Abstract
G protein-coupled receptors (GPCRs) are implicated in the regulation of fear and anxiety. GPCR signaling involves canonical G protein pathways but can also engage downstream kinases and effectors through scaffolding interactions mediated by β-arrestin. Here, we investigated whether β-arrestin signaling regulates anxiety-like and fear-related behavior in mice in response to activation of the GPCR δ-opioid receptor (δOR or DOR). Applying β-arrestin–biased δOR agonists to mouse brain slices revealed β-arrestin 2–dependent activation of extracellular signal–regulated kinases 1 and 2 (ERK1/2) in the dorsal hippocampus and amygdala and β-arrestin 1–dependent activation of ERK1/2 in the nucleus accumbens. In mice, β-arrestin–biased agonist treatment was associated with reduced anxiety-like and fear-related behaviors, with some overlapping and isoform-specific input. In contrast, applying a G-protein–biased δOR agonist decreased ERK1/2 activity in all three regions as well as the dorsal striatum, and was associated with increased fear-related behavior without effects on baseline anxiety. Our results indicate a complex picture of δOR neuromodulation in which β-arrestin 1- and 2-dependent ERK signaling in specific brain subregions suppresses behaviors associated with anxiety and fear and opposes the effects of G protein-biased signaling. Overall, our findings highlight the importance of noncanonical β-arrestin–dependent GPCR signaling in the regulation of these interrelated emotions.
Introduction
Anxiety- and fear-related behaviors are evolutionary adaptive behaviors important for human survival. However, aberrant stimulation of the neural circuits that regulate such behaviors can manifest as psychiatric disorders, such as post-traumatic stress disorder and phobias. The expression of anxiety- and fear-related behaviors is regulated by complex integration of both internal and external physiological and sensory cues that influence reflexive behavior, cognitive control, and executive functions. Accordingly, behavioral correlates of anxiety and fear are regulated by a harmonious activity of neurotransmitters and cellular actions across many overlapping and distinct neural circuits (1).
With regard to cellular actions underlying physiological and pathophysiological behavior, G protein-coupled receptors (GPCRs) play an important role in neuronal signaling. GPCRs bind neurotransmitters and initiate intracellular signal transduction pathways which ultimately affect neuronal excitability, neurotransmitter release and synaptic plasticity. GPCRs—including serotonergic (2), dopaminergic (3), adrenergic (4), opioidergic (5) and corticotropin-releasing factor receptors (6)—have well-documented roles in the modulation of anxiety and fear and thus are attractive drug targets for treating anxiety disorders.
Although drug development at GPCRs has traditionally focused on the canonical G protein pathways, the prior two decades have introduced arrestin-dependent signaling as a new concept of GPCR signal transduction. In particular, the “non-visual” arrestins 2 and 3 (referred to here as β-arrestin 1 and 2, respectively) have been studied for their ability to drive G-protein–independent signaling (7). The primary role of β-arrestins is to desensitize GPCRs; for example, increased β-arrestin 2 expression in the mouse amygdala, as is caused by HIV1-tat infection, leads to significant reduction in morphine efficacy in this region (8). Beyond desensitization, β-arrestin 2 may also partake in receptor signaling by scaffolding with various kinases. For example, β-arrestin 2 to p38 mitogen-activated protein kinase (MAPK) signaling has been linked to the aversive effects of κ-opioid receptor (κOR) agonists (5, 9), whereas a scaffold comprised of β-arrestin 2 and the kinases GSK3β and AKT appears to mediate the antipsychotic effects of dopamine D2 receptor agonists (10, 11). However, to date, few studies have investigated how signaling scaffolds involving the β-arrestin isoforms may influence anxiety- and fear-like behavior. It is important to begin to address this gap in our current knowledge of the GPCR modulation of psychiatric behavior, especially since most medications that target GPCRs were developed without consideration of the potential adverse or therapeutic effects of β-arrestin signaling. Yet, it is now possible to develop molecules that preferentially activate or avoid β-arrestin signaling and, thus, have the potential to treat psychiatric disorders more effectively and with a wider therapeutic window (12, 13).
Here, we explored the roles of β-arrestin isoforms in mediating GPCR signaling in relation to the modulation of anxiety and fear-like behavior. For this study, we chose to utilize the δ-opioid receptor (δOR, also known as DOR) as a model GPCR for multiple reasons. Previous studies have shown that the δOR-selective agonist SNC80 effectively induces the recruitment of β-arrestins 1 and 2 (14-16) and has anxiolytic-like (17, 18) and fear-reducing effects (17, 19). Moreover, the δOR-selective agonist TAN67, which is a poor recruiter or β-arrestin 2, does not reduce anxiety-like behavior in naïve mice (20), providing support for our hypothesis that β-arrestin 2 signaling is correlated with anxiolytic-like effects.
MAPKs have been implicated in mood disorders and can scaffold with β-arrestin (21, 22). Studies have suggested that MAPK signaling, specifically ERK1/2, in the hippocampus and the basolateral amygdala is required for the acquisition and extinction of fear memory (23, 24). Therefore, we further hypothesize that β-arrestin–dependent MAPK signaling may contribute to anxiety-like and fear-related behavior. To test our hypotheses, we assessed the degree to which β-arrestin isoforms and MAPK activation were involved in δOR agonist-mediated modulation of unconditioned anxiety-related behavior and cued-induced, fear-related behavior. Our results suggest that ERK1/2 activity is differentially modulated by G protein and β-arrestin signaling and is correlated with anxiety-like and fear-related responses in C57BL/6 mice. Notably, different β-arrestin isoforms were involved in the activation of ERK1/2 across various brain regions, including the dorsal striatum, hippocampus, and amygdala.
Results
Involvement of β-arrestin 2 in the modulation of anxiety-like behavior
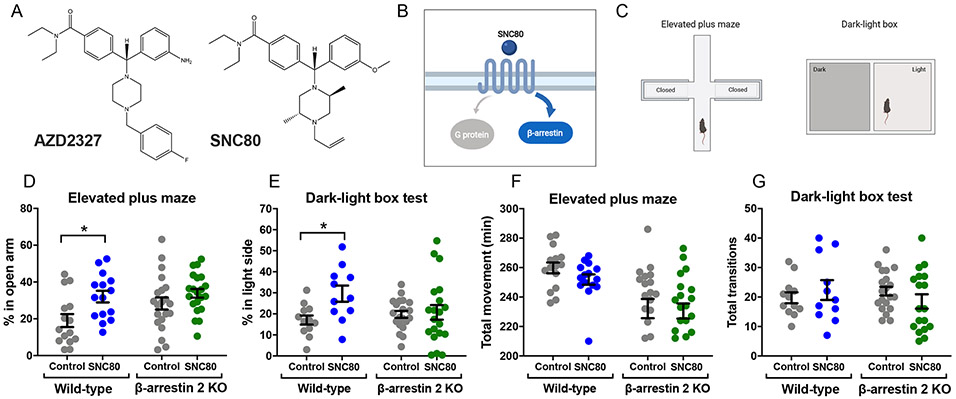
In 2016, Astra Zeneca revealed that their novel δOR-selective agonist AZD2327 (Fig. 1A) reduced anxiety-like behavior in mice (25). AZD2327 is not commercially available; however, it is structurally similar to the commercially available δOR-selective agonist SNC80 (Fig. 1A), a known “super-recruiter” of β-arrestin 2 (14) (Fig. 1B) that, similar to AZD2327, exhibits anxiolytic-like effects in rodents (17, 20). These previous findings led us to hypothesize that β-arrestin 2 may be required for the anxiolytic effects of SNC80 and AZD2327. Using two model tests of anxiety-like behavior—the elevated plus maze (EPM) test and dark-light box transition test (Fig. 1C), we measured the behavioral effects of SNC80 in β-arrestin 2 KO mice at a dose known to produce anxiolytic-like effects in wild-type (WT) mice (20). As expected, peripheral administration of SNC80 through subcutaneous injection (s.c., at 20 mg/kg) increased the time WT mice spent in the open arm of the elevated plus maze (Fig. 1D and table S1) and the light chamber of dark-light transition box (Fig. 1E and table S1). As we predicted, the anxiolytic effects of SNC80 were attenuated in β-arrestin 2 KO mice (Fig. 1, D and E, and table S1). Although the total movement in the elevated plus maze was slightly lower in β-arrestin 2 KO mice than WT mice, no drug effects were observed in both genotypes (Fig. 1F and table S1). Likewise, no statistical difference in total transition was observed in the dark-light transition box test (Fig. 1G and table S1). However, as also previously described (14), SNC80 produced hyperlocomotive behavior in mice (fig. S1 and table S1).
