Abstract
Aims
Risk stratification algorithms for sudden cardiac death (SCD) in hypertrophic cardiomyopathy (HCM) and regional differences in clinical practice have evolved over time. We sought to compare primary prevention implantable cardioverter defibrillator (ICD) implantation rates and associated clinical outcomes in US vs. non-US tertiary HCM centres within the international Sarcomeric Human Cardiomyopathy Registry.
Methods and results
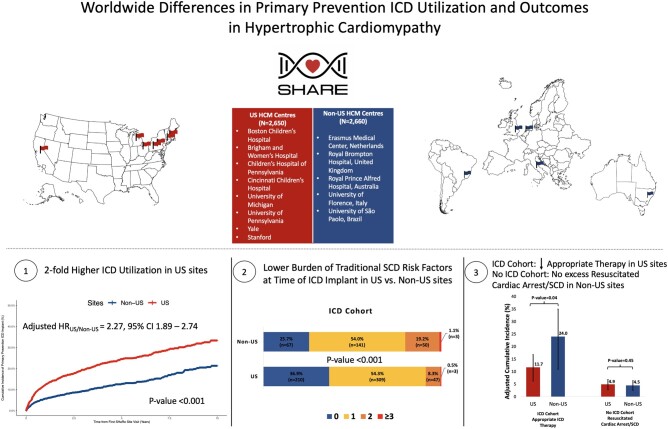
We included patients with HCM enrolled from eight US sites (n = 2650) and five non-US (n = 2660) sites and used multivariable Cox-proportional hazards models to compare outcomes between sites. Primary prevention ICD implantation rates in US sites were two-fold higher than non-US sites (hazard ratio (HR) 2.27 [1.89–2.74]), including in individuals deemed at high 5-year SCD risk (≥6%) based on the HCM risk-SCD score (HR 3.27 [1.76–6.05]). US ICD recipients also had fewer traditional SCD risk factors. Among ICD recipients, rates of appropriate ICD therapy were significantly lower in US vs. non-US sites (HR 0.52 [0.28–0.97]). No significant difference was identified in the incidence of SCD/resuscitated cardiac arrest among non-recipients of ICDs in US vs. non-US sites (HR 1.21 [0.74–1.97]).
Conclusion
Primary prevention ICDs are implanted more frequently in patients with HCM in US vs. non-US sites across the spectrum of SCD risk. There was a lower rate of appropriate ICD therapy in US sites, consistent with a lower-risk population, and no significant difference in SCD in US vs. non-US patients who did not receive an ICD. Further studies are needed to understand what drives malignant arrhythmias, optimize ICD allocation, and examine the impact of different ICD utilization strategies on long-term outcomes in HCM.
Keywords: Hypertrophic cardiomyopathy, Sudden cardiac death, Primary prevention, Risk stratification, Implantable cardioverter defibrillator, Outcomes
Graphical Abstract
See the editorial comment for this article ‘Sudden cardiac death in hypertrophic cardiomyopathy: time to change the narrative’, by P. Elliott, doi:10.1093/eurheartj/ehab608.
Introduction
Sudden cardiac death (SCD) is a grave complication of hypertrophic cardiomyopathy (HCM), disproportionately affecting young adults.1 Primary prevention implantable cardioverter defibrillators (ICD) effectively decrease the incidence of SCD;2 however, because many patients with HCM are relatively young at ICD implantation, they bear a higher life-time risk of ICD-related complications including device malfunction, infection, and inappropriate ICD therapy.3 Accurate assessment of risk is essential to ensure the optimal allocation of ICD devices to patients who stand to benefit the most.4 Risk stratification algorithms for SCD in HCM have evolved over time with notable differences in current US and European guidelines.5–8 Whereas the most recent 2014 European Society of Cardiology (ESC) guidelines endorse a summative approach to risk assessment focusing on a quantitative predicted risk of SCD using the HCM risk-SCD calculator,7 , 9 the 2020 American Heart Association (AHA)/American College of Cardiology (ACC) guidelines emphasize identifying individual risk factors associated with SCD and recommend considering primary prevention ICD implantation if a single major risk factor is present.8 These differing approaches emphasize ongoing challenges of accurate risk stratification and uncertainty about best practice.10 In this study, we sought to leverage the international Sarcomeric Human Cardiomyopathy Registry (SHaRe) to compare primary prevention ICD implantation rates and associated clinical outcomes in US vs. non-US tertiary HCM centres.
Methods
SHaRe is an international collaborative consortium of high-volume tertiary HCM centres that houses granular longitudinal clinical, imaging, genetic, and outcome data on patients with HCM and their families. The registry design and initial findings have been previously reported.11 Briefly, a core set of phenotypic variables and outcomes are harmonized across all participating sites and stored in a secure centralized database. At initial visit to a SHaRe site, patients undergo a detailed patient history and medical record review to ascertain clinical diagnoses and historical events. Prospective longitudinal data are captured by sites as they occur or during clinical encounters. Every quarter each participating site submits updated deidentified data to the central database where it is subjected to auditing tools for checking the completeness and accuracy of the data. This allows for prospective data capture and the amendment of missing or inaccurate data prior to incorporation into the registry and maintenance of the fidelity of the data. Each site maintains appropriate institutional review and ethics approval. Depending on individual site practices, informed consent is obtained or waived based on the de-identified nature of the study. This study was conducted in compliance with the Declaration of Helsinki.
Study design and patient population
This study is a retrospective observational study comparing primary prevention ICD utilization and outcomes in US vs. non-US sites within the SHaRe registry from January 2000 through June 2020. Patients were included if they were adults, 18 years or older, at the time of initial visit at a SHaRe site. Only patients diagnosed with HCM after the year 2000, at which time contemporary ICDs were readily clinically available, were included in this study. Patients who had secondary prevention ICDs implanted prior to their first SHaRe site visit were excluded. The study population included patients with HCM enrolled from eight US sites (n = 2650) [Brigham and Women’s Hospital, MA (n = 486); Boston Children’s Hospital, MA (n = 19); University of Michigan, MI (n = 791); Cincinnati Children’s Hospital, OH (n = 70); University of Pennsylvania, PA (n = 41), Children’s Hospital of Philadelphia, PA (n = 2); Yale-New Haven Hospital, CT (n = 462); Stanford University, CA (n = 779)] and five non-US sites (n = 2660) [Cardiomyopathy Unit, University of Florence, Florence, Italy (n = 1220); Erasmus Medical Center, Rotterdam, the Netherlands (n = 777); Royal Brompton Hospital, London, United Kingdom (n = 57); University of São Paulo, Brazil (n = 180); Royal Prince Alfred Hospital, University of Sydney, Sydney, Australia (n = 426)] (Supplementary material online, Figure S1).
Exposures
Clinical covariates were modelled as time-varying covariates to capture change in exposure status over time and to allow for accurate adjustment for risk profile at time of primary prevention ICD implantation. For instance, patients who developed syncope during follow-up were considered free of syncope until the time of event occurrence and carried forward thereafter. Detailed echocardiographic data were available in a subset of the study population (n = 4743, 89.3%). Baseline echocardiographic data were carried forward and updated when new echocardiographic data were clinically obtained. Definitions of traditional risk factors for SCD were harmonized across sites in accordance with guidelines.6–8 Unexplained syncope was defined as one or more recent (within <6 months) episodes of acute transient loss of consciousness deemed not secondary to neurocardiogenic syncope or attributable to left ventricular outflow tract obstruction. Family history of SCD was defined as SCD in ≥1 first degree relative under the age of 40 or in a first degree relative with confirmed HCM diagnosis regardless of age. Non-sustained ventricular tachycardia was defined as at least three consecutive ventricular beats at a rate ≥120 b.p.m., lasting <30 s without associated symptoms. Maximal left ventricular wall thickness was categorized according to the guideline-defined cut-off of 30 mm. Left atrial anteroposterior diameter was measured from a parasternal long window of the heart. Finally, left ventricular outflow tract gradient was defined as maximal rest, Valsalva or exercise gradient measured by 2D Doppler echocardiography. The higher of the rest or Valsalva left ventricular outflow tract gradient estimates was included in calculation of the HCM risk-SCD score. In calculating the HCM risk-SCD score, echocardiographic parameters from the closest echocardiogram performed before the SHaRe site visit were used. In addition, in instances where the upper or lower bounds of the range accepted by the model for individual predictors were violated, we imputed the value of the upper or lower limit of the model range, as appropriate (e.g. maximal left ventricular wall thickness of 37 mm was imputed to 35 mm and maximal left ventricular wall thickness of 8 mm was imputed to 10 mm). If left ventricular outflow tract gradient was noted as absent in an echocardiogram without a numerical estimate of the outflow tract gradient, a value of 10 mmHg was imputed to calculate the HCM risk-SCD score. This approach is commensurate with real-world clinical use of the HCM risk-SCD score. Data on left ventricular aneurysm or quantification of late gadolinium enhancement on cardiac magnetic resonance imaging are not currently systematically captured in SHaRe.
