Abstract
Background
Despite advances in antiretroviral therapy (ART), people living with human immunodeficiency virus (HIV) continue to be at increased risk of cardiometabolic complications compared to HIV-uninfected individuals. Advanced glycation end products (AGEs) are implicated in the development and progression of cardiometabolic complications in the general population. Their role in HIV remains unclear.
Methods
ACTG A5260s is a prospective open-label randomized trial in which ART-naive people living with HIV were randomized to tenofovir disoproxil fumarate /emtricitabine plus atazanavir/ritonavir, darunavir/ritonavir, or raltegravir over 96 weeks. Changes in circulating AGEs with ART initiation were assessed, and linear regression was used to examine the associations between serum AGEs with carotid intima-media thickness (cIMT), visceral and subcutaneous adipose tissue, total fat, lean mass, body mass index, insulin resistance, leptin, and adiponectin.
Results
Overall, 214 participants were included. Ninety percent were male, 48% were White, the median age was 36 years, median HIV-1 RNA was 4.58 log10 copies/mL, and median CD4 count was 338 cells/µL. Most AGEs remained relatively unchanged following 96 weeks of ART initiation, except for methylglyoxal-derived hydroimidazolone 1 (MG-H1), which increased following 96 weeks of ART (mean fold change, 1.15 [95% confidence interval, 1.02–1.30]). No differences were detected across ART regimens. Increases in AGE levels over time were associated with worsening body fat composition measures, insulin resistance, and cIMT, even after adjusting for clinically relevant factors.
Conclusions
AGE levels did not decrease following ART initiation. Most AGE levels remained stable, except for MG-H1, which increased. In people with HIV on ART, the accumulation of circulating AGEs over time appears to be independently associated with worsening cardiometabolic biomarkers.
Summary: Antiretroviral therapy (ART) does not appear to be effective in reducing advanced glycation end product (AGE) levels. On the contrary, AGE levels seem to increase following ART initiation. Accumulation of AGEs was found to be independently associated with cardiometabolic complications in treated people living with HIV.
Keywords: advanced glycation end products, body composition, inflammation, immune activation, insulin resistance
Advanced glycation end products (AGEs) are produced from nonenzymatic reactions between proteins, lipids, or nucleic acid with reducing sugars [1]. These reactions take place with normal aging and are also produced at an accelerated rate under hyperglycemic, inflammatory, and oxidative stress conditions [2]. Accumulation of AGEs on long-lived proteins results in persistent inflammation and tissue damage [3] and is implicated in the progression of different pathological conditions, including diabetes, renal, cardiovascular, and neurological disorders [4]. The buildup of AGEs occurs endogenously through a series of complex reactions as part of normal body metabolism [5], and can also accumulate from exogenous sources in the environment such as diet or tobacco smoking [6]. Many functionally and structurally different AGEs are observed under various physiological and pathological conditions. For example, methylglyoxal-derived hydroimidazolone 1 (MG-H1)—the most common AGE in serum and tissue—is formed from methylglyoxal and arginine [7].
Despite advances in antiretroviral therapy (ART), people living with human immunodeficiency virus (PLWH) continue to be at increased risk of cardiometabolic complications and multisystem decline that arise with aging compared to matched human immunodeficiency virus (HIV)–uninfected individuals [8]. The mechanism leading to these complications is not well characterized [9], and finding ways to halt the progression of these processes is a priority in the management of PLWH [10]. Little is known about the role of AGEs in HIV disease. We have recently found that PLWH have higher circulating levels of AGEs compared to HIV-uninfected matched controls [11]; higher AGE levels were also found to be independently associated with increased systemic inflammation, immune activation, and cardiovascular markers [11]. One other study measured skin AGE levels in PLWH and found them to be predictors of cardiovascular disease over a 5-year follow-up period [12].
These findings call for further investigation of the role of AGEs in PLWH, which might provide a new avenue for the prevention or treatment of non-HIV-associated comorbidities. The aims of our study were to (1) understand the effect of antiretroviral therapy on circulating AGE levels in the AIDS Clinical Trials Group (ACTG) A5260s cohort after initiating tenofovir disoproxil fumarate/emtricitabine (TDF/FTC) plus atazanavir/ritonavir (ATV/r), darunavir/ritonavir (DRV/r), or raltegravir (RAL) in PLWH naive to ART over 96 weeks; (2) identify whether there are differences between the 3 regimens; (3) explore the relationship between circulating AGEs, cardiometabolic factors (body fat composition, insulin resistance, carotid intima-media thickness [cIMT]), and markers of inflammation and immune activation at baseline and at week 96. Our hypotheses were (1) that AGE levels would decrease over time, (2) regardless of ART regimen used, and (3) that increased AGE levels would be associated with increased cardiometabolic biomarkers before and after ART initiation.
METHODS
Study Population
We accessed data from ACTG A5260s, a substudy of parent study A5257, a phase 3, prospective trial where treatment-naive PLWH, aged ≥18 years with HIV-1 RNA ≥1000 copies/mL, were randomized in an open-label fashion to receive TDF/FTC plus either ATV/r, DRV/r, or RAL for at least 96 weeks. Enrolled participants had no known cardiovascular disease, diabetes mellitus, or uncontrolled thyroid disease. A total of 328 participants entered A5260s. In this analysis, we considered a subset of 234 participants, referred to as the successfully treated population, who completed A5260s on their A5257 randomized treatment (n = 283), achieved virological suppression (HIV-1 RNA <50 copies/mL) by week 24 and at subsequent time points (n = 249), and had no treatment interruptions >7 days (n = 234). Of these participants, 214 had at least 1 AGE measure at both baseline and week 96. Both the parent study and substudy were approved by the institutional review boards at all participating sites, and all participants provided written informed consent. Further details of the parent study and substudy have been previously described [13, 14].
The institutional review boards at all participating institutions approved the parent study A5257 and substudy A5260 (NCT00811954 and NCT00851799), and all participants provided written informed consent.
Study Evaluations
The AGEs were measured on stored samples from A5260s. These samples were from 2 different time points: pre-ART (baseline) and week 96. All remaining data come from data sources that were measured during A5260s.
AGEs were measured by liquid chromatography–mass spectrometry using internal stable heavy isotope–substituted standards (PreventAGE Health Care, Lebanon, New Hampshire). Five dicarbonyl-derived circulating AGEs compounds were measured: MG-H1, Nε-carboxymethyl lysine (CML), Nε-carboxyethyl lysine (CEL), glyoxal-derived hydroimidazolone 1 (G-H1), and 3-deoxyglucosone hydroimidazolone (3DG-H). The analysis was performed on the serum filtrate after centrifugation through 10k cutoff Amicon filters in a blinded fashion. An Agilent model 6490 Triple Quadrupole MS System with a 1290 Rapid Resolution LC System was used for analyte detection. All AGEs were separated and analyzed in a single run using a single Waters X-select HSS T3 2.5 µm, 2.1 mm × 150 mm column with a mobile phase gradient of methanol/water with 0.20% heptafluorobutyric acid and a total analysis time of 19 minutes. The interassay coefficient of variation ranged from 3.6% to 9.6%, as previously described [11, 15].
