Abstract
Wound healing is a complex process with many interdependent pathophysiological and immunological mediators to restore the cellular integrity of damaged tissue. Cutaneous wound healing is the repair response to a multitude of pathologies induced by trauma, surgery, and burn leading to the restoration and functionality of the compromised cells. Many different methods have been employed to treat acute and chronic wounds, such as antimicrobial therapy, as most wounds are susceptible to infection from microbes and are difficult to treat. However, many antimicrobial agents have become ineffective in wound treatment due to the emergence of multiple drug-resistant bacteria, and failures in current wound treatment methods have been widely reported. For this reason, alternative therapies have been sought, one of which is the use of honey as a wound treatment agent. The use of honey has recently gained clinical popularity for possible use in wound treatment and regenerative medicine. With this high demand, a better delivery and application procedure is required, as well as research aiming at its bioactivity. Honey is a safe natural substance, effective in the inhibition of bacterial growth and the treatment of a broad range of wound types, including burns, scratches, diabetic boils (Skin abscesses associated with diabetic), malignancies, leprosy, fistulas, leg ulcers, traumatic boils, cervical and varicose ulcers, amputation, burst abdominal wounds, septic and surgical wounds, cracked nipples, and wounds in the abdominal wall. Honey comprises a wide variety of active compounds, including flavonoids, phenolic acid, organic acids, enzymes, and vitamins, that may act to improve the wound healing process. Tissue-engineered scaffolds have recently attracted a great deal of attention, and various scaffold fabrication techniques are being researched. Some incorporate honey to improve their delivery during wound treatment. Hence, the aim of this review is to summarize recent studies on the wound healing properties of honey.
Keywords: wound healing, honey, multidrug-resistant bacteria, antibacterial effects, antifungal properties
1. Introduction
A wound is a disturbance in the normal structure and function of the epidermis. The epidermis is considered the first line of defense and protection against trauma. Various mechanisms can cause wounds, such as acute injury (abrasion, puncture, and/or crushing), surgery, and physiological conditions that compromise the skin (e.g., ischemia and pressure). Wound healing is a complex process with many interdependent immunological and pathophysiological mediators to restore the cellular integrity of the damaged tissue [1]. Wound healing depends on the presence of multiple types of cells, the extracellular matrix (ECM), cytokines, and growth factors, in addition to restoring the functionality of the compromised cells. Four distinct and overlapping stages are involved – inflammation, proliferation/regeneration, and tissue fibroplasia [1]. Recently, there has been a major increase in the burden of wound healing management due to the presence of multiple drug-resistant bacteria that can interfere with the wound repair process. Therefore, alternative natural compounds have been sought. Among those compounds is honey. The therapeutic potential of honey in the treatment of wounds and ulcers was initially recognized by the Sumerians and was known as far back as 2100–2000 BC [2]. The beneficial properties of honey have been known since ancient times [3], and its therapeutic use remained popular until the advent of antibiotics [4]. Published data show that honey benefits wound healing in the chronic inflammatory phase via the scavenging of reactive oxygen species produced by neutrophils [5,6].
With the emergence of drug-resistant bacteria, many antimicrobial agents have become ineffective in wound treatment, and many failures in current wound treatment methods have been reported. For this reason, alternative therapies have been sought, one of which is the use of honey as a wound treatment agent [7,8]. The use of honey has recently gained clinical popularity for possible use in wound treatment and in regenerative medicine.
Honey is made from the nectar of flowers collected by honeybees and is composed mostly of glucose and fructose. However, it also contains vitamins, minerals, amino acids, enzymes, organic acids, and other compounds. Its composition is affected by seasonal variations as well as the geographic location where the nectar was gathered by the bees. The moisture content of the deposited nectar mixture reduces and dries out, becoming more concentrated and producing viscous honey [9,10,11,12].
Natural honey is composed of around 82% of water, carbohydrates, proteins, phytochemicals, antioxidants, and minerals. It has been proven that few of the ingredients that determine the biological and medical potential of this substance are likely to vary among the various types of honey [13]. The sugars in honey include, in descending order, the following: “fructose (38.2%), glucose (31.2%), disaccharides and some other tri-saccharides and higher saccharides (9%) and sucrose (0.7–1%)” [14]. Honey containing a wide range of active compounds, including flavonoids, organic acids, phenolic acid, vitamins, and enzymes, may improve wound healing [14]. The deposition of fibroblasts and collagen formation may also be promoted by the large amount of amino acids found in honey [15].
The natural properties of honey as well as its active compounds are crucial for the wound healing process (Figure 1). Natural honey is a viscous fluid; its jelly consistency creates a surface layer over the wound that inhibits the entrance of bacteria and protects the wound from dehydration [2]. Its high sugar content creates a higher osmotic gradient that pulls fluid up through the subdermal tissue and offers an additional glucose source for flourishing cellular components in the wounded area [2,16]. The water activity of honey is less than 0.91 aw, which prevents and controls the growth of bacteria on the wound surface [17,18] and causes fluid flow that flushes slough, debris, and necrotic tissue as well as microorganisms out of the wound. Apart from this, the low water activity of honey helps transport oxygen and nutrients from the deep tissue into the wound area. In addition, the low pH of honey increases tissue oxygenation, while free radicals, which lead to tissue damage, are removed by flavonoids and aromatic acids [19,20].
Figure 1.
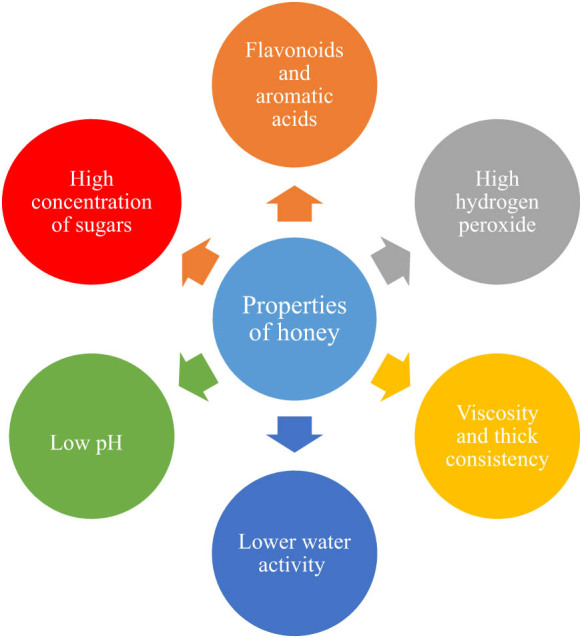
Some physicochemical properties of honey.
Another property that triggers antimicrobial activity in honey is the production of hydrogen peroxide on the glucose [5]. Certain types of honey do not rely on hydrogen peroxide for their antimicrobial activity but probably rely more on pH change and osmolarity for their bactericidal capability [5]. The unique Manuka factor (UMF) in Manuka honey (MH) is the methylglyoxal (MGO) level which is responsible for its antibacterial effect [21]. However, hydrogen peroxide-dependent honey stimulates the production of vascular endothelial growth factor (VEGF) and sterilizes the wound site [3,22]. In addition to glucose oxidase, the invertase produced by bees strengthens the osmotic potential of honey, dividing sucrose into fructose and glucose [3,22].
Two principal types of honey that have been researched are non-peroxidase MH and peroxidase based honey, both known for their efficacy in wound management [15]. Hence, the aim of this review is to summarize recent studies on the wound healing properties of honey.
2. The cascade of wound healing
The natural wound healing process includes a chain of events involving proteins, proteases, blood cells, growth factors, and ECM. The process comprises four successive and overlapping phases – hemostasis (blood vessels constrict to restrict blood flow), inflammation (controls bleeding and prevents infection), proliferation (rebuilding new tissue made up of ECM and collagen), and re-modeling (maturation) [23]. An imbalance in any one of these phases can result in over-induction of wound healing or attenuation of the healing process. In diseases, such as peripheral vascular diseases or type 2 diabetes, excessive inflammation can lead to a reduction or delay in the wound healing process [24]. On the other hand, over-induction of the healing process can be caused by excessive proliferation, resulting in scar or keloid formation.
3. Properties of honey
The therapeutic potency of honey is complex due to the presence of many compounds as well as variations in the composition of different types of honey [12,25]. It has specific physicochemical properties (Figure 1) that favor its use as a therapeutic agent to combat several microbial infections. These properties of honey are also associated with its wound healing effect, anti-inflammatory potency, antioxidant, and free radical scavenging ability. It is an immunomodulator with the power to enhance the immune system. It can be applied in the treatment of gastric ulcers, recurrent canine dermatitis, arthritis, diarrhea, tumors, and ulcers in diabetic patients; it can also be used for skin disinfection and wound healing [26,27]. In addition to its anti-inflammatory and antibacterial properties, honey enhances the wound healing process [7].
