Abstract
Brain abnormalities in the reading network have been repeatedly reported in individuals with developmental dyslexia (DD); however, it is still not totally understood where the structural and functional abnormalities are consistent/inconsistent across languages. In the current multimodal meta-analysis, we found convergent structural and functional alterations in the left superior temporal gyrus across languages, suggesting a neural signature of DD. We found greater reduction in grey matter volume and brain activation in the left inferior frontal gyrus in morpho-syllabic languages (e.g. Chinese) than in alphabetic languages, and greater reduction in brain activation in the left middle temporal gyrus and fusiform gyrus in alphabetic languages than in morpho-syllabic languages. These language differences are explained as consequences of being DD while learning a specific language. In addition, we also found brain regions that showed increased grey matter volume and brain activation, presumably suggesting compensations and brain regions that showed inconsistent alterations in brain structure and function. Our study provides important insights about the etiology of DD from a cross-linguistic perspective with considerations of consistency/inconsistency between structural and functional alterations.
Research organism: Human
Introduction
Individuals with developmental dyslexia (DD) encounter difficulty in learning to read even with normal intelligence and adequate educational guidance (Peterson and Pennington, 2012). DD affects a large number of individuals across writing systems, and the prevalence is about 5–10% in alphabetic writing systems (e.g. English and German) (Döhla and Heim, 2015; Katusic et al., 2001; Shaywitz, 1996) and about 4–7% in morpho-syllabic writing systems (e.g. Chinese and Japanese Kanji) (Sun et al., 2013; Uno et al., 2009; Zhao et al., 2016). Multiple deficits have been identified to be associated with DD (Ring and Black, 2018), among which phonological deficit has been well-documented across languages (Gu and Bi, 2020; Snowling and Melby-Lervag, 2016). Individuals with DD show deficient phonological ability including phonological representation, manipulation, and retrieval even when compared to reading-level controls (Melby-Lervag et al., 2012; Parrila et al., 2020). However, the common phonological deficit may manifest differently in reading behavior depending on the specific requirements of the writing system. For example, phonological deficit in English is associated with lower accuracy in phonological decoding (Landerl et al., 1997; Ziegler et al., 2003), and it is associated with slower reading speed in transparent orthographies with relatively intact accuracy in phonological decoding (Wimmer and Schurz, 2010). In Chinese, phonological deficit is associated with a higher rate of semantic errors during character reading (Shu et al., 2005), because children with DD over-rely on the semantic cue in the character during reading due to the inability to use the phonological cue. According to research, 80% of Chinese characters have a semantic radical and a phonetic radical providing semantic cues and phonological cues of the character, respectively (Honorof and Feldman, 2006).
At the neurological level, the reading network in the left hemisphere has often been found to show alterations in individuals with DD (Pugh et al., 2000; Richlan, 2012; Richlan, 2014), including the temporoparietal cortex (TP), the occipitotemporal cortex (OT), and the inferior frontal cortex. The left TP area is further subdivided into the posterior superior temporal gyrus (STG), which is involved in fine phonological analysis (Petersen and Fiez, 1993; Richlan, 2012) and the inferior parietal lobule (IPL) which is associated with general attention control (Richlan, 2014). This TP region tends to show reduced brain activation in individuals with DD in alphabetic languages as demonstrated in a cross-linguistic study of English, Italian, and French (Paulesu et al., 2001) and several meta-analysis studies in alphabetic languages (Maisog et al., 2008; Martin et al., 2016; Paulesu et al., 2014; Richlan et al., 2009; Richlan et al., 2011). The left OT area, including the middle occipital gyrus (MOG), inferior temporal gyrus (ITG) and fusiform gyrus, has been consistently found to show reduced activation in individuals with DD across morpho-syllabic and alphabetic languages (Bolger et al., 2008; Cao et al., 2020; Centanni et al., 2019; Chyl et al., 2019; Paz-Alonso et al., 2018). This region is associated with visuo-orthographic processing during reading (Glezer et al., 2016; Glezer et al., 2019). The left inferior frontal cortex is further subdivided into the inferior frontal gyrus (IFG) and the precentral gyrus (Richlan, 2014). The precentral gyrus may relate to compensatory articulatory processes in dyslexia (Hancock et al., 2017), whereas the IFG has been known to be involved in phonological and semantic retrieval, lexical selection and integration (Booth et al., 2007a; Booth et al., 2007b; Costafreda et al., 2006; Szatkowska et al., 2000). However, the nature of dysfunction in the left IFG in individuals with DD remains controversial. Although reduced activation in the left IFG was confirmed by many fMRI studies and meta-analysis studies (Booth et al., 2007a; Cao et al., 2006; Richlan et al., 2010; Wimmer et al., 2010), increased activation in the left IFG was also reported in many fMRI studies (Grunling et al., 2004; Kronbichler et al., 2006; Waldie et al., 2013; Wimmer et al., 2010). The inconsistent results may be related to task and task difficulty (Waldie et al., 2013; Wimmer et al., 2010), orthographic transparency (Martin et al., 2016), and age of participants (Chyl et al., 2019).
There is a sparsity in research investigating whether the deficits associated with DD are language-universal. Paulesu et al., 2001 found that readers with DD in English, Italian, and French showed similar brain abnormality during an explicit word reading task and an implicit reading task. Hu et al., 2010 found that Chinese and English children with DD showed language-universal deficits. Feng et al., 2020 found that children with DD in both Chinese and French showed common reduction of brain activation in the left fusiform gyrus and STG. In summary, meta-analytic studies should make a greater contribution in such a topic by gathering studies from different languages and comparing them.
Even though language-universal deficits in the brain have been suggested in several studies (Feng et al., 2020; Hu et al., 2010; Paulesu et al., 2001), language specificity has been demonstrated as well (Martin et al., 2016; Siok et al., 2004). In a meta-analysis study (Martin et al., 2016), researchers directly compared brain deficits associated with DD between transparent and opaque orthographies and found that functional abnormalities in the brain vary with orthographic depth in alphabetic languages. Specifically, consistent reduction of brain activation was found in a left OT area regardless of orthographic depth, whereas greater reduction was found in the left fusiform gyrus, left TP and left IFG pars orbitalis in transparent orthographies than in opaque orthographies, and greater reduction in the bilateral intraparietal sulcus, left precuneus and left IFG pars triangularis was found in opaque orthographies than in transparent orthographies. In a recent study on Chinese-English bilingual children with DD, researchers also found both language-universal and language-specific deficits for Chinese and English (Cao et al., 2020). These findings suggest that there are both language-universal and language-specific deficits across languages. The language-universal deficits might be related to the causal risk of DD while the language-specific deficits tend to be interpreted as a result of interaction between DD and the specific language system that one studies.
Alphabetic and morpho-syllabic languages make a contrastive cross-linguistic comparison. As a representative morpho-syllabic language, Chinese character represents a morpheme and a syllable rather than a phoneme, even though a small percent of Chinese characters are logographic. Research on DD in Chinese has revealed different patterns of brain abnormalities from alphabetic languages. Significant alteration in the left middle frontal gyrus (MFG) or dorsal IFG has been consistently reported in different studies (Cao et al., 2017; Cao et al., 2020; Liu et al., 2012; Liu et al., 2013a; Siok et al., 2004; Siok et al., 2008), while the alteration in the left TP areas has been reported in only a few studies (Cao et al., 2017; Cao et al., 2018; Hu et al., 2010). This might be because the left dorsal IFG plays an essential role in Chinese. It has been found that the left dorsal IFG is more involved in Chinese reading than in English reading, while the left TP is more involved in English reading than in Chinese reading in typical readers (Bolger et al., 2005; Tan et al., 2005). Therefore, the greater deficit in the left dorsal IFG suggests a Chinese-specific deficit.
In addition to functional studies, there have also been a large number of studies with a focus on structural alterations associated with DD. Even though brain structural alterations may cause DD, it is equally possible that altered brain structure is a result of being DD, since learning experience shapes brain development. However, only one of these studies has taken language difference into account (Silani et al., 2005). Previous meta-analysis studies on alphabetic languages have found grey matter reduction in the left TP area (Linkersdörfer et al., 2012; McGrath and Stoodley, 2019; Richlan et al., 2013) as well as the left OT area (Linkersdörfer et al., 2012). These three meta-analytic studies echo findings from functional studies by showing abnormal brain structures within the classic reading network in alphabetic languages. In consistent, studies on morpho-syllabic languages have also found grey matter reduction within the classic reading network (Liu et al., 2013b; Siok et al., 2008; Wang et al., 2019; Xia et al., 2016). However, there are also studies that found abnormal brain structures outside the classic reading network, for example, in putamen, cerebellum, thalamus, and caudate etc. (Adrian-Ventura et al., 2020; Brambati et al., 2004; Brown et al., 2001; Jagger-Rickels et al., 2018; Jednorog et al., 2015; Wang et al., 2019), suggesting that these regions are also affected in this condition. Taken together, both classic reading regions and other regions have been found to show structural alterations in DD, and the previous inconsistent findings in brain structure might be due to the lack of differentiation in participants’ language. It is important to differentiate language-universal structural alterations as a core deficit which might be related to the cause of DD and language-specific structural alterations as a consequence of being DD in a specific language. Learning a specific language with DD may affect brain development in regions that are specifically important for that language and related functions (Mechelli et al., 2004).
DD is associated with altered brain structure and function, but very few studies have investigated whether brain structural and functional alterations are consistent or inconsistent. In a study by Siok et al., 2008, researchers examined both structural and functional alterations in Chinese children with DD, and found reduced GMV and brain activation in the left MFG, which underscores the association between the left MFG and DD in Chinese. Another study located a key region in the left IPL, which showed reduced GMV and activation in English-speaking readers with DD (Hoeft et al., 2007). A recent study, from a developmental perspective, found a dissociation between brain structural development and brain functional development in some brain regions (e.g. left fusiform gyrus) in the context of reading development (Siok et al., 2020), suggesting that learning experience may significantly shape brain function independent of brain structure. However, more research is sorely needed to examine whether there are brain regions that show increased GMV but decreased brain function or vice versa, and to understand the neurocognitive implications of such patterns. Simultaneously considering structural and functional abnormalities with a focus on cross-linguistic comparison would provide a comprehensive perspective to understand the neural mechanisms of DD.
In this meta-analysis study, we aimed to explore how structural and functional impairment of DD converge or diverge and whether this pattern is similar or different across writing systems. We expected to find brain regions that show decreased brain structure and function, indicating insufficient neuronal resources for certain cognitive computations. For regions that show increased brain structure and function, we believe they develop to an unusually high degree for compensation. For brain regions with increased structure but decreased function or decreased structure and increased function, it may be due to brain structures receiving inhibitory input from other regions. We also expected to find language-universal as well as language-specific neurological abnormalities. For language-universal deficits, we tend to believe that they are related to the cause of DD, while the language-specific deficits tend to be consequences of DD in different languages.
Results
Description of the included studies
For the functional studies, a total of 2728 participants (controls:1370, DD:1358) were included, and the mean age was 16.56 years for controls and 16.26 years for participants with DD. Specifically, there were 79 functional experiments in alphabetic languages, including 31 experiments on adults (N = 434, mean age = 26.12 for controls, N = 411, mean age = for 25.86 for DD), 36 experiments on children (N = 553, mean age = 10.59 for controls, N = 586, mean age = for 10.54 for DD), 7 experiments on adolescents (N = 131, mean age = 14.44 for controls, N = 108, mean age = for 14.30 for DD), and 5 studies of mixed ages. There were 12 functional experiments in morpho-syllabic languages (N = 164, mean age = 11.48 for controls, N = 162, mean age = for 11.45 for DD), including 11 experiments on children and 1 experiment on adolescents.
For the structural studies, there were 21 experiments in alphabetic languages, including 10 experiments on adults (N = 209, mean age = 26.68 for controls, N = 193, mean age = for 27.15 for DD), 8 experiments on children (N = 245, mean age = 10.17 for controls, N = 266, mean age = for 10.28 for DD), 1 experiment on adolescents and 2 studies of mixed ages. There were six structural experiments on children in morpho-syllabic languages (N = 89, mean age = 11.82 for controls, N = 94, mean age = for 11.74 for DD).
Meta-analysis results
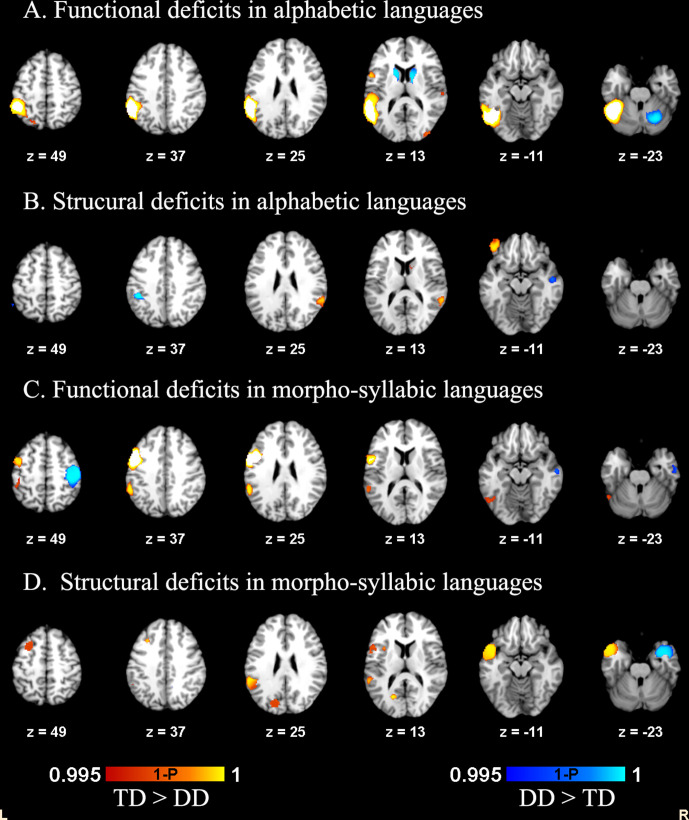
Functional deficits in alphabetic languages and morpho-syllabic languages
In the meta-analysis of functional studies in alphabetic languages, hypoactivation in DD was found in a large cluster peaked at the left supramarginal gyrus which extended to the inferior frontal cortex, occipitotemporal cortex and cerebellum, a cluster peaked at the right MOG and a cluster peaked at right STG (Table 1 and Figure 1A). Hyperactivation in DD was found in the right cerebellum and bilateral caudate nucleus.
Table 1. Functional deficits in individuals with DD in alphabetic languages (JK represents the results of jack-knife sensitivity analysis).
| Regions | MNI coordinate | SDM-Z | p | Voxels | Cluster breakdown (Voxels) | JK |
|---|---|---|---|---|---|---|
| Hypoactivation in DD | ||||||
| Left supramarginal gyrus | −56,–46,30 | 4.919 | 0.0000 | 10,742 | Left IPL, BA 40 (986)Left MTG, BA 21 (719)Left MTG, BA 37 (634)Left ITG, BA 37 (601)Left fusiform gyrus, BA 37 (479)Left ITG, BA 20 (414)Left STG, BA 48 (377)Left supramarginal gyrus, BA 48 (329)Left angular gyrus, BA 39 (307)Left MTG, BA 22 (300)Left cerebellum, lobule VI, BA 37 (285)Left STG, BA 42 (281)Left rolandic operculum, BA 48 (261)Left arcuate network (255)Left cerebellum, crus I, BA 37 (210)Left STG, BA 22 (201)Left supramarginal gyrus, BA 40 (198)Left superior longitudinal fasciculus III (182)Left IPL, BA 2 (167)Left inferior occipital gyrus, BA 19 (156) | 79/79 |
| Right MOG | 42,–86,6 | 2.244 | 0.0002 | 361 | Right MOG, BA19 (208) | 76/79 |
| Right STG | 60,–16,4 | 1.936 | 0.0011 | 358 | 73/79 | |
| Hyperactivation in DD | ||||||
| Right cerebellum | 26,–60,–28 | –1.526 | 0.0000 | 1,559 | Right cerebellum, lobule VI, BA 37 (352)Right cerebellum, lobule VI, BA 19 (233)Middle cerebellar peduncles (212) | 79/79 |
| Left caudate nucleus | –16,12,6 | –1.459 | 0.0000 | 611 | Left anterior thalamic projections (364) | 79/79 |
| Right caudate nucleus | 10,2,14 | –1.317 | 0.0001 | 520 | Right anterior thalamic projections (185)Right caudate nucleus (184) | 79/79 |
Figure 1. Functional and structural deficits related to DD in alphabetic languages and morpho-syllabic languages.
In the meta-analysis of functional studies in morpho-syllabic languages, hypoactivation in DD was found in left IFG opercular part, left supramarginal gyrus and left ITG. Hyperactivation in DD was found in right precentral gyrus and right middle temporal gyrus (MTG) (Table 2, Table 3 and Figure 1C). The jack-knife sensitivity analysis showed that all results reported above were replicable (Table 1; Table 3).
Table 2. Structural deficits in individuals with DD in alphabetic languages (JK represents the results of jack-knife sensitivity analysis).
| Regions | MNI coordinate | SDM-Z | p | Voxels | Cluster breakdown (Voxels) | JK |
|---|---|---|---|---|---|---|
| Decreased GMV in DD | ||||||
| Left IFG orbital part | −38,42,–16 | 2.306 | 0.0001 | 611 | Left IFG orbital part, BA 47 (217) | 20/21 |
| Right STG | 56,–44,18 | 2.024 | 0.0003 | 560 | Right STG, BA 42 (156) | 20/21 |
| Right caudate | 6,14,2 | 1.695 | 0.0022 | 166 | 21/21 | |
| Increased GMV in DD | ||||||
| Left IPL | −42,–36,36 | –1.976 | 0.0000 | 237 | Left IPL, BA 40 (237) | 20/21 |
| Right MTG | 50,–12,–14 | –1.040 | 0.0014 | 174 | 21/21 | |
Table 3. Functional deficits in individuals with DD in morpho-syllabic languages (JK represents the results of jack-knife sensitivity analysis).
| Regions | MNI coordinate | SDM-Z | p | Voxels | Cluster breakdown (Voxels) | JK |
|---|---|---|---|---|---|---|
| Hypoactivation in DD | ||||||
| Left IFG opercular part | –48,10,28 | 4.071 | 0.0000 | 2527 | Left precentral gyrus, BA 6 (623)Left IFG opercular part, BA 44 (278)Left precentral gyrus, BA 44 (195)Corpus callosum (178)Left MFG, BA 44 (162) | 12/12 |
| Left supramarginal gyrus | −58,–42,26 | 2.149 | 0.0001 | 1001 | Left IPL, BA 40 (271)Left STG, BA 42 (153)Left supramarginal gyrus, BA 48 (144) | 11/12 |
| Left ITG | −48,–56,–18 | 1.761 | 0.0008 | 326 | Left ITG, BA37 (166) | 9/12 |
| Hyperactivation in DD | ||||||
| Right precentral gyrus | 52,–16,44 | –2.035 | 0.0000 | 2201 | Right precentral gyrus, BA 6 (640)Right postcentral gyrus, BA 3 (447)Right precentral gyrus, BA 4 (350)Right postcentral gyrus, BA 4 (215) | 12/12 |
| Right MTG | 56,–10,–18 | –1.453 | 0.0013 | 298 | 10/12 | |
Structural deficits in alphabetic languages and morpho-syllabic languages
In the meta-analysis of structural studies in alphabetic languages, readers with DD showed a decrease in GMV in the left IFG orbital part, right STG and right caudate nucleus (Table 2 and Figure 1B). In contrast, readers with DD showed an increase in GMV in the left IPL and right MTG. In the meta-analysis of structural studies in morpho-syllabic languages, readers with DD showed a decrease in GMV in the left temporoparietal cortex, left calcarine cortex and left MFG. Readers with DD showed an increase in GMV in the right STG (Table 4 and Figure 1D). The jack-knife sensitivity analysis showed that all results reported were replicable (Table 2; Table 4).
