Abstract
Background:
IgE to α-Gal is a cause of mammalian meat allergy and has been linked to tick bites in North America, Australia, and Eurasia. Reports from the developing world indicate that α-Gal sensitization is prevalent but has been little investigated.
Objective:
We sought evidence for the cause(s) of α-Gal sensitization and lack of reported meat allergy among children in less developed settings in Ecuador and Kenya.
Methods:
IgE to α-Gal and total IgE were assessed in children from Ecuador (n = 599) and Kenya (n = 254) and compared with children with (n = 42) and without known (n = 63) mammalian meat allergy from the southeastern United States. Information on diet, potential risk factors, and helminth infections was available for children from Ecuador. IgG4 to α-Gal and antibodies to regionally representative parasites were assessed in a subset of children.
Results:
In Ecuador (32%) and Kenya (54%), α-Gal specific IgE was prevalent, but levels were lower than in children with meat allergy from the United States. Sensitization was associated with rural living, antibody markers of Ascaris exposure, and total IgE, but not active infections with Ascaris or Trichuris species. In Ecuador, 87.5% reported consuming beef at least once per week, including 83.9% of those who had α-Gal specific IgE. Levels of α-Gal specific IgG4 were not high in Ecuador, but were greater than in children from the United States.
Conclusions:
These results suggest that in areas of the developing world with endemic parasitism, α-Gal sensitization is (1) common, (2) associated with Ascaris exposure, and (3) distinguished by a low percentage of specific/total IgE compared with individuals with meat allergy in the United States.
Keywords: IgE, alphα-Gal, mammalian meat allergy, Ascaris, parasite, sensitization
GRAPHICAL ABSTRACT
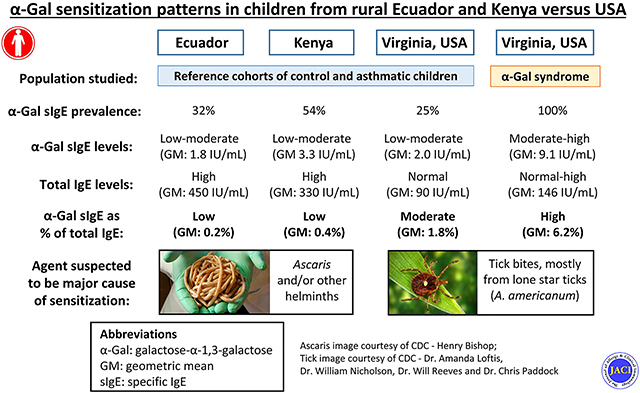
Galactose-α-1,3-galactose (α-Gal) is an oligosaccharide of nonprimate mammals and the target of IgE antibodies in subjects with the “α-Gal syndrome” (AGS).1 The syndrome, which commonly manifests with delayed reactions to mammalian meat, has been well described in the southeastern United States and also parts of Australia, Europe, and Asia. In these regions the dominant cause of sensitization has been related to tick bites, though other ectoparasites such as chiggers could be involved.2 Although there has been little information on the incidence or prevalence of the syndrome, population-based screening has revealed that the frequency of sensitization to α-Gal can approach 20% in some areas of the United States and Europe where certain species of ticks are endemic.3,4 Interestingly, we and others have reported striking rates of IgE sensitization to α-Gal in studies conducted in developing countries such as Kenya, Zimbabwe, and Ecuador.3,5 For example, 76% of children from a village in rural Kenya and 47% of children from a rural area in Esmeraldas province of Ecuador were reported to have specific IgE (sIgE) to α-Gal.3 Because these studies were originally designed to study asthma, information about food allergy is lacking in these 2 studies. Nonetheless, we are unaware of an epidemic of allergy to mammalian meat in these populations.
We have considered several possibilities that could explain the high rates of α-Gal sensitization without a corresponding high rate of mammalian meat allergy. As compared with children with symptomatic mammalian meat allergy, IgE-positive children from Ecuador or Kenya could have (1) lower levels of α-Gal sIgE, (2) similar absolute α-Gal sIgE levels, but lower as a percentage of total IgE, and/or (3) higher levels of IgG4, an antibody subclass that is often associated with tolerance. An additional question relates to the factors that could contribute to the high rate of α-Gal sensitization reported in developing regions. A role for endoparasitic exposures is suggested by the fact that (1) α-Gal has now been identified in species of Plasmodium6 and Leishmania,7 and also members of the nematode and platyhelminth phyla8–10 and (2) malaria and geohelminths, such as Ascaris, Trichuris, and hookworm, are endemic in many of these areas. Thus, here we sought to determine whether the high prevalence of α-Gal sensitization in Ecuador and Kenya could be explained by geohelminth or Plasmodium exposures. We hypothesized that endoparasite exposure(s) is associated with low levels of α-Gal sIgE and concomitant high levels of α-Gal IgG4—an immune signature often associated with tolerance. To address these questions, we compared serologic responses in children from Ecuador and Kenya with responses in age-comparable reference populations, including children in the United States with the α-Gal–related mammalian meat allergy.
METHODS
Study samples
Ecuadorian schoolchildren.
Children living in riverine communities in rural districts of Eloy Alfaro and San Lorenzo (n = 376) and the city of Esmeraldas (n = 223), Esmeraldas Province in northwest Ecuador, were recruited in a case-control study of asthma/wheeze, nested within a larger cross-sectional survey, as previously described.11,12 Esmeraldas is at sea level, equatorial, and with a tropical rainforest climate.13 Cases (n = 251) were defined by a parental report of recent wheeze (ie, wheeze within the past 12 months), whereas controls (n = 348) had no history of wheeze symptoms. Urban children were from neighborhoods on the periphery of the city of Esmeraldas to which immigrants from northern rural districts of the province had migrated over the last 40 or so years. Questionnaires were administered to the parent or guardian of each child and included detailed questions about exposures and diet. Blood samples were drawn and plasma stored for later analysis. Stool samples were collected and examined for presence and intensity of geohelminth eggs and larvae using Kato Katz and formol-ether methods, as previously described.12 All children in the nested case-control study with complete data and sample collection were included in the analysis. Approval for this study was obtained locally in the area where subjects were enrolled and from the University of Virginia Institutional Review Board (UVA-IRB).
Kenyan schoolchildren.
Children were enrolled from the fourth grade of elementary schools in the rural village of Kabati (n = 131) and the industrialized town of Thika (n = 123), which were in proximity to each other and approximately 50 to 80 km north of Nairobi, as previously described.14 This region is approximately 5000 to 6000 feet above sea level and is near the equator with a subtropical highland climate.13 Blood samples were drawn and plasma stored for later analysis. Approval for this study was obtained from the Kenya Medical Research Institute ethical review board and from the UVA-IRB.
Children in southeastern United States with α-Gal meat allergy.
This population included 42 children from central Virginia (age <18 years) who reported hives and/or anaphylaxis to mammalian meat and had sIgE testing consistent with AGS (ie, α-Gal sIgE > 0.35 IU/mL). Twenty-seven of the cases were part of a cohort of 261 children and adults with mammalian meat allergy who were prospectively enrolled and described in a recent report.15 Fifteen of the children were identified through a retrospective chart review of AGS cases managed at UVA between 2015 and 2020. Both the prospective and retrospective arms were approved by the UVA-IRB.
Children from central Virginia unselected for food allergy.
This population included children enrolled from the emergency department or a pediatrician’s office in Charlottesville, Va, as part of a case-control study of pediatric asthma, as previously described.16,17 This area is at approximately 500 feet above sea level and has a humid subtropical climate.13 Cases were defined as subjects who were seen for acute wheezing symptoms; control children were evaluated for problems such as trauma, gastrointestinal distress, or fever but did not have acute respiratory symptoms or signs. No information about diet or food allergy was collected as part of this investigation. Of the 74 subjects in the parent study, sera were available from 63 for additional serologic investigation. This study was approved by the UVA-IRB.
