Abstract
Prosthetic joint infection caused by Mycobacterium tuberculosis (TBPJI) is uncommon but can be encountered in immunocompromised patients or those from tuberculosis-endemic regions. A lack of clinical suspicion and experience with TBPJI often leads to a delay in diagnosis. We report 2 cases of TBPJI in a Hungarian-Canadian and Iranian-Canadian immigrant, respectively. Both were treated with concurrent surgical and medical therapy. We also performed a literature review on TBPJI case reports, outlining their diagnosis and management.
Keywords: extrapulmonary TB, Mycobacterium tuberculosis, prosthetic joint infection, PJI
Tuberculosis remains a major global health problem with an estimated 10 million incident cases worldwide in 2019 [1]. In Canada, 1796 cases of active tuberculosis were reported in 2017, with an incidence of 4.9 per 100 000 population [2]. Most cases were from foreign-born (71.8%) and Canadian-born Indigenous populations (17.4%) [2].
In 2017, extrapulmonary tuberculosis accounted for 21.4% of all tuberculosis cases reported in Canada [2]. In 2010, osteoarticular tuberculosis (eg, Pott disease, tuberculous arthritis) accounted for 2.5% of all tuberculosis cases reported in Canada [3], a proportion essentially unchanged for years and similar to that of neighboring countries (eg, United States) [4, 5].
Prosthetic joint infections caused by Mycobacterium tuberculosis (TBPJI) are rare [6, 7]. Similar to native joint tuberculous arthritis, the varied clinical and radiographic presentation of TBPJI, as well as lack of consideration by clinicians, often leads to delays in diagnosis and subsequent disease progression [8–10].
To date, there are no established, evidence-based recommendations on the diagnosis and management of TBPJI. We report 2 cases of TBPJI and review the current literature on this topic.
CASE 1
A 71-year-old immunocompetent, nondiabetic woman had progressive left hip pain since 2013; initial workup demonstrated normal inflammatory markers, no metabolic bone diseases, and no underlying malignancy by whole-body computed tomography. Her medical history included cardiac bypass surgery.
In April 2017, increasing difficulty ambulating and radiographic signs of severe arthropathy with acetabular erosion prompted surgical intervention. Preoperatively, her C-reactive protein level was elevated (141.6 mg/L), but image-guided hip aspiration for routine bacterial culture was negative.
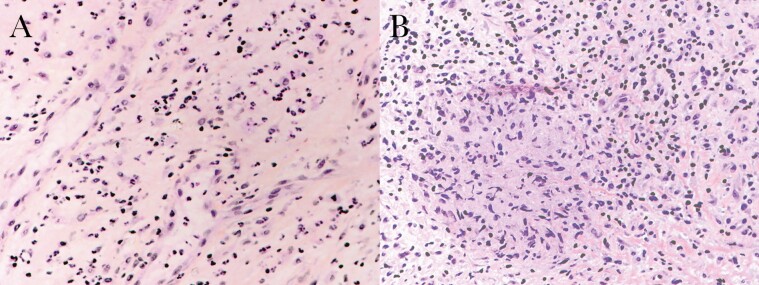
Intraoperative findings revealed a necrotic femoral head with acetabular erosion, raising concerns for severe inflammatory arthropathy vs occult/past infection; a cement spacer was therefore inserted rather than total hip arthroplasty until infection was ruled out. Intraoperative samples for routine bacterial cultures were ultimately negative. Histopathology demonstrated scattered multinucleated giant cells with granulomatous reaction (Figure 1), though fungal and bacterial (including acid-fast) stains were negative.
Figure 1.
Histopathology of left acetabulum tissue from case-patient 1. A, Acute neutrophilic inflammation (hematoxylin and eosin [H&E] stain, ×400). B, Granulomatous reaction with scattered multinucleated giant cells (H&E stain, ×400).
She was well until 2 months postoperatively, when a sinus tract developed with serosanguinous discharge from her left hip. A repeat hip aspirate was again negative for bacterial growth. A Mantoux skin test done was positive at 32 mm. An additional hip aspirate was positive for acid-fast bacilli (AFB), later identified as Mycobacterium tuberculosis complex at a provincial reference laboratory by mycobacterial culture and in-house–developed nucleic acid amplification testing (NAAT)/polymerase chain reaction (PCR). Recent chest computed tomography revealed old granulomatous disease.
She was referred to Infectious Diseases for management. Further history revealed possible childhood exposure to active pulmonary tuberculosis from her stepmother when the patient emigrated from Hungary to Austria in 1987, before permanently residing in Canada in 1989.
