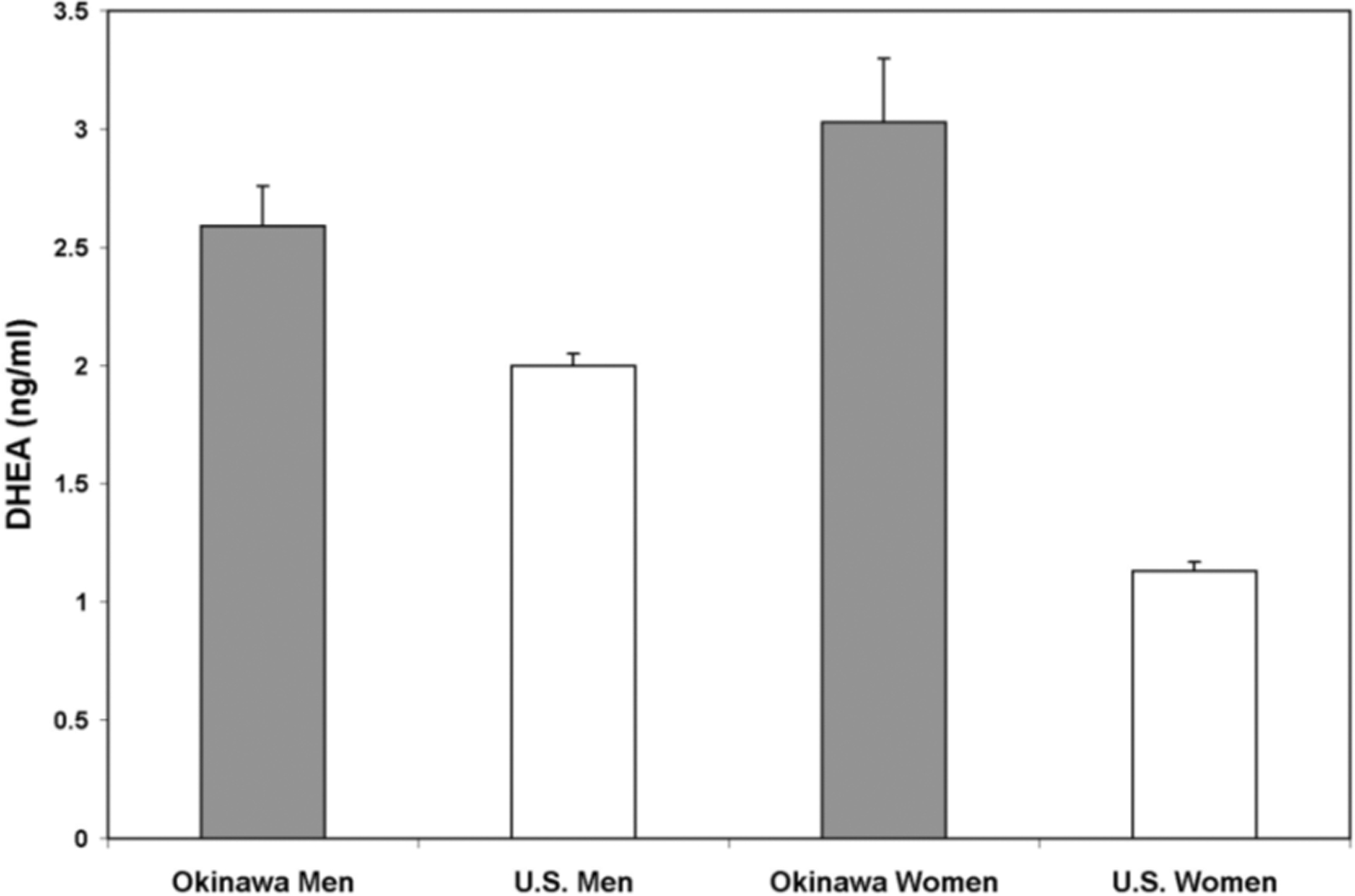
Mean values of DHEA for Okinawan men were higher than American men (p<0.001). Larger differences were seen in similarly aged women from the two populations (p<0.001).
