Abstract
Nopp140 is thought to shuttle between nucleolus and cytoplasm. However, the predominant nucleolar localization of Nopp140 homologues from different species suggests that Nopp140 is also involved in events occurring within the nucleolus. In this study, we demonstrated that the largest subunit of RNA polymerase I, RPA194, was coimmunoprecipitated with the human Nopp140 (hNopp140). Such an interaction is mediated through amino acids 204 to 382 of hNopp140. By double immunofluorescence, hNopp140 was colocalized with RNA polymerase I at the rDNA (rRNA genes) transcription active foci in the nucleolus. These results suggest that Nopp140 can interact with RNA polymerase I in vivo. Transfected cells expressing the amino-terminal half of hNopp140, hNopp140N382 (amino acids 1 to 382), displayed altered nucleoli with crescent-shaped structures. This phenotype is reminiscent of the segregated nucleoli induced by actinomycin D treatment, which is known to inhibit rRNA synthesis. Consistently, the hNopp140N382 protein mislocalized the endogenous RNA polymerase I and shut off cellular rRNA gene transcription as revealed by an in situ run-on assay. These dominant negative effects of the mutant hNopp140N382 suggest that Nopp140 plays an essential role in rDNA transcription. Interestingly, ectopic expression of hNopp140 to a very high level caused the formation of a transcriptionally inactive spherical structure occupying the entire nucleolar area which trapped the RNA polymerase I, fibrillarin, and hNopp140 but excluded the nucleolin. The mislocalizations of these nucleolar proteins after hNopp140 overexpression imply that Nopp140 may also play roles in maintenance of nucleolar integrity.
The nucleolus in eukaryotic cells carries out most of the important reactions in ribosome biogenesis, including rDNA (rRNA genes) transcription, rRNA processing, and preribosome assembly (34, 47, 49, 50). Elements of nucleolar architecture mediate a tight temporal and topological orchestration of these processes. In higher eukaryotic cells, nucleoli exhibit a common organization consisting of three ultrastructurally distinct regions, the fibrillar center (FC), the dense fibrillar component (DFC), and the granular component (GC). The main body of the nucleolus is made up of the GC, embedded in this granular mass are several islets of rounded structures, FCs, each surrounded by a compact layer of the DFC. Tandemly repeated rRNA genes are clustered mostly in the FCs (42, 57), with transcription occurring largely at the boundary between the FC and the DFC (13, 20, 42, 58). Nascent rRNA transcripts are processed in the DFC (25, 38, 59). Some processing steps may also occur in the GC, together with the assembly of the mature rRNA and ribosomal proteins into preribosomal subunits (42, 47). In addition to rDNA, rRNA, and ribosomal proteins, the nucleolus harbors a large number of nonribosomal proteins and small nucleolar RNAs, which mediate the transcriptional and posttranscriptional reactions of ribosome biogenesis (49, 54). Many nucleolar proteins locate in particular nucleolar regions, which may reflect functional compartmentalization of the nucleolus. For example, RNA polymerase I localizes to the FC and catalyzes rDNA transcription (48), whereas fibrillarin is a well-established DFC constituent and is involved in modification and processing of pre-rRNA (38, 59). On the other hand, nucleolin, which is abundant in the entire nucleolus, has been widely implicated in rDNA transcription, rRNA processing, and ribosome assembly (18).
RNA polymerases are large multisubunit enzymes. Although three classes of RNA polymerases exist in eukaryotic cells, they are structurally closely related. For example, the largest subunit of RNA polymerase I shares conserved regions with the cognate subunits of RNA polymerases II and III and the β′ subunit of RNA polymerase from Escherichia coli, which serve basic functions in transcription (2, 5, 35, 51). RNA polymerase I is the central machinery of rDNA transcription. In mammals, RNA polymerase I core enzyme consists of at least 11 subunits (55). RNA polymerase I and basal factors such as TIF-IA, TIF-IB, TIF-IC, and UBF exist as a preassembled complex, the polymerase I holoenzyme, which appears to be transcription initiation competent (1, 45, 52). Moreover, casein kinase II and histone acetyltransferase are also present in the RNA polymerase I complex, suggesting that transcription-regulating and chromatin-modifying activities are associated with RNA polymerase I (1, 19, 61, 62). Several lines of evidence suggest that the structural integrity of a nucleolus relies on the transcriptional activities of the rRNA genes (47, 50). Inhibition of rDNA transcription by actinomycin D or microinjection of anti-RNA polymerase I antibody results in alterations of nucleolar structure (4, 43). However, details of the molecular architecture of the nucleolus and its relationship to nucleolar activities are still unclear.
We have previously identified a human nucleolar phosphoprotein of 130 kDa (p130) localized mainly in the DFC of nucleoli (39) and defined two isoforms of p130 whose expression is proliferation dependent (40). Human p130 shares high sequence homology with the well-characterized rat nucleolar phosphoprotein Nopp140, which is thought to shuttle between nucleolus and cytoplasm (32). Unlike most other nucleolar proteins, neither p130 nor Nopp140 carries RNA-binding motifs or glycine/arginine-rich stretches in the deduced amino acid sequences. Instead, both exhibit a unique three-domain structure: the evolutionarily conserved amino- and carboxyl-terminal domains and the central repeated region which contains 10 acidic serine clusters alternating with basic proline-rich stretches. The negatively and positively charged central repeats can provide potentials for protein-protein interactions (34, 36, 39). We have previously shown that immunoprecipitated p130 can be triggered by Mg2+ and F− to form large protein complexes (12). Furthermore, p130 is characterized as a GTP/ATP-binding protein with associated GTPase/ATPase activities (12). Despite these biochemical characteristics, the biological functions of Nopp140/p130 are still the subject of intensive study.