Figure 1. Beneficial role for β-arrestin 2 in reducing anxiety-like behavior.
(A) Chemical structures of AZD2327 (a δOR agonist used in phase II clinical trials for anxious major depressive disorder) and SNC80. (B) Schematic depicting SNC80 as a δOR selective agonist that activates Gi proteins but also strongly recruits β-arrestin 2. (C) Schematic of the elevated plus maze test and dark-light box test. (D) Percentage of time spent in open arms pf the maze by WT mice (Control: N = 15, SNC80: N = 15) and β-arrestin 2 KO mice (Control: N = 21, SNC80: N = 21) 30 minutes after subcutaneous administration of β-arrestin–biased δOR agonist SNC80 (20 mg/kg). (E) Percentage of time spent in the light box by WT mice (Control: N = 12, SNC80: N = 11) and β-arrestin 2 KO mice (Control: N = 20, SNC80: N = 20) 30 minutes after subcutaneous administration of SNC80 (20 mg/kg). (F and G) Total movent time as recorded for the control and SNC80-treated mice in the elevated plus maze (F) and dark-light transition box (G) described in (D) and (E), respectively. See table S1 for two-way ANOVA and post-hoc multiple comparisons.
The β-arrestin recruiting δOR agonist SNC80 strongly activates ERK1/2 in vitro and in vivo
Activation of κOR has been associated with β-arrestin 2-mediated p38 phosphorylation (9). To determine if δOR agonism similarly stimulates MAPKs, we measured p38, JNK, and ERK1/2 activation in Chinese hamster ovary cells stably expressing δOR and β-arrestin 2 (CHO-δOR-βArr2) following stimulation with 10 μM SNC80, a concentration that will fully activate G-protein signaling and induce β-arrestin 2 recruitment (26). We found that SNC80 led to a rapid increase in ERK1/2 activation within 3 minutes in CHO-δOR-βArr2 cells, which lasted until 60 minutes (Fig. 2A), in agreement with previous δOR-mediated ERK activation in CHO cells (27). We did not observe strong activation of p38 and JNK by SNC80 (Fig. 2A). The δOR mediated ERK1/2 signaling in these cells was not an artifact of the recombinant overexpression of δOR and β-arrestin 2 in the CHO cells as we observed a comparable profile for ERK1/2 activation in NG108-15 neuroblastoma cells endogenously expressing δOR and β-arrestin (28-30) (Fig. 2B). We similarly found ERK1/2 activation in several mouse brain regions, known to express δORs, including the dorsal hippocampus, the amygdala and the dorsal striatum (31, 32) (Fig. 2C). The SNC80-induced ERK1/2 activation in these regions was confirmed and quantified by the Western blot analysis of flash-frozen tissue punches upon collection (Fig. 2D). Here, we observed that SNC80 (20 mg/kg, i.p.) significantly increased ERK1/2 activation at the 10-minute time point in all tested brain regions except for the ventral hippocampus of WT mice (Fig. 2, E to I, and table S2), and these activations returned to basal levels by the 30-minute time point. The SNC80-induced ERK1/2 activation was not observed in the dorsal hippocampus and the amygdala of δOR KO mice (fig. S2), further confirming this unique profile of ERK1/2 activation by SNC80 is mediated by δOR.
Figure 2. The anxiolytic agonist SNC80 activates ERK1/2 in vitro and in the limbic and striatal regions of the brain.
(A) Time course analysis of the phosphorylation of ERK1/2, p38, and JNK in CHO cells stably expressing δOR and β-arrestin 2 and stimulated with 10 μM SNC80. Data are from N = 3 experiments; a representative blot is shown, right. (B) Time course analysis of the phosphorylation of ERK1/2 in NG108-15 cells that endogenously express δOR and were stimulated with 10 μM SNC80. Data are from N = 3 experiments; a representative blot is shown, right. (C) Immunofluorescence staining for ERK (green), phosphorylated ERK (red), and nuclear DNA marker DAPI (blue) in limbic and striatal regions of WT mouse brain tissues that were perfused 10 min after i.p. administration with saline or SNC80 (20 mg/kg). 4x-Magnification images (left; scale bars, 1000 μm) were used to stitch together the whole brain-slice images shown. Enlarged images (right; scale bars, 100 μm) are 20x magnification that correspond to the regions marked (white boxes) in the 4x images. (D) Representative images of brain sections with micropunctures of five specified brain regions for the Western blot analysis. (E to I) Western blot analysis of the activation of ERK1/2 at 10 and 30 min after systemic administration of saline (Con) or SNC80 in five brain regions from WT mice (N = 3 to 13 mice; see table S2). Representative images below the respective bar graph. See table S2 for one-way and two-way ANOVA and post-hoc multiple comparisons.
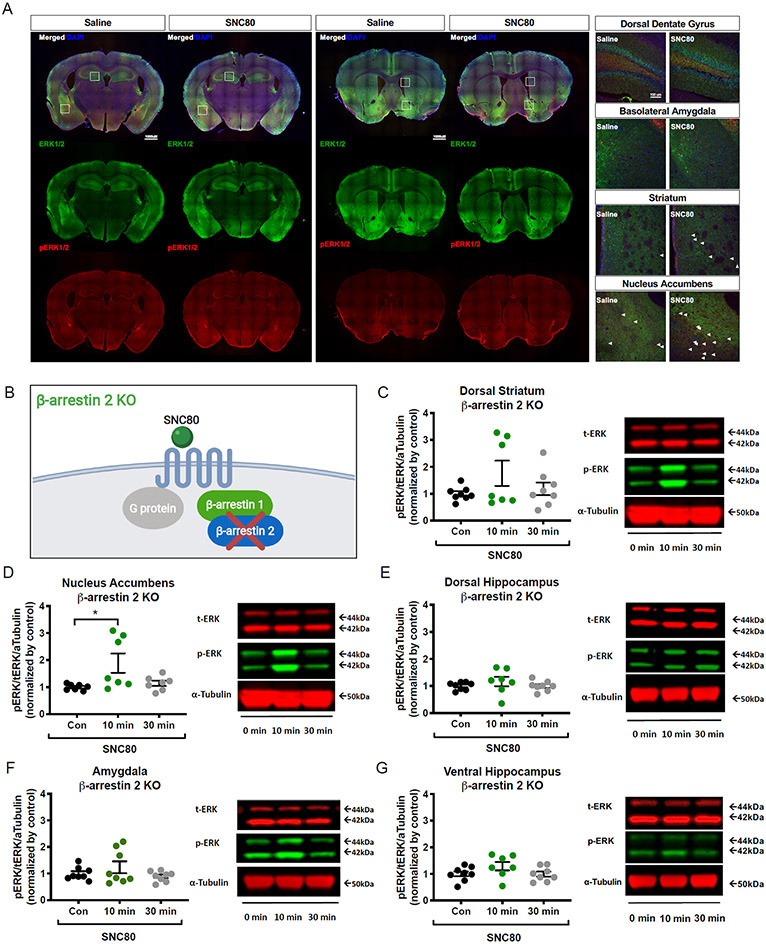
β-arrestin 2 is required to activate ERK1/2 signaling in the limbic structures of the brain
To determine if β-arrestin 2 is responsible for the ERK1/2 activation observed with SNC80 in WT mice, we quantified ERK1/2 activation across the same hippocampal, striatal and amygdalar regions of β-arrestin 2 KO mice upon systemic administration of SNC80 (20 mg/kg, i.p.; Fig. 3, A and B). In these β-arrestin 2 KO mice, SNC80 induced the activation of ERK1/2 in a statistically significant manner only in the nucleus accumbens (Fig. 3, C to G and table S2).
Figure 3. ERK1/2 activation in the amygdala and the dorsal hippocampus are β-arrestin 2 dependent.
