Abstract
Aims
Ivabradine has been used in patients who have chronic heart failure (HF) with reduced ejection fraction (HFrEF) and concomitant sinus heart rate ≥70 bpm. This administration for acute HFrEF remains a concern. This study used a real‐world multicentre database to investigate the effects of ivabradine among patients with acute decompensated HFrEF before discharge.
Methods and results
This study retrospectively identified patients with acute decompensated HFrEF who were administered ivabradine at discharge from two multicentre HF databases. Propensity score matching was performed to adjust for confounders. Cardiovascular mortality, all‐cause mortality, and recurrent HF rehospitalization risks were then compared between those with and without ivabradine treatment. After 1:2 propensity score matching, 876 patients (age, 60.7 ± 14.6 years; female, 23.2%; left ventricular ejection fraction, 28.2% ± 7.8%; and heart rate at discharge, 84.3 ± 13.8 bpm) were included in the final analysis, including 292 and 584 patients with and without ivabradine treatment at discharge, respectively. No significant differences were observed in baseline characteristics between the two groups. At 1 year follow‐up, patients in the ivabradine group had significantly lower heart rates (77.6 ± 14.7 vs. 81.1 ± 16.3 bpm; P = 0.005) and lower HF severity symptoms (New York Heart Association Functional class, 2.1 ± 0.7 vs. 2.3 ± 0.9; P < 0.001) than those from the non‐ivabradine group. Ivabradine users had significantly lower risks of 1 year cardiovascular mortality (5.8 vs. 12.2 per 100‐person year; P = 0.003), all‐cause mortality (7.2 vs. 14.0 per 100‐person year; P = 0.003), and total HF rehospitalization (42.3 vs. 72.6 per 100‐person year; P < 0.001) than non‐ivabradine users. Following multivariate analysis, the predischarge prescription of ivabradine remained independently associated with lower 1 year all‐cause mortality (hazard ratio, 0.45; 95% confidence interval, 0.28–0.74; P = 0.002) and cardiovascular mortality (hazard ratio, 0.41; 95% confidence interval, 0.24–0.72; P = 0.002).
Conclusions
The current study findings suggest that ivabradine treatment is associated with reduced risks of cardiovascular mortality, all‐cause mortality, and HF rehospitalization within 1 year among patients with acute decompensated HFrEF in real‐world populations.
Keywords: Heart failure, Hospitalization, Ivabradine, Mortality, Real‐world
Introduction
Heart failure (HF) is a global public health concern owing to substantial resource consumption. In addition to inflicting high mortality, HF adversely impacts the quality of life. 1 Repeated hospitalizations for HF that occur shortly after discharge has become a particularly troublesome problem. 2 Despite advances in HF treatment, rehospitalization rates and mortality remain high, resulting in a heavy social and economic burden. 3 , 4 , 5 Recent data demonstrated that the prevalence of HF in Southeast Asia is similar to that in Western countries, with 30‐day rehospitalization rates ranging from 3% to 15%. 6 In addition, 1 year all‐cause mortality rates after acute decompensated HF hospitalization ranged between 9.2% and 37.5%. 7 In a recently published United States Registry enrolling >10 000 patients, 56% of the patients were rehospitalized within 30 days because of worsening HF events. However, the use of standard‐of‐care therapies both before and after the onset of worsening HF is low. 8 These findings highlight the importance of adequate patient education, greater optimizations of existing guideline‐recommended therapy, and novel pharmacological strategies.
Oral disease‐modifying HF therapy, including angiotensin‐converting enzyme inhibitors (ACEi), angiotensin receptor blockers (ARB), beta‐blockers, and mineralocorticoid receptor antagonists (MRA), should be continued and/or initiated once achieving haemodynamic stabilization during acute HF hospitalization, based on guideline recommendation. 9 The SHIFT (Systolic Heart failure treatment with If inhibitor ivabradine Trial) trial demonstrated that ivabradine contributes to beneficial effects in patients with sinus rhythm and a heart rate of ≥70 beats per minute (bpm). 10 Nevertheless, the SHIFT study enrolled patients who had been hospitalized for HF within the previous 12 months but not within the preceding 4 weeks. Approximately 73% of patients hospitalized because of HF and reduced ejection fraction (HFrEF) had a heart rate of ≥70 bpm at discharge and significantly higher 1 year all‐cause mortality, rehospitalization rate, and corresponding medical costs. 11 Hence, the optimization of heart rate control in the post‐acute phase of HFrEF is a crucial issue. Because the SHIFT trial did not include patients who were discharged from hospitalization for acute decompensated HF, the clinical benefits of ivabradine on these patients were less clear. Against this background, two Taiwanese multicentre cohorts of patients with HF were utilized to evaluate the effects of ivabradine prescribed before discharge among patients who were hospitalized for acute decompensated HFrEF.
Methods
Study designs and patient characteristics
The present study extracted and analysed data from two multicentre HF cohorts in Taiwan: (1) the TSOC‐HFrEF registry initiated by the Taiwan Society of Cardiology, which contains data on a prospective, multicentre, and observational survey of 1,509 patients with HFrEF recently admitted in 21 hospitals in Taiwan for HF from 2013 to 2014, 12 and (2) a principal investigator‐initiated multicentre and retrospective HF study, which comprised 1845 patients with HFrEF from 10 hospitals between 2016 and 2018. 13 The definition of acute decompensated HF refers to the rapid onset or worsening of symptoms and/or signs of HF (e.g. fluid retention and/or reduced cardiac output with peripheral hypoperfusion). 9 The inclusion criteria for the current study were (i) >20‐year‐old male or female patients with symptomatic HFrEF, (ii) patients discharged from hospitalization for acute decompensated HF, and (iii) patients having a sinus rhythm with a resting heart rate of ≥70 bpm at discharge. The exclusion criteria included patients who refused medical advice or were lost to follow‐up and had a non‐sinus rhythm (atrial pacing, atrial fibrillation, or atrial flutter) or a sinus rhythm with a resting heart rate of <70 bpm. The eligible patients were further divided into two groups according to ivabradine prescription at discharge (ivabradine and non‐ivabradine groups). The flowchart of the current study is shown in Figure 1 .
Figure 1.
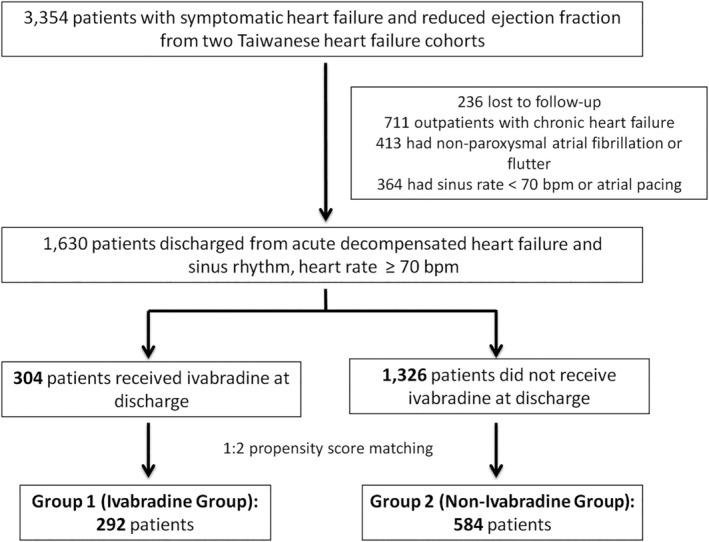
The flowchart of current study.
