Abstract
Models of reading emphasize that visual (orthographic) processing provides input to phonological as well as lexical–semantic processing. Neurobiological models of reading have mapped these processes to distributed regions across occipital–temporal, temporal–parietal, and frontal cortices. However, the role of the precentral gyrus in these models is ambiguous. Articulatory phonemic representations in the precentral gyrus are obviously involved in reading aloud, but it is unclear if the precentral gyrus is recruited during reading silently in a time window consistent with participation in phonological processing contributions. Here, we recorded intracranial electrophysiology during a speeded semantic decision task from 24 patients to map the spatio-temporal flow of information across the cortex during silent reading. Patients selected animate nouns from a stream of nonanimate words, letter strings, and false-font stimuli. We characterized the distribution and timing of evoked high-gamma power (70–170 Hz) as well as phase-locking between electrodes. The precentral gyrus showed a proportion of electrodes responsive to linguistic stimuli (27%) that was at least as high as those of surrounding peri-sylvian regions. These precentral gyrus electrodes had significantly greater high-gamma power for words compared to both false-font and letter-string stimuli. In a patient with word-selective effects in the fusiform, superior temporal, and precentral gyri, there was significant phase-locking between the fusiform and precentral gyri starting at ∼180 msec and between the precentral and superior temporal gyri starting at ∼220 msec. Finally, our large patient cohort allowed exploratory analyses of the spatio-temporal reading network underlying silent reading. The distribution, timing, and connectivity results place the precentral gyrus as an important hub in the silent reading network.
INTRODUCTION
The neurobiology of reading has generated interest since lesion studies in the late 1800s (Dejerine, 1892). Early theories posited a visual letter identification system that then used the existing peri-sylvian auditory language network for lexical–semantic encoding through visual-to-auditory stimulus conversion (Geschwind, 1974). Since then, lesion (Coltheart, 1980), neuroimaging (Price, 2012), developmental (Grainger, Lété, Bertand, Dufau, & Ziegler, 2012), and modeling (Coltheart, Rastle, Perry, Langdon, & Ziegler, 2001) studies support a visual stream of processing (orthographic), which in addition to providing input to lexical–semantic processing, also provides input to an auditory stream of processing (phonological). Both the orthographic and phonological routes concurrently operate in an integrative manner, working toward lexical–semantic encoding (Perry, Ziegler, & Zorzi, 2007; Harm & Seidenberg, 2004; Coltheart et al., 2001). Neurobiological studies have been mapping the proposed orthographic, phonological, and lexical–semantic processes onto the cortex, finding evidence of a distributed reading network across large portions of the occipital–temporal, temporal–parietal, and frontal cortices (Carreiras, Armstrong, Perea, & Frost, 2014; Taylor, Rastle, & Davis, 2013; Price, 2012; Jobard, Crivello, & Tzourio-Mazoyer, 2003; Fiez & Petersen, 1998). The orthographic to lexical–semantic processing stream is mainly associated with the ventral occipital–temporal cortex. Orthographic to phonological processing is instead associated with lateral temporal–parietal and inferior frontal cortices. Here, we focus on the role of the precentral gyrus in reading, examining whether this region, which is associated with engagement in articulatory processes, is also important during silent reading.
The precentral gyrus is linked with articulatory phonemes. Therefore, when reading aloud, the precentral gyrus is an obvious contributor to the reading network, but when reading silently, it is less clear whether the precentral gyrus is a contributor. Early psychological theory emphasized articulatory cognitive operations in silent reading (Allport, 1979), providing a rationale for the precentral gyrus to be involved in the neurobiology of reading. This theory was based on evidence from the articulatory suppression paradigm in which participants repeat a nonsense phrase to occupy the articulatory cognitive operations while performing a reading task, resulting in suppressed phonological behavioral effects during reading (Burani, Vallar, & Bottini, 1991; Barron & Baron, 1977; Kleiman, 1975), but not if mouth movements were nonarticulatory (Burani et al., 1991). Critically, articulatory suppression removed the phonological similarity effect for visual but not auditory words (Peterson & Johnson, 1971), implying a contribution of subvocal articulation in silent reading. Modern neurobiological models of reading emphasize regions associated with phoneme encoding, such as the superior temporal gyrus (STG) and adjoining regions in the inferior parietal cortex such as the supramarginal gyrus (Carreiras et al., 2014; Taylor et al., 2013), as contributing to phonological processing during reading. This is a continuation of lesion studies on visual language, which posited a critical relationship between visual processing and Wernicke's area, linking visual text encoding in the occipital lobe and the peri-sylvian auditory language network (Geschwind, 1974). Lesion studies continue to find associations between lesions in temporal–parietal regions and phonological processing during visual language encoding (Pillay, Stengel, Humphries, Book, & Binder, 2014). In addition, modern neurobiological models also emphasize the contributions from frontal regions such as the pars opercularis, which adjoins the precentral gyrus, suggesting that there may be a role for both articulatory and encoding phonemic representations to be automatically recruited during the process of silent reading.
The automaticity of phonological processing during silent reading is attested to by phonological effects on reading from unconscious priming (Frost, 1998). Behavioral studies using an unconscious priming methodology demonstrate that auditory linguistic information derived from text presented as short as 15–60 msec affects reading (Rastle & Brysbaert, 2006; Berent & Perfetti, 1995; Perfetti & Bell, 1991; Perfetti, Bell, & Delaney, 1988). In a blood oxygen level dependent (BOLD) study using masked phonological priming, the masked presentation of visual text activated the left precentral gyrus (Dehaene et al., 2001), which suggests that precentral activity during reading is an automatic part of visual word encoding. Neuroimaging and lesion studies provide additional empirical evidence to support a role for the precentral gyrus. There was greater precentral gyrus activity when making phonological than semantic judgments (Price, Moore, Humphreys, & Wise, 1997), differential activation based on the spelling–sound consistency of a word (Fiez, Balota, Raichle, & Petersen, 1999), and increased activation with increasing grapheme-to-phoneme conversion difficulty (Binder, Medler, Desai, Conant, & Liebenthal, 2005). In a study of a patient with a lesion centered on the precentral gyrus, the patient's ability to make phonological judgments about visual words was impaired (Vallar, Di Betta, & Silveri, 1997). Another patient with a similar lesion could not make rhyming judgments or manipulate pronounceable pseudowords (Vallar & Cappa, 1987). Taken together, these theories hint at a link between reading, the precentral gyrus, and articulatory phonemes.
A fuller understanding of the relationship of the precentral gyrus to the reading network will require an understanding of the timing of precentral gyrus activity during silent reading. Although the BOLD and lesion studies support that the precentral gyrus is involved in phonological processing in a variety of tasks, these methodologies do not have the temporal resolution to observe at what time this activity may occur during silent reading. Specifically at question is whether activity in the precentral gyrus during silent reading occurs at a time consistent with participation in phonological processing. This timing question can be investigated with intracranial electrophysiology (iEEG). iEEG has documented that visual information begins at ∼60 msec in the visual cortex (Foxe & Simpson, 2002), followed first by orthographic processing in the posterior ventral visual route at ∼160–180 msec (Thesen et al., 2012; Allison, Puce, Spencer, & McCarthy, 1999; Allison, McCarthy, Nobre, Puce, & Belger, 1994) and then lexical–semantic effects in the anterior–ventral temporal lobe beginning at ∼250–300 msec and continuing for several hundred milliseconds (Chan et al., 2011; Nobre & McCarthy, 1995; Nobre, Allison, & McCarthy, 1994). This lexical–semantic processing time window from iEEG studies aligns well with the lexical–semantic period of the N400 complex found between 250 and 500 msec (Kutas & Federmeier, 2011; Marinković, 2004). Therefore, supporting the hypothesis that precentral gyrus processing during silent reading participates in phonological processing necessitates finding activity in the precentral gyrus before or overlapping with the time window from 250 to 500 msec. However, iEEG has not been used to examine the temporal relationship between the precentral gyrus and the dorsal and ventral routes during silent reading.
The Current Study
Here, we seek to understand the flow of information during silent reading across both the ventral and dorsal reading routes using the spatio-temporal precision afforded by a large cohort of patients with intracranial electrodes. A high proportion of the electrodes placed for clinical monitoring were localized in peri-sylvian regions, providing excellent spatial coverage to investigate the evoked activity in the dorsal reading route. Because of the empirical literature strongly supporting precentral gyrus involvement in reading, we place particular focus on the relationship of the precentral gyrus and posterior temporal–parietal regions. Our findings provide evidence that the precentral gyrus is involved at an early stage of silent reading.
METHODS
Participants and Recordings
Electrocorticographic recordings were obtained from 24 patients (15 female, mean age = 36.6 [range = 16–53] years, mean onset age = 17 [range = 1–42] years; patient information contained in Table 1) undergoing iEEG monitoring to treat drug-resistant epilepsy. All patients were either right-handed, confirmed to be left-hemisphere language by Wada testing, or both. All procedures were approved by the institutional review board at New York University, and written informed consent was obtained from all participants. Electrode placement was determined by clinical criteria to identify seizure activity and eloquent tissue. Each patient was implanted with subdural platinum–iridium electrode arrays embedded in silastic sheets (AdTech Medical Instrument Corp.). Data included arrays of grids (8 × 8 contacts) and strips (1 × 4 to 1 × 12 contacts). Contacts had a diameter of 4 mm with 2.3-mm exposure. Center-to-center spacing between contacts was 10 mm for grids. In total, 13 patients had predominantly left-sided implantations, nine patients had predominantly right-sided implantations, and two had bilateral implantations (i.e., strips distributed around both hemispheres). Recordings were acquired using a Nicolet One EEG system sampled at 512 Hz (i.e., a temporal resolution of 1.95 msec) and bandpass filtered between 0.5 and 250 Hz.
Table 1. .
Patient Clinical Information
| Patient | Age (Years) | Onset | Sex | Handedness | Wada | Predominant Implantation |
|---|---|---|---|---|---|---|
| P1 | 37 | 34 | F | R | L | L |
| P2 | 27 | 22 | M | L | L | R |
| P3 | 27 | 15 | M | R | L | L |
| P4 | 53 | 32 | F | R | L | L |
| P5 | 36 | 27 | F | R | L | L |
| P6 | 42 | 13 | M | R | L | R |
| P7 | 40 | 3.5 | F | R | L | L |
| P8 | 53 | 12 | M | R | L | L |
| P9 | 51 | 37 | F | R | L | L |
| P10 | 43 | 42 | M | R | L | L |
| P11 | 45 | 4 | F | R | – | L |
| P12 | 18 | 7 | M | R | L | R |
| P13 | 24 | 4 | F | R | – | L |
| P14 | 41 | 18 | F | R | L | R |
| P15 | 27 | 4.5 | F | R | L | L |
| P16 | 39 | 5 | F | R | L | L |
| P17 | 48 | 3 | F | R | L | B |
| P18 | 16 | 11 | F | R | L | R |
| P19 | 20 | – | F | – | L | B |
| P20 | 50 | 8 | F | – | L | R |
| P21 | 51 | 38 | F | R | L | R |
| P22 | 36 | 29 | M | R | L | R |
| P23 | 26 | 19 | M | R | L | L |
| P24 | 29 | 0.8 | M | R | – | R |
F = female; L = left; M = male; R = right.
