Abstract
Aims
Telemedical emergency services for heart failure (HF) patients are usually provided during business hours. However, many emergencies occur outside of business hours. This study evaluates if a 24/7 telemedical emergency service is needed for the remote management of high‐risk HF patients.
Methods and results
The study included 1119 patients merged from the TIM‐HF and TIM‐HF2 trials [age 69 ± 11, 73% male, left ventricular ejection fraction 37% ± 13, 557 New York Heart Association (NYHA) II/562 NYHA III]. Patients received a 24/7 physician‐guided emergency service provided by the telemedical centre (TMC) in addition to remote management within business hours. During emergency calls, patient status, symptoms, electronic patient record, and instant telemonitoring data were evaluated by the TMC physician. Following diagnosis, patients were referred for hospital admission or instructed to stay at home. Apart from the TMC, patients could place a call to the public emergency service at any time.
Seven hundred sixty‐eight emergency calls were placed over 1383 patient years (0.56 calls/patient year). Five hundred twenty‐six calls (69%) occurred outside business hours. There were 146 (19%) emergency calls for worsening HF, 297 (39%) other cardiovascular, and 325 (42%) non‐cardiac causes, with a similar pattern inside and outside business hours. Of the 1119 patients, 417 (37%) placed at least one emergency call. Patients with NYHA Class III, higher N‐terminal prohormone of brain natriuretic peptide (>1.400 pg/mL) levels, ischaemic aetiology of HF, implanted defibrillator, and impaired renal function had a higher probability of placing emergency calls. During study follow‐up, patients who made an emergency call had a higher all‐cause mortality (22% vs. 11%, P = 0.007 in TIM‐HF; 16% vs. 4%, P < 0.001 in TIM‐HF2) and more unplanned hospitalizations (324 vs. 162, P < 0.001 in TIM‐HF; 545 vs. 180, P < 0.001 in TIM‐HF2). Of the total 1,211 unplanned hospital admissions, 492 (41%) were initiated by a patient emergency call.
Three hundred seventy‐nine calls (49%) were placed to the TMC, whereas 389 calls (51%) were made to the public emergency service. Three hundred twenty‐six (84%) of the calls to the public emergency service resulted in acute hospitalizations.
The TMC initiated 202 (53%) hospital admissions; 177 (47%) patients were advised to stay at home. All patients that remained at home were alive during a prespecified safety period of 7 days post‐call. Diagnoses made by the TMC physician were confirmed in 83% of cases by the hospital.
Conclusion
A telemedical emergency service for high‐risk HF patients is safe and should operate 24/7 to reduce unplanned hospitalizations. Emergency calls could be considered as a marker for higher morbidity and mortality.
Keywords: Remote patient management, Heart failure, Telemonitoring, Electrocardiogram, Emergency
Introduction
There is growing evidence that structured heart failure (HF) care, including telemonitoring, is able to stabilize individual health status, reducing HF readmissions and lowering mortality rates. 1 , 2 , 3 , 4 , 5 Telemedical approaches in HF care differ in regards to the types of telemedical devices and types of services.
Telemonitoring of implantable devices 3 , 4 or non‐invasive devices 5 is used for a daily transfer of vital parameters to detect the onset of congestion, arrhythmia, and critical changes in blood pressure and to enable a tailored before/afterload management. Structured telephone support has demonstrated improved outcomes through coaching, lifestyle intervention, titration of guideline‐based medical therapy, and improved adherence. 1 , 2
In most of the randomized telemedical trials and in real‐world settings, HF nurses are providing routine telemonitoring during business hours only. 6 Nevertheless, acute worsening of symptoms with urgent need for treatment and unplanned hospital admissions can occur at any time. 7
Telemedical emergency service has shown to be effective in improving clinical outcomes in acute coronary syndromes and stroke by shortening time to diagnosis and enabling early treatment. 8 , 9 , 10 , 11 , 12 The European Union offers a medical emergency telephone number for the general public. However, a specified help line or emergency service for HF patients has not yet been implemented in Germany. In two randomized controlled telemedical interventional management in HF trials (TIM‐HF and TIM‐HF2) conducted in Germany, a 24/7 telemedical emergency service provided by physicians was added to the routine telemanagement by the telemedical centre (TMC). The assessment of this service is the objective of this study. 5 , 13
Methods
The TIM‐HF and TIM‐HF2 trials investigated the effects of telemedicine in hear failure patients using a comparable non‐invasive technology
In the TIM‐HF trial (clinicaltrials.gov identifier: NCT00543881) conducted between 2008 and 2010 in four German states, 354 out of 710 patients were randomized to the remote patient management (RPM) group. The TIM‐HF2 trial (clinicaltrials.gov identifier: NCT01878630) was conducted between 2013 and 2018 in 14 German states; 765 out of 1538 patients were assigned to the RPM group. For both studies, patients were managed by a dedicated TMC, staffed with HF nurses and physicians.
