Abstract
Objectives
To review prevalence studies of multimorbidity in South Africa to identify prevalence estimates, common disease clusters and factors associated with multimorbidity.
Design
Systematic review.
Setting
South Africa (general community and healthcare facilities).
Data sources
Articles were retrieved from electronic databases (PubMed, Web of Science, Scopus, CINAHL, Science Direct and JSTOR).
Eligibility criteria
Studies addressing the prevalence of multimorbidity in South Africa were eligible for inclusion. A systematic search was done in various databases up to December 2020. A risk of bias assessment was conducted for each article using a modified checklist.
Study selection
Two researchers independently screened titles and abstracts; assessed the risk of bias of each study and extracted data. Included studies were described using a narrative synthesis.
Results
In total, 1407 titles were retrieved; of which 10 articles were included in the narrative synthesis. Six studies had a low risk of bias and three had a moderate risk of bias. One study was not assessed for risk of bias, because there was no criteria that apply to routine health information systems. Three of the included studies were population-based surveys, four were community-based cohorts and three cross-sectional studies of health facility data. The prevalence of multimorbidity was low to moderate (3%–23%) in studies that included younger people or had a wide range of selected age groups; and moderate to high (30%–87%) in studies of older adults. The common disease clusters were hypertension and diabetes, hypertension and HIV, and TB and HIV.
Conclusion
All studies indicated that multimorbidity is a norm in South Africa, especially among older adults. Hypertension is the main driver of multimorbidity. Research on multimorbidity in South Africa needs to be strengthened with high-quality study designs.
PROSPERO registration number
CRD42020196895.
Keywords: public health, epidemiology, epidemiology
Strengths and limitations of this study.
To our knowledge, this is the first systematic review of multimorbidity prevalence studies in South Africa and of an African country.
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.
This review includes studies conducted in general community and healthcare settings.
A limitation of this study was that it excludes studies conducted in subpopulations with one specific disease (eg, multimorbidity in patients with cancer).
Grey literature (non-academic literature) was excluded.
Introduction
One-third of adults residing in low-income and middle-income countries (LMICs) are thought to be afflicted by two or more coexisting health conditions; also known as multimorbidity.1 The last two decades have seen an exponential growth in the number of studies about multimorbidity.2 This can be attributed to more research into ageing populations,2 and the recognition that multimorbidity impacts patient care and healthcare systems.3 Other consequences of multimorbidity include increased mortality levels,4 lowered quality of life,5 the risk of polypharmacy6 and intensified utilisation of health services and associated costs.7 8 More recently, multimorbidity was implicated as a risk factor for COVID-19 mortality.9 10
Most research to date has been conducted in high-income countries; sparking calls for similar research in LMICs.2 11 12 Research is needed into multimorbidity in LMICs, like South Africa, where disease burdens differ to those in high-income countries. South Africa has a unique disease burden—it has the largest number of people living with HIV in the world.13 With the availability of antiretrovirals, people with HIV are living longer and developing age-related non-communicable diseases (NCDs).14 At the same time, the burden of disease due to NCDs is increasing in the country; giving rise to a disease pattern of coexisting infectious diseases and NCDs.15 16
In resource-constrained health settings, it is imperative that we estimate the magnitude of multimorbidity as well as the nature and type of disease clusters to more efficiently manage patients and organise health service delivery. South Africa lacks a robust national routine health information system (RHIS) to inform its morbidity profile. Countries with less robust RHISs need to rely on smaller-scale studies and surveys to better understand the scale and impact of the problem of multimorbidity. This has led to numerous studies focused on quantifying the prevalence of multimorbidity and studies focused on integrated care in South Africa.17–21 However, many of these studies suffer from the methodological problems that tend to plague multimorbidity studies elsewhere, which is a lack of standardisation.22 This makes it difficult to compare and interpret studies, given their varying estimates and methodologies. This study set out to systematically assess multimorbidity prevalence studies in South Africa, to report on common disease clusters and factors associated with multimorbidity in South Africa.
Methods
Search strategy and database search
The protocol for this study was published elsewhere.23 The Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines24 guided this study (online supplemental appendix 1). One researcher experienced in systematic review methodology (EBT), performed a systematic literature search in PubMed, Web of Science, Scopus, CINAHL, Science Direct and JSTOR to identify articles reporting epidemiological data on multimorbidity in the adult population of South Africa. The search strategy was reviewed by an expert librarian (online supplemental appendix 2). The time frame of the search was not restricted and covered a period up to December 2020.
bmjopen-2021-048676supp001.pdf (167.7KB, pdf)
Study selection and data extraction
The search output citations were downloaded and saved to EndNote V.X8.25 The EndNote deduplication function was employed, and remaining citations were uploaded into an electronic screening website, Rayyan.26 Two researchers (RAR and EBT) independently screened the titles and abstracts and studies deemed irrelevant were discarded. A third researcher (BvW) assisted with conflicts. Case reports, reviews, editorials, letters, studies among children, studies not conducted in South Africa, study designs that were not cross-sectional or cohorts, studies where it was not possible to calculate the prevalence of multimorbidity in the general population (eg, studies only examining multimorbidity in cancer patients) were excluded. Where multiple studies reported on the same source of data (eg, one national survey), only the most relevant study was included.
The full texts were independently assessed by two researchers (RAR and EBT) using the electronic data capture system, the Burden of Disease Review Manager (BODREVMAN).27 BODREVMAN facilitates the independent data collection of study characteristics (study design, sample size, geographical location, whether a study is community based or facility based). Also, data on the definition of multimorbidity used, the disease conditions included in the study and the prevalence of multimorbidity (by age and sex where possible) were extracted. Disagreements were discussed and resolved. The reference lists of included articles were screened for additional studies.
