Abstract
Introduction
The emergence of heated tobacco products (HTPs) in the US marks a critical time for identifying those most likely to use, particularly among young adults.
Aims and Methods
We analyzed Fall 2019 data from a longitudinal study of young adults (ages 18–34; n = 2375, Mage=24.66±4.68) in 6 US cities, 24.1% of whom used cigarettes and 32.7% e-cigarettes. We assessed HTP awareness, use, and sources, as well as perceived risk, social acceptability, and the likelihood of future use.
Results
In this sample, 9.7% (n = 230) heard of HTPs, 3.5% (n = 84) ever used them, and 2.4% (n = 56) reported past-year purchases (tobacco shops, 66.1%; traditional retailers, 60.7%; online, 39.3%; IQOS specialty stores, 35.7%). In multivariable analyses, having heard of HTPs correlated with being an older, male, and current cigarette and e-cigarette users; among those ever hearing of them, using HTPs correlated with being non-Hispanic and current cigarette and e-cigarette users. Greater likelihood of future use correlated with being older, male, sexual minority, non-Hispanic, and current cigarette and e-cigarette users. Among past-month users (n = 78), the average number of days used was 5.48 (SD = 5.54). Past-month cigarette and e-cigarette users, respectively, who tried HTPs were more likely to report consistent or more frequent use of their respective products than a year ago (p < .001). HTPs were perceived as less addictive than cigarettes, smokeless tobacco, and e-cigarettes, and less harmful and more socially acceptable than other tobacco products except for e-cigarettes and hookah.
Conclusions
The relatively positive perceptions of HTPs and access via various channels underscores the potential penetration of HTPs among US young adults.
Implications
In Fall 2019, as IQOS was launching in the US, there were relatively low rates of awareness, use, and use intentions in this sample of young adults with high proportions of other tobacco use. However, this sample reported relatively positive perceptions of HTPs with regard to potential addiction and harm, as well as social acceptability. They also reported accessing HTPs via various channels, underscoring how pervasive the availability to HTPs already has become and may increasingly become. Moreover, certain subgroups (ie, other tobacco users, men) are particularly likely to use HTPs.
Introduction
Heated tobacco products (HTPs) are marketed as a less harmful alternative to combustible cigarettes.1,2 HTPs are currently sold in >57 countries, and awareness and use of HTPs has grown recently, with greater use among current smokers, men, and racial/ethnic minorities in various contexts/countries.3–7
Among the HTPs relevant to the US market are: (1) RJ Reynolds’ Eclipse, which was granted US Food and Drug Administration’s (FDA) substantial equivalence approval in 2018,8 and (2) Philip Morris International’s (PMI) IQOS, which has the largest global market share9 and launched in 2019 after receiving FDA authorization in 2019. In July 2020, FDA approved IQOS as a “reduced exposure” product despite insufficient evidence that IQOS reduces harm or risk of tobacco‐related disease.1,10,11
During HTPs early expansion in the US market, it is critical to examine who is most likely to try or use HTPs, especially among young people. Applications to the FDA must include data regarding consumer perceptions (eg, health harms, addictiveness); however, there is limited evidence that young adults are not enticed by HTPs.12,13 Thus, we examined HTP awareness, use, sources, perceived risk and social acceptability, and the likelihood of future use among young adults (ages 18–34) across 6 US metropolitan statistical areas (MSAs).
Methods
Study Design
We analyzed data from 3006 young adults (aged 18–34) participating in a 2-year, 5-wave longitudinal study, the Vape shop Advertising, Place characteristics, and Effects Surveillance (VAPES) study. VAPES examines vape retail and its impact across 6 MSAs (Atlanta, Boston, Minneapolis, Oklahoma City, San Diego, Seattle) that vary in tobacco control. This study was approved by the Emory University Institutional Review Board.14
Participants and Recruitment
Participants were recruited via social media in Fall 2018. Eligibility criteria were: (1) ages 18–34; (2) residing in the six MSAs; and (3) English-speaking. Purposive, quota-based sampling was used to ensure sufficient representation of e-cigarette and cigarette users (~1/3 each), sexes, and racial/ethnic minorities. Advertisements posted on Facebook and Reddit targeted individuals of the eligible age range and MSAs (eg, targeting followers of sports/athletics, entertainment, technology, or tobacco-related interest pages/groups; using images of diverse young adults in various settings).
