Abstract
Aim
We wanted to examine survival in patients with resected colorectal cancer (CRC) whose lung metastases are or are not resected.
Methods
Teams participating in the study of Pulmonary Metastasectomy in Colorectal Cancer (PulMiCC) identified potential candidates for lung metastasectomy and invited their consent to join Stage 1. Baseline data related to CRC and fitness for surgery were collected. Eligible patients were invited to consent for randomization in the PulMiCC randomized controlled trial (Stage 2). Sites were provided with case report forms for non‐randomized patients to record adverse events and death at any time. They were all reviewed at 1 year. Baseline and survival data were analysed for the full cohort.
Results
Twenty‐five clinical sites recruited 512 patients from October 2010 to January 2017. Data collection closed in October 2020. Before analysis, 28 patients with non‐CRC lung lesions were excluded and three had withdrawn consent leaving 481. The date of death was known for 292 patients, 136 were alive in 2020 and 53 at earlier time points. Baseline factors and 5‐year survival were analysed in three strata: 128 non‐randomized patients did not have metastasectomy; 263 had elective metastasectomy; 90 were from the randomized trial. The proportions of solitary metastases for electively operated and non‐operated patients were 69% and 35%. Their respective 5‐year survivals were 47% and 22%.
Conclusion
Survival without metastasectomy was greater than widely presumed. Difference in survival appeared to be largely related to selection. No inference can be drawn about the effect of metastasectomy on survival in this observational study.
Keywords: lung metastasectomy, prospective observational study
What does this paper add to the literature?
The assumed near‐zero survival without resection of patients with lung metastases from colorectal cancer was not supported by this study. It seems likely that a much smaller part of the 40% observed 5‐year survival can be attributed to lung metastasectomy than is widely believed.
INTRODUCTION
The Pulmonary Metastasectomy in Colorectal Cancer (PulMiCC) study enrolled 512 patients who were being considered for lung metastasectomy from October 2010 to January 2017. Nested within this observational study was the PulMiCC randomized controlled trial (RCT) of 93 patients. The RCT was reported in Colorectal Disease in 2020 [1]. The widely believed large survival benefit from lung metastasectomy was not evident although a small late effect cannot be excluded. Patient‐reported outcomes—quality of life and health utility—declined at a similar rate in the metastasectomy and the unoperated control arms [2, 3].
The PulMiCC study was run in the context of a firm belief in the clinical effectiveness of lung metastasectomy. In response to a paper arguing the case for a trial [4], the European Journal of Cardio‐Thoracic Surgery published an Editorial in 2017 proclaiming ‘Surgery for pulmonary metastases is a pillar of modern thoracic surgery’ [5]. In support of lung metastasectomy for colorectal cancer (CRC), the authors cited two retrospective single‐institution studies [6, 7]. These provided a pooled 5‐year survival of 60% in groups of 165 and 113 patients collected over 10 and 12 years. The Work Force of Evidence Based Surgery of the Society of Thoracic Surgeons based its recommendations for lung metastasectomy on the assumption that 5‐year survival without resection is zero [8]. Neither statement was supported by control data. These publications in the leading thoracic surgical journals invite the conclusion that lung metastasectomy provides 60% survival benefit. This widely held belief and the climate of certainty resulted in multidisciplinary teams (MDTs) having difficulty randomizing patients into the PulMiCC trial (Figure 1).
FIGURE 1.

Concerned by the low rate of randomization into the RCT the Independent Data Monitoring Committee asked for an investigation. The three largest recruiting centres (Bristol, Liverpool and Sheffield) provided reasons for not randomizing in 155 patients. Among 78 patients for whom the MDT overrode the patients' wishes to be in a controlled trial, 77 (99%) were operated on. When patients made their own decision they were more evenly divided, demonstrating group equipoise. The results are given in the Sankey chart. Diagram created using SankeyMATIC (http://sankeymatic.com/)
Although PulMiCC found no difference in survival, a small survival advantage due to resection of lung metastases that prove to be the only site of residual CRC cannot be discounted. But the trial was large enough to refute the improbable 0% [8] and the less extreme estimate of 5% more generally cited [9]. The 5‐year survival in the control group was 30% (95% CI 15.3–45.7%), significantly above 5% (P < 0.001) [1].
Colorectal Disease has recently published a big data study of pulmonary metastasectomy for CRC [10]. Among 173 354 patients who had a CRC resection from 2005 to 2013, lung resection was subsequently reported in 3434 patients which averages 2% over the time span of the study. From this an average number of pulmonary metastasectomy operations for CRC can be estimated at about 380 per annum in the National Health Service (NHS) in England. During an overlapping period from late 2010 to early 2017, the PulMiCC cohort recruited more than 300 patients who had a lung metastasectomy for CRC which is equivalent to about 14% of all patients having this procedure in England.
Here we report data from the full PulMiCC cohort. The large majority of the patients were not randomized and so we cannot draw any valid inferences about survival attributable to lung metastasectomy. Nevertheless, the characteristics of the large sample of patients and their outcomes are available to inform the Association of Coloproctology of Great Britain and Ireland (ACPGBI) IMPACT initiative and the design of future research in this uncertain area [11, 12].
GENERAL METHODS
Recruitment
The PulMiCC cohort study was planned around the PulMiCC RCT. The study was administered by clinical trials staff based at the recruiting hospitals under the direction of the site's principal investigator (PI) who was either a thoracic surgeon or an oncologist. Patients who were potential candidates for lung metastasectomy were given written information and an explanatory DVD to take home. A healthcare professional training DVD was also available for clinicians to aid their discussions with patients.