Electrode Localization
Electrode localization was done through coregistration of preimplant and postimplant MRIs, followed by manual and automatic localization of electrodes (Yang et al., 2012). Anatomical parcellations were determined using a modified Desikan–Killiany atlas (Desikan et al., 2006). Three-dimensional reconstructions of cortical surfaces in figures were created using FreeSurfer (Dale, Fischl, & Sereno, 1999). Localization into a brain region was performed in each participant's native brain.
Task Design
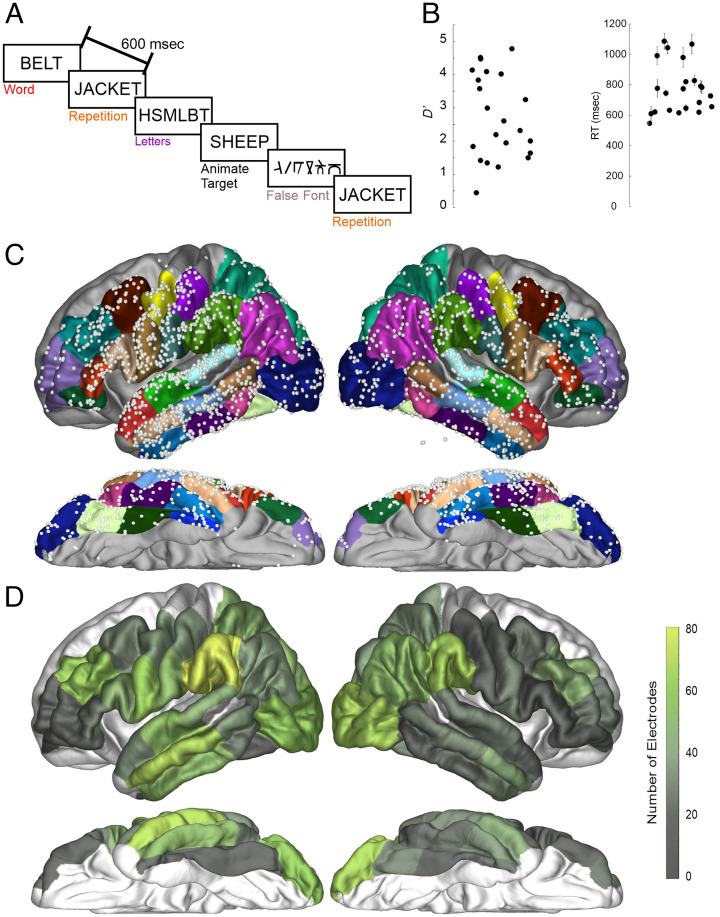
Patients performed a semantic judgment task in which they were instructed to respond by pressing a button to rare animal target items (e.g., SHEEP), which were ∼5% of the total stimuli (target trials were not included in the analysis). Patients responded with their left hand to ensure that no left precentral gyrus activation could be attributed to hand motor movements. The task used semantic judgment to ensure completion of lexical–semantic integration, as opposed to the less-complete familiarity judgments possible in a lexical decision task (Balota & Chumbley, 1984). Participants were asked to press a button if the word was an animate noun and to not press the button if the target was not an animate noun. Stimuli were “words,” consonant strings (i.e., “letters”), and “false fonts.” False fonts, matched to alphabetic letters on a variety of sensory characteristics, allowed us to discriminate visual sensory processing from orthographic, phonological, and lexical–semantic processing. These false fonts were created by researchers at New York University and the University of California San Diego (Thesen et al., 2012). Each false-font character was matched to a real letter in the English alphabet in size, number of strokes, total line length, and curvature (e.g., see Figure 1A). Consonant strings isolated orthographic from phonological and lexical–semantic operations. Unpronounceable consonant strings (“BRZ”) were chosen to provide a control for orthographic versus phonological and lexical–semantic processes. We were unable to find any literature on whether consonant strings are phonologically recoded, but as they are unpronounceable and cannot be broken down in syllables, it is assumed they evoke no more than cursory phonological recoding. These three stimulus types allowed us to split word processing into sensory, orthographic, and phonological/lexical–semantic operations. Also included are words that were repeated throughout the task, which evoked repetition effects and serve as an assay of if and when information regarding linguistic identities (e.g., letters, phonemes, and/or words) begins to affect processing in a region (Gotts, Chow, & Martin, 2012). Because our repetition is a whole-word repetition, we cannot say for certain which linguistic level leads to the repetition effects.
Figure 1. .
Task design and electrode coverage. (A) Patients detected animate nouns amid four other stimulus types: novel words, letters, repeated words, and false fonts. Stimuli were presented every 600 msec. (B) Patient performance as expressed by d′ and RT. Each dot represents the performance of one patient. (C) Electrode coverage across the included ROIs presented on an average brain for illustration purposes. Colors on the brain highlight the ROIs involved in the study from the Desikan atlas. Gray regions were not included in analyses because of lack of electrode coverage in ROI. (D) Electrode coverage expressed as the total number of electrodes within each ROI.
Stimuli were presented visually as white letters on a black background in Arial font. Stimuli consisted of 400 novel object words that were presented only once (e.g., “BELT”), 400 repeated object words (20 words repeated 20 times each), 400 unpronounceable consonant letter strings (e.g., “HSMBLT”), 400 false-font stimuli, and 80 target animal words. The false-font stimuli were alphabet-like characters that matched a real letter in the English alphabet in size, number of strokes, total line length, and curvature. The repeated word trials were interleaved with normal trials and spaced with an average of 4.2 intervening stimuli between instances of repetition (∼2520 msec), with a range of 1–10 (600–6000 msec). Individual repeated words were spaced with an average of 42 intervening stimuli (25,200 msec), with a range of 29–59 (17,400–35,400 msec). Figure 1A shows the task design and sample stimuli. All stimuli were four to eight characters in length, with a written lexical frequency of 3–80 per 10 million (Frances & Kucera, 1982). As a post hoc comparison, we were interested in examining word frequency effects. We obtained word frequency from the MCWord database (Medler & Binder, 2005). Words were then split into high- and low-frequency categories, with the bottom 40% of novel words labeled “Low-Frequency” and the top 40% of novel words labeled “High-Frequency” (excluding the middle 20% of words from the analysis).
Data were collected using a rapid stimulus onset asynchrony (600 msec) and a very large number of trials per condition to obtain electrophysiological data with a high signal-to-noise ratio in a short time frame. The experimental task was organized into two separate lists, each list taking approximately 10 min to complete. The tasks were programmed using Presentation software (Neurobehavioral Systems, Inc.).
Data Processing
Data were preprocessed using MATLAB (MathWorks, Inc.), the Fieldtrip toolbox (Oostenveld, Fries, Maris, & Schoffelen, 2011), and custom scripts. We used an average subtraction reference for each patient to remove global artifacts and noise followed by a bandstop around line noise and its harmonics (60, 120, and 180 Hz). Data were epoched to the onset of stimulus presentation from −1400 to +2200 msec to provide sufficient temporal padding to avoid epoch-edge artifacts introduced by converting from the time domain to the frequency domain. Temporal padding was removed at the end of preprocessing for finalized epochs from −400 to 1200 msec. To calculate high-gamma power (HGP), epochs were transformed from the time domain to the time–frequency domain using the complex Morlet wavelet transform from 70 to 170 Hz in 10-Hz increments. The wavelet widths increase linearly from 14 to 38 as frequency increased from 70 to 170 Hz, resulting in a standard deviation (i.e., wavelet width) of 16 msec and a frequency resolution of 10 Hz. For each epoch, spectral power was calculated from the wavelet spectra, normalized by the inverse square frequency to adjust for the rapid drop-off in the EEG power spectrum with frequency, and averaged from 70 to 170 Hz, excluding line noise harmonics. These data were smoothed by a moving Gaussian window exactly matching the temporal characteristics of the wavelet (i.e., a standard deviation of 16 msec). Baseline subtraction was performed on each trial epoch with a baseline from −75 to 0 msec. Trials containing artifacts were identified by outlier amplitude and variance, visually inspected for artifacts, and removed from further analysis.
Task Effect Analysis
Behavior
Behavior was characterized with both measures of performance (d′) and speed (response time [RT]).
Identifying Task-modulated Electrodes
Electrodes that had significantly increased activity from a baseline of 0 to any of the four stimulus conditions between 50 and 600 msec were identified using a timepoint-by-timepoint t test (i.e., running a t test at each observation from 50 to 600 msec) corrected for temporal false discovery rate at p < .05 (Benjamini & Hochberg, 1995). Next, electrodes were run through a one-way ANOVA between the four stimulus conditions between 50 and 600 msec at p < .01 temporally corrected using a bootstrapped shuffling of trial identity 1000 times (Maris & Oostenveld, 2007). This correction was performed by using temporal clustering as the criterion for significance. Only electrodes that were significant in both these tests (i.e., both a significant increase from baseline in HGP and a significant difference during this increase among the four stimulus classes) were included in further analysis (task-modulated electrodes). To emphasize, an electrode had to have both a significant evoked response and that response had to be modulated by a linguistic stimulus class for further inclusion in the study.
Characterizing Task-modulated Electrodes
To understand which stimuli were driving the significant differences between conditions identified by the ANOVA, pairwise one-way ANOVAs were run to determine if electrodes were letter specific (i.e., words > false fonts but words = letters) or word specific (i.e., words > false fonts, words > letters). ANOVAs were run timepoint-by-timepoint, once again corrected using the bootstrapped shuffling method. The “Task-Modulated” ANOVA results were used to mask significant periods to ensure differences found between conditions were part of the originally identified task-modulated period.
