Abstract
Invasive fungal disease often plays an important role in the morbidity and mortality of immunocompromised patients. The poor sensitivity of current fungal blood culture and histological practices has led to the development of highly sensitive and specific molecular techniques, such as the PCR. Sequence variability of the internal transcribed spacer 2 (ITS2) region of fungi is potentially useful in rapid and accurate diagnosis of clinical fungal isolates. PCR with fungus-specific primers targeted toward conserved sequences of the 5.8S and 28S ribosomal DNA (rDNA) results in amplification of the species-specific ITS2 regions, which are variable in amplicon length. We have made use of the ABI PRISM 310 genetic analyzer and the ABI PRISM 310 GeneScan analysis software for the determination of variable size differences of the ITS2 region of clinically important fungi, including Candida and non-Candida yeasts, Aspergillus species, and a variety of dermatophytes. No cross-reaction occurred when samples were tested against human and bacterial genomic DNA. We have found that most clinically significant fungal isolates can be differentiated by this method, and it therefore serves to be a promising tool for the rapid (<7 h) diagnosis of fungemia and other invasive fungal infections.
Advances in medicine contributing to the increased survival of immunocompromised patients, including oncology, human immunodeficiency virus-infected, diabetes, and transplant patients, have also brought forth an increase in the prevalence of nosocomial fungal infections (1, 2, 11). These infections carry a high mortality, ranging from 30 to 60% (2, 9, 11, 18), depending on the underlying condition and whether effective antifungal therapy was administered. Tissue involvement can occur in up to 36% of fungemic episodes, which has been associated with an even higher mortality rate of 47 to 88% (8, 9, 28). Disseminated infections due to some organisms such as Aspergillus and Fusarium species have a mortality rate close to 100% (9, 18).
Candida species now rank fourth among the most commonly isolated organisms from bloodstream infections (1, 3, 19). There has also been a rise in the incidence of disease caused by non-albicans Candida species (17, 20). While Candida albicans (∼60%) and Candida species (∼20%) are responsible for most fungal infections (2, 9), up to 150 fungal species have been demonstrated to be primary pathogens of humans, involving all body sites (6). Furthermore, this problem is compounded by an increase in resistance to antifungal agents, particularly the azoles (20, 21) and amphotericin B (17), and an increase in the empirical use of these agents.
Early detection of infection has a great impact on the clinical outcome of many infectious diseases. Unfortunately, the identification of fungi by traditional morphologic and metabolic characteristics may take days to weeks. For molds in particular, these methods are laborious, time-consuming, and require significant technological expertise. Blood culture systems may fail to detect as many as 45 to 75% of cases of disseminated candidiasis (9, 23) and most cases of invasive aspergillosis (25). Consequently, when a blood culture result is positive for pathogenic and opportunistic fungi, far too often it is obtained just prior to death, when it is too late. Therefore, a high index of suspicion is required, leading to the empiric use of antifungal therapy. While the choice of treatment is speculative, based on the most probably pathogens involved, the standard choice of antifungal treatment often remains amphotericin B. However, as more alternative antifungal agents with various spectra of activity are being developed, specific identification of pathogenic fungi will become even more important in the near future. Investigators have attempted to overcome these difficulties by developing rapid, sensitive detection and identification methods with the intent of improving patient outcome and reducing costs.
At the molecular level, genetic sequence variation offers an alternative to culturing for detection and identification of fungi. For example, the ribosomal genes demonstrate conserved sequence regions ideal for primer targeting as well as regions of variability useful for species identification. Amplification techniques, with subsequent probing of the amplicons with species-specific probes or in a PCR-enzyme immunoassay format, have been utilized to overcome the problems of sensitivity, specificity, and delay encountered with conventional methodology (4, 5, 7, 10, 22, 24, 29). These methods have already shown great promise in the field of diagnostics. However, the use of species-specific probes is not always an efficient approach in mycology, given the large number of potentially pathogenic fungi.
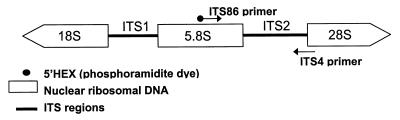
PCR primers that target conserved regions of fungal rRNA genes, amplifying sequence-variable fragments of genes or intervening noncoding regions (26), have been used for sequence comparisons for phylogenetic analyses of a variety of fungal groups. Interspecies variability is also manifested in the fragment size of the internal transcribed spacer 1 and 2 (ITS1 and ITS2, respectively) regions (15, 27). We have utilized the variability in length of the ITS2 region to make specific diagnosis of pathogenic fungal isolates from blood and tissues (Fig. 1). This is a promising method; however, the size differences of the amplicons from different species may not be detectable by agarose gel electrophoresis. In this study, we used the ABI PRISM 310 genetic analyzer, an automated capillary electrophoresis sequencer, to detect fluorescently tagged amplicons from the ITS2 region. The basic method for this application is described as follows. A single capillary is filled with a replaceable liquid polymer for high resolution under denaturing conditions between each sample test. The sample containing fluorescent PCR product is injected into the capillary and is subject to constant voltage and temperature (60°C). The fluorescently tagged fragments of DNA resulting from amplification by fluorescent primers are detected by a laser beam and automatically analyzed by the instrument. Between 1 and 96 samples can be loaded at any one time on the instrument, and each run is completed within 30 min. Results can be viewed as soon as each sample has been processed. The instrument detects up to four various fluorescent “colors,” allowing for the inclusion of an internal standard with each run. Accurate fragment size analysis based on the electrophoretic mobility of the sample relative to the internal standard is determined by using the ABI PRISM 310 GeneScan analysis software. This method has enabled us to generate a profile of expected amplicon sizes for a broad range of yeasts and molds, allowing for their rapid identification.
