Abstract
Background
In the midst of the COVID-19 pandemic, there has been an information overload of health data (both accurate and inaccurate) available to the public. With vitamins and supplements being readily accessible, many have turned to using them in an effort to combat the virus. The purpose of this review was to analyse clinical trials regarding vitamins and supplements for the treatment of COVID-19 infections.
Methods
Articles were identified through a literature search utilizing online databases and bibliographic review.
Results
A total of seven articles were identified for review. All articles evaluated the use of vitamins and supplements for the treatment of COVID-19. Drug therapies included oral vitamin D, intravenous and oral vitamin C, oral vitamin D/magnesium/vitamin B12, oral zinc, oral combination zinc/ascorbic acid, and intravenous alpha-lipoic acid. The end points of each study varied, including the Sequential Organ Failure Assessment score, mortality, rate of intensive care unit (ICU) admissions, negativity of COVID-19 tests, oxygen requirements, and symptom burden.
Conclusion
Of the vitamins and supplements that were studied, vitamin D presented the most promising data demonstrating significant decreases in oxygen requirements, need for ICU treatment, SARS-CoV-2 RNA test positivity, and mortality. All of these benefits were exhibited in hospitalized patients. Other vitamins and supplements that were evaluated in studies did not demonstrate any statistically significant benefits. Common shortcomings of the articles included generally small sample sizes, varying sites of study (which could determine the virus variant), a lack of standard of care as background therapy, and utilization of doses that were higher than standard.
Keywords: coronavirus, COVID-19, SARS-COV-2, severe acute respiratory syndrome coronavirus, supplement, vitamin
Introduction
SARS-CoV-2, the virus causing COVID-19, was first reported to the WHO on 31 December 2019 and was declared a global pandemic on 11 March 2020.1–3 To date, there have been more than 229 million reported cases and 4.7 million deaths globally.4
Whilst the fight against the COVID-19 pandemic has persisted for more than 18 months at the time of writing, few therapies have proven effective in the management or prevention of COVID-19 infections, with the exception of vaccines.5–7 Throughout the course of this pandemic, many therapies have been proposed as having utility, with many, but not all of them, falling short of providing meaningful results in clinical trials.8–16 Some proposed therapies have never undergone clinical trials, and medical claims are being made based on theoretical or anecdotal evidence.17 Since the publication of the preceding article that reviewed in-progress studies on vitamins and supplements in COVID-19,18 various vaccines have been developed and used globally, with others in the pipeline.5–7,19–24
The National Institutes of Health (NIH) released and regularly updates a set of guideline recommendations based on evolving evidence. As of this writing, remdesivir is the only formally FDA-approved drug for the treatment of COVID-19 in patients meeting certain criteria,25 including hospitalized patients requiring supplemental oxygen but who do not require high-flow oxygen, ventilatory support or extracorporeal membrane oxygenation.25 Additionally, the NIH recommended against any medication, pre-exposure or post-exposure prophylaxis, for COVID-19.26 The NIH guidelines also stated that there are insufficient data regarding the use of supplements for the treatment of COVID-19.27
For COVID-19 management in the outpatient setting, the NIH recommended bamlanivimab plus etesevimab28 or casirivimab plus imdevimab29 in certain populations as defined by the Emergency Use Authorization (EUA) criteria.30 Previously, bamlanivimab alone had received an EUA in the outpatient setting.31 For COVID-19 management in the inpatient setting, the NIH recommended remdesivir, dexamethasone, and/or tocilizumab, depending on oxygen requirements and risk of disease progression.32 Several other immunomodulators are currently in the pipeline.33 The Infectious Diseases Society of America,34 the Society for Critical Care Medicine35 and the WHO36 have each published their own set of fluid guideline recommendations that are generally in accordance with the NIH recommendations. The CDC did not recommend specific therapies but instead deferred to the NIH guidance.37 Whilst there is no universal standard of care at the time of this publication, most institutions have protocolized COVID-19 management, with recommendations evolving with changing evidence.
With an abundance of news outlets and means of communication, there has been ample misinformation circulating amongst the public regarding the dos and don’ts of combatting this novel virus.17 With vitamins and supplements being readily accessible to the general public without provider oversight, it is important to address their role in this pandemic as there has been much discussion surrounding their use. The purpose of this review was to analyse completed and published clinical trials regarding vitamins and supplements for the treatment and/or prevention of COVID-19 infections.
Methods
We performed a literature search using PubMed, Google Scholar and bibliography review using the National Clinical Trials (NCT) numbers from previous manuscripts and the following search terms: coronavirus/COVID-19/SARS-CoV-2/COVID and vitamins/supplements. The results were filtered to “clinical trial” and “randomized controlled trial”. Both prospective and retrospective studies evaluating the use of vitamins and/or supplements for the prevention or treatment of COVID-19 and published on or before 26 February 2021 were included. Studies were excluded if they did not report on an intervention or if complete/final results were not available. This manuscript was exempt from ethics review and Institutional Review Board approval as it did not involve human subject research.
