Abstract
Background
The glycolytic nature of cancer cells presents a potential treatment target that may be addressed by a ketogenic diet (KD).
Objective
We hypothesized that a KD would improve body composition and lower serum insulin and insulin-like growth factor-I (IGF-I) in women with ovarian or endometrial cancer.
Methods
In this randomized controlled trial, women with ovarian or endometrial cancer [age: ≥19 y; body mass index (kg/m2): ≥18.5] were randomly assigned to a KD (70:25:5 energy from fat, protein, and carbohydrate) or the American Cancer Society diet (ACS; high-fiber, low-fat). Body composition (DXA) and fasting serum insulin, IGF-I, and β-hydroxybutyrate were obtained at baseline and at 12 wk; urinary ketones were also measured throughout the intervention. We assessed differences between the diets with ANCOVA and independent t tests. We used correlation analyses to estimate associations between changes in serum analytes and body composition.
Results
After 12 wk, the KD (compared with ACS) group had lower adjusted total (35.3 compared with 38.0 kg, P < 0.05) and android (3.0 compared with 3.3 kg, P < 0.05) fat mass. Percentage of change in visceral fat was greater in the KD group (compared with the ACS group; –21.2% compared with –4.6%, P < 0.05). Adjusted total lean mass did not differ between the groups. The KD (compared with ACS) group had lower adjusted fasting serum insulin (7.6 compared with 11.2 µU/mL, P < 0.01). There was a significant inverse association between the changes in serum β-hydroxybutyrate and IGF-I concentrations (r = –0.57; P < 0.0001).
Conclusions
In women with ovarian or endometrial cancer, a KD results in selective loss of fat mass and retention of lean mass. Visceral fat mass and fasting serum insulin also are reduced by the KD, perhaps owing to enhanced insulin sensitivity. Elevated serum β-hydroxybutyrate may reflect a metabolic environment inhospitable to cancer proliferation. This trial was registered at www.clinicaltrials.gov as NCT03171506.
Keywords: ketogenic diet, body composition, insulin, carbohydrate, ovarian cancer, endometrial cancer
Introduction
A growing body of research suggests that cancer is a metabolic disease. Cancer cells preferentially metabolize glucose for energy via glycolysis, even in the presence of oxygen (i.e., the Warburg effect). Compared with oxidative phosphorylation used by normal cells, aerobic glycolysis is relatively inefficient in terms of ATP produced per mole of glucose (1, 2). Accordingly, cancer cells require high amounts of glucose for proliferation, making them vulnerable under conditions of glucose deprivation.
Although cancer cells rely on glucose for fuel, blood glucose concentrations in healthy individuals are tightly regulated, suggesting that there are other factors facilitating cancer cells’ uptake of glucose. Insulin is perhaps one such factor. This hormone is secreted in response to carbohydrate intake and induces the uptake of glucose by normal and cancer cells alike (3). Cancer cells are disproportionately populated by insulin receptors, which enables them to obtain sufficient glucose, even in the presence of normal glucose concentrations (4). In addition, insulin and insulin-like growth factor I (IGF-I), a hormone that has sequence homology with insulin, also may promote cancer cell proliferation via insulin receptor–mediated activation of MAPK and phosphatydalinositol-3 kinase (5, 6).
Cancer cells’ dependence on glucose and insulin for growth and replication represents a cancer treatment target that may be addressed by a very low-carbohydrate, high-fat (i.e., ketogenic) diet. This manipulation of macronutrients shifts the body's fuel source from carbohydrate to fat, which presents a 3-fold disadvantage to cancer cells: 1) owing to their dependence on aerobic glycolysis, many types of cancer cells lack the capacity to use fat as an energy source; 2) most cancer cells are also unable to metabolize the ketone bodies produced as a by-product of fatty acid metabolism (7); and 3) these ketone bodies also may inhibit cancer cells’ glycolytic metabolism, thereby diminishing growth (8).
Ketogenic diets (KDs) have been used as a therapy for a variety of neurologic diseases in humans, most extensively in epilepsy, as well as for metabolic disorders such as type 2 diabetes (T2D) (9). These diets generally produce a more favorable metabolic profile than low-fat diets (2, 10–13). In particular, KDs have been shown to decrease IGF-I and 24-h plasma glucose and increase insulin sensitivity (11, 12). Carbohydrate restriction also selectively diminishes metabolically hazardous adipose depots while maintaining lean mass (14, 15).
Although animal models have demonstrated that diet modification can yield profound effects, such as preventing cancer initiation, inhibiting tumor growth, enhancing the effects of radiation and chemotherapy, and prolonging survival (16–22), few studies have assessed the effects of KDs in humans with cancer. The limited evidence available suggests that KDs are safe, feasible, and may contribute to improved or stable disease status among cancer patients (23–27).
Ovarian and endometrial cancer are among the most deadly female cancer types in the United States (28) and both are associated with obesity (29, 30). The link between obesity and some cancer types involves glucose, insulin, and IGF-I, all of which are increased in obesity (31, 32). Indeed, epidemiologic studies provide strong evidence for the involvement of the insulin-IGF axis in several obesity-related cancers (33–37), although data are somewhat limited for endometrial and ovarian cancer specifically. However, overexpression of insulin and IGF-I receptors has been observed in both of these cancer types (38–40). Accordingly, an insulin-lowering KD may diminish growth factors needed for proliferation in ovarian and endometrial cancers. The objective of this study was to examine the effects of 12 wk of treatment with either the KD or the lower-fat American Cancer Society diet (ACS) on body composition and biochemical analytes in women with ovarian or endometrial cancer. Biochemical analytes included: glucose, insulin, C-peptide (a gauge of pancreatic insulin production), β-hydroxybutyrate (a ketone indicative of fatty acid metabolism), IGF-I, and insulin-like growth factor binding protein 1 (IGFBP-1). We tested the hypothesis that a KD would improve body composition and lower serum insulin and IGF-I in this patient population.
Methods
Participants
This study was conducted at the University of Alabama at Birmingham (UAB, Birmingham, AL). Participants were women with ovarian or endometrial cancer, recruited between October 2015 and April 2017, from the UAB Gynecologic Oncology clinic and from other treatment centers via physician referral, flyers, local television advertisements, and news articles. Eligibility criteria were BMI (kg/m2) ≥18.5, age ≥19 y, no pre-existing medical conditions affecting body weight (other than cancer and associated treatment), not actively attempting weight loss/gain or diet modification, and no medical history contraindicating enrollment. Women with T2D were eligible to participate (4 with T2D completed the study; 3 managed blood glucose for the duration of the study using only metformin, and 1 used only insulin glargine at baseline but discontinued use after initiating the KD). UAB's Institutional Review Board approved the study, and all participants provided written informed consent prior to enrollment.