The patient was treated with isoniazid, rifampin, and ethambutol. Pyrazinamide was avoided due to age and the increased risk for drug-induced hepatitis. Her isolate was pan-susceptible to first-line agents; she continued triple therapy for 2 months followed by isoniazid and rifampin for an additional 10 months. She responded well to antimycobacterial therapy (AMT) alone without any adverse reactions. In September 2018 (2 months after completing AMT), she underwent a revision total hip arthroplasty to improve mobility (from a cement spacer) and remains clinically well postoperatively.
CASE 2
A 50-year-old man was seen for worsening mobility and inability to work due to prosthetic hip infection in 2016. His past history included left hip osteomyelitis as a child, complicated by osteoarthritis requiring multiple surgeries. He underwent total hip arthroplasty in 2013 at age 47. Preoperative imaging showed a grossly deformed femoral head, neck, and intertrochanteric region. During arthroplasty, tissue samples sent for routine bacterial cultures and histopathology for standard (ie, bacterial, fungal) stains were negative. In 2014, he underwent a washout with head/liner exchange and 6 weeks of ceftriaxone, vancomycin, and rifampin for culture-negative prosthetic joint infection (intraoperative samples negative for routine bacterial cultures). For reasons unexplained, he continued rifampin monotherapy until reassessment in June 2016 for increased swelling and sinus drainage from his surgical wound. A superficial wound swab grew Peptostreptococcus spp, which was interpreted as a contaminant.
He underwent single-stage revision arthroplasty with operative cultures yielding no bacterial growth. Suspecting an indolent infection, additional specimens were sent for AFB and returned positive with confirmed M tuberculosis complex by NAAT/PCR and mycobacterial culture. Histopathology demonstrated necrotizing granulomas and presence of AFB. Sputum acid-fast staining was negative; the patient denied any respiratory symptoms. Recent chest radiography showed healed granulomatous disease.
Infectious Diseases was consulted for management. The patient was born in Iran but lived in Afghanistan and Uzbekistan during childhood before permanently residing in Canada. Of interest, his wife was treated for tuberculous mastitis in 2010; the patient received a 9-month course of isoniazid for latent tuberculosis treatment in 2011.
He was treated with isoniazid, rifampin, ethambutol, and pyrazinamide (isolate was pan-susceptible to first-line agents) for 2 months, followed by isoniazid and rifampin for an additional 10 months. He responded well to AMT with only mild transient thrombocytopenia and lymphopenia during therapy. Six months after completion of AMT and following rehabilitation, he successfully returned to work with full capacity and remained clinically well.
REVIEW OF THE LITERATURE
We undertook a comprehensive search of English-language articles from inception to February 2021 through Embase, Ovid Medline, PubMed, and Google Scholar, using keywords “Mycobacterium tuberculosis” and “prosthetic joint infection” including medical subject heading (MeSH) terms (Supplementary Files 1 and 2). Nonhuman infections were excluded. Native joint septic arthritis, infections not involving prosthetic joints, infections by pathogens other than M tuberculosis, and non-English articles were excluded. Our search expanded by reviewing citations from included studies. We identified a total of 107 cases of TBPJI from 50 published articles (Supplementary Table 1) [7, 11–59].
Median age was 71 years (interquartile range [IQR], 60.3–79 years), with male-to-female ratio approaching 1:1. The most common were hip (52%) and knee (43%) infections; other affected joints were also reported. Patients initially received arthroplasty for osteoarthritis (70%), inflammatory arthritis (9.5%), or fracture involving the joint (12.3%).
Twenty-two (20.6%) cases reported underlying comorbidities (Table 1). Twenty-nine (27.1%) cases reported prior tuberculous infections, including pulmonary (n = 10), osteoarticular (n = 13), and latent infection (n = 2). Only 3 cases documented prior treatment. Ten patients reported having lived in or traveled to endemic countries. Only 1 case confirmed prior household exposure to tuberculosis.
Table 1.