Quality assessment
Two researchers (RAR and EBT) independently assessed and appraised each article. BODREVMAN contains a modified checklist based on the Newcastle Ottawa28 and Hoy checklist.29 The tool has been described elsewhere.30 Each article was independently scored and categorised as either having a high, moderate or low risk of bias. Studies based on RHIS did not undergo a risk of bias assessment due to a lack of assessment criteria for this study type.
Data extraction and analysis
Information on multimorbidity definitions, disease conditions included and the proportion of the sample with more than one condition, was extracted. Authors were contacted for data by age and sex breakdowns. Studies were categorised by study type (cohort or cross-sectional), and study setting (community or facility based). It was noted whether disease conditions included were self-reported or biologically assessed.
The mean and SD, or the absolute number and the percentage were recorded, as appropriate. The age range and sex for each category were recorded. Where data appeared in graphical formats, authors were contacted for the original data or WebPlotDigitizer V.4.3 (California, USA)31 was used to extract data. STATA V.15 (StataCorp) was used to calculate standard errors using the sample size and prevalence estimates where possible.
Patient and public involvement
Patients and the public were not involved in this study.
Results
Search results
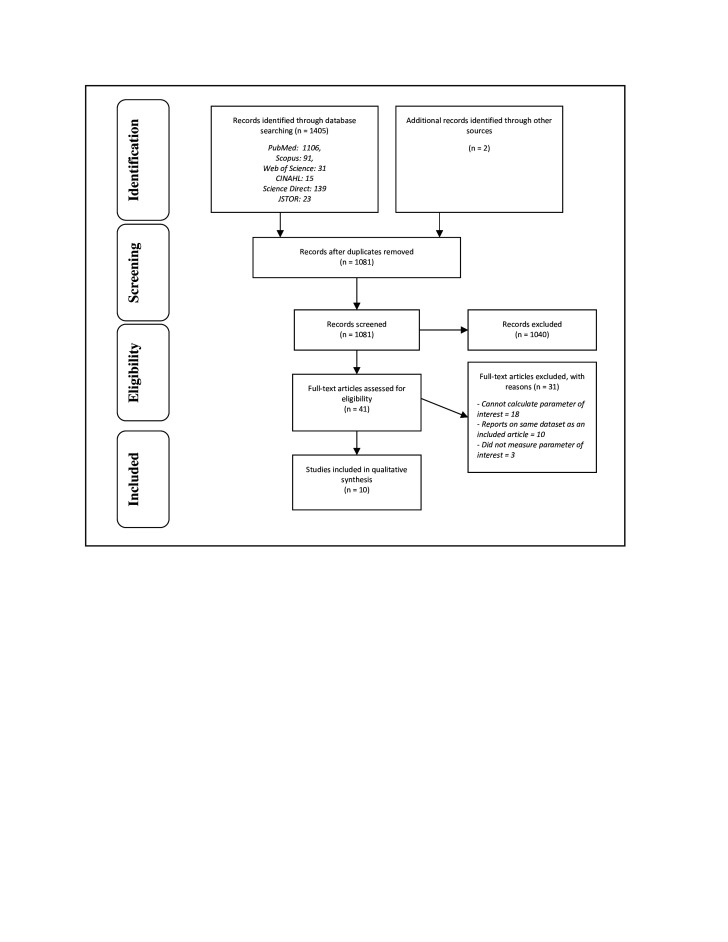
In total, 1407 titles were retrieved, and 1081 records were screened after deduplication (figure 1). By screening titles and abstracts, 1040 articles were excluded. Forty-one full-text articles were assessed for eligibility, of which 10 were included in a narrative synthesis.32–41 In the title and abstract screening phase, reviewers conflicted on 2.9% of the articles. In the full-text phase, the reviewers had conflicts in 2 of the 41 articles. All conflicts were resolved.
Figure 1.
PRISMA flow diagram. PRISMA, Preferred Reporting Items for Systematic reviews and Meta-Analyses.
Study characteristics
The sample sizes of included studies ranged from 42235 to 47 334 participants38 (table 1). All included studies were published after 2015 but the period of data collected ranged from 200332 to 2015.37 38 40 Three studies conducted a secondary data analysis of population-based surveys.32–34 The surveys analysed were the 2003 World Health Survey,32 2007 and 2010 WHO Study on global AGEing and adult health,33 35 and the 2008 and 2012 South African National Income Dynamics Survey.34 Three studies were cross-sectional analyses of community-based cohorts and surveys.36–38 The remaining three studies were of a cross-sectional nature and based in health facilities.39–41
Table 1.
Characteristics of included studies
| Study type | Study | Study population and size | Year | Location | Risk of bias (score) |
| Population-based survey | Afshar et al32 | N=2629. Adults 18 years and older in the 2003 World Health Survey. |
2003 | South Africa (Urban and rural areas included) |
Low (14) |
| Garin et al33 | N=3836. Adults 50 years and older in the 2007 WHO Study on global AGEing and adult health (SAGE). |
2007–2008 | South Africa (Urban and rural areas included) |
Low (15) | |
| Weimann et al34 | N=18 526 in 2008. N=20 015 in 2012. Participants 15 years and older in the National Income Dynamic Survey wave 1 (2008) and wave 3 (2012). |
2008, 2012 | South Africa (Urban and rural areas included) |
Low (17) | |
| Cross-sectional study (community based) | Ghose and Razak35 | N=422. Adults 50 years and older infected and/or affected by HIV in the SAGE Well-being of Older People Study 2010. |
2010 | Hlabisa subdistrict, KwaZulu-Natal (Rural) |
Moderate (12) |
| van Heerden et al36 | N=570. Adults older than 18 years enrolled in a cohort study to increase engagement in HIV care and testing. |
November 2011–June 2012 | KwaZulu-Natal (Rural) |
Moderate (13) | |
| Chang et al37 | N=3889. Adults enrolled in the Health and Ageing in Africa: A longitudinal study of an INDEPTH Community in South Africa Programme. |
2014–2015 | Agincourt Health and Demographic Surveillance System, Bushbuckridge subdistrict, Mpumalanga (Rural) |
Low (17) | |
| Sharman and Bachmann38 | N=47 334. Participants 15 years and older enrolled in the population-based HIV and health surveillance study, conducted by the Africa Health Research Institute. |
2009–2015 | uMkhanyakude district, KwaZulu-Natal (Rural) |
Low (14) | |
| Cross-sectional study (health facility based) |
Lalkhen and Mash39 | N=5793. Primary healthcare users where all participants had at least one NCD (hypertension, diabetes, asthma, epilepsy, COPD, osteoarthritis). |
2010 | Primary healthcare facilities in the Western Cape, North West, Northern Cape and Limpopo (Urban and rural areas included) |
Low (16) |
| Roche and de Vries40 | N=491. Consecutive admissions to an internal medicine department of a large district hospital. |
2015 | District hospital, Cape Town, Western Cape (Urban) |
Moderate (13) | |
| Routine health information systems | Oni et al41 | N=14 364. Chronic disease patients (adults) with at least one disease (HIV, TB, diabetes, and hypertension) identified using the Western Cape Department of Health Data Repository and the Electronic prescription system. |
September 2012–May 2013 | Michael Mapongwana clinic, Khayelitsha, Cape Town, Western Cape (Periurban) |
NA |
COPD, chronic obstructive pulmonary disease; NA, not available; NCD, non-communicable disease; TB, tuberculosis.