Protocol and procedures
This study was conducted as a randomized clinical trial (NCT03171506) with parallel arm design. Fasting serum concentrations of glucose, insulin, C-peptide, IGF-I, IGFBP-1, and β-hydroxybutyrate were obtained at baseline and at 12 wk; body composition was measured at these same time points. Participants were randomly assigned, through the use of a computer-generated blocked randomization scheme (created by KRF), to either the ACS or the KD group. Because this was a diet intervention study, it was not possible for participants or study personnel to be blinded to group assignment. The project coordinator enrolled and assigned participants to their interventions.
Diet interventions
The ACS diet consisted of general guidelines to encourage intake of antioxidants and fiber, while reducing consumption of saturated fat and added sugars; these guidelines were based on recommendations established by the American Cancer Society and the Academy of Nutrition and Dietetics (41, 42). The KD had a macronutrient distribution of ∼5% of energy from carbohydrate (≤20 g/d), 25% energy from protein (≤100 g/d), and 70% energy from fat (≥125 g/d). Carbohydrate foods were limited to nonstarchy vegetables such as salad greens, broccoli, and summer squash. Permitted protein foods included meat, poultry, eggs, and fish, provided that they were neither breaded nor battered. Fat-containing foods included olive and coconut oils, avocados, butter, olives, cheese, cream, and small amounts of nuts. KD participants were instructed to avoid all grains and grain products, starchy vegetables, and fruit. Total energy intake was not restricted for either the ACS or KD.
A registered dietitian provided diet-specific nutrition education to each participant immediately after baseline testing. Additional counseling was provided via phone and e-mail on a weekly basis, and included distribution of sample meal plans and recipes. When necessary, based on feedback from the participant, diet recommendations were further individualized to enhance adherence, assessed via weekly food record reviews. In addition, KD participants were also asked to measure urinary ketones with Ketostix (Bayer AG, Leverkusen, Germany). They submitted smartphone photographs of their strips via e-mail daily for the first 2 wk, and thereafter on a weekly basis.
Body composition
Total body fat, lean mass, and visceral fat were measured by DXA with the use of a Lunar iDXA densitometer [GE Medical Systems, Madison, WI, enCORE Version 15 (SP2)]. Participants were required to wear light clothing, remove all metal objects, and lie supine with arms at their sides during the scan.
Serum chemistry
Glucose was measured with a SIRRUS analyzer (Stanbio Laboratory, Boerne, TX); intra-assay CV of 1.3% and interassay CV of 2.5%. Insulin was assayed by immunofluorescence through the use of the TOSOH AIA-II analyzer (TOSOH Corp, South San Francisco, CA); intra-assay CV of 1.5% and interassay CV of 4.0%. C-peptide was assayed by immunofluorescence through the use of the TOSOH analyzer; intra-assay CV of 1.7% and interassay CV of 6.8%. β-hydroxybutyrate was measured with a SIRRUS analyzer (Stanbio Laboratory, Boerne, TX) by colorimetric assay; intra-assay CV of 5.2% and interassay CV of 3.0%. IGF-I was measured by ELISA (American Laboratory Products Company, Salem, NH); intra-assay CV of 4.4% and interassay CV of 6.1%. IGFBP-1 was also measured by ELISA (American Laboratory Products Company, Salem, NH); intra-assay CV of 4.9% and interassay CV of 4.7%.
Statistical methods
Descriptive statistics were calculated for all study variables. Visceral fat was log10 transformed to ensure a normal distribution. All statistical tests were 2-sided, with an α level of 0.05 denoting statistical significance. Statistical analyses were performed with SAS (version 9.4; SAS Institute, Cary, NC). Sample size calculations were based on the change in fasting insulin during a previous diet intervention in which a decrease of 2.7 ± 4.6 µU/mL was detected after 8 wk of consumption of a reduced-carbohydrate diet (43). Statistical power analysis indicated that 25 individuals per group were required to have 80% power to detect a 2.7 ± 4.6 µU/mL difference in fasting insulin, through the use of a 2-sided paired t test and a significance alpha level of 0.05. ANCOVA was used to compare the effects of the study diets on body composition and biochemical analytes, through the use of baseline values and the change in total fat mass and/or the change in weight as covariates where appropriate. Independent t tests were used to determine the effect of the diet on percentage of change in visceral fat, to assess differences between diet groups for baseline demographic variables, and to compare participants who did with those who did not complete the study. Pearson correlation analyses were used to estimate associations between body composition and biochemical analytes. One outlier (>3 SDs above the mean on fasting insulin concentration) was excluded from insulin-related analyses. In addition, 1 participant who was using exogenous insulin at baseline but not at 12 wk was excluded from all insulin analyses. One outlier (>3 SDs above the mean on IGFBP-1 and IGF-I) was also excluded from analyses involving IGFBP-1 and IGF-I. Visceral fat mass data were not available for 5 participants who exceeded the DXA limits for body width. Serum samples were not available for 2 participants in the KD group.