Mean values of DHEA for Okinawan men were 2.59 ng/ml (95%CI: 2.24–2.94) vs. 2.00 ng/ml (95%CI: 1.91–2.10) in American men (p<0.001). Similarly aged women from the two populations exhibited larger differences than men. Men DHEA values were 3.03 ng/ml (95%CI: 2.48–3.58) in Okinawans vs. 1.13 ng/ml (95%CI: 1.06–1.20) in Americans (p<0.001).
Testosterone Levels (Figure 3)
Figure 3. Testosterone Levels.
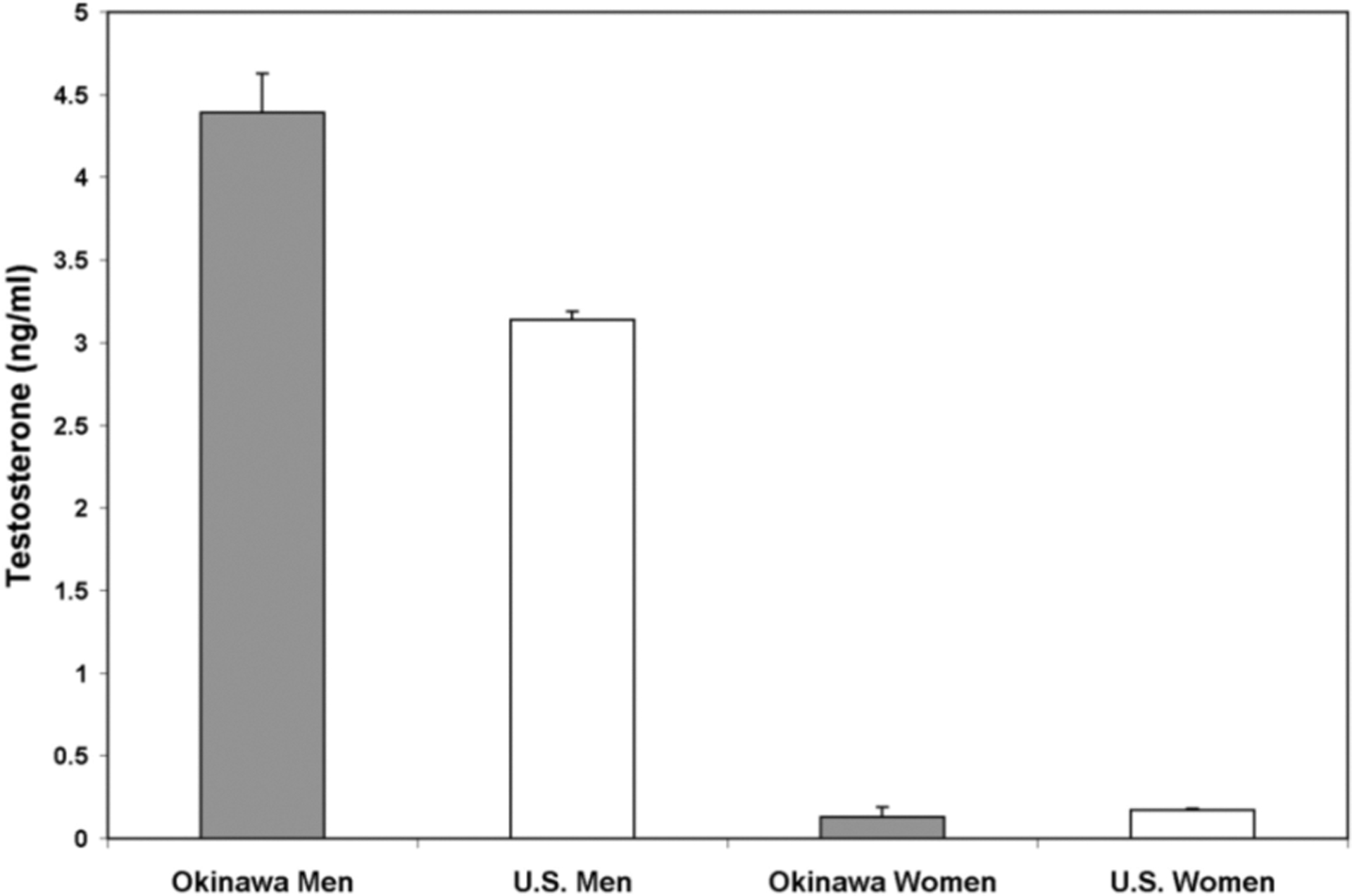
Mean values of testosterone for Okinawan men were significantly higher than American men (p<0.001). No differences were seen in similarly aged women from the two populations (p>0.73).