ImmunoCAP IgE and IgG4 assays
Sera were tested for total IgE, sIgE, and specific IgG4 using the ImmunoCAP 250 instrument (Thermo-Fisher/Phadia U.S., Portage, Mich). The IgE results are expressed as international units (IU) per milliliter, with 1 IU equivalent to approximately 2.4 ng. A positive reaction to α-Gal was considered as 0.35 IU/mL or greater. Using a previously described technique, sIgE to α-Gal was assayed using cetuximab on the solid phase and specific IgG4 to α-Gal was assayed with α-Gal-HSA on the solid phase.18 All other IgE and IgG4 assays used commercial ImmunoCAPs (Thermo-Fisher/Phadia).
IgG assays to Ascaris, malaria, Toxocara, and Strongyloides
ELISA kits were purchased from commercial vendors and used according to manufacturer’s instructions: Ascaris IgG and Malaria IgG/IgM kits were from IBL International (Hamburg, Germany); Strongyloides IgG/IgM kit was from IVD Research (Carlsbad, Calif); Toxocara canis IgG to secretory-excretory antigens of T canis larvae were measured as described.19 Positive responses were determined by comparison with an internal calibrator control or predetermined OD cutoff.
Statistical analysis
The central tendency of antibody levels was assessed with geometric means and 95% CIs. Comparisons between 2 groups were analyzed using Student t test for parametric data and Mann-Whitney U test for nonparametric data. Correlations between variables were analyzed using Spearman rank correlation coefficients (rs). Categorical variables were compared using chi-square or Fisher exact test, as appropriate. The Kruskal-Wallis statistic was used for multiple comparisons of continuous nonparametric data, and chi-square for trend was used for multiple comparisons of categorical data. Statistical analysis was performed using GraphPad Prism, version 7 (GraphPad Software Inc, La Jolla, Calif). Logistic regression was conducted using SPSS v25 (IBM Inc, Armonk, NY).
RESULTS
Characteristics of study samples
The median age of the children in Ecuador (11 years; range, 6–19 years) and Kenya (11 years; range, 8–15 years) was little different than the age of children who had AGS in the United States (12 years; range, 5–15 years) (Table I). The children who had AGS were predominantly male (74% males and 26% females), whereas the children from Ecuador (56% males and 44% females) and Kenya (51% males and 49% females) had a more balanced representation of girls and boys. In Ecuador, 99% of the children identified as Afro-Ecuadorian. In Kenya, all of the children were indigenous Africans. The children with AGS were predominantly Caucasian (88%). The unselected children from the United States trended younger than their counterparts with AGS (median age, 8 years; range, 4–18 years), were more frequently girls, and 56% were African American and 35% Caucasian. Among the different samples, recurrent wheeze/asthma was present in 29% from Ecuador, 7% from Kenya, 19% with AGS, and 40% among children from the United States not selected on the basis of food allergy.
TABLE I.
Characteristics of subjects in the major cohorts
| Clinical and immune characteristic | Ecuador (n = 599) | Kenya (n = 254) | USA | |
|---|---|---|---|---|
| α-Gal syndrome (n = 42) | Controls (n = 63) | |||
| Age (y), median (range)* | 11 (6–19) | 11 (8–15)† | 12 (5–15) | 8 (4–18) |
| Sex: male, n (%)‡ | 337 (56) | 130 (51)† | 31 (74) | 28 (44)† |
| Recurrent wheeze/asthma, n (%)‡ | 170 (29) | 19 (7)† | 8 (19) | 25 (40)† |
| Consume beef ≥1X per week, n (%) | 524 (87.5) | NA | NA§ | NA |
| Total IgE, (IU/mL), GM (95% CI)‖ | 450 (404–502)¶ | 330 (261–418)† | 146 (104–204) | 90 (58–140) |
| α-Gal sIgE, n > 0.35 (%)‡ | 194 (32)¶ | 137 (54)¶ | 42 (100) | 16 (25)¶ |
| α-Gal sIgE (IU/mL), GM (95% CI)‖ | 1.8 (1.5–2.1)¶ | 3.3 (2.7–4.1)¶ | 9.1 (6.1–13.5) | 2.0 (1.1–3.6)¶ |
| α-Gal sIgE as % of total, GM (95% CI)‖ | 0.2 (0.1–0.2)¶ | 0.4 (0.3–0.5)¶ | 6.2 (4.5–8.6) | 1.8 (0.8–3.9)† |
GM, Geometric mean.
Student t test, compared with AGS.
P value in relation to AGS < .05.
Fisher exact test, compared with AGS.
Most of these children were not consuming mammalian meat, but detailed dietary history was not available
Mann-Whitney U test, compared with AGS.
P value in relation to α-Gal syndrome < .001.
Comparison of α-Gal sensitization across samples
In regard to α-Gal sensitization, 32% of the Ecuadorian children, 54% of the Kenyan children, and 25% of control children from the southeastern United States were positive for IgE to α-Gal (Fig 1, A). The levels of α-Gal sIgE were lower in all 3 of these populations compared with the children from Virginia who had symptomatic AGS (Fig 1, B). Not surprisingly, total IgE levels were higher in the serum of children from Kenya and Ecuador than in the sera of children from the United States (Table I). Accordingly, α-Gal sIgE as a percentage of total IgE was markedly lower in children from Kenya and Ecuador as compared with children from the United States with symptomatic AGS (Fig 1, C).
FIG 1.
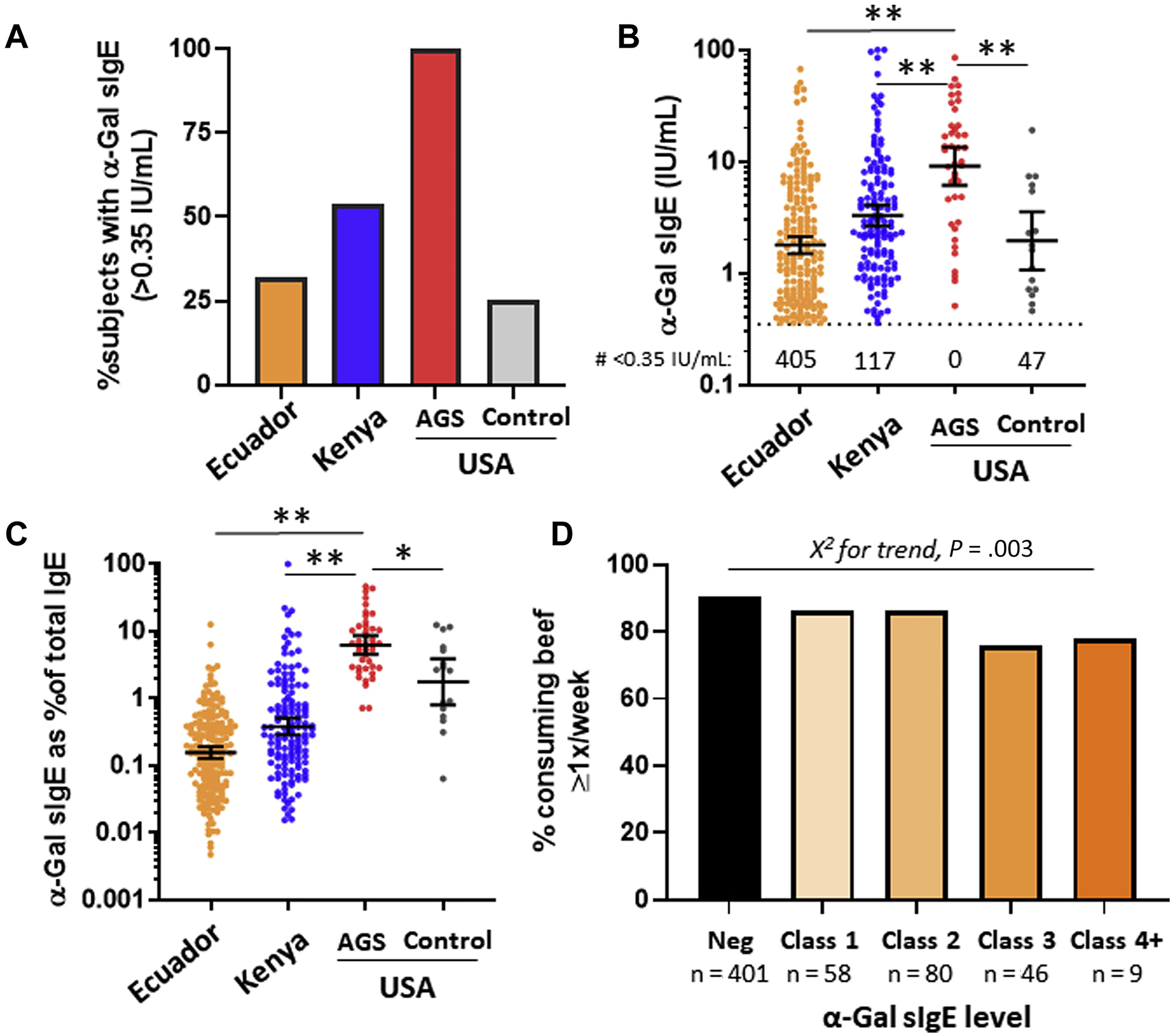
(A) Prevalence, (B) levels, and (C) levels in relation to total IgE of α-Gal sIgE (>0.35 IU/mL) among children from population-based samples in Ecuador and Kenya as compared with control children or children with mammalian meat allergy in the southeastern United States. D, Beef consumption in children from Ecuador in relation to α-Gal sIgE level. Neg, Negative.