Three studies were conducted nationally32–34 with others conducted in Kwa-Zulu Natal province (n=3),35 36 38 the Western Cape province (n=2)40 41 and Mpumalanga province (n=1).37 One study was conducted in primary healthcare facilities in the Western Cape, North West, Northern Cape and Limpopo provinces.39 Four studies were conducted in rural areas,35–38 two studies were conducted in urban areas40 41 and the remaining studies were conducted in both urban and rural areas.32 33 39 42 Six studies had a low risk of bias,32–34 37–39 three had a moderate risk of bias35 36 40 and one based on a RHIS was not assessed for risk of bias due to a lack of assessment criteria for this study type.
Disease conditions assessed
Study findings on the prevalence of multimorbidity can be influenced by (1) the definition of multimorbidity used, (2) the number of disease conditions included in the study, (3) the actual disease conditions included and (4) how the disease conditions were measured.
All included studies used a ‘count’ of the number of diseases to define multimorbidity, that is, multimorbidity was defined by having two or more diseases (online supplemental appendix 3). Half of these studies specified they were only focused on chronic conditions.32–34 37 41 Two health facility-based studies included acute conditions such as lower respiratory infections.39 40 The inclusion of acute disease conditions could inflate the prevalence of multimorbidity. The full list of disease conditions included can be found in online supplemental appendix 3.
One study included two definitions of multimorbidity—a ‘count’ definition (as described above) and another more detailed definition. The detailed definition specified multimorbidity as the presence of conditions from more than one of the following categories of disease: cardiometabolic conditions, mental disorders or HIV and anaemia.37 When using this definition, the prevalence of multimorbidity was lowered as it only includes discordant diseases (ie, excludes diseases that belong to the same category such as hypertension and diabetes). For this review, we used their results from the ‘count’ definition, unless otherwise stated.
The number of disease conditions included in each study ranged from 441 to 2440 (table 2). Diabetes was included as a disease condition in all 10 studies. Most studies included hypertension (n=9) in their assessment of multimorbidity. HIV (n=5), asthma (n=5) and heart disease (n=5) were also commonly included disease conditions.
Table 2.
Ten common disease conditions reported in articles
| Disease conditions included | Studies | Total articles included in | |||||||||
| Afshar et al32 | Garin et al33 | Weimann et al34 | Ghose and Abdoul Razak35 | 36van Heerden et al36 | Chang et al37 | Sharman and Bachmann38 | Lalkhen and Mash39 | Roche and de Vries40 | Oni et al41 | ||
| Diabetes | x | x | x | x | x† | x | x | x | x | x | 10 |
| Hypertension | x | x | x | x | x | x | x | x | x | 9 | |
| HIV | x | x | x¶ | x | x | x | x | 5 | |||
| Asthma | x | x | x | x | x | 5 | |||||
| IHD/heart disease/ angina | x | x | x | x | x | 5 | |||||
| Depression | x§ | x | x¶ | x | x* | 4 | |||||
| COPD | x | x‡ | x | x | 4 | ||||||
| Arthritis/osteoarthritis | x | x | x | x | 4 | ||||||
| TB/current TB | x | x | x | x | 4 | ||||||
| Lipid disorder | x | x | x | 3 | |||||||
*Depression, post-traumatic stress disorder, alcohol dependence.
†Hyperglycaemia.
‡Chronic lung disease.
§Depression, schizophrenia or psychosis.
¶Assessed condition but was not able to incorporate into multimorbidity calculation based on the way study reported it.
COPD, chronic obstructive pulmonary disease; IHD, ischaemic heart disease; TB, tuberculosis.
The study design and setting influenced how disease conditions were measured (online supplemental appendix 3). Population-based surveys tended to use self-reported data, although some included measurements of blood pressure and obesity. Studies based on cohorts tended to use a mix of measured (biomarkers) and self-reported disease conditions. Facility-based studies tended to use medical records and biomarkers to determine the disease burden in their samples.
Patterns of disease clusters observed
The studies reported on common disease clusters using bubble charts of pairwise comorbid conditions,33 37 reporting each disease with their most common comorbid condition38 39 or schematics detailing double and triple morbidities.34 37 41 The results of the studies were difficult to compare due to how the data were reported. Four studies did not describe common disease clusters found in their study populations.32 35 36 40
While it was not possible to ascertain the largest disease cluster in one study, Garin, Koyanagi33 found hypertension featured strongly with diabetes, stroke, angina, cataract, cognitive impairment and all other conditions examined in their analysis. Arthritis and obesity were also commonly listed as comorbid conditions for all other disease conditions.