Interested patients were invited to sign Stage 1 to be assessed for lung metastasectomy within PulMiCC and to be registered by the trials unit. They consented to collection of baseline information: sex, age, height and weight to derive body mass index, the interval since primary CRC resection, whether they had prior liver metastasectomy, the number of lung metastases, carcinoembryonic antigen assay (CEA) and tests of lung function.
Stage 1 consent included 1‐year follow‐up by a Case Report Form (CRF) to record treatments since evaluation, including lung metastasectomy and other cancer directed local interventions. The sites were given CRFs to report death, serious adverse events, new lung metastases, new liver metastases and other cancer recurrence.
The local MDT considered whether patients should have lung metastasectomy and, if they were uncertain, agreed to offer patients randomization in the PulMiCC RCT. When the PulMiCC was closed, in view of the size and wealth of baseline data about the 82.2% non‐randomized patients we sought Research Ethics Committee agreement to approach sites for follow‐up data. Approval was readily granted as an audit of practice on 11 February 2019. All patients who gave consent on entry to Stage 1 are accounted for in this analysis.
From 11 February 2019, all the site PIs and trials staff were approached with an individualized CRF request for each patient in Stage 1 asking for a date of death or when last known alive. We repeated the requests for missing survival information until the end of October 2020.
Statistical methods
Data from the full PulMiCC cohort were used to investigate baseline factors that influenced survival. Patients not known to have died were censored at the last time they were known to be alive in all analyses of survival. These investigations were based on Cox's relative risk model with time to death as the outcome and time from cohort entry as the time scale. The observational nature of the cohort does not support the estimation of treatment effects. Three strata were defined and all analyses were stratified on this basis.
The first stratum included all patients who were not randomized and did not have a metastasectomy. They entered the analysis from their time of cohort entry.
Patients receiving an elective metastasectomy formed the second stratum, entered at the time of their operation.
Patients in the RCT formed the third stratum entering at the time of randomization.
Results are summarized as hazard ratios (HRs) with confidence intervals (CIs). An HR greater than 1 indicates that larger values of the risk factor are associated with poorer survival and an HR less than 1 indicates that larger values are associated with higher survival. For categorical risk factors, an HR >1 indicates that poorer survival is associated with the presence of the risk factor (e.g., for male sex) or with a risk factor category compared to the reference category as for performance status using the Eastern Cooperative Oncology Group (ECOG) scores.
For descriptive purposes, survival curves were also estimated based on the fixed grouping of the patients into these three strata. The times of origin for these three curves are different and are the dates of cohort entry. These classifications are retrospective and therefore analyses using these from the time of cohort entry are ad hoc because the classes are determined after cohort entry and, more importantly, by decisions made by the patient and MDT following cohort entry.
RESULTS
Sources of patients and composition of the three strata
In all, 512 patients were recruited into Stage 1 of the PulMiCC trial from October 2010 to January 2017 from 25 clinical sites, mainly in England but also in China, Scotland, Serbia and Sicily (Table 1). Of the 512 patients, 31 were excluded for reasons given in Table 2. This left 481 patients in the cohort, of whom 90 had been randomized in the PulMiCC RCT. Of these, 41 never had a metastasectomy and 49 had a lung metastasectomy 0 to 627 days after randomization (median 51; interquartile range 27–160 days) including some late crossovers.
TABLE 1.
Trials sites and numbers of participants included at each site
| Site | City and institution | Participants enrolled |
|---|---|---|
| 076 | Sheffield, Northern General Hospital | 101 |
| 073 | Liverpool, Heart and Chest Hospital | 88 |
| 062 |
Bristol, Royal Infirmary Serbia, Institute for Lung Diseases of Vojvodina Middlesbrough, James Cook Hospital Cambridge, Papworth Hospital |
77 |
| 078 | 41 | |
| 070 | 29 | |
| 029 | 29 | |
| 028 | London Guy's Hospital | 22 |
| 060 | Basildon, Basildon Hospital | 15 |
| 065 | Plymouth, Derriford Hospital | 15 |
| 068 | Glasgow, Golden Jubilee Hospital | 15 |
| 071 | Sutton in Ashfield, King's Mill Hospital | 12 |
| 063 | Manchester, Christie | 11 |
| 067 | Leicester, Glenfield Hospital | 10 |
| 074 | Wolverhampton, New Cross Hospital | 9 |
| 105 | Zhengzhou, Henan Cancer Hospital | 6 |
| 066 | Newcastle, Royal Victoria Hospital | 5 |
| 075 | Norwich, Norfolk and Norwich Hospital | 5 |
| 080 | Catania, Policlinico Hospital | 5 |
| 082 |
London, Royal Brompton Hospital London, Royal Free Hospital Belfast, City Hospital |
5 |
| 018 | 4 | |
| 061 | 2 | |
| 069 | Birmingham, Heartlands Hospital | 2 |
| 084 | London, St George's Hospital | 2 |
| 072 | Leeds, St James' Hospital | 1 |
| 081 | Burton, New Cross Hospital | 1 |
| Total patients | 512 |
TABLE 2.
