Abstract
To detect and identify adenovirus (Ad), we investigated hypervariable regions (HVRs) of Ad by using a combination of PCR and direct sequencing (PCR-sequence) method. Primers for nested PCR to amplify the conserved region in the hexon protein containing HVRs were designed based on hexon gene sequences derived from GenBank. These two primer sets amplified a DNA fragment of 7 HVRs from 16 prototypes of Ad, which were divided into five subgenera, including seven serotypes that are the predominant causative agents of acute conjunctivitis in Japan, and from 31 recent conjunctival scraping specimens from patients with adenoviral conjunctivitis. HVR DNA sequences were determined by means of universal sequence primers. Analysis of the predicted amino acid homology of HVRs among Ad prototypes suggested three regions, HVR4, -5, and -7, to be candidates for the neutralization epitopes. The clinical serotype of specimens was determined by the PCR-sequence method with reference to these three HVRs. The serotype determined according to this method was identical to that obtained by culture isolation and the neutralization test (NT) in all scraping samples, whereas the results of this method did not match PCR and restriction fragment length polymorphism (PCR-RFLP) analysis in five samples. It took only three days to detect Ad and to identify the serotype, in contrast to culture isolation-NT, which took at least 2 weeks. These findings indicate that our newly developed PCR-sequence method is applicable for the detection and serotyping of human Ads.
Adenoviruses (Ads) are characterized by reproducing well in specific cell lines (32), and Ad serotypes have been determined based on the fact that immune sera specifically inhibit their reproduction (9, 24, 38). At present there are 49 serotypes of Ad (15, 31, 38), and they have been classified into six subgenera, A to F, based on DNA homology and the physical, biochemical, and biological properties of the viruses (36). However, differences in virus reproduction rates in cultured cells (14, 28) and ease of adsorption and penetration of host cells according to Ad serotype (11, 37) create problems in attempting to identify virus serotypes. In addition, determination of neutralization and determination of virus titer endpoints depend on the evaluation of cytopathic effects (CPE) and are subjective and time-consuming (9). For these reasons, standard sera cannot always be expected to perform neutralization under stable reproducible conditions and so details concerning the epitopes that govern Ad neutralization still remain unknown. Furthermore, many of the serotypes produce proteins that inhibit host cell resistance (10, 39, 40), and this modifies the pathological picture, which is not only related to neutralization but to both tissue affinity for the host and host resistance as well.
We have previously developed a PCR and restriction fragment length polymorphism (PCR-RFLP) analysis based on PCR of the hexon region combined with restriction fragment patterns (30). However, this method has limitations, since it targets the downstream conserved region of the hexon structural gene which has not been specified as a region determining the serotype.
Recently, it has been reported that by hexon X-ray crystallography analysis (3, 29) and sequencing (19, 26, 27, 34, 35), hypervariable regions (HVRs) that participate in type-specific neutralization have been found to exist on the surface of Ads (6, 7). We therefore focused our attention on these HVRs and, by combining nested PCR, which amplifies these regions, with the direct sequencing method (PCR-sequence method), we have developed a new method that detects Ads in conjunctival scrapings and identifies their serotype. We compared the results of this method with those obtained by conventional serotype identification methods (9, 30).
MATERIALS AND METHODS
Study group and specimens.
The Ad prototypes used were obtained from the American Type Culture Collection (ATCC), Rockville, Md. A total of 16 serotypes were studied. They consisted of the seven strains that cause Ad conjunctivitis in Japan: Ad types 3, 4, 7, 8, 11, 19, and 37 (1, 2, 12, 16) and, in addition, Ad types 11, 14, 34, and 35, which belong to subgenus B2; Ad types 1, 2, 5, and 6 in subgenus C; and Ad types 40 and 41 in subgenus F. The study materials consisted of 31 conjunctival scraping specimens collected from patients with Ad conjunctivitis in Japan from 1994 through 1998. The lower palpebral conjunctiva was scraped with two cottonwool swabs, and the virus was extracted by immediately placing each sample in 1.5 ml of Eagle minimal essential medium (MEM) solution (Nissui, Tokyo, Japan). One swab was used for culture isolation, and the other was used for PCR.
Culture isolation-neutralization test (NT).
Swabs from the conjunctiva were collected and inoculated onto HEp-2 cells and HEL cells. The viruses were passaged in cell cultures, maintained under Eagle MEM with 2% fetal calf serum, and subpassaged as described previously (13). Infected cells were identified by the immunofluorescent-antibody technique with the mouse monoclonal antibody of Ad (Chemicon International, Inc., Temecula, Calif.). All virus preparations were tested in a preliminary logarithmic serial dilution to determine an optimum working dilution of 100 50% tissue culture infective doses by using four replicate wells per dilution (5). Neutralization tests were performed on HEp-2 cells and HEL cells in 96-well microtiter plates to provide an index of CPE. Antiserum used in this study was obtained from the ATCC.
PCR-RFLP analysis.
Serotype identification was performed by PCR-RFLP analysis of all of the specimens prior to carrying out the PCR-sequence method. We described the details of PCR-RFLP analysis previously (30). Briefly, the 1,004-bp conserved region for the hexon was amplified in the first-step PCR and, in the second step, nested PCR was performed to amplify the 956-bp DNA fragment. Differences in the restriction patterns obtained with three restriction enzymes, EcoT14I, HaeIII, and HinfI, were then evaluated, and the serotypes of the clinical specimens were identified by comparisons with the Ad prototypes.
PCR-sequence method. (i) DNA extraction from prototypes and clinical specimens.
A 500-μl sample of each of the prototype-infected cell suspensions and conjunctival scrapings extracted with MEM solution was collected in a 1.5-ml microtube and centrifuged at 12,000 × g (High-Speed Microcentrifuge MRX-15; Tomy Seiko Co., Ltd., Tokyo, Japan) at 4°C for 15 min. The pellets were used as samples, and DNA was extracted with SepaGene (Sanko Junyaku Co., Ltd., Tokyo, Japan). After the addition of 50 μl of Tris buffer, 50 μl of guanidine thiocyanate, 350 μl of chloroform containing a protein absorbant, and 200 μl of sodium acetate to the pellet with stirring, the mixture was centrifuged at 12,000 × g at 15°C for 10 min. The supernatant was recovered, 24 μl of acetate buffer and 600 μl of 100% ethanol were added, and the solution was centrifuged at 12,000 × g at 4°C for 10 min. The supernatant was discarded, and 380 μl of 70% ethanol was added to the pellet and, after centrifugation at 12,000 × g at 4°C for 1 min, the supernatant was discarded again. When the DNA pellet had dried, it was dissolved in 25 μl of sterile water.
(ii) PCR primers.
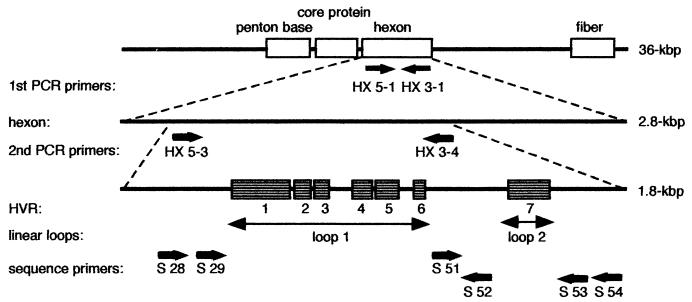
Ad is a double-stranded DNA virus, and its full length is approximately 36 kbp (33), with the hexon accounting for about 2.8 kbp (19). The primers to amplify the hexon HVRs were designed for the sites with the highest homology. The first set of primers included HX5-1 (forward primer), 5′-AAGATGGCCACCCCCTCGATGATGCCGCAGT-3′, and HX3-1 (reverse primer), 5′-CACTTATGTGGTGGCGTTGCCGGCCGAGAACGG-3′, which were designed to amplify the region that corresponds to 1 to 2,829 bp in the hexon base sequence of Ad type 3. The second PCR primer set included HX5-3 (forward primer), 5′-CACATCGCCGGACAGGATGCTTCGGAGTA-3′, and HX3-4 (reverse primer), 5′-GTGTTGTGAGCCATGGGGAAGAAGGTGGC-3′. In the second-step PCR, the primers were designed to amplify the region that contains all seven HVRs, which corresponds to bp 40 to 1847 in the Ad type 3 hexon base sequence (Fig. 1).