Individuals who clicked on ads were directed to a webpage with a study description, consent form, and eligibility screener. Subgroup enrollment was capped by MSA. Overall, 65 843 Facebook/Reddit users viewed study ads, 10 433 clicked ads, and 9847 consented. Of the 9874, 2751 were not allowed to advance to the baseline survey (1427 ineligible; 1279 excluded to reach recruitment targets). Of the 7096 allowed to advance to the baseline survey, 3460 (48.8%) completed the full survey, and 3636 (51.2%) partially completed it (not enrolled). Participants were required to confirm their participation 7-days postbaseline by clicking a “confirm” button included in an email, after which they would be enrolled and emailed their first incentive ($10 e-gift card). Participation was confirmed at a 7-day follow-up among 3006 (86.9%). This study uses data from Wave 3 (Fall 2019), which included 2375 participants with complete data (79.0% of the baseline sample).14
Measures
Sociodemographics
Participants reported their age, sex, sexual orientation, race, and ethnicity.
Tobacco Use
Participants reported past 30-day (current) use of cigarettes, e-cigarettes, large cigars, little cigars/cigarillos, smokeless tobacco (SLT), and hookah/waterpipe (operationalized as dichotomous variables). Current cigarette and e-cigarette users, were asked, “Compared to a year ago, do you use [product] less, more, or about the same?”
HTP Awareness and Use
Participants were instructed, “The following questions are aimed at learning more about your awareness of, interest in, and use of a new category of tobacco products, sometimes referred to as heat-not-burn tobacco, which heat sticks of tobacco instead of burning it. Two common brands are IQOS and Eclipse. A picture of the IQOS product is shown below.” We then asked: (1) “Have you heard of heat-not-burn products, like IQOS or Eclipse?” (2) “In your lifetime, have you ever tried a heat-not-burn product?” and (3) “In the past 12 months, have you purchased an IQOS or Eclipse?” with response options of: No; Yes, IQOS only; Yes, Eclipse only; or Yes, both IQOS and Eclipse. If they reported a purchase, we asked, “Where did you purchase your product? IQOS specialty store; vape shop; online via a Philip Morris, IQOS, Altria, RJ Reynolds, or Eclipse website; online via a vendor not connected to Philip Morris, IQOS, Altria, RJ Reynolds, or Eclipse; gas station; convenience store; grocery store; pharmacy; tobacco specialty store (eg, smoke shop); liquor store; and other.” We also asked, “How many days of the past 6 months did you use a heat-not-burn product?” and, among those reporting past 6-month use, “How many days of the past 30 days did you use a heat-not-burn product?”
Tobacco Product Perceptions and Likelihood of Future Use
Participants were asked to rate HTPs, cigarettes, e-cigarettes, large cigars, little cigars/cigarillos, SLT, and hookah on a scale of 1 = not at all to 7 = extremely with regard to (1) “How addictive do you think the following products are?” (2) “How harmful to your health do you think the following products are?” (3) “How harmful to your health do you think breathing in the smoke or vapor of the following products is?” (4) “How socially acceptable among your peers do you think the following products are?” and (5) “How likely are you to try or continue to use the following in the next year?”
Data Analysis
Participants were characterized using descriptive statistics; key outcomes of awareness, use, and the likelihood of future use were examined using bivariate and multivariate analyses (including sociodemographics, cigarette/e-cigarette use), using SPSS version 2643 and alpha set at .05.
Results
Table 1 provides participant sociodemographic and tobacco use characteristics, as well as bivariate results examining those who had (vs. not) heard of HTPs and, among those who had, those who had tried (vs. not) HTPs. In this sample, 9.7% (n = 230) heard of IQOS (4.0%, n = 94), Eclipse (2.2%, n = 52), or both (3.5%, n = 84); 3.5% (n = 84) ever used IQOS (0.8%, n = 19), Eclipse (1.5%, n = 35), or both (1.3%, n = 30). In multivariate analyses, having heard of HTPs correlated with being older (OR = 1.10, CI:1.07–1.13), male (OR = 2.05, CI:1.53–2.76), and current users of cigarettes (OR = 1.85, CI:1.34–2.54) and e-cigarettes (OR = 2.54, CI:1.86–3.49; p < .001; Nagelkerke R-square = 0.144); among those ever hearing of HTPs, ever use correlated with being non-Hispanic (OR = 3.57, CI:1.51–8.33) and current users of cigarettes (OR = 4.96, CI:2.53–9.76) and e-cigarettes (OR = 2.44, CI:1.18–5.07; p < .01; Nagelkerke R-square = 0.327). Among past 6-month users (n = 82), 13 (0.5%) used on at least half of the days; among past-month users (n = 78), the average number of days used was 5.48 (SD = 5.54), with 90.4% using ≤10 days.