FIG. 1.
Schematic representation of the fungal ribosomal genes containing the primer target areas used in the amplification of the ITS2 region.
MATERIALS AND METHODS
Fungal strains.
For the testing of primer specificity, a reference strain of Candida albicans (ATCC 10231) was used. For the determination of the ITS2 PCR fragment length, a wide collection of reference strains, American Type Culture Collection or College of American Pathologist specimens were used, as well as patient strains obtained from the Health Sciences Centre Clinical Microbiology Laboratory stock culture collection. Yeast organisms were grown on Sabouraud dextrose agar plates (BBL, Becton-Dickinson, Cockeysville, Md.) for 24 h at 37°C, and molds were grown on potato dextrose agar (16) for up to 7 days. Species identification was established by using the API 20C kit (BioMerieux, Hazelwood, Mo.) or by conventional morphological analysis (14).
DNA isolation from molds.
Mold scrapings were suspended in 1 ml of T10E1 buffer (10 mM Tris-HCl, 1 mM EDTA [pH 8.0]) followed by centrifugation at 13,000 rpm for 5 min. The pellet was resuspended in 200 μl of 50 mM NaOH, vortexed, and incubated at 95°C for 10 min. The mixture was then neutralized with 200 μl of 0.1 mM Tris-HCl (pH 7.0) and centrifuged for 5 min at 13,000 rpm. The pellet was resuspended in 500 μl of sterile H2O and centrifuged at 13,000 rpm for 5 min. The supernatant was removed, followed by the addition of 200 ml of yeast cracking buffer (2% Triton X-100, 1% sodium dodecyl sulfate, 100 mM NaCl, 20 mM Tris [pH 8.0], 10 mM EDTA [pH 8.0]). Extraction of genomic DNA was achieved by addition of glass beads to 3/4 of the liquid volume followed by the addition of 200 ml of cold phenol-chloroform-isoamyl alcohol in a 25:24:1 ratio. The mixture was subjected to constant vortexing for 30 min. The sample was then centrifuged at 13,000 rpm for 5 min. The aqueous (top) layer was transferred into a new tube, and 1 ml of cold (−20°C) 100% ethanol was added for DNA precipitation. The sample was centrifuged at 13,000 rpm for 2 min. The supernatant was removed, and the pellet was resuspended in 400 μl of T10E1 buffer and 30 μg of RNase A (Sigma, St. Louis, Mo.) for a 1-h water bath incubation at 35°C. We then added 10 μl of 3 M sodium acetate and 1,000 μl of cold 100% ethanol. The sample was centrifuged at 13,000 rpm for 2 min, the supernatant was removed, and the pellet was air dried. The DNA pellet was resuspended in 50 μl of TE buffer, and was used as DNA template for amplification after 30 min or stored at −20°C for future use. This method, which can be used with all fungi we have tested, is necessary for the extraction of DNA from certain molds, such as Aspergillus terreus, Penicillium sp., Paecilomyces sp., and Sporothrix schenckii.
DNA isolation from yeast.
Steps in the method described above were omitted or reduced in duration if the organism was a yeast. Resuspension and incubation of the fungal pellet in 50 mM NaOH were not necessary. The vortexing with glass beads was reduced to three times for 30 s, and the mixture was held on ice for short periods in between. Finally, the RNase A incubation period was reduced to 5 min.
PCR amplification.
The primers used for universal fungal amplification were ITS4 (reverse primer [5′-tcc tcc gct tat tga tag c-3′]) obtained from White et al. and fluorescently labeled (5′HEX) ITS86 (forward primer [5′-gtg aat cat cga atc ttt gaa c-3′]) (Life Technologies, Burlington, Ontario, Canada) derived from sequence comparison of various fungi from GenBank databases by using PCGene software (University of Geneva, Geneva, Switzerland). The 50-μl PCR reaction mixture contained 5 μl of DNA template; 5 μl of 25 mM MgCl2–10× PCR buffer; 1.25 mM deoxynucleoside triphosphate; dATP, dGTP, dCTP, and an 8:1 ratio of dUTP to dTTP; 0.5 μl of 100 μM each primer; 0.5 U of uracil DNA glycosylase (UDG) (Gibco BRL); 2.5 U of Taq DNA polymerase (Pharmacia Biotech, Baie d’Urfé, Québec, Canada); and 30 ml of sterile distilled H2O. The PCR was performed in a GeneAmp PCR system 9600 (Perkin-Elmer Applied Biosystems, Foster City, Calif.) with cycles of 37°C for 10 min (UDG activation) and 94°C for 10 min (UDG inactivation) and 30 cycles of 94, 55, and 72°C for 1 min each, and then the mixture was incubated at 72°C for 10 min for final extension. Amplification of all fungi tested with these primers yielded fragments of 200 to 500 bp in length.