The protocols of the two HF cohorts were similar, and 50 variables per patient in both cohorts were obtained during index HF hospitalization, including age, gender, body mass index, HF aetiologies, systolic blood pressure, heart rate, New York Heart Association (NYHA) functional class, left ventricular ejection fraction (LVEF), estimated glomerular filtration rate (eGFR), comorbidities, drug therapy, laboratory data, and cardiac device use. This study complied with the ethical principles of the Declaration of Helsinki and was approved by the institutional ethics committee of each hospital.
Study outcomes
Three clinical outcomes were identified in the study during 1 year follow‐up as follows: mortality from cardiovascular causes, all‐cause mortality, and hospital rehospitalization owing to HF. Data on hospital rehospitalization for HF were collected within 6 months and between 6 and 12 months after discharge. The frequencies of HF rehospitalization were categorized into 0, 1, 2, and ≥3 times.
Statistical analyses
Continuous and categorical variables are expressed as the mean values ± standard deviations and percentages, respectively. Propensity score matching was performed to adjust for confounders. Propensity was estimated using a logistic regression model with the following covariates: age, gender, body mass index, systolic blood pressure, heart rate, and NYHA functional class at discharge, LVEF, eGFR, HF aetiology, and 13 comorbidities (hypertension, diabetes mellitus, dyslipidaemia, peripheral arterial disease, atrial fibrillation, chronic obstructive pulmonary disease (COPD) or asthma, chronic kidney disease, sleep apnoea, history of stroke, thyroid disorder, prior history of myocardial infarction, malignancy, and depression). Because more patients did not receive ivabradine, each patient in the ivabradine group was matched with two patients in the non‐ivabradine group (1:2 matching). In the matching process, the greedy, nearest‐neighbour method without replacement and with a calliper of 0.01 of the propensity score was used.
Differences in baseline characteristics and clinical parameters were tested using the χ 2 test for categorical variables, and continuous data were compared using Student's t test or the Mann–Whitney U test. The risks of cardiovascular mortality and all‐cause mortality were analysed using survival analysis with the Kaplan–Meier method and log‐rank test. Because the baseline HF treatment between the two groups was significantly different, subgroup and multivariate Cox regression analyses were performed to assess the consistency of the treatment effects of ivabradine and evaluate the influence of each treatment on clinical outcomes. A P value of <0.05 was considered statistically significant, and statistical analyses were performed using IBM SPSS Statistics 24.0 software (IBM SPSS, IBM Corp., Armonk, NY, USA).
Results
Baseline characteristics
This study included 1,630 patients with HFrEF discharged from hospitalization for acute decompensated HF with a sinus rate of ≥70 bpm. Among these patients, 304 received ivabradine at discharge (ivabradine group), whereas 1,326 patients did not receive ivabradine at discharge (non‐ivabradine group). Patients in the non‐ivabradine group were significantly older, had a higher measurement of heart rate at discharge, and were prone to have an associated history of paroxysmal atrial fibrillation before propensity score matching. By contrast, patients in the ivabradine group were more likely to have a history of myocardial infarction and dyslipidaemia. After 1:2 propensity score matching, 876 patients were included in the final analysis. The mean age of the study subjects and the mean LVEF were 60.7 years and 28.2%, respectively. Overall, the two matched cohorts were well balanced. Table 1 shows the detailed baseline characteristics of both cohorts before and after propensity score matching.
Table 1.
Baseline characteristics among patients with different groups
| Before propensity score matching | After propensity score matching | |||||
|---|---|---|---|---|---|---|
| IVA group (n = 304) | Non‐IVA group (n = 1326) | P value | IVA group (n = 292) | Non‐IVA group (n = 584) | P value | |
| Age (year) | 60.1 ± 14.9 | 62.4 ± 15.3 | 0.018 | 60.5 ± 14.9 | 60.8 ± 14.4 | 0.764 |
| Male gender, n (%) | 226 (76.9) | 967 (72.9) | 0.165 | 224 (76.7) | 449 (76.9) | 0.955 |
| BMI (kg/m2) | 25.4 ± 5.6 | 25.3 ± 4.9 | 0.659 | 25.4 ± 5.6 | 25.1 ± 5.1 | 0.433 |
| Length of stay (day) | 13.6 ± 24.4 | 12.7 ± 13.1 | 0.437 | 13.6 ± 24.6 | 12.4 ± 11.9 | 0.342 |
| Admission SBP (mmHg) | 129.1 ± 21.2 | 130.6 ± 25.2 | 0.286 | 129.2 ± 21.2 | 129.9 ± 25.5 | 0.675 |
| Admission HR (bpm) | 92.9 ± 16.1 | 94.7 ± 19.9 | 0.092 | 92.8 ± 16.1 | 93.4 ± 20.0 | 0.622 |
| Discharge SBP (mmHg) | 119.5 ± 20.7 | 120.4 ± 19.0 | 0.487 | 119.6 ± 20.7 | 119.7 ± 20.2 | 0.967 |
| Discharge HR (bpm) | 83.7 ± 14.7 | 86.0 ± 12.2 | 0.010 | 83.5 ± 15.0 | 84.7 ± 13.1 | 0.248 |
| Discharge NYHA Fc | 2.6 ± 0.7 | 2.6 ± 0.7 | 0.101 | 2.6 ± 0.7 | 2.6 ± 0.7 | 0.225 |
| LVEF (%) | 28.2 ± 7.2 | 28.5 ± 8.1 | 0.488 | 28.2 ± 7.3 | 28.2 ± 8.1 | 0.930 |