Table 1.
Participant Characteristics, N = 2375
| Heard of HTPs | Ever Used HTPs | ||||||
|---|---|---|---|---|---|---|---|
| N = 2375 | No N = 2145 (90.3%) | Yes N = 230 (9.7%) | No N = 146 (63.5%) | Yes N = 84 (36.5%) | |||
| Variable | n (%) or M (SD) | n (%) or M (SD) | n (%) or M (SD) | p | n (%) or M (SD) | n (%) or M (SD) | p |
| MSA, N (%) | .822 | .071 | |||||
| Atlanta | 501 (21.1) | 449 (20.9) | 52 (22.6) | 39 (26.7) | 13 (15.5) | ||
| Boston | 489 (20.6) | 440 (20.5) | 49 (21.3) | 25 (17.1) | 24 (28.6) | ||
| Minneapolis-St. Paul | 422 (17.8) | 388 (18.1) | 34 (14.8) | 25 (17.1) | 9 (10.7) | ||
| Oklahoma City | 237 (10.0) | 216 (10.1) | 21 (9.1) | 15 (10.3) | 6 (7.1) | ||
| San Diego | 376 (15.8) | 336 (14.7) | 40 (17.4) | 24 (16.4) | 16 (19.0) | ||
| Seattle | 350 (14.7) | 316 (14.7) | 34 (14.8) | 18 (12.3) | 16 (19.0) | ||
| Sociodemographics | |||||||
| Age, M (SD) | 24.66 (4.68) | 24.45 (4.64) | 26.62 (4.49) | <.001 | 26.60 (4.67) | 26.68 (4.47) | .911 |
| Male, N (%)* | 980 (41.3) | 841 (40.3) | 139 (61.0) | <.001 | 85 (59.0) | 54 (64.3) | .260 |
| Sexual minority, N (%) | 741 (31.2) | 688 (32.1) | 53 (23.0) | .005 | 37 (25.3) | 16 (19.0) | .177 |
| Race, N (%) | .132 | .128 | |||||
| White | 1705 (71.8) | 1547 (72.1) | 158 (68.7) | 105 (71.9) | 53 (63.1) | ||
| Black | 122 (5.1) | 104 (4.8) | 18 (7.8) | 9 (6.2) | 9 (10.7) | ||
| Asian | 299 (12.6) | 274 (12.8) | 25 (10.9) | 18 (12.3) | 7 (8.3) | ||
| Other | 249 (10.5) | 220 (10.3) | 29 (12.6) | 14 (9.6) | 15 (17.9) | ||
| Hispanic, N (%) | 268 (11.3) | 231 (10.8) | 37 (16.1) | .015 | 14 (9.6) | 23 (27.4) | <.001 |
| Past 30-day use, N (%) | |||||||
| Cigarettes | 572 (24.1) | 466 (21.7) | 106 (46.1) | <.001 | 43 (29.5) | 63 (75.0) | <.001 |
| E-cigarettes | 776 (32.7) | 647 (30.2) | 129 (56.1) | <.001 | 65 (44.5) | 64 (76.2) | <.001 |
| Large cigars | 149 (6.3) | 118 (5.5) | 31 (13.5) | <.001 | 8 (5.5) | 23 (27.4) | <.001 |
| Little cigars/cigarillos | 194 (8.2) | 155 (7.2) | 39 (17.0) | <.001 | 18 (12.3) | 21 (25.0) | .012 |
| Smokeless tobacco | 69 (2.9) | 44 (2.1) | 25 (10.9) | <.001 | 9 (6.2) | 16 (19.0) | .003 |
| Hookah | 200 (8.4) | 157 (7.3) | 43 (18.7) | <.001 | 22 (15.1) | 21 (25.0) | .047 |
Italic p-values indicate p < .05.