Plasma biomarkers of systemic inflammation (high-sensitivity C-reactive protein, D-dimer, interleukin 6), monocyte activation (soluble CD14 and soluble CD163, CD14+CD16+, and CD14dimCD16+), immune activation (CD4:CD38+HLA-DR+, CD8:CD38+HLA-DR+), immunosenescence (CD4:PD1+ and CD28–CD57+, CD8:PD1+ and CD38–CD57+), oxidized low-density and high-density lipoproteins, and adipocytokines (adiponectin and leptin), which were previously measured, were included in this analysis [14, 16, 17]. cIMT, a marker of cardiovascular disease risk measured using a high-resolution linear array ultrasound transducer, was also part of this analysis [17]. Previously collected markers of body composition [13], including whole-body dual-energy x-ray absorptiometry (DXA) measurements of the trunk and total limb fat, and single/slice computed tomography scan measurements of abdominal visceral adipose tissue (VAT), subcutaneous abdominal tissue (SAT), and total adipose tissue, as well as measures of bone mineral density (BMD) using DXA [18], including whole-body and site-specific BMD, were also used in this analysis. The homeostatic model assessment for insulin resistance (HOMA-IR) value was obtained using the following calculation: log10 (HOMA-IR) = log10 (glucose [mg/dL] × log10 (insulin [IU] / 405)).
Statistical Analysis
Nonnormal data (including all AGEs) were log-transformed on the log10 scale. All treatment group comparisons were done pairwise between each of the 3 treatment groups using a 2-sided α value of 2.5%. All additional statistical tests used a 2-sided 5% significance level. This analysis was exploratory in nature, and no adjustments were made for multiple testing. Emphasis was placed on the magnitude of effect sizes, consistency of observed effects, and hypothesis generation for future study. Descriptive summaries of absolute levels (log10 transformed) and changes over time in serum AGEs were generated by treatment arm at week 0 and week 96. Changes in the log10 AGEs were converted to fold changes for presentation. The mean fold changes were determined by calculating the mean absolute difference between levels on the log10 scale and then back-transforming. Wilcoxon rank-sum tests were used to contrast distribution shifts in the changes in AGEs (from week 0 to week 96) between treatment groups.
Cross-sectional (Table 1) and longitudinal (Table 2, Supplementary Table 2) associations between AGE levels and cardiometabolic factors were examined. Body composition measures (VAT, SAT, lean mass, total fat, and BMI), cardiovascular markers (measured by cIMT), insulin resistance (measured by HOMA-IR), and adipokines (leptin and adiponectin) were the biomarkers studied. Numerical summaries of these cardiometabolic factors at baseline and week 96 are shown in Supplementary Table 2. At least 95% of participants had observed measures at both time points. These associations were also examined while adjusting for potential confounders (including age, sex, race/ethnicity, smoking status, current alcohol use, illicit drug use history, physical activity level, and baseline HIV RNA load).
Table 1.
Regression Estimates Between Advanced Glycation End Products and Cardiometabolic Factors at Baseline and Week 96
| Adjustment | Adiponectin | Leptin | L1–L4 BMD | Hip BMD | BMI | cIMT | HOMA-IR | Lean Mass | SAT | VAT | Total Fat | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Week 0 Levels | ||||||||||||
| 3DG-H | Estimate | 0.03 | –0.05 | –0.02 | –0.02 | –0.88 | 0.02 | 0.04 | 0.06 | –22.80 | 2.79 | –1.32 |
| 95% CI | (–.02, .08) | (–.14, .04) | (–.05, .02) | (–.05, .01) | (–1.89, .13) | (–.00, .03) | (–.03, .12) | (–1.91, 2.04) | (–51.91, 6.30) | (–7.00, 12.59) | (–3.30, .66) | |
| P value | 0.3 | 0.24 | 0.29 | 0.25 | 0.09 | 0.07 | 0.24 | 0.95 | 0.12 | 0.58 | 0.19 | |
| CEL | Estimate | 0.01 | 0.1 | –0.02 | –0.01 | 0.57 | 0.03 | 0.14 | 1.56 | 2.93 | 12.26 | 0.78 |
| 95% CI | (–.05, .07) | (–.01, .21) | (–.06, .02) | (–.04, .03) | (–.63, 1.77) | (.00–.05) | (.06–.22) | (–.78, 3.91) | (–31.85, 37.71) | (.73–23.79) | (–1.57, 3.12) | |
| P value | 0.69 | 0.07 | 0.41 | 0.65 | 0.35 | 0.022 | <.001 | 0.19 | 0.87 | 0.037 | 0.52 | |
| CML | Estimate | 0.01 | 0.08 | –0.00 | 0.01 | 0.58 | 0.01 | 0.1 | 1.79 | 3.1 | 5.77 | 0.17 |
| 95% CI | (–.05, .06) | (–.02, .17) | (–.04, .04) | (–.02, .04) | (–.48, 1.64) | (–.00, .03) | (.02–.17) | (–.27, 3.85) | (–27.89, 34.09) | (–4.46, 15.99) | (–1.93, 2.26) | |
| P value | 0.85 | 0.11 | 0.99 | 0.5 | 0.28 | 0.12 | 0.009 | 0.09 | 0.84 | 0.27 | 0.88 | |
| G-H1 | Estimate | 0.04 | –0.06 | –0.04 | –0.03 | –1.57 | 0.02 | 0.13 | –2.30 | –44.35 | –5.84 | –2.48 |
| 95% CI | (–.05, .12) | (–.20, .09) | (–.09, .02) | (–.08, .02) | (–3.23, .09) | (–.01, .05) | (.02–.25) | (–5.58, .98) | (–92.26, 3.56) | (–21.99, 10.30) | (–5.74, .77) | |
| P value | 0.41 | 0.46 | 0.19 | 0.21 | 0.06 | 0.16 | 0.02 | 0.17 | 0.07 | 0.48 | 0.14 | |
| MG-H1 | Estimate | 0 | 0.02 | –0.01 | –0.00 | –0.16 | 0.01 | 0.1 | 0.29 | –9.51 | 0.33 | –0.40 |
| 95% CI | (–.03, .04) | (–.04, .08) | (–.03, .02) | (–.02, .02) | (–.83, .51) | (–.00, .02) | (.05–.14) | (–1.02, 1.61) | (–28.90, 9.88) | (–6.17, 6.84) | (–1.71, .91) | |