| eGFR (ml/min/1.73m2) | 65.7 ± 47.4 | 61.4 ± 35.1 | 0.073 | 65.5 ± 48.0 | 63.9 ± 32.6 | 0.573 |
| ICMP, n (%) | 152 (50.0) | 628 (47.4) | 0.406 | 140 (47.9) | 280 (47.9) | 1.000 |
| Comorbidities, n (%) | ||||||
| Diabetes mellitus | 156 (51.3) | 611 (46.1) | 0.099 | 150 (51.4) | 301 (51.5) | 0.962 |
| Hypertension | 166 (54.6) | 719 (54.3) | 0.904 | 160 (54.8) | 312 (53.4) | 0.701 |
| Prior myocardial infarction | 103 (33.9) | 364 (27.5) | 0.025 | 97 (33.2) | 173 (29.6) | 0.277 |
| PAD | 24 (7.9) | 99 (7.5) | 0.799 | 24 (8.2) | 45 (7.7) | 0.790 |
| Prior stroke | 27 (8.9) | 128 (9.7) | 0.679 | 27 (9.2) | 51 (8.7) | 0.801 |
| Paroxysmal atrial fibrillation | 40 (13.2) | 249 (18.8) | 0.019 | 40 (13.7) | 89 (15.2) | 0.544 |
| Dyslipidaemia | 141 (46.4) | 519 (39.2) | 0.020 | 129 (44.2) | 269 (46.1) | 0.598 |
| COPD | 36 (11.8) | 157 (11.8) | 0.999 | 36 (12.3) | 84 (14.4) | 0.404 |
| Chronic kidney disease | 113 (37.2) | 456 (34.4) | 0.359 | 107 (36.6) | 219 (37.5) | 0.805 |
| History of thyroid disease | 21 (6.9) | 69 (5.2) | 0.241 | 21 (7.2) | 42 (7.2) | 1.000 |
| Sleep apnoea | 10 (3.3) | 36 (2.7) | 0.585 | 10 (3.4) | 17 (2.9) | 0.678 |
| History of malignancy | 21 (6.9) | 62 (4.7) | 0.110 | 19 (6.5) | 40 (6.8) | 0.849 |
| Depression | 9 (3.0) | 28 (2.1) | 0.370 | 9 (3.1) | 10 (1.7) | 0.189 |
| Heart failure treatment, n (%) | ||||||
| RASi | 241 (79.3) | 943 (71.1) | 0.004 | 235 (80.5) | 415 (71.1) | 0.003 |
| ACEi/ARB | 125 (41.1) | 713 (53.8) | <0.001 | 124 (42.5) | 310 (53.1) | 0.003 |
| Sacubitril/valsartan | 116 (38.2) | 230 (17.3) | <0.001 | 111 (38.0) | 105 (18.0) | <0.001 |
| Beta blocker | 209 (68.8) | 856 (64.6) | 0.124 | 205 (70.2) | 398 (68.2) | 0.536 |
| MRA | 215 (70.7) | 689 (52.0) | <0.001 | 209 (71.6) | 303 (51.9) | <0.001 |
| Digoxin | 47 (15.5) | 310 (23.4) | 0.003 | 47 (16.1) | 140 (24.0) | 0.007 |
| CRT | 21 (6.9) | 33 (2.5) | <0.001 | 21 (7.2) | 14 (2.4) | 0.001 |
| ICD | 32 (10.5) | 48 (3.6) | <0.001 | 32 (11.0) | 24 (4.1) | <0.001 |
Abbreviations: ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; COPD, chronic obstructive pulmonary diseases; CRT, cardiac resynchronization therapy; eGFR, estimated glomerular filtration rate; HR, heart rate; ICD, Implantable cardioverter defibrillator; ICMP, ischaemic cardiomyopathy; IVA, ivabradine; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NYHA Fc, New York Heart Association Functional class; PAD, peripheral artery diseases; RASi, renin–angiotensin system inhibitor; SBP, systolic blood pressure.
Heart failure medications and device therapies at discharge
The prescription rates of beta‐blockers (70.2% vs. 68.2%, P = 0.536), loop diuretics (68.8% vs. 73.1%, P = 0.185), and anticoagulants (15.4% vs. 15.2%, P = 0.947) were similar between the two groups at discharge. Patients in the matched ivabradine group were more likely to receive ACEi, ARB, or sacubitril/valsartan (80.5% vs. 71.1%, P = 0.003); sacubitril/valsartan (38.0% vs. 18.0%, P < 0.001); or MRA (71.6% vs. 51.9%, P < 0.001), whereas patients in the non‐ivabradine group were more likely to receive ACEi/ARB (53.1% vs. 42.5%, P = 0.003), digoxin (24.0% vs. 16.1%, P = 0.007), and amiodarone (14.2% vs. 8.9%, P = 0.025). Compared with patients in the non‐ivabradine group, patients in the ivabradine group were more likely to receive cardiac device implantation, including cardiac resynchronization therapy (7.2% vs. 2.4%, P = 0.001) and implantable cardioverter defibrillator (11.0% vs. 4.1%, P < 0.001). Despite discrepancies in heart rate‐lowering regimens at discharge, patients in both groups had comparable heart rate measurements at discharge (83.5 ± 15.0 vs. 84.7 ± 13.1 bpm, P = 0.248).
The prescription rates and dosages of renin–angiotensin system inhibitors, beta‐blockers, MRAs, and ivabradine at discharge, 6 months, and 12 months after index hospitalization are shown in Figure 2 . Renin–angiotensin system inhibitor and beta‐blocker uptitrations were significant in both groups at 1 year follow‐up (all P values <0.01). There was no significant change in the prescription patterns of MRA at 1 year follow‐up in both groups. Ivabradine was initiated in 5.3% of the patients in the non‐ivabradine group and discontinued in 16.8% of the patients in the ivabradine group at 1‐year follow‐up.
Figure 2.
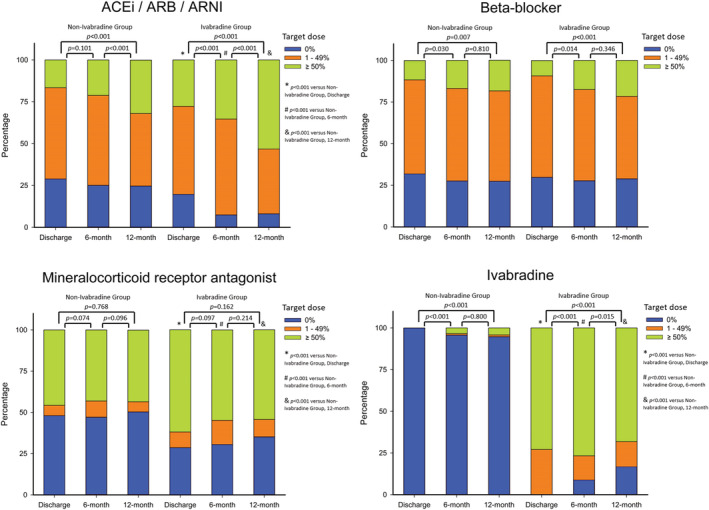
Types and dosages of disease‐modifying medications for heart failure among study patients over time. ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor.
Clinical outcomes
The overall incidence of cardiovascular mortality was 10.0 per 100‐person year at 1 year follow‐up. The incidences of cardiovascular mortality were 5.8 and 12.2 per 100‐person year for the matched ivabradine and non‐ivabradine groups, respectively [hazard ratio (HR), 0.45; 95% confidence interval (CI), 0.26–0.76; P = 0.003; Figure 3 A ]. The incidences of mortality from any causes in patients in the matched ivabradine and non‐ivabradine groups were 7.2 and 14.0 per 100‐person year, respectively (HR, 0.48; 95% CI, 0.30–0.77; P = 0.003; Figure 3 B ).
