Abstract
Fatigue is a dominant feature of both acute and convalescent coronavirus disease 2019 (COVID-19) (sometimes termed “long-COVID”), with up to 46% of patients reporting fatigue that lasts from weeks to months. The investigators of the international Collaborative on Fatigue Following Infection (COFFI) conducted a systematic review of post-COVID fatigue and a narrative review on fatigue after other infections, and made recommendations for clinical and research approaches to assessing fatigue after COVID-19.
In the majority of COVID-19 cohort studies, persistent fatigue was reported by a significant minority of patients, ranging from 13% to 33% at 16–20 weeks post-symptom onset. Data from the prospective cohort studies in COFFI and others indicate that fatigue is also a prevalent outcome from many acute systemic infections, notably infectious mononucleosis, with a case rate for clinically significant Post-infective fatigue after exclusion of recognized medical and psychiatric causes, ranging from 10%–35% at 6 months.
To better characterize post-COVID fatigue, the COFFI investigators recommend the following: application of validated screening questionnaires for case detection; standardized interviews encompassing fatigue, mood, and other symptoms; and investigative approaches to identify end-organ damage and mental health conditions.
Keywords: assessment, cohorts, COVID-19, fatigue, post-viral
Fatigue after COVID-19 is common but generally resolves over months, like other postinfective fatigue states. Post-COVID fatigue results from end-organ injury, mental health conditions, or idiopathic post-COVID fatigue. Post-COVID fatigue should be assessed with validated questionnaires, interviews, and protocolized investigations.
Emerging data suggest that some patients fail to fully recover after acute coronavirus disease 2019 (COVID-19) infection. Patients who report symptoms persisting for weeks or months after the acute illness have been termed “long haulers” or described as having “long-COVID” [1]. Although a case definition of “long-COVID” has not been established, fatigue is a dominant feature, along with other symptoms reminiscent of the acute infection. The condition has gained attention from the media, the public, as well as the scientific and medical communities [2].
The term “fatigue” has diverse meanings, including that experienced by people as part of daily living (“physiological” or “everyday” fatigue) or in disease (eg, anemia) (“pathological fatigue”). The fatigue state may be objectively measurable as a reduction in the efficiency of force generation recorded on physical examination as weakness (as in myopathy), or it may be a purely subjective sensation (ie, fatigue as a symptom). More importantly, when patients complain of fatigue, they may actually be referring to weakness, dyspnea, difficulties in concentration, somnolence, or low mood. Hence, careful delineation of the nature of the symptom complaint(s) is key in both clinical and research settings. The subjective experience of fatigue (as with pain) is automatically interpreted in view of other concomitant brain processes, such as perceptions, emotions, and cognitions [3].
Evolutionarily, fatigue might be considered as a homeostatic alarm directed towards energy preservation [3], which is well exemplified in the acute sickness response to a wide range of pathogens. This response features a stereotyped collection of physiological, behavioral, and psychological manifestations including fever, fatigue, hypersomnia, musculoskeletal pain, anorexia, mood disturbance, and cognitive impairment [4]. Persistence of 1 or more of these symptoms for weeks or months beyond the acute phase of infection is common [5]. In this context, patients describe the persistent fatigue as having both “physical” components (loss of energy and a feeling of heaviness) and “mental” components (a feeling of brain fog). Another characteristic feature is that relatively minor physical or cognitive activity triggers a prolonged exacerbation of the fatigue and other symptoms [6].
When fatigue persists for 6 months or more, it is termed “chronic” [7]. When thorough clinical assessments and investigations do not reveal alternative explanations for chronic fatigue, and if other typical symptoms such as musculoskeletal pain and cognitive difficulties are present, a diagnosis of chronic fatigue syndrome (CFS), or more specifically post-infective fatigue syndrome (PIFS), may be considered [5, 7].
The investigators of the international Collaborative on Fatigue Following Infection (COFFI) [5] have sought to provide guidance on these complexities of fatigue after COVID-19 infection by the following: (1) conducting a systematic review on the emerging data on the epidemiology of fatigue after COVID-19 infection and (2) comparing the literature regarding fatigue after other infections through a narrative review. Recommendations for clinical and research approaches to assessment of fatigue after COVID-19 are provided.