Primer specificity.
A variety or bacterial organisms were used to determine the specificity of the fungal primers: Staphylococcus epidermidis (ATCC 12228), Escherichia coli (ATCC 25922), Staphylococcus aureus (ATCC 25923), Pseudomonas aeruginosa (ATCC 27853), and Clostridium perfringens (ATCC 13124). Bacterial DNA was isolated by using the QIAamp tissue kit (Qiagen, Santa Clarita, Calif.). The primers were also tested against genomic DNA deriving from human whole blood, concentrated leukocytes, and liver tissue. The PCR conditions were as described above.
Agarose gel electrophoresis.
Detection of PCR-amplified product was performed by electrophoresis on a 2% (wt/vol) agarose gel stained with ethidium bromide. A volume of 20 μl of PCR product and 2.2 μl of Ficoll dye was loaded in each lane. Electrophoretic conditions were 100 V for 45 min in 0.5× Tris-borate-EDTA buffer. A 123-bp ladder was also run in parallel for approximate PCR product band sizing.
Fragment analysis of the ITS2 region.
The ABI PRISM 310 genetic analyzer was used for determination of the precise lengths of the PCR fragments containing the ITS2 regions. Fragment analysis was done by using the ABI PRISM 310 GeneScan analysis software (Perkin-Elmer Applied Biosystems). Sample preparation for capillary electrophoresis involved the addition of 1 μl of diluted PCR product (approximately 5 to 20 ng) to the capillary electrophoresis mix (12 μl of deionized formamide and 0.5 μl of GeneScan-500 (ROX) size standard (Perkin-Elmer Applied Biosystems). A dilution of the PCR product in TE buffer to a concentration of 5 to 20 ng/μl resulted in an ideal peak intensity analyzable on the instrument relative to the internal standard peaks. The capillary sample mix was denatured for 2 min at 95°C and rapidly cooled on ice prior to loading the instrument. Subsequent preparation such as the setup of the ABI PRISM 310 genetic analyzer was done according to the manufacturer’s instructions with reference to using Performance Optimized Polymer 4 (POP-4) for microsatellite analysis (Perkin-Elmer Applied Biosystems). A capillary (47 cm by 50 μm inside diameter) was installed, and POP-4 was used as the replaceable liquid polymer matrix. Electrophoresis conditions were set on the instrument at a 5-s injection time, 15-kV injection voltage, 15-kV electrophoresis voltage, 150-s syringe pump time, 120-s preinjection electrophoresis, temperature constant of 60°C, and a 28-min collection time to ensure the detection of PCR fragments under 450 bp in length. The matrix file (for POP-4, filter set A, under the conditions described above) was created according to the manufacturer’s instructions to account for spectral overlap of the various fluorescent molecules under the specific conditions used for this application. Successful analysis was derived from the red peaks being assigned to the ROX-labeled internal standard, for which the user indicated in the program the appropriate fragment lengths, with the green peak being assigned to HEX-labeled PCR fragments from fungal ITS2 amplification.
RESULTS
Primer specificity.
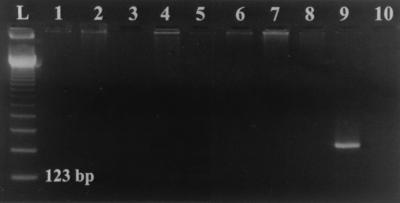
No amplification product was detected by using the ITS86 and ITS4 primers with template from human leukocytes, human whole blood, or human liver or against any of the following bacterial organisms: Staphylococcus epidermidis, Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, and Bacteroides fragilis (Fig. 2).
FIG. 2.
Specificity of universal ITS2 primers against bacteria and human genomic DNA. PCR amplification using the ITS4 and ITS86 primer pair was performed as described in Materials and Methods. The following DNA templates were used for PCR (by lane): 1, Staphylococcus epidermidis ATCC 12228; 2, Escherichia coli ATCC 25922; 3, Staphylococcus aureus ATCC 25923; 4, Pseudomonas aeruginosa ATCC 27853; 5, Clostridium perfringens ATCC 13124; 6, human whole blood; 7, human leukocytes; 8, human liver; 9, Candida albicans ATCC 10231; and 10, H2O contamination control.
Fragment analysis information.