| P value | 0.92 | 0.61 | 0.53 | 0.98 | 0.64 | 0.08 | <.001 | 0.66 | 0.34 | 0.92 | 0.55 | |
| Week 96 Levels | ||||||||||||
| 3DG-H | Estimate | –0.04 | 0.07 | 0.01 | –0.00 | 0.41 | 0.02 | 0.03 | –0.36 | 28.38 | 7.46 | 1.74 |
| 95% CI | (–.09, .02) | (–.02, .16) | (–.02, .04) | (–.03, .02) | (–.60, 1.42) | (.00–.04) | (–.04, .11) | (–2.26, 1.54) | (–.93, 57.68) | (–4.51, 19.44) | (–.35, 3.82) | |
| P value | 0.19 | 0.12 | 0.56 | 0.83 | 0.42 | 0.022 | 0.35 | 0.71 | 0.06 | 0.22 | 0.1 | |
| CEL | Estimate | –0.04 | 0.2 | –0.03 | –0.02 | 0.76 | 0.01 | 0.14 | –0.42 | 55.43 | 28.46 | 3.27 |
| 95% CI | (–.11, .03) | (.10–.31) | (–.07, .01) | (–.05, .01) | (–.44, 1.96) | (–.01, .04) | (.06–.23) | (–2.73, 1.89) | (20.36–90.51) | (14.29–42.64) | (.79–5.76) | |
| P value | 0.23 | <.001 | 0.14 | 0.29 | 0.22 | 0.29 | 0.001 | 0.72 | 0.002 | <.001 | 0.01 | |
| CML | Estimate | –0.02 | 0.13 | –0.00 | 0 | 0.39 | 0.03 | 0.09 | 0.29 | 35.96 | 14.48 | 1.83 |
| 95% CI | (–.08, .04) | (.04–.23) | (–.04, .03) | (–.03, .03) | (–.67, 1.46) | (.00–.05) | (.02–.17) | (–1.75, 2.33) | (4.25–67.67) | (1.43–27.54) | (–.37, 4.03) | |
| P value | 0.47 | 0.007 | 0.81 | 0.86 | 0.47 | 0.016 | 0.016 | 0.78 | 0.026 | 0.03 | 0.1 | |
| G-H1 | Estimate | –0.01 | 0.08 | –0.03 | –0.03 | –0.09 | 0.02 | 0.11 | –1.14 | 11.81 | 18.84 | 1.21 |
| 95% CI | (–.11, .08) | (–.08, .24) | (–.08, .03) | (–.07, .02) | (–1.88, 1.69) | (–.02, .05) | (–.02, .24) | (–4.56, 2.28) | (–40.21, 63.83) | (–2.34, 40.02) | (–2.42, 4.83) | |
| P value | 0.77 | 0.34 | 0.35 | 0.29 | 0.92 | 0.35 | 0.1 | 0.51 | 0.66 | 0.08 | 0.51 | |
| MG-H1 | Estimate | –0.03 | 0.08 | –0.01 | –0.01 | 0.45 | 0.01 | 0.07 | 0.07 | 25.86 | 8.25 | 1.67 |
| 95% CI | (–.07, .01) | (.02–.15) | (–.03, .01) | (–.03, .01) | (–.28, 1.17) | (–.00, .02) | (.01–.12) | (–1.31, 1.46) | (4.66–47.06) | (–.47, 16.97) | (.17–3.17) | |
| P value | 0.16 | 0.014 | 0.41 | 0.51 | 0.23 | 0.17 | 0.014 | 0.92 | 0.017 | 0.06 | 0.029 |
Unadjusted estimates are presented as 0.3 log10 units, which is equivalent to a 2-fold difference in AGEs. Values in parentheses indicate the 95% confidence interval. Adiponectin, leptin, and HOMA-IR are analyzed on the log10 scale.
Abbreviations: 3DG-H, 3-deoxyglucosone hydroimidazolone; BMD, bone mineral density; BMI, body mass index; CEL, Nε-carboxyethyl lysine; CI, confidence interval; cIMT, carotid intima-media thickness; CML, Nε-carboxymethyl lysine; G-H1, glyoxal-derived hydroimidazolone 1; HOMA-IR, homeostatic model assessment for insulin resistance; MG-H1, methylglyoxal hydroimidazolone 1; SAT, subcutaneous abdominal tissue; VAT, visceral adipose tissue.
Table 2.
Linear Regression Estimates for Changes in Advanced Glycation End Products and Cardiometabolic Biomarkers Over 96 Weeks
| Adjustment | Week 96 Changes | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adiponectin | Leptin | L1–L4 BMD | Hip BMD | BMI | cIMT | HOMA-IR | Lean Mass | SAT | VAT | Total Fat | ||
| 3DG-H | Estimate | 0.03 | 0.05 | –0.32 | –0.07 | 0.16 | 0.64 | 0.11 | 0.91 | 0.69 | 3.40 | –1.04 |
| 95% CI | (.00–.06) | (.00–.10) | (–.97, .32) | (–.64, .51) | (–1.34, 1.66) | (–.07, 1.36) | (.05–.17) | (.02–1.81) | (–6.90, 8.28) | (–5.25, 12.06) | (–6.31, 4.24) | |
| P value | .032 | .038 | .33 | .82 | .83 | .08 | <.001 | .045 | .86 | .44 | .70 | |
| CEL | Estimate | 0.05 | 0.10 | –0.12 | –0.22 | 1.39 | 0.31 | 0.12 | 1.97 | 6.05 | 12.67 | 1.92 |
| 95% CI | (.01–.08) | (.04–.15) | (–.88, .64) | (–.90, .46) | (–.35, 3.13) | (–.54, 1.16) | (.06–.19) | (.95–3.00) | (–2.83, 14.94) | (2.59–22.75) | (–4.28, 8.12) | |
| P value | .007 | <.001 | .76 | .52 | .12 | .47 | <.001 | <.001 | .18 | .014 | .54 | |
| CML | Estimate | 0.03 | 0.09 | 0.05 | –0.17 | 0.29 | 0.68 | 0.10 | 0.98 | 2.73 | 6.50 | 0.51 |
| 95% CI | (–.00, .06) | (.04–.13) | (–.65, .76) | (–.80, .45) | (–1.32, 1.91) | (–.09, 1.44) | (.04–.16) | (.02–1.94) | (–5.61, 11.06) | (–3.07, 16.07) | (–5.22, 6.25) | |
| P value | .06 | <.001 | .88 | .59 | .72 | .08 | .002 | .046 | .52 | .18 | .86 | |
| G-H1 | Estimate | 0.05 | –0.03 | 0.62 | 0.75 | –3.00 | 1.35 | 0.11 | –1.59 | –16.27 | –7.08 | –8.99 |
| 95% CI | (–.01, .11) | (–.13, .07) | (–.69, 1.93) | (–.42, 1.91) | (–6.08, .08) | (–.10, 2.79) | (–.01, .23) | (–3.45, .27) | (–31.06, –1.49) | (–25.03, 10.88) | (–19.12, 1.15) | |
| P value | .08 | .59 | .36 | .21 | .06 | .07 | .06 | .09 | .031 | .44 | .08 | |
| MG-H1 | Estimate | 0.01 | 0.05 | –0.15 | –0.20 | 0.52 | 0.48 | 0.09 | 0.64 | 0.53 | 4.13 | 0.75 |
| 95% CI | (–.00, .03) | (.02–.08) | (–.57, .28) | (–.58, .18) | (–.47, 1.51) | (.01–.95) | (.05–.13) | (.05–1.24) | (–4.50, 5.56) | (–1.61, 9.87) | (–2.74, 4.25) | |
| P value | .13 | .001 | .50 | .31 | .30 | .047 | <.001 | .033 | .84 | .16 | .67 |
Adiponectin, leptin, and HOMA-IR are analyzed on the log10 scale. Changes for HOMA-IR, adiponectin, and leptin are analyzed as absolute difference of log10-transformed markers. All other changes are calculated as percentage change. Associations did not change after adjusting for different factors including age, sex, race/ethnicity, smoking, alcohol, drugs, physical activity, CD4+ T-cell count, and human immunodeficiency virus RNA as further detailed in Supplement 5.