Summary of Cases and Patient Characteristics From Literature Review, by Medical/Surgical Management
| Characteristic | All Patients (N = 107a) |
AMT Only (n = 21) | Surgery Only (n = 4) | AMT + DAIR (n = 27b) | AMT + Staged Revisionc (n = 36b) | AMT + Hardware Removal (n = 22b) |
|---|---|---|---|---|---|---|
| Age, y, median (IQR) | 71 (60.3–79) | 65.5 (60–77.5) | 59.5 (46–73.8) | 73.5 (67.8–80) | 69 (56–77) | 71.5 (60.3–75) |
| Female sex | 52 | 7 | 3 | 15 | 18 | 11 |
| Risk factor | ||||||
| Diabetes mellitus | 12 | 2 | 1 | 2 | 5 | 3 |
| Steroid use | 6 | 0 | 0 | 2 | 1 | 2 |
| Anti-TNF use | 2 | 2 | 0 | 0 | 0 | 0 |
| HIV/AIDS | 1 | 1 | 0 | 0 | 0 | 0 |
| Chemotherapy | 1 | 0 | 0 | 0 | 0 | 1 |
| Preoperative diagnosis | ||||||
| Osteoarthritis | 51/73 (70) | 13/17 (76.5) | 3/3 | 10/16 (62.5) | 21/28 (75) | 8/13 (61.5) |
| Inflammatory arthritis | 7/73 (9.5) | 4/17 (23.5) | 0/3 | 2/16 (12.5) | 0/28 | 1/13 (7.7) |
| Fracture | 9/73 (12.3) | 0/17 | 0/3 | 4/16 (25) | 4/28 (14.3) | 1/13 (7.7) |
| Other | 6/73 (8.2) | 0/17 | 0/3 | 0/16 | 3/28 (10.7) | 3/13 (23.1) |
| Arthroplasty | ||||||
| Hip | 56 | 8 | 3 | 12 | 22 | 13 |
| Knee | 46 | 13 | 1 | 14 | 12 | 7 |
| Other | 5 | 0 | 0 | 1 | 2 | 2 |
| Other bacteria isolated from joint aspirate | 26 | 0 | 1 | 5 | 12 | 9 |
| AMT duration, mo, median (IQR) | 12 (11.3–18) | 15 (12–18) | NA | 12 (9–15.5) | 13 (11–15.8) | 12 (12–15.8) |
| Outcome | ||||||
| Clinical resolution | 81/102 (79.4) | 18/20 (90) | 0/3 | 21/27 (77.8) | 29/35 (82.9) | 17/21 (81) |
| Functional lossd | 6/102 (5.9) | 1/20 (5) | 1/3 (33.3) | 0/27 | 2/35 (5.7) | 2/21 (9.5) |
| Clinical failuree | 4/102 (3.9) | 0/20 | 0/3 | 3/27 (11.1) | 1/35 (2.9) | 0/21 |
| Overall mortality | 11/102 (10.8) | 1/20 (5) | 2/3 (66.7) | 3/27 (11.1) | 3/35 (8.5) | 2/21 (9.5) |
| TB-related death | 4/102 (3.9) | 1/20 (5) | 0/3 | 1/27 (3.7) | 1/35 (2.9) | 0/21 |
| Additional surgery required | 5/102 (4.9) | 0/20 | 0/3 | 4/27 (14.8) | 1/35 (2.9) | 0/21 |
Data are presented as No. or as no./No. (%) unless otherwise indicated.
Abbreviations: AMT, antimycobacterial therapy; DAIR, debridement and implant retention; HIV, human immunodeficiency virus; IQR, interquartile range; NA, not applicable; TB, Mycobacterium tuberculosis; TNF, tumor necrosis factor.
aTwo patients received no interventions (1 was lost to follow-up, 1 had a postmortem diagnosis of prosthetic joint infection caused by Mycobacterium tuberculosis).
bFive cases received 2 surgical interventions: DAIR + 2-stage revision (n = 2); DAIR + hardware removal (n = 2); and 2-stage revision + hardware removal (n = 1).
cStaged revision includes 1-stage and 2-stage revision arthroplasty.
dFunctional loss defined here as limitations or inability to use affected joint to carry out activities or functions of daily living (in relation to unaffected joint).
eClinical failure includes chronic joint infection, relapse of infection, etc.
Clinical presentations varied and were nonspecific. Localized (eg, pain, swelling) and constitutional (eg, fever, night sweats, weight loss) symptoms were described, including development of sinus drainage (n = 33) or abscesses (n = 14). Symptom onset following arthroplasty ranged from 10 days to 38 years (median, 2 years); 4 cases had a presumptive diagnosis of TBPJI made intraoperatively.
Diagnosis was confirmed from joint aspirate or tissue sent for 1 or more of the following: AFB staining (n = 19), mycobacterial culture (n = 91), NAAT/PCR (n = 23), or histopathology (n = 32). Twenty patients had a tuberculin skin test done (16 positive; 4 negative). One or multiple bacterial pathogens were isolated in at least 26 cases prior to confirmation of TBPJI, including Staphylococcus aureus (n = 10), coagulase-negative staphylococci (n = 10), Corynebacterium spp (n = 3), and Pseudomonas aeruginosa (n = 3).
Table 1 summarizes patient characteristics and outcomes based on the treatment modality received. Excluding patients receiving no interventions, almost all patients (96%) received AMT. The 4 cases receiving surgery only (2 hardware removal, 2 staged revisions) have no explanation available for omission of AMT, although 2 patients died shortly from unrelated causes and 1 was lost to follow-up. For the 21 medically managed cases, 2 were clinically unstable to undergo surgery, and 8 had stable prosthesis or recent surgical intervention for the joint.