FIG. 1.
Ad genome. Gene map of the regions surrounding the Ad hexon protein showing the seven HVRs, the primers, and the regions sequenced.
(iii) Nested PCR.
In the first-step PCR, 1 μl of a 100-fold dilution was used when the sample was an Ad prototype whose template DNA was extracted from infected cells, and 2 μl was used when the sample was a clinical specimen extracted from conjunctival scrapings. A 20-μl volume of PCR reaction solution contained Long and Accurate (LA) PCR Buffer II (LA PCR Kit, Ver. 2; Takara Shuzo Biomedicals Co., Ltd., Shiga, Japan), deoxynucleoside triphosphate (dNTP; i.e., dATP, dGTP, dCTP, and dTTP) mixture containing 400 μM concentrations of each, 0.2 μM concentrations of each primer, and 1 U of LA Taq DNA polymerase (Takara Shuzo Biomedicals). Shuttle PCR consisting of a total of 40 cycles of denaturing at 98°C for 10 s, with annealing and extension at 65°C for 6 min, was carried out (Gene Amp PCR System 9600; Perkin-Elmer, Norwalk, Conn.).
In the second-step PCR, the DNA amplified in the first-step PCR (1 μl for the prototypes and 2 μl for the clinical specimens) was placed in 20 μl of PCR solution. The reaction solution contained buffer (10 mM Tris-HCl, pH 8.3; 50 mM KCl; 1.5 mM MgCl2), dNTP mixture containing 200 μM concentrations of each, 0.2 μM concentrations of each primer, and 1 U of Taq DNA polymerase (TaKaRa Taq; Takara Shuzo Biomedicals). The reaction conditions consisted of conventional PCR in a thermal sequencer (Thermal Sequencer TSR-300; Iwaki Glass Co., Ltd., Chiba, Japan) with 40 cycles of denaturing at 94°C for 1 min, annealing at 40°C for 1 min, and extension at 72°C for 2 min.
Each PCR test included a sample of double-distilled water that was treated identically to the virus samples throughout as a negative control. As positive controls, 10 pg of Ad2 or Ad41 DNA was used. Furthermore, to avoid the potential for contamination for DNA amplification, DNA-free handling for reaction mixture preparation and specimen addition for first- and second-step PCRs were performed in separate rooms. Plugged pipette tips, tubes, and other materials used for PCR were all disposable.
(iv) Purification of PCR products.
A 1-μl sample of the nested-PCR amplification product was subjected to 1.5% agarose gel electrophoresis and, after being stained with 0.5 mg of ethidium bromide per ml, an approximately 1.8-kbp band of Ad DNA fragment was confirmed under UV light. The confirmed amplified DNA in the PCR products of the specimens was concentrated with Microcon-100 (Amicon, Inc.), and the unreacted primers were removed. After the buffer was completely replaced with deionized water, the purified DNA was electrophoresed, and the DNA concentration was adjusted to approximately 100 ng/ml based on the density of the band.
(v) Sequence primers.
The sequences of the 12 serotypes whose base sequences upstream of the hexon had already been elucidated among the 16 standard serotype strains that were the subject of the study were compared, and universal primers were designed in the conserved region present between the individual HVRs (Fig. 1). A total of six primers was used: for sense, S-28 (5′-ACCCACGATGTGACCAC-3′; bp 152 to 172 in the base sequence of Ad type 3), S-29 (5′-GCCAGCACRTWCTTTGACAT-3′; bp 289 to 308), and S-51 (5′-CCCAACAGACCCAAYTACAT-3′; bp 937 to 956); and for antisense, S-52 (5′-CCCATGTTGCCAGTGCTGTTGTARTACA-3′; bp 986 to 1013), S-53 (5′-AAGGGGTTGACGTTGTCCAT-3′; bp 1555 to 1574), and S-54 (5′-CCAGCATTGCGGTGGTGRTT-3′; bp 1576 to 1595). The melting points (Tms) of all of the primers were calculated from their percent GC content and primer length, and they were designed to have a Tm of ∼60°C.
(vi) Cycle sequence.
A total volume of 20 μl, consisting of 8.0 μl of Terminator Ready Reaction Mix (DNA Sequencing Kit; Perkin-Elmer Applied Biosystems, Warrington, Great Britain), 1.0 μl (3.2 μM) of primers for each sequence, 1.0 μl of purified DNA, and 10 μl of sterile water, was placed in a 0.2-ml Gene Amp microtube, and a cycle sequence with a total of 25 cycles at 96°C for 10 s, 50°C for 5 s, and 60°C for 4 min was carried out (Gene Amp PCR System 9600). The samples were analyzed by means of an autosequencer (ABI Prism 310 Genetic Analyzer; Perkin-Elmer).
Analysis of PCR-sequence method. (i) Homology between prototypes.
The sequences of a total of 16 standard serotype strains were compared. The sequences of prototypes previously reported were derived from GenBank. DNASIS (Hitachi Software Engineering Co., Ltd., Tokyo, Japan) was used for sequence alignment and analysis. After referring to the HVR sites described by Crawford-Miksza et al. (7), we compared the HVRs of the 16 serotypes, and the range of each of the new HVRs was reestablished. The rate of homology of each HVR was evaluated.
(ii) Serotype identification of clinical specimens.
The predicted amino acid sequences of the clinical specimens were aligned according to the sequences of the 16 serotype prototypes. The homology rate between clinical specimens and Ad prototypes was determined. The serotypes of the clinical specimens were determined by comparing the homology rate. The serotypes identified by the PCR-sequence method were compared with the results of serotype identification by culture isolation-NT and PCR-RFLP analysis.
(iii) Nucleotide sequence accession numbers.
Sequence data from this article have been deposited with the GenBank/EMBL/DDBJ data libraries under the following accession numbers: hexon genes Ad11 (AB018424), Ad14 (AB018425), Ad34 (AB018426), and Ad35 (AB018427). The amino acid sequences of these residues were deduced.
RESULTS
PCR of Ad hexons and determination of HVR sequences.
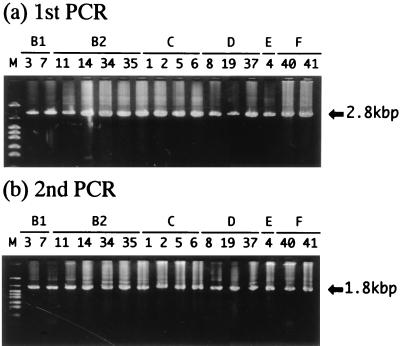
The entire approximately 2.8-kbp hexon region of all 16 prototypes could be amplified by LA PCR with the first-step PCR primer set. In addition, the approximately 1.8-kbp region that includes the hexon HVR1 to HVR7 regions could be amplified by conventional PCR with the second-step PCR primer set (Fig. 2). The size of the amplified product of the second-step PCR matched the size predicted from the known sequence (corresponding to 1,808 bp in the base sequence of Ad type 3).