Significant advances have been made recently in the identification of proteins associated with Nopp140. The interaction of Nopp140 with a constituent of the coiled body, p80-coilin (3), implies that Nopp140 can function as a molecular link between the nucleolus and the coiled bodies (21). In addition, Nopp140 was found to associate with casein kinase II (29), a protein kinase involved in growth control and also in the regulation of rDNA transcription (1, 19, 56, 61, 62). While casein kinase II heavily phosphorylates both Nopp140 and p130 (32, 39), the significance of their constant interactions remains unclear. A mammalian nucleolar protein, NAP57, has been identified as a Nopp140-associated protein that colocalizes with Nopp140 in the DFC of nucleolus and in the coiled bodies (33). Implications of roles for NAP57 came mostly from analysis of its yeast homologue Cbf5p, which is characterized as a putative rRNA pseudouridine synthase involved in rRNA synthesis and pre-rRNA processing (7, 23, 27). Intriguingly, Nopp140 has been shown recently to function as an RNA polymerase II transcription coactivator and to interact with the general transcription factor TFIIB and a specific DNA motif-binding transcription factor (36). However, the predominant nucleolar localization of the Nopp140 homologous proteins from different species (8, 32, 39) implies the involvement of these proteins in activities carried out within the nucleolus. For consistency with the literature, we rename our p130 as human Nopp140 (hNopp140). Here, we report the identification of the largest subunit of RNA polymerase I (RPA194) as an hNopp140-interacting protein and the analysis of roles for hNopp140 in rRNA synthesis and in nucleolar structural organization.
MATERIALS AND METHODS
Antibodies.
Monoclonal antibodies used included CP2 (immunoglobulin G2a [IgG2a]) and HC2 (IgG2b) (anti-hNopp140) (12, 39), CC98 (IgG1; antinucleolin (11), M2 (IgG1; anti-FLAG; Eastman Kodak, New Haven, Conn.), and an anti-bromodeoxyuridine antibody (IgG1; Sigma, St. Louis, Mo.). Rabbit antiserum specific to RPA194 (51) was a generous gift from I. Grummt (German Cancer Research Center, Heidelburg, Germany). A human autoimmune serum against fibrillarin (S4) was kindly provided by U. Scheer (University of Würzburg, Würzburg, Germany).
Cell culture, actinomycin D treatment, and metabolic labeling.
HeLa, CEM, COS7, and hybridoma cell lines were grown as described previously (39). For actinomycin D treatment, cells were incubated in culture medium containing a low concentration (0.08 μg/ml) of actinomycin D (Sigma) for 6 h. For metabolic labeling, cells grown on 15-cm-diameter petri dishes were washed twice with Hanks’ balanced salt solution (GIBCO BRL, Gaithersburg, Md.) and then incubated in 10 ml of methionine-free medium (Sigma) containing 10% dialyzed fetal calf serum for 1 h. After addition of 0.5 mCi of [35S]methionine (1,000 Ci/mmol; NEN Life Science Products, Boston, Mass.) to the culture medium in each dish, cells were subjected to an additional incubation at 37°C for 4 h.
Cell lysis, immunoprecipitation, and Western blot analysis.
To prepare whole-cell lysates, 2 × 107 cells were lysed in 1 ml of lysis buffer (50 mM Tris-HCl [pH 7.5], 0.15 M NaCl, 1% Triton X-100, 2 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 1 μg of aprotinin per ml, 1 μg of leupeptin per ml) at 4°C for 1 h as described previously (12). For immunoprecipitation experiments, anti-FLAG M2 affinity gels (Eastman Kodak) or protein A-Sepharose beads (Amersham Pharmacia Biotech, Uppsala, Sweden) preadsorbed with anti-hNopp140 monoclonal antibody HC2, anti-major histocompatibility complex class I monoclonal antibody Y16 (antibody control), or NS1 culture supernatant (mock control) were incubated with cell lysates at 4°C for 2 h. The bound proteins were eluted as described previously (12). For detection with Coomassie blue staining, immunoprecipitates from about 3 × 108 CEM cells were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). For Western blotting or autoradiography, immunoprecipitates from about 2 × 107 HeLa cells were applied. Western blot analysis was performed essentially as described previously (39) except that the blots were developed by enhanced chemiluminescence detection reagents (Amersham Pharmacia Biotech) according to the manufacturer’s instructions.
Protein sequencing.
For purification of hNopp140-associated proteins, HeLa cell lysates from about 5 × 108 cells were subjected to immunoprecipitation with anti-hNopp140 antibody HC2. Bound proteins were separated by SDS-PAGE and electrophoretically transferred to polyvinylidene difluoride membranes (Micron Separations Inc., Westborough, Mass.). Protein sequencing was performed by J. Leszyk (Core laboratory for protein chemistry, University of Massachusetts). The proteins were digested in situ with trypsin followed by matrix-assisted laser desorption/ionization–time-of-flight mass spectrometry using a Perseptive Biosystems (Framingham, Mass.) liner biospectrometry workstation and α-cyano-4-hydroxy cinnamic acid as the matrix (17). Based on the mass data, the National Center for Biotechnology Information nonredundant database was searched by using the University of California at San Francisco mass spectrometry facility’s MSFIT program. In the case of p40, the search was restricted to mammalian proteins in the size range of 1,000 to 100,000 Da, using a 0.1% peptide mass tolerance and a minimum requirement of 10 mass matches. The only hit observed matched (15 of 15 masses) the α-chain of human casein kinase II. For p190, the search was restricted to Homo sapiens proteins in the range of 1,000 to 200,000 Da, using a 0.1% peptide mass tolerance and a minimum requirement of 15 mass matches. Only one hit was found, with 18 of 22 masses matching the largest subunit of human RNA polymerase I. To further verify this identification, a single mass fraction (1,830 Da) from the digest mixture of p190 was selected for Edman degradation using an Applied Biosystems 494 Procise protein sequencer.
Mammalian expression constructs.