Choice of AMT combinations varied depending on clinician and drug adverse events; generally, a combination of first-line antimycobacterials was used unless drug resistance was detected. Duration ranged from 6 months to 3 years, including 1 case considering lifelong suppression (drug not specified) following debridement and implant retention surgery [55]. Slightly longer courses (median, 15 months [IQR, 12–18 months]) appeared to be used in those receiving AMT only, compared to those undergoing combined medical/surgical management (eg, median, 12 months [IQR, 12–15.8 months]).
The follow-up period ranged from immediately completing AMT to 20 years. Most cases (81 of 102 [79.4%]) had clinical resolution. Four of 11 deaths were attributed to tuberculosis, including disseminated or pulmonary tuberculosis (n = 2), multidrug resistance (n = 1), and 1 case of postmortem-confirmed TBPJI without receiving treatment. Five unrelated deaths were due to preexisting conditions (eg, cancer, cirrhosis); 2 cases did not report cause of death.
DISCUSSION
Mycobacterium tuberculosis is an acid-fast, aerobic bacillus with humans as its only known reservoir [60]. It has a wide spectrum of clinical manifestations, including pulmonary, disseminated, and osteoarticular tuberculosis [61].
Prosthetic joint infections caused by M tuberculosis are uncommon [6, 7]. One explanation is that the population more often undergoing joint replacement surgery (ie, higher income or socioeconomic status) [62] is less likely affected by tuberculosis, which largely impacts marginalized persons or those from tuberculosis-endemic countries, though this trend may change as access to health care improves with time [63].
Based on our literature review, the nonspecific clinical presentation and variable timeline for symptom onset following arthroplasty suggest the need for a detailed history by the clinician suspecting TBPJI. This includes outlining any relevant risk factors including immunocompromised status, travel to or living in endemic regions, or prior active or latent tuberculosis infection, as well as an exposure history that raises suspicion and need to obtain samples for microbiologic testing of TBPJI [6, 64]. Although the tuberculin skin test was used in cases (19%) from our literature review, it should not be relied upon to diagnose or exclude active infection [3, 65].
Clinicians should consider atypical pathogens (eg, fungal, M tuberculosis) when patients with PJI fail a trial of empiric antibiotics with negative synovial or tissue cultures for common bacteria. However, 24% of cases in our literature review had other bacteria isolated, further delaying diagnosis of TBPJI. Possible coinfections with intracellular bacteria (eg, Salmonella spp, Listeria spp) via survival within macrophages through a cell-mediated response (Th1) was proposed in case reports/series [66–68]; however, a population-based study found no associated infection risk with intracellular pathogens in patients with active tuberculosis vs the general population [69]. Additionally, we searched for M tuberculosis and coinfection with bacteria reported in our literature review; few case reports were found [70, 71]. The relation between tuberculosis and other bacteria causing PJI may be of interest for future research.
As with native joint tuberculous arthritis and extrapulmonary tuberculosis, diagnostic confirmation of TBPJI is challenged by poor diagnostic yield and low sensitivity (19%) of AFB smear microscopy on extrapulmonary samples, which are often paucibacillary [3, 65]. As such, synovial fluid (sensitivity, 64%–79%) or tissue (sensitivity, 94%) for mycobacterial culture is often required [3], although the incorporation of NAAT/PCR testing (if available) offers rapid identification of M tuberculosis [72], as in our 2 presented cases. The advent of next-generation sequencing offers greater versatility in diagnosis of tuberculosis, drug resistance detection, and typing of M tuberculosis [73]. In England, whole-genome sequencing available as part of routine testing for tuberculosis along with rapid molecular diagnostics (NAAT/PCR) allows for effective public health outbreak investigation and earlier genotypic susceptibilities [73]. Certainly, considering use of whole-genome sequencing depends on cost-effectiveness in comparison to standard culture and molecular testing methods, availability of trained personnel and local infrastructure, and local prevalence of tuberculosis.
The patient characteristics, stratified by treatment modality (Table 1), appeared to be similar between groups. Duration of AMT (median, 12 months [IQR, 11.3–18 months]) may have been extrapolated from guidelines for native joint tuberculous arthritis [6, 74], where 6–12 months of isoniazid- and rifampin-based regimen is recommended, with longer durations preferred for complicated cases [3]. The optimal AMT duration for TBPJI remains to be determined, although based on our literature review, slightly longer courses were used in patients receiving AMT alone vs combined medical/surgical management. Though not explicitly mentioned, clinicians likely preferred longer courses to achieve remission in patients where hardware retention is part of the management strategy.
Comparing outcomes between treatment groups, rate of clinical resolution seemed to be higher in patients on AMT only. Approximately 40% of patients on AMT alone were assessed by their surgeon to have stable prosthesis (ie, better prognosis) without further surgical intervention needed. Conversely, patients requiring surgery likely had severe hardware-related complications contributing to less favorable outcomes. Although overall clinical success appeared similar between groups receiving combined medical/surgical management, patients undergoing debridement and implant retention surgery were more likely (15%) to require definitive surgery afterward (ie, staged revision, hardware removal) to achieve clinical remission and regain function/mobility. As choice of optimal medical and/or combined surgical management remains tentatively a case-by-case decision in consultation with Infectious Diseases and Orthopedics, larger observational studies comparing treatment strategies may be conducive to developing best practices for TBPJI.