Figure 3.
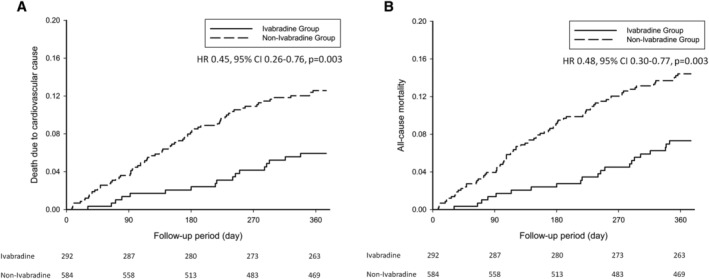
Kaplan–Meier curves of death from cardiovascular causes (A), and death from any causes (B) within 1 year in study patients.CI, confidence interval; HR, hazard ratio.
During the first 6 months after index HF hospitalization, 319 rehospitalizations for HF occurred in 221 patients. Moreover, 20.3% and 27.7% of the patients in the matched ivabradine and non‐ivabradine groups experienced rehospitalization for HF at least once within 6 months after index hospitalization, respectively (P = 0.004). Between 6 and 12 months after index HF hospitalization, 187 rehospitalizations for HF occurred in 132 patients. Furthermore, 12.2% and 19.5% of the patients in the matched ivabradine and non‐ivabradine groups, respectively, experienced rehospitalization for HF at least once between 6 and 12 months following index hospitalization (P = 0.007). Figure 4 A shows the significantly lower incidence of first and repeated HF rehospitalizations in patients in the matched ivabradine group than that in patients in the non‐ivabradine group (the odds ratio for the first unplanned HF rehospitalization within 1 year was 0.65; 95% CI, 0.48–0.89; P = 0.006).
Figure 4.
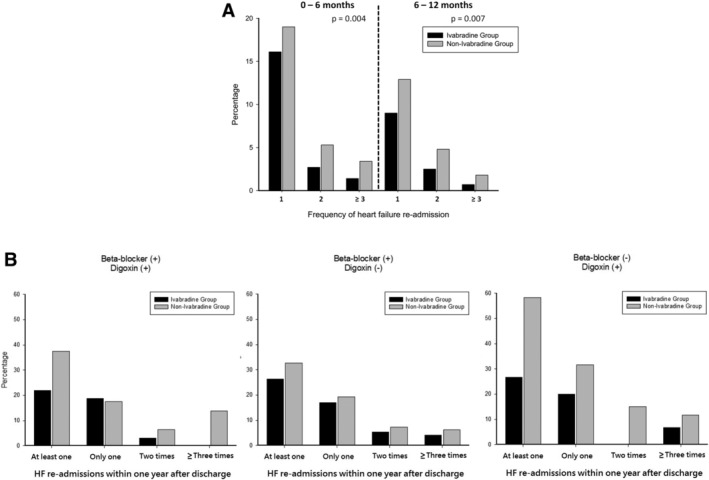
Frequencies of heart failure (HF) re‐admission following index hospitalization within 1 year (A), and stratified by baseline prescription of beta‐blocker and digoxin (B).
Clinical outcomes in different background HF therapies
Among patients with concomitant background beta‐blocker treatment, patients who received ivabradine treatment had a significantly lower risk of cardiovascular mortality than those who did not receive ivabradine (3.9 vs. 9.5 per 100‐person year; HR, 0.39; 95% CI, 0.18–0.83; P = 0.011). The favourable outcomes of ivabradine in cardiovascular mortality were consistent across the variable examined subgroups of different background HF medications and implantable devices (Figure 5 ). The incidences of cardiovascular mortality were similar among patients not on ivabradine treatment between the 2013–2014 and 2016–2018 cohorts (P = 0.540).
Figure 5.
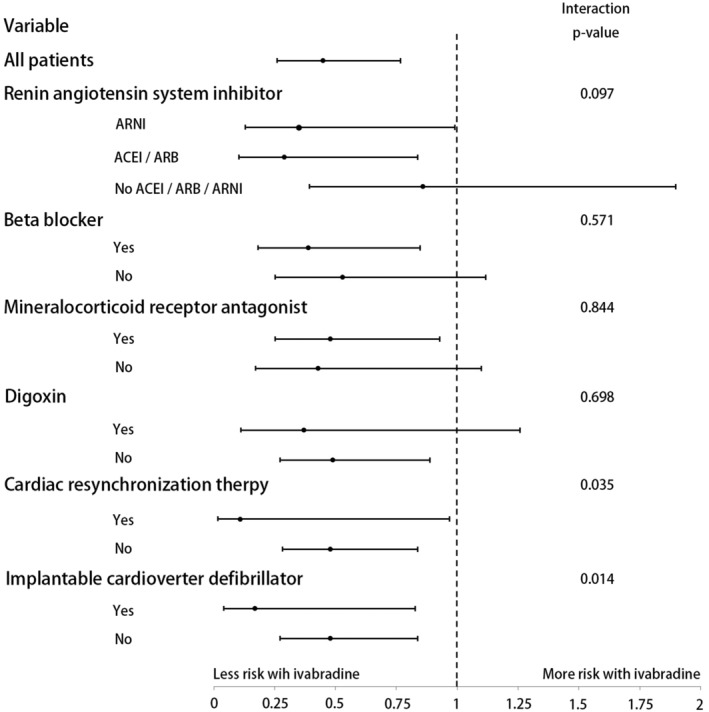
Hazard ratio of cardiovascular death according to heart failure treatment subgroups in two cohorts. ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor.
Table 2 demonstrates the univariate and multivariate Cox regression analyses for baseline HF treatments associated with 1‐year outcomes. The prescription of ivabradine at discharge was independently associated with a lower risk of 1‐year all‐cause mortality (HR, 0.45; 95% CI, 0.28–0.74; P = 0.002). Beta‐blocker and renin–angiotensin system inhibitor use at discharge were also independently associated with better survival (HR, 0.59; 95% CI, 0.40–0.88; P = 0.009 for beta‐blockers and HR, 0.58; 95% CI, 0.38–0.86; P = 0.008 for renin–angiotensin system inhibitors). Furthermore, the prescriptions of ivabradine, renin–angiotensin system inhibitors, and beta‐blockers at discharge were independently associated with a lower risk of 1 year cardiovascular mortality (Table 2 ).
Table 2.