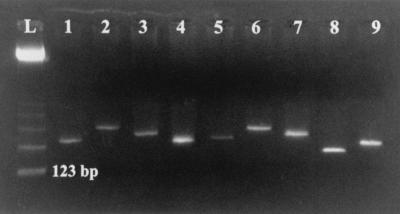
The PCR products of all species tested were initially visualized by UV illumination on ethidium bromide-stained agarose gel to confirm successful amplification and crude variability in length of the final product. The yeast family demonstrated the highest level of interspecies variability (Fig. 3) compared to other fungi (data not shown); however, size determination based on agarose gel electrophoresis is not precise enough to unmistakably confirm species identification.
FIG. 3.
Detection of fungal species by agarose gel electrophoresis. PCR amplification of variable Candida species from culture colonies using the ITS4 and ITS86 universal fungal primers was performed as described in Materials and Methods. Lanes: L, 123-bp ladder; 1, Candida albicans; 2, Candida kefyr; 3, Candida zeylanoides; 4, Candida tropicalis; 5, Candida krusei; 6, Candida glabrata; 7, Candida guilliermondii; 8, Candida lusitaniae; 9, Candida parapsilosis.
To determine the degree of accuracy of the fragment analysis, multiple strains of Candida species were analyzed in multiple runs on the ABI PRISM 310 genetic analyzer, and the standard deviation was then calculated. The run-to-run variation resulted in a standard deviation ≤0.5 bp (Table 1). Although the variation between different strains of a species was not extensively tested, it was comparable to the run-to-run variation of a single strain, indicating that the different strains tested were of the same ITS2 length. The documented instrument resolution provided by the manufacturer indicated a 1-base detection to 250 bp and a 2-base detection from 251 to 350 bp.
TABLE 1.
Base pair determination and associated standard deviation of the ITS2-containing PCR fragments of various species during multiple fragment analysis runs
| Organism | Strain (n)a | ITS2 fragment length (SD)b |
|---|---|---|
| Candida albicans | ATCC 10231 (42) | 279.15 (0.4) |
| Candida tropicalis | ATCC 66029 (8) | 268.61 (0.5) |
| Candida glabrata | ATCC 90030 (7) | 359.47 (0.4) |
| Candida parapsilosis | ATCC 22019 (7) | 251.33 (0.4) |
| Candida krusei | ATCC 6258 (6) | 282.21 (0.2) |
| Candida lusitaniae | ATCC 42720 (8) | 198.67 (0.5) |
| Candida guilliermondii | CAP F1-95 (9) | 320.87 (0.5) |
| Candida kefyr | ATCC 4135 (6) | 372.35 (0.2) |
| Saccharomyces cerevisiae | ATCC 9763 (8) | 363.94 (0.3) |
n, number of runs (including PCR amplification and detection by capillary electrophoresis).
Lengths are in base pairs. SD, standard deviation.
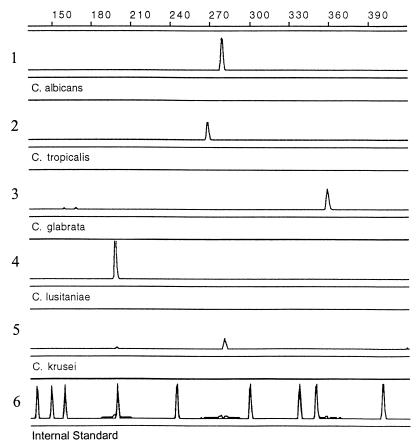
The length of the ITS2 region amplicons was determined for 12 Candida species, 9 non-Candida yeasts, 14 dermatophytes, and 21 other opportunistic and dematiaceous fungi (Table 2). Reference strains were used for the majority of the specimens, as well as some clinical strains to test for intraspecies consistency. Examples of the electropherograms produced from the GeneScan analysis are shown in Fig. 4. Within 21 species of the yeast group, 18 ITS2 fragment lengths were determined. Candida stellatoides had the same fragment length as Candida albicans (279 bp). Candida zeylanoides, Cryptococcus neoformans, and Candida pelliculosa (H. anomala) differed by only 1 bp (316, 315, and 317 bp respectively).
TABLE 2.