Expression vectors pFLAG-CMV2 (Eastman Kodak) and pEGFP-C3 (Clontech Laboratories, Palo Alto, Calif.), both driven by the cytomegalovirus promoter, were used to express protein fused N terminally with a FLAG epitope tag or green fluorescent protein (GFP) tag, respectively. A SalI-BamHI (2.5-kb) fragment of hNopp140 cDNA which encompasses the entire coding region and the stop codon was cloned in frame to the pFLAG-CMV2 vector to produce plasmid F-hNopp140. The C-terminally deleted constructs of hNopp140 cDNA were generated by restriction enzyme digestions to remove the hNopp140 cDNA sequence downstream of the HindIII, KpnI, and XhoI sites, which gave the partial hNopp140 constructs containing amino acids 1 to 605 (F-hNopp140N605), 1 to 382 (F-hNopp140N382), and 1 to 204 (F-hNopp140N204), respectively. The N-terminally deleted constructs F-hNopp140C316 and F-hNopp140C285 were created by inserting the KpnI-BamHI (1.3-kb) or HindIII-BamHI (1.2-kb) fragments from F-hNopp140 into the pFLAG-CMV2 vector in frame with the FLAG epitope at the 5′ end. The XhoI-PstI (0.6-kb) fragment of hNopp140 cDNA was subcloned in frame to the pFLAG-CMV2 vector to make the F-hNopp140M204-398. A 68-bp BglII-KpnI fragment containing the nuclear localization signal (NLS; SPKKKRKV) of simian virus 40 (SV40) large T antigen from pSV-NLS-LacZ (30) was subcloned into the pFLAG-CMV2 vector in frame with the FLAG-tag at the 5′ end to make plasmid pF-NLS. The construct F-NLS-hNopp140M204-383 was generated by inserting the sequence spanning the XhoI-KpnI (0.5-kb) fragment of hNopp140 into plasmid pF-NLS downstream of the NLS. The inserts containing the partial hNopp140 cDNAs from the F-hNopp140N382 and F-hNopp140C285 were subcloned in frame to the pEGFP-C3 vector to make G-hNopp140N382 and G-hNopp140C285, respectively.
Transfections.
Primarily HeLa cells but also COS7 cells were used for the transfection studies and gave identical results. Subconfluent cells grown on glass coverslips or 15-cm-diameter petri dishes were transiently transfected with purified plasmid DNA (2 μg per coverslip or 20 μg per dish) by mixing with Lipofectamine (GIBCO BRL) according to the manufacturer’s protocol. The cells were analyzed approximately 48 h after transfection unless otherwise specified.
Immunofluorescence microscopy.
The procedure for indirect immunofluorescence staining was modified from a method described previously (11). Cells were usually fixed with 2% formaldehyde in phosphate-buffered saline (PBS) for 20 min at room temperature, followed by permeabilization with −20°C acetone for 3 min and washing once with PBS. For staining with anti-RPA194 antibody, cells were fixed with 1% paraformaldehyde in PBS at room temperature for 20 min, and then permeabilized with 0.5% Triton X-100 in PBS for 5 min. Cells were rinsed twice with PBS and then incubated with primary antibodies for 1 h. After being washed with PBS three times, cells were incubated with secondary antibodies conjugated with a fluorescent dye or biotin for 1 h. Then the coverslips were washed three times in PBS. An additional incubation with UltraAvidin-rhodamine conjugate (Leinco, Ballwin, Mo.) was performed to detect the biotin-conjugated secondary antibody. For costaining of DNA, Hoechst 33258 (0.4 μg/ml; Sigma) was added to the last staining solution.
Different sets of antibodies were used for double immunofluorescence. For double staining of RNA polymerase I and hNopp140, anti-RPA194 antiserum was detected by fluorescein isothiocyanate-conjugated goat anti-rabbit antibody (Jackson ImmunoResearch, West Grove, Pa.), while anti-hNopp140 antibody CP2 was recognized by Alexa 594-conjugated goat anti-mouse IgG (Molecular Probes, Eugene, Oreg.). For costaining of fibrillarin and hNopp140, human antifibrillarin autoimmune serum S4 was detected by rhodamine-conjugated rabbit anti-human IgG (Jackson), while anti-hNopp140 CP2 antibody was recognized by Alexa 488-conjugated goat anti-mouse IgG (Molecular Probes). For double staining by monoclonal antibodies of subclasses IgG1 and IgG2a, the primary IgG1 antibody (antinucleolin CC98, anti-FLAG, or antibromodeoxyuridine) was detected by biotin-conjugated goat anti-mouse IgG1 (Caltag, San Francisco, Calif.) followed by UltraAvidin-rhodamine conjugate (Leinco), whereas the primary IgG2a antibody (anti-hNopp140 CP2) was detected by fluorescein isothiocyanate-conjugated goat anti-mouse IgG2a (Caltag). Control experiments showed that no significant background was observed in the absence of primary antibody, and no bleed-through fluorescence was detected in samples labeled singly with either primary antibody.
Samples mounted in antifade fluid (150 mM Tris-HCl [pH 8.8], 90% glycerol, 1 mg of p-phenylenediamine per ml) were examined under an Olympus BX50 microscope equipped with BX-FLA epifluorescence optics and photographed on Kodak Elite Chrome 400 films. The digital images were obtained by scanning the films with a film scanner (LS-2000; Nikon, Tokyo, Japan).
Confocal laser scanning microscopy and image processing.
Confocal laser scanning microscopy was performed with a Leica TCS-NT confocal microscope (Leica Lasertechnik GmbH, Heidelberg, Germany). For double labeling experiments, images from the same confocal plane were recorded. The images were assembled on a Macintosh computer equipped with an Adobe Photoshop 4.0 software program.
In situ run-on transcription assay.