Multivariate analysis for heart failure treatments associated with one‐year outcomes following index heart failure hospitalization
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% confidence interval | P value | Hazard ratio | 95% confidence interval | P value | |
| All‐cause mortality | ||||||
| Ivabradine | 0.48 | 0.30–0.77 | 0.003 | 0.45 | 0.28–0.74 | 0.002 |
| RASi | 0.51 | 0.35–0.76 | 0.001 | 0.58 | 0.38–0.86 | 0.008 |
| Beta‐blocker | 0.45 | 0.31–0.66 | <0.001 | 0.59 | 0.40–0.88 | 0.009 |
| MRA | 0.66 | 0.45–0.97 | 0.034 | NS | NS | NS |
| Digoxin | 1.33 | 0.86–2.06 | 0.206 | NS | NS | NS |
| CRT | 1.49 | 0.65–3.39 | 0.345 | NS | NS | NS |
| ICD | 1.50 | 0.76–2.97 | 0.246 | NS | NS | NS |
| Cardiovascular death | ||||||
| Ivabradine | 0.45 | 0.26–0.76 | 0.003 | 0.41 | 0.24–0.72 | 0.002 |
| RASi | 0.49 | 0.32–0.76 | 0.001 | 0.54 | 0.35–0.84 | 0.006 |
| Beta‐blocker | 0.48 | 0.32–0.73 | 0.001 | 0.65 | 0.42–0.99 | 0.047 |
| MRA | 0.77 | 0.51–1.17 | 0.225 | NS | NS | NS |
| Digoxin | 1.48 | 0.93–2.35 | 0.097 | NS | NS | NS |
| CRT | 1.76 | 0.77–4.02 | 0.183 | NS | NS | NS |
| ICD | 1.78 | 0.90–3.56 | 0.100 | NS | NS | NS |
Multivariate analysis was adjusted for age, gender, heart failure aetiology, body mass index, length of stay, left ventricular ejection fraction, systolic blood pressure, heart rate, estimated glomerular filtration rate, New York Heart Association functional class at discharge, history of heart failure hospitalization, atrial fibrillation, hypertension, diabetes, chronic obstructive pulmonary disease, peripheral arterial disease, prior stroke, history of thyroid disease, sleep apnoea, history of malignancy, depression, device therapies, and prescriptions of heart failure medications at discharge.
Abbreviations: CRT, cardiac resynchronization therapy; ICD, implantable cardioverter defibrillator; MRA, mineralocorticoid receptor antagonist; NS, not significant; RASi, renin–angiotensin system inhibitor.
Figure 4 B shows the percentages of total HF rehospitalizations 1 year after discharge, stratified by different heart rate‐lowering regimens. Irrespective of the different combinations of beta‐blockers and digoxin at discharge, add‐on ivabradine showed reduced risks of first and/or repeated HF rehospitalization within 1 year after discharge from index hospitalization.
Alternations of blood pressure, heart rate, and left ventricular ejection fraction
Table 3 shows the alternations of vital signs and clinical outcomes within 1 year. At 1 year follow‐up, patients in the ivabradine group had significantly lower heart rates (77.6 ± 14.7 vs. 81.1 ± 16.3 bpm; P = 0.005) and lower severity of HF symptoms (NYHA functional class, 2.1 ± 0.7 vs. 2.3 ± 0.9; P < 0.001) than those from the non‐ivabradine group. Moreover, patients in the ivabradine group had numerically higher LVEF measurements than those from the non‐ivabradine group, although not statistically significant (39.2% ± 14.0% vs. 37.3% ± 15.2%; P = 0.104).
Table 3.
Patient characteristics at discharge and in those who survived to 12 months after discharge
| IVA group (n = 292) | Non‐IVA group (n = 584) | P value | |
|---|---|---|---|
| Baseline | |||
| Systolic blood pressure (mmHg) | 129.2 ± 21.2 | 129.9 ± 25.5 | 0.675 |
| Heart rate (bpm) | 83.5 ± 15.0 | 84.7 ± 13.1 | 0.248 |
| LVEF (%) | 28.2 ± 7.3 | 28.2 ± 8.1 | 0.930 |
| NYHA Fc | 2.6 ± 0.7 | 2.6 ± 0.7 | 0.225 |
| At 12 months a | |||
| Systolic blood pressure (mmHg) | 120.4 ± 19.4 | 121.5 ± 21.5 | 0.489 |
| Heart rate (bpm) | 77.6 ± 14.7 | 81.1 ± 16.3 | 0.005 |
| LVEF (%) | 39.2 ± 14.0 | 37.3 ± 15.2 | 0.104 |
| NYHA Fc | 2.1 ± 0.7 | 2.3 ± 0.9 | <0.001 |
| Cardiovascular death, n (%) | 17 (5.8%) | 71 (12.2%) | 0.002 |
| All‐cause mortality, n (%) | 21 (7.2%) | 82 (14.0%) | 0.002 |
| At least 1 HF re‐admission, n (%) | 79 (27.1%) | 212 (36.3%) | <0.001 |
Systolic blood pressure, heart rate and left ventricular ejection fraction were collected from those who survived to 12 months after discharge (IVA group n = 271, non‐IVA group n = 502).
Abbreviations: HF, heart failure; IVA, ivabradine; LVEF, left ventricular ejection fraction; NYHA Fc, New York Heart Association functional class.
Discussion
Adverse events frequently occurred following discharge from hospitalization for acute decompensated HF. In the current study, one‐fourth of the patients suffered from HF rehospitalization within 6 months and one‐tenth died because of cardiovascular causes within 1 year after index HF hospitalization, suggesting that timely and appropriate treatment should be provided to these high‐risk patients.