Mean values of testosterone for Okinawan men were 4.39 ng/ml (95%CI: 3.90–4.89) vs. 3.14 ng/ml (95%CI: 3.05–3.23) in American men (p<0.001). No differences were seen in similarly aged women from the two populations with levels of 0.13 ng/ml (95%CI: <0.005–0.26) found in Okinawans vs. 0.17 ng/ml (95%CI: 0.16–0.18) in Americans (p>0.73).
Estrogen Levels (Figure 4)
Figure 4. Estrogen Levels.
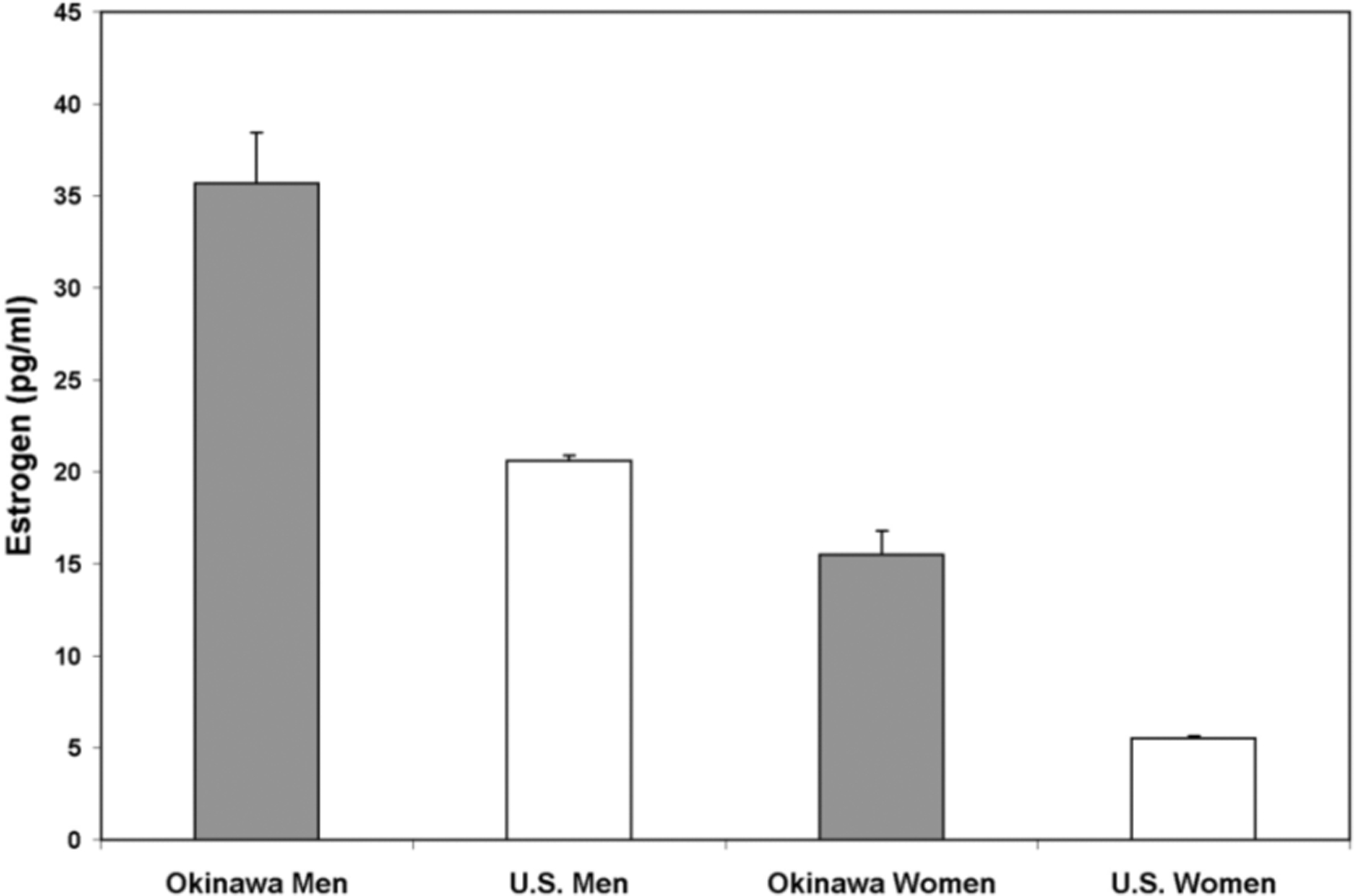
Mean values of estrogen for Okinawan men were significantly higher than American men (p<0.001). Large differences were seen in similarly aged women from the two populations with significantly greater levels found in Okinawans vs. Americans (p<0.001).
