Abstract
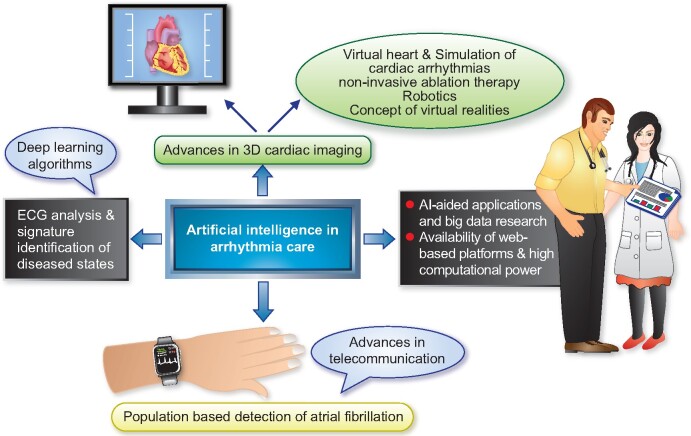
The field of cardiac electrophysiology (EP) had adopted simple artificial intelligence (AI) methodologies for decades. Recent renewed interest in deep learning techniques has opened new frontiers in electrocardiography analysis including signature identification of diseased states. Artificial intelligence advances coupled with simultaneous rapid growth in computational power, sensor technology, and availability of web-based platforms have seen the rapid growth of AI-aided applications and big data research. Changing lifestyles with an expansion of the concept of internet of things and advancements in telecommunication technology have opened doors to population-based detection of atrial fibrillation in ways, which were previously unimaginable. Artificial intelligence-aided advances in 3D cardiac imaging heralded the concept of virtual hearts and the simulation of cardiac arrhythmias. Robotics, completely non-invasive ablation therapy, and the concept of extended realities show promise to revolutionize the future of EP. In this review, we discuss the impact of AI and recent technological advances in all aspects of arrhythmia care.
Keywords: Artificial intelligence, Machine learning, Electrophysiology, Atrial fibrillation, Ablation
Graphical Abstract
Introduction
As artificial intelligence (AI) has entered the medical field in recent years, machine learning (ML) approaches have made progress in assisting healthcare professionals in optimizing personalized treatment in a given situation, in particular in electrocardiography and image interpretation. Artificial intelligence methodologies are increasingly being adopted into all aspects of patient care and are paving the way to minimally invasive or non-invasive treatment modalities. This article offers a state-of-the-art overview on milestones achieved, but also on future integration of this information into diagnostic and therapeutic measures, and its likely impact on all aspects of arrhythmia care. Integration of all individual information in combination with AI solutions is likely to revolutionize electrophysiology (EP) interventions in the near future (Figure 1 and Graphical Abstract).
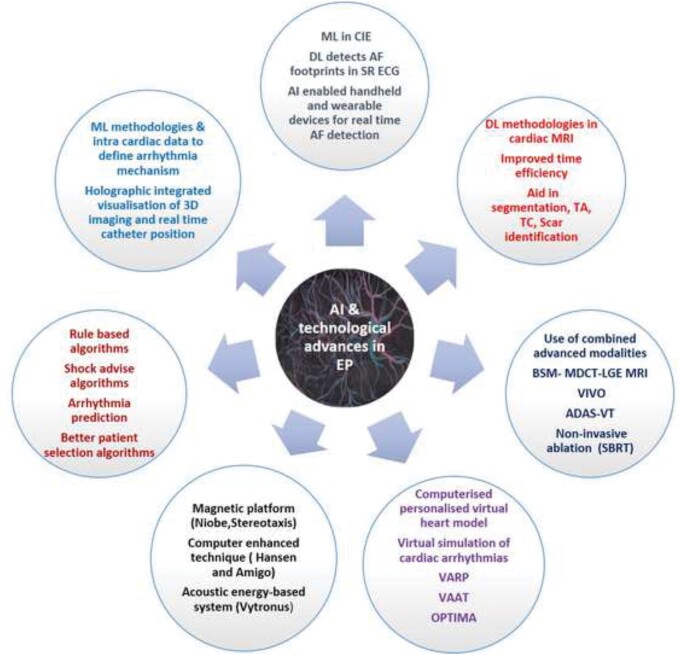
Figure 1.
An illustration highlighting the impact of artificial intelligence and recent technological advancements on all aspects of patient care in the field of cardiac electrophysiology. ADAS, Automatic Detection of Arrhythmic Substrate; AF, atrial fibrillation; BSM, body surface mapping; CIE, computerized interpretation of electrocardiography; DL, deep learning; EAM, electro anatomical mapping; EP, electrophysiology; LGE, late gadolinium enhancement; MDCT, multidetector computed tomography; ML, machine learning; MRI, magnetic resonance imaging; OPTIMA, optimal target identification via modelling of arrhythmogenesis; SBRT, stereotactic body radiotherapy; SR, sinus rhythm; TA, texture analysis; TC, tissue characterization; VAAT, virtual heart arrhythmia ablation targeting; VARP, virtual heart arrhythmia risk predictor approach; VIVO, view into ventricular onset; VT, ventricular tachycardia.
Artificial intelligence-enhanced arrhythmia care.
Cardiac electrical signal analysis using artificial intelligence methodologies
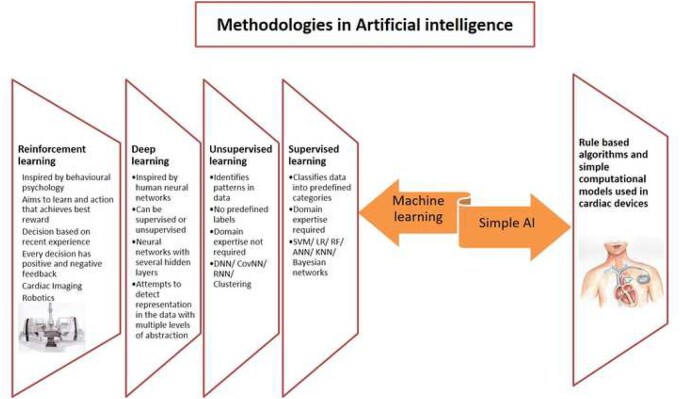
Human intelligence is characterized by the capability of learning, reasoning, analysing, and decision-making. When machines mimic the use of these capabilities, it can be termed AI. Although the concept and the term AI have been used for over six decades,1–3 its usage has skyrocketed over the last decade. Whilst ML is the most commonly used term for AI, it only denotes one of the methodologies of AI (Figure 2 and Supplementary material online, Section S1).
Figure 2.
Artificial intelligence methodologies with their individual characteristics. AI, artificial intelligence; ANN, artificial neural network; CovNN, convolutional neural network; DNN, deep neural network; KNN, K-nearest neighbours; LR, logistic regression; RF, random forest; RNN, recurrent neural network; SVM, support vector machine.
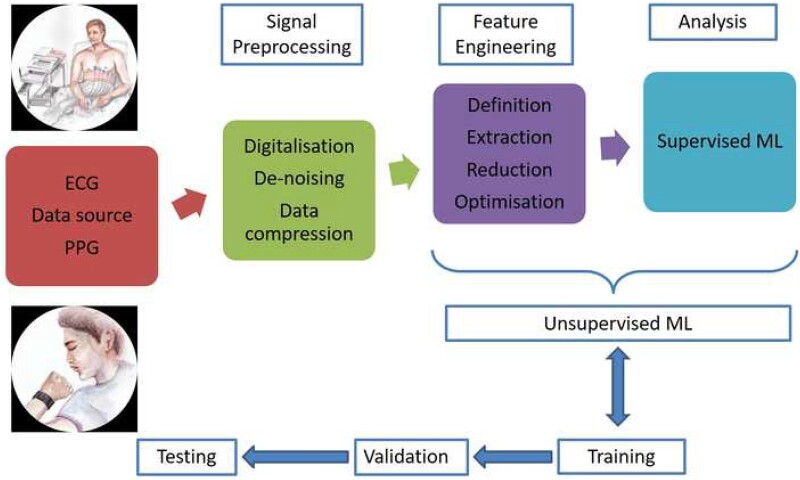
Most of the AI applications in EP are based on the analysis of signals that represent cardiac electrical activity, with the signal varying with the type of sensor and underlying technology. Two of the most commonly used signals are electrocardiogram (ECG) and photo plethysmography (PPG). Photo plethysmography is more contemporary and has been used in some of the wearable devices including watches, wrist bands, and smartphones. Fundamental AI processes employed in analysing data obtained from these devices are essentially similar (Figure 3).
Figure 3.
Schematic representation of steps involved in cardiac impulse analysis from the data acquisition to analysis by machine learning algorithm. ECG, electrocardiogram; ML, machine learning; PPG, photo plethysmography.
Following data collection, data are pre-processed, feature engineering (Table 1) carried out, and followed by classification by one of the ML methodologies (Table 2).The ML methodology is first trained using the ‘training data’ with appropriate labels. The second stage involves ML methodology assessment using a ‘validation data set’ and fine tuning the algorithm. Following these two steps, the ML algorithm would then be ready to be used with a third ‘test set’. Performance of the algorithm is expressed using values such as sensitivity, specificity, accuracy, receiver operating curve (ROC), and area under ROC (AUC).4
Table 1.