Outcomes
The primary endpoint of this study was time-to-primary prevention ICD implantation. Primary prevention ICD implantation was defined as prophylactic ICD implantation in individuals who have not experienced symptomatic sustained ventricular arrhythmias or resuscitated cardiac arrest. We additionally examined a number of secondary endpoints in clinical outcome analyses comparing outcomes between sites among recipients (ICD cohort) and non-recipients (No ICD cohort) of primary prevention ICDs. In the ICD cohort, we compared the incidence of appropriate ICD therapy, inappropriate ICD therapy, and all-cause mortality between US and non-US sites. Appropriate therapy was defined as ICD shocks or anti-tachycardia pacing delivered for clinically adjudicated ventricular tachyarrhythmia. Inappropriate therapy was defined as ICD therapy in the setting of supraventricular tachyarrhythmia or device malfunction. In the No ICD cohort, we examined two composite endpoints: SCD composite (SCD or resuscitated cardiac arrest) and non-SCD composite (non-SCD or heart transplant/ventricular assist device implant) across US vs. non-US sites. SCD was defined as death within 1 h of onset of cardiac symptoms when witnessed or within 24 h of last being known alive and well when unwitnessed.
Statistical analysis
Baseline characteristics of US vs. non-US sites are presented as means ± standard deviation or median (interquartile range) for continuous variables and frequency and percent for categorical variables. Unpaired Student’s t-test and Pearson chi-squared tests were used to compare continuous and categorical variables across the two groups, respectively. Time-to-event analysis with time-varying covariates was implemented to compare the primary and secondary endpoints between US and non-US sites. Follow-up time for the primary endpoint analysis was defined as time from initial SHaRe site visit to either primary prevention ICD implantation, death, or last follow-up visit, whichever came first. Study participants were censored at time of last follow-up visit. Study participants who had an ICD implanted prior to first SHaRe site visit (n = 221) were excluded as they were not at risk for the outcome of interest. In addition, participants who received secondary prevention ICDs during follow-up (n = 98) were excluded from this analysis focused on comparing rates of primary prevention ICD utilization. Kaplan–Meier estimates with log-rank significance test were used to compare the cumulative incidence of primary prevention ICD implantation across US vs. non-US sites. Multivariable Cox-proportional hazards models were implemented to assess the association of US vs. non-US sites with the primary endpoint of primary prevention ICD implantation adjusting for age at first SHaRe site visit, year of first visit, sex, race, atrial fibrillation, coronary artery disease, septal reduction therapy (surgical myectomy or alcohol ablation), unexplained syncope, family history of SCD, and non-sustained ventricular tachycardia. Two sensitivity analyses evaluating the primary endpoint across sites were performed on subgroups of the study sample with non-missing echocardiographic and HCM risk-SCD data. First, we additionally adjusted our primary endpoint analysis for echocardiographic parameters when available including left ventricular ejection fraction, left atrial diameter, maximal left ventricular wall thickness, and left ventricular outflow tract gradient. Second, we calculated the HCM risk-SCD score9 in the subset of patients with complete requisite component data (n = 4183/5310, 78.8%) and compared the primary endpoint between sites stratified by low- (<4%), intermediate- (4–6%), and high-risk (≥6%) categories of SCD. In addition, tests of interaction on the multiplicative scale were performed to assess for heterogeneity of the impact of SCD risk factors on the decision to implant a primary prevention ICD in US vs. non-US sites. To examine the effect of introduction of the HCM risk-SCD score in 2014 on ICD implantation rates, we performed a stratified analysis by era: 2000–13 and 2014–20.
We then focused on examining outcomes across sites among recipients and non-recipients of primary prevention ICDs. Among primary prevention ICD recipients, follow-up time for the secondary endpoint analysis was defined as time from ICD implant to first occurrence of any of the secondary endpoints, death or last follow-up visit, whichever came first. In the No ICD group, follow-up time was calculated from time of initial SHaRe site visit to first occurrence of any of the components of the SCD or non-SCD composite secondary endpoints, death or last follow-up visit. Individuals who received ICDs during follow-up contributed follow-up time to the No ICD group until the time they received the ICD. Multivariable Cox-proportional hazards models were fit to compare secondary endpoints in the ICD and No ICD groups across sites adjusting for age, sex, race, year of ICD implant (ICD cohort), year of first SHaRe site visit (No ICD cohort), coronary artery disease, atrial fibrillation, syncope, septal reduction therapy, non-sustained ventricular tachycardia, family history of SCD, ejection fraction, and maximal left ventricular wall thickness. Multivariable analysis comparing rates of inappropriate ICD therapy between US and non-US sites was adjusted for age, sex, race, year of ICD implant, coronary artery disease, atrial fibrillation, ejection fraction, and left atrial diameter. The validity of the proportional hazards assumption was verified by examining the Schoenfeld residuals. All tests were two-sided and P-values <0.05 were considered statistically significant. All statistical analyses were performed using R version 4.0.2 (R Core Team 2020).
Results
Baseline characteristics of the study population
Our study sample included 5310 patients with HCM enrolled from eight US (n = 2650) and five non-US (n = 2660) tertiary HCM centres (Table 1). Although the study period spanned 2000–20, the majority of patients (68%, n = 3626/5310) had their first visit to a SHaRe site between 2010 and 2020. The median study follow-up from initial SHaRe visit to time of death or last SHaRe visit was 3.0 years (interquartile range, 0.6–6.6). The mean age at initial visit at a SHaRe site was 51 and 52 years for US and non-US site patients, respectively. Self-reported white race constituted a larger proportion of the patient population in non-US sites (91.6%) compared to US sites (71.9%). Additional notable differences in baseline characteristics between the two groups included a higher prevalence of hypertension, larger left atrial diameter, and higher maximum left ventricular outflow tract gradient among patients with HCM in US sites. Conversely, patients with HCM from non-US sites were more likely to have a family history of SCD, and syncope.
Table 1.