Table 4. Structural deficits in individuals with DD in morpho-syllabic languages (JK represents the results of jack-knife sensitivity analysis).
| Regions | MNI coordinate | SDM-Z | p | Voxels | Cluster breakdown (Voxels) | JK |
|---|---|---|---|---|---|---|
| Decreased GMV in DD | ||||||
| Left STG | −50,4,–4 | 2.466 | 0.0000 | 2,948 | Left insula, BA 48 (539)Left STG, BA 38 (392)Left rolandic operculum, BA 48 (226)Left MTG, BA 21 (215)Left STG, BA 48 (186) | 6/6 |
| Left temporoparietal cortex | −56,–40,18 | 2.102 | 0.0002 | 900 | Left supramarginal gyrus, BA 48 (188)Left STG, BA 42 (171) | 6/6 |
| Left calcarine cortex | −20,–66,14 | 2.447 | 0.0000 | 449 | Corpus callosum (297) | 6/6 |
| Left MFG | –32,26,40 | 2.319 | 0.0001 | 438 | 6/6 | |
| Increased GMV in DD | ||||||
| Right STG | 34,6,–26 | –1.572 | 0.0001 | 1,829 | Right STG, BA 38 (261)Right ITG, BA 20 (250)Right MTG, BA 20 (212) | 6/6 |
| Right precuneus | 12,–52,42 | –1.254 | 0.0014 | 156 | 2/6 | |
In the supplementary materials, we reported whether the structural and functional deficits found in the current study were reported in each study included in the meta-analysis (Supplementary file 1f-1i).
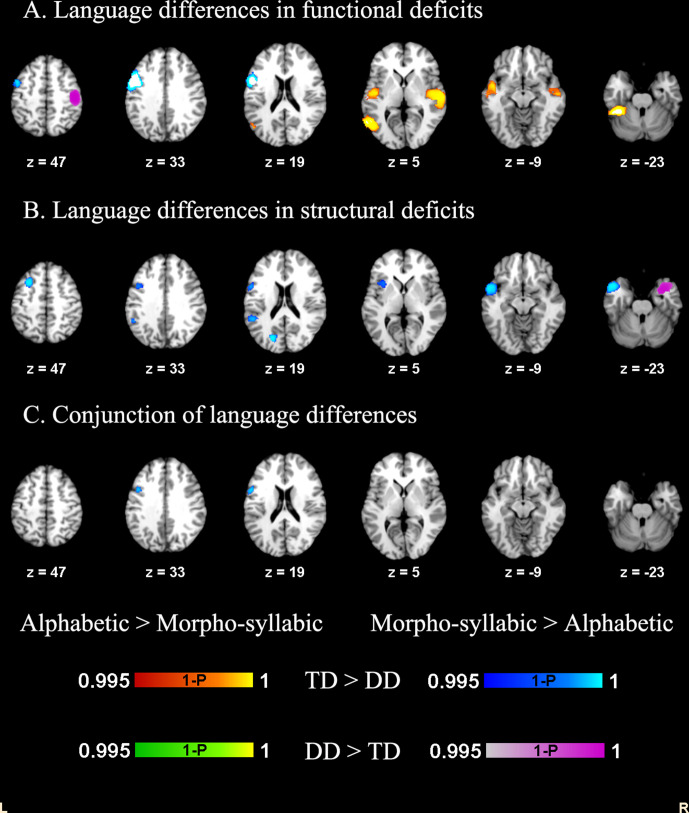
Comparison between alphabetic and morpho-syllabic languages
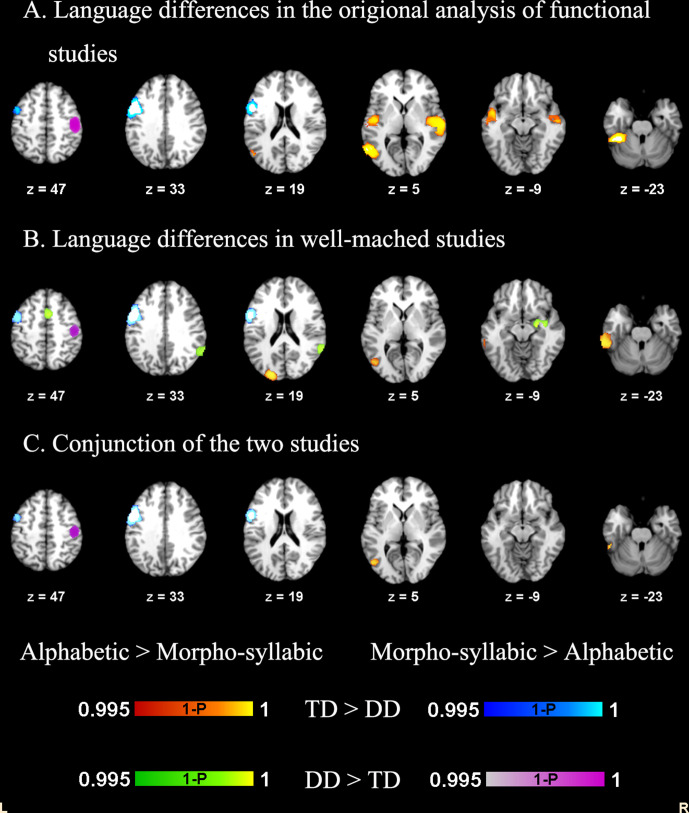
For the direct comparison between the morpho-syllabic and alphabetic groups in functional studies, we found greater reduction of brain activation in alphabetic languages than in morpho-syllabic languages in the left MTG, right STG and left fusiform gyrus. We found greater reduction of brain activation in morpho-syllabic languages than in alphabetic languages in the left IFG, opercular part and greater increase of brain activation in DD in morpho-syllabic languages than in alphabetic languages in the right precentral gyrus (Table 5, Figure 2A).
Table 5. Direct comparisons between alphabetic languages and morpho-syllabic languages in functional studies.
| Regions | MNI coordinate | SDM-Z | p | Voxels | Cluster breakdown (Voxels) |
|---|---|---|---|---|---|
| Hypoactivation in DD | |||||
| Alphabetic languages > Morpho-syllabic languages | |||||
| Left MTG | −54,–62,8 | 1.203 | 0.0000 | 2,173 | Left MTG, BA 37 (453)Left MTG, BA 48 (294)Left MTG, BA 21 (293)Corpus callosum (184) |
| Right STG | 60,–18,4 | 1.080 | 0.0000 | 2047 | Right STG, BA 22 (400)Corpus callosum (373)Right insula, BA 48 (278)Right STG, BA 48 (259)Right rolandic operculum, BA 48 (193) |
| Left fusiform gyrus | −40,–42,–24 | 1.279 | 0.0000 | 924 | Left fusiform gyrus, BA 37 (198)Left ITG, BA 20 (198) |
| Morpho-syllabic languages > Alphabetic languages | |||||
| Left IFG opercular part | –48,8,30 | –3.945 | 0.0000 | 2093 | Left precentral gyrus, BA 6 (512)Left IFG opercular part, BA 44 (274)Left precentral gyrus, BA 44 (191)Corpus callosum (161)Left IFG, triangular part, BA 48 (159) |
| Hyperactivation in DD | |||||
| Morpho-syllabic languages > Alphabetic languages | |||||
| - | - | - | - | - | - |
| Morpho-syllabic languages > Alphabetic languages | |||||
| Right precentral gyrus | 40,–20,54 | –2.262 | 0.0000 | 1,518 | Right precentral gyrus, BA 6 (525)Right precentral gyrus, BA 4 (306)Right postcentral gyrus, BA 3 (286)Right postcentral gyrus, BA 4 (171) |
Figure 2. Direct comparisons between the alphabetic group and the morpho-syllabic group in structural and functional deficits.
Conjunction analysis showed greater reduction of both GMV and brain activation in the left dorsal IFG in morpho-syllabic languages than alphabetic languages.
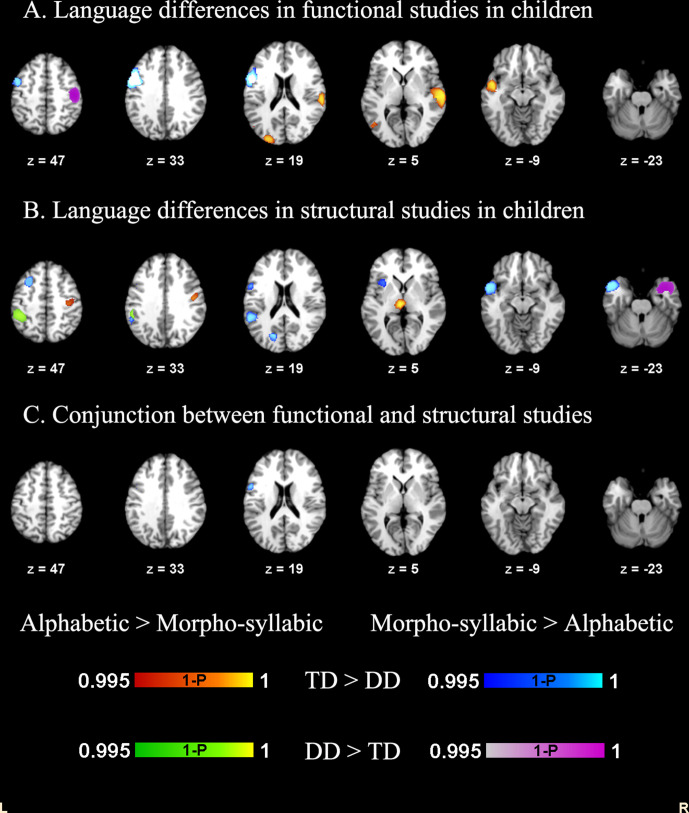
Figure 2—figure supplement 1. Language differences in functional studies and structural studies in children.
Figure 2—figure supplement 2. Functional deficits in children with DD in each group and common deficits between them.
Figure 2—figure supplement 3. Language differences between the two well-matched groups in the confirmation analysis and how the current results overlap with the original results.
For the direct comparison between the morpho-syllabic and alphabetic groups in structural studies, we found greater reduction of GMV in DD in morpho-syllabic languages than in alphabetic languages in the left STG, left IFG opercular part, left MFG, left supramarginal gyrus, left superior occipital gyrus (SOG) and left insula. We also found greater increase of GMV in DD in morpho-syllabic languages than in alphabetic languages in the right STG and left ITG (Table 6, Figure 2B). We found no regions that showed greater GMV alterations in alphabetic languages than in morpho-syllabic languages.
Table 6. Direct comparisons between alphabetic languages and morpho-syllabic languages in structural studies.
| Regions | MNI coordinate | SDM-Z | P | Voxels | Cluster breakdown (Voxels) |
|---|---|---|---|---|---|
| Decreased GMV in DD | |||||
| Alphabetic languages > Morpho-syllabic languages | |||||
| - | - | - | - | - | - |
| Morpho-syllabic languages > Alphabetic languages | |||||
| Left STG | −50,10,–18 | –3.139 | 0.0000 | 1,218 | Left STG, BA 38 (322) |
| Left IFG opercular part | –52,10,24 | –2.253 | 0.0008 | 409 | |
| Left MFG | –28,16,44 | –2.888 | 0.0001 | 346 | |
| Left supramarginal gyrus | −48,–44,24 | –2.761 | 0.0001 | 344 | |
| Left SOG | −20,–72,20 | –2.911 | 0.0001 | 277 | Corpus callosum (187) |
| Left insula | –32,14,8 | –2.046 | 0.0015 | 179 | |
| Increased GMV in DD | |||||
| Alphabetic languages > Morpho-syllabic languages | |||||
| - | - | - | - | - | - |
| Morpho-syllabic languages > Alphabetic languages | |||||
| Right STG | 34,6,–28 | –1.893 | 0.0001 | 1,397 | Right STG, BA 38 (246) |
| Left ITG | −54,–58,–16 | –1.112 | 0.0018 | 161 | |
To identify the common language differences between the structural and functional studies, we conducted a conjunction analysis between the language differences in structural studies and functional studies. This produced an overlap of 377 voxels in the left IFG opercular part, with a peak at (–52, 10, 24), indicating greater reduction of both GMV and brain activation in morpho-syllabic languages than in alphabetic languages (Figure 2C).
Multimodal analysis results in alphabetic and morpho-syllabic languages
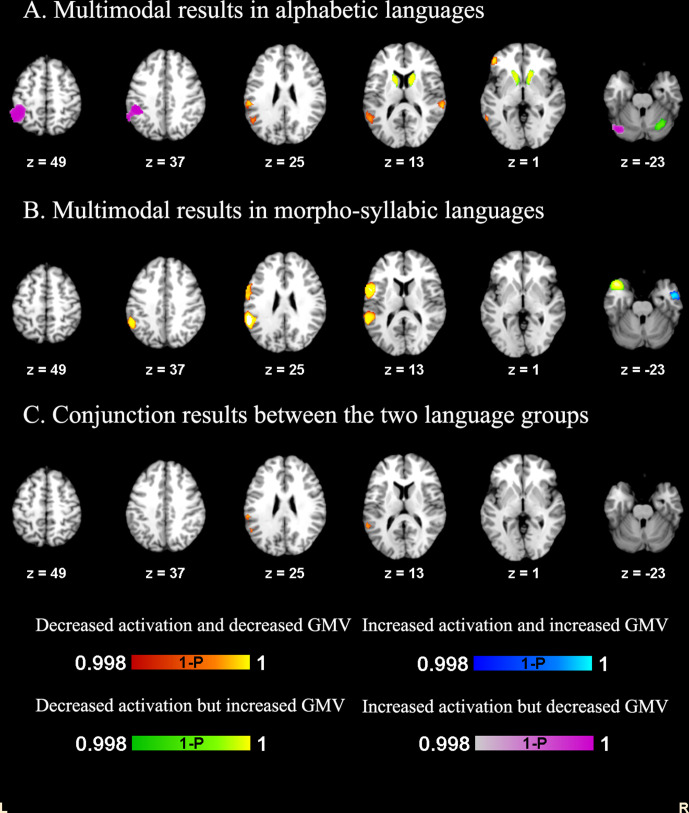
Multimodal meta-analysis in alphabetic languages showed that decreased GMV and hypoactivation in DD were found in the bilateral STG and left IFG triangular part; no regions showed increased GMV and hyperactivation; increased GMV and hypoactivation in DD were found in left IPL and left cerebellum; decreased GMV and hyperactivation in DD were found in bilateral caudate and right cerebellum (Table 7, Figure 3A). Multimodal meta-analysis in morpho-syllabic languages showed that decreased GMV and hypoactivation in DD were found in the left STG and left IFG opercular part; increased GMV and hyperactivation in DD were found in the right MTG; decreased GMV and hyperactivation in DD were found in left STG; no regions showed increased GMV and hypoactivation (Table 8, Figure 3B).
Table 7. Multimodal structural and functional abnormalities in individuals with DD in alphabetic languages.
| Regions | MNI coordinate | Voxels | Cluster breakdown (Voxels) |
|---|---|---|---|
| Decreases of GMV and hypoactivation in DD | |||
| Left STG | −52,–30,20 | 1,099 | Left MTG, BA 21 (237) |
| Right STG | 62,–32,14 | 322 | |
| Left IFG, triangular part | –46,42,0 | 219 | |
| Increases of GMV and hypoactivation in DD | |||
| Left IPL | −46,–40,38 | 1,689 | Left IPL, BA 40 (941) |
| Left cerebellum | −40,–70,–24 | 446 | Left cerebellum, crus I, BA 19 (159) |
| Decreases of GMV and hyperactivation in DD | |||
| Right cerebellum | 28,–52,–34 | 1,286 | Right cerebellum, lobule VI, BA 37 (273)Middle cerebellar peduncles (267)Right cerebellum, lobule VI, BA 19 (204) |
| Right caudate | 8,8,12 | 600 | Right anterior thalamic projections (214)Right caudate nucleus (189) |
| Left caudate | –16,8,14 | 595 | Left anterior thalamic projections (353) |
Figure 3. Structural and functional deficits in DD for alphabetic languages and morpho-syllabic languages.
Decreased GMV and brain activation were found in both groups in the left STG.
Table 8. Multimodal structural and functional abnormalities in individuals with DD in morpho-syllabic languages.
| Regions | MNI coordinate | Voxels | Cluster breakdown (Voxels) | |
|---|---|---|---|---|
| Decreases of GMV and hypoactivation in DD | ||||
| Left STG | −58,–38,22 | 1,566 | Left supramarginal gyrus, BA 48 (254)Left STG, BA 42 (253)Left supramarginal gyrus, BA 40 (153) | |
| Left IFG opercular part | –56,2,10 | 1,052 | Left IFG opercular part, BA 44 (151) | |
| Decreases of GMV and hyperactivation in DD | ||||
| Left STG | −44,16,–22 | 854 | Left STG, BA 38 (394) | |
| Increases of GMV and hyperactivation in DD | ||||
| Right MTG | 48,–6,–26 | 493 | Right MTG, BA 21 (178) | |
To identify the common multimodal deficits in alphabetic languages and morpho-syllabic languages, we conducted a conjunction analysis of the thresholded multimodal maps of the two types of writing systems. This procedure produced an overlap of 482 voxels in the left STG, which peaked at (−54,–34, 20), indicating shared reduction of GMV and hypoactivation in both types of writing systems (Figure 3C).