Table 3 summarises the top five disease clusters from the five remaining studies. The number of disease combinations varied in each study with some studies reporting less than 10 disease clusters34 41 and others reporting more than 20 disease clusters37–39 (online supplemental appendix 4).
Table 3.
Top five disease clusters in each study
| Disease combinations/clusters | Total studies reported (n=5) | Study citation | ||
| Disease 1 | Disease 2 | Disease 3 | ||
| Hypertension | Diabetes | 4 | 34 38 39 41 | |
| Hypertension | HIV | 3 | 34 38 41 | |
| TB | HIV | 3 | 34 38 41 | |
| Hypertension | TB | 2 | 34 41 | |
| Diabetes | HIV | 2 | 38 41 | |
| TB | Diabetes | 1 | 34 | |
| Hypertension | Osteoarthritis | 1 | 39 | |
| Asthma | Hypertension | 1 | 39 | |
| Hypertension | COPD | 1 | 39 | |
| Hypertension | IHD | 1 | 39 | |
| Hypertension | Dyslipidaemia | 1 | 37 | |
| Hypertension | Anaemia | 1 | 37 | |
| Hypertension | Dyslipidaemia | Anaemia | 1 | 37 |
| Anaemia | HIV | 1 | 37 | |
| Hypertension | Anaemia | HIV | 1 | 37 |
COPD, chronic obstructive pulmonary disease; IHD, ischaemic heart disease; TB, tuberculosis.
Hypertension was frequently comorbid with other diseases (table 3). Weimann et al34 and Oni et al41 showed similar patterns of disease—with hypertension and diabetes being the most common disease cluster. In these studies, the disease cluster hypertension and HIV ranked highly, followed by TB and HIV. In terms of having three co-occurring diseases, both ranked the combination of TB, diabetes and hypertension highest, followed by the combination of hypertension, HIV and TB. Lalkhen and Mash39 also found hypertension and diabetes to be the largest disease cluster in their study. While Chang et al37 found the largest disease cluster was hypertension and dyslipidaemia, followed by hypertension and anaemia; and the combination of hypertension, dyslipidaemia and anaemia. Anaemia and HIV also commonly co-occurred.
Age and sex tend to influence the susceptibility of an individual to certain diseases. However, studies generally did not report disease clusters by these breakdowns. Two studies reported that HIV was more prevalent in their younger participants37 38; while hypertension affected those over the age of 40 years, and diabetes and angina affected people above the age of 60 years. One study also noted that hypertension and diabetes were more common in females compared with males, and TB was more common in males.38 One study noted that multimorbidity was lower in patients with HIV that were on ART (compared with patients not on ART or with unknown ART status) but the association did not hold when broken down by age group.41
These results must be interpreted with caution as each study included different disease conditions; and even when the same disease conditions were included, these could differ in the way they were measured for example, self-reported or biologically measured.
Multimorbidity prevalence
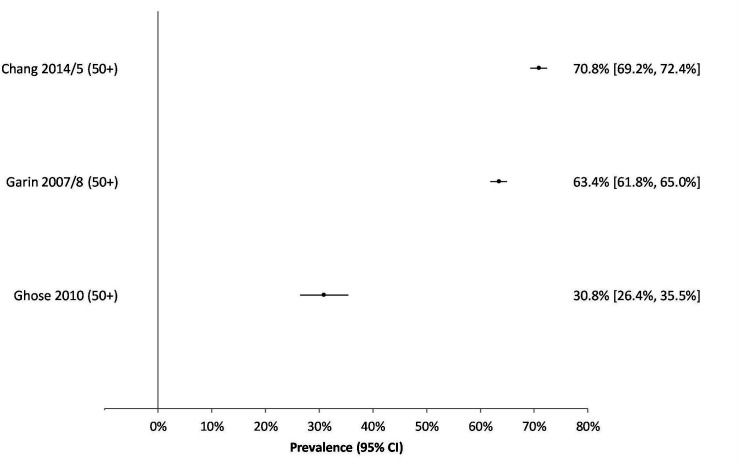
Due to study heterogeneity, it was not possible to do a meta-analysis. Studies reported multimorbidity prevalence by varying age breakdowns making direct comparison difficult. Several studies reported multimorbidity by age group and/or sex (online supplemental appendix 5). Two studies reported the median/mean age of participants but the age range of participants was not included39 40 and one did not report an overall multimorbidity prevalence for their study.36 From the remaining studies, multimorbidity prevalence tended to be low to moderate in studies which included younger people or had a wide range of age groups (3%–23%) (figure 2); and moderate to high in studies reporting on adults aged 50 years and older (30%–71%) (figure 3).
Figure 2.
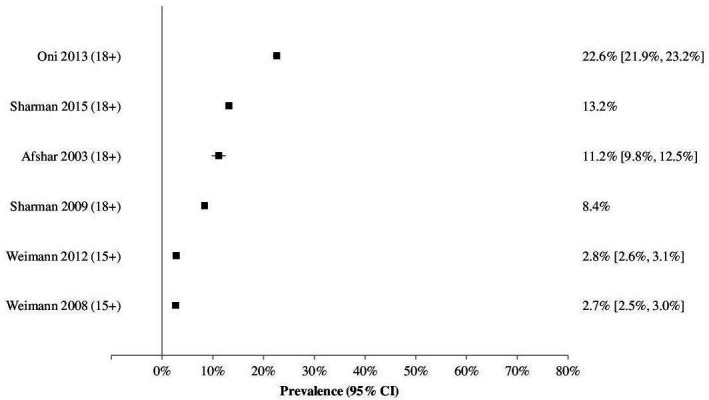
Graph of multimorbidity prevalence estimates for studies that include younger age groups.
Figure 3.
Graph of multimorbidity prevalence in studies including persons aged 50 years and older.