Honey is effective in curing a wide range of wound types, including trauma, burns, malignancy, leprosy, diabetic ulcers, boils, cervical varicose ulcers, scratches, leg ulcers, gastric ulcers, fistulas, amputation, burst abdominal wounds, septic and surgical wounds, cracked nipples, and wounds in the abdominal wall [28].
Many flavonoids, including pinobanksin, chrysin, and pinocembrin, as well as certain other compounds in lower levels, such as luteolin, quercetin, 8-methoxykaempferol, isorhamnetin, kaempferol, and galangin, are found in MH. Flavonoids provide honey with antioxidants and anti-inflammatory efficacy. Catechins, which are members of the flavone group of polyphenols, are often present in honey. Catechins have the potential to scavenge both superoxide and hydroxyl radicals [29] as well as the 1,1-diphenyl 1,3-picrylhydrazyl radical [30], proxy radicals [31], nitric oxide [32], carbon-centered free radicals, singlet oxygen and lipid free radicals [29], and also peroxynitrite by preventing the nitration of tyrosine [33].
4. Antimicrobial activities
According to the international guidelines on the proper use of antimicrobials in medicine, honey and other alternative therapeutics were used for the treatment of skin lesions in both humans and animals [34]. The antibacterial effect of honey has been reported in numerous studies [35,36,37]. Honey exerts bacteriostatic and bactericidal actions [26,37,38]. Many enzymes are present in an internal pouch of the bee called the crop and are transferred to the honey.
The antibacterial activity of non-peroxide honey is related to the presence of glyoxal, 3-deoxyglucosulose, and MGO. The concentration of MGO in honey is dependent on the geographic location and the kind of honey. However, it is well-known that MH has the highest concentrations of MGO compared to other types of honey. MGO is present in all kinds of honey, with levels ranging from 3 to 800 µg/gram, depending on the type of MH. The antibacterial efficacy of honey is dependent on the MGO content; honey will have a weaker or stronger effect on a narrower or wider spectrum of bacteria, particularly on the methicillin-resistant Staphylococcus aureus strains, vancomycin-resistant enterococci, and Pseudomonas aeruginosa. Nevertheless, studies have shown that a high MGO concentration is not required to exert antibacterial efficacy. For instance, in a study by Girma et al. (2019) [39], MH of lower UMF grade demonstrated significantly increased antimicrobial activity compared to higher UMF grade honey against tested S. aureus and E. coli. An MGO of 10+ UMF values were sufficient to provide antibacterial efficacy. It has been reported that high MGO may cause damage at the cellular level either through blood leading to its glycation or via other external pathways leading to malignant young cell degeneration [40].
The antimicrobial effects of honey have also been studied in various in vivo experiments, suggesting that this property of honey is crucial in reducing secondary bacterial contamination of the wound area and hastening the healing process [41].
Fluids in the wound are drawn out of the damaged tissues, leading to drying of cellular tissues and bacterial death [3]. In addition, phenolic compounds, organic acids, vitamins, and flavonoids exert antioxidant action and boost the antibacterial effect of honey. Flavonoids neutralize free radicals produced by hydrogen peroxide [3,22]. However, despite the increase in studies on the use of honey for wound healing, whether traumatic or surgical in origin, only a few studies on its use on infected wounds have been published. Some authors analyzed honey’s potential on the growth of selected intestinal bacteria [42] and in combatting pathogenic bacteria frequently isolated from skin wounds of mammals, including humans [35]. In a rat model, the topical application of honey on a dorsal wound resulted in an increase in both salt- and acid-soluble collagens by 107 and 117%, respectively. Further, it potentiated the levels of insoluble collagen to achieve a 109% increase after seven days of treatment compared to the untreated control [22].
Medical-grade honey (MGH) is seen as promising wound therapy because it has a wide spectrum of antimicrobial efficacy with no known resistant strains. It has been effective against clinical isolates of Pseudomonas aeruginosa and their associated biofilm formation [43].
Studies have shown that the supplements in the MGH formulation such as vitamins (C and E) enhanced the antimicrobial activity of pure honey. Supplementation of honey with other additives may, therefore, be a promising approach to further improve the antimicrobial activity of honey [44,45,46].
Another study evaluated the antibacterial efficacy of 57 Slovak blossom honeys against Pseudomonas aeruginosa and Staphylococcus aureus. Their data showed that different types of honey had different antibacterial potentials. Between acacia, wildflower and rapeseed honeys, the wildflower honey samples showed the greatest antibacterial activity, while rapeseed honeys had the highest level of minimal inhibitory concentration. There was a statistically significant association between the antibacterial activity of the honeys and their H2O2 and turgor pressure content. However, there was no correlation between glucose oxidase (GOX) and H2O2 content [47].
The results of another in vitro investigation revealed that lower supplemented honey at a lower percentage (40%) is more effective than 80% of MH against a wide range of commonly present cutaneous pathogens, including Methicillin-resistant Staphylococci and Pseudomonas species. Yet again, the supplements may have resulted in the improvement of the antimicrobial activity of honey [48].
5. MGH and its wound healing effects
Several types of honey, including MGH, have recently been re-introduced into modern medicine. There is no clear definition of MGH, but according to Hermann et al. (2020) [49], MGH must fulfill the following criteria:
Purity and being organic.
Free from toxic substances and contaminants.
Sterilized by gamma radiation under standardized procedures and having no pathogenic microorganisms.
Suitable for use in medical therapies.
Adheres to standardized production and storage methods, and all legal and safety regulations;
Satisfies physicochemical criteria necessary for use in wound treatment.
MH reduces inflammation and stimulates fibroblast migration and collagen deposition that support regeneration and hasten healing of the injured area [6]. Clinical studies have proven definitively that multiple varieties of honey, including MH, have the potential to close various types of infected non-healing ulcers [14,50,51,52]. In a study by Ranzato et al. (2012) [9], a 180% rate increase in keratinocyte closure and 150–240% fibroblast migration were recorded using 0.1% MH. Likewise, Efem [53] stated that application of honey on wounds caused rapid tissue debridement, stimulated quick epithelialization, and decreased the development of edema, causing quicker healing.
Bucekova et al. (2017) [54] reported that Def-1 peptide found in honey had a positive impact on cutaneous wound closure and exerted its effect by potentiating Keratinocyte migration and MMP-9 secretion.
MGH has attracted a great deal of interest recently; the term is used by healthcare professionals to refer to honey used in wound treatment [55]. In recent years, there has been a resurgence of interest in the use of MGH in the management of wounds. MGH possesses antibacterial efficacy and wound healing potential, including for mucositis in pediatric patients [26]. Some advantages of using MGH are that it is safe, easy to apply, and cost-effective for the treatment of severe wounds, burns, ulcers, oral mucositis in young pediatric patients, and in those suffering from cancer [56,57]. Honey’s anti-inflammatory effect and ability to treat local infections, promote autolytic debridement, disinfect wounds, and promote granulation tissue, which have been confirmed in previous literature [58]. A study evaluated the efficacy of honey in the treatment of wounds of lower leg and diabetic ulcers. The results showed a reduction in healing time, a much higher percentage of completely healed wounds, and better success in wound infections [50]. The therapeutic and wound healing effects of honey have been reported by others [59,60].
In a comparative study on topical application of MH and acacia honey in diabetic and normal rats, it was reported that MH achieved up to 80% wound contraction after nine days of treatment. In the MH-treated group, complete epithelialization was evident two days earlier than usual epithelialization [61].
The combined effect of topical honey with silver nanoparticles was assessed in an experimental wound healing process in rats, and the data showed that multiflora honey with silver nanoparticles enhanced the efficacy of wound contraction compared to honey alone [62].
The efficacy of topical application of mad honey, a rhododendron honey, was evaluated in wound healing in diabetic rats. The data showed that mad honey enhanced the healing process in diabetic rats and caused a significant decline in the levels of TNF-α, malondialdehyde, and MMP-9 expression. This was accompanied by an increase in the activities of the antioxidant enzyme and IL-10 expression in comparison to the untreated controls [63].
In addition, honey reduced excessive scar formation, thus improving the outcome of wound healing [64]. Based on that, the use of honey, which is a natural product, can be cost effective, safe, and efficient in the treatment of large and complicated wounds [65].
The mixture of bees’ honey and N. sativa is a relatively cheap and safe natural preparation used traditionally to cure human diseases since ancient times [66]. The combination enhanced the wound healing process by inhibiting the toxic effect of N. sativa, especially when the seed extract is used, in chronic and large surface area application [67].
Another study reported that the localized application of a mixture of honey and black seed oil in experimentally induced wounds on the ears of rabbits showed enhanced wound healing and a significant reduction in the wound area by the end of the fourth week. This finding suggests that the mixture of black seed oil and honey enhances wound healing without producing toxicity to the cells [68]. In a study on the synergistic effect of honey and N. sativa on wound healing was assessed in a rat model, and the results indicated that the mixture of honey and N. sativa seed oil significantly reduced the wound surface area compared to the control group [69].