Results
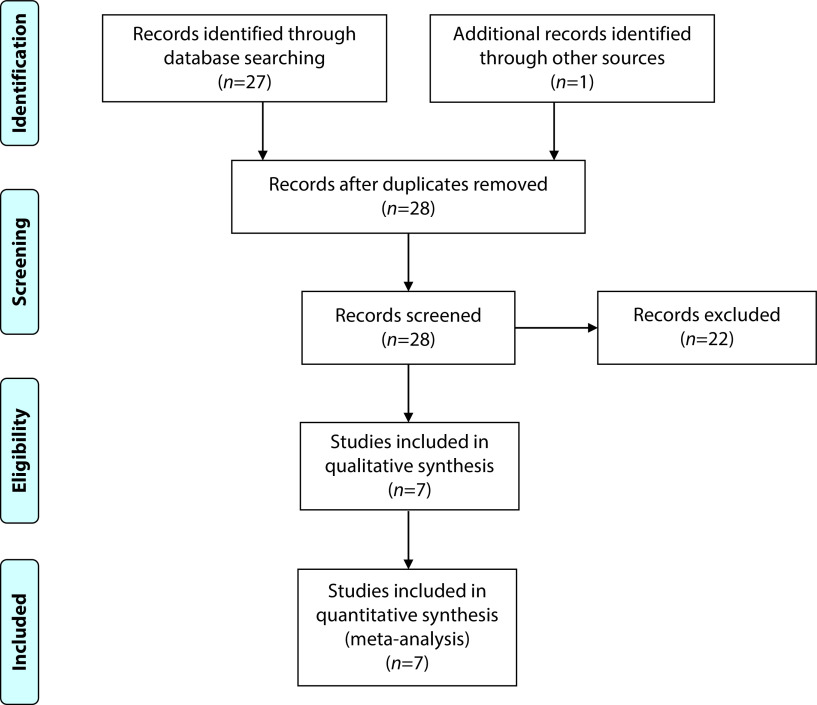
Twenty-seven manuscripts were identified from an initial database search, with six of which qualified for inclusion in this analysis (Figure 1). Reasons for study exclusion were non-interventional study (n=2), erroneous search result (n=11), inability to obtain access to paper (n=1) and study in progress (n=7). An additional China-based study that was not available through online databases was identified through a bibliography review and included in this review, making a total of seven qualifying trials for this paper.
Figure 1.
Clinical studies included in review.100
All identified studies involved the treatment of COVID-19 and did not address prophylaxis therapies for COVID-19. The interventions in the studies were oral vitamin D, intravenous (IV) and oral vitamin C, oral combination vitamin D/magnesium/vitamin B12, oral zinc, oral combination zinc/ascorbic acid, and IV alpha-lipoic acid (ALA), with the majority of the studies investigating the use of vitamin D (n=4). Of the identified trials, two were retrospective, and five were prospective with randomization. Of the randomized trials, three were open-label, one was single-blind, and the other was unspecified. The proposed utility of each of the vitamins and supplements and available data are summarized below and in Table 1.
Table 1.
Summary of clinical trial evidence for vitamins and supplements.
| Trial title | Location, study period (publication date) | Study design | Treatment arms | Background therapies | Inclusion/exclusion | End points | Main patient characteristics | Results | Conclusion |
|---|---|---|---|---|---|---|---|---|---|
| Safety and effectiveness of high-dose vitamin C in patients with COVID-19: a randomized open-label clinical trial68 |
|
Open-label, randomized, controlled trial |
|
All participants received oral lopinavir/ritonavir 400/100 mg twice daily and a single stat dose of oral hydroxychloroquine ( 400 mg) on the first day of hospitalization according to the Iranian COVID-19 treatment protocol at time of study |
Inclusion:
|
Primary: Duration of hospitalization
|
|
Primary:
|
There were improvements in peripheral oxygen saturation and body temperature in both groups during the time of admission, but there were no significantly better outcomes in the group that was treated with high-dose vitamin C at discharge |
Exclusion:
|
Secondary:
|
Secondary:
|
|||||||
| A randomized, single-blind, group sequential, active-controlled study to evaluate the clinical efficacy and safety of α-lipoic acid for critically ill patients with coronavirus disease 2019 (COVID-19)87* |
|
Randomized, single-blind, group sequential, active-controlled trial |
|
Not specified |
Inclusion:
|
Primary:
|
|
Primary: SOFA score at day 7:
|
ALA treatment did not significantly improve 30-day survival rate of patients with critically ill COVID-19, nor did it significantly slow down the increase in SOFA score |
Exclusion:
|
Secondary:
|
Secondary: 30-day all-cause mortality:
|
|||||||
| Cohort study to evaluate the effect of vitamin D, magnesium, and vitamin B12 in combination on progression to severe outcomes in older patients with coronavirus (COVID-19)71 |
|
Retrospective, cohort observational study |
|
Treatment with oral lopinavir/ritonavir or IV remdesivir or oral hydroxychloroquine (unspecified doses)
|
Inclusion:
|
Primary:
|
|
Primary end point:
|
DMB-treated patients were significantly less likely to require oxygen therapy than controls; however, comorbidities and background therapies were not equally matched at baseline |
| Exclusion: none stated |
|
||||||||
| Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: a pilot randomized clinical study51 |
|
Randomized open-label, double-blind clinical trial |
|
All patients received background therapy of combination of oral hydroxychloroquine ( 400 mg every 12 hours on the first day and 200 mg every 12 hours for the following 5 days), oral azithromycin (500 mg for 5 days, unspecified frequency) ± broad-spectrum antibiotic |
Inclusion:
|
|
|
|
Administration of a high dose of calcifediol or 25(OH)D significantly reduced the need for ICU treatment of patients requiring hospitalization due to proven COVID-19 |
Exclusion:
| |||||||||
| Short term, high-dose vitamin D supplementation for COVID-19 disease: a randomised, placebo-controlled, study (SHADE study)52 |
|
Randomized, placebo controlled study (unspecified whether blinded or not) |
|
All the participants received standard care for the SARSCoV- 2 infection as per institute protocol (unspecified) |
Inclusion:
|
Primary:
|
|
Primary:
|
A greater proportion of patients could attain SARS CoV-2 RNA negativity on high-dose vitamin D supplementation at 25(OH)D > 50 ng/ml compared to vitamin D-deficient individuals |
Exclusion:
|
Secondary:
|
Secondary:
|
|||||||
| High-dose cholecalciferol booster therapy is associated with a reduced risk of mortality in patients with COVID-19: a cross-sectional multi-centre observational study53 |
|
Retrospective, multi-centre, cross-sectional observational study | Patients received cholecalciferol booster therapy if they were vitamin D insufficient (serum 25(OH)D 25–50 nmol/L) or deficient (<25 nmol/L) as part of routine clinical care; dosing regimens varied from 40,000 IU daily to 20,000 IU every 2 weeks (n=984) | Not specified |
Inclusion:
|
|
|
The following were significantly associated with reduced risk of COVID-19 mortality:
|
Treatment with cholecalciferol appeared to be protective against mortality, regardless of baseline serum 25(OH)D levels |
Exclusion:
| |||||||||
| Effect of high-dose zinc and ascorbic acid supplementation vs usual care on symptom length and reduction among ambulatory patients with SARS-CoV-2 infection: the COVID A to Z randomized clinical trial69 |
|
Prospective, randomized, clinical open-label trial | Each of the following was for a duration of 10 days:
|
Overall:
|
Inclusion:
|
Primary:
|
|
Primary:
|
A significantly faster reduction in symptoms was not observed in any of the active treatment groups versus usual care |
Exclusion:
|
Secondary:
|
Secondary:
|
This article is pre-print and has not yet been peer-reviewed.