(A) for ERK (green), phosphorylated ERK (red), and nuclear DNA marker DAPI (blue) in limbic and striatal regions of β-arrestin 2 KO mouse brain tissues that were perfused 10 min after i.p. administration with saline or SNC80 (20 mg/kg). 10x-Magnification images (left; scale bars, 1000 μm) were used to stitch together the whole brain-slice images shown. Enlarged images (right; scale bars, 100 μm) are 20x magnification that correspond to the regions marked (white boxes) in the 10x images. (B) Schematic of the cellular context in β-arrestin 2 KO mice. (C to G) Blotting analysis of SNC80-induced ERK1/2 activation in the dorsal striatum (C), nucleus accumbens (D), dorsal hippocampus (E), amygdala (F), and ventral hippocampus (G) at 10 and 30 minutes after i.p. administration with SNC80 (20 mg/kg). Con, 0 min: saline administered. Data are from N = 7 to 8 mice; see table S2. Representative blots are shown to the right of the related bar graph. See table S2 for one-way ANOVA and post-hoc multiple comparisons.
ERK1/2 signaling plays a key role in the SNC80-mediated anxiolytic-like effects
We next assessed if the anxiolytic effects of SNC80 were dependent on ERK1/2 activation. We administered WT mice with SL327, a MEK1/2 inhibitor that indirectly prevents ERK1/2 activation (Fig. 4A) (33). We found that pre-administration of SL327 (50 mg/kg, s.c.) ablated the anxiolytic-like effects of SNC80 (20 mg/kg, s.c.) in WT mice (Fig. 4B and table S3). The hippocampus is a brain region associated with anxiety-like behavior in the elevated plus maze (34) and δOR-agonism in the dorsal hippocampus, and the amygdala is associated with reduced anxiety-like behavior in the open field test (18, 35). These published findings agree with our observation of SNC80-induced β-arrestin 2-dependent ERK1/2 activity specifically in these two brain regions (Fig. 3, E and F). Therefore, if the β-arrestin-mediated ERK1/2 signaling in these two regions was critical for the anxiolytic-like effects of SNC80, we would expect ERK1/2 activity to be abolished in these regions in the mice with SNC80 (20 mg/kg, i.p.) and SL327 (50 mg/kg, s.c.). Indeed, we found that SL327 effectively decreased SNC80-induced ERK1/2 activity in the dorsal hippocampus and the amygdala (Fig. 4, C and D, and table S3), but not in the dorsal striatum, the nucleus accumbens, and the ventral hippocampus (fig. S3 and table S3). As an additional approach to investigate the importance of β-arrestin 2 signaling on ERK1/2 signaling and anxiety-like behavior, we utilized a δOR agonist that is a weaker β-arrestin recruiter than SNC80. Specifically, we utilized ADL5859, which affinity (Ki = 0.84 nM) and G-protein potency (EC50=20 nM) at δOR is not significantly different from SNC80 (Ki = 1.2 nM, EC50=10 nM, (36)), but has 50-fold lower potency (pEC50=6.6±0.1, fig. S4A) and slightly lower efficacy (Emax = 119% ±5, relative to Leu-enkephalin fig. S4A) than SNC80 (pEC50=8.2±0.1, Emax =142%±9, (14)). Systemic administration of ADL5859 (30 mg/kg, p.o.) at a dose that produces robust δOR-mediated analgesia (37) did not result in ERK1/2 activation in the dorsal hippocampus and the amygdala (fig. S4, B and C) nor reduce anxiety-like behavior (fig. S4D), suggesting that ERK1/2 activation in the dorsal hippocampus and the amygdala as well as δOR-mediated anxiolytic effects require a strong recruitment of β-arrestin 2.
Figure 4. ERK1/2 is required for the anxiolytic-like effects induced by SNC80.
(A) Schematic of SL327-induced inhibition of SNC80-mediated ERK1/2 signaling. (B) Time spent in the light side of the dark-light-transition box by WT mice administered SL327 (50 mg/kg, s.c.) 60 min before testing, and SNC80 (20 mg/kg i.p.) administered 30 min before testing. Control, N = 8; SNC80, N = 12; SNC+SL, N = 12; and SL327, N = 12 mice. (C and D) Blotting analysis of SNC80-induced ERK1/2 phosphorylation in the dorsal hippocamus and the amygdala in WT mice administered SL327 (50 mg/kg, s.c.) 40 min before collection and SNC80 (20 mg/kg i.p.) 10 min before perfusion. Data are from N = 9 to 10 mice (see table S3). Note that the SNC80 dataset here includes part of the data collected for the 0 and 10 min SNC80 shown in Fig. 2, panels G and H. See table S3 for one-way ANOVA and post-hoc multiple comparisons.
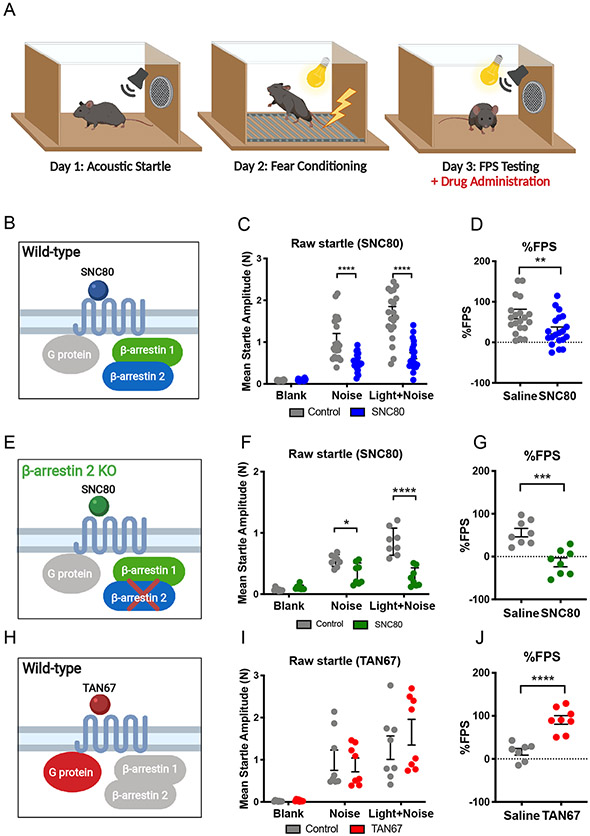
Fear-potentiated startle behavior is alleviated by β-arrestin–biased δOR agonism but facilitated by G protein-biased δOR agonism
Besides reducing anxiety-like behavior, δOR activation can also alleviate conditioned fear-related behavior (17, 38). Based on our results and previous studies, we hypothesized that SNC80 may also reduce fear-related behavior through a mechanism that involves β-arrestin 2. To measure conditioned fear, we utilized a mouse behavior paradigm of fear-potentiated startle (FPS) (Fig. 5A). Mice were grouped in a counterbalanced manner, such that no statistically significant differences between startle reflexes were observed between groups (fig. S5). In WT mice (Fig. 5B), SNC80 (20 mg/kg, i.p.) significantly reduced startle responses to the unconditioned ‘noise’ cue as well as to the conditioned ‘light plus noise’ cue (Fig. 5C and table S4). The reduction produced by ‘light plus +noise’ is larger than the reduction produced by ‘noise’ and resulting in a significant reduction in % FPS response (Fig. 5D). We also assessed whether SNC80 would alter % FPS at a dose of 10 mg/kg (i.p.) in WT mice (fig. S6A; groups were assigned based on counterbalanced baseline startle as shown in fig. S6B), as this dose of SNC80 has been shown to be effective in other studies (17, 39); however, in our hands we did not observe any significant effects in WT mice (fig. S6, C and D). To our surprise, we found that SNC80 (20 mg/kg i.p.) was equally effective in reducing % FPS responses in β-arrestin 2 KO mice as we had observed in WT mice (Fig. 5, E to G, and table S4). While SNC80 is an efficacious recruiter of β-arrestin, it still also activates Gi protein signaling (14, 26). Thus, we next hypothesized that the observed fear-reducing effects of SNC80 could be mediated through Gi protein signaling. To address this hypothesis, we utilized the δOR selective agonist TAN67, which is a poor recruiter of β-arrestin and is considered Gi protein-biased (14, 26). When we administered TAN67 (25 mg/kg, i.p.) to our WT mice (Fig. 5H) did not significantly change the raw startle to the ‘noise’ or to the ‘light+noise’ cue (Fig. 5I and table S4). Yet, the ratio of the raw startle response to these two cues resulted in a significant increase in % FPS (Fig. 5J). The lack of a direct effect of TAN67 on noise-alone startle is in agreement with our previous finding that TAN67 does not change basal anxiety-like behavior in the elevated plus maze and dark-light transition test (20). Our Western blot analysis of ERK1/2 activities in WT mice revealed that TAN67 decreased basal ERK1/2 activity in the dorsal striatum, the nucleus accumbens, the dorsal hippocampus, and the amygdala (Fig. 6, A to D). In the ventral hippocampus, however, TAN67 did not alter ERK1/2 activity (Fig. 6E), which was similar to the lack of ERK1/2 modulation by SNC80 in this region (described above; Fig. 2I) and may be indicative of low δOR expression in the ventral region (40).