Frequency of beef consumption in relation to α-Gal sensitization
Among study samples, dietary information was available only for children from Ecuador. Of the 594 children in Ecuador whose parents or guardians answered a question about frequency of beef (“carne”) consumption, 524 (87.5%) reported consuming at least once a week. Although information about allergic reactions to beef or other mammalian products was not addressed in the questionnaire, only 3 (0.5%) children reported never consuming beef. The frequency of children who consumed beef at least once a week was lower in children who were sensitized (83.9%) versus nonsensitized (90.3%), but even among those with α-Gal sIgE levels of class 4 or above (ie, >17.5 IU/mL), 77.8% reported eating beef at least once a week (Fig 1, D).
Factors associated with α-Gal sensitization
In the cohort from Ecuador, detailed information was available to investigate risk factors associated with α-Gal sensitization including exposures to geohelminths and other relevant parasites. Where complementary information was available, we compared findings from Ecuador with results from Kenya and other reference samples.
Demographic, clinical, and socioeconomic characteristics.
Male sex, rural living, and contact with farm animals were associated with α-Gal sensitization in Ecuadorian children (Table II). The association between rural living and α-Gal sIgE prevalence was also seen in children from Kenya (Fig 2, A; see Table E1 in this article’s Online Repository at www.jacionline.org). Levels of α-Gal sIgE were higher in the children from Kenya who lived in the rural village of Kabati as compared with their counterparts who lived in the industrial town of Thika (Fig 2, B); a similar trend in α-Gal sIgE levels in relation to rural living was observed in Ecuador. Interestingly, α-Gal sIgE as a percentage of total IgE was lower in the rural versus urban Kenyan children, but this was not true in Ecuador (Fig 2, C). Monthly income and presence of cat or dog in the house were not associated with sensitization (Table II). Subjects with histories of wheeze were neither more likely to be positive nor have higher antibody levels for α-Gal sIgE (see Fig E1 in this article’s Online Repository at www.jacionline.org).
TABLE II.
Characteristics of subjects in Ecuador (n = 599) in relation to α-Gal sensitization
| Characteristic | α-Gal sIgE positive (n = 194) | α-Gal IgE negative (n = 405) | OR (95% CI) | P |
|---|---|---|---|---|
| Demographic and clinical characteristic | ||||
| Age (y), mean (range)* | 11.3 (7–19) | 11 (6–19) | NA | .18 |
| Sex: male† | 136 (70.1) | 201 (49.6) | 2.4 (1.6–3.4) | <.001 |
| Rural† | 142 (73.2) | 234 (57.8) | 2.0 (1.4–2.9) | <.001 |
| Monthly income >$250† | 33 (17.0) | 80 (19.8) | 0.8 (0.5–1.3) | .42 |
| Current wheeze† | 57 of 192 (29.7) | 113 of 404 (28.1) | 1.1 (0.7–1.6) | .69 |
| Diet and exposures | ||||
| Never consume beef† | 2 of 192 (1.0) | 1 of 402 (0.3) | 4.2 (0.5–61) | .20 |
| Consume beef ≥1X per week† | 161 of 192 (83.9) | 363 of 402 (90.3) | 0.6 (0.3–0.9) | .02 |
| Never consume milk† | 4 (2.1) | 4 (1.0) | 2.1 (0.6–7.3) | .28 |
| Consume milk ≥1X per week† | 156 (80.4) | 347 (85.7) | 0.7 (0.4–1.1) | .1 |
| Consume unpasteurized milk† | 75 (38.7) | 153 (37.8) | 1.0 (0.7–1.5) | .84 |
| Cat in house† | 72 of 193 (37.3) | 155 (38.3) | 1.0 (0.7–1.4) | .82 |
| Dog in house† | 147 of 193 (76.2) | 324 (80) | 0.8 (0.5–1.2) | .28 |
| Farm animal contact† | 56 of 193 (29.0) | 68 (16.8) | 2.0 (1.3–3.0) | <.001 |
| Ascaris in stool† | 96 (49.5) | 178 (44.0) | 1.2 (0.9–1.8) | .2 |
| Trichuris in stool† | 113 (58.2) | 224 (55.3) | 1.1 (0.8–1.6) | .5 |
| Hookworm in stool† | 20 (10.3) | 17 (4.2) | 2.6 (1.4–5.2) | .004 |
| Any worm in stool† | 140 (72.2) | 264 (65.2) | 1.4 (1.0–2.0) | .09 |
| Immune marker | ||||
| Total IgE, GM (95% CI)‡ | 1141 (975–1335) | 288 (255–326) | NA | <.001 |
| Total IgE >500 IU/mL† | 152 (78.4) | 141 (34.8) | 6.8 (4.6–10.1) | <.001 |
| Ascaris IgE ≥0.7 IU/mL† | 162 (83.5) | 165 (40.7) | 7.4 (4.8–11.2) | <.001 |
| Positive skin test result (any allergen)† | 54 (27.8) | 71 (17.5%) | 1.8 (1.2–2.7) | .004 |
GM, Geometric mean; NA, not applicable/available; OR, odds ratio.
Compared with Student t test.
Compared with χ2 test.
Compared with Mann-Whitney U test.
FIG 2.
