Abstract
Schwann cell precursors (SCPs) are a transient population in the embryo, closely associated with nerves along which they migrate into the periphery of the body. Long considered to be progenitors that only form Schwann cells--the myelinating cells of nerves, current evidence suggests that SCPs have much broader developmental potential. Indeed, different cell marking techniques employed over the past 20 years have identified multiple novel SCP derivatives throughout the body. It is now clear that SCPs represent a multipotent progenitor population, which also display a level of plasticity in response to injury. Moreover, they originate from multiple origins in the embryo and may reflect several distinct subpopulations in terms of molecular identity and fate. Here we review SCP origins, derivatives and plasticity in development, growth and repair.
Keywords: Schwann cell precursor, peripheral nervous system, cell lineage, neural crest
1. Introduction
Schwann cell precursors (SCPs) are glial progenitors, closely associated with developing nerves of the peripheral nervous system along which they migrate, sometimes long distances, throughout the body. SCPs are derived from neural crest cells that emigrate from the neural tube and migrate into the periphery. Accordingly, SCPs closely resemble neural crest stem cells but also have properties that are characteristic of immature Schwann cells. For this reason, dogma had it that these precursors were fated to differentiate into mature Schwann cells, the myelinating glia that wrap peripheral axons thus enabling saltatory conduction as well as protection of peripheral nerves. More recently however, in addition to both myelinating and non-myelinating Schwann cells, SCPs have been shown to give rise to a diverse range of other cell types.
The first demonstration that SCPs could form non-glial cell types came from cell lineage labelling experiments that demonstrated that they gave rise to endoneurial fibroblasts in the nerve bundle [1]. Since then, SCPs have been shown to give rise to a variety of cell types throughout the body including melanocytes [2], parasympathetic neurons [3, 4], tooth pulp cells and odontoblasts [5], enteric neurons [6, 7], chromaffin cells [8, 9], chondrocytes and osteoblasts [10], Thus, SCPs, like the neural crest cells, have a broad developmental potential.
Indeed, SCPs have even been referred to as a continuation of the multipotent neural crest cell population from which they originate [11] and have sometimes been called nerve associated ‘neural crest stem cells’. It has also been proposed that subpopulations with different properties exist for SCPs. However, the changes that occur during the course of SCP cell lineage segregation are not fully understood. Moreover, there are several documented yet contested origins for SCPs in the embryo, adding further complexity to the understanding of SCP cell lineages. Here we review current thinking about the origins of this fascinating cell type, consider the function of cells at different stages of the SCP development and discuss the diversity and plasticity of this lineage in development, growth and repair.
2. Origin of Schwann cell precursors
In mice, SCPs are first apparent from E12-13. Behaviourally, onset of SCP identity is inferred by the arrival of a cell at and in close association with axons. SCPs form sheet-like processes that connect to other SCPs via adherens junctions and together envelope axons, along which they then migrate to more distal destinations [12]. SCPs are also closely associated with growth cones as first shown for growth cone advance in the developing limb mesenchyme [13]. Molecularly, SCPs and their neural crest progenitors share a number of features but can be distinguished by the gain and loss of expression of a number of genes [14, 15]. For most genes whose expression increases in SCPs, expression also persists in the cell lineage later into development (e.g. the myelin protein 0, also associated with immature Schwann cells) [16], while for other genes, expression is unique to SCPs (e.g. Cadherin 19) [17]. Another feature specific to SCPs when compared to their neural crest progeny is their dependence on axonal signals. Degeneration of axons has been shown to cause death of mouse SCPs in vivo [18] while culture of rat SCPs in the absence of axons, results in SCP apoptosis [19]. In both cases, application of the axonal signal neuregulin 1 (NRG1), prevents death of SCPs in the absence of axons [18, 20]. Furthermore, in mouse homozygous neuregulin mutants (−/−), numbers of SCPs in the trunk were found to be severely reduced [21]. In rat, NRG1 has also been shown to be required for SCP proliferation [20], and inhibition of neurogenesis [22], thus indirectly increasing the glial population size. Collectively this illustrates the dependence of SCPs on NRG1 signalling from axons for survival and proliferation. In turn, SCPs (and their derivatives, immature Schwann cells) appear to play a role in survival of motor and sensory neurons which in their absence, undergo eventual cell death, as shown in mouse [23, 24]. Therefore, for a period of development, neurons and glia are dependent upon one another for survival.
During the 20th century, there was debate around possible origins of sheath cells in the periphery of the embryo and whether they were derived from the neural crest, neural tube or mesoderm. While a mesodermal origin was ruled out early on and there is general agreement that Schwann cells arise from the neural crest [25, 26], an additional potential contribution from the neuroepithelium has remained controversial [27–30]. Furthermore, within the neural crest derived cell population, SCPs are derived from at least two separate subpopulations, directly from the neural crest in the initial wave of migration, and indirectly via the boundary cap cells in what has been termed the ‘secondary wave’ of migration [30] (overview in Figure 1).
Figure 1: Embryonic origins of Schwann cell precursors.
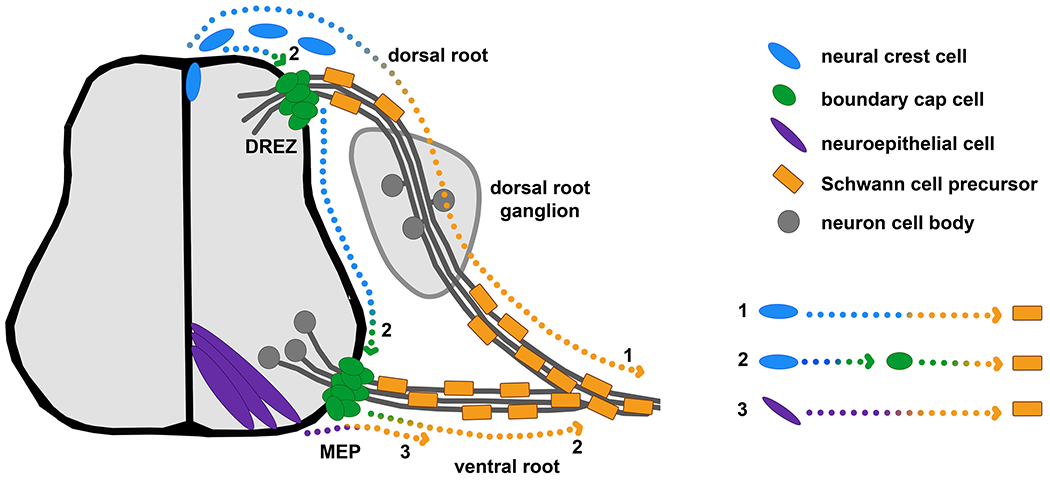
Transverse cross-section through the neural tube showing three pathways giving rise to Schwann cell precursors (orange) that have been discussed in the literature: 1. Neural crest cells (blue) migrate from the dorsal neural tube and give rise to Schwann cell precursors along the dorsal root along which they migrate into the periphery. 2. Neural crest cells (blue) migrate to the site of the future dorsal root entry zone (DREZ) or future motor exit point (MEP) where they give rise to boundary cap cells (green). These boundary cap cells then give rise to Schwann cell precursors along the dorsal and ventral roots. 3. The neuroepithelium (purple) is a currently contested source of Schwann cell precursors along the ventral and possibly dorsal roots. Arrows show direction of migration.