Study sample characteristics across US vs. non-US sites
| Overall cohort |
ICD cohort |
No ICD cohort |
||||
|---|---|---|---|---|---|---|
| US sites (n = 2650) | Non-US sites (n = 2660) | US sites (n = 611) | Non-US sites (n = 335) | US sites (n = 2512) | Non-US sites (n = 2577) | |
| Age (years)a,c | 51.1 ± 15.5 | 52.3 ± 15.3 | 49.3 ± 15.2 | 50.3 ± 14.5 | 51.5 ± 15.3 | 52.4 ± 15.2 |
| Male sex (%) | 1592 (60.1) | 1664 (62.6) | 380 (62.2) | 227 (67.8) | 1509 (60.1) | 1608 (62.4) |
| White (%)a,b,c | 1905 (71.9) | 2437 (91.6) | 463 (75.8) | 312 (93.1) | 1799 (71.6) | 2359 (91.5) |
| Year of initial visit to SHaRe site (%)a,c, † | ||||||
| 2000–2005 | 242 (9.1) | 346 (13.0) | 58 (9.5) | 28 (8.4) | 207 (8.3) | 338 (13.1) |
| 2006–2009 | 485 (18.3) | 612 (23.0) | 117 (19.2) | 59 (17.6) | 442 (17.6) | 595 (23.1) |
| 2010–2014 | 1008 (38.1) | 858 (32.3) | 214 (35.0) | 134 (40.0) | 965 (38.4) | 827 (32.1) |
| 2015–2020 | 915 (34.5) | 844 (31.7) | 222 (36.3) | 114 (34.0) | 898 (35.7) | 817 (31.7) |
| HCM risk-SCD categoriesa,c | ||||||
| Low (<4%) | 1626 (85.9) | 2044 (89.2) | 296 (63.1) | 200 (66.4) | 1551 (87.0) | 2004 (90.0) |
| Intermediate (4–6%) | 178 (9.4) | 164 (7.2) | 104 (22.2) | 61 (20.3) | 157 (8.8) | 149 (6.7) |
| High (≥6%) | 88 (4.7) | 83 (3.6) | 69 (14.7) | 40 (13.3) | 74 (4.2) | 73 (3.3) |
| Atrial fibrillation (%) | 254 (9.6) | 244 (9.2) | 113 (18.5) | 62 (18.5) | 242 (9.6) | 238 (9.2) |
| Hypertension (%)a,b,c | 298 (11.2) | 74 (2.8) | 68 (11.1) | 10 (3.0) | 291 (11.6) | 71 (2.8) |
| Coronary artery disease (%) | 61 (2.3) | 71 (2.7) | 25 (4.1) | 12 (3.6) | 57 (2.3) | 65 (2.5) |
| P/LP variant carrier in sarcomere gene (%)a,b,c, ‡ | 497/1428 (34.8) | 834/1807 (46.2) | 208/411 (50.6) | 163/270 (60.4) | 435/1328 (32.8) | 795/1749 (45.5) |
| Primary prevention ICD implanted at or prior to first SHaRe site visit (%)a, * | 138 (5.2) | 83 (3.1) | 138 (22.6) | 83 (24.8) | – | – |
| Subcutaneous ICD (%)b | – | – | 60 (9.8) | 10 (3.0) | – | – |
| Family history of SCD (%)a | 138 (5.2) | 176 (6.6) | 85 (13.9) | 60 (17.9) | 116 (4.6) | 151 (5.9) |
| Non-sustained ventricular tachycardia (%) | 109 (4.1) | 103 (3.9) | 173 (28.3) | 95 (28.4) | 98 (3.9) | 92 (3.6) |
| Syncope (%)a,b | 188 (7.1) | 236 (8.9) | 112 (18.3) | 105 (31.3) | 171 (6.8) | 209 (8.1) |
| Surgical septal myectomy (%)a,c | 38 (1.4) | 65 (2.4) | 59 (9.7) | 25 (7.5) | 37 (1.5) | 63 (2.4) |
| Alcohol septal ablation (%) | 33 (1.2) | 35 (1.3) | 20 (3.3) | 15 (4.5) | 29 (1.2) | 33 (1.3) |
| Ejection fraction (%)a,b,c | 67 ± 9 | 65 ± 9 | 66 ± 10 | 61 ± 10 | 67 ± 9 | 65 ± 9 |
| Left atrial diameter (mm)a,b,c | 45 ± 10 | 40 ± 10 | 47 ± 10 | 38 ± 12 | 45 ± 10 | 40 ± 10 |
| Maximum LVOT gradient (mmHg)a,b,c | 55 ± 50 | 36 ± 40 | 45 ± 45 | 29 ± 36 | 55 ± 50 | 36 ± 40 |
| Maximum left ventricular wall thickness (mm)a,b,c | 17 ± 5 | 18 ± 5 | 19 ± 6 | 20 ± 6 | 17 ± 5 | 18 ± 5 |
Characteristics of the overall cohort reflect study sample characteristics at time of first visit to SHaRe site. Characteristics of the ICD cohort reflect patient profile at time of ICD implant or at first visit to SHaRe site if ICD was implanted prior to first visit. Characteristics of the No ICD cohort reflect characteristics at time of first visit to SHaRe site inclusive of individuals who contributed person-time to the No ICD group until time of ICD implant during follow-up. Superscript letters a, b, and c denote statistically significant differences in baseline characteristics between US and non-US sites for the overall cohort, ICD cohort, and No ICD cohort, respectively.
HCM, hypertrophic cardiomyopathy; ICD, implantable cardioverter defibrillator; LVOT, left ventricular outflow tract; P/LP, pathogenic/likely pathogenic; SCD, sudden cardiac death; SHaRe, Sarcomeric Human Cardiomyopathy Registry.
A total of 118/138 and 40/83 ICDs were implanted at first SHaRe site visit in US and non-US sites, respectively, with the remaining 20/138 and 43/83 being implanted prior to first SHaRe site visit in US and non-US sites, respectively.
Reflects year of ICD implant for the ICD group.
Genetic testing was performed and available in 3235/5310. Echocardiographic parameters were available in a subset of the overall population (ejection fraction, n = 4598/5310; left atrial diameter, n = 4239/5310; maximum LVOT gradient, n = 3980/5310; maximum left ventricular wall thickness, n = 4743/5310). HCM risk-SCD score could be calculated in 4183/5310.
Primary prevention ICD implantation: US vs. non-US
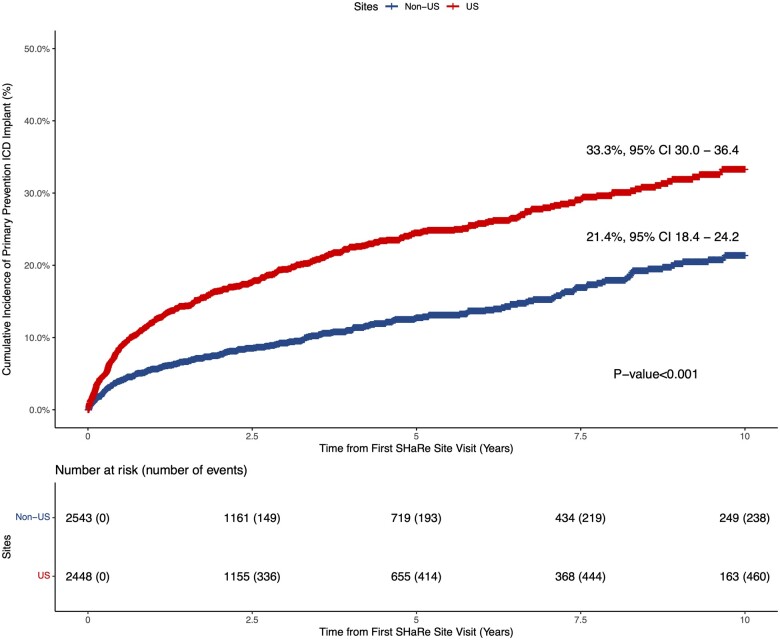
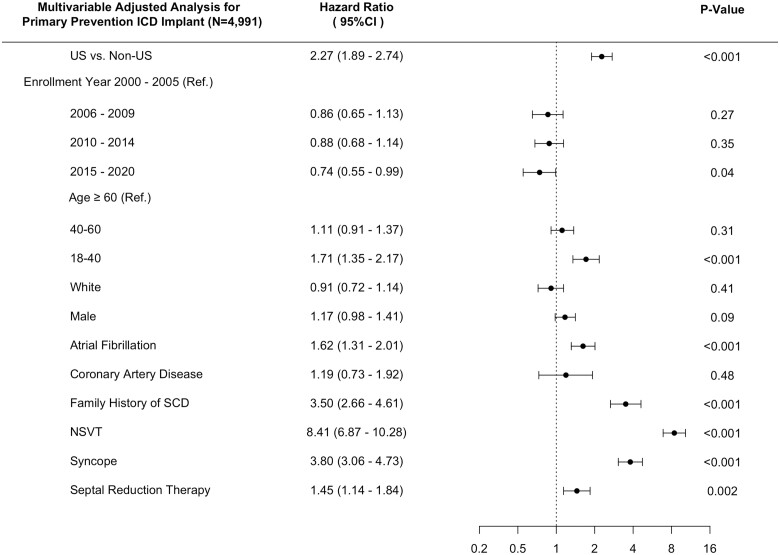
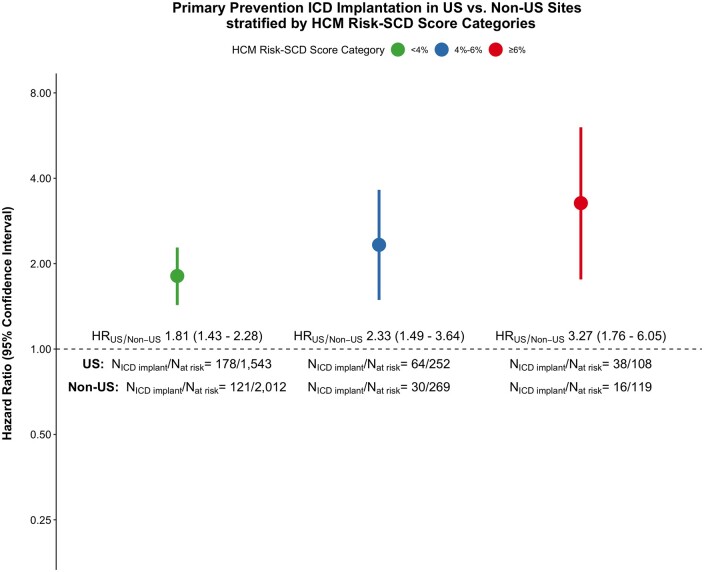
Among study participants without an ICD implanted at time of initial SHaRe site visit, 460/2448 and 238/2543 received a primary prevention ICD during follow-up in US and non-US sites, respectively. The cumulative incidence of primary prevention ICD implantation was 33.3% [95% confidence interval (CI), 30.0–36.4] in US centres vs. 21.4% (95% CI, 18.4–24.2) in non-US centres (log-rank P-value < 0.001) over the study follow-up period (Figure 1). Within the US and non-US groups, primary prevention ICD utilization rates across centres were overall similar with the notable exception of Royal Prince Alfred Hospital having a significantly higher rate of primary prevention ICD implantation as compared to the other non-US centres (Supplementary material online, Figure S2). In multivariable analysis using Cox-proportional hazards models and adjusting for clinical comorbidities and risk factors for SCD, US sites were associated with a 2.27-fold increased rate of primary prevention ICD implant as compared to non-US sites (HR 2.27, 95% CI 1.89–2.74; P < 0.001) (Figure 2). Our findings remained unchanged in a sensitivity analysis after incremental adjustment for echocardiographic parameters in the subgroup of patients where that data were available (HRUS vs. non-US 3.32, 95% CI 2.48–4.44; P < 0.001) (Supplementary material online, Figure S3). The HCM risk-SCD score was calculated on a subset of the study sample with data available for all score parameters. Stratifying the study sample into low (<4%), intermediate (4–6%) and high (≥6%) 5-year risk for SCD using this summative metric of SCD risk, revealed a similar two- to three-fold increased rate of primary prevention ICD implantation in US sites compared to non-US sites independent of SCD risk category (Figure 3).