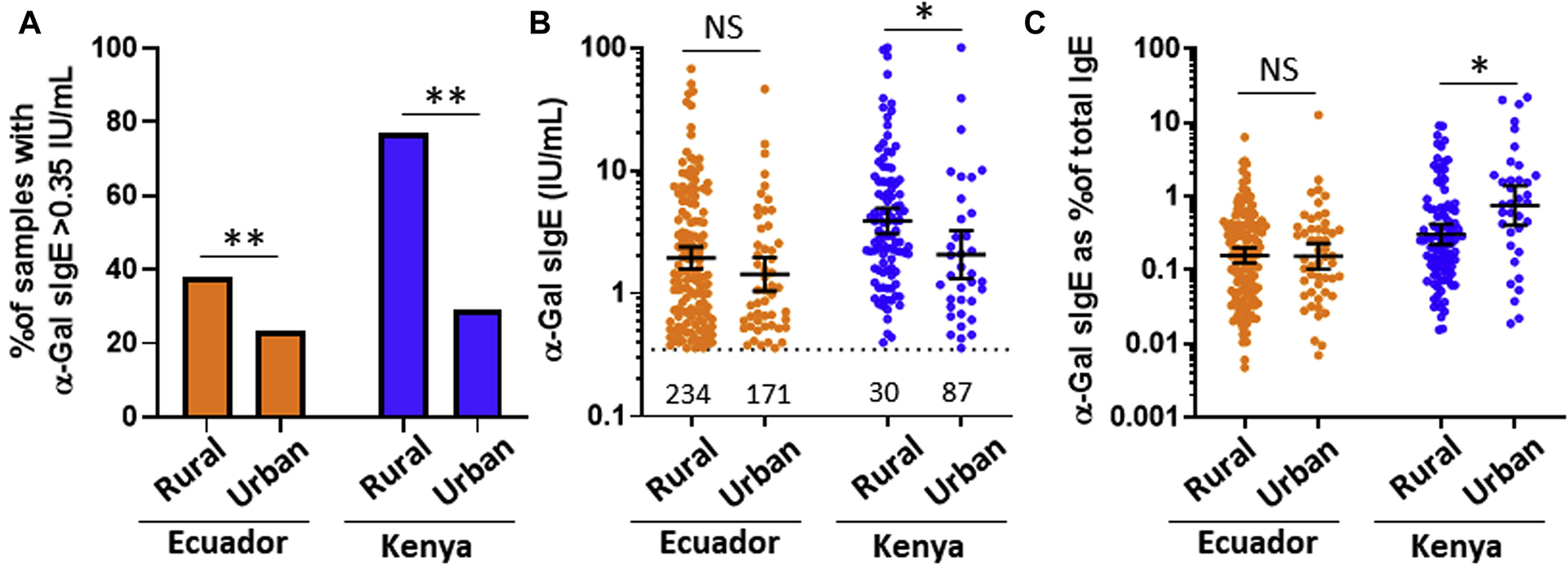
Comparison of (A) prevalence, (B) levels, and (C) levels in relation to total IgE for α-Gal sIgE in children from rural and less rural areas (“urban”) of Esmeraldas province of Ecuador and the rural community of Kabati compared with the industrialized “urban” town of Thika, Kenya. NS, Nonsignificant. The values in Fig 2, B, reflect the number of samples that were less than 0.35 IU/mL. Prevalence values were compared using χ2. Levels of α-Gal sIgE were expressed as geometric mean of positives (95% CI) and compared by the Mann-Whitney U test, *P < .05, NS P > .05.
Exposure to parasites.
Ascaris and Trichuris were present in the stool of approximately 50% of Ecuadorian children at the time of the blood draw. These geohelminths were found in similar frequency among α-Gal–sensitized and nonsensitized subjects (Table II), and there was no correlation between parasite burden of Ascaris or Trichuris and α-Gal sIgE (Fig 3, A). The frequency of samples with hookworm was higher in α-Gal–sensitized subjects (χ2, P < .01), but hookworm was present in only 6% of the sample. Accordingly, the correlation between hookworm burden and α-Gal sIgE was weak (rs = 0.14; P < .001). In a subset of the Ecuadorian children, we also measured specific IgG and IgG4 as markers for previous exposures to Ascaris. The association of α-Gal sIgE with Ascaris sIgG was moderate (rs = 0.31; P < .001). Among 15 children from the United States with AGS, only a single serum had detectable IgG to Ascaris (Fig 3, B). In the Ecuadorian children, there was also a moderate association between Ascaris sIgG4 and α-Gal sIgE (rs = 0.43; P < .001) (Fig 3, C). Because malaria is present in Esmeraldas Province in Ecuador and there have been reports that Plasmodium species (which cause malaria) can express α-Gal, we also measured antibodies specific for Plasmodium as a marker of exposure. Serologic evidence of malaria exposure was present in 43.9% of the children, but there was no association between IgG/IgM to Plasmodium species and α-Gal sIgE (rs = 0.02; P = .79); none of the children with AGS tested positive for Plasmodium spp antibodies (see Fig E2, A, in this article’s Online Repository at www.jacionline.org). We also assessed the relationship of α-Gal sIgE with serologic markers of Strongyloides stercoralis and Toxocara canis exposure, but no association was observed (Fig E2, B and C).
FIG 3.
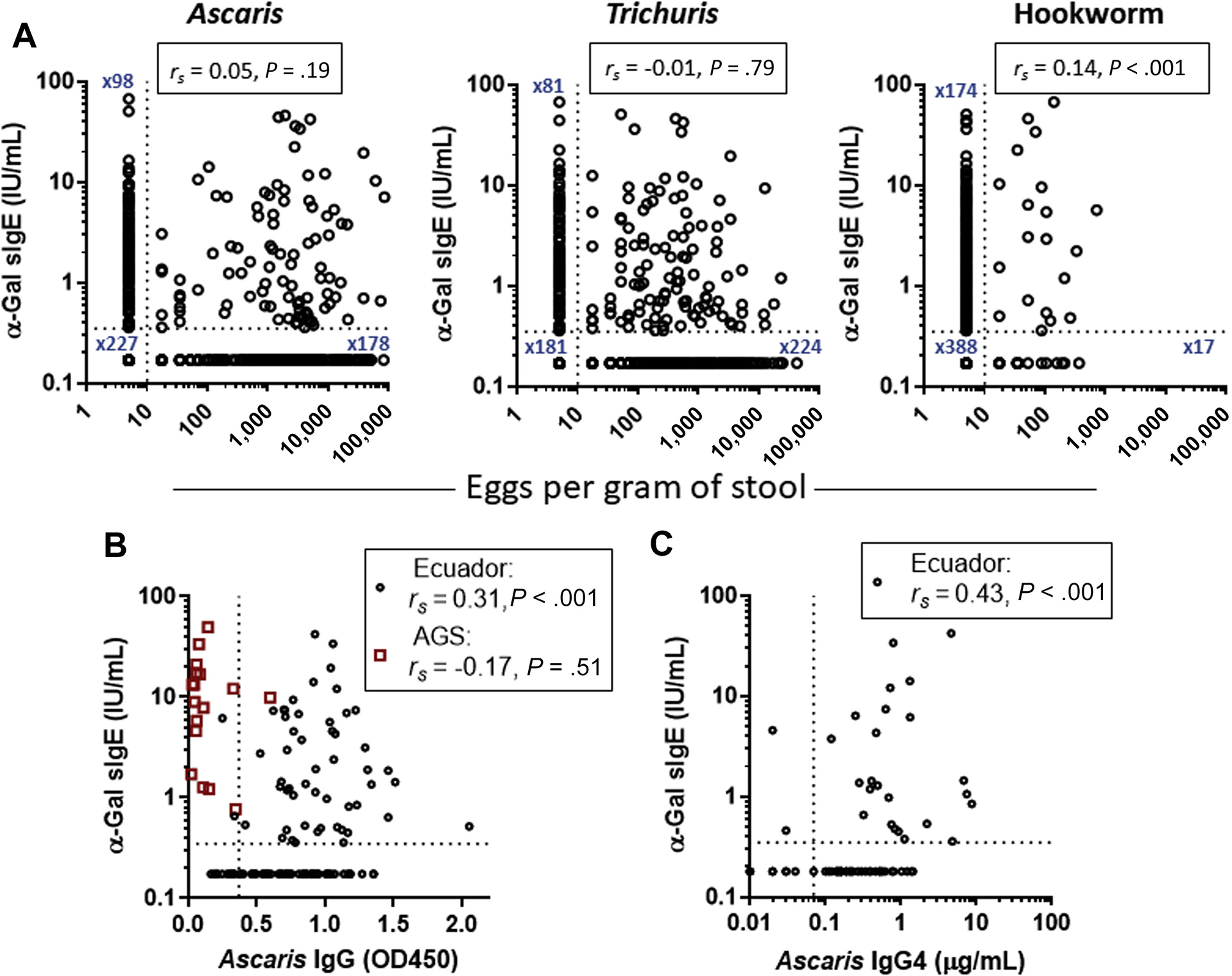
A, Relationship between geohelminth parasite burden and α-Gal sIgE in children from Ecuador (n = 599). Relationship of α-Gal sIgE with (B) Ascaris IgG and (C) Ascaris IgG4 in children from Ecuador (n = 123) and children with AGS in the United States (n = 18). rs, Spearman rank correlation. For α-Gal sIgE and eggs per gram of stool, dotted lines reflect threshold of detection. For Ascaris IgG and IgG4, the dotted line reflects approximation of calibrator cutoff (with minor interassay variation) for distinguishing positive and negative values.