Figure 5. Modulation of fear-potentiated startle (FPS) by β-arrestin–biased and G protein-biased δOR agonists.
(A) Schematic of the experimental paradigm of the FPS test, wherein drugs were administered prior to the tests on the third day. See fig. S5 for the Day 1 acoustic startle test. (B to G) Schematics of the conditions (B and C) in which raw startle amplitudes to “noise” and “light+noise” and % FPS during the test diagrammed in (A) were measured in WT mice (C and D; control, N = 21; SNC80, N = 21) and β-arrestin 2 KO mice (F and G; control, N = 8; SNC80, N = 8) 20 to 30 min after i.p. administration of 20 mg/kg SNC80. (H to J) As described in (B to G), in WT mice 30 to 40 min after i.p. administration of 25 mg/kg TAN-67 (control, N = 8; TAN-67, N = 8). See table S4 for two-way ANOVA and post-hoc multiple comparisons.
Figure 6. Differential roles for G protein and β-arrestin 1 in δOR agonist-induced ERK1/2 activation.
(A to E) Schematic of the experimental conditions (A) and Western blotting analysis for ERK1/2 activation in the dorsal straitum (A), nucleus accumbens (B), dorsal hippocampus (C), amygdala (D) and ventral hippocampus (E) of WT mice 10 and 30 min after i.p. adminstration of 25 mg/kg TAN67 compared with that after saline (Con). (F to J) As described in (A to E), in β-arrestin 1 KO mice administered SNC80 (20 mg/kg) or saline. Data are from N = 6 to 7 mice (see table S5); a representative blot is beside the associated bar graph. See table S5 for one-way ANOVA and post-hoc multiple comparisons.
Deletion of one single β-arrestin isoform is not sufficient to abolish the inhibition of conditioned-fear behavior by β-arrestin–biased δOR agonism.
The decrease in ERK1/2 activation, combined with increased FPS response produced by the G protein-biased agonist TAN67, stands in stark contrast with the observed increase in ERK1/2 activation and decreased FPS response by SNC80. However, since our results suggest that SNC80 reduces conditioned fear through a mechanism that does not involve β-arrestin 2 signaling, we next hypothesized that the effect may be mediated instead by β-arrestin 1. SNC80 is a potent and efficacious recruiter of β-arrestin 1 in vitro, particularly compared to TAN67, which recruits β-arrestin1 with much lower efficacy (fig. S7). However, SNC80 is known to induce severe seizures in β-arrestin1 KO mice (16). Indeed, injection of SNC80 (20 mg/kg, i.p.) into β-arrestin1 KO mice produced notable tonic seizures. In comparison to WT mice (described above, Fig. 2, E to H), genetic knockout of β-arrestin 1 prevented SNC80 (20 mg/kg i.p.)-mediated activation of ERK1/2 in the dorsal striatum and nucleus accumbens (Fig. 6, F and G, and table S5), but not in the amygdala and dorsal hippocampus (Fig. 6, H and I). Additionally, we observed a prolonged ERK1/2 activation in the ventral hippocampus (Fig. 6J), a region where no δOR agonist-mediated ERK1/2 activation was observed in WT mice (described above; Figs. 2I and 6E), suggesting this pattern of ERK1/2 activity in the hippocampus may not be exclusively mediated by δOR and is most likely a result of seizure activity. Indeed, the hippocampus has previously been reported as the main site for the control of tonic-clonic seizure mediated by δOR (41). The pronounced seizure activity with 20 mg/kg SNC80 in β-arrestin 1 KO mice prevented us from testing the FPS response at this dose. Instead, we tested FPS response in β-arrestin 1 KO mice with a 10 mg/kg SNC80 (i.p.) dose, because at this dose seizure activity was noticeably reduced, and we also did not observe robust ERK1/2 activation in the hippocampus (fig. S8A). Here, β-arrestin 1 KO mice groups were first assigned based on counterbalanced baseline startle (fig. S6, E and F). While 10 mg/kg SNC80 did not significantly alter % FPS in this strain (fig. S6H), SNC80 reduced the raw startle response to ‘noise’ and ‘light+noise’ cue (fig. S6G and table S4), similar to those seen in WT and β-arrestin 2 KO mice when i.p.-injected with 20 mg/kg SNC80 (described above; Fig. 5, C and F). Furthermore, in contrast to ERK1/2 activation patterns in the dorsal striatum and nucleus accumbens of β-arrestin 1 KO mice in response to i.p. injection with 20 mg/kg SNC80, we detected a statistically significant increase in ERK1/2 activation in the dorsal striatum, but not the nucleus accumbens, of these mice in response to i.p. injection of 10 mg/kg SNC80 (fig. S8, B and C, and table S5), suggesting that β-arrestin 1 may be required for ERK1/2 activation in the nucleus accumbens. The observation of residual fear-reducing effects for SNC80 in each of the β-arrestin knockout strains could indicate that either isoform may be able to compensate for the absence of the other isoform in the mechanism of modulating fear-related behaviors.
Discussion
The β-arrestin proteins were discovered in quick succession (42, 43), but surprisingly, and despite the availability of genetic KO mice for each isoform (44, 45), studies investigating β-arrestin in the central nervous system (CNS) had for some time largely focused on β-arrestin 2 (46-49). More recent research has shown the importance of studying both β-arrestin isoforms for their differential roles in mediating the biochemical, neurological, and behavioral effects of GPCR signaling (of δOR and others) (15, 50). Here, using G protein- or β-arrestin–biased δOR agonists with β-arrestin isoform-selective knockout mice, we discovered that G proteins, β-arrestin 1, and β-arrestin 2 uniquely modulated ERK1/2 activity that resulted in differential effects on anxiety- and fear-related behaviors in mice (Fig. 7). The data suggest that there are both isoform-specific roles and, in the case of regulating fear-related behavior, possibly functionally compensatory roles between the β-arrestin isoforms. Because the β-arrestin–biased δOR agonist used here (SNC80) produces severe seizures at certain doses in β-arrestin 1 KO mice (16), it is uncertain to what degree β-arrestin 1–mediated ERK1/2 is directly involved in the fear-reducing effects. Thus, further investigation of the roles of ERK1/2 in the nucleus accumbens and β-arrestin1 in neuropsychiatric behavior would be required using a conditional knockout approach. Unfortunately, while conditional β-arrestin 2 knockout mice exist (51), no conditional β-arrestin1 knockout mice had been reported. Nonetheless, our results begin to reveal the complex- and context-specific nature of GPCR biased signaling in modulation of fear-related and anxiety-like behavior. These results expand our current understanding of therapeutic effects of β-arrestin signaling in mood disorders, which ultimately may aid the development of more efficacious pharmacological treatment options for these disorders.
Figure 7. Graphical summary.
δOR agonists activate downstream signaling through G protein- and/or β-arrestin-mediated pathway. Our study discovered that the β-arrestin 2 isoform mediates anxiolytic-like effects in mice through ERK1/2 activation in limbic regions of the brain (dorsal hippocampus and amygdala; blue). Notably, both isoforms of β-arrestin appear to be involved in reducing fear-related behaviors and have compensatory function (green), which may be mediated by ERK1/2 activation. G protein-mediated pathways appear to suppress ERK1/2 activation and increase fear-related behaviors (red), suggesting opposing effects between G-protein–biased and β-arrestin–biased signaling in modulating fear-associated behaviors.
Processing and executing emotional behaviors in tasks such as the elevated plus maze and FPS tests engages multiple overlapping, yet distinct, brain regions and circuits involved in memory retrieval, locomotion, decision making, reward, and mood (52). Our study also observed a unique activation profile of ERK1/2 in different brain regions between β-arrestin 1 KO and β-arrestin 2 KO mice in response to the β-arrestin–biased δOR agonist SNC80. The data suggest that β-arrestin2 was critical for this agonist-induced response in the dorsal hippocampus and amygdala, whereas β-arrestin 1 was critical in the dorsal striatum and nucleus accumbens. This finding concurs with reports showing differential β-arrestin isoform expression in neonatal and postnatal rats (53, 54); these and others have shown relatively high β-arrestin 1 and low β-arrestin 2 expressions in the striatal regions (43, 53, 55). It is possible that such expression profiles of may be linked to the functional differences between the β-arrestin isoforms in regulating anxiety- and fear-related behaviors.