In population-based surveys, each study reported a different age group (table 4). In those 18 years and above, Afshar et al32 reported an overall prevalence of 11%, however, this was age-standardised against the WHO Standard Population which means it uses a standardised age structure rather than the one found in South Africa. Another study reported the results of a panel survey in 2008 and 2012 and showed a rather low prevalence of multimorbidity (2.7%) for those aged over 15 years old.34 The study showed a negligible increase (0.1%) during a 4-year period. A study that only reported on those aged above 50 years of age showed a very high overall prevalence of multimorbidity (63.4%).33
Table 4.
Multimorbidity prevalence by age group
| Study | Year | Age band (years) | Prevalence of multimorbidity | ||
| n/N | % (95% CI)* | ||||
| Population-based surveys | Afshar et al32‡ | 2003 | Overall (18+) | – | 11.2 (9.8 to 12.5) |
| Garin et al33 | 2007/8 | Overall (50+) | 2376/3747† | 63.4 | |
| Weimann et al34 | 2008 | Overall (15+) | – | 2.7 (2.5 to 3.0) | |
| 2012 | Overall (15+) | – | 2.8 (2.6 to 3.1) | ||
| Cross-sectional study (community based) | Ghose et al35 | 2010 | Overall (50+) | 130/422 | 30.8 |
| Chang et al37 | 2014/15 | Overall (40+) | 2700/3889 | 69.4 | |
| Sharman et al38 | 2009 | Overall (18+) | – | 8.4 | |
| 2015 | Overall (40+) | – | 18.4 | ||
| 2015 | Overall (18+) | – | 13.2 | ||
| Cross-sectional study (health facility based) | Lalkhen et al39 | 2010 | Overall (mean age§) | 2806/5793 | 48.4 |
| Roche and De Vries40 | 2015 | Overall (mean age 49 years) | 371/427 | 87.0 | |
| Routine health information systems | Oni et al41 | 2012/13 | Overall (18+) | 3246/14 364 | 22.6 |
*Not all studies reported a 95% CI and there was insufficient information to calculate this.
†Estimated from available information.
‡Reports a standardised multimorbidity prevalence.
§Mean age of patients with osteoarthritis (56.9 years), COPD (56.8 years), diabetes (56.6 years), hypertension (56.4 years), asthma (45.5 years), epilepsy (37.9 years).
COPD, chronic obstructive pulmonary disease.
Among community-based cross-sectional studies, the prevalence among older adults ranged from 18%38 to 69%.37 However, Chang et al37 used two definitions of multimorbidity and when applying the second definition (categories of discordant disease groups), they estimated a lower prevalence of 54%. One study that included younger people noted a 5% increase in multimorbidity prevalence between the period 2009 to 2015.37
In health facilities, two studies found moderate levels of multimorbidity (14.4% and 22.6%).39 41 One study based in a health facility found an extremely high prevalence of multimorbidity (87.0%), however, this study included both chronic and acute health conditions.40
Factors associated with multimorbidity
Most of the included studies reported on factors they found to be associated with multimorbidity (online supplemental appendix 3). Multimorbidity was frequently associated with increasing age.32–34 37 38 41 However, Garin et al33 noted a decrease in the prevalence of multimorbidity in the age group 60+ years and Chang et al37 noted a decrease from the age group 69+ years.
Being female was inconsistently linked to a high prevalence of multimorbidity. The pattern was noted in two studies33 34; although another study reported it was not statistically significant37; while one found no distinction between males and females.41 One study found that living in urban areas was a risk factor for multimorbidity34 while another found that living in rural areas was associated with multimorbidity.33 Other factors found to be associated with multimorbidity were: a lower level of education32 33; being separated, divorced or widowed33 37; living in KwaZulu-Natal or the Eastern Cape provinces, being Indian/Asian or being obese.34 Socioeconomic deprivation was found to be associated with multimorbidity in one study,34 but another found no association between wealth and multimorbidity.37
Other studies identified the effects of multimorbidity such as having memory complaints (in women), suffering from depression,35 decreased well-being and self-reported health.37 38 One study found that length of stay in hospital was not related to multimorbidity and also did not link lifestyle risk factors to multimorbidity.40
Discussion
This study set out to assess the prevalence of multimorbidity in adults in South Africa using systematic review methodology. This study found considerable heterogeneity among included articles, which stemmed from differences in study design, disease conditions assessed and how study results were reported. Despite this, we found a low to moderate multimorbidity prevalence in studies including younger people and a moderate to high prevalence in studies including older adults. Due to study heterogeneity, it is difficult to compare these results to the findings of a recent systematic review which estimated a pooled multimorbidity prevalence of 30% for LMICs.1
Three of our included studies reported fairly low levels of multimorbidity prevalence.32 34 38 One study standardised the prevalence to the world population which may have resulted in a lower prevalence estimate (11.2%).32 The other study reported an overall prevalence of less than 3% among people 15 years and older; and in people over the age of 65 years, they estimated a prevalence of only 10%.34 The same 2008 dataset from a population-based survey was used in another study and found a similar prevalence of multimorbidity, despite using different methods (4.0%).43 The low prevalence found in this survey could be attributed to a healthier population being sampled or as the authors suggested, under-reporting of self-reported data due to stigma around HIV and TB.34 The study also included only four disease conditions which may have resulted in a lower prevalence. In contrast, a study that included many acute and chronic conditions resulted in a very high prevalence estimate.40 This highlights the significant impact of study design on the estimates produced. The third study had a large sample size but may have underestimated the burden of multimorbidity due to the use of self-reported data.38 Also, they had missing data on HIV due to additional consent being required.
Age is accepted to be an important predictor of multimorbidity.40 Most studies showed that the prevalence of multimorbidity increased with age, however, two studies observed decreases in the oldest age groups. This needs further investigation. What also remains unclear is whether multimorbidity does in fact affect people at younger ages in LMICs.12 Based on this systematic review, more studies need to interrogate multimorbidity by age group as the lack of reporting makes it difficult to monitor. Age and sex are both important predictors of multimorbidity and multimorbidity should be reported in a disaggregated manner where possible.44
The common diseases assessed in our included studies (diabetes and hypertension) have a high prevalence in South Africa. It was surprising that only half of the studies included HIV as a condition of interest; given the high prevalence of HIV in the country. However, many of the studies were based on secondary data analysis and were limited to the conditions that were included. Future primary studies in South Africa should plan to incorporate infectious diseases (HIV and TB) into studies of multimorbidity where possible.