The main differences between both studies were the patient characteristics, the duration of follow‐up, and the design of home care devices for the telemedical emergency system. 14 , 15 The technical settings within the TMC and the standard operating procedures (SOPs) were identical in both trials.
The investigation conforms with the principles outlined in the Declaration of Helsinki and the laws and regulations applicable in Germany. Written approval from the appropriate ethics committees was obtained.
Study population and follow‐up
Patients with chronic HF in functional Classes II or III, according to the classification of the New York Heart Association (NYHA), were included in both studies.
Main inclusion criteria for the TIM‐HF trial were a left ventricular ejection fraction (LVEF) ≤ 35% and at least one HF hospitalization within 24 months prior to randomization. In patients with an LVEF ≤25%, which was measured twice within 6 months, a previous HF hospitalization was not mandatory.
In the TIM‐HF2 trial, HF patients with reduced as well as with preserved LVEF were included, but at least one HF hospitalization within 12 months prior to randomization was mandatory. Patients with major depression measured by Patient Health Questionnaire‐9 (PHQ‐9) questionnaire were excluded according to the subgroup analysis of TIM‐HF. 16
The TIM‐HF trial used a fixed stopping date, resulting in a minimal follow‐up period of 12 months for all patients, the median follow‐up was 26 months resulting in 641 patient years.
The follow‐up period in the TIM‐HF2 trial was 12 months resulting in 742 patient years.
According to the study protocol, the telemedical emergency service was part of the intervention in the RPM group.
Telemedical emergency system
For the daily routine telemonitoring, patients in both trials measured body weight, blood pressure, peripheral oxygen saturation (SpO2), electrocardiogram, and subjective well‐being with non‐invasive telemedical devices. Measurements were transferred wirelessly via a mobile phone network connectivity from a patients' home to the TMC. A detailed description of the system has been published elsewhere. 14 , 15
In TIM‐HF, a fixed emergency response system was installed at the patient's home, requiring a landline. This system had a big red one‐touch button to establish a bi‐directional voice link to the TMC, without using a phone. In the TIM‐HF2 trial, all patients received a mobile phone with a button to start a direct telephone link to the TMC.
In addition, both studies featured a free call phone number for all patients to get in contact with the TMC in case of emergencies.
The analysis included only calls due to medical emergencies. Accidental activation of the emergency system or calls for non‐medical reasons were excluded from the analysis.
Structure of the telemedical emergency service
The TMC was located at a tertiary care centre (Charité–Universitätsmedizin Berlin). It was connected to all rescue services and emergency departments within Germany, with the option to open a direct line to the emergency medical service nearest to the patient. Physicians with expertise in cardiology and emergency medicine conducted the triage and management of incoming emergency calls.
According to the study protocols of both trials, the physicians worked in shifts at the TMC. The service was available 24/7 for the entire study period. SOPs were implemented.
The handling of an incoming emergency call included a clinical assessment of patient symptoms and a review of information from the electronic patient record containing transferred telemedical vital parameters, reports about HF status, course of the disease, recent events, comorbidities, medical therapy, and implantable cardiac device programming. Thus, a virtual emergency room was established at the TMC. According to the SOPs, the telemedical management of an emergency call resulted in (i) acute admission to the next available emergency room or (ii) in a ‘stay at home’ policy. The ‘stay at home’ policy included the following options: changes in patient's medication and/or referral to the general practitioner within the next 24 h.
Evaluation of the emergency service
Emergency calls were classified by the on‐duty physician as HF, other cardiovascular, or non‐cardiac events. To evaluate the diagnostic accuracy of the triage conducted by the TMC physician, the TMC‐classification of the case was compared with the reports of the hospitalization or emergency room visit. This process of adjudication was performed by an independent clinical endpoint committee in both studies.
For safety reasons, we defined a period of 7 days for unplanned hospitalization or death to be potentially associated with the emergency call.
Statistical analysis
Statistical analysis was performed using SPSS (version 25.0, IBM corp.). Data were descriptively analysed, reporting mean ± standard deviation (SD) for quantitative measurements. Differences were compared using a two‐tailed t test for normally distributed variables, and Mann–Whitney U test for non‐parametric variables. For all data analyses, significance level was set at P < 0.05 and was reported in an explorative manner. Mean bias and 95% confidence interval were calculated as mean ± 1.96 SD between test differences.