*59 indicated other sex.
Among past-month cigarette smokers, those ever trying HTPs (vs. not) were more likely to report using more (14.3% vs. 11.8%) or about the same (68.3% vs. 32.6%) compared to a year ago and less likely to report using less (17.5% vs. 48.5%, p < .001). Among past-month e-cigarette users, those ever trying HTPs (vs. not) were more likely to report using more (20.3% vs. 19.1%) or about the same (59.4% vs. 38.3%) compared to a year ago and less likely to report they used less (20.3% vs. 34.7%, p < .001).
Of the 2.4% (n = 56) who purchased HTPs in the past 12 months (IQOS, n = 25; Eclipse, n = 13; both, n = 18), the largest proportion purchased from tobacco specialty stores (66.1%, n = 37, including vape shops [n = 31] and smoke/head shops [n = 6]), followed by: traditional brick-and-mortar retailers (60.7%, n = 34, including gas stations, grocery stores [n = 11, respectively], convenience stores [n = 7], liquor stores [n = 3], and pharmacies [n = 2]); online (39.3%, n = 22 via industry websites or via websites not connected to the industry [n = 11, respectively]); and IQOS specialty stores (35.7%, n = 20).
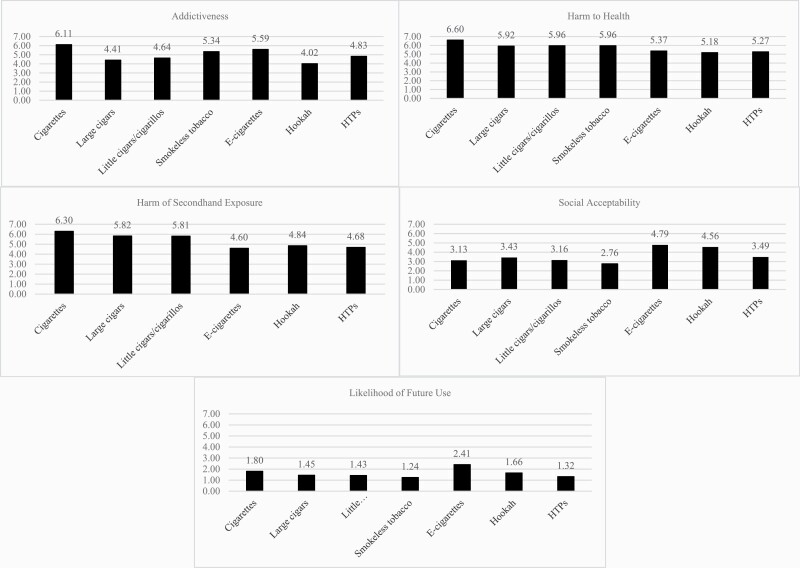
HTPs were perceived as more addictive than cigars, little cigars/cigarillos, and hookah but less addictive than cigarettes, SLT, and e-cigarettes (Figure 1). HTPs were perceived as more harmful to health than hookah but less harmful than all other products. Secondhand exposure to HTPs was perceived as more harmful than e-cigarettes but less harmful than all other tobacco products. HTPs were perceived as more socially acceptable than cigarettes, cigars, little cigars/cigarillos, and SLT but less acceptable than e-cigarettes and hookah. HTPs were rated as less likely to be used in the next year compared to all products except SLT. Greater likelihood of future use was associated with being older (B = 0.02, CI:0.08–0.24), male (B = 0.12, CI: 0.05–0.20), sexual minority (B = 0.09, CI: 0.01–0.17), non-Hispanic (B = 0.44, CI: 0.32–0.56), and current users of cigarettes (B = 0.64, CI: 0.54–0.73) and e-cigarettes (B = 0.25, CI: 0.16–0.34; p < .001; Adjusted R-square = 0.158).
Figure 1.
Perceptions and likelihood of future use of HTPs versus other tobacco products (ave. ratings, “1=not at all” to “7=extremely”).