Abbreviations: 3DG-H, 3-deoxyglucosone hydroimidazolone; BMD, bone mineral density; CEL, Nε-carboxyethyl lysine; CI, confidence interval; cIMT, carotid intima-media thickness; CML, Nε-carboxymethyl lysine; G-H1, glyoxal-derived hydroimidazolone 1; HOMA-IR, homeostatic model assessment for insulin resistance; MG-H1, methylglyoxal hydroimidazolone 1; SAT, subcutaneous abdominal tissue; VAT, visceral adipose tissue.
aEstimates are presented as 0.3 log10 units, which is equivalent to a 2-fold difference in the advanced glycation end product level.
We then used linear regression to examine the relationship between changes in circulating AGEs and changes in cardiometabolic markers following 96 weeks of suppressive ART. These associations are shown in Table 2 (with adjusted models presented in Supplementary Table 2).
Heat maps depicting Spearman correlation coefficients between AGEs with inflammatory markers and immune activation markers (including monocyte and immune activation markers, oxidized lipoproteins, and inflammation and immunosenesence markers), CD4+ T cells, and HIV RNA load (baseline only), as well as between changes in these biomarkers over time, were generated for visualization.
RESULTS
Baseline Characteristics
Of the 234 participants who maintained virological suppression and remained on their treatment in A5260s, 214 participants had available plasma samples at both weeks 0 and 96 and were included in this analysis. Sixty-four participants were randomized to ATV/r, 75 to DRV/r, and 75 to RAL. Participants had similar baseline demographics across treatment groups. Overall, 10% of participants were female. Participants were asked to self-identify their race/ethnicity, and overall, 29% identified as Black (non-Hispanic) and 19% identified as Hispanic (regardless of race). The median age was 36 years. Forty-five percent of the population reported no history of smoking, and 23% reported no current alcohol use. Physical activity was assessed using the International Physical Activity Questionnaire (IPAQ); activity was stratified into low, medium, and high based on IPAQ definitions [19, 20]. Seventy-nine percent of participants self-reported moderate physical activity, while 17% reported low physical activity. Only 2% were hepatitis B surface antigen positive, and 7% had a positive hepatitis C serology. The median baseline HIV-1 RNA was 4.58 log10 copies/mL, and the median CD4 count was 338 cells/μL, as seen in Table 3.
Table 3.
Baseline Characteristics by Randomized Treatment Group
| Characteristics | All (N = 214) |
Atazanavir/Ritonavir (n = 64) |
Raltegravir (n = 75) |
Darunavir/Ritonavir (n = 75) |
|---|---|---|---|---|
| Age, y, median (1st, 3rd quartiles) | 36 (29, 45) | 38 (31, 45) | 36 (27, 44) | 36 (28, 47) |
| Sex, male | 193 (90) | 60 (94) | 67 (89) | 66 (88) |
| Race/ethnicity | ||||
| White, non-Hispanic | 102 (48) | 33 (52) | 34 (45) | 35 (47) |
| Black, non-Hispanic | 62 (29) | 21 (33) | 20 (27) | 21 (28) |
| Hispanic, regardless of race | 41 (19) | 9 (14) | 14 (19) | 18 (24) |
| Current smoking, yes | 117 (55) | 36 (56) | 41 (55) | 40 (53) |
| Alcohol usage | ||||
| Never drink | 50 (23) | 16 (25) | 15 (20) | 19 (25) |
| Light, moderate, or heavy drinker | 159 (74) | 48 (75) | 58 (77) | 53 (71) |
| Missing | 5 (2) | 0 (0) | 2 (3) | 3 (4) |
| Physical activity level | ||||
| Low | 36 (17) | 11 (17) | 14 (19) | 11 (15) |
| Moderate | 169 (79) | 50 (78) | 60 (80) | 59 (79) |
| Missing | 9 (4) | 3 (5) | 1 (1) | 5 (7) |
| Hepatitis B | ||||
| Negative | 205 (96) | 62 (97) | 71 (95) | 72 (96) |
| Positive | 5 (2) | 2 (3) | 1 (1) | 2 (3) |
| Missing | 2 (1) | 0 (0) | 1 (1) | 2 (1) |
| Hepatitis C | ||||
| Negative | 199 (93) | 57 (89) | 68 (91) | 74 (99) |
| Positive | 14 (7) | 7 (11) | 6 (8) | 1 (1) |
| Missing | 1 (0) | 0 (0) | 1 (1) | 0 (0) |
| CD4+ count, cells/µL, median (1st, 3rd quartiles) | 338 (185, 451) | 317 (180, 467) | 348 (241, 451) | 336 (166, 423) |
| HIV-1 RNA, log10 copies/mL, median (1st, 3rd quartiles) | 4.6 (4.0, 5.04) | 4.7 (4.0, 5.1) | 4.5 (4.0, 5.0) | 4.7 (4.1, 5.0) |
| AGE, log10 nmoL/L, median (1st, 3rd quartiles) | ||||
| 3DG-H | 2.4 (2.3, 2.6) | 2.4 (2.3, 2.6) | 2.4 (2.3, 2.5) | 2.4 (2.3, 2.6) |
| CEL | 1.7 (1.7, 1.8) | 1.7 (1.6, 1.8) | 1.7 (1.7, 1.9) | 1.8 (1.7, 1.9) |
| CML | 1.9 (1.8, 2.0) | 1.9 (1.8, 2.0) | 1.9 (1.8, 2.0) | 1.9 (1.8, 2.0) |
| G-H1 | 1.0 (0.9, 1.0) | 0.9 (0.9, 1.0) | 1.0 (0.9, 1.0) | 1.0 (0.9, 1.0) |
| MG-H1 | 2.0 (2.0, 2.2) | 2.0 (1.8, 2.2) | 2.0 (1.9, 2.2) | 2.0 (1.9, 2.3) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: 3DG-H, 3-deoxyglucosone hydroimidazolone; AGE, advanced glycation end product; CEL, Nε-carboxyethyl lysine; CML, Nε-carboxymethyl lysine; G-H1, glyoxal-derived hydroimidazolone 1; HIV-1, human immunodeficiency virus type 1; MG-H1, methylglyoxal hydroimidazolone 1.