In contrast to the β-arrestin-mediated activation of ERK1/2, we found that selective G protein signaling at the δOR by TAN67 decreased ERK1/2 activation and was associated with increased fear-associated behavior. TAN67 is a known weak recruiter of β-arrestins 1 and 2 (14). This result is in agreement with the observation and that blocking Gi/o protein signaling using pertussis toxin in the basolateral amygdala reduced FPS (56) and of decreased ERK in the hippocampus of ovariectomized mice exposed to the anxiogenic estradiol benzoate (57, 58). One explanation for our observation is that TAN67 competes with the endogenous δOR agonist Leu-enkephalin, which is anxiolytic-like and a much more efficacious recruiter of β-arrestin (14, 59, 60) (fig. S9). In this manner, TAN-67 acts as a functional antagonist, matching the anxiogenic effects of the δOR antagonist, naltrindole (61).
β-arrestin 2-mediated signaling in the CNS is not limited to ERK1/2 signaling; and while in this study we report δORs require β-arrestin-dependent ERK signaling for reduction in anxiety-like behavior, it is certainly possible that other GPCRs may engage different intracellular signaling pathways following β-arrestin recruitment.
The basolateral amygdala (BLA) also plays an important role in fear conditioning (52), including FPS (62) and thus it was not surprising to find that the anxiolytic-like effects of SNC80 may also involve β-arrestin 2-dependent ERK1/2 signaling in the amygdala, a region more commonly associated with innate anxiety-like behavior (63, 64). Still, our finding that δOR signaling in the dorsal hippocampus is connected to the anxiolytic-like effects of SNC80, agrees with a study showing that intra-dorsal CA1 injection of the δOR antagonist naltrindole is anxiogenic (35). Further studies with circuit-based approaches are necessary to assess the role of biased signaling pathways in the acquisition and expression of conditioned fear-related behavior.
For some time now, β-arrestin 2 has been associated solely with adverse effects of opioid activation, including tolerance, constipation, respiratory depression, aversion and alcohol use (20, 48, 65). These studies fueled a drive to develop G protein-biased opioids to treat pain and other disorders with an improved therapeutic window (66, 67); yet, a number of studies have started to push back against this narrative (68-72). Clearly, β-arrestin signaling is not inherently negative, as the therapeutic effects of lithium and fluoxetine and D2R agonists seem to depend on β-arrestin 2 (73-75). The increased propensity for β-arrestin 1 KO mice to experience SNC80-induced seizure points to a potential beneficial role for this isoform in maintaining seizure threshold, which could be of use in the treatment of epilepsy. In this study, we provide additional insights regarding potential therapeutic benefits of β-arrestin signaling in reducing anxiety-like behavior. Providing adequate relief of chronic pain is not trivial, partly because it is often associated with negative affect (76, 77) including anxiety, which may exacerbate pain (78). δOR agonists have been proposed as potential treatment for chronic pain disorders (79), partly because they have the ability to not only provide analgesia, but also treat comorbid anxiety and depression (17, 20, 80). However, our results would argue that developing G protein-biased δOR agonists may produce drugs that are suboptimal for the treatment of complex chronic pain; and that suggest such a drug would not alleviate co-morbid fear and anxiety, but potentially even worsen these symptoms. Thus, our findings argue in favor of a reassessment of drug development efforts that seek solely to identify G protein-biased drugs. Instead, we propose that efforts should be directed towards the development of drugs with finely tuned bias and, if possible, towards the development of molecules that are biased against a single β-arrestin isoform rather than both isoforms and are circuit-specific.
Materials and Methods
Animals
Wild-type (WT) C57BL/6 male mice were purchased from Envigo (Indianapolis, IN), and β-arrestin 1 or 2 global knockout (KO) mice as well as δOR KO mice were bred in our facility (14, 81, 82). The knockout mice have been rederived every three years by crossing the KO mice with WT C57BL/6 mice from Envigo to reduce genetic drift. Adult mice (8-10 weeks, 25 ± 3g) were group housed (3-5 mice) in a single ventilated Plexiglas cage. Mice were maintained at ambient temperature (21°C) in an animal housing facility approved by the Association for Assessment and Accreditation of Laboratory Animal Care and animals were kept on a reversed 12-hour dark-light cycle (lights off at 10:00, lights on at 22:00). Food and water were provided ad libitum. Purchased mice were acclimated for one week prior to the experiments. All animal protocols (#1305000864 by RMvR) were preapproved by Purdue Animal Care and Use Committee and were in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals.
Drug preparation and administration
SNC80 (#076410, Tocris, Thermo Fisher Scientific, Waltham, MA) was diluted in slightly acidic saline pH5-6. TAN67 (#092110, Tocris, Thermo Fisher Scientific) was diluted in sterile saline. SL327 (#19691, Tocris, Thermo Fisher Scientific) was diluted in 5% DMSO, 10% Cremophore (Millipore Sigma, Burlington, MA) and 85% saline. ADL5859 (#1751, Axon Medchem, Reston, VA) was diluted in 0.5 % methylcellulose and 0.1 % tween 80 as indicated in (37). To investigate our hypotheses, we utilized several δOR agonists: SNC80, TAN67, and ADL5859. Doses for each drug were based on those that proved to impact behavior through δOR (14, 20, 37). For SNC80, we used a dose of 20 mg/kg (14, 20). We have also tested 10 mg/kg SNC80 (i.p.). While this dose was effective in other studies effect (17, 39), in our hands, we did not observe any significant effects (fig. S6, C and D). TAN67 was tested at a dose of 25 mg/kg (14, 20), whereas ADL5859 was administered at a dose of 30 mg/kg (37). We also used the MEK inhibitor SL327 at a 50mg/kg dose to prevent ERK1/2 activation, as this inhibitor has been reported to cross the blood-brain barrier (83, 84). Except for ADL5859, which was administered with per oral (p.o.), all drugs were administered either intraperitoneal (i.p.) or subcutaneous (s.c.), and specific times prior to behavior or brain tissue extraction can be found in figure legends. Additionally, separate batches of mice with no prior history of drug injection were used for the brain collection and behavioral tests to test earlier time-points of ERK1/2 signaling (such as 10 minutes).
Elevated-plus maze test
The elevated-plus maze test was performed as previously described (20). Mice were allowed to explore the maze for 5 minutes, and arm entries and time spent in each arm were recorded with a camera positioned above the maze. Drugs and vehicle were administered 30 minutes before the test.
Dark–light transition box test
The test was performed based on previously established protocols (20, 81) Testing was conducted without a habituation session to the boxes and a ½-area dark insert was placed in the locomotor boxes, leaving the remaining ½ of the area lit as described previously (85). Two LED lights were inserted above the light portion of the testing chamber where the lux of the light region ranged from 390-540 lumens and dark chamber lux ranged from 0-12 lumens. For testing, animals were placed in the light portion of the chamber and testing began upon animal entry. Time spent in the dark and light chambers as well as their locomotor activity was recorded for 5 minutes with a photobeam-based tracking system. SNC80 and vehicle were administered 30 minutes before the test. SL327 was administered 60 minutes before the test (30 minutes before SNC80 administration).
Fear-potentiated startle (FPS) test
Startle reflexes of mice were recorded in the startle reflex chambers (25.8 x 25 x 26.5 cm) using the Hamilton Kinder Startle Monitor system (Kinder Scientific). On the conditioning day, all subjects were conditioned with 40 conditioning trials by a fixed 2-minute inter-trial interval (ITI), and FPS responses were tested on the following day. The fear conditioning and FPS parameters were based on a previously established protocol (86). In this system, all 8 test chambers are started synchronously once the first mouse is placed in the first box, but we aimed to have all mice receive drugs on average 30 minutes prior to testing.