Despite few studies reporting on which disease clusters were largest, hypertension appeared to be the biggest contributor to the burden of multimorbidity, particularly the co-occurrence of hypertension with diabetes. That said, hypertension and diabetes were also among the most widely included conditions in studies of multimorbidity. Hence, these findings may be biased to conditions that are included in studies and not necessarily the reality of the situation. Given that the prevalence of hypertension is high in South Africa (44% of men and 46% of women aged 15 years and older, as high as 84% in people aged above 65 years),45 it does hold weight that it would be a common comorbid condition. A recent study on COVID-19 mortality in South Africa found the combination of hypertension and diabetes was a common disease cluster in people who had succumbed to the disease.46 This cluster of disease was more prevalent than having hypertension or diabetes only. Information on the prevalence of comorbidities and multimorbidities may prove very important in light of the COVID-19 pandemic.
We mainly included three types of studies in our analysis; studies based on the secondary data analysis of national surveys, studies based on community cohorts and studies based in health facilities. All three types of studies have strengths. National survey data can provide an overall picture of what is happening in the general population. However, they tend to use self-reported data which may result in an underestimation of the burden of disease; as a large percentage of NCDs are underdiagnosed. Nevertheless, there are many more national surveys that could be analysed to provide an overview of multimorbidity from these sources. Studies based on cohorts generated rich information, tended to have large sample sizes and had a mixture of self-report data and measure biological samples. These studies were mostly limited to rural areas. Whether multimorbidity is more common in rural or urban areas in South Africa remains unclear. Existing cohorts will continue to provide a good source of information on multimorbidity and we can expect more data to come out of planned urban cohorts.47 Studies based in health facilities tended to include more health conditions (both acute and chronic diseases) and tended to report higher levels of multimorbidity. This may be due to people who require healthcare (ill individuals) accessing these facilities. However, these studies provide an important source of information that is highly relevant to the management and planning for multimorbidities. For example, a recent study by Mannie and Kharrazi48 assessed the geographical distribution of comorbidities among 2.6 million commercially insured individuals in South Africa using a comorbidity index that highlighted healthcare utilisation. Using this score, they were able to identify areas of high utilisation and underserved individuals; although they did not provide detail on the types of services needed. Multimorbidity is known to increase the costs to healthcare systems.49
Prevalence estimates from systematic reviews can provide an important source of information that is used for evidence-based health decision making—especially in LMICs that have constrained health information systems. A multimorbidity prevalence systematic review conducted for South Asia highlighted the insufficient work conducted in the area of multimorbidity and called for greater methodological rigour to better build scientific evidence in this domain.50 In a similar vein, we also advocate for more studies to be conducted and with rigorous study designs. A recent report by the Academy of Science of South Africa,51 highlighted the problematic nature of multimorbidity research in sub-Saharan Africa as: funding provided for only specific diseases; lack of health system preparedness; and low prioritisation of multimorbidity due to a lack of political commitment to implement concomitant heath reforms. Research into multimorbidity is crucial for better understanding of the nature of the problem in the sub-Saharan African region, and to identify ways to introduce comprehensive health service delivery.51
This systematic review was limited in that it excluded studies conducted with subpopulations that had one specific disease (eg, multimorbidity in patients with cancer). While these studies are very important, their inclusion would require different search strategies. This study differed from the protocol in that it includes age groups of 15 years plus as the age 15 years is commonly reported as adults in population-based surveys.
Conclusion
To our knowledge, this is the first systematic review of multimorbidity prevalence for an African country and one of the few focused on an LMIC. This systematic review set out to determine the prevalence of multimorbidity of adults in South Africa, ideally stratified by age and sex. We found that there was a low number of studies focused on multimorbidity in South Africa. Studies with data available indicated many people aged 50 years and older are afflicted with more than one long-term disease condition. These findings are significant as they support the notion that multimorbidity is the norm and not an exception; which has strong implications for how healthcare is organised and utilised. These findings may also be reflective of the situation in other LMICs.
Our study indicated that a large component of multimorbidity was attributed to hypertension. While HIV did contribute to multimorbidity, NCDs were the most common source, even in environments with a high HIV prevalence. However, these results should be interpreted with caution as many studies focused only on older adults and did not give disease clusters using age breakdowns. Heterogeneity in studies also made it difficult to detect trends.
More studies are needed in the general population to determine which disease clusters are most prevalent and could potentially be targeted for intervention. Sources of secondary data could be further explored to answer this question. Studies at health facilities would help to provide information regarding multimorbidity’s effect on quality of life indicators, to assess whether people are receiving optimal treatment; and to identify the ways that multimorbidity might be impacting healthcare utilisation.
Supplementary Material
Acknowledgments
We would like to thank Dr Annibale Cois for statistical input, Ms Elizabeth Pienaar (from Cochrane South Africa at the South African Medical Research Council) for reviewing our search strategy and Dr Angela Y Chang for providing clarification regarding information of interest. We would also like to thank the reviewers for their helpful comments.
Footnotes
Contributors: RAR, VP-vW, BvW and EBT conceptualised the study. RAR and EBT conducted screening and data extraction. RAR wrote the first draft. All authors reviewed and gave input into subsequent drafts.
Funding: The work reported herein was made possible through funding by the Burden of Disease Research Unit at the South African Medical Research Council. RAR is funded through the South African Medical Research Council through its Division of Research Capacity Development under the Internship Scholarship Programme from funding received from the South African National Treasury. The content hereof is the sole responsibility of the authors and does not necessarily represent the official views of the South African Medical Research Council or the funders. Grant number: NA
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study was given ethics approval by the Biomedical Research Ethics Committee of the University of the Western Cape (BM20/5/8).