25(OH)D, 25-hydroxyvitamin D3 ALA, alpha-lipoic acid; ARDS, acute respiratory distress syndrome; CRP, c-reactive protein; CT, computed tomography; DMB, vitamin D, magnesium, vitamin B; ESRD, end-stage renal disease; GI, gastrointestinal; ICU, intensive care unit; IU, international units; IV, intravenous; NSAIDs, non-steroidal anti-inflammatory drugs; PCR, polymerase chain reaction; SOFA, Sequential Organ Failure Assessment; SpO2, saturation of peripheral oxygen; y/o, years old.
Vitamin D (cholecalciferol, calcifediol)
Vitamin D has previously been proposed to have antiviral effects, which led to a theoretical benefit of its use as an adjuvant in treating COVID-19 infections.38–43 Several retrospective studies have addressed an observed correlation between low serum vitamin D levels and severity of the course of COVID-19 disease symptoms, which is evaluated later in this paper.44–50 Amongst the vitamin D interventional trials assessed in this review, calcifediol use showed significant decreases in intensive care unit (ICU) admission rates, from 50% without therapy to 2% with therapy (p<0.001).51 Additionally, patients receiving high-dose cholecalciferol showed significantly more negative SARS-CoV-2 tests prior to week 3 (p=0.018).52 A retrospective study involving various dosing strategies of cholecalciferol was associated with decreased risk of COVID-19-related mortality (p<0.001).53 With regard to vitamin D levels, in the SHADE study, the cholecalciferol group had achieved significantly higher vitamin D levels (>50 ng/mL) compared to the placebo group (p<0.001)52 by day 14.52
Vitamin C (ascorbic acid)
Vitamin C, a water-soluble vitamin, plays various roles, including supporting connective tissues through collagen synthesis, wound healing, and enhancing the immune system through its bactericidal properties and antibody boosting.54 It has previously been proposed as having a theoretical benefit in immune defence against COVID-19 infection, based on its known properties and hypothetical, inconsistent evidence supporting its role in symptom mitigation in the common cold.55–57 Additionally, various studies have demonstrated the positive effects of vitamin C against Epstein–Barr virus, enterovirus/rhinovirus-induced acute respiratory distress syndrome, and severe sepsis and in mechanically ventilated patients with acute respiratory distress syndrome in the ICU.58–66 IV vitamin C was investigated based on variable evidence of its use in critically ill patients and showed no mortality benefit but some symptom management benefit.67 One study involving high-dose vitamin C in the setting of COVID-19 demonstrated a significantly longer hospital stay than the non-vitamin C arm. Additionally, there were no significant differences in mortality or ICU length of stay.68 Vitamin C, alone and in combination with zinc, showed no significant decreases in COVID-19-related symptoms compared to no study intervention.69
Magnesium
Magnesium has previously been shown to increase 25-hydroxyvitamin D levels when they are <30 ng/mL at baseline;70 thus, if vitamin D helps protect against COVID-19, magnesium could in turn also be beneficial. So far, magnesium has only been studied in combination with vitamins B and D. The combination therapy showed significant decreases in oxygen support (including ICU support) (p=0.006); however, there were no significant differences in the outcome of oxygen support, excluding any ICU support.71
Vitamin B12
Vitamin B12 has been observed to play a fundamental role in gut microbiome,72 which can affect the innate immune response.73 Some data report that SARS-CoV-2 RNA was found in the stool of patients testing positive for COVID-19, implying that there could be involvement of the gut–lung axis in COVID-19 infections.74 Additionally, one study demonstrated that the faecal microbiome of patients testing positive for COVID-19 was significantly altered compared to a control group.75 Similar to magnesium, vitamin B has only been studied in combination with vitamin D and magnesium. As stated above, this combination therapy showed significant decreases in oxygen support (including ICU support) (p=0.006); however, there were no significant differences in the outcome of oxygen support, excluding any ICU support.71
Zinc
The proposed immune-related mechanism of action of zinc is through enhancement of the innate anti-infective properties of basophils, eosinophils, and neutrophils.76 Some weak evidence supports the use of zinc in mitigating symptoms of the common cold.77–80 Additionally, zinc has demonstrated inhibition of RNA polymerase in vitro but this has not been studied in SARS-CoV-2.81,82 Zinc supplementation has been minimally studied in COVID-19; however, one trial demonstrates that zinc, both alone and in combination with vitamin C, showed no significant decreases in COVID-19-related symptoms compared to no study intervention.69
Alpha-lipoic acid
ALA is an anti-inflammatory and antioxidant. It has previously been shown to decrease the levels of serum inflammatory cytokines and inflammatory-related symptoms in patients with acute coronary syndrome, liver transplantation, and kidney–pancreas combined transplantation.83–86 Only one study investigated the use of ALA in COVID-19, and this study demonstrated no significant differences in the Sequential Organ Failure Assessment (SOFA) score by day 7 of therapy or mortality.87 SOFA is a validated scoring system used to predict mortality in ICU patients.88
Vitamin, mineral and nutrient deficiency in COVID-19
