Abstract
Bioluminescent tools have been used for decades to image processes in complex tissues and preclinical models. However, few distinct probes are available to probe multicellular interactions. We and others are addressing this limitation by engineering new luciferases that can selectively process synthetic luciferin analogs. In this work, we explored naphthylamino luciferins as orthogonal bioluminescent probes. Three analogs were prepared using an optimized synthetic route. The luciferins were found to be robust emitters with native luciferase in vitro and in cellulo. We further screened the analogs against libraries of luciferase mutants to identify unique enzyme-substrate pairs. The new probes can be used in conjunction with existing bioluminescent tools for multi-component imaging.
Keywords: bioluminescence, imaging, luciferase, luciferin, orthogonal
Graphical Abstract
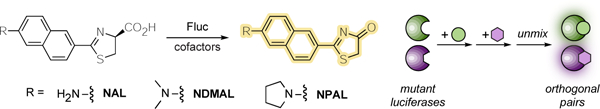
Naphthylamino luciferins were used for rapid, multi-component bioluminescent imaging. The analogs were synthesized using a modular route, and activities were measured in vitro and in cellulo. Mutant luciferases were identified that could differentiate the substrates, and the unique enzyme-substrate pairs were compatible with existing bioluminescent probes. These substrates add to the growing set of bioluminescent tools for multiplexed imaging.
Introduction
Bioluminescence imaging (BLI) is a versatile method for monitoring cellular processes.[1–4] Among the most popular BLI probes are derivatives of firefly luciferase (Fluc), an enzyme that catalyzes the activation and oxidation of a small molecule substrate, d-luciferin (d-luc, Figure 1A).[5,6] Fluc/d-luc and related pairs are routinely used for monitoring biological events, including stem cell proliferation[7] and cancer metastasis.[8] While powerful, many of these probes cannot be deployed simultaneously owing to overlapping emission spectra or substrate cross-reactivities.[9]
Figure 1. Bioluminescence imaging with unique luciferases and luciferins.
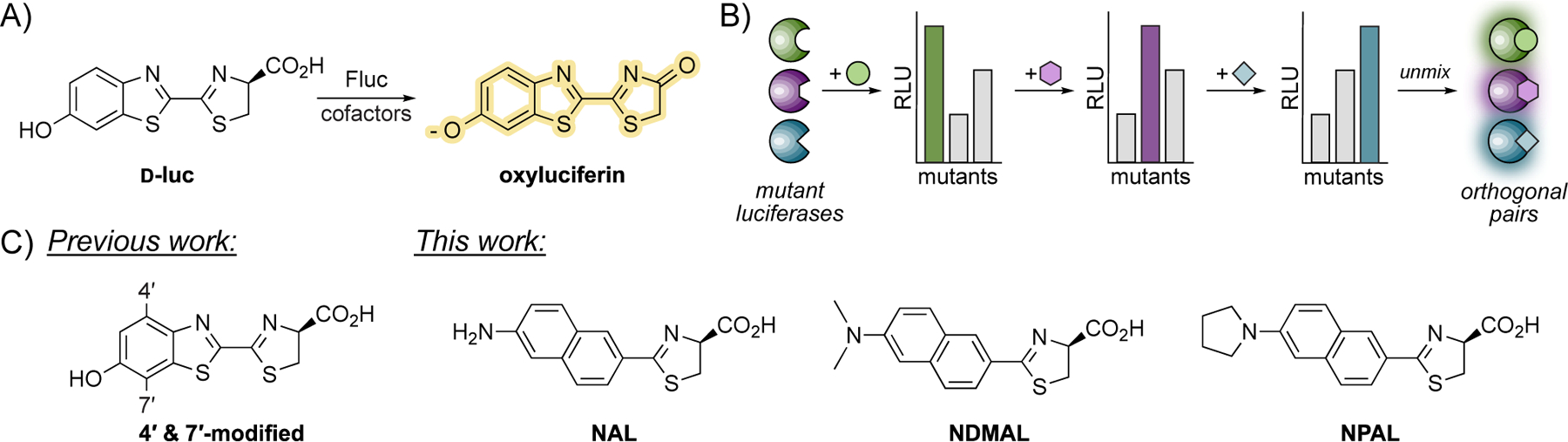
(A) Firefly luciferase (Fluc) catalyzes the transformation of d-luciferin (d-luc) to oxyluciferin, resulting in light emission. (B) Distinct luciferases can be differentiated upon sequential luciferin administration. Changes in bioluminescent intensity can be used to resolve the identities of unique luciferin-luciferase pairs. (C) Previous work evaluated sterically modified luciferins. This work showcases naphthylamino luciferins for multi-component imaging.