References
- 1.Nguyen H, Manolova G, Daskalopoulou C, et al. Prevalence of multimorbidity in community settings: a systematic review and meta-analysis of observational studies. J Comorb 2019;9:2235042X1987093–15. 10.1177/2235042X19870934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu X, Mishra GD, Jones M. Mapping the global research landscape and knowledge gaps on multimorbidity: a bibliometric study. J Glob Health 2017;7:010414. 10.7189/jogh.07.010414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . Multimorbidity: technical series on safer primary care; report No.: 9241511656. Geneva: World Health Organization, 2016. http://apps.who.int/iris/bitstream/10665/252275/1/9789241511650-eng.pdf [Google Scholar]
- 4.Wei MY, Mukamal KJ, Multimorbidity MKJ. Multimorbidity, mortality, and long-term physical functioning in 3 prospective cohorts of community-dwelling adults. Am J Epidemiol 2018;187:103–12. 10.1093/aje/kwx198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanesarajah J, Waller M, Whitty JA, et al. Multimorbidity and quality of life at mid-life: a systematic review of general population studies. Maturitas 2018;109:53–62. 10.1016/j.maturitas.2017.12.004 [DOI] [PubMed] [Google Scholar]
- 6.Calderón-Larrañaga A, Poblador-Plou B, González-Rubio F, et al. Multimorbidity, polypharmacy, referrals, and adverse drug events: are we doing things well? Br J Gen Pract 2012;62:e821–6. 10.3399/bjgp12X659295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sum G, Salisbury C, Koh GC-H, et al. Implications of multimorbidity patterns on health care utilisation and quality of life in middle-income countries: cross-sectional analysis. J Glob Health 2019;9:020413. 10.7189/jogh.09.020413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frølich A, Ghith N, Schiøtz M, et al. Multimorbidity, healthcare utilization and socioeconomic status: a register-based study in Denmark. PLoS One 2019;14:e0214183. 10.1371/journal.pone.0214183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maddaloni E, D’Onofrio L, Alessandri F, et al. Cardiometabolic multimorbidity is associated with a worse Covid-19 prognosis than individual cardiometabolic risk factors: a multicentre retrospective study (CoViDiab II). Cardiovasc Diabetol 2020;19:1–11. 10.1186/s12933-020-01140-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iaccarino G, Grassi G, Borghi C, et al. Age and multimorbidity predict death among COVID-19 patients: results of the SARS-RAS study of the Italian Society of hypertension. Hypertension 2020;76:366–72. 10.1161/HYPERTENSIONAHA.120.15324 [DOI] [PubMed] [Google Scholar]
- 11.The Lancet . Making more of multimorbidity: an emerging priority. Lancet 2018;391:1637. 10.1016/S0140-6736(18)30941-3 [DOI] [PubMed] [Google Scholar]
- 12.The Academy of Medical Sciences . Multimorbidity: a priority for global health research 2018, 2020. Available: https://acmedsci.ac.uk/file-download/82222577
- 13.Simbayi L, Zuma K, Moyo S. South African national HIV prevalence, incidence, behaviour and communication survey, 2017; report No.: 978-0-7969-2444-5. Cape Town: HSRC Press, 2019. https://www.hsrcpress.ac.za/books/south-african-national-hiv-prevalence-incidence-behaviour-and-communication-survey-2017 [Google Scholar]
- 14.Chang AY, Gómez-Olivé FX, Manne-Goehler J, et al. Multimorbidity and care for hypertension, diabetes and HIV among older adults in rural South Africa. Bull World Health Organ 2019;97:10–23. 10.2471/BLT.18.217000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nojilana B, Bradshaw D, Pillay-van Wyk V, et al. Emerging trends in non-communicable disease mortality in South Africa, 1997 - 2010. S Afr Med J 2016;106:477–84. 10.7196/SAMJ.2016.v106i5.10674 [DOI] [PubMed] [Google Scholar]
- 16.Mudie K, Jin MM, Kendall L, et al. Non-Communicable diseases in sub-Saharan Africa: a scoping review of large cohort studies. J Glob Health 2019;9:020409. 10.7189/jogh.09.020409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oni T, McGrath N, BeLue R, et al. Chronic diseases and multi-morbidity-a conceptual modification to the WHO ICCC model for countries in health transition. BMC Public Health 2014;14:575. 10.1186/1471-2458-14-575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahomed OH, Asmall S. Professional nurses' perceptions and experiences with the implementation of an integrated chronic care model at primary healthcare clinics in South Africa. Curationis 2017;40:1–6. 10.4102/curationis.v40i1.1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahomed OH, Asmall S. Development and implementation of an integrated chronic disease model in South Africa: lessons in the management of change through improving the quality of clinical practice. Int J Integr Care 2015;15:e038. 10.5334/ijic.1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Limbani F, Thorogood M, Gómez-Olivé FX, et al. Task shifting to improve the provision of integrated chronic care: realist evaluation of a lay health worker intervention in rural South Africa. BMJ Glob Health 2019;4:e001084. 10.1136/bmjgh-2018-001084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ameh S, Klipstein-Grobusch K, D'ambruoso L, et al. Quality of integrated chronic disease care in rural South Africa: user and provider perspectives. Health Policy Plan 2017;32:257–66. 10.1093/heapol/czw118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fortin M, Stewart M, Poitras M-E, et al. A systematic review of prevalence studies on multimorbidity: toward a more uniform methodology. Ann Fam Med 2012;10:142–51. 10.1370/afm.1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roomaney RA, van Wyk B, Turawa EB, et al. Prevalence of multimorbidity in South Africa: a systematic review protocol. BMJ Open 2020;10:e042889. 10.1136/bmjopen-2020-042889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The EndNote Team . Endnote. EndNote X8 ed. Philadelphia, PA: Clarivate, 2013. [Google Scholar]
- 26.Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan-a web and mobile APP for systematic reviews. Syst Rev 2016;5:210. 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roomaney RA, Awotiwon OF. Burden of disease review manager for systematic review of observational studies: technical report version 1. Cape Town: South African Medical Research Council, 2017. [Google Scholar]
- 28.Wells GA, Tugwell P, O’Connell D, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses, 2015. Available: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 29.Hoy D, Brooks P, Woolf A, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol 2012;65:934–9. 10.1016/j.jclinepi.2011.11.014 [DOI] [PubMed] [Google Scholar]
- 30.Pheiffer C, Pillay-van Wyk V, Joubert JD, et al. The prevalence of type 2 diabetes in South Africa: a systematic review protocol. BMJ Open 2018;8:e021029. 10.1136/bmjopen-2017-021029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rohatgi A. WebPlotDigitizer version: 4.3 2020.