Results
Of the 1119 RPM patients of both trials, 417 (37%) initiated emergency calls, 251 patients called once, 166 patients called several times, with up to 19 calls per patient. While 702 (63%) patients made no emergency calls.
Both studies showed similar baseline characteristics for those patients who made an emergency call. NYHA Class III, higher N‐terminal prohormone of brain natriuretic peptide (NT‐proBNP) levels (>1.400 pg/mL), ischaemic aetiology of HF, history of coronary revascularisation, implanted defibrillator, and impaired renal function were conditions with higher probability for emergencies (Table 1 ). In the TIM‐HF trial, 256 (72%) of the 354 patients in the RPM group received a fixed emergency response system. The system could not be installed at the home of 98 patients due to a lack of a landline at the patient's home. In TIM‐HF2, the dedicated emergency mobile phone was provided to all 765 patients in the RPM group. In both studies the free call phone number to the TMC was tested successfully by the patients during an initial home visit from the instructing HF nurse.
Table 1.
Baseline characteristics of the patients with or without an emergency call (EC) in TIM‐HF and TIM‐HF2
| TIM‐HF | TIM‐HF2 | |||||
|---|---|---|---|---|---|---|
| Without EC | With EC | P | Without EC | With EC | P | |
| n = 216 | n = 138 | n = 486 | n = 279 | |||
| Age, years | 66 ± 11 | 68 ± 10 | 0.04 | 69 ± 11 | 72 ± 10 | <0.001 |
| Male sex, n (%) | 173 (80) | 112 (81) | 0.80 | 342 (70) | 191 (69) | 0.58 |
| Living alone, n (%) | 45 (21) | 30 (22) | 0.84 | 140 (29) | 73 (26) | 0.43 |
| NYHA class, n (%) | 0.006 | <0.001 | ||||
| I | 0 | 0 | 3 (0.6) | 0 | ||
| II | 120 (55) | 56 (41) | 281 (57.8) | 119 (42.7) | ||
| III | 96 (45) | 82 (59) | 200 (41.2) | 159 (57.0) | ||
| IV | 0 | 0 | 2 (0.4) | 1 (0.4) | ||
| LVEF, % | 27 ± 6 | 26 ± 6 | 0.07 | 41 ± 13 | 42 ± 14 | 0.27 |
| BMI, kg/m2 | 29 ± 5 | 28 ± 5 | 0.09 | 30 ± 6 | 29 ± 6 | 0.41 |
| BP, mmHg systolic | 121 ± 15 | 120 ± 18 | 0.47 | 127 ± 19 | 124 ± 18 | 0.16 |
| Heart rate, bpm | 72 ± 14 | 69 ± 11 | 0.23 | 72 ± 14 | 73 ± 14 | 0.62 |
| Diabetes mellitus | 85 (39) | 56 (41) | 0.78 | 217 (45) | 130 (47) | 0.60 |
| HF hospitalization <24 month before, n (%) | 155 (72) | 104 (75) | 0.45 | |||
| Primary cause of heart failure, n (%) | 0.014 | 0.001 | ||||
| Ischaemic | 111 (51) | 91 (66) | 169 (35) | 132 (47) | ||
| Non‐ischaemic | 87 (40) | 37 (27) | 128 (26) | 48 (17) | ||
| Hypertension | 6 (3) | 2 (1) | 84 (17) | 44 (16) | ||
| Other | 12 (6) | 8 (6) | 105 (22) | 55 (20) | ||
| Medical history, n (%) | ||||||
| Hyperuricaemia | 52 (24) | 57 (41) | 0.001 | |||
| Myocardial infarction | 97 (45) | 79 (57) | 0.06 | 110 (23) | 95 (34) | 0.001 |
| Coronary revascularization (PCI or CABG) | 131 (61) | 118 (86) | 0.008 | 187 (38) | 146 (52) | <0.001 |