Results
Patient characteristics
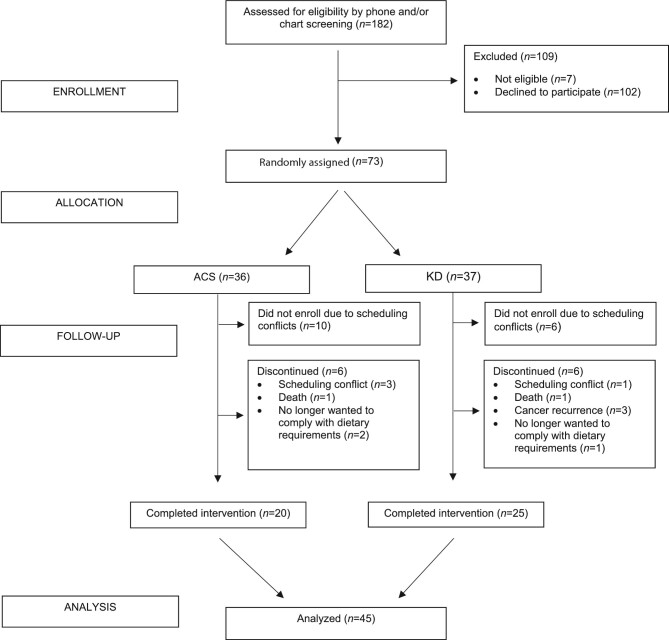
Of the 182 women approached for enrollment, 73 women were randomly assigned in this study (Figure 1). However, for a variety of reasons, only 57 went on their randomized diet, 45 of whom completed the trial. Four women withdrew during the study owing to scheduling conflicts, 2 women died during the study, 3 women experienced a cancer recurrence and no longer wished to participate, and 3 women no longer wanted to comply with their assigned diet. In all, 6 women from each diet group discontinued the study. Those who withdrew did not differ from participants who completed the trial on BMI or fat mass at baseline. However, dropouts were younger (P = 0.01) than those who completed the study. Baseline characteristics of the 45 study participants by diet group are shown in Table 1. The groups did not differ in age, racial distribution, BMI, cancer type, percentage of patients receiving concurrent chemotherapy, number of previous chemotherapies, or time since initial cancer diagnosis.
FIGURE 1.
Participant flow diagram. ACS, American Cancer Society diet; KD, ketogenic diet.
TABLE 1.
Baseline characteristics of study participants by diet1
| ACS (n = 20) | KD (n = 25) | |
|---|---|---|
| Age, y | 58.6 ± 11.7 | 61.5 ± 8.5 |
| Race, white/black/Asian, n/n/n | 17/3/0 | 22/2/1 |
| BMI, kg/m2 | 33.0 ± 10.7 | 30.7 ± 8.0 |
| Ovarian cancer, stage I/II/III/IV, n/n/n/n | 3/3/6/1 | 2/2/10/1 |
| Endometrial cancer, stage I/II/III/IV, n/n/n/n | 6/0/1/0 | 7/2/1/0 |
| Receiving concurrent chemotherapy, % | 4 (20.0) | 7 (28.0) |
| Previous chemotherapies, n | 2.4 ± 1.6 | 1.6 ± 1.4 |
| Time since initial diagnosis, y | 4.9 ± 5.0 | 2.4 ± 3.0 |
Values are means ± SDs unless otherwise indicated. ACS, American Cancer Society diet; KD, ketogenic diet.
Dietary adherence
KD participants were expected to achieve urinary ketone concentrations of ∼0.5 mmol/L, as indicated by a relative color change on the Ketostix; 20 of the 25 KD participants (80%) achieved this level of ketosis within the first 3 wk of the diet intervention. Based on communications with the study dietitian and regular food record reviews, ∼80% of all participants were considered adherent to their assigned diets.
Body composition
Table 2 and Figure 2 show body composition results by diet. At 12 wk, those randomly assigned to the KD had significantly less total body fat, android fat, and visceral fat after adjusting for the baseline condition (P < 0.05 for all). The percentage of change in visceral fat mass was also greater in the KD group than in the ACS group (–21.2% compared with –4.6%, P < 0.05). In contrast, adjusted lean mass did not differ significantly between groups at 12 wk. Although there were modest reductions in fat mass in the ACS group, adjusted fat mass was lower in the KD group in all regions (P < 0.05 for all).
TABLE 2.
Baseline and 12-wk body composition outcomes in women with ovarian or endometrial cancer who consumed the ACS and KD1
| ACS (n = 20) | KD (n = 25) | |||
|---|---|---|---|---|
| Week 0 | Week 12 | Week 0 | Week 12 | |
| Total fat mass, kg | 44.1 ± 4.6 | 41.2 ± 4.4 | 37.9 ± 3.2 | 32.7 ± 3.1* |
| Android fat, kg | 3.9 ± 0.5 | 3.6 ± 0.5 | 3.4 ± 0.4 | 2.8 ± 0.4* |
| Visceral fat,2 g | 1150 ± 210.0 | 1024 ± 175.6 | 1152 ± 164.6 | 975 ± 150.9* |
| Trunk fat, kg | 22.2 ± 2.6 | 20.9 ± 2.4 | 19.4 ± 1.8 | 16.3 ± 1.8*** |
| Gynoid fat, kg | 7.7 ± 0.9 | 7.3 ± 0.8 | 6.6 ± 0.5 | 5.7 ± .05*** |
| Arm fat, kg | 4.8 ± 0.5 | 4.6 ± 0.5 | 4.2 ± 0.4 | 3.7 ± 0.4* |
| Leg fat, kg | 15.5 ± 1.7 | 15.0 ± 1.6 | 13.4 ± 1.1 | 11.8 ± 1.1** |
| Total lean mass, kg | 44.9 ± 2.5 | 44.8 ± 2.8 | 43.3 ± 1.3 | 42.4 ± 1.4 |
Values are means ± SEMS. *P < 0.05; **P < 0.01; ***P < 0.001 compared with week 12 ACS. P values from ANCOVA, adjusted for baseline condition. ACS, American Cancer Society diet; KD, ketogenic diet.
Visceral fat mass data were not available for 5 participants.
FIGURE 2.