Overview of feature engineering processes employed during development of machine learning methods
| Feature engineering | ||
|---|---|---|
| Definition | Extraction | Optimization |
|
|
|
DL, deep learning; ML, machine learning.
Table 2.
Commonly used machine learning classification algorithms
| Algorithm | Learning method | Description | Utility in EP ML |
|---|---|---|---|
| Support vector machine |
|
|
|
| Random Forest |
|
|
|
| Bayesian networks |
|
|
|
| Neural networks |
|
|
|
| Convolutional neural networks |
|
|
|
BN, bayesian networks; CRT, cardiac resynchronization therapy; DL, deep learning; ECG, electrocardiogram; EP, electrophysiology; HMM, Hidden Markov Models; ML, machine learning; VF, ventricular fibrillation.
Arrhythmia detection using artificial intelligence
Since digitalization of ECG, AI methods have been employed in computerized interpretation of ECGs. Whilst ML methods revealed high sensitivity and specificity for detecting normal sinus rhythm, their abilities were lower than expert cardiologists for the identification of cardiac arrhythmias.5 One of the main deterrents have been the presence of noise, small or varying P waves resulting in over diagnosis of atrial fibrillation (AF), paced rhythms, poor-quality ECGs, tremor, and previously untrained rhythms. With better ML algorithms, noise reduction techniques and advanced feature extraction, selection and reduction methods [including use of the unsupervised deep neural network (DNN)], computerized interpretation of ECGs has clearly improved arrhythmia detection achieving an accuracy close to 95%.6 , 7
Hannun et al. 8 developed an end to end deep learning (DL) approach for ECG analysis by using a DNN for identifying 12 rhythm abnormalities by using 91 232 single-lead ECGs. When validated against independent data reported by a committee of certified cardiologists, their algorithm was shown to be superior to an average cardiologist in identifying these rhythm abnormalities (ROC 0.97 vs. 0.78).
With the development of unsupervised DNN algorithms, more interest has been generated amongst researchers for identifying hidden diseased state signatures in a 12-lead ECG. So far, it has been shown to be feasible to detect hyperkalaemia,9 heart failure,10 hypoglycaemia,11 and even changes in emotional states12 using 12-lead ECG. Attia et al. 13 at Mayo Clinic Rochester assessed the feasibility of identifying previous episodes of or impending AF using an AI-enabled 12-lead ECG in normal sinus rhythm. They used 0.65 million ECGs to train, validate, and test the AI algorithm in a 7:1:2 ratio and found that AI-enabled ECG recorded during normal sinus rhythm performed well as a screening test to identify AF with an accuracy of 79.4% and this improved to 83.4% when it was a first ECG following an episode of AF. Additional multiple ECGs improved the model accuracy leading to the hypothesis that structural changes that may precede AF including myocyte hypertrophy, fibrosis, or chamber dilatation may result in subtle multifaceted changes in ECG that may otherwise be unrecognized by the human eye but are detectable by a DNN. This observation has important clinical implications with potential point-of-care identification of individuals at the risk of AF and for the embolic stroke of undetermined source.
Deep neural network has also been used to predict hypertrophic cardiomyopathy (HCM),14 age and sex,15 and plasma dofetilide concentration from ECG.16
Advancements in sensor technology, telecommunications (increased availability of wireless, Wi-Fi, Bluetooth and smartphone technologies), availability of web-based data storage, and AI-aided analysis have seen rapid growth of handheld and wearable cardiac monitoring systems (Table 3).
Table 3.
Diagnostic accuracy of artificial intelligence-aided devices in identifying atrial fibrillation
| Study | Device and AI algorithm | Signal analysed | AF detection |
|---|---|---|---|
|
Algorithm using smartphone (Kardia Mobile Cardiac Monitor) and handheld cardiac rhythm recorder vs. physician-interpreted ECG | ECG | 96.6% sensitivity and 94.1% specificity for AF detection |
|
Wristband/wristwatch-based irregular pulse notification algorithm | PPG | Positive predictive value of PPG signals being 91.6% (95% CI 91.5–91.8%) |
|
Smartwatch-based irregular pulse notification algorithm vs. subsequent monitoring with ECG patch | Initial PPG followed by simultaneous PPG and ECG | Smartwatch-based algorithm had a positive predictive value of 0.84 (95% CI 0.76–0.92) for observing AF during the simultaneous monitoring period |
| Chen et al.20 | Smart wristband device enabled by AF-identifying AI algorithm vs. wristband ECG reviewed by physicians | PPG and ECG | Sensitivity, specificity, and accuracy were 88.00%, 96.41%, and 93.27%, respectively, for PPG and 87.33%, 99.20%, and 94.76% for ECG |
| Wasserlauf et al.21 | Apple Watch with KardiaBand (enabled by convoluted neural network algorithm) vs. insertable cardiac monitor | ECG | 97.5% and 97.7% for episode sensitivity and duration sensitivity, respectively |
|
Smartwatch-based algorithm vs. cardiologists’ diagnosis by electrocardiography | PPG | Sensitivity of 93.7% (95% CI 89.8–96.4%), specificity of 98.2% (95% CI 95.8–99.4%), and 96.1% accuracy (95% CI 94.0–97.5%) |
AF, atrial fibrillation; AI, artificial intelligence; CI, confidence interval; ECG, electrocardiogram; PPG, photo plethysmography.
Whilst several handheld devices are available, the AliveCor Heart Monitor, an ECG-based system, has been studied extensively for AF detection in symptomatic patients.17 , 23–27 In most of these studies, AliveCor Heart Monitor achieved well over 90% sensitivity and specificity for AF detection both in outpatient and hospital settings.28 In a comparison with traditional transtelephonic monitor, AliveCor Heart Monitor achieved 100% sensitivity and 97% specificity for AF and atrial flutter detection.29 Similar results were seen with the use of PPG-based systems coupled with AI-aided analysis.30 , 31 In the WATCH AF trial, Dörr et al. 22 studied the efficacy of a smartphone PPG-based algorithm in AF detection. The PPG algorithm achieved a sensitivity of 93.7%, a specificity of 98.2%, and an accuracy of 96.1% to detect AF.
In a recent community-based trial on the utility of a smartwatch in AF detection (Apple Heart Study),19 smartwatch in conjunction with a pulse notification algorithm showed promising results in 0.41 million participants with no prior history of AF. The smartwatch application collected single 1-min tachograms every 2 h. If a smartwatch-based irregular pulse notification algorithm identified possible AF, the participant was notified to have further simultaneous 7-day monitoring using an ECG patch. The smartwatch-based algorithm had a positive predictive value of 0.84 (95% confidence interval 0.76–0.92) for identifying AF during the simultaneous monitoring period. Similar results were shown in yet another population-based AF screening study using a PPG algorithm in conjunction with smart devices.18
Chen et al. 20 studied AF detection using a smart wristband equipped with both ECG and PPG sensors, comparing ECG and PPG individually as well as in combination. They demonstrated higher accuracy of 97.5% for AF detection with the combination against 94.7% and 93.2% with ECG and PPG, respectively.
Wasserlauf et al. 21 compared smartwatch detection of AF using a DL algorithm (episodes lasting ≥1 h) with an insertable loop recorder. They analysed 31 348 h of simultaneously recorded data. The smartwatch algorithm achieved 97.5% and 97.7% for episode sensitivity and duration sensitivity, respectively.
Artificial intelligence-enabled monitoring systems are affordable and reliable, can be used for continuous ambulatory monitoring, and will facilitate the detection of vulnerable groups.32 A step closer to the holy grail of early AF detection within the community in otherwise asymptomatic patients may in fact initiate a paradigm shift in arrhythmia detection.
Artificial intelligence and cardiac devices
Most of the pacemaker and defibrillator functions use rule-based algorithms. Rate response feature in a pacemaker, which incorporates the ability of the device to vary the pacing rate based on an input from a biosensor; tachycardia detection and deliverance of an appropriate therapy by an implantable defibrillator, are some of the illustrations of the rule-based decision-making. A rule-based algorithm is referred to as a simplest form of AI but it differs from ML in its inability to learn.33 In rule-based algorithms, rules are laid down by humans based on the domain expertise, whereas ML methodologies learn actively from the training data and create their own rules for decision-making, which may not be transparent in some instances (Supplementary material online, Section S2 and Table S1).
