Abstract
Background
Immune checkpoint inhibitor-associated acute kidney injury (ICPi-AKI) has emerged as an important toxicity among patients with cancer.
Methods
We collected data on 429 patients with ICPi-AKI and 429 control patients who received ICPis contemporaneously but who did not develop ICPi-AKI from 30 sites in 10 countries. Multivariable logistic regression was used to identify predictors of ICPi-AKI and its recovery. A multivariable Cox model was used to estimate the effect of ICPi rechallenge versus no rechallenge on survival following ICPi-AKI.
Results
ICPi-AKI occurred at a median of 16 weeks (IQR 8–32) following ICPi initiation. Lower baseline estimated glomerular filtration rate, proton pump inhibitor (PPI) use, and extrarenal immune-related adverse events (irAEs) were each associated with a higher risk of ICPi-AKI. Acute tubulointerstitial nephritis was the most common lesion on kidney biopsy (125/151 biopsied patients [82.7%]). Renal recovery occurred in 276 patients (64.3%) at a median of 7 weeks (IQR 3–10) following ICPi-AKI. Treatment with corticosteroids within 14 days following ICPi-AKI diagnosis was associated with higher odds of renal recovery (adjusted OR 2.64; 95% CI 1.58 to 4.41). Among patients treated with corticosteroids, early initiation of corticosteroids (within 3 days of ICPi-AKI) was associated with a higher odds of renal recovery compared with later initiation (more than 3 days following ICPi-AKI) (adjusted OR 2.09; 95% CI 1.16 to 3.79). Of 121 patients rechallenged, 20 (16.5%) developed recurrent ICPi-AKI. There was no difference in survival among patients rechallenged versus those not rechallenged following ICPi-AKI.
Conclusions
Patients who developed ICPi-AKI were more likely to have impaired renal function at baseline, use a PPI, and have extrarenal irAEs. Two-thirds of patients had renal recovery following ICPi-AKI. Treatment with corticosteroids was associated with improved renal recovery.
Keywords: immunotherapy, CTLA-4 antigen, programmed cell death 1 receptor
Background
Immune checkpoint inhibitors (ICPis) have become some of the most widely prescribed anticancer treatments in current use.1 Despite their proven efficacy across a wide range of malignancies, ICPis can cause a unique spectrum of autoimmune toxicities known as immune-related adverse events (irAEs). These irAEs can affect virtually any organ in the body, including the kidneys.
Direct renal toxicity from ICPis, referred to here as ICPi-associated acute kidney injury (ICPi-AKI), occurs with an estimated incidence of 3%–5%.2–9 ICPi-AKI can have serious consequences for patients including dose delay or permanent discontinuation of ICPi therapy, irreversible loss of kidney function (which can impact eligibility to receive other anticancer treatments), and prolonged courses of immunosuppression. Additionally, when a patient undergoing treatment with ICPis develops AKI, there is often uncertainty regarding its etiology, since AKI occurs commonly in patients with cancer and can be due to a variety of causes.10 11
Despite these challenges in diagnosing and treating ICPi-AKI, existing data are largely limited to case reports and small single-center case series.5 6 12–15 Key questions, therefore, remain unanswered regarding the risk factors, clinical features, histopathological findings, renal outcomes, and overall survival in patients with ICPi-AKI. Further, very limited data are available regarding the safety of rechallenging patients with ICPis after an episode of ICPi-AKI. To address these and other critical knowledge gaps, we conducted an international multicenter cohort study of patients with ICPi-AKI.
Methods
Study design and oversight
We conducted a multicenter cohort study of adults diagnosed with ICPi-AKI between 2012 and 2020. We contacted nephrologists and oncologists at 40 major academic cancer centers across North America, Europe, and Asia to identify cases of ICPi-AKI, 30 of which provided data on 429 patients with ICPi-AKI (online supplemental table S1 and figure S1). Of the 429 patients with ICPi-AKI in this study, 100 (23.3%) were described previously in 12 publications (online supplemental appendix).
jitc-2021-003467supp001.pdf (952.7KB, pdf)
Data collection
Study personnel at each site collected data by detailed chart review. Data were entered into a standardised case report form using a secure, web-based platform (online supplemental appendix),16 and included the following: demographics and comorbidities; concomitant treatment with nephrotoxic chemotherapies and medications associated with acute tubulointerstitial nephritis (ATIN), including proton pump inhibitors (PPIs), non-steroidal anti-inflammatory drugs, and antibiotics; prior or concomitant extrarenal irAEs; laboratory data at baseline and at the time of ICPi-AKI; kidney biopsy data; treatments received for ICPi-AKI; and data on renal recovery, ICPi rechallenge, and overall survival.
Definition of ICPi-AKI
Patients were eligible for inclusion if they had AKI that was directly attributed to the ICPi by the treating provider and if they met either of the following criteria: (1) an increase in serum creatinine (SCr) ≥100% from baseline or treatment with renal replacement therapy (RRT); (2) an increase in SCr ≥50% from baseline and at least one of the following: ATIN on kidney biopsy; ICPi therapy held for at least once cycle due to concern for ICPi-AKI; or treatment with corticosteroids due to concern for ICPi-AKI (online supplemental table S2). Baseline SCr was defined as the closest value prior to ICPi initiation. We excluded kidney transplant patients and those with end stage kidney disease.
Definitions of AKI severity, renal recovery, and recurrent ICPi-AKI
AKI severity was based on the maximum SCr achieved in the 4 weeks following ICPi-AKI and staged according to the Kidney Disease: Improving Global Outcomes criteria (online supplemental table S3).17 Renal recovery was defined as a nadir SCr ≤1.5 times the baseline value within 90 days following ICPi-AKI.18 Recurrent ICPi-AKI after ICPi rechallenge was defined as an increase in SCr ≥50% from the new baseline (at the time of rechallenge) and attributed to the ICPi by the treating provider.
Patients without ICPi-AKI
To identify risk factors for ICPi-AKI, we also collected data on control patients who received ICPis contemporaneously but who did not develop ICPi-AKI (defined as absence of an increase in SCr ≥50% from baseline or treatment with RRT that was definitely or probably ICPi related). Each collaborating institution provided data on one control patient for every patient with ICPi-AKI from that site to maintain a 1:1 ratio of cases:controls. Control patients were selected at random to preserve the ability to investigate all characteristics as potential risk factors for ICPi-AKI.
Statistical analyses
Continuous and categorical data were compared using the Wilcoxon rank-sum and Fisher’s exact test, respectively. Multivariable logistic regression was used to identify risk factors for ICPi-AKI, and a sensitivity analysis was performed in patients with stage 2 or 3 ICPi-AKI.
Multivariable logistic regression was also used to identify predictors of renal recovery in patients with ICPi-AKI, including treatment with corticosteroids within 14 days following ICPi-AKI. A sensitivity analysis limited to patients treated with corticosteroids at any time following ICPi-AKI was conducted to assess the effect of early corticosteroid initiation (within 3 days following ICPi-AKI) versus later corticosteroid initiation (anytime after 3 days following ICPi-AKI) on renal recovery. To minimize the potential for confounding due to terminal illness, which could affect the decision to prescribe corticosteroids, each of these analyses were limited to patients who survived at least 14 days following ICPi-AKI.