FATIGUE AFTER COVID-19: A SYSTEMATIC REVIEW
A meta-analysis of studies in acute COVID-19 infection revealed an overall prevalence of fatigue of 23% (95% confidence interval, 15%–33%) [8]. The current review focused on persistent fatigue after acute COVID-19 infection, defined here as 21 days or greater post-symptom onset. The review aimed to describe the incidence, natural history, and predictors of such post-COVID fatigue.
Methods
References were identified through searches of PubMed for articles published from January 2020 to January 2021, using terms “fatigue”, “malaise”, or “tired” and “COVID-19” or “COVID19” or “SARS-CoV-2”. Additional articles were identified by searching reference lists and citations of included articles. In addition, MedRxiv (a preprint server for health sciences) was also searched using terms “fatigue”, “tired”, “persistent symptoms”, and “COVID-19” to identify relevant prepublication manuscripts. Prospective cohort studies and cross-sectional studies were included, provided that they (1) specifically reported the rates of fatigue in the convalescent phase after confirmed acute COVID-19 infection, (2) included a minimum of 10 participants, and (3) were written in English. Almost all studies used those who had completed follow-up as the denominator for symptom prevalence rates. Accordingly, data were extracted from each study to recalculate the proportion of patients reporting fatigue using all eligible COVID-19-confirmed subjects as the modified denominator (including those who refused, were lost to follow-up, or died).
Results
Study and Patient Characteristics
The search until January 2021 yielded 914 articles from PubMed, an additional 208 records identified through MedRxiv, and 6 additional papers through reference lists and citations. A total of 1117 records were screened by title and abstract, and 154 articles were subjected to full text review. The reasons for exclusion of these full-text articles (n = 133) are outlined in the PRISMA flowchart (Supplementary Figure 1). The final list of included articles (n = 21) described 3 prospective studies [9–11] and 18 cross-sectional studies [12–29]. The sample sizes ranged from 33 to 4182 participants (median n = 131, total n = 7639), with an age range between 32 and 71 years (median 50 years), 52% of whom were male (median 52%; range, 28%–70%). Most studies (15 of 21) only included patients who had been admitted to the hospital [11–18, 20, 21, 23, 25, 26, 28, 29], with the remaining 6 studies including a mixture of hospitalized and nonhospitalized patients [9, 10, 19, 22, 24, 27]. Ten studies included patients who were admitted to the intensive care unit (ICU) [12–15, 19, 22, 24–26, 28], and 3 studies specifically excluded ICU patients [17, 20, 21]. To ensure consistent reporting of observation periods, “time since symptom onset” was used as the anchor point. If the authors only provided the time since hospitalization or the time since discharge, it was assumed that subjects were symptomatic for 7 days before hospitalization, and the duration of hospitalization was taken as the median reported for each study.
Prevalence of Fatigue
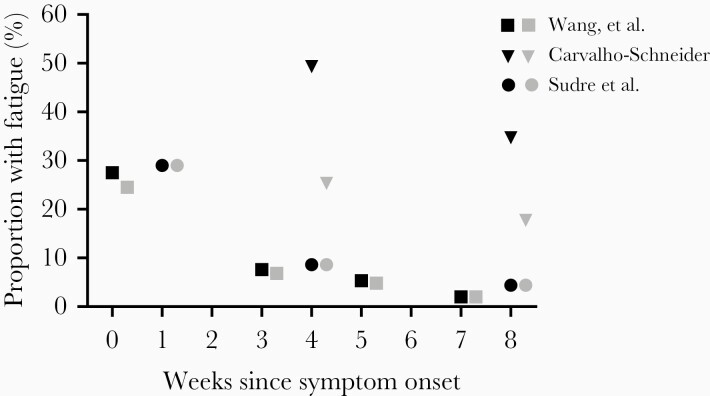
The average period of observation across all studies was 82 days since symptom onset (range, 27–199 days). To date, only a single study has conducted follow-up beyond 129 days [29]. Three prospective cohort studies assessed rates of fatigue from symptom onset [11] to 60 days post-symptom onset [9, 10]. In the acute phase, the peak fatigue rates in these studies ranged from 8% [11] to 29% [10] (Figure 1). At 4 weeks post-symptom onset, rates of fatigue ranged from 9% [10] to 49% [9]. A trend of resolution was evident within the individual cohorts with falling rates of fatigue reported at 8 weeks (4% [10] to 35% [9]) after symptom onset. When the modified denominator was considered including all eligible subjects with confirmed COVID-19 infection, the recalculated rates of fatigue were lower, ranging from 7% [11] to 29% [10] in the acute phase, 9% [10] to 25% [9] at week 4, and 4% [10] to 18% [9] at week 8. None of these prospective cohort studies collected data beyond 8 weeks.
