Hypertrophic growth of the heart in response to pathogenic stress has historically been regarded as a compensatory mechanism to reduce wall stress and maximize ventricular performance. However, epidemiological studies have illustrated that chronic hypertrophy increases the risk of poor outcomes in patients with cardiovascular disease, and in pre-clinical models of heart failure (HF), suppression of hypertrophy is beneficial rather than detrimental1. As such, there is great interest in defining the molecular underpinnings of cardiomyocyte hypertrophy to facilitate the development of targeted anti-hypertrophic therapies for HF.
In 1998, Molkentin, Olson, and colleagues published a landmark paper describing a crucial role for the Ca2+/calmodulin-dependent protein phosphatase, calcineurin, in the control of pathological cardiac hypertrophy2. Prior to this discovery, calcineurin had primarily been studied in lymphocytes, where it promotes cellular activation through dephosphorylation of the nuclear factor of activated T cells (NFAT) transcription factor3. Enthusiasm for the discovery of the link between calcineurin signaling and hypertrophy was bolstered by the existence of agents that inhibit calcineurin catalytic activity, such as the immunosuppressants FK506 and cyclosporine A, suggesting potential repurposing of these drugs to treat HF. However, given the diverse actions of calcineurin in immune as well as non-immune cells, it is now widely accepted that selective inhibition of calcineurin in cardiomyocytes will be better tolerated and more efficacious than systemic inhibition in patients with HF. Thus, there is a need to better understand the molecular details of calcineurin regulation in the heart in order to illuminate cardiac-specific approaches for targeting this phosphatase as an anti-hypertrophic therapy for HF.
Calcineurin exists as a heterodimer consisting of a catalytic subunit (CaNA) and a 19-kDa regulatory subunit (CaNB). Of the three CaNA isoforms (α, β and γ), CaNAβ appears to be the most important for the development of cardiac hypertrophy4. Binding of Ca2+ to CaNB enables binding of a Ca2+/calmodulin complex to CaNA, thereby releasing auto-inhibition and freeing the enzyme to dephosphorylate downstream substrates5. As detailed below, in the current issue of Circulation, Li et al describe the discovery of a CaNAβ binding protein, CDC42 interacting protein 4 (CIP4), which functions as a scaffold to sequester of pool of calcineurin near the sarcolemma of cardiomyocytes, where it regulates pro-hypertrophic signaling6. The findings have important implications for understanding how cardiac calcineurin is selectively activated by stress signals as opposed to the Ca2+ that floods cardiomyocytes during each contractile cycle. Furthermore, the data provide proof-of-concept for an innovative therapeutic approach whereby CIP4 anchoring activity is selectively inhibited to block the action of a small, pathogenic pool of calcineurin as a means of treating HF.
The importance of calcineurin scaffolding in the heart is not a novel concept. Kapiloff and colleagues have spent nearly two decades exploring the functionality of A-kinase anchoring protein (mAKAP)-mediated scaffolding in the development of cardiac hypertrophy7. The AKAP family of proteins share the ability to directly bind protein kinase A (PKA), and in particular, mAKAPβ is found predominantly in striated muscle at the nuclear envelope of myocytes8. It is now appreciated that mAKAPβ orchestrates an entire ‘signalosome’ to integrate upstream signals and regulate downstream effectors, in particular through stress-induced transcription factors including NFAT and myocyte enhancer factor-2 (MEF2)8. mAKAPβ anchors calcineurin to the perinuclear region of cardiomyocytes (Figure 1), where it has been shown to be necessary for NFAT dephosphorylation and subsequent nuclear translocation, resulting in the expression of a gene program associated with cardiomyocyte hypertrophy9. Furthermore, conditional, cardiomyocyte-specific ablation of mAKAPβ was demonstrated to be cardioprotective in the setting of left ventricular (LV) pressure overload, ameliorating myocardial apoptosis and interstitial fibrosis as well as improving overall survival10. Importantly, however, the perinuclear scaffolding role of mAKAPβ cannot sufficiently explain the paradoxical manner by which CaNAβ responds solely to pathological stimuli, as opposed to physiological Ca2+ cycling as a component of excitation-contraction coupling, to initiate a signaling cascade culminating in cardiac hypertrophy.
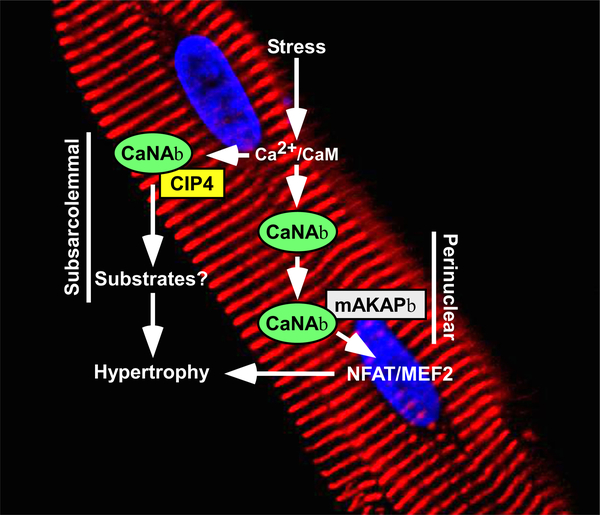
Figure 1. A model for regulation of cardiomyocyte hypertrophy by distinct pools of calcineurin.