Characterizing Sensitivity to Item Repetition and Lexical Frequency
Electrodes were examined for differential responses to repetition and lexical frequency. The full repetition of items leads to multiple well-documented empirical regularities, with decreased neuronal activity previously found using functional magnetic resonance imaging (van Turennout, Ellmore, & Martin, 2000), unit firing (Desimone, 1996), and iEEG (McDonald et al., 2010). Repetition sensitivity (novel words > repeated words) were identified using the same methods as discussed above. Because repetition effects are related to decreased neuronal firing, this was why only one effect direction was accepted. Word frequency affects naming speed (Forster & Chambers, 1973) and eye fixation time (Sereno, Rayner, & Posner, 1998). Several behavioral effects in reading are only found with low-frequency words (Jared & Seidenberg, 1991; Seidenberg, Waters, Barnes, & Tanenhaus, 1984), and extracranial EEG reports effects based on word frequency (Hauk & Pulvermüller, 2004). The same ANOVA procedure, corrected with the shuffled method, was performed to identify frequency-sensitive electrodes (low frequency > high frequency).
Regional Comparisons: A Priori Comparison of Precentral and Temporal–Parietal Regions
The critical question of this study was whether the precentral gyrus shows evidence of involvement in silent reading. However, comparisons between regions are difficult in iEEG because of sparse coverage that varies between patients because of clinical considerations. Studies with large numbers of patients note that, although locations of interest, such as language, vary in precise location, they occur in broadly defined regions according to neuroanatomical landmarks (Ojemann, Ojemann, Lettich, & Berger, 1989). For this reason, this study used nonparametric statistics to compare both proportion of electrodes and timing of electrodes grouped into regions (i.e., FreeSurfer parcellations). Our first approach was to compare the precentral region of the Desikan atlas to the STG, supramarginal, and inferior parietal parcellations from the same atlas. This was chosen because temporal–parietal regions' involvement in phonological processing is well established. Finding that, during silent reading, the precentral gyrus has activity at least as involved as these regions would constitute evidence of the precentral gyrus' similar involvement in silent reading. With three comparisons, the corrected p value threshold was <.016. All tests run were a Fisher's exact nonparametric test. Because of the a priori assumption from neurobiological models of reading that the network is left-lateralized, we will restrict our regional comparisons to the left hemisphere (Carreiras et al., 2014; Taylor et al., 2013).
Regional Comparisons: Exploratory Analysis of the Reading Network
The second approach was a broader post hoc comparison between regions of interest (ROIs) across the entire cortical reading network. We performed exploratory comparisons including seven Desikan atlas candidate regions from neurobiological models of reading (fusiform, lateral occipital, STG, middle temporal gyrus (MTG), supramarginal, pars opercularis, and pars triangularis parcellations) as well as the precentral parcellation, for a total of eight regions. To ascertain whether regions stood out relative to the network, each region was compared to the rest of the network to understand if the region in question differed significantly in proportion or timing of effects. These analyses compared each region to a “pool” of the other seven regions' electrodes using a Fisher's exact nonparametric test (proportion) or rank-sum (RS) test (timing). Given the eight regions, the corrected p value was <.006. Because this was a stringent threshold and we used nonparametric statistics with lower power, we still reported p values greater than .006 but below .05 and marked them as uncorrected. Investigating timing between regions statistically was difficult because the variable number of effects per region causes differences in power. For example, a critical question was the timing of the precentral gyrus versus the STG, but the STG contained only nine task-modulated electrodes compared to 27 for the precentral gyrus. However, despite these difficulties, some regularities emerged. Comparisons will again be in the left hemisphere.
Electrode Display
Participant average electrode locations, used for display purposes only, were obtained using FreeSurfer surface-to-surface calculations with the fsaverage brain. Regions with less than five electrodes were excluded from visualization. For display purposes of the proportions across the brain, long gyri were split into three equal parts, inferior/middle/superior (precentral gyrus, postcentral gyrus) or caudal/middle/rostral (fusiform, inferior temporal gyrus, MTG, STG, middle frontal gyrus), using FreeSurfer.
Connectivity
We used phase-locking value (PLV) calculated pairwise between electrodes, as described in Lachaux, Rodriguez, Martinerie, and Varela (1999), to test whether functional connectivity could be inferred between electrodes. PLV measures the consistency of the relative phase of local field potentials in two locations. High PLV indicates consistent synchronization of the synaptic currents in pyramidal apical dendrites between the cortical locations underlying the intracranial sensors. Significant PLV was determined by creating a distribution of all PLVs from a baseline period (−200 to 0 msec) for each participant. Only if the obtained PLV after stimulus presentation was p < .00005 based on the participant's own baseline distribution was a pairwise connection judged to be significant.
RESULTS
Behavior
Behavioral data were available for 19 of the 24 patients because of technical issues with five of the patients. Figure 1B shows d′ and RT across participants. Average d′ was 2.79 (range = 0.5–4.84), and average correct RT was 787 msec (range = 560–1099 msec). This demonstrates participants were able to perform the task effectively, and the RTs were equivalent for a similar task in a healthy control behavioral database (Pexman, Heard, Lloyd, & Yap, 2017).
Dorsal Route Comparison: Precentral Gyrus versus Posterior Temporal–Parietal Regions
Task-modulated Effects
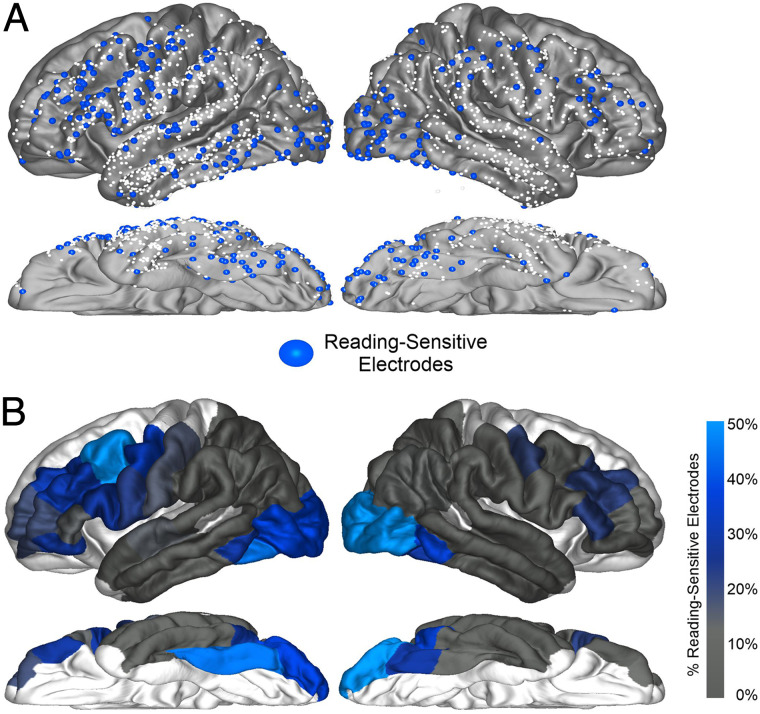
Our central analysis was a comparison of the distribution and timing of language effects in the putative visual language “dorsal route.” Figure 1C and D displays our electrode coverage and ROIs. Table 2 and Figure 2 display the parcellation regions and their task-modulated electrodes. In the left hemisphere, our test of whether the precentral gyrus had at least as much activity as posterior temporal–parietal regions revealed that the precentral gyrus had a greater proportion of task-modulated electrodes (27%) than the STG (8%; Fisher's exact test [FET]: p < .001), supramarginal (8%; FET: p < .001), and inferior parietal (5%; FET: p = .004) parcellations.
Table 2. .
Distribution of Task-modulated Electrodes, and How Many Patients Were Contributed Task-modulated Electrodes, In Each Region
| Region | Left Hemisphere | Right Hemisphere | ||
|---|---|---|---|---|
| Task-modulated | Participants | Task-modulated | Participants | |
| Occipital–temporal | ||||
| Lateral occipital | 38% (23/61) | 10/14 | 62% (44/71) | 10/10 |
| Fusiform | 45% (18/40) | 6/10 | 23% (11/48) | 6/11 |
| Inferior temporal gyrus | 15% (12/81) | 6/12 | 18% (8/44) | 4/8 |
| Parietal | ||||
| Inferior parietal | 5% (2/41) | 2/8 | 13% (8/62) | 5/11 |
| Superior parietal | 6% (3/52) | 1/9 | 10% (4/39) | 3/7 |
| Supramarginal | 8% (8/99) | 6/16 | 12% (8/69) | 6/11 |
| Lateral temporal | ||||
| MTG | 8% (8/106) | 3/14 | 3% (2/64) | 2/7 |
| STG | 8% (9/106) | 5/14 | 3% (2/60) | 1/8 |
| Rolandic | ||||
| Precentral | 27% (27/101) | 7/15 | 15% (8/55) | 3/8 |
| Postcentral | 16% (14/89) | 6/11 | 7% (3/43) | 1/6 |
| Frontal | ||||
| Pars opercularis | 28% (14/50) | 8/13 | 13% (2/16) | 1/7 |
| Pars triangularis | 13% (4/30) | 2/10 | 22% (5/23) | 4/8 |
| Pars orbitalis | 28% (5/18) | 5/13 | 0% (0/19) | 0/9 |
| Middle frontal | 28% (27/95) | 3/9 | 14% (10/72) | 0/7 |
“Task-modulated” columns: #% (#/#) = proportion of electrodes (electrodes showing effect / total electrodes). “Participants” columns: # / # = number of patients with ≥1 electrode showing effect / total patients with electrodes in region.
Figure 2. .
Task-modulated electrode distribution across the cortex. Electrodes were identified, which displayed both a significant increase from baseline and a significant difference between conditions for HGP (70–170 Hz). (A) Electrodes meeting criteria for a task-modulated effect displayed on an average brain for illustration purposes. Smaller white dots represent electrodes recorded, which did not meet criteria for being task-modulated. (B) Electrodes meeting criteria for a task-modulated effect displayed as percentages out of total electrodes in an ROI.