| Implantable cardioverter defibrillator (ICD) | 87 (40) | 77 (56) | 0.004 | 121 (25) | 101 (36) | 0.001 |
| Cardiac resynchronization therapy (CRT) | 34 (16) | 20 (14) | 0.75 | 71 (15) | 47 (17) | 0.38 |
| TAVI | 11 (2) | 12 (4) | 0.09 | |||
| MitraClip | 16 (3) | 10 (4) | 0.63 | |||
| Laboratory measurements | ||||||
| Haemoglobin, mmol/L | 13.5 (11.0–14.7) | 13.4 (11.5–14.4) | 0.07 | 8.3 (7.6–9.0) | 8.1 (7.2–8.8) | 0.004 |
| Serum sodium, mmol/L | 140 (138–142) | 140 (138–142) | 0.55 | 140 (137–142) | 139 (137–142) | 0.76 |
| Potassium, mmol/L | 4.6 (4.2–5.0) | 4.6 (4.3–5.0) | 0.45 | 4.5 (4.2–4.9) | 4.5 (4.2–4.9) | 0.64 |
| eGFR, mL/min per 1.73 m2 of BSA | 64 (46–79) | 55 (40–72) | 0.012 | 61 (46–80) | 52 (40–71) | <0.001 |
| NT proBNP, pg/mL | 1,177 (571–2,565) | 1756 (873–4,036) | <0.001 | 1,249 (527–2,596) | 1909 (930–3,999) | <0.001 |
| Concomitant treatment, n (%) | ||||||
| ACE inhibitor or ARB | 208 (96) | 134 (97) | 0.68 | 410 (84) | 218 (78) | 0.03 |
| ARN inhibitors | 25 (5) | 19 (7) | 0.34 | |||
| Beta‐blocker | 197 (91) | 129 (93) | 0.44 | 445 (92) | 257 (92) | 0.79 |
| MRA | 143 (66) | 88 (64) | 0.64 | 290 (60) | 151 (54) | 0.14 |
| Loop diuretic | 179 (83) | 122 (88) | 0.16 | 449 (92) | 268 (96) | 0.04 |
| Thiazides | 78 (36) | 51 (37) | 0.87 | 110 (23) | 81 (29) | 0.049 |
| Digitalis glycoside | 61 (28) | 47 (34) | 0.25 | 73 (15) | 46 (16) | 0.12 |
| Insulin | 32 (15) | 31 (22) | 0.07 | 96 (20) | 74 (27) | 0.03 |
| Oral hypoglycaemic | 40 (19) | 18 (13) | 0.17 | 142 (29) | 64 (23) | 0.06 |
| Nitrate | 18 (8) | 23 (17) | 0.02 | 19 (4) | 18 (6) | 0.11 |
| Antiarrhythmic | 30 (14) | 22 (16) | 0.59 | 56 (12) | 43 (15) | 0.12 |
ACE, angiotensin‐converting enzyme; ARB, angiotensin‐receptor blocker; ARN, angiotensin receptor‐neprilysin; BMI, body mass index; BP, blood pressure; BSA, body surface area; CABG, coronary artery bypass grafting; EC, emergency call; eGFR, estimated glomerular filtration rate; HF, heart failure; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal prohormone of brain natriuretic peptide; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; TAVI, transcatheter aortic valve implantation.
Data are mean (SD) or n (%), median (interquartile range) for all laboratory tests.
Pattern and causes of emergency calls
A total of 768 emergency calls occurred during 1383 patient years (0.56 calls per patient year). Two hundred eighteen emergency calls (29%) were made during business hours (Monday to Friday 9 a.m. to 5 p.m.), and 526 emergency calls (68%) were made outside business hours or on bank holidays. Twenty‐four emergency calls (2%) were incompletely recorded in terms of the time stamp of the incoming call (Figure 1 ). There were two calls from relatives after a sudden death of two patients, which were omitted from the analysis.
Figure 1.
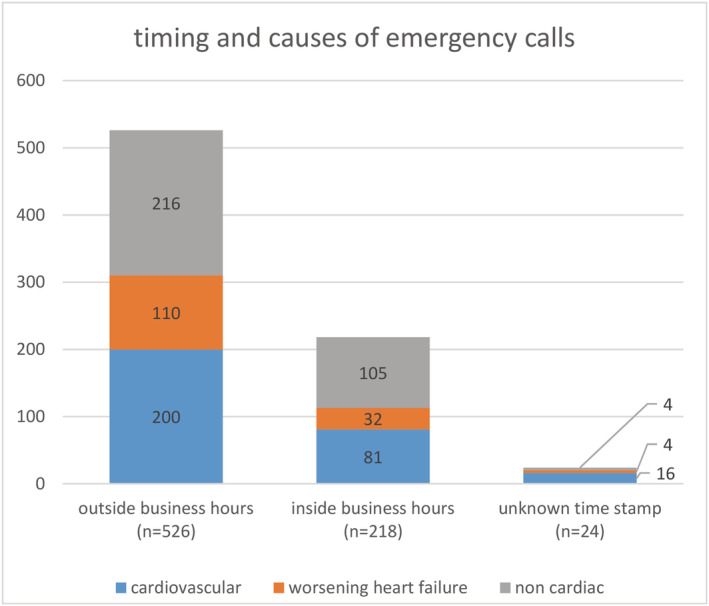
Timing around the clock and causes of emergency calls.