2.1. Neural crest origin of Schwann cell precursors
Neural crest cells originate from the dorsal portion of the neural tube and then undergo an epithelial to mesenchymal transition to initiate migration into the periphery. They follow several different migratory pathways to give rise to a diverse set of peripheral nervous system derivatives (neurons and glial cells), mesectodermal derivatives (skeletal, connective tissue and muscle) and other cell types including endocrine and para-endocrine cells and melanocytes [31]. Some of these derivatives differ along the rostro-caudal axis, while others are shared by all neural crest populations. For example, only cranial neural crest cells contribute to bone and cartilage of the face whereas trunk neural crest cells in vivo lack cartilage-forming ability. Similarly, cardiac neural crest cells appear to have the unique ability to contribute to the cardiovascular system. On the other hand, neural crest cells at all axial levels form melanocytes, glial cells and some type of neuron.
At the single cell level, many neural crest precursors appear to be multipotent but others may have a restricted ability with respect to the cell types into which they can differentiate. Analysis of clones of individual neural crest cells in culture [32, 33] or in vivo [34, 35] show that many individual neural crest cells can give rise to a wide range of different cell types. However, others contribute to single derivatives. Schwann cells have been suggested to derive from both multipotent and restricted neural crest progenitors. For example, some in vitro clones of quail mesencephalic neural crest differentiate into homogenous populations of Schwann cells; however, the majority of clones contain mixed populations of Schwann cells alongside neurons, melanocytes and/or cartilage [32].
2.2. Boundary cap cells are similar to SCPs
Boundary cap cells originate from the neural crest, migrating ventrally to the future nerve exit points of the neural tube, as demonstrated by following GFP labelled neural crest in the chick hindbrain [36]. In the rat it was demonstrated that neural crest associated glycoprotein Po and transcription factor Krox20 were present at the dorsal root entry zones along the trunk, also supporting a neural crest origin of boundary cap cells at these more caudal axial levels [37]. Once at the entry/exit zones, boundary cap cells form discrete clusters 3-5 cells deep [37]. Their arrival at these sites prior to the formation of the entry/exit points led to the suggestion that boundary cap cells might be involved in positioning these future entry/exit points.
Ablation of boundary cap cells via surgical or genetic techniques (using chick and mouse models respectively) revealed that positioning of motor exit points was unaffected [38]. Furthermore, using the chick for transplantation of trunk or midbrain-level pre-migratory neural crest into hindbrain did not affect positioning of hindbrain level root entry/exit points. As trunk and midbrain have separate dorsal and ventral entry/exit points whereas hindbrain has a common sensory and motor axons exit point, this suggests that boundary cap cells do not play a role in positioning of these sites [36]. However, in the absence of boundary caps, cell bodies of motor neurons were found to leave the central nervous system (CNS) and invade the periphery via motor exit points (which they normally do not do), and undergo cell death shortly afterwards [38]. This suggests a role for boundary cap cells as a ‘boundary’ between the CNS and the peripheral nervous system (PNS), allowing axons, but not cell bodies, to pass through. A number of molecular markers found to be associated with boundary cap cells have been used to identify their progeny. One such gene is Krox20, expressed at both dorsal and ventral exit/entry sites (Egr2 in mouse). Using a cre-mediated recombination line with EYFP or LacZ to follow Krox20 positive cells and their progeny in the mouse [30], it was reported that the majority of glia in the dorsal and ventral root are derived from these Krox20 positive boundary cap cells. Based on their close association with axons, their morphology and expression of the PNS glial marker ErbB3, these glia are likely to be Schwann cell precursors [30].
The boundary caps therefore appear to give rise to the majority of SCPs along the proximal parts of the dorsal and ventral roots. In contrast, SCPs derived directly from the neural crest contribute to more distal regions as demonstrated by the boundary cap ablation experiment, where SCPs are found only at more distal parts of the ventral root and peripheral nerve [38].
2.3. Neural tube origin for some Schwann cells?
A third, yet contested, cellular origin for glial cells is the neural tube itself. Support for this possibility comes from DiI labelling of the neural tube in chick spinal cord after the completion of neural crest emigration (HH24/5), which results in labelling of the dorsal root and dorsal root ganglia [27]. These cells were shown to exit the neural tube specifically at the level of the dorsal root entry zone. Furthermore, similar fates were observed following quail-into-chick grafts of the dorsal neural tube [27]. In contrast to these findings, electroporation of a GFP expressing plasmid into the neural tube of chick hindbrain between stages HH23-26 resulted in no GFP labelling in the dorsal root ganglia and thus does not support a neuroepithelial origin for these cells [30]. However, it is important to note that the two studies were done at different axial levels so results may represent different cellular contributions across different rostro-caudal levels. An alternative explanation could be that labelling experiments of the neural tube also label the boundary cap cells, which are tightly associated with the future entry/exit points and have even been shown to span the CNS-PNS border with cytoplasmic processes extending into the CNS in both chick and mouse [36, 39]. Such extension into the neural tube could result in inadvertent labelling of boundary cap cells in addition to neural tube, thus resulting in labelling of boundary cap progeny. However, some studies in chick where neural crest has been removed, thus removing both the direct (neural crest) and indirect (via boundary cap) contribution to SCPs, still appeared to develop glial cells along the motor axons [28, 29].