Letter- and Word-Selective Effects
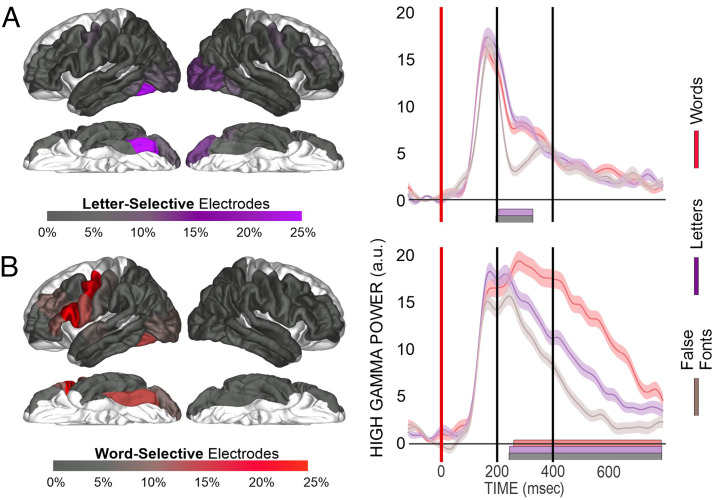
Next, we sought to characterize the linguistic processing level in the “dorsal reading route.” This involved finding the preferred stimuli (i.e., letters and/or words) of the task-modulated electrodes identified in the previous section. Figure 3 and Table 3 provide the distribution and number of the task-modulated electrodes, which displayed “letter-specific” (i.e., words > false fonts but words = letters) or “word-specific” (i.e., both words > false fonts and words > letters) effects. Figure 4 displays onset timings for the effects across parcellations. Table 4 contains the median and range of effect onset timings for each parcellation.
Figure 3. .
Characterizing task-modulated electrodes. Language-sensitive electrodes were characterized as either letter-selective (words > false fonts) or word-selective (words > false fonts and words > letters). (A) The left panel displays the proportion of electrodes in an ROI displaying a letter-selective effect. The right panel displays an example fusiform electrode from Patient P15 displaying a letter-selective effect. Vertical axis for HGP is in arbitrary units (a.u.). The gray bar highlights a significant ANOVA effect between conditions, and the purple bar highlights a significant difference between words and false fonts. (B) The left panel displays the proportion of electrodes in a region displaying a word-selective effect. The right panel displays an example fusiform electrode from Patient P16 displaying a word-selective effect. Vertical axis for HGP is in a.u. The gray bar highlights a significant ANOVA effect between conditions; the purple bar highlights a significant difference between words and false fonts, and the red bar highlights a significant difference between words and letters.
Table 3. .
Number of Electrodes Displaying Each Effect Divided in Region
| Region | Left Hemisphere | |||||
|---|---|---|---|---|---|---|
| Total Electrodes | Letter-selective | Word-selective | Word Frequency | Repetition | Nonspecific | |
| Occipital–temporal | ||||||
| Lateral occipital | 61 | 6 | 6 | 0 | 6 | 9 |
| Fusiform gyrus | 40 | 6 | 6 | 2 | 7 | 6 |
| Inferior temporal gyrus | 81 | 3 | 4 | 1 | 4 | 1 |
| Parietal | ||||||
| Inferior parietal | 41 | 0 | 2 | 1 | 2 | 0 |
| Superior parietal | 52 | 0 | 2 | 0 | 0 | 0 |
| Supramarginal | 99 | 3 | 4 | 1 | 3 | 0 |
| Lateral temporal | ||||||
| MTG | 106 | 1 | 3 | 1 | 2 | 2 |
| STG | 106 | 1 | 6 | 4 | 6 | 1 |
| Rolandic | ||||||
| Precentral gyrus | 101 | 5 | 17 | 4 | 8 | 3 |
| Postcentral gyrus | 89 | 5 | 7 | 1 | 4 | 1 |
| Frontal | ||||||
| Pars opercularis | 50 | 0 | 10 | 0 | 3 | 3 |
| Pars triangularis | 30 | 0 | 4 | 1 | 1 | 0 |
| Pars orbitalis | 18 | 1 | 1 | 0 | 2 | 1 |
| Middle frontal gyrus | 95 | 5 | 8 | 1 | 1 | 7 |
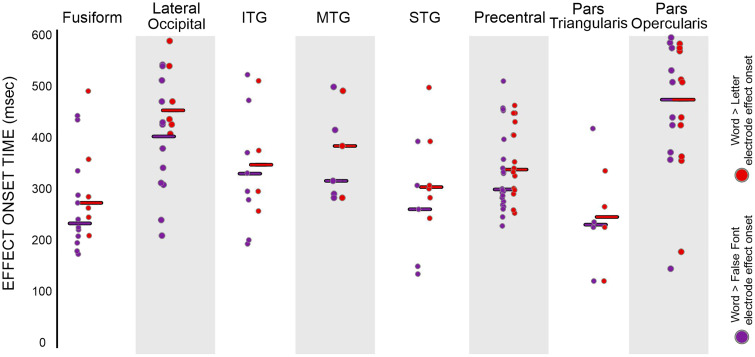
Figure 4. .
Timing of letter-specific and word-specific effect onsets across regions: Circles are the onset of significant letter-specific (word > false font) and word-specific effects (word > letter), with each dot representing an electrode. Purple circles are the onset of a letter-specific effect, and red circles are the onset of a word-specific effect. The line is the median for each effect in the region.
Table 4. .
Timing of Effect Onset Divided in Each Cortical Region
| Region | Letter-selective (msec) | Word-selective (msec) | Word Frequency (msec) | Repetition (msec) | False Font (msec) |
|---|---|---|---|---|---|
| Fusiform | 230 (180–440) | 280 (220–500) | 460 (360–560) | 460 (340–520) | 180 (180–600) |
| Lateral occipital | 400 (220–540) | 460 (420–580) | 520 (360–560) | 180 (140–200) | |
| Inferior temporal gyrus | 340 (200–520) | 360 (260–520) | 280 (280) | 350 (280–440) | 160 (160–280) |
| MTG | 300 (280–500) | 390 (280–500) | 420 (420) | 360 (280–440) | 250 (220–600) |
| STG | 260 (140–400) | 310 (240–500) | 360 (340–540) | 180 (120–320) | 380 (380) |
| Precentral | 300 (240–520) | 340 (260–460) | 490 (400–500) | 390 (220–500) | 300 (300–400) |
| Pars triangularis | 240 (120–420) | 260 (120–340) | 460 (460) | 380 (380) | |
| Pars opercularis | 480 (160–600) | 480 (180–580) | 400 (140–520) | 300 (200–400) |
For letter-specific electrodes, there were no significant differences between the precentral gyrus and the temporal–parietal regions as none of the comparisons reached family-wise error rate (FWER-corrected) significance. The precentral gyrus had a nonsignificantly different proportion (5%) to the STG (1%; FET: p = .11), supramarginal (3%; FET: p = .72), and inferior parietal (0%, FET: p = .32) parcellations. The letter effect onset timings in the precentral gyrus (∼300 msec) did not significantly differ from the STG (∼260 msec; RS: p = .34), supramarginal (∼240 msec; RS: p = .24), or inferior parietal (∼350 msec; RS: p = .28) parcellations.
For word-specific electrodes, the precentral gyrus again had at least as high a proportion as the surrounding temporal–parietal regions. The proportion of word-specific responses (17%) in the precentral gyrus was higher than the STG (6%, FET: p = .014, FWER-uncorrected) and supramarginal (4%; FET: p = .004) parcellations and not significantly different from the inferior parietal parcellations (4%; FET: p = .09). The word effect onset timings (i.e., words > letters) in the precentral gyrus (∼340 msec) did not differ from the STG (∼310 msec; RS: p = .13), supramarginal (∼410 msec; RS: p = .08), or inferior parietal (∼410 msec; RS: p = .26) parcellations. Taken together, the precentral gyrus showed at least as many of task-modulated, letter-specific, and word-specific electrodes as temporal–parietal regions. There was no evidence of differences in the timing of these effects between the precentral gyrus and the temporal–parietal regions.
Exploratory Analysis: Ventral Visual Stream (Fusiform Gyrus and Lateral Occipital Parcellations)
The two ventral parcellations showed strong involvement during our reading task; both the lateral occipital (37%; FET: p < .001) and fusiform gyrus (45%; FET: p < .001) had a greater proportion of task-modulated electrodes than the pooled electrodes (see Regional Comparisons: Exploratory Analysis of the Reading Network under the Methods section). This increased proportion was primarily driven by greater proportions of letter-selective electrodes. The fusiform gyrus (15%; FET: p = .002) and lateral occipital (10%; FET: p = .018, FWER-uncorrected) had greater proportions of letter-specific electrodes than other parcellations. These letter-specific effects in the fusiform were significantly faster than the pooled effects (∼230 msec; RS: p < .001), but the lateral occipital onsets did not significantly differ (∼400 msec; RS: p = .036, FWER-uncorrected). For word-specific effect proportions, both the fusiform (15%; FET: p = .26) and lateral occipital (10%; FET: p = 1.0) parcellations were not significantly different than the pooled estimates. The word onset timings had a trend toward being faster in the fusiform than the pooled onsets (∼280 msec; RS: p = .008, FWER-uncorrected); however, the lateral occipital parcellation showed no significant difference (∼460 msec; RS: p = .09, FWER-uncorrected) from the pooled responses.
Exploratory Analysis: The Posterior Peri-sylvian Regions (STG, MTG, and Supramarginal Parcellations)
For task-modulated electrodes, the STG (8%, FET: p = .004), MTG (8%; FET: p = .002), and supramarginal gyrus (8%; FET: p = .004) all had lower proportions than the pooled electrodes. Breaking down these effects, letter-specific electrodes were not significantly different from the pooled reading network for the STG (1%; FET: p = .15), MTG (1%; FET: p = .15), or supramarginal (3%; FET: p = .78) parcellations. For word-specific effects, the MTG (2%; FET: p = .009, FWER-uncorrected) had a trend toward a lower proportion of word-specific responses. The STG (6%; FET: p = .15) and supramarginal gyrus (4%; FET: p = .057) did not significantly differ from the pooled electrodes. The overall low number of effects in these regions made any tests of onset timing uninformative.
Exploratory Analysis: Frontal Regions (Pars Opercularis, Pars Triangularis, and Precentral Parcellations)
The frontal parcellations showed a generally strong involvement in the reading task. The proportion of task-modulated electrodes was greater in the precentral gyrus (27%; FET: p = .025, not FWER-corrected), with no significant difference for the pars opercularis (28%, FET: p = .08) or pars triangularis (13%, FET: p = .48). The frontal regions did not differ from the pooled electrodes in letter-specific electrode proportion for the precentral gyrus (5%, FET: p = .56), pars opercularis (0%, FET: p = .14), or pars triangularis (0%, FET: p = .40). In contrast, for word-specific effect proportions, the precentral gyrus had a significantly higher proportion than the pooled proportion (17%, FET: p = .008) with a trend in the pars opercularis (20%; FET: p = .012, not FWER-corrected). The pars triangularis did not significantly differ (13%, FET: p = .51). For onset timing, the pars opercularis showed a trend toward slower letter-specific effect onsets (∼480 msec; RS: p = .009, FWER-uncorrected) and word-specific effect onsets (∼480 msec; RS: p = .003, FWER-uncorrected). For letter-specific effects, neither the precentral (∼300 msec; RS: p = .12) nor pars triangularis (∼240 msec; RS: p = .22) showed any significant differences from the pooled network timings. Similarly, there were no differences for word-specific effects in either the precentral gyrus (∼340 msec; RS: p = .88) or the pars triangularis (∼260 msec; RS: p = .12).