The use of MGH as a therapeutic agent decreased malodor in a few days and stopped the infection within 2–3 weeks. MGH promoted wound healing by enhancing granulation tissue formation, angiogenesis, and re-epithelialization by reducing oxidative stress and providing nutrients.
Yilmaz and Aygin (2020) [70] conducted a systematic review in a randomized controlled study to evaluate the efficacy of honey in the wound treatment process. Their data showed that honey resulted in rapid epithelialization and wound contraction in wound healing, and reduced pain, inflammation, and debridement, ensuring control of infection and reducing the time of wound healing, and was cost-effective. The authors advanced the idea that MGH improved wound healing and patient’s quality of life. MGH is safe and cost-effective, especially when dealing with complicated diabetic wounds (antibiotic-resistant) with infections and the risk of amputation.
In their study, Smaropoulos and Cremers (2020) [57] reported the safety, efficacy, and usefulness of MGH in the treatment of abdominal wounds in pediatric patients. All treated wounds rapidly revealed granulation tissue formation and underwent re-epithelialization. There was a noticeable reduction in peripheral edema and inflammation upon initial application. An effective debriding of necrotic tissue was observed, and the same applied to sloughs, which were easily removed when detected with no sign of infection, regardless of initial wound presentation. There was minimal scarring with full preservation of movement in all cases.
In a systematic review carried out by Jull et al. (2015) [71] on the efficacy of honey in comparison with alternative wound dressings and topical treatment of acute burns, lacerations, and/or chronic wounds (e.g., venous ulcers), results suggested that honey healed partial thickness burns more quickly than conventional treatments (e.g., polyurethane film, paraffin gauze, soframycin-impregnated gauze, sterile linen, and leaving the burn exposed), and infection resulting from post-operative wounds healed faster than with antiseptics and gauze.
The results of a comparative study using topical applications of Acemannan gel (AG), hyaluronic acid, and MH on bilateral wounds introduced on the backs of six sheep indicated that treatment with AG resulted in wound dehydration and stimulated late granulation tissue and cell proliferation. Moreover, the AG-treated wounds had a mild late pro-inflammatory and neovascularization effect and a positive influence on moist wounds with abundant granulation tissue and exudate [72]. In contrast, the MH-treated wounds were slightly dry. The main effect of MH was to promote cell proliferation and neovascularization, with an overall pro-inflammatory effect. Results suggest that MH treatment enhanced the healing process [72].
Table 1 summarizes the effects of different types of honey, including Manuka honey, in acute, chronic, and mixed types of malignant wounds.
Table 1.
Effects of various types of honey on classes of wound
| Wound class | Wound type | Type of honey | Effect of honey | References |
|---|---|---|---|---|
| Acute | Burns | Manuka | Control of infection and inflammation, shorter healing time | [73] |
| Surgical and traumatic | Indian Syzygium cumini | No significant difference | [74] | |
| Chronic | Infected, surgical and traumatic | Manuka | Significant clinical improvement | [75] |
| Pressure ulcers | Medihoney | Shorter healing time | [76] | |
| Lower extremity ulcers | Pakistani Beri (Ziziphus jujuba)-honey | Shorter median healing time | [77] | |
| Mixed acute and chronic | — | MedihoneyTM | Significant decrease in wound size, perceived pain levels, and wound sloughing/necrosis | [78] |
| Malignant | — | Manuka honey | Decreased odor and inflammation | [79] |
6. Underlying mechanisms of honey in wound healing
The antibacterial activity of honey has been well documented [35,36,37,80]. Honey activity can be either bacteriostatic or bactericidal, depending on the kind of honey [37]. The internal pouch of the honeybee known as the crop is considered the reservoir for many enzymes that are added to honey.
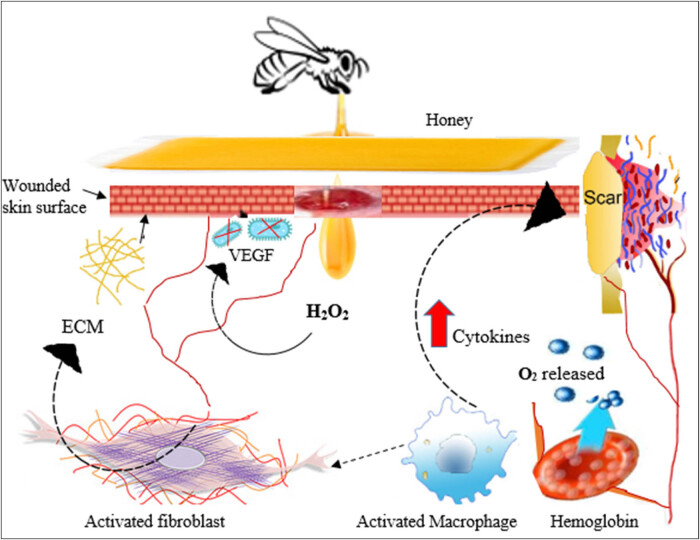
One of these enzymes is glucose oxidase, which catalyzes glucose oxidation to form gluconic acid and hydrogen peroxide. The production of gluconic acid results in lowering the pH, and hydrogen peroxide enhances its bactericidal efficacy [3,22]. Therefore, to lower the pH levels between 3.5–4, a series of events essential for the process of tissue repair takes place: reduction in protease activity in the wound site, increase in the oxygen release from hemoglobin, and stimulation of fibroblast and macrophage activity. Furthermore, the production of hydrogen peroxide stimulates VEGF and sterilizes the wound (Figure 2) [3,22]. Another important enzyme produced by the honeybee is invertase, which provides the honey with stronger osmotic potential by hydrolyzing sucrose into fructose and glucose [3,22]. Fluids in the wound are oozed out of damaged tissues, leading to drying of cellular tissues and bacterial death [3].
Figure 2.
Antibacterial and wound healing effects of honey (ECM, extracellular matrix; H2O2, hydrogen peroxide; VEGF, vascular endothelial growth factor; O2, oxygen).
Notably, phenolic compounds, organic acids, vitamins, and flavonoids exert antioxidant activities and boost the antimicrobial effect of honey. Flavonoids neutralize free radicals produced by the hydrogen peroxide [3,22]. In addition, the immunomodulatory effects of honey enhance wound healing, and various ingredients in honey contribute to its anti-inflammatory and antioxidant properties [81]. Moreover, the high concentration of nutrients promotes epithelialization and angiogenesis [2]. An important source of nutrients for the tissues is derived from the presence of carbohydrates, especially glucose and fructose, with maltose, sucrose, and isomaltose in smaller quantities. Carbohydrates represent about 80% of honey’s components [22,82,83,84]. Certain types of honey exert their bactericidal efficacy primarily by bee defensin-1 and the glucose oxidase enzyme. The latter enzyme changes honey sugar into gluconic acid and 3% of hydrogen peroxide, tolerated by tissues and effective against bacteria [85].
7. Future directions with tissue-engineered honey-impregnated scaffolds
Despite the increase in studies on the use of honey for wound healing, whether of traumatic or surgical origin, only a few studies on its use on infected wounds have been done. Some authors have analyzed honey’s potential on the growth of selected intestinal bacteria [42] and against pathologic bacteria frequently isolated from the skin wounds of mammals, including humans [35].
Nanotechnology is an emerging field that has found its way into many applications, including medicine, drug delivery, and cosmetics, and its application in medicine is growing very rapidly [86]. In the past decade, tissue has been widely investigated. Honey initially used in wound healing was an 80–100% MH and a gelling agent; however, due to its high osmolarity, it tended to leak out of the bed of the wound. To resolve this issue, scaffolds were implemented to provide slow and controllable release of honey to maintain the absorption of wound exudate (Table 2). Currently, tissue-engineered honey-infused scaffolds include mostly electrospun fibers, cryogels, and hydrogels. Such scaffolds may provide a better honey delivery system [7,87]. In addition, several presented clinical cases illustrated that honey formulation comprising natural wound care products such as L-Mesitran Ointment, L-Mesitran Soft, and the associated ingredients (vitamins, polyethylene glycol, etc.) enhance the healing properties of honey [43].
Table 2.
Currently researched types of honey-infused scaffolds
| No. | Techniques | Characteristics | Drawbacks | References |
|---|---|---|---|---|
| 1 | Electrospinning | Manufactured by impregnating honey into electrospun nanofibers. High surface area-to-volume ratio enables bio-resorption and a permeable structure. Considered easiest to apply, most efficient, and cost‐effective | Fiber morphology can be affected by the addition of honey | [7] |
| 2 | Hydrogels | A solid gel-like structure made from a polymer solution cross-linked at ambient temperature | Many types of honey and polymers are used to produce these hydrogels | [7] |
| 3 | Cryogels | Produced by freezing a polymer solution immediately after cross-linking, causing ice crystals to form surrounded by the formation of gel-like matrices | Such scaffolds have a wide range of active ingredients, making it difficult to evaluate the efficacy of individual honey types | [88] |
8. Conclusion
MGH is a promising wound healing agent because it has a broad spectrum of antimicrobial efficacy with no known resistant pathogens. It has been shown to be effective against clinical bacterial and fungal isolates and their associated biofilm formation in a dose-dependent manner. It is safe and cost-effective, especially in the treatment of different types of wounds. MGH should be considered a potential alternative to antibiotics or complementary therapy for treating locally infected wounds. An improved delivery system and a structure to support wound healing could tremendously enhance the treatment process and result in better outcomes.