Machine learning algorithms are finding their use with cardiac devices both in arrhythmia detection and prediction of future events. Machine learning methods have been employed in automated external defibrillators in the development of shock advice algorithms.7 , 34–36 Recently, Nguyen et al. 37 developed an algorithm for the detection of shockable and non-shockable rhythms using ML. They used both boosting classifier and convolutional neural network as a feature extractor with a sensitivity and specificity of 95.21% and 99.31%, respectively. This shock advice algorithm was a considerable improvement over existing algorithms and in keeping with the standards set by the American Heart Association guidelines.38 More recently, a DL technique was introduced to identify any cardiac device model from a chest radiograph.39
Machine learning methods have been used to improve cardiac resynchronization therapy (CRT) outcomes prediction, paving the way for better patient selection.40 , 41 In a proof of concept, single centre study, ML algorithm in conjunction with natural language processing was applied to electronic health records.42 This model successfully identified subgroups of patients who were unlikely to benefit from CRT. An ML algorithm using naive Bayes classifier using patient variables including age, sex, QRS duration and morphology, left ventricular ejection fraction and end-diastolic diameter, New York Heart Association functional class, presence of AF, and epicardial left ventricular lead was superior to existing guidelines in predicting event-free survival post-CRT.43
Machine learning algorithms including random forests and convoluted neural networks, when applied to the AF signature burden obtained using continuous remote monitoring data in patients with cardiac implantable electronic devices, were superior in predicting stroke compared to the widely used CHA2DS2-VASc score. An ensemble method using ML model in conjunction with CHA2DS2-VASc score had better sensitivity and specificity when compared to using CHA2DS2-VASc score alone and improved AUC from 0.52 to 0.63.44
Multimodal integrative approach to predict sites of arrhythmia origins and role of machine learning
Non-invasive characterization of arrhythmia prior to attempting ablative therapy is gaining favour amongst electrophysiologists. This approach aids in focused targeting of the cardiac region of interest. One of the important contributors to this being ML aided advances in cardiac 3D imaging.
Application of DL methods including convolutional neural network has improved the speed of acquisition,45 time efficiency, reconstruction quality of images,46 , 47 and accuracy of cardiac magnetic resonance (CMR) segmentation.48 , 49 Machine learning techniques have been applied to improve myocardial tissue characterization and texture analysis50 , 51 and define the heterogeneous nature of the scarred myocardium in late gadolinium enhancement (LGE) CMR images in patients post-myocardial infarction.52
Automated CMR analysis using a convolutional neural network algorithm was shown to be similar in precision to human analysis for measuring left ventricular ejection fraction and left ventricular mass but was 186 times faster.53
In a proof of concept study, Fahmy et al. used U-Net deep convoluted networks with 150 operational layers to quantify scar volumes in patients with HCM. A strong correlation was observed between the manually and automatically segmented scar volumes.54
Cardiac magnetic resonance-defined scar regions have gained considerable importance and form the basis of some of the ablation strategies including scar homogenization and scar de-channelling in ventricular tachycardia (VT) ablation. These advancements have also paved the way for the concept of targeting fibrotic substrate that can perpetuate rotors, in addition to pulmonary vein isolation in patients with persistent AF.
Advances of ML-aided imaging55 have set the stage for the development of several novel concepts in EP including non-invasive localization of arrhythmia foci with high precision, personalized virtual heart modelling including simulation of cardiac arrhythmias and concept of non-invasive ablation.
In parallel with developments in cardiac imaging, further advancements have been made in ECG acquisition with the development of body surface mapping (using up to 252 electrodes instead of standard 12 leads). Electrocardiography imaging systems that integrate body surface mapping with non-contrast computed tomography (CT) that simultaneously records electrode location and geometry of cardiac surface can localize focal activation of atrial or ventricular ectopy on the 3D reconstruction of the patient’s heart using an inverse solution approach.
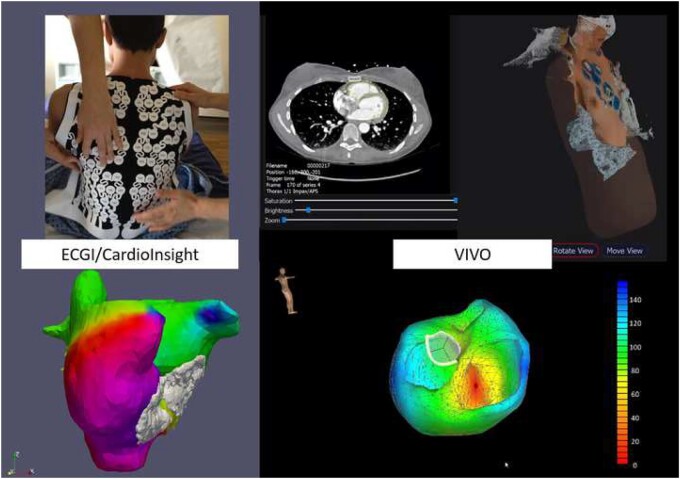
The Amycard 01C (EP Solutions SA, Yverdon-les-Bains, Switzerland)56 and ECVUE (CardioInsight Technologies Inc., Cleveland, OH, USA)57 systems are now commercially available and are able to locate atrial and ventricular arrhythmias. As they provide a simultaneous, quasi global view of the entire atrial or ventricular activation, these systems allow to visualize even AF. Using this technique, focal trigger and rotor sites are identified, which is impossible using the conventional sequential mapping techniques (Figure 4, left).
Figure 4.
Left panel: Example of non-invasive simultaneous mapping of atrial fibrillation of both the right and left atrium using the electrocardiogram imaging technology. Several mechanisms occur in various areas of the atria simultaneously and thereby maintain atrial fibrillation. Right panel: Example of non-invasive simultaneous mapping of ventricular ectopy using the view into ventricular onset technology.
View into ventricular onset (VIVO, Catheter Precision) is a next-generation non-invasive mapping system that combines knowledge of the exact location of the surface 12-lead ECG stickers with carefully reconstructed cardiac anatomy from either CMR or CT imaging (Figure 4, right). With the VIVO platform, prediction of the focus of premature ventricular electrical activity and VT focus is correct in 85% and 88% of patients, respectively.58
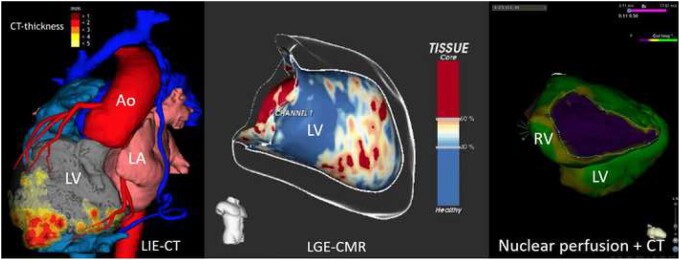
Using a multimodal integrative approach, feasibility of combining body surface mapping, cardiac gated multidetector CT, and/or delayed contrast-enhanced magnetic resonance (MR) imaging on a common platform is shown in Figure 5 (right); this approach was useful in understanding complex accessory pathway previously resistant to ablation and to identify rotor trajectories in patients with AF.59
Figure 5.
Left panel: 3D image information from computed tomography and myocardial thickness in a patient with coronary artery disease and apical scar after myocardial infarction. Middle panel: Image information of late gadolinium enhancement from cardiac magnetic resonance imaging of the left ventricle with identification of the potentially arrhythmogenic channels within the scar responsible for ventricular re-entrant tachycardia. Right panel: Example of perfusion information from functional nuclear imaging superimposed on a contrast computed tomography scan in a patient with arrhythmogenic right ventricular disease. Ao, aorta; CMR, cardiac magnetic resonance; LA, left atrium; LGE, late gadolinium enhancement; LV, left ventricle; RV, right ventricle.
Software (Automatic Detection of Arrhythmic Substrate, ADAS-VT, Galgo Medical SL, Barcelona, Spain), which processes LGE CMR images offline to characterize 3D scar architecture to be merged with electro anatomical mapping (EAM) during the ablation procedure, has been shown to facilitate the ablation procedure60 (Figure 5, left).
With the development of methods for non-invasive localization of arrhythmia focus, several groups have reported on their experience of using radiation therapy in patients with mostly ischaemic ventricular arrhythmia.61–65 Robinson et al. 66 reported successful utilization of radiotherapy in a cohort of patients with treatment-refractory episodes of VT or cardiomyopathy related to premature ventricular contractions. They identified scar regions using ECG imaging, cardiac anatomical imaging and delivered Focused stereotactic body radiation therapy (SBRT) with marked reduction in arrhythmia burden, reduced use of anti-arrhythmic medication and improved quality of life following therapy. Overall survival was 89% and 72% at the end of 6 and 12 months, respectively.
In addition, first-in-man treatment of paroxysmal AF using SBRT in two patients has been reported67 with the demonstration of safety and efficacy of delivering SBRT lesion set in the left atrium confirmed by presence of fibrosis. Results from these initial pilot studies and case reports are encouraging. However, more robust data are required from larger clinical trials with longer follow-up to show long-term safety of these techniques, which are of great interest but still under evaluation.
Personalized virtual heart modelling—a new paradigm
Considerable advances in cellular modelling technology have paved the way for the development of a computerized human cardiac myocyte.68 Virtual ventricular myocytes, with predefined electrophysiological properties, and predictable functional changes in response to surroundings including ion channel changes and myocardial ischaemia have been developed.69–74
Trayanova et al. envisaged the creation of a computerized but personalized virtual heart model.69 , 75–77 Contrast-enhanced MR images of the patient’s heart were used to create near identical geometrical models of the cardiac chambers and were populated with virtual cardiac myocytes with physiological properties pertaining to the cells from a designated location. Previously validated rule-based algorithms were applied for fibre orientation to compute heart models. These computed virtual hearts were electrophysiological twins to the patient’s heart on which various stimulation protocols could be applied to induce ventricular arrhythmias of different morphologies and identify critical isthmus zones for these arrhythmias.