Figure 1.
Prevalence of fatigue in COVID-19 from prospective studies. Black symbols refer to the original rate reported by each study. Gray symbols refer to rate recalculated with all eligible individuals included in the denominator.
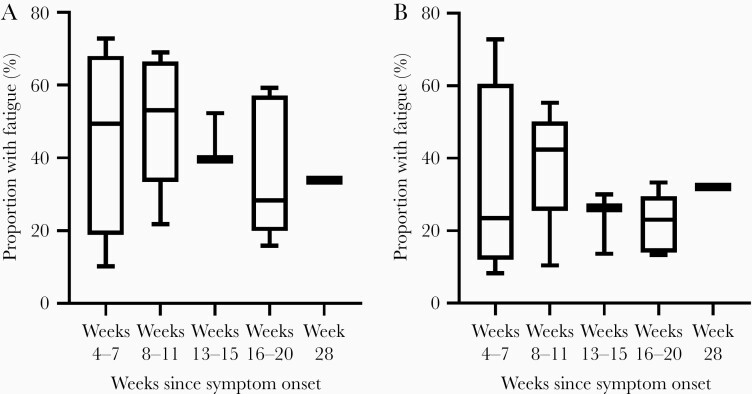
The 18 cross-sectional studies [12–20] assessed fatigue at various time windows ranging from 4 weeks to 28 weeks post-symptom onset. The median proportion of patients reporting fatigue were as follows: 50% at 4–7 weeks [17, 23, 24, 28], 53% at 8–11 weeks [13, 15, 21, 22, 26], 40% at 12–15 weeks [12, 18, 19], 28% at 16–20 weeks [14, 16, 20, 25, 27], and 34% at 28 weeks from symptom onset [29]. When the rates of fatigue were recalculated using the more inclusive denominator, the median rates were as follows: 23% at 4–7 weeks [17, 23, 24, 28], 42% at 8–11 weeks [13, 15, 21, 22, 26], 26% at 12–15 weeks [12, 18, 19], 23% between weeks 16 and 20 [14, 16, 20, 25, 27], and 32% at 28 weeks from symptom onset. The ranges of fatigue prevalence from each time window are reported in Figure 2 [29]. In several studies, patients reported additional symptoms such as dyspnea [12–16, 24–26, 28] and/or cognitive difficulties [14, 15, 18, 29] at similar but somewhat lower rates than fatigue.
Figure 2.
Prevalence of fatigue in COVID-19 from cross-sectional studies. The box extends from the 25th to 75th percentiles, the line represents the median, and the whiskers show the minimum and maximum. Week 28 is represented by a single study. (A) shows the original rates reported by the included studies. The proportion of patients reporting fatigue were as follows: 10%–73% at 4–7 weeks [17, 23, 24, 28], 22%–69% at 8–11 weeks [13, 15, 21, 22, 26], 39%–52% at 12–15 weeks [12, 18, 19], 16%–59% at 16–20 weeks [14, 16, 20, 25, 27], and 34% at 28 weeks from symptom onset [29]. (B) shows these rates recalculated with all eligible individuals included in the denominator: 8%–24% at 4–7 weeks [17, 23, 24, 28], 10%–55% at 8–11 weeks [13, 15, 21, 22, 26], 14%–26% at 12–15 weeks [12, 18, 19], 13%–33% between weeks 16 and 20 [14, 16, 20, 25, 27], and 32% at 28 weeks from symptom onset [29].
Functional Impact and Predictors of Long COVID
In 3 studies that measured the functional impact of persistent symptoms, there was evidence of associated disability with 40% [15], 31% [19], and 9%–15% [14, 25] of patients unable to return to work, at 2, 3, and 4 months post-symptom onset, respectively. Although no studies were sufficiently powered to run multivariable regression analysis, exploratory analyses found that severity of illness as measured by hospitalization [9], ICU [24], duration of stay in hospital [20], duration of viral shedding [20], and dyspnea during hospitalization [9, 20] were associated with fatigue at follow-up.