Reasons for exclusion
| Total Stage 1 Recruitment | 512 | |
| Reasons for exclusion | ||
| Non‐neoplastic nodules | 13 | |
| Primary lung cancer | 13 | |
| Carcinoid tumour | 1 | |
| Hamartoma | 1 | |
| Consent withdrawn | 3 | |
| Total exclusions | 31 | |
| Included patients | 481 |
We received a record of the date of death for 292 of them. Four patients who had metastasectomy died on the day of surgery. For the 189 patients without death dates, 136 were known to be alive in 2020, 33 in 2019 and for 20 patients last alive dates were longer ago than the end of December 2018 (Figure 2, Table 3). Data collected up to October 2020 are used in this analysis. The source, mix and eventual outcome of the three strata are shown in Figure 3.
FIGURE 2.

For 189 patients where a death form had not been returned this chart gives the date at which they were last known to be alive in order from the earliest to the most recent. Requests continued until October 2020 when we closed the study for analysis. Some centres regarded the close of the RCT as the end of their commitment to give time to the PulMiCC study
TABLE 3.
Recorded date of alive or dead status in all 481 cohort patients
| Status | From | To | Number |
|---|---|---|---|
| Known dates of death | 31 July 2012 | 11 August 2020 | 292 |
| Known to be alive to 2020 | 01 January 2020 | 29 October 2020 | 136 |
| Known to be alive to 2019 | 03 January 2019 | 30 December 2019 | 33 |
| Last known to be alive <2019 | 22 October 2014 | 10 December 2018 | 20 |
| Total | 481 |
FIGURE 3.

The total 512 Stage 1 enrolled patients divide into 93 in the RCT and the remaining 419 in the observational study. After 31 exclusions for the reasons given in Table 2, 481 patients from the PulMiCC cohort are analysed in three strata: elective non‐metastasectomy 128, elective metastasectomy 263 and the 90 patients who were randomized. Diagram created using SankeyMATIC (http://sankeymatic.com/)
Mortality risk factors
The baseline risk factors for the patients in the three strata are shown in Table 4. The proportions of metastases in the three strata (Figure 4) show the predominance of solitary metastases (69%) in the elective metastasectomy group (Table 5).
TABLE 4.
Data availability and median, full range and quartile values in the three strata
| Data available a | Percentage | Minimum | 0.25 | Median | 0.75 | Maximum | |
|---|---|---|---|---|---|---|---|
| Age in years | |||||||
| Elective no metastasectomy | 128 | 99.2 | 42.4 | 65.7 | 71.9 | 77.7 | 87.9 |
| Elective metastasectomy | 263 | 100.0 | 30.8 | 60.0 | 67.0 | 72.8 | 85.6 |
| Randomized | 90 | 100.0 | 35.3 | 59.8 | 67.1 | 73.8 | 86.5 |
| Body mass index | |||||||
| Elective no metastasectomy | 105 | 81.4 | 17.9 | 25.0 | 27.8 | 30.9 | 57.8 |
| Elective metastasectomy | 259 | 98.5 | 17.0 | 24.6 | 27.7 | 31.4 | 56.8 |
| Randomized | 86 | 95.6 | 18.7 | 26.1 | 28.6 | 31.7 | 40.9 |
| Carcinoembryonic antigen, ng/l | |||||||
| Elective no metastasectomy | 82 | 63.6 | 0.3 | 1.7 | 3.0 | 5.9 | 57.0 |
| Elective metastasectomy | 144 | 54.8 | 0.3 | 1.3 | 2.0 | 4.0 | 151.0 |
| Randomized | 78 | 86.7 | 0.3 | 1.9 | 3.0 | 4.6 | 74.0 |
| Forced expiratory volume, % predicted | |||||||
| Elective no metastasectomy | 90 | 70.3 | 44.0 | 76.0 | 86.6 | 101.5 | 145.0 |
| Elective metastasectomy | 233 | 88.6 | 26.0 | 82.3 | 96.0 | 111.0 | 153.0 |
| Randomized | 84 | 93.3 | 46.0 | 78.0 | 93.1 | 109.3 | 139.0 |
| Months from primary CRC resection to metastasectomy or registration | |||||||
| Elective no metastasectomy | 117 | 91.4 | 1.3 | 13.9 | 25.6 | 49.7 | 145.4 |
| Elective metastasectomy | 253 | 96.2 | 0.0 | 14.3 | 24.7 | 37.1 | 138.7 |
| Randomized | 84 | 93.3 | 0.8 | 13.8 | 25.6 | 36.9 | 130.3 |
| Number of metastases | |||||||
| Elective no metastasectomy | 107 | 82.9 | 1 | 1 | 2 | 3 | 17 |
| Elective metastasectomy | 245 | 93.2 | 1 | 1 | 1 | 2 | 8 |
| Randomized | 90 | 100.0 | 1 | 1 | 2 | 3 | 9 |
| % solitary | Missing | Solitary | Multiple | % solitary | |||
| Elective no metastasectomy | 107 | 83.6 | 22 | 38 | 69 | 35.5% | |
| Elective metastasectomy | 245 | 93.2 | 18 | 169 | 76 | 69.0% | |
| Randomized | 90 | 100.0 | 0 | 30 | 60 | 33.3% | |
| ECOG | Missing | 0 | 1 | 2 | |||
| Elective no metastasectomy | 90 | 70.3 | 29.7% | 35.9% | 29.7% | 4.7% | |
| Elective metastasectomy | 215 | 81.7 | 18.3% | 67.9% | 31.6% | 0.5% | |
| Randomized | 74 | 82.2 | 21.6% | 73.0% | 25.7% | 1.4% | |
| Sex | Women | Men | % women | ||||
| Elective no metastasectomy | 128 | 100.0 | 42 | 87 | 36.4% | ||
| Elective metastasectomy | 263 | 100.0 | 100 | 163 | 38.0% | ||
| Randomized | 90 | 100.0 | 34 | 56 | 37.8% | ||
| Prior liver metastasectomy | Yes | No | % previous | ||||
| Elective no metastasectomy | 121 | 94.5 | 44 | 77 | 36.4% | ||
| Elective metastasectomy | 256 | 97.3 | 71 | 185 | 27.7% | ||
| Randomized | 83 | 92.2 | 26 | 57 | 31.3% | ||
Abbreviations: CEA, carcinoembryonic antigen; CRC, colorectal cancer; ECOG, Eastern Cooperative Oncology Group.