Length determination (in base pairs) of the PCR fragment containing the ITS2 of known fungal isolates
| Organism | Strain(s)a | Conventional ID | PCR fragment length (bp) |
|---|---|---|---|
| Yeasts | |||
| Candida albicans | ATCC 10231, SCC, HSC1159, HSC67442, HSC67630, HSC68392, HSC81363 | API 20C-AUX | 279 |
| Candida kefyr | ATCC 4135, SCC | API 20C-AUX | 372 |
| Candida guilliermondii | CAP F1-95, SCC | API 20C-AUX | 321 |
| Candida tropicalis | ATCC 66029, SCC, HSC75317, HSC75418, HSC75422, HSC78308, HSC78408, HSC81682, HSC81701, HSC71273, HSC71278, HSC29977, HSC71277 | API 20C-AUX | 269 |
| Candida krusei | ATCC 6258, SCC, CT1, K1865, CA37, E175 | API 20C-AUX | 282 |
| Candida glabrata | ATCC 90030, SCC, HSC17253, HSC20818, HSC20819 | API 20C-AUX | 360 |
| Candida zeylanoides | CAP F18-93 | API 20C-AUX | 316 |
| Candida lusitaniae | ATCC 42720 | API 20C-AUX | 199 |
| Candida parapsilosis | ATCC 22019, SCC, HSC5966, HSC21193 | API 20C-AUX | 251 |
| Candida pelliculosa | ATCC 8168 | API 20C-AUX | 317 |
| Candida pseudotropicalis | ATCC 66028 | API 20C-AUX | 372 |
| Candida stellatoidea | ATCC 36232 | API 20C-AUX | 279 |
| Trichosporon beigelii | ATCC 28592 | API 20C-AUX | 295 |
| HSC6088 | API20C-AUX | 298 | |
| Geotrichum candidum | ATCC 34614 | API 20C-AUX, mannitol | 192 |
| Cryptococcus neoformans | ATCC 90112 | API 20C-AUX, urease, phenoloxidase | 315 |
| Cryptococcus albidus | ATCC 10666 | API 20C-AUX | 350 |
| Cryptococcus laurentii | ATCC 18803 | API 20C-AUX | 306 |
| Rhodotorula rubra | ATCC 9449, SCC HSC20116, HSC37777 | API 20C-AUX | 348 |
| Blastoschizomyces capitatus | ATCC 10663 | API 20C-AUX | 248 |
| Saccharomyces cerevisiae | ATCC 9763 | API 20C-AUX | 363 |
| Malassezia furfur | ATCC 14521 | API 20C-AUX | 494 |
| Other opportunistic fungi | |||
| Aspergillus fumigatus | CAP F6-91 | Morphology | 284 |
| Aspergillus flavus | ATCC 10124 | Morphology | 288 |
| Aspergillus niger | ATCC 16404 | Morphology | 290 |
| Aspergillus terreus | ATCC 28301 | Morphology | 292 |
| Alternaria alternata | CAP F13-93 | Morphology | 285 |
| Penicillium sp. | SCC | Morphology | 283 |
| Acremonium strictum | CAP F4-96 | Morphology | 294 |
| Fusarium solani | CAP F15-95 | Morphology | 286 |
| Paecilomyces sp. | SCC | Morphology | 287 |
| Rhizopus sp. | SCC | Morphology | 319 |
| Absidia corymbifera | ATCC 66271 | Morphology | 410 |
| Mucor sp. | SCC | Morphology | 312 |
| Cunninghamella bertholletiae | ATCC 42115 | Morphology | 347 |
| Pseudallescheria boydii | ATCC 58085 | Morphology | 321 |
| Scedosporium prolificans | SCC | Morphology | 303 |
| Phialophora verrucosa | ATCC 28181 | Morphology | 308 |
| Phialophora richardsiae | ATCC 26465 | Morphology | 276 |
| Fonsecaea pedrosoi | ATCC 28174 | Morphology | 319 |
| Cladosporium carrionii | ATCC 32279 | Morphology | 303 |
| Wangiella dermatitidis | SCC | Morphology | 334 |
| Exophiala jeanselmei | ATCC 10224 | Morphology | 311 |
| Stemphylium ilicis | ATCC 18160 | Morphology | 280 |
| Dimorphic fungi | |||
| Penicillium marneffei | ATCC 18224 | Morphology | 280 |
| Sporothrix schenckii | ATCC 10212 | Morphology, conversion to yeast phase | 310 |
| Blastomyces dermatitidis | HSC patient isolate | Nucleic acid probe | 315 |
| Histoplasma capsulatum | HSC patient isolate | Nucleic acid probe | 299 |
| Dermatophytes | |||
| Microsporum canis | CAP F4-90 | Morphology | 321 |
| Microsporum gypseum | CAP F20-90 | Morphology | 321 |
| Microsporum audouinii | ATCC 42558 | Morphology | 318 |
| Microsporum nanum | SCC | Morphology | 324 |
| Microsporum gallinae | SCC | Morphology | 325 |
| Microsporum cookei | SCC | Morphology | 314 |
| Trichophyton schoenleinii | SCC | Morphology, nutritional and temp (°C) requirements | 306 |
| Trichophyton terrestre | SCC | Morphology, nutritional and temp (°C) requirements | 298 |
| Trichophyton violaceum | SCC | Morphology | 315 |
| Trichophyton mentagrophytes | ATCC 28185 | Morphology, urease, hair performation | 303 |
| Trichophyton rubrum | ATCC 28188 | Morphology | 307 |
| Trichophyton tonsurans | ATCC 28942 | Morphology and nutritional requirements | 302 |
| Trichophyton verrucosum | CAP F15-1994, SSC | Morphology, nutritional and temp (°C) requirements | 307 |
| Epidermophyton floccosum | ATCC 52066 | Morphology | 366 |
SCC and HSC, stock culture collection and patient isolates, respectively, obtained from the Health Sciences Centre, Winnipeg, Manitoba, Canada.
FIG. 4.