Critique
It should be noted that almost all studies (20 of 21) were likely to be influenced by ascertainment bias (because not all of those with confirmed COVID-19 and eligible were included in the reported denominators) [30]. As expected, the rates of fatigue reported from cross-sectional studies were higher than those from prospective studies, which is likely to reflect the greater selection bias in those who remain unwell and elect to respond to cross-sectional surveys. Further bias was introduced by studies that excluded those who were severely unwell [9, 14, 17, 21]. By contrast, the largest study was an observational cohort of a subset of individuals (n = 4182) utilizing the COVID Symptom Study online app, which has been taken up by several million individuals in the United Kingdom and United States [10]. Although a convenient method of assessment, computer literacy may have restricted the participating population, and this cohort had an unusually high number of female participants (72%), whereas epidemiological studies show no gender difference in the prevalence of acute COVID-19 infection [31].
The measurement of fatigue was generally poorly described, with most studies providing little detail on the instrumentation used. Most studies used either only a “customized questionnaire” [9, 11, 13, 21, 24–26, 28, 29], “telephone interview” [12, 15, 16, 20], “medical records” [14], or a mobile phone application [10], with no further details provided. Only 5 studies administered validated multi-item fatigue questionnaires, using the Chalder Fatigue scale [17, 19], the Fatigue Severity Scale [18], the Somatic and Psychological HEalth Report (SPHERE) [22], the Fatigue Impact Scale [27], or the PROMIS Scale-Global Health [23].
Multiple studies have identified significant long-term complications of severe acute COVID-19 infection and the associated hospitalization, including pulmonary, cardiac, neurological, and psychiatric conditions—many of which may manifest with the complaint of persistent fatigue [32, 33]. In the follow-up studies reviewed here that identified persistent fatigue, very few conducted systematic clinical or laboratory assessments to consider these possibilities, with those doing so including a full blood count [11, 12, 21], chest x-ray [12, 26], chest computed tomography [11, 17, 25], or lung function tests [21, 25]. Only 1 study described cardiac investigations (eg, electrocardiography or echocardiography) to screen for cardiac pathology [21]. Mental health status and social supports were only assessed in 1 study [28].
Summary
From this review, it is clear that fatigue is a dominant complaint in “long COVID” and that larger prospective studies with longer follow-up, using more comprehensive and well validated methods for the assessment of fatigue and related conditions, are needed. Previous studies of fatigue after other infections may help guide the choice of measures.
POST-INFECTIVE FATIGUE STATES AFTER OTHER INFECTIONS: A NARRATIVE REVIEW
Fatigue is a very common symptom in primary care where it is generally short-lived and attributable to infective illnesses or minor psychiatric disorders [34]. Several acute infections are also a well established trigger for the onset of chronic fatigue.
Methods
In addition to consideration of data from the COFFI cohorts, a narrative review was conducted searching PubMed for prospective cohort, observational, or case-control studies that observed individuals from acute infection for chronic fatigue.
Results
Fifteen studies were identified following from several different viral, bacterial, or protozoal pathogens, including Epstein-Barr virus (EBV), dengue virus, chikungunya virus, Ebola virus, Coxiella burnetii (the causative agent of Q fever), and Giardia lamblia. These studies documented a prevalent complaint of post-infective fatigue persisting in disabling degree for 6 months or more in 10%–35% of adolescents or adults (see Supplementary Table 1 for cohort summaries and references). In all of these studies, multi-item validated questionnaires were used to characterize the fatigue state. In 6 studies, a case definition for CFS was applied at 6 months, which necessitated a clinical assessment including a medical history, physical examination, mental health assessment, and laboratory investigations leading to a designation of PIFS, after exclusion of other medical or psychiatric conditions (Supplementary Table 1) [7]. By contrast, a prospective case-control cohort study in general practice found that patients presenting with minor symptomatic infections, such as common colds, did not experience an increased likelihood of developing chronic fatigue [35].