Changes in AGEs and Cardiometabolic Markers Following 96 Weeks of ART
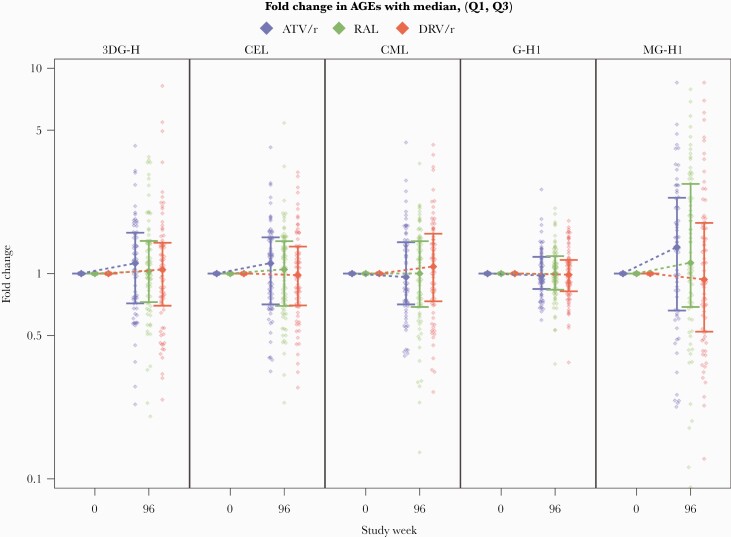
Overall circulating levels of MG-H1 increased following 96 weeks of ART initiation, with a median fold-change (Q1, Q3) of 1.13 (0.64, 2.14) from baseline. This increase was most pronounced among participants in the ATV/r arm, with a median fold change of 1.35 (0.66, 2.34), and in the RAL arm, with a median fold change of 1.13 (0.69, 2.73). We did not find any substantive changes among the remaining AGEs (3DG-H, G-H1, CEL, and CML) following 96 weeks of ART initiation. There were no differences between treatment groups in the change of all the AGEs over the study period (P > .14; Figure 1 and Supplementary Table 1).
Figure 1.
Changes in advanced glycation end product (AGE) levels over time by antiretroviral therapy arm. Abbreviations: 3DG-H, 3-deoxyglucosone hydroimidazolone; AGEs, advanced glycation end products; ATV/r, atazanavir/ritonavir; CEL, Nε-carboxyethyl lysine; CML, Nε-carboxymethyl lysine; DRV/r, darunavir/ritonavir; G-H1, glyoxal-derived hydroimidazolone 1; MG-H1, methylglyoxal hydroimidazolone 1; RAL, raltegravir.
Changes in insulin resistance, body composition measures, and cardiovascular risk (measured by cIMT) from the A5260s cohort have been previously published [13, 16, 21, 22].
Associations Between AGEs With Cardiometabolic Factors
Cross-Sectional Associations at Baseline (Pre-ART) and 96 Weeks After ART
Associations between AGE levels and cardiometabolic factors at baseline and at week 96 are presented in Table 1. At baseline, all AGEs, except 3DG-H1, were positively associated with insulin resistance (measured by HOMA-IR). These associations remained of approximately the same magnitude after adjusting for the potential confounders. Furthermore, CEL was positively associated with cIMT and VAT at baseline. After adjusting for age, the association of CEL with cIMT was attenuated.
Associations between the AGEs and HOMA-IR were relatively unchanged at week 96. Interestingly, new positive associations emerged between various AGEs and cIMT, body composition measures (VAT, SAT, total fat), and adipokines (leptin), as seen in Table 1. Associations between AGEs and some body composition measures were diminished when adjusted for age.
Changes Over 96 Weeks
Similar to what was observed in the cross-sectional analysis, a 2-fold increase in circulating levels of all AGEs, except G-H1, was associated with a 0.10–0.13 log10 increase in HOMA-IR following 96 weeks of ART. This association remained of a similar magnitude after adjusting for potential confounders.
Additionally, a 2-fold increase in CEL levels was associated with a 13% increase in VAT; a 2-fold increase in G-H1 was associated with a 16% decrease in SAT; and a 2-fold increase in levels of 3DG-H, CEL, CML, and MG-H1 was associated with a 1%–2% increase in lean mass. These associations remained relatively unchanged after adjusting for clinically relevant factors (Supplementary Table 2).
As for other the remaining biomarkers, we found that a 2-fold increase in MG-H1 levels was associated with a 0.5% increase in cIMT, although this was diminished after adjusting for age. A 2-fold increase in 3DG-H, CEL, CML, and MG-H1 was associated with a 0.05 to 0.1 increase in log10 leptin levels, and a 2-fold increase in 3DG-H and CEL was associated with a 0.03 to 0.05 increase in log10 adiponectin levels.