Item Repetition and Lexical-Frequency Effects Across the Network
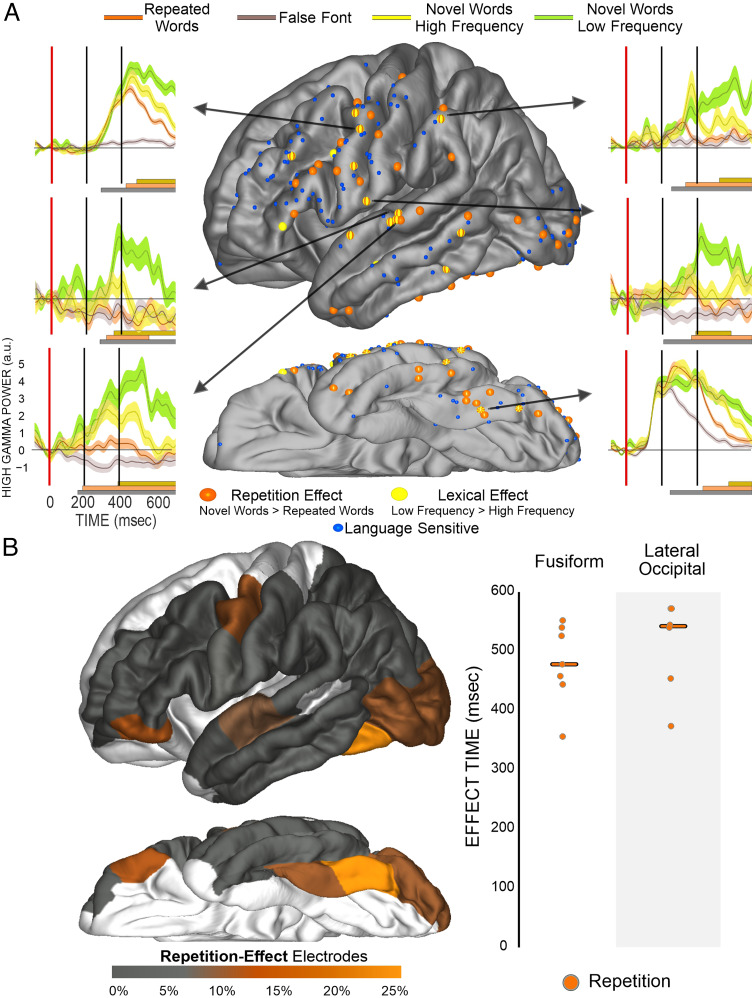
Figure 5 displays the location and example waveforms for electrodes displaying frequency-sensitive and repetition-sensitive effects. Repetition-sensitive effects (49 electrodes) were more numerous than frequency-sensitive effects (17 electrodes). To assess whether these effects were significantly more overlapping than would be expected by chance, we ran a post hoc binomial test that demonstrated that frequency-sensitive electrodes were significantly more overlapping with repetition-sensitive electrodes than would be expected by chance (observed overlap: 78%; binomial test: p < .001). There were not enough frequency-sensitive electrodes to make statistical claims regarding the distribution of these effects across regions, but effects tended to cluster in the STG (4), precentral gyrus (4), MTG (2), and fusiform (2). Examining the distribution of repetition-sensitive effects, in the exploratory eight-region analysis, only the fusiform (17%, FET: p = .007) showed increased repetition-sensitive effects relative to the pooled regions (Figure 4B). In raw numbers, the greatest concentration of repetition-sensitive effects was found in the precentral gyrus (8), fusiform (7), and STG (6) and also in the lateral occipital (6). Onset timing of these effects was too distributed to make any regional claims. The median onset timing of repetition-sensitive effects across the cortex (∼400 msec) and frequency-sensitive effects (∼420 msec) was not significantly different (RS: p = .32).
Figure 5. .
The distribution and timing of repetition and lexical-frequency effects. (A) Display of electrode location (approximate, morphed to an average brain for display purposes), which demonstrates both a significant effect for orange repetition (novel > repeated) or yellow lexical-frequency (low frequency > high frequency) effects. Arrows from electrodes point to plots of HGP for low-frequency novel words (greenish-yellow), high-frequency novel words (bright yellow), repeated words (orange), and false fonts (gray). The dark gray bar at the bottom notes periods of a significant ANOVA effect between conditions, the orange bar notes periods of significant repetition effects, and the yellow bar notes periods of significant lexical-frequency effects. (B) The left panel shows a brain displaying the proportion of repetition effects, most prevalent in the caudal fusiform. On the right is displayed repetition effect onset times. Orange circles are onset of a repetition effect (novel > repeated) at a specific electrode. The line is the median for each effect in the region.
Connectivity Results
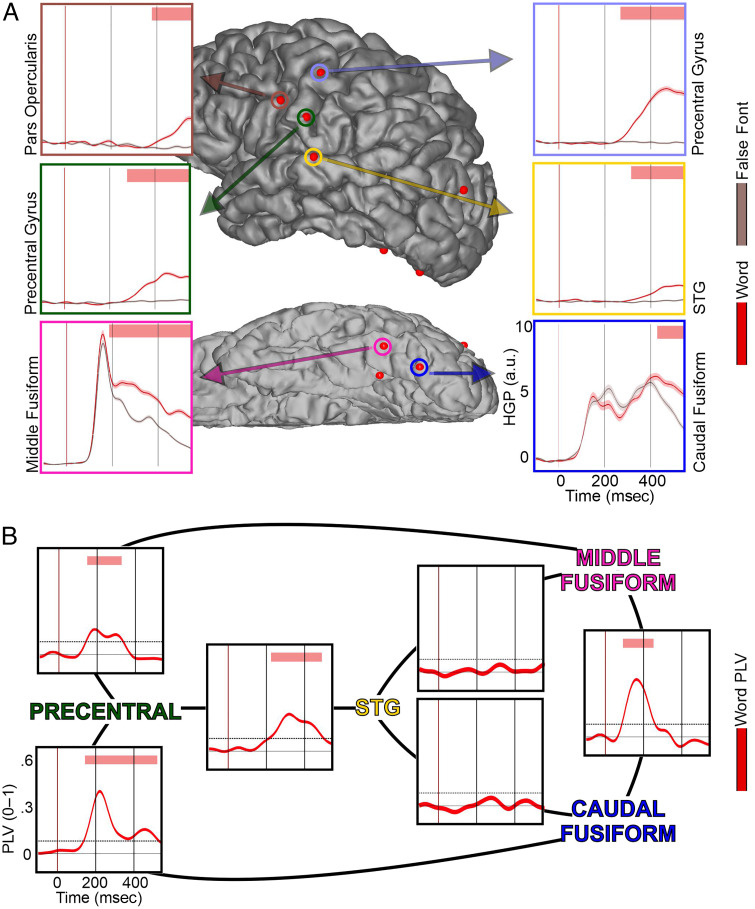
One participant was found to have word-specific responses in the caudal fusiform, STG, and precentral parcellations, which served as a case-study participant to add complementary connectivity grounding to the evoked responses (Patient P16). Figure 6A displays the HGP responses in these electrodes to give an idea of the underlying time course of the neural activity at the same time as the PLV results. Figure 6B displays the average PLV connections (4–12 Hz) between four electrodes: a caudal fusiform, a middle fusiform, an STG, and a precentral.
Figure 6. .
Fusiform and precentral gyrus display phase-locking during silent reading. (A) HGP responses from the only patient with word-selective effects (words > false fonts and words > letters) in the fusiform, STG, and precentral gyrus (Patient P16). Red bar at the top displays periods of significant difference in HGP between word (red line) and false font (reddish-gray line). Robust differences are observed beginning at ∼190 msec in fusiform, ∼260 msec in precentral, and ∼320 msec in STG. Vertical axis for HGP is in arbitrary units (a.u.). (B) PLVs centered on 4–12 Hz for word trials between the fusiform, STG, and precentral gyrus. Red bar at the top displays periods of PLV that were significantly above chance (p < .001). Words evoke strong phase-locking beginning at ∼180 msec between fusiform and precentral and then follow at ∼220 msec between precentral and STG. No direct phase-locking was observed between precentral and fusiform.
The PLV results show an early phase-locking between fusiform sites, between fusiform sites and the precentral gyrus, and between the precentral gyrus and STG, but not between the fusiform and the STG. The earliest significant PLV was between the fusiform sites, beginning at ∼100 msec and lasting till ∼225 msec. Neither of the fusiform sites had a significant PLV connection with the STG site. However, both fusiform sites did display phase-locking with the precentral gyrus beginning at ∼180 msec. The fusiform–precentral phase-locking with the middle fusiform site lasted until ∼320 msec; and that with the caudal fusiform, to ∼520 msec. Finally, there was also prolonged connectivity between the STG and the precentral gyrus from ∼220 to 500 msec.
DISCUSSION
The role of the precentral gyrus in silent reading is often ambiguous in neurobiological models of reading (Carreiras et al., 2014; Taylor et al., 2013; Jobard et al., 2003; Fiez & Petersen, 1998). Whether a region associated primarily with articulatory phonemes is recruited during silent reading, when articulation is not required, was the focus of this study. We present evidence from electrophysiology recorded from the cortical surface during speeded semantic decision-making demonstrating that the precentral gyrus shows word-selective, word-frequency, and repetition effects during a time window (∼250–500 msec) that is early enough to participate in and contribute to phonological processing. The location, timing, and connectivity of evoked electrophysiological activity strongly implicate the precentral gyrus as an important node in the silent reading network.
The Peri-sylvian Dorsal Route: Candidate Regions for Phonological Recoding
Neurobiological models of reading seeking to understand how a visual orthographic code is neurally represented phonologically have two candidate representations, articulatory and encoding phonemes. Articulatory phonemes are associated with the frontal regions such as the precentral gyrus, whereas encoding phonemes are associated with temporal–parietal regions such as the STG. On the basis of evidence from lesion (Pillay et al., 2014), neuroimaging (Booth et al., 2002), and iEEG (Chan et al., 2014; Perrone-Bertolotti et al., 2012) studies, neurobiological models of reading have emphasized the importance of temporal–parietal regions as phonological processing hubs during silent reading (Carreiras et al., 2014; Taylor et al., 2013; Jobard et al., 2003; Fiez & Petersen, 1998). Here, we demonstrate that the precentral gyrus shows at least as much involvement as these temporal–parietal regions for task-modulated, letter-selective, and word-selective effects during a silent reading task. In addition, electrodes in both regions were sensitive to lexical frequency and item repetition. Across these regions, the available evidence was that the language effect onset timing did not significantly differ, suggesting simultaneous processing across regions.