Electropherograms of five Candida species as analyzed by the ABI PRISM 310 genetic analyzer. Control strains (graphs 1 to 5) were amplified by using ITS4 and fluorescently labeled ITS86 and dUTP as described in Materials and Methods. Each was run separately on the capillary electrophoresis system along with an internal size standard (GeneScan ROX-500). Standard peaks are shown as a separate electropherogram (graph 6) for clarity of illustration. The standard peak sizes are 139, 150, 150, 200, 240, 300, 340, 350, and 400 bp. Graphs: 1, Candida albicans ATCC 10231; 2, Candida tropicalis ATCC 66029; 3, Candida glabrata ATCC 90030; 4, Candida lusitaniae ATCC 42720; 5, Candida krusei ATCC 6258.
With the exception of Epidermophyton floccosum (366 bp), all of the dermatophytes were situated in a range between 298 and 325 bp, with Microsporum spp. in the higher range (313 to 325 bp) and the Trichophyton spp. in the lower range (298 to 315 bp). Trichophyton rubrum and Trichophyton verrucosum had the same fragment length, whereas the other dermatophytes tested had a unique ITS2 length.
In addition to the yeasts and dermatophytes, 21 other medically important fungi were tested and included opportunistic and pathogenic fungi. The four Aspergillus species tested had close fragment length sizes: Aspergillus fumigatus, 284 bp; Aspergillus flavus, 288 bp; Aspergillus niger, 290 bp; and Aspergillus terreus, 292 bp. Other opportunistic fungi tested included Fusarium solani (286 bp), Absidia corymbifera (410 bp), and Cunninghamella bertholletiae (347 bp). The majority of the other molds had unique ITS2 lengths; however, a few fungi demonstrated fragments of equal length or nearly equal length. These included Scedosporum prolificans and Cladophialophora carrionii, which were both 303 bp; Penicillium marneffei, which had the same length as Candida albicans; and Exophiala jeanselmei and Sporothrix schenckii, which differed by 1 bp.
DISCUSSION
The use of ribosomal DNA (rDNA) genes for identification of fungal species is based on the detection of conserved sequences in 5.8S rDNA and 28S rDNA that enable the amplification of the ITS2 region between these two genes. In this study, we PCR amplified, by using a fluorescent primer pair, the ITS2 regions of 56 fungal species of clinical significance. The amplicons were rapidly and accurately sized with an automated capillary electrophoresis system, ABI PRISM 310 genetic analyzer. No intraspecies variability was observed among species for which more than one strain was tested. These species included Candida albicans, Candida guilliermondii, Candida tropicalis, Candida krusei, Candida glabrata, Candida parapsilosis, Rhodotorula rubra, and Trichophyton verrucosum. An exception was Trichosporon beigelii, of which a reference strain and a clinical strain were tested. Kemker et al. (12) previously demonstrated, by using restriction fragment length polymorphism of a segment of the ribosomal genes including the ITS2 region, that isolates identified as Trichosporon beigelii are genetically distinct from each other, depending on the source of the organism. The authors suggest that Trichosporon beigelii may represent several distinct entities and that, for the purpose of molecular diagnosis research, isolates from invasive disease must be used as opposed to those from culture collections.
The length of the amplicon containing the ITS2 region that has been determined for each control organism may serve as reference for specific fungal identification from clinical specimens, such as blood and fresh tissue and paraffin-embedded specimens. Specific identification may not always be possible, since even with the precision of capillary electrophoresis, some ITS2 lengths are very similar. However, even with a short list of causative organisms, combined with the clinical setting, site of infection, and histologic information, a presumptive identification can often be made. Of those species likely to be isolated in blood specimens (Candida albicans, Candida tropicalis, Candida glabrata, Candida parapsilosis, Candida krusei, Candida lusitaniae, and Candida pseudotropicalis [Candida kefyr]), all have a unique ITS2 amplicon length. This may have implications for selection of antifungal therapy. While fluconazole has become a drug of choice due to its low level of toxicity, some non-albicans Candida species, particularly Candida krusei, Candida glabrata, and Candida parapsilosis (17, 21), have demonstrated fluconazole resistance, and another agent, such as amphotericin B, should be chosen for empirical therapy. The ITS2 length similarity of Candida stellatoidea and Candida albicans is not surprising, since the genetically distinct Candida stellatoidea type II has been classified as a sucrose-negative variant of Candida albicans (13), and this species is normally reported as Candida albicans. It is important to keep in mind the clinical importance of the organisms that need to be identified. The fact that the dermatophytes have very similar ITS2 lengths is not as great a concern as, for example, the differentiation of dematiaceous from opportunistic organisms. Thus, the ITS2 length in association with the clinical picture can be an effective method for early diagnosis of fungal infection.
We are in the process of applying this method to clinical specimens. In cases in which the ITS2 lengths may be similar, it is highly probable that their sequences differ and may potentially be differentiated by single-stranded conformation polymorphism (SSCP). This can also be performed on a capillary electrophoresis system in the same time frame. However, SSCP may detect intraspecies variations in the sequence and therefore limit the specificity of the test. Another possibility may be to include the amplification of the ITS1 fragment in a multiplex PCR reaction, although this also increases the chance of detecting intraspecies length differences if they occur. Further work is necessary in a clinical setting to determine the specificity at which our method will provide optimal clinically significant data.