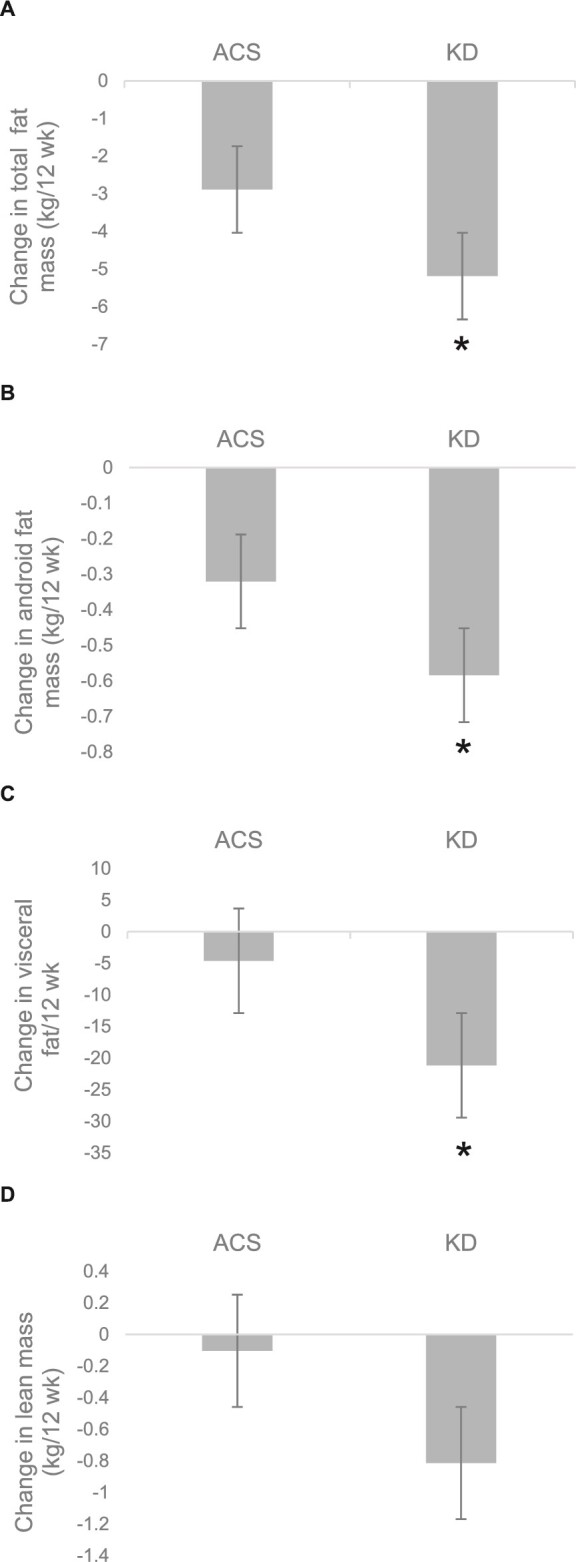
Changes in (A) total fat, (B) android fat, (C) visceral fat, and (D) lean mass in women with ovarian or endometrial cancer who consumed the ACS and KD. Values are means ± SEMs, n = 20 (ACS) or 25 (KD). *Different from ACS by ANCOVA, with the use of baseline values and baseline total fat mass as covariates where appropriate, P < 0.05. ACS, American Cancer Society diet; KD, ketogenic diet.
Metabolic effects
Compared with the ACS group, the KD group demonstrated lower fasting serum concentrations of insulin (P < 0.01) and C-peptide (P < 0.01) at 12 wk, after adjusting for weight loss and baseline values (see Table 3). In addition, the adjusted serum β-hydroxybutyrate concentration after the diet intervention was significantly higher in the KD group than in the ACS group (P < 0.001). The groups did not significantly differ in the adjusted mean at 12 wk for IGF-I or IGFBP-1.
TABLE 3.
Baseline and 12-wk fasting serum analytes in women with ovarian or endometrial cancer who consumed the ACS and KD1
| ACS (n = 20) | KD (n = 23)2 | |||
|---|---|---|---|---|
| Week 0 | Week 12 | Week 0 | Week 12 | |
| Glucose, mg/dL | 102.2 ± 3.6 | 98.7 ± 2.5 | 102.3 ± 3.9 | 93.0 ± 3.3 |
| Insulin,3 µU/mL | 14.2 ± 2.2 | 12.1 ± 1.5 | 10.5 ± 1.4 | 6.7 ± 0.9** |
| C-peptide, ng/mL | 3.2 ± 0.3 | 3.0 ± 0.3 | 2.8 ± 0.3 | 2.0 ± 0.3** |
| β-hydroxybutyrate, mmol/L | 0.20 ± 0.02 | 0.25 ± 0.04 | 0.36 ± 0.15 | 0.91 ± 0.16*** |
| IGF-I,4 ng/mL | 116 ± 13.7 | 111 ± 13.4 | 120.3 ± 8.9 | 100.7 ± 9.6 |
| IGFBP-1,4 µg/mL | 1.8 ± 0.2 | 1.7 ± 0.2 | 1.8 ± 0.2 | 2.1 ± 0.3 |
Values are means ± SEMs. **P < 0.01; ***P < 0.001 compared with week 12 ACS. P values from ANCOVA, adjusted for baseline condition. ACS, American Cancer Society diet; IGF-I, insulin-like growth factor I; IFGBP-1, insulin-like growth factor binding protein 1; KD, ketogenic diet.
Serum samples were not available for 2 participants.
One participant receiving exogenous insulin and 1 outlier excluded.
One outlier excluded.
Correlation analyses
Correlation analyses revealed a significant positive association between the change in android fat and change in the fasting serum insulin concentration (r = 0.32, P < 0.05) (Figure 3). A similar positive association was observed between the change in visceral fat and change in fasting serum insulin concentration (r = 0.33, P < 0.05). In addition, there was a significant inverse association between the change in serum β-hydroxybutyrate and the change in IGF-I (r = –0.57, P < 0.0001).
FIGURE 3.
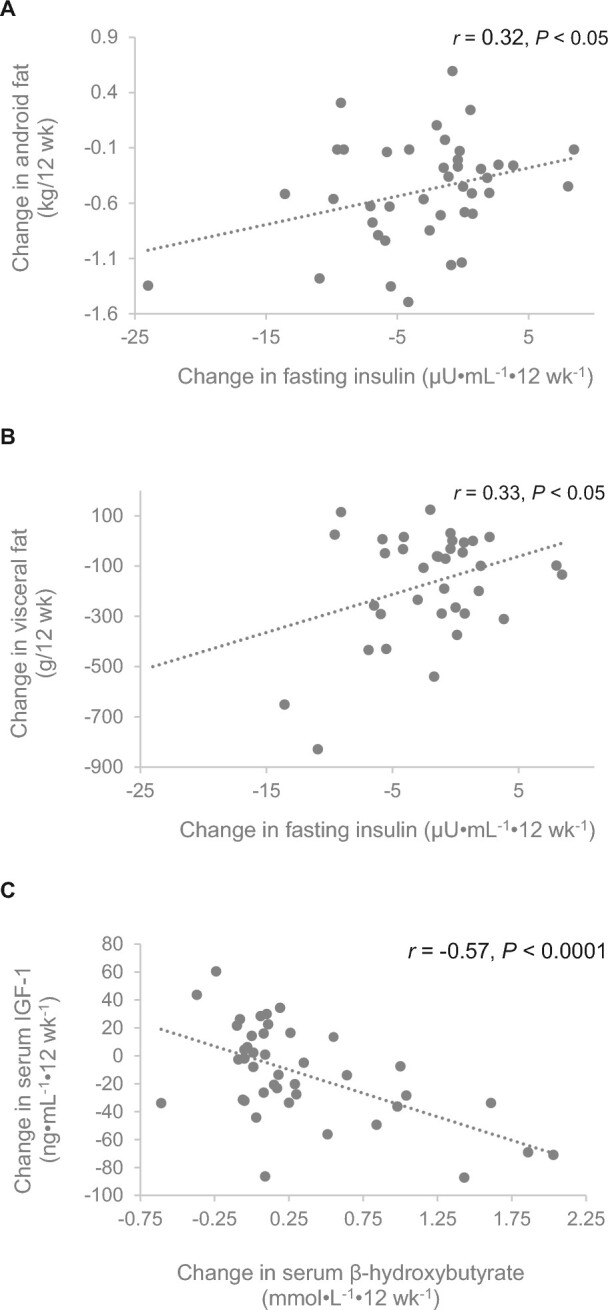
Associations of changes in (A) insulin and android fat mass, n = 20 (ACS) or 21 (KD), (B) insulin and visceral fat mass, n = 16 (ACS) or 20 (KD), and (C) β-hydroxybutyrate and IGF-I, n = 20 (ACS) or 23 (KD) in women with ovarian or endometrial cancer who consumed the ACS and KD. Data from both arms combined. ACS, American Cancer Society diet; KD, ketogenic diet.