While boundary cap cells have been documented in birds and mammals, they have not yet been described in zebrafish. However, a population of myelinating cells at the CNS-PNS border and along the ventral motor root has been documented and are termed Motor Exit Point Glia (MEP glia). These MEP glia appear to function as a ‘boundary’ between the CNS and the PNS, preventing oligodendrocyte cells from migrating out into the PNS [40, 41] and their function is therefore comparable to the ‘boundary’ role of boundary cap cells in birds and mammals [38]. However, MEP glia arise in the CNS rather than in the neural crest, and do not express boundary cap markers such as Krox20 [41]. Although boundary cap cells in birds and mammals are currently considered to arise from the neural crest [36, 37], the observation that they extend cytoplasmic processes into the neural tube [36, 39] raises the possibility that boundary cap cells might have more intimate roles with the CNS than previously thought or perhaps even that some might arise from the neural tube, as MEP glia do in fish [41, 42]. This in turn would suggest a neuroepithelial origin for boundary cap derived SCPs.
3. Development into Schwann cells
As their name suggests, SCPs can give rise to Schwann cells. However, Schwann cells are not derived directly from SCPs, but instead arise via intermediate cell states. A subset of SCPs first gives rise to immature Schwann cells, which then in turn give rise to myelinating Schwann cells (via a pro-myelin Schwann cell) or to non-myelinating (Remak) Schwann cells. The conversion of SCPs into immature Schwann cells (from E15-16 in mouse) occurs at a time when the structure of nerves with which they associate changes from one that is tightly packed to one that is more loose, with the addition of extracellular matrix [12]. The nerves become vascularized, gain endoneurial fibroblast cells and become ensheathed by perineurial cells. Immature Schwann cells, like SCPs, remain closely associated with nerve axons but unlike SCPs, begin to gain a basal lamina which persists in mature Schwann cells [15, 43]. Survival of immature Schwann cells is no longer dependent on axons [44] and their molecular signature changes from that of SCPs, with increase in expression of genes including GFAP and S100. The fate of immature Schwann cells then depends upon the type of axons with which they associate. Immature Schwann cells in contact with ‘large-diameter’ axons, undergo a process known as ‘radial sorting’ in which immature Schwann cells form a 1:1 ratio with these axons and proceed to develop into mature myelinating Schwann cells, which, in rodents, begins around birth [45]. To achieve this 1:1 ratio, Schwann cells increase their proliferation [46, 47]. In contrast, immature Schwann cells in contact with ‘small-diameter’ axons do not become myelinating, instead developing into mature non-myelinating Schwann cells, in varying axon-to-Schwann cell ratios.
4. SCPs differentiate into multiple cell types
Until the beginning of this century, SCPs were considered to give rise only to glial fates [48, 49]. However, the observation in rat sciatic nerve that some SCPs or nerve associated ‘neural crest stem cells’ as they are sometimes referred to, persisted into later developmental stages [50], raised the question of whether this cell population could give rise to derivatives other than Schwann cells. It was shown that single ‘neural crest stem cells’ taken from the sciatic rat nerve from E14.5-E17.5 and cultured ex vivo could give rise to colonies which sometimes contained multiple cell types including Schwann cells, neurons and myofibroblasts [50]. Could SCPs also give rise to non-glial cell types during normal development in vivo? Here we provide an overview of the SCP derived cell types documented to date (overview in Figure 2).
Figure 2: Schwann cell precursor derivatives.
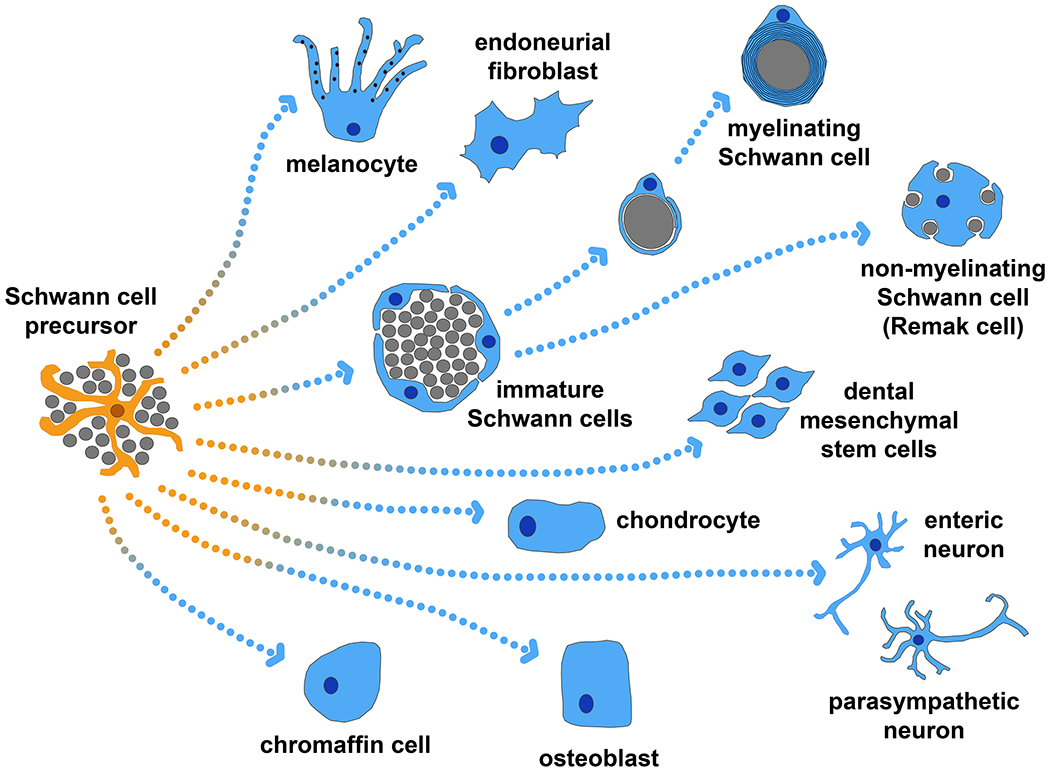
Schwann cell precursors (orange) have been shown to give rise to a diverse range of cell types (blue). Grey circles represent axons, viewed in transverse cross-section. Arrows show direction of differentiation.