IgE to Ascaris and total IgE.
IgE to Ascaris was more frequent in α-Gal–sensitized than nonsensitized Ecuadorian children (80.5% vs 40.7%; P <.001) (Table II), with a correlation coefficient between Ascaris sIgE and α-Gal sIgE of 0.45 (P < .001) (Fig 4, A). When Ascaris IgE was stratified into 5 groups on the basis of level, there was a significant difference in α-Gal sIgE prevalence, ranging from 7.3% in the group lacking Ascaris IgE up to 67% in the group with the highest Ascaris IgE levels (χ2 for trend, P <.001), but median α-Gal sIgE levels among positives were similar across all Ascaris IgE levels (Fig 4, B). Consistent with the findings in Ecuador, the relationship between IgE to Ascaris and α-Gal sIgE was moderately strong in the children from Kenya, with a correlation coefficient of 0.56 (P < .001) (see Fig E3 in this article’s Online Repository at www.jacionline.org).
FIG 4.
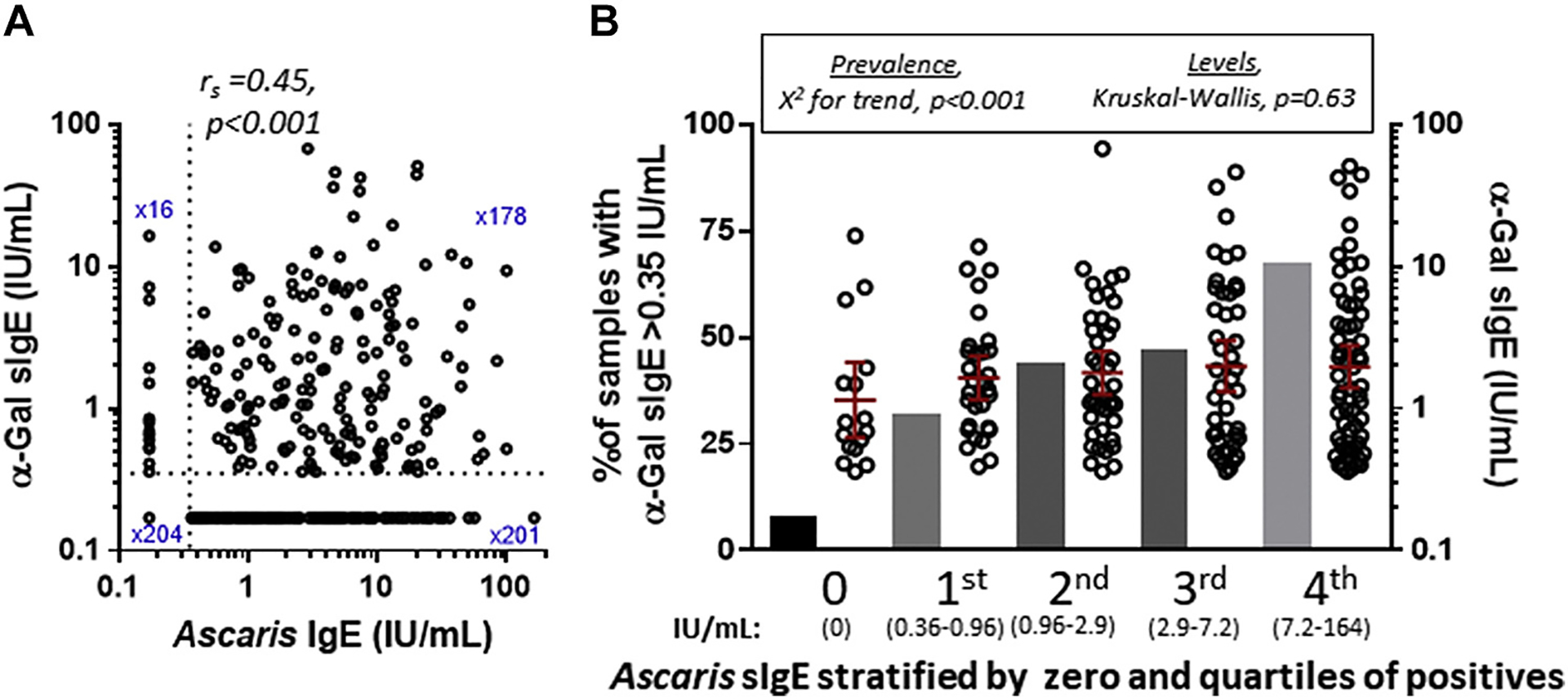
A, Ascaris sIgE vs α-Gal sIgE in children from Ecuador (n = 599). B, Relationship between α-Gal sIgE prevalence (bars, left y-axis) and levels (scatter plot, right y-axis) in the cohort when stratified on the basis of Ascaris sIgE status, where n ≤ 0.35 IU/mL = 220 and n > 0.35 IU/mL = 379.
Total IgE was much higher in α-Gal–sensitized children compared with nonsensitized children in children from Ecuador (Table II) and Kenya (Table E1). The correlation coefficient between total IgE and α-Gal sIgE was 0.49 (P < .001) in Ecuador and 0.59 (P < .001) in Kenya (see Fig E4 in this article’s Online Repository at www.jacionline.org). Not surprisingly, α-Gal sIgE as a % of total IgE decreased as levels of total IgE increased in children in Ecuador and Kenya (Kruskal-Wallis, P < .001) (see Fig E5 in this article’s Online Repository at www.jacionline.org). The correlation of α-Gal sIgE with total IgE levels raised the possibility that nonspecific binding could occur with the ImmunoCAP in situations with high total IgE. To investigate this possibility, we assessed α-Gal sIgE prevalence in 2 reference populations in relation to total IgE levels—children from an urban area of Costa Rica where there are effective public health programs for geohelminth control and children from northern Sweden where the climate and social conditions are unsuitable for geohelminth transmission. Additional details on these samples, which we have previously investigated in relation to asthma, are available in this article’s Online Repository at www.jacionline.org.20,21 In Costa Rica and Northern Sweden, there were 63 children who had total IgE levels more than 1000 IU/mL, but only 6 of those were sensitized to α-Gal (Table III).
TABLE III.