Predictors of Post-infective Fatigue Syndrome
A systematic review of biological, psychological, and social predictors of chronic fatigue or PIFS 6 months after onset in the prospective cohort studies revealed that clinical and laboratory features indicative of the severity of the acute infection were the most consistent predictors, including the following: the presence of markers of the host immune response, including biochemical hepatitis; self-reported severity of acute illness, and of fatigue in particular; and associated functional impairment such as the number of days in bed or days off school. In addition, there was some evidence across studies for self-reported anxiety, perceived stress, neuroticism, negative beliefs about the acute illness, and premorbid distress, as risk factors [36]. A notable exception to the latter was the sole prospective cohort that collected data before the acute illness to characterize mental health and personality characteristics [37]. This study observed US college students (n = 4501) for asymptomatic seroconversion or symptomatic acute EBV, revealed a case rate for PIFS of 23% at 6 months, and showed that premorbid psychological factors did not predict PIFS [37]. Nested case-control studies from the prospective cohorts have investigated subjects with well characterized PIFS and matched control subjects who recovered uneventfully from the same acute infection, and they have not found evidence of (1) ongoing replication of the pathogen beyond several weeks (although persistent detection of nucleic acids is recognized) or (2) a consistent pattern of ongoing immune activation [38–42].
Summary
Taken together, these findings from post-infective cohorts show that (1) fatigue is a common and sometimes disabling symptom after a diverse range of infections, (2) the natural history of persistent fatigue is often of slow resolution over months or longer, (3) the severity of the acute illness, psychological status at baseline, and the cognitive and behavioral responses to the acute illness predict PIFS, (4) and structured medical and psychiatric assessments of those with self-reported chronic fatigue will identify a subset with explanatory diagnoses such as residual lung injury.
DISCUSSION
Clinical and Research Approaches to the Assessment of Post-infective Fatigue
In combination, the limitations of the studies in COVID-19 and the evidence from studies in other post-infective cohorts argue that a validated case definition for chronic fatigue after COVID-19 infection is needed for both clinical and research purposes. In line with current definitions of post-infective fatigue [5], we suggest that the label “post-COVID fatigue” should be applied when the fatigue is as follows: a dominant symptom; chronic; disabling to an extent that it interrupts all or a majority of normal activities (such as work/school attendance, social activities, etc); persistent for 6 months or more (3 months in children/adolescents); and emerged during confirmed acute COVID-19 (ie, with a positive severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2] test), without symptom-free interval since onset.
If a case of post-COVID fatigue is identified, a search for underlying diagnoses should be initiated, including the following: end-organ sequelae of the acute COVID-19 illness and hospitalization; mental health conditions precipitated or exacerbated by COVID-19; and other (non-COVID-related) premorbid or intercurrent disorders of which fatigue is a feature. We recommend a structured diagnostic work-up (see Supplementary Tables 2 and 3 for summaries of instruments and references). In both clinical and research settings, brief screening questionnaires to characterize the fatigue state, such as the Chalder Fatigue Scale or the SPHERE (Supplementary Table 2), provide a systematic approach to identify “clinically-significant” fatigue, in line with the disease-specific recommendations from the National Institute of Neurological Disorders and Stroke Common Data Elements. Because the symptom of fatigue is often part of a multisymptom cluster, it is appropriate to include other validated questionnaires to screen for the following: related physical symptoms (such as the SPHERE) and mental health (such as the Patient Health Questionnaire-9 or the Hospital Anxiety and Depression Scale) (Supplementary Table 2). Screening for other relevant symptom domains may also be undertaken with validated instruments to assess pain and sleep quality. Clinically significant fatigue is usually taken to be associated with disability, and so concurrent assessment of functional status using an instrument such as the SF-36 is strongly recommended (Supplementary Table 2).
Because both medical and mental health conditions may manifest with fatigue, or co-occur with a post-infective fatigue state, for research purposes in particular, the validated, clinician-administered, semistructured diagnostic interview schedules for (1) fatigue states (Structured Clinical Interview for Neurasthenia [SCIN]) [6] and (2) psychiatric disorders (Composite International Diagnostic Interview [CIDI]) offer an ideal approach to further assessment. In addition, if screening questionnaires raise of the possibility of sleep disturbance as a contributor, the Structured Diagnostic Interview for Sleep patterns and Disorders may be used (Supplementary Table 3).
In clinical practice, patients with persistent fatigue after COVID should have a careful history to elucidate the nature of the symptoms, the timing of onset, and their impact on functional status, as well as a physical examination with particular emphasis on respiratory, cardiac, and neurological findings. This clinical assessment should include review of premorbid and intercurrent mental health with a particular emphasis on depression, anxiety, and posttraumatic stress disorder. In addition, a restricted list of laboratory tests should be ordered, such as a full blood count, kidney, liver, and thyroid function tests, C-reactive protein, blood glucose, ferritin, B-type natriuretic peptide, as well as a chest x-ray [43]. Additional investigations or specialist referral may be considered if the history or examination raises concerns. Children and adolescents with post-COVID fatigue should be referred to a pediatric service for assessment.