4.1. Endoneurial fibroblasts
One of the earliest studies to test whether non-glial cell types were also derivatives of SCPs, focused on a subpopulation of neural crest derivatives along the peripheral nerves using the Dhh-Cre+loxpRosa+ mouse line, where Dhh marks a subset of SCPs [1]. Analysis of postnatal nerves at P11 revealed that Bluo-gal labelled cells represented not just Schwann cells but also 60% of endoneurial fibroblasts in the nerves, suggesting that SCPs give rise to both of these cell types [1]. These fibroblasts contribute to the nerve structure, filling the gaps of the endoneurial space, between the bundles of axons ensheathed by myelinating and non-myelinating Schwann cells.
4.2. Melanocytes
Perhaps one of the more striking cell derivatives described for SCPs are melanocytes (pigment forming cells). Traditionally, melanocytes have been considered to arise entirely from the dorsolateral migrating neural crest cells, originating in the dorsal neural tube and ending up at the skin [51]. However, a second population of melanocyte progenitors has been identified along the ventral nerve in chick and mouse [2]. These putative progenitor cells were first identified in chick based on their co-expression of Mitf (a central regulator of pigment development) and Sox10 (a gene associated with many neural crest lineages including SCPs). These Mitf+/Sox10+ cells were closely associated with the nerve, and lineage tracing by GFP electroporation of the neural tube revealed that some of these Mitf+/Sox10+ cells were derived from the neural crest, further supporting that these were SCPs. These cells also expressed melanosome matrix protein (MEB-L), which is a marker for avian melanocytes and their precursors, reinforcing their melanocyte precursor identity. Additionally, ablation of the ventral migratory pathway led to a significant decrease in Mitf+ melanoblasts in the dorsal and lateral body wall as well as in the limb, suggesting that a substantial number of melanoblasts in the body originate from this newly identified ventral migratory pathway [2]. Genetic tracing in mouse revealed that SCPs give rise to melanocytes in the hair follicle and dermis but, following their change from SCPs into pro-myelinating and myelinating Schwann cells, no longer do so during normal development. However, these Krox20+ myelinating Schwann cells are able to give rise to melanocytes if forced to detach from the nerve, for example following nerve damage [2]. This not only demonstrates a novel population of melanocyte progenitors during development, but also illustrates that derivatives of this population (myelinating Schwann cells) can act as a source of melanocytes, even in the adult.
4.3. Chondrocytes and Osteoblasts
Cartilage and bone has traditionally been thought to originate from three embryonic tissues; paraxial mesoderm (axial and cranial skeleton) [52], lateral plate mesoderm (skeleton in limbs) [53] and neural crest (contribution to skeleton in cranial region only) [54]. However, contribution to chondrocytes and osteoblasts from SCPs has recently been demonstrated in mouse and fish [10]. In mouse this was investigated by genetic tracing using a tamoxifen inducible proteolipid protein-1 (PLP1) line (Plp1CreERT2) [10]. PLP1 is a major myelin protein, in mouse expressed predominantly in SCPs from E9.5 in the cranium and E10.5 in the trunk. Visualisation of the lineage was enabled by crossing with a YFP reporter line (R26RYFP). Tamoxifen labelling from E11.5 and analysis at E15.5 and E17.5 revealed that some YFP+ cells in the cranium were found alongside nerves (TUJI+), while some YFP+ cells dissociated from nerves and were found to express the chondrocyte marker SOX9, suggesting that SCPs (YFP+ cells) contribute to chondrocytes (SOX9+ cells) in the cranium. In the trunk, YFP+ cells also overlapped with SOX9+ cells in the rib and scapula, suggesting that SCPs also contribute to chondrocytes in more caudal axial positions. Furthermore, some YFP+ cells at E17.5 were also found to overlap with the markers for osteoblast progenitors (Runx2 and Osterix) in both cranium (mandible) and trunk (scapula and rib) regions, while some YFP+ cells were found in bone regions, expressing the osteocyte marker DMP1 [10]. This suggests that SCPs contribute to osteocytes as well as osteoblast progenitors in the head and the trunk. Contribution to chondrocytes and osteoblasts in the trunk is particularly interesting since at these trunk axial locations chondrocytes and osteoblasts typically originate from mesoderm and not neural crest [52].
However, not all SCP populations contribute to skeletal derivatives. DHH is expressed in a subset of SCPs from E12, and a DhhCre line revealed that by E17.5, no DHH+ cells were found to contribute to cartilage but instead were confined to nerve bundles as Schwann cells [10]. This supports the hypothesis that there may be several subpopulations of SCPs, each with different prospective fates. Genetic tracing of SCPs was also performed in the zebrafish, using a SOX10 line (Sox10CreERT2;Ubi:zebrabrow). Recombination at 24 or 48 hours post fertilization and analysis at 44 days post fertilization (dpf), which is well past stages of neural crest emigration, revealed labelled cells (SCP derivatives) contributing to skeletal structures in the craniofacial region and overlapping with SOX9a antibody staining (a marker for cartilage) [10]. Therefore, in evolutionary terms, contribution of SCPs to skeletal elements appears to be conserved across mouse and fish.
4.4. Tooth development
Tooth development involves reciprocal interactions between epithelial and mesenchymal tissues, where the former gives rise to enamel secreting ameloblasts and the latter gives rise to dentine secreting odontoblasts. Within the dental tooth mesenchyme, the odontoblasts and tooth pulp arise from dental mesenchymal stem cells. Most of the dental organ, excluding the enamel, has been thought to derive from neural crest [55]. However, more recently a considerable contribution has also been traced to nerve associated SCPs in mouse [5]. SCPs were followed by genetic tracing using a Plp1CreERT2/R26RYFP mouse line, restricting recombination to SCPs by applying tamoxifen at E12.5, when PLP1 is restricted to SCPs. Analysis at E15.5 and E17.5 revealed YFP+ cells along nerves (corresponding to SCPs) and also forming streams of cells inside the incisors, corresponding to the region of pulp cells and to the odontoblast layer. Comparable patterns of YFP+ cells were also observed in the Sox10CreERT2/R26RYFP line, which also labels SCPs and their derivatives [5]. These results suggest that nerve associated SCPs can give rise to mesenchymal stem cells of the tooth, contributing both to tooth pulp and odontoblast development.