Prevalence of α-Gal sensitization in relation to total IgE levels in reference populations as compared with children from Ecuador
| Population | Entire cohort | Total IgE: 1000–2000 IU/mL | Total IgE: >2000 IU/mL | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | α-Gal positive (%) | P * | n | α-Gal positive (%) | P * | n | α-Gal positive (%) | P * | |
| Ecuador | 599 | 194 (32.4) | NA | 104 | 57 (55) | NA | 89 | 65 (73) | NA |
| Reference cohort | |||||||||
| Costa Rica | 277 | 13 (5) | <.001 | 28 | 3 (11) | <.001 | 18 | 2 (11) | <.001 |
| Northern Sweden | 413 | 1 (0.2) | <.001 | 11 | 0 (0) | <.001 | 6 | 1 (17) | .01 |
NA, Not applicable/available.
Comparison of reference population with Ecuador using Fisher exact test.
Levels of IgG4 to α-Gal are higher in children from Ecuador and Kenya compared with children in the United States
Elevated levels of α-Gal sIgG4 could be expected (1) in a situation in which allergic sensitization to α-Gal was driven by prolonged exposure(s), such as occurs with geohelminths and other endoparasites (as opposed to tick bite exposure, which is intermittent), and (2) to be associated with tolerance to mammalian meat. This hypothesis was addressed by assaying α-Gal specific IgG4 in a subset of the sera from Ecuadorian children using ImmunoCAP. The sera were chosen to be representative of the overall sample with respect to sex, area of residence (urban vs rural), and current wheeze. As shown in Fig 5, A, levels of IgG4 to α-Gal were all less than 1 μg/mL and were lower than levels of IgG4 to 2 allergens that children in Ecuador are commonly exposed to—casein (in the form of cow’s milk) and Ascaris. Moreover, among the children with detectable IgE to the respective allergens, the IgG4/IgE ratio was lower for α-Gal than for casein (Fig 5, B). Nonetheless, Ecuadorian children had higher levels of α-Gal specific IgG4 than reference children from the United States (Fig 5, C).
FIG 5.
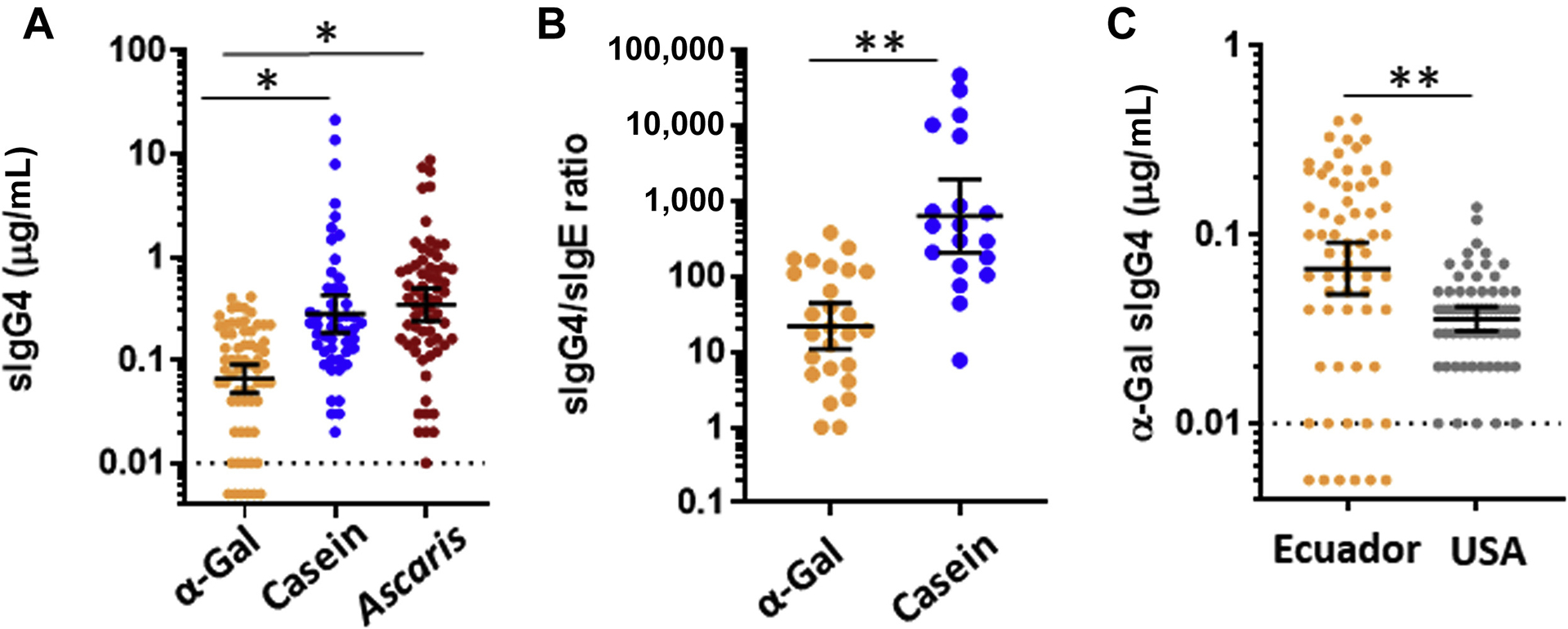
A, IgG4 to α-Gal (n = 65), casein (n = 50), and Ascaris (n = 65) in children from Ecuador. B, Ratio of specific IgG4/sIgE (normalized to ng/ng) in children from Ecuador who had detectable sIgE to the respective IgG4 component. C, IgG4 to α-Gal in children from Ecuador (n = 63) compared with children from the United States who were unselected for food allergy (n = 63). Values expressed as geometric mean of positives (95% CI) and compared by the Mann-Whitney U test, *P < .05, **P < .001.
DISCUSSION
In contrast to those IgE responses that are important causes of allergic diseases in industrialized societies (eg, dust mite, cat, and peanut), α-Gal sensitization was positively associated with rural living and farm animal contact. Similar findings, including the higher rates in males than females, have also been reported in North America.22 In the United States, these variables lose significance when accounting for tick exposure,22 which is consistent with a large body of evidence that supports the connection between tick bites and α-Gal sensitization in North America, Europe, Australia, and Japan.1 Unfortunately, tick history was not available in the current studies and there are no validated serologic assays to determine previous tick exposure. Although ticks, and also other ectoparasites such as chiggers,2 cannot be excluded as a cause of sensitization, there are good reasons to consider that endoparasites could contribute to induction of α-Gal sIgE in developing areas.
It is well established that chronic parasitism, particularly to geohelminths, is endemic among many children who reside in rural Ecuador and Kenya. In the current cohort, 67% of children from Ecuador had geohelminth larvae in their stool at the time of enrollment and IgG to Ascaris, a biomarker of previous Ascaris exposure, was detected in 113 of 123 screened (92%). Serologic markers reflecting previous exposure were also common for malaria (44%), Strongyloides (47%), and Toxocara (32%). Notably, α-Gal has been identified on several species of parasites, including Plasmodium spp (causal agent of malaria),6 Leishmania spp,7 and helminths of the nematode, trematode, and platyhelminth phyla.8–10 Although the frequency of stool geohelminth burden was similar in children with and without α-Gal sensitization, α-Gal sensitization was associated with each of IgG, IgG4, and IgE to Ascaris. In contrast, serologic markers of previous exposure to Plasmodium, Toxocara, and Strongyloides were not associated with α-Gal sensitization. When the relationship between Ascaris sIgE and α-Gal sIgE was stratified by quintile of Ascaris sIgE, it was apparent that the frequency of α-Gal sensitization, but not the level of IgE to α-Gal, was strongly associated with the level of IgE to Ascaris (Fig 4, B). Such a finding could reflect a situation in which α-Gal is a minor allergen and/or where α-Gal is present only transiently during the parasite lifecycle. To date we are unaware that α-Gal has been identified in Ascaris species. Hodzic et al10 recently probed for α-Gal in soluble crude extracts prepared from adult A suum using the M86 mAb but did not detect the oligosaccharide by ELISA or Western blot. Poltl et al23 carried out mass spectrometry using similar source material. Although a number of glycans were identified, galactosyl groups were rare and were suggested to have β-linkages.23 Given that it is the larvae (rather than the adults) that transit through the lungs as part of the Ascaris lifecycle, we speculate that α-Gal may be selectively expressed during the larval stage. This would also be consistent with the idea that the respiratory mucosa may be more favorable for IgE induction than the gastrointestinal tract.