Associations Between AGE Levels, HIV Variables, and Markers of Inflammation and Immune Activation
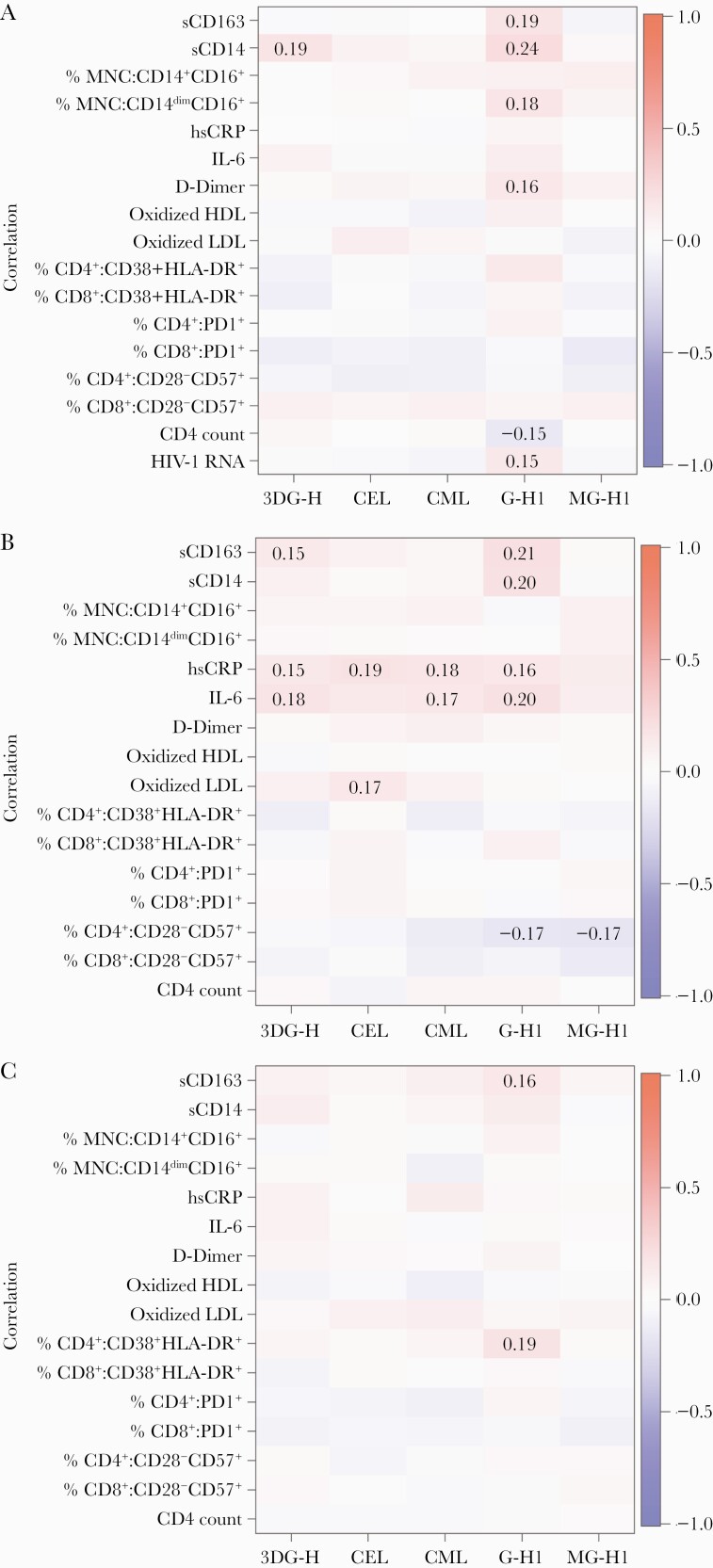
Associations between AGE levels with inflammatory and immune activation markers are shown in Figure 2. A few modest positive correlations emerged between circulating AGEs and markers of monocyte activation and inflammation at baseline (Figure 2A) and week 96 (Figure 2B) (ρ = 0.15–0.24). The correlations between the changes in the AGEs and the changes in these markers (Figure 2C) appeared to be approximately consistent with the 2 cross-sectional time points. The associations remained in similar directions, although many were weakened when considering the change over time.
Figure 2.
Correlations between advanced glycation end product with inflammatory markers and lymphocytes and monocyte activation markers. A, Baseline. B, Week 96. C, Changes over time. Dark red shading indicates positive correlations close to 1, while dark blue shading indicates negative correlations close to –1. Colors close to white indicate low correlation, around 0. Only correlations reaching statistical significance (P ≤ .05) are included in these figures. Abbreviations: 3DG-H, 3-deoxyglucosone hydroimidazolone; CEL, Nε-carboxyethyl lysine; CML, Nε-carboxymethyl lysine; G-H1, glyoxal-derived hydroimidazolone 1; HDL, high-density lipoprotein; HIV-1, human immunodeficiency virus type 1; hsCRP, high-sensitivity C-reactive protein; IL-6, interleukin 6; LDL, low-density lipoprotein; MG-H1, methylglyoxal hydroimidazolone 1; MNC, mononuclear cells; sCD14, soluble CD14; sCD163, soluble CD163.
When looking at the associations between AGEs and HIV-related variables (HIV viral load [baseline only, Figure 2A] and CD4 cell count), a modest negative correlation was observed between CD4+ T-cell count and G-H1 levels at baseline (Figure 2A, ρ = –0.15), but not at week 96 (Figure 2B). Furthermore, a weak positive association was observed between circulating G-H1 levels and HIV-1 RNA level at baseline (ρ = 0.15), but not with other AGEs.
DISCUSSION
In the context of a large prospective randomized clinical trial of ART initiation with TDF/FTC plus either ATV/r, DRV/r, or RAL, we evaluated, for the first time in PLWH, changes in circulating levels of AGEs over 96 weeks. We looked at the associations between AGE levels and cardiovascular disease, body composition, insulin resistance, inflammation, and immune activation markers. Our results showed an increase in MG-H1 levels over 96 weeks of ART initiation, with no differences between treatment arms. A key finding in our exploratory analysis was that increases in AGE level after ART initiation are potentially associated with worsening body fat composition measures (SAT, VAT, and total fat), insulin resistance (HOMA-IR), and cIMT following 96 weeks of treatment.
Glycation is an irreversible, nonenzymatic reaction between carbohydrates and free amino groups and is responsible for AGE formation [23]. Accumulation of AGEs occurs as part of normal aging, but can also occur at an accelerated rate with high inflammatory and oxidative stress conditions, as well as with hyperglycemia, and high protein turnover state [2, 23]. Very little is known about AGE levels in HIV, but recent studies suggested that PLWH have increased levels of circulating AGEs compared to matched HIV-uninfected controls [11]. Contrary to our initial hypothesis, initiation of ART in treatment-naive individuals leads to an increase in AGE levels; this could result from high protein/DNA turnover and increase anaerobic glycolysis and lipid peroxidation [24, 25], all contributing to the increase in endogenous AGE formation [24, 26, 27]. Our results showed that among the 5 AGEs measured, only MG-H1 levels significantly increased over time. One possible explanation is that MG-H1 is a more sensitive marker among measured AGEs. MG-H1 is reported as 1 of the most abundant circulating AGEs [28]. MG-H1 is derived from methylglyoxal, which has been recognized as one of the most reactive AGE precursors and the most potent glycating agent in the generation of AGEs on short-lived proteins and DNA [29].