In mouse, incisors grow continuously in the adult, and so can also be used to look at post-embryonic tooth development. These two mouse lines (Plp1CreERT2/R26RYF and Sox10CreERT2/R26RYFP) were used to trace contribution of PLP1+ or SOX10+ nerve associated cells in 60-85 postnatal day mice. These nerve associated PLP1+ and SOX10+ cells in the adult were found to express S100, P0, p75 and Krox20, and therefore likely represent Schwann cells. After 30 days, YFP+ cells from these lines were found to contribute to the dental mesenchymal stem cells, pulp cells and odontoblasts [5]. Furthermore, Schwann cell derived cells were also shown to contribute to tooth regeneration in the adult mouse following injury [5]. Collectively, these results illustrate a role for SCPs and Schwann cells in tooth development, growth and repair, both in the embryo and in the adult.
4.5. Chromaffin cells
Chromaffin cells are neuroendocrine cells that produce, store and release catecholamines, which are then secreted into the blood and are involved in mediating physiological responses across different organs. The adrenal medulla is comprised of chromaffin cells at the center of the adrenal gland, which have previously been considered to originate from neural crest cells [56]. However, another source of chromaffin cells has been revealed in mouse using genetic tracing of SCPs using tamoxifen inducible Sox10CreERT2/R26RYFP and Plp1CreERT2/R26RYF lines [8]. Inducing recombination from E11.5 or E12.5, which is well after neural crest migration is complete, and analysing contribution of YFP+ cells at E17.5 revealed that both lines contribute to TH+ chromaffin cells in the adrenal medulla, thus illustrating a SCP contribution to these chromaffin cells. Since SCPs are closely associated with nerves, it was also tested whether development of chromaffin cells was dependent on the preganglionic sympathetic motor neurons within the adrenal gland. Genetic ablation of preganglionic nerves using diphtheria toxin in Hb9Cre;Isl2DTA mouse embryos resulted in a 78% reduction in the number of adrenal medulla cells [8]. This demonstrates the dependence of normal adrenal medulla development on innervation with preganglionic nerves and suggests that the SCPs that give rise to chromaffin cells are associated with preganglionic nerves. In further support of a SCP origin of chromaffin cells, mice deficient in a factor crucial for chromaffin differentiation (Ascl1), were found to contain an accumulation of glial cells and a reduction in TH+ chromaffin cells [8]. Preganglionic nerve associated SCPs therefore contribute a substantial proportion of the chromaffin cells to the adrenal medulla, at least in mice.
Could other chromaffin cells also be SCP derived? In mammals there is a transient chromaffin organ adjacent to the dorsal aorta during development called the ‘Zuckerkandl organ’, thought to be involved in homeostasis during fetal development. A genetic tracing approach, similar to the one used to investigate SCP contribution to the adrenal medulla, was used for the Zuckerkandl organ. First, genetic tracing using the Plp1CreERT2/R26RYPF mouse line with recombination at E11.5 and analysis at E15.5 revealed YFP+ chromaffin cells in the Zuckerkandl organ [9]. Next, to test whether the SCPs giving rise to chromaffin cells were those attached to the preganglionic neurons that associate with the Zuckerkandl organ, these neurons were genetically ablated using diphtheria toxin in Hb9Cre;Isl2DTA mouse embryos. Loss of these preganglionic neurons led to a ~53% reduction in the number of TH+ chromaffin cells when analysed at E14.5 [9]. In addition, mice deficient in Ascl1 (a factor crucial for chromaffin differentiation), were found to contain an accumulation of glial cells and a reduction in TH+ chromaffin cells [9]. Therefore, taken together, these results demonstrate that nerve associated SCPs contribute to a substantial population of chromaffin cells in both the adrenal medulla and Zuckerkandl organs.
4.6. Autonomic neurons
The autonomic nervous system is composed of three parts; sympathetic, parasympathetic and enteric nervous systems. Most neurons of the autonomic system were thought to be derived from the neural crest [57, 58]; however, subsequent reports show that SCPs also form a substantial contribution.
Parasympathetic neurons:
Developmental origins of parasympathetic nervous system were investigated using the Sox10CreERT2/+;R26RYFP/+ mouse line, with which SCP derivatives could be traced by inducing recombination at E11.5-E12.5 [3]. Analysis at E17.5 revealed that most parasympathetic neurons were YFP+, suggesting that the majority of parasympathetic neurons are derived from nerve associated SOX10+ cells. Furthermore, depletion of SCPs in an ErbB3−/− mouse line resulted in a loss of parasympathetic neurons, further supporting that a substantial contribution to parasympathetic neurons comes from nerve associated SCPs [3]. Use of a multicolour Cre reporter line R26R-Confetti crossed with a PLPCreERT2 mouse line enabled clonal analysis of SCPs when recombined from E11.5 or E12.5. This revealed that individual SCPs at E11.5-E12.5 give rise to both neurons and glia and are therefore bipotent [3].
Enteric neurons:
The enteric nervous system is found in the lining of the gastrointestinal system and controls gastrointestinal behaviour. The consensus was that neurons of the enteric nervous system were derived entirely from the vagal and sacral neural crest cells which invade the gut wall and migrate until the entire length of the gut is colonized [59, 60]. However, in Ret- or Gfrα1-deficient mouse embryos, which lack enteric neural crest cell-derived intrinsic innervation, some neurons still develop in the submucosal layer of the small intestine [6]. These neurons were always associated with extrinsic nerves raising the possibility that they were SCP derived. This hypothesis was tested by visualising SCP derived neural cells (using a Dhh::Cre transgene and Retfl-CFP reporter allele) in a mouse deficient for Gfrα1 (and therefore lacking neural crest derived enteric neurons). In this experiment, CFP+ enteric neurons were found in the hindgut, indicating that they were SCP-derived, thus suggesting that SCPs contribute to neurogenesis in the gut [6]. Quantification of SCP derived neurons to the adult enteric nervous system following normal development in mouse revealed that SCPs contributed 5% of the submucosal neurons in the small intestine and 20% of both the submucosal and myenteric neurons in the large intestine [6]. Contribution of SCPs to enteric neurons also appears to be conserved in fish. Lineage tracing in zebrafish using an inducible Sox10-Cre line was used to test whether glial cells extrinsic to the intestine could be a source of neurogenesis within the intestine: SOX10 is no longer expressed in the zebrafish intestine from 3.5 dpf. Inducing the Sox10-Cre line at 3.5 dpf (with SOX10+ cells labelled permanently with mCherry) and analysis at 5.5 dpf revealed cells positive for the mCherry reporter and the neuronal marker HuC/D within the intestine [61]. This demonstrates that de novo neurogenesis occurs from progenitors, likely SCPs, external to the intestine. Furthermore, it has been demonstrated that neurogenesis from such external sources in the zebrafish persists in the post-embryonic intestine, during both normal development and in response to injury, as demonstrated at 5 dpf [61].