We and others are addressing the need for more distinguishable bioluminescent tools by developing substrate-selective (i.e., “orthogonal”) luciferases.[10–20] In this strategy, enzymes are engineered to process chemically distinct luciferin analogs. We previously targeted luciferins comprising steric appendages at C4ʹ and C7ʹ (Figure 1C).[10] Iterative screening of the scaffolds with mutant luciferase libraries revealed unique combinations of enzymes and substrates for multi-component imaging.[12] Other luciferase-luciferin pairs have been similarly identified and are now used in a variety of applications.[17–19]
Selectivity between orthogonal luciferase-luciferin pairs has been historically achieved via mutual exclusion: “unmatched” luciferins and luciferases do not interact. We have recently found, though, that perfect selectivity among the probes is not required for multi-component imaging.[11,12] Rather, unique patterns of light emission are sufficient. If the dimmer substrate is imaged first, the change in brightness when the second substrate is added allows for two unique luciferases to be resolved (Figure 1B).[11] Multi-component imaging via this approach still requires diverse substrates, though, as distinct emission profiles are required. Unique patterns of photon output are most readily achieved using molecules that “look” different.
To broaden the scope of multi-component imaging, we set out to identify additional luciferins that could be differentially processed by mutant luciferases. Herein we report that naphthylamino luciferins are useful scaffolds for orthogonal imaging. These classic motifs are robust emitters and structurally distinct from other popular luciferins, making them attractive probes for multi-component studies.[21,22] Three naphthylamino luciferins were synthesized and evaluated in vitro and in cellulo. The luciferins were also screened against a library of mutant luciferases to identify complementary enzymes. The luciferase hits were capable of discriminating subtle changes in luciferin structure. The naphthylamino luciferins were also readily applied in tandem with other established analogs. Overall, this work expands the number of distinct and bright luciferins to facilitate multi-component imaging applications.
Results and Discussion
Design and synthesis of luciferin analogs
We were drawn to naphthylamino luciferins for a variety of reasons. The naphthalene core is small and rigid, and the light emitter can adopt a planar structure in the enzyme active site, ensuring efficient photon output. Previous studies have also shown that naphthalene can substitute for the benzothiazole unit in d-luc. For example, Branchini and co-workers synthesized a naphthylluciferin variant (Scheme 1, R = OH) and demonstrated its ability to produce light with Fluc.[21] Additional modifications to the naphthalene core were also pursued. Nagano and colleagues synthesized naphthylluciferins bearing amine substituents in place of the C6′ hydroxy group (NAL, NDMAL).[22] NAL and NDMAL were robust emitters with Fluc, making them well suited for biological application. The probes also exhibited red-shifted emission compared to the parent naphthylluciferin. Similar changes in spectral output and binding have been observed upon amination of other Fluc substrates.[13, 23, 24]
Scheme 1.