In ACTG A5260s and other ART initiation trials, markers of insulin resistance, body composition measures, and cardiovascular disease were found to worsen after ART initiation [13, 21, 22], which has become a major concern with newer ART regimens. The mechanism contributing to these complications remains unknown and has been the focus of researchers for the last 2 decades. Persistent inflammation and immune activation were initially thought to be driving these complications; however, inflammation and immune activation indices often improve with ART initiation [16, 30], and no clear associations were identified between markers of inflammation, immune activation, and cardiometabolic factors in multiple studies [10, 31, 32]. Our findings suggest that AGEs may play a role in the development of cardiometabolic complications following ART initiation. Previous data have shown that the accumulation of AGEs has many consequences that can lead to a wide variety of pathological effects [26, 33]. Recent and ongoing studies have shown that AGEs are implicated in diabetic complications [28], cardiovascular disease [34], cancer [35], and disorders of the central nervous system [36]. Additionally, recent evidence suggested that MG-H1–derived compounds are implicated in the development of obesity [37] and metabolic disorders [38], and were independent predictors of changes in intima-media thickening [39]. In accordance with these findings, we found that increases in the circulating levels of AGEs over time were associated with worsening cardiometabolic factors following 96 weeks of ART initiation. AGEs can exert deleterious effects on the structure and function of intracellular and extracellular proteins [40], and can also bind to their specific receptors (RAGE), inducing inflammation, cytokine formation, and macrophage activations [41, 42]. Further understanding of the exact pathophysiology by which AGEs are involved in cardiometabolic complications is important to provide new therapeutic opportunities to reduce these pathologies.
Despite the novelty of our findings, our study has some limitations that should be acknowledged. First, dietary intake was not assessed. Dietary AGE assessment, in particular, would have helped answer whether dietary factors across the 96-week period contributed to an increase in AGEs. Second, a control group consisting of individuals who were not started on ART would have helped ensure that the observed increases could not be explained by a natural progression of HIV infection, despite ART. Also, it is important to note that we did not correct for multiple comparisons due to the exploratory nature of our analysis. Furthermore, our study population was limited by design to those who were able to suppress and remain suppressed on their ART regimen over 96 weeks and had available samples for analysis from both pre-ART initiations (week 0/baseline) and at week 96. Further studies are warranted to understand better the pathogenesis leading to the elevated AGE levels in this population. Furthermore, a definitive claim regarding the causation between AGEs with cardiovascular and metabolic complications cannot be made, and this will require a concerted effort from multiple fields, including in vitro work and controlled experiments in animal models.
Our results may have important clinical relevance, suggesting that the accumulation of AGEs is associated with increased cardiometabolic biomarkers in PLWH and that targeting AGEs might be a potential therapeutic target for treating these comorbidities in HIV.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. AIDS Clinical Trials Group (ACTG) 5260s team members: H. Hodis, C. Godfrey, B. Jarocki, A. Benns, and K. Braun. We thank the staff and patients from the following hospitals who participate in ACTG (in alphabetical order): Beth Israel Deaconess Medical Center; Brigham and Womens Hospital; Case Western Reserve University/University Hospitals Clinical Research Site (CRS); Duke University Medical Center; Harbor–University of California, Los Angeles (UCLA) Medical Center; Houston AIDS Research Team CRS; Johns Hopkins Adult AIDS CRS; Metrohealth; New Jersey Medical School; New York University HIV/AIDS CRS; Northwestern University; Rush University Medical Center ACTG; Ohio State University; Ponce De Leon Center CRS; UCLA Care Center; University of California, San Francisco AIDS CRS; University of Cincinnati; University of Colorado; University of North Carolina AIDS CRS; University of Pittsburg CRS; University of Rochester ACTG AIDS Care; University of Southern California; University of Washington; Vanderbilt Therapeutics CRS; Washington University.
Financial support. This work was supported by the National Institutes of Health (grant numbers HL095132, HL095126, AI069501, AI068636, AI068634, AI69471, and AI56933), and by University Hospitals Cleveland Medical Center and the Clinical and Translational Science Collaborative of Cleveland (4UL1TR000439 from the National Center for Advancing Translational Sciences).
Potential conflicts of interest. G. A. M. has served as a consultant for Gilead, ViiV, Janssen, and Merck and has received research funding from Merck, ViiV, Astellas, Roche, Gilead, and Tetraphase. J. H. S. has received research funding from Merck and Company. T. T. B. has received research funding from Gilead, Merck, ViiV Healthcare, Theratechnologies, and Janssen. All other authors report no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Schmidt AM, Hori O, Brett J, et al. Cellular receptors for advanced glycation end products. Implications for induction of oxidant stress and cellular dysfunction in the pathogenesis of vascular lesions. Arterioscler Thromb 1994; 14:1521–8. [DOI] [PubMed] [Google Scholar]
- 2. Monnier VM. Nonenzymatic glycosylation, the Maillard reaction and the aging process. J Gerontol 1990; 45:B105–11. [DOI] [PubMed] [Google Scholar]
- 3. Ulrich P, Cerami A. Protein glycation, diabetes, and aging. Recent Prog Horm Res 2001; 56:1–21. [DOI] [PubMed] [Google Scholar]
- 4. Van Puyvelde K, Mets T, Njemini R, et al. Effect of advanced glycation end product intake on inflammation and aging: a systematic review. Nutr Rev 2014; 72:638–50. [DOI] [PubMed] [Google Scholar]
- 5. Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia 2001; 44:129–46. [DOI] [PubMed] [Google Scholar]
- 6. O’Brien J, Morrissey PA. Nutritional and toxicological aspects of the Maillard browning reaction in foods. Crit Rev Food Sci Nutr 1989; 28:211–48. [DOI] [PubMed] [Google Scholar]
- 7. Arakawa S, Suzuki R, Kurosaka D, et al. Mass spectrometric quantitation of AGEs and enzymatic crosslinks in human cancellous bone. Sci Rep 2020; 10:18774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shah ASV, Stelzle D, Lee KK, et al. Global burden of atherosclerotic cardiovascular disease in people living with HIV: systematic review and meta-analysis. Circulation 2018; 138:1100–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tabib A, Leroux C, Mornex JF, Loire R. Accelerated coronary atherosclerosis and arteriosclerosis in young human-immunodeficiency-virus-positive patients. Coron Artery Dis 2000; 11:41–6. [DOI] [PubMed] [Google Scholar]