For those cases in whom this process does not reveal an explanatory condition, we recommend making a diagnosis of “idiopathic post-COVID fatigue”. These patients may satisfy diagnostic criteria for PIFS—that is, a post-infective fatigue syndrome after COVID-19 [7]. In terms of clinical care, provision of such a diagnosis is a key starting point for reassurance of a generally self-limiting natural history and supportive care [44]. For research purposes, we recommend that additional symptoms and comorbid conditions are well charted, enabling statistical analyses that control for these factors.
Pathophysiology
Because the pathophysiology of PIFS remains unresolved, a biopsychosocial approach to conceptualizing research approaches to idiopathic post-COVID fatigue is recommended, incorporating predisposing, precipitating, and perpetuating factors. Predisposing factors in PIFS may include genetic [45] as well as psychosocial vulnerabilities [46]. COVID-19 is the precipitating factor, but it may well act in concert with other concomitant triggers, such as distressing life events (eg, death of a relative from COVID-19, loss of employment) [47]. Perpetuating factors may include the advent of sleep disturbance [48], autonomic dysfunction with sympathetic predominance [49], endocrine disturbance with hypothalamus-pituitary-adrenal axis attenuation [50], reactive mood disorder such as depression or anxiety [51], as well as abnormal illness beliefs and behavioral changes such as activity patterns that are boom-bust or avoidant [52], resulting in a complex set of determinants of illness and disability [36]. It is likely that idiopathic post-COVID fatigue will have comparable pathophysiology to PIFS. For research investigations of the predictors or associations of post-COVID fatigue, large sample sizes and stratification by the multiple contributory variables are recommended, and careful matching by, or controlling for, these variables in case-control designs.
CONCLUSIONS
Although there are many unknown factors to be resolved about long COVID for both clinical and research contexts, the lessons learned from several decades of investigation of fatigue states after other infections highlight the need for careful clinical characterization, protocolized investigations, and a broad bio-psychosocial approach.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We are grateful to Professor Peter White for the review of the manuscript.
Author contributions. All authors contributed to the manuscript conception and design. C. X. S. and A. R. L. performed the literature search, data extraction, and analysis. All authors have contributed to and approved the final manuscript.
Disclaimer. The views expressed are those of R.M.-M and not necessarily those of the National Institute for Health Research (NHS), the National Institute for Health Research (NIHR), or the Department of Health and Social Care.
Financial support . A. R. L. is supported by a National Health and Medical Research Council Practitioner Fellowship (Grant 1041897). C. X. S. is supported by the Mason Foundation National Medical Program. R. M.-M. is partly funded by the NIHR Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London.
Potential conflicts of interest. All authors: No reported conflicts of interest.
References
- 1. Baig AM. Chronic COVID syndrome: need for an appropriate medical terminology for long-COVID and COVID long-haulers. J Med Virol 2020; 93:2555–6. [DOI] [PubMed] [Google Scholar]
- 2. Time. Have We Been Thinking About Long-Haul Coronavirus All Wrong? Available at: https://time.com/5897992/long-haul-coronavirus-me-cfs/. Accessed 28 October 2020.