Figure 1.
Cumulative incidence of primary prevention implantable cardioverter defibrillator implantation in US vs. non-US sites.
Figure 2.
Forest plot of multivariable association of US vs. non-US sites with primary prevention implantable cardioverter defibrillator implantation adjusting for clinical covariates. SCD, sudden cardiac death.
Figure 3.
Hazard ratios of primary prevention implantable cardioverter defibrillator implantation for US vs. non-US sites across hypertrophic cardiomyopathy risk-sudden cardiac death score categories. HCM, hypertrophic cardiomyopathy; SCD, sudden cardiac death.
To examine whether the introduction of the HCM risk-SCD score in 2014 impacted ICD implantation rates in US and non-US sites, we performed a stratified analysis by era: 2000–13 (n = 2904) vs. 2014–20 (n = 3463). We found no evidence of change in overall utilization of primary prevention ICDs before and after introduction of the HCM risk-SCD score (2000–13: US N ICD = 219/1343; non-US N ICD = 142/1561; 2014–20: US N ICD = 241/1825; non-US N ICD = 96/1638) (Supplementary material online, Figure S4). The 5-year cumulative incidence of primary prevention ICD implantation was similar across eras for US sites (2000–13: 19.7%, 95% CI [17.1–22.3]; 2014–20: 22.4%, 95% CI [19.5–25.2]) as well as non-US sites (2000–13: 12.0%, 95% CI [9.9–14.1]; 2014–20: 11.7%, 95% CI [8.8–14.5]). In multivariable analysis stratified by era and adjusting for clinical covariates, we found a similar two-fold excess of primary prevention ICD implantation in US sites during both eras (HRUS vs. non-US (2000 – 13) 2.29, 95% CI 1.78–2.96; HRUS vs. non-US (2014 – 20) 2.27, 95% CI 1.74–2.96; P-interaction = 0.74).
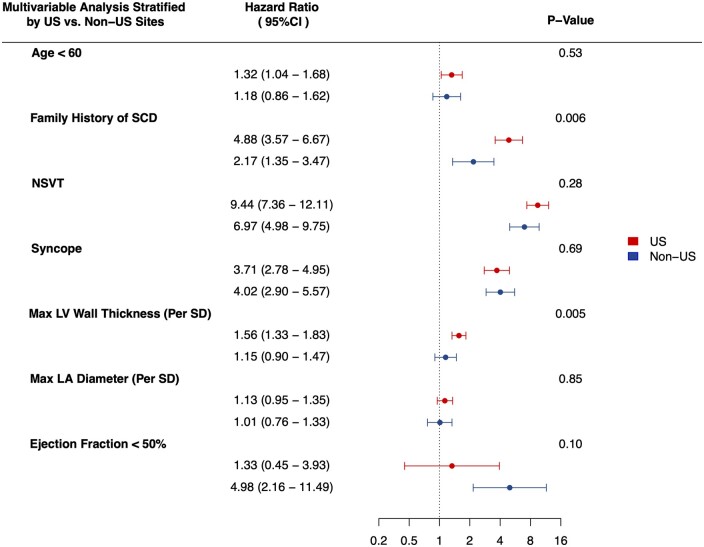
To investigate potential drivers of differential primary prevention ICD implantation rates among US vs. non-US sites, we tested for effect modification of US vs. non-US sites on the association of SCD risk factors with primary prevention ICD implantation. The direction of the association of SCD risk factors with ICD implantation was consistent across US and non-US sites, albeit with varying effect size magnitudes (Figure 4). Maximal left ventricular wall thickness was found to have a stronger association with primary prevention ICD implantation in US sites (HRper standard deviation 1.56, 95% CI 1.33–1.83) vs. non-US sites (HRper standard deviation 1.15, 95% CI 0.90–1.47) (P-interaction = 0.005). Family history of SCD also showed evidence of a stronger association with primary prevention ICD implantation in US sites (HR 4.88, 95% CI 3.57–6.67) compared to non-US sites (HR 2.17, 95% CI 1.35–3.47) (P-interaction = 0.006).
Figure 4.
Forest plot of multivariable association of sudden cardiac death risk factors with primary prevention implantable cardioverter defibrillator implantation stratified by US vs. non-US sites. P-values correspond to tests of interaction between sites (US vs. non-US) and corresponding sudden cardiac death risk factor. Tests of interaction for clinical sudden cardiac death risk factors were performed in the full cohort adjusting for age at first Sarcomeric Human Cardiomyopathy Registry site visit, year of first visit, sex, race, atrial fibrillation, coronary artery disease, septal reduction therapy, unexplained syncope, family history of sudden cardiac death, and non-sustained ventricular tachycardia. Tests of interaction between sites and echocardiographic parameters were performed among study participants with available echocardiographic data further adjusting for maximum left ventricular wall thickness, maximum left ventricular outflow tract gradient, left atrial diameter and ejection fraction. CI, confidence interval; SD, standard deviation.
ICD cohort outcomes: US vs. non-US
We examined outcomes of 946 patients who received a primary prevention ICD, 611 from US sites and 335 from non-US sites (Table 1). ICD recipients in US sites overall had a smaller number of traditional SCD risk factors. Of US ICD recipients with complete data on SCD risk factors (n = 569/611), 210 (36.9%), 309 (54.3%), and 50 (8.8%) had 0, 1, or 2 or more traditional SCD risk factors, respectively. In contrast, for non-US ICD recipients (n = 261/335 with complete data), 67 (25.7%), 141 (54%), and 53 (20.3%) had 0, 1, or 2 or more traditional ICD risk factors, respectively (P < 0.001) (Figure 5). In examining the reasons cited for ICD implant among recipients with zero risk factors (n = 277), we found that most either fell slightly short of the threshold defined for a continuous risk factor (ex: left ventricular wall thickness) or had multiple risk enhancers that did not meet the guideline definitions for traditional risk factors. Among the most commonly cited reasons for ICD implant in this zero-risk factor group were family history of SCD in second degree relatives or first-degree relatives without confirmed HCM and at age >40 (n = 65/277, 23.5%), presence of late gadolinium enhancement on cardiac magnetic resonance imaging (n = 56/277, 20.2%), maximal left ventricular wall thickness between 25 and 30 mm (n = 35/277, 12.6%), remote (>6 months) or non-arrhythmogenic syncope (n = 30/277, 10.8%), and implantation of an ICD in the setting of a clinical indication for a permanent pacemaker (n = 30/277, 10.8%) (Supplementary material online, Figure S5). Notably, 46/277 (16.6%) patients with zero traditional SCD risk factors had their ICDs implanted prior to their first SHaRe site visit and no clear indications for ICD implant were identified by medical record review.