As a proof of concept, this study of virtual heart modelling was used for non-invasive risk assessment of sudden cardiac death in a high-risk population undergoing cardioverter defibrillator implantation. The virtual heart arrhythmia risk predictor approach (VARP) was evaluated retrospectively in a cohort of 41 patients executing simulations to evaluate patient specific VT inducibility and found to be superior to other predictors including left ventricular ejection fraction.78 Predictive capability of this novel targeted approach needs further evaluation in larger studies.
Yet another proof of concept study evaluated the use of virtual heart modelling in patients undergoing VT ablation.77 Virtual hearts were modelled from patients’ contrast-enhanced MR images and VT induction carried out as in the VARP study. Once VT induction was carried out, the optimal ablation strategy was performed virtually. This technique was termed virtual heart arrhythmia ablation targeting (VAAT). When compared retrospectively in 21 patients undergoing VT ablation, it was found to correspond well with real ablation lesions. The VAAT strategy was further tested prospectively in 5 patients undergoing VT ablation in two different centres. VAAT lesions were merged with an EAM system and an ablation was carried out at these sites without further prior mapping. The clinical outcomes for these patients were encouraging with no further VT episodes post-ablation.79
Virtual hearts and machine learning in atrial fibrillation
Machine learning methodologies in conjunction with atrial computational models were used to define re-entrant driver locations in AF.80 Segmented LGE CMR scans were used to identify atrial fibrosis in 21 patients with persistent AF. Fibrotic and non-fibrotic regions were identified and were assigned with region-specific tissue properties. Atrial fibrillation was induced using multisite atrial pacing in these virtual atrial models. Phase mapping with an unsupervised density-based spatial cluster algorithm was used to define re-entrant driver locations. Over 80% of re-entrant driver locations matched to the fibrosis border zones.
The first-in-human clinical study of virtual heart models to guide ablation in patients with persistent AF used personalized atrial geometric models created using segmented LGE MR scans done prior to the procedure.81 Rapid pacing was carried out from 40 uniformly distributed bi-atrial sites. The model response was analysed to determine the optimal ablation lesion set to eliminate all possible persistent re-entrant drivers (identified using above mentioned ML methodologies) sustaining AF and other atrial arrhythmias; the optimal ablation lesion set created using this approach was called OPTIMA: OPtimal Target Identification via Modelling of Arrhythmogenesis was then loaded onto the EAM mapping system and the ablation was carried out without prior mapping. This was a proof of concept feasibility study and was not designed to evaluate procedure outcomes. In fact, outcomes reported from 10 patients were encouraging with no further recurrence of persistent AF.
In a study combining ML and personalized computational modelling, an ML algorithm was shown to predict AF recurrence post-pulmonary vein isolation in patients with paroxysmal AF.82 In this proof of concept study, features were derived from patient’s pre-pulmonary vein isolation LGE MR images and also from the results of AF simulations carried out on their personalized computational model. Random forests were used for unbiased feature selection, and ten-fold nested cross-validation was used to train, validate, and test quadratic discriminant analysis ML classifier. Most predictive features were used as input to this classifier. This ML algorithm predicted post-pulmonary vein isolation AF recurrence with an average validation sensitivity and specificity of 82% and 89%, respectively, and a validation AUC of 0.82.
Intracardiac data and machine learning applications in atrial fibrillation
Large quantities of intracardiac data are recorded during EP procedures. Recent advances in ML methodologies have encouraged researchers to apply these techniques to the intracardiac electrograms and EAM data with a view to define extra pulmonary ablation sites in AF.
Schilling et al. 83 showed the feasibility of classifying complex fractionated atrial electrograms in an objective way using fuzzy decision tree algorithm retrospectively on intracardiac electrograms. Atrial electrograms were classified into four subgroups ranging from non-fractionated with high frequency to continuous activity achieving a correct rate of 81 ± 3%. Electrograms with continuous activity were detected correctly 100% of the time.
In a proof of concept study, McGillivray et al. 84 developed random forest supervised ML algorithm to locate re-entrant circuits driving AF using indirect feature measurements, derived from electrograms in a simulated model. The model correctly identified 95.4% of drivers in the simulation model.
In a recent study, Alhusseini et al. 85 developed an ML algorithm to classify intracardiac electrical patterns during AF. They used a convoluted neural network DL approach to analyse EAM data obtained from bi-atrial sites using basket catheters. Spatial maps of activation were created to identify the presence of rotational activation features. Algorithm compared well with a team of experts with an accuracy of 97.3% when the experts were in unanimous agreement and 85.1% in more difficult instances. Convoluted neural network accuracy in the test set was similar for locations containing termination sites (95.6%) or otherwise (94.2%).
With ML-guided ablation strategies becoming a possibility for the near future, electrophysiologists would need real-time access to integrated data from different sources including EAM and 3D cardiac imaging. Availability of this information with the ability to manipulate the data for better visualization whilst still operating in a sterile field would enhance operator dexterity and procedural efficacy during complex ablations. Holographic visualization of real-time catheter position, cardiac geometry, EAM, and ablation data in an EP lab has been shown to be feasible.86 Systems to provide augmented reality solutions in EP labs are currently being developed for future use87 and would certainly aid in better and efficient work flow.
Robotics in electrophysiology and potential role of machine learning
To deliver catheter ablation safely to a high degree of precision reducing the impact of operator variability in level of training and technical skill, robotic ablation can be seen as a valuable tool to help improve access to the same level of accuracy in a reproducible fashion.
In the field of EP, robotic navigation was introduced 20 years ago. The two concepts proposed were either a mechanical sheath system guiding a conventional ablation catheter via computer-enhanced technique (Hansen & Amigo)88–90 or a magnetic platform (Stereotaxis).89 , 91
A more recent contender is another mechanical system, which uses acoustic energy for both imaging and lesion deployment (Vytronus). 3D reconstruction using ultrasound imaging is performed automatically and the robotic system deploys acoustic energy completely automatically along an operator designed ablation line. First-in-human experience was reported in a cohort of 52 patients with paroxysmal AF undergoing pulmonary vein isolation using low intensity collimated ultrasound (LICU). Acute pulmonary vein isolation was achieved in 77.3% and 94.2% of patients using LICU only and LICU with enhanced software, respectively, with continued freedom from atrial arrhythmia recurrence at 12 months.92
A vast body of evidence has been published for the magnetic navigation system (Niobe, Stereotaxis), which in combination with 3D EAM systems (CARTO or ACUTUS) plus 3D image integration, can be applied to all arrhythmias.93 , 94
Feasibility of using an ML algorithm to guide automated electro-anatomical voltage mapping was previously demonstrated using remote magnetic navigation system. The ML algorithm used learning from demonstration framework utilizing prior knowledge from expert mapping procedures and Gaussian process model-based active learning.95
Non-invasive ML-aided identification of the ablation targets using 3D imaging and personalized heart modelling followed by robotic ablation of these pre-defined locations appears to be an exciting prospect for the future. This approach, if successful, could limit the number of catheters to a minimum and could be both more time and cost-efficient.
Is artificial intelligence bridging the gaps in arrhythmia care?
Recent research into AI-enabled ECG has rekindled interest into observational-based research. Artificial intelligence-enabled ECG has been shown to identify patients with persistent AF, left ventricular systolic dysfunction, and HCM and the list is likely to grow in time with emerging evidence from ongoing research. This ubiquitous cardiac investigation has a potential to be a powerful screening tool at point of care, an innovation that may have a significant impact on community-based diagnosis of latent cardiac conditions, even more so in the under privileged parts of the world.
In an acute setting, AI-enabled ECG may aid in the rapid identification of life threatening electrolyte imbalance and patients at the imminent risk of cardiac arrest who may need more intensive monitoring. Severe restrictions imposed by the recent COVID-19 pandemic resulted in some of the AI-enabled technologies such as QT interval monitoring using AI-enabled mobile devices, approved by regulatory authorities to see the light of the day in clinical practice.96
Advancements in sensor technology and wireless communications with ability to link devices over internet of things have made continuous heart rhythm monitoring feasible, albeit resulting in an exponential increase in data to be analysed. Review of such data by a skilled personnel is nearly impossible due to time and resource constraints. Artificial intelligence solutions can effectively analyse these data in a time and a resource efficient manner. Artificial intelligence-assisted near real-time analysis of data from wearable devices has prompted the contemplation of newer research into novel treatment strategies such as pill in the pocket anticoagulation following an episode of AF.97
Machine learning algorithms have shown their utility to further personalized patient care by improving existing guidelines, which aid in clinical decision-making regarding anticoagulation in the at-risk population and patient selection for cardiac device therapy. Superiority of ML methodologies over traditional rule-based algorithms in handling big data may facilitate data analysis from multiple data sources to identify the impending risk of life threatening arrhythmia or heart failure episodes in a timely manner.