Kaplan-Meier curves and multivariable Cox regression models were used to assess the association between ICPi-AKI stage, treatment with corticosteroids within 14 days following ICPi-AKI, and other factors with survival, with ICPi-AKI diagnosis serving as time 0. Similar to the analyses above, these analyses were also limited to those who survived at least 14 days following ICPi-AKI.
Kaplan-Meier curves and multivariable Cox regression models were used to estimate the effect of ICPi rechallenge versus no ICPi rechallenge on survival. To eliminate the potential for immortal time bias, we limited this analysis to patients who survived at least 90 days after the initial ICPi-AKI event, and we compared the survival of patients rechallenged in the first 90 days to those not rechallenged in the first 90 days. We repeated this analysis in patients who survived at least 180 days following the initial ICPi-AKI event.
For each of the multivariable analyses above, covariate selection was based on univariate associations, biological plausibility, prior knowledge,3 4 and parsimony. Missing data were not imputed, as less than 1% of data were missing for all variables. Rather, missing data categories were used in multivariable models. Analyses were performed using SAS V.9.5 (SAS Institute), R V.3.6.3 (R Foundation), and GraphPad PRISM V.9.1.0 (GraphPad Software). All comparisons are two tailed, with p<0.05 considered significant.
Results
Baseline characteristics
In total, 429 patients with ICPi-AKI and 429 without ICPi-AKI from 30 institutions were included. Baseline characteristics are shown in table 1. Patients with ICPi-AKI were older, had lower baseline estimated glomerular filtration rate (eGFR), and were more likely to have genitourinary cancer, PPI exposure, and to have received combination ICPi therapy compared with patients without ICPi-AKI (table 1). The temporal distribution of ICPi initiation was similar in patients with and without ICPi-AKI (online supplemental figure S2). Baseline characteristics among biopsied (n=151) and non-biopsied patients (n=278) with ICPi-AKI were largely similar, though biopsied patients were more likely to have more severe AKI and less likely to have extrarenal irAEs (online supplemental table S4).
Table 1.
Baseline characteristics
| Variable | ICPi-AKI (n=429) | No ICPi-AKI (n=429) | P value |
| Age at ICPi initiation, years, median (IQR) | 68 (59–75) | 65 (58–73) | 0.02 |
| Male, n (%) | 266 (62.0) | 251 (58.5) | 0.32 |
| Race, n (%) | 0.99 | ||
| White | 351 (81.8) | 350 (81.6) | |
| Black | 27 (6.3) | 24 (5.6) | |
| Asian | 21 (4.9) | 21 (4.9) | |
| Other/unknown | 30 (7.0) | 34 (7.9) | |
| Comorbidities, n (%) | |||
| Hypertension | 251 (58.5) | 229 (53.4) | 0.15 |
| Diabetes | 77 (17.9) | 61 (14.2) | 0.16 |
| CHF | 17 (4.0) | 9 (2.1) | 0.16 |
| COPD | 45 (10.5) | 46 (10.7) | 0.99 |
| Cirrhosis | 11 (2.6) | 10 (2.3) | 0.99 |
| Body mass index, median (IQR) | 26 (23–30) | 26 (22–29) | 0.12 |
| Baseline SCr, mg/dL, median (IQR) | 0.97 (0.80–1.21) | 0.88 (0.73–1.07) | <0.001 |
| Baseline eGFR,*(mL/min per 1.73 m2 | |||
| Median (IQR) | 73 (57–90) | 83 (66–97) | <0.001 |
| eGFR categories, n (%) | <0.001 | ||
| ≥90 | 111 (25.9) | 168 (39.2) | |
| 60–89 | 192 (44.8) | 189 (44.1) | |
| 45–59 | 72 (16.8) | 44 (10.3) | |
| 30–44 | 43 (10.0) | 23 (5.4) | |
| <30 | 11 (2.6) | 5 (1.2) | |
| Autoimmune disease, n (%) | 42 (9.8) | 56 (13.1) | 0.16 |
| Extrarenal irAE,† n (%) | 201 (46.9) | 123 (28.7) | <0.001 |
| Malignancy, n (%) | 0.01 | ||
| Melanoma | 104 (24.2) | 93 (21.7) | |
| Lung | 126 (29.4) | 133 (31.0) | |
| Genitourinary | 100 (23.8) | 70 (16.7) | |
| Other | 99 (23.6) | 133 (31.7) | |
| PPI,‡ n (%) | 208 (45.5) | 115 (26.8) | <0.001 |
| Concomitant nephrotoxic chemotherapy,§ n (%) | |||
| Cisplatin | 7 (1.6) | NA | |
| VEGF/TKI | 23 (5.4) | NA | |
| Other¶ | 43 (10.0) | NA | |
| ICPi class, n (%) | |||
| Anti-CTLA-4 | 103 (24.0) | 95 (22.1) | 0.57 |
| Anti-PD-1 | 347 (80.9) | 355 (82.8) | 0.54 |
| Anti-PD-L1 | 42 (9.8) | 30 (7.0) | 0.18 |
| Combo anti-CTLA-4+ anti-PD-1/PD-L1 | 99 (23.1) | 75 (17.5) | 0.05 |
Data are shown as median (IQR) and n (%).
Data on body mass index are missing in one patient with ICPi-AKI and one without ICPi-AKI. Data on PPI use are missing in two patients without ICPi-AKI. All other data are complete.
*Baseline eGFR calculated based on Chronic Kidney Disease-Epidemiology Collaboration equation.33
†Extrarenal irAEs were assessed prior to (>14 days) or concomitant (within 14 days before or after) with ICPi-AKI diagnosis among patients with ICPi-AKI, and at any time after ICPi initiation among patients without ICPi-AKI.
‡PPIs were assessed in the 14 days preceding AKI among patients with ICPi-AKI, and were assessed at ICPi initiation in patients without ICPi-AKI.
§Concomitant chemotherapies were assessed in the 30 days preceding ICPi-AKI.
¶Includes pemetrexed (n=28), carboplatin (n=23), BRAF inhibitors (n=2), and paclitaxel (n=1).
AKI, acute kidney injury; BRAF, v-raf murine sarcoma viral oncogene homolog B1; CHF, congestive heart failure; Combo, combination therapy; COPD, chronic obstructive pulmonary disease; CTLA-4, cytotoxic T lymphocyte-associated antigen 4; eGFR, estimated glomerular filtration rate; ICPi, immune checkpoint inhibitor; irAEs, immune-related adverse events; NA, not assessed; PD-1, programmed cell death 1; PD-L1, programmed death-ligand 1; PPI, proton pump inhibitor; SCr, serum creatinine; TKI, tyrosine kinase inhibitor; VEGF, vascular endothelial growth factor.
Risk factors for ICPi-AKI
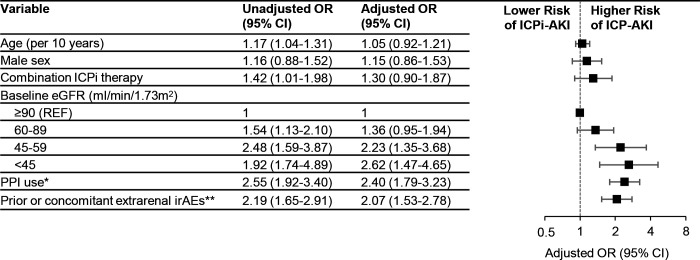
Lower baseline eGFR, PPI use, and prior or concomitant extrarenal irAEs were each associated with a higher risk of ICPi-AKI in multivariable models (figure 1). When limited to patients with stage 2 or 3 ICPi-AKI, PPI use and extrarenal irAEs remained associated with ICPi-AKI, whereas baseline eGFR did not (online supplemental figure S3).