Patients admitted for acute HF are often fragile owing to more comorbidities, for example, renal impairment and COPD, haemodynamic instability, and need for vasopressors and inotrope treatment. These conditions usually limit the initiation and titration of guideline‐recommended therapies, that is, MRA, renin–angiotensin system inhibitors, and beta‐blockers. 14 Nevertheless, different from the above medications, ivabradine highly and specifically works at the If current in the sinoatrial node and does not adversely affect renal and bronchial systems. The subgroup analysis of the SHIFT trial produced promising evidence in terms of consistent cardiovascular benefits and safety in patients with HF and renal dysfunction or COPD. 14 , 15 Regarding treating patients with HF and hypotension or haemodynamic instability, using ivabradine (compared with beta‐blockers) may be more appropriate in these situations because of its distinguishing feature in heart rate reduction without inducing negative inotropy or hypotension. 16 , 17 , 18 A case series presented the safety and effectiveness of ivabradine in five patients with cardiogenic shock. 18 Another study enrolling 10 patients with advanced HF with pulmonary capillary wedge pressure ≥15 mmHg and sinus tachycardia demonstrated that intravenous ivabradine significantly reduced heart rate and increased stroke volume and LV systolic work. 16 Likewise, 52 patients with decompensated HF on dobutamine treatment were found to have lower heart rates and better stroke volume after ivabradine treatment. 17 Apart from these advantages, ivabradine has another specific effect on improving coronary blood flow and contractile function without affecting adrenoceptors. 19 A post hoc analysis of the SHIFT study showed that patients in ivabradine and non‐ivabradine groups experienced an increase in blood pressure by 12 and 11 mmHg after 24 months, respectively, and the baseline blood pressure did not affect the impact of heart rate reduction on clinical outcomes. 20 Our results echoed the aforementioned study and showed no differences in systolic blood pressure at 1 year follow‐up between the two groups. These advantages and specific pharmacological properties of ivabradine may support its utilization in patients with acute HF with high comorbidity burden and haemodynamic instability and even enable an early initiation or uptitration of beta‐blocker dose in real‐world practice. 21
A rise in heart rate increases myocardial oxygen consumption, exacerbates myocardial injury, and results in negative ventricular remodelling. 19 Moreover, in contrast to the increased contraction force accompanied with an increased frequency of muscle depolarization in a normal heart, a negative force–frequency relationship was noted in the failing myocardium caused by decreased coronary blood flow, defective calcium transient and sarcoplasmic reticulum activity, and increased oxidative stress. 19 , 22 , 23 Therefore, when managing patients with acute decompensated HF, the occurrence of tachycardia is regarded as a red flag because elevated heart rate is associated with unfavourable cardiovascular outcomes at different stages during HF hospitalization. 11 , 24 , 25 , 26 Of note, although our data showed that the absolute difference in heart rates between the two groups was smaller than that observed in the SHIFT study (3.5 vs. 8 bpm), 10 the additional ivabradine treatment was still associated with a significantly lower 1‐year cardiovascular mortality and fewer recurrent hospitalizations for HF. This phenomenon may imply other cardioprotective effects from ivabradine beyond that induced by reducing heart rates. First, when beta‐blockade‐associated heart rate reduction is prevented by pacing, the alpha‐adrenergic coronary vasoconstriction is unmasked, which may deteriorate coronary flow and heart function. 27 Different from beta‐blockades, ivabradine can simultaneously reduce heart rates and improve coronary flow and cardiac function by the preservation of the endothelium‐mediated vasodilation and lack of unmasked alpha‐adrenergic coronary vasoconstriction or negative inotropic action. 28 , 29 This would permit a better performance in heart function during daily life activity or exercise. 30 Second, the benefits of ivabradine against myocardial infarction are beyond heart rate reduction. 31 Studies revealed that ivabradine may decrease cardiac infarction size and preserve more cardiomyocyte viability after ischaemia or reperfusion by reducing mitochondrial reactive oxygen species formation and increasing adenosine triphosphate production and calcium retention capacity. 31 , 32 In addition, ivabradine was observed to attenuate adverse cardiac remodelling and improve angiogenesis after myocardial infarction. 33 These pleiotropically cardioprotective effects may provide plausible explanations why the patients treated with ivabradine had a significant improvement in clinical outcomes even with the small heart rate reduction.
Thus far, the rate of ivabradine utilization is still low in real‐world practice, although the ESC‐HF long‐term registry reported that the ivabradine prescription rate increased from 1.2% to 3.2% before HF hospitalization and at discharge. 34 In theory, it is suggested that ivabradine be used as an add‐on heart rate‐lowering regimen following a beta‐blocker. However, although relevant studies suggested that beta‐blocker therapy should be continued in patients with acute decompensated HF if their clinical condition permits, 35 the negative inotropic effect of beta‐blockers on cardiovascular haemodynamics causes reluctance among some physicians when prescribing them, particularly during the acute decompensated period. In the real‐world setting, the use of beta‐blockers significantly decreased from 89.9% to 69.1% at 6 months after the worsening of HF event. 8 Hence, it is unrealistic to assume that all hospitalized patients with HFrEF can receive and tolerate beta‐blocker therapy first and gradually be introduced to ivabradine over subsequent months because many patients are still at a high risk of rehospitalization during the vulnerable phase. This obstacle may limit the timely administration of ivabradine. A post hoc analysis from the SHIFT study showed that continuous ivabradine therapy was associated with fewer all‐cause hospitalizations at 1, 2, and 3 months. 36 Moreover, the ETHIC‐AHF trial demonstrated that patients treated with ivabradine and beta‐blockers at discharge had significantly lower heart rates and better LVEF at 4 months than patients treated with beta‐blockers alone. 37 Likewise, a study from the post‐Soviet states showed that add‐on ivabradine to beta‐blocker therapy for patients with acute HF during hospitalization contributed to a reduction in all‐cause mortality and HF rehospitalization within 1 year compared with using beta‐blockers alone. 38 In accordance, owing to the beneficial effects of the ivabradine and beta‐blocker combination in patients with acute decompensated HF, some clinicians started to advocate the non‐stepped approach rather than introducing each class in a stepwise manner. 39 This real‐world study supports this approach and provides favourable results that ivabradine could be effectively used in any clinical circumstances as long as the HF patients have been discharged with a sinus rhythm and heart rate of ≥70 bpm.
Several limitations inherent in the retrospective design of this study should be mentioned. First, treatment decisions were based on real‐world practice by the participating cardiologists. This type of retrospective study might have potential unmeasured biases. However, this study aimed to include a broad range of patients reflecting the current reality of post‐acute practice for ivabradine and not to enrol the narrowly defined HF population included in clinical trials. Second, the number of patients was relatively small. An ongoing randomized, placebo‐controlled trial evaluating the efficacy and safety of ivabradine in 674 patients with acute HF may ascertain the role of early heart rate reduction by ivabradine among these patients. 40
In conclusion, among real‐world Asian populations with acute decompensated HF and reduced LVEF, treatment with ivabradine was associated with a reduced risk of cardiovascular mortality, all‐cause mortality, and HF rehospitalization within 1 year. These benefits of ivabradine were consistent across various background HF medications. Additional large‐scale clinical trials are needed to confirm the benefits of ivabradine among patients with acute decompensated HFrEF.
Conflict of interest
None declared.
Funding
The present work was supported by the Cheng Hsin General Hospital Foundation [CHGH109‐(IP)1‐01] & [CHGH109‐(IP)1‐02]
Acknowledgements
We are grateful to Dr Chao‐Wen Hsueh, Dr Wei‐Tsung Lai, Dr Hao‐Neng Fu, Ms Yi‐Hua Lin, Ms I‐Ching Liu, Ms Wei‐Ting Huang, Ms Yu‐Ping Lin, Ms Yu‐Ching Lai, Ms Fang‐Hsiu Kou, and Mr Tzu‐Yuan Sung for their effort of data collection. We greatly appreciate Santina l for English editing.
Liao, C.‐T. , Huang, J.‐L. , Liang, H.‐W. , Chung, F.‐P. , Lee, Y.‐H. , Lin, P.‐L. , Chiou, W.‐R. , Lin, W.‐Y. , Hsu, C.‐Y. , and Chang, H.‐Y. (2021) The association between ivabradine and adverse cardiovascular events in acute decompensated HFrEF patients. ESC Heart Failure, 8: 4199–4210. 10.1002/ehf2.13536.