In situ run-on transcription was performed as previously described (22, 63), with a few modifications. Cells grown as monolayers on coverslips were washed twice in PBS and permeabilized with digitonin (150 μg/ml; Sigma) in PB buffer (22 mM NaCl, 1 mM MgCl2, 8 mM KCl, 11 mM K2HPO4, 100 mM CH3COOK, 1 mM ATP, 1 mM dithiothreitol, 0.2 mM phenylmethylsulfonyl fluoride [pH 7.4]) for 4 min on ice. Then the coverslips were washed once with PB buffer and incubated on ice for 10 min with PB buffer supplemented with α-amanitin (100 μg/ml; Sigma) to inhibit activities of RNA polymerases II and III. Subsequently, transcription mix (10× concentrate) was added to give final concentrations of 2 mM ATP, 0.1 mM CTP, 0.1 mM GTP, 0.2 mM 5-bromouridine 5′-triphosphate (Br-UTP; Sigma), and 2 mM MgCl2. The run-on transcription was carried out at 33°C for 20 min and was terminated by rinsing the coverslips in ice-cold PBS. For control experiments, actinomycin D (0.2 μg/ml) was added in the transcription reaction mixture to inhibit transcription. Cells were then fixed with 2% formaldehyde in PBS at room temperature for 20 min, followed by permeabilization with 0.5% Triton X-100 in PBS for 3 min on ice. Br-UTP labeling was detected by immunofluorescence staining with an antibromodeoxyuridine monoclonal antibody (Sigma), referred to here as anti-Br-UTP antibody for its cross-reactivity with Br-UTP.
RESULTS
RPA194 and the α-chain of casein kinase II can be coimmunoprecipitated with hNopp140.
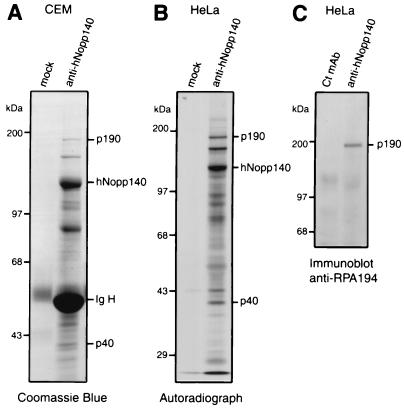
To investigate proteins that interact with hNopp140, we performed immunoprecipitation with anti-hNopp140 monoclonal antibody from cell lysates of unlabeled CEM or [35S]methionine-labeled HeLa cells. Subsequent SDS-PAGE analysis showed that several polypeptides together with hNopp140 were precipitated by anti-hNopp140 antibody but not by mock controls (Fig. 1A and B). Two proteins with apparent molecular masses of 190 and 40 kDa (p190 and p40) were consistently detected in the hNopp140 immunoprecipitates. From lysates of cells transfected with F-hNopp140 (Fig. 4), p190 and p40 were also coimmunoprecipitated with the recombinant hNopp140 by anti-FLAG antibody (data not shown). These results suggest that p190 and p40 can form a stable complex with hNopp140. To identify these putative hNopp140-interacting proteins, the purified p190 and p40 were subjected to trypsin digestion followed by mass spectrometry. Interestingly, the mass values of p190-derived peptides matched that of the largest subunit of human RNA polymerase I, RPA194. To verify its identity, one tryptic peptide of p190 was selected for Edman degradation; this analysis resulted in the sequence ELVLNTEGINLPELFK, which is identical to that of human RPA194 (amino acids 1573 to 1588). Coimmunoprecipitation of RPA194 with hNopp140 was further confirmed by Western blotting with a known antibody that recognizes human RPA194 (Fig. 1C). On the other hand, p40 was identified on the basis of its mass as the α chain of human casein kinase II, consistent with a previous report that casein kinase II binds to rat Nopp140 (29). Our results suggest that hNopp140 interacts with both RNA polymerase I and casein kinase II.
FIG. 1.
Identification of cellular proteins that interact with hNopp140. (A) The hNopp140-coimmunoprecipitated complex detected by Coomassie blue staining. Total lysates of CEM cells were immunoprecipitated with either anti-hNopp140 monoclonal antibody HC2 or a mock control immobilized on protein A beads. Bound proteins were revealed by Coomassie blue staining after SDS-PAGE. The identity of hNopp140 was confirmed by Western blotting in a parallel experiment. p190 and p40 were subjected to trypsin digestion, followed by mass spectrometry and peptide sequencing. Ig H, immunoglobulin heavy chain. (B) The hNopp140-interacting proteins revealed by radioimmunoprecipitation assay. HeLa cells were metabolically labeled with [35S]methionine. Immunoprecipitation was performed on the cell lysates with either anti-hNopp140 antibody or a mock control, followed by SDS-PAGE and autoradiography. (C) Identification of p190 as the RPA194, the largest subunit of RNA polymerase I. HeLa cell lysates were immunoprecipitated by either anti-hNopp140 antibody or a subclass-matched control monoclonal antibody, Y16 (Ct mAb). The p190 copurified with hNopp140 was immunoreactive with anti-RPA194 antibody by Western blot analysis.
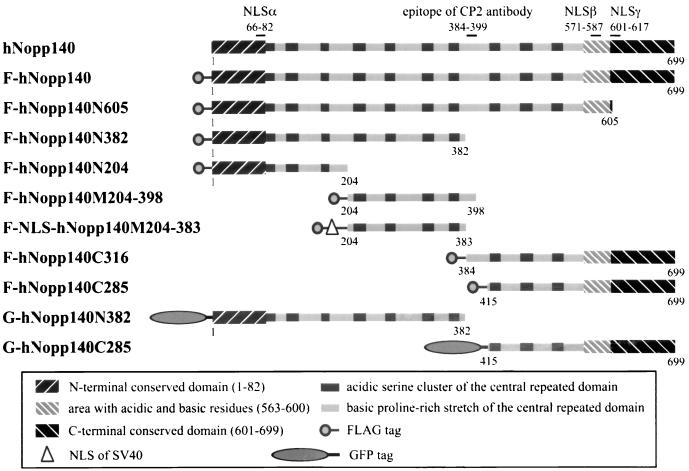
FIG. 4.