FIG. 2.
Electrophoresis of PCR-amplified products. Agarose gel electrophoresis shows the specific band of ca. 2.8 kbp obtained in the first PCR (a) and of ca. 1.8 kbp obtained in the second PCR (b) from DNA samples of all 16 prototypes. Numbers above the lanes correspond to the Ad serotypes, and lane M contains molecular weight standards (pHY).
In addition, it was also possible to determine the HVR sequences of all of the newly sequenced Ad prototypes (Ad types 11, 14, 34, and 35) and the clinical specimens by using the six sequence primers described above. The region containing seven HVRs was amplified and sequenced directly from as few as 103 virus particles by two-step PCR (data not shown). On the other hand, by using single amplification products, it was not possible to obtain complete or clear sequences of these regions, particularly in clinical specimens (data not shown). The minimum limit of detection of 103 copies is sufficient because the nested PCR assay is highly sensitive.
Comparison of sequences between Ad prototypes.
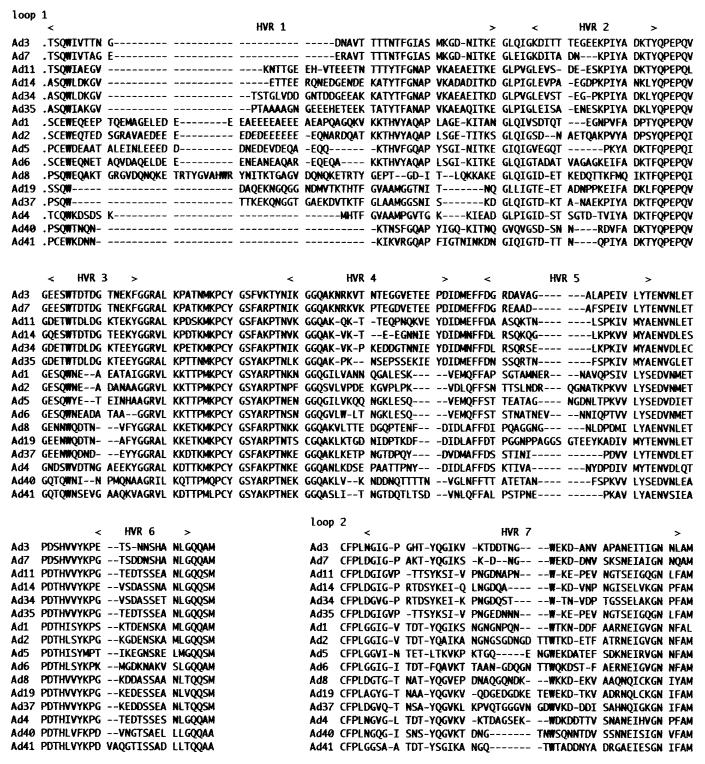
The amino acid sequences derived from the nucleotide sequences of the 16 serotype prototypes are shown in Fig. 3, divided into two loops: loop 1 and loop 2. The results of aligning the 16 different serotypes of the prototype Ad strains revealed that six of the seven HVRs, HVR6 being the only exception, had changed. As an example of the reason for this, in HVR1 the sequence was expanded by four amino acids upstream, but the homology of the prototypes of the 16 serotypes at these four amino acids was at least 25%. In contrast, the homology of the four amino acids immediately preceding them was at least 50%. In HVR2, the sequence was expanded by six amino acids downstream. The homology of this region in the prototypes of the 16 serotypes was low (16.7%). In contrast, at least 83.3% homology was conserved in the next six amino acids. The newly designed HVRs were as follows (amino acids): HVR1 (132 to 189), HVR2 (195 to 211), HVR3 (219 to 230), HVR4 (254 to 272), HVR5 (279 to 300), HVR6 (317 to 328), and HVR7 (430 to 474) (amino acid numbers according to Crawford-Miksza et al. [7].
FIG. 3.
Comparison of predicted amino acid sequences of seven hexon HVRs among 16 prototypes. The sequences of the region of loop 1 and loop 2 have been aligned to obtain maximal homology. Deduced amino acid sequences taken from previous reports were as follows (accession number): Ad1 (X67709) (25), Ad2 (J 01917) (17), Ad5 (X76550) (19), Ad6 (X67710) (25), Ad3 (X76549) (27), Ad7 (X76551) (26), Ad8 (X74663), Ad19 (X98359), Ad37 (X98360), Ad4 (X84646) (27), Ad40 (X51782) (35), and Ad41 (X51783) (34).
The amino acid homology rate in all seven HVRs among the prototypes are shown in Table 1. The six pairs of serotypes that showed highest amino acid homology in whole HVRs were: Ad3 and Ad7, 77.5%; Ad11 and Ad35, 74.0%; Ad14 and Ad34, 69.0%, Ad11 and Ad34, 58.0%; Ad11 and Ad14, 57.5%; and Ad1 and Ad2, 56.3%. Each of these prototypes belongs in the same subgenus. The homology rate of each HVRs, that is, HVR1 to HVR7, among the prototypes is shown in Tables 1 to 4.
TABLE 1.
Homology according to HVR between 16 prototypes (all 7 HVRs and HVR1)a
| Homology in HVR1 between prototypesc | % Homology in all 7 HVRs between prototypesb
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ad3 | Ad7 | Ad11 | Ad14 | Ad34 | Ad35 | Ad1 | Ad2 | Ad5 | Ad6 | Ad8 | Ad19 | Ad37 | Ad4 | Ad40 | Ad41 | |
| Ad3 | 77.5 | 43.4 | 41.5 | 37.9 | 40.3 | 25.3 | 25.9 | 25.5 | 24.9 | 24.7 | 24.9 | 29.1 | 36.4 | 29.3 | 26.5 | |
| Ad7 | 85.3 | 44.2 | 40.8 | 38.0 | 42.2 | 25.9 | 25.9 | 25.3 | 23.4 | 25.4 | 26.3 | 33.1 | 36.3 | 30.8 | 25.5 | |
| Ad11 | 38.6 | 40.0 | 57.5 | 58.0 | 74.0 | 23.3 | 22.8 | 21.0 | 20.8 | 25.1 | 27.4 | 29.2 | 34.9 | 27.2 | 23.0 | |
| Ad14 | 29.5 | 31.8 | 51.1 | 69.0 | 53.3 | 19.3 | 23.0 | 20.2 | 20.0 | 23.6 | 25.3 | 29.2 | 29.8 | 24.0 | 23.1 | |
| Ad34 | 29.8 | 29.8 | 51.1 | 66.0 | 59.4 | 19.9 | 21.9 | 19.8 | 17.3 | 19.6 | 24.3 | 28.8 | 28.7 | 27.0 | 24.0 | |
| Ad35 | 29.8 | 31.9 | 57.4 | 48.9 | 55.3 | 23.4 | 24.4 | 20.0 | 22.3 | 21.8 | 26.5 | 29.9 | 29.1 | 26.0 | 20.8 | |
| Ad1 | 10.0 | 10.0 | 14.8 | 14.8 | 13.1 | 14.8 | 56.3 | 36.9 | 50.8 | 18.8 | 19.5 | 22.1 | 22.8 | 26.1 | 27.2 | |
| Ad2 | 15.5 | 15.5 | 15.0 | 16.7 | 16.7 | 16.7 | 56.7 | 37.2 | 48.7 | 19.5 | 19.7 | 22.2 | 23.2 | 25.3 | 27.2 | |
| Ad5 | 19.3 | 21.1 | 16.7 | 15.0 | 15.0 | 15.0 | 35.0 | 39.7 | 41.7 | 18.8 | 17.0 | 21.6 | 21.9 | 29.9 | 25.7 | |
| Ad6 | 13.8 | 13.8 | 13.3 | 15.0 | 13.3 | 18.3 | 56.7 | 53.4 | 43.6 | 20.4 | 21.0 | 23.7 | 23.2 | 27.4 | 25.4 | |
| Ad8 | 11.6 | 13.0 | 13.0 | 11.6 | 7.2 | 8.8 | 5.8 | 7.2 | 13.0 | 14.5 | 38.4 | 34.4 | 26.7 | 18.1 | 17.0 | |
| Ad19 | 9.1 | 9.3 | 20.5 | 9.1 | 8.5 | 14.9 | 6.7 | 3.4 | 5.2 | 5.1 | 22.4 | 45.8 | 35.1 | 21.2 | 16.0 | |
| Ad37 | 11.4 | 11.6 | 13.3 | 18.2 | 17.0 | 19.1 | 3.3 | 3.4 | 6.7 | 3.4 | 19.4 | 43.2 | 39.8 | 23.4 | 23.9 | |
| Ad4 | 14.7 | 14.7 | 15.9 | 13.6 | 12.8 | 12.8 | 8.3 | 5.2 | 7.1 | 6.9 | 6.0 | 25.0 | 25.0 | 28.0 | 26.4 | |
| Ad40 | 32.4 | 32.4 | 22.7 | 22.7 | 21.3 | 19.1 | 16.7 | 15.5 | 25.5 | 18.5 | 8.7 | 12.8 | 10.6 | 15.2 | 36.6 | |
| Ad41 | 11.4 | 11.4 | 9.1 | 11.1 | 10.6 | 6.4 | 16.7 | 15.3 | 21.2 | 18.2 | 4.3 | 4.2 | 6.3 | 14.7 | 37.0 | |
Viewed from the standpoint of one of the serotypes. Boldface numbers indicate the highest value compared with the other serotype.