CONCLUSIONS
TBPJI is a rare manifestation of tuberculosis, but clinical suspicion should be raised in those with epidemiologic and clinical risk factors for active tuberculosis infection, which may include failure to respond to prior antibiotics for treatment of PJI. Though medical therapy alone has shown promising outcomes based on our literature review, further studies comparing treatment strategies (AMT and/or surgical management) are required to better define groups of patients who require either or both as their optimal management for TBPJI.
Supplementary Material
Notes
Author contributions. G. C. A. W. and E. W. W. cared for the patient in case 1. J. G. cared for the patient in case 2. C. K. L. L. and E. W. W. conceived of the study. C. K. L. L. performed the literature search and wrote the original draft. L. C. and S. V. prepared the pathology slides for figures and visualization. All authors critically reviewed the manuscript and read and approved the final manuscript.
Patient consent statement. The design of the work was approved by the local Queen’s University Health Sciences and Affiliated Teaching Hospitals Research Ethics Board (HSREB DMED-2470-21). Signed consent to publish this case report was obtained from the patient’s family in case 1. Verbal consent to publish this case report was obtained from the patient in case 2, but we could not subsequently reach the patient through all available contact information provided in his medical record to obtain signed authorization. All identifiers were removed from the medical information of the presented cases prior to publication, according to the Personal Information Protection and Electronic Document Act (PIPEDA). To protect the patients’ identity, no images of the patients were used.
Potential conflicts of interest. G. C. A. W. holds educational contracts with Stryker, Zimmer Biomet, and Depuy Synthes, outside the submitted work. All other authors report no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Global Tuberculosis Report 2020. Geneva, Switzerland: World Health Organization; 2020. [Google Scholar]
- 2. LaFreniere M, Hussain H, He N, McGuire M. Tuberculosis in Canada: 2017. Can Commun Dis Rep 2019; 45(2/3):68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fisher D, Elwood K. Nonrespiratory tuberculosis. In: Canadian Tuberculosis Standards. 7th ed. Ottawa: Public Health Agency of Canada; 2014. [Google Scholar]
- 4. Peto HM, Pratt RH, Harrington TA, et al. Epidemiology of extrapulmonary tuberculosis in the United States, 1993–2006. Clin Infect Dis 2009; 49:1350–7. [DOI] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention. Reported tuberculosis in the United States, 2019. 2020. Available at: https://www.cdc.gov/tb/statistics/reports/2019/table15.htm. Accessed 16 January 2021.
- 6. Kim SJ, Kim JH. Late onset Mycobacterium tuberculosis infection after total knee arthroplasty: a systematic review and pooled analysis. Scand J Infect Dis 2013; 45:907–14. [DOI] [PubMed] [Google Scholar]
- 7. Veloci S, Mencarini J, Lagi F, et al. Tubercular prosthetic joint infection: two case reports and literature review. Infection 2018; 46:55–68. [DOI] [PubMed] [Google Scholar]
- 8. Al-Sayyad MJ, Abumunaser LA. Tuberculous arthritis revisited as a forgotten cause of monoarticular arthritis. Ann Saudi Med 2011; 31:398–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Broderick C, Hopkins S, Mack DJF, et al. Delays in the diagnosis and treatment of bone and joint tuberculosis in the United Kingdom. Bone Joint J 2018; 100-B:119–24. [DOI] [PubMed] [Google Scholar]
- 10. Stanish W, Hyndman J, Forsythe M. Skeletal tuberculosis: the great imitator. J Bone Joint Surg Br 1977; 59B:511. [Google Scholar]
- 11. Asopa V, Wallace AL. Case report: management of occult tuberculosis infection by 2-stage arthroplasty of the elbow. J Shoulder Elbow Surg 2004; 13:364–5. [DOI] [PubMed] [Google Scholar]
- 12. Baldini N, Toni A, Greggi T, Giunti A. Deep sepsis from Mycobacterium tuberculosis after total hip replacement. Case report. Arch Orthop Trauma Surg 1988; 107:186–8. [DOI] [PubMed] [Google Scholar]
- 13. Barry M, Akkielah L, Askar MA, Bin Nasser AS. Miliary tuberculosis with delayed-onset total knee arthroplasty mycobacteria tuberculosis infection successfully treated with medical therapy alone: a case report and literature review. Knee 2019; 26:1152–8. [DOI] [PubMed] [Google Scholar]