Footnotes
Funding information: The authors state no funding involved.
Conflict of interest: The authors state no conflict of interest.
Data availability statement: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- [1].Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89(3):219–29. [DOI] [PMC free article] [PubMed]; Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89(3):219–29. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Molan PC. Potential of honey in the treatment of wounds and burns. Am J Clin Dermatol. 2001;2(1):13–9. [DOI] [PubMed]; Molan PC. Potential of honey in the treatment of wounds and burns. Am J Clin Dermatol. 2001;2(1):13–9. doi: 10.2165/00128071-200102010-00003. [DOI] [PubMed] [Google Scholar]
- [3].Molan P, Rhodes T. Honey: a biologic wound dressing. Wounds. 2015;27(6):141–51. [PubMed]; Molan P, Rhodes T. Honey: a biologic wound dressing. Wounds. 2015;27(6):141–51. [PubMed] [Google Scholar]
- [4].Langemo DK, Hanson D, Anderson J, Thompson P, Hunter S. Use of honey for wound healing. Adv Skin Wound Care. 2009;22(3):113–8. [DOI] [PubMed]; Langemo DK, Hanson D, Anderson J, Thompson P, Hunter S. Use of honey for wound healing. Adv Skin Wound Care. 2009;22(3):113–8. doi: 10.1097/01.ASW.0000305460.87058.42. [DOI] [PubMed] [Google Scholar]
- [5].Sell SA, Wolfe PS, Spence AJ, Rodriguez IA, McCool JM, Petrella RL, et al. A preliminary study on the potential of manuka honey and platelet-rich plasma in wound healing. Int J Biomater. 2012;2012:2012–14. [DOI] [PMC free article] [PubMed]; Sell SA, Wolfe PS, Spence AJ, Rodriguez IA, McCool JM, Petrella RL. et al. A preliminary study on the potential of manuka honey and platelet-rich plasma in wound healing. Int J Biomater. 2012;2012:2012–14. doi: 10.1155/2012/313781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Leong AG, Herst PM, Harper JL. Indigenous New Zealand honeys exhibit multiple anti-inflammatory activities. Innate Immun. 2012;18(3):459–66. [DOI] [PubMed]; Leong AG, Herst PM, Harper JL. Indigenous New Zealand honeys exhibit multiple anti-inflammatory activities. Innate Immun. 2012;18(3):459–66. doi: 10.1177/1753425911422263. [DOI] [PubMed] [Google Scholar]
- [7].Hixon KR, Klein RC, Eberlin CT, Linder HR, Ona WJ, Gonzalez H, et al. A critical review and perspective of honey in tissue engineering and clinical wound healing. Adv Wound Care. 2019;8(8):403–15. [DOI] [PMC free article] [PubMed]; Hixon KR, Klein RC, Eberlin CT, Linder HR, Ona WJ, Gonzalez H. et al. A critical review and perspective of honey in tissue engineering and clinical wound healing. Adv Wound Care. 2019;8(8):403–15. doi: 10.1089/wound.2018.0848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hixon KR, Lu T, Carletta MN, McBride-Gagyi SH, Janowiak BE, Sell SA. A preliminary in vitro evaluation of the bioactive potential of cryogel scaffolds incorporated with Manuka honey for the treatment of chronic bone infections. J Biomed Mater Res B Appl Biomater. 2018;106(5):1918–33. [DOI] [PubMed]; Hixon KR, Lu T, Carletta MN, McBride-Gagyi SH, Janowiak BE, Sell SA. A preliminary in vitro evaluation of the bioactive potential of cryogel scaffolds incorporated with Manuka honey for the treatment of chronic bone infections. J Biomed Mater Res B Appl Biomater. 2018;106(5):1918–33. doi: 10.1002/jbm.b.34002. [DOI] [PubMed] [Google Scholar]
- [9].Ranzato E, Martinotti S, Burlando B. Epithelial mesenchymal transition traits in honey-driven keratinocyte wound healing: comparison among different honeys. Wound Repair Regen. 2012;20(5):778–85. [DOI] [PubMed]; Ranzato E, Martinotti S, Burlando B. Epithelial mesenchymal transition traits in honey-driven keratinocyte wound healing: comparison among different honeys. Wound Repair Regen. 2012;20(5):778–85. doi: 10.1111/j.1524-475X.2012.00825.x. [DOI] [PubMed] [Google Scholar]
- [10].Ranzato E, Martinotti S, Burlando B. Honey exposure stimulates wound repair of human dermal fibroblasts. Burn Trauma. 2013;1(1):32–8. [DOI] [PMC free article] [PubMed]; Ranzato E, Martinotti S, Burlando B. Honey exposure stimulates wound repair of human dermal fibroblasts. Burn Trauma. 2013;1(1):32–8. doi: 10.4103/2321-3868.113333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Khan KA, Ansari MJ, Al-Ghamdi A, Nuru A, Harakeh S, Iqbal J. Investigation of gut microbial communities associated with indigenous honeybee (Apis mellifera jemenitica) from two different eco-regions of Saudi Arabia. Saudi J Biol Sci. 2017;24(5):1061–8. [DOI] [PMC free article] [PubMed]; Khan KA, Ansari MJ, Al-Ghamdi A, Nuru A, Harakeh S, Iqbal J. Investigation of gut microbial communities associated with indigenous honeybee (Apis mellifera jemenitica) from two different eco-regions of Saudi Arabia. Saudi J Biol Sci. 2017;24(5):1061–8. doi: 10.1016/j.sjbs.2017.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Alotibi IA, Harakeh SM, Al-Mamary M, Mariod AA, Al-Jaouni SK, Al-Masaud S, et al. Floral markers and biological activity of Saudi honey. Saudi J Biol Sci. 2018;25(7):1369–74. [DOI] [PMC free article] [PubMed]; Alotibi IA, Harakeh SM, Al-Mamary M, Mariod AA, Al-Jaouni SK, Al-Masaud S. et al. Floral markers and biological activity of Saudi honey. Saudi J Biol Sci. 2018;25(7):1369–74. doi: 10.1016/j.sjbs.2018.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].White JD, Doner LW. Honey composition and properties. Beekeeping in the United States agriculture handbook. Washington, DC, USA: USDA; 1980. p. 82–91; White JD, Doner LW. Beekeeping in the United States agriculture handbook. Washington, DC, USA: USDA; 1980. Honey composition and properties; pp. p. 82–91. [Google Scholar]
- [14].Visavadia BG, Honeysett J, Danford M. Manuka honey dressing: an effective treatment for chronic wound infections. Br J Oral Maxillofac Surg. 2008;46(8):696–7. [DOI] [PubMed]; Visavadia BG, Honeysett J, Danford M. Manuka honey dressing: an effective treatment for chronic wound infections. Br J Oral Maxillofac Surg. 2008;46(8):696–7. doi: 10.1016/j.bjoms.2007.12.014. [DOI] [PubMed] [Google Scholar]
- [15].Dryden M, Goddard C, Madadi A, Heard M, Saeed K, Cooke J. Using antimicrobial Surgi honey to prevent caesarean wound infection. Br J Midwifery. 2014;22(2):111–5.; Dryden M, Goddard C, Madadi A, Heard M, Saeed K, Cooke J. Using antimicrobial Surgi honey to prevent caesarean wound infection. Br J Midwifery. 2014;22(2):111–5. [Google Scholar]
- [16].Molan PC. Re-introducing honey in the management of wounds and ulcers – theory and practice. Ostomy Wound Manage. 2002;48(11):28–40. [PubMed]; Molan PC. Re-introducing honey in the management of wounds and ulcers – theory and practice. Ostomy Wound Manage. 2002;48(11):28–40. [PubMed] [Google Scholar]
- [17].Gleiter R, Horn H, Isengard H. Influence of type and state of crystallisation on the water activity of honey. Food Chem. 2006;96(3):441–5.; Gleiter R, Horn H, Isengard H. Influence of type and state of crystallisation on the water activity of honey. Food Chem. 2006;96(3):441–5. [Google Scholar]
- [18].Sundoro A, Nadia K, Nur A, Sudjatmiko G, Tedjo A. Comparison of physical–chemical characteristic and antibacterial effect between Manuka honey and local honey. J Plastik Rekonstruksi. 2012;1:3.; Sundoro A, Nadia K, Nur A, Sudjatmiko G, Tedjo A. Comparison of physical–chemical characteristic and antibacterial effect between Manuka honey and local honey. J Plastik Rekonstruksi. 2012;1:3. [Google Scholar]