Figure 5.
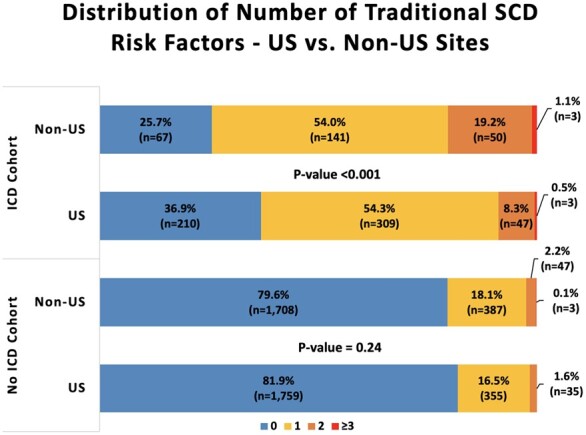
Distribution of number of traditional sudden cardiac death risk factors in US vs. non-US sites stratified by implantable cardioverter defibrillator vs. no implantable cardioverter defibrillator cohort. Risk factors included ejection fraction <50%, syncope, family history of sudden cardiac death, non-sustained ventricular tachycardia, and left ventricular wall thickness >30 mm. Data on late gadolinium enhancement and left ventricular aneurysm were not available in the Sarcomeric Human Cardiomyopathy Registry. Only individuals with non-missing data for all risk factors are included (n = 830/946 in implantable cardioverter defibrillator cohort and n = 4294/5089 in no implantable cardioverter defibrillator cohort). P-values are derived from Chi-square tests comparing the proportion of study participants in risk factor categories between US and non-US sites.
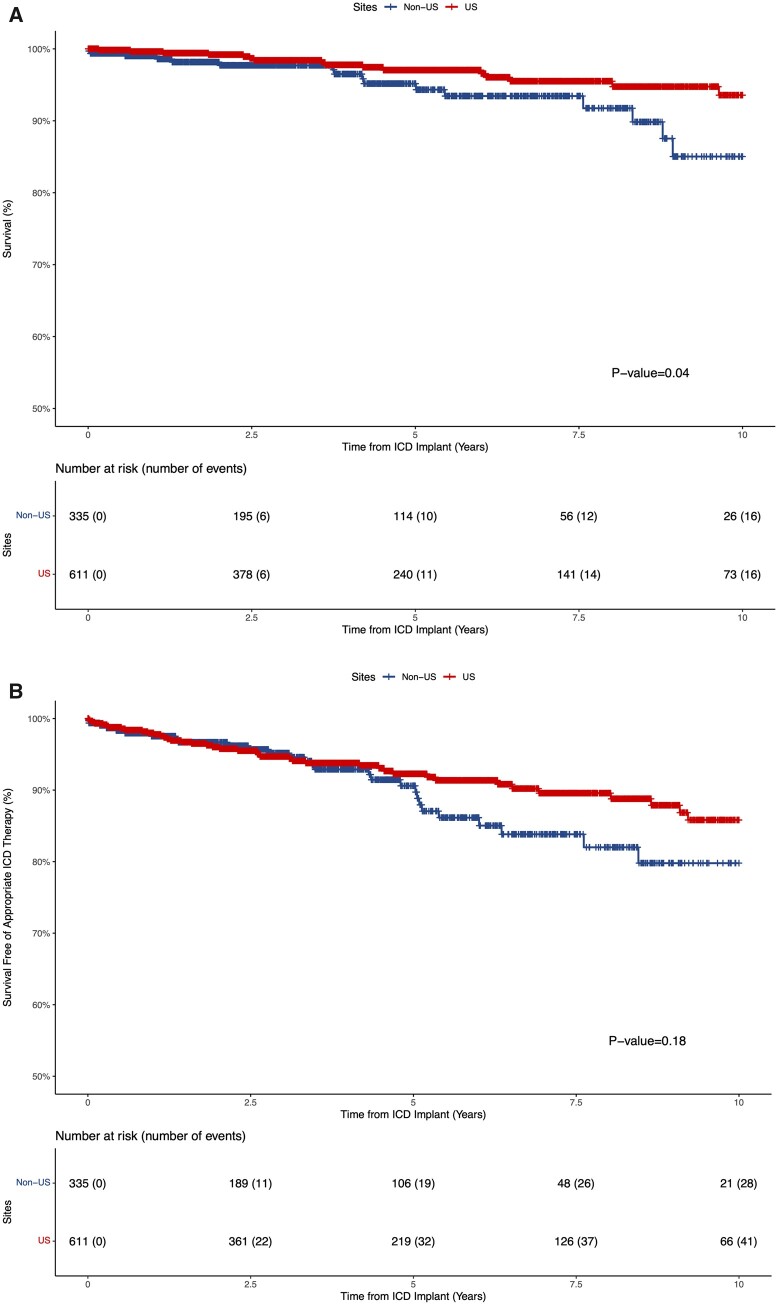
The Kaplan–Meier unadjusted survival estimates among primary prevention ICD recipients revealed a lower incidence of mortality (N US = 16/611; N non-US = 16/335) and appropriate ICD therapy (N US = 41/611; N non-US = 28/335) in US vs. non-US sites with no difference in incidence of inappropriate therapy (N US = 43/611; N non-US = 30/335) (Figure 6 and Supplementary material online, Figure S6). In multivariable analysis, ICD recipients in US sites had a significantly lower incidence of appropriate ICD therapy (HR 0.52, 95% CI 0.28–0.97; P = 0.04) that manifested approximately 5 years following ICD implant. In the multivariable analysis examining all-cause mortality, there was an effect suggesting reduced mortality among ICD recipients in US sites that did not reach the threshold for statistical significance (HR 0.42, 95% CI 0.17–1.02; P = 0.06). Finally, there was no difference in inappropriate ICD therapy among the two groups in multivariable analysis (HR 0.63, 95% CI 0.38–1.06; P = 0.10).
Figure 6.
Kaplan–Meier estimates of overall survival (A) and survival free of appropriate implantable cardioverter defibrillator therapy (B) in the primary prevention implantable cardioverter defibrillator cohort stratified by US vs. non-US sites. Out of 69 appropriate therapy events, 64 were implantable cardioverter defibrillator shocks and 5 were anti-tachycardia pacing. Time-zero reflects time of implantable cardioverter defibrillator implant during follow-up or first Sarcomeric Human Cardiomyopathy Registry site visit among participants who had a primary prevention implantable cardioverter defibrillator device implanted prior to first Sarcomeric Human Cardiomyopathy Registry site visit.