Preparation of tissue homogenates
After drug injections, mice were euthanized by carbon dioxide asphyxiation and rapidly decapitated after 0, 10 or 30 minutes [other euthanasia methods that may increase basal ERK1/2 activity in the brain (87)]. The collected brains were first sliced as coronal sections (1.5-2.0 mm) with a brain matrix (#RBMS-205C, Kent Scientific), and then flash-frozen in dry-ice-chilled 2-methylbutane (−40 °C; #03551-4, Fisher Scientific). Regions of interest were collected from these slices using a 1 mm biopsy micropunch (#15110-10, Miltex) as follows: dorsal striatum and nucleus accumbens (A/P: +0.5 mm to +1.5 mm), dorsal hippocampus and amygdala (A/P: −1 mm to −2 mm), and ventral hippocampus (A/P: −2 mm to −4 mm) (88). The punches targeted a specific region and produced enough tissue to run several blots. However, it is noteworthy that the extracted tissue may contain small amounts of tissue from neighboring regions. For example, while the punches for the amygdala primarily consisted of the BLA, the tissue will also have included a small portion of the central amygdala. Collected tissues were further homogenized with a tissue grinder (#357535 nda 357537, DWK Life Sciences) in RIPA buffer mixed Halt™ Protease and Phosphatase Inhibitor Cocktail (#1861280, Thermo Fisher Scientific). Samples were further prepared based on previously established protocols (87). Data depicted in Fig. 2, E to I also includes part of the data collected for SNC80 at the 0 min or 10 min time points in the experiment depicted in Fig. 4, C and D to represent the full range of observed SNC80 induced ERK1/2 activation in mice tested in separate cohorts at different occasions.
Cell culture
Chinese hamster ovary cells expressing δOR and β-arrestin 2 (CHO-δOR-βArr2; DiscoverX), human osteosarcoma U2OS cells expressing δOR and β-arrestin 1 (U2OS-δOR-βArr1; DiscoverX), and NG-108-15 cells (HB-12317™, ATCC) were cultured as recommended by the manufacturer and maintained at 37° C/5 % CO2. Cells were seeded in a clear 6-well plate (Corning™, Thermo Fisher Scientific) with 250,000 cells per 2 mL per well. On the following day, all growth media was aspirated and changed into 1 mL serum-free Opti-MEM (#31985070, Gibco®, Thermo Fisher Scientific). The next day, cells were challenged with 10 μM drugs (SNC80) for a specific duration (0, 3, 6, 20, and 60 minutes). All drugs were diluted in Opti-MEM prior to administration. The media was aspirated following the challenge and 100 μL RIPA buffer was added to collect the samples on ice. Using cell scrapers (#353089, Thermo Fisher Scientific), all samples were dislodged from the 6-well plate, collected and stored at −30° C until usage. For the Western blot, the collected samples were quantified with the Bradford assay and samples were prepared with 4 x Laemmli and boiled at 95° C for 5 minutes. The CHO-δOR-βArr2 cells were also used to measure β-arrestin recruitment using the DiscoverX PathHunter Assay as previously described (14).
SDS-Page and Western blotting
Samples (20 μL containing 10 μg protein) were loaded per well of a NuPage 4-12 % Bis-Tris gradient gels (#NP0336BOX, Thermo Fisher Scientific), and the SDS-Page gel was subsequently transferred to nitrocellulose membranes (#1620115, BioRad) by the Western blot. Membranes were incubated following previously established protocols (87). For reproducibility, detailed information regarding the antibodies used in the study are listed in table S6. Prepared samples were scanned using the LiCor Odyssey® CLx Scanner (Li-Cor). By utilizing the Li-Cor secondary antibodies, we were able to detect the MAPK, pMAPK, and α-tubulin on the same blot without the need of stripping/reblotting. In the same membrane, each band was cut based on their size. For instance, total-ERK1/2 and phospho-ERK1/2 bands were collected around 42/44 kDa and α-Tubulin band was collected around 50 kDa in the same membrane. For statistical analysis, we normalized the pMAPK/MAPK ratio to α-tubulin in case drug treatment changed ERK1/2 levels.
Preparation of tissue for immunofluorescence
To preserve the intact ERK1/2 activity in vivo for fluorescence microscopy, mice were transcardially perfused before isolation of brain tissue. Thirty minutes prior to transcardiac perfusion, mice were administered with 100/10 mg/kg ketamine/xylazine. Ten minutes prior to perfusion, 20 mg/kg SNC80 (i.p.) or a corresponding volume of saline was administered to the mice. Mice were then perfused with 30 mL of cold PBS and 4% paraformaldehyde (#100503-916, VWR) and were immediately decapitated to collect the brains. The brains were fixated in 4% paraformaldehyde overnight, dehydrated in 30% sucrose, and then embedded in Frozen Section Compound (#3801480, Leica). Frozen brains were sliced at a width of 30 μm using the Leica cryostat and permeabilized in 100% methanol at - 20 °C for 10 minutes. The slices were blocked in 5 % Normal goat serum (#S26-100ml, Millipore Sigma) for an hour then stained with primary antibodies as listed in table S6. For immunofluorescence labeling, the sections were incubated in the secondary antibodies according to the previously established protocol (89) and as listed in table S6. After the final washing, the nuclei of the sections were stained and the slices were mounted on a glass slide with Vectashield® (#H-1200, Vector lab). Images were acquired with a Nikon confocal microscope and assembled in Adobe Photoshop CS6 (Adobe).
Statistical analysis
The maximum amplitude of the startle response was measured from the average of all responses for each trial type (12 noise-alone, 12 light+noise) of each mouse. Fear-potentiated startle response was analyzed using raw (maximum) startle amplitudes and proportional changes of each trial type (noise-alone, light+noise), which is shown as % FPS in the graphs. The proportional change score (% FPS) was calculated as follows: (startle response to light and noise – startle response to noise)/startle response to noise x 100. Thus, % FPS is a sensitive measure that adjusts for individual and group differences (such as possible non-specific effects of drug treatment) in startle response magnitude that may be observed on noise-alone and light + noise trials (90).
All data are presented as individual data points (or means) ± standard error of the mean (S.E.M.). Assays with one independent variable were analyzed for statistical significance using a one-way Analysis of Variance (ANOVA), whereas assays with two independent variables were analyzed using a two-way ANOVA. If a significant deviation of the mean was identified, an appropriate post-hoc analysis was performed as indicated in the supplemental table or the corresponding figure legends. Gaussian distribution of our datasets was assessed using the D’Augostino and Pearson analysis. We excluded one outlier in our WT cohort that received SNC80 and one that received TAN67 in the FPS assay based on the Grubbs’ test (α = 0.05). In the dark-light and elevated plus maze tests we excluded subjects that were frozen/stationary for >95% of the experimental time. All statistical analysis was conducted using GraphPad Prism 7 (GraphPad Software). The appropriateness of each test was confirmed through consultation with the Purdue Statistical Consulting Service.
Supplementary Material
Acknowledgements:
We appreciate Dr. Marcus M. Weera for technical assistance with the fear-conditioning experiments. Included diagrams were created with BioRender. We thank Dr. Sabbaghi and Mudit Gaur for consulting on the statistical analysis of our data sets.
Funding:
This work was funded by a NARSAD Young Investigator Award from the Brain and Behavior Research Foundation (#23603 to RMvR) and the National Institute on Alcohol Abuse and Alcoholism (AA025368, AA026949, AA026675) and Drug Abuse (DA045897).
Footnotes
Competing interests: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper or the Supplementary Materials.