Artificial intelligence-enabled technological advancements are aiding in arrhythmia focus identification prior to EP procedures. In time, AI-enabled ECG may better contribute to accurate localization of accessory pathway or arrhythmia focus. There is a vast potential for the application of ML methodologies to intracardiac data including EAM, for better characterization of an arrhythmia to aid in selection of the ideal ablation strategy.
Limitations and challenges
Machine learning methodologies are not error free, best example being overfitting, a phenomenon resulting from a disproportionate number of features in comparison to the amount of data in the training set.98 As a result of overfitting, the ML algorithm performs very well on the training set whilst performing poorly on the test set with poor generalizability. It has to be appreciated that traditional statistical methods may be superior in analysing lesser amount of data whilst ML offers the ability to handle high computational power to classify large amounts of data efficiently.
Some of the ML methodologies are opaque, and as a consequence, it may not possible to verify how an algorithm arrives at its conclusions. This current lack of transparency in methodology, often referred to as black box nature of ML,99 can affect clinicians’ confidence when applying ML-based technologies in active clinical decision-making. Defining regulatory guidelines for these self-learning, non-transparent yet accurate ML methods can be challenging.
Artificial intelligence is a data science, and hence, the importance of the quality of the data used to train and validate ML algorithms cannot be overstated.100 There is a greater need for collaboration and data sharing between research centres to collate large quantities of robust healthy data to improve the generalizability of ML methodologies. This highlights yet another challenge relating to data security and privacy. More transparency about how data are shared and stricter adherence to data management laws is essential in research involving ML methodologies.
Increasing use of ML algorithms in clinical decision-making101 is likely to challenge the concept of personal responsibility and a physicians fiduciary relationship towards patients,102 necessitating regulatory guidelines to clarify the distribution of liability in the event of mishaps involving AI-aided technologies. Cardiologists in the near term are likely to be keen on AI-aided rather than AI-dictated clinical decision-making for patient management.
Conclusion
Artificial intelligence has considerable impact on all aspects of patient management in the field of cardiac EP from the identification of arrhythmia to therapy (invasively and non-invasively). Simple AI techniques have been established in the form of computerized interpretation of electrocardiography and rule-based algorithms for cardiac devices. Recent advances with the use of DL techniques are paving the way for newer research in arrhythmia detection and arrhythmogenic focus identification. The most recent AI-aided advancements in cardiac imaging invigorated the attempts to develop better non-invasive mapping techniques to guide targeted ablation therapies (invasive and non-invasive). This is a new paradigm shift and promises personalized ‘state of the art’ precision care for patients with complex cardiac arrhythmias. The combination of advanced communication and imaging technologies have helped the rapid adoption of AI techniques and big data research as evidenced by the exponential growth of literature on AI-aided research. It may well be that we are in the midst of an AI-led profound change in patient care. Cardiologists are invited to this new paradigm shift whilst cautiously evaluating the fallout. As is well said ‘with great power comes great responsibility’, it ultimately rests upon the EP community to take responsibility and engage in collaboration.
Supplementary material
Supplementary material is available at European Heart Journal online.
Conflict of interest: J.-L.R. is a Director and shares capital holder of AEMEC—Smart in Media Ltd. S.E. is a consultant to Biosense Webster & Stereotaxis Inc. and has received grants from Catheter Precision and Baylis Medical. All other authors declared no conflict of interest.
Supplementary Material
Contributor Information
Venkat D Nagarajan, Department of Cardiology, Royal Brompton and Harefield NHS Foundation Trust, Sydney Street, London SW3 6NP, UK; Department of Cardiology, Doncaster and Bassetlaw Hospitals, NHS Foundation Trust, Thorne Road, Doncaster DN2 5LT, UK.
Su-Lin Lee, Wellcome/EPSRC Centre for Interventional and Surgical Sciences (WEISS), UCL, Foley Street, London W1W 7TS, UK.
Jan-Lukas Robertus, Department of Pathology, Royal Brompton and Harefield NHS Foundation Trust, Sydney Street, London SW3 6NP, UK; National Heart and Lung Institute, Imperial College London, Guy Scadding Building, Dovehouse St, London SW3 6LY, UK.
Christoph A Nienaber, Department of Cardiology, Royal Brompton and Harefield NHS Foundation Trust, Sydney Street, London SW3 6NP, UK; National Heart and Lung Institute, Imperial College London, Guy Scadding Building, Dovehouse St, London SW3 6LY, UK.
Natalia A Trayanova, Department of Biomedical Engineering, Johns Hopkins University, Charles Street, Baltimore, MD 21218, USA.
Sabine Ernst, Department of Cardiology, Royal Brompton and Harefield NHS Foundation Trust, Sydney Street, London SW3 6NP, UK; National Heart and Lung Institute, Imperial College London, Guy Scadding Building, Dovehouse St, London SW3 6LY, UK.
References
- 1. Turing A. Computing machinery and intelligence. Mind 1950;LIX:433–460. [Google Scholar]
- 2. Moore J. The Dartmouth College Artificial Intelligence Conference: the next fifty years. AI Mag 2006;27:87–91. [Google Scholar]
- 3. Marques E, Filho DS, Fernandes FDA, Lacerda C, Soares DA, Seixas L, Augusto A, Sarmet MD, Gismondi RA, Mesquita ET, Mesquita CT. Artificial intelligence in cardiology: concepts, tools and challenges—“the horse is the one who runs, you must be the jockey”. Arq Bras Cardiol 2020;114:718–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Martin-Isla C, Campello VM, Izquierdo C, Raisi-Estabragh Z, Baeßler B, Petersen SE, Lekadir K. Image-based cardiac diagnosis with machine learning: a review. Front Cardiovasc Med 2020;7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schläpfer J, Wellens HJ. Computer-interpreted electrocardiograms benefits and limitations. J Am Coll Cardiol 2017;70:1183–1192. [DOI] [PubMed] [Google Scholar]
- 6. Acharya UR, Oh SL, Hagiwara Y, Tan JH, Adam M, Gertych A, Tan R. A deep convolutional neural network model to classify heartbeats. Comput Biol Med 2017;89:389–396. [DOI] [PubMed] [Google Scholar]
- 7. Li Q, Rajagopalan C, Clifford G. Ventricular fibrillation and tachycardia classification using a machine learning approach. IEEE Trans Biomed Eng 2014;61:1607–1613. [DOI] [PubMed] [Google Scholar]
- 8. Hannun AY, Rajpurkar P, Haghpanahi M, Tison GH, Bourn C, Turakhia MP, Ng AY. Cardiologist-level arrhythmia detection and classification in ambulatory electrocardiograms using a deep neural network. Nat Med 2019;25:65–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Galloway CD, Valys AV, Shreibati JB, Treiman DL, Petterson FL, Gundotra VP, Albert DE, Attia ZI, Carter RE, Asirvatham SJ, Ackerman MJ, Noseworthy PA, Dillon JJ, Friedman P. Development and validation of a deep-learning model to screen for hyperkalemia from the electrocardiogram. JAMA Cardiol 2019;4:428–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Attia ZI, Kapa S, Lopez-Jimenez F, McKie PM, Ladewig DJ, Satam G, Pellikka PA, Enriquez-Sarano M, Noseworthy PA, Munger TM, Asirvatham SJ, Scott CG, Carter RE, Friedman PA. Screening for cardiac contractile dysfunction using an artificial intelligence-enabled electrocardiogram. Nat Med 2019;25:70–74. [DOI] [PubMed] [Google Scholar]
- 11. Porumb M, Stranges S, Pescapè A, Pecchia L. Precision medicine and artificial intelligence: a pilot study on deep learning for hypoglycemic events detection based on ECG. Sci Rep 2020;10:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dissanayake T, Rajapaksha Y, Ragel R, Nawinne I. An ensemble learning approach for electrocardiogram sensor based human emotion recognition. Sensors 2019;19:4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Attia ZI, Noseworthy PA, Lopez-Jimenez F, Asirvatham SJ, Deshmukh AJ, Gersh BJ, Carter RE, Yao X, Rabinstein AA, Erickson BJ, Kapa S, Friedman P. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: a retrospective analysis of outcome prediction. Lancet 2019;394:861–867. [DOI] [PubMed] [Google Scholar]