In the TIM‐HF trial, 281 emergency calls (92%) were placed to the TMC and subsequently managed by the telemedical emergency service, whereas 23 calls (8%) were made directly to the general public emergency services and thus not managed by the TMC.
In the TIM‐HF2 trial, in 98 (21%) emergency cases, the patient contacted the TMC, while 366 calls (79%) were made directly to the general public emergency service and thus not managed by the TMC.
Summarized, almost half of all emergency calls (n = 379; 49%) were primarily managed by the TMC. Two hundred seventy‐three (72%) calls to the TMC occurred outside business hours, and 97 calls (26%) inside business hours; nine calls (2%) had a missing time stamp. From the 389 emergency calls to the public emergency number, 253 (65%) calls occurred outside business hours and 121 (31%) inside business hours; 15 (4%) had a missing time stamp.
There were 146 (19%) emergency calls for worsening HF, 297 (39%) for other cardiovascular, and 325 (42%) for non‐cardiac causes with a similar pattern inside and outside business hours (Figure 1).
Emergency calls managed by the telemedical centre
After the diagnosis and triage by the TMC physician, 202 (53%) patients were immediately transferred to the next available emergency room; 177 patients (47%) were left at home. Of those patients acutely sent to an emergency room, 166 patients (82%) were hospitalized, while 36 (18%) patients were sent home from the emergency room after ambulatory treatment.
All patients left at home after triage from the TMC were alive within the prespecified safety period of 7 days; seven patients (4%) were hospitalized within the next 7 days after placing an emergency call.
The initial diagnosis made by the TMC physician was confirmed in 83% when compared with the on‐site diagnosis at the emergency room by adjudication of the clinical endpoint committee.
Emergency calls managed by the public emergency service
All 389 calls to the public emergency number resulted in immediate admission by an ambulance to the next available emergency room. In TIM‐HF, all of the 23 calls resulted in an acute hospitalization, 303 (83%) of 366 calls in TIM‐HF2, respectively. In total, 326 (84%) calls, which were directed to the public emergency service, resulted in a hospitalization.
Emergency calls and outcomes during follow‐up
Patients who made an emergency call had a higher all‐cause mortality (22% vs. 11%, P = 0.007 in TIM‐HF; 16% vs. 4%, P < 0.001 in TIM‐HF2), more events of unplanned hospitalization (324 vs. 162, P < 0.001 in TIM‐HF; 545 vs. 180, P < 0.001 in TIM‐HF2), and more days lost due to cardiovascular hospitalization or all‐cause death (13.9 vs. 3.6, P < 0.001 in TIM‐HF2) during study follow‐up (Table 2). Of all 1211 unplanned hospitalizations in the RPM group, 492 (41%) were directly triggered by an emergency call.
Table 2.
Mortality and unplanned hospitalizations of the patients with or without an emergency call (EC) in TIM‐HF and TIM‐HF2
| TIM HF | TIM HF2 | |||||
|---|---|---|---|---|---|---|
| Without EC n = 216 | With EC n = 138 | P | Without EC n = 468 | With EC n = 279 | P | |
| All‐cause death | 24 (11%) | 30 (22%) | 0.007 | 22 (4%) | 45 (16%) | <0.001 |
| Total number of hospitalization | 162 | 324 | <0.001 | 180 | 545 | <0.001 |
| Total number of cardiovascular hospitalization | 80 | 208 | <0.001 | 109 | 279 | <0.001 |
| Number of hospitalization per patient | 0.6 (0.5–0.7) | 2.6 (2.2–3.0) | <0.001 | 0.4 (0.3–0.4) | 2.0 (1.8–2.2) | <0.001 |
| Days lost due to all‐cause hospitalization | 9.2 (6.0–12.3) | 31.3 (23.9–38.8) | <0.001 | 6.3 (5.1–7.6) | 23.3 (20.2–26.5) | <0.001 |
| Days lost due to cardiovascular hospitalization | 3.2 (1.9–4.6) | 22 (15.3–28.6) | <0.001 | 2.9 (2.1–3.7) | 11.5 (9.4–13.6) | <0.001 |
| Days lost due to cardiovascular hospitalization or all‐cause death during 1 year follow‐up | * | * | 3.6 | 13.9 | <0.001 | |
EC, emergency call.
TIM‐HF had a fixed stopping date resulting in an individualized patient follow‐up time.
Discussion
The TIM‐HF and TIM‐HF2 trials were the only randomized controlled trials that conducted 24/7 telemedical emergency services in a high‐risk HF population.