Mean values of estrogen for Okinawan men were 35.7 pg/ml (95%CI: 30.07–41.33) vs. 20.6 pg/ml (95%CI: 20.02–21.18) in American men (p<0.001). Large differences were seen in similarly aged women from the two populations with levels of 15.5 pg/ml (95%CI: 12.86–18.14) found in Okinawans vs. 5.5 pg/ml (95%CI: 5.21–5.79) in Americans (p<0.001).
DISCUSSION
Caloric Restriction in Older Okinawans
The septuagenarian cohort of Okinawans had an approximate caloric deficit in the range of 10–15% compared to their estimated energy requirements, from youth until middle age. This figure should be viewed as an approximation only since it was obtained from energy balance estimates using the whole Okinawan population. The energy deficit was due, in large part, to high occupational energy expenditure due to labor intensive occupations as farmers. Concomitantly, this generation of Okinawans had a low calorie intake from an energy poor but nutrient dense diet. This diet was dominated by sweet potatoes, other vegetables, legumes and many plant foods that are typically low in energy density [24].
The relative importance of physical activity vs. diet-induced energy deficits to the CR phenotype is an area that requires further study. Currently, it is not known whether a “negative” energy balance caused by increased physical activity, by diet, or by a combination modifies the biological effects observed with CR. A recent study of mice found that individuals with greater resting and total daily energy expenditure survived longer than those with lower energy expenditure [31]. This was ascribed to higher levels of endogenous activators of proton leak through adenine nucleotide translocase and uncoupling protein-3 pathways in the long-lived group. CR also lowers the inner mitochondrial membrane potential, reducing protein leak, and thus reduces free radical damage to mitochondria but the relationship of energy expenditure to this phenomenon or how energy production and consumption appear to be related is an area of continuing research [31]. Recent studies of human CR that lasted 6 months suggest that CR induced by a combination of increased physical activity and low energy intake might have similar effects to CR achieved by diet alone on some candidate biomarkers of aging [4].
CR is among the most effective means to extend average lifespan and the only non-genetic means to extend maximum lifespan and to reduce age-related chronic disease in experimental animals. CR also appears to affect several experimental biomarkers of aging in animal studies, among the most robust being blood DHEA(S) levels.
Dehydroepiandrosterone (DHEA) is the most abundant circulating steroid hormone in the human body, mainly of adrenal origin, and is found in relatively high concentrations in human plasma in sulfated form (DHEAS) [32,33]. Approximately 50% of the total androgens in men and 75% to 100% of total estrogens in women are derived from this steroid precursor but whether DHEA performs an active role as a hormone or is simply an inactive steroid precursor is unknown [32,33].
Despite the fact that its physiological role and mechanism of action are unclear, it has received much attention for purported protection against age-related morbidity. Elevated blood levels have been associated with reduced risk for obesity, diabetes, heart disease, and cancer among other diseases [32,33].
Perhaps the most compelling role for DHEA(S) may be as a biomarker of aging. A steady, age-related decline of DHEA(S) is seen in both monkeys and humans. For example, DHEAS declined approximately 30% over 4 years in one prospective study of adult male monkeys [22]. In prospective and cross-sectional studies of humans, the blood level of DHEA(S) peaks by the second decade and then declines in a linear fashion by approximately 10–20% per decade such that by age seventy human blood levels are negligible [34]. The rate of decline of DHEAS appears to be about 2–2.5 times more rapid in rhesus monkeys than in humans, consistent with an approximately 3-fold greater life span of the latter [6,22].
Human Studies on Caloric Restriction and DHEA(S)
Recently, DHEA(S) was linked to human survival in a prospective study of the Baltimore Longitudinal Study of Aging (BLSA) cohort although caloric intake was not reported [35]. In the BLSA, men who were in the upper half of plasma DHEAS at study onset had longer survival than their respective counterparts in the lower half of DHEAS over the twenty-plus year follow-up. This was consistent with other measured biomarkers linked to a CR phenotype in the longer-lived men of the BLSA cohort, including lower plasma insulin and body temperature.