Upper right triangle of data shows percent homology in all seven HVRs.
Lower left triangle of data shows percent homology in HVR1.
TABLE 4.
Homology according to HVR between 16 prototypes (HVR6 and HVR7)a
| % Homology in HVR7 between prototypes | % Homology in HVR6 between prototypesb
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ad3 | Ad7 | Ad11 | Ad14 | Ad34 | Ad35 | Ad1 | Ad2 | Ad5 | Ad6 | Ad8 | Ad19 | Ad37 | Ad4 | Ad40 | Ad41 | |
| Ad3 | 75.0 | 50.0 | 58.3 | 41.7 | 50.0 | 41.7 | 41.7 | 25.0 | 25.0 | 33.3 | 33.3 | 33.3 | 41.7 | 16.7 | 14.3 | |
| Ad7 | 67.5 | 66.7 | 58.3 | 58.3 | 66.7 | 50.0 | 58.3 | 25.0 | 33.3 | 50.0 | 50.0 | 58.3 | 58.3 | 16.7 | 14.3 | |
| Ad11 | 37.2 | 38.1 | 58.3 | 66.7 | 100 | 41.7 | 50.0 | 16.7 | 25.0 | 58.3 | 75.0 | 75.0 | 91.7 | 41.7 | 21.4 | |
| Ad14 | 34.1 | 36.6 | 56.1 | 75.0 | 58.3 | 41.7 | 41.7 | 16.7 | 25.0 | 58.3 | 50.0 | 50.0 | 50.0 | 33.3 | 21.4 | |
| Ad34 | 26.8 | 34.1 | 48.8 | 71.8 | 66.7 | 33.3 | 41.7 | 16.7 | 25.0 | 58.3 | 58.3 | 58.3 | 66.7 | 41.7 | 21.4 | |
| Ad35 | 39.5 | 38.1 | 87.5 | 51.2 | 46.3 | 41.7 | 50.0 | 16.7 | 25.0 | 58.3 | 75.0 | 75.0 | 91.7 | 41.7 | 21.4 | |
| Ad1 | 43.9 | 46.3 | 37.2 | 26.2 | 28.6 | 34.9 | 83.3 | 25.0 | 41.7 | 41.7 | 50.0 | 41.7 | 33.3 | 16.7 | 14.3 | |
| Ad2 | 40.0 | 37.8 | 31.9 | 26.1 | 21.7 | 31.9 | 71.1 | 25.0 | 50.0 | 50.0 | 58.3 | 50.0 | 41.7 | 16.7 | 21.4 | |
| Ad5 | 28.3 | 32.6 | 23.4 | 20.5 | 20.0 | 23.4 | 35.6 | 37.0 | 16.7 | 8.3 | 8.3 | 8.3 | 16.7 | 16.7 | 14.3 | |
| Ad6 | 34.8 | 33.3 | 25.0 | 21.3 | 17.0 | 25.0 | 50.0 | 65.2 | 42.2 | 16.7 | 16.7 | 16.7 | 25.0 | 25.0 | 14.3 | |
| Ad8 | 38.1 | 38.1 | 27.9 | 25.6 | 20.9 | 30.2 | 33.3 | 31.1 | 26.1 | 30.4 | 66.7 | 75.0 | 50.0 | 16.7 | 35.7 | |
| Ad19 | 36.4 | 31.8 | 21.7 | 24.4 | 22.2 | 21.7 | 33.3 | 35.6 | 30.4 | 34.8 | 53.3 | 83.3 | 66.7 | 25.0 | 21.4 | |
| Ad37 | 28.9 | 35.6 | 21.7 | 21.7 | 23.9 | 21.7 | 34.8 | 31.1 | 32.6 | 32.6 | 44.4 | 44.4 | 66.7 | 25.0 | 28.6 | |
| Ad4 | 52.4 | 45.2 | 31.8 | 27.9 | 27.9 | 29.5 | 41.9 | 43.5 | 39.1 | 43.5 | 35.6 | 40.0 | 37.0 | 41.7 | 21.4 | |
| Ad40 | 34.1 | 35.7 | 26.1 | 23.3 | 29.5 | 26.1 | 34.1 | 28.3 | 31.7 | 35.6 | 31.1 | 32.6 | 30.4 | 40.0 | 28.6 | |
| Ad41 | 27.9 | 29.3 | 22.2 | 21.4 | 20.9 | 22.2 | 41.9 | 43.5 | 29.3 | 31.1 | 22.7 | 21.7 | 23.9 | 34.1 | 25.6 | |
Viewed from the standpoint of one of the serotypes. Boldface numbers indicate the highest value compared with the other serotype.
Upper right triangle of data shows percent homology in HVR6.
Lower left triangle of data shows percent homology in HVR7.
Serotype identification of clinical specimens.
The results of serotyping by the culture isolation-NT, PCR-RFLP, and PCR-sequence methods for 31 clinical specimens are shown in Table 5. The serotype determined by the PCR-sequence method was based on homology with the prototypes in HVR regions 4, 5, and 7. With the exception of the three specimens in subgenus D, in which the results of the culture isolation-NT and PCR-RFLP analysis conflicted, the homology of the clinical specimens in this study was 83.1% for Ad7 and 90% or more for the other serotypes. In the 26 specimens, the results of serotyping by culture isolation-NT and PCR-RFLP analysis and those obtained by PCR-sequence method were identical. In the five specimens in which there was a discrepancy between the results of the culture isolation-NT and PCR-RFLP analysis, the serotype determined by the PCR-sequence method was identical to that determined by culture isolation-NT.