To understand the relationship of the activity in the precentral gyrus and the rest of the distributed reading network, we examined the time course of PLV (Lachaux et al., 1999) in a patient with a fortuitous clinical electrode placement. This patient had word-specific effects in all three critical parcellations: fusiform, STG, and precentral. Both fusiform sites showed significant phase-locking with the precentral gyrus starting at ∼180 msec (the median time of word-specific onsets in the fusiform) and that lasted for several hundred milliseconds. Neither fusiform site displayed significant PLV with the STG site. However, starting at ∼220 msec, there was significant phase-locking between the STG and the precentral gyrus, which lasted for several hundred milliseconds. Similarly, previous studies using MEG have demonstrated early phonological effects in frontal regions such as the pars opercularis and precentral gyrus that support this pattern of early coordination between frontal and visual regions during reading (Wheat, Cornelissen, Frost, & Hansen, 2010; Cornelissen et al., 2009; Pammer et al., 2004). This pattern of results, early connectivity between ventral occipital–temporal areas and the precentral gyrus at the time of letter identification, is surprisingly in line with early psychological theories of reading that emphasized articulatory phonemes in silent reading (Allport, 1979).
The Temporal Flow of the Distributed Reading Network
Neuroanatomical models of reading emphasize the multistream nature of information flow. Debates have often centered on the temporal sequencing of this flow as well as whether regions associated with phonology are automatically recruited during reading (Frost, 1998). Visual information reaches posterior visual cortex at ∼60 msec (Foxe & Simpson, 2002), followed by the onset of orthographic processing in the posterior ventral visual route at ∼160–180 msec (Thesen et al., 2012; Allison et al., 1994, 1999). Lexical–semantic effects in the more anterior–ventral temporal lobe begin soon after at ∼250–300 msec (Chan et al., 2011; Nobre & McCarthy, 1995; Nobre et al., 1994). However, these neural onsets are followed by a prolonged period of processing as behavioral responses typically take another 500–700 msec (Pexman et al., 2017; Balota et al., 2007). During these hundreds of milliseconds, there is ample time for sustained integration across the whole reading network, including the dorsal route.
The ∼250-msec anterior–ventral temporal onset of lexical–semantic processing is aligned well with the ∼250-msec onset of the widespread N400 complex. The N400 is taken to index lexical–semantic integration across a wide variety of paradigms (Marinković, 2004), which begins at ∼250 msec, peaks at ∼400 msec, and “ends” at ∼550–600 msec. The theorized widespread and simultaneous processing supporting the N400 complex could be understood as a prolonged period of feedforward/feedback integration. Evidence from activity across cortical layers in the anterior–ventral temporal lobe confirms the presence of such feedforward/feedback activity. Here, a feedforward period of activity in the deeper layers beginning at ∼120 msec is followed by alternating superficial (feedback) and deeper layer (feedforward) activity with flips every ∼100–200 msec (Halgren et al., 2006, 2015). Therefore, the N400 complex period has alternating periods of information flow during which coordination likely occurs between ventral and dorsal portions of the lexical–semantic network.
Our data provide supporting evidence that emphasizes early fusiform processing followed by widespread and sustained activity across the wider distributed network. The fusiform parcellation had a high proportion of language effects with early effect onsets. Letter-specific responses were higher in proportion in both the lateral occipital and the fusiform than the wider reading network, with fusiform letter-specific effect onset significantly earlier than the rest of the network. However, the word-specific onset timings in these visual regions were not significantly earlier and were even delayed in the lateral occipital region. For repetition-sensitive effects, both the fusiform and lateral occipital regions had a median repetition-effect onset of ∼450–500 msec. This late onset provides ample time for inputs from the lateral areas of the STG and precentral gyrus, where the median word-specific effects were ∼300–350 msec. The PLV results support this theory further, with sustained precentral–fusiform and precentral–STG phase-locking during the critical period from 200 to 600 msec after stimulus onset. These timings provide strong evidence that processing in the precentral gyrus occurs during a period consistent with its involvement in the distributed and integrative processing that leads to successful lexical–semantic processing in other regions such as the anterior–ventral temporal cortex. Previous functional magnetic resonance imaging and lesion studies did not have the temporal specificity to fully ground this assertion.
A Place for the Precentral Gyrus in Silent Reading
Precentral activation is often associated with articulatory activity during reading aloud, but its role during reading silently is more ambiguous (Carreiras et al., 2014; Taylor et al., 2013; Fiez & Petersen, 1998; but see Price, 2012). Here, we demonstrate strong evidence in the distribution and timing of effects that the precentral acts as a hub in the reading network. On the basis of evidence from BOLD neuroimaging (Binder et al., 2005; Dehaene et al., 2001; Fiez et al., 1999; Price et al., 1997) and lesion studies (Vallar et al., 1997; Vallar & Cappa, 1987), the precentral gyrus is likely to contribute to phonological processing by involvement in grapheme-to-phoneme conversion (Allport, 1979).
If the precentral gyrus does play a role in mediating graphemic and phonological representations, it is theorized that the relationship begins in early reading development. Articulatory activity is crucial in learning to read, a theory called the “self-teaching hypothesis” (Share, 1995). Phonemic awareness is a key determinant in the ability to learn how to read (Jorm & Share, 1983), and readers exhibit a strong reliance upon the phonological route at low reading levels (Grainger et al., 2012). Articulating words aloud leads to better word knowledge (Cunningham, Perry, Stanovich, & Share, 2002), and articulatory suppression impairs word knowledge (Kyte & Johnson, 2006). Further evidence for the importance of articulation during early reading development comes from studies showing that disruption of the motor cortex during childhood interferes with learning to read. Benign epilepsy with centrotemporal spikes is associated with lower face motor seizures (Wirrell, 1998). Traditionally, this syndrome has been associated with no overall cognitive impairments (Commission on Classification and Terminology of the ILAE, 1989), but evidence has emerged for a variety of specific learning deficits including reading (Clarke et al., 2007; Staden, Isaacs, Boyd, Brandl, & Neville, 1998), phonological awareness (Northcott et al., 2005), and lexical–semantic but not morphosyntactic knowledge (Riva et al., 2007). Earlier onset of these seizures increases the chance of developing a learning disability (Piccinelli et al., 2008). A neuroimaging review of reading disorders tied an overactive precentral gyrus in disordered reading individuals to compensatory mechanisms (Hancock, Richlan, & Hoeft, 2017).
It therefore seems that articulatory processing in the precentral gyrus plays a crucial role in early reading development and that this region continues to be engaged during skilled silent reading. This suggests a transition in this region's function that is similar to the “neuronal recycling” that is proposed to occur in the left fusiform gyrus. This “recycling” involves the transition of the left fusiform face area into an orthographic processing hub (Dehaene & Cohen, 2007). Given the widespread nature of cortical involvement in reading, it is likely that additional cortical areas undergo similar functional changes as a result of learning to read, potentially including the precentral gyrus. Evidence from developing readers shows that, as reading proficiency improves, the relationship between orthographic and phonological processing is modified (Grainger et al., 2012). It is quite possible that an initially motor–articulatory contribution to phonological processing by the precentral gyrus may subtly recycle as orthographic proficiency increases and the network matures.
Limitations and Future Directions
There are two main limitations to our approach, both related to the iEEG environment. First, in consideration of patients' time and comfort, our task could not be optimized for all considerations so we did not have definitive evidence to answer questions regarding the linguistic level of several of our effects, such as word frequency. However, we believe that the strong overlap between repetition, low frequency, and responses to linguistic stimuli (i.e., letter-specific and word-specific effects) suggests that the electrodes with these effects do at least reflect linguistic processing. Second, as the electrodes were placed for clinical rather than research reasons, we ended up with an uneven number of electrodes within each region. This presents a drawback for interpretations of between-region comparison, in terms of both power and sampling.
Finally, although not included in our a priori comparisons, an additional frontal region that stood out for involvement in silent reading is the middle frontal gyrus. This region had a high proportion of task-modulated effects (28%) and word-selective effects (8%). Although less prominently featured in neurobiological models of reading, it has been considered a candidate involved in lexical–semantic processing (Taylor et al., 2013; Price, 2012). On the basis of our exploratory results, the middle frontal gyrus should be considered in future studies on the silent reading network. Second, future studies of reading can directly test the articulatory phoneme hypothesis with a coordination of stimulation mapping for speech movements and measurement of activity during a silent reading test in the precentral gyrus. Whether the precentral electrodes that show effects during silent reading overlap with articulatory motor areas is a critical part of grounding the articulatory phoneme hypothesis.
Conclusion
Here, we present evidence from a silent reading paradigm implicating the precentral gyrus as a contributor to the reading network. Further studies will be required to elucidate the exact mechanistic contribution of the precentral gyrus to reading.
Acknowledgments
This work was supported by NIH R01 NS018741, Kavli Institute for Brain and Mind, and Chancellor's Collaboratories Award (University of California San Diego). We are grateful for the insightful comments of the reviewers in helping to improve this article.
Reprint requests should be sent to Eric Halgren, Radiology Imaging Laboratory (MC0852), University of California at San Diego, 9500 Gilman Dr., La Jolla, CA 92093, or via e-mail: ehalgren@health.ucsd.edu.
Diversity in Citation Practices
A retrospective analysis of the citations in every article published in this journal from 2010 to 2020 has revealed a persistent pattern of gender imbalance: Although the proportions of authorship teams (categorized by estimated gender identification of first author/last author) publishing in the Journal of Cognitive Neuroscience (JoCN) during this period were M(an)/M = .408, W(oman)/M = .335, M/W = .108, and W/W = .149, the comparable proportions for the articles that these authorship teams cited were M/M = .579, W/M = .243, M/W = .102, and W/W = .076 (Fulvio et al., JoCN, 33:1, pp. 3–7). Consequently, JoCN encourages all authors to consider gender balance explicitly when selecting which articles to cite and gives them the opportunity to report their article's gender citation balance.