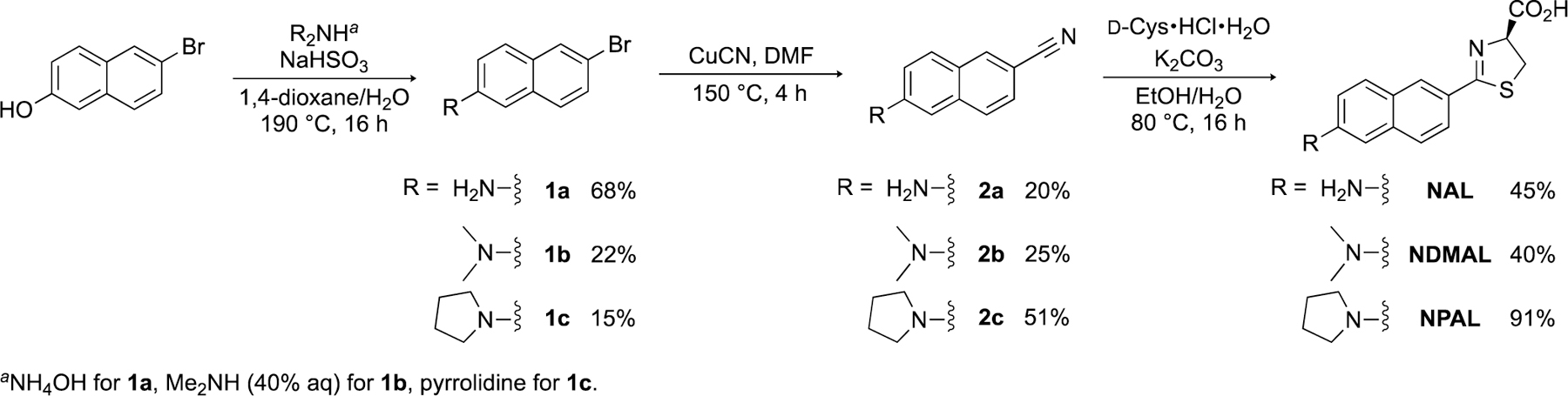
Synthesis of naphthylamino luciferins.
Given the robustness of the naphthylamino analogs and their unique aromatic cores, we reasoned that they would be useful for orthogonal imaging. To our knowledge, though, NAL and NDMAL have yet to be applied in cellular contexts. We thus aimed to prepare these luciferins and examine their utility for multi-component cellular imaging. We also decided to synthesize an analog comprising a pyrrolidine (NPAL) substituent. Such cyclic amino motifs have been previously shown to enhance luciferin binding to complementary luciferases.[13,14,25] To access the panel of probes, we devised a short and modular route. Previous attempts to prepare N-substituted naphthylamino luciferins relied on double reductive amination of 1a,[22] yielding complex mixtures of both mono- and di-functionalized products. To circumvent this issue, we envisioned using a Bucherer reaction to install diverse groups at C6′ (Scheme 1).[26] Simply changing the amine base could thus provide the desired structures. Using this approach, dimethylamine and pyrrolidine were successfully leveraged to prepare analogs 1b and 1c, respectively. The amino products were then subjected to copper-mediated cyanation to install the requisite nitriles. Condensation with d-cysteine ultimately afforded the panel of desired probes.
Naphthylamino luciferins are competent light emitters in vitro and in cellulo
Once synthesized, the naphthylamino luciferins were tested with purified Fluc to verify activity. All three probes provided robust photon outputs (Figure 2), corroborating previous observations. NDMAL and NPAL emitted the most light with the native enzyme. The light output from NPAL was detected even at picomolar concentrations (Figure S1), suggesting that the probe is suitable for sensitive imaging applications. Collectively, these data indicated that the naphthylamino luciferins were robust emitters and suitable candidates for developing unique bioluminescent pairs.