Since a SCP origin of enteric neurons appears to be conserved across mammals and fish, could it be an ancestral mechanism of neurogenesis in vertebrate guts? The basal jawless vertebrate, the lamprey, is a great model to address this question. Strikingly, lineage tracing experiments along the neural tube using DiI suggest that lampreys lack a classic vagal neural crest population [62]. However, DiI labelling of the neural tube along the trunk axial levels gives rise to DiI labelled cells closely associated with nerve processes and to 5-HT+ and acetylated-tubulin+ neurons in the anterior gut, oesophagus and typhlosole [62]. This demonstrates that enteric neurons of the gut in lamprey originate from the trunk, and raises the possibility that these trunk derived cells may be nerve associated SCPs, similar to the SCP-derived enteric neurons documented in mouse [6] and fish [61]. This in turn raises the possibility that trunk, rather than vagal, neural crest-derived cells may be an evolutionarily conserved source of enteric neurons, possibly via a glial intermediate stage.
5. Post-natal plasticity of the Schwann cell lineage
In addition to the diverse cell types to which SCPs can give rise in early development, it appears that some SCP-derived cells maintain this ability during later postnatal stages. For example in the adult mouse incisor, which undergoes continual growth, nerve associated Schwann cells contribute to the dental mesenchymal stem cell pool, from which they give rise to tooth pulp and odontoblasts, thus contributing to adult dental growth [5]. Another documented case is in the gut of fish, where nerve associated glial cells external to the gut contribute to enteric neurons within the gut in the ongoing process of post-natal enteric neurogenesis [61]. In both these cases, the glial cells have also been able to give rise to their non-glial progeny (mesenchymal stem cells or enteric neurons) following injury [5, 61]. Injury to the sciatic nerve in the adult mouse, where glial cells become detached from the nerve, has been shown to result in Schwann cells differentiating into melanocytes, producing clusters of pigmentation at the site of nerve damage [2]. However, Schwann cells detaching from a damaged nerve in the skin of mouse were instead observed to de-differentiate into ‘injury-activated glia’, helping with the healing process [63]. Further work is required to understand the mechanisms underlying such variation in Schwann cell fate following detachment from damaged nerves.
Myelinating Schwann cells in the adult are quiescent and do not undergo cell division under normal conditions. Following nerve damage, however, almost all myelinating Schwann cells distal to a damaged nerve can turn into proliferating, progenitor-like repair cells facilitating nerve regeneration [64]. Such repair Schwann cell phenotypes (‘Bungner cells’) are thought to help guide the regenerating axon [65–67] and to promote its growth and survival [68, 69] before re-differentiating back into Schwann cells in response to axonal signals. While these cells retain their Schwann cell lineage identify, they are able to differentiate into either myelinating or non-myelinating Schwann cell types following nerve regeneration [64]. Collectively, these examples illustrate a considerable plasticity among SCP derivatives during postnatal development and repair and raise intriguing questions regarding the extent of this plasticity and underlying mechanisms that warrant further investigation.
6. Conclusions
SCPs are a transient and multipotent progenitor population, associated with developing nerves in the embryo that gives rise to a diverse range of cell types throughout the body. Given the multiple embryonic origins of SCPs, their varied migratory routes and the apparent differences in molecular signatures, it is likely that subpopulations of SCPs exist. It would be interesting to characterize these populations and to ascertain how these correlate with cell fate and developmental potential. The observations that some SCP derivatives maintain the ability to give rise to other cell types during growth and in response to injury also raises the possibility that these cells represent a peripheral nerve associated ‘stem cell’ population that persists in the adult. With such diverse developmental potential and distribution across many different structures throughout the body, a better understanding of SCP lineages could also hold therapeutic implications for processes such as nerve regeneration.
Highlights.
Schwann cell precursors (SCP) are a transient and multipotent progenitor population
There are multiple embryonic origins documented for SCPs
SCPs migrate and give rise to a diverse range of cell types throughout the body
SCP derived cells can show plasticity following damage in the adult
Acknowledgements
This work was supported by grants from the National Institute of Health (R01DE027568 and R35NS111564).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
There are no competing interests.
References
- 1.Joseph NM, et al. , Neural crest stem cells undergo multilineage differentiation in developing peripheral nerves to generate endoneurial fibroblasts in addition to Schwann cells. Development, 2004. 131(22): p. 5599–5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adameyko I, et al. , Schwann cell precursors from nerve innervation are a cellular origin of melanocytes in skin. Cell, 2009. 139(2): p. 366–379. [DOI] [PubMed] [Google Scholar]
- 3.Dyachuk V, et al. , Parasympathetic neurons originate from nerve-associated peripheral glial progenitors. Science, 2014. 345(6192): p. 82–87. [DOI] [PubMed] [Google Scholar]
- 4.Espinosa-Medina I, et al. , Parasympathetic ganglia derive from Schwann cell precursors. Science, 2014. 345(6192): p. 87–90. [DOI] [PubMed] [Google Scholar]
- 5.Kaukua N, et al. , Glial origin of mesenchymal stem cells in a tooth model system. Nature, 2014. 513(7519): p. 551–554. [DOI] [PubMed] [Google Scholar]