References and Notes
- 1.Tovote P, Fadok JP, Luthi A, Neuronal circuits for fear and anxiety. Nat Rev Neurosci 16, 317–331 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Akimova E, Lanzenberger R, Kasper S, The serotonin-1A receptor in anxiety disorders. Biol Psychiatry 66, 627–635 (2009). [DOI] [PubMed] [Google Scholar]
- 3.de la Mora MP, Gallegos-Cari A, Arizmendi-Garcia Y, Marcellino D, Fuxe K, Role of dopamine receptor mechanisms in the amygdaloid modulation of fear and anxiety: Structural and functional analysis. Prog Neurobiol 90, 198–216 (2010). [DOI] [PubMed] [Google Scholar]
- 4.Kindt M, Soeter M, Vervliet B, Beyond extinction: erasing human fear responses and preventing the return of fear. Nat Neurosci 12, 256–258 (2009). [DOI] [PubMed] [Google Scholar]
- 5.Land BB et al. Activation of the kappa opioid receptor in the dorsal raphe nucleus mediates the aversive effects of stress and reinstates drug seeking. Proc Natl Acad Sci U S A 106, 19168–19173 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takahashi LK, Role of CRF(1) and CRF(2) receptors in fear and anxiety. Neurosci Biobehav Rev 25, 627–636 (2001). [DOI] [PubMed] [Google Scholar]
- 7.Pierce KL, Premont RT, Lefkowitz RJ, Seven-transmembrane receptors. Nat Rev Mol Cell Biol 3, 639–650 (2002). [DOI] [PubMed] [Google Scholar]
- 8.Hahn YK et al. Central HIV-1 Tat exposure elevates anxiety and fear conditioned responses of male mice concurrent with altered mu-opioid receptor-mediated G-protein activation and beta-arrestin 2 activity in the forebrain. Neurobiol Dis 92, 124–136 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruchas MR, Macey TA, Lowe JD, Chavkin C, Kappa opioid receptor activation of p38 MAPK is GRK3- and arrestin-dependent in neurons and astrocytes. J Biol Chem 281, 18081–18089 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beaulieu JM et al. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell 122, 261–273 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Allen JA et al. Discovery of beta-arrestin-biased dopamine D2 ligands for probing signal transduction pathways essential for antipsychotic efficacy. Proc Natl Acad Sci U S A 108, 18488–18493 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wisler JW, Xiao K, Thomsen AR, Lefkowitz RJ, Recent developments in biased agonism. Curr Opin Cell Biol 27, 18–24 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Urs NM, Peterson SM, Caron MG, New Concepts in Dopamine D2 Receptor Biased Signaling and Implications for Schizophrenia Therapy. Biol Psychiatry 81, 78–85 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiang T, Sansuk K, van Rijn RM, beta-Arrestin 2 dependence of delta opioid receptor agonists is correlated with alcohol intake. Br J Pharmacol 173, 332–343 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pradhan AA et al. Agonist-Specific Recruitment of Arrestin Isoforms Differentially Modify Delta Opioid Receptor Function. J Neurosci 36, 3541–3551 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vicente-Sanchez A et al. Tolerance to high-internalizing delta opioid receptor agonist is critically mediated by arrestin 2. Br J Pharmacol 175, 3050–3059 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saitoh A et al. Potential anxiolytic and antidepressant-like activities of SNC80, a selective delta-opioid agonist, in behavioral models in rodents. J Pharmacol Sci 95, 374–380 (2004). [DOI] [PubMed] [Google Scholar]
- 18.Saitoh A et al. The delta opioid receptor agonist KNT-127 in the prelimbic medial prefrontal cortex attenuates veratrine-induced anxiety-like behaviors in mice. Behav Brain Res 336, 77–84 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Li Y et al. Regulation of amygdalar PKA by beta-arrestin-2/phosphodiesterase-4 complex is critical for fear conditioning. Proc Natl Acad Sci U S A 106, 21918–21923 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Rijn RM, Brissett DI, Whistler JL, Dual efficacy of delta opioid receptor-selective ligands for ethanol drinking and anxiety. J Pharmacol Exp Ther 335, 133–139 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lefkowitz RJ, Shenoy SK, Transduction of receptor signals by beta-arrestins. Science 308, 512–517 (2005). [DOI] [PubMed] [Google Scholar]
- 22.Coyle JT, Duman RS, Finding the intracellular signaling pathways affected by mood disorder treatments. Neuron 38, 157–160 (2003). [DOI] [PubMed] [Google Scholar]
- 23.Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD, The MAPK cascade is required for mammalian associative learning. Nat Neurosci 1, 602–609 (1998). [DOI] [PubMed] [Google Scholar]
- 24.Herry C, Trifilieff P, Micheau J, Luthi A, Mons N, Extinction of auditory fear conditioning requires MAPK/ERK activation in the basolateral amygdala. Eur J Neurosci 24, 261–269 (2006). [DOI] [PubMed] [Google Scholar]
- 25.Richards EM et al. A randomized, placebo-controlled pilot trial of the delta opioid receptor agonist AZD2327 in anxious depression. Psychopharmacology (Berl) 233, 1119–1130 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robins MT, Chiang T, Mores KL, Alongkronrusmee D, van Rijn RM, Critical Role for Gi/o-Protein Activity in the Dorsal Striatum in the Reduction of Voluntary Alcohol Intake in C57Bl/6 Mice. Front Psychiatry 9, 112 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rozenfeld R, Devi LA, Receptor heterodimerization leads to a switch in signaling: beta-arrestin2-mediated ERK activation by mu-delta opioid receptor heterodimers. FASEB J 21, 2455–2465 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cen B et al. Direct binding of beta-arrestins to two distinct intracellular domains of the delta opioid receptor. J Neurochem 76, 1887–1894 (2001). [DOI] [PubMed] [Google Scholar]
- 29.Klee WA et al. Identification of a Mr 58 000 glycoprotein subunit of the opiate receptor. FEBS Lett 150, 125–128 (1982). [DOI] [PubMed] [Google Scholar]
- 30.Eisinger DA, Ammer H, Schulz R, Chronic morphine treatment inhibits opioid receptor desensitization and internalization. J Neurosci 22, 10192–10200 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erbs E et al. A mu-delta opioid receptor brain atlas reveals neuronal co-occurrence in subcortical networks. Brain Struct Funct 220, 677–702 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chu Sin Chung P et al. A novel anxiogenic role for the delta opioid receptor expressed in GABAergic forebrain neurons. Biol Psychiatry 77, 404–415 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tohgo A, Pierce KL, Choy EW, Lefkowitz RJ, Luttrell LM, beta-Arrestin scaffolding of the ERK cascade enhances cytosolic ERK activity but inhibits ERK-mediated transcription following angiotensin AT1a receptor stimulation. J Biol Chem 277, 9429–9436 (2002). [DOI] [PubMed] [Google Scholar]
- 34.File SE, Kenny PJ, Cheeta S, The role of the dorsal hippocampal serotonergic and cholinergic systems in the modulation of anxiety. Pharmacol Biochem Behav 66, 65–72 (2000). [DOI] [PubMed] [Google Scholar]
- 35.Solati J, Zarrindast MR, Salari AA, Dorsal hippocampal opioidergic system modulates anxiety-like behaviors in adult male Wistar rats. Psychiatry Clin Neurosci 64, 634–641 (2010). [DOI] [PubMed] [Google Scholar]
- 36.Le Bourdonnec B et al. Potent, orally bioavailable delta opioid receptor agonists for the treatment of pain: discovery of N,N-diethyl-4-(5-hydroxyspiro[chromene-2,4'-piperidine]-4-yl)benzamide (ADL5859). J Med Chem 51, 5893–5896 (2008). [DOI] [PubMed] [Google Scholar]
- 37.Nozaki C et al. delta-Opioid mechanisms for ADL5747 and ADL5859 effects in mice: analgesia, locomotion, and receptor internalization. J Pharmacol Exp Ther 342, 799–807 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sugiyama A et al. Systemic administration of a delta opioid receptor agonist, KNT-127, facilitates extinction learning of fear memory in rats. J Pharmacol Sci 139, 174–179 (2019). [DOI] [PubMed] [Google Scholar]
- 39.Saitoh A et al. Antidepressant-like effects of the delta-opioid receptor agonist SNC80 ([(+)-4-[(alphaR)-alpha-[(2S,5R)-2,5-dimethyl-4-(2-propenyl)-1-piperazinyl]-(3-me thoxyphenyl)methyl]-N,N-diethylbenzamide) in an olfactory bulbectomized rat model. Brain Res 1208, 160–169 (2008). [DOI] [PubMed] [Google Scholar]
- 40.Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ, Autoradiographic differentiation of mu, delta, and kappa opioid receptors in the rat forebrain and midbrain. J Neurosci 7, 2445–2464 (1987). [PMC free article] [PubMed] [Google Scholar]
- 41.Chung PC et al. Delta opioid receptors expressed in forebrain GABAergic neurons are responsible for SNC80-induced seizures. Behav Brain Res 278, 429–434 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lohse MJ, Benovic JL, Codina J, Caron MG, Lefkowitz RJ, beta-Arrestin: a protein that regulates beta-adrenergic receptor function. Science 248, 1547–1550 (1990). [DOI] [PubMed] [Google Scholar]
- 43.Attramadal H et al. Beta-arrestin2, a novel member of the arrestin/beta-arrestin gene family. J Biol Chem 267, 17882–17890 (1992). [PubMed] [Google Scholar]