Both studies revealed a similar pattern of emergency cases with respect to the cause of emergency, as well as a similar distribution of emergency calls over time. Most of the emergency calls happened outside business hours.
While there were slight differences in the inclusion criteria between TIM‐HF and TIM‐HF2, similar baseline characteristics were found in both studies for those patients who initiated emergency calls. Although two‐thirds of patients in both studies had never placed an emergency call during follow‐up, one‐third of the study population initiated at least one alarm in both studies. There were predisposing characteristics for patients to make an emergency call: (i) ischaemic aetiology of HF, (ii) advanced HF status (functional Class NYHA III and NT‐proBNP level >1.400 pg/mL), (iii) impaired renal function as a relevant comorbidity, which would define a patient population with a potential need for a 24/7 RPM.
Patients' emergency calls triggered a significant number (492; 41%) of all unplanned hospitalizations during follow‐up. Moreover, those patients who initiated alarms had a higher mortality rate during further study follow‐up. Thus, emergency calls could be considered as a marker for a higher morbidity and mortality. Caregivers should acknowledge the occurrence of an emergency call as an alert to intensify medical therapy and to reconsider other interventional options for long‐term care.
In both studies, patients were equipped with a specific hardware for direct contact to the TMC in case of an emergency. However, patients were free to use the latter or to call the public emergency number.
In TIM‐HF, 92% of all emergency calls were placed to the TMC, whereas in TIM‐HF2, only every fifth emergency call (21%) was placed to the TMC. The study procedures, SOPs in the TMC, and home monitoring systems were identical in both trials, with the exception of the emergency hardware in the patients home. While landline technology was still the accepted method during the TIM‐HF trial, technological advances in patient mobility lead to the implementation of a new dedicated emergency mobile phone for the TIM‐HF2 trial. We therefore attribute the low adherence rate in TIM‐HF2 to a poor usability of the emergency device. Patients reported that in case of an emergency, they were unable to find the mobile phone or it was not charged. We consider that an adequate design of the emergency device is crucial for the efficacy of a 24/7 telemedical emergency service.
Almost half of the TMC‐managed emergencies (47%) allowed patients to stay at home without harm. No patient died within the predefined safety period of 7 days after a call. Only a few patients were hospitalized during these safety period. Thus, telemedical triage by physicians is safe. The diagnostic accuracy of initial telediagnosis was comparable with the on‐site diagnosis at the hospital (83%), due to the high level of medical expertise of the TMC physicians and predefined SOP's.
Based on the data collected for the TIM‐HF and TIM‐HF2 studies, we propose that a 24/7 telemedical emergency service should be considered as a component for telemonitoring of high‐risk HF patients.
We recognize that the technology and 24/7 service used in both trials require significant health care and financial resources. In order to present a more cost‐effective solution for real‐life setting, the patients who require 24/7 telemedical emergency services could be forwarded to a regional TMC, while local TMC's would only provide routine RPM during regular business hours. Within a HF network, this would provide a routine 24/7 emergency support for large HF populations with a high risk of life‐threatening cardiac events. In December 2020, a new regulation was passed regarding the reimbursement of telemonitoring in Germany for HF patients with reduced ejection fraction <40%. 17 The new regulation requires primary care physicians (general practitioner and/or cardiologist) to decide whether a HF patient requires a 24/7 telemonitoring or telemonitoring during office hours. Therefore, TMCs should work in close cooperation with the primary care physicians in order to optimize the management of the 24/7 telemonitoring services and provide the best care for the patient.
Limitations
Our study was a post‐hoc analysis. Because the emergency calls were not prespecified as endpoints in both trials, a direct comparison between the emergency calls in the intervention group and the emergency calls in the control group was not possible. The RPM patients who placed emergency calls to the public emergency service could be considered behaviourally comparable with the usual care group in regards to medical emergencies. The key findings in this subset of patients in the intervention arm was a high rate of hospitalization after initiating an emergency call (84%). Presumably, a similar hospitalization rate after initiating an emergency call can be expected in the control group.
Both studies were conducted within the German health care system. We are therefore unable to forecast how a telemedical emergency management system would function in other health care settings.
Conclusion
A telemedical emergency service for high‐risk HF patients is safe and should operate 24/7 to reduce unplanned hospitalizations. Emergency calls could be considered as a marker for higher morbidity and mortality. Furthermore, we could show that a physician‐guided telemedical emergency service provides a very high diagnostic accuracy for telediagnosis compared with on‐site hospital diagnostics.