TABLE 5.
Percent homology between clinical isolates and prototypes in HVR4, -5, and -7 by the PCR-sequence methoda
| Subgenus | Prototype | % Homology of clinical isolate (NT/PCR-RFLP [no.]) with subgenus:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B1 (Ad7/ Ad7 [4]) | B2
|
D
|
E (Ad/Ad4 [7]) | |||||||||
| Ad11/Ad11 (1) | Ad11/Ad11 (1) | Ad11/Ad11 (1) | Ad11/Ad11 (3) | Ad34/Ad14 (2) | Ad8/Ad8 (2) | Ad8/Ad37 (3) | Ad19/Ad19 (5) | Ad37/Ad37 (2) | ||||
| B1 | Ad3 | 69.1 | 39.3 | 39.3 | 40.5 | 40.5 | 27.7 | 33.3 | 34.9 | 29.0 | 29.1 | 44.3 |
| Ad7 | 83.1 | 38.1 | 38.1 | 36.9 | 36.9 | 28.9 | 32.1 | 39.0 | 26.9 | 31.8 | 38.0 | |
| B2 | Ad11 | 38.6 | 98.7 | 97.4 | 96.1 | 98.7 | 46.8 | 27.6 | 29.3 | 22.1 | 23.0 | 32.9 |
| Ad14 | 35.4 | 57.0 | 57.1 | 57.7 | 57.7 | 64.9 | 27.1 | 29.1 | 23.4 | 23.3 | 32.5 | |
| Ad34 | 35.4 | 48.8 | 48.8 | 47.5 | 48.8 | 92.2 | 24.7 | 25.0 | 24.5 | 25.6 | 29.8 | |
| Ad35 | 36.9 | 73.8 | 75.0 | 75.6 | 75.6 | 46.8 | 29.1 | 30.2 | 24.0 | 24.1 | 31.8 | |
| C | Ad1 | 30.6 | 27.6 | 27.6 | 27.6 | 27.6 | 22.1 | 27.7 | 31.3 | 25.3 | 30.6 | 33.3 |
| Ad2 | 27.8 | 23.9 | 23.9 | 23.9 | 23.9 | 19.8 | 26.1 | 30.0 | 26.7 | 29.1 | 34.1 | |
| Ad5 | 28.7 | 23.1 | 23.1 | 23.1 | 23.1 | 20.2 | 25.0 | 24.7 | 24.2 | 30.2 | 31.0 | |
| Ad6 | 27.0 | 23.9 | 23.9 | 23.9 | 23.9 | 14.3 | 26.4 | 30.2 | 26.4 | 32.6 | 32.2 | |
| D | Ad8 | 31.8 | 27.9 | 27.9 | 26.7 | 26.7 | 22.4 | 98.8 | 60.5 | 42.2 | 39.3 | 36.6 |
| Ad19 | 26.9 | 20.0 | 20.0 | 20.0 | 20.0 | 20.2 | 40.0 | 42.2 | 96.6 | 37.7 | 35.6 | |
| Ad37 | 29.1 | 23.0 | 23.0 | 21.8 | 21.8 | 23.3 | 39.3 | 41.7 | 38.9 | 98.7 | 42.9 | |
| E | Ad4 | 36.6 | 27.9 | 27.9 | 29.1 | 29.1 | 23.5 | 36.6 | 35.4 | 36.7 | 41.0 | 94.9 |
| F | Ad40 | 34.9 | 29.4 | 29.4 | 30.6 | 30.6 | 25.3 | 27.9 | 28.9 | 28.0 | 31.8 | 31.8 |
| Ad41 | 25.6 | 26.2 | 26.2 | 26.2 | 26.2 | 24.4 | 24.7 | 28.0 | 21.5 | 30.1 | 27.4 | |
Boldface numbers indicate the most probable serotype as determined by the PCR-sequence method.
DISCUSSION
In this study we developed a method of rapidly and accurately identifying the serotype of Ad by using a new technique, the PCR-sequence method. The serotypes identified by comparing the hexon HVR sequences were identical to the results obtained by the neutralization assay, which is the conventional serotyping method. This new method saves time and labor compared to culture isolation-NT, and it is a more objective and accurate method than PCR-RFLP analysis.
The results obtained by recent molecular biological approaches have now demonstrated the structure of the Ad hexon (3, 29) and the existence of HVRs (6, 7), and serotype-specific HVRs are thought to be important regions for serotype identification. The fact that serotype-specific sequences are restricted to seven independent HVRs (6, 7) means that the neutralization epitopes are located in one to several sites in these regions. The comparison of hexon HVRs in this study revealed homology within the same subgenus to be high. These results mean that the HVRs reflect the subgenus, which is a group that has similar DNA properties (36). In particular, in subgenus B, whose sequence we elucidated in this study, B1 and B2 were both restricted to within their respective individual subgenus. B1 is known to cause acute respiratory tract infections, and B2 is said to be common in urinary tract infection (20). Our results suggested that the hexon that contains the HVRs may also be associated with disease specificity.
Neutralization epitopes are the optimal regions for serotype identification by the PCR-sequence method and therefore it was necessary to elucidate the regions where neutralization is governed in the seven HVRs. Neutralization epitopes may be regions where there is low homology. The mean maximum homology rate between any two serotypes was 74.0% for HVR3 and 66.9% for HVR6. This indicates that HVR3 and HVR6 may not be involved in the neutralization regions which should reflect the serotype specificity, and this finding is consistent with the report by Crawford-Miksza et al. (6), who compared HVRs in subgenus D. HVR3 had the highest mean maximum homology rate with other serotypes, 74.0%, but it was demonstrated that high rates are restricted to within the same subgenus. Actually, both types Ad3 and Ad7 and types Ad34 and Ad35 had 100% homology in HVR3. Since subgenera are groups that have similar genomic properties (36), members of the same subgenus tend to show high homology. In HVR2, high homology was observed outside the subgenus, as shown by the 66.7% homology between Ad7 (subgenus B) and Ad37 (subgenus D), the 62.5% homology between Ad7 (subgenus B) and Ad41 (subgenus F), and the 52.6% homology between Ad19 (subgenus D) and Ad6 (subgenus C). HVR6 had high homology outside the subgenus, in particular with subgenera B, D, and E, where it was greater than 50%. Collectively, HVR2 and HVR6 are not candidates for neutralization epitopes. In contrast, HVR4, -5, and -7 are the HVRs with the mean maximum homologies with other serotypes—54.3, 55.9, and 58.0%, respectively—and there were few serotypes with a maximum homology outside the subgenus. These findings suggest that these three HVRs are serotype specific.
Homology can be compared by substituting amino acids for nucleic acids, but since neutralization is based on the recognition of proteins, it seems valid to make comparisons based on amino acids. Moreover, at present, since it is not clear whether neutralization epitopes are involved within HVRs alone, further assessment is needed to determine whether the PCR-sequence method, which only compares hexon HVRs, can be used to determine serotype. However, Ad serotypes are not only determined by NT of the antigenic determinant ɛ, which is present on the external, exposed portion of the hexon, but by hemagglutination inhibition tests for the antigenic determinant γ, which is present at the outer end of the fibers (22, 24). Recently, molecular biological approaches examining the fiber regions were reported (8), and we are currently analyzing the serotype-specific regions within fibers (23).