Despite the fact that 32% of the Ecuadorian children were sensitized to α-Gal, nearly 90% of the total cohort, and 83% of those who were sensitized, reported eating mammalian meat at least once a week. This finding is consistent with our clinical experience. We have seen many individuals who have positive α-Gal sIgE test results but nonetheless routinely consume mammalian meat without subjective symptoms. This is also supported by the work of Fischer et al4 in Germany who investigated a cohort with high risk for tick bites, that is, forest workers and hunters. They found that more than 90% of those who were sensitized to α-Gal nonetheless tolerated mammalian meat without overt allergic reactions.4 A relevant question is whether the level of IgE to α-Gal can predict clinical reactivity to mammalian meat. Mabelane et al24 reported on the utility of α-Gal sIgE levels in predicting clinical reactions to mammalian meat in a cohort of children and adults in the Eastern Cape of South Africa. They found a significant overlap in sIgE levels among individuals who reacted and tolerated mammalian meat; however, α-Gal sIgE of 5.5 IU/mL and specific/total IgE ratio of 2.1% were associated with a 95% probability of clinical allergy to mammalian meat.24 In the current report, levels of IgE to α-Gal were lower, and IgE to α-Gal expressed as a percentage of total IgE strikingly lower, in children from Ecuador and Kenya as compared with age-similar children in the United States with AGS. The levels were also less than the cutoffs described in the study from South Africa. It is also important to consider that affinity/avidity of α-Gal sIgE could vary between individuals and/or different populations.
Tolerance is favored in situations with a relatively high ratio of specific IgG4 to specific IgE.25,26 For example, we have previously shown that cow’s milk–tolerant children from an unselected birth cohort had IgG4/IgE ratios to cow’s milk proteins that exceeded 1000:1.27 In the current study, IgG4 to α-Gal was not present at high levels in children from Ecuador and the ratio of α-Gal specific IgG4/IgE was only approximately 20:1, as compared with a ratio of approximately 500:1 for casein. Hence, IgG4 to α-Gal is unlikely to explain tolerance to mammalian meat despite wide-spread IgE sensitization. Nonetheless, IgG4 to α-Gal was higher in the children from Ecuador than in age-similar children from the United States. A logical explanation, and one that is consistent with our understanding that IgG4 results from chronic allergen exposure, is that elevated IgG4 is an indicator of chronic/recurrent exposure to the relevant sensitizing agent.28 This contrasts with the situation in North America, Europe, Japan, and Australia, where the relevant exposure is from occasional tick bites and there is little or no IgG4 to α-Gal.29–31
There are several limitations to this report. The study samples from Ecuador and Kenya were originally recruited to study asthma and information about allergic reactions to food was not collected. Although we do not know how many, if any, subjects from these cohorts experienced allergic reactions to mammalian meat, the data from Ecuador indicate that most children routinely consumed mammalian meat. The current data support our hypothesis that geohelminths contribute to α-Gal sensitization, but cannot exclude a role for tick bites. Additional information about local tick populations and prospective questioning about tick exposures would be required to better address this possibility. We hypothesize that the reason IgG4 levels to α-Gal were higher in children from Ecuador than the United States relates to differences in the nature of the sensitizing exposure, that is, chronic exposure to a geohelminth in Ecuador versus intermittent exposure to tick bites in the United States, but this is indirect evidence. We did not carry out inhibition assays to confirm specificity of IgE to α-Gal, but the results from Sweden and Costa Rica indicate that high levels of total IgE are not sufficient to explain α-Gal sensitization. For the investigation into geohelminth exposures, we focused on parasites in which serologic assays were available. A role for other geohelminths or parasites, especially those that could cross-react with the Ascaris assay, cannot be excluded. However, the relation of α-Gal sIgE was evident for Ascaris but not Toxocara or Strongyloides, which are also nematodes. There is some evidence that Echinococcus or Schistosoma may contain α-Gal glycans, but these helminths are not thought to be of relevance in our study populations.10
Conclusions
IgE to α-Gal was more prevalent among children from rural Ecuador and Kenya living in an area with endemic parasitism than children from an area in the United States where mammalian meat allergy is widely reported. Geohelminth eggs were present in stool samples at a similar frequency among sensitized and nonsensitized children; however, IgE, IgG, and IgG4 to Ascaris were all associated with α-Gal sIgE. To our knowledge, α-Gal has not been reported in Ascaris species, but nonetheless it is likely that Ascaris and/or other endoparasites that are endemic in developing regions contribute to sensitization to this blood-group–like glycan. An intriguing possibility is that IgE to α-Gal represents an adaptive response that evolved in higher primates as a means of recognizing and mounting rapid defense against ecto- and endo-parasites. This hypothesis would be consistent with emerging ideas on the evolutionary role of allergic immunity in defending against parasites and venomous organisms.25,32,33 This hypothesis would also distinguish α-Gal from most other forms of food allergy, where the adaptive benefit of IgE is not clear.
Supplementary Material
Key messages.
The prevalence of α-Gal sIgE in areas of the developing world with endemic parasitism can exceed the prevalence reported in areas of the United States and Europe where tick-acquired mammalian meat allergy is commonly recognized.
The lack of cases of reported mammalian meat allergy in Ecuador and Kenya is likely explained by the fact that levels of IgE specific for α-Gal are low, particularly in relation to total IgE.
An association between antibodies to Ascaris and α-Gal sIgE, and also higher levels of IgG4 to α-Gal, suggests that chronic exposure to Ascaris contributes to sensitization in areas where this helminth is endemic.
Acknowledgments
This study was supported by the Wellcome Trust grant (grant no. 088862/Z/09/Z to P.C.), National Institutes of Health (grant no. R37 AI-20565 to T.P.-M.) and AAAAI Faculty Development Award (J.W.).
Disclosure of potential conflict of interest:
J. Wilson has received research support from Thermo-Fisher/Phadia. T. A. E. Platts-Mills has a patent on an IgE assay to α-Gal and has received assay support from Thermo-Fisher/Phadia. The rest of the authors declare that they have no relevant conflicts of interest.