Aside from interventional trials involving vitamins and supplements in COVID-19, data have also been published regarding serum levels of vitamins, minerals, and nutrients and their role in COVID-19.89,90 Most of the data involve vitamin D levels. A full review of deficiencies in COVID-19 is beyond the scope of this article, but representative studies are discussed below to better contextualize supplementation in COVID-19. Interested readers can find a more in-depth analysis on this topic in the cited review articles.91–94
Several retrospective studies found a relationship between vitamin D levels and COVID-19 positivity rate. Amongst patients aged >70 years old, one study showed that patients positive for COVID-19 had significantly lower median vitamin D levels compared to those negative for COVID-19 (9.3 ng/mL versus 23.1 ng/mL, respectively; p=0.037).48 Similarly, another study found positive COVID-19 tests were associated with deficient vitamin D status (defined as <20 ng/mL) at the time of testing (relative risk 1.77, 95% CI 1.12–2.81; p=0.02).49 Moreover, a third study demonstrated an association between low vitamin D levels (defined as <30 ng/mL) and an increased likelihood of COVID-19 infection (p<0.001).50
Additional retrospective studies found vitamin D was also related to the severity and outcomes of COVID-19. Amongst patients who were positive for COVID-19, in both inpatient and outpatient settings and equally treated at a single site in Germany, those who had vitamin D deficiency (<12 ng/mL) had significantly higher hospitalization rates (p=0.004), required intensive oxygen therapy (p<0.001), and had significantly higher rates of invasive mechanical ventilation and/or death (p<0.001) or death alone (p<0.001). Insufficient levels of vitamin D (<20 ng/mL) were also associated with higher rates of invasive mechanical ventilation and/or death (p=0.004) or death alone (p=0.2).95 In contrast, another study did not show a difference in mortality between vitamin D deficiency (≤30 nmol/L) and replete inpatient adults ≥65 years old in the United Kingdom. However, vitamin D deficiency was associated with significantly higher ventilation requirements (p=0.042).96 In an Italian study, patients with severe vitamin D deficiency (<10 ng/mL) had higher median respiratory intermediate care unit stays compared to those with vitamin D levels ≥10 ng/mL (8 versus 12.5 days). Additionally, those with severe vitamin D deficiency had higher mortality rates (50% versus 5%; p=0.019).97
Minimal data exist regarding supplements or vitamins, besides vitamin D; however, there are some data on selenium and potassium. In one study, 64.7% of COVID-19 non-survivors had selenium levels <45.7 μg/L, whereas 39.3% of COVID-19 survivors had these levels. Additionally, the COVID-19 non-survivors had significantly lower selenium serum levels than the survivors (p<0.001).98 In another study of 197 inpatients with COVID-19, those who were normokalaemic (K >3.5 mmol/L) had significantly fewer complications (including respiratory failure, sepsis, liver damage, respiratory distress and cardiac damage) than those with severe hypokalaemia (K <3 mmol/L) (p=0.006). Additionally, normokalaemic patients were less likely to be critically ill compared to severely hypokalaemic patients (p=0.03).99
Discussion
Of the vitamins and supplements that were studied, vitamin D presents the most promising data demonstrating significant decreases in oxygen requirements (p=0.006),71 need for ICU treatment (p<0.001),51 SARS-CoV-2 RNA test positivity (p=0.018)52 and mortality (p<0.001).53 All of these benefits were exhibited in hospitalized patients; no studies were conducted in the outpatient setting to demonstrate similar results. A shortcoming of most of the identified trials is the small sample size, with the exception of a large, retrospective trial evaluating various dosing strategies of cholecalciferol and its impact on COVID-19 mortality.53 The end points of each study varied, including SOFA score, mortality, rate of ICU admissions, negativity of COVID-19 tests, oxygen requirements and symptom burden. Additionally, with each study taking place in different parts of the world, the study populations were likely affected by different virus variants. The lack of a global standard of care meant that background therapy varied from trial to trial. In many instances, the dose of the vitamin or supplement utilized in these trials was higher than standard over-the-counter doses,89,90 making it unlikely that patients would take the doses that were studied in these trials without the supervision of a clinician.
Conclusion
With the lack of large randomized controlled trials, results from the studies to date must be interpreted cautiously. At this time, studies involving vitamins and supplements do not provide enough evidence to justify their use over other established pharmacological therapies and prevention techniques that have been proven for use in COVID-19 management and prevention.
Additionally, current data regarding vitamin D levels and COVID-19 suggest that low vitamin D levels are associated with increased risk of COVID-19 infection as well as with more complications during infection and higher rates of death. However, from these data alone, it cannot be deducted that vitamin D supplementation is beneficial in the setting of COVID-19 infections. More data are needed regarding other vitamins and minerals to deduct further effects of serum levels on COVID-19. Finally, with regard to selenium levels, the challenge for most institutions would be limited access to selenium testing.
Acknowledgements
None.