- 14. Berbari EF, Hanssen AD, Duffy MC, et al. Prosthetic joint infection due to Mycobacterium tuberculosis: a case series and review of the literature. Am J Orthop (Belle Mead NJ) 1998; 27:219–27. [PubMed] [Google Scholar]
- 15. Brown S, Berger R, Ramgopal M, Dahya V. Tuberculosis infection status post partial knee arthroplasty: a novel treatment approach. Infect Dis Clin Pract 2016; 24:350–1. [Google Scholar]
- 16. Carrega G, Bartolacci V, Burastero G, et al. Prosthetic joint infections due to Mycobacterium tuberculosis: a report of 5 cases. Int J Surg Case Rep 2013; 4:178–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chang CH, Hu CC, Chang Y, et al. Two-stage revision arthroplasty for Mycobacterium tuberculosis periprosthetic joint infection: an outcome analysis. PLoS One 2018; 13:e0203585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Congia S, Puddu G, Sorrentino G, et al. Conservative treatment of early-onset tubercular periprosthetic joint infection following total knee arthroplasty. J Infect Dev Ctries 2020; 14:223–7. [DOI] [PubMed] [Google Scholar]
- 19. de Haan J, Vreeling AW, van Hellemondt GG. Reactivation of ancient joint tuberculosis of the knee following total knee arthroplasty after 61 years: a case report. Knee 2008; 15:336–8. [DOI] [PubMed] [Google Scholar]
- 20. De Nardo P, Corpolongo A, Conte A, et al. Total hip replacement infected with Mycobacterium tuberculosis complicated by Addison disease and psoas muscle abscess: a case report. J Med Case Rep 2012; 6:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Egües Dubuc C, Uriarte Ecenarro M, Errazquin Aguirre N, Belzunegui Otano J. Prosthesis infection by Mycobacterium tuberculosis in a patient with rheumatoid arthritis: a case report and literature review. Reumatol Clin 2014; 10:347–9. [DOI] [PubMed] [Google Scholar]
- 22. Elzein FE, Haris M, Alolayan SS, Al Sherbini N. Total knee prosthesis infected with Mycobacterium tuberculosis. BMJ Case Rep 2017; 2017:bcr2017220596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fernández-Valencia JA, García S, Riba J. Presumptive infection of a total hip prosthesis by Mycobacterium tuberculosis: a case report. Acta Orthop Belg 2003; 69:193–6. [PubMed] [Google Scholar]
- 24. Harwin SF, Banerjee S, Issa K, et al. Tubercular prosthetic knee joint infection. Orthopedics 2013; 36:e1464–9. [DOI] [PubMed] [Google Scholar]
- 25. Hattrup SJ, Bhagia UT. Shoulder arthroplasty complicated by Mycobacterium tuberculosis infection: a case report. J Shoulder Elbow Surg 2008; 17:e5–7. [DOI] [PubMed] [Google Scholar]
- 26. Hugate RJ, Pellegrini VDJ. Reactivation of ancient tuberculous arthritis of the hip following total hip arthroplasty: a case report. J Bone Joint Surg Br 2002; 84:101–5. [DOI] [PubMed] [Google Scholar]
- 27. Jitmuang A, Yuenyongviwat V, Charoencholvanich K, Chayakulkeeree M. Rapidly-growing mycobacterial infection: a recognized cause of early-onset prosthetic joint infection. BMC Infect Dis 2017; 17:802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kadakia AP, Williams R, Langkamer VG. Tuberculous infection in a total knee replacement performed for medial tibial plateau fracture: a case report. Acta Orthop Belg 2007; 73:661–4. [PubMed] [Google Scholar]
- 29. Kaya M, Nagoya S, Yamashita T, et al. Peri-prosthetic tuberculous infection of the hip in a patient with no previous history of tuberculosis. J Bone Joint Surg Br 2006; 88:394–5. [DOI] [PubMed] [Google Scholar]
- 30. Khater FJ, Samnani IQ, Mehta JB, et al. Prosthetic joint infection by Mycobacterium tuberculosis: an unusual case report with literature review. South Med J 2007; 100:66–9. [DOI] [PubMed] [Google Scholar]
- 31. Klein GR, Jacquette GM. Prosthetic knee infection in the young immigrant patient—do not forget tuberculosis! J Arthroplasty 2012; 27:1414.e1–4. [DOI] [PubMed] [Google Scholar]
- 32. Krappel FA, Harland U. Failure of osteosynthesis and prosthetic joint infection due to Mycobacterium tuberculosis following a subtrochanteric fracture: a case report and review of the literature. Arch Orthop Trauma Surg 2000; 120:470–2. [DOI] [PubMed] [Google Scholar]