- 6.Uesaka T, Nagashimada M, and Enomoto H, Neuronal differentiation in Schwann cell lineage underlies postnatal neurogenesis in the enteric nervous system. Journal of Neuroscience, 2015. 35(27): p. 9879–9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Espinosa-Medina I, et al. , Dual origin of enteric neurons in vagal Schwann cell precursors and the sympathetic neural crest. Proceedings of the National Academy of Sciences, 2017. 114(45): p. 11980–11985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furlan A, et al. , Multipotent peripheral glial cells generate neuroendocrine cells of the adrenal medulla. Science, 2017. 357(6346). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kastriti ME, et al. , Schwann cell precursors generate the majority of chromaffin cells in Zuckerkandl organ and some sympathetic neurons in paraganglia. Frontiers in molecular neuroscience, 2019. 12: p. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie M, et al. , Schwann cell precursors contribute to skeletal formation during embryonic development in mice and zebrafish. Proceedings of the National Academy of Sciences, 2019. 116(30): p. 15068–15073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furlan A and Adameyko I, Schwann cell precursor: a neural crest cell in disguise? Developmental biology, 2018. 444: p. S25–S35. [DOI] [PubMed] [Google Scholar]
- 12.Wanner IB, et al. , Role of N-cadherin in Schwann cell precursors of growing nerves. Glia, 2006. 54(5): p. 439–459. [DOI] [PubMed] [Google Scholar]
- 13.Wanner IB, et al. , Invariant mantling of growth cones by Schwann cell precursors characterize growing peripheral nerve fronts. Glia, 2006. 54(5): p. 424–438. [DOI] [PubMed] [Google Scholar]
- 14.Buchstaller J, et al. , Efficient isolation and gene expression profiling of small numbers of neural crest stem cells and developing Schwann cells. Journal of Neuroscience, 2004. 24(10): p. 2357–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jessen KR and Mirsky R, The origin and development of glial cells in peripheral nerves. Nature Reviews Neuroscience, 2005. 6(9): p. 671–682. [DOI] [PubMed] [Google Scholar]
- 16.Lee M-J, et al. , P0Is Constitutively Expressed in the Rat Neural Crest and Embryonic Nerves and Is Negatively and Positively Regulated by Axons to Generate Non-Myelin-Forming and Myelin-Forming Schwann Cells, Respectively. Molecular and Cellular Neuroscience, 1997. 8(5): p. 336–350. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi M and Osumi N, Identification of a novel type II classical cadherin: rat cadherin19 is expressed in the cranial ganglia and Schwann cell precursors during development. Developmental dynamics: an official publication of the American Association of Anatomists, 2005. 232(1): p. 200–208. [DOI] [PubMed] [Google Scholar]
- 18.Winseck AK and Oppenheim RW, An in vivo analysis of Schwann cell programmed cell death in embryonic mice: the role of axons, glial growth factor, and the pro-apoptotic gene Bax. European Journal of Neuroscience, 2006. 24(8): p. 2105–2117. [DOI] [PubMed] [Google Scholar]
- 19.Jessen K, et al. , The Schwann cell precursor and its fate: a study of cell death and differentiation during gliogenesis in rat embryonic nerves. Neuron, 1994. 12(3): p. 509–527. [DOI] [PubMed] [Google Scholar]
- 20.Dong Z, et al. , Neu differentiation factor is a neuron-glia signal and regulates survival, proliferation, and maturation of rat Schwann cell precursors. Neuron, 1995. 15(3): p. 585–596. [DOI] [PubMed] [Google Scholar]
- 21.Meyer D and Birchmeier C, Multiple essential functions of neuregulin in development. Nature, 1995. 378(6555): p. 386–390. [DOI] [PubMed] [Google Scholar]
- 22.Shah NM, et al. , Glial growth factor restricts mammalian neural crest stem cells to a glial fate. Cell, 1994. 77(3): p. 349–360. [DOI] [PubMed] [Google Scholar]
- 23.Riethmacher D, et al. , Severe neuropathies in mice with targeted mutations in the ErbB3 receptor. Nature, 1997. 389(6652): p. 725–730. [DOI] [PubMed] [Google Scholar]
- 24.Britsch S, et al. , The transcription factor Sox10 is a key regulator of peripheral glial development. Genes & development, 2001. 15(1): p. 66–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noden DM, The control of avian cephalic neural crest cytodifferentiation: II. Neural tissues. Developmental biology, 1978. 67(2): p. 313–329. [DOI] [PubMed] [Google Scholar]
- 26.Le Douarin NM, Cell line segregation during peripheral nervous system ontogeny. Science, 1986. 231(4745): p. 1515–1522. [DOI] [PubMed] [Google Scholar]
- 27.Sharma K, Korade Z, and Frank E, Late-migrating neuroepithelial cells from the spinal cord differentiate into sensory ganglion cells and melanocytes. Neuron, 1995. 14(1): p. 143–152. [DOI] [PubMed] [Google Scholar]
- 28.Rickmann M, Fawcett J, and Keynes R, The migration of neural crest cells and the growth of motor axons through the rostral half of the chick somite. Development, 1985. 90(1): p. 437–455. [PubMed] [Google Scholar]
- 29.Lunn E, et al. , The neural tube origin of ventral root sheath cells in the chick embryo. Development, 1987. 101(2): p. 247–254. [DOI] [PubMed] [Google Scholar]
- 30.Maro GS, et al. , Neural crest boundary cap cells constitute a source of neuronal and glial cells of the PNS. Nature neuroscience, 2004. 7(9): p. 930–938. [DOI] [PubMed] [Google Scholar]
- 31.Le Douarin NM and Smith J, Development of the peripheral nervous system from the neural crest. Annual review of cell biology, 1988. 4(1): p. 375–404. [DOI] [PubMed] [Google Scholar]
- 32.Dupin E, et al. , Schwann-cell differentiation in clonal cultures of the neural crest, as evidenced by the anti-Schwann cell myelin protein monoclonal antibody. Proceedings of the National Academy of Sciences, 1990. 87(3): p. 1119–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baroffio A, Dupin E, and Le Douarin NM, Clone-forming ability and differentiation potential of migratory neural crest cells. Proceedings of the National Academy of Sciences, 1988. 85(14): p. 5325–5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bronner-Fraser M and Fraser SE, Cell lineage analysis reveals multipotency of some avian neural crest cells. Nature, 1988. 335(6186): p. 161–164. [DOI] [PubMed] [Google Scholar]
- 35.Bronner-Fraser M and Fraser S, Developmental potential of avian trunk neural crest cells in situ. Neuron, 1989. 3(6): p. 755–766. [DOI] [PubMed] [Google Scholar]
- 36.Niederlander C and Lumsden A, Late emigrating neural crest cells migrate specifically to the exit points of cranial branchiomotor nerves. Development, 1996. 122(8): p. 2367–2374. [DOI] [PubMed] [Google Scholar]
- 37.Golding JP and Cohen J, Border controls at the mammalian spinal cord: late-surviving neural crest boundary cap cells at dorsal root entry sites may regulate sensory afferent ingrowth and entry zone morphogenesis. Molecular and Cellular Neuroscience, 1997. 9(5–6): p. 381–396. [DOI] [PubMed] [Google Scholar]
- 38.Vermeren M, et al. , Integrity of developing spinal motor columns is regulated by neural crest derivatives at motor exit points. Neuron, 2003. 37(3): p. 403–415. [DOI] [PubMed] [Google Scholar]