Footnotes
Contributions: LS, SM and MB developed the concept for this manuscript and equally contributed to the research, analysis, and writing of the manuscript and development of tables and figures. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosure and potential conflicts of interest: Dr Michienzi has received grants or contracts from Moderna outside of the work. The authors declare that they have no other conflicts of interest relevant to this manuscript. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at: https://www.drugsincontext.com/wp-content/uploads/2021/09/dic.2021-6-2-COI.pdf
Funding declaration: There was no funding associated with the preparation of this article.
Correct attribution: Copyright © 2021 Speakman LL, Michienzi SM, Badowski ME. https://doi.org/10.7573/dic.2021-6-2. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Article URL: https://www.drugsincontext.com/vitamins-supplements-and-covid-19-a-review-of-currently-available-evidence
Provenance: Invited; externally peer reviewed.
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: Plaza Building, Lee High Road, London, England, SE13 5PT.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editorial office editorial@drugsincontext.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
References
- 1.World Health Organization (WHO) Naming the coronavirus disease (COVID-19) and the virus that causes it. [Accessed March 4, 2021]. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it.
- 2.World Health Organization (WHO) Rolling updates on coronavirus disease (COVID-19) [Accessed March 4, 2021]. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen.
- 3.World Health Organization (WHO) Virtual press conference on COVID-19. Mar 11, 2020. [Accessed March 4, 2021]. https://www.who.int/docs/default-source/coronaviruse/transcripts/who-audio-emergencies-coronavirus-press-conference-full-and-final-11mar2020.pdf?sfvrsn=cb432bb3_2.
- 4.Johns Hopkins University and Medicine. Coronavirus Resource Center; [Accessed June 3, 2021]. https://coronavirus.jhu.edu/map.html. [Google Scholar]
- 5.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sadoff J, Le Gars M, Shukarev G, et al. Interim results of a phase 1–2a trial of Ad26.COV2.S Covid-19 vaccine. N Engl J Med. 2021;384:1824–1835. doi: 10.1056/NEJMoa2034201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavalcanti AB, Zampieri FG, Rosa RG, et al. Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19 [published correction appears in N Engl J Med. 2020;383(21):e119] N Engl J Med. 2020;383(21):2041–2052. doi: 10.1056/NEJMoa2019014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 – final report. N Engl J Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomazini BM, Maia IS, Cavalcanti AB, et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA. 2020;324(13):1307–1316. doi: 10.1001/jama.2020.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.RECOVERY Collaborative Group. Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen P, Nirula A, Heller B, et al. SARS-CoV-2 Neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med. 2021;384(3):229–237. doi: 10.1056/NEJMoa2029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.RECOVERY Collaborative Group. Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial. Lancet. 2021;397(10289):P2049–2059. doi: 10.1016/S0140-6736(21)00897-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deftereos SG, Giannopoulos G, Vrachatis DA, et al. Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: the GRECCO-19 randomized clinical trial. JAMA Netw Open. 2020;3(6):e2013136. doi: 10.1001/jamanetworkopen.2020.13136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lenze EJ, Mattar C, Zorumski CF, et al. Fluvoxamine vs placebo and clinical deterioration in outpatients with symptomatic COVID-19: a randomized clinical trial. JAMA. 2020;324(22):2292–2300. doi: 10.1001/jama.2020.22760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tasnim S, Hossain MM, Mazumder H. Impact of rumors and misinformation on COVID-19 in social media. J Prev Med Public Health. 2020;53(3):171–174. doi: 10.3961/jpmph.20.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michienzi SM, Badowski ME. Can vitamins and/or supplements provide hope against coronavirus? Drugs Context. 2020;9 doi: 10.7573/dic.2020-5-7. 2020-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knoll MD, Wonodi C. Oxford-AstraZeneca COVID-19 vaccine efficacy. Lancet. 2021;397(10269):72–74. doi: 10.1016/S0140-6736(20)32623-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamilton J, Diaz J, Wamsley L. France and Germany pause use of AstraZeneca’s COVID-19 vaccine. NPR. [Accessed September 21, 2021]. https://www.npr.org/sections/coronavirus-live-updates/2021/03/14/976994771/ireland-joins-list-of-countries-pausing-use-of-astrazenecas-covid-19-vaccine.
- 21.Reuters. AstraZeneca to seek US authorization for Covid vaccine this month or early next. NBCNews.com. [Accessed March 16, 2021]. https://www.nbcnews.com/health/health-news/astrazeneca-seek-u-s-authorization-covid-vaccine-month-or-early-n1260987.
- 22.AstraZeneca COVID-19 vaccine: review of very rare cases unusual blood clots continues. European Medicines Agency; [Accessed September 21, 2021]. https://www.ema.europa.eu/en/news/astrazeneca-covid-19-vaccine-review-very-rare-cases-unusual-blood-clots-continues. [Google Scholar]
- 23.Ritchie H, Ortiz-Ospina E, Beltekian D. Coronavirus (COVID-19) vaccinations – statistics and research. Our World in Data. [Accessed April 14, 2021]. https://ourworldindata.org/covid-vaccinations.
- 24.Vaccine Roll COVAX-Out. Gavi, the vaccine alliance. [Accessed September 21, 2021]. https://www.gavi.org/covax-vaccine-roll-out.