Confirmation analysis results
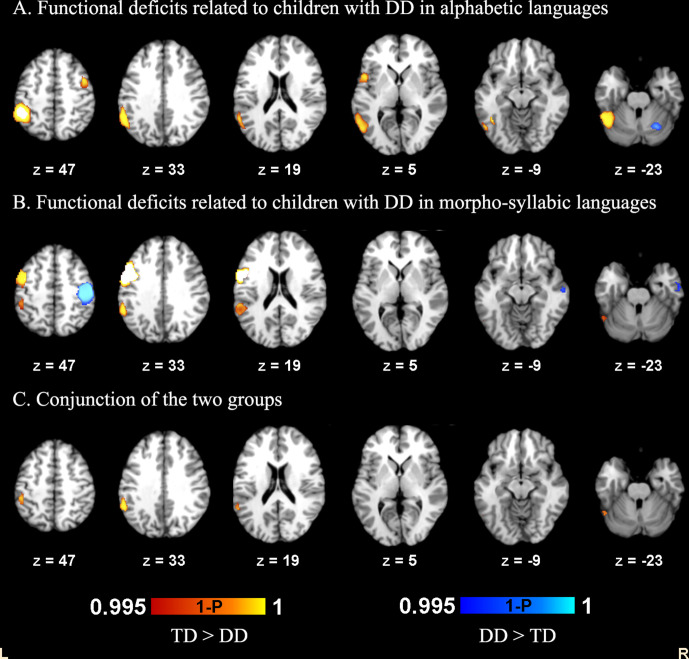
For the confirmation analysis on children only in the alphabetic group, when we compared children in alphabetic languages to children in morpho-syllabic languages, we found a conjunction of the language differences in the functional studies and the structural studies, which was greater reduction of brain activation and GMV in morpho-syllabic languages than in alphabetic languages in the left IFG opercular part (–54, 10, 20) with a cluster of 273 voxels (Figure 2—figure supplement 1C), which is consistent with the original result. Conjunction analysis of the functional alterations in the alphabetic group and the morpho-syllabic group revealed common reduction of brain activation in children with DD in the left ITG (−48,–56, –18) and the left TP area (−56,–44, 32) with a cluster size of 291 voxels and 778 voxels, respectively. The TP area overlapped with the original multimodal result at the left STG (−56,–48, 22) where both language groups showed reduced brain activation and GMV. Taken together, these results are consistent with the original results, suggesting that the language differences are not due to unmatched age range. For detailed results of the confirmation analysis, please see Supplementary file 1a-1d, Figure 2—figure supplement 1, Figure 2—figure supplement 2.
Furthermore, the other confirmation analysis on functional studies of two well-matched subgroups on age, task and number of studies also confirmed our original findings. Conjunction analysis between the original results and the current functional differences between English and Chinese showed consistent greater reduction of brain activation in DD in morpho-syllabic languages/Chinese than in alphabetic languages/English in the left dorsal IFG (–52, 6, 16) with a cluster size of 1966 voxels. There was consistent greater reduction of brain activation in DD in alphabetic languages/English than in morpho-syllabic languages/Chinese in the left MTG (−48,–68, 4) and left MOG (−51,–41, –24) with a cluster size of 166 voxels and 90 voxels, respectively. Consistent greater increase of brain activation in morpho-syllabic languages/Chinese than in alphabetic languages/English was also found in the right precentral gyrus (44, -22, 50), and the cluster size was 792 voxels (Figure 2—figure supplement 3C). For detailed results of this confirmation analysis, please see Supplementary file 1e, Figure 2—figure supplement 3.
Discussion
In this meta-analysis study, we examined the convergence and divergence between the brain structural and functional deficits associated with DD as well as whether the deficits are consistent across languages. We found that readers with DD showed both GMV reduction and functional hypoactivation in the left TP and ventral IFG in alphabetic languages, readers with DD showed both GMV reduction and functional hypoactivation in the left TP and dorsal IFG in morpho-syllabic languages, among which, the left STG was a shared impairment across all languages, and the dorsal left IFG showed a greater impairment in morpho-syllabic languages than in alphabetic languages, suggesting both language-universal and language-specific deficits in the brain. We also found GMV increase and functional hyperactivation in the right anterior MTG/ITG region in morpho-syllabic languages; however, conjunction analysis between morpho-syllabic languages and alphabetic languages did not reveal any overlap. In addition to the consistent structural and functional alterations, we also detected inconsistent structural and functional alterations. Individuals with DD showed increased GMV and hypoactivation in the left IPL and left cerebellum, and decreased GMV and hyperactivation in the bilateral caudate in alphabetic languages, but decreased GMV and hyperactivation in left STG in morpho-syllabic languages. However, conjunction analysis between morpho-syllabic languages and alphabetic languages did not reveal any overlap.
Convergent structural and functional impairment across writing systems
Across writing systems, convergent structural and functional deficit was found in the left STG due to reduced GMV and brain activation in both alphabetic languages and morpho-syllabic languages. Further confirmation analysis confirmed that this is a stable deficit across children and adults. This is consistent with previous meta-analysis studies (Maisog et al., 2008; McGrath and Stoodley, 2019; Paulesu et al., 2014; Richlan et al., 2013). The left STG is a very important component in the language network (Friederici, 2012; Hickok and Poeppel, 2007) as well a key region in the reading network (Pugh et al., 2000; Richlan, 2014). It is involved in phonological representation and phonological processing during both spoken language processing and reading (Bolger et al., 2005; Enge et al., 2020; Tan et al., 2005). Recent research has suggested that proficient reading is characterized by convergence between speech and print at this region regardless of languages, as multivariate brain activity patterns are similar for speech and print at this region (Chyl et al., 2021). Therefore, the reading network may develop based on the built-in language circuit, as reading is a skill that humans acquire too late in the course of human evolution to have a brain network dedicated to it. Recently, a growing number of studies have investigated early signs of dyslexia before the onset of reading and found that structural and functional deficits in the left TP area and left inferior frontal cortex appear before reading onset (Clark et al., 2014; Hosseini et al., 2013; Plewko et al., 2018; Raschle et al., 2012; Raschle et al., 2014; Vandermosten et al., 2019). It further suggests that DD might be due to early abnormality in the language network. Specifically, Skeide et al., 2018 found hypermyelination in the left auditory cortex in readers with DD using ultra-high-field MRI at 7T, and disrupted neural firing induced by hypermyelination in the layer IV of the auditory cortex, which may cause hypoactivation in the left STG. The left STG actually serves as an important hub in the language and reading network (Fernández et al., 2020), which connects the inferior frontal network and the OT network through a dorsal pathway and a ventral pathway (Brauer et al., 2013; Cummine et al., 2015). Disconnection with the left STG has been verified in task-related functional connectivity studies (Boets et al., 2013; Cao et al., 2017; Schurz et al., 2015) and a resting-state functional connectivity study (Schurz et al., 2015), as well as in a meta-analysis of DTI studies (Vandermosten et al., 2012). Our finding suggests that dyslexia is associated with structural and functional abnormalities of the left STG regardless of language. The evidence suggests that this is a neural signature of DD, which supports the phonological deficit hypothesis (Shaywitz et al., 1998).
However, we failed to find consistent structural and functional deficits in the OT area. The main reason was that there was no structural alteration but only functional reduction at this area. The OT area is a key region for orthographic recognition during visual word processing (Glezer et al., 2009; Glezer et al., 2016; Hirshorn et al., 2016; Nobre et al., 1994) and was reported to be impaired in individuals with DD (McCrory et al., 2005; Richlan et al., 2010; Wandell et al., 2012). The specialization of this region for orthographic processing is developed along with reading acquisition (Brem et al., 2010), and the dysfunction of the OT area in DD is possibly a result of reading failure (Pugh et al., 2000). A recent meta-analysis of VBM studies (McGrath and Stoodley, 2019) also failed to detect structural deficit in the OT area, which is consistent with our finding. Taken together, the lack of structural deficits with only hypoactivation at the OT area appears to suggest that the visuo-orthographic deficits at the OT might be a consequence of being DD. In contrast, the left STG which was discussed above, appears to be associated with the cause of DD. Our results provide further support for the phonological deficit hypothesis that phonological deficit is the primary deficit and other deficits may be a result of the phonological deficit (Pugh et al., 2000).
Language differences in structural and functional alterations
The left dorsal IFG which peaked at (–52, 10, 24) showed greater reduction in both GMV and brain activation in morpho-syllabic languages than in alphabetic languages, suggesting greater impairment in this region in morpho-syllabic languages than in alphabetic languages. This finding is also verified in the confirmation analyses. Previously, many Chinese studies have reported impairment at the dorsal left IFG, for example, reduced brain activation in an auditory rhyming judgment task in children with DD at (–44, 10, 26) (Cao et al., 2017), in a lexical decision task at (–44, 3, 29) (Siok et al., 2004), in a homophone judgment task at (–55, 5, 22) (Siok et al., 2008), and in a morphological task at (–36, 8, 26) (Liu et al., 2013a). This left dorsal IFG has been believed to be more involved in Chinese reading than in alphabetic languages, with a peak at the left MFG (–46, 18, 28) as reported in a previous meta-analysis study (Tan et al., 2005). The dorsal IFG was found to be involved in phonological processing in Chinese reading (Wu et al., 2012), and it is thought to be related to addressed phonology during Chinese character reading (Tan et al., 2005). Our study adds to the literature that by direct comparison, this region does show greater deficit in individuals with DD in morpho-syllabic languages than in alphabetic languages in terms of both brain activation and GMV. This might be due to the fact that healthy Chinese readers have increased GMV and brain activation in the left dorsal IFG than healthy alphabetic readers, because the features of Chinese require greater involvement of this region in reading than alphabetic languages due to the whole-character-to-whole-syllable mapping. Actually, two cross-linguistic studies have argued that different findings of DD in different languages are actually driven by the fact that control readers show language-specific brain activation patterns (Feng et al., 2020; Hu et al., 2010), and that brain activation in individuals with DD is actually the same across languages. For example, Hu et al., 2010 found that Chinese control readers showed greater activation in the left IFG, and English control readers showed greater activation in the left superior temporal sulcus; however, children with DD in Chinese and English showed similar brain activation in these two regions. Therefore, readers with DD fail to show language specialization due to their limited reading experience and skills. In summary, this language-specific deficit is believed to be a consequence of being DD in learning morpho-syllabic languages, indicating their inability to accommodate to their own writing system.
In the direct comparison between alphabetic and morpho-syllabic languages, we also found greater hypoactivation in DD in alphabetic languages than in morpho-syllabic languages in the left MTG, right STG and left fusiform gyrus, which was verified in the well-matched confirmation analysis, suggesting that the language difference should not be due to differences in age, tasks, and number of studies in the two language groups. Our finding is consistent with previous neuroimaging studies that revealed reduced activation associated with DD in the posterior reading network in alphabetic languages (Paulesu et al., 2001; Richlan et al., 2010; Vandermosten et al., 2019), suggesting deficient orthographic and phonological processing. However, the novelty of the current study is to demonstrate greater severity of deficit in these regions in alphabetic languages than in morpho-syllabic languages. In a previous meta-analysis study of alphabetic languages, it was found that there was greater hypoactivation in the left fusiform gyrus (−40,–42, –16) in shallow orthographies than in deep orthographies (Martin et al., 2016). The explanation is that this region is associated with bottom-up rapid processing of letters, because it was found that at a proximal region (−38,–50, –16), there was a word length effect for German nonwords in non-impaired readers (Schurz et al., 2010). Moreover, the left fusiform gyrus has been found to be more involved in English reading than in Chinese reading in typical mature readers with a peak of the effect at (−44,–56, –12) (Tan et al., 2005). Therefore, the left fusiform gyrus is important for letter-by-letter orthographic recognition in alphabetic languages, and this explains why we found greater deficit in this region in alphabetic languages than in morpho-syllabic languages. As for the right STG, the previous meta-analysis found greater hypoactivation in deep orthographies than shallow orthographies (Martin et al., 2016). Together with our finding, it suggests that the right STG might be associated with the inconsistent mapping between graphemes and phonemes in deep orthographic alphabetic languages. In summary, these greater deficits in alphabetic languages than in morpho-syllabic languages might be due to the inability to adapt to the special features of alphabetic languages in individuals with DD.
For the structural studies, we found greater GMV alterations in morpho-syllabic languages than in alphabetic languages, including greater GMV reduction in the left STG, left MFG, left supramarginal gyrus, left SOG and left insula, as well as greater GMV increase in the right STG and ITG. However, considering the limited number of studies included in the morpho-syllabic language group and inconsistent results with functional studies, the results should be interpreted with caution.
Increased GMV and hyperactivation
In the multi-modal meta-analysis, we found increased GMV and hyperactivation in participants with DD in the right MTG which was driven by the morpho-syllabic languages. For the functional studies, we also found greater hyperactivation in the right precentral gyrus in morpho-syllabic languages than in alphabetic languages. These alterations might be related to the compensation mechanism of the right hemisphere. As precentral gyri play an important role in articulation (Dronkers, 1996), overactivation in the precentral gyrus is interpreted as an articulation strategy used by individuals with DD to compensate for their deficient phonological processing (Cao et al., 2018; Shaywitz et al., 1998; Waldie et al., 2013). The compensation in the right MTG is developed in morpho-syllabic languages presumably due to the tight connection between orthography and semantics (Wang et al., 2015). Substantial evidence has shown that dyslexia was often accompanied by excessive activation of the right hemisphere (Cao et al., 2017; Cao et al., 2018; Kovelman et al., 2012; Kronschnabel et al., 2014; Yang and Tan, 2020) and reduced left lateralization of the language network (Altarelli et al., 2014; Bloom et al., 2013). Furthermore, training studies have also found increased activation in many regions in the right hemisphere in individuals with DD after reading intervention (Barquero et al., 2014; Meyler et al., 2008), suggesting the compensatory role of the right hemisphere when the left language/reading network is deficient (Coslett and Monsul, 1994; Weiller et al., 1995). However, according to the previous meta-analysis study, different regions showed overactivation in different writing systems (Martin et al., 2016). In particular, the left anterior insula showed greater overactivation in deep orthographies while the left precentral gyrus showed greater overactivation in shallow orthographies in individuals with DD. Taken together, it suggests that different compensatory mechanisms are developed depending on the characteristics of the writing system as well as learning experiences, and the compensation in the right MTG and right precentral gyrus appears to be particularly salient in morpho-syllabic languages. However, it is also possible that the increased GMV and hyperactivation in individuals with DD are due to some fundamental deficits rather than compensation. Further research is needed to understand the nature of these alterations by running brain-behavioral correlation and/or employing longitudinal designs.
Divergent structural and functional alterations in DD
In the multimodal analysis, we also found divergent structural and functional changes related to DD, including the left IPL and left cerebellum where there was increased GMV and hypoactivation and bilateral caudate where there was reduced GMV and hyperactivation in alphabetic languages; There was decreased GMV and hyperactivation in left STG in morpho-syllabic languages. This is in line with a recent study which found a dissociation between the developmental changes of brain structure and function (Siok et al., 2020), suggesting that learning experience may sometimes shape the brain function independent of the brain structure.
The left IPL
We found increased GMV and hypoactivation in DD in the left IPL in alphabetic languages. Consistent hypoactivation in the left IPL in DD has been documented in previous studies (Maisog et al., 2008; Martin et al., 2016). Furthermore, it was found that the deficit of the left IPL was greater in children than in adults with DD (Richlan et al., 2011), suggesting that the functional impairment of the left IPL may gradually recover with development. This may be related to the transfer of the reading circuit from the dorsal pathway to the ventral pathway over development (Younger et al., 2017). Control children activate the left IPL to a greater degree than control adults because they rely more on the dorsal pathway. Therefore, children with DD show a great reduction in the left IPL in comparison to adults with DD. Alternatively, the left IPL has been found to be deactivated during language tasks (Cao et al., 2008; Cao et al., 2017; Meyler et al., 2008; Schulz et al., 2009), and this is due to the nature of the default mode network (Laird et al., 2009), which is deactivated during active tasks. Therefore, it might be the case that the increased GMV in individuals with DD increases inhibitory inputs received by the IPL, which results in greater deactivation.
For structural studies involving the left IPL, the results are inconsistent. The GMV of the left supramarginal gyrus around the posterior part of perisylvian cortex was found to be reduced in individuals with DD (Linkersdörfer et al., 2012; McGrath and Stoodley, 2019) and it showed a positive correlation with reading accuracy only in normal readers (Jednorog et al., 2015). However, the GMV of the left inferior parietal cortex excluding the supramarginal and the angular was found to increase in individuals with DD (McGrath and Stoodley, 2019) and a study showed that the volume of the left inferior parietal cortex in control readers was negatively correlated with reading level (Houston et al., 2014). The IPL in the current study is outside the supramarginal and angular gyrus; therefore, it is consistent with the previous findings that there is increased GMV in individuals with DD.
The cerebella
Increased GMV and hypoactivation in DD were also found in the left cerebellum in alphabetic languages; however, in the right cerebellum, we found decreased GMV and hyperactivation. Previously, it was found that the right cerebellum is greater in size than the left cerebellum in healthy controls while the asymmetry is reduced in individuals with DD (Kibby et al., 2008; Rae et al., 2002). This is consistent with our finding of increased GMV in the left cerebellum and decreased GMV in the right cerebellum in individuals with DD, suggesting reduced asymmetry in cerebellum.
The cerebella have been found to play an important role in inner speech, automatization in reading and suppression of overt articulatory movement in silent reading (Ait Khelifa-Gallois et al., 2015). Functional abnormality of cerebellum in DD has been reported repeatedly; however, hyperactivation was reported more often in the right cerebellum (Feng et al., 2017; Hernandez et al., 2013; Kronschnabel et al., 2014; Richlan et al., 2010; Rumsey et al., 1997; van Ermingen-Marbach et al., 2013a), while hypoactivation was reported more often in the left cerebellum (Christodoulou et al., 2014; McCrory et al., 2000; Olulade et al., 2012; Reilhac et al., 2013; Siok et al., 2008). This is consistent with our finding of hyperactivation in the right cerebellum and hypoactivation in the left cerebellum. Different alteration patterns in the left and right cerebellum suggest that they may play different roles in reading and dyslexia. The right cerebellum has been found to be connected with the left frontal-parietal pathway for phonological processing and with the left frontal-temporal pathway for semantic processing (Alvarez and Fiez, 2018; Gatti et al., 2020). The left cerebellum, however, is involved in error monitoring during reading unfamiliar non-words (Ben-Yehudah and Fiez, 2008), as well as articulation related movement process, since it is activated in reading aloud but not in lexical decision (Carreiras et al., 2007). Richards et al., 2006 argued that the left cerebellum is involved in processing the morphology of word forms, and the right cerebellum is involved in phonological processing. Therefore, hyperactivation in the right cerebellum in readers with DD suggests that they may use it as a compensation for their deficient phonological processing, while hypoactivation in the left cerebellum may suggest reduced error monitoring in readers with DD. The finding of neurological alterations in the cerebellum is consistent with the previous findings of cerebellar deficit (Menghini et al., 2006; Yang et al., 2013) for which, some researchers argued that impaired articulatory motor control in the cerebellum leads to reading impairment (Nicolson et al., 2001; Stoodley and Stein, 2011). It also should be noticed that the cerebellum showed divergent patterns in structural studies and functional studies. Taken together, these results implicate the necessity of considering DD from a broader spectrum of developmental disorders.
It is still unclear why there is increased GMV but decreased activation in some brain regions. It may be due to the following reasons: (1) increased dendrites receiving more inhibitory input from other neurons; (2) abnormal neuronal migration deactivated the firing of neurons as a result of disrupted local microcircuits (Giraud and Ramus, 2013); (3) weaker input from other regions deactivated the target region and changed the structure of the region (Wang et al., 2019).