References
- 1. Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart 2007; 93: 1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gheorghiade M. Rehospitalization for heart failure: problems and perspectives. J Am Coll Cardiol 2013; 61: 391–403. [DOI] [PubMed] [Google Scholar]
- 3. Sakata Y, Shimokawa H. Epidemiology of heart failure in Asia. Circ J 2013; 77: 2209–2217. [DOI] [PubMed] [Google Scholar]
- 4. Cleland JG, Swedberg K, Follath F, Komajda M, Cohen‐Solal A, Aguilar JC, Dietz R, Gavazzi A, Hobbs R, Korewicki J, Madeira HC, Moiseyev VS, Preda I, van Gilst WH, Widimsky J, Freemantle N, Eastaugh J, Mason J, Study Group on Diagnosis of the Working Group on Heart Failure of the European Society of Cardiology . The EuroHeart Failure survey programme‐‐ a survey on the quality of care among patients with heart failure in Europe. Part 1: patient characteristics and diagnosis. Eur Heart J 2003; 24: 442–463. [DOI] [PubMed] [Google Scholar]
- 5. Shimokawa H, Miura M, Nochioka K, Sakata Y. Heart failure as a general pandemic in Asia. Eur J Heart Fail 2015; 17: 884–892. [DOI] [PubMed] [Google Scholar]
- 6. Reyes EB, Ha JW, Firdaus I, Ghazi AM, Phrommintikul A, Sim D, Vu QN, Siu CW, Yin WH, Cowie MR. Heart failure across Asia: Same healthcare burden but differences in organization of care. Int J Cardiol 2016; 223: 163–167. [DOI] [PubMed] [Google Scholar]
- 7. Wang CC, Wu CK, Tsai ML, Lee CM, Huang WC, Chou HH, Huang JL, Chi NH, Yen HW, Tzeng BH, Chang WT, Chang HY, Wang CH, Lu YY, Tsai JP, Su CH, Cherng WJ, Yin WH, Tsai CT, Wu YW, Lin JL, Hwang JJ. 2019 Focused Update of the Guidelines of the Taiwan Society of Cardiology for the Diagnosis and Treatment of Heart Failure. Acta Cardiol Sin 2019; 35: 244–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Butler J, Yang M, Manzi MA, Hess GP, Patel MJ, Rhodes T, Givertz MM. Clinical course of patients with worsening heart failure with reduced ejection fraction. J Am Coll Cardiol 2019; 73: 935–944. [DOI] [PubMed] [Google Scholar]
- 9. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force Members; Document Reviewers . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 10. Swedberg K, Komajda M, Böhm M, Borer JS, Ford I, Dubost‐Brama A, Lerebours G, Tavazzi L, SHIFT Investigators . Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo‐controlled study. Lancet 2010; 376: 875–885. [DOI] [PubMed] [Google Scholar]
- 11. DeVore AD, Schulte PJ, Mentz RJ, Hardy NC, Kelly JP, Velazquez EJ, Maya JF, Kielhorn A, Patel HK, Reed SD, Hernandez AF. Relation of elevated heart rate in patients with heart failure with reduced ejection fraction to one‐year outcomes and costs. Am J Cardiol 2016; 117: 946–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang CC, Chang HY, Yin WH, Wu YW, Chu PH, Wu CC, Hsu CH, Wen MS, Voon WC, Lin WS, Huang JL, Chen SM, Yang NI, Chang HC, Chang KC, Sung SH, Shyu KG, Lin JL, Mar GY, Chan KC, Kuo JY, Wang JH, Chen ZC, Tseng WK, Cherng WJ. TSOC‐HFrEF registry: a registry of hospitalized patients with decompensated systolic heart failure: description of population and management. Acta Cardiol Sin 2016; 32: 400–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee YH, Lin PL, Chiou WR, Huang JL, Lin WY, Liao CT, Chung FP, Liang HW, Hsu CY, Chang HY. Combination of ivabradine and sacubitril/valsartan in patients with heart failure and reduced ejection fraction. ESC Heart Fail 2021; 8: 1204–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tavazzi L, Swedberg K, Komajda M, Böhm M, Borer JS, Lainscak M, Robertson M, Ford I, SHIFT Investigators . Clinical profiles and outcomes in patients with chronic heart failure and chronic obstructive pulmonary disease: an efficacy and safety analysis of SHIFT study. Int J Cardiol 2013; 170: 182–188. [DOI] [PubMed] [Google Scholar]
- 15. Voors AA, van Veldhuisen DJ, Robertson M, Ford I, Borer JS, Böhm M, Komajda M, Swedberg K, Tavazzi L, SHIFT investigators . The effect of heart rate reduction with ivabradine on renal function in patients with chronic heart failure: an analysis from SHIFT. Eur J Heart Fail 2014; 16: 426–434. [DOI] [PubMed] [Google Scholar]
- 16. De Ferrari GM, Mazzuero A, Agnesina L, Bertoletti A, Lettino M, Campana C, Schwartz PJ, Tavazzi L. Favourable effects of heart rate reduction with intravenous administration of ivabradine in patients with advanced heart failure. Eur J Heart Fail 2008; 10: 550–555. [DOI] [PubMed] [Google Scholar]
- 17. Porcile R, Levin R, Fridman O, Baztarrica GP, Villeco S, Salvaggio F, Blanco N. Safety, tolerability and efficacy of ivabradine for control of sinus tachycardia in patients undergoing inotropic therapy. Curr Res Cardiol 2016; 3: 13–16. [Google Scholar]
- 18. Chiu MH, Howlett JG, Sharma NC. Initiation of ivabradine in cardiogenic shock. ESC Heart Fail 2019; 6: 1088–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heusch G. Heart rate in the pathophysiology of coronary blood flow and myocardial ischaemia: benefit from selective bradycardic agents. Br J Pharmacol 2008; 153: 1589–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Komajda M, Böhm M, Borer JS, Ford I, Robertson M, Manolis AJ, Tavazzi L, Swedberg K, SHIFT Investigators . Efficacy and safety of ivabradine in patients with chronic systolic heart failure according to blood pressure level in SHIFT. Eur J Heart Fail 2014; 16: 810–816. [DOI] [PubMed] [Google Scholar]
- 21. Barry AE, Schukina EV, Samoilova OV, Pricolota OA, Malovichko SI, Pricolota AV, Bagriy EA. The addition of ivabradine to beta‐blocker improves exercise capacity in systolic heart failure patients in a prospective, open‐label study. Adv Ther 2015; 32: 108–119. [DOI] [PubMed] [Google Scholar]