Discussion
Cancer cells’ dependence on aerobic glycolysis renders them vulnerable in situations of glucose and insulin deprivation. Accordingly, diets that limit carbohydrates such as a KD may provide a nonpharmacologic means of disrupting cancer cell growth and replication. In this investigation, we tested the hypothesis that a KD would improve body composition and lower insulin and IGF-I in women with ovarian or endometrial cancer. Our results indicated that, compared with the ACS diet, the KD resulted in lower total body fat, android fat, and visceral fat, while maintaining lean mass. Those in the KD group also demonstrated lower serum insulin and C-peptide, independent of weight loss. Finally, a greater degree of ketosis, as indicated by higher concentrations of serum β-hydroxybutyrate, was associated with lower IGF-I. In aggregate, these findings suggest that the KD creates a metabolic environment that is not supportive of cancer proliferation.
We found that the KD decreased fat mass in several fat depots, including visceral fat. Previous studies examining the effects of carbohydrate restriction on body composition have generated similar results in various patient populations. Specifically, a lower-carbohydrate diet (40–43% of total energy) decreased total fat and intra-abdominal adipose tissue to a greater extent than a low-fat diet in women with polycystic ovary syndrome as well as in overweight adults at risk of T2D (14, 15). Studies examining KDs have also demonstrated significant reductions in fat mass, particularly in the visceral and android regions, in overweight and obese adults (44–46). Visceral fat is widely noted for its association with increased cardiometabolic risk, but recent evidence also suggests that this type of adipose tissue may likewise increase cancer risk (47, 48). A potential mechanism connecting visceral adiposity and cancer could relate to visceral fat's secretion of proinflammatory cytokines, which may contribute to insulin resistance and a subsequent elevation of proliferative growth factors (49). Thus, given the potential adverse effects associated with central obesity, depletion of visceral and android fat may shift body composition toward a profile that coincides with favorable metabolic health.
The substantial losses of total and visceral fat in the KD group were not accompanied by a concurrent loss of lean mass. We found no significant difference between groups in lean mass after 12 wk of the dietary intervention. Accordingly, the KD appeared to have induced a selective depletion of fat mass and retention of lean mass. These findings contribute to a growing body of literature indicating that the maintenance of lean body mass during weight loss may respond in part to diet composition. For example, in obese young men, progressively greater total and relative body fat were lost as the carbohydrate content of the diet was decreased, such that at the highest level of carbohydrate restriction, 95% of the weight lost was fat, and thus loss of lean mass was minimal (50). Furthermore, 6 wk of low-carbohydrate diet resulted in an increase in lean body mass in healthy men, despite no change in physical activity; the entire loss of body weight was from body fat (51). Similarly, in cancer patients receiving concurrent radiotherapy, a KD induced a loss of fat mass while preserving fat-free mass (52). A lower-carbohydrate diet may protect lean mass by lowering insulin, which is associated with greater loss of lean mass during energy deficit (53). In addition, the elevation of blood ketones may contribute to the preservation of lean mass by providing an alternate fuel source to the brain and other tissues, thereby limiting the use of amino acids from muscle as a substrate for gluconeogenesis (54). It is worth noting that such preservation of lean mass is of particular importance in cancer patients, a population in which the incidence of sarcopenia is high (55).
In addition to these improvements in body composition, we also found significant changes in biochemical parameters, including fasting insulin and C-peptide. The KD resulted in lower fasting insulin in comparison to the ACS diet, a trend that has been observed previously in murine models (56, 57). This reduction in fasting insulin suggests that a KD may improve insulin sensitivity. We also observed that there was a significant positive association between the change in fasting insulin concentration and the change in android fat. The precise mechanism underlying this relation is unclear, but it may involve improved insulin sensitivity, which has been observed with the KD (11). Increased insulin sensitivity, as observed with use of the insulin-sensitizing thiazolidinedione drugs, results in a redistribution of lipid from ectopic to peripheral adipose tissue depots (58). Thus, it is plausible that both the decrease in insulin and the selective depletion of visceral fat are manifestations of improved insulin sensitivity (i.e., share a common antecedent).
Elevated concentrations of IGF-I have been associated with several common cancer types (34, 36), potentially due to its mitogenic and antiapoptotic effects. In our sample, there was a trend toward significance for lower IGF-I on the KD compared with the ACS diet. Further, as seen in Figure 3, there was a significant inverse association between the change in serum β-hydroxybutyrate and the change in IGF-I. Variation in serum β-hydroxybutyrate among participants may have been due in part to variations in dietary intake (e.g., degree of carbohydrate restriction or glycemic load), or perhaps in part to individuals’ unique metabolic proclivity or resistance to achieving higher levels of ketosis. The importance of achieving ketosis was demonstrated in a small pilot study where cancer remission by 18F-2-fluoro-2-deoxyglucose positron emission tomography was directly related to blood ketone concentrations (25). Hence, research is needed to understand variation in the serum β-hydroxybutyrate response to the KD.