Figure 1.
Risk factors for ICPi-AKI. Total n=856, of whom 429 had ICPi-AKI and 427 did not have ICPi-AKI. All model covariates are shown in the figure. *Denotes PPI use in the 14 days preceding ICPi-AKI among those with ICPi-AKI, and PPI use at the time of ICPi initiation among patients without ICPi-AKI. **Extrarenal irAEs were assessed prior to (>14 days) or concomitant (within 14 days before or after) with ICPi-AKI diagnosis among patients with ICPi-AKI, and at any time after ICPi initiation among patients without ICPi-AKI. eGFR, estimated glomerular filtration rate; ICPi, immune checkpoint inhibitor; irAEs, immune-related adverse events; PPI, proton pump inhibitor.
Clinical features of ICPi-AKI
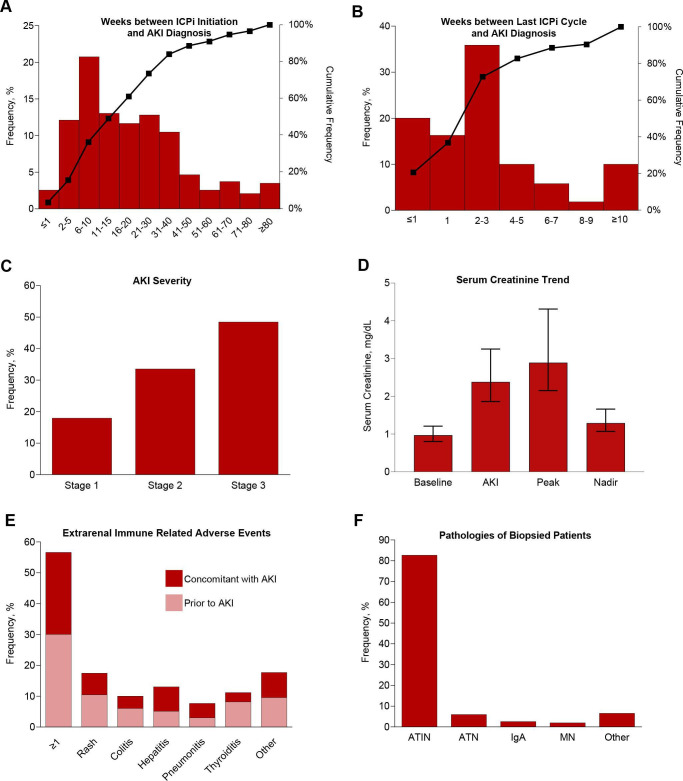
ICPi-AKI developed at a median of 16 weeks (IQR 8–32) after ICPi initiation, with 49 of 429 patients (11.4%) developing ICPi-AKI more than a year after ICPi initiation (figure 2A). Patients developed ICPi-AKI at a median of 3 weeks (IQR 1–4) after the last ICPi cycle (figure 2B). A total of 77 of the 429 patients (17.9%) with ICPi-AKI had stage 1 AKI, 144 (33.6%) had stage 2, and 208 (48.5%) had stage 3, including 33 who received RRT (15.8% of those with stage 3 AKI; 7.7% overall) (figure 2C). SCr at baseline, at ICPi-AKI diagnosis, the peak level within 4 weeks of ICPi-AKI, and the nadir within 90 days following ICPi-AKI are shown in figure 2D. Prior or concomitant extrarenal irAEs occurred in 243 patients (56.6%), with rash and hepatitis being the most common (figure 2E). Among patients who underwent kidney biopsy, ATIN was the most common primary lesion (125/151; 82.7%) (figure 2F).
Figure 2.
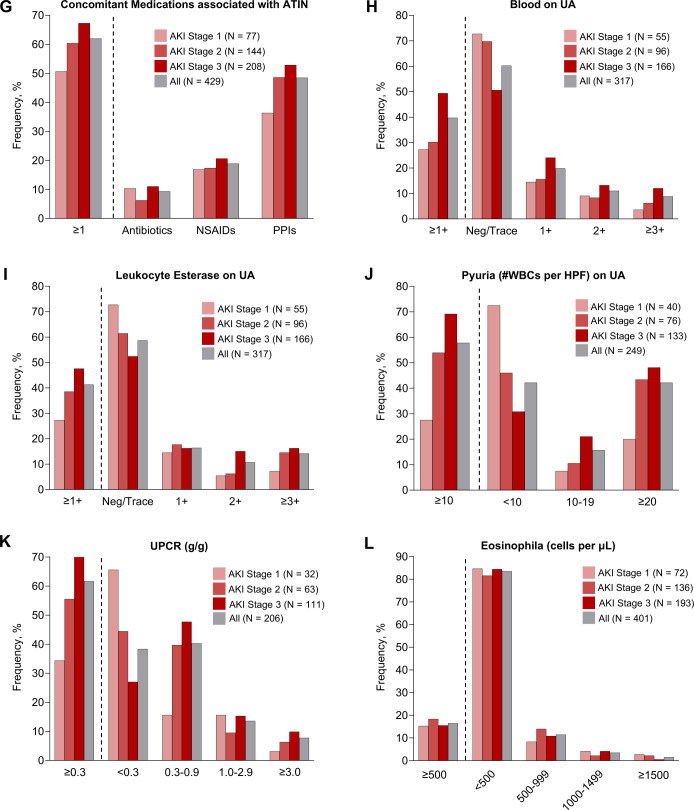
Clinical features of ICPi-AKI. (A) The number of weeks between ICPi initiation and ICPi-AKI diagnosis. (B) The number of weeks between the last ICPi cycle and ICPi-AKI diagnosis. (C) Distribution of AKI severity. (D) Serum creatinine trend (median, IQR). (E) Frequency of extrarenal irAEs occurring before (>14 days) or concomitant (within 14 days before or after) with ICPi-AKI diagnosis. Other irAEs include hypophysitis (0.7% prior, 1.4% concomitantly), adrenalitis (0.2% prior, 1.4% concomitantly), type 1 diabetes mellitus (0% prior, 0.5% concomitantly), and myocarditis (1.2% prior, 0.2% concomitantly). (F) Distribution of pathologies among the 151 patients who underwent biopsy. Other includes 2 patients with FSGS and one patient with each of the following: reactive amyloidosis, AA amyloidosis, focal proliferative glomerulonephritis with C3 deposits, immune complex deposition disease not otherwise specified, mesangial proliferative immune complex mediated glomerulonephritis, pauci-immune glomerulonephritis, minimal change disease and thrombotic microangiopathy. (G) Frequency of potential ATIN-causing medications taken within 14 days before ICPi-AKI diagnosis. (H) Frequency of blood on UA at the time of ICPi-AKI. (I) Frequency of leukocyte esterase on UA at the time of ICPi-AKI. (J) Frequency of pyuria on UA at the time of ICPi-AKI. (K) Frequency of proteinuria at the time of ICPi-AKI. (L) Frequency of eosinophilia at the time of ICPi-AKI. AKI, acute kidney injury; ATIN, acute tubulointerstitial nephritis; ATN, acute tubular necrosis; FSGS, focal segmental glomerulosclerosis; HPF, high-power field; ICPi, immune checkpoint inhibitor; MN, membranous nephropathy; UA, urinalysis; UPCR, urine protein-to-creatinine ratio; WBCs, white blood cells.