REFERENCES
- Allison, T., McCarthy, G., Nobre, A., Puce, A., & Belger, A. (1994). Human extrastriate visual cortex and the perception of faces, words, numbers, and colors. Cerebral Cortex, 4, 544–554. 10.1093/cercor/4.5.544, [DOI] [PubMed] [Google Scholar]
- Allison, T., Puce, A., Spencer, D. D., & McCarthy, G. (1999). Electrophysiological studies of human face perception. I: Potentials generated in occipitotemporal cortex by face and non-face stimuli. Cerebral Cortex, 9, 415–430. 10.1093/cercor/9.5.415, [DOI] [PubMed] [Google Scholar]
- Allport, A. (1979). Word recognition in reading (Tutorial paper). In Kolers Paul A., Wrolstad Merald E., & Bouma Herman (Eds.), Processing of visible language (pp. 227–257). Boston: Springer. 10.1007/978-1-4684-0994-9_14 [DOI] [Google Scholar]
- Balota, D. A., & Chumbley, J. I. (1984). Are lexical decisions a good measure of lexical access? The role of word frequency in the neglected decision stage. Journal of Experimental Psychology: Human Perception and Performance, 10, 340–357. 10.1037/0096-1523.10.3.340, [DOI] [PubMed] [Google Scholar]
- Balota, D. A., Yap, M. J., Hutchison, K. A., Cortese, M. J., Kessler, B., Loftis, B., et al. (2007). The English lexicon project. Behavior Research Methods, 39, 445–459. 10.3758/BF03193014, [DOI] [PubMed] [Google Scholar]
- Barron, R. W., & Baron, J. (1977). How children get meaning from printed words. Child Development, 48, 587–594. 10.2307/1128657 [DOI] [Google Scholar]
- Benjamini, Y., & Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B: (Methodological), 57, 289–300. 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- Berent, I., & Perfetti, C. A. (1995). A rose is a REEZ: The two-cycles model of phonology assembly in reading English. Psychological Review, 102, 146–184. 10.1037/0033-295X.102.1.146 [DOI] [Google Scholar]
- Binder, J. R., Medler, D. A., Desai, R., Conant, L. L., & Liebenthal, E. (2005). Some neurophysiological constraints on models of word naming. Neuroimage, 27, 677–693. 10.1016/j.neuroimage.2005.04.029, [DOI] [PubMed] [Google Scholar]
- Booth, J. R., Burman, D. D., Meyer, J. R., Gitelman, D. R., Parrish, T. B., & Mesulam, M. M. (2002). Functional anatomy of intra- and cross-modal lexical tasks. Neuroimage, 16, 7–22. 10.1006/nimg.2002.1081, [DOI] [PubMed] [Google Scholar]
- Burani, C., Vallar, G., & Bottini, G. (1991). Articulatory coding and phonological judgements on written words and pictures: The role of the phonological output buffer. European Journal of Cognitive Psychology, 3, 379–398. 10.1080/09541449108406235 [DOI] [Google Scholar]
- Carreiras, M., Armstrong, B. C., Perea, M., & Frost, R. (2014). The what, when, where, and how of visual word recognition. Trends in Cognitive Sciences, 18, 90–98. 10.1016/j.tics.2013.11.005, [DOI] [PubMed] [Google Scholar]
- Chan, A. M., Baker, J. M., Eskandar, E., Schomer, D., Ulbert, I., Marinkovic, K., et al. (2011). First-pass selectivity for semantic categories in human anteroventral temporal lobe. Journal of Neuroscience, 31, 18119–18129. 10.1523/JNEUROSCI.3122-11.2011, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, A. M., Dykstra, A. R., Jayaram, V., Leonard, M. K., Travis, K. E., Gygi, B., et al. (2014). Speech-specific tuning of neurons in human superior temporal gyrus. Cerebral Cortex, 24, 2679–2693. 10.1093/cercor/bht127, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke, T., Strug, L. J., Murphy, P. L., Bali, B., Carvalho, J., Foster, S., et al. (2007). High risk of reading disability and speech sound disorder in rolandic epilepsy families: Case–control study. Epilepsia, 48, 2258–2265. 10.1111/j.1528-1167.2007.01276.x, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coltheart, M. (1980). Reading, phonological reading and deep dyslexia. In Coltheart M., Patterson K., & Marshal J. C. (Eds.), Deep dyslexia (pp. 197–226). London: Routledge & Keegan Paul. [Google Scholar]
- Coltheart, M., Rastle, K., Perry, C., Langdon, R., & Ziegler, J. (2001). DRC: A dual route cascaded model of visual word recognition and reading aloud. Psychological Review, 108, 204–256. 10.1037/0033-295X.108.1.204, [DOI] [PubMed] [Google Scholar]
- Commission on Classification and Terminology of the ILAE. (1989). Proposal for a revised classification of epilepsies and epileptic syndromes. Epilepsia, 30, 389–399. 10.1111/j.1528-1157.1989.tb05316.x, [DOI] [PubMed] [Google Scholar]
- Cornelissen, P. L., Kringelbach, M. L., Ellis, A. W., Whitney, C., Holliday, I. E., & Hansen, P. C. (2009). Activation of the left inferior frontal gyrus in the first 200 ms of reading: Evidence from magnetoencephalography (MEG). PLoS One, 4, e5359. 10.1371/journal.pone.0005359, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham, A. E., Perry, K. E., Stanovich, K. E., & Share, D. L. (2002). Orthographic learning during reading: Examining the role of self-teaching. Journal of Experimental Child Psychology, 82, 185–199. 10.1016/S0022-0965(02)00008-5, [DOI] [PubMed] [Google Scholar]
- Dale, A. M., Fischl, B., & Sereno, M. I. (1999). Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage, 9, 179–194. 10.1006/nimg.1998.0395, [DOI] [PubMed] [Google Scholar]
- Dehaene, S., & Cohen, L. (2007). Cultural recycling of cortical maps. Neuron, 56, 384–398. 10.1016/j.neuron.2007.10.004, [DOI] [PubMed] [Google Scholar]
- Dehaene, S., Naccache, L., Cohen, L., Bihan, D. L., Mangin, J.-F., Poline, J.-B., et al. (2001). Cerebral mechanisms of word masking and unconscious repetition priming. Nature Neuroscience, 4, 752–758. 10.1038/89551, [DOI] [PubMed] [Google Scholar]
- Dejerine, J. (1892). Contribution à l'étude anatomopathologique et clinique des différents variétés de cécité verbale. Mémoires de la Societé de Biologie, 4, 61–90. [Google Scholar]
- Desikan, R. S., Ségonne, F., Fischl, B., Quinn, B. T., Dickerson, B. C., Blacker, D., et al. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage, 31, 968–980. 10.1016/j.neuroimage.2006.01.021, [DOI] [PubMed] [Google Scholar]
- Desimone, R. (1996). Neural mechanisms for visual memory and their role in attention. Proceedings of the National Academy of Sciences, U.S.A., 93, 13494–13499. 10.1073/pnas.93.24.13494, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiez, J. A., Balota, D. A., Raichle, M. E., & Petersen, S. E. (1999). Effects of lexicality, frequency, and spelling-to-sound consistency on the functional anatomy of reading. Neuron, 24, 205–218. 10.1016/S0896-6273(00)80833-8, [DOI] [PubMed] [Google Scholar]
- Fiez, J. A., & Petersen, S. E. (1998). Neuroimaging studies of word reading. Proceedings of the National Academy of Sciences, U.S.A., 95, 914–921. 10.1073/pnas.95.3.914, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster, K. I., & Chambers, S. M. (1973). Lexical access and naming time. Journal of Verbal Learning and Verbal Behavior, 12, 627–635. 10.1016/S0022-5371(73)80042-8 [DOI] [Google Scholar]
- Foxe, J. J., & Simpson, G. V. (2002). Flow of activation from V1 to frontal cortex in humans. Experimental Brain Research, 142, 139–150. 10.1007/s00221-001-0906-7, [DOI] [PubMed] [Google Scholar]
- Frances, N., & Kucera, H. (1982). Frequency analysis of English usage. Boston: Houghton Mifflin. [Google Scholar]
- Frost, R. (1998). Toward a strong phonological theory of visual word recognition: True issues and false trails. Psychological Bulletin, 123, 71–99. 10.1037/0033-2909.123.1.71, [DOI] [PubMed] [Google Scholar]
- Geschwind, N. (1974). The organization of language and the brain. In Selected papers on language and the brain (pp. 452–466). Dordrecht, The Netherlands: Springer. 10.1007/978-94-010-2093-0_21 [DOI] [Google Scholar]
- Gotts, S. J., Chow, C. C., & Martin, A. (2012). Repetition priming and repetition suppression: A case for enhanced efficiency through neural synchronization. Cognitive Neuroscience, 3, 227–237. 10.1080/17588928.2012.670617, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainger, J., Lété, B., Bertand, D., Dufau, S., & Ziegler, J. C. (2012). Evidence for multiple routes in learning to read. Cognition, 123, 280–292. 10.1016/j.cognition.2012.01.003, [DOI] [PubMed] [Google Scholar]
- Halgren, E., Kaestner, E., Marinkovic, K., Cash, S. S., Wang, C., Schomer, D. L., et al. (2015). Laminar profile of spontaneous and evoked theta: Rhythmic modulation of cortical processing during word integration. Neuropsychologia, 76, 108–124. 10.1016/j.neuropsychologia.2015.03.021, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halgren, E., Wang, C., Schomer, D. L., Knake, S., Marinkovic, K., Wu, J., et al. (2006). Processing stages underlying word recognition in the anteroventral temporal lobe. Neuroimage, 30, 1401–1413. 10.1016/j.neuroimage.2005.10.053, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock, R., Richlan, F., & Hoeft, F. (2017). Possible roles for fronto-striatal circuits in reading disorder. Neuroscience & Biobehavioral Reviews, 72, 243–260. 10.1016/j.neubiorev.2016.10.025, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harm, M. W., & Seidenberg, M. S. (2004). Computing the meanings of words in reading: Cooperative division of labor between visual and phonological processes. Psychological Review, 111, 662–720. 10.1037/0033-295X.111.3.662, [DOI] [PubMed] [Google Scholar]
- Hauk, O., & Pulvermüller, F. (2004). Effects of word length and frequency on the human event-related potential. Clinical Neurophysiology, 115, 1090–1103. 10.1016/j.clinph.2003.12.020, [DOI] [PubMed] [Google Scholar]
- Jared, D., & Seidenberg, M. S. (1991). Does word identification proceed from spelling to sound to meaning? Journal of Experimental Psychology: General, 120, 358–394. 10.1037/0096-3445.120.4.358 [DOI] [Google Scholar]