This study has several strengths. To our knowledge, this is the first randomized controlled trial examining the effects of a KD for cancer in humans. Furthermore, we conducted DXA scans on all participants, allowing us to quantify diet effects on total and regional body composition. The primary limitation of this study was the heterogeneous nature of the sample. Participants varied in cancer type, stage, treatment history, and concurrent chemotherapy status, which may have influenced the results. In addition, we did not provide the food for participants, which detracts from our ability to better control dietary intake and adherence but enhances the generalizability of our results. This study also did not include healthy control participants, which would have allowed for the comparison of the KD's effects in cancer-free women. Finally, our protocol lacked a measure of insulin sensitivity.
In summary, among women with ovarian or endometrial cancer, a 12-wk KD produced selective loss of total and visceral fat, maintenance of lean body mass, and decreases in cancer-related growth factors. Further study in a clinical setting is needed to determine whether the KD may be an effective nonpharmacologic adjuvant therapy for cancer.
Acknowledgments
The authors thank Maryellen Williams, Cindy Zeng, Nikki Bush, and Heather Hunter from the Metabolism/Human Physiology Core Laboratory of the Nutrition Obesity Research Center, Diabetes Research Center, and Center for Clinical and Translational Science for laboratory analyses. We also acknowledge Mary Kat Smith from UAB Gynecologic Oncology for assistance with participant recruitment. The authors’ contributions were as follows—BAG, KRF, and RDA: designed the research project; CWC, RCA, RDA, CAL, WKH, KSB, KHK, and JMS: conducted the research; BAG and CWC: analyzed the data; CWC, KRF, and BAG: wrote the manuscript; BAG and CWC: had primary responsibility for final content; and all authors: read and approved the final manuscript.
Notes
Supported by American Institute for Cancer Research, UAB Comprehensive Cancer Center, Nutrition Obesity Research Center grant P30DK56336, and Diabetes Research Center grant P60DK079626.
Author disclosures: CWC, KRF, RCA, RDA, CAL, WKH, KSB, KHK, JMS, and BAG, no conflicts of interest.
Abbreviations used:
- ACS
American Cancer Society diet
- IGF-I
insulin-like growth factor I
- IGFBP-1
insulin-like growth factor binding protein 1
- KD
ketogenic diet
- T2D
type 2 diabetes
Contributor Information
Caroline W Cohen, Departments of Nutrition Sciences, University of Alabama at Birmingham, Birmingham, AL.
Kevin R Fontaine, Departments of Health Behavior, University of Alabama at Birmingham, Birmingham, AL.
Rebecca C Arend, Obstetrics and Gynecology, University of Alabama at Birmingham, Birmingham, AL.
Ronald D Alvarez, Obstetrics and Gynecology, University of Alabama at Birmingham, Birmingham, AL.
Charles A Leath III, Obstetrics and Gynecology, University of Alabama at Birmingham, Birmingham, AL.
Warner K Huh, Obstetrics and Gynecology, University of Alabama at Birmingham, Birmingham, AL.
Kerri S Bevis, Obstetrics and Gynecology, University of Alabama at Birmingham, Birmingham, AL.
Kenneth H Kim, Obstetrics and Gynecology, University of Alabama at Birmingham, Birmingham, AL.
John M Straughn, Jr, Obstetrics and Gynecology, University of Alabama at Birmingham, Birmingham, AL.
Barbara A Gower, Departments of Nutrition Sciences, University of Alabama at Birmingham, Birmingham, AL.
References
- 1. Taubes G. Cancer research. Unraveling the obesity-cancer connection. Science 2012;335(6064):28, 30–2. [DOI] [PubMed] [Google Scholar]
- 2. Klement RJ, Kammerer U. Is there a role for carbohydrate restriction in the treatment and prevention of cancer? Nutr Metab (Lond) 2011;8:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dowling RJ, Goodwin PJ, Stambolic V. Understanding the benefit of metformin use in cancer treatment. BMC Med 2011;9:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Simone BA, Champ CE, Rosenberg AL, Berger AC, Monti DA, Dicker AP, Simone NL. Selectively starving cancer cells through dietary manipulation: methods and clinical implications. Future Oncol 2013;9(7):959–76. [DOI] [PubMed] [Google Scholar]
- 5. Siddle K. Molecular basis of signaling specificity of insulin and IGF receptors: neglected corners and recent advances. Front Endocrinol (Lausanne) 2012;3:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. D'Esposito V, Passaretti F, Hammarstedt A, Liguoro D, Terracciano D, Molea G, Canta L, Miele C, Smith U, Beguinot F, et al. Adipocyte-released insulin-like growth factor-1 is regulated by glucose and fatty acids and controls breast cancer cell growth in vitro. Diabetologia 2012;55(10):2811–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zuccoli G, Marcello N, Pisanello A, Servadei F, Vaccaro S, Mukherjee P, Seyfried TN. Metabolic management of glioblastoma multiforme using standard therapy together with a restricted ketogenic diet: case report. Nutr Metab (Lond) 2010;7:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fine EJ, Miller A, Quadros EV, Sequeira JM, Feinman RD. Acetoacetate reduces growth and ATP concentration in cancer cell lines which over-express uncoupling protein 2. Cancer Cell Int 2009;9:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Paoli A, Rubini A, Volek JS, Grimaldi KA. Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur J Clin Nutr 2013;67(8):789–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Volek JS, Phinney SD, Forsythe CE, Quann EE, Wood RJ, Puglisi MJ, Kraemer WJ, Bibus DM, Fernandez ML, Feinman RD. Carbohydrate restriction has a more favorable impact on the metabolic syndrome than a low fat diet. Lipids 2009;44(4):297–309. [DOI] [PubMed] [Google Scholar]
- 11. Boden G, Sargrad K, Homko C, Mozzoli M, Stein TP. Effect of a low-carbohydrate diet on appetite, blood glucose levels, and insulin resistance in obese patients with type 2 diabetes. Ann Intern Med 2005;142(6):403–11. [DOI] [PubMed] [Google Scholar]