Additional clinical features of ICPi-AKI stratified by AKI stage are shown in figure 2G–L. At the time of ICPi-AKI, 62% of patients were receiving concomitant medications associated with ATIN, with PPIs being the most common (figure 2G); 39.7% had ≥1+ blood on urinalysis (figure 2H); 41.3% had ≥1+ leukocyte esterase on urinalysis (figure 2I); 57.8% had pyuria (≥10 white blood cells per high power field) (figure 2J); 61.7% had a urine protein-to-creatinine ratio ≥0.3 g/g (figure 2K); and 16.5% had ≥500 eosinophils /µL (figure 2L). Patients with higher stages of AKI were more likely to be receiving concomitant medications associated with ATIN and to have hematuria, proteinuria, and leukocyte esterase on urinalysis.
Treatment of ICPi-AKI
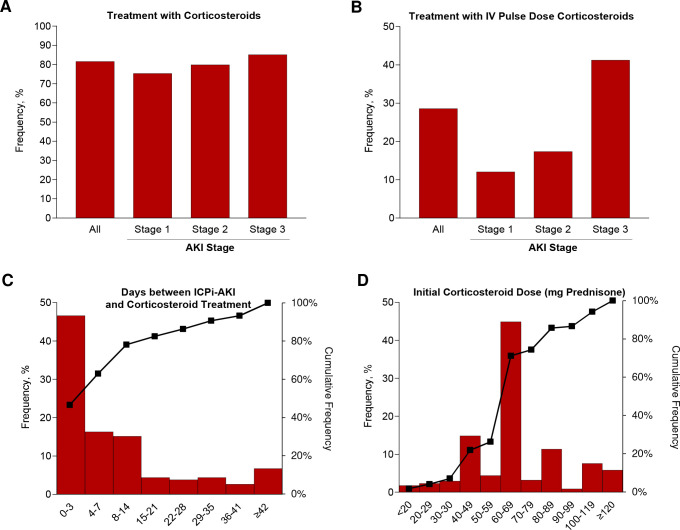
ICPi therapy was held for at least one cycle in 390 of the 429 patients (90.9%) with ICPi-AKI. Most patients (350/429 [81.6%]) were treated with corticosteroids (figure 3A), including 100 patients treated with intravenous pulse dose corticosteroids (figure 3B). Patients were initiated on corticosteroids at a median of 4 days (IQR 1–13) following ICPi-AKI diagnosis, and 273 of the 350 patients treated with corticosteroids (78.0%) initiated this treatment in the first 14 days following ICPi-AKI (figure 3C). The median initial corticosteroid dose was 60 mg in prednisone equivalent units (IQR 50–80) (figure 3D). Patients were treated with corticosteroids for a median of 41 days (IQR 26–75) before tapering to ≤10 mg of prednisone (or the equivalent). A total of 22 patients (5.1%) were treated with additional or alternative immunosuppressive agents, most commonly mycophenolate mofetil (online supplemental table S5).
Figure 3.
Treatment of ICPi-AKI. (A) Frequency of treatment with oral or intravenous corticosteroids by stage of initial ICPi-AKI. (B) Frequency of treatment with intravenous pulse dose corticosteroids by stage of initial ICPi-AKI. (C) Distribution of days between ICPi-AKI diagnosis and initiation of corticosteroids. (D) Distribution of initial corticosteroid dose (in prednisone equivalent units [mg]). AKI, acute kidney injury; ICPi, immune checkpoint inhibitor.
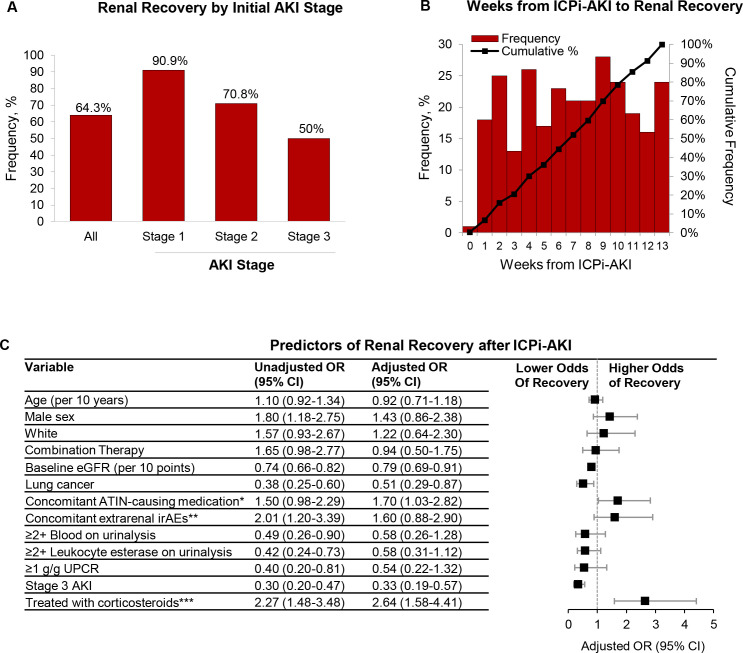
Renal recovery
Renal recovery occurred in 276 patients (64.3%) overall, including 90.9% of patients who initially had ICPi-AKI stage 1, 70.8% with stage 2, and 50.0% with stage 3 (figure 4A). Renal recovery occurred at a median of 7 weeks (IQR 3–10) following ICPi-AKI diagnosis (figure 4B). The characteristics of patients with versus without renal recovery are shown in online supplemental table S6). In a multivariable model, higher baseline eGFR, lung cancer, and stage 3 AKI were each associated with a lower odds of renal recovery, whereas concomitant treatment with ATIN-causing medications was associated with higher odds of renal recovery (figure 4C). Importantly, treatment with corticosteroids within 14 days following ICPi-AKI was also associated with a higher odds of renal recovery (OR 2.64; 95% CI 1.58 to 4.41; figure 4C). In a sensitivity analysis limited to patients treated with corticosteroids at any time following ICPi-AKI, initiation of corticosteroids within 3 days of ICPi-AKI was associated with a higher odds of renal recovery compared with later initiation (OR 2.09; 95% CI 1.16 to 3.79; online supplemental figure S4).
Figure 4.
Characteristics of renal recovery among patients with ICPi-AKI. (A) Renal recovery overall and according to initial ICPi-AKI stage. (B) Time (in weeks) from ICPi-AKI diagnosis to renal recovery. (C) Predictors of renal recovery (total n=405, of whom 270 (66.7%) had renal recovery and 135 (33.3%) did not). Renal recovery was defined as a return of serum creatinine to ≤50% of the baseline value within 90 days of ICPi-AKI. Patients who died within 14 days of ICPi-AKI (n=24) were excluded. All model covariates are shown in the figure. *Denotes receipt of NSAIDs, PPIs, or antibiotics in the 14 days preceding ICPi-AKI. **Extrarenal irAEs were assessed concomitantly (within 14 days before or after) with ICPi-AKI diagnosis. ***Refers to oral or intravenous corticosteroids initiated within 14 days following ICPi-AKI. AKI, acute kidney injury; ATIN, acute tubulointerstitial nephritis; eGFR, estimated glomerular filtration rate; ICPi, immune checkpoint inhibitor; irAEs, immune-related adverse events; NSAIDs, non-steroidal anti-inflammatory drugs; PPI, proton pump inhibitor; UPCR, urine protein-to-creatinine ratio.