Figure 2. Naphthylamino luciferins are competent light emitters.
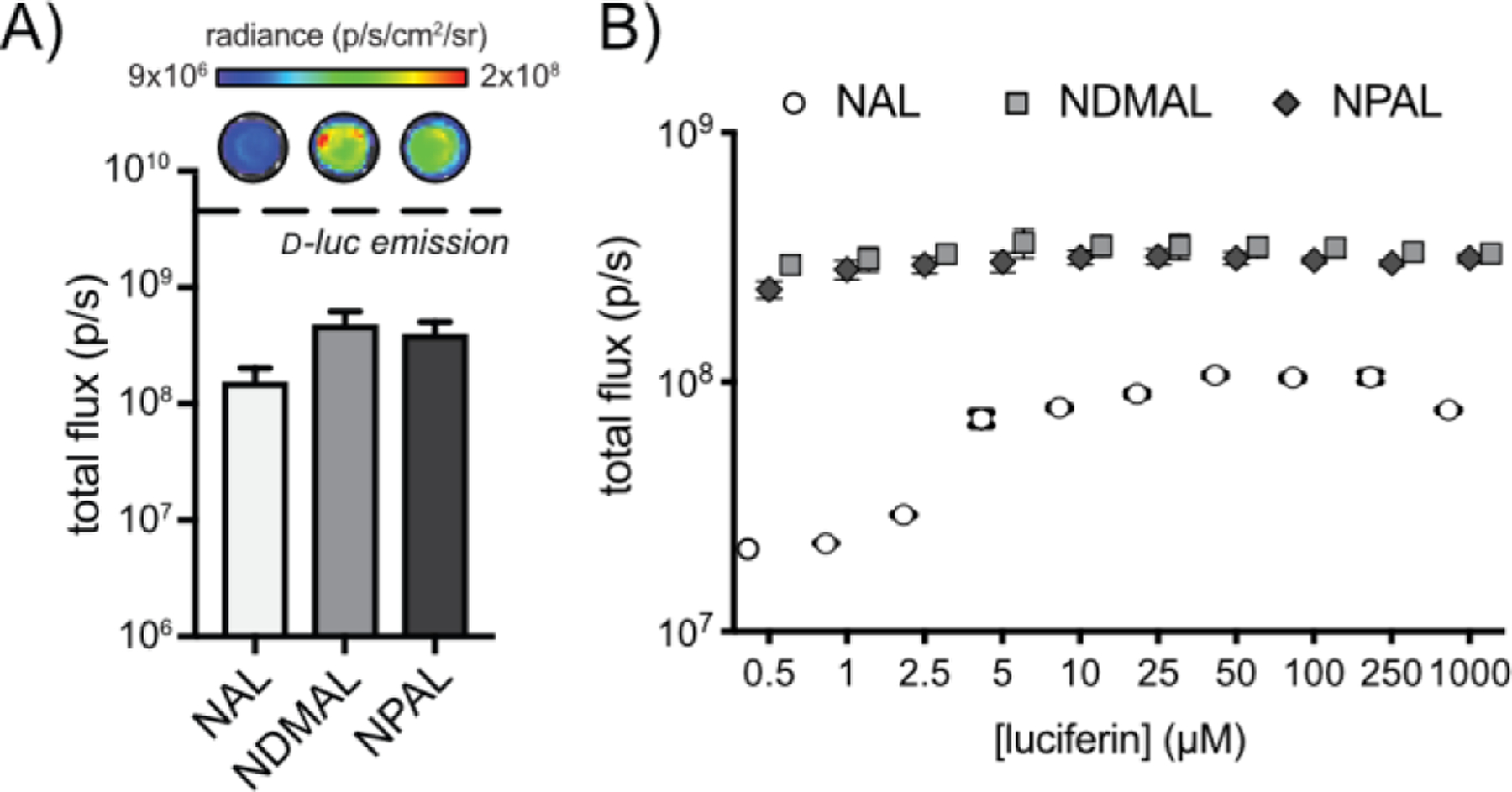
(A) Bioluminescent light emission from naphthylamino luciferins and d-luc (100 µM) incubated with Fluc (1.2 µg). Error bars represent the standard deviation of the mean for n=3 measurements. (B) Naphthylamino luciferins emit light over a broad range of concentrations. NAL, NDMAL, and NPAL (0–1 mM) were incubated with recombinant Fluc (1.2 µg) and light emission was recorded. Error values represent the standard deviation of the mean for n=3 measurements.
We next investigated whether the naphthylamino luciferins would be sufficiently cell permeable for biological use. The scaffolds were incubated with multiple cell types stably expressing Fluc, and bioluminescent outputs were recorded (Figure 3, S2-S3). Light emission was observed with all three substrates. NPAL and NDMAL exhibited light on par with d-luc at saturating concentrations. At low concentrations, photon outputs for NPAL and NDMAL were larger than those of d-luc (Figure S2-S3). These results are significant as several luciferin analogs are orders of magnitude dimmer than the native substrate and thus not suitable for the most sensitive imaging applications. High concentrations of NDMAL resulted in decreased photon outputs likely due to product inhibition. NAL exhibited the least amount of light among the naphthylamino analogs, likely due to reduced permeability. Cellular light emission of the analogs was also monitored over time (Figure 3B) and in a tissue mimic (Figure S4). In all cases, sustained photon outputs were observed.
Figure 3. Naphthylamino luciferins emit light in cellulo.