- 44.Bohn LM et al. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science 286, 2495–2498 (1999). [DOI] [PubMed] [Google Scholar]
- 45.Conner DA et al. beta-Arrestin1 knockout mice appear normal but demonstrate altered cardiac responses to beta-adrenergic stimulation. Circ Res 81, 1021–1026 (1997). [DOI] [PubMed] [Google Scholar]
- 46.Latapy C, Beaulieu JM, beta-Arrestins in the central nervous system. Prog Mol Biol Transl Sci 118, 267–295 (2013). [DOI] [PubMed] [Google Scholar]
- 47.Whalen EJ, Rajagopal S, Lefkowitz RJ, Therapeutic potential of beta-arrestin- and G protein-biased agonists. Trends Mol Med 17, 126–139 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raehal KM, Walker JK, Bohn LM, Morphine side effects in beta-arrestin 2 knockout mice. J Pharmacol Exp Ther 314, 1195–1201 (2005). [DOI] [PubMed] [Google Scholar]
- 49.Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG, Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature 408, 720–723 (2000). [DOI] [PubMed] [Google Scholar]
- 50.Zurkovsky L, Sedaghat K, Ahmed MR, Gurevich VV, Gurevich EV, Arrestin-2 and arrestin-3 differentially modulate locomotor responses and sensitization to amphetamine. Neuropharmacology 121, 20–29 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang B et al. beta-Arrestin-biased beta-adrenergic signaling promotes extinction learning of cocaine reward memory. Sci Signal 11, (2018). [DOI] [PubMed] [Google Scholar]
- 52.Janak PH, Tye KM, From circuits to behaviour in the amygdala. Nature 517, 284–292 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gurevich EV, Benovic JL, Gurevich VV, Arrestin2 and arrestin3 are differentially expressed in the rat brain during postnatal development. Neuroscience 109, 421–436 (2002). [DOI] [PubMed] [Google Scholar]
- 54.Gurevich EV, Benovic JL, Gurevich VV, Arrestin2 expression selectively increases during neural differentiation. J Neurochem 91, 1404–1416 (2004). [DOI] [PubMed] [Google Scholar]
- 55.Bjork K et al. Modulation of voluntary ethanol consumption by beta-arrestin 2. FASEB J 22, 2552–2560 (2008). [DOI] [PubMed] [Google Scholar]
- 56.Melia KR, Falls WA, Davis M, Involvement of pertussis toxin sensitive G-proteins in conditioned fear-potentiated startle: possible involvement of the amygdala. Brain Res 584, 141–148 (1992). [DOI] [PubMed] [Google Scholar]
- 57.Morgan MA, Pfaff DW, Estrogen's effects on activity, anxiety, and fear in two mouse strains. Behav Brain Res 132, 85–93 (2002). [DOI] [PubMed] [Google Scholar]
- 58.Anchan D, Clark S, Pollard K, Vasudevan N, GPR30 activation decreases anxiety in the open field test but not in the elevated plus maze test in female mice. Brain Behav 4, 51–59 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ragnauth A et al. Female preproenkephalin-knockout mice display altered emotional responses. Proc Natl Acad Sci U S A 98, 1958–1963 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kung JC, Chen TC, Shyu BC, Hsiao S, Huang AC, Anxiety- and depressive-like responses and c-fos activity in preproenkephalin knockout mice: oversensitivity hypothesis of enkephalin deficit-induced posttraumatic stress disorder. J Biomed Sci 17, 29 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Narita M et al. Chronic pain induces anxiety with concomitant changes in opioidergic function in the amygdala. Neuropsychopharmacology 31, 739–750 (2006). [DOI] [PubMed] [Google Scholar]
- 62.Terburg D et al. The Basolateral Amygdala Is Essential for Rapid Escape: A Human and Rodent Study. Cell 175, 723–735 e716 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tye KM et al. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature 471, 358–362 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Felix-Ortiz AC et al. BLA to vHPC inputs modulate anxiety-related behaviors. Neuron 79, 658–664 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Raehal KM, Bohn LM, The role of beta-arrestin2 in the severity of antinociceptive tolerance and physical dependence induced by different opioid pain therapeutics. Neuropharmacology 60, 58–65 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Manglik A et al. Structure-based discovery of opioid analgesics with reduced side effects. Nature 537, 185–190 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mores KL, Cummins BR, Cassell RJ, van Rijn RM, A Review of the Therapeutic Potential of Recently Developed G Protein-Biased Kappa Agonists. Front Pharmacol 10, 407 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kliewer A et al. Phosphorylation-deficient G-protein-biased mu-opioid receptors improve analgesia and diminish tolerance but worsen opioid side effects. Nat Commun 10, 367 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hill R et al. The novel mu-opioid receptor agonist PZM21 depresses respiration and induces tolerance to antinociception. Br J Pharmacol 175, 2653–2661 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Austin Zamarripa C et al. The G-protein biased mu-opioid agonist, TRV130, produces reinforcing and antinociceptive effects that are comparable to oxycodone in rats. Drug Alcohol Depend 192, 158–162 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kliewer A et al. Morphine-induced respiratory depression is independent of beta-arrestin2 signalling. Br J Pharmacol, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gillis A et al. Low intrinsic efficacy for G protein activation can explain the improved side-effect profile of new opioid agonists. Science Signaling, (2020). [DOI] [PubMed] [Google Scholar]
- 73.David DJ et al. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron 62, 479–493 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Urs NM et al. Distinct cortical and striatal actions of a beta-arrestin-biased dopamine D2 receptor ligand reveal unique antipsychotic-like properties. Proc Natl Acad Sci U S A 113, E8178–E8186 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Beaulieu JM et al. A beta-arrestin 2 signaling complex mediates lithium action on behavior. Cell 132, 125–136 (2008). [DOI] [PubMed] [Google Scholar]
- 76.Corder G et al. An amygdalar neural ensemble that encodes the unpleasantness of pain. Science 363, 276–281 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Massaly N et al. Pain-Induced Negative Affect Is Mediated via Recruitment of The Nucleus Accumbens Kappa Opioid System. Neuron 102, 564–573 e566 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.al Absi M, Rokke PD, Can anxiety help us tolerate pain? Pain 46, 43–51 (1991). [DOI] [PubMed] [Google Scholar]
- 79.Pradhan AA, Befort K, Nozaki C, Gaveriaux-Ruff C, Kieffer BL, The delta opioid receptor: an evolving target for the treatment of brain disorders. Trends Pharmacol Sci 32, 581–590 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Perrine SA, Hoshaw BA, Unterwald EM, Delta opioid receptor ligands modulate anxiety-like behaviors in the rat. Br J Pharmacol 147, 864–872 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Robins MT et al. Behavioral Characterization of beta-Arrestin 1 Knockout Mice in Anxiety-Like and Alcohol Behaviors. Front Behav Neurosci 12, 54 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van Rijn RM, Whistler JL, The delta(1) opioid receptor is a heterodimer that opposes the actions of the delta(2) receptor on alcohol intake. Biol Psychiatry 66, 777–784 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Selcher JC, Atkins CM, Trzaskos JM, Paylor R, Sweatt JD, A necessity for MAP kinase activation in mammalian spatial learning. Learn Mem 6, 478–490 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Valjent E, Corvol JC, Trzaskos JM, Girault JA, Herve D, Role of the ERK pathway in psychostimulant-induced locomotor sensitization. BMC Neurosci 7, 20 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bourin M, Hascoet M, The mouse light/dark box test. Eur J Pharmacol 463, 55–65 (2003). [DOI] [PubMed] [Google Scholar]
- 86.Barrenha GD, Chester JA, Genetic correlation between innate alcohol preference and fear-potentiated startle in selected mouse lines. Alcohol Clin Exp Res 31, 1081–1088 (2007). [DOI] [PubMed] [Google Scholar]
- 87.Ko MJ, Mulia GE, van Rijn RM, Commonly Used Anesthesia/Euthanasia Methods for Brain Collection Differentially Impact MAPK Activity in Male and Female C57BL/6 Mice. Front Cell Neurosci 13, 96 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Paxinos G, Franklin KB, The mouse brain in stereotaxic coordinates. (Gulf professional publishing, 2004). [Google Scholar]
- 89.Kim JW et al. Social support rescues acute stress-induced cognitive impairments by modulating ERK1/2 phosphorylation in adolescent mice. Sci Rep 8, 12003 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Walker DL, Davis M, Quantifying fear potentiated startle using absolute versus proportional increase scoring methods: implications for the neurocircuitry of fear and anxiety. Psychopharmacology (Berl) 164, 318–328 (2002). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.