While 49 human Ad serotypes are currently known (15, 31, 38), the clinical specimens in this study were compared with 16 different prototypes. Our objective was to detect Ad in the conjunctival scrapings of conjunctivitis patients and to develop a new method of identifying the associated serotypes. There have been seven principal etiologic serotypes of conjunctivitis in Japan recently: Ad types 3, 4, 7, 8, 11, 19, and 37 (1, 2, 12, 16). In the western hemisphere, subgenus C Ad serotypes 1, 2, 5, and 6 are also known to cause acute conjunctivitis (4, 18, 21). Application of the PCR-sequence method to specimens collected from Ad conjunctivitis patients in Asia and western countries is currently being attempted, but the Ad prototypes prepared in this study are almost enough for the identification of ordinary serotypes that cause adenoviral conjunctivitis. However, an extremely rare virus to cause conjunctivitis, Ad34, was discovered in a conjunctivitis patient in Japan in this study. In the future, the HVR sequences of all 49 serotypes currently known should be determined for accurate identification of serotypes that have not been known as causes of conjunctivitis in the past. Ad is also a cause of acute pharyngitis, acute hemorrhagic cystitis, and infantile intussusception (20). The PCR-sequence method is not restricted to the field of ophthalmology but should also prove useful as a method of Ad detection and serotyping in specimens related to a variety of diseases.
LA PCR and conventional PCR were compared in the first-step PCR, but there was more nonspecific amplification in the low-molecular-weight region with conventional PCR than with LA PCR (data not shown). Furthermore, since the reaction conditions of LA PCR included a higher Tm value when the primers were long and the difference of temperatures between annealing and DNA extension was smaller, there was less nonspecific reaction with shuttle PCR, which uses two different temperatures, than with conventional PCR, which uses three (data not shown).
These findings show that the results obtained by the PCR-sequence method coincided with the results of culture isolation-NT, and it was demonstrated that the PCR-sequence method is useful as a method of serotype identification. The series of processes, i.e., sequence alignment, comparison of HVR homology, and serotype identification, were all performed manually in this study, but it took only 3 days for the determination of the serotype. If advances could be made to simplify the process in order to reduce the time required for sequencing and if new analysis software that enables calculation of the homology based on amino acid alignment is developed, serotype identification will require even less time. The PCR-sequence method targeting HVR4, -5, and -7 could become a standard method of detecting and identifying Ads in clinical specimens.
TABLE 2.
Homology according to HVR between 16 prototypes (HVR2 and HVR3)a
| % Homology in HVR3 between prototypes | % Homology in HVR2 between prototypesb
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ad3 | Ad7 | Ad11 | Ad14 | Ad34 | Ad35 | Ad1 | Ad2 | Ad5 | Ad6 | Ad8 | Ad19 | Ad37 | Ad4 | Ad40 | Ad41 | |
| Ad3 | 68.4 | 57.9 | 47.4 | 63.2 | 57.9 | 42.1 | 42.1 | 36.8 | 42.1 | 26.3 | 31.6 | 57.9 | 42.1 | 31.6 | 57.9 | |
| Ad7 | 100 | 52.6 | 44.4 | 47.1 | 55.6 | 33.3 | 36.8 | 37.5 | 31.6 | 22.2 | 50.0 | 66.7 | 47.1 | 37.5 | 62.5 | |
| Ad11 | 57.1 | 57.1 | 61.1 | 76.5 | 73.7 | 31.6 | 36.8 | 38.9 | 31.6 | 16.7 | 33.3 | 47.1 | 38.9 | 33.3 | 44.4 | |
| Ad14 | 78.6 | 78.6 | 64.3 | 77.8 | 61.1 | 15.8 | 36.8 | 26.3 | 26.3 | 11.1 | 44.4 | 38.9 | 27.8 | 15.8 | 31.6 | |
| Ad34 | 50.0 | 50.0 | 92.9 | 57.1 | 72.2 | 27.8 | 36.8 | 41.2 | 36.8 | 15.8 | 47.4 | 44.4 | 44.4 | 27.8 | 44.4 | |
| Ad35 | 50.0 | 50.0 | 92.9 | 57.1 | 100 | 27.8 | 36.8 | 33.3 | 26.3 | 11.1 | 44.4 | 44.4 | 33.3 | 26.3 | 36.8 | |
| Ad1 | 14.3 | 14.3 | 7.1 | 7.1 | 7.1 | 7.1 | 42.1 | 37.5 | 31.6 | 15.8 | 21.1 | 33.3 | 26.3 | 47.1 | 41.2 | |
| Ad2 | 14.3 | 14.3 | 7.1 | 7.1 | 7.1 | 7.1 | 75.0 | 21.1 | 15.8 | 16.7 | 22.2 | 38.9 | 27.8 | 38.9 | 33.3 | |
| Ad5 | 14.3 | 14.3 | 7.1 | 7.1 | 7.1 | 7.1 | 50.0 | 58.3 | 31.6 | 21.1 | 31.6 | 44.4 | 38.9 | 26.7 | 46.7 | |
| Ad6 | 28.6 | 28.6 | 14.3 | 21.4 | 14.3 | 14.3 | 57.1 | 57.1 | 35.7 | 21.1 | 52.6 | 52.6 | 36.8 | 26.3 | 36.8 | |
| Ad8 | 28.6 | 28.6 | 21.4 | 28.6 | 21.4 | 21.4 | 14.3 | 14.3 | 14.3 | 14.3 | 22.2 | 22.2 | 38.9 | 16.7 | 22.2 | |
| Ad19 | 35.7 | 35.7 | 28.6 | 35.7 | 28.6 | 28.6 | 14.3 | 14.3 | 14.3 | 21.4 | 81.8 | 55.6 | 33.3 | 22.2 | 33.3 | |
| Ad37 | 42.9 | 42.9 | 42.9 | 42.9 | 42.9 | 42.9 | 14.3 | 14.3 | 14.3 | 21.4 | 54.5 | 63.6 | 52.9 | 29.4 | 58.8 | |
| Ad4 | 50.0 | 50.0 | 42.9 | 64.3 | 35.7 | 35.7 | 7.1 | 7.1 | 7.1 | 7.1 | 35.7 | 35.7 | 28.6 | 35.3 | 47.1 | |
| Ad40 | 7.1 | 7.1 | 7.1 | 14.3 | 7.1 | 7.1 | 25.0 | 33.3 | 25.0 | 21.4 | 7.1 | 7.1 | 7.1 | 7.1 | 50.0 | |
| Ad41 | 21.4 | 21.4 | 21.4 | 28.6 | 14.3 | 14.3 | 28.6 | 28.6 | 14.3 | 28.6 | 7.1 | 7.1 | 7.1 | 28.6 | 42.9 | |
Viewed from the standpoint of one of the serotypes. Boldface numbers indicate the highest value compared with the other serotype.
Upper right triangle of data shows percent homology in HVR2.
Lower left triangle of data shows percent homology in HVR3.
TABLE 3.