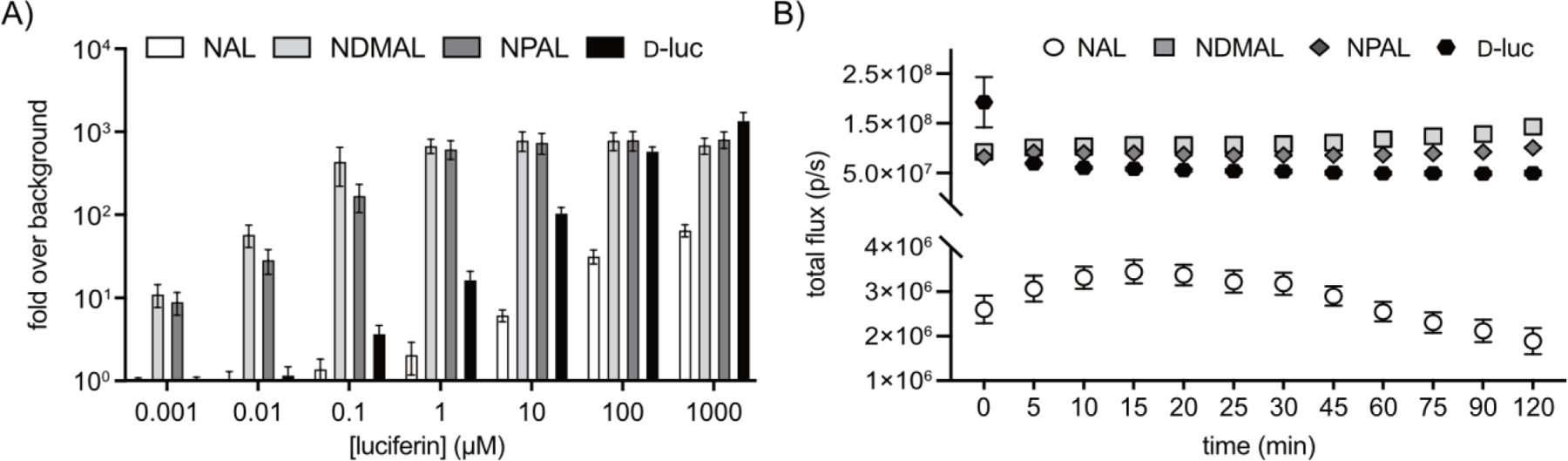
(A) NAL, NDMAL, NPAL, and d-luc were incubated with Fluc-expressing HEK cells at multiple concentrations. Data represent light emission measured 20 minutes post-luciferin administration. Error bars represent the standard deviation of the mean for n=3 measurements. (B) Light emission was sustained for >2 h at all concentrations examined. Data shown are from cells treated with 100 µM luciferin. Error values represent the standard deviation of the mean for n=3 measurements.
Structurally diverse analogs enable orthogonal imaging
The robust activity of naphthylamino luciferins in cellulo suggested that they were good candidates for multiplexed imaging, provided that unique (i.e., orthogonal) enzymes to process the luciferins could be identified. Toward this end, the luciferins were screened against a 220-member library of engineered luciferases (Figure S6).[12] Each mutant was tested and light emission was recorded (Figure S7). Several enzymes capable of discriminating the naphthylamino luciferins were identified using a cross-compare algorithm (Figure S7).[12] One set of orthogonal enzymes (mutants 20 and 83) was further analyzed in a multi-component format. Each of the luciferases was treated with the distinct luciferins (Figure 4). NAL (preferentially processed by mutant 20) was administered first, and signal was recorded. NDMAL (preferentially processed by mutant 83) was then immediately added, and a second image was acquired. The change in light emission upon NDMAL addition enabled the pairs to be readily resolved using a published algorithm.[27] Interestingly, the same set of mutants could not be distinguished using 6′-aminoluciferin and 6′-dimethylamino luciferin (the benzothiazole equivalents of NAL and NDMAL, Figure S8). These results suggest a key role for the naphthalene ring in selective enzyme processing. The naphthylamino probes are also structurally distinct from (and thus compatible with) previously synthesized analogs, enabling expanded multiplexed imaging. As shown in Figure 5, NDMAL and one of its complementary luciferases (mutant 12) could be used in conjunction with two existing luciferins (PhOH-Luc[20] and 7′Pyr-Luc[16]) and their associated luciferases (mutants 207 and 213, respectively). Sequential addition of the unique substrates to the mutant samples enabled triple-component imaging in under 30 minutes.
Figure 4. Mutant luciferases can discriminate naphthylamino luciferins.