Schematic representation of hNopp140 domain structure and a series of truncated forms of hNopp140. The hNopp140 cDNA or its deletion mutants were cloned into the expression vectors pFLAG-CMV2 and the pEGFP-C3 in frame with the N-terminal FLAG epitope (denoted by prefix “F”) or GFP (denoted by prefix “G”), respectively. To construct F-NLS-hNopp140M204-383, the NLS from the SV40 large T antigen was inserted in frame downstream of the FLAG tag and upstream of the sequence from hNopp140. Numbering refers to the amino acid residues on hNopp140 (39). N, N-terminal portion of hNopp140; M, middle portion of hNopp140; C, C-terminal portion of hNopp140.
RNA polymerase I colocalizes with hNopp140 in the nucleolus.
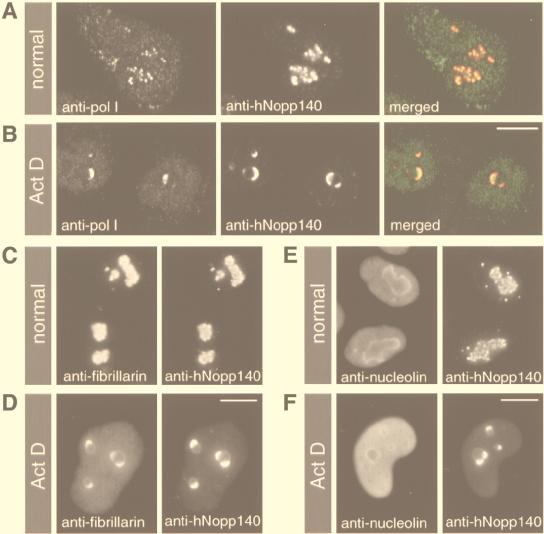
The RPA194 subunit is an integral component of RNA polymerase I core enzyme (51) that localizes in nucleoli (43). While hNopp140 is also a nucleolar protein, the interaction of hNopp140 with RNA polymerase I would be physiologically meaningful. We carefully examined the localization of RNA polymerase I compared with that of hNopp140 by indirect double immunofluorescence. The staining of RNA polymerase I and hNopp140 revealed similar dot-like structures in nucleoli (Fig. 2A), with their images overlapped under the confocal microscopy (Fig. 2A, merged; the weak staining in the nucleoplasm and cytoplasm was derived from the rabbit antiserum nonspecifically). This result suggests that RNA polymerase I colocalizes with hNopp140 in the nucleolus. For comparison, the distributions of fibrillarin and nucleolin in relation to hNopp140 were also analyzed. Fibrillarin was concentrated in the same hNopp140-containing granules in nucleoli (Fig. 2C), whereas nucleolin was distributed mostly within the entire nucleoli and also within nucleoplasm in a diffuse pattern (Fig. 2E). Those dot-like structures containing RNA polymerase I, hNopp140, and fibrillarin represent the fibrillar components of the nucleolus, i.e., the FC together with the surrounding DFC. It is known that blocking rDNA transcription by actinomycin D (41) leads to segregation of the nucleolar fibrillar components from the granular component (38, 43). Whether hNopp140 and RNA polymerase I remain together in the altered nucleoli was further examined. After actinomycin D treatment, both RNA polymerase I and hNopp140 were redistributed as crescent-shaped structures in the segregated nucleoli (Fig. 2B), with their staining images matched precisely (Fig. 2B, merged). Fibrillarin was also relocated to these crescent-shaped structures, while most nucleolin was dispersed into the nucleoplasm (Fig. 2D and F). Taken together with the coimmunoprecipitation and the colocalization of RNA polymerase I with hNopp140, our results suggest that the interaction between RNA polymerase I and hNopp140 exists in vivo.
FIG. 2.
Subcellular distributions of RNA polymerase I, fibrillarin, and nucleolin relative to hNopp140. HeLa cells without (A, C, and E) or with (B, D, and F) actinomycin D (0.08 μg/ml) treatment for 6 h were subjected to double immunofluorescence staining. (A and B) Colocalization of RNA polymerase I and hNopp140 before or after actinomycin D treatment. Cells were costained with anti-RPA194 antiserum (anti-pol I) and CP2 monoclonal antibody (anti-hNopp140). Images from the same confocal plane were analyzed by confocal laser scanning microscopy. In the merged image, RNA polymerase I (green) and hNopp140 (red) are extensively overlapped (yellow). (C and D) Colocalization of fibrillarin and hNopp140 regardless of the actinomycin D treatment. Cells were double stained with the S4 autoimmune serum (anti-fibrillarin) and CP2 monoclonal antibody (anti-hNopp140). (E and F) Distinct distribution patterns of nucleolin and hNopp140 in normal and actinomycin D-treated cells. Nucleolin and hNopp140 were detected by monoclonal antibodies CC98 and CP2, respectively. Preparations in panels C to F were examined by fluorescence microscopy. Images from the same microscopic field are shown side by side. Act D, actinomycin D. Bars, 10 μm.
Overexpression of hNopp140 mislocalizes RNA polymerase I and disturbs nucleolar integrity.