Homology according to HVR between 16 prototypes (HVR4 and HVR5)a
| % Homology in HVR5 between prototypes | % Homology in HVR4 between prototypesb
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ad3 | Ad7 | Ad11 | Ad14 | Ad34 | Ad35 | Ad1 | Ad2 | Ad5 | Ad6 | Ad8 | Ad19 | Ad37 | Ad4 | Ad40 | Ad41 | |
| Ad3 | 87.0 | 47.8 | 43.5 | 43.5 | 47.8 | 13.0 | 13.0 | 21.7 | 21.7 | 26.1 | 21.7 | 26.1 | 17.4 | 34.8 | 34.8 | |
| Ad7 | 66.7 | 43.5 | 39.1 | 39.1 | 43.5 | 13.0 | 13.0 | 17.4 | 13.0 | 26.1 | 17.4 | 21.7 | 21.7 | 34.8 | 26.1 | |
| Ad11 | 33.3 | 35.3 | 60.0 | 42.9 | 55.0 | 13.0 | 13.0 | 21.7 | 21.7 | 45.0 | 30.0 | 35.0 | 35.0 | 27.3 | 27.3 | |
| Ad14 | 38.9 | 29.4 | 64.7 | 61.9 | 55.0 | 21.7 | 17.4 | 26.1 | 21.7 | 45.0 | 30.0 | 40.0 | 35.0 | 31.8 | 27.3 | |
| Ad34 | 38.9 | 35.3 | 64.7 | 76.5 | 47.6 | 17.4 | 21.7 | 21.7 | 13.0 | 30.4 | 21.7 | 30.4 | 21.7 | 40.9 | 31.8 | |
| Ad35 | 27.8 | 41.2 | 76.5 | 52.9 | 64.7 | 17.4 | 17.4 | 26.1 | 21.7 | 30.4 | 26.1 | 26.1 | 17.4 | 31.8 | 27.3 | |
| Ad1 | 28.6 | 33.3 | 23.8 | 14.3 | 19.0 | 28.6 | 36.8 | 63.2 | 52.6 | 25.0 | 15.0 | 31.6 | 30.0 | 22.7 | 22.7 | |
| Ad2 | 22.7 | 22.7 | 18.2 | 27.3 | 22.7 | 22.7 | 30.4 | 31.6 | 31.6 | 30.0 | 20.0 | 26.3 | 30.0 | 22.7 | 22.7 | |
| Ad5 | 38.1 | 28.6 | 23.8 | 33.3 | 23.8 | 19.0 | 21.7 | 45.5 | 68.4 | 20.0 | 20.0 | 31.6 | 20.0 | 31.8 | 31.8 | |
| Ad6 | 19.0 | 23.8 | 23.8 | 19.0 | 14.3 | 28.6 | 52.4 | 39.1 | 39.1 | 20.0 | 20.0 | 31.6 | 20.0 | 23.8 | 33.3 | |
| Ad8 | 31.6 | 26.3 | 31.6 | 26.3 | 26.3 | 26.3 | 19.0 | 13.6 | 27.3 | 23.8 | 40.0 | 40.0 | 40.0 | 27.3 | 27.3 | |
| Ad19 | 20.0 | 20.0 | 20.0 | 20.0 | 24.0 | 20.0 | 16.0 | 12.0 | 12.0 | 12.0 | 32.0 | 35.0 | 30.0 | 31.8 | 22.7 | |
| Ad37 | 33.3 | 35.3 | 23.5 | 29.4 | 23.5 | 29.4 | 23.8 | 27.3 | 23.8 | 33.3 | 31.6 | 28.0 | 40.0 | 31.8 | 40.9 | |
| Ad4 | 44.4 | 35.3 | 41.2 | 29.4 | 35.3 | 35.3 | 19.0 | 18.2 | 23.8 | 23.8 | 31.6 | 32.0 | 58.8 | 22.7 | 22.7 | |
| Ad40 | 27.8 | 33.3 | 41.2 | 29.4 | 23.5 | 41.2 | 28.6 | 36.4 | 47.6 | 42.9 | 21.1 | 12.0 | 35.3 | 27.8 | 55.0 | |
| Ad41 | 22.2 | 22.2 | 35.3 | 41.2 | 47.1 | 35.3 | 28.6 | 27.3 | 23.8 | 19.0 | 26.3 | 12.0 | 26.7 | 16.7 | 29.4 | |
Viewed from the standpoint of one of the serotypes. Boldface numbers indicate the highest value compared with the other serotype.
Upper right triangle of data shows percent homology in HVR4.
Lower left triangle of data shows percent homology in HVR5.
ACKNOWLEDGMENTS
The skillful technical assistance by Akira Oshima, from Mitsubishi Kagaku Bio-Clinical Laboratories, Inc., is gratefully acknowledged.
REFERENCES
- 1.Aoki K, Kato M, Ohtsuka H, Ishii K, Nakazono N, Sawada H. Clinical and aetiological study of adenoviral conjunctivitis, with special reference to adenovirus type 4 and 19 infections. Br J Ophthalmol. 1982;66:776–780. doi: 10.1136/bjo.66.12.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aoki K, Kawana R, Matsumoto I, Wadell G, de Jong J C. Viral conjunctivitis with special reference to adenovirus type 37 and enterovirus 70 infection. Jpn J Ophthalmol. 1986;30:158–164. [PubMed] [Google Scholar]
- 3.Athappilly F K, Murali R, Rux J J, Cai Z, Burnett R M. The refined crystal structure of hexon, the major coat protein of adenovirus type 2, at 2.9 Å resolution. J Mol Biol. 1994;242:430–455. doi: 10.1006/jmbi.1994.1593. [DOI] [PubMed] [Google Scholar]
- 4.British Journal of Ophthalmology. Adenovirus keratoconjunctivitis. Br J Ophthalmol. 1977;61:73–75. doi: 10.1136/bjo.61.2.73. . (Editorial.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crawford-Miksza L, Schnurr D P. Quantitative colorimetric microneutralization assay for characterization of adenoviruses. J Clin Microbiol. 1994;32:2331–2334. doi: 10.1128/jcm.32.9.2331-2334.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crawford-Miksza L K, Schnurr D. Adenovirus serotype evolution is driven by illegitimate recombination in the hypervariable regions of the hexon protein. Virology. 1996;224:357–367. doi: 10.1006/viro.1996.0543. [DOI] [PubMed] [Google Scholar]
- 7.Crawford-Miksza L, Schnurr D P. Analysis of 15 adenovirus hexon proteins reveals the location and structure of seven hypervariable regions containing serotype-specific residues. J Virol. 1996;70:1836–1844. doi: 10.1128/jvi.70.3.1836-1844.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eiz B, Adrian T, Pring-Åkerblom P. Immunological adenovirus variant strains of subgenus D: comparison of the hexon and fiber sequences. Virology. 1995;213:313–320. doi: 10.1006/viro.1995.0004. [DOI] [PubMed] [Google Scholar]
- 9.Fife K H, Ashley R, Shields A F, Salter D, Meyers J D, Corey L. Comparison of neutralization and DNA restriction enzyme methods for typing clinical isolates of human adenovirus. J Clin Microbiol. 1985;22:95–100. doi: 10.1128/jcm.22.1.95-100.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gooding L R. Virus proteins that counteract host immune defenses. Cell. 1992;71:5–7. doi: 10.1016/0092-8674(92)90259-f. [DOI] [PubMed] [Google Scholar]
- 11.Greber U F, Willetts M, Webster P, Helenius A. Stepwise dismantling of adenovirus 2 during entry into cells. Cell. 1993;75:477–486. doi: 10.1016/0092-8674(93)90382-z. [DOI] [PubMed] [Google Scholar]
- 12.Guo D F, Shinagawa M, Aoki K, Sawada H, Itakura S, Sato G. Genome typing of adenovirus strains isolated from conjunctivitis in Japan, Australia, and the Philippines. Microbiol Immunol. 1988;32:1107–1118. doi: 10.1111/j.1348-0421.1988.tb01475.x. [DOI] [PubMed] [Google Scholar]
- 13.Hierholzer J C, Atuk N O, Gwaltney J M. New human adenovirus isolated from a renal transplant recipient: description and characterization of candidate adenovirus type 34. J Clin Microbiol. 1975;1:366–376. doi: 10.1128/jcm.1.4.366-376.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hierholzer J C, Stone Y O, Broderson J R. Antigenic relationships among the 47 human adenoviruses determined in reference horse antisera. Arch Virol. 1991;121:179–197. doi: 10.1007/BF01316753. [DOI] [PubMed] [Google Scholar]