- 14. Ko W-Y, Siontis KC, Attia ZI, Carter RE, Kapa S, Ommen SR, Demuth SJ, Ackerman MJ, Gersh BJ, Arruda-Olson AM, Geske JB, Asirvatham SJ, Lopez-Jimenez F, Nishimura RA, Friedman PA, Noseworthy P. Detection of hypertrophic cardiomyopathy using a convolutional neural network-enabled electrocardiogram. J Am Coll Cardiol 2020;75:722–733. [DOI] [PubMed] [Google Scholar]
- 15. Attia ZI, Friedman PA, Noseworthy PA, Lopez-Jimenez F, Ladewig DJ, Satam G, Pellikka PA, Munger TM, Asirvatham SJ, Scott CG, Carter RE, Kapa S. Age and sex estimation using artificial intelligence from standard 12-lead ECGs. Circ Arrhythmia Electrophysiol 2019;12:e007284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Attia ZI, Sugrue A, Asirvatham SJ, Ackerman MJ, Kapa S, Friedman PA, Noseworthy P. Noninvasive assessment of dofetilide plasma concentration using a deep learning (neural network) analysis of the surface electrocardiogram: a proof of concept study. PLoS One 2018;13:e0201059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. William AD, Kanbour M, Callahan T, Bhargava M, Varma N, Rickard J, Saliba W, Wolski K, Hussein A, Lindsay BD, Wazni OM, Tarakji KG. Assessing the accuracy of an automated atrial fibrillation detection algorithm using smartphone technology: the iREAD study. Heart Rhythm 2018;15:1561–1565. [DOI] [PubMed] [Google Scholar]
- 18. Guo Y, Wang H, Zhang H, Liu T, Liang Z, Xia Y, Yan L, Xing Y, Shi H, Li S, Liu Y, Liu F, Feng M, Chen Y, Lip GYH; MAFA II Investigators. Mobile photoplethysmographic technology to detect atrial fibrillation. J Am Coll Cardiol 2019;74:2365–2375. [DOI] [PubMed] [Google Scholar]
- 19. Perez MV, Mahaffey KW, Hedlin H, Rumsfeld JS, Garcia A, Ferris T, Balasubramanian V, Russo AM, Rajmane A, Cheung L, Hung G, Lee J, Kowey P, Talati N, Nag D, Gummidipundi SE, Beatty A, Hills MT, Desai S, Granger CB, Desai M, Turakhia M; Apple Heart Study Investigators. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med 2019;381:1909–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen E, Jiang J, Su R, Gao M, Zhu S, Zhou J, Huo Y. A new smart wristband equipped with an artificial intelligence algorithm to detect atrial fibrillation. Heart Rhythm 2020;17:847–853. [DOI] [PubMed] [Google Scholar]
- 21. Wasserlauf J, You C, Patel R, Valys A, Albert D, Passman R. Smartwatch performance for the detection and quantification of atrial fibrillation. Circ Arrhythmia Electrophysiol 2019;12:e006834. [DOI] [PubMed] [Google Scholar]
- 22. Dörr M, Nohturfft V, Brasier N, Bosshard E, Djurdjevic A, Gross S, Raichle CJ, Rhinisperger M, Stöckli R, Eckstein J. The WATCH AF trial: SmartWATCHes for detection of atrial fibrillation. JACC Clin Electrophysiol 2019;5:199–208. [DOI] [PubMed] [Google Scholar]
- 23. Goldenthal IL, Sciacca RR, Riga T, Bakken S, Baumeister M, Biviano AB, Dizon JM, Wang D, Wang KC, Whang W, Hickey KT, Garan H. Recurrent atrial fibrillation/flutter detection after ablation or cardioversion using the AliveCor KardiaMobile device: iHEART results. Cardiovasc Electrophysiol 2019;30:2220–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reed MJ, Grubb NR, Lang CC, O'Brien R, Simpson K, Padarenga M, Grant A, Tuck S, Keating L, Coffey F, Jones L, Harris T, Lloyd G, Gagg J, Smith JE, Coats T. Multi-centre randomised controlled trial of a smartphone-based event recorder alongside standard care versus standard care for patients presenting to the emergency department with palpitations and pre-syncope: the IPED (Investigation of Palpitations in the ED) study. EClinicalMedicine 2019;8:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Himmelreich JCL, Karregat EPM, Lucassen WAM, van Weert HCPM, de Groot JR, Handoko ML, Nijveldt R, Harskamp R. Diagnostic accuracy of a smartphone-operated, single-lead electrocardiography device for detection of rhythm and conduction abnormalities in primary care. Ann Fam Med 2019;17:403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lau JK, Lowres N, Neubeck L, Brieger DB, Sy RW, Galloway CD, Albert DE, Freedman S. iPhone ECG application for community screening to detect silent atrial fibrillation: a novel technology to prevent stroke. Int J Cardiol 2013;165:193–194. [DOI] [PubMed] [Google Scholar]
- 27. Lowres N, Neubeck L, Salkeld G, Krass I, McLachlan AJ, Redfern J, Bennett AA, Briffa T, Bauman A, Martinez C, Wallenhorst C, Lau JK, Brieger DB, Sy RW, Freedman S. Feasibility and cost-effectiveness of stroke prevention through community screening for atrial fibrillation using iPhone ECG in pharmacies. The SEARCH-AF study. Thromb Haemost 2014;111:1167–1176. [DOI] [PubMed] [Google Scholar]
- 28. Desteghe L, Raymaekers Z, Lutin M, Vijgen J, Dilling-Boer D, Koopman P, Schurmans J, Vanduynhoven P, Dendale P, Heidbuchel H. Performance of handheld electrocardiogram devices to detect atrial fibrillation in a cardiology and geriatric ward setting. Europace 2017;19:29–39. [DOI] [PubMed] [Google Scholar]
- 29. Tarakji KG, Wazni OM, Callahan T, Kanj M, Hakim AH, Wolski K, Wilkoff BL, Saliba W, Lindsay B. Using a novel wireless system for monitoring patients after the atrial fibrillation ablation procedure: the iTransmit study. Heart Rhythm 2015;12:554–559. [DOI] [PubMed] [Google Scholar]
- 30. Tison GH, Sanchez JM, Ballinger B, Singh A, Olgin JE, Pletcher MJ, Vittinghoff E, Lee ES, Fan SM, Gladstone RA, Mikell C, Sohoni N, Hsieh J, Marcus G. Passive detection of atrial fibrillation using a commercially available smartwatch. JAMA Cardiol 2018;3:409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fan YY, Li YG, Li J, Cheng WK, Shan ZL, Wang YT, Guo Y. Diagnostic performance of a smart device with photoplethysmography technology for atrial fibrillation detection: pilot study (Pre-mAFA II Registry). JMIR Mhealth Uhealth 2019;7:e11437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sana F, Isselbacher EM, Singh JP, Heist EK, Pathik B, Armoundas A. Wearable devices for ambulatory cardiac monitoring: JACC state-of-the-art review. J Am Coll Cardiol 2020;75:1582–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Grosan C, Abraham A. Intelligent systems. In: Rule-Based Expert Systems. ISRL. Vol. 17. Berlin, Heidelberg: Springer-Verlag; 2011. p.149–187. [Google Scholar]
- 34. Alonso-Atienza F, Morgado E, Fernández-Martínez L, García-Alberola A, Rojo-Álvarez JL. Detection of life-threatening arrhythmias using feature selection and support vector machines. IEEE Trans Biomed Eng 2014;61:832–840. [DOI] [PubMed] [Google Scholar]
- 35. Li Y, Bisera J, Weil MH, Member S, Tang W. An algorithm used for ventricular fibrillation detection without interrupting chest compression. IEEE Trans Biomed Eng 2012;59:78–86. [DOI] [PubMed] [Google Scholar]
- 36. Figuera C, Irusta U, Morgado E, Aramendi E, Ayala U, Wik L, Kramer-Johansen J, Eftestøl T, Alonso-Atienza F. Machine learning techniques for the detection of shockable rhythms in automated external defibrillators. PLoS One 2016;11:e0159654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nguyen MT, Nguyen BV, Kim K. Deep feature learning for sudden cardiac arrest detection in automated external defibrillators. Sci Rep 2018;8:17196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC, Gillis AM, Granger CB, Hammill SC, Hlatky MA, Joglar JA, Kay GN, Matlock DD, Myerburg RJ, Page R. 2017 AHA/ACC/HRS Guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2018;72:e91–e220. [DOI] [PubMed] [Google Scholar]