- 33. Kreder HJ, Davey JR. Total hip arthroplasty complicated by tuberculous infection. J Arthroplasty 1996; 11:111–4. [DOI] [PubMed] [Google Scholar]
- 34. Lederman E, Kweon C, Chhabra A. Late Mycobacterium tuberculosis infection in the shoulder of an immunocompromised host after hemiarthroplasty: a case report. J Bone Joint Surg Br 2011; 93:e67.1–4. [DOI] [PubMed] [Google Scholar]
- 35. Lee CL, Wei YS, Ho YJ, Lee CH. Postoperative Mycobacterium tuberculosis infection after total knee arthroplasty. Knee 2009; 16:87–9. [DOI] [PubMed] [Google Scholar]
- 36. Lee H-J, Kim K-W, Kim KS, et al. Primary musculoskeletal Mycobacterium infection with large cystic masses after total hip arthroplasty. J Arthroplasty 2013; 28:374.e1–3. [DOI] [PubMed] [Google Scholar]
- 37. Lusk RH, Wienke EC, Milligan TW, Albus TE. Tuberculous and foreign-body granulomatous reactions involving a total knee prosthesis. Arthritis Rheum 1995; 38:1325–7. [DOI] [PubMed] [Google Scholar]
- 38. Mahale YJ, Aga N. Implant-associated Mycobacterium tuberculosis infection following surgical management of fractures: a retrospective observational study. Bone Joint J 2015; 97-B:1279–83. [DOI] [PubMed] [Google Scholar]
- 39. Malhotra R, Gautam D, Wahal N. Tuberculous periprosthetic infection precipitated by infliximab therapy. BMJ Case Rep 2017; 2017:bcr2016218726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Maricevic A, Dogas Z, Goic-Barisić I, Barisić I. Reactivation of tuberculosis after total hip replacement—58 years after primary infection. Wien Klin Wochenschr 2008; 120:642–3. [DOI] [PubMed] [Google Scholar]
- 41. Marmor M, Parnes N, Dekel S. Tuberculosis infection complicating total knee arthroplasty: report of 3 cases and review of the literature. J Arthroplasty 2004; 19:397–400. [DOI] [PubMed] [Google Scholar]
- 42. Marschall J, Evison JM, Droz S, et al. Disseminated tuberculosis following total knee arthroplasty in an HIV patient. Infection 2008; 36:274–8. [DOI] [PubMed] [Google Scholar]
- 43. Meyssonnier V, Zeller V, Malbos S, et al. Prosthetic joint infections due to Mycobacterium tuberculosis: a retrospective study. Joint Bone Spine 2019; 86:239–43. [DOI] [PubMed] [Google Scholar]
- 44. Neogi DS, Kumar A, Yadav CS, Singh S. Delayed periprosthetic tuberculosis after total knee replacement: is conservative treatment possible? Acta Orthop Belg 2009; 75:136–40. [PubMed] [Google Scholar]
- 45. Ribeiro AF, Inacio Oliveira M, Jordão P, et al. Mycobacterium tuberculosis prosthesis joint infection. Pediatr Int 2020; 62:97–9. [DOI] [PubMed] [Google Scholar]
- 46. Seng P, Honnorat E, Loffeier V, et al. Mycobacterium tuberculosis and prosthetic joint infection. Lancet Infect Dis 2016; 16:894. [DOI] [PubMed] [Google Scholar]
- 47. Shanbhag V, Kotwal R, Gaitonde A, Singhal K. Total hip replacement infected with Mycobacterium tuberculosis. A case report with review of literature. Acta Orthop Belg 2007; 73:268–74. [PubMed] [Google Scholar]
- 48. Spinner RJ, Sexton DJ, Goldner RD, Levin LS. Periprosthetic infections due to Mycobacterium tuberculosis in patients with no prior history of tuberculosis. J Arthroplasty 1996; 11:217–22. [DOI] [PubMed] [Google Scholar]
- 49. Tekin Koruk S, Sipahioğlu S, Calişir C. Periprosthetic tuberculosis of the knee joint treated with antituberculosis drugs: a case report. Acta Orthop Traumatol Turc 2013; 47:440–3. [DOI] [PubMed] [Google Scholar]
- 50. Tokumoto JI, Follansbee SE, Jacobs RA. Prosthetic joint infection due to Mycobacterium tuberculosis: report of three cases. Clin Infect Dis 1995; 21:134–6. [DOI] [PubMed] [Google Scholar]
- 51. Ueng WN, Shih CH, Hseuh S. Pulmonary tuberculosis as a source of infection after total hip arthroplasty. A report of two cases. Int Orthop 1995; 19:55–9. [DOI] [PubMed] [Google Scholar]
- 52. Uhel F, Corvaisier G, Poinsignon Y, et al. ; Groupe d’Epidémiologie et Recherche en Infectiologie Clinique Centre-Ouest (GERICCO). Mycobacterium tuberculosis prosthetic joint infections: a case series and literature review. J Infect 2019; 78:27–34. [DOI] [PubMed] [Google Scholar]