We hypothesize that DHEA(S) may serve as an important biomarker of aging in humans, that it may be responsive to caloric restriction, but human data are lacking. Both cross-sectional and longitudinal studies are needed to answer this question. To date no study has reported on DHEA(S) levels in a human population that has been subject to long-term caloric restriction. Therefore, DHEA levels in older Okinawans are of considerable interest since all birth cohorts in Okinawa aged 65-plus years appear to have been mildly calorically restricted for much of their lives.
In this cross-sectional study of elderly individuals in Okinawa and Rancho Bernardo County, California, DHEA levels were significantly higher in Okinawans who had eaten a calorically restricted diet for most of their lives than Americans of comparable chronological age. While population data on calorie intake is only an approximation of what is happening at the individual level, other aging-related effects linked to CR in animal studies support a CR phenotype in older Okinawans. This includes markedly lower age-specific mortality rates from CR-linked diseases in Okinawans versus other Japanese and Americans, smaller body stature, lower body weight, very long life expectancy of older Okinawans and the high prevalence of exceptionally aged individuals, particularly centenarians [18,19].
The two populations were of different ethnic origin so genetic differences might account for the differences in DHEA levels [36] and/or the health attributes of the populations [37,38]. Recent work on the genetics of Okinawan longevity supports a substantial familial component [39]. However, this does not rule out an additional longevity-enhancing effect due to CR. DHEA was also measured in separate laboratories, which might have affected the results. Nevertheless, similar study protocols were used and the operating characteristics of the DHEA assays are known in each laboratory and are unlikely to have been responsible for more than 10–15% of the difference.
While the small sample size and the cross sectional nature of the study are somewhat limiting, another study of DHEA in community-dwelling white Americans that included septuagenarians reported DHEA levels that were close to the values obtained for similarly aged whites in the Rancho Bernardo Study. Plasma levels in this study were 1.6 ng/ml for septuagenarians with no significant differences between genders [34].
More support of a CR phenotype in elderly Okinawans can be derived from cross-population comparisons of plasma hormone levels between Japanese and Americans. Cross-sectional comparisons demonstrate that DHEAS appears to be higher at younger ages in Americans compared to Japanese [40]. Therefore, higher hormone levels in Okinawan septuagenarians would not be expected without a slower age-related decline. Thus, these biomarker data support a crossover effect, possibly due to caloric restriction-mediated slower physiological aging in Okinawans, which may have resulted in slower aging of the adrenal axis.
Support of the current CR-linked DHEA findings may also be derived from a previous study of DHEAS and cardiovascular disease in a group of Japanese-American men (including Okinawans) in the Honolulu Heart Program (HHP) where nutritional information was collected [40]. In a cross-sectional analysis of the control group after adjusting for age, subjects with the lowest caloric intake at study onset had significantly higher DHEAS levels. Since the diet records were from middle-aged men, and are thought to reflect long-term dietary habits, this also supports a potential link between eating fewer calories and slower physiological aging of the adrenal glands.
Testosterone and Estrogen as Biomarkers of Aging
There has been little study of testosterone and estrogen as biomarkers of human aging although it is well known that both hormones decline markedly with age [41–45]. This is particularly so for estrogen since rapid postmenopausal decline occurs. There does appear to be some support for higher plasma levels of these sex hormones at older ages correlating with better health [41–45]. Recent work suggests that estrogen may independently influence insulin signaling in mice, a potential pathway linked to the rate of aging [46]. The fact that both of these hormones are downstream physiological products of DHEA, and that they both are present in higher levels in Okinawans than similarly aged Americans lends further support to the hypothesis that the Okinawans may experienced delayed aging of their hypothalamic-adrenal-pituitary/gonadal axis (reproductive axis) through caloric restriction.