Conflict of interest
F. K. reports grants from the German Federal Ministry of Education and Research and grants from Federal Ministry of Economic Affairs and Energy for conducting the clinical trials. He reports personal fees for advisory board from Abbott and personal fees for lectures from Boston Scientific, Sanofi‐Aventis Deutschland GmbH, Novartis, Linde/Saúde, Roche Pharma AG, Amgen GmbH, and Astra Zeneca outside the submitted work. Further, he is a member of the Commission Digital Health of the German Association for Internal Medicine (DGIM).
All other authors have nothing to declare.
Funding
This work was supported by a research grant of the German Federal Ministry of Education and Research (grant numbers 13KQ0904A, 13KQ0904B, and 13KQ1104A) and a research grant of the German Federal Ministry of Economic Affairs and Energy (grant number: 01MG531).
The TIM‐HF study was a part of the research and development project ‘Partnership for the heart’. The TIM‐HF2 study was a part of the research and development project Gesundheitsregion der Zukunft–Nordbrandenburg. Open access funding enabled and organized by Projekt DEAL.
Acknowledgements
We would like to thank Jan Wiemer for his helpful advice regarding the statistical analysis and Volker Moeller for his support with database issues.
Furthermore, we thank all the physicians and nurses who provided 24/7 telemedical services in both trials. We thank Priyanka Shah from Ahmedabad, India, for supporting the preparation of the manuscript and Sheila Rieger for revising the manuscript.
Winkler, S. , Koehler, K. , Prescher, S. , Koehler, M. , Kirwan, B.‐A. , Tajsic, M. , and Koehler, F. (2021) Is 24/7 remote patient management in heart failure necessary? Results of the telemedical emergency service used in the TIM‐HF and in the TIM‐HF2 trials. ESC Heart Failure, 8: 3613–3620. 10.1002/ehf2.13413.
References
- 1. Inglis SC, Clark RA, Dierckx R, Prieto‐Merino D, Cleland JG. Structured telephone support or non‐invasive telemonitoring for patients with heart failure. Heart 2017; 103: 255–257. [DOI] [PubMed] [Google Scholar]
- 2. Angermann CE, Stork S, Gelbrich G, Faller H, Jahns R, Frantz S, Loeffler M, Ertl G. Mode of action and effects of standardized collaborative disease management on mortality and morbidity in patients with systolic heart failure: the Interdisciplinary Network for Heart Failure (INH) study. Circ Heart Fail 2012; 5: 25–35. [DOI] [PubMed] [Google Scholar]
- 3. Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, Strickland W, Neelagaru S, Raval N, Krueger S, Weiner S, Shavelle D, Jeffries B, Yadav JS. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 2011; 377: 658–666. [DOI] [PubMed] [Google Scholar]
- 4. Hindricks G, Taborsky M, Glikson M, Heinrich U, Schumacher B, Katz A, Brachmann J, Lewalter T, Goette A, Block M, Kautzner J, Sack S, Husser D, Piorkowski C, Sogaard P. Implant‐based multiparameter telemonitoring of patients with heart failure (IN‐TIME): a randomised controlled trial. Lancet 2014; 384: 583–590. [DOI] [PubMed] [Google Scholar]
- 5. Koehler F, Koehler K, Deckwart O, Prescher S, Wegscheider K, Kirwan BA, Winkler S, Vettorazzi E, Bruch L, Oeff M, Zugck C, Doerr G, Naegele H, Stork S, Butter C, Sechtem U, Angermann C, Gola G, Prondzinsky R, Edelmann F, Spethmann S, Schellong SM, Schulze PC, Bauersachs J, Wellge B, Schoebel C, Tajsic M, Dreger H, Anker SD, Stangl K. Efficacy of telemedical interventional management in patients with heart failure (TIM‐HF2): a randomised, controlled, parallel‐group, unmasked trial. Lancet 2018; 392: 1047–1057. [DOI] [PubMed] [Google Scholar]
- 6. Seferovic PM, Ponikowski P, Anker SD, Bauersachs J, Chioncel O, Cleland JGF, de Boer RA, Drexel H, Ben Gal T, Hill L, Jaarsma T, Jankowska EA, Anker MS, Lainscak M, Lewis BS, McDonagh T, Metra M, Milicic D, Mullens W, Piepoli MF, Rosano G, Ruschitzka F, Volterrani M, Voors AA, Filippatos G, Coats AJS. Clinical practice update on heart failure 2019: pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2019; 21: 1169–1186. [DOI] [PubMed] [Google Scholar]