The greatest impact this method may have in the clinical laboratory is upon the time saved from sample collection to a final diagnosis. The time to identification from specimen collection can be as little as 6.5 h, including the DNA extraction, amplification, and processing of the first sample by the capillary electrophoresis. While at present, the ABI PRISM 310 genetic analyzer is a single-capillary system which runs one sample at a time (∼30 min) for analysis, we anticipate the availability of a multicapillary system in the near future which can process multiple samples concurrently. Conventional blood cultures, on the other hand, take an average of 3 to 7 days before positive fungal identification. In addition, this technique is independent of organism viability, growth, and biochemical and morphological phenotypes. This technique requires only minimal technological expertise, in contrast to conventional morphological methods. However, this method does not establish antifungal susceptibilities, which must still be determined by conventional methodology requiring growth of the organism. In summary, we have developed a method for the comprehensive identification of clinically relevant fungal isolates, which is sensitive, rapid, and specific for most organisms tested.
ACKNOWLEDGMENTS
We thank Assunta Rendina and Evelyn Witwicki for their technical assistance and expertise.
This project was supported in part by the Children’s Hospital Research Foundation.
REFERENCES
- 1.Banerjee S N, Emori T G, Culver D H, Gaynes R P, Jarvis W R, Horan T, Edwards J R, Tolson J, Henderson T, Martone W J the National Nosocomial Infections Surveillance System. Secular trends in nosocomial primary bloodstream infections in the United States, 1980–1989. Am J Med. 1991;91(Suppl. 3B):86S–89S. doi: 10.1016/0002-9343(91)90349-3. [DOI] [PubMed] [Google Scholar]
- 2.Beck-Sagué C M, Jarvis W R the National Nosocomial Infection Surveillance System. Secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980–1990. J Infect Dis. 1993;167:1247–1251. doi: 10.1093/infdis/167.5.1247. [DOI] [PubMed] [Google Scholar]
- 3.CDC NNIS System. National Nosocomial Infection Surveillance (NNIS) Report, data summary from October 1986–April 1996, issued May 1996. Am J Infect Control. 1996;24:380–388. [PubMed] [Google Scholar]
- 4.Einsele H, Hebart H, Roller G, Löeffler J, Rothenhöfer I, Müller C A, Bowden R A, van Burik J-A, Engelhard D, Kanz L, Schumacher U. Detection and identification of fungal pathogens in blood by using molecular probes. J Clin Microbiol. 1997;35:1353–1360. doi: 10.1128/jcm.35.6.1353-1360.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elie C M, Lott T J, Reiss E, Morrisson C J. Rapid identification of Candida species with species-specific DNA probes. J Clin Microbiol. 1998;36:3260–3265. doi: 10.1128/jcm.36.11.3260-3265.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fromtling R A . (section ed.) Mycology. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C: ASM Press; 1995. pp. 697–855. [Google Scholar]
- 7.Fujita S-I, Lasker B A, Lott T J, Reiss E, Morrison C J. Microtitration plate enzyme immunoassay to detect PCR-amplified DNA from Candida species in blood. J Clin Microbiol. 1995;33:962–967. doi: 10.1128/jcm.33.4.962-967.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodman J L, Winston D J, Greenfield R A, Chandrasekar P H, Fox B, Kaizer H, et al. A controlled trial of fluconazole to prevent fungal infections in patients undergoing bone marrow transplantation. N Engl J Med. 1992;326:845–851. doi: 10.1056/NEJM199203263261301. [DOI] [PubMed] [Google Scholar]
- 9.Goodrich J M, Reed E C, Mori M, Fisher L D, Skerrett S, Dandliker P S, Klis B, Counts G W, Meyers J D. Clinical features and analysis of risk factors for invasive candidal infection after marrow transplantation. J Infect Dis. 1991;164:731–740. doi: 10.1093/infdis/164.4.731. [DOI] [PubMed] [Google Scholar]
- 10.Hee Shin J, Nolte F S, Morrison C J. Rapid identification of Candida species in blood cultures by a clinically useful PCR method. J Clin Microbiol. 1997;35:1454–1459. doi: 10.1128/jcm.35.6.1454-1459.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karlowsky J A, Zhanel G G, Klym K A, Hoban D J, Kabani A M. Candidemia in a Canadian tertiary care hospital from 1976 to 1996. Diagn Microbiol Infect Dis. 1997;28:5–9. doi: 10.1016/s0732-8893(97)00068-0. [DOI] [PubMed] [Google Scholar]