Phenotypic Changes in Post-World-War 2 Birth Cohorts
These data are further supported by phenotypic changes suggesting positive energy balance in cohorts of Okinawans born after World War 2. The significant increases in BMI seen in these post-war generations, who underwent large changes in lifestyle, suggest at least a partial loss of the CR phenotype. Cultural influences on dietary habits due to American influence (bread and processed meat) and Japanese influence (white rice) led to replacement of the main food staple (sweet potatoes) resulting in a higher calorie density diet and higher overall calorie intake [47–49]. This has been accompanied by reduced physical activity due to vocational change from blue collar work to white collar work. The resultant shift in energy balance has been associated with increased risk for chronic disease and a relative slowing of life expectancy gains compared with other Japanese prefectures [48]. However, not all of the CR phenotype has been lost. For example, Okinawans are still noticeably shorter than other Japanese [48,49] suggesting some possible genetic influence over this phenotype.
Conclusion
In conclusion, we observed higher plasma hormone levels in elderly Okinawans who appear to have undergone long-term caloric restriction versus non-restricted Americans of similar chronological age. CR in the pre-World War II birth cohorts of Okinawans may have contributed to low chronic disease prevalence and the exceptional longevity seen in this population. Prior studies support a beneficial effect of CR on candidate biomarkers of aging, morbidity and lifespan in animal models. The current study suggests that the biological effects of CR on some candidate biomarkers of aging in animal studies may also extend to humans. Further studies, particularly prospective studies, are needed to define the biological effects of CR in humans.
References
- 1.McCay CM, Cromwell MF, Maynard LA: The effect of retarded growth upon the length of the life span and upon the ultimate body size. J Nutr 1935;10:63–79. [PubMed] [Google Scholar]
- 2.Lane MA, de Cabo R, Mattison J, Anson RM, Roth GS, Ingram DK: The Roy Walford legacy: diet restriction from molecules to mice to monkeys to man and onto mimetics. Exp Gerontol 2004;39:897–902. [DOI] [PubMed] [Google Scholar]
- 3.Fontana L, Klein S: Aging, adiposity, and calorie restriction. JAMA 2007;297:986–994. [DOI] [PubMed] [Google Scholar]
- 4.Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, Nguyen T, Martin CK, Volaufova J, Most MM, Greenway FL, Smith SR, Deutsch WA, Williamson DA, Ravussin E: Pennington CALERIE Team: Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA 2006;295:1539–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hadley EC, Lakatta EG, Morrison-Bogorad M, Warner HR, Hodes RJ: The future of aging therapies. Cell 2005;120:557–567. [DOI] [PubMed] [Google Scholar]
- 6.Mattison JA, Lane MA, Roth GS, Ingram DK: Calorie restriction in rhesus monkeys. Exp Gerontol 2003;38:35–46. [DOI] [PubMed] [Google Scholar]
- 7.Masoro EJ: Overview of caloric restriction and ageing. Mech Ageing Dev 2005;126:913–922. [DOI] [PubMed] [Google Scholar]
- 8.Roth GS, Mattison JA, Ottinger MA, Chachich ME, Lane MA, Ingram DK: Aging in rhesus monkeys: relevance to human health interventions. Science 2004;305:1423–1426. [DOI] [PubMed] [Google Scholar]
- 9.Heilbronn LK, Ravussin E: Calorie restriction and aging: review of the literature and implications for studies in humans. Am J Clin Nutr 2003;78:361–369. [DOI] [PubMed] [Google Scholar]
- 10.Walford RL, Mock D, Verdery R, MacCallum T: Energy restriction in biosphere 2; alterations in physiologic, hematologic, hormonal, and biochemical parameters in humans restricted for a 2-year period. J Gerontol A Biol Sci Med Sci 2002;57:B211–B224. [DOI] [PubMed] [Google Scholar]