- 39. Howard JP, Fisher L, Shun-Shin MJ, Keene D, Arnold AD, Ahmad Y, Cook CM, Moon JC, Manisty CH, Whinnett ZI, Cole GD, Rueckert D, Francis DP. Cardiac rhythm device identification using neural networks. JACC Clin Electrophysiol 2019;5:576–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kalscheur MM, Kipp RT, Tattersall MC, Mei C, Buhr KA, DeMets DL, Field ME, Eckhardt LL, Page C. Machine learning algorithm predicts cardiac resynchronization therapy outcomes: lessons from the COMPANION trial. Circ Arrhythm Electrophysiol 2018;11:e005499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tokodi M, Schwertner WR, Kovács A, Tősér Z, Staub L, Sárkány A, Lakatos BK, Behon A, Boros AM, Perge P, Kutyifa V, Széplaki G, Gellér L, Merkely B, Kosztin A. Machine learning-based mortality prediction of patients undergoing cardiac resynchronization therapy: the SEMMELWEIS-CRT score. Eur Heart J 2020;41:1747–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hu SY, Santus E, Forsyth AW, Malhotra D, Haimson J, Chatterjee NA, Kramer DB, Barzilay R, Tulsky JA, Lindvall C. Can machine learning improve patient selection for cardiac resynchronization therapy? PLoS One 2019;14:e0222397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Feeny AK, Rickard J, Patel D, Toro S, Trulock KM, Park CJ, LaBarbera MA, Varma N, Niebauer MJ, Sinha S, Gorodeski EZ, Grimm RA, Ji X, Barnard J, Madabhushi A, Spragg DD, Chung M. Machine learning prediction of response to cardiac resynchronization therapy: improvement versus current guidelines. Circ Arrhythm Electrophysiol 2019;12:e00731625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Han L, Askari M, Altman RB, Schmitt SK, Fan J, Bentley JP, Narayan SM, Turakhia MP. Atrial fibrillation burden signature and near-term prediction of stroke: a machine learning analysis. Circ Cardiovasc Qual Outcomes 2019;12:e005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Petersen SE, Abdulkareem M, Leiner T. Artificial intelligence will transform cardiac imaging—opportunities and challenges. Front Cardiovasc Med 2019;6:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Qin C, Schlemper J, Caballero J, Price AN, Hajnal JV, Rueckert D. Convolutional recurrent neural networks for dynamic MR image reconstruction. IEEE Trans Med Imaging 2019;38:280–290. [DOI] [PubMed] [Google Scholar]
- 47. Schlemper J, Caballero J, Hajnal JV, Price AN, Rueckert D. A deep cascade of convolutional neural networks for dynamic MR image reconstruction. IEEE Trans Med Imaging 2018;37:491–503. [DOI] [PubMed] [Google Scholar]
- 48. Avendi MR, Kheradvar A, Jafarkhani H. A combined deep-learning and deformable-model approach to fully automatic segmentation of the left ventricle in cardiac MRI. Med Image Anal 2016;30:108–119. [DOI] [PubMed] [Google Scholar]
- 49. Leiner T, Rueckert D, Suinesiaputra A, Baeßler B, Nezafat R, Išgum I, Young AA. Machine learning in cardiovascular magnetic resonance: basic concepts and applications. J Cardiovasc Magn Reson 2019;21:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fahmy AS, El-Rewaidy H, Nezafat M, Nakamori S, Nezafat R. Automated analysis of cardiovascular magnetic resonance myocardial native T1 mapping images using fully convolutional neural networks. J Cardiovasc Magn Reson 2019;21:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fahmy AS, Rausch J, Neisius U, Chan RH, Maron MS, Appelbaum E, Menze B, Nezafat R. Automated cardiac MR scar quantification in hypertrophic cardiomyopathy using deep convolutional neural networks. JACC Cardiovasc Imaging 2018;11:1917–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kotu LP, Engan K, Skretting K, Måløy F, Ørn S, Woie L, Eftestøl T. Probability mapping of scarred myocardium using texture and intensity features in CMR images. Biomed Eng Online 2013;12:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bhuva AN, Bai W, Lau C, Davies RH, Ye Y, Bulluck H, McAlindon E, Culotta V, Swoboda PP, Captur G, Treibel TA, Augusto JB, Knott KD, Seraphim A, Cole GD, Petersen SE, Edwards NC, Greenwood JP, Bucciarelli-Ducci C, Hughes AD, Rueckert D, Moon JC, Manisty C. A multicenter, scan-rescan, human and machine learning CMR study to test generalizability and precision in imaging biomarker analysis. Circ Cardiovasc Imaging 2019;12:e009214. [DOI] [PubMed] [Google Scholar]
- 54. Fahmy AS, Neisius U, Chan RH, Rowin EJ, Manning WJ, Maron MS, Nezafat R. Three-dimensional deep convolutional neural networks for automated myocardial scar quantification in hypertrophic cardiomyopathy: a multicenter multivendor study. Radiology 2020;294:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Al'Aref SJ, Anchouche K, Singh G, Slomka PJ, Kolli KK, Kumar A, Pandey M, Maliakal G, van Rosendael AR, Beecy AN, Berman DS, Leipsic J, Nieman K, Andreini D, Pontone G, Schoepf UJ, Shaw LJ, Chang HJ, Narula J, Bax JJ, Guan Y, Min JK. Clinical applications of machine learning in cardiovascular disease and its relevance to cardiac imaging. Eur Heart J 2019;40:1975–1986. [DOI] [PubMed] [Google Scholar]
- 56. Tsyganov A, Wissner E, Metzner A, Mironovich S, Chaykovskaya M, Kalinin V, Chmelevsky M, Lemes C, Kuck K. Mapping of ventricular arrhythmias using a novel noninvasive epicardial and endocardial electrophysiology system. J Electrocardiol 2018;51:92–98. [DOI] [PubMed] [Google Scholar]
- 57. Cheniti G, Puyo S, Martin CA, Frontera A, Vlachos K, Takigawa M, Bourier F, Kitamura T, Lam A, Dumas-Pommier C, Pillois X, Pambrun T, Duchateau J, Klotz N, Denis A, Derval N, Cochet H, Sacher F, Dubois R, Jais P, Hocini M, Haissaguerre M. Noninvasive mapping and electrocardiographic imaging in atrial and ventricular arrhythmias (CardioInsight). Card Electrophysiol Clin 2019;11:459–471. [DOI] [PubMed] [Google Scholar]
- 58. Misra S, van Dam P, Chrispin J, Assis F, Keramati A, Kolandaivelu A, Berger R, Tandri H. Initial validation of a novel ECGI system for localization of premature ventricular contractions and ventricular tachycardia in structurally normal and abnormal hearts. J Electrocardiol 2018;51:801–808. [DOI] [PubMed] [Google Scholar]
- 59. Cochet H, Dubois R, Sacher F, Derval N, Sermesant M, Hocini M, Montaudon M, Haïssaguerre M, Laurent F, Jaïs P. Cardiac arrhythmias: multimodal assessment integrating body surface ECG mapping into cardiac imaging. Radiology 2014;271:239–247. [DOI] [PubMed] [Google Scholar]
- 60. Bertagnolli L, Torri F, Paetsch I, Jahnke C, Hindricks G, Arya A, Dinov B. Cardiac magnetic resonance imaging for coregistration during ablation of ischemic ventricular tachycardia for identification of the critical isthmus. HeartRhythm Case Rep 2018;4:70–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kim EJ, Davogustto G, Stevenson WG, John RM. Non-invasive cardiac radiation for ablation of ventricular tachycardia: A new therapeutic paradigm in electrophysiology. Arrhythmia Electrophysiol Rev 2018;7:8–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bhaskaran A, Downar E, Chauhan VS, Lindsay P, Nair K, Ha A, Hope A, Nanthakumar K. Electroanatomical mapping–guided stereotactic radiotherapy for right ventricular tachycardia storm. HeartRhythm Case Rep 2019;5:590–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cuculich PS, Schill MR, Kashani R, Mutic S, Lang A, Cooper D, Faddis M, Gleva M, Noheria A, Smith TW, Hallahan D, Rudy Y, Robinson CG. Noninvasive cardiac radiation for ablation of ventricular tachycardia. N Engl J Med 2017;377:2325–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Scholz EP, Seidensaal K, Naumann P, André F, Katus HA, Debus J. Risen from the dead: cardiac stereotactic ablative radiotherapy as last rescue in a patient with refractory ventricular fibrillation storm. HeartRhythm Case Rep 2019;5:329–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Loo BW, Soltys SG, Wang L, Lo A, Fahimian BP, Iagaru A, Norton L, Shan X, Gardner E, Fogarty T, Maguire P, Al-Ahmad A, Zei P. Stereotactic ablative radiotherapy for the treatment of refractory cardiac ventricular arrhythmia. Circ Arrhythmia Electrophysiol 2015;8:748–750. [DOI] [PubMed] [Google Scholar]
- 66. Robinson CG, Samson PP, Moore KMS, Hugo GD, Knutson N, Mutic S, Goddu SM, Lang A, Cooper DH, Faddis M, Noheria A, Smith TW, Woodard PK, Gropler RJ, Hallahan DE, Rudy Y, Cuculich PS. Phase I/II trial of electrophysiology-guided noninvasive cardiac radioablation for ventricular tachycardia. Circulation 2019;139:313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Qian PC, Azpiri JR, Assad J, Gonzales Aceves EN, Cardona Ibarra CE, de la Pena C, Hinojosa M, Wong D, Fogarty T, Maguire P, Jack A, Gardner EA, Zei PC. Noninvasive stereotactic radioablation for the treatment of atrial fibrillation: first-in-man experience. J Arrhythmia 2020;36:67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. O'Hara T, Virág L, Varró A, Rudy Y. Simulation of the undiseased human cardiac ventricular action potential: model formulation and experimental validation. PLoS Comput Biol 2011;7:e1002061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Niederer SA, Lumens J, Trayanova N. Computational models in cardiology. Nat Rev Cardiol 2019;16:100–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Luo CH, Rudy Y. A model of the ventricular cardiac action potential. Depolarization, repolarization, and their interaction. Circ Res 1991;68:1501–1526. [DOI] [PubMed] [Google Scholar]
- 71. Koivumäki JT, Korhonen T, Tavi P. Impact of sarcoplasmic reticulum calcium release on calcium dynamics and action potential morphology in human atrial myocytes: a computational study. PLoS Comput Biol 2011;7:e1001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lascano EC, Said M, Vittone L, Mattiazzi A, Mundiña-Weilenmann C, Negroni J. Role of CaMKII in post acidosis arrhythmias: a simulation study using a human myocyte model. J Mol Cell Cardiol 2013;60:172–183. [DOI] [PubMed] [Google Scholar]