Discussion
Among these young adults with high other tobacco use rates, only 10% had heard of HTPs. Among those who had, ~40% ever used them, but very few were ongoing and/or frequent users. In addition, self-reported likelihood of future use was low in general—lower than all other tobacco products with the exception of SLT. Moreover, fewer cigarette and e-cigarette users who had tried them reported reducing their own product use behaviors in the past year, which is particularly concerning given that ~10% of current cigarette and e-cigarette users had used HTPs.
Findings regarding those more likely to be aware of and use HTPs coincide with prior research documenting that, among US young adults ages 18–30, being older, male, racial/ethnic minorities, and other tobacco users (particularly cigarette and e-cigarette users) correlated with greater curiosity, interest, and likelihood to use IQOS.15 This prior study15 also documented that greater perceived risks of IQOS were negatively associated with curiosity, interest, and likelihood of use. Research among adults in Canada16 indicated that IQOS was perceived as less harmful than cigarettes among 48% and as less harmful than e-cigarettes among 23%; also noteworthy is that 54% perceived IQOS as equally or more harmful than e-cigarettes. Current findings provide a broader perspective regarding how participants perceive HTPs across different dimensions of risk (ie, addictiveness, health harm, secondhand exposure) and within the broader tobacco landscape. We found that HTPs were perceived as more addictive than cigars, little cigars/cigarillos, and hookah; more harmful to one’s health than hookah; and more harmful to other’s health than e-cigarettes. On the other hand, HTPs were perceived as less addictive than cigarettes, SLT, and e-cigarettes; less harmful to one’s health than all other products except hookah; and less harmful to other’s health than all tobacco products except e-cigarettes. Moreover, HTPs were perceived as more socially acceptable than cigarettes, cigars, little cigars/cigarillos, and SLT but less acceptable than e-cigarettes and hookah. Findings might suggest that, based on perceived risk and evaluations of social acceptability, HTPs could have substantial penetration in the US young adult market, despite the self-reported low likelihood of future use. This is concerning given that as IQOS was launching in the US, Philip Morris already used covert marketing strategies that implied the FDA endorsed its product, violated FDA tobacco product regulations, and circumvented the terms of the media channel it advertised on.17
Low likelihood of future use may be an indicator of limited HTP availability, as IQOS was just launching in the US at the time of data collection. However, there were no differences in awareness, use, interest, and purchase history across cities, even controlling for tobacco use (data not shown). Notably, sources of IQOS were diverse, including tobacco specialty shops (particularly vape shops), as well as traditional brick-and-mortar retailers (eg, convenience stores), online, and in IQOS specialty stores. Findings highlight the various channels used by Philip Morris to penetrate the US market, underscoring concerns about underage access given the low rates of age verification compliance among tobacco specialty stores18,19 and online.20
Limitations
Limitations include generalizability to other young adults in these MSAs or the US more broadly. Rates of tobacco and HTP use should not be interpreted as use prevalence rates, given the purposive sampling design used. In addition, cross-sectional data precludes determining the directionality of these associations, and low rates of HTP use/awareness limited power for subgroup analyses.
Conclusion
Despite relatively low rates of awareness, use, and use intentions in Fall 2019 as IQOS launched in the US, participants reported relatively positive perceptions of HTPs regarding addiction, harm, and social acceptability. They also accessed HTPs via various channels, underscoring their pervasive availability and likely increasing availability. Moreover, certain subgroups (ie, other tobacco users, men) are particularly likely to use HTPs.
Supplementary Material
A Contributorship Form detailing each author’s specific involvement with this content, as well as any supplementary data, are available online at https://academic.oup.com/ntr.
Funding
This publication was supported by the US National Cancer Institute (R01CA215155-01A1; PI: Berg). Dr. Berg is also supported by other US National Cancer Institute funding (R01CA179422-01; PI: Berg; R01CA239178-01A1; MPIs: Berg, Levine), the US National Institutes of Health/Fogarty International Center (1R01TW010664-01; MPIs: Berg, Kegler), and the US National Institute of Environmental Health Sciences/Fogarty International Center (D43ES030927-01; MPIs: Berg, Marsit, Sturua).
Declaration of Interests
The authors declare no conflicts of interests.