- 11.Lane MA, Mattison JA, Roth GS, Brant LJ, Ingram DK: Effects of long-term diet restriction on aging and longevity in primates remain uncertain. J Gerontol A Biol Sci Med Sci. 2004;59:405–407. [DOI] [PubMed] [Google Scholar]
- 12.Fontana L, Meyer TE, Klein S, Holloszy JO: Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci USA 2004;101:6659–6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ingram DK, Nakamura E, Smucny D, Roth GS, Lane MA: Strategy for identifying biomarkers of aging in long-lived species. Exp Gerontol 2001;36:1025–1034. [DOI] [PubMed] [Google Scholar]
- 14.Butler RN, Sprott R, Warner H, Bland J, Feuers R, Forster M, Fillit H, Harman SM, Hewitt M, Hyman M, Johnson K, Kligman E, McClearn G, Nelson J, Richardson A, Sonntag W, Weindruch R, Wolf N: Biomarkers of aging: from primitive organisms to humans. J Gerontol A Biol Sci Med Sci 2004:59:B560–B567. [DOI] [PubMed] [Google Scholar]
- 15.de Grey AD: The unfortunate influence of the weather on the rate of ageing: why human caloric restriction or its emulation may only extend life expectancy by 2–3 years. Gerontology 2005;51:73–82. [DOI] [PubMed] [Google Scholar]
- 16.Lee IM, Blair SN, Allison DB, Folsom AR, Harris TB, Manson JE, Wing RR: Epidemiological data on the relationships of energy intake, energy balance, and weight gain over the life span with longevity and morbidity. J Gerontol A Biol Sci Med Sci 2001;56A:7–19. [DOI] [PubMed] [Google Scholar]
- 17.Willcox BJ, Yano K, Chen R, Willcox DC, Rodriguez BL, Masaki KH, Donlon T, Tanaka B, Curb JD: How much should we eat? The association between energy intake and mortality in a 36-year follow-up study of Japanese American men. J Gerontol A Biol Sci Med Sci 2004;59:789–795. [DOI] [PubMed] [Google Scholar]
- 18.Kagawa Y: Impact of Westernization on the nutrition of Japanese: changes in physique, cancer, longevity and centenarians. Prev Med 1978;7:205–217. [DOI] [PubMed] [Google Scholar]
- 19.Chan YC, Suzuki M, Yamamoto S: Dietary, anthropometric, hematological and biochemical assessment of the nutritional status of centenarians and elderly people in Okinawa, Japan. J Am Coll Nutr 1997;16:229–235. [DOI] [PubMed] [Google Scholar]
- 20.Statistics and Information Division: Japan Ministry of Health, Labor and Welfare, 2000. [Google Scholar]
- 21.World Health Organization: World Health Statistics Annual. WHO, 2000. [Google Scholar]
- 22.Lane MA, Ingram DK, Ball SS, Roth GS: Dehydroepiandrosterone sulfate: a biomarker of primate aging slowed by calorie restriction. J Clin Endocrinol Metab 1997;82:2093–2096. [DOI] [PubMed] [Google Scholar]
- 23.Sanabe E, Ashitomi I, Suzuki M: Social and medical survey of centenarians. Okinawa J Public Health 1977;9:98–106 [Japanese]. [Google Scholar]
- 24.National Archives: Records of U.S. Occupation Headquarters, World War II. Record Group 260.12.5, 1949. (Records of the Health, Education and Welfare Department of the Office of the Civil Administrator of the Ryukyu Islands). [Google Scholar]
- 25.Division of Health, Labor and Welfare: Health and Welfare Dataset 1880–1976. Okinawa Prefectural Government. [Google Scholar]
- 26.Health and Nutritional Information Research Association, Japan Ministry of Health, Labor and Welfare: 1998 National Nutrition Survey Report. Daiichi Shuppan, 1999. [Google Scholar]
- 27.Putnam J, Allshouse J, Kantor LS: U.S. per capita food supply trends: more calories, refined carbohydrates, and fats. Food Review 2002;25:2–15. [Google Scholar]
- 28.Ogden CL, Fryar CD, Carrol MD, Flegal KM: Mean body weight, height, and body mass index, United States 1960–2002. Advance Data from Vital and Health Statistics, CDC Publication; 2004;347. [PubMed] [Google Scholar]
- 29.Frankenfield DC, Muth ER, Rowe WA: The Harris-Benedict studies of human basal metabolism: history and limitations. J Am Diet Assoc 1998;98:439–445. [DOI] [PubMed] [Google Scholar]