- Jobard, G., Crivello, F., & Tzourio-Mazoyer, N. (2003). Evaluation of the dual route theory of reading: A metanalysis of 35 neuroimaging studies. Neuroimage, 20, 693–712. 10.1016/S1053-8119(03)00343-4, [DOI] [PubMed] [Google Scholar]
- Jorm, A. F., & Share, D. L. (1983). An invited article: Phonological recoding and reading acquisition. Applied PsychoLinguistics, 4, 103–147. 10.1017/S0142716400004380 [DOI] [Google Scholar]
- Kleiman, G. M. (1975). Speech recoding in reading. Journal of Verbal Learning and Verbal Behavior, 14, 323–339. 10.1016/S0022-5371(75)80013-2 [DOI] [Google Scholar]
- Kutas, M., & Federmeier, K. D. (2011). Thirty years and counting: Finding meaning in the N400 component of the event-related brain potential (ERP). Annual Review of Psychology, 62, 621–647. 10.1146/annurev.psych.093008.131123, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte, C. S., & Johnson, C. J. (2006). The role of phonological recoding in orthographic learning. Journal of Experimental Child Psychology, 93, 166–185. 10.1016/j.jecp.2005.09.003, [DOI] [PubMed] [Google Scholar]
- Lachaux, J.-P., Rodriguez, E., Martinerie, J., & Varela, F. J. (1999). Measuring phase synchrony in brain signals. Human Brain Mapping, 8, 194–208. , [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinković, K. (2004). Spatiotemporal dynamics of word processing in the human cortex. Neuroscientist, 10, 142–152. 10.1177/1073858403261018, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris, E., & Oostenveld, R. (2007). Nonparametric statistical testing of EEG-and MEG-data. Journal of Neuroscience Methods, 164, 177–190. 10.1016/j.jneumeth.2007.03.024, [DOI] [PubMed] [Google Scholar]
- McDonald, C. R., Thesen, T., Carlson, C., Blumberg, M., Girard, H. M., Trongnetrpunya, A., et al. (2010). Multimodal imaging of repetition priming: Using fMRI, MEG, and intracranial EEG to reveal spatiotemporal profiles of word processing. Neuroimage, 53, 707–717. 10.1016/j.neuroimage.2010.06.069, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medler, D. A., & Binder, J. R. (2005). MCWord: An on-line orthographic database of the English language. http://www.neuro.mcw.edu/mcword/ [Google Scholar]
- Nobre, A. C., Allison, T., & McCarthy, G. (1994). Word recognition in the human inferior temporal lobe. Nature, 372, 260–263. 10.1038/372260a0, [DOI] [PubMed] [Google Scholar]
- Nobre, A. C., & McCarthy, G. (1995). Language-related field potentials in the anterior-medial temporal lobe: II. Effects of word type and semantic priming. Journal of Neuroscience, 15, 1090–1098. 10.1523/JNEUROSCI.15-02-01090.1995, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northcott, E., Connolly, A. M., Berroya, A., Sabaz, M., McIntyre, J., Christie, J., et al. (2005). The neuropsychological and language profile of children with benign rolandic epilepsy. Epilepsia, 46, 924–930. 10.1111/j.1528-1167.2005.62304.x, [DOI] [PubMed] [Google Scholar]
- Ojemann, G., Ojemann, J., Lettich, E., & Berger, M. (1989). Cortical language localization in left, dominant hemisphere: An electrical stimulation mapping investigation in 117 patients. Journal of Neurosurgery, 71, 316–326. 10.3171/jns.1989.71.3.0316, [DOI] [PubMed] [Google Scholar]
- Oostenveld, R., Fries, P., Maris, E., & Schoffelen, J.-M. (2011). FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Computational Intelligence and Neuroscience, 2011, 156869. 10.1155/2011/156869, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pammer, K., Hansen, P. C., Kringelbach, M. L., Holliday, I., Barnes, G., Hillebrand, A., et al. (2004). Visual word recognition: The first half second. Neuroimage, 22, 1819–1825. 10.1016/j.neuroimage.2004.05.004, [DOI] [PubMed] [Google Scholar]
- Perfetti, C. A., & Bell, L. (1991). Phonemic activation during the first 40 ms of word identification: Evidence from backward masking and priming. Journal of Memory and Language, 30, 473–485. 10.1016/0749-596X(91)90017-E [DOI] [Google Scholar]
- Perfetti, C. A., Bell, L. C., & Delaney, S. M. (1988). Automatic (prelexical) phonetic activation in silent word reading: Evidence from backward masking. Journal of Memory and Language, 27, 59–70. 10.1016/0749-596X(88)90048-4 [DOI] [Google Scholar]
- Perrone-Bertolotti, M., Kujala, J., Vidal, J. R., Hamame, C. M., Ossandon, T., Bertrand, O., et al. (2012). How silent is silent reading? Intracerebral evidence for top–down activation of temporal voice areas during reading. Journal of Neuroscience, 32, 17554–17562. 10.1523/JNEUROSCI.2982-12.2012, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry, C., Ziegler, J. C., & Zorzi, M. (2007). Nested incremental modeling in the development of computational theories: The CDP+ model of reading aloud. Psychological Review, 114, 273–315. 10.1037/0033-295X.114.2.273, [DOI] [PubMed] [Google Scholar]
- Peterson, L. R., & Johnson, S. T. (1971). Some effects of minimizing articulation on short-term retention. Journal of Verbal Learning and Verbal Behavior, 10, 346–354. 10.1016/S0022-5371(71)80033-6 [DOI] [Google Scholar]
- Pexman, P. M., Heard, A., Lloyd, E., & Yap, M. J. (2017). The Calgary semantic decision project: Concrete/abstract decision data for 10,000 English words. Behavior Research Methods, 49, 407–417. 10.3758/s13428-016-0720-6, [DOI] [PubMed] [Google Scholar]
- Piccinelli, P., Borgatti, R., Aldini, A., Bindelli, D., Ferri, M., Perna, S., et al. (2008). Academic performance in children with rolandic epilepsy. Developmental Medicine & Child Neurology, 50, 353–356. 10.1111/j.1469-8749.2008.02040.x, [DOI] [PubMed] [Google Scholar]
- Pillay, S. B., Stengel, B. C., Humphries, C., Book, D. S., & Binder, J. R. (2014). Cerebral localization of impaired phonological retrieval during rhyme judgment. Annals of Neurology, 76, 738–746. 10.1002/ana.24266, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price, C. J. (2012). A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. Neuroimage, 62, 816–847. 10.1016/j.neuroimage.2012.04.062, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price, C. J., Moore, C. J., Humphreys, G. W., & Wise, R. J. S. (1997). Segregating semantic from phonological processes during reading. Journal of Cognitive Neuroscience, 9, 727–733. 10.1162/jocn.1997.9.6.727, [DOI] [PubMed] [Google Scholar]
- Rastle, K., & Brysbaert, M. (2006). Masked phonological priming effects in English: Are they real? Do they matter? Cognitive Psychology, 53, 97–145. 10.1016/j.cogpsych.2006.01.002, [DOI] [PubMed] [Google Scholar]
- Riva, D., Vago, C., Franceschetti, S., Pantaleoni, C., D'Arrigo, S., Granata, T., et al. (2007). Intellectual and language findings and their relationship to EEG characteristics in benign childhood epilepsy with centrotemporal spikes. Epilepsy & Behavior, 10, 278–285. 10.1016/j.yebeh.2006.12.003, [DOI] [PubMed] [Google Scholar]
- Seidenberg, M. S., Waters, G. S., Barnes, M. A., & Tanenhaus, M. K. (1984). When does irregular spelling or pronunciation influence word recognition? Journal of Verbal Learning and Verbal Behavior, 23, 383–404. 10.1016/S0022-5371(84)90270-6 [DOI] [Google Scholar]
- Sereno, S. C., Rayner, K., & Posner, M. I. (1998). Establishing a time-line of word recognition: Evidence from eye movements and event-related potentials. NeuroReport, 9, 2195–2200. 10.1097/00001756-199807130-00009, [DOI] [PubMed] [Google Scholar]
- Share, D. L. (1995). Phonological recoding and self-teaching: Sine qua non of reading acquisition. Cognition, 55, 151–218. 10.1016/0010-0277(94)00645-2, [DOI] [PubMed] [Google Scholar]
- Staden, U., Isaacs, E., Boyd, S. C., Brandl, U., & Neville, B. G. R. (1998). Language dysfunction in children with rolandic epilepsy. Neuropediatrics, 29, 242–248. 10.1055/s-2007-973569, [DOI] [PubMed] [Google Scholar]
- Taylor, J. S. H., Rastle, K., & Davis, M. H. (2013). Can cognitive models explain brain activation during word and pseudoword reading? A meta-analysis of 36 neuroimaging studies. Psychological Bulletin, 139, 766–791. 10.1037/a0030266, [DOI] [PubMed] [Google Scholar]
- Thesen, T., McDonald, C. R., Carlson, C., Doyle, W., Cash, S., Sherfey, J., et al. (2012). Sequential then interactive processing of letters and words in the left fusiform gyrus. Nature Communications, 3, 1284. 10.1038/ncomms2220, [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Turennout, M., Ellmore, T., & Martin, A. (2000). Long-lasting cortical plasticity in the object naming system. Nature Neuroscience, 3, 1329–1334. 10.1038/81873, [DOI] [PubMed] [Google Scholar]
- Vallar, G., & Cappa, S. F. (1987). Articulation and verbal short-term memory: Evidence from anarthria. Cognitive Neuropsychology, 4, 55–77. 10.1080/02643298708252035 [DOI] [Google Scholar]
- Vallar, G., Di Betta, A. M., & Silveri, M. C. (1997). The phonological short-term store-rehearsal system: Patterns of impairment and neural correlates. Neuropsychologia, 35, 795–812. 10.1016/S0028-3932(96)00127-3, [DOI] [PubMed] [Google Scholar]
- Wheat, K. L., Cornelissen, P. L., Frost, S. J., & Hansen, P. C. (2010). During visual word recognition, phonology is accessed within 100 ms and may be mediated by a speech production code: Evidence from magnetoencephalography. Journal of Neuroscience, 30, 5229–5233. 10.1523/JNEUROSCI.4448-09.2010, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirrell, E. C. (1998). Benign epilepsy of childhood with centrotemporal spikes. Epilepsia, 39, S32–S41. 10.1111/j.1528-1157.1998.tb05123.x, [DOI] [PubMed] [Google Scholar]
- Yang, A. I., Wang, X., Doyle, W. K., Halgren, E., Carlson, C., Belcher, T. L., et al. (2012). Localization of dense intracranial electrode arrays using magnetic resonance imaging. Neuroimage, 63, 157–165. 10.1016/j.neuroimage.2012.06.039, [DOI] [PMC free article] [PubMed] [Google Scholar]