References
- 1. St Helen G, Jacob Iii P, Nardone N, Benowitz NL. IQOS: examination of Philip Morris International’s claim of reduced exposure. Tob Control. 2018;27(Suppl 1):s30–s36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jackler RK, Ramamurthi D, Axelrod A, et al. Global Marketing of IQOS The Philip Morris Campaign to Popularize “Heat Not Burn” Tobacco. Palo Alto, CA: Stanford University School of Medicine; February 21, 2020. 2020. [Google Scholar]
- 3. Nyman AL, Weaver SR, Popova L, et al. Awareness and use of heated tobacco products among US adults, 2016–2017. Tob Control. 2018;27(Suppl 1):s55–s61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sutanto E, Miller C, Smith DM, et al. Prevalence, use behaviors, and preferences among users of heated tobacco products: findings from the 2018 ITC Japan Survey. Int J Environ Res Public Health. 2019;16(23):4630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu X, Lugo A, Spizzichino L, Tabuchi T, Pacifici R, Gallus S. Heat-not-burn tobacco products: concerns from the Italian experience. Tob Control. 2019;28(1):113–114. [DOI] [PubMed] [Google Scholar]
- 6. Czoli CD, White CM, Reid JL, OConnor RJ, Hammond D. Awareness and interest in IQOS heated tobacco products among youth in Canada, England and the USA. Tob Control. 2020;29(1):89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ratajczak A, Jankowski P, Strus P, Feleszko W. Heat not burn tobacco product—a new global trend: impact of heat-not-burn tobacco products on public health, a systematic review. Int J Environ Res Public Health. 2020;17(2):409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. US Food and Drug Administration. FDA Submission Tracking Number (STN): SE0006177.2018. Available at: https://www.fda.gov/media/114729/download
- 9. Berg CJ, Bar-Zeev Y, Levine H. Informing iQOS regulations in the United States: a synthesis of what we know. SAGE Open. 2020;10(1):2158244019898823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. US Food and Drug Administration. Scientific Review of Modified Risk Tobacco Product Application (MRTPA) Under Section 911(d) of the FD&C Act. 07/07/2020 2020. Available at: https://www.fda.gov/tobacco-products/rules-regulations-and-guidance/section-911-federal-food-drug-and-cosmetic-act-modified-risk-tobacco-products
- 11. Moazed F, Chun L, Matthay MA, Calfee CS, Gotts J. Assessment of industry data on pulmonary and immunosuppressive effects of IQOS. Tob Control. 2018;27(Suppl 1):s20–s25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lempert LK, Glantz S. Analysis of FDA’s IQOS marketing authorisation and its policy impacts. Tob Control. 2020. doi: 10.1136/tobaccocontrol-2019-055585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McKelvey K, Popova L, Kim M, et al. Heated tobacco products likely appeal to adolescents and young adults. Tob Control. 2018;27(Suppl 1):s41–s47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Berg CJ, Duan X, Getachew B, et al. Young adult e-cigarette use and retail exposure in 6 US metropolitan areas. Tob Reg Sci. 2020;7(1):59–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Phan L, Strasser AA, Johnson AC, et al. Young adult correlates of IQOS curiosity, interest, and likelihood of use. Tob Reg Sci. 2020;6(2):81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sutanto E, Miller CR, Smith DM, et al. Perceived relative harm of heated tobacco products (IQOS), e-cigarettes, and cigarettes among adults in Canada: Findings from the ITC Project. Tob Induc Dis. 2020;18:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Leas EC, Cohen JE, Ayers JW. A Philip Morris advertisement for its heated tobacco product IQOS sets a troubling precedent. Tob Control. 2020. doi: 10.1136/tobaccocontrol-2019-055363. [DOI] [PubMed] [Google Scholar]
- 18. Roeseler A, Vuong TD, Henriksen L, Zhang X. Assessment of underage sales violations in tobacco stores and vape shops. JAMA Pediatr. 2019;173(8):795–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Berg CJ, Barker DC, Meyers C, et al. Exploring the point-of-sale among vape shops across the US: audits integrating a mystery shopper approach. Nicotine Tob Res. 2021;23(3):495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Williams RS, Derrick J, Liebman AK, LaFleur K, Ribisl KM. Content analysis of age verification, purchase and delivery methods of internet e-cigarette vendors, 2013 and 2014. Tob Control. 2018;27(3):287–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.