- 25.Therapeutic Management. National Institutes of Health; [Accessed March 3, 2021]. https://www.covid19treatmentguidelines.nih.gov/therapeutic-management/ [Google Scholar]
- 26.Prevention and Prophylaxis of SARS-CoV-2 Infection. National Institutes of Health; [Accessed March 3, 2021]. https://www.covid19treatmentguidelines.nih.gov/overview/prevention-of-sars-cov-2/ [Google Scholar]
- 27.Supplements. National Institutes of Health; [Accessed March 3, 2021]. https://www.covid19treatmentguidelines.nih.gov/supplements/ [Google Scholar]
- 28.Gottlieb RL, Nirula A, Chen P, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2021;325(7):632–644. doi: 10.1001/jama.2021.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim YJ, Jang US, Soh SM, Lee JY, Lee HR. The impact on infectivity and neutralization efficiency of SARS-CoV-2 lineage B.1.351 Pseudovirus. Viruses. 2021;13(4):633. doi: 10.3390/v13040633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anti-SARS-CoV-2 Monoclonal Antibodies. National Institutes of Health, US Department of Health and Human Services; [Accessed April 21, 2021]. www.covid19treatmentguidelines.nih.gov/anti-sars-cov-2-antibody-products/anti-sars-cov-2-monoclonal-antibodies. [Google Scholar]
- 31.Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibody for Treatment of COVID-19. US Food and Drug Administration; [Accessed April 14, 2021]. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibody-treatment-covid-19. [Google Scholar]
- 32.Pharmacologic Interventions. National Institutes of Health, US Department of Health and Human Services; [Accessed October 9, 2020]. www.covid19treatmentguidelines.nih.gov/critical-care/pharmacologic-interventions/ [Google Scholar]
- 33.Immunomodulators. National Institutes of Health; [Accessed March 3, 2021]. https://www.covid19treatmentguidelines.nih.gov/immunomodulators/ [Google Scholar]
- 34.Bhimraj A, Morgan R, Hirsch Shumaker A. IDSA Guidelines on the treatment and management of patients with COVID-19. IDSA; [Accessed March 3, 2021]. https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/ [Google Scholar]
- 35.Alhazzani W, Evans L, Alshamsi F. Surviving sepsis campaign guidelines on the management of adults with coronavirus disease 2019 (COVID-19) in the ICU: first update. Crit Care Med. [Accessed March 3, 2021]. https://journals.lww.com/ccmjournal/Fulltext/2021/03000/Surviving_Sepsis_Campaign_Guidelines_on_the.21.aspx. [DOI] [PubMed]
- 36.World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. [Accessed March 3, 2021]. https://www.who.int/publications/i/item/10665-332299.
- 37.Centers for Disease Control and Prevention. Information for clinicians on investigational therapeutics for patients with COVID-19. [Accessed March 3, 2021]. https://www.cdc.gov/coronavirus/2019-ncov/hcp/therapeutic-options.html.
- 38.Jakovac H. COVID-19 and vitamin D-is there a link and an opportunity for intervention? Am J Physiol Endocrinol Metab. 2020;318:E589. doi: 10.1152/ajpendo.00138.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caccialanza R, Laviano A, Lobascio F, et al. Early nutritional supplementation in non-critically ill patients hospitalized for the 2019 novel coronavirus disease (COVID-19): rationale and feasibility of a shared pragmatic protocol. Nutrition. 2020;74:110835. doi: 10.1016/j.nut.2020.110835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carter SJ, Baranauskas MN, Fly AD. Considerations for obesity, vitamin D, and physical activity amid the COVID-19 pandemic. Obesity. 2020;28:1176–1177. doi: 10.1002/oby.22838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malaguarnera L. Vitamin D3 as potential treatment adjuncts for COVID-19. Nutrients. 2020;12:3512. doi: 10.3390/nu12113512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Teymoori-Rad M, Shokri F, Salimi V, Marashi SM. The interplay between vitamin D and viral infections. Rev Med Virol. 2019;29:e2032. doi: 10.1002/rmv.2032. [DOI] [PubMed] [Google Scholar]
- 43.Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. doi: 10.1136/bmj.i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Panagiotou G, Tee SA, Ihsan Y, et al. Low serum 25-hydroxyvitamin D (25[OH]D) levels in patients hospitalized with COVID-19 are associated with greater disease severity. Clin Endocrinol. 2020;93:508–511. doi: 10.1111/cen.14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ilie PC, Stefanescu S, Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin Exp Res. 2020;32:1195–1198. doi: 10.1007/s40520-020-01570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Munshi R, Hussein MH, Toraih EA, et al. Vitamin D insufficiency as a potential culprit in critical COVID-19 patients. J Med Virol. 2021;93:733–740. doi: 10.1002/jmv.26360. [DOI] [PubMed] [Google Scholar]
- 47.Laird E, Rhodes J, Kenny RA. Vitamin D and inflammation: potential implications for severity of Covid-19. Ir Med J. 2020;113(5):81. [PubMed] [Google Scholar]
- 48.D’Avolio A, Avataneo V, Manca A, et al. 25-Hydroxyvitamin D concentrations are lower in patients with positive PCR for SARS-CoV-2. Nutrients. 2020;12(5):1359. doi: 10.3390/nu12051359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meltzer DO, Best TJ, Zhang H, Vokes T, Arora V, Solway J. Association of vitamin D status and other clinical characteristics with COVID-19 test results. JAMA Netw Open. 2020;3(9):e2019722. doi: 10.1001/jamanetworkopen.2020.19722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Merzon E, Tworowski D, Gorohovski A, et al. Low plasma 25(OH) vitamin D level is associated with increased risk of COVID-19 infection: an Israeli population-based study. FEBS J. 2020;287(17):3693–3702. doi: 10.1111/febs.15495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Entrenas Castillo M, Entrenas Costa LM, Vaquero Barrios JM, et al. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: a pilot randomized clinical study. J Steroid Biochem Mol Biol. 2020;203:105751. doi: 10.1016/j.jsbmb.2020.105751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rastogi A, Bhansali A, Khare N, et al. Short term, high-dose vitamin D supplementation for COVID-19 disease: a randomised, placebo-controlled, study (SHADE study) Postgrad Med J. 2020. postgradmedj-2020-139065. [DOI] [PubMed]