The caudate
We also found decreased GMV and hyperactivation in readers with DD in the bilateral caudate in alphabetic languages. Previous studies have observed decreased GMV (Brown et al., 2001; Jagger-Rickels et al., 2018; McGrath and Stoodley, 2019; Tamboer et al., 2015) and hyperactivation in bilateral caudate in individuals with DD (Martin et al., 2016; Olulade et al., 2012; Pekkola et al., 2006; Richlan et al., 2010; Richlan et al., 2011; Rumsey et al., 1997). However, there are also studies that observed different patterns (Cheema et al., 2018), such as decreased activation in caudate (Perrachione et al., 2016). Furthermore, the GMV volume of the caudate in individuals with DD was found to be positively correlated with reading performance (Pernet et al., 2009; Tamboer et al., 2015), and the left caudate’s activation was correlated with longer reaction time in word reading only in individuals with DD (Cheema et al., 2018). The caudate plays an important role in procedural learning and phonological processing (Grahn et al., 2008; Tettamanti et al., 2005; Ullman et al., 2020). Decreased GMV and increased activation at the bilateral caudate might be caused by reduced dendrites and reduced inhibitory inputs received in individuals with DD (Achal et al., 2016; Finn et al., 2014). It may also be due to pre-existing local structural deficit leading to compensatory hyperactivation of the remaining part of the caudate. GMV reduction in basal ganglia was found in many other neuropsychiatric disorders, such as attention-deficit hyperactivity disorder (Frodl and Skokauskas, 2012; Mous et al., 2015; Nakao et al., 2011), autism spectrum disorder (Nickl-Jockschat et al., 2012) and major depression disorder (Husain et al., 1991; Lu et al., 2016). Altered myelination and neurotransmitters may contribute to the structural and functional alterations related to basal ganglia (Nord et al., 2019; Wichmann and DeLong, 2012).
Conclusion
We found convergent functional and structural alterations in the left STG across different writing systems, suggesting a neural signature of DD, which might be associated with phonological deficit. We also found greater functional and structural alteration in the left dorsal IFG in morpho-syllabic languages than alphabetic languages, suggesting a language-specific effect of DD, which might be related to the special feature of whole-character-to-whole-syllable mapping in morpho-syllabic languages.
Limitation
In this meta-analysis, we found convergent and divergent functional and structural alterations across writing systems. However, due to the limitations of voxel-based neuroimaging meta-analysis, the peak coordinate only provides limited information, therefore, future image-based meta-analysis studies should be conducted with full statistical images of the original studies (Muller et al., 2018).
Materials and methods
Literature retrieval and data extraction
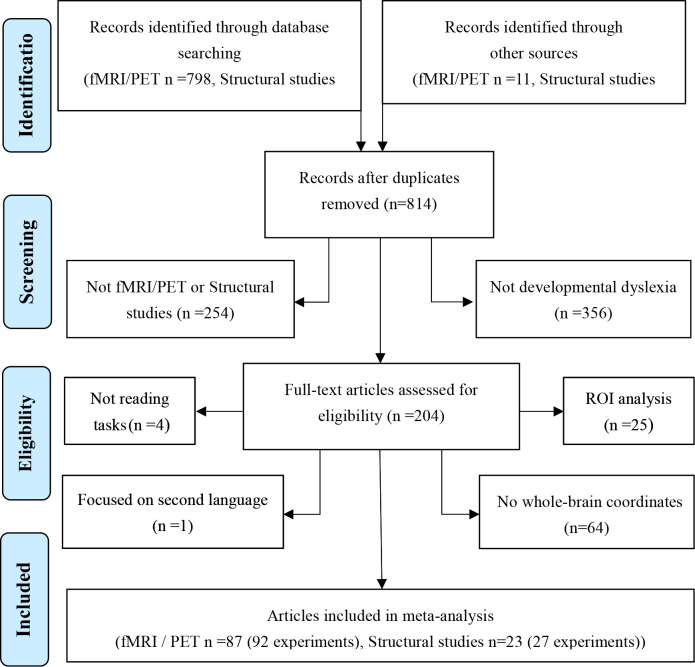
We searched in ‘PubMed’ (http://www.pubmed.org) and ‘Web of science’ for neuroimaging studies published from January 1986 to January 2020 using a combination of a condition term (i.e. dyslexia, reading disorder, reading impairment, reading difficulty or reading disability) and a technical term (i.e. fMRI, PET, voxel-based morphometry, VBM, or neuroimaging), for example, ‘dyslexia’ and ‘fMRI’. See the full list of key word combinations in the Supplementary file 2. Additionally, we manually added studies by checking the references of the selected papers that were missed in the search. The inclusion criteria were: (1) PET, fMRI, voxel-based morphometry (VBM) studies or structural studies using a volumetric FreeSurfer pipeline, (2) whole-brain results were reported, (3) direct group comparisons between readers with DD and age control readers were reported, (4) coordinates were reported in Talairach or MNI stereotactic space, and (5) studies on DD in the first language. The exclusion criteria were (1) studies with only ROI analysis, (2) resting-state studies, (3) studies that only included readers with DD or did not report group differences, (4) studies with direct group comparisons only between readers with DD and reading level control readers, (5) studies on children at risk for dyslexia, and (6) studies focused on non-linguistic tasks (Evans et al., 2014b; Margolis et al., 2020; Menghini et al., 2006; Yang et al., 2013). Finally, 119 experiments from 110 papers were included in this meta-analysis comprising 92 brain functional experiments (from 87 papers) and 27 brain structural experiments (from 23 papers) (see Table 9; Table 10 and Figure 4 for detail). From the original publications, we extracted peak coordinates, where there is a significant difference between controls and individuals with DD either in brain activation or regional GMV. We also extracted effect sizes and other information from the publications. In order to explore the language effect, we subdivided these studies into two groups according to the native language of the participants, namely, an alphabetic language group in which writing symbols represent phonemes, and a morpho-syllabic language group in which each writing symbol represents a morpheme with a syllable. This procedure resulted in 79 functional and 21 structural experiments for the alphabetic language group and 12 functional and six structural experiments for the morpho-syllabic language group.
Table 9. Functional studies included in the meta-analysis.
| Studies | N(TD) | N(DD) | Age in months | Language | Writing system | Subject type | Tasks | Threshold | |
|---|---|---|---|---|---|---|---|---|---|
| Voxel-wise | Cluster-wise | ||||||||
| Bach et al., 2010 | 18 | 14 | 99.6 | German | Alphabetic | Children | Covert reading task | p < 0.005 | 24 voxels* |
| Beneventi et al., 2009 | 13 | 11 | 160.4 | Norwegian | Alphabetic | Children | Sequential verbal working memory task | p < 0.001 | 10 voxels |
| Beneventi et al., 2010a; | 13 | 11 | 160.4 | Norwegian | Alphabetic | Children | n-back task (Letter) | FDR p < 0.05 | 5 voxels |
| Beneventi et al., 2010b | 14 | 12 | 160.3 | Norwegian | Alphabetic | Children | n-back task (Picture) | FDR p < 0.05 | |
| Blau et al., 2009 | 13 | 13 | 301.8 | Dutch | Alphabetic | Adults | Letter–speech-sound integration task | p < 0.001 | 160 mm3* |
| Booth et al., 2007a | 13 | 13 | 126.0 | English | Alphabetic | Children | Word judgment task | p < 0.001 | 15 voxels |
| Boros et al., 2016 | 17 | 12 | 129.7 | French | Alphabetic | Children | String detection and passive reading task | p < 0.001 | FDR p < 0.05 |
| Brambati et al., 2006 | 11 | 13 | 368.5 | Italian | Alphabetic | Adults and adolescents | Word reading and pseudoword reading | p < 0.001 | 20 voxels |
| Brunswick et al., 1999 | 6 | 6 | 277.2 | English | Alphabetic | Adults | Explicit reading task | p < 0.001 | |
| Brunswick et al., 1999 | 6 | 6 | 294.0 | English | Alphabetic | Adults | Implicit reading task | p < 0.001 | |
| Cao et al., 2008 | 12 | 12 | 148.2 | English | Alphabetic | Children | Visual word rhyming task | p < 0.001 | 10 voxels |
| Cao et al., 2017 | 13 | 17 | 134.0 | Chinese | Morpho-syllabic | Children | Auditory rhyming task | p < 0.001 | FDR p < 0.05 |
| Cao et al., 2018 | 19 | 23 | 132.9 | Chinese | Morpho-syllabic | Children | Visual spelling task | p < 0.001 | FDR p < 0.05 |
| Cao et al., 2020 | 17 | 16 | 137.3 | Chinese | Morpho-syllabic | Children | Visual rhyming task | p < 0.001 | FDR p < 0.05 |
| Christodoulou et al., 2014 | 12 | 12 | 274.8 | English | Alphabetic | Adults | Sentence reading | p < 0.001 | FDR corrected |
| Chyl et al., 2019 | 24 | 24 | 105.4 | Polish | Alphabetic | Children | Visual word reading | p < 0.001 | 50 voxels* |
| Conway et al., 2008 | 11 | 11 | 420.0 | English | Alphabetic | Adults | Auditory working memory task | p < 0.005 | 150 mL |
| Cutting et al., 2013 | 19 | 20 | 147.02 | English | Alphabetic | Adolescents | Lexical decision task | p < 0.005 | 34 voxels |
| Danelli et al., 2017 | 23 | 20 | 250.55 | Italian | Alphabetic | Adults | Pseudoword reading, auditory letter-name rhyming task, visual motion stimulation task and motor sequence learning task | p < 0.001 | FWE p < 0.05 |
| Desroches et al., 2010 | 12 | 12 | 137.4 | English | Alphabetic | Children | Auditory rhyming task | p < 0.001 | 15 voxels |
| Dufor et al., 2007 | 16 | 14 | 344.6 | French | Alphabetic | Adult | Auditory phoneme categorization task | p < 0.01 | |
| Eden et al., 2004 | 19 | 19 | 512.4 | English | Alphabetic | Adults | Word repetition task and initial sound deletion task | p < 0.001 | 80 voxels |
| Farris et al., 2016 | 16 | 15 | 112.2 | English | Alphabetic | Children | Object rhyming task | p < 0.001 | 10 voxels** |
| Feng et al., 2017 | 20 | 14 | 123.1 | Chinese | Morpho-syllabic | Children | Character spelling task and character rhyming task | p < 0.001 | 12 voxels* |
| Francisco et al., 2018 | 20 | 21 | 303.7 | Dutch | Alphabetic | Adults | 1-back task | p < 0.001 | FWE p < 0.05 |
| Gaab et al., 2007 | 23 | 22 | 127.8 | English | Alphabetic | Children | Sound discrimination task | p < 0.01 | 20 voxels |
| Georgiewa et al., 1999 | 17 | 17 | 168.0 | German | Alphabetic | Children | Letter reading task, nonwords reading task, words reading task and phonological transformation task | p < 0.05 | p < 0.05 |
| Grande et al., 2011 | 25 | 20 | 115.1 | German | Alphabetic | Children | Picture naming task and words reading task | p < 0.001 | t10 voxels |
| Grunling et al., 2004 | 21 | 17 | 162.8 | German | Alphabetic | Adolescents | Slash patterns matching task, letters matching task, words matching task, pseudoword matching task and pseudoword rhyming task | p < 0.01 | 10 voxels |
| Hancock et al., 2016 | 11 | 16 | 125.0 | English | Alphabetic | Children | Word rhyming task | p < 0.01 | 50 voxels |
| Heim et al., 2010 | 20 | 16 | 113.9 | German | Alphabetic | Children | First sound detection task, motion detection task, Posner attention task,auditory discrimination task | p < 0.001 | p < 0.05 |
| Heim et al., 2013 | 15 | 11 | 435.4 | German | Alphabetic | Adults | Overt word reading | p < 0.05 | |
| Heim et al., 2015 | 10 | 33 | 118.9 | German | Alphabetic | Children | Overt word reading | FWE p < 0.05 | 100 voxels |
| Hernandez et al., 2013 | 16 | 15 | 252.7 | French | Alphabetic | Adults | Word rhyming task and font matching task | p < 0.001* | |
| Higuchi et al., 2020 | 14 | 11 | 172.7 | Japanese | Morpho-syllabic | Adolescents | Character/picture passive viewing task | p < 0.005 | 20 voxels |
| Hoeft et al., 2006 | 10 | 10 | 133.9 | English | Alphabetic | Children | Visual word rhyming task | p < 0.001 | 10 voxels |
| Hoeft et al., 2007 | 19 | 19 | 172.8 | English | Alphabetic | Adolescents | Visual word rhyming task | p < 0.001 | 10 voxels |
| Horowitz-Kraus et al., 2016 | 9 | 10 | 120.5 | English | Alphabetic | Children | Narrative comprehension task | p < 0.001 | FWE corrected |
| Hu et al., 2010 | 8 | 8 | 171.6 | Chinese | Morpho-syllabic | Children | Sematic match task, word/ picture naming task | p < 0.001* | |
| Hu et al., 2010 | 10 | 11 | 164.5 | English | Alphabetic | Children | Sematic match task, word/ picture naming task | p < 0.001* | |
| Ingvar et al., 2002 | 9 | 9 | 287.0 | Swedish | Alphabetic | Adults | Word reading task and nonword reading task | p < 0.001 | |
| Jaffe-Dax et al., 2018 | 19 | 20 | 302.2 | Hebrew | Alphabetic | Adults | Tone frequency discrimination task | p < 0.05 corrected | |
| Kast et al., 2011 | 13 | 12 | 314.5 | German | Alphabetic | Adults | Lexical decision task | p < 0.001 | 30 voxels |
| Kovelman et al., 2012 | 12 | 12 | 108.4 | English | Alphabetic | Children | Auditory words rhyming task and auditory words matching task | p < 0.001 | 25 voxels* |
| Kronbichler et al., 2006 | 15 | 13 | 187.9 | German | Alphabetic | Adolescents | Sentence verification task | FDR p < 0.05 | 4 voxels |
| Kronschnabel et al., 2013 | 22 | 13 | 191.7 | German | Alphabetic | Adolescents | Rapid serial visual stimulation detect task | p < 0.005 | 160 voxels* |
| Kronschnabel et al., 2014 | 22 | 13 | 190.9 | German | Alphabetic | Adolescents | Target detection task | p < 0.005 | 160 voxels* |
| Landi et al., 2010 | 13 | 13 | 157.8 | English | Alphabetic | Adolescents | Rhyming task and semantic categorization task | FDR p < 0.01 | 20 voxels |
| Langer et al., 2015 | 15 | 15 | 119.4 | English | Alphabetic | Children | Sentence reading task | p < 0.005 | 50 voxels |
| Liu et al., 2012 | 11 | 11 | 142.8 | Chinese | Morpho-syllabic | Children | Word rhyming task and semantic judgment task | p < 0.0002 | 18 voxels* |
| Liu et al., 2013a | 14 | 14 | 141.8 | Chinese | Morpho-syllabic | Children | Lexical match task and character rhyming task | p < 0.001 | 10 voxels |
| Lobier et al., 2014 | 12 | 12 | 129.6 | French | Alphabetic | Adults | visual categorization of character task | p < 0.001 | 20 voxels |
| MacSweeney et al., 2009 | 7 | 7 | 343.5 | English | Alphabetic | Adults | Picture rhyming task | p < 0.01 | 20 voxels |
| Maurer et al., 2011 | 16 | 11 | 136.6 | German | Alphabetic | Children | Word matching task, pseudoword matching task, picture matching task | p < 0.01 | 30 voxels* |
| McCrory et al., 2000 | 6 | 8 | 275.0 | English | Alphabetic | Adults | Words and pseudowords production | p < 0.001, | |
| McCrory et al., 2005 | 10 | 8 | 242.0 | English | Alphabetic | Adults | Words reading and pictures naming | p < 0.05 corrected | |
| Meyler et al., 2008 | 12 | 23 | 129.6 | English | Alphabetic | Children | Sentence comprehension | p < 0.002 | 10 voxels |
| Monzalvo et al., 2012 | 23 | 23 | 130.0 | French | Alphabetic | Children | Passive picture/word viewing task and passive sentence listening task | p < 0.001 | p < 0.05 corrected |
| Olulade et al., 2012 | 9 | 6 | 247.7 | English | Alphabetic | Adults | Word rhyme task3-D spatial rotations | p < 0.005 | 10 voxels |
| Olulade et al., 2015 | 12 | 16 | 120.5 | English | Alphabetic | Children | Implicit word reading | p < 0.001 | 20 voxels |
| Paulesu et al., 2001 | 36 | 36 | 286.4 | English, French, Italian | Alphabetic | Adults | Word and non-word reading task | p< 0.001 | corrected |
| Paulesu et al., 1996 | 5 | 5 | 314.5 | English | Alphabetic | Adults | Letter rhyming and letter memory | p < 0.001 | |
| Pecini et al., 2011 | 13 | 13 | 276.0 | Italian | Alphabetic | Adults and adolescents | Rhyme-generation task | p < 0.05 corrected | 100 mm3 |
| Pekkola et al., 2006 | 10 | 10 | 330.6 | Finnish | Alphabetic | Adults | Audio-visual speech perception | z > 1.8 | p < 0.05 corrected |
| Perrachione et al., 2016 | 19 | 19 | 279.6 | English | Alphabetic | Adults | Speech perception | p < 0.001 | p < 0.001 FDR |
| Perrachione et al., 2016 | 24 | 23 | 267.0 | English | Alphabetic | Adults | Spoken words listening, Written words, objects, and faces viewing | p < 0.001 | p < 0.001 FDR |
| Perrachione et al., 2016 | 25 | 26 | 95.4 | English | Alphabetic | Children | Spoken words listening, Written words, objects, and faces viewing | p < 0.001 | p < 0.05 FDR |
| Peyrin et al., 2011 | 12 | 12 | 120.0 | French | Alphabetic | Children | Categorical matching task | p < 0.001 | 15 voxels |
| Prasad et al., 2020 | 15 | 16 | 144.0 | Hindi | Syllabic | Children and adolescents | Auditory rhyming task, picture-naming task and semantic tasks | p < 0.001 | |
| Reilhac et al., 2013 | 12 | 12 | 306.6 | French | Alphabetic | Adults | Perceptual matching task | p < 0.001 | 15 voxels |
| Richlan et al., 2010 | 18 | 15 | 215.8 | German | Alphabetic | Adults and adolescents | phonological decision task | p < 0.005 | 20 voxels |
| Rimrodt et al., 2009 | 15 | 14 | 141.0 | English | Alphabetic | Children | Sentence comprehension task | p < 0.001 | 78 voxels* |
| Ruff et al., 2002 | 11 | 6 | 348.7 | French | Alphabetic | Aadults | Passive listening task | p < 0.01 | 38 voxels |
| Rumsey et al., 1997 | 14 | 17 | 313.2 | English | Alphabetic | Adults | Pronunciation task and lexical decision task | p < 0.01 | |
| Schulz et al., 2008 | 21 | 12 | 137.7 | German | Alphabetic | Children | Sentence reading task | p < 0.001 | |
| Schulz et al., 2009 | 15 | 15 | 138.0 | German | Alphabetic | Children | Sentence reading task | p < 0.001 | |
| Siok et al., 2004 | 8 | 8 | 132.0 | Chinese | Morpho-syllabic | Children | Homophone judgement task and lexical decision task | p < 0.001 | 20 voxels |
| Siok et al., 2008 | 12 | 12 | 131.5 | Chinese | Morpho-syllabic | Children | Character rhyming task | p < 0.005 | 10 voxels |
| Siok et al., 2009 | 12 | 12 | 131.5 | Chinese | Morpho-syllabic | Children | Font size judgment task | p < 0.05 FDR corrected | 10 voxels |
| Steinbrink et al., 2012 | 12 | 14 | 223.7 | German | Alphabetic | Adults and adolescents | Syllable discrimination | p < 0.05 FWE corrected | |
| Temple et al., 2000 | 10 | 8 | 362.7 | English | Alphabetic | Adults | Pitch discrimination task | p < 0.001 | |
| Temple et al., 2001 | 15 | 24 | 127.5 | English | Alphabetic | Children | Letter rhyming task, letter matching task | p < 0.001 | 20 voxels |
| van der Mark et al., 2009 | 24 | 18 | 136.1 | German | Alphabetic | Children | Phonological lexical decision task | p < 0.001 | 10 voxels |
| van Ermingen-Marbach et al., 2013a | 13 | 17 | 117.2 | German | Alphabetic | Children | Phoneme detection task | p < 0.001 | 10 voxels |
| van Ermingen-Marbach et al., 2013a | 13 | 14 | 116.4 | German | Alphabetic | Children | Phoneme detection task | p < 0.001 | 10 voxels |
| van Ermingen-Marbach et al., 2013b | 10 | 32 | 117.0 | German | Alphabetic | Children | Initial phoneme deletion task | p < 0.01 | 30 voxels |
| Vasic et al., 2008 | 13 | 12 | 219.6 | German | Alphabetic | Adults | Verbal working memory task | p < 0.005 | p < 0.05 |
| Waldie et al., 2013 | 16 | 12 | 365.1 | English | Alphabetic | Adults | Go/no-go lexical decision task | p < 0.001 | |
| Weiss et al., 2016 | 22 | 21 | 325.1 | Hebrew | Alphabetic | Adults | Word reading task | p < 0.001 | 50 voxels |
| Wimmer et al., 2010 | 19 | 20 | 247.6 | German | Alphabetic | Adults and adolescents | Phonological lexical decision task | p < 0.005 | 10 voxels |
| Yang and Tan, 2020 | 16 | 16 | 123.5 | Chinese | Morpho-syllabic | Children | Homophone judgments task and component judgments task | p < 0.005 | 25 voxels* |
| Zuk et al., 2018 | 13 | 11 | 114.0 | English | Alphabetic | Children | First sound matching task | p < 0.005 | 50 voxels |
equivalent to p < 0.05 corrected; ** equivalent to p < 0.01 corrected.