There are usually more missing data in the elective non‐metastasectomy group because, if metastasectomy is decided against for any reason, other investigations may serve no purpose. CEA is an exception. It was required for minimization but the value was not always in the record. It is a paradoxical marker because its elevation on screening may prompt a search for metastases but its elevation is a negative predictor for survival after metastasectomy and may contribute to a decision against operating.
FIGURE 4.
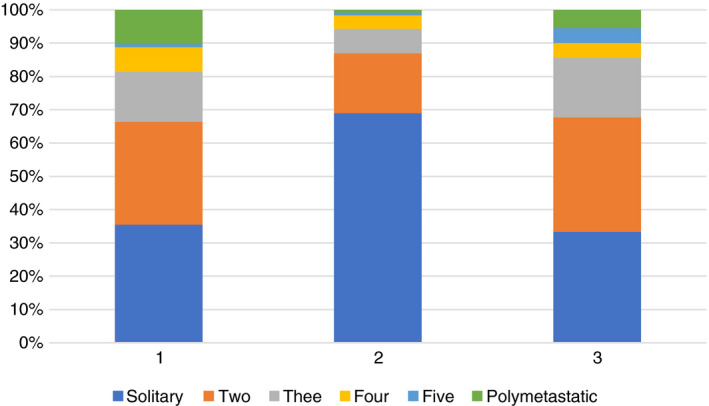
Three numbers of lung metastases in the three strata based on available data as in Table 5: (1) N = 128 non‐randomized patients who did not have a metastasectomy; (2) N = 263 non‐randomized patients who had a metastasectomy; (3) N = 90 randomized patients
TABLE 5.
Numbers of patients with a solitary metastasis and 2, 3, 4, 5 and polymetastatic disease in each stratum
| Solitary | Two | Three | Four | Five | Polymetastatic | Available | |
|---|---|---|---|---|---|---|---|
| Elective no metastasectomy | 38 | 33 | 16 | 8 | 1 | 11 | 107/128 |
| Elective metastasectomy | 169 | 44 | 18 | 10 | 2 | 2 | 245/263 |
| Randomized | 30 | 31 | 16 | 4 | 4 | 5 | 90/90 |
Table 6 presents the results from single‐factor analyses of baseline factors derived from stratified Cox regression models. The table includes the number of deaths examined in each analysis as these vary depending on the number of patients for whom information is available on the various potential risk factors. The factors demonstrating evidence of a higher mortality risk were higher values of log(CEA), a shorter interval from CRC to cohort entry and higher ECOG classes with a suggestive effect for male sex. Multiple lung metastases and prior liver metastases also demonstrated limited potential for an increase in mortality so were also considered for multi‐factor analyses. There was some evidence that the effect for log(CEA) was different in different strata. Estimated HRs and 95% CIs were 1.73 (1.37, 2.19), 1.16 (0.93, 1.44) and 1.58 (1.22, 2.05) in the elective no metastasectomy, elective metastasectomy and randomized groups respectively with no effect being demonstrable in the elective metastasectomy group.
TABLE 6.
Results from single‐factor analyses using a stratified Cox regression model
| Variable | Categories | No. of deaths | HR (95% CI) | P value |
|---|---|---|---|---|
| Age (continuous) | 292 | 1.00 (0.99,1.01) | 0.73 | |
| Male sex | 292 | 1.24 (0.98,1.58) | 0.08 | |
| Log(CEA) | 200 | 1.42 (1.25,1.62) a | <0.001 | |
| Liver metastasectomy (Y/N) | 280 | 1.22 (0.95,1.56) | 0.12 | |
| No. of lung metastasectomies > 1 | 265 | 1.19 (0.92,1.54) | 0.181 | |
| Interval CRC to start (years) | 274 | 0.90 (0.84,0.97) | 0.003 | |
| BMI | 269 | 1.00 (0.98,1.02) | 0.99 | |
| ECOG | 0 | 219 | 1.00 | |
| 1 | 1.28 (0.97,1.69) | 0.09 | ||
| 2 | 1.11 (0.49,2.56) | 0.80 | ||
| %FEV | 247 | 1.00 (0.99,1.00) | 0.20 |
Strata: elective no metastasectomy or randomization; elective metastasectomy; randomized.
Abbreviations: BMI, body mass index; CEA, carcinoembryonic antigen; CRC, colorectal cancer; ECOG, Eastern Cooperative Oncology Group; FEV, forced expiratory volume in the 1st second; HR, hazard ratio.
Some evidence of effects being different in different strata.
Table 7 presents results from multi‐factor stratified Cox regression models including variables demonstrating some potential for a relationship in single‐factor analyses. The set of models presented is based on gradually decreasing numbers of deaths depending on the availability of risk factor information for the variables included with a final model with the four most significant variables. These were male sex, interval (shorter) from CRC to cohort entry, prior liver metastases and log(CEA). Again, there was evidence of differential effects for log(CEA) with estimated HRs and 95% CIs of 1.57 (1.20, 2.04), 1.14 (0.92, 1.43) and 1.52 (1.16, 1.98) in the elective no metastasectomy, elective metastasectomy and randomized groups respectively.