A perinuclear pool of CaNAβ associated with mAKAPβ is stimulated by stress-induced Ca2+/calmodulin (CaM) signaling, resulting in activation of the pro-hypertrophic transcription factors nuclear factor of activated T cells (NFAT) and myocyte enhancer factor-2 (MEF2). Stimulation of a pool of CaNAβ that is anchored to the subsarcolemmal region of cardiomyocytes by CIP4 also triggers hypertrophy through dephosphorylation of substrates that remain unknown.
In the current study, Li et al present a compelling demonstration of the mechanisms by which CaNAβ is further compartmentalized through association with another membrane-bound scaffolding protein that is critical for CaNAβ-dependent hypertrophy in response to pathologic stimuli6. Initially, employing a yeast two-hybrid screen, the authors identified 10 candidate scaffold proteins that interact with the unique, amino-terminal polyproline (PP) domain present in CaNAβ but not CaNAα. Of these, the authors chose to focus the remainder of the study on CIP4, presumably based on their prior demonstration that CIP4 promotes cardiomyocyte hypertrophy through an undefined mechanism11. CIP4-CaNAβ interactions were validated using both co-immunoprecipitation and proximity ligation assays, confirming the specificity and physiologic binding of CaNAβ to CIP4 through the PP domain. While the precise localization of CIP4-CaNAβ complexes in cardiomyocytes was not determined, the authors propose that CIP4 anchors the phosphatase near plasma membrane receptors and ion channels to mediate cytoskeletal-based signaling networks (Figure 1).
The authors demonstrate that conditional, cardiomyocyte-specific knockout (KO) of CIP4 in adult mice attenuates pathologic hypertrophy in a 2-week model of LV pressure overload induced by transverse aortic constriction (TAC). Of note, the reduction of hypertrophy in CIP4 KO mice was not as dramatic as the phenotype observed in CaNAβ knockout mouse models4, likely highlighting the involvement of distinct pools of calcineurin in the control of stress-dependent cardiac growth (e.g. mAKAPβ-bound calcineurin). In a 4-week TAC model, CIP4 KO mice had improved systolic function and reduced lung congestion compared to WT controls, indicating a role for this anchoring protein in cardiac dysfunction in pressure-overload induced HF. Importantly for clinical translation, a peptide-based competitive disruptor of the CIP4-CaNAβ complex was shown to block agonist-dependent hypertrophy of cultured adult rat ventricular myocytes (ARVMs), and expression of this peptide in mouse cardiomyocytes in vivo using adeno-associated-virus 9 also blunted cardiac hypertrophy and modestly improved LV systolic function in a 2-week TAC model.
In order to further interrogate and visualize the CaNAβ-CIP4 interaction, the authors leveraged a FRET-based calcineurin reporter fused to CIP4. Stimulation of ARVMs using phenylephrine, isoproterenol, or angiotensin II increased the signal of the fusion sensor by 4–5%, indicating activation of CIP4-bound CaNAβ. In contrast, electrical pacing failed to alter the signal. This suggests that CaNAβ associated with CIP4 is compartmentalized such that it is specifically activated by increased Ca2+ driven by pathologic stimuli, while being shielded from physiological Ca2+ released during excitation-contraction coupling.
This tour de force study by Li et al further highlights the complexity of cardiomyocyte signal transduction networks and the therapeutic potential of targeting compartmentalized signaling mediators6. Some subtle findings not highlighted above warrant further investigation. For example, CIP4 KO reduced TAC-mediated increases in atrial mass, most notably in the 4-week model. Increased left atrial volume is a surrogate for elevated LV end diastolic pressure (LVEDP) and diastolic dysfunction (DD)12. It is not known if the TAC-induced increase in atrial mass is due to higher LVEDP. However, if it is, the data suggest that the CIP4-associated pool of CaNAβ is exquisitely involved in regulating diastolic function through an as-yet-unidentified mechanism, which would have profound implications for the development of therapies for conditions with underlying DD, such as HF with preserved ejection fraction.
CIP4 KO reduced LV hypertrophy in the 2-week but not the 4-week TAC model. Does this mean that the CIP4-associated pool of CaNAβ drives early, but not late hypertrophic growth in response to pressure overload? If so, what are the relevant substrates? The CIP4-bound pool of CaNAβ did not appear to reduce activation of canonical, pro-hypertrophic calcineurin substrates such as NFAT and myocyte enhancer factor-2. Thus, there must be subsarcolemmal targets of calcineurin in the CIP4 compartment that regulate early hypertrophy of the LV in response to pressure overload. Future phosphoproteomic studies comparing WT and CIP4 KO hearts should reveal these substrates.