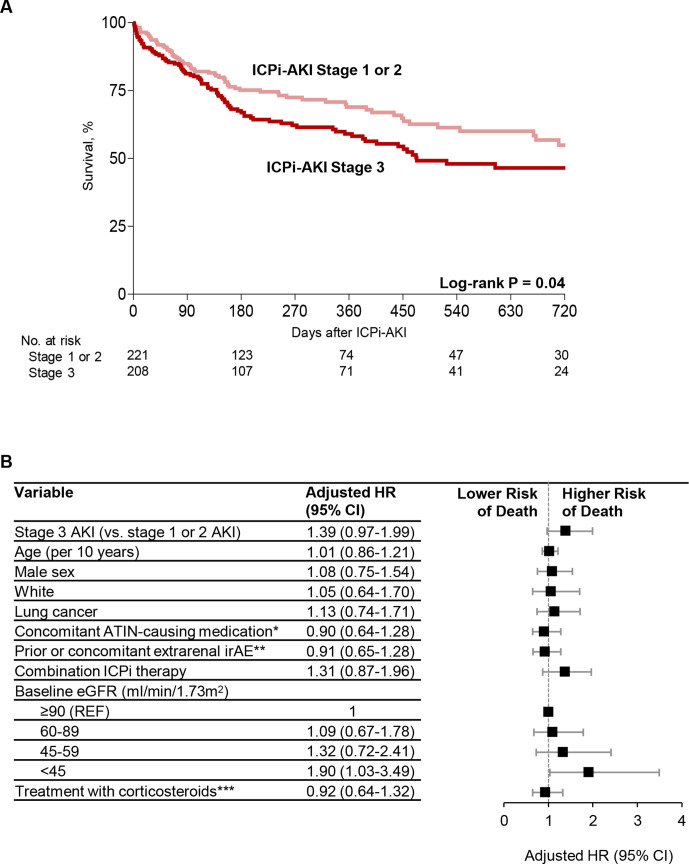
Survival in patients with ICPi-AKI
During a median follow-up of 30 weeks (IQR 15–66) from ICPi-AKI diagnosis, 168 of 429 patients (39.2%) died. Survival was lower in patients with ICPi-AKI stage 3 compared with those with stages 1 and 2 in univariate analyses (figure 5A). In a multivariable model, only lower baseline eGFR was associated with a higher risk of death (figure 5B).
Figure 5.
Risk factors for death in patients with ICPi-AKI. (A) Survival among patients with stages 1 and 2 ICPi-AKI versus stage 3. (B) Multivariable Cox regression model showing predictors of death among patients with ICPi-AKI (total n=405, of whom 144 (35.6% (died)). Patients who died within 14 days of ICPi-AKI (n=24) were excluded. All model covariates are shown in the figure. *Denotes receipt of NSAIDs, PPIs, or antibiotics in the 14 days preceding ICPi-AKI. **Extrarenal irAEs were assessed prior to (>14 days) or concomitant (within 14 days before or after) with ICPi-AKI. ***Refers to oral or intravenous corticosteroids initiated within 14 days following ICPi-AKI. AKI, acute kidney injury; ATIN, acute tubulointerstitial nephritis; eGFR, estimated glomerular filtration rate; ICPi, immune checkpoint inhibitor; irAEs, immune-related adverse events; NSAIDs, non-steroidal anti-inflammatory drugs; PPI, proton pump inhibitor.
ICPi rechallenge
A total of 121 of the 429 patients (28.2%) with ICPi-AKI were rechallenged with an ICPi, including 93 (76.9%) who had renal recovery and 28 (23.1%) who did not (online supplemental figure S5). Rechallenge occurred at a median of 1.9 months (IQR 1.1–4.0) after the initial ICPi-AKI episode (online supplemental figure S6). Rechallenged patients were less likely to have had ICPi-AKI stage 3 initially compared with non-rechallenged patients (34.7% vs 53.9%, respectively) and were more likely to have had renal recovery following the initial ICPi-AKI episode (76.8% vs 59.4%, respectively) (online supplemental table S7). Survival was similar among patients rechallenged versus not rechallenged in the first 90 days (online supplemental figure S7, panels A and B) and in the first 180 days following ICPi-AKI (online supplemental figure S7, panels C and D).
Recurrent ICPi-AKI after rechallenge
Of the 121 patients rechallenged, 20 (16.5%) developed recurrent ICPi-AKI, including 4 patients (20%) who developed AKI stage 1, 8 (40%) who developed AKI stage 2, and 8 (40%) who developed AKI stage 3 (online supplemental figure S8). Recurrent ICPi-AKI occurred at a median of 10 weeks (IQR 3–17) following rechallenge. There were no significant differences in the characteristics of rechallenged patients who developed recurrent ICPi-AKI versus those who did not, including receipt of corticosteroids at the time of rechallenge (online supplemental table S8).
All patients with recurrent ICPi-AKI had their ICPi therapy held, and most (14/20 [70%]) were treated with corticosteroids (online supplemental table S8). A total of 12 of the 20 patients (60%) with recurrent ICPi-AKI had renal recovery (online supplemental figure S5), which occurred at a median of 34 days (IQR 27–38) following diagnosis of recurrent ICPi-AKI. Patients who developed recurrent ICPi-AKI had higher mortality compared with those who did not (12/20 [60%] vs 32/101 [31.7%]; p=0.02).
Discussion
In this international multicenter cohort study of over 400 patients with ICPi-AKI treated at 30 sites, we identified key risk factors, clinical features, and outcomes associated with ICPi-AKI. First, we found that lower baseline eGFR, PPI use, and extrarenal irAEs are each independently associated with a higher risk of ICPi-AKI. Second, we expand on initial observations by our group3 19 and others5–7 12–15 20 regarding the clinicopathological features of ICPi-AKI, including the variable and often prolonged delay between ICPi initiation and ICPi-AKI, the high frequency of extrarenal irAEs in patients with ICPi-AKI, and ATIN as the most common histopathological lesion. Third, we found that renal recovery occurs in approximately two thirds of patients, and that early initiation of corticosteroids is associated with a higher likelihood of renal recovery. Fourth, among patients with ICPi-AKI who are rechallenged, fewer than one in five develop recurrent ICPi-AKI, half of whom subsequently have renal recovery.