- 22. Hasenfuss G, Holubarsch C, Hermann HP, Astheimer K, Pieske B, Just H. Influence of the force–frequency relationship on haemodynamics and left ventricular function in patients with nonfailing hearts and in patients with dilated cardiomyopathy. Eur Heart J 1994; 15: 164–170. [DOI] [PubMed] [Google Scholar]
- 23. Heusch G. Heart rate and heart failure. Not a simple relationship. Circ J 2011; 75: 229–236. [DOI] [PubMed] [Google Scholar]
- 24. Claret PG, Stiell IG, Yan JW, Clement CM, Rowe BH, Calder LA, Perry JJ. Hemodynamic, management, and outcomes of patients admitted to emergency department with heart failure. Scand J Trauma Resusc Emerg Med 2016; 24: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bui AL, Grau‐Sepulveda MV, Hernandez AF, Peterson ED, Yancy CW, Bhatt DL, Fonarow GC. Admission heart rate and in‐hospital outcomes in patients hospitalized for heart failure in sinus rhythm and in atrial fibrillation. Am Heart J 2013; 165: 567–574.e6. [DOI] [PubMed] [Google Scholar]
- 26. Lan WR, Lin SI, Liao FC, Chang HY, Tsai CT, Wu YJ, Liu PY, Chen CH, Lee YH. Effect of reducing heart rate on outcomes in patients with reduced ejection fraction. Am J Cardiol 2021; 150: 77–81. [DOI] [PubMed] [Google Scholar]
- 27. Seitelberger R, Guth BD, Heusch G, Lee JD, Katayama K, Ross J Jr. Intracoronary alpha 2‐adrenergic receptor blockade attenuates ischemia in conscious dogs during exercise. Circ Res 1998; 62: 436–442. [DOI] [PubMed] [Google Scholar]
- 28. Simon L, Ghaleh B, Puybasset L, Giudicelli J‐F, Berdeaux A. Coronary and hemodynamic effects of S 16257, a new bradycardic agent, in resting and exercising conscious dogs. J Pharmacol Exp Ther 1995; 275: 659–666. [PubMed] [Google Scholar]
- 29. Monnet X, Ghaleh B, Colin P, Parent De Curzon O, Giudicelli J‐F, Berdeaux A. Effects of heart rate reduction with ivabradine on exercise induced myocardial ischemia and stunning. J Pharmacol Exp Ther 2001; 299: 1133–1139. [PubMed] [Google Scholar]
- 30. Colin P, Ghaleh B, Monnet X, Hittinger L, Berdeaux A. Effect of graded heart rate reduction with ivabradine on myocardial oxygen consumption and diastolic time in exercising dogs. J Pharmacol Exp Ther 2004; 308: 236–240. [DOI] [PubMed] [Google Scholar]
- 31. Heusch G, Skyschally A, Gres P, van Caster P, Schilawa D, Schulz R. Improvement of regional myocardial blood flow and function and reduction of infarct size with ivabradine: Protection beyond heart rate reduction. Eur Heart J 2008; 29: 2265–2275. [DOI] [PubMed] [Google Scholar]
- 32. Heusch G. Pleiotropic action(s) of the bradycardic agent ivabradine: Cardiovascular protection beyond heart rate reduction. Br J Pharmacol 2008; 155: 970–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mulder P, Barbier S, Chagraoui A, Richard V, Henry JP, Lallemand F, Renet S, Lerebours G, Mahlberg‐Gaudin F, Thuillez C. Long‐term heart rate reduction induced by the selective If current inhibitor ivabradine improves left ventricular function and intrinsic myocardial structure in congestive heart failure. Circulation 2004; 109: 1674–1679. [DOI] [PubMed] [Google Scholar]
- 34. Crespo‐Leiro MG, Anker SD, Maggioni AP, Coats AJ, Filippatos G, Ruschitzka F, Ferrari R, Piepoli MF, Delgado Jimenez JF, Metra M, Fonseca C, Hradec J, Amir O, Logeart D, Dahlström U, Merkely B, Drozdz J, Goncalvesova E, Hassanein M, Chioncel O, Lainscak M, Seferovic PM, Tousoulis D, Kavoliuniene A, Fruhwald F, Fazlibegovic E, Temizhan A, Gatzov P, Erglis A, Laroche C, Mebazaa A, Heart Failure Association (HFA) of the European Society of Cardiology (ESC) . European Society of Cardiology Heart Failure Long‐Term Registry (ESC‐HF‐LT): 1‐year follow‐up outcomes and differences across regions. Eur J Heart Fail 2016; 18: 613–625. [DOI] [PubMed] [Google Scholar]
- 35. Komajda M, Tavazzi L, Swedberg K, Böhm M, Borer JS, Moyne A, Ford I, Investigators SHIFT. Chronic exposure to ivabradine reduces readmissions in the vulnerable phase after hospitalization for worsening systolic heart failure: a post‐hoc analysis of SHIFT. Eur J Heart Fail 2016; 18: 1182–1189. [DOI] [PubMed] [Google Scholar]
- 36. Hidalgo FJ, Anguita M, Castillo JC, Rodríguez S, Pardo L, Durán E, Sánchez JJ, Ferreiro C, Pan M, Mesa D, Delgado M, Ruiz M. Effect of early treatment with ivabradine combined with beta‐blockers versus beta‐blockers alone in patients hospitalised with heart failure and reduced left ventricular ejection fraction (ETHIC‐AHF): A randomised study. Int J Cardiol 2016; 217: 7–11. [DOI] [PubMed] [Google Scholar]
- 37. Lopatin YM, Cowie MR, Grebennikova AA, Sisakian HS, Pagava ZM, Hayrapetyan HG, Abdullaev TA, Voronkov LG, Chesnikova AI, Tseluyko VI, Tarlovskaya EI, Dadashova GM, Berkinbaev SF, Glezer MG, Koziolova NA, Rakisheva AG, Kipiani ZV, Kurlyanskaya AK. Optimization of heart rate lowering therapy in hospitalized patients with heart failure: Insights from the Optimize Heart Failure Care Program. Int J Cardiol 2018; 260: 113–117. [DOI] [PubMed] [Google Scholar]
- 38. Prins KW, Neill JM, Tyler JO, Eckman PM, Duval S. Effects of Beta‐Blocker withdrawal in acute decompensated heart failure: A systematic review and meta‐analysis. JACC Heart failure 2015; 3: 647–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lam CSP, Butler J. Victims of Success in Failure. Circulation 2020; 142: 1129–1131. [DOI] [PubMed] [Google Scholar]
- 40. Su Y, Ma T, Wang Z, Dong B, Tai C, Wang H, Zhang F, Yan C, Chen W, Xu Y, Ye L, Tye GJ, Ong SB, Zhang J, Xu D. Efficacy of early initiation of ivabradine treatment in patients with acute heart failure: rationale and design of SHIFT‐AHF trial. ESC Heart Fail 2020; 7: 4465–4471. [DOI] [PMC free article] [PubMed] [Google Scholar]