- 15.Hierholzer J C, Wigand R, Anderson L J, Adrian T, Gold J W M. Adenoviruses from patients with AIDS: a plethora of serotypes and a description of five new serotypes of subgenus D (type 43-47) J Infect Dis. 1988;158:804–813. doi: 10.1093/infdis/158.4.804. [DOI] [PubMed] [Google Scholar]
- 16.Ishii K, Nakazono N, Fujinaga K, Fujii S, Kato M, Ohtsuka H, Aoki K, Chen C W, Lin C C, Sheu M M, Lin K H, Oum B S, Lee S H, Chun C H, Yoshii T, Yamazaki S. Comparative studies on aetiology and epidemiology of viral conjunctivitis in three countries of east Asia-Japan, Taiwan and South Korea. Int J Epidemiol. 1987;16:98–103. doi: 10.1093/ije/16.1.98. [DOI] [PubMed] [Google Scholar]
- 17.Jörnvall H, Akusjärvi G, Aleström P, von Bahr-Lindström H, Pettersson U, Appella E, Fowler A V, Philipson L. The adenovirus hexon protein. The primary structure of the polypeptide and its correlation with the hexon gene. J Biol Chem. 1981;256:6181–6186. [PubMed] [Google Scholar]
- 18.Kinchington P R, Turse S E, Kowalski R P, Gordon Y J. Use of polymerase chain amplification reaction for the detection of adenoviruses in ocular swab specimens. Invest Ophthalmol Vis Sci. 1994;35:4126–4134. [PubMed] [Google Scholar]
- 19.Kinloch R, Mackay N, Mautner V. Adenovirus hexon: sequence comparison of subgroup C serotypes 2 and 5. J Biol Chem. 1984;259:6431–6436. [PubMed] [Google Scholar]
- 20.Mei Y-F, Wadell G. Molecular determinants of adenovirus tropism. Curr Top Microbiol Immunol. 1995;199(Pt. 3):213–228. doi: 10.1007/978-3-642-79586-2_11. [DOI] [PubMed] [Google Scholar]
- 21.Morris D J, Bailey A S, Cooper R J, Turner P C, Jackson R, Corbitt G, Tullo A B. Polymerase chain reaction for rapid detection of ocular adenovirus infection. J Med Virol. 1995;46:126–132. doi: 10.1002/jmv.1890460208. [DOI] [PubMed] [Google Scholar]
- 22.Norby E. The structural and functional diversity of adenovirus capsid components. J Gen Virol. 1969;5:221–236. doi: 10.1099/0022-1317-5-2-221. [DOI] [PubMed] [Google Scholar]
- 23.Oshima A, Itoh N, Tanaka K, Ishiko H, Ohno S, Aoki K. The sequence analysis of fiber knob genomic region on adenovirus types 19 and 37. Invest Ophthalmol Vis Sci. 1996;37:S1030. [Google Scholar]
- 24.Philipson L. Structure and assembly of adenoviruses. Curr Top Microbiol Immunol. 1984;109:1–52. doi: 10.1007/978-3-642-69460-8_1. [DOI] [PubMed] [Google Scholar]
- 25.Pring-Åkerblom P, Adrian T. The hexon genes of adenoviruses of subgenus C: comparison of the variable regions. Res Virol. 1993;144:117–127. doi: 10.1016/s0923-2516(06)80020-8. [DOI] [PubMed] [Google Scholar]
- 26.Pring-Åkerblom P, Trijssenaar F E J, Adrian T. Hexon sequence of adenovirus type 7 and comparison with other serotypes of subgenus B. Res Virol. 1995;146:383–388. doi: 10.1016/0923-2516(96)80897-1. [DOI] [PubMed] [Google Scholar]
- 27.Pring-Åkerblom P, Trijssenaar F E J, Adrian T. Sequence characterization and comparison of human adenovirus subgenus B and E hexons. Virology. 1995;212:232–236. doi: 10.1006/viro.1995.1474. [DOI] [PubMed] [Google Scholar]
- 28.Roba L A, Kowalski R P, Gordon A T, Romanowski E G, Gordon Y J. Adenoviral ocular isolates demonstrate serotype-dependent differences in in vitro infectivity titers and clinical course. Cornea. 1995;14:388–393. doi: 10.1097/00003226-199507000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Roberts M M, White J L, Grüter M G, Burnett R M. Three-dimensional structure of the adenovirus major coat protein hexon. Science. 1986;232:1148–1151. doi: 10.1126/science.3704642. [DOI] [PubMed] [Google Scholar]
- 30.Saitoh-Inagawa W, Oshima A, Aoki K, Itoh N, Isobe K, Uchio E, Ohno S, Nakajima H, Hata K, Ishiko H. Rapid diagnosis of adenoviral conjunctivitis by PCR and restriction fragment length polymorphism analysis. J Clin Microbiol. 1996;34:2113–2116. doi: 10.1128/jcm.34.9.2113-2116.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schnurr D P, Dondero M E. Two new candidate adenovirus serotypes. Intervirology. 1993;36:79–83. doi: 10.1159/000150325. [DOI] [PubMed] [Google Scholar]
- 32.Takiff H E, Straus S E, Gordon C F. Propagation and in vitro studies of previously non-cultivable enteral adenoviruses in 293 cells. Lancet. 1981;ii:832–834. doi: 10.1016/s0140-6736(81)91104-1. [DOI] [PubMed] [Google Scholar]
- 33.Toogood C I A, Crompton J, Hay R T. Antipeptide antisera define neutralizing epitopes on the adenovirus hexon. J Gen Virol. 1992;73:1429–1435. doi: 10.1099/0022-1317-73-6-1429. [DOI] [PubMed] [Google Scholar]
- 34.Toogood C I A, Hay R T. DNA sequence of the adenovirus type 41 hexon gene and predicted structure of the protein. J Gen Virol. 1988;69:2291–2301. doi: 10.1099/0022-1317-69-9-2291. [DOI] [PubMed] [Google Scholar]
- 35.Toogood C I A, Murali R, Burnett R M, Hay R T. The adenovirus type 40 hexon: sequence, predicted structure and relationship to other adenovirus hexons. J Gen Virol. 1989;70:3203–3214. doi: 10.1099/0022-1317-70-12-3203. [DOI] [PubMed] [Google Scholar]
- 36.Wadell G. Molecular epidemiology of human adenoviruses. Curr Top Microbiol Immunol. 1984;110:191–220. doi: 10.1007/978-3-642-46494-2_7. [DOI] [PubMed] [Google Scholar]
- 37.Wickham T J, Mathias P, Cheresh D A, Nemerow G R. Integrins αvβ3 and αvβ5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 38.Wigand R, Adrian T, Bricout F. A new human adenovirus of subgenus D: candidate adenovirus type 42. Arch Virol. 1987;94:283–286. doi: 10.1007/BF01310720. [DOI] [PubMed] [Google Scholar]
- 39.Wold W S M, Gooding L R. Region E3 of adenovirus: a cassette of genes involved in host immunosurveillance and virus-cell interactions. Virology. 1991;184:1–8. doi: 10.1016/0042-6822(91)90815-s. [DOI] [PubMed] [Google Scholar]
- 40.Wold W S M, Tollefson A E, Hermiston T W. E3 transcription unit of adenovirus. Curr Top Microbiol Immunol. 1995;199(Pt. 1):237–274. doi: 10.1007/978-3-642-79496-4_13. [DOI] [PubMed] [Google Scholar]