TABLE 7.
Results from multi‐factor analyses using a stratified Cox regression model
| Model A | Model B | Model C | Model D | Model E | |
|---|---|---|---|---|---|
| No. deaths | 274 | 270 | 251 | 191 | 192 |
| Male sex | 1.30 (1.02, 1.67) (P = 0.036) | 1.29 (1.00, 1.65) (P = 0.051) | 1.20 (0.93, 1.55) (P = 0.167) | 1.25 (0.93, 1.69) (P = 0.144) | 1.45 (1.07, 1.98) (P = 0.018) |
|
Interval CRC to start |
0.90 (0.84, 0.96) (P = 0.002) | 0.88 (0.81, 0.94) (P < 0.001) | 0.89 (0.82, 0.95) (P < 0.001) | 0.86 (0.79, 0.94) (P < 0.001) | 0.84 (0.77, 0.92) (P < 0.001) |
| Liver metastasectomy (Y/N) | 1.33 (1.02, 1.74) (P = 0.035) | 1.27 (0.96, 1.67) (P = 0.092) | 1.23 (0.89, 1.70) (P = 0.210) | 1.29 (0.94, 1.76) (P = 0.113) | |
| No. of lung metastasectomies >1 | 1.19 (0.91, 1.55) (P = 0.208) | 1.23 (0.90, 1.68) (P = 0.191) | |||
| ECOG (0) | 1.00 | ||||
| ECOG (1) | 1.31 (0.97, 1.78) (P = 0.081) | ||||
| ECOG (2) | 0.96 (0.38, 2.44) (P = 0.938) | ||||
| Log(CEA) | 1.35 (1.17, 1.55) (P < 0.001) |
Strata: elective no metastasectomy or randomization; elective metastasectomy; randomized. HR (95% CI) (P value).
Abbreviations: CEA, carcinoembryonic antigen; CRC, colorectal cancer; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio.
Survival
With a time origin of cohort entry, Figure 5 displays Kaplan–Meier estimated survival curves and 95% CIs of three groups of patients retrospectively classified in the strata: non‐randomized and no metastasectomy, elective metastasectomy, and randomized.
FIGURE 5.

Survival in three strata with 95% confidence intervals. The curves are displayed separately because the entry points into the analyses and the baseline prognostic factors of those in the three strata are different. No direct comparison of these curves is appropriate
Figure 6 displays the estimated survival after metastasectomy curves for randomized and elective metastasectomy patients. The curve in Figure 6 begins at a value of 0.989 for elective patients because there were three patients with a death date on the same day as the day of metastasectomy. The curve for randomized patients begins at 0.980 with one death on the day of metastasectomy. Overall, in the 6 months after metastasectomy there were 9/288 deaths compared with 2/166 among those who did not have an operation in the 6 months after cohort entry.
FIGURE 6.

Survival curves for all 312 patients who had pulmonary metastasectomy separated into those in the elective and randomized strata
Over the time period after metastasectomy, the overall estimated HR (95% CI) for multiple metastases vs. a single metastasis, stratified by elective/randomized status, in PulMICC patients having a metastasectomy is 1.33 (0.94, 1.88) (P = 0.10). The separate estimates based on elective and randomized metastasectomies are 1.18 (0.80, 1.76) (P = 0.40) and 2.15 (0.93, 4.99) (P = 0.07) respectively providing suggestive evidence for a much more marked effect in the randomized patients. The difference in survival for those having elective resection of solitary and multiple metastases is shown in Figure 7. The number of lung metastases is a risk factor of particular relevance for metastasectomy patients and is considered further in the next section.
FIGURE 7.

Survival for elective metastasectomy patients with solitary or multiple metastases. The difference in survival does not appear until nearly 2 years
Interpretation of results
All the curves in Figure 5 show a relatively slow rate of decline initially. This is a hallmark of many cancer survival studies—whether randomized or observational—in contrast to cancer registry data presentations when there is characteristically an early sharp fall which becomes progressively flatter. For randomized patients, the plateau may be because patients have to have a good probability of short‐term survival (1–2 years) often defined by entry criteria. This is also seen among patients in whom the decision is individualized. All patients in the cohort were being considered for lung metastasectomy and therefore had favourable features. This provides for a further bias in addition to the selection of patients on prognostic features. The median interval from registration to elective metastasectomy was 21 days with the upper quartile of delayed decisions ranging from 39 to 431 days. Lung metastasectomy is used highly selectively, not undertaken for imminently life‐threatening disease. The time needed to check that there are no further metastases or disease at other sites allows for a further manifestation of selection bias seen in the initial parts of the curves in Figure 5B,C. This effect carries over to the initial period after the metastasectomy as seen by the initial plateaus in Figure 6 after deaths on the day of operation. We suggest that this is a form of guarantee‐time bias [13]. It can occur when survival is timed from enrolment, and is compared across groups defined by a classifying event occurring sometime during the subsequent passage of time. In this case, patients who show other sites of disease, or other adverse features, fall outside the standard criteria for metastasectomy [14]. But metastasectomy remains an option for patients whose progression trajectory indicates the likelihood of longer survival. It is carried out if survival appears to be ‘guaranteed’ for a reasonable length of time. In assessment for liver metastasectomy there is a period of assessment before the final decision is made. It is a conscious policy referred to by liver surgeons as the ‘test of time’ [15, 16].