- 12. Fraser DA, Thoen J, Bondhus S, Haugen M, Reseland JE, Djoseland O, Forre O, Kjeldsen-Kragh J. Reduction in serum leptin and IGF-1 but preserved T-lymphocyte numbers and activation after a ketogenic diet in rheumatoid arthritis patients. Clin Exp Rheumatol 2000;18(2):209–14. [PubMed] [Google Scholar]
- 13. Young LR, Kurzer MS, Thomas W, Redmon JB, Raatz SK. Low-fat diet with omega-3 fatty acids increases plasma insulin-like growth factor concentration in healthy postmenopausal women. Nutr Res 2013;33(7):565–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goss AM, Goree LL, Ellis AC, Chandler-Laney PC, Casazza K, Lockhart ME, Gower BA. Effects of diet macronutrient composition on body composition and fat distribution during weight maintenance and weight loss. Obesity (Silver Spring) 2013;21(6):1139–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goss AM, Chandler-Laney PC, Ovalle F, Goree LL, Azziz R, Desmond RA, Wright Bates G, Gower BA. Effects of a eucaloric reduced-carbohydrate diet on body composition and fat distribution in women with PCOS. Metabolism 2014;63(10):1257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ho VW, Leung K, Hsu A, Luk B, Lai J, Shen SY, Minchinton AI, Waterhouse D, Bally MB, Lin W, et al. A low carbohydrate, high protein diet slows tumor growth and prevents cancer initiation. Cancer Res 2011;71(13):4484–93. [DOI] [PubMed] [Google Scholar]
- 17. Abdelwahab MG, Fenton KE, Preul MC, Rho JM, Lynch A, Stafford P, Scheck AC. The ketogenic diet is an effective adjuvant to radiation therapy for the treatment of malignant glioma. PLoS One 2012;7(5):e36197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Allen BG, Bhatia SK, Buatti JM, Brandt KE, Lindholm KE, Button AM, Szweda LI, Smith BJ, Spitz DR, Fath MA. Ketogenic diets enhance oxidative stress and radio-chemo-therapy responses in lung cancer xenografts. Clin Cancer Res 2013;19(14):3905–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stafford P, Abdelwahab MG, Kim DY, Preul MC, Rho JM, Scheck AC. The ketogenic diet reverses gene expression patterns and reduces reactive oxygen species levels when used as an adjuvant therapy for glioma. Nutr Metab (Lond) 2010;7:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Seyfried TN, Sanderson TM, El-Abbadi MM, McGowan R, Mukherjee P. Role of glucose and ketone bodies in the metabolic control of experimental brain cancer. Br J Cancer 2003;89(7):1375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Allen BG, Bhatia SK, Anderson CM, Eichenberger-Gilmore JM, Sibenaller ZA, Mapuskar KA, Schoenfeld JD, Buatti JM, Spitz DR, Fath MA. Ketogenic diets as an adjuvant cancer therapy: history and potential mechanism. Redox Biol 2014;2:963–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Poff AM, Ari C, Seyfried TN, D'Agostino DP. The ketogenic diet and hyperbaric oxygen therapy prolong survival in mice with systemic metastatic cancer. PLoS One 2013;8(6):e65522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schmidt M, Pfetzer N, Schwab M, Strauss I, Kammerer U. Effects of a ketogenic diet on the quality of life in 16 patients with advanced cancer: a pilot trial. Nutr Metab (Lond) 2011;8(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Champ CE, Palmer JD, Volek JS, Werner-Wasik M, Andrews DW, Evans JJ, Glass J, Kim L, Shi W. Targeting metabolism with a ketogenic diet during the treatment of glioblastoma multiforme. J Neurooncol 2014;117(1):125–31. [DOI] [PubMed] [Google Scholar]
- 25. Fine EJ, Segal-Isaacson CJ, Feinman RD, Herszkopf S, Romano MC, Tomuta N, Bontempo AF, Negassa A, Sparano JA. Targeting insulin inhibition as a metabolic therapy in advanced cancer: a pilot safety and feasibility dietary trial in 10 patients. Nutrition 2012;28(10):1028–35. [DOI] [PubMed] [Google Scholar]
- 26. Jansen N, Walach H. The development of tumours under a ketogenic diet in association with the novel tumour marker TKTL1: a case series in general practice. Oncol Lett 2016;11(1):584–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tan-Shalaby JL, Carrick J, Edinger K, Genovese D, Liman AD, Passero VA, Shah RB. Modified Atkins diet in advanced malignancies - final results of a safety and feasibility trial within the Veterans Affairs Pittsburgh Healthcare System. Nutr Metab (Lond) 2016;13:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. American Cancer Society. Cancer facts and figures 2017 [Internet]. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures-2017.pdf.
- 29. Collaborative Group on Epidemiological Studies of Ovarian Cancer. Ovarian cancer and body size: individual participant meta-analysis including 25,157 women with ovarian cancer from 47 epidemiological studies. PLoS Med 2012;9(4):e1001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dougan MM, Hankinson SE, Vivo ID, Tworoger SS, Glynn RJ, Michels KB. Prospective study of body size throughout the life-course and the incidence of endometrial cancer among premenopausal and postmenopausal women. Int J Cancer 2015;137(3):625–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Belardi V, Gallagher EJ, Novosyadlyy R, LeRoith D. Insulin and IGFs in obesity-related breast cancer. J Mammary Gland Biol Neoplasia 2013;18(3–4):277–89. [DOI] [PubMed] [Google Scholar]
- 32. Hursting SD, DiGiovanni J, Dannenberg AJ, Azrad M, Leroith D, Demark-Wahnefried W, Kakarala M, Brodie A, Berger NA. Obesity, energy balance, and cancer: new opportunities for prevention. Cancer Prev Res (Phila) 2012;5(11):1260–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cohen DH, LeRoith D. Obesity, type 2 diabetes, and cancer: the insulin and IGF connection. Endocr Relat Cancer 2012;19(5):F27–45. [DOI] [PubMed] [Google Scholar]
- 34. Kaaks R. Nutrition, insulin, IGF-1 metabolism and cancer risk: a summary of epidemiological evidence. Novartis Found Symp 2004;262:247–60,discussion 260–8. [PubMed] [Google Scholar]
- 35. Rinaldi S, Cleveland R, Norat T, Biessy C, Rohrmann S, Linseisen J, Boeing H, Pischon T, Panico S, Agnoli C, et al. Serum levels of IGF-I, IGFBP-3 and colorectal cancer risk: results from the EPIC cohort, plus a meta-analysis of prospective studies. Int J Cancer 2010;126(7):1702–15. [DOI] [PubMed] [Google Scholar]