- [19].Molan PC, White R. Honey in modern wound management. Aberdeen, UK: Wounds UK Ltd; 2009.; Molan PC, White R. Honey in modern wound management. Aberdeen, UK: Wounds UK Ltd; 2009. [Google Scholar]
- [20].Molan PC. The evidence supporting the use of honey as a wound dressing. Int J Low Extrem Wounds. 2006;5(1):40–54. [DOI] [PubMed]; Molan PC. The evidence supporting the use of honey as a wound dressing. Int J Low Extrem Wounds. 2006;5(1):40–54. doi: 10.1177/1534734605286014. [DOI] [PubMed] [Google Scholar]
- [21].Speer SL, Schreyack GE. Manuka honey: a tissue engineering essential ingredient. J Tissue Sci Eng. 2015;6(2):1.; Speer SL, Schreyack GE. Manuka honey: a tissue engineering essential ingredient. J Tissue Sci Eng. 2015;6(2):1. [Google Scholar]
- [22].Minden-Birkenmaier BA, Bowlin GL. Honey-based templates in wound healing and tissue engineering. Bioeng (Basel). 2018;5(2):46. [DOI] [PMC free article] [PubMed]; Minden-Birkenmaier BA, Bowlin GL. Honey-based templates in wound healing and tissue engineering. Bioeng (Basel) 2018;5(2):46. doi: 10.3390/bioengineering5020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Singh SY, Young A, McNaught CE. The physiology of wound healing. Surgery. 2017;35(9):473–7.; Singh SY, Young A, McNaught CE. The physiology of wound healing. Surgery. 2017;35(9):473–7. [Google Scholar]
- [24].Sorg H, Tilkorn DJ, Hager S, Hauser J, Mirastschijski U. Skin wound healing: an update on the current knowledge and concepts. Eur Surg Res. 2017;58(1–2):81–94. [DOI] [PubMed]; Sorg H, Tilkorn DJ, Hager S, Hauser J, Mirastschijski U. Skin wound healing: an update on the current knowledge and concepts. Eur Surg Res. 2017;58(1–2):81–94. doi: 10.1159/000454919. [DOI] [PubMed] [Google Scholar]
- [25].Afrin S, Haneefa SM, Fernandez-Cabezudo MJ, Giampieri F, Al-Ramadi BK, Battino M. Therapeutic and preventive properties of honey and its bioactive compounds in cancer: an evidence-based review. Nutr Res Rev. 2019;33:1–27. [DOI] [PubMed]; Afrin S, Haneefa SM, Fernandez-Cabezudo MJ, Giampieri F, Al-Ramadi BK, Battino M. Therapeutic and preventive properties of honey and its bioactive compounds in cancer: an evidence-based review. Nutr Res Rev. 2019;33:1–27. doi: 10.1017/S0954422419000192. [DOI] [PubMed] [Google Scholar]
- [26].Almasaudi SB, El-Shitany NA, Abbas AT, Abdel-dayem UA, Ali SS, Al Jaouni SK, et al. Antioxidant, anti-inflammatory, and antiulcer potential of manuka honey against gastric ulcer in rats. Oxid Med Cell Longev. 2016;2016:3643824. [DOI] [PMC free article] [PubMed]; Almasaudi SB, El-Shitany NA, Abbas AT, Abdel-dayem UA, Ali SS, Al Jaouni SK. et al. Antioxidant, anti-inflammatory, and antiulcer potential of manuka honey against gastric ulcer in rats. Oxid Med Cell Longev. 2016;2016:3643824. doi: 10.1155/2016/3643824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Almasaudi SB, Abbas AT, Al-Hindi RR, El-Shitany NA, Abdel-Dayem UA, Ali SS, et al. Manuka honey exerts antioxidant and anti-inflammatory activities that promote healing of acetic acid-induced gastric ulcer in rats. Evid Based Compl Altern Med. 2017;2017:5413917. [DOI] [PMC free article] [PubMed]; Almasaudi SB, Abbas AT, Al-Hindi RR, El-Shitany NA, Abdel-Dayem UA, Ali SS. et al. Manuka honey exerts antioxidant and anti-inflammatory activities that promote healing of acetic acid-induced gastric ulcer in rats. Evid Based Compl Altern Med. 2017;2017:5413917. doi: 10.1155/2017/5413917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Eteraf-Oskouei T, Najafi M. Traditional and modern uses of natural honey in human diseases: a review. Iran J Basic Med Sci. 2013;16(6):731–42. [PMC free article] [PubMed]; Eteraf-Oskouei T, Najafi M. Traditional and modern uses of natural honey in human diseases: a review. Iran J Basic Med Sci. 2013;16(6):731–42. [PMC free article] [PubMed] [Google Scholar]
- [29].Maresso AW, Schneewind O. Sortase as a target of anti-infective therapy. Pharmacol Rev. 2008;60(1):128–41. [DOI] [PubMed]; Maresso AW, Schneewind O. Sortase as a target of anti-infective therapy. Pharmacol Rev. 2008;60(1):128–41. doi: 10.1124/pr.107.07110. [DOI] [PubMed] [Google Scholar]
- [30].Wang G, Yao S, Zhang X-X, Song H. Rapid screening and structural characterization of antioxidants from the extract of Selaginella doederleinii Hieron with DPPH-UPLC-Q-TOF/MS method. Int J Anal Chem. 2015;2015:849769. [DOI] [PMC free article] [PubMed]; Wang G, Yao S, Zhang X-X, Song H. Rapid screening and structural characterization of antioxidants from the extract of Selaginella doederleinii Hieron with DPPH-UPLC-Q-TOF/MS method. Int J Anal Chem. 2015;2015:849769. doi: 10.1155/2015/849769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Li G, Qiao M, Guo Y, Wang X, Xu Y, Xia X. Effect of subinhibitory concentrations of chlorogenic acid on reducing the virulence factor production by Staphylococcus aureus. Foodborne Pathog Dis. 2014;11(9):677–83. [DOI] [PubMed]; Li G, Qiao M, Guo Y, Wang X, Xu Y, Xia X. Effect of subinhibitory concentrations of chlorogenic acid on reducing the virulence factor production by Staphylococcus aureus. Foodborne Pathog Dis. 2014;11(9):677–83. doi: 10.1089/fpd.2013.1731. [DOI] [PubMed] [Google Scholar]
- [32].White Jr JW. Physical characteristics of honey. In: E. Crane, editor. Honey: A comprehensive survey. London: Heinemann; 1975.; White Jr JW. Crane E. Honey: A comprehensive survey. London: Heinemann; 1975. Physical characteristics of honey. editor. [Google Scholar]
- [33].El Denshary ES, Al-Gahazali MA, Mannaa FA, Salem HA, Hassan NS, Abdel-Wahhab MA. Dietary honey and ginseng protect against carbon tetrachloride-induced hepatonephrotoxicity in rats. Exp Toxicol Pathol. 2012;64(7–8):753–60. [DOI] [PubMed]; El Denshary ES, Al-Gahazali MA, Mannaa FA, Salem HA, Hassan NS, Abdel-Wahhab MA. Dietary honey and ginseng protect against carbon tetrachloride-induced hepatonephrotoxicity in rats. Exp Toxicol Pathol. 2012;64(7–8):753–60. doi: 10.1016/j.etp.2011.01.012. [DOI] [PubMed] [Google Scholar]
- [34].Olofsson TC, Butler É, Lindholm C, Nilson B, Michanek P, Vásquez A. Fighting off wound pathogens in horses with honeybee lactic acid bacteria. Curr Microbiol. 2016;73(4):463–73. [DOI] [PMC free article] [PubMed]; Olofsson TC, Butler É, Lindholm C, Nilson B, Michanek P, Vásquez A. Fighting off wound pathogens in horses with honeybee lactic acid bacteria. Curr Microbiol. 2016;73(4):463–73. doi: 10.1007/s00284-016-1080-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Basualdo C, Sgroy V, Finola MS, Marioli JM. Comparison of the antibacterial activity of honey from different provenance against bacteria usually isolated from skin wounds. Vet Microbiol. 2007;124(3–4):375–81. [DOI] [PubMed]; Basualdo C, Sgroy V, Finola MS, Marioli JM. Comparison of the antibacterial activity of honey from different provenance against bacteria usually isolated from skin wounds. Vet Microbiol. 2007;124(3–4):375–81. doi: 10.1016/j.vetmic.2007.04.039. [DOI] [PubMed] [Google Scholar]
- [36].Mandal MD, Mandal S. Honey: its medicinal property and antibacterial activity. Asian Pac J Trop Biomed. 2011;1(2):154–60. [DOI] [PMC free article] [PubMed]; Mandal MD, Mandal S. Honey: its medicinal property and antibacterial activity. Asian Pac J Trop Biomed. 2011;1(2):154–60. doi: 10.1016/S2221-1691(11)60016-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Vandamme L, Heyneman A, Hoeksema H, Verbelen J, Monstrey S. Honey in modern wound care: a systematic review. Burns. 2013;39(8):1514–25. [DOI] [PubMed]; Vandamme L, Heyneman A, Hoeksema H, Verbelen J, Monstrey S. Honey in modern wound care: a systematic review. Burns. 2013;39(8):1514–25. doi: 10.1016/j.burns.2013.06.014. [DOI] [PubMed] [Google Scholar]