- 53.Ling SF, Broad E, Murphy R, et al. High-dose cholecalciferol booster therapy is associated with a reduced risk of mortality in patients with COVID-19: a cross-sectional multi-centre observational study. Nutrients. 2020;12(12):3799. doi: 10.3390/nu12123799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carr AC, Maggini S. Vitamin C and immune function. Nutrients. 2017;9(11):1211. doi: 10.3390/nu9111211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hemilä H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2013;1:CD000980. doi: 10.1002/14651858.CD000980.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Farjana M, Moni A, Sohag AAM, et al. Repositioning vitamin C as a promising option to alleviate complications associated with COVID-19. Infect Chemother. 2020;52(4):461–477. doi: 10.3947/ic.2020.52.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hemilä H. Vitamin C and infections. Nutrients. 2017;9(4):E339. doi: 10.3390/nu9040339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mikirova NA, Hunninghake R. Effect of high dose vitamin C on Epstein-Barr viral infection. Med Sci Monitor. 2014;20:725. doi: 10.12659/MSM.890423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fowler AA, III, Kim C, Lepler L, et al. Intravenous vitamin C as adjunctive therapy for enterovirus/rhinovirus induced acute respiratory distress syndrome. World J Critical Care Med. 2017;6(1):85. doi: 10.5492/wjccm.v6.i1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fisher BJ, Seropian IM, Kraskauskas D, Thakkar JN, Voelkel NF, Natarajan R. Ascorbic acid attenuates lipopolysaccharide-induced acute lung injury. Crit Care Med. 2011;39(6):1454–1460. doi: 10.1097/CCM.0b013e3182120cb8. [DOI] [PubMed] [Google Scholar]
- 61.Fowler AA, III, Syed AA, Knowlson S, et al. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J Trans Med. 2014;12(1):32. doi: 10.1186/1479-5876-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Borrelli E, Roux-Lombard P, Grau GE, et al. Plasma concentrations of cytokines, their soluble receptors, and antioxidant vitamins can predict the development of multiple organ failure in patients at risk. Crit Care Med. 1996;24(3):392–397. doi: 10.1097/00003246-199603000-00006. [DOI] [PubMed] [Google Scholar]
- 63.Fisher BJ, Kraskauskas D, Martin EJ, et al. Attenuation of sepsis-induced organ injury in mice by vitamin C. J Parenteral Enteral Nutr. 2014;38(7):825–839. doi: 10.1177/0148607113497760. [DOI] [PubMed] [Google Scholar]
- 64.Hemilä H, Chalker E. Vitamin C as a possible therapy for COVID-19. Infect Chemother. 2020;52:222–223. doi: 10.3947/ic.2020.52.2.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hemilä H, Chalker E. Vitamin C can shorten the length of stay in the ICU: a meta-analysis. Nutrients. 2019;11(4):708. doi: 10.3390/nu11040708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hemilä H, Chalker E. Vitamin C may reduce the duration of mechanical ventilation in critically ill patients: a meta-regression analysis. J Intensive Care. 2020;8(1):15. doi: 10.1186/s40560-020-0432-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carr AC. Vitamin C administration in the critically ill: a summary of recent meta-analyses. Crit Care. 2019;23(1):265. doi: 10.1186/s13054-019-2538-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jamali Moghadam Siahkali S, Zarezade B, Koolaji S, et al. Safety and effectiveness of high-dose vitamin C in patients with COVID-19: a randomized open-label clinical trial. Eur J Med Res. 2021;26(1):20. doi: 10.1186/s40001-021-00490-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thomas S, Patel D, Bittel B, et al. Effect of high-dose zinc and ascorbic acid supplementation vs usual care on symptom length and reduction among ambulatory patients with SARS-CoV-2 infection: the COVID A to Z randomized clinical trial. JAMA Netw Open. 2021;4(2):e210369. doi: 10.1001/jamanetworkopen.2021.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dai Q, Zhu X, Manson JE, et al. Magnesium status and supplementation influence vitamin D status and metabolism: results from a randomized trial. Am J Clin Nutr. 2018;108:1249–1258. doi: 10.1093/ajcn/nqy274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tan CW, Ho LP, Kalimuddin S, et al. Cohort study to evaluate the effect of vitamin D, magnesium, and vitamin B12 in combination on progression to severe outcomes in older patients with coronavirus (COVID-19) Nutrition. 2020;79–80:111017. doi: 10.1016/j.nut.2020.111017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Degnan PH, Taga ME, Goodman AL. Vitamin B12 as a modulator of gut microbial ecology. Cell Metab. 2014;20:769–778. doi: 10.1016/j.cmet.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Negi S, Das DK, Pahari S, Nadeem S, Agrewala JN. Potential role of gut micro- biota in induction and regulation of innate immune memory. Front Immunol. 2019;10:2441. doi: 10.3389/fimmu.2019.02441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dhar D, Mohanty A. Gut microbiota and Covid-19 – possible link and implications. Virus Res. 2020;285:198018. doi: 10.1016/j.virusres.2020.198018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zuo T, Zhang F, Lui GCY, et al. Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology. 2020;159(3):944–955.e8. doi: 10.1053/j.gastro.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gammoh NZ, Rink L. Zinc in infection and inflammation. Nutrients. 2017;9(6):E624. doi: 10.3390/nu9060624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Singh M, Das RR. Zinc for the common cold. Cochrane Datab Syst Rev. 2011;2:CD001364. doi: 10.1002/14651858.CD001364.pub3. [DOI] [PubMed] [Google Scholar]