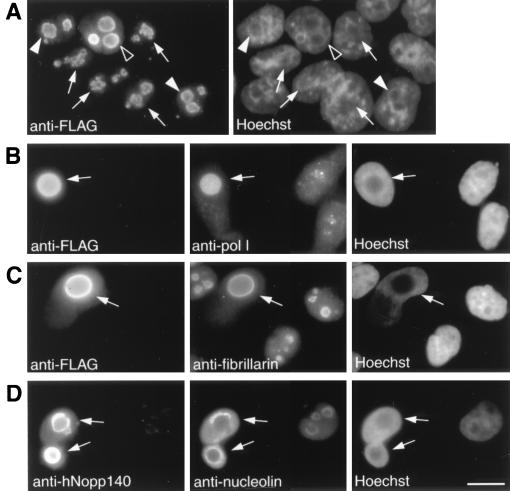
The interaction of hNopp140 with RNA polymerase I was investigated in transfection studies. F-hNopp140 was expressed in either HeLa (Fig. 3) or COS7 (data not shown) cells. Subsequent immunofluorescence analysis using anti-FLAG antibody and the DNA dye Hoechst 33258 showed that typically 10 to 40% of the cells were transfected (Fig. 3A to C; untransfected cells were defined as cells stained positively by Hoechst 33258 but negatively by anti-FLAG antibody). With Hoechst staining, the nucleolar region can be recognized as the area with a relatively low density of DNA. Transfectants expressing small amounts of exogenous hNopp140, as judged by intensities of anti-FLAG immunofluorescence, displayed a dot-like nucleolar staining (Fig. 3A, arrows) indistinguishable from that of the endogenous hNopp140 in normal cells (Fig. 2C and E, right). However, those nucleolar granular pattern became less discrete with a higher level of hNopp140 expression (Fig. 3A, filled arrowheads). Finally, the sphere-like structures with a diffuse and somewhat circular staining were observed in the nucleolar regions which harbored large amounts of ectopically expressed hNopp140 (Fig. 3A, open arrowheads).
FIG. 3.
Effects of ectopic expression of hNopp140. After 48 h of transfection with the FLAG-tagged hNopp140 construct, HeLa cells were subjected to indirect immunofluorescence. The corresponding staining of nuclear DNA by Hoechst 33258 is also displayed. (A) Levels of ectopic expression of hNopp140 monitored by anti-FLAG antibody. Cells expressing small, moderate, and large amounts of FLAG-tagged hNopp140 are marked by arrows, filled arrowheads, and open arrowheads, respectively. (B to D) Localizations of RNA polymerase I, fibrillarin, and nucleolin in relation to hNopp140. The transfectants expressing large amounts of hNopp140 (arrows) were recognized by features of being heavily stained with anti-FLAG antibody (B and C, left) or by the appearance of the large sphere-like structure positively stained with anti-hNopp140 antibody CP2 (D, left). Double staining of the same transfected and the neighboring untransfected cells with antibodies against RNA polymerase I, fibrillarin, and nucleolin is shown in the middle images of panels B to D. Bars, 10 μm.
We next examined whether other nucleolar proteins were affected by overexpression of hNopp140. In hNopp140 transfectants with sphere-like nucleoli, both RNA polymerase I and fibrillarin were redistributed to fill up the whole nucleolar area similarly to the overexpressed hNopp140 (Fig. 3B and C, arrows). In contrast, nucleolin was dispersed into the nucleoplasm away from those bulky sphere-like nucleoli (Fig. 3D, arrows). It seems that those sphere-like nucleoli induced by overexpression of hNopp140 retain only a certain subset of nucleolar proteins such as hNopp140 itself (Fig. 3D, left), RNA polymerase I, and fibrillarin but not nucleolin. The mislocalization of these essential nucleolar proteins indicates that the nucleolar integrity was disrupted. Nevertheless, RNA polymerase I, fibrillarin, and hNopp140 remained colocalized in these altered nucleoli.
hNopp140 interacts with the largest subunit of RNA polymerase I through amino acids 204 to 382.
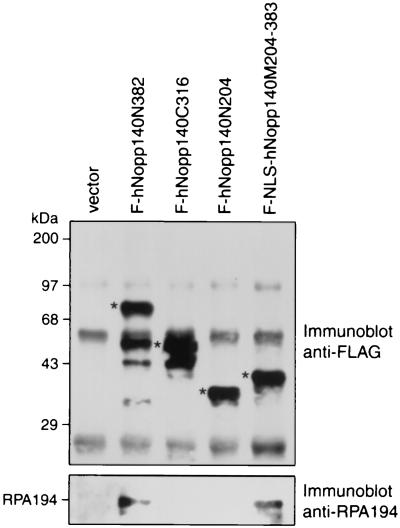
To further study the interaction between hNopp140 and RNA polymerase I, we proceeded to map the domain on hNopp140 for interaction with RPA194. We constructed a series of deletion mutants of hNopp140 and fused each at its amino terminus to the FLAG epitope (Fig. 4). The deduced amino acid sequence of hNopp140 exhibited three conventional bipartite NLSs (44) (Fig. 4, NLSα, NLSβ, and NLSγ). Since no NLS resides in amino acids 204 to 383, an NLS of SV40 large T antigen was added in frame between the FLAG epitope and the sequence encoding amino acids 204 to 383 of hNopp140 to create the F-NLS-hNopp140M204-383 construct for nuclear expression. Mutant proteins representing different regions of hNopp140 were expressed in HeLa cells and immunoprecipitated with anti-FLAG antibody. Subsequent Western blot analysis with anti-FLAG (Fig. 5, top) and anti-RPA194 (Fig. 5, bottom) antibodies showed that deletion mutants F-hNopp140N382 and F-NLS-hNopp140M204-383 retained the ability to pull down RPA194. In contrast, neither F-hNopp140C316 nor F-hNopp140N204 could interact with RPA194. Similar results were obtained for radioimmunoprecipitation experiments using HeLa cells transfected with various hNopp140 deletion mutants (data not shown). From these observations, it is clear that amino acids 204 to 382 of hNopp140 are responsible for interaction with RPA194.
FIG. 5.
Determination of the domain of hNopp140 for binding of RPA194. HeLa cells were transiently transfected with partial constructs of hNopp140 or vector alone as indicated. The cell lysates were immunoprecipitated with anti-FLAG antibody followed by SDS-PAGE and Western blotting. Interactions of truncated hNopp140 with RPA194 were analyzed on blots probed with anti-FLAG (top) and anti-RPA194 (bottom) antibodies. The asterisks mark the truncated hNopp140 proteins with expected molecular masses. Several smaller forms are probably proteolytic breakdown products. Common signals, also seen in the control transfected with vector alone, were generated from the dissociated heavy and light chains of anti-FLAG antibody that had been covalently linked to the beads. Positions of molecular mass standards and RPA194 are indicated on the left.
Nucleolar structure altered by truncated hNopp140.