- 30.Greendale GA, Edelstein S, Barrett-Connor E: Endogenous sex steroids and bone mineral density in older women and men: the Rancho Bernardo Study. J Bone Miner Res 1997;12:1833–1843. [DOI] [PubMed] [Google Scholar]
- 31.Speakman JR, Talbot DA, Selman C, Snart S, McLaren JS, Redman P, Krol E, Jackson DM, Johnson MS, Brand MD: Uncoupled and surviving: individual mice with high metabolism have greater mitochondrial uncoupling and live longer. Aging Cell 2004;3:87–95. [DOI] [PubMed] [Google Scholar]
- 32.Herrington DM: DHEA: a biologic conundrum. J Lab Clin Med 1998;131:292–294. [DOI] [PubMed] [Google Scholar]
- 33.Buffington CK: DHEA: elixir of youth or mirror of age? J Am Geriatr Soc 1998;46:391–392. [DOI] [PubMed] [Google Scholar]
- 34.Nafziger AN, Bowlin SJ, Jenkins PL, Pearson TA: Longitudinal changes in dehydroepiandrosterone concentrations in men and women. J Lab Clin Med 1998;131:316–323. [DOI] [PubMed] [Google Scholar]
- 35.Roth GS, Lane MA, Ingram DK, Mattison JA, Mahi D, Tobin JD, Muller D, Metter EJ: Biomarkers of caloric restriction may predict longevity in humans. Science 2002;297:811. [DOI] [PubMed] [Google Scholar]
- 36.Rotter JI, Wong FL, Lifrak ET, Parker LN: A genetic component to the variation of dehydroepiandrosterone sulfate. Metabolism 1985;34:731–736. [DOI] [PubMed] [Google Scholar]
- 37.Takata H, Suzuki M, Ishii T, Sekiguchi S, Iri H: Influence of major histocompatibility complex region genes on human longevity among Okinawan-Japanese centenarians and nonagenarians. Lancet 1987;2:824–826. [DOI] [PubMed] [Google Scholar]
- 38.Akisaka M, Suzuki M, Inoko H: Molecular genetic studies on DNA polymorphism of the HLA class II genes associated with human longevity. Tissue Antigens 1997;50:489–493. [DOI] [PubMed] [Google Scholar]
- 39.Willcox BJ, Hsueh WC, He Q, Willcox DC, Curb JD, Suzuki M: Substantial advantage for longevity in siblings of Okinawan centenarians. Genetic Epidemiology 2005;29:286–287. [Google Scholar]
- 40.LaCroix AZ, Yano K, Reed DM: Dehydroepiandrosterone sulfate, incidence of myocardial infarction, and extent of atherosclerosis in men. Circulation 1992;86:1529–1535. [DOI] [PubMed] [Google Scholar]
- 41.Nieschlag E, Nieschlag S, behre HM: Lifespan and testosterone. Nature 1993;366:215. [DOI] [PubMed] [Google Scholar]
- 42.Muller M, den Tonkelaar I, Thijssen JH, Grobbee DE, van der Schouw YT: Endogenous sex hormones in men aged 40–80 years. Eur J Endocrinol 2003;149:583–589. [DOI] [PubMed] [Google Scholar]
- 43.Derry PS: Hormones, menopause, and heart disease: making sense of the Women’s Health Initiative. Women’s Health Issues 2004;14:212–219 [DOI] [PubMed] [Google Scholar]
- 44.Barrett-Connor E: Hormones and the health of women: past, present, and future. Keynote address. Menopause: 2002;9:23–31. [DOI] [PubMed] [Google Scholar]
- 45.Labrie F, Belanger A, Luu-The V, Labrie C, Simard J, Cusan L, Gomez JL, Candas B: DHEA and the intracrine formation of androgens and estrogens in peripheral target tissues: its role during aging. Steroids 1998;63:322–328. [DOI] [PubMed] [Google Scholar]
- 46.Baba T, Shimizu T, Suzuki Y, Ogawara M, Isono K, Koseki H, Kurosawa H, Shirasawa T: Estrogen, insulin, and dietary signals cooperatively regulate longevity signals to enhance resistance to oxidative stress in mice. J Biol Chem 2005;280:16417–16426. [DOI] [PubMed] [Google Scholar]
- 47.Willcox BJ, Willcox DC, Suzuki M. Okinawa Diet Plan. Random House, New York, NY: 2004 [Google Scholar]
- 48.Todoriki H, Willcox DC, Willcox BJ. The Effects of Post-War Dietary change on longevity and health in Okinawa. Oki J Amer Studies 2004;1:55–64. [Google Scholar]
- 49.Willcox DC, Willcox BJ, Todoriki H, Curb JD, Suzuki M. Caloric restriction and human longevity: what can we learn from the Okinawans? Biogerontology 2006;7:173–77. [DOI] [PubMed] [Google Scholar]