No ICD cohort outcomes: US vs. non-US
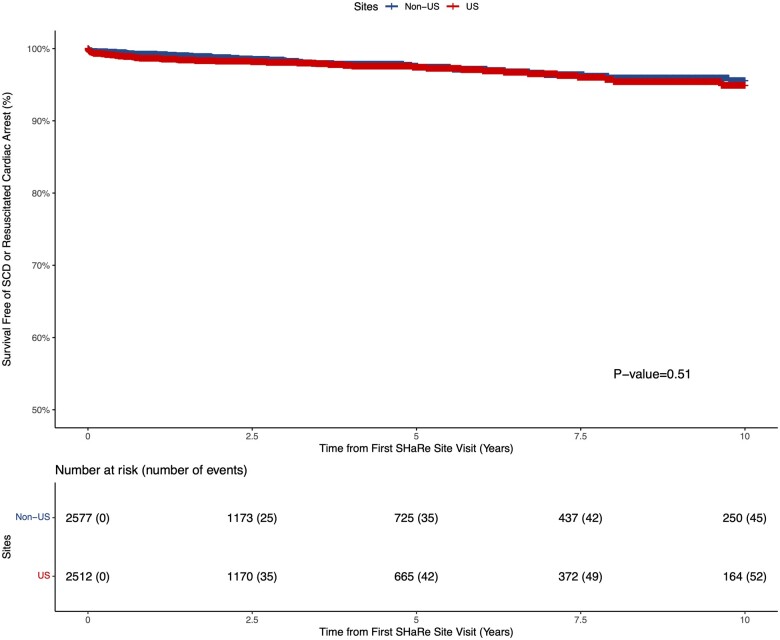
A total of 2512 and 2577 patients from US and non-US sites, respectively, contributed person-time to the No ICD cohort (Table 1). The majority of patients who did not receive a primary prevention ICD in both US (81.9%) and non-US sites (79.6%) had no traditional SCD risk factors (Figure 5). However, non-US sites had a trend towards a higher proportion of non-recipients of ICDs with at least one SCD risk factor as compared to US sites (20.4% vs. 18.1%; P = 0.07). Despite the higher proportion of individuals with at least one SCD risk factor in non-US sites, survival free of the composite SCD (SCD or resuscitated cardiac arrest; Figure 7) and the composite non-SCD (non-SCD, heart transplant or ventricular assist device implant; Supplementary material online, Figure S7) endpoint was similar between US and non-US sites. Fifty-two (N SCD = 17, N resuscitated cardiac arrest = 35) and 45 (N SCD = 16, N resuscitated cardiac arrest = 29) composite SCD events occurred over follow-up in US and non-US sites in the No ICD cohort, respectively. In the multivariable analysis, we found no association between US vs. non-US sites and the SCD (HR 1.21, 95% CI 0.74–1.97; P = 0.45) or non-SCD (HR 1.02, 95% CI 0.73–1.41; P = 0.91) composite endpoints.
Figure 7.
Kaplan–Meier estimates of survival free of sudden cardiac death or resuscitated cardiac arrest composite endpoint in the no implantable cardioverter defibrillator cohort stratified by US vs. non-US sites. Out of 97 composite sudden cardiac death events 34 were sudden cardiac death and 63 were resuscitated cardiac arrest.
Discussion
In this international multi-centre study of tertiary HCM centres, we found that primary prevention ICD utilization was approximately two-fold higher in US sites compared to non-US sites, even after accounting for well-established risk factors for SCD. Recipients of primary prevention ICDs in US sites had a lower burden of SCD risk factors than non-US sites. Consistent with the milder SCD risk profile of ICD recipients in US sites, we found a significantly lower incidence of appropriate therapy, predominantly ICD shocks, among ICD recipients in US vs. non-US sites. The higher rate of ICD implantation in US vs. non-US sites was seen even among individuals deemed at highest risk for SCD (5-year risk of SCD ≥6%) based on the HCM risk-SCD score. Despite the higher burden of SCD risk factors among patients who did not receive ICDs in non-US sites, we did not detect a significant excess of SCD or resuscitated cardiac arrest among non-recipients of primary prevention ICDs in non-US sites (Graphical Abstract). These results highlight the limitations of current strategies for risk stratification, as well as regional heterogeneity in practice trends, patient and provider preferences, and risk tolerance. While ICDs have been successfully deployed to reduce the burden of SCD in patients with HCM, further collaborative efforts are needed to improve the accuracy of risk stratification of SCD in HCM and to optimize healthcare resource utilization and patient outcomes.
Since the advent of ICDs, it has been a high priority to accurately identify patients with HCM at risk of SCD to prevent the tragic loss of young lives. However, the low rate of SCD in HCM creates significant challenges in devising accurate risk-stratification schemes that maximize both sensitivity and specificity.12 A number of risk-stratification algorithms for SCD in patients with HCM have been proposed and highlighted in iterations of treatment guidelines and consensus documents.5–8 Two main paradigms have arisen over time with varying emphasis on sensitivity and specificity.10 The first, endorsed by the AHA/ACC guidelines, recommends a single risk factor approach for identifying candidates for primary prevention ICDs.5 , 6 , 8 The second, endorsed by the ESC, recommends quantitative SCD risk assessment using the HCM risk-SCD score to bin patients into risk categories that guide the decision to implant a primary prevention ICD.7 At the heart of both paradigms is shared decision-making with an adequately informed patient because real-world decision-making for ICD utilization is nuanced and not uncommonly forced to deviate from strict adherence to guideline recommendations.
Despite numerous studies confirming the increased risk of SCD associated with established SCD risk factors, the positive predictive value of individual risk factors remains low, typically <20%.12 As a result, pursuing ICD implantation based on the presence of a single risk factor may result in exposing patients with HCM to possible adverse effects associated with ICDs3 with limited benefit. Indeed, in this study, we identified a two-fold higher rate of ICD implantation and lower rate of appropriate ICD therapy in US sites. On the other hand, O’Mahony et al. 9 developed a 5-year SCD risk calculator from a longitudinal multi-centre study of European HCM centres, assigning weighted coefficients to individual risk factors and assessing risk across the spectrum of continuous variables. The HCM risk-SCD score was externally validated in an international cohort with reasonably good discrimination (c-index 0.70), but it tended to over-estimate observed SCD risk in those with predicted intermediate and high SCD risk.13 The risk score was incorporated into the 2014 ESC HCM guidelines and expert consensus-driven risk categories were defined in an effort to optimize allocation of primary prevention ICDs.7 In our current study, we did not identify an obvious change in primary prevention ICD utilization in non-US sites following the 2014 ESC guidelines. In addition, primary prevention ICDs appeared to be under-utilized in non-US sites even among the high SCD risk category (HCM risk-SCD score ≥6%) where ICDs are recommended by the ESC guidelines. It remains to be determined whether this reflects a slow adoption of the risk score into clinical practice in non-US sites, or that the score is used as a component of a multi-pronged approach to clinical risk assessment. Despite the lower rate of ICD implantation across the spectrum of SCD risk in non-US sites, no significant excess SCD or resuscitated cardiac arrest was identified among HCM patients who did not receive a primary prevention ICD in non-US sites. Thus, neither approach is yet optimized and our study findings suggest that there is an opportunity to improve guideline recommendations and primary prevention ICD allocation without compromising SCD outcomes in patients with HCM.
The higher ICD implantation rate seen in the USA was independent of quantifiable SCD risk and definable risk factors. In exploring the association of individual risk factors with primary prevention ICD implantation, separately, in US vs. non-US sites, we found that maximum left ventricular wall thickness and family history of SCD were stronger predictors of ICD implantation in US than in non-US sites. Thus, the difference in primary prevention ICD utilization between US and non-US sites may be partly secondary to differences in perceived risk attributed to individual risk factors. In particular, a real-life experience with SCD in a family member may be perceived differently in different cultures and may trigger different patient preferences. The true dilemma is trying to balance the degree of SCD risk individual patients and clinicians are willing to tolerate against the cost deemed justifiable in unnecessary primary prevention ICD implantations to prevent an SCD event. This balance, and therefore practice, will vary based on patient/provider preferences and risk perception/tolerance, as well as societal, cultural, economic, and healthcare system differences between US and non-US sites. Indeed, higher ICD utilization in the USA is not restricted to HCM. Similar trends have been reported for ICD utilization in heart failure patients. Seidl and Senges14 found a four-fold higher rate of overall ICD implantation in the USA compared to Western Europe, Canada, and Australia. This pattern of relative over-utilization of ICDs in heart failure patients in US sites13 and under-utilization in non-US sites15–17 is present despite overlapping international guideline criteria for ICD implantation,18 , 19 supporting the theory that inherent non-clinical factors contribute to the differences in ICD utilization. However, we were unable to examine the impact of these factors on ICD utilization in this study as these data are not captured in the SHaRe registry.