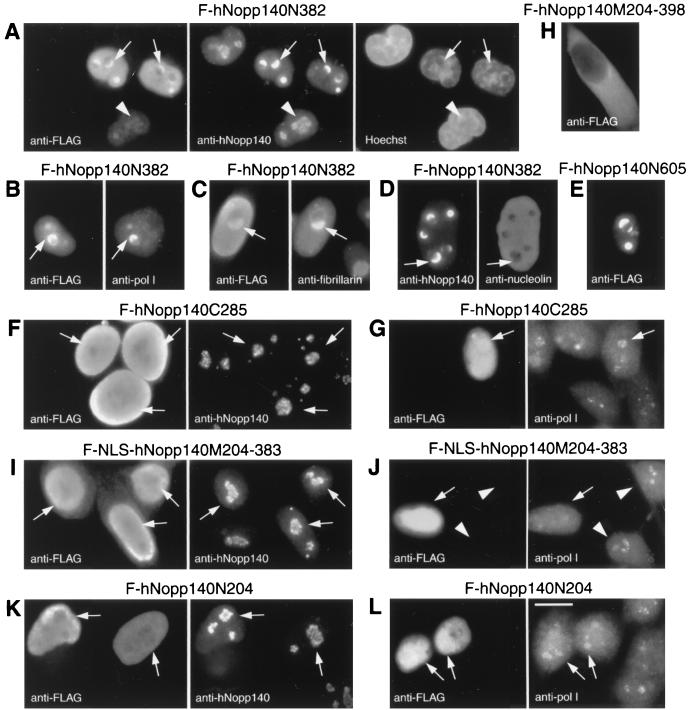
To investigate effects of different regions of hNopp140, we carried out transfection of HeLa cells with partial hNopp140 constructs F-hNopp140N382, F-hNopp140N204, F-NLS-hNopp140M204-383, and F-hNopp140C285 (Fig. 4) followed by indirect immunofluorescence. These constructs avoid the epitope region (amino acids 384 to 399) of anti-hNopp140 monoclonal antibody CP2 to allow detection of the endogenous hNopp140 by this antibody without contamination with ectopically expressed partial hNopp140. Proteins derived from the N-terminal half of hNopp140, hNopp140N382 (amino acids 1 to 382), were initially localized to the nucleolus and gave a dot-like staining pattern (Fig. 6A, arrowheads). Surprisingly, a higher level of hNopp140N382 expression induced formation of the crescent-shaped structures in nucleoli (Fig. 6A to D, arrows). The crescent-shaped structures induced by hNopp140N382 could recruit the endogenous hNopp140, RNA polymerase I, and fibrillarin (Fig. 6A to C; compare signals marked by arrows in each of the paired panels). In contrast, most nucleolin was chased out of the nucleolus into the nucleoplasm (Fig. 6D, right). These observations indicate that ectopic expression of hNopp140N382 can lead to alterations of the nucleolar structure in a particular way. Similar results were obtained from transfection carried out in COS7 cells (data not shown). In addition, stable transfectants of hNopp140N382 under a Tet-Off inducible promoter (18a) gave similar phenotypic changes after induction (data not shown). Interestingly, these effects of hNopp140N382 expression were analogous to events caused by actinomycin D (Fig. 2B, D, and F). It seems that hNopp140N382 exerts a dominant negative effect on the endogenous hNopp140, resulting in mislocalization of RNA polymerase I, fibrillarin, and nucleolin. A larger construct of hNopp140, mutant hNopp140N605 (amino acids 1 to 605), caused the formation of similar crescent-shaped structures in the nucleolus (Fig. 6E). On the other hand, expression of the C-terminal part of hNopp140, hNopp140C285 (amino acids 415 to 699), had no significant effects on both endogenous hNopp140 and RNA polymerase I (Fig. 6F and G, arrows).
FIG. 6.
Mislocalizations of nucleolar proteins by expressing truncated hNopp140. HeLa cells were transfected with FLAG-tagged hNopp140-partial constructs F-hNopp140N382 (A to D), F-hNopp140N605 (E), F-hNopp140C285 (F and G), F-hNopp140M204-398 (H), F-NLS-hNopp140M204-383 (I and J), and F-hNopp140N204 (K and L). After 48 h of transfection, cells were examined by indirect double immunofluorescence. Ectopic expression of FLAG-tagged mutants of hNopp140 was monitored by anti-FLAG antibody, whereas the endogenous hNopp140, RNA polymerase I (pol I), fibrillarin, and nucleolin were visualized with their specific antibodies as indicated. Nuclear DNA was stained with Hoechst 33258. Arrows in panels A to D indicate the crescent-shaped structures formed in nucleoli of cells expressing large amounts of F-hNopp140N382; arrowheads in panel A point out one cell expressing only a small amount of F-hNopp140N382 without forming the crescents. The transfected cells in images in panels F, G, I, J, K, and L are marked by arrows. Untransfected cells are those stained by antibodies to nucleolar protein but not by anti-FLAG (e.g., arrowheads in images in panel J). Bars, 10 μm.
Proteins derived from mutant hNopp140M204-398 (amino acids 204 to 398) were retained only in the cytoplasm possibly due to the lack of an NLS (Fig. 6H). Therefore, F-NLS-hNopp140M204-383 (amino acids 204 to 383) with an additional NLS was constructed as described above. Since proteins expressed from F-NLS-hNopp140M204-383 were sufficient to interact with RNA polymerase I (Fig. 5), we examined whether such a mutant might affect the localization of RNA polymerase I. Exogenous NLS-hNopp140M204-383 was distributed throughout the whole nuclei (Fig. 6I, left) without significant effects on the endogenous hNopp140 (Fig. 6I, arrows in the right-hand image). However, the nucleolar staining of RNA polymerase I, which no longer displayed discrete nucleolar granules, was diminished (Fig. 6J, arrows; compare with that of the untransfected cells noted by arrowheads). It is likely that a portion of RNA polymerase I molecules were mislocalized through their interactions with the nucleoplasmic NLS-hNopp140M204-383. On the other hand, expression of hNopp140N204 (amino acids 1 to 204), which lost the ability to interact with RNA polymerase I (Fig. 5), showed no effect on both RNA polymerase I and endogenous hNopp140 (Fig. 6K and L, arrows). From observations for all hNopp140 mutants, we conclude that the region from amino acids 204 to 382 is responsible for association of hNopp140 with RNA polymerase I in vivo, in good agreement with a role for this domain of hNopp140 in interaction with RNA polymerase I.
rDNA transcription is shut off by overexpression of the full-sized hNopp140 or its truncated mutant with dominant negative effect.