The early deaths in the elective metastasectomy stratum merit attention. There were three deaths on the day of surgery and a further five in the 6 months after operation. Although lung metastasectomy is comparatively low risk, thoracic surgery is associated with non‐trivial rates of pneumothorax, persistent air leak, bleeding, lung infection and pleural space infection, all of which take their toll on wellbeing and may contribute to deterioration and earlier death than would otherwise have been the case. Deaths in the months after operation for selected patients with very limited CRC are probably related to surgery. A similar inference was drawn in the analysis of deaths within 90 days after primary resection in the National Bowel Cancer Audit [17].
The relatively low hazard ratio of 1.19 for multiple vs. single metastases in Table 6 is not directly comparable with results derived only from metastasectomy patients. In the meta‐analysis of 24 reports including 2589 patients already referred to, the hazard ratio for multiple vs. solitary metastases, for patients having a metastasectomy, was 2.04 [9]. The better survival of patients with a solitary metastasis has been found repeatedly in case series, in the International Registry of Lung Metastases [18] and in systematic reviews [19, 20]. Figure 4 shows that among patients selected for the elective metastasectomy stratum the solitary metastasis rate was nearly double that in the unoperated patients (69% vs. 35%). A large difference was seen in survival after 2 years among patients with a difference in this risk factor.
The analysis of the randomized stratum in PulMICC produced an HR of 2.15 which is comparable with that in the meta‐analysis, while there was no evidence of an effect in the elective metastasectomy patients. A likely explanation is that in more recent practice, aware of the hazard of multiple metastases, the MDT will only recommend metastasectomy if the balance of other risk factors is favourable. In randomized patients, minimization prevents trading off risk factors.
However, erosion of the effect of a risk factor as the practice becomes more selective has been observed in the use of risk factors in case selection [21]. A simple analogy might help explain. Looking at performance data of a large pool of high school basketball players an analyst noted that youths ≥2 m tall were higher scorers. They were preferentially picked for the county team. When the analysis was run for elite teams, height was no longer such a strong discriminator. They were nearly all very tall. Blackstone and Lauer have called this effect ‘work up bias’ [22]. The coach, however, continued to select for the squad one or two players ≥1.7 m tall who could almost unerringly shoot and score from 15 to 20 m away. As does the coach, an MDT looks at all the factors. If a patient has two to three metastases that have remained much the same over a year of observation, they know that patient is likely to have a longer survival.
DISCUSSION
The major limitation of the PulMiCC cohort study is that it is not a randomized comparison and cannot provide evidence about survival benefit attributable to metastasectomy. The best currently available data are in the PulMiCC RCT report [1].
We can confidently state that the 5‐year survival of people with lung metastases from CRC is not zero. Among 481 patients in the cohort, 169 patients did not have a metastasectomy and 37 of them were 5‐year survivors (22%, 95% CI 16%–29%). It is possible but very unlikely that a few of these survivors did not in fact have malignant lung lesions. We specifically sought information on long survivors, however treated, and the PIs did not report any case in whom the diagnosis of CRC had been wrong.
In most cases after lung metastasectomy CRC recurs sooner or later, but it is quite feasible that in some cases the lung metastases are the only residual site of disease and metastasectomy is curative. We hear anecdotal accounts [23] but documented proof of disease‐free survival at a long interval after lung metastasectomy are yet to be seen. Reported long‐term survivors have usually also had systemic treatments. The majority of patients in this cohort with and without metastasectomy had systemic treatments. An analysis of those treatments is the subject of a further report.
The big data study in the English NHS already referred to showed that lung metastasectomy is highly selective, being used on only 2.3% of patients with resected CRC in 2013 [10]. A study in the Korean National Health Insurance Database of 2573 CRC lung metastasectomy patients found a similar rate of 2.5% [24]. The lung metastasectomy operations referred to as ‘a pillar of modern thoracic surgery’ were performed at a rate of about one a month in the two series cited in support [5]—more a flying buttress than a pillar. This is a highly selective practice.
At this point, discussants of observational studies tend to point to ‘the need for trials’. In this instance we refer to the PulMiCC RCT, a trial already done. It refutes the zero assumption and substantially narrows the plausible effect size from the assumed 40% difference.
The National Institute for Health and Care Excellence (NICE) has considered the question of lung metastasectomies and the recommendation was to ‘consider’ metastasectomy. While it might have been intended as a weak recommendation, it sends out a signal for ‘business as usual’. The guideline development group advising NICE discounted PulMiCC as too small [25]. Instead, they chose to use a non‐randomized follow‐up study in which 48 had metastasectomy. PulMiCC's metastasectomy arm fell short of their unspecified cut‐off with only 46 patients but there was a control group. Also recommended for consideration was peritoneal surgery. They cited an RCT in support. It had the same surgery in both arms but the authors concluded ‘that high‐quality surgery is of value’ which cannot be derived from the abstract cited. The position with respect to liver resection was summed up by surgeons at Memorial Sloan Kettering: ‘We took as our point of departure the assumption that there will never be an RCT to answer the question of if liver resection has a role in the management of CRLM [colorectal liver metastases], or even to quantify its exact benefits’ [26] It seems questionable that the practitioners of a particular form of surgery should seek to bar the way to its evaluation. We are aware that the IMPACT initiative of ACPGBI includes active detection of metastases for resection but meta‐analysis of the many trials of more vs. less intensive screening protocols have found no overall survival benefit [27, 28].