- 10. Zicari S, Sessa L, Cotugno N, et al. Immune activation, inflammation, and non-AIDS co-morbidities in HIV-infected patients under long-term ART. Viruses 2019; 11:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. El Kamari V, Thomas A, Shan L, et al. Advanced glycation end products are associated with inflammation and endothelial dysfunction in HIV. J Acquir Immune Defic Syndr 2019; 81:e55–62. [DOI] [PubMed] [Google Scholar]
- 12. Sprenger HG, Bierman WF, Martes MI, et al. Skin advanced glycation end products in HIV infection are increased and predictive of development of cardiovascular events. AIDS 2017; 31:241–6. [DOI] [PubMed] [Google Scholar]
- 13. McComsey GA, Moser C, Currier J, et al. Body composition changes after initiation of raltegravir or protease inhibitors: ACTG A5260s. Clin Infect Dis 2016; 62:853–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kelesidis T, Jackson N, McComsey GA, et al. Oxidized lipoproteins are associated with markers of inflammation and immune activation in HIV-1 infection. AIDS 2016; 30:2625–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Saremi A, Howell S, Schwenke DC, et al. ; VADT Investigators. Advanced glycation end products, oxidation products, and the extent of atherosclerosis during the VA diabetes trial and follow-up study. Diabetes Care 2017; 40:591–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kelesidis T, Tran TT, Stein JH, et al. Changes in inflammation and immune activation with atazanavir-, raltegravir-, darunavir-based initial antiviral therapy: ACTG 5260s. Clin Infect Dis 2015; 61:651–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stein JH, Brown TT, Ribaudo HJ, et al. Ultrasonographic measures of cardiovascular disease risk in antiretroviral treatment-naive individuals with HIV infection. AIDS 2013; 27:929–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brown TT, Moser C, Currier JS, et al. Changes in bone mineral density after initiation of antiretroviral treatment with tenofovir disoproxil fumarate/emtricitabine plus atazanavir/ritonavir, darunavir/ritonavir, or raltegravir. J Infect Dis 2015; 212:1241–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003; 35:1381–95. [DOI] [PubMed] [Google Scholar]
- 20. Brown TT, Chen Y, Currier JS, et al. Body composition, soluble markers of inflammation, and bone mineral density in antiretroviral therapy-naive HIV-1–infected individuals. J Acquir Immune Defic Syndr 2013; 63:323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dirajlal-Fargo S, Moser C, Brown TT, et al. Changes in insulin resistance after initiation of raltegravir or protease inhibitors with tenofovir-emtricitabine: AIDS Clinical Trials Group A5260s. Open Forum Infect Dis 2016; 3:ofw174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stein JH, Ribaudo HJ, Hodis HN, et al. A prospective, randomized clinical trial of antiretroviral therapies on carotid wall thickness. AIDS 2015; 29:1775–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fournet M, Bonté F, Desmoulière A. Glycation damage: a possible hub for major pathophysiological disorders and aging. Aging Dis 2018; 9:880–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Korencak M, Byrne M, Richter E, et al. Effect of HIV infection and antiretroviral therapy on immune cellular functions. JCI Insight 2019; 4:e126675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Palmer CS, Ostrowski M, Balderson B, et al. Glucose metabolism regulates T cell activation, differentiation, and functions. Front Immunol 2015; 6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chaudhuri J, Bains Y, Guha S, et al. The role of advanced glycation end products in aging and metabolic diseases: bridging association and causality. Cell Metab 2018; 28:337–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wilson EM, Sereti I. Immune restoration after antiretroviral therapy: the pitfalls of hasty or incomplete repairs. Immunol Rev 2013; 254:343–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brings S, Fleming T, Freichel M, et al. Dicarbonyls and advanced glycation end-products in the development of diabetic complications and targets for intervention. Int J Mol Sci 2017; 18:984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maessen DE, Stehouwer CD, Schalkwijk CG. The role of methylglyoxal and the glyoxalase system in diabetes and other age-related diseases. Clin Sci (Lond) 2015; 128:839–61. [DOI] [PubMed] [Google Scholar]
- 30. Hileman CO, Funderburg NT. Inflammation, immune activation, and antiretroviral therapy in HIV. Curr HIV/AIDS Rep 2017; 14:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vos AG, Hulzebosch A, Grobbee DE, et al. Association between immune markers and surrogate markers of cardiovascular disease in HIV positive patients: a systematic review. PLoS One 2017; 12:e0169986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vos AG, Idris NS, Barth RE, et al. Pro-inflammatory markers in relation to cardiovascular disease in HIV infection. a systematic review. PLoS One 2016; 11:e0147484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Monnier VM, Sun W, Sell DR, et al. Glucosepane: a poorly understood advanced glycation end product of growing importance for diabetes and its complications. Clin Chem Lab Med 2014; 52:21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hanssen NM, Stehouwer CD, Schalkwijk CG. Methylglyoxal and glyoxalase I in atherosclerosis. Biochem Soc Trans 2014; 42:443–9. [DOI] [PubMed] [Google Scholar]
- 35. Thornalley PJ, Rabbani N. Glyoxalase in tumourigenesis and multidrug resistance. Semin Cell Dev Biol 2011; 22:318–25. [DOI] [PubMed] [Google Scholar]
- 36. Srikanth V, Westcott B, Forbes J, et al. Methylglyoxal, cognitive function and cerebral atrophy in older people. J Gerontol A Biol Sci Med Sci 2013; 68: 68–73. [DOI] [PubMed] [Google Scholar]
- 37. Matafome P, Sena C, Seiça R. Methylglyoxal, obesity, and diabetes. Endocrine 2013; 43:472–84. [DOI] [PubMed] [Google Scholar]
- 38. Matafome P, Rodrigues T, Sena C, Seiça R. Methylglyoxal in metabolic disorders: facts, myths, and promises. Med Res Rev 2017; 37:368–403. [DOI] [PubMed] [Google Scholar]
- 39. Ogawa S, Nakayama K, Nakayama M, et al. Methylglyoxal is a predictor in type 2 diabetic patients of intima-media thickening and elevation of blood pressure. Hypertension 2010; 56:471–6. [DOI] [PubMed] [Google Scholar]
- 40. DeGroot J. The AGE of the matrix: chemistry, consequence and cure. Curr Opin Pharmacol 2004; 4:301–5. [DOI] [PubMed] [Google Scholar]
- 41. Bierhaus A, Humpert PM, Morcos M, et al. Understanding RAGE, the receptor for advanced glycation end products. J Mol Med (Berl) 2005; 83:876–86. [DOI] [PubMed] [Google Scholar]
- 42. Kelesidis T, Kendall MA, Danoff A, et al. Soluble levels of receptor for advanced glycation endproducts and dysfunctional high-density lipoprotein in persons infected with human immunodeficiency virus: ACTG NWCS332. Medicine (Baltimore) 2018; 97:e10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.