- [38].Al-Nahari AA, Almasaudi SB, Abd El-Ghany el SM, Barbour E, Al Jaouni SK, Harakeh S. Antimicrobial activities of Saudi honey against Pseudomonas aeruginosa. Saudi J Biol Sci. 2015;22(5):521–5. [DOI] [PMC free article] [PubMed]; Al-Nahari AA, Almasaudi SB, Abd El-Ghany el SM, Barbour E, Al Jaouni SK, Harakeh S. Antimicrobial activities of Saudi honey against Pseudomonas aeruginosa. Saudi J Biol Sci. 2015;22(5):521–5. doi: 10.1016/j.sjbs.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Girma A, Seo W, She RC. Antibacterial activity of varying UMF-graded Manuka honeys. PLoS One. 2019;14(10):e0224495. [DOI] [PMC free article] [PubMed]; Girma A, Seo W, She RC. Antibacterial activity of varying UMF-graded Manuka honeys. PLoS One. 2019;14(10):e0224495. doi: 10.1371/journal.pone.0224495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Alvarez-Suarez JM, Gasparrini M, Forbes-Hernández TY, Mazzoni L, Giampieri F. The composition and biological activity of honey: a focus on Manuka honey. Foods. 2014;3(3):420–32. [DOI] [PMC free article] [PubMed]; Alvarez-Suarez JM, Gasparrini M, Forbes-Hernández TY, Mazzoni L, Giampieri F. The composition and biological activity of honey: a focus on Manuka honey. Foods. 2014;3(3):420–32. doi: 10.3390/foods3030420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Moghazy AM, Shams ME, Adly OA, Abbas AH, El-Badawy MA, Elsakka DM, et al. The clinical and cost effectiveness of bee honey dressing in the treatment of diabetic foot ulcers. Diab Res Clin Pract. 2010;89(3):276–81. [DOI] [PubMed]; Moghazy AM, Shams ME, Adly OA, Abbas AH, El-Badawy MA, Elsakka DM. et al. The clinical and cost effectiveness of bee honey dressing in the treatment of diabetic foot ulcers. Diab Res Clin Pract. 2010;89(3):276–81. doi: 10.1016/j.diabres.2010.05.021. [DOI] [PubMed] [Google Scholar]
- [42].Shin SH, Kim JS, Kim HR, Lim JK, Choi BK, Yeo YK. Carbohydrate composition of honey from different floral sources and their influence on growth of selected intestinal bacteria. Food Res Int. 2005;38(6):721–8.; Shin SH, Kim JS, Kim HR, Lim JK, Choi BK, Yeo YK. Carbohydrate composition of honey from different floral sources and their influence on growth of selected intestinal bacteria. Food Res Int. 2005;38(6):721–8. [Google Scholar]
- [43].Pleeging C, Coenye T, Mossialos D, De Rooster H, Chrysostomou D, Wagener F, et al. Synergistic antimicrobial activity of supplemented medical-grade honey against Pseudomonas aeruginosa biofilm formation and eradication. Antibiotics. 2020;9(12):866. [DOI] [PMC free article] [PubMed]; Pleeging C, Coenye T, Mossialos D, De Rooster H, Chrysostomou D, Wagener F. et al. Synergistic antimicrobial activity of supplemented medical-grade honey against Pseudomonas aeruginosa biofilm formation and eradication. Antibiotics. 2020;9(12):866. doi: 10.3390/antibiotics9120866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].de Groot T, Janssen T, Faro D, Cremers NA, Chowdhary A, Meis JF. Antifungal activity of a medical-grade honey formulation against candida auris. J Fungi. 2021;7(1):50. [DOI] [PMC free article] [PubMed]; de Groot T, Janssen T, Faro D, Cremers NA, Chowdhary A, Meis JF. Antifungal activity of a medical-grade honey formulation against candida auris. J Fungi. 2021;7(1):50. doi: 10.3390/jof7010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Oliveira AM, Devesa JS, Hill PB. In vitro efficacy of a honey‐based gel against canine clinical isolates of Staphylococcus pseudintermedius and Malassezia pachydermatis. Vet Dermatol. 2018;29(3):180-e65. [DOI] [PubMed]; Oliveira AM, Devesa JS, Hill PB. In vitro efficacy of a honey‐based gel against canine clinical isolates of Staphylococcus pseudintermedius and Malassezia pachydermatis. Vet Dermatol. 2018;29(3):180-e65. doi: 10.1111/vde.12533. [DOI] [PubMed] [Google Scholar]
- [46].Hermanns R, Cremers NA, Leeming JP, van der Werf ET. Sweet relief: determining the antimicrobial activity of medical grade honey against vaginal isolates of candida albicans. J Fungi. 2019;5(3):85. [DOI] [PMC free article] [PubMed]; Hermanns R, Cremers NA, Leeming JP, van der Werf ET. Sweet relief: determining the antimicrobial activity of medical grade honey against vaginal isolates of candida albicans. J Fungi. 2019;5(3):85. doi: 10.3390/jof5030085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Bucekova M, Jardekova L, Juricova V, Bugarova V, Di Marco G, Gismondi A, et al. Antibacterial activity of different blossom honeys: new findings. Molecules. 2019;24(8):1573. [DOI] [PMC free article] [PubMed]; Bucekova M, Jardekova L, Juricova V, Bugarova V, Di Marco G, Gismondi A. et al. Antibacterial activity of different blossom honeys: new findings. Molecules. 2019;24(8):1573. doi: 10.3390/molecules24081573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Cremers N, Belas A, Santos Costa S, Couto I, De Rooster H, Pomba C. In vitro antimicrobial efficacy of two medical grade honey formulations against common high‐risk methicillin‐resistant staphylococci and Pseudomonas spp. pathogens. Vet Dermatol. 2020;31(2):90-e10. [DOI] [PubMed]; Cremers N, Belas A, Santos Costa S, Couto I, De Rooster H, Pomba C. In vitro antimicrobial efficacy of two medical grade honey formulations against common high‐risk methicillin‐resistant staphylococci and Pseudomonas spp. pathogens. Vet Dermatol. 2020;31(2):90–e10. doi: 10.1111/vde.12811. [DOI] [PubMed] [Google Scholar]
- [49].Hermanns R, Mateescu C, Thrasyvoulou A, Tananaki C, Wagener FA, Cremers NA. Defining the standards for medical grade honey. J Apicult Res. 2020;59(2):125–35.; Hermanns R, Mateescu C, Thrasyvoulou A, Tananaki C, Wagener FA, Cremers NA. Defining the standards for medical grade honey. J Apicult Res. 2020;59(2):125–35. [Google Scholar]
- [50].Gethin G, Cowman S. Case series of use of Manuka honey in leg ulceration. Int Wound J. 2005;2(1):10–5. [DOI] [PMC free article] [PubMed]; Gethin G, Cowman S. Case series of use of Manuka honey in leg ulceration. Int Wound J. 2005;2(1):10–5. doi: 10.1111/j.1742-4801.2005.00078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Al-Waili N, Al-Ghamdi A, Ansari MJ, Al-Attal Y, Salom K. Synergistic effects of honey and propolis toward drug multi-resistant Staphylococcus aureus, Escherichia coli and Candida albicans isolates in single and polymicrobial cultures. Int J Med Sci. 2012;9(9):793–800. [DOI] [PMC free article] [PubMed]; Al-Waili N, Al-Ghamdi A, Ansari MJ, Al-Attal Y, Salom K. Synergistic effects of honey and propolis toward drug multi-resistant Staphylococcus aureus, Escherichia coli and Candida albicans isolates in single and polymicrobial cultures. Int J Med Sci. 2012;9(9):793–800. doi: 10.7150/ijms.4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Simon A, Sofka K, Wiszniewsky G, Blaser G, Bode U, Fleischhack G. Wound care with antibacterial honey (Medihoney) in pediatric hematology-oncology. Supp Care Cancer. 2006;14(1):91–7. [DOI] [PubMed]; Simon A, Sofka K, Wiszniewsky G, Blaser G, Bode U, Fleischhack G. Wound care with antibacterial honey (Medihoney) in pediatric hematology-oncology. Supp Care Cancer. 2006;14(1):91–7. doi: 10.1007/s00520-005-0874-8. [DOI] [PubMed] [Google Scholar]