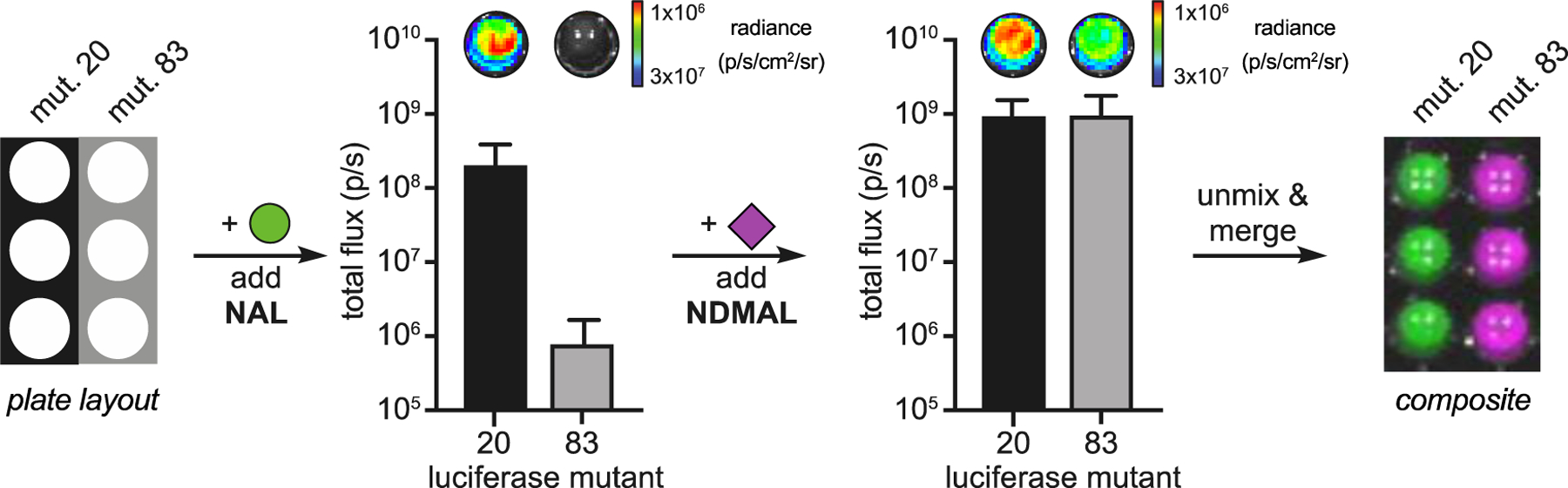
(A) E. coli cells expressing mutant 20 or 83 were lysed, treated with luciferin analogs, and imaged. The mutants were resolved upon sequential addition of the corresponding substrates. NAL was administered to both mutants first, followed by NDMAL. The images were unmixed and false colored to create a composite image. Error bars represent the standard deviation of the mean for n=3 measurements.
Figure 5: Triplet set of orthogonal pairs.
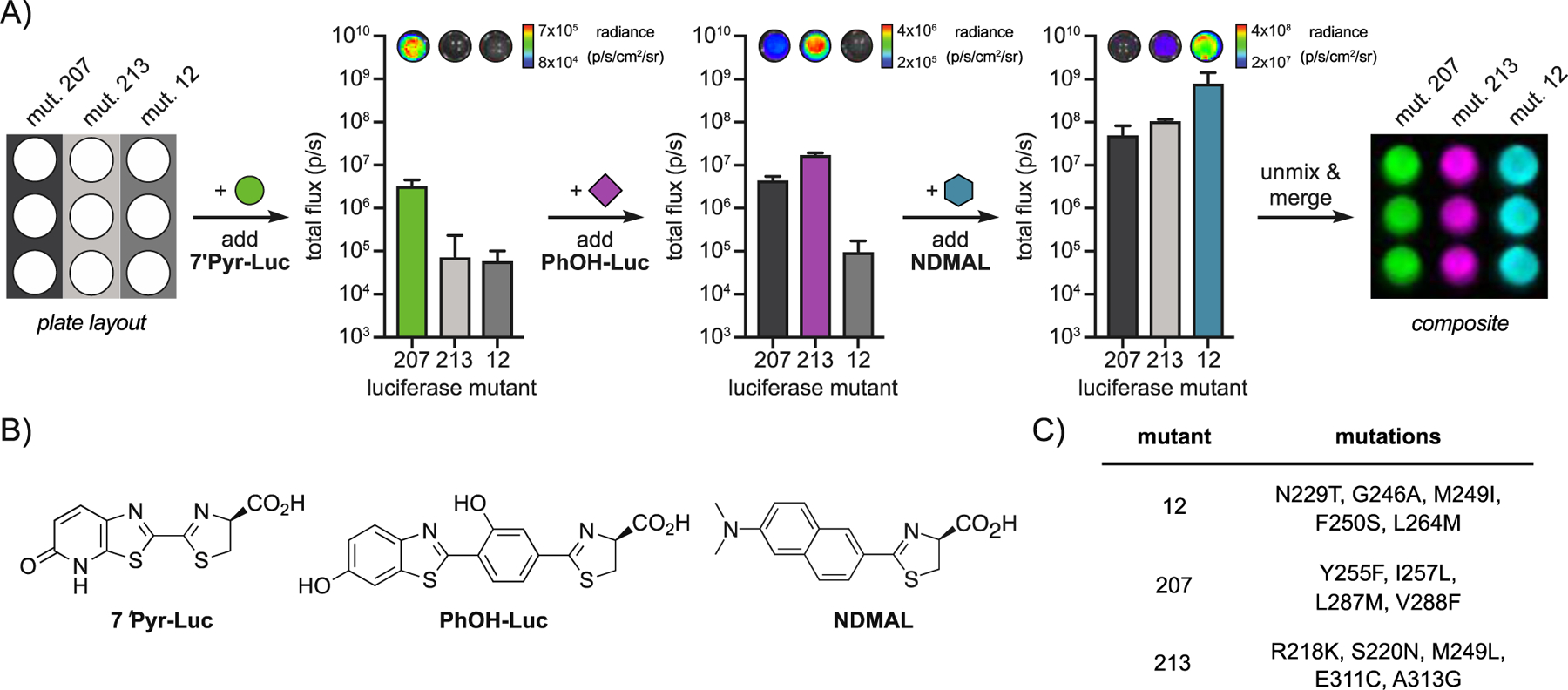
(A) E. coli cells expressing mutant 207, 213, or 12 were lysed and imaged. The mutants were resolved upon sequential addition of the corresponding substrates. 7ʹPyr-Luc was administered to all mutants first, followed by PhOH-Luc and NDMAL. These images were unmixed and false colored to create a composite image. Error bars represent the standard deviation of the mean for n=3 measurements. (B) Structures of luciferin analogs used in this experiment. (C) Mutants used for tri-component imaging.
Conclusion
We demonstrated that naphthylamino luciferins are viable substrates for multi-component imaging with orthogonal luciferases. These probes comprise unique structures and exhibit robust light emission profiles, making them well suited for cellular application. In this work, three analogs were synthesized and characterized using both in vitro and cell-based assays. Screening studies revealed luciferase mutants capable of selectively processing the analogs. Pairs of the mutants could be discriminated based on their unique patterns of light emission with the analogs. The naphthylamino luciferins were also used in conjunction with existing bioluminescent probes for rapid triple-component imaging. Collectively, the naphthylamino probes add to the growing collection of bioluminescent probes for multiplexed detection.
Supplementary Material
Acknowledgements
This work was supported by the U.S. National Institutes of Health (R01-GM107630 to J.A.P.) and the University of California, Irvine (UCI) School of Physical Sciences. Z.W.Y. was supported by the National Science Foundation Graduate Research Fellowship under Grant No. DGE-1321846. Some experiments were performed at the Laser Spectroscopy Laboratories (LSL) at UCI. We thank members of the Martin, Nowick, Weiss, and Jarvo laboratories for providing reagents and experimental assistance. We also thank members of the Prescher laboratory for experimental advice and helpful discussions.
Footnotes
Supporting information for this article is given via a link at the end of the document.