Thoracic surgeons have addressed the question of the clinical effectiveness of lung metastasectomy by participating in the PulMiCC studies, the RCT and this cohort, but resolution of this matter is unlikely to come from specialist thoracic surgeons engaged in a very small part of the overall treatment of advanced CRC. ACPGBI have made a commitment to improving the care of patients with advanced CRC [11]. That should include reducing and avoiding the use of ineffective treatments if only to make room in the budget for adopting new ones. It is noteworthy that, in the process of prioritizing patients for cancer treatments during the COVID‐19 pandemic, metastasectomy was deemed low priority. The use of stereotactic radiotherapy to treat ‘oligometastases’ has been commissioned by NHS England on the basis of very weak evidence, and this may well supersede the use of surgical metastasectomy [29].
Colorectal MDTs are in the best position to implement trials of treatments for advanced CRC. Commenting on yet another round of the homoeopathy vs. allopathy debate The Lancet recognized that allopathy might have RCT evidence but homoeopathy has a following and while ‘doctors need to be bold and honest with their patients about homoeopathy's lack of benefit’ they should also be honest ‘with themselves about the failings of modern medicine to address patients' needs for personalized care’. The IMPACT initiative seeks to personalize care [11] but all systemic treatments have been introduced on the basis of controlled trial evidence. To paraphrase The Lancet, doctors need to be bold and honest with themselves about the failure to even seek proof of the effectiveness of local treatments. This is a declared research priority of the ACPGBI [12].
CONFLICTS OF INTEREST
None of the authors has a conflict of interest with respect to any of the content of this submission.
ETHICS STATEMENT
Ethics approval was granted by the National Research Ethics Committee London—Hampstead 10/H0720/5. Approval for follow‐up as an audit of practice 11 Feb 2019 HAMPSTEAD, NRESCommittee. London‐(HEALTH RESEARCH AUTHORITY).
AUTHOR CONTRIBUTION
Trial design, leadership and management: TT, VF, FM, LF, PL. Principle investigators TB, MM, JK, YZ. Trial coordination CNG NRW. Manuscript drafting and editing TT, VF, FM. All authors have seen draft versions and the final version as submitted.
ACKNOWLEDGEMENTS
Adrian Marchbank, Alison Hardwick, Aman Coonar, Amanda Stone, Belinda Lees, Benjamin Hyams, Bina Shah, Brian Davidson, Charlotte Jacobs, Christopher Rollinson, Cinzia Baldini, David Tsang, Elinor Pegg, Elizabeth Radford, Ian Morgan, Ingrid Potyka, Jacqueline Connell, Jakub Kablec, Jaqui Currie, Jo Allen, Joel Dunning, John Edwards, Julie Alderton, Jurjees Hasan, Kay Cash, Kerry Goodsell, Lucy Stelfox, Mark Cutting, Michael Shackcloth, Monica Narasingham, Rebecca Boyles, Robert Rintoul, Roxanne Todd, Sarah Feeney, Sarah Milgate, Simon Grumett, Trevor Thompson, Victoria Hughes, Victoria Lake.
Funding information
PulMiCC was funded by Cancer Research UK Grant no. C7678/A1139.
DATA AVAILABILITY STATEMENT
Data available on request from the authors.
REFERENCES
- 1. Milosevic M, Edwards J, Tsang D, Dunning J, Shackcloth M, Batchelor T, et al. Pulmonary Metastasectomy in Colorectal Cancer: updated analysis of 93 randomized patients—control survival is much better than previously assumed. Colorectal Dis. 2020;22(10):1314–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Treasure T, Farewell V, Macbeth F, Monson K, Williams NR, Brew‐Graves C, et al. Pulmonary Metastasectomy versus Continued Active Monitoring in Colorectal Cancer (PulMiCC): a multicentre randomised clinical trial. Trials. 2019;20(1):718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brew‐Graves C, Farewell V, Monson K, Milosevic M, Williams N, Morris E, et al. Pulmonary Metastasectomy in Colorectal Cancer: health utility scores by EQ‐5D‐3L in a randomised controlled trial show no benefit from lung metastasectomy. Colorectal Dis. 2021;23(1):200–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aberg T, Treasure T. Analysis of pulmonary metastasis as an indication for operation: an evidence‐based approach. Eur J Cardiothorac Surg. 2016;50(5):792–8. [DOI] [PubMed] [Google Scholar]
- 5. Schirren J, Schirren M, Lampl L, Editorial SS. Surgery for pulmonary metastases: quo vadis? Eur J Cardiothorac Surg. 2017;51(3):408–10. [DOI] [PubMed] [Google Scholar]
- 6. Watanabe K, Nagai K, Kobayashi A, Sugito M, Saito N. Factors influencing survival after complete resection of pulmonary metastases from colorectal cancer. Br J Surg. 2009;96(9):1058–65. [DOI] [PubMed] [Google Scholar]
- 7. Bolukbas S, Sponholz S, Kudelin N, Eberlein M, Schirren J. Risk factors for lymph node metastases and prognosticators of survival in patients undergoing pulmonary metastasectomy for colorectal cancer. Ann Thorac Surg. 2014;97(6):1926–32. [DOI] [PubMed] [Google Scholar]
- 8. Handy JR, Bremner RM, Crocenzi TS, Detterbeck FC, Fernando HC, Fidias PM, et al. Expert consensus document on pulmonary metastasectomy. Ann Thorac Surg. 2019;107(2):631–49. [DOI] [PubMed] [Google Scholar]
- 9. Gonzalez M, Poncet A, Combescure C, Robert J, Ris HB, Gervaz P. Risk factors for survival after lung metastasectomy in colorectal cancer patients: a systematic review and meta‐analysis. Ann Surg Oncol. 2013;20(2):572–9. [DOI] [PubMed] [Google Scholar]
- 10. Fenton HM, Finan PJ, Milton R, Shackcloth M, Taylor JC, Treasure T, et al. National variation in pulmonary metastasectomy for colorectal cancer. Colorectal Dis. 2020. 10.1111/codi.15506. https://www.ncbi.nlm.nih.gov/pubmed/33368958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dawson P. ACPGBI IMPACT Initiative: Improving Management of Patients with Advanced Colorectal Tumours: Association of Coloproctology of Great Britain and Ireland; 2017. updated 5/15/2017. https://www.acpgbi.org.uk/content/uploads/2017/02/ACPGBI‐Advanced‐Malignancy‐Initiative‐15‐May‐2017‐v4.pdf. Accessed 14 April 2021.