- 3. Kuppuswamy A. The fatigue conundrum. Brain 2017; 140:2240–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vollmer-Conna U. Acute sickness behaviour: an immune system-to-brain communication? Psychol Med 2001; 31:761–7. [DOI] [PubMed] [Google Scholar]
- 5. Katz BZ, Collin SM, Murphy G, et al. . The international Collaborative on Fatigue Following Infection (COFFI). Fatigue 2018; 6:106–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bennett BK, Goldstein D, Chen M, et al. . Characterization of fatigue states in medicine and psychiatry by structured interview. Psychosom Med 2014; 76:379–88. [DOI] [PubMed] [Google Scholar]
- 7. Fukuda K, Straus SE, Hickie I, et al. . The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med 1994; 121:953–9. [DOI] [PubMed] [Google Scholar]
- 8. Borges do Nascimento IJ, von Groote TC, O’Mathuna DP, et al. . Clinical, laboratory and radiological characteristics and outcomes of novel coronavirus (SARS-CoV-2) infection in humans: a systematic review and series of meta-analyses. PLoS One 2020; 15:e0239235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carvalho-Schneider C, Laurent E, Lemaignen A, et al. . Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin Microbiol Infect 2021; 27:258–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sudre CH, Murray B, Varsavsky T, et al. . Attributes and predictors of long COVID. Nat Med 2021; 27:626–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang X, Xu H, Jiang H, et al. . Clinical features and outcomes of discharged coronavirus disease 2019 patients: a prospective cohort study. QJM 2020; 113:657–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arnold DT, Hamilton FW, Milne A, et al. . Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: results from a prospective UK cohort. Thorax 2020. doi: 10.1101/2020.08.12.20173526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carfì A, Bernabei R, Landi F; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA 2020; 324:603–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Garrigues E, Janvier P, Kherabi Y, et al. . Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect 2020; 81:e4–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Halpin SJ, McIvor C, Whyatt G, et al. . Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J Med Virol 2021; 93:1013–22. [DOI] [PubMed] [Google Scholar]
- 16. Miyazato Y, Morioka S, Tsuzuki S, et al. . Prolonged and late-onset symptoms of coronavirus disease 2019. Open Forum Infect Dis 2020; 7:ofaa507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Qi R, Chen W, Liu S, et al. . Psychological morbidities and fatigue in patients with confirmed COVID-19 during disease outbreak: prevalence and associated biopsychosocial risk factors. Preprint. medRxiv. 2020;2020.05.08.20031666. Published May 11 2020. doi: 10.1101/2020.05.08.20031666 [DOI] [Google Scholar]
- 18. Savarraj JPJ, Burkett AB, Hinds SN, et al. . Three-month outcomes in hospitalized COVID-19 patients [preprint ]. medRxiv 2020.10.16.20211029; doi: 10.1101/2020.10.16.20211029. [DOI] [Google Scholar]
- 19. Townsend L, Dyer AH, Jones K, et al. . Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS One 2020; 15:e0240784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xiong Q, Xu M, Li J, et al. . Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study. Clin Microbiol Infect 2021; 27:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Daher A, Balfanz P, Cornelissen C, et al. . Follow up of patients with severe coronavirus disease 2019 (COVID-19): pulmonary and extrapulmonary disease sequelae. Respir Med 2020; 174:106197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Darley DR, Dore GJ, Cysique L, et al. . High rate of persistent symptoms up to 4 months after community and hospital-managed SARS-CoV-2 infection. Med J Aust 2020; 214:279–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jacobs LG, Gourna Paleoudis E, Lesky-Di Bari D, et al. . Persistence of symptoms and quality of life at 35 days after hospitalization for COVID-19 infection. PLoS One 2020; 15:e0243882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kamal M, Abo Omirah M, Hussein A, Saeed H. Assessment and characterisation of post-COVID-19 manifestations. Int J Clin Pract 2021; 75:e13746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liang L, Yang B, Jiang N, et al. . Three-month follow-up study of survivors of Coronavirus disease 2019 after discharge. J Korean Med Sci 2020; 35:e418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mandal S, Barnett J, Brill SE, et al. ; ARC Study Group. ‘Long-COVID’: a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax 2021; 76:396–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Petersen MS, Kristiansen MF, Hanusson KD, et al. . Long COVID in the Faroe Islands - a longitudinal study among non-hospitalized patients. Clin Infect Dis 2020. doi: 10.1093/cid/ciaa1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sami R, Soltaninejad F, Amra B, et al. . A one-year hospital-based prospective COVID-19 open-cohort in the Eastern Mediterranean region: the Khorshid COVID Cohort (KCC) study. PLoS One 2020; 15:e0241537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pilotto A, Cristillo V, Cotti Piccinelli S, et al. . Long-term neurological manifestations of COVID-19: prevalence and predictive factors. Neurol Sci 2021:1–5. doi: 10.1101/2020.12.27.20248903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Delgado-Rodríguez M, Llorca J. Bias. J Epidemiol Community Health 2004; 58:635–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jin JM, Bai P, He W, et al. . Gender differences in patients with COVID-19: focus on severity and mortality. Front Public Health 2020; 8:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Taquet M, Geddes JR, Husain M, et al. . 