Abbreviations used
- α-Gal
Galactose-α-1,3-galactose
- AGS
α-Gal syndrome
- IRB
Institutional Review Board
- sIgE
Specific IgE
- UVA
University of Virginia
REFERENCES
- 1.Platts-Mills TAE, Commins SP, Biedermann T, van Hage M, Levin M, Beck LA, et al. On the cause and consequences of IgE to galactose-alpha-1,3-galactose: a report from the National Institute of Allergy and Infectious Diseases Workshop on Understanding IgE-Mediated Mammalian Meat Allergy. J Allergy Clin Immunol 2020;145:1061–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stoltz LP, Cristiano LM, Dowling APG, Wilson JM, Platts-Mills TAE, Traister RS. Could chiggers be contributing to the prevalence of galactose-alpha-1,3-galactose sensitization and mammalian meat allergy? J Allergy Clin Immunol Pract 2019;7:664–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Commins SP, James HR, Kelly LA, Pochan SL, Workman LJ, Perzanowski MS, et al. The relevance of tick bites to the production of IgE antibodies to the mammalian oligosaccharide galactose-alpha-1,3-galactose. J Allergy Clin Immunol 2011;127:1286–93.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fischer J, Lupberger E, Hebsaker J, Blumenstock G, Aichinger E, Yazdi AS, et al. Prevalence of type I sensitization to alpha-gal in forest service employees and hunters. Allergy 2017;72:1540–7. [DOI] [PubMed] [Google Scholar]
- 5.Arkestal K, Sibanda E, Thors C, Troye-Blomberg M, Mduluza T, Valenta R, et al. Impaired allergy diagnostics among parasite-infected patients caused by IgE antibodies to the carbohydrate epitope galactose-alpha 1,3-galactose. J Allergy Clin Immunol 2011;127:1024–8. [DOI] [PubMed] [Google Scholar]
- 6.Yilmaz B, Portugal S, Tran TM, Gozzelino R, Ramos S, Gomes J, et al. Gut micro-biota elicits a protective immune response against malaria transmission. Cell 2014; 159:1277–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avila JL, Rojas M, Galili U. Immunogenic Gal alpha 1——3Gal carbohydrate epitopes are present on pathogenic American Trypanosoma and Leishmania. J Immunol 1989;142:2828–34. [PubMed] [Google Scholar]
- 8.Wuhrer M, Grimm C, Dennis RD, Idris MA, Geyer R. The parasitic trematode Fasciola hepatica exhibits mammalian-type glycolipids as well as Gal(beta1-6)Gal-terminating glycolipids that account for cestode serological cross-reactivity. Glycobiology 2004;14:115–26. [DOI] [PubMed] [Google Scholar]
- 9.Duffy MS, Morris HR, Dell A, Appleton JA, Haslam SM. Protein glycosylation in Parelaphostrongylus tenuis–first description of the Galalpha1-3Gal sequence in a nematode. Glycobiology 2006;16:854–62. [DOI] [PubMed] [Google Scholar]
- 10.Hodzic A, Mateos-Hernandez L, Frealle E, Roman-Carrasco P, Alberdi P, Pichavant M, et al. Infection with Toxocara canis inhibits the production of IgE antibodies to alpha-Gal in humans: towards a conceptual framework of the hygiene hypothesis? Vaccines (Basel) 2020;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooper PJ, Chico ME, Vaca MG, Rodriguez A, Alcantara-Neves NM, Genser B, et al. Risk factors for asthma and allergy associated with urban migration: background and methodology of a cross-sectional study in Afro-Ecuadorian school children in Northeastern Ecuador (Esmeraldas-SCAALA Study). BMC Pulm Med 2006;6:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Endara P, Vaca M, Platts-Mills TA, Workman L, Chico ME, Barreto ML, et al. Effect of urban vs. rural residence on the association between atopy and wheeze in Latin America: findings from a case-control analysis. Clin Exp Allergy 2015;45:438–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beck HE, Zimmermann NE, McVicar TR, Vergopolan N, Berg A, Wood EF. Present and future Koppen-Geiger climate classification maps at 1-km resolution. Sci Data 2018;5:180214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perzanowski MS, Ng’ang’a LW, Carter MC, Odhiambo J, Ngari P, Vaughan JW, et al. Atopy, asthma, and antibodies to Ascaris among rural and urban children in Kenya. J Pediatr 2002;140:582–8. [DOI] [PubMed] [Google Scholar]
- 15.Wilson JM, Schuyler AJ, Workman L, Gupta M, James HR, Posthumus J, et al. Investigation into the alpha-Gal syndrome: characteristics of 261 children and adults reporting red meat allergy. J Allergy Clin Immunol Pract 2019;7:2348–58.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kennedy JL, Shaker M, McMeen V, Gern J, Carper H, Murphy D, et al. Comparison of viral load in individuals with and without asthma during infections with rhinovirus. Am J Respir Crit Care Med 2014;189:532–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kennedy JL, Stallings AP, Platts-Mills TA, Oliveira WM, Workman L, James HR, et al. Galactose-alpha-1,3-galactose and delayed anaphylaxis, angioedema, and urticaria in children. Pediatrics 2013;131:e1545–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schuyler AJ, Wilson JM, Tripathi A, Commins SP, Ogbogu PU, Kruzsewski PG, et al. Specific IgG4 antibodies to cow’s milk proteins in pediatric patients with eosinophilic esophagitis. J Allergy Clin Immunol 2018;142:139–48.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mendonca LR, Veiga RV, Dattoli VC, Figueiredo CA, Fiaccone R, Santos J, et al. Toxocara seropositivity, atopy and wheezing in children living in poor neighbourhoods in urban Latin American. PLoS Negl Trop Dis 2012;6:e1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soto-Quiros M, Avila L, Platts-Mills TA, Hunt JF, Erdman DD, Carper H, et al. High titers of IgE antibody to dust mite allergen and risk for wheezing among asthmatic children infected with rhinovirus. J Allergy Clin Immunol 2012;129:1499–505.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perzanowski MS, Ronmark E, James HR, Hedman L, Schuyler AJ, Bjerg A, et al. Relevance of specific IgE antibody titer to the prevalence, severity, and persistence of asthma among 19-year-olds in northern Sweden. J Allergy Clin Immunol 2016;138:1582–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson JM, Keshavarz B, Retterer M, Workman LJ, Schuyler AJ, McGowan EC, et al. A dynamic relationship between two regional causes of IgE-mediated anaphylaxis: alpha-Gal syndrome and imported fire ant. J Allergy Clin Immunol 2021;147:643–52.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poltl G, Kerner D, Paschinger K, Wilson IB. N-glycans of the porcine nematode parasite Ascaris suum are modified with phosphorylcholine and core fucose residues. FEBS J 2007;274:714–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mabelane T, Basera W, Botha M, Thomas HF, Ramjith J, Levin ME. Predictive values of alpha-gal IgE levels and alpha-gal IgE: total IgE ratio and oral food challenge-proven meat allergy in a population with a high prevalence of reported red meat allergy. Pediatr Allergy Immunol 2018;29:841–9. [DOI] [PubMed] [Google Scholar]
- 25.Wilson JM, Platts-Mills TAE. alpha-Gal and other recent findings that have informed our understanding of anaphylaxis. Ann Allergy Asthma Immunol 2020;124:135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aalberse RC, Platts-Mills TA, Rispens T. The developmental history of IgE and IgG4 antibodies in relation to atopy, eosinophilic esophagitis, and the modified TH2 response. Curr Allergy Asthma Rep 2016;16:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson JM, Workman L, Schuyler AJ, Rifas-Shiman SL, McGowan EC, Oken E, et al. Allergen sensitization in a birth cohort at midchildhood: focus on food component IgE and IgG4 responses. J Allergy Clin Immunol 2018;141:419–23.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGowan EC, Platts-Mills TAE, Wilson JM. Food allergy, eosinophilic esophagitis, and the enigma of IgG4. Ann Allergy Asthma Immunol 2019;122:563–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rispens T, Derksen NI, Commins SP, Platts-Mills TA, Aalberse RC. IgE production to alpha-gal is accompanied by elevated levels of specific IgG1 antibodies and low amounts of IgE to blood group B. PLoS One 2013;8:e55566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kollmann D, Nagl B, Ebner C, Emminger W, Wohrl S, Kitzmuller C, et al. The quantity and quality of alpha-gal-specific antibodies differ in individuals with and without delayed red meat allergy. Allergy 2017;72:266–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Apostolovic D, Rodrigues R, Thomas P, Starkhammar M, Hamsten C, van Hage M. Immunoprofile of alpha-Gal- and B-antigen-specific responses differentiates red meat-allergic patients from healthy individuals. Allergy 2018;73:1525–31. [DOI] [PubMed] [Google Scholar]
- 32.Palm NW, Rosenstein RK, Medzhitov R. Allergic host defences. Nature 2012;484:465–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galli SJ, Starkl P, Marichal T, Tsai M. Mast cells and IgE in defense against venoms: possible “good side” of allergy? Allergol Int 2016;65:3–15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