- 78.Eby GA, Davis DR, Halcomb WW. Reduction in duration of common colds by zinc gluconate lozenges in a double-blind study. Antimicrob Agents Chemother. 1984;25(1):20–24. doi: 10.1128/AAC.25.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hemilä H. Zinc lozenges and the common cold: a meta-analysis comparing zinc acetate and zinc gluconate, and the role of zinc dosage. JRSM Open. 2017;8(5):2054270417694291. doi: 10.1177/2054270417694291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Weismann K, Jakobsen JP, Weismann JE, et al. Zinc gluconate lozenges for common cold: a double-blind clinical trial. Dan Med Bull. 1990;37(3):279–281. [PubMed] [Google Scholar]
- 81.Te Velthuis AJ, van den Worm SH, Sims AC, Baric RS, Snijder EJ, van Hemert MJ. Zn(2+) inhibits corona virus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6(11):e1001176. doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Krenn BM, Gaudernak E, Holzer B, Lanke K, Van Kuppeveld FJ, Seipelt J. Antiviral activity of the zinc ionophores pyrithione and hinokitiol against picornavirus infections. J Virol. 2009;83(1):58–64. doi: 10.1128/JVI.01543-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li RJ, Ji WQ, Pang JJ, et al. Alpha-lipoic acid ameliorates oxidative stress by increasing aldehyde dehydrogenase-2 activity in patients with acute coronary syndrome. Tohoku J Exp Med. 2013;229(1):45–51. doi: 10.1620/tjem.229.45. [DOI] [PubMed] [Google Scholar]
- 84.Sardu C, Santulli G, Santamaria M, et al. Effects of alpha lipoic acid on multiple cytokines and biomarkers and recurrence of atrial fibrillation within 1 year of catheter ablation. Am J Cardiol. 2017;119(9):1382–1386. doi: 10.1016/j.amjcard.2017.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Casciato P, Ambrosi N, Caro F, et al. Alpha-lipoic acid reduces postreperfusion syndrome in human liver transplantation – a pilot study. Transpl Int. 2018;31(12):1357–1368. doi: 10.1111/tri.13314. [DOI] [PubMed] [Google Scholar]
- 86.Bacchetti P, Leung JM. Sample size calculations in clinical research. Anesthesiology. 2002;97(4):1028–1029. doi: 10.1097/00000542-200210000-00050. author reply 1029–1032. [DOI] [PubMed] [Google Scholar]
- 87.Zhong M, Sun A, Xiao T, et al. A randomized, single-blind, group sequential, active-controlled study to evaluate the clinical efficacy and safety of α-Lipoic acid for critically ill patients with coronavirus disease 2019 (COVID-19) medRxiv. 2020 doi: 10.1101/2020.04.15.20066266. 04.15.20066266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ferreira FL, Bota DP, Bross A, Mélot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286(14):1754–1758. doi: 10.1001/jama.286.14.1754. [DOI] [PubMed] [Google Scholar]
- 89.Ross AC, Taylor CL, Yaktine AL, Del Valle HB, editors. Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: National Academies Press (US); 2011. [PubMed] [Google Scholar]
- 90.Vitamin C Fact Sheet for Consumer. National Institutes of Health: Office of Dietary Supplements; Dec 10, 2019. [Accessed September 13, 2021]. https://ods.od.nih.gov/pdf/factsheets/VitaminC-Consumer.pdf. [Google Scholar]
- 91.Wang MX, Gwee SXW, Pang J. Micronutrients deficiency, supplementation and novel coronavirus infections-a systematic review and meta-analysis. Nutrients. 2021;13(5):1589. doi: 10.3390/nu13051589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gorji A, Khaleghi Ghadiri M. Potential roles of micronutrient deficiency and immune system dysfunction in the coronavirus disease 2019 (COVID-19) pandemic. Nutrition. 2021;82:111047. doi: 10.1016/j.nut.2020.111047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Teshome A, Adane A, Girma B, Mekonnen ZA. The impact of vitamin D level on COVID-19 infection: systematic review and meta-analysis. Front Public Health. 2021;9:624559. doi: 10.3389/fpubh.2021.624559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Petrelli F, Luciani A, Perego G, Dognini G, Colombelli PL, Ghidini A. Therapeutic and prognostic role of vitamin D for COVID-19 infection: A systematic review and meta-analysis of 43 observational studies. J Steroid Biochem Mol Biol. 2021;211:105883. doi: 10.1016/j.jsbmb.2021.105883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Radujkovic A, Hippchen T, Tiwari-Heckler S, Dreher S, Boxberger M, Merle U. Vitamin D deficiency and outcome of COVID-19 Patients. Nutrients. 2020;12(9):2757. doi: 10.3390/nu12092757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Baktash V, Hosack T, Patel N, et al. Vitamin D status and outcomes for hospitalised older patients with COVID-19. Postgrad Med J. 2021;97(1149):442–447. doi: 10.1136/postgradmedj-2020-138712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Carpagnano GE, Di Lecce V, Quaranta VN, et al. Vitamin D deficiency as a predictor of poor prognosis in patients with acute respiratory failure due to COVID-19. J Endocrinol Invest. 2021;44(4):765–771. doi: 10.1007/s40618-020-01370-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Moghaddam A, Heller RA, Sun Q, et al. Selenium deficiency is associated with mortality risk from COVID-19. Nutrients. 2020;12(7):2098. doi: 10.3390/nu12072098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen D, Li X, Song Q, et al. Assessment of hypokalemia and clinical characteristics in patients with coronavirus disease 2019 in Wenzhou, China. JAMA Netw Open. 2020;3(6):e2011122. doi: 10.1001/jamanetworkopen.2020.11122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]