- 32.Afshar S, Roderick PJ, Kowal P, et al. Multimorbidity and the inequalities of global ageing: a cross-sectional study of 28 countries using the world health surveys. BMC Public Health 2015;15:776. 10.1186/s12889-015-2008-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garin N, Koyanagi A, Chatterji S, et al. Global multimorbidity patterns: a cross-sectional, population-based, Multi-Country study. J Gerontol A Biol Sci Med Sci 2016;71:205–14. 10.1093/gerona/glv128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weimann A, Dai D, Oni T. A cross-sectional and spatial analysis of the prevalence of multimorbidity and its association with socioeconomic disadvantage in South Africa: a comparison between 2008 and 2012. Soc Sci Med 2016;163:144–56. 10.1016/j.socscimed.2016.06.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghose B, Abdoul Razak MY. Memory and learning complaints in relation to depression among elderly people with multimorbidity. Geriatrics 2017;2. 10.3390/geriatrics2020015. [Epub ahead of print: 09 May 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Heerden A, Barnabas RV, Norris SA, et al. High prevalence of HIV and non-communicable disease (Ncd) risk factors in rural KwaZulu-Natal, South Africa. J Int AIDS Soc 2017;20:e25012. 10.1002/jia2.25012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang AY, Gómez-Olivé FX, Payne C, et al. Chronic multimorbidity among older adults in rural South Africa. BMJ Glob Health 2019;4:e001386. 10.1136/bmjgh-2018-001386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharman M, Bachmann M. Prevalence and health effects of communicable and non-communicable disease comorbidity in rural KwaZulu-Natal, South Africa. Trop Med Int Health 2019;24:1198–207. 10.1111/tmi.13297 [DOI] [PubMed] [Google Scholar]
- 39.Lalkhen H, Mash R. Multimorbidity in non-communicable diseases in South African primary healthcare. S Afr Med J 2015;105:134–8. 10.7196/SAMJ.8696 [DOI] [PubMed] [Google Scholar]
- 40.Roche S, De Vries E. Multimorbidity in a large district Hospital: a descriptive cross-sectional study. S Afr Med J 2017;107:1110–5. 10.7196/SAMJ.2017.v107i12.12397 [DOI] [PubMed] [Google Scholar]
- 41.Oni T, Youngblood E, Boulle A, et al. Patterns of HIV, TB, and non-communicable disease multi-morbidity in peri-urban South Africa- a cross sectional study. BMC Infect Dis 2015;15:20. 10.1186/s12879-015-0750-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weimann A, Dai D, Oni T. A cross-sectional and spatial analysis of the prevalence of multimorbidity and its association with socioeconomic disadvantage in South Africa: a comparison between 2008 and 2012. Soc Sci Med 2016;163:144–56. 10.1016/j.socscimed.2016.06.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alaba O, Chola L. The social determinants of multimorbidity in South Africa. Int J Equity Health 2013;12:1. 10.1186/1475-9276-12-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Griffith LE, Gruneir A, Fisher KA, et al. Key factors to consider when measuring multimorbidity: results from an expert panel and online survey. J Comorb 2018;8:2235042x18795306. 10.1177/2235042X18795306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.National Department of Health, Statistics South Africa, South African Medical Research Council, and ICF . South Africa demographic and health survey 2016. Pretoria, South Africa, and Rockville, Maryland, USA: NDoH, Stats SA, SAMRC, and ICF, 2019. https://dhsprogram.com/pubs/pdf/FR337/FR337.pdf [Google Scholar]
- 46.Pillay-van Wyk V, Bradshaw D, Groenewald P, et al. COVID deaths in South Africa: 99 days since South Africa's first death. S Afr Med J 2020;110:1093–9. [PubMed] [Google Scholar]
- 47.South African Medical Research Council, Department of Science and Innovation . South African population research infrastructure network launches two new urban nodes to expand to a nationwide network and improve response to COVID-19 and other epidemics, 2020. Available: http://saprin.mrc.ac.za/1press2020.html
- 48.Mannie C, Kharrazi H. Assessing the geographical distribution of comorbidity among commercially insured individuals in South Africa. BMC Public Health 2020;20:1709. 10.1186/s12889-020-09771-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sum G, Hone T, Atun R, et al. Multimorbidity and out-of-pocket expenditure on medicines: a systematic review. BMJ Glob Health 2018;3:e000505. 10.1136/bmjgh-2017-000505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pati S, Swain S, Hussain MA, et al. Prevalence and outcomes of multimorbidity in South Asia: a systematic review. BMJ Open 2015;5:e007235. 10.1136/bmjopen-2014-007235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Academy of Science of South Africa . Improving the prevention and management of multimorbidity in sub-Saharan Africa, 2020. Available: https://research.assaf.org.za/bitstream/handle/20.500.11911/139/2020_assaf_ams_multimorbidity_subsaharan_africa.pdf?sequence=1&isAllowed=y
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-048676supp001.pdf (167.7KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information.