- 12.Kemker B J, Lehmann P F, Lee J W, Walsh T J. Distinction of deep versus superficial clinical and nonclinical isolates of Trichsporon beigelii by isoenzymes and restriction fragment length polymorphism of rDNA generated by polymerase chain reaction. J Clin Microbiol. 1991;29:1677–1683. doi: 10.1128/jcm.29.8.1677-1683.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwon-Chung K J, Hicks J B, Lipke P N. Evidence that Candida stellatoidea type II is a mutant of Candida albicans that does not express sucrose-inhibitable α-glucosidase. Infect Immun. 1990;58:2804–2808. doi: 10.1128/iai.58.9.2804-2808.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larone D H. Medically important fungi: a guide to identification. 3rd ed. Washington, D.C: ASM Press; 1995. [Google Scholar]
- 15.Lott T J, Kuykendall R J, Reiss E. Nucleotide sequence analysis of the 5.8S rDNA and adjacent ITS2 region of Candida albicans and related species. Yeast. 1993;9:1199–1206. doi: 10.1002/yea.320091106. [DOI] [PubMed] [Google Scholar]
- 16.McGinnis M R. Laboratory handbook of medical mycology. New York, N.Y: Academic Press, Inc.; 1980. p. 570. [Google Scholar]
- 17.Nguyen M H, Peacock J E, Morris A J, Tanner D C, Nguyen M L, Syndman D R, Wagener M M, Rinaldi M G, Yu V L. The changing face of candidemia: emergence of non-Candida albicans species and antifungal resistance. Am J Med. 1996;100:617–623. doi: 10.1016/s0002-9343(95)00010-0. [DOI] [PubMed] [Google Scholar]
- 18.Nucci M, Spector N, Bueno A, Solza C, Perecmanis T, Bacha P C, Pulcheri W. Risk factors and attributable mortality associated with superinfections in neutropenic patients with cancer. Clin Infect Dis. 1997;24:575–579. doi: 10.1093/clind/24.4.575. [DOI] [PubMed] [Google Scholar]
- 19.Pittet D, Li N, Woolson R F, Wenzel R P. Microbiological factors influencing the outcome of nosocomial bloodstream infections: a 6-year validated, population-based model. Clin Infect Dis. 1997;24:1068–1078. doi: 10.1086/513640. [DOI] [PubMed] [Google Scholar]
- 20.Price M F, LaRocco M T, Gentry L O. Fluconazole susceptibilities of Candida species and distribution of species recovered from blood cultures over a 5-year period. Antimicrob Agents Chemother. 1994;38:1422–1424. doi: 10.1128/aac.38.6.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rex J H, Rinaldi M G, Pfaller M A. Resistance of Candida species to fluconazole. Antimicrob Agents Chemother. 1995;39:1–8. doi: 10.1128/aac.39.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sandhu G S, Kline B C, Stockman L, Roberts G D. Molecular probes for diagnosis of fungal infections. J Clin Microbiol. 1995;33:2913–2919. doi: 10.1128/jcm.33.11.2913-2919.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thaler M, Pastakia B, Shawker T H, O’Leary T, Pizzo P A. Hepatic candidiasis in cancer patients: the evolving picture of the syndrome. Ann Intern Med. 1988;108:88–100. doi: 10.7326/0003-4819-108-1-88. [DOI] [PubMed] [Google Scholar]
- 24.Van Burik J-A, Myerson D, Schreckhise R W, Bowden R A. Panfungal PCR assay for detection of fungal infection in human blood specimens. J Clin Microbiol. 1998;36:1169–1175. doi: 10.1128/jcm.36.5.1169-1175.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wald A, Leisenring W, van Burik J, Bowden R A. Natural history of Aspergillus infections in a large cohort of patients undergoing bone marrow transplantation. J Infect Dis. 1997;175:1459–1466. doi: 10.1086/516480. [DOI] [PubMed] [Google Scholar]
- 26.White T J, Bruns T D, Lee S B, Taylor J W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M A, Gefland D H, Sninsky J J, White T J, editors. PCR protocols: a guide to methods and applications. San Diego, Calif: Academic Press, Inc.; 1990. pp. 315–322. [Google Scholar]
- 27.Williams D W, Wilson M J, Lewis M A O, Potts A J C. Identification of Candida species by PCR and restriction fragment length polymorphism analysis of intergenic spacer regions of ribosomal DNA. J Clin Microbiol. 1995;33:2476–2479. doi: 10.1128/jcm.33.9.2476-2479.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winston D J, Chandrasekar P H, Lazarus H M, Goodman J L, Silber J L, Horowitz H H, Shadduck R K, Rosenfeld C S, Ho W G, Islam M Z, Buell D N. Fluconazole prophylaxis of fungal infections in patients with acute leukemia. Ann Intern Med. 1993;118:495–503. doi: 10.7326/0003-4819-118-7-199304010-00003. [DOI] [PubMed] [Google Scholar]
- 29.Yamakami Y, Hashimoto A, Tokimatsu I, Nasu M. PCR detection of DNA specific for Aspergillus species in serum of patients with invasive aspergillosis. J Clin Microbiol. 1996;34:2464–2468. doi: 10.1128/jcm.34.10.2464-2468.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]