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry 2021; 8:416–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 2021; 594:259–64. [DOI] [PubMed] [Google Scholar]
- 34. Bates DW, Schmitt W, Buchwald D, et al. . Prevalence of fatigue and chronic fatigue syndrome in a primary care practice. Arch Intern Med 1993; 153:2759–65. [PubMed] [Google Scholar]
- 35. Wessely S, Chalder T, Hirsch S, et al. . Postinfectious fatigue: prospective cohort study in primary care. Lancet 1995; 345:1333–8. [DOI] [PubMed] [Google Scholar]
- 36. Hulme K, Hudson JL, Rojczyk P, et al. . Biopsychosocial risk factors of persistent fatigue after acute infection: a systematic review to inform interventions. J Psychosom Res 2017; 99:120–9. [DOI] [PubMed] [Google Scholar]
- 37. Jason LA, Cotler J, Islam MF, et al. . Risks for developing ME/CFS in college students following infectious mononucleosis: a prospective cohort study. Clin Infect Dis 2020. doi: 10.1093/cid/ciaa1886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hopper B, Cameron B, Li H, et al. . The natural history of acute Q fever: a prospective Australian cohort. QJM 2016; 109:661–8. [DOI] [PubMed] [Google Scholar]
- 39. Cameron B, Bharadwaj M, Burrows J, et al. ; Dubbo Infection Outcomes Study. Prolonged illness after infectious mononucleosis is associated with altered immunity but not with increased viral load. J Infect Dis 2006; 193:664–71. [DOI] [PubMed] [Google Scholar]
- 40. Broderick G, Katz BZ, Fernandes H, et al. . Cytokine expression profiles of immune imbalance in post-mononucleosis chronic fatigue. J Transl Med 2012; 10:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hanevik K, Kristoffersen E, Mørch K, et al. . Giardia-specific cellular immune responses in post-giardiasis chronic fatigue syndrome. BMC Immunol 2017; 18:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cameron B, Flamand L, Juwana H, et al. . Serological and virological investigation of the role of the herpesviruses EBV, CMV and HHV-6 in post-infective fatigue syndrome. J Med Virol 2010; 82:1684–8. [DOI] [PubMed] [Google Scholar]
- 43. National Institute for Health and Care Excellence. COVID-19 rapid guideline: managing the long-term effects of COVID-19. Available at: https://www.nice.org.uk/guidance/ng188. Accessed 18 January 2021. [PubMed]
- 44. Kingstone T, Taylor AK, O’Donnell CA, et al. . Finding the ‘right’ GP: a qualitative study of the experiences of people with long-COVID. BJGP Open 2020; 4:bjgpopen20X101143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Piraino B, Vollmer-Conna U, Lloyd AR. Genetic associations of fatigue and other symptom domains of the acute sickness response to infection. Brain Behav Immun 2012; 26:552–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cvejic E, Li H, Hickie IB, et al. . Contribution of individual psychological and psychosocial factors to symptom severity and time-to-recovery after naturally-occurring acute infective illness: the Dubbo Infection Outcomes Study (DIOS). Brain Behav Immun 2019; 82:76–83. [DOI] [PubMed] [Google Scholar]
- 47. Theorell T, Blomkvist V, Lindh G, Evengård B. Critical life events, infections, and symptoms during the year preceding chronic fatigue syndrome (CFS): an examination of CFS patients and subjects with a nonspecific life crisis. Psychosom Med 1999; 61:304–10. [DOI] [PubMed] [Google Scholar]
- 48. Jackson ML, Bruck D. Sleep abnormalities in chronic fatigue syndrome/myalgic encephalomyelitis: a review. J Clin Sleep Med 2012; 8:719–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nelson MJ, Bahl JS, Buckley JD, et al. . Evidence of altered cardiac autonomic regulation in myalgic encephalomyelitis/chronic fatigue syndrome: a systematic review and meta-analysis. Medicine (Baltimore) 2019; 98:e17600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Papadopoulos AS, Cleare AJ. Hypothalamic-pituitary-adrenal axis dysfunction in chronic fatigue syndrome. Nat Rev Endocrinol 2011; 8:22–32. [DOI] [PubMed] [Google Scholar]
- 51. Afari N, Buchwald D. Chronic fatigue syndrome: a review. Am J Psychiatry 2003; 160:221–36. [DOI] [PubMed] [Google Scholar]
- 52. King E, Beynon M, Chalder T, et al. . Patterns of daytime physical activity in patients with chronic fatigue syndrome. J Psychosom Res 2020; 135:110154. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.