- 12. Tiernan J, Cook A, Geh I, George B, Magill L, Northover J, et al. Use of a modified Delphi approach to develop research priorities for the Association of Coloproctology of Great Britain and Ireland. Colorectal Dis. 2014;16(12):965–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Giobbie‐Hurder A, Gelber RD, Regan MM. Challenges of guarantee‐time bias. J Clin Oncol. 2013;31(23):2963–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thomford NR, Woolner L, Clagett O. The surgical treatment of metastatic tumours in the lung. J Thorac Cardiovasc Surg. 1965;49:357–63. [PubMed] [Google Scholar]
- 15. Morris E, Treasure T. Surgical management and outcomes of colorectal cancer liver metastases. Cancer Epidemiol. 2018;52:160–1. [DOI] [PubMed] [Google Scholar]
- 16. Morris E, Treasure T. If a picture is worth a thousand words, take a good look at the picture: survival after liver metastasectomy for colorectal cancer. Cancer Epidemiol. 2017;49:152–5. [DOI] [PubMed] [Google Scholar]
- 17. Vallance AE, Fearnhead NS, Kuryba A, Hill J, Maxwell‐Armstrong C, Braun M, et al. Effect of public reporting of surgeons’ outcomes on patient selection, "gaming," and mortality in colorectal cancer surgery in England: population based cohort study. BMJ. 2018;361:k1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pastorino U, Buyse M, Friedel G, Ginsberg RJ, Girard P, Goldstraw P, et al. Long‐term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg. 1997;113:37–49. [DOI] [PubMed] [Google Scholar]
- 19. Fiorentino F, Hunt I, Teoh K, Treasure T, Utley M. Pulmonary metastasectomy in colorectal cancer: a systematic review and quantitative synthesis. J R Soc Med. 2010;103(2):60–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pfannschmidt J, Hoffmann H, Dienemann H. Reported outcome factors for pulmonary resection in metastatic colorectal cancer. J Thorac Oncol. 2010;5(6 Suppl 2):S172–S178. [DOI] [PubMed] [Google Scholar]
- 21. Utley M, Treasure T. The use of scoring systems in selecting patients for lung resection: work‐up bias comes full‐circle. Thorac Surg Clin. 2008;18(1):107–12. [DOI] [PubMed] [Google Scholar]
- 22. Blackstone EH, Lauer MS. Caveat emptor: the treachery of work‐up bias. J Thorac Cardiovasc Surg. 2004;128(3):341–4. [DOI] [PubMed] [Google Scholar]
- 23. Van Raemdonck D, Treasure T, Van Cutsem E, Macbeth F. Pulmonary Metastasectomy in Colorectal Cancer: has the randomized controlled trial brought enough reliable evidence to convince believers in metastasectomy to reconsider their oncological practice? Eur J Cardiothorac Surg. 2020. 10.1093/ejcts/ezaa450. https://www.ncbi.nlm.nih.gov/pubmed/33332567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yu WS, Bae MK, Choi JK, Hong YK, Park IK. Pulmonary metastasectomy in colorectal cancer: a population‐based retrospective cohort study using the Korean National Health Insurance Database. Cancer Res Treat. 2021. 10.4143/crt.2020.1213. https://www.ncbi.nlm.nih.gov/pubmed/33494126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mohamed F, Kallioinen M, Braun M, Fenwick S, Shackcloth M, Davies RJ, et al. Management of colorectal cancer metastases to the liver, lung or peritoneum suitable for curative intent: summary of NICE guidance. Br J Surg. 2020;107:943–5. [DOI] [PubMed] [Google Scholar]
- 26. Wei AC, Jarnagin WR. Questioning why more patients with colorectal liver metastases are not referred for metastasectomy. JAMA Surg. 2020;155:909. [DOI] [PubMed] [Google Scholar]
- 27. Mokhles S, Macbeth F, Farewell V, Fiorentino F, Williams NR, Younes RN, et al. Meta‐analysis of colorectal cancer follow‐up after potentially curative resection. Br J Surg. 2016;103(10):1259–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jeffery M, Hickey BE, Hider PN, See AM. Follow‐up strategies for patients treated for non‐metastatic colorectal cancer. Cochrane Database Syst Rev. 2016;11:CD002200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chalkidou A, Macmillan T, Grzeda MT, Peacock J, Summers J, Eddy S, et al. Stereotactic ablative body radiotherapy in patients with oligometastatic cancers: a prospective, registry‐based, single‐arm, observational, evaluation study. Lancet Oncol. 2021;22(1):98–106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request from the authors.