Table 10. Structural studies included in the meta-analysis.
| Study | N(TD) | N(DD) | Age in months | Language | Writing system | Subject type | Threshold | |
|---|---|---|---|---|---|---|---|---|
| Voxel-wise | Cluster-wise | |||||||
| Adrian-Ventura et al., 2020 | 12 | 13 | 146.2 | Spanish | Alphabetic | Children | p < 0.05 FWE corrected | 750 voxels |
| Brambati et al., 2004 | 11 | 10 | 352.8 | Italian | Alphabetic | Adults and adolescents | p < 0.05 corrected for small brain volume | |
| Brown et al., 2001 | 14 | 16 | 288.0 | English | Alphabetic | Adults | p < 0.05 | p < 0.05 |
| Eckert et al., 2005 | 13 | 13 | 136.5 | English | Alphabetic | Children | p < 0.001 | |
| Evans et al., 2014a | 14 | 14 | 505.8 | English | Alphabetic | Adults | Pp< 0.001 | p < 0.05 |
| Evans et al., 2014a | 13 | 13 | 371.4 | English | Alphabetic | Adults | p < 0.001 | p< 0.05 |
| Evans et al., 2014a | 15 | 15 | 107.4 | English | Alphabetic | Children | p < 0.001 | p < 0.05 |
| Evans et al., 2014a | 17 | 17 | 115.2 | English | Alphabetic | Children | p < 0.001 | p < 0.05 |
| Hoeft et al., 2007 | 19 | 19 | 172.8 | English | Alphabetic | Children and adolescents | p < 0.01 | p < 0.01 |
| Jagger-Rickels et al., 2018 | 32 | 17 | 114.2 | English | Alphabetic | Children | p < 0.001 | |
| Jednorog et al., 2014 | 35 | 46 | 123.5 | Polish | Alphabetic | Children | p < 0.001 | p < 0.05 |
| Jednorog et al., 2015 | 106 | 130 | 123.9 | French, German, Polish | Alphabetic | Children | p < 0.001 | 150 voxels* |
| Krafnick et al., 2014 | 15 | 15 | 118.2 | English | Alphabetic | Children | Pp< 0.01 | p < 0.01 FWE corrected |
| Kronbichler et al., 2008 | 15 | 13 | 187.9 | German | Alphabetic | Adolescents | p < 0.005 | |
| Liu et al., 2013b | 18 | 18 | 141.4 | Chinese | Morpho-syllabic | Cchildren | p < 0.001 | 196 voxels |
| Menghini et al., 2008 | 10 | 10 | 489.0 | Italian | Alphabetic | Adult | p < 0.005 | p < 0.05 corrected |
| Moreau et al., 2019 | 12 | 12 | 352.2 | English | Alphabetic | Adult | p < 0.05 FWE corrected | |
| Pernet et al., 2009 | 39 | 38 | 336.0 | French | Alphabetic | Adult | p< 0.05 FDR corrected | |
| Silani et al., 2005 | 32 | 32 | 304.5 | Italian, French, English | Alphabetic | Adults | ||
| Siok et al., 2008 | 16 | 16 | 132.0 | Chinese | Morpho-syllabic | Children | p < 0.05 FWE corrected | 50 voxels |
| Steinbrink et al., 2008 | 8 | 8 | 262.8 | German | Alphabetic | Adults | p < 0.05 FDR corrected | |
| Tamboer et al., 2015 | 57 | 37 | 245.3 | Dutch | Alphabetic | Adults | p < 0.05 | 200 voxels |
| Vinckenbosch et al., 2005 | 10 | 13 | 282.0 | French | Alphabetic | Adults | p < 0.01 | p < 0.05 |
| Wang et al., 2019 | 17 | 27 | 134.0 | Chinese | Morpho-syllabic | Children | p < 0.001 | p < 0.05 FWE corrected |
| Xia et al., 2016 | 12 | 12 | 132.0 | Chinese | Morpho-syllabic | Children | p < 0.001 | |
| Xia et al., 2016 | 12 | 12 | 169.2 | Chinese | Morpho-syllabic | Children | p < 0.001 | |
| Yang et al., 2016 | 14 | 9 | 149.0 | Chinese | Morpho-syllabic | Children | p < 0.001 | 111 voxels* |
equivalent to 0.05 corrected; ** equivalent to 0.01 corrected.
Figure 4. PRISMA flowchart of the selection process for included articles.
Voxel-wise meta-analysis
After data acquisition, we conducted a voxel-wise meta-analysis using the anisotropic effect-size version of Signed Differential Mapping software (AES-SDM version 5.14, see http://www.sdmproject.com) separately for functional studies and structural studies in alphabetic languages and morpho-syllabic languages. Unlike other coordinate-based meta-analysis methods such as Activation likelihood estimation (ALE) or Multilevel peak Kernel density analysis (MKDA), AES-SDM combined the peak coordinates with the statistical parameter maps to increase the sensitivity of the analysis (Radua et al., 2012a). Data were first preprocessed with the statistical parameter maps and the peak coordinates were convolved with a fully anisotropy un-normalized Gaussian kernel (ɑ = 1) (full width at half maximum = 20 mm) to recreate the effect size map and the corresponding variance map for each study (Radua et al., 2012a; Radua et al., 2014). Then, a random-effect model was set up to calculate the differences between the DD group and the control group. Five hundred permutations were performed to ensure the stability of the analysis. Finally, the results of the standard meta-analysis were thresholded at peak height of the mean effect size SDM-Z = 1, uncorrected p = 0.005 at the voxel level and 150 voxels at the cluster level, which is stricter than the threshold suggested by Radua et al., 2012a (peak height SDM-Z = 1, uncorrected p = 0.005 at the voxel level and 10 voxels at the cluster level) in order to avoid false-positive results and gain enough sensitivity.
In order to identify differences between the two language groups, we conducted a direct comparison between the alphabetic language group and the morpho-syllabic language group for functional studies and structural studies separately, using SDM linear model function. The threshold was set at peak height SDM-Z = 1, voxel level uncorrected p = 0.005 and 150 voxels at the cluster level.
To find out the common language difference between the structural and functional studies, we conducted a conjunction minimum analysis (Friston et al., 1999; Nichols et al., 2005) using the image calculation function of SPM12 (https://www.fil.ion.ucl.ac.uk) between the language differences in the structural studies and the language differences in the functional studies.
To test the stability of the meta-analysis results, we conducted a whole-brain jack-knife sensitivity analysis. The standard meta-analysis was repeated n times (n = 79 for functional experiments in alphabetic languages, n = 12 for functional experiments in morpho-syllabic languages, n = 21 for structural experiments in alphabetic languages, n = 6 for structural experiments in morpho-syllabic languages) but leaving out one experiments each time, to determine whether the results remained significant.
Multimodal meta-analysis
Because we were interested in the convergence between functional deficits and structural deficits, a multimodal meta-analysis was conducted in alphabetic languages and morpho-syllabic languages separately, which provided an efficient way to combine two meta-analyses in different modalities. The union probabilities of the meta-analytical maps of functional studies and structural studies were estimated and then thresholded at the peak height p = 0.00025, with a voxel level uncorrected p = 0.0025 and 150 voxels at the cluster level, which was stricter than the one suggested by Radua et al., 2012b; Radua et al., 2013 (peak height p = 0.00025, with a voxel level uncorrected p = 0.0025 and 10 voxels at cluster level).
To find out the common multimodal deficits in the two language groups, we conducted a conjunction minimum analysis (Friston et al., 1999; Nichols et al., 2005) using the image calculation function of SPM12 (https://www.fil.ion.ucl.ac.uk) between the multimodal deficits in the alphabetic group and the morpho-syllabic group.
Confirmation study
Because there were studies on both adults and children in the alphabetic group, whereas most of the morpho-syllabic studies were on children, the language difference may be due to the unmatched age range in the two groups of studies. In order to eliminate the influence of the confound, we conducted a confirmation analysis with only studies on children in the alphabetic group (n = 36 for the functional studies and n = 8 for the structure studies). Then, we compared the alphabetic and morpho-syllabic groups for functional studies and structural studies separately.
We conducted another confirmation analysis with 10 English studies (Booth et al., 2007a; Cao et al., 2008; Farris et al., 2016; Hancock et al., 2016; Hu et al., 2010; Langer et al., 2015; Meyler et al., 2008; Olulade et al., 2015; Rimrodt et al., 2009; Temple et al., 2001) and 10 Chinese studies (Cao et al., 2018; Cao et al., 2020; Feng et al., 2017; Hu et al., 2010; Liu et al., 2012; Liu et al., 2013a; Siok et al., 2004; Siok et al., 2008; Siok et al., 2009; Yang and Tan, 2020), because different languages were included in the alphabetic language group and orthographic depth makes a difference as found in previous research (Martin et al., 2016). Therefore, we only included English studies in the alphabetic group in this confirmation analysis. The two subgroups were also matched on participants’ age (mean age = 10.95 years for English studies, mean age = 11.40 years for Chinese studies), number of studies and task (visual word tasks). Then, we conducted a direct comparison between the Chinese studies and English studies. The threshold was set at peak height SDM-Z = 1 and voxel level uncorrected p = 0.005 with 150 voxels at the cluster level.
Acknowledgements
This work was supported by the “Fundamental Research Funds for the Central Universities” awarded to Dr. Fan Cao, “Guangdong Planning Office of Philosophy and Social Science” (GD19CXL05) awarded to Dr. Fan Cao, “Science and Technology Program of Guangzhou, China, Key Area Research and Development Program (202007030011)”,and by “The national social science fund of China" (21BYY204) awarded to Dr. Fan Cao.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Contributor Information
Fan Cao, Email: caofan3@mail.sysu.edu.cn.
Ruth de Diego-Balaguer, Universitat de Barcelona, Spain.
Chris I Baker, National Institute of Mental Health, National Institutes of Health, United States.
Funding Information
This paper was supported by the following grants:
Fundamental Research Funds for the Central Universities to Fan Cao.
Guangdong Planning Office of Philosophy and Social Science GD19CXL05 to Fan Cao.
Science and Technology Program of Guangzhou, China, Key Area Research and Development Program 202007030011 to Fan Cao.
The National Social Science Fund of China 21BYY204 to Fan Cao.
Additional information
Competing interests
No competing interests declared.
No competing interests declared.
Author contributions
Data curation, Methodology, Visualization, Writing – review and editing.
Writing – original draft, Data acquisition, Data acquisition, Data acquisition.
Writing – original draft, Data acquisition, Data acquisition, Data acquisition.
Writing – original draft, Data acquisition, Data acquisition, Data acquisition.
Validation.
Conceptualization, Funding acquisition, Supervision, Validation, Writing – review and editing.
Additional files
(a) Functional deficits in children with DD in alphabetic languages (b) Structural deficits in children with DD in alphabetic languages (c) Direct comparations between functional studies in alphabetic languages and morpho-syllabic languages for children with DD (d) Direct comparations between structural studies in alphabetic languages and morpho-syllabic languages for children with DD (e) Language differences in functional studies between the two well-matched groups in the confirmation analysis (f) Abnormal brain functions found in the current study that were reported in each functional study of the alphabetic language group (g) Abnormal brain structures found in the current study that were reported in each structural study of the alphabetic group (h) Abnormal brain functions found in the current study that were reported in each functional study of the morpho-syllabic group (i) Abnormal brain structures found in the current study that were reported in each structural study of the morpho-syllabic group.
Data availability
All data generated or analysed during this study are included in the manuscript and supporting files. Meta-analysis data is deposited to Dryad.
The following dataset was generated:
Yan X. 2021. meta-analysis data. Dryad Digital Repository.