Our findings regarding risk factors for development of ICPi-AKI, including lower baseline eGFR, PPI use, and extrarenal irAEs, are consistent with and expand on prior studies.3–5 We previously reported an association between lower baseline eGFR and higher risk of ICPi-AKI.3 However, it is unclear whether the risk of immunological injury to the kidneys from ICPis is truly increased in patients with underlying chronic kidney disease, or if lower renal reserve simply facilitates the crossing of a threshold increase in SCr (eg, ≥50%) considered to be ‘AKI’ in response to an insult such as ATIN. Our finding that baseline eGFR was no longer associated with ICPi-AKI when limited to patients with moderate-to-severe kidney injury suggests the latter. PPIs have been recognised as an important cause of drug-induced ATIN in both the general population21–23 and in patients receiving ICPis.3 4 PPIs and other medications associated with ATIN may predispose to ICPi-AKI through loss of tolerance via activation or reactivation of drug-specific T cells. Thus, PPIs should be used with caution in patients receiving ICPi therapy. Finally, prior or concomitant extrarenal irAEs were present in over half of the patients with ICPi-AKI in our cohort. Thus, the presence of extrarenal irAEs should raise clinical suspicion for ICPi-AKI in patients receiving ICPi therapy who develop AKI.24
We did not identify any clinical features that were reliably present or absent in patients with ICPi-AKI. The latency period between ICPi initiation and ICPi-AKI was highly variable, with some patients developing ICPi-AKI more than a year after ICPi initiation, suggesting that clinicians should remain vigilant regarding ICPi-AKI even when it occurs late. The finding that ICPi-AKI occurred at a median of 16 weeks suggests there is a longer latency period with ICPi-AKI than extrarenal irAEs.25–27 Urinary findings such as pyuria, hematuria, and proteinuria were present in only 40%–60% of the patients in our cohort, and thus cannot be used in isolation to differentiate ICPi-AKI from other causes of AKI. Among the patients biopsied, ATIN was by far the most common lesion (occurring in 83% of patients), as has been described in previous reports.3 4 12 19 However, one in six patients had an alternative primary lesion, including glomerulonephritis and other lesions that have been described previously.14 28 The lack of clinical features that distinguish ICPi-AKI from other causes of AKI, as well as the wide spectrum of histopathological findings in patients with ICPi-AKI, underscores the importance of performing renal biopsy in patients without contraindications, particularly in patients with atypical features (eg, nephrotic range proteinuria) or those who do not respond to corticosteroids.24
We found that approximately two-thirds of patients with ICPi-AKI have renal recovery, occurring at a median of 7 weeks. Patients taking concomitant ATIN-causing medications had a higher rate of renal recovery, consistent with our previous findings.3 This may be a reflection of T-cell reactivity to the exogenous drug, whereby cessation of the causative agent in conjunction with corticosteroids can rapidly attenuate the immune response. We paradoxically found that higher baseline eGFR was associated with a lower likelihood of renal recovery. One possible explanation for this finding is that patients with more renal reserve would need to sustain a greater renal insult in order to achieve the same relative increase in SCr as a patient with less renal reserve.29 We also found that patients with higher stages of ICPi-AKI were less likely to have renal recovery, highlighting the importance of early recognition and treatment of ICPi-AKI. This is further supported by the finding that early initiation of corticosteroids (within 3 days of ICPi-AKI diagnosis) was associated with a higher rate of renal recovery. Similar findings have been observed with extrarenal irAEs, including myocarditis,30 as well as with other forms of drug-induced AKI.31
Data on renal and overall outcomes in patients with ICPi-AKI who are rechallenged are sparse.4 15 32 A total of 121 patients in the current study were rechallenged, and fewer than one in five developed recurrent ICPi-AKI. We did not detect a survival difference in patients rechallenged versus those not rechallenged; however, patients with more aggressive malignancies may have been preferentially selected for rechallenge, which could have obscured our ability to identify a survival benefit. Given the low incidence of recurrent ICPi-AKI, it seems reasonable to consider rechallenge in patients for whom ICPis are the optimal therapy.
Although this is the largest study of ICPi-AKI to date, we acknowledge several limitations. First, not all patients had a kidney biopsy to confirm the diagnosis, which reflects clinical practice in which patients are often treated empirically. Second, our outcomes analyses were focused on renal recovery and overall survival, and we did not collect data on tumor response to ICPi therapy. We, therefore, do not have data on cancer status at the time of ICPi-AKI or rechallenge. Third, patients in our cohort were disproportionately treated at sites in the USA, which may affect the generalizability of our findings.
In this international multicenter cohort study, we identified risk factors, clinical features, and histopathological findings associated with ICPi-AKI, predictors, rates, and timing of renal recovery following ICPi-AKI, and the incidence of recurrent ICPi-AKI after rechallenge. Future studies with longitudinal biospecimen collection are needed to provide additional insight into the mechanisms of ICPi-AKI, and to aid clinicians in differentiating it from other causes of AKI. Further, randomized clinical trials are needed to evaluate the efficacy and safety of different corticosteroid dosing regimens and other forms of immunosuppression for optimal management of ICPi-AKI.
Footnotes
Twitter: @ShrutiGKidney, @HerrmannMd, @DavidLeaf9
Correction notice: This article has been corrected since it was first published online. Figure 2 has been updated, there was a calculation error in panels 2J and 2K. The authors had indicated that 56.2% of patients had pyuria, when it was in fact 57.8%. The definition of pyuria has been updated to be ≥10 WBCs per high power field from ≥5 WBCs per high power field since 10 is the cutoff used more often than 5 in the literature. Furthermore, 61.7% of patients had a urine protein-to-creatinine ratio ≥0.3 g/g, not 58.7%.
Collaborators: ICPi-AKI Consortium Investigators: Assistance Publique-Hôpitaux de Paris (AP-HP), Sorbonne Université, Hôpital Pitié-Salpêtrière: Luca Campedel, Joe-Elie Salem, Corinne Isnard Bagnis. Brigham and Women’s Hospital/Dana-Farber Cancer Institute: Shruti Gupta, David E. Leaf, Harkarandeep Singh, Shveta S. Motwani, Naoka Murakami, Maria C. Tio, Osama E. Rahma, Suraj S. Mothi, Umut Selamet, Deborah Schrag. Charité – Universitätsmedizin Berlin: Sebastian Loew, Kai M. Schmidt-Ott. Chi-Mei Medical Center: Weiting Chang. Donald and Barbara Zucker School of Medicine: Kenar D. Jhaveri, Rimda Wanchoo, Yuriy Khanin, Jamie S. Hirsch, Vipulbhai Sakhiya, Daniel Stalbow, Sylvia Wu. Duke University Medical Center: David I. Ortiz-Melo. Guy’s and St. Thomas NHS Hospital: Marlies Ostermann, Nuttha Lumlertgul, Nina Seylanova, Lucy Flanders, Armando Cennamo, Sophie Papa, Anne Rigg, Nisha Shaunak. Harvard Medical School: Zoe A. Kibbelaar. Heidelberg University Hospital: Karolina Benesova. Icahn School of Medicine at Mount Sinai Hospital: Priya Deshpande. Massachusetts General Hospital: Meghan E. Sise, Kerry L. Reynolds, Harish S. Seethapathy, Meghan Lee, Ian A. Strohbhen. Mayo Clinic: Sandra M. Herrmann, Busra Isik. Memorial Sloan Kettering Cancer Center: Ilya G. Glezerman. New York Nephrology Vasculitis and Glomerular Center: Frank B. Cortazar. Northwestern University: Vikram Aggarwal, Sunandana Chandra. Ohio State University: Jason M. Prosek, Sethu M. Madhavan, Dwight H. Owen, Marium Husain. Sheba Medical Center: Pazit Beckerman, Sharon Mini. Stanford University School of Medicine: Shuchi Anand, Pablo Garcia, Aydin Kaghazchi. University of Alabama at Birmingham: Sunil Rangarajan. University of California-Los Angeles: Daniel Sanghoon Shin, Grace Cherry. University of California-San Francisco: Christopher A. Carlos, Raymond K. Hsu, Andrey Kisel. University Hospitals Cleveland Medical Center: Arash Rashidi, Sheru K. Kansal, Nicole Albert, Katherine Carter, Vicki Donley, Tricia Young, Heather Cigoi. University Hospital of Geneva: Sophie De Seigneux, Thibaud Koessler. University Hospitals Leuven: Ben Sprangers, Els Wauters. University of Florida: Chintan V. Shah. University Medical Center Groningen: Mark Eijgelsheim. University of Miami Miller School of Medicine: Zain Mithani, Javier A. Pagan. University of Pennsylvania Health System: Gaia Coppock, Jonathan J. Hogan. University of Texas MD Anderson Cancer Center: Ala Abudayyeh, Omar Mamlouk, Jamie S. Lin, Valda Page. University of Toronto: Abhijat Kitchlu. University of Vermont Larner College of Medicine: Samuel AP Short. University of Virginia Health System: Amanda D. Renaghan, Elizabeth M. Gaughan. University of Washington: A. Bilal Malik. Vall d’Hebron University Hospital: Maria Jose Soler, Clara García-Carro, Sheila Bermejo, Enriqueta Felip, Eva Muñoz-Couselo, Maria Josep Carreras.