- 53. Uppal S, Garg R. Tubercular infection presenting as sinus over ankle joint after knee replacement surgery. J Glob Infect Dis 2010; 2:71–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Upton A, Woodhouse A, Vaughan R, et al. Evolution of central nervous system multidrug-resistant Mycobacterium tuberculosis and late relapse of cryptic prosthetic hip joint tuberculosis: complications during treatment of disseminated isoniazid-resistant tuberculosis in an immunocompromised host. J Clin Microbiol 2009; 47:507–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. von Keudell A, Nathavitharana R, Yassa D, Abdeen A. An unusual pathogen for prosthetic joint infection. Lancet Infect Dis 2016; 16:506. [DOI] [PubMed] [Google Scholar]
- 56. Walczak P, Rąpała K, Nowak-Misiak M, et al. Recurrence of tuberculosis after hip replacement 58 years after primary infection. Ortop Traumatol Rehabil 2012; 14:189–96. [DOI] [PubMed] [Google Scholar]
- 57. Wang PH, Shih KS, Tsai CC, Wang HC. Pulmonary tuberculosis with delayed tuberculosis infection of total knee arthroplasty. J Formos Med Assoc 2007; 106:82–5. [DOI] [PubMed] [Google Scholar]
- 58. Wray CC, Roy S. Arthroplasty in tuberculosis of the knee. Two cases of missed diagnosis. Acta Orthop Scand 1987; 58:296–8. [DOI] [PubMed] [Google Scholar]
- 59. Zeiger LS, Watters W, Sherk H. Scintigraphic detection of prosthetic joint and soft tissue sepsis secondary to tuberculosis. Clin Nucl Med 1984; 9:638–9. [DOI] [PubMed] [Google Scholar]
- 60. Talip BA, Sleator RD, Lowery CJ, et al. An update on global tuberculosis (TB). Infect Dis (Auckl) 2013; 6:39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Heemskerk D, Caws M, Marais B, Farrar J.. Tuberculosis in Adults and Children. London, UK: Springer Nature; 2015. [PubMed] [Google Scholar]
- 62. Wetterholm M, Turkiewicz A, Stigmar K, et al. The rate of joint replacement in osteoarthritis depends on the patient’s socioeconomic status. Acta Orthop 2016; 87:245–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Furin J, Cox H, Pai M. Tuberculosis. Lancet 2019; 393:1642–56. [DOI] [PubMed] [Google Scholar]
- 64. Reichler MR, Khan A, Sterling TR, et al. Risk factors for tuberculosis and effect of preventive therapy among close contacts of persons with infectious tuberculosis. Clin Infect Dis 2019; 70:1562–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lewinsohn DM, Leonard MK, LoBue PA, et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention clinical practice guidelines: diagnosis of tuberculosis in adults and children. Clin Infect Dis 2017; 64:e1–33. [DOI] [PubMed] [Google Scholar]
- 66. Monno R, Maggi P, Carbonara S, et al. Chlamydia trachomatis and Mycobacterium tuberculosis lung infection in an HIV-positive homosexual man. AIDS Patient Care STDS 2001; 15:607–10. [DOI] [PubMed] [Google Scholar]
- 67. Trauner M, Grasmug E, Stauber RE, et al. Recurrent Salmonella enteritidis sepsis and hepatic tuberculosis. Gut 1995; 37:136–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Whittaker E, López-Varela E, Broderick C, Seddon JA. Examining the complex relationship between tuberculosis and other infectious diseases in children. Front Pediatr 2019; 7:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Huaman MA, Fiske CT, Jones TF, et al. Tuberculosis and the risk of infection with other intracellular bacteria: a population-based study. Epidemiol Infect 2015; 143:951–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Attia EF, Pho Y, Nhem S, et al. Tuberculosis and other bacterial co-infection in Cambodia: a single center retrospective cross-sectional study. BMC Pulm Med 2019; 19:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lamas ES, Bononi RJR, Bernardes MVAA, et al. Acute purulent pericarditis due co-infection with Staphylococcus aureus and Mycobacterium tuberculosis as first manifestation of HIV infection. Oxf Med Case Reports 2019; 2019:omy127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mehta PK, Raj A, Singh N, Khuller GK. Diagnosis of extrapulmonary tuberculosis by PCR. FEMS Immunol Med Microbiol 2012; 66:20–36. [DOI] [PubMed] [Google Scholar]
- 73. Satta G, Lipman M, Smith GP, et al. Mycobacterium tuberculosis and whole-genome sequencing: how close are we to unleashing its full potential? Clin Microbiol Infect 2018; 24:604–9. [DOI] [PubMed] [Google Scholar]
- 74. Nahid P, Dorman SE, Alipanah N, et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America clinical practice guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis 2016; 63:e147–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.