References
- Achal S, Hoeft F, Bray S. Individual differences in adult reading are associated with left temporo-parietal to dorsal striatal functional connectivity. Cerebral Cortex. 2016;26:4069–4081. doi: 10.1093/cercor/bhv214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian-Ventura J, Soriano-Ferrer M, Fuentes-Claramonte P, Morte-Soriano M, Parcet MA, Avila C. Grey matter reduction in the occipitotemporal cortex in spanish children with dyslexia: A voxel-based morphometry study. Journal of Neurolinguistics. 2020;53:100873. doi: 10.1016/j.jneuroling.2019.100873. [DOI] [Google Scholar]
- Ait Khelifa-Gallois N, Puget S, Longaud A, Laroussinie F, Soria C, Sainte-Rose C, Dellatolas G. Clinical evidence of the role of the cerebellum in the suppression of overt articulatory movements during reading. A study of reading in children and adolescents treated for cerebellar pilocytic astrocytoma. Cerebellum. 2015;14:97–105. doi: 10.1007/s12311-014-0612-1. [DOI] [PubMed] [Google Scholar]
- Altarelli I, Leroy F, Monzalvo K, Fluss J, Billard C, Dehaene-Lambertz G, Galaburda AM, Ramus F. Planum temporale asymmetry in developmental dyslexia: Revisiting an old question. Human Brain Mapping. 2014;35:5717–5735. doi: 10.1002/hbm.22579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez TA, Fiez JA. Current perspectives on the cerebellum and reading development. Neuroscience and Biobehavioral Reviews. 2018;92:55–66. doi: 10.1016/j.neubiorev.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach S, Brandeis D, Hofstetter C, Martin E, Richardson U, Brem S. Early emergence of deviant frontal fmri activity for phonological processes in poor beginning readers. NeuroImage. 2010;53:682–693. doi: 10.1016/j.neuroimage.2010.06.039. [DOI] [PubMed] [Google Scholar]
- Barquero LA, Davis N, Cutting LE. Neuroimaging of reading intervention: A systematic review and activation likelihood estimate meta-analysis. PLOS ONE. 2014;9:e83668. doi: 10.1371/journal.pone.0083668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Yehudah G, Fiez JA. Impact of cerebellar lesions on reading and phonological processing. Annals of the New York Academy of Sciences. 2008;1145:260–274. doi: 10.1196/annals.1416.015. [DOI] [PubMed] [Google Scholar]
- Beneventi H, Tønnessen FE, Ersland L. Dyslexic children show short-term memory deficits in phonological storage and serial rehearsal: an fMRI study. The International Journal of Neuroscience. 2009;119:2017–2043. doi: 10.1080/00207450903139671. [DOI] [PubMed] [Google Scholar]
- Beneventi H, Tønnessen FE, Ersland L, Hugdahl K. Executive working memory processes in dyslexia: Behavioral and FMRI evidence. Scandinavian Journal of Psychology. 2010a;51:192–202. doi: 10.1111/j.1467-9450.2010.00808.x. [DOI] [PubMed] [Google Scholar]
- Beneventi H, Tønnessen FE, Ersland L, Hugdahl K. Working memory deficit in dyslexia: Behavioral and FMRI evidence. International Journal of Neuroscience. 2010b;120:51–59. doi: 10.3109/00207450903275129. [DOI] [PubMed] [Google Scholar]
- Blau V, van Atteveldt N, Ekkebus M, Goebel R, Blomert L. Reduced neural integration of letters and speech sounds links phonological and reading deficits in adult dyslexia. Current Biology. 2009;19:503–508. doi: 10.1016/j.cub.2009.01.065. [DOI] [PubMed] [Google Scholar]
- Bloom JS, Garcia-Barrera MA, Miller CJ, Miller SR, Hynd GW. Planum temporale morphology in children with developmental dyslexia. Neuropsychologia. 2013;51:1684–1692. doi: 10.1016/j.neuropsychologia.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boets B, Op de Beeck HP, Vandermosten M, Scott SK, Gillebert CR, Mantini D, Bulthe J, Sunaert S, Wouters J, Ghesquiere P. Intact but less accessible phonetic representations in adults with dyslexia. Science. 2013;342:1251–1254. doi: 10.1126/science.1244333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger DJ, Perfetti CA, Schneider W. Cross-cultural effect on the brain revisited: Universal structures plus writing system variation. Human Brain Mapping. 2005;25:92–104. doi: 10.1002/hbm.20124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger DJ, Minas J, Burman DD, Booth JR. Differential effects of orthographic and phonological consistency in cortex for children with and without reading impairment. Neuropsychologia. 2008;46:3210–3224. doi: 10.1016/j.neuropsychologia.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Bebko G, Burman DD, Bitan T. Children with reading disorder show modality independent brain abnormalities during semantic tasks. Neuropsychologia. 2007a;45:775–783. doi: 10.1016/j.neuropsychologia.2006.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Cho S, Burman DD, Bitan T. Neural correlates of mapping from phonology to orthography in children performing an auditory spelling task. Developmental Science. 2007b;10:441–451. doi: 10.1111/j.1467-7687.2007.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boros M, Anton JL, Pech-Georgel C, Grainger J, Szwed M, Ziegler JC. Orthographic processing deficits in developmental dyslexia: Beyond the ventral visual stream. NeuroImage. 2016;128:316–327. doi: 10.1016/j.neuroimage.2016.01.014. [DOI] [PubMed] [Google Scholar]
- Brambati SM, Termine C, Ruffino M, Stella G, Fazio F, Cappa SF, Perani D. Regional reductions of gray matter volume in familial dyslexia. Neurology. 2004;63:742–745. doi: 10.1212/01.wnl.0000134673.95020.ee. [DOI] [PubMed] [Google Scholar]
- Brambati SM, Termine C, Ruffino M, Danna M, Lanzi G, Stella G, Cappa SF, Perani D. Neuropsychological deficits and neural dysfunction in familial dyslexia. Brain Research. 2006;1113:174–185. doi: 10.1016/j.brainres.2006.06.099. [DOI] [PubMed] [Google Scholar]
- Brauer J, Anwander A, Perani D, Friederici AD. Dorsal and ventral pathways in language development. Brain and Language. 2013;127:289–295. doi: 10.1016/j.bandl.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Brem S, Bach S, Kucian K, Guttorm TK, Martin E, Lyytinen H, Brandeis D, Richardson U. Brain sensitivity to print emerges when children learn letter-speech sound correspondences. PNAS. 2010;107:7939–7944. doi: 10.1073/pnas.0904402107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown WE, Eliez S, Menon V, Rumsey JM, White CD, Reiss AL. Preliminary evidence of widespread morphological variations of the brain in dyslexia. Neurology. 2001;56:781–783. doi: 10.1212/wnl.56.6.781. [DOI] [PubMed] [Google Scholar]
- Brunswick N, McCrory E, Price CJ, Frith CD, Frith U. Explicit and implicit processing of words and pseudowords by adult developmental dyslexics: A search for Wernicke’s Wortschatz? Brain. 1999;122:1901–1917. doi: 10.1093/brain/122.10.1901. [DOI] [PubMed] [Google Scholar]
- Cao F, Bitan T, Chou TL, Burman DD, Booth JR. Deficient orthographic and phonological representations in children with dyslexia revealed by brain activation patterns. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2006;47:1041–1050. doi: 10.1111/j.1469-7610.2006.01684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao F, Bitan T, Booth JR. Effective brain connectivity in children with reading difficulties during phonological processing. Brain and Language. 2008;107:91–101. doi: 10.1016/j.bandl.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao F, Yan X, Wang Z, Liu YN, Wang J, Spray GJ, Deng Y. Neural signatures of phonological deficits in Chinese developmental dyslexia. NeuroImage. 2017;146:301–311. doi: 10.1016/j.neuroimage.2016.11.051. [DOI] [PubMed] [Google Scholar]
- Cao F, Yan X, Spray GJ, Liu Y, Deng Y. Brain mechanisms underlying visuo-orthographic deficits in children with developmental dyslexia. Frontiers in Human Neuroscience. 2018;12:490. doi: 10.3389/fnhum.2018.00490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao F, Yan X, Yan X, Zhou H, Booth JR. Reading Disability in Chinese Children Learning English as an L2. Child Development; 2020. [DOI] [PubMed] [Google Scholar]
- Carreiras M, Mechelli A, Estévez A, Price CJ. Brain activation for lexical decision and reading aloud: two sides of the same coin? Journal of Cognitive Neuroscience. 2007;19:433–444. doi: 10.1162/jocn.2007.19.3.433. [DOI] [PubMed] [Google Scholar]
- Centanni TM, Norton ES, Ozernov-Palchik O, Park A, Beach SD, Halverson K, Gaab N, Gabrieli JDE. Disrupted left fusiform response to print in beginning kindergartners is associated with subsequent reading. NeuroImage. Clinical. 2019;22:715. doi: 10.1016/j.nicl.2019.101715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheema K, Lantz N, Cummine J. Exploring the role of subcortical structures in developmental reading impairments: Evidence for subgroups differentiated by caudate activity. Neuroreport. 2018;29:271–279. doi: 10.1097/WNR.0000000000000938. [DOI] [PubMed] [Google Scholar]
- Christodoulou JA, Del Tufo SN, Lymberis J, Saxler PK, Ghosh SS, Triantafyllou C, Whitfield-Gabrieli S, Gabrieli JDE. Brain bases of reading fluency in typical reading and impaired fluency in dyslexia. PLOS ONE. 2014;9:e100552. doi: 10.1371/journal.pone.0100552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chyl K, Kossowski B, Dębska A, Łuniewska M, Marchewka A, Pugh KR, Jednoróg K. Reading acquisition in children: Developmental processes and dyslexia-specific effects. Journal of the American Academy of Child and Adolescent Psychiatry. 2019;58:948–960. doi: 10.1016/j.jaac.2018.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chyl K, Kossowski B, Wang S, Dębska A, Łuniewska M, Marchewka A, Wypych M, van den Bunt M, Mencl W, Pugh K, Jednoróg K. The brain signature of emerging reading in two contrasting languages. NeuroImage. 2021;225:117503. doi: 10.1016/j.neuroimage.2020.117503. [DOI] [PubMed] [Google Scholar]
- Clark KA, Helland T, Specht K, Narr KL, Manis FR, Toga AW, Hugdahl K. Neuroanatomical precursors of dyslexia identified from pre-reading through to age 11. Brain. 2014;137:3136–3141. doi: 10.1093/brain/awu229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway T, Heilman KM, Gopinath K, Peck K, Bauer R, Briggs RW, Torgesen JK, Crosson B. Neural substrates related to auditory working memory comparisons in dyslexia: An FMRI study. Journal of the International Neuropsychological Society. 2008;14:629–639. doi: 10.1017/S1355617708080867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coslett HB, Monsul N. Reading with the right hemisphere: evidence from transcranial magnetic stimulation. Brain and Language. 1994;46:198–211. doi: 10.1006/brln.1994.1012. [DOI] [PubMed] [Google Scholar]
- Costafreda SG, Fu CHY, Lee L, Everitt B, Brammer MJ, David AS. A systematic review and quantitative appraisal of FMRI studies of verbal fluency: Role of the left inferior frontal gyrus. Human Brain Mapping. 2006;27:799–810. doi: 10.1002/hbm.20221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummine J, Dai W, Borowsky R, Gould L, Rollans C, Boliek C. Investigating the ventral-lexical, dorsal-sublexical model of basic reading processes using diffusion tensor imaging. Brain Structure and Function. 2015;220:445–455. doi: 10.1007/s00429-013-0666-8. [DOI] [PubMed] [Google Scholar]
- Cutting LE, Clements-Stephens A, Pugh KR, Burns S, Cao A, Pekar JJ, Davis N, Rimrodt SL. Not all reading disabilities are dyslexia: distinct neurobiology of specific comprehension deficits. Brain Connectivity. 2013;3:199–211. doi: 10.1089/brain.2012.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danelli L, Berlingeri M, Bottini G, Borghese NA, Lucchese M, Sberna M, Price CJ, Paulesu E. How many deficits in the same dyslexic brains? A behavioural and fmri assessment of comorbidity in adult dyslexics. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior. 2017;97:125–142. doi: 10.1016/j.cortex.2017.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desroches AS, Cone NE, Bolger DJ, Bitan T, Burman DD, Booth JR. Children with reading difficulties show differences in brain regions associated with orthographic processing during spoken language processing. Brain Research. 2010;1356:73–84. doi: 10.1016/j.brainres.2010.07.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döhla D, Heim S. Developmental Dyslexia and Dysgraphia: What can We Learn from the One About the Other? Frontiers in Psychology. 2015;6:2045. doi: 10.3389/fpsyg.2015.02045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dronkers NF. A new brain region for coordinating speech articulation. Nature. 1996;384:159–161. doi: 10.1038/384159a0. [DOI] [PubMed] [Google Scholar]
- Dufor O, Serniclaes W, Sprenger-Charolles L, Démonet J-F. Top-down processes during auditory phoneme categorization in dyslexia: A pet study. NeuroImage. 2007;34:1692–1707. doi: 10.1016/j.neuroimage.2006.10.034. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Leonard CM, Wilke M, Eckert M, Richards T, Richards A, Berninger V. Anatomical signatures of dyslexia in children: Unique information from manual and voxel based morphometry brain measures. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior. 2005;41:304–315. doi: 10.1016/S0010-9452(08)70268-5. [DOI] [PubMed] [Google Scholar]
- Eden GF, Jones KM, Cappell K, Gareau L, Wood FB, Zeffiro TA, Dietz NAE, Agnew JA, Flowers DL. Neural changes following remediation in adult developmental dyslexia. Neuron. 2004;44:411–422. doi: 10.1016/j.neuron.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Enge A, Friederici AD, Skeide MA. A meta-analysis of FMRI studies of language comprehension in children. NeuroImage. 2020;215:116858. doi: 10.1016/j.neuroimage.2020.116858. [DOI] [PubMed] [Google Scholar]
- Evans TM, Flowers DL, Napoliello EM, Eden GF. Sex-specific gray matter volume differences in females with developmental dyslexia. Brain Structure & Function. 2014a;219:1041–1054. doi: 10.1007/s00429-013-0552-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans TM, Flowers DL, Napoliello EM, Olulade OA, Eden GF. The functional anatomy of single-digit arithmetic in children with developmental dyslexia. NeuroImage. 2014b;101:644–652. doi: 10.1016/j.neuroimage.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris EA, Ring J, Black J, Lyon GR, Odegard TN. Predicting growth in word level reading skills in children with developmental dyslexia using an object rhyming functional neuroimaging task. Developmental Neuropsychology. 2016;41:145–161. doi: 10.1080/87565641.2016.1158264. [DOI] [PubMed] [Google Scholar]
- Feng X, Li L, Zhang M, Yang X, Tian M, Xie W, Lu Y, Liu L, Bélanger NN, Meng X, Ding G. Dyslexic children show atypical cerebellar activation and cerebro-cerebellar functional connectivity in orthographic and phonological processing. Cerebellum. 2017;16:496–507. doi: 10.1007/s12311-016-0829-2. [DOI] [PubMed] [Google Scholar]
- Feng X, Altarelli I, Monzalvo K, Ding G, Ramus F, Shu H, Dehaene S, Meng X, Dehaene-Lambertz G. A universal reading network and its modulation by writing system and reading ability in French and chinese children. eLife. 2020;9:e54591. doi: 10.7554/eLife.54591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández L, Velásquez C, García Porrero JA, de Lucas EM, Martino J. Heschl’s gyrus fiber intersection area: a new insight on the connectivity of the auditory-language hub. Neurosurgical Focus. 2020;48:2019.11.FOCUS19778. doi: 10.3171/2019.11.Focus19778. [DOI] [PubMed] [Google Scholar]
- Finn ES, Shen X, Holahan JM, Scheinost D, Lacadie C, Papademetris X, Shaywitz SE, Shaywitz BA, Constable RT. Disruption of functional networks in dyslexia: A whole-brain, data-driven analysis of connectivity. Biological Psychiatry. 2014;76:397–404. doi: 10.1016/j.biopsych.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francisco AA, Takashima A, McQueen JM, van den Bunt M, Jesse A, Groen MA. Adult dyslexic readers benefit less from visual input during audiovisual speech processing: fMRI evidence. Neuropsychologia. 2018;117:454–471. doi: 10.1016/j.neuropsychologia.2018.07.009. [DOI] [PubMed] [Google Scholar]
- Friederici AD. The cortical language circuit: From auditory perception to sentence comprehension. Trends in Cognitive Sciences. 2012;16:262–268. doi: 10.1016/j.tics.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Price CJ, Buchel C, Worsley KJ. Multisubject fMRI studies and conjunction analyses. NeuroImage. 1999;10:385–396. doi: 10.1006/nimg.1999.0484. [DOI] [PubMed] [Google Scholar]
- Frodl T, Skokauskas N. Meta-analysis of structural MRI studies in children and adults with attention deficit hyperactivity disorder indicates treatment effects. Acta Psychiatrica Scandinavica. 2012;125:114–126. doi: 10.1111/j.1600-0447.2011.01786.x. [DOI] [PubMed] [Google Scholar]
- Gaab N, Gabrieli JD, Deutsch GK, Tallal P, Temple E. Neural correlates of rapid auditory processing are disrupted in children with developmental dyslexia and ameliorated with training: An fMRI study. Restorative Neurology and Neuroscience. 2007;25:295–310. [PubMed] [Google Scholar]
- Gatti D, Van Vugt F, Vecchi T. A causal role for the cerebellum in semantic integration: A transcranial magnetic stimulation study. Scientific Reports. 2020;10:18139. doi: 10.1038/s41598-020-75287-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiewa P, Rzanny R, Hopf JM, Knab R, Glauche V, Kaiser WA, Blanz B. fMRI during word processing in dyslexic and normal reading children. Neuroreport. 1999;10:3459–3465. doi: 10.1097/00001756-199911080-00036. [DOI] [PubMed] [Google Scholar]
- Giraud AL, Ramus F. Neurogenetics and auditory processing in developmental dyslexia. Current Opinion in Neurobiology. 2013;23:37–42. doi: 10.1016/j.conb.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Glezer LS, Jiang X, Riesenhuber M. Evidence for highly selective neuronal tuning to whole words in the “Visual Word Form Area. Neuron. 2009;62:199–204. doi: 10.1016/j.neuron.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glezer LS, Eden G, Jiang X, Luetje M, Napoliello E, Kima J, Riesenhuber M. Uncovering phonological and orthographic selectivity across the reading network using fMRI-RA. NeuroImage. 2016;138:248–256. doi: 10.1016/j.neuroimage.2016.05.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glezer LS, Jiang X, Luetje MM, Napoliello EM, Kim J, Riesenhuber M, Eden GF. An fMRI-adaptation study of phonological and orthographic selectivity to written words in adults with poor reading skills. Brain and Language. 2019;191:1–8. doi: 10.1016/j.bandl.2019.01.002. [DOI] [PubMed] [Google Scholar]
- Grahn JA, Parkinson JA, Owen AM. The cognitive functions of the caudate nucleus. Progress in Neurobiology. 2008;86:141–155. doi: 10.1016/j.pneurobio.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Grande M, Meffert E, Huber W, Amunts K, Heim S. Word frequency effects in the left IFG in dyslexic and normally reading children during picture naming and reading. NeuroImage. 2011;57:1212–1220. doi: 10.1016/j.neuroimage.2011.05.033. [DOI] [PubMed] [Google Scholar]
- Grunling C, Ligges M, Huonker R, Klingert M, Mentzel HJ, Rzanny R, Kaiser WA, Witte H, Blanz B. Dyslexia: The possible benefit of multimodal integration of fMRI- and EEG-data. Journal of Neural Transmission. 2004;111:951–969. doi: 10.1007/s00702-004-0117-z. [DOI] [PubMed] [Google Scholar]
- Gu CY, Bi HY. Auditory processing deficit in individuals with dyslexia: A meta-analysis of mismatch negativity. Neuroscience and Biobehavioral Reviews. 2020;116:396–405. doi: 10.1016/j.neubiorev.2020.06.032. [DOI] [PubMed] [Google Scholar]
- Hancock R, Gabrieli JDE, Hoeft F. Shared temporoparietal dysfunction in dyslexia and typical readers with discrepantly high IQ. Trends in Neuroscience and Education. 2016;5:173–177. doi: 10.1016/j.tine.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R, Richlan F, Hoeft F. Possible roles for fronto-striatal circuits in reading disorder. Neuroscience and Biobehavioral Reviews. 2017;72:243–260. doi: 10.1016/j.neubiorev.2016.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim S, Grande M, Pape-Neumann J, van Ermingen M, Meffert E, Grabowska A, Huber W, Amunts K. Interaction of phonological awareness and “magnocellular” processing during normal and dyslexic reading: Behavioural and fMRI investigations. Dyslexia. 2010;16:258–282. doi: 10.1002/dys.409. [DOI] [PubMed] [Google Scholar]