- 36. Renehan AG, Zwahlen M, Minder C, O'Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet 2004;363(9418):1346–53. [DOI] [PubMed] [Google Scholar]
- 37. Endogenous Hormones Breast Cancer Collaborative Group, Key TJ, Appleby PN, Reeves GK, Roddam AW. Insulin-like growth factor 1 (IGF1), IGF binding protein 3 (IGFBP3), and breast cancer risk: pooled individual data analysis of 17 prospective studies. Lancet Oncol 2010;11(6):530–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Frasca F, Pandini G, Sciacca L, Pezzino V, Squatrito S, Belfiore A, Vigneri R. The role of insulin receptors and IGF-I receptors in cancer and other diseases. Arch Physiol Biochem 2008;114(1):23–37. [DOI] [PubMed] [Google Scholar]
- 39. Ouban A, Muraca P, Yeatman T, Coppola D. Expression and distribution of insulin-like growth factor-1 receptor in human carcinomas. Hum Pathol 2003;34(8):803–8. [DOI] [PubMed] [Google Scholar]
- 40. Wang CF, Zhang G, Zhao LJ, Qi WJ, Li XP, Wang JL, Wei LH. Overexpression of the insulin receptor isoform A promotes endometrial carcinoma cell growth. PLoS One 2013;8(8):e69001. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41. Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, Bandera EV, Hamilton KK, Grant B, McCullough M, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin 2012;62(4):243–74. [DOI] [PubMed] [Google Scholar]
- 42. Hamilton KK. Nutritional needs of the adult oncology patient. In: Leser M, Ledesma N, Bergerson S, Trujillo E, editors. Oncology nutrition for clinical practice. Chicago, IL: Oncology Nutrition Dietetic Practice Group of the Academy of Nutrition and Dietetics, 2013:33–9. [Google Scholar]
- 43. Gower BA, Chandler-Laney PC, Ovalle F, Goree LL, Azziz R, Desmond RA, Granger WM, Goss AM, Bates GW. Favourable metabolic effects of a eucaloric lower-carbohydrate diet in women with PCOS. Clin Endocrinol (Oxf) 2013;79(4):550–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gomez-Arbelaez D, Bellido D, Castro AI, Ordonez-Mayan L, Carreira J, Galban C, Martinez-Olmos MA, Crujeiras AB, Sajoux I, Casanueva FF. Body composition changes after very-low-calorie ketogenic diet in obesity evaluated by 3 standardized methods. J Clin Endocrinol Metab 2017;102(2):488–98. [DOI] [PubMed] [Google Scholar]
- 45. Moreno B, Crujeiras AB, Bellido D, Sajoux I, Casanueva FF. Obesity treatment by very low-calorie-ketogenic diet at two years: reduction in visceral fat and on the burden of disease. Endocrine 2016;54(3):681–90. [DOI] [PubMed] [Google Scholar]
- 46. Colica C, Merra G, Gasbarrini A, De Lorenzo A, Cioccoloni G, Gualtieri P, Perrone MA, Bernardini S, Bernardo V, Di Renzo L, et al. Efficacy and safety of very-low-calorie ketogenic diet: a double blind randomized crossover study. Eur Rev Med Pharmacol Sci 2017;21(9):2274–89. [PubMed] [Google Scholar]
- 47. Murphy RA, Bureyko TF, Miljkovic I, Cauley JA, Satterfield S, Hue TF, Klepin HD, Cummings SR, Newman AB, Harris TB. Association of total adiposity and computed tomographic measures of regional adiposity with incident cancer risk: a prospective population-based study of older adults. Appl Physiol Nutr Metab 2014;39(6):687–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kim B, Kim BC, Nam SY, Nam JH, Ryu KH, Park BJ, Sohn DK, Hong CW, Han KS, Kim HB. Visceral adipose tissue volume and the occurrence of colorectal adenoma in follow-up colonoscopy for screening and surveillance. Nutr Cancer 2017;69(5):739–45. [DOI] [PubMed] [Google Scholar]
- 49. Donohoe CL, Doyle SL, Reynolds JV. Visceral adiposity, insulin resistance and cancer risk. Diabetol Metab Syndr 2011;3:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Young CM, Scanlan SS, Im HS, Lutwak L. Effect on body composition and other parameters in obese young men of carbohydrate level of reduction diet. Am J Clin Nutr 1971;24(3):290–6. [DOI] [PubMed] [Google Scholar]
- 51. Volek JS, Sharman MJ, Love DM, Avery NG, Gomez AL, Scheett TP, Kraemer WJ. Body composition and hormonal responses to a carbohydrate-restricted diet. Metabolism 2002;51(7):864–70. [DOI] [PubMed] [Google Scholar]
- 52. Klement RJ, Sweeney RA. Impact of a ketogenic diet intervention during radiotherapy on body composition: I. Initial clinical experience with six prospectively studied patients. BMC Res Notes 2016;9:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hron BM, Ebbeling CB, Feldman HA, Ludwig DS. Relationship of insulin dynamics to body composition and resting energy expenditure following weight loss. Obesity (Silver Spring) 2015;23(11):2216–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Manninen AH. Very-low-carbohydrate diets and preservation of muscle mass. Nutr Metab (Lond) 2006;3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhang G, Li X, Sui C, Zhao H, Zhao J, Hou Y, Du Y. Incidence and risk factor analysis for sarcopenia in patients with cancer. Oncol Lett 2016;11(2):1230–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bielohuby M, Sawitzky M, Stoehr BJ, Stock P, Menhofer D, Ebensing S, Bjerre M, Frystyk J, Binder G, Strasburger C, et al. Lack of dietary carbohydrates induces hepatic growth hormone (GH) resistance in rats. Endocrinology 2011;152(5):1948–60. [DOI] [PubMed] [Google Scholar]
- 57. Garbow JR, Doherty JM, Schugar RC, Travers S, Weber ML, Wentz AE, Ezenwajiaku N, Cotter DG, Brunt EM, Crawford PA. Hepatic steatosis, inflammation, and ER stress in mice maintained long term on a very low-carbohydrate ketogenic diet. Am J Physiol Gastrointest Liver Physiol 2011;300(6):G956–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nakamura T, Funahashi T, Yamashita S, Nishida M, Nishida Y, Takahashi M, Hotta K, Kuriyama H, Kihara S, Ohuchi N, et al. Thiazolidinedione derivative improves fat distribution and multiple risk factors in subjects with visceral fat accumulation—double-blind placebo-controlled trial. Diabetes Res Clin Pract 2001;54(3):181–90. [DOI] [PubMed] [Google Scholar]