- 73. Saucerman JJ, Healy SN, Belik ME, Puglisi JL, McCulloch A. Proarrhythmic consequences of a KCNQ1 AKAP-binding domain mutation: computational models of whole cells and heterogeneous tissue. Circ Res 2004;95:1216–1224. [DOI] [PubMed] [Google Scholar]
- 74. Nygren A, Fiset C, Firek L, Clark JW, Lindblad DS, Clark RB, Giles WR. Mathematical model of an adult human atrial cell: the role of K+ currents in repolarization. Circ Res 1998;82:63–81. [DOI] [PubMed] [Google Scholar]
- 75. Aronis KN, Ali R, Trayanova N. The role of personalized atrial modeling in understanding atrial fibrillation mechanisms and improving treatment. Int J Cardiol 2019;287:139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Aronis KN, Ali RL, Liang JA, Zhou S, Trayanova N. Understanding AF mechanisms through computational modelling and simulations. Arrhythm Electrophysiol Rev 2019;8:210–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Trayanova NA. How personalized heart modeling can help treatment of lethal arrhythmias: a focus on ventricular tachycardia ablation strategies in post-infarction patients. Wiley Interdiscip Rev Syst Biol Med 2020;12:e1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Arevalo HJ, Vadakkumpadan F, Guallar E, Jebb A, Malamas P, Wu KC, Trayanova N. Arrhythmia risk stratification of patients after myocardial infarction using personalized heart models. Nat Commun 2016;7:11437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Prakosa A, Arevalo HJ, Deng D, Boyle PM, Nikolov PP, Ashikaga H, Blauer JJE, Ghafoori E, Park CJ, Blake RC 3rd, Han FT, MacLeod RS, Halperin HR, Callans DJ, Ranjan R, Chrispin J, Nazarian S, Trayanova N. Personalized virtual-heart technology for guiding the ablation of infarct-related ventricular tachycardia. Nat Biomed Eng 2018;2:732–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zahid S, Cochet H, Boyle PM, Schwarz EL, Whyte KN, Vigmond EJ, Dubois R, Hocini M, Haïssaguerre M, Jaïs P, Trayanova N. Patient-derived models link re-entrant driver localization in atrial fibrillation to fibrosis spatial pattern. Cardiovasc Res 2016;110:443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Boyle PM, Zghaib T, Zahid S, Ali RL, Deng D, Franceschi WH, Hakim JB, Murphy MJ, Prakosa A, Zimmerman SL, Ashikaga H, Marine JE, Kolandaivelu A, Nazarian S, Spragg DD, Calkins H, Trayanova N. Computationally guided personalized targeted ablation of persistent atrial fibrillation. Nat Biomed Eng 2019;3:870–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Shade JK, Ali RL, Basile D, Popescu D, Akhtar T, Marine JE, Spragg DD, Calkins H, Trayanova N. Pre-procedure application of machine learning and mechanistic simulations predicts likelihood of paroxysmal atrial fibrillation recurrence following pulmonary vein isolation. Circ Arrhythm Electrophysiol 2020;13:e008213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Schilling C, Keller M, Scherr D, Oesterlein T, Haïssaguerre M, Schmitt C, Dössel O, Luik A. Fuzzy decision tree to classify complex fractionated atrial electrograms. Biomed Tech 2015;60:245–255. [DOI] [PubMed] [Google Scholar]
- 84. McGillivray MF, Cheng W, Peters NS, Christensen K. Machine learning methods for locating re-entrant drivers from electrograms in a model of atrial fibrillation. R Soc Open Sci 2018;5:172434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Alhusseini MI, Abuzaid F, Rogers AJ, Zaman JAB, Baykaner T, Clopton P, Bailis P, Zaharia M, Wang PJ, Rappel WJ, Narayan S. Machine learning to classify intracardiac electrical patterns during atrial fibrillation: machine learning of atrial fibrillation. Circ Arrhythm Electrophysiol 2020;13:e008160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Silva JNA, Southworth M, Raptis C, Silva J. Emerging applications of virtual reality in cardiovascular medicine. JACC Basic Transl Sci 2018;3:420–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Southworth MK, Silva JR, Silva JNA. Use of extended realities in cardiology. Trends Cardiovasc Med 2020;30:143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Al-Ahmad A, Grossman JD, Wang PJ. Early experience with a computerized robotically controlled catheter system. J Interv Card Electrophysiol 2005;12:199–202. [DOI] [PubMed] [Google Scholar]
- 89. Faddis MN, Blume W, Finney J, Hall A, Rauch J, Sell J, Bae KT, Talcott M, Lindsay B. Novel, magnetically guided catheter for endocardial mapping and radiofrequency catheter ablation. Circulation 2002;106:2980–2985. [DOI] [PubMed] [Google Scholar]
- 90. Scarà A, Sciarra L, De Ruvo E, Borrelli A, Grieco D, Palamà Z, Golia P, De Luca L, Rebecchi M, Calò L. Safety and feasibility of atrial fibrillation ablation using Amigo® system versus manual approach: a pilot study. Indian Pacing Electrophysiol J 2018;18:61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ernst S, Ouyang F, Linder C, Hertting K, Stahl F, Chun J, Hachiya H, Bänsch D, Antz M, Kuck KH. Initial experience with remote catheter ablation using a novel magnetic navigation system: magnetic remote catheter ablation. Circulation 2004;109:1472–1475. [DOI] [PubMed] [Google Scholar]
- 92. Turagam MK, Petru J, Neuzil P, Kakita K, Kralovec S, Harari D, Phillips P, Piazza D, Whang W, Dukkipati SR, Reddy V. Automated noncontact ultrasound imaging and ablation system for the treatment of atrial fibrillation: outcomes of the first-in-human VALUE trial. Circ Arrhythm Electrophysiol 2020;13:e007917. [DOI] [PubMed] [Google Scholar]
- 93. Ernst S, Babu-Narayan SV, Keegan J, Horduna I, Lyne J, Till J, Kilner PJ, Pennell D, Rigby ML, Gatzoulis MA. Remote-controlled magnetic navigation and ablation with 3D image integration as an alternative approach in patients with intra-atrial baffle anatomy. Circ Arrhythmia Electrophysiol 2012;5:131–139. [DOI] [PubMed] [Google Scholar]
- 94. Reddy VY, Neuzil P, Malchano ZJ, Vijaykumar R, Cury R, Abbara S, Weichet J, McPherson CD, Ruskin JN. View-synchronized robotic image-guided therapy for atrial fibrillation ablation: experimental validation and clinical feasibility. Circulation 2007;115:2705–2714. [DOI] [PubMed] [Google Scholar]
- 95. Feng Y, Guo Z, Dong Z, Zhou XY, Kwok KW, Ernst S, Lee SL. An efficient cardiac mapping strategy for radiofrequency catheter ablation with active learning. Int J Comput Assist Radiol Surg 2017;12:1199–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Krittanawong C, Rogers AJ, Johnson KW, Wang Z, Turakhia MP, Halperin JL, Narayan SM. Integration of novel monitoring devices with machine learning technology for scalable cardiovascular management. Nat Rev Cardiol 2021;18:75–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Stavrakis S, Stoner JA, Kardokus J, Garabelli PJ, Po SS, Lazzara R. Intermittent vs. Continuous Anticoagulation theRapy in patiEnts with Atrial Fibrillation (iCARE-AF): a randomized pilot study. J Interv Card Electrophysiol 2017;48:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Krittanawong C, Johnson KW, Rosenson RS, Wang Z, Aydar M, Baber U, Min JK, Tang WHW, Halperin JL, Narayan SM. Deep learning for cardiovascular medicine: a practical primer. Eur Heart J 2019;40:2058–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Castelvecchi D. Can we open the black box of AI? Nature 2016;538:20–23. [DOI] [PubMed] [Google Scholar]
- 100. Goldstein BA, Navar AM, Carter RE. Moving beyond regression techniques in cardiovascular risk prediction: applying machine learning to address analytic challenges. Eur Heart J 2017;38:1805–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Motwani M, Dey D, Berman DS, Germano G, Achenbach S, Al-Mallah MH, Andreini D, Budoff MJ, Cademartiri F, Callister TQ, Chang HJ, Chinnaiyan K, Chow BJ, Cury RC, Delago A, Gomez M, Gransar H, Hadamitzky M, Hausleiter J, Hindoyan N, Feuchtner G, Kaufmann PA, Kim YJ, Leipsic J, Lin FY, Maffei E, Marques H, Pontone G, Raff G, Rubinshtein R, Shaw LJ, Stehli J, Villines TC, Dunning A, Min JK, Slomka PJ. Machine learning for prediction of all-cause mortality in patients with suspected coronary artery disease: a 5-year multicentre prospective registry analysis. Eur Heart J 2017;38:500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Char DS, Shah NH, Magnus D. Implementing machine learning in health care—addressing ethical challenges. N Engl J Med 2018;378:981–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.