- Heim S, Wehnelt A, Grande M, Huber W, Amunts K. Effects of lexicality and word frequency on brain activation in dyslexic readers. Brain and Language. 2013;125:194–202. doi: 10.1016/j.bandl.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Heim S, Pape-Neumann J, van Ermingen-Marbach M, Brinkhaus M, Grande M. Shared vs. specific brain activation changes in dyslexia after training of phonology, attention, or reading. Brain Structue and Function. 2015;220:2191–2207. doi: 10.1007/s00429-014-0784-y. [DOI] [PubMed] [Google Scholar]
- Hernandez N, Andersson F, Edjlali M, Hommet C, Cottier JP, Destrieux C, Bonnet-Brilhault F. Cerebral functional asymmetry and phonological performance in dyslexic adults. Psychophysiology. 2013;50:1226–1238. doi: 10.1111/psyp.12141. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nature Reviews Neuroscience. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Higuchi H, Iwaki S, Uno A. Altered visual character and object recognition in Japanese-speaking adolescents with developmental dyslexia. Neuroscience Letters. 2020;723:134841. doi: 10.1016/j.neulet.2020.134841. [DOI] [PubMed] [Google Scholar]
- Hirshorn EA, Li Y, Ward MJ, Richardson RM, Fiez JA, Ghuman AS. Decoding and disrupting left midfusiform gyrus activity during word reading. PNAS. 2016;113:8162–8167. doi: 10.1073/pnas.1604126113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, Hernandez A, McMillon G, Taylor-Hill H, Martindale JL, Meyler A, Keller TA, Siok WT, Deutsch GK, Just MA, Whitfield-Gabrieli S, Gabrieli JD. Neural basis of dyslexia: A comparison between dyslexic and nondyslexic children equated for reading ability. Journal of Neuroscience. 2006;26:10700–10708. doi: 10.1523/JNEUROSCI.4931-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, Meyler A, Hernandez A, Juel C, Taylor-Hill H, Martindale JL, McMillon G, Kolchugina G, Black JM, Faizi A, Deutsch GK, Siok WT, Reiss AL, Whitfield-Gabrieli S, Gabrieli JD. Functional and morphometric brain dissociation between dyslexia and reading ability. PNAS. 2007;104:4234–4239. doi: 10.1073/pnas.0609399104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honorof D, Feldman L. In: The Handbook of East Asian Psycholinguistics. Li P, Tan LH, Bates E, Tzeng OJL, editors. Cambridge University Press; 2006. The chinese character in psycholinguistic research: Form, structure, and the reader; pp. 195–208. [Google Scholar]
- Horowitz-Kraus T, Buck C, Dorrmann D. Altered neural circuits accompany lower performance during narrative comprehension in children with reading difficulties: An fMRI study. Annals of Dyslexia. 2016;66:301–318. doi: 10.1007/s11881-016-0124-4. [DOI] [PubMed] [Google Scholar]
- Hosseini SMH, Black JM, Soriano T, Bugescu N, Martinez R, Raman MM, Kesler SR, Hoeft F. Topological properties of large-scale structural brain networks in children with familial risk for reading difficulties. NeuroImage. 2013;71:260–274. doi: 10.1016/j.neuroimage.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston SM, Lebel C, Katzir T, Manis FR, Kan E, Rodriguez GG, Sowell ER. Reading skill and structural brain development. Neuroreport. 2014;25:347–352. doi: 10.1097/Wnr.0000000000000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Lee HL, Zhang Q, Liu T, Geng LB, Seghier ML, Shakeshaft C, Twomey T, Green DW, Yang YM, Price CJ. Developmental dyslexia in Chinese and English populations: Dissociating the effect of dyslexia from language differences. Brain. 2010;133:1694–1706. doi: 10.1093/brain/awq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain MM, McDonald WM, Doraiswamy PM, Figiel GS, Na C, Escalona PR, Boyko OB, Nemeroff CB, Krishnan KRR. A magnetic resonance imaging study of putamen nuclei in major depression. Psychiatry Research. 1991;40:95–99. doi: 10.1016/0925-4927(91)90001-7. [DOI] [PubMed] [Google Scholar]
- Ingvar M, af Trampe P, Greitz T, Eriksson L, Stone-Elander S, von Euler C. Residual differences in language processing in compensated dyslexics revealed in simple word reading tasks. Brain and Language. 2002;83:249–267. doi: 10.1016/S0093-934x(02)00055-X. [DOI] [PubMed] [Google Scholar]
- Jaffe-Dax S, Kimel E, Ahissar M. Shorter cortical adaptation in dyslexia is broadly distributed in the superior temporal lobe and includes the primary auditory cortex. eLife. 2018;7:e30018. doi: 10.7554/eLife.30018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagger-Rickels AC, Kibby MY, Constance JM. Global gray matter morphometry differences between children with reading disability, ADHD, and comorbid reading disability/ADHD. Brain and Language. 2018;185:54–66. doi: 10.1016/j.bandl.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jednorog K, Gawron N, Marchewka A, Heim S, Grabowska A. Cognitive subtypes of dyslexia are characterized by distinct patterns of grey matter volume. Brain Structure and Function. 2014;219:1697–1707. doi: 10.1007/s00429-013-0595-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jednorog K, Marchewka A, Altarelli I, Lopez AKM, van Ermingen-Marbach M, Grande M, Grabowska A, Heim S, Ramus F. How reliable are gray matter disruptions in specific reading disability across multiple countries and languages? Insights from a large-scale Voxel-Based Morphometry study. Human Brain Mapping. 2015;36:1741–1754. doi: 10.1002/hbm.22734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kast M, Bezzola L, Jancke L, Meyer M. Multi- and unisensory decoding of words and nonwords result in differential brain responses in dyslexic and nondyslexic adults. Brain and Language. 2011;119:136–148. doi: 10.1016/j.bandl.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Katusic SK, Colligan RC, Barbaresi WJ, Schaid DJ, Jacobsen SJ. Incidence of reading disability in a population-based birth Cohort, 1976–1982. Rochester, Minn. Mayo Clinic Proceedings. 2001;76:1081–1092. doi: 10.4065/76.11.1081. [DOI] [PubMed] [Google Scholar]
- Kibby MY, Fancher JB, Markanen R, Hynd GW. A quantitative magnetic resonance imaging analysis of the cerebellar deficit hypothesis of dyslexia. Journal of Child Neurology. 2008;23:368–380. doi: 10.1177/08883073807309235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovelman I, Norton ES, Christodoulou JA, Gaab N, Lieberman DA, Triantafyllou C, Wolf M, Whitfield-Gabrieli S, Gabrieli JD. Brain basis of phonological awareness for spoken language in children and its disruption in dyslexia. Cerebral Cortex. 2012;22:754–764. doi: 10.1093/cercor/bhr094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krafnick AJ, Flowers DL, Luetje MM, Napoliello EM, Eden GF. An investigation into the origin of anatomical differences in dyslexia. Journal of Neuroscience. 2014;34:901–908. doi: 10.1523/JNEUROSCI.2092-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronbichler M, Hutzler F, Staffen W, Mair A, Ladurner G, Wimmer H. Evidence for a dysfunction of left posterior reading areas in German dyslexic readers. Neuropsychologia. 2006;44:1822–1832. doi: 10.1016/j.neuropsychologia.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Kronbichler M, Wimmer H, Staffen W, Hutzler F, Mair A, Ladurner G. Developmental dyslexia: Gray matter abnormalities in the occipitotemporal cortex. Human Brain Mapping. 2008;29:613–625. doi: 10.1002/hbm.20425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronschnabel J, Schmid R, Maurer U, Brandeis D. Visual print tuning deficits in dyslexic adolescents under minimized phonological demands. NeuroImage. 2013;74:58–69. doi: 10.1016/j.neuroimage.2013.02.014. [DOI] [PubMed] [Google Scholar]
- Kronschnabel J, Brem S, Maurer U, Brandeis D. The level of audiovisual print-speech integration deficits in dyslexia. Neuropsychologia. 2014;62:245–261. doi: 10.1016/j.neuropsychologia.2014.07.024. [DOI] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Li K, Robin DA, Glahn DC, Fox PT. Investigating the functional heterogeneity of the default mode network using coordinate-based meta-analytic modeling. The Journal of Neuroscience. 2009;29:14496. doi: 10.1523/JNEUROSCI.4004-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landerl K, Wimmer H, Frith U. The impact of orthographic consistency on dyslexia: A German-English comparison. Cognition. 1997;63:315–334. doi: 10.1016/S0010-0277(97)00005-X. [DOI] [PubMed] [Google Scholar]
- Landi N, Mencl WE, Frost SJ, Sandak R, Pugh KR. An fMRI study of multimodal semantic and phonological processing in reading disabled adolescents. Annals of Dyslexia. 2010;60:102–121. doi: 10.1007/s11881-009-0029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer N, Benjamin C, Minas J, Gaab N. The neural correlates of reading fluency deficits in children. Cerebral Cortex. 2015;25:1441–1453. doi: 10.1093/cercor/bht330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkersdörfer J, Lonnemann J, Lindberg S, Hasselhorn M, Fiebach CJ. Grey matter alterations co-localize with functional abnormalities in developmental dyslexia: An ALE meta-analysis. PLOS ONE. 2012;7:e43122. doi: 10.1371/journal.pone.0043122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Wang W, You W, Li Y, Awati N, Zhao X, Booth JR, Peng D. Similar alterations in brain function for phonological and semantic processing to visual characters in Chinese dyslexia. Neuropsychologia. 2012;50:2224–2232. doi: 10.1016/j.neuropsychologia.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Tao R, Wang WJ, You WP, Peng DL, Booth JR. Chinese dyslexics show neural differences in morphological processing. Developmental Cognitive Neuroscience. 2013a;6:40–50. doi: 10.1016/j.dcn.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, You W, Wang W, Guo X, Peng D, Booth J. Altered brain structure in Chinese dyslexic children. Neuropsychologia. 2013b;51:1169–1176. doi: 10.1016/j.neuropsychologia.2013.03.010. [DOI] [PubMed] [Google Scholar]
- Lobier MA, Peyrin C, Pichat C, Le Bas JF, Valdois S. Visual processing of multiple elements in the dyslexic brain: evidence for a superior parietal dysfunction. Frontiers in Human Neuroscience. 2014;8:479. doi: 10.3389/fnhum.2014.00479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Liang H, Han D, Mo Y, Li Z, Cheng Y, Xu X, Shen Z, Tan C, Zhao W, Zhu Y, Sun X. The volumetric and shape changes of the putamen and thalamus in first episode, untreated major depressive disorder. NeuroImage. 2016;11:658–666. doi: 10.1016/j.nicl.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacSweeney M, Brammer MJ, Waters D, Goswami U. Enhanced activation of the left inferior frontal gyrus in deaf and dyslexic adults during rhyming. Brain. 2009;132:1928–1940. doi: 10.1093/brain/awp129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisog JM, Einbinder ER, Flowers DL, Turkeltaub PE, Eden GF. A meta-analysis of functional neuroimaging studies of dyslexia. Annals of the New York Academy of Sciences. 2008;1145:237–259. doi: 10.1196/annals.1416.024. [DOI] [PubMed] [Google Scholar]
- Margolis AE, Pagliaccio D, Davis KS, Thomas L, Banker SM, Cyr M, Marsh R. Neural correlates of cognitive control deficits in children with reading disorder. Brain Imaging and Behavior. 2020;14:1531–1542. doi: 10.1007/s11682-019-00083-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Kronbichler M, Richlan F. Dyslexic brain activation abnormalities in deep and shallow orthographies: A meta-analysis of 28 functional neuroimaging studies. Human Brain Mapping. 2016;37:2676–2699. doi: 10.1002/hbm.23202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer U, Schulz E, Brem S, der Mark S, Bucher K, Martin E, Brandeis D. The development of print tuning in children with dyslexia: Evidence from longitudinal ERP data supported by fMRI. NeuroImage. 2011;57:714–722. doi: 10.1016/j.neuroimage.2010.10.055. [DOI] [PubMed] [Google Scholar]
- McCrory E, Frith U, Brunswick N, Price C. Abnormal functional activation during a simple word repetition task: A PET study of adult dyslexics. Journal of Cognitive Neuroscience. 2000;12:753–762. doi: 10.1162/089892900562570. [DOI] [PubMed] [Google Scholar]
- McCrory EJ, Mechelli A, Frith U, Price CJ. More than words: A common neural basis for reading and naming deficits in developmental dyslexia. Brain. 2005;128:261–267. doi: 10.1093/brain/awh340. [DOI] [PubMed] [Google Scholar]
- McGrath LM, Stoodley CJ. Are there shared neural correlates between dyslexia and ADHD? A meta-analysis of voxel-based morphometry studies. Journal of Neurodevelopmental Disorders. 2019;11:31. doi: 10.1186/s11689-019-9287-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechelli A, Crinion JT, Noppeney U, O’Doherty J, Ashburner J, Frackowiak RS, Price CJ. Neurolinguistics: Structural plasticity in the bilingual brain. Nature. 2004;431:757. doi: 10.1038/431757a. [DOI] [PubMed] [Google Scholar]
- Melby-Lervag M, Lyster SAH, Hulme C. Phonological skills and their role in learning to read: A meta-analytic review. Psychological Bulletin. 2012;138:322–352. doi: 10.1037/a0026744. [DOI] [PubMed] [Google Scholar]
- Menghini D, Hagberg GE, Caltagirone C, Petrosini L, Vicari S. Implicit learning deficits in dyslexic adults: An fMRI study. NeuroImage. 2006;33:1218–1226. doi: 10.1016/j.neuroimage.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Menghini D, Hagberg GE, Petrosini L, Bozzali M, Macaluso E, Caltagirone C, Vicari S. Structural correlates of implicit learning deficits in subjects with developmental dyslexia. Annals of the New York Academy of Sciences. 2008;1145:212–221. doi: 10.1196/annals.1416.010. [DOI] [PubMed] [Google Scholar]
- Meyler A, Keller TA, Cherkassky VL, Gabrieli JD, Just MA. Modifying the brain activation of poor readers during sentence comprehension with extended remedial instruction: A longitudinal study of neuroplasticity. Neuropsychologia. 2008;46:2580–2592. doi: 10.1016/j.neuropsychologia.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monzalvo K, Fluss J, Billard C, Dehaene S, Dehaene-Lambertz G. Cortical networks for vision and language in dyslexic and normal children of variable socio-economic status. NeuroImage. 2012;61:258–274. doi: 10.1016/j.neuroimage.2012.02.035. [DOI] [PubMed] [Google Scholar]
- Moreau D, Wiebels K, Wilson AJ, Waldie KE. Volumetric and surface characteristics of gray matter in adult dyslexia and dyscalculia. Neuropsychologia. 2019;127:204–210. doi: 10.1016/j.neuropsychologia.2019.02.002. [DOI] [PubMed] [Google Scholar]
- Mous SE, Hammerschlag AR, Polderman TJC, Verhulst FC, Tiemeier H, van der Lugt A, Jaddoe VW, Hofman A, White T, Posthuma D. A population-based imaging genetics study of inattention/hyperactivity: Basal ganglia and genetic pathways. Journal of the American Academy of Child and Adolescent Psychiatry. 2015;54:745–752. doi: 10.1016/j.jaac.2015.05.018. [DOI] [PubMed] [Google Scholar]
- Muller V, Cieslik EC, Laird AR, Fox PT, Radua J, Mataix-Cols D, Tench CR, Yarkoni T, Nichols TE, Turkeltaub PE, Wager TD, Eickhoff SB. Ten simple rules for neuroimaging meta-analysis. Neurosciece and Biobehavioral Reviews. 2018;84:151–161. doi: 10.1016/j.neubiorev.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao T, Radua J, Rubia K, Mataix-Cols D. Gray matter volume abnormalities in ADHD: Voxel-based meta-analysis exploring the effects of age and stimulant medication. American Journal of Psychiatry. 2011;168:1154–1163. doi: 10.1176/appi.ajp.2011.11020281. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. NeuroImage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Nickl-Jockschat T, Habel U, Maria Michel T, Manning J, Laird AR, Fox PT, Schneider F, Eickhoff SB. Brain structure anomalies in autism spectrum disorder—a meta-analysis of VBM studies using anatomic likelihood estimation. Human Brain Mapping. 2012;33:1470–1489. doi: 10.1002/hbm.21299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson R, Fawcett AJ, Dean P. Developmental dyslexia: the cerebellar deficit hypothesis. Trends in Neurosciences. 2001;24:508–511. doi: 10.1016/S0166-2236(00)01896-8. [DOI] [PubMed] [Google Scholar]
- Nobre AC, Allison T, McCarthy G. Word recognition in the human inferior temporal lobe. Nature. 1994;372:260–263. doi: 10.1038/372260a0. [DOI] [PubMed] [Google Scholar]
- Nord CL, Kim SG, Callesen MB, Kvamme TL, Jensen M, Pedersen MU, Thomsen KR, Voon V. The myeloarchitecture of impulsivity: Premature responding in youth is associated with decreased myelination of ventral putamen. Neuropsychopharmacology. 2019;44:1216–1223. doi: 10.1038/s41386-019-0343-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olulade OA, Gilger JW, Talavage TM, Hynd GW, McAteer C. Beyond phonological processing deficits in adult dyslexics: Atypical fMRI activation patterns for spatial problem solving. Developmental Neuropsychology. 2012;37:617–635. doi: 10.1080/87565641.2012.702826. [DOI] [PubMed] [Google Scholar]
- Olulade OA, Flowers DL, Napoliello EM, Eden GF. Dyslexic children lack word selectivity gradients in occipito-temporal and inferior frontal cortex. NeuroImage. 2015;7:742–754. doi: 10.1016/j.nicl.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrila R, Dudley D, Song S, Georgiou GK. A meta-analysis of reading-level match dyslexia studies in consistent alphabetic orthographies. Annals of Dyslexia. 2020;70:1–26. doi: 10.1007/s11881-019-00187-5. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Frith U, Snowling M, Gallagher A, Morton J, Frackowiak RSJ, Frith CD. Is developmental dyslexia a disconnection syndrome?: Evidence from PET scanning. Brain. 1996;119:143–157. doi: 10.1093/brain/119.1.143. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Démonet JF, Fazio F, McCrory E, Chanoine V, Brunswick N, Cappa SF, Cossu G, Habib M, Frith CD, Frith U. Dyslexia: Cultural diversity and biological unity. Science. 2001;291:2165–2167. doi: 10.1126/science.1057179. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Danelli L, Berlingeri M. Reading the dyslexic brain: Multiple dysfunctional routes revealed by a new meta-analysis of PET and fMRI activation studies. Frontiers in Human Neuroscience. 2014;8:830. doi: 10.3389/fnhum.2014.00830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz-Alonso PM, Oliver M, Lerma-Usabiaga G, Caballero-Gaudes C, Quinones I, Suarez-Coalla P, Dunabeitia JA, Cuetos F, Carreiras M. Neural correlates of phonological, orthographic and semantic reading processing in dyslexia. NeuroImage. 2018;20:433–447. doi: 10.1016/j.nicl.2018.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecini C, Biagi L, Brizzolara D, Cipriani P, Di Lieto MC, Guzzetta A, Tosetti M, Chilosi AM. How many functional brains in developmental dyslexia? When the history of language delay makes the difference. Cognitive and Behavioral Neurology. 2011;24:85–92. doi: 10.1097/WNN.0b013e318222a4c2. [DOI] [PubMed] [Google Scholar]
- Pekkola J, Laasonen M, Ojanen V, Autti T, Jaaskelainen IP, Kujala T, Sams M. Perception of matching and conflicting audiovisual speech in dyslexic and fluent readers: An fMRI study at 3 T. NeuroImage. 2006;29:797–807. doi: 10.1016/j.neuroimage.2005.09.069. [DOI] [PubMed] [Google Scholar]
- Pernet C, Andersson J, Paulesu E, Demonet JF. When all hypotheses are right: A multifocal account of dyslexia. Human Brain Mapping. 2009;30:2278–2292. doi: 10.1002/hbm.20670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrachione TK, Del Tufo SN, Winter R, Murtagh J, Cyr A, Chang P, Halverson K, Ghosh SS, Christodoulou JA, Gabrieli JDE. Dysfunction of rapid neural adaptation in dyslexia. Neuron. 2016;92:1383–1397. doi: 10.1016/j.neuron.2016.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SE, Fiez JA. The processing of single words studied with positron emission tomography. Annual Review of Neuroscience. 1993;16:509–530. doi: 10.1146/annurev.ne.16.030193.002453. [DOI] [PubMed] [Google Scholar]