- 39.Radomska KJ and Topilko P, Boundary cap cells in development and disease. Current opinion in neurobiology, 2017. 47: p. 209–215. [DOI] [PubMed] [Google Scholar]
- 40.Kucenas S, et al. , A selective glial barrier at motor axon exit points prevents oligodendrocyte migration from the spinal cord. Journal of Neuroscience, 2009. 29(48): p. 15187–15194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith CJ, et al. , Contact-mediated inhibition between oligodendrocyte progenitor cells and motor exit point glia establishes the spinal cord transition zone. PLoS Biol, 2014. 12(9): p. e1001961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fontenas L and Kucenas S, Motor exit point (MEP) glia: Novel myelinating glia that bridge CNS and PNS myelin. Frontiers in cellular neuroscience, 2018. 12: p. 333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Webster H.d., The geometry of peripheral myelin sheaths during their formation and growth in rat sciatic nerves. The Journal of cell biology, 1971. 48(2): p. 348–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meier C, et al. , Developing Schwann cells acquire the ability to survive without axons by establishing an autocrine circuit involving insulin-like growth factor, neurotrophin-3, and platelet-derived growth factor-BB. Journal of Neuroscience, 1999. 19(10): p. 3847–3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feltri ML, Poitelon Y, and Previtali SC, How Schwann cells sort axons: new concepts. The Neuroscientist, 2016. 22(3): p. 252–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stewart HJ, et al. , Changes in DNA synthesis rate in the Schwann cell lineage in vivo are correlated with the precursor—Schwann cell transition and myelination. European Journal of Neuroscience, 1993. 5(9): p. 1136–1144. [DOI] [PubMed] [Google Scholar]
- 47.Yu W-M, et al. , Schwann cell-specific ablation of laminin γ1 causes apoptosis and prevents proliferation. Journal of Neuroscience, 2005. 25(18): p. 4463–4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jessen KR and Mirsky R, Schwann cells: early lineage, regulation of proliferation and control of myelin formation. Current opinion in neurobiology, 1992. 2(5): p. 575–581. [DOI] [PubMed] [Google Scholar]
- 49.Mirsky R and Jessen KR, Schwann cell development, differentiation and myelination. Current opinion in neurobiology, 1996. 6(1): p. 89–96. [DOI] [PubMed] [Google Scholar]
- 50.Morrison SJ, et al. , Prospective identification, isolation by flow cytometry, and in vivo self-renewal of multipotent mammalian neural crest stem cells. Cell, 1999. 96(5): p. 737–749. [DOI] [PubMed] [Google Scholar]
- 51.Dupin E and Le Douarin NM, Development of melanocyte precursors from the vertebrate neural crest. Oncogene, 2003. 22(20): p. 3016–3023. [DOI] [PubMed] [Google Scholar]
- 52.Tam PP and Trainor PA, Specification and segmentation of the paraxial mesoderm. Anatomy and embryology, 1994. 189(4): p. 275–305. [DOI] [PubMed] [Google Scholar]
- 53.Cohn MJ and Tickle C, Limbs: a model for pattern formation within the vertebrate body plan. Trends in Genetics, 1996. 12(7): p. 253–257. [DOI] [PubMed] [Google Scholar]
- 54.Noden D, Cell movements and control of patterned tissue assembly during craniofacial development. Journal of craniofacial genetics and developmental biology, 1991. 11(4): p. 192–213. [PubMed] [Google Scholar]
- 55.Miletich I and Sharpe PT, Neural crest contribution to mammalian tooth formation. Birth Defects Research Part C: Embryo Today: Reviews, 2004. 72(2): p. 200–212. [DOI] [PubMed] [Google Scholar]
- 56.Huber K, Kalcheim C, and Unsicker K, The development of the chromaffin cell lineage from the neural crest. Autonomic Neuroscience, 2009. 151(1): p. 10–16. [DOI] [PubMed] [Google Scholar]
- 57.D’amico-Martel A and Noden DM, Contributions of placodal and neural crest cells to avian cranial peripheral ganglia. American Journal of Anatomy, 1983. 166(4): p. 445–468. [DOI] [PubMed] [Google Scholar]
- 58.Lee VM, et al. , Both neural crest and placode contribute to the ciliary ganglion and oculomotor nerve. Developmental biology, 2003. 263(2): p. 176–190. [DOI] [PubMed] [Google Scholar]
- 59.Yntema CL and Hammond WS, The origin of intrinsic ganglia of trunk viscera from vagal neural crest in the chick embryo. Journal of Comparative Neurology, 1954. 101(2): p. 515–541. [DOI] [PubMed] [Google Scholar]
- 60.Le Douarin NM and Teillet M-A, The migration of neural crest cells to the wall of the digestive tract in avian embryo. Development, 1973. 30(1): p. 31–48. [PubMed] [Google Scholar]
- 61.El-Nachef WN and Bronner ME, De novo enteric neurogenesis in post-embryonic zebrafish from Schwann cell precursors rather than resident cell types. Development, 2020. 147(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Green SA, Uy BR, and Bronner ME, Ancient evolutionary origin of vertebrate enteric neurons from trunk-derived neural crest. Nature, 2017. 544(7648): p. 88–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Parfejevs V, et al. , Injury-activated glial cells promote wound healing of the adult skin in mice. Nature communications, 2018. 9(1): p. 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stierli S, et al. , The regulation of the homeostasis and regeneration of peripheral nerve is distinct from the CNS and independent of a stem cell population. Development, 2018. 145(24). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cattin A-L, et al. , Macrophage-induced blood vessels guide Schwann cell-mediated regeneration of peripheral nerves. Cell, 2015. 162(5): p. 1127–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Parrinello S, et al. , EphB signaling directs peripheral nerve regeneration through Sox2-dependent Schwann cell sorting. Cell, 2010. 143(1): p. 145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Clements MP, et al. , The wound microenvironment reprograms Schwann cells to invasive mesenchymal-like cells to drive peripheral nerve regeneration. Neuron, 2017. 96(1): p. 98–114. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Arthur-Farraj PJ, et al. , c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron, 2012. 75(4): p. 633–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fontana X, et al. , c-Jun in Schwann cells promotes axonal regeneration and motoneuron survival via paracrine signaling. Journal of Cell Biology, 2012. 198(1): p. 127–141. [DOI] [PMC free article] [PubMed] [Google Scholar]