Limitations
This is a largely retrospective observational study and despite adjusting for a comprehensive array of clinical and echocardiographic parameters associated with SCD, we cannot exclude the presence of residual confounding. The overall study follow-up time was relatively short and event rates for SCD and cardiac arrest are low, limiting our ability to detect differences in outcomes between US and non-US sites. Thus, findings from the outcome analysis should be interpreted with caution. Further studies of large cohorts with long-term follow-up are needed to more definitively examine how differences in ICD utilization impact clinical outcomes. Echocardiographic parameters and HCM risk-SCD score were not available for all patients. Analyses that adjusted for these covariates were thus limited to a subgroup of the overall population and may be susceptible to bias. However, our main analysis findings are congruent with sensitivity analyses that further adjusted for echocardiographic and HCM risk-SCD score, suggesting that there is no significant selection bias in the subgroup with non-missing parameters. Furthermore, several risk factors associated with SCD, including late gadolinium enhancement and left ventricular apical aneurysm, were not systematically available in SHaRe, which prevented us from accounting for these risk factors in our analysis. Our findings reflect practice trends in select HCM tertiary centres in US and non-US sites (tertiary centres from Europe, South America, and Australia) participating in SHaRe. Practice at these sites is not necessarily fully representative of national/continental practices and likely does not exclusively draw from guideline recommendations. While ICD implantation rates were overall similar comparing US sites to each other and non-US sites to each other, Royal Prince Alfred Hospital in Sydney, Australia, was an outlier compared to other non-US sites and had ICD implantation rates on par with US sites (Supplementary material online, Figure S2). Although this would be expected to drive our results closer to the null, we nonetheless detected a significantly higher rate of primary prevention ICD implantation and lower rate of appropriate ICD therapy in US vs. non-US sites. Moreover, in a sensitivity analysis excluding Royal Prince Alfred Hospital in Sydney, Australia, we found no difference in the SCD composite endpoint between US and non-US sites (HRUS vs. non-US 1.20, 95% CI 0.71–2.02; P = 0.49). Data on ICD programming were not available; thus, we cannot exclude that differences in rates of ICD therapy were not due to heterogeneity in device programming between sites. However, of the 69 appropriate therapies, 64 were ICD shocks that usually reflect underlying life-threatening sustained ventricular arrhythmias. Finally, this is a registry-based study and while subject to the limitations of non-uniform clinical follow-up and data accrual, it affords a unique opportunity to examine real-world practices and outcomes.
Conclusions
In this large, multicentre registry of patients with HCM receiving care at tertiary care centres, we identified a two-fold higher rate of primary prevention ICD implantation in US sites vs. non-US sites, independent of SCD risk. The relative under-utilization of ICDs in non-US sites persisted in the highest SCD risk group. However, the increased rate of ICD use in US sites was not associated with a significant reduction in overall SCD. Moreover, a smaller proportion of ICD recipients in US sites received potentially life-saving appropriate ICD therapy. These findings highlight the need to more reliably and objectively identify individuals who stand to benefit the most from primary prevention ICD implantation. More efforts are needed to better understand factors that drive risk for malignant arrhythmias to ultimately improve patient management, outcomes, and healthcare utilization.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
V.N. is funded by a training grant in cardiovascular research from the National Institutes of Health (T32HL007604). C.S. is the recipient of a National Health and Medical Research Council (NHMRC) Practitioner Fellowship (# 1154992). This work was supported by the Wellcome Trust [107469/Z/15/Z], Medical Research Council (UK), National Institute for Health Research (NIHR) Royal Brompton Cardiovascular Biomedical Research Unit, and the NIHR Imperial College Biomedical Research Centre. P.M. has received funding through an Elite Research Scholarship from the Danish Ministry of Science (8068-00018B), the Lundbeck Foundation (R358-2020-2343), and the Danish Heart Foundation (17-R115-A7532-22065).
This research was funded in part by the Wellcome Trust [107469/Z/15/Z]. For the purpose of open access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission.
Conflict of interest: A.S.H. receives support from Myokardia and Tenaya. J.I. receives research grant support from Myokardia. N.K. is a consultant for Myokardia, Tenaya, Pfizer, Array, and Serapta. A.T.O. receives support from Myokardia and Cytokinetics. J.W.R. is a consultant for Novartis, Bayer, MyoKardia, Cytokinetics, and Abiomed. S.S. is a consultant for Myokardia. J.S.W. receives research grant support and is a consultant for Myokardia. S.D. receives support from Myokardia and Tenaya. I.O. receives support from Myokardia, Cytokinetics, Amicus, Sanofi Genzyme, Shire Takeda, Bayer, and Boston Scientific. C.Y.H. receives support from MyoKardia, Novartis, and Tenaya. The other authors have no relevant disclosures to report.
Data availability
The data underlying this article cannot be shared publicly to protect the privacy of individuals that participated in the study. The data will be shared on reasonable request to the corresponding author.
Supplementary Material
Contributor Information
Victor Nauffal, Department of Medicine, Brigham and Women’s Hospital, Cardiovascular Medicine Division, 75 Francis Street, Boston, MA 02115, USA.
Peter Marstrand, Department of Cardiology, Herlev-Gentofte Hospital, University Hospital of Copenhagen, Gentofte Hospitalsvej 1, Hellerup 2900, Denmark.
Larry Han, Department of Biostatistics, Harvard T.H. Chan School of Public Health, 677 Huntington Ave, Boston, MA 02115, USA.
Victoria N Parikh, Division of Cardiovascular Medicine, Department of Medicine, Stanford University School of Medicine, 291 Campus Drive, Stanford, CA 94305, USA.
Adam S Helms, Department of Medicine, Cardiovascular Medicine Division, University of Michigan, 1500 E Medical Center Dr, Ann Arbor, MI 48109, USA.
Jodie Ingles, Department of Cardiology, Cardio Genomics Program at Centenary Institute, The University of Sydney, Royal Prince Alfred Hospital, Missenden Rd, Sydney NSW 2050, Australia; Department of Cardiology, Royal Prince Alfred Hospital, Missenden Rd, Sydney NSW 2050, Australia.
Daniel Jacoby, Department of Internal Medicine, Section of Cardiovascular Medicine, Yale University, 20 York St, New Haven, CT 06510, USA.
Neal K Lakdawala, Department of Medicine, Brigham and Women’s Hospital, Cardiovascular Medicine Division, 75 Francis Street, Boston, MA 02115, USA.
Sunil Kapur, Department of Medicine, Brigham and Women’s Hospital, Cardiovascular Medicine Division, 75 Francis Street, Boston, MA 02115, USA.
Michelle Michels, Department of Cardiology, Thoraxcenter, Erasmus, Dr. Molewaterplein 40, Rotterdam 3015 GD, the Netherlands.
Anjali T Owens, Division of Cardiovascular Medicine, Department of Medicine, Center for Inherited Cardiovascular Disease, University of Pennsylvania Perelman School of Medicine, 3400 Spruce St, Philadelphia, PA 19104, USA.
Euan A Ashley, Division of Cardiovascular Medicine, Department of Medicine, Stanford Center for Inherited Cardiovascular Disease, Stanford University School of Medicine, 291 Campus Drive, Stanford, CA 94305, USA.
Alexandre C Pereira, Department of Cardiology, Heart Institute (InCor), University of Sao Paulo Medical School, Av. Dr. Enéas Carvalho de Aguiar, 44 - Cerqueira César, São Paulo - SP, 05403-900, Brazil.
Joseph W Rossano, Department of Pediatrics, Division of Cardiology, Children's Hospital of Philadelphia, Philadelphia, PA, USA.
Sara Saberi, Department of Medicine, Cardiovascular Medicine Division, University of Michigan, 1500 E Medical Center Dr, Ann Arbor, MI 48109, USA.
Christopher Semsarian, Department of Cardiology, Royal Prince Alfred Hospital, Missenden Rd, Sydney NSW 2050, Australia; Department of Cardiology, Agnes Ginges Centre for Molecular Cardiology at Centenary Institute, The University of Sydney, Australia.
James S Ware, Department of Medicine, National Heart & Lung Institute & MRC London Institute of Medical Sciences, Imperial College London, Du Cane Rd, London W12 0NN, UK; Division of Cardiovascular Medicine, Department of Medicine, Royal Brompton & Harefield Hospitals, Sydney St, London SW3 6NP, UK.
Samuel G Wittekind, Department of Pediatrics, University of Cincinnati College of Medicine, 3230 Eden Ave, Cincinnati, OH 45267, USA; The Heart Institute, Cincinnati Children’s, 3333 Burnet Ave, Cincinnati, OH 45229, USA.