Contributors: Concept and design: all authors. Acquisition, analysis, or interpretation of data: all authors. Drafting of the manuscript: SG and DEL. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: SG, SAPS, and DEL. Preparation of figures and tables: SG, SAPS, and DEL. All authors approved the final version of the manuscript, and accept responsibility to submit for publication. SG and DEL have verified the underlying data and are accountable for all aspects of the submitted work.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Disclaimer: Shruti Gupta is a Scientific Coordinator for GlaxoSmithKline’s ASCEND Trial and receives funding from GE Healthcare. Sunil Rangarajan is supported by the 2020 Young Investigator Award from the Conquer Cancer Foundation of the American Society of Clinical Oncology and by the UAB-UCSD O'Brien Center for Acute Kidney Injury Research (NIH P30-DK079337). Kenar D. Jhaveri is a consultant for Astex Pharmaceuticals, Natera, GlaxoSmithKline, ChemoCentryx, and Chinook, a paid contributor to Uptodate.com, and receives honorarium from ISN and ASN. Daniel Sanghoon Shin participates in the speakers’ bureau at Genentech. Sunandana Chandra is on the Advisory Board or Bristol Myers Squibb, EMD Serono, Regeneron, Sanofi Genzyme, Exicure, Pfizer, and Novartis, and on the steering committee for Genentech. Shveta S. Motwani is a Deputy Editor at UpToDate. Naoka Murakami reports funding from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; K08DK12068) and from the American Society of Nephrology (Carl W. Gottschalk Research Scholar Grant). Frank B. Cortazar is a consultant for ChemoCentryx and Retrophin. Maria J. Soler reports personal fees from NovoNordisk, Jansen, Mundipharma, AstraZeneca, Esteve, Fresenius, Ingelheim Lilly, Vifor, ICU and grants and personal fees from Boehringer. Clara García-Carro has given scientific lectures and participated in advisory boards organized by Astra-Zeneca, Boehringer-Ingelheim Lilly, Mundipharma and NovoNordisk. Ala Abudayyeh and Jamie S. Lin are supported by the Division of Internal Medicine Immuno-Oncology Toxicity Award Program of the University of Texas MD Anderson Cancer Center. Jamie S. Lin is supported by a grant from the National Institutes of Health (NIH; K08DK119466). Ben Sprangers is a senior clinical investigator at the Research Foundation Flanders (F.W.O.) (1842919N) and is supported by Stichting tegen Kanker (grant C/2020/1380). Arash Rashidi is a consultant for Otsuka pharmaceutical company. Ilya Glezerman’s spouse has stock ownership in Pfizer. Sethu M. Madhavan is supported by NIH grant K08 DK123411. Dwight H. Owen is a Paul Calabresi Scholar supported by the OSU K12 Training Grant for Clinical Faculty Investigators (K12 CA133250) and reports research support (to institution) from Bristol Myers Squibb, Merck, Palobiofarma, Genentech. Dr. Owen is also supported in part by The Ohio State University Comprehensive Cancer Center and by the NIH (P30 CA016058). Els Wauters is supported by Stichting tegen Kanker (Mandate for basic & clinical oncology research). David E. Leaf is supported by NIH grants R01HL144566 and R01DK125786. Sandra M. Herrmann is supported by NIH grant K08 DK118120, the Mary Kathryn and Michael B. Panitch Career Development Award, and Mayo Clinic K2R award.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
ICPi-AKI Consortium Investigators:
Luca Campedel, Joe-Elie Salem, Corinne Isnard Bagnis, Shruti Gupta, David E Leaf, Harkarandeep Singh, Shveta S Motwani, Naoka Murakami, Maria C Tio, Osama E Rahma, Suraj S Mothi, Umut Selamet, Deborah Schrag, Sebastian Loew, Kai M Schmidt-Ott, Weiting Chang, Kenar D Jhaveri, Rimda Wanchoo, Yuriy Khanin, Jamie S Hirsch, Vipulbhai Sakhiya, Daniel Stalbow, Sylvia Wu, David I Ortiz-Melo, Marlies Ostermann, Nuttha Lumlertgul, Nina Seylanova, Lucy Flanders, Armando Cennamo, Sophie Papa, Anne Rigg, Nisha Shaunak, Zoe A Kibbelaar, Karolina Benesova, Priya Deshpande, Meghan E Sise, Kerry L Reynolds, Harish S Seethapathy, Meghan Lee, Ian A Strohbhen, Sandra M Herrmann, Busra Isik, Ilya G Glezerman, Frank B Cortazar, Vikram Aggarwal, Sunandana Chandra, Jason M Prosek, Sethu M Madhavan, Dwight H Owen, Marium Husain, Pazit Beckerman, Sharon Mini, Shuchi Anand, Pablo Garcia, Aydin Kaghazchi, Sunil Rangarajan, Daniel Sanghoon Shin, Grace Cherry, Christopher A Carlos, Raymond K Hsu, Andrey Kisel, Arash Rashidi, Sheru K Kansal, Nicole Albert, Katherine Carter, Vicki Donley, Tricia Young, Heather Cigoi, Sophie De Seigneux, Thibaud Koessler, Els Wauters Ben Sprangers, Chintan V Shah, Mark Eijgelsheim, Zain Mithani, Javier A Pagan, Gaia Coppock, Jonathan J Hogan, Ala Abudayyeh, Omar Mamlouk, Jamie S Lin, Valda Page, Abhijat Kitchlu, Samuel AP Short, Amanda D Renaghan, Elizabeth M Gaughan, A Bilal Malik, Maria Jose Soler, Clara García-Carro, Sheila Bermejo, Enriqueta Felip, Eva Muñoz-Couselo, and Maria Josep Carreras
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
All protocols were approved by the Mass General Brigham Institutional Review Board (IRB), and by the IRBs of participating sites.