- [53].Efem SE. Clinical observations on the wound healing properties of honey. Br J Surg. 1988;75(7):679–81. [DOI] [PubMed]; Efem SE. Clinical observations on the wound healing properties of honey. Br J Surg. 1988;75(7):679–81. doi: 10.1002/bjs.1800750718. [DOI] [PubMed] [Google Scholar]
- [54].Bucekova M, Sojka M, Valachova I, Martinotti S, Ranzato E, Szep Z, et al. Bee-derived antibacterial peptide, defensin-1, promotes wound re-epithelialisation in vitro and in vivo. Sci Rep. 2017;7(1):1–13. [DOI] [PMC free article] [PubMed]; Bucekova M, Sojka M, Valachova I, Martinotti S, Ranzato E, Szep Z. et al. Bee-derived antibacterial peptide, defensin-1, promotes wound re-epithelialisation in vitro and in vivo. Sci Rep. 2017;7(1):1–13. doi: 10.1038/s41598-017-07494-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Maruhashi E. Honey in wound healing. In: Boateng J, editor. Therapeutic dressings and wound healing applications. US: Wiley; 2020. p. 235–54.; Maruhashi E. Boateng J. Therapeutic dressings and wound healing applications. US: Wiley; 2020. Honey in wound healing; pp. p. 235–54. , editor. [Google Scholar]
- [56].Al Jaouni SK, Al Muhayawi MS, Hussein A, Elfiki I, Al-Raddadi R, Al Muhayawi SM, et al. Effects of honey on oral mucositis among pediatric cancer patients undergoing chemo/radiotherapy treatment at King Abdulaziz University Hospital in Jeddah, Kingdom of Saudi Arabia. Evid Based Compl Altern Med. 2017;2017:2017. [DOI] [PMC free article] [PubMed]; Al Jaouni SK, Al Muhayawi MS, Hussein A, Elfiki I, Al-Raddadi R, Al Muhayawi SM. et al. Effects of honey on oral mucositis among pediatric cancer patients undergoing chemo/radiotherapy treatment at King Abdulaziz University Hospital in Jeddah, Kingdom of Saudi Arabia. Evid Based Compl Altern Med. 2017;2017:2017. doi: 10.1155/2017/5861024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Smaropoulos E, Cremers NAJ. Treating severe wounds in pediatrics with medical grade honey: a case series. Clin Case Rep. 2020;8(3):469–76. [DOI] [PMC free article] [PubMed]; Smaropoulos E, Cremers NAJ. Treating severe wounds in pediatrics with medical grade honey: a case series. Clin Case Rep. 2020;8(3):469–76. doi: 10.1002/ccr3.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Henry N, Jeffery S, Radotra I. Properties and use of a honey dressing and gel in wound management. Br J Nurs. 2019;28(6):S30–5. [DOI] [PubMed]; Henry N, Jeffery S, Radotra I. Properties and use of a honey dressing and gel in wound management. Br J Nurs. 2019;28(6):S30–5. doi: 10.12968/bjon.2019.28.6.S30. [DOI] [PubMed] [Google Scholar]
- [59].Martinotti S, Ranzato E. Honey, wound repair and regenerative medicine. J Funct Biomater. 2018;9(2):34. [DOI] [PMC free article] [PubMed]; Martinotti S, Ranzato E. Honey, wound repair and regenerative medicine. J Funct Biomater. 2018;9(2):34. doi: 10.3390/jfb9020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Nweze AJ, Olovo CV, Nweze EI, John OO, Paul C. Therapeutic properties of honey. Honey analysis. UK: IntechOpen; 2019.; Nweze AJ, Olovo CV, Nweze EI, John OO, Paul C. Therapeutic properties of honey. Honey analysis. UK: IntechOpen; 2019. [Google Scholar]
- [61].Gill R, Poojar B, Bairy LK, Praveen KSE. Comparative evaluation of wound healing potential of manuka and acacia honey in diabetic and nondiabetic rats. J Pharm Bioallied Sci. 2019;11(2):116–26. [DOI] [PMC free article] [PubMed]; Gill R, Poojar B, Bairy LK, Praveen KSE. Comparative evaluation of wound healing potential of manuka and acacia honey in diabetic and nondiabetic rats. J Pharm Bioallied Sci. 2019;11(2):116–26. doi: 10.4103/jpbs.JPBS_257_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Rani GN, Rao BN, Shamili M, Padmaja J. Combined effect of silver nanoparticles and honey in experimental wound healing process in rats. Biomed Res. 2018;29(15):3074–78.; Rani GN, Rao BN, Shamili M, Padmaja J. Combined effect of silver nanoparticles and honey in experimental wound healing process in rats. Biomed Res. 2018;29(15):3074–78. [Google Scholar]
- [63].Malkoç M, Yaman SÖ, Imamoğlu Y, İnce İ, Kural BV, Mungan S, et al. Anti-inflammatory, antioxidant and wound-healing effects of mad honey in streptozotocin-induced diabetic rats. J Apicult Res. 2019;59(4):426–36.; Malkoç M, Yaman SÖ, Imamoğlu Y, İnce İ, Kural BV, Mungan S. et al. Anti-inflammatory, antioxidant and wound-healing effects of mad honey in streptozotocin-induced diabetic rats. J Apicult Res. 2019;59(4):426–36. [Google Scholar]
- [64].Saikaly SK, Khachemoune A. Honey and wound healing: an update. Am J Clin Dermatol. 2017;18(2):237–51. [DOI] [PubMed]; Saikaly SK, Khachemoune A. Honey and wound healing: an update. Am J Clin Dermatol. 2017;18(2):237–51. doi: 10.1007/s40257-016-0247-8. [DOI] [PubMed] [Google Scholar]
- [65].Oryan A, Alemzadeh E, Moshiri A. Biological properties and therapeutic activities of honey in wound healing: a narrative review and meta-analysis. J Tissue Viabil. 2016;25(2):98–118. [DOI] [PubMed]; Oryan A, Alemzadeh E, Moshiri A. Biological properties and therapeutic activities of honey in wound healing: a narrative review and meta-analysis. J Tissue Viabil. 2016;25(2):98–118. doi: 10.1016/j.jtv.2015.12.002. [DOI] [PubMed] [Google Scholar]
- [66].Abdelrahman JE, Magzoub AA, Ibrahim RE, Elnoor MA, Musa OA. Effect of combination of Nigella sativa and Bee’s honey on lung function, respiratory muscle power, and asthma control in patients with persistent asthma. Int J Res Med Sci. 2016;5(1):236–9.; Abdelrahman JE, Magzoub AA, Ibrahim RE, Elnoor MA, Musa OA. Effect of combination of Nigella sativa and Bee’s honey on lung function, respiratory muscle power, and asthma control in patients with persistent asthma. Int J Res Med Sci. 2016;5(1):236–9. [Google Scholar]
- [67].Salem ML. Immunomodulatory and therapeutic properties of the Nigella sativa L. seed. Int Immunopharmacol. 2005;5(13–14):1749–70. [DOI] [PubMed]; Salem ML. Immunomodulatory and therapeutic properties of the Nigella sativa L. seed. Int Immunopharmacol. 2005;5(13–14):1749–70. doi: 10.1016/j.intimp.2005.06.008. [DOI] [PubMed] [Google Scholar]
- [68].Allwayzy KR. Effect of locally applied black-seed oil and honey mixture on wound healing. Int J Sci Technol Res. 2013;2:31–4.; Allwayzy KR. Effect of locally applied black-seed oil and honey mixture on wound healing. Int J Sci Technol Res. 2013;2:31–4. [Google Scholar]
- [69].Javadi SMRH M, Mohammadi Y, MamMohammadi A, Sharifi A, Makarchian HR. Synergistic effect of honey and Nigella sativa on wound healing in rats. Acta Cir Brasil. 2018;33(6):518–23. [DOI] [PubMed]; Javadi SMRH M, Mohammadi Y, MamMohammadi A, Sharifi A, Makarchian HR. Synergistic effect of honey and Nigella sativa on wound healing in rats. Acta Cir Brasil. 2018;33(6):518–23. doi: 10.1590/s0102-865020180060000006. [DOI] [PubMed] [Google Scholar]
- [70].Yilmaz AC, Aygin D. Honey dressing in wound treatment: a systematic review. Compl Ther Med. 2020;51:102388. [DOI] [PubMed]; Yilmaz AC, Aygin D. Honey dressing in wound treatment: a systematic review. Compl Ther Med. 2020;51:102388. doi: 10.1016/j.ctim.2020.102388. [DOI] [PubMed] [Google Scholar]
