ABSTRACT
All membrane-bound organelles are degraded during the terminal differentiation of lens fiber cells. How these organelles are degraded has been a long-standing question in biology. We recently revealed that PLAAT (phospholipase A and acyltransferase)-family phospholipases degrade organelles in the lens independently of macroautophagy. Here, we discuss the mechanism and physiological relevance of this new mode of intracellular degradation.
KEYWORDS: Autophagy, HRASLS, lens, mice, organelle degradation, phospholipase, PLA2G16, PLAAT, zebrafish
The eye lens of vertebrates is composed of fiber cells that differentiate from epithelial cells and constitute the bulk of the lens by overlaying onto preexisting fiber cells (Figure 1A). The differentiation of lens fiber cells is accompanied by cell elongation, the expression of crystallins, and the degradation of intracellular organelles. The disappearance of the nuclei in the lens was first described by Carl Rabl in 1899. Later studies revealed that all membrane-bound organelles including mitochondria and the endoplasmic reticulum (ER) are also degraded during terminal differentiation. Although previous studies identified lysosomal DNases that degrade nuclear DNA in the lens, the mechanism of the degradation of other organelles such as mitochondria and the ER remained largely unknown. We previously showed that macroautophagy is dispensable for lens organelle degradation, suggesting the presence of a yet unknown mechanism.
Figure 1.
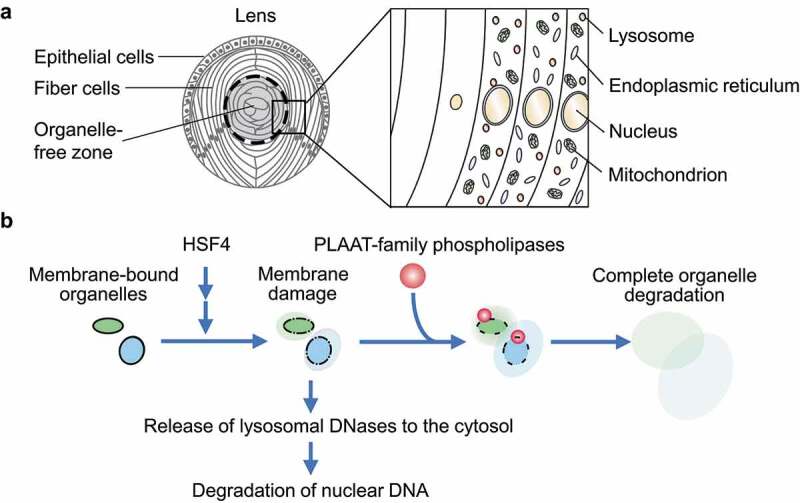
Model of organelle degradation in the lens by PLAAT-family phospholipases. (A) A schematic representation of zebrafish lens and organelle degradation. (B) A model of PLAAT-family phospholipase-mediated organelle degradation in the lens
In our recent work, we revealed that PLAAT (phospholipase A and acyltransferase)-family phospholipases, which are conserved among vertebrates, degrade lens organelles such as mitochondria, the ER, and lysosomes (Figure 1B). Among the PLAAT homologs, Plaat1/Hrasls in zebrafish and PLAAT3/ HRASLS3/PLA2G16/H-rev107/AdPLA in mice are highly expressed in the lens and essential for lens organelle degradation. PLAAT phospholipases have a PLA1 (phospholipase A1)-PLA2 activity toward various glycerophospholipids.
How do PLAAT-family phospholipases degrade organelles?
Although Plaat1 and PLAAT3 have a predicted transmembrane domain in their C terminus, they exist in the cytosol before organelle degradation. During differentiation of the lens, organellar membranes are partially damaged by a currently unknown mechanism, but it does not sufficiently induce organelle degradation. Then, the partial membrane damage recruits PLAAT-family phospholipases to induce complete degradation of these organelles (Figure 1B). In vitro experiments using giant unilamellar vesicles revealed that small pore formation in membranes is sufficient for the recruitment of PLAAT3. A PLAAT3 mutant that is forced to localize to mitochondria in cultured cells also causes mitochondrial membrane rupture. Thus, PLAAT3 selectively targets and degrades partially damaged organelles. Degradation products such as lysophospholipids may also function as a natural detergent to further solubilize membranes.
What factors regulate the organelle targeting of PLAAT-family phospholipases?
The formation of initiating membrane damage and subsequent organelle degradation by PLAAT-family phospholipases in the lens depends on HSF4 (heat shock transcription factor 4), which regulates lens fiber differentiation (Figure 1B). HSF4 is transcribed and activated from the center to the periphery of the lens. This activation pattern of HSF4 explains why organelle degradation takes place in this order. However, it is currently unknown how HSF4 activation leads to membrane damage. Highly expressed intracellular proteins such as crystallins might damage membranes.
How is the nucleus degraded?
Nuclear DNA degradation occurs almost normally, even in the absence of Plaat1 or PLAAT3. It was already known that nuclear DNA is degraded by lysosomal DNases. How can lysosomal enzymes attack nuclear DNA? We found that lysosomal membranes are also partially damaged in a manner dependent on HSF4, but independent of PLAAT, causing the leakage of lysosomal DNases (Figure 1B). We suppose that the leaked DNases would degrade nuclear DNA. Nevertheless, PLAAT3 is partially required for nuclear DNA degradation in the mouse lens. It may be because PLAAT3 releases more DNases from lysosomes and/or degrades the nuclear envelope to facilitate access to DNA.
What is the physiological significance of lens organelle degradation?
Our study demonstrated that PLAAT-family phospholipase-dependent organelle degradation is required for the acquisition of lens transparency and refractive functions. In general, the refractive index of membrane-bound organelles and the cytoplasm differs, in part due to the high lipid content of organelles. Thus, lens organelle degradation would be important for abolishing the difference in the refractive index between the organelles and the surrounding cytosol, which will be filled with crystallins to achieve a high refractive index and transparency.
How are organellar components other than membranes eliminated?
We revealed that PLAAT-family phospholipases are critical for the degradation of not only organellar membranes but also matrix proteins and transmembrane proteins. We speculate that membrane permeabilization by PLAAT-family phospholipases enables other cytosolic degradation systems, such as the ubiquitin-proteasome system, to access the other organellar components. It is unknown whether lysosomal hydrolases also contribute to the degradation of organelles in addition to nuclear DNA. The identification of responsible hydrolases and ubiquitin ligases will reveal the events downstream of PLAAT-mediated membrane degradation.
Does PLAAT-dependent organelle degradation occur in non-lens tissues?
PLAAT-family phospholipases are abundantly expressed in several tissues, suggesting their possible roles in organelle degradation in non-lens tissues. In non-lens cells (e.g., HEK293T cells), exogenously overexpressed PLAAT3 specifically localizes to and ruptures peroxisomes, suggesting the role of PLAAT3 in peroxisomal homeostasis in non-lens tissues. This function of PLAAT3 is likely due to its interaction with peroxisomal PEX19 as previously reported by Uyama et al. In mammals, all organelles are eliminated not only in lens cells but also in erythrocytes and keratinocytes. Further studies on the other members of PLAAT-family phospholipases will clarify the general function of this new type of organelle degradation [1].
Funding Statement
This work was supported by Exploratory Research for Advanced Technology (ERATO; grant [JPMJER1702] to N.M.) from the Japan Science and Technology Agency (JST).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Reference
- [1].Morishita H, Eguchi T, Tsukamoto S, et al. Organelle degradation in the lens by PLAAT phospholipases. Nature. 2021. Apr 14;592(7855):634–638. [DOI] [PubMed] [Google Scholar]