Along with CIP4, the yeast two-hybrid screen identified other interesting proteins as potential binding partners for the amino-terminus of CaNAβ, including the z-disc-associated factor Sorbin and SH3 domain-containing protein 2 (SORBS2)/ArgBP2. Prior studies showed that z-disc protein calsarcin-1 sequesters and inhibits a pool of calcineurin at the sarcomere13. Does SORBS2 function in a similar manner to selectively inhibit a pool of CaNAβ that is deployed in response to pathological stress?
In addition to addressing these questions, it will be essential to test the therapeutic potential of inhibitory peptides of the CIP4-CaNAβ complex, or small molecules that mimic their action, in a large animal model of HF to establish whether this approach provides the safety and efficacy needed for advancement to human clinical trials.
Even 22 years after the publication of the Molkentin and Olson paper2, scientists are still discovering important nuances about calcineurin regulation and function in cardiac muscle. The elegant work by Li et al adds an intricate piece to the puzzle, defining a mechanism by which an anchor that tethers calcineurin to a subsarcolemmal region of cardiomyocytes weighs on the heart by triggering hypertrophic growth in response to pathological stress6.
Acknowledgements
T.A.M. was supported by the National Institute of Health by grants HL116848, HL147558, DK119594, HL127240, HL150225, and by the American Heart Association (16SFRN31400013). J.G.T was supported by the National Institute of Health by grant HL147463. K.C.W was supported by the National Institute of Health by grant K01AG066845 and the Lorna Grindlay Moore Faculty Launch Award (University of Colorado Anschutz Medical Campus).
Footnotes
Conflict of Interest Disclosures
T.A.M. received support from Italfarmaco for an unrelated project. K.C.W. and J.G.T. have no conflicts to disclose.
References
- 1.Schiattarella GG and Hill JA. Inhibition of hypertrophy is a good therapeutic strategy in ventricular pressure overload. Circulation. 2015;131:1435–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR and Olson EN. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crabtree GR and Olson EN. NFAT signaling: choreographing the social lives of cells. Cell. 2002;109 Suppl:S67–79. [DOI] [PubMed] [Google Scholar]
- 4.Bueno OF, Wilkins BJ, Tymitz KM, Glascock BJ, Kimball TF, Lorenz JN and Molkentin JD. Impaired cardiac hypertrophic response in Calcineurin Abeta -deficient mice. Proc Natl Acad Sci U S A. 2002;99:4586–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parra V and Rothermel BA. Calcineurin signaling in the heart: The importance of time and place. J Mol Cell Cardiol. 2017;103:121–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X, Li J, Martinez EC, Froese A, Passariello CL, Henshaw K, Rusconi F, Li Y, Yu Q, Thakur H, Nikolaev VO and Kapiloff MS. Calcineurin Abeta-Specific Anchoring Confers Isoform-Specific Compartmentation and Function in Pathological Cardiac Myocyte Hypertrophy. Circulation. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pare GC, Bauman AL, McHenry M, Michel JJ, Dodge-Kafka KL and Kapiloff MS. The mAKAP complex participates in the induction of cardiac myocyte hypertrophy by adrenergic receptor signaling. J Cell Sci. 2005;118:5637–46. [DOI] [PubMed] [Google Scholar]
- 8.Dodge-Kafka K, Gildart M, Tokarski K and Kapiloff MS. mAKAPbeta signalosomes - A nodal regulator of gene transcription associated with pathological cardiac remodeling. Cell Signal. 2019;63:109357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J, Negro A, Lopez J, Bauman AL, Henson E, Dodge-Kafka K and Kapiloff MS. The mAKAPbeta scaffold regulates cardiac myocyte hypertrophy via recruitment of activated calcineurin. J Mol Cell Cardiol. 2010;48:387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kritzer MD, Li J, Passariello CL, Gayanilo M, Thakur H, Dayan J, Dodge-Kafka K and Kapiloff MS. The scaffold protein muscle A-kinase anchoring protein beta orchestrates cardiac myocyte hypertrophic signaling required for the development of heart failure. Circ Heart Fail. 2014;7:663–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rusconi F, Thakur H, Li J and Kapiloff MS. CIP4 is required for the hypertrophic growth of neonatal cardiac myocytes. J Biomed Sci. 2013;20:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuspidi C, Negri F, Sala C, Valerio C and Mancia G. Association of left atrial enlargement with left ventricular hypertrophy and diastolic dysfunction: a tissue Doppler study in echocardiographic practice. Blood Press. 2012;21:24–30. [DOI] [PubMed] [Google Scholar]
- 13.Frey N, Barrientos T, Shelton JM, Frank D, Rutten H, Gehring D, Kuhn C, Lutz M, Rothermel B, Bassel-Duby R, Richardson JA, Katus HA, Hill JA and Olson EN. Mice lacking calsarcin-1 are sensitized to calcineurin signaling and show accelerated cardiomyopathy in response to pathological biomechanical stress. Nat Med. 2004;10:1336–43. [DOI] [PubMed] [Google Scholar]