- 7. Fonarow GC, Abraham WT, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, O'Connor CM, Pieper K, Sun JL, Yancy CW, Young JB. Factors identified as precipitating hospital admissions for heart failure and clinical outcomes: findings from OPTIMIZE‐HF. Arch Intern Med 2008; 168: 847–854. [DOI] [PubMed] [Google Scholar]
- 8. Yagi N, Otsuka Y, Oe Y, Yamane T, Yamanaka F, Tada E, Kasahara Y, Kataoka Y, Yokoyama H, Nonogi H. Initial experience of the novel mobile telemedicine system in real‐time transmission of prehospital 12‐lead ECG for cardiac emergency. J Am Coll Cardiol 2010; 55: A13.E123. [Google Scholar]
- 9. Sejersten M, Sillesen M, Hansen PR, Nielsen SL, Nielsen H, Trautner S, Hampton D, Wagner GS, Clemmensen P. Effect on treatment delay of prehospital teletransmission of 12‐lead electrocardiogram to a cardiologist for immediate triage and direct referral of patients with ST‐segment elevation acute myocardial infarction to primary percutaneous coronary intervention. Am J Cardiol 2008; 101: 941–946. [DOI] [PubMed] [Google Scholar]
- 10. Brunetti ND, De Gennaro L, Amodio G, Dellegrottaglie G, Pellegrino PL, Di Biase M, Antonelli G. Telecardiology improves quality of diagnosis and reduces delay to treatment in elderly patients with acute myocardial infarction and atypical presentation. Eur J Cardiovasc Prev Rehabil 2010; 17: 615–620. [DOI] [PubMed] [Google Scholar]
- 11. Levine SR, Gorman M. “Telestroke”: the application of telemedicine for stroke. Stroke 1999; 30: 464–469. [DOI] [PubMed] [Google Scholar]
- 12. Kunz A, Ebinger M, Geisler F, Rozanski M, Waldschmidt C, Weber JE, Wendt M, Winter B, Zieschang K, Fiebach JB, Villringer K, Erdur H, Scheitz JF, Tütüncü S, Bollweg K, Grittner U, Kaczmarek S, Endres M, Nolte CH, Audebert HJ. Functional outcomes of pre‐hospital thrombolysis in a mobile stroke treatment unit compared with conventional care: an observational registry study. Lancet Neurol 2016; 15: 1035–1043. [DOI] [PubMed] [Google Scholar]
- 13. Koehler F, Winkler S, Schieber M, Sechtem U, Stangl K, Bohm M, Boll H, Baumann G, Honold M, Koehler K, Gelbrich G, Kirwan BA, Anker SD. Impact of remote telemedical management on mortality and hospitalizations in ambulatory patients with chronic heart failure: the telemedical interventional monitoring in heart failure study. Circulation 2011; 123: 1873–1880. [DOI] [PubMed] [Google Scholar]
- 14. Koehler F, Winkler S, Schieber M, Sechtem U, Stangl K, Bohm M, Boll H, Kim SS, Koehler K, Luecke S, Honold M, Heinze P, Schweizer T, Braecklein M, Kirwan BA, Gelbrich G, Anker SD. Telemedical Interventional Monitoring in Heart Failure (TIM‐HF), a randomized, controlled intervention trial investigating the impact of telemedicine on mortality in ambulatory patients with heart failure: study design. Eur J Heart Fail 2010; 12: 1354–1362. [DOI] [PubMed] [Google Scholar]
- 15. Koehler F, Koehler K, Deckwart O, Prescher S, Wegscheider K, Winkler S, Vettorazzi E, Polze A, Stangl K, Hartmann O, Marx A, Neuhaus P, Scherf M, Kirwan BA, Anker SD. Telemedical Interventional Management in Heart Failure II (TIM‐HF2), a randomised, controlled trial investigating the impact of telemedicine on unplanned cardiovascular hospitalisations and mortality in heart failure patients: study design and description of the intervention. Eur J Heart Fail 2018; 20: 1485–1493. [DOI] [PubMed] [Google Scholar]
- 16. Koehler F, Winkler S, Schieber M, Sechtem U, Stangl K, Bohm M, de Brouwer S, Perrin E, Baumann G, Gelbrich G, Boll H, Honold M, Koehler K, Kirwan BA, Anker SD. Telemedicine in heart failure: pre‐specified and exploratory subgroup analyses from the TIM‐HF trial. Int J Cardiol 2012; 161: 143–150. [DOI] [PubMed] [Google Scholar]
- 17. Gemeinsamer Bundesausschuss . Beschluss des Gemeinsamen Bundesausschusses über eine Änderung der Richtlinie Methoden vertragsärztliche Versorgung: Telemonitoring bei Herzinsuffizienz. 2020.