References
- 1. Robert C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun 2020;11. 10.1038/s41467-020-17670-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Manohar S, Kompotiatis P, Thongprayoon C, et al. Programmed cell death protein 1 inhibitor treatment is associated with acute kidney injury and hypocalcemia: meta-analysis. Nephrol Dial Transplant 2019;34:108–17. 10.1093/ndt/gfy105 [DOI] [PubMed] [Google Scholar]
- 3. Cortazar FB, Kibbelaar ZA, Glezerman IG, et al. Clinical features and outcomes of immune checkpoint Inhibitor–Associated AKI: a multicenter study. JASN 2020;31:435–46. 10.1681/ASN.2019070676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Seethapathy H, Zhao S, Chute DF, et al. The incidence, causes, and risk factors of acute kidney injury in patients receiving immune checkpoint inhibitors. CJASN 2019;14:1692–700. 10.2215/CJN.00990119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Meraz-Muñoz A, Amir E, Ng P, et al. Acute kidney injury associated with immune checkpoint inhibitor therapy: incidence, risk factors and outcomes. J Immunother Cancer 2020;8:e000467. 10.1136/jitc-2019-000467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Espi M, Teuma C, Novel-Catin E, et al. Renal adverse effects of immune checkpoints inhibitors in clinical practice: ImmuNoTox study. Eur J Cancer 2021;147:29–39. 10.1016/j.ejca.2021.01.005 [DOI] [PubMed] [Google Scholar]
- 7. García-Carro C, Bolufer M, Bury R, et al. Acute kidney injury as a risk factor for mortality in oncological patients receiving check-point inhibitors. Nephrol Dial Transplant 2021. 10.1093/ndt/gfab034 [DOI] [PubMed] [Google Scholar]
- 8. Stein C, Burtey S, Mancini J. Acute kidney injury in patients treated with anti-programmed death receptor-1 for advanced melanoma: a real-life study in a single-centre cohort. Nephrol Dial Transplant 2020. [DOI] [PubMed] [Google Scholar]
- 9. Hultin S, Nahar K, Menzies AM, et al. Histological diagnosis of immune checkpoint inhibitor induced acute renal injury in patients with metastatic melanoma: a retrospective case series report. BMC Nephrol 2020;21. 10.1186/s12882-020-02044-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Darmon M, Vincent F, Canet E. Acute kidney injury in critically ill patients with haematological malignancies: results of a multicentre cohort study from the Groupe de Recherche en Rnimation Respiratoire en Onco-Hatologie. Nephrol Dial Transplant 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Luciano RL, Brewster UC. Kidney involvement in leukemia and lymphoma. Adv Chronic Kidney Dis 2014;21:27–35. 10.1053/j.ackd.2013.07.004 [DOI] [PubMed] [Google Scholar]
- 12. Shirali AC, Perazella MA, Gettinger S. Association of acute interstitial nephritis with programmed cell death 1 inhibitor therapy in lung cancer patients. Am J Kidney Dis 2016;68:287–91. 10.1053/j.ajkd.2016.02.057 [DOI] [PubMed] [Google Scholar]
- 13. Oleas D, Bolufer M, Agraz I, et al. Acute interstitial nephritis associated with immune checkpoint inhibitors: a single-centre experience. Clin Kidney J 2021;14:1364–70. 10.1093/ckj/sfaa008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mamlouk O, Selamet U, Machado S, et al. Nephrotoxicity of immune checkpoint inhibitors beyond tubulointerstitial nephritis: single-center experience. j. immunotherapy cancer 2019;7. 10.1186/s40425-018-0478-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Isik B, Alexander MP, Manohar S, et al. Biomarkers, clinical features, and rechallenge for immune checkpoint inhibitor renal immune-related adverse events. Kidney Int Rep 2021;6:1022–31. 10.1016/j.ekir.2021.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harris PA, Taylor R, Minor BL, et al. The REDCap Consortium: building an international community of software platform partners. J Biomed Inform 2019;95:103208. 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kellum JA, Lameire N, Aspelin P. Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2012. [Google Scholar]
- 18. Bellomo R, Ronco C, Kellum JA. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. In: Critical Care (London, England) 2004. [DOI] [PMC free article] [PubMed]
- 19. Cortazar FB, Marrone KA, Troxell ML, et al. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int 2016;90:638–47. 10.1016/j.kint.2016.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Seethapathy H, Zhao S, Strohbehn IA, et al. Incidence and clinical features of immune-related acute kidney injury in patients receiving programmed cell death ligand-1 inhibitors. Kidney Int Rep 2020;5:1700–5. 10.1016/j.ekir.2020.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Blank M-L, Parkin L, Paul C, et al. A nationwide nested case-control study indicates an increased risk of acute interstitial nephritis with proton pump inhibitor use. Kidney Int 2014;86:837–44. 10.1038/ki.2014.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Qiu T, Zhou J, Zhang C. Acid-suppressive drugs and risk of kidney disease: a systematic review and meta-analysis. J Gastroenterol Hepatol 2018;33:1566–73. 10.1111/jgh.14157 [DOI] [PubMed] [Google Scholar]
- 23. Hart E, Dunn TE, Feuerstein S, et al. Proton pump inhibitors and risk of acute and chronic kidney disease: a retrospective cohort study. Pharmacotherapy 2019;39:443–53. 10.1002/phar.2235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gupta S, Cortazar FB, Riella LV, et al. Immune checkpoint inhibitor nephrotoxicity: update 2020. Kidney360 2020;1:130–40. 10.34067/KID.0000852019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol 2012. [DOI] [PubMed] [Google Scholar]
- 26. Weber JS, Dummer R, de Pril V, et al. Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer 2013;119:1675–82. 10.1002/cncr.27969 [DOI] [PubMed] [Google Scholar]
- 27. Weber JS, Hodi FS, Wolchok JD, et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol 2017;35:785–92. 10.1200/JCO.2015.66.1389 [DOI] [PubMed] [Google Scholar]
- 28. Gallan AJ, Alexander E, Reid P, et al. Renal vasculitis and Pauci-immune glomerulonephritis associated with immune checkpoint inhibitors. Am J Kidney Dis 2019;74:853–6. 10.1053/j.ajkd.2019.04.016 [DOI] [PubMed] [Google Scholar]
- 29. Husain-Syed F, Ferrari F, Sharma A, et al. Preoperative renal functional reserve predicts risk of acute kidney injury after cardiac operation. Ann Thorac Surg 2018;105:1094–101. 10.1016/j.athoracsur.2017.12.034 [DOI] [PubMed] [Google Scholar]
- 30. Zhang L, Zlotoff DA, Awadalla M, et al. Major adverse cardiovascular events and the timing and dose of corticosteroids in immune checkpoint inhibitor-associated myocarditis. Circulation 2020;141:2031–4. 10.1161/CIRCULATIONAHA.119.044703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. González E, Gutiérrez E, Galeano C, et al. Early steroid treatment improves the recovery of renal function in patients with drug-induced acute interstitial nephritis. Kidney Int 2008;73:940–6. 10.1038/sj.ki.5002776 [DOI] [PubMed] [Google Scholar]
- 32. Manohar S, Ghamrawi R, Chengappa M, et al. Acute interstitial nephritis and checkpoint inhibitor therapy. Kidney360 2020;1:16–24. 10.34067/KID.0000152019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2021-003467supp001.pdf (952.7KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.