- [71].Jull AB, Cullum N, Dumville JC, Westby MJ, Deshpande S, Walker N. Honey as a topical treatment for wounds. Cochrane Database Syst Rev. 2015;3:005083. [DOI] [PMC free article] [PubMed]; Jull AB, Cullum N, Dumville JC, Westby MJ, Deshpande S, Walker N. Honey as a topical treatment for wounds. Cochrane Database Syst Rev. 2015;3:005083. doi: 10.1002/14651858.CD005083.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Iacopetti I, Perazzi A, Martinello T, Gemignani F, Patruno M. Hyaluronic acid, Manuka honey and Acemannan gel: wound-specific applications for skin lesions. Res Vet Sci. 2020;129:82–9. [DOI] [PubMed]; Iacopetti I, Perazzi A, Martinello T, Gemignani F, Patruno M. Hyaluronic acid, Manuka honey and Acemannan gel: wound-specific applications for skin lesions. Res Vet Sci. 2020;129:82–9. doi: 10.1016/j.rvsc.2020.01.009. [DOI] [PubMed] [Google Scholar]
- [73].Maghsoudi H, Salehi F, Khosrowshahi MK, Baghaei M, Nasirzadeh M, Shams R. Comparison between topical honey and mafenide acetate in treatment of burn wounds. Ann Burn Fire Disasters. 2011;24(3):132–7. [PMC free article] [PubMed]; Maghsoudi H, Salehi F, Khosrowshahi MK, Baghaei M, Nasirzadeh M, Shams R. Comparison between topical honey and mafenide acetate in treatment of burn wounds. Ann Burn Fire Disasters. 2011;24(3):132–7. [PMC free article] [PubMed] [Google Scholar]
- [74].Subrahmanyam M. Honey dressing accelerates split-thickness skin graft donor site healing. Indian J Surg. 2015;77(Suppl 2):261–3. [DOI] [PMC free article] [PubMed]; Subrahmanyam M. Honey dressing accelerates split-thickness skin graft donor site healing. Indian J Surg. 2015;77(Suppl 2):261–3. doi: 10.1007/s12262-012-0789-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Dina Jarjis R, Thomas Crewe B, Henrik Matzen S. Post-bariatric abdominoplasty resulting in wound infection and dehiscence-conservative treatment with medical grade honey: a case report and review of literature. Int J Surg Case Rep. 2016;20:1–3. [DOI] [PMC free article] [PubMed]; Dina Jarjis R, Thomas Crewe B, Henrik Matzen S. Post-bariatric abdominoplasty resulting in wound infection and dehiscence-conservative treatment with medical grade honey: a case report and review of literature. Int J Surg Case Rep. 2016;20:1–3. doi: 10.1016/j.ijscr.2015.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Biglari B, vd Linden PH, Simon A, Aytac S, Gerner HJ, Moghaddam A. Use of Medihoney as a non-surgical therapy for chronic pressure ulcers in patients with spinal cord injury. Spinal Cord. 2012;50(2):165–9. [DOI] [PubMed]; Biglari B, vd Linden PH, Simon A, Aytac S, Gerner HJ, Moghaddam A. Use of Medihoney as a non-surgical therapy for chronic pressure ulcers in patients with spinal cord injury. Spinal Cord. 2012;50(2):165–9. doi: 10.1038/sc.2011.87. [DOI] [PubMed] [Google Scholar]
- [77].Imran M, Hussain MB, Baig M. A randomized, controlled clinical trial of honey-impregnated dressing for treating diabetic foot ulcer. J Coll Phys Surg Pak. 2015;25(10):721–5. [DOI] [PubMed]; Imran M, Hussain MB, Baig M. A randomized, controlled clinical trial of honey-impregnated dressing for treating diabetic foot ulcer. J Coll Phys Surg Pak. 2015;25(10):721–5. doi: 10.2015/JCPSP.721725. [DOI] [PubMed] [Google Scholar]
- [78].Biglari B, Moghaddam A, Santos K, Blaser G, Büchler A, Jansen G, et al. Multicentre prospective observational study on professional wound care using honey (Medihoney™). Int Wound J. 2013;10(3):252–9. [DOI] [PMC free article] [PubMed]; Biglari B, Moghaddam A, Santos K, Blaser G, Büchler A, Jansen G. et al. Multicentre prospective observational study on professional wound care using honey (Medihoney™) Int Wound J. 2013;10(3):252–9. doi: 10.1111/j.1742-481X.2012.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Drain J, Fleming MO. Palliative management of malodorous squamous cell carcinoma of the oral cavity with Manuka honey. J Wound Ostomy Cont Nurs. 2015;42(2):190–2. [DOI] [PubMed]; Drain J, Fleming MO. Palliative management of malodorous squamous cell carcinoma of the oral cavity with Manuka honey. J Wound Ostomy Cont Nurs. 2015;42(2):190–2. doi: 10.1097/WON.0000000000000114. [DOI] [PubMed] [Google Scholar]
- [80].Hashim GM, Almasaudi SB, Azhar E, Al Jaouni SK, Harakeh S. Biological activity of Cymbopogon schoenanthus essential oil. Saudi J Biol Sci. 2017;24(7):1458–64. [DOI] [PMC free article] [PubMed]; Hashim GM, Almasaudi SB, Azhar E, Al Jaouni SK, Harakeh S. Biological activity of Cymbopogon schoenanthus essential oil. Saudi J Biol Sci. 2017;24(7):1458–64. doi: 10.1016/j.sjbs.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Majtan J. Honey: an immunomodulator in wound healing. Wound Repair Regen. 2014;22(2):187–92. [DOI] [PubMed]; Majtan J. Honey: an immunomodulator in wound healing. Wound Repair Regen. 2014;22(2):187–92. doi: 10.1111/wrr.12117. [DOI] [PubMed] [Google Scholar]
- [82].Carnwath R, Graham EM, Reynolds K, Pollock PJ. The antimicrobial activity of honey against common equine wound bacterial isolates. Vet J. 2014;199(1):110–4. [DOI] [PubMed]; Carnwath R, Graham EM, Reynolds K, Pollock PJ. The antimicrobial activity of honey against common equine wound bacterial isolates. Vet J. 2014;199(1):110–4. doi: 10.1016/j.tvjl.2013.07.003. [DOI] [PubMed] [Google Scholar]
- [83].Cavanagh D, Beazley J, Ostapowicz F. Radical operation for carcinoma of the vulva. A new approach to wound healing. J Obstet Gynaecol Br Commonw. 1970;77(11):1037–40. [DOI] [PubMed]; Cavanagh D, Beazley J, Ostapowicz F. Radical operation for carcinoma of the vulva. A new approach to wound healing. J Obstet Gynaecol Br Commonw. 1970;77(11):1037–40. doi: 10.1111/j.1471-0528.1970.tb03455.x. [DOI] [PubMed] [Google Scholar]
- [84].Cooper RA, Molan PC, Harding KG. The sensitivity to honey of Gram-positive cocci of clinical significance isolated from wounds. J Appl Microbiol. 2002;93(5):857–63. [DOI] [PubMed]; Cooper RA, Molan PC, Harding KG. The sensitivity to honey of Gram-positive cocci of clinical significance isolated from wounds. J Appl Microbiol. 2002;93(5):857–63. doi: 10.1046/j.1365-2672.2002.01761.x. [DOI] [PubMed] [Google Scholar]
- [85].Szweda P. Antimicrobial activity of honey. Honey Anal. 2017;1:215–32.; Szweda P. Antimicrobial activity of honey. Honey Anal. 2017;1:215–32. [Google Scholar]
- [86].Singla R, Abidi SMS, Dar AI, Acharya A. Nanomaterials as potential and versatile platform for next generation tissue engineering applications. J Biomed Mater Res B Appl Biomater. 2019;107(7):2433–49. [DOI] [PubMed]; Singla R, Abidi SMS, Dar AI, Acharya A. Nanomaterials as potential and versatile platform for next generation tissue engineering applications. J Biomed Mater Res B Appl Biomater. 2019;107(7):2433–49. doi: 10.1002/jbm.b.34327. [DOI] [PubMed] [Google Scholar]
- [87].Kalaf EG, Hixon KR, Kadakia PU, Dunn AJ, Sell SA. Electrospun biomaterials for dermal regeneration. Electrospun materials for tissue engineering and biomedical applications. UK/England: Woodhead Publishing; 2017. p. 179–231.; Kalaf EG, Hixon KR, Kadakia PU, Dunn AJ, Sell SA. Electrospun materials for tissue engineering and biomedical applications. UK/England: Woodhead Publishing; 2017. Electrospun biomaterials for dermal regeneration; pp. p. 179–231. [Google Scholar]
- [88].Varalakshmi V, Suganiya SA, Mala R. Fabrication and characterization of hybrid sponge for healing of infectious burn wound. Recent Pat Nanotechnol. 2015;9(3):212–21. [DOI] [PubMed]; Varalakshmi V, Suganiya SA, Mala R. Fabrication and characterization of hybrid sponge for healing of infectious burn wound. Recent Pat Nanotechnol. 2015;9(3):212–21. doi: 10.2174/1872210510999151126112122. [DOI] [PubMed] [Google Scholar]