References
- [1].Zambito G, Chawda C, Mezzanotte L, Curr. Opin. Chem. Biol 2021, 63, 86. [DOI] [PubMed] [Google Scholar]
- [2].Syed AJ, Anderson JC, Chem. Soc. Rev 2021, 50, 5668. [DOI] [PubMed] [Google Scholar]
- [3].Thorne N; Inglese J; Auld DS Chem. Biol 2010, 17, 646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yeh H-W; Ai H-W Annu. Rev. Anal. Chem 2019, 12, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rathbun CM; Prescher JA Biochemistry 2017, 56, 5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yao Z; Zhang BS; Prescher JA Curr. Opin. Chem. Biol 2018, 45, 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cao Y-A; Wagers AJ; Beilhack A; Dusich J; Bachmann MH; Negrin RS; Weissman IL; Contag CH Proc. Natl. Acad. Sci. U. S. A 2004, 101, 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Liu H; Patel MR; Prescher JA; Patsialou A; Qian D; Lin J; Wen S; Chang Y-F; Bachmann MH; Shimono Y; Dalerba P; Adorno M; Lobo N; Bueno J; Dirbas FM; Goswami S; Somlo G; Condeelis J; Contag CH; Gambhir SS; Clarke MF Proc. Natl. Acad. Sci. U. S. A 2010, 107, 18115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Williams SJ; Prescher JA Acc. Chem. Res 2019, 52, 3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jones KA; Porterfield WB; Rathbun CM; McCutcheon DC; Paley MA; Prescher JA J. Am. Chem. Soc 2017, 139, 2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rathbun CM; Ionkina AA; Yao Z; Jones KA; Porterfield WB; Prescher JA, ACS Chem. Biol 2021, DOI: 10.1021/acschembio.0c00959. [DOI] [PMC free article] [PubMed]
- [12].Rathbun CM; Porterfield WB; Jones KA; Sagoe MJ; Reyes MR; Hua CT; Prescher JA ACS Cent. Sci 2017, 3, 1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mofford DM, Reddy GR, Miller SC, J. Am. Chem. Soc 2014, 136, 13277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Adams ST, Mofford DM, Reddy GS, Miller SC, Angew. Chem. Int. Ed 2016, 55, 4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Su Y, Walker JR, Park Y, Smith TP, Liu LX, Hall MP, Labanieh L, Hurst R, Wang DC, Encell LP, Kim N, Zhang F, Kay MA, Casey KM, Majzner RG, Cochran JR, Mackall CL, Kirkland TA, Lin MZ, Nat. Methods 2020, 17, 852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhang BS; Jones KA; McCutcheon DC; Prescher JA ChemBioChem 2018, 19, 470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Iwano S; Obata R; Miura C; Kiyama M; Hama K; Nakamura M; Amano Y; Kojima S; Hirano T; Maki S; Niwa H. Tetrahedron 2013, 69, 3847. [Google Scholar]
- [18].Jathoul AP, Grounds H, Anderson JC, Pule MA, Angew. Chem. Int. Ed 2014, 53, 13059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hall MP; Woodroofe CC; Wood MG; Que I; van’t Root M; Ridwan Y; Shi C; Kirkland TA; Encell LP; Wood KV; Löwik C; Mezzanotte L. Nat. Commun 2018, 9, 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yao Z; Zhang BS; Steinhardt RC; Mills JH; Prescher JA J. Am. Chem. Soc 2020, 142, 14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Branchini BR; Hayward MM; Bamford S; Brennan M, P.; Lajiness EJ Photochem. and Photobiol 1989, 49, 689. [DOI] [PubMed] [Google Scholar]
- [22].Takakura H; Sasakura K; Ueno T; Urano Y; Terai T; Hanaoka K; Tsuboi T; Nagano T. Chem. - Asian J 2010, 2053. [DOI] [PubMed]
- [23].Shinde R; Perkins J; Contag CH Biochemistry 2006, 45, 11103. [DOI] [PubMed] [Google Scholar]
- [24].White EH; Wörther H; Seliger HH; McElroy WD J. Am. Chem. Soc 1966, 88, 2015. [Google Scholar]
- [25].Kakiuchi M; Ito S; Kiyama M; Goto F; Matsuhashi T; Yamaji M; Maki S; Hirano T. Chem. Lett 2017, 46, 1090. [Google Scholar]
- [26].Seeboth H. Angew. Chem., Int. Ed. Engl 1967, 6, 307. [Google Scholar]
- [27].Gammon ST; Leevy WM; Gross S; Gokel GW; Piwnica-Worms D. Anal. Chem 2006, 78, 1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