- Peterson RL, Pennington BF. Developmental dyslexia. Lancet. 2012;379:1997–2007. doi: 10.1016/S0140-6736(12)60198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyrin C, Demonet JF, N’Guyen-Morel MA, Le Bas JF, Valdois S. Superior parietal lobule dysfunction in a homogeneous group of dyslexic children with a visual attention span disorder. Brain and Language. 2011;118:128–138. doi: 10.1016/j.bandl.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Plewko J, Chyl K, Bola L, Luniewska M, Debska A, Banaszkiewicz A, Wypych M, Marchewka A, van Atteveldt N, Jednorog K. Letter and speech sound association in emerging readers with familial risk of dyslexia. Frontiers in Human Neuroscience. 2018;12:393. doi: 10.3389/fnhum.2018.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad S, Sagar R, Kumaran SS, Mehta M. Study of functional magnetic resonance imaging (fMRI) in children and adolescents with specific learning disorder (dyslexia. Asian Journal of Psychiatry. 2020;50:e101945. doi: 10.1016/j.ajp.2020.101945. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Jenner AR, Katz L, Frost SJ, Lee JR, Shaywitz SE, Shaywitz BA. Functional neuroimaging studies of reading and reading disability (developmental dyslexia. Mental Retardation and Developmental Disabilities Research Reviews. 2000;6:207–213. doi: 10.1002/1098-2779(2000)6:3<207::AID-MRDD8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Radua J, Mataix-Cols D, Phillips ML, El-Hage W, Kronhaus DM, Cardoner N, Surguladze S. A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. European Psychiatry. 2012a;27:605–611. doi: 10.1016/j.eurpsy.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Radua J, Borgwardt S, Crescini A, Mataix-Cols D, Meyer-Lindenberg A, McGuire PK, Fusar-Poli P. Multimodal meta-analysis of structural and functional brain changes in first episode psychosis and the effects of antipsychotic medication. Neuroscience and Biobehavioral Reviews. 2012b;36:2325–2333. doi: 10.1016/j.neubiorev.2012.07.012. [DOI] [PubMed] [Google Scholar]
- Radua J, Romeo M, Mataix-Cols D, Fusar-Poli P. A general approach for combining voxel-based meta-analyses conducted in different neuroimaging modalities. Current Medicinal Chemistry. 2013;20:462–466. doi: 10.2174/0929867311320030017. [DOI] [PubMed] [Google Scholar]
- Radua J, Rubia K, Canales-Rodriguez EJ, Pomarol-Clotet E, Fusar-Poli P, Mataix-Cols D. Anisotropic kernels for coordinate-based meta-analyses of neuroimaging studies. Frontiers in Psychiatry. 2014;5:13. doi: 10.3389/fpsyt.2014.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae C, Harasty JA, Dzendrowskyj TE, Talcott JB, Simpson JM, Blamire AM, Dixon RM, Lee MA, Thompson CH, Styles P, Richardson AJ, Stein JF. Cerebellar morphology in developmental dyslexia. Neuropsychologia. 2002;40:1285–1292. doi: 10.1016/S0028-3932(01)00216-0. [DOI] [PubMed] [Google Scholar]
- Raschle NM, Zuk J, Gaab N. Functional characteristics of developmental dyslexia in left-hemispheric posterior brain regions predate reading onset. PNAS. 2012;109:2156–2161. doi: 10.1073/pnas.1107721109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschle NM, Stering PL, Meissner SN, Gaab N. Altered neuronal response during rapid auditory processing and its relation to phonological processing in prereading children at familial risk for dyslexia. Cerebral Cortex. 2014;24:2489–2501. doi: 10.1093/cercor/bht104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilhac C, Peyrin C, Demonet JF, Valdois S. Role of the superior parietal lobules in letter-identity processing within strings: fMRI evidence from skilled and dyslexic readers. Neuropsychologia. 2013;51:601–612. doi: 10.1016/j.neuropsychologia.2012.12.010. [DOI] [PubMed] [Google Scholar]
- Richards TL, Aylward EH, Berninger VW, Field KM, Grimme AC, Richards AL, Nagy W. Individual fMRI activation in orthographic mapping and morpheme mapping after orthographic or morphological spelling treatment in child dyslexics. Journal of Neurolinguistics. 2006;19:56–86. doi: 10.1016/j.jneuroling.2005.07.003. [DOI] [Google Scholar]
- Richlan F, Kronbichler M, Wimmer H. Functional abnormalities in the dyslexic brain: A quantitative meta-analysis of neuroimaging studies. Human Brain Mapping. 2009;30:3299–3308. doi: 10.1002/hbm.20752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F, Sturm D, Schurz M, Kronbichler M, Ladurner G, Wimmer H. A common left occipito-temporal dysfunction in developmental dyslexia and acquired letter-by-letter reading. PLOS ONE. 2010;5:e12073. doi: 10.1371/journal.pone.0012073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F, Kronbichler M, Wimmer H. Meta-analyzing brain dysfunctions in dyslexic children and adults. NeuroImage. 2011;56:1735–1742. doi: 10.1016/j.neuroimage.2011.02.040. [DOI] [PubMed] [Google Scholar]
- Richlan F. Developmental dyslexia: Dysfunction of a left hemisphere reading network. Frontiers in Human Neuroscience. 2012;6:120. doi: 10.3389/fnhum.2012.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F, Kronbichler M, Wimmer H. Structural abnormalities in the dyslexic brain: A meta-analysis of voxel-based morphometry studies. Human Brain Mapping. 2013;34:3055–3065. doi: 10.1002/hbm.22127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F. Functional neuroanatomy of developmental dyslexia: The role of orthographic depth. Frontiers in Human Neuroscience. 2014;8:347. doi: 10.3389/fnhum.2014.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimrodt SL, Clements-Stephens AM, Pugh KR, Courtney SM, Gaur P, Pekar JJ, Cutting LE. Functional MRI of sentence comprehension in children with dyslexia: Beyond word recognition. Cerebral Cortex. 2009;19:402–413. doi: 10.1093/cercor/bhn092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring J, Black JL. The multiple deficit model of dyslexia: What does it mean for identification and intervention. Annals of Dyslexia. 2018;68:104–125. doi: 10.1007/s11881-018-0157-y. [DOI] [PubMed] [Google Scholar]
- Ruff S, Cardebat D, Marie N, Demonet JF. Enhanced response of the left frontal cortex to slowed down speech in dyslexia: An fMRI study. Neuroreport. 2002;13:1285–1289. doi: 10.1097/00001756-200207190-00014. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, Nace K, Donohue B, Wise D, Maisog JM, Andreason P. A positron emission tomographic study of impaired word recognition and phonological processing in dyslexic men. Archives of Neurology. 1997;54:562–573. doi: 10.1001/archneur.1997.00550170042013. [DOI] [PubMed] [Google Scholar]
- Schulz E, Maurer U, van der Mark S, Bucher K, Brem S, Martin E, Brandeis D. Impaired semantic processing during sentence reading in children with dyslexia: Combined fMRI and ERP evidence. NeuroImage. 2008;41:153–168. doi: 10.1016/j.neuroimage.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Schulz E, Maurer U, van der Mark S, Bucher K, Brem S, Martin E, Brandeis D. Reading for meaning in dyslexic and young children: Distinct neural pathways but common endpoints. Neuropsychologia. 2009;47:2544–2557. doi: 10.1016/j.neuropsychologia.2009.04.028. [DOI] [PubMed] [Google Scholar]
- Schurz M, Sturm D, Richlan F, Kronbichler M, Ladurner G, Wimmer H. A dual-route perspective on brain activation in response to visual words: Evidence for a length by lexicality interaction in the visual word form area (VWFA. NeuroImage. 2010;49:2649–2661. doi: 10.1016/j.neuroimage.2009.10.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurz M, Wimmer H, Richlan F, Ludersdorfer P, Klackl J, Kronbichler M. Resting-state and task-based functional brain connectivity in developmental dyslexia. Cerebral Cortex. 2015;25:3502–3514. doi: 10.1093/cercor/bhu184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz SE. Dyslexia. Scientific American. 1996;275:98–104. doi: 10.1038/scientificamerican1196-98. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Pugh KR, Fulbright RK, Constable RT, Mencl WE, Shankweiler DP, Liberman AM, Skudlarski P, Fletcher JM, Katz L, Marchione KE, Lacadie C, Gatenby C, Gore JC. Functional disruption in the organization of the brain for reading in dyslexia. PNAS. 1998;95:2636–2641. doi: 10.1073/pnas.95.5.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu H, Meng XZ, Chen X, Luan H, Cao F. The subtypes of developmental dyslexia in Chinese: Evidence from three cases. Dyslexia. 2005;11:311–329. doi: 10.1002/dys.310. [DOI] [PubMed] [Google Scholar]
- Silani G, Frith U, Demonet JF, Fazio F, Perani D, Price C, Frith CD, Paulesu E. Brain abnormalities underlying altered activation in dyslexia: A voxel based morphometry study. Brain. 2005;128:2453–2461. doi: 10.1093/brain/awh579. [DOI] [PubMed] [Google Scholar]
- Siok WT, Perfetti CA, Jin Z, Tan LH. Biological abnormality of impaired reading is constrained by culture. Nature. 2004;431:71–76. doi: 10.1038/nature02865. [DOI] [PubMed] [Google Scholar]
- Siok WT, Niu Z, Jin Z, Perfetti CA, Tan LH. A structural-functional basis for dyslexia in the cortex of Chinese readers. PNAS. 2008;105:5561–5566. doi: 10.1073/pnas.0801750105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siok WT, Spinks JA, Jin Z, Tan LH. Developmental dyslexia is characterized by the co-existence of visuospatial and phonological disorders in Chinese children. Current Biology. 2009;19:R890–R892. doi: 10.1016/j.cub.2009.08.014. [DOI] [PubMed] [Google Scholar]
- Siok WT, Jia FL, Liu CY, Perfetti CA, Tan LH. A lifespan fMRI study of neurodevelopment associated with reading Chinese. Cerebral Cortex. 2020;30:4140–4157. doi: 10.1093/cercor/bhaa038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeide MA, Bazin PL, Trampel R, Schafer A, Mannel C, von Kriegstein K, Friederici AD. Hypermyelination of the left auditory cortex in developmental dyslexia. Neurology. 2018;90:e492–e497. doi: 10.1212/WNL.0000000000004931. [DOI] [PubMed] [Google Scholar]
- Snowling MJ, Melby-Lervag M. Oral language deficits in familial dyslexia: A meta-analysis and review. Psychological Bulletin. 2016;142:498–545. doi: 10.1037/bul0000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbrink C, Vogt K, Kastrup A, Muller HP, Juengling FD, Kassubeek J, Riecker A. The contribution of white and gray matter differences to developmental dyslexia: Insights from DTI and VBM at 3.0 T. Neuropsychologia. 2008;46:3170–3178. doi: 10.1016/j.neuropsychologia.2008.07.015. [DOI] [PubMed] [Google Scholar]
- Steinbrink C, Groth K, Lachmann T, Riecker A. Neural correlates of temporal auditory processing in developmental dyslexia during German vowel length discrimination: An fMRI study. Brain and Language. 2012;121:1–11. doi: 10.1016/j.bandl.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Stein JF. The cerebellum and dyslexia. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior. 2011;47:101–116. doi: 10.1016/j.cortex.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Sun Z, Zou L, Zhang JJ, Mo SN, Shao SS, Zhong R, Ke JT, Lu XZ, Miao XP, Song RR. Prevalence and associated risk factors of dyslexic children in a middle-sized city of China: A cross-sectional study. PLOS ONE. 2013;8:e56688. doi: 10.1371/journal.pone.0056688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatkowska I, Grabowska A, Szymańska O. Phonological and semantic fluencies are mediated by different regions of the prefrontal cortex. Acta Neurobiol Exp. 2000;60:503–508. doi: 10.55782/ane-2000-1370. [DOI] [PubMed] [Google Scholar]
- Tamboer P, Scholte HS, Vorst HC. Dyslexia and voxel-based morphometry: Correlations between five behavioural measures of dyslexia and gray and white matter volumes. Annals of Dyslexia. 2015;65:121–141. doi: 10.1007/s11881-015-0102-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan LH, Laird AR, Li K, Fox PT. Neuroanatomical correlates of phonological processing of Chinese characters and alphabetic words: A meta-analysis. Human Brain Mapping. 2005;25:83–91. doi: 10.1002/hbm.20134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple E, Poldrack RA, Protopapas A, Nagarajan S, Salz T, Tallal P, Merzenich MM, Gabrieli JD. Disruption of the neural response to rapid acoustic stimuli in dyslexia: Evidence from functional MRI. PNAS. 2000;97:13907–13912. doi: 10.1073/pnas.240461697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple E, Poldrack RA, Salidis J, Deutsch GK, Tallal P, Merzenich MM, Gabrieli JD. Disrupted neural responses to phonological and orthographic processing in dyslexic children: An fMRI study. Neuroreport. 2001;12:299–307. doi: 10.1097/00001756-200102120-00024. [DOI] [PubMed] [Google Scholar]
- Tettamanti M, Moro A, Messa C, Moresco RM, Rizzo G, Carpinelli A, Matarrese M, Fazio F, Perani D. Basal ganglia and language: Phonology modulates dopaminergic release. Neuroreport. 2005;16:397–401. doi: 10.1097/00001756-200503150-00018. [DOI] [PubMed] [Google Scholar]
- Ullman MT, Earle FS, Walenski M, Janacsek K. The neurocognition of developmental disorders of language. Annual Review of Psychology. 2020;71:389–417. doi: 10.1146/annurev-psych-122216-011555. [DOI] [PubMed] [Google Scholar]
- Uno A, Wydell TN, Haruhara N, Kaneko M, Shinya N. Relationship between reading/writing skills and cognitive abilities among Japanese primary-school children: Normal readers versus poor readers (dyslexics. Reading and Writing. 2009;22:755–789. doi: 10.1007/s11145-008-9128-8. [DOI] [Google Scholar]
- van der Mark S, Bucher K, Maurer U, Schulz E, Brem S, Buckelmuller J, Kronbichler M, Loenneker T, Klaver P, Martin E, Brandeis D. Children with dyslexia lack multiple specializations along the visual word-form (VWF) system. NeuroImage. 2009;47:1940–1949. doi: 10.1016/j.neuroimage.2009.05.021. [DOI] [PubMed] [Google Scholar]
- van Ermingen-Marbach M, Grande M, Pape-Neumann J, Sass K, Heim S. Distinct neural signatures of cognitive subtypes of dyslexia with and without phonological deficits. NeuroImage. 2013a;2:477–490. doi: 10.1016/j.nicl.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ermingen-Marbach M, Pape-Neumann J, Grande M, Grabowska A, Heim S. Distinct neural signatures of cognitive subtypes of dyslexia: Effects of lexicality during phonological processing. Acta Neurobiologiae Experimentalis. 2013b;73:404–416. doi: 10.55782/ane-2013-1947. [DOI] [PubMed] [Google Scholar]
- Vandermosten M, Boets B, Wouters J, Ghesquiere P. A qualitative and quantitative review of diffusion tensor imaging studies in reading and dyslexia. Neuroscience and Biobehavioral Reviews. 2012;36:1532–1552. doi: 10.1016/j.neubiorev.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Vandermosten M, Correia J, Vanderauwera J, Wouters J, Ghesquiere P, Bonte M. Brain activity patterns of phonemic representations are atypical in beginning readers with family risk for dyslexia. Developmental Science. 2019;10:e12857. doi: 10.1111/desc.12857. [DOI] [PubMed] [Google Scholar]
- Vasic N, Lohr C, Steinbrink C, Martin C, Wolf RC. Neural correlates of working memory performance in adolescents and young adults with dyslexia. Neuropsychologia. 2008;46:640–648. doi: 10.1016/j.neuropsychologia.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Vinckenbosch E, Robichon F, Eliez S. Gray matter alteration in dyslexia: Converging evidence from volumetric and voxel-by-voxel MRI analyses. Neuropsychologia. 2005;43:324–331. doi: 10.1016/j.neuropsychologia.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Waldie KE, Haigh CE, Badzakova-Trajkov G, Buckley J, Kirk IJ. Reading the wrong way with the right hemisphere. Brain Sciences. 2013;3:1060–1075. doi: 10.3390/brainsci3031060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandell BA, Rauschecker AM, Yeatman JD. Learning to see words. Annual Review of Psychology. 2012;63:31–53. doi: 10.1146/annurev-psych-120710-100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ, Yang JF, Yang J, Mencl WE, Shu H, Zevin JD. Language differences in the brain network for reading in naturalistic story reading and lexical decision. PLOS ONE. 2015;10:e0124388. doi: 10.1371/journal.pone.0124388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Yan X, Liu Y, Spray GJ, Deng Y, Cao F. Structural and functional abnormality of the putamen in children with developmental dyslexia. Neuropsychologia. 2019;130:26–37. doi: 10.1016/j.neuropsychologia.2018.07.014. [DOI] [PubMed] [Google Scholar]
- Weiller C, Isensee C, Rijntjes M, Huber W, Müller S, Bier D, Dutschka K, Woods RP, Noth J, Diener HC. Recovery from wernicke’s aphasia: A positron emission tomographic study. Annals of Neurology. 1995;37:723–732. doi: 10.1002/ana.410370605. [DOI] [PubMed] [Google Scholar]
- Weiss Y, Katzir T, Bitan T. When transparency is opaque: Effects of diacritic marks and vowel letters on dyslexic Hebrew readers. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior. 2016;83:145–159. doi: 10.1016/j.cortex.2016.07.017. [DOI] [PubMed] [Google Scholar]
- Wichmann T, DeLong MR. In: Basic Neurochemistry. Brady ST, Siegel GJ, Albers RW, Price DL, editors. Academic Press; 2012. Neurotransmitters and disorders of the basal ganglia; pp. 856–871. [Google Scholar]
- Wimmer H, Schurz M. Dyslexia in regular orthographies: Manifestation and causation. Dyslexia. 2010;16:283–299. doi: 10.1002/dys.411. [DOI] [PubMed] [Google Scholar]
- Wimmer H, Schurz M, Sturm D, Richlan F, Klackl J, Kronbichler M, Ladurner G. A dual-route perspective on poor reading in a regular orthography: An fMRI study. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior. 2010;46:1284–1298. doi: 10.1016/j.cortex.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CY, Ho MHR, Chen SHA. A meta-analysis of fMRI studies on Chinese orthographic, phonological, and semantic processing. NeuroImage. 2012;63:381–391. doi: 10.1016/j.neuroimage.2012.06.047. [DOI] [PubMed] [Google Scholar]
- Xia Z, Hoeft F, Zhang L, Shu H. Neuroanatomical anomalies of dyslexia: Disambiguating the effects of disorder, performance, and maturation. Neuropsychologia. 2016;81:68–78. doi: 10.1016/j.neuropsychologia.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Bi HY, Long ZY, Tao S. Evidence for cerebellar dysfunction in Chinese children with developmental dyslexia: An fMRI study. International Journal of Neuroscience. 2013;123:300–310. doi: 10.3109/00207454.2012.756484. [DOI] [PubMed] [Google Scholar]
- Yang YH, Yang Y, Chen BG, Zhang YW, Bi HY. Anomalous cerebellar anatomy in Chinese children with dyslexia. Frontiers in Psychology. 2016;7:324. doi: 10.3389/Fpsyg.2016.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Tan LH. Whole-brain functional networks for phonological and orthographic processing in Chinese good and poor readers. Frontiers in Psychology. 2020;10:2945. doi: 10.3389/fpsyg.2019.02945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younger JW, Tucker-Drob E, Booth JR. Longitudinal changes in reading network connectivity related to skill improvement. NeuroImage. 2017;158:90–98. doi: 10.1016/j.neuroimage.2017.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Zhang BP, Chen Y, Zhou X, Zuo P. Environmental risk factors in Han and Uyghur children with dyslexia: A comparative study. PLOS ONE. 2016;11:e0159042. doi: 10.1371/journal.pone.0159042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler JC, Perry C, Ma-Wyatt A, Ladner D, Schulte-Korne G. Developmental dyslexia in different languages: Language-specific or universal. Journal of Experimental Child Psychology. 2003;86:169–193. doi: 10.1016/S0022-0965(03)00139-5. [DOI] [PubMed] [Google Scholar]
- Zuk J, Perdue M, Becker B, Yu X, Chang M, Raschle NM, Gaab N. Neural correlates of phonological processing: Disrupted in children with dyslexia and enhanced in musically trained children. Developmental Cognitive Neuroscience. 2018;34:82–91. doi: 10.1016/j.dcn.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]