Sharlene Day, Division of Cardiovascular Medicine, Department of Medicine, Center for Inherited Cardiovascular Disease, University of Pennsylvania Perelman School of Medicine, 3400 Spruce St, Philadelphia, PA 19104, USA.
Iacopo Olivotto, Department of Experimental and Clinical Medicine, Careggi University Hospital, Largo Giovanni Alessandro Brambilla, 3, 50134 Firenze FI, Italy.
Carolyn Y Ho, Department of Medicine, Brigham and Women’s Hospital, Cardiovascular Medicine Division, 75 Francis Street, Boston, MA 02115, USA.
References
- 1. Maron BJ. Clinical course and management of hypertrophic cardiomyopathy. N Engl J Med 2018;379:655–668. [DOI] [PubMed] [Google Scholar]
- 2. Maron BJ, Shen WK, Link MS, Epstein AE, Almquist AK, Daubert JP, Bardy GH, Favale S, Rea RF, Boriani G, Estes NM 3rd, Spirito P. Efficacy of implantable cardioverter-defibrillators for the prevention of sudden death in patients with hypertrophic cardiomyopathy. N Engl J Med 2000;342:365–373. [DOI] [PubMed] [Google Scholar]
- 3. O'Mahony C, Lambiase PD, Quarta G, Cardona M, Calcagnino M, Tsovolas K, Al-Shaikh S, Rahman SM, Arnous S, Jones S, McKenna W, Elliott P. The long-term survival and the risks and benefits of implantable cardioverter defibrillators in patients with hypertrophic cardiomyopathy. Heart 2012;98:116–125. [DOI] [PubMed] [Google Scholar]
- 4. Kanterakis A, Sharma S. Cardiomyopathy and heart failure conventional risk factors for SCD in HCM. Eur Cardiol Rev 2015;10:31–36. [Google Scholar]
- 5. Maron B, McKenna WJ, Danielson GK, Kappenberger LJ, Kuhn HJ, Seidman CE, Shah PM, Spencer WH 3rd, Spirito P, Ten Cate FJ, Wigle ED; American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents; European Society of Cardiology Committee for Practice Guidelines American College of Cardiology/European Society of Cardiology Clinical Expert Consensus Document on Hypertrophic Cardiomyopathy. A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the European Society of Cardiology Committee for Practice Guidelines. Eur Heart J 2003;24:1965–1991. [DOI] [PubMed] [Google Scholar]
- 6. Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, Naidu SS, Nishimura RA, Ommen SR, Rakowski H, Seidman CE, Towbin JA, Udelson JE, Yancy CW, Jacobs AK, Smith SC, Anderson JL, Albert NM, Buller CE, Creager MA, Ettinger SM, Guyton RA, Halperin JL, Hochman JS, Krumholz HM, Kushner FG, Nishimura RA, Ohman EM, Page RL, Stevenson WG, Tarkington LG, Yancy CW; Society of Thoracic Surgeons. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy. J Thorac Cardiovasc Surg 2011;142:e153–e203. [DOI] [PubMed] [Google Scholar]
- 7. Zamorano JL, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege AA, Lafont A, Limongelli G, Mahrholdt H, McKenna WJ, Mogensen J, Nihoyannopoulos P, Nistri S, Piepe PG, Pieske B, Rapezzi C, Rutten FH, Tillmanns C, Watkins H. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the task force for the diagnosis and management of hypertrophic cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2733–2779. [DOI] [PubMed] [Google Scholar]
- 8. Ommen SR, Mital S, Burke MA, Day SM, Deswal A, Elliott P, Evanovich LL, Hung J, Joglar JA, Kantor P, Kimmelstiel C, Kittleson M, Link MS, Maron MS, Martinez MW, Miyake CY, Schaff HV, Semsarian C, Paul S. 2020 AHA/ACC Guideline for the Diagnosis and Treatment of Patients With Hypertrophic Cardiomyopathy: executive Summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2020;142:e533–e557. [DOI] [PubMed] [Google Scholar]
- 9. O'Mahony C, Jichi F, Pavlou M, Monserrat L, Anastasakis A, Rapezzi C, Biagini E, Gimeno JR, Limongelli G, McKenna WJ, Omar RZ, Elliott PM; Hypertrophic Cardiomyopathy Outcomes Investigators. A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM Risk-SCD). Eur Heart J 2014;35:2010–2020. [DOI] [PubMed] [Google Scholar]
- 10. Udelson JE. Evaluating and reducing the risk of sudden death in hypertrophic cardiomyopathy: a trans-Atlantic divergence? Circulation 2019;139:727–729. [DOI] [PubMed] [Google Scholar]
- 11. Ho CY, Day SM, Ashley EA, Michels M, Pereira AC, Jacoby D, Cirino AL, Fox JC, Lakdawala NK, Ware JS, Caleshu CA, Helms AS, Colan SD, Girolami F, Cecchi F, Seidman CE, Sajeev G, Signorovitch J, Green EM, Olivotto I; for the SHaRe Investigators. Genotype and lifetime burden of disease in hypertrophic cardiomyopathy insights from the Sarcomeric Human Cardiomyopathy Registry (SHaRe). Circulation 2018;138:1387–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maron BJ, Spirito P, Shen W-K, Haas TS, Formisano F, Link MS, Epstein AE, Almquist AK, Daubert JP, Lawrenz T, Boriani G, Estes NAM, Favale S, Piccininno M, Winters SL, Santini M, Betocchi S, Arribas F, Sherrid MV, Buja G, Semsarian C, Bruzzi P. Implantable cardioverter-defibrillators and prevention of sudden cardiac death in hypertrophic cardiomyopathy. JAMA 2007;298:405–412. [DOI] [PubMed] [Google Scholar]
- 13. O’Mahony C, Jichi F, Ommen SR, Christiaans I, Arbustini E, Garcia-Pavia P, Cecchi F, Olivotto I, Kitaoka H, Gotsman I, Carr-White G, Mogensen J, Antoniades L, Mohiddin SA, Maurer MS, Tang HC, Geske JB, Siontis KC, Mahmoud KD, Vermeer A, Wilde A, Favalli V, Guttmann OP, Gallego-Delgado M, Dominguez F, Tanini I, Kubo T, Keren A, Bueser T, Waters S, Issa IF, Malcolmson J, Burns T, Sekhri N, Hoeger CW, Omar RZ, Elliott PM. International external validation study of the 2014 European Society of Cardiology guidelines on sudden cardiac death prevention in hypertrophic cardiomyopathy (EVIDENCE-HCM). Circulation 2018;137:1015–1023. [DOI] [PubMed] [Google Scholar]
- 14. Seidl K, Senges J. Worldwide utilization of implantable cardioverter/defibrillators now and in the future. Card Electrophysiol Rev 2003;7:5–13. [DOI] [PubMed] [Google Scholar]
- 15. Camm AJ, Nisam S. European utilization of the implantable defibrillator: has 10 years changed the enigma? Europace 2010;12:1063–1069. [DOI] [PubMed] [Google Scholar]
- 16. Raatikainen MJP, Arnar DO, Zeppenfeld K, Merino JL, Levya F, Hindriks G, Kuck KH. Statistics on the use of cardiac electronic devices and electrophysiological procedures in the European Society of Cardiology countries: 2014 report from the European Heart Rhythm Association. Europace 2015;17:i1–i75. [DOI] [PubMed] [Google Scholar]
- 17. Schrage B, Uijl A, Benson L, Westermann D, Ståhlberg M, Stolfo D, Dahlström U, Linde C, Braunschweig F, Savarese G. Association between use of primary-prevention implantable cardioverter-defibrillators and mortality in patients with heart failure. Circulation 2019;140:1530–1539. [DOI] [PubMed] [Google Scholar]
- 18. Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, Kirchhof P, Kjeldsen K, Kuck KH, Hernandez-Madrid A, Nikolaou N, Norekvål TM, Spaulding C, Van Veldhuisen DJ; Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J 2015;36:2793–2867l.26320108 [Google Scholar]
- 19. Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC, Gillis AM, Granger CB, Hammill SC, Hlatky MA, Joglar JA, Kay GN, Matlock DD, Myerburg RJ, Page RL. 2017 AHA/ACC/HRS Guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 2018;138:e210–e271. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly to protect the privacy of individuals that participated in the study. The data will be shared on reasonable request to the corresponding author.