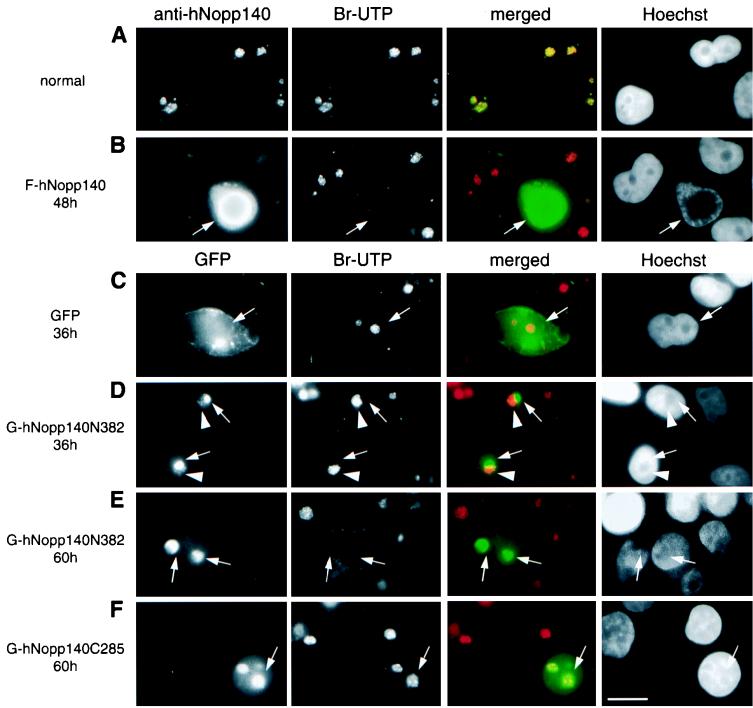
As mentioned above, ectopic expression of the dominant negative mutant hNopp140N382, or large amounts of full-sized hNopp140, results in mislocalization of RNA polymerase I accompanied by alterations in nucleolar structure. Whether rDNA transcription was affected in these transfectants was examined by in situ run-on assays. In such a transcription assay, the activity of RNA polymerase I was promoted by supplementation with α-amanitin to suppress transcriptions from RNA polymerases II and III. Thus, Br-UTP was preferentially incorporated into the newly synthesized rRNA, and the modified transcripts were detected by anti-Br-UTP antibody. In normal cells, run-on labeling was observed predominantly as intense fluorescent spots within nucleoli representing active sites of rDNA transcription (Fig. 7A, Br-UTP channel). Interestingly, the nucleolar staining of hNopp140 coincided with the uptake of Br-UTP (Fig. 7A, merged channel) at the foci for rDNA transcription. This result is consistent with colocalization of hNopp140 with RNA polymerase I (Fig. 2A). With the formation of the sphere-like structure by hNopp140 overexpression, the nucleolar uptake of Br-UTP no longer occurred (Fig. 7B, arrows; compare with the surrounding cells with normal uptake of Br-UTP), suggesting that rRNA synthesis was hampered.
FIG. 7.
Effects on rDNA transcription after ectopic expression of hNopp140 or its derivatives. HeLa cells grown under normal conditions or after transfection with constructs of GFP alone, full-sized hNopp140, or hNopp140-partial mutants tagged with GFP (marked on the right) were subjected to in situ run-on assay (see Materials and Methods). Cells were either double stained by anti-hNopp140 and anti-Br-UTP (A and B) or single stained by anti-Br-UTP combined with the direct observation of the GFP signals (C to F). Nucleolar labeling of Br-UTP indicates active sites of rDNA transcription (Br-UTP channel). The nucleolar regions can be recognized as areas with relatively low densities of DNA (Hoechst channel, DNA staining with Hoechst 33258). Localizations of the endogenous and exogenous full-sized hNopp140 were detected by anti-hNopp140 antibody CP2 (anti-hNopp140 channels of panels A and B). The truncated hNopp140 was revealed by its tagged GFP (GFP channels of panels D to F). Signals from anti-hNopp140 and GFP (green) and from anti-Br-UTP (red) were merged (merged channels of all panels). In normally growing HeLa cells (A), hNopp140 foci coincide with sites of Br-UTP uptake (yellow in merged channel) within nucleolar areas. However, in an hNopp140-overexpressing cell with a bulky sphere-like nucleolus (arrows in panel B; hNopp140 was overexpressed at least 10-fold as estimated by the fluorescence intensities of the transfected cell and the neighboring untransfected cell), nucleolar labeling of Br-UTP was eliminated (Br-UTP channel), while the surrounding untransfected cells exhibited the normal uptake of Br-UTP (note that the green fluorescence from the left image of panel B was exposed for a shorter time due to the presence of a high level of hNopp140 in the transfected cell; therefore, the merged images for these untransfected cells could not turn entirely yellow). Expression of GFP alone (C) or G-hNopp140C285 (F) had no obvious effects on rDNA transcription (arrows). At 36 h after transfection with G-hNopp140N382 (D), the partially formed crescent-shaped structures (arrows) failed to incorporate Br-UTP, whereas the residual granular parts of the same nucleolus (arrowheads) showed normal Br-UTP uptake. At 60 h after transfection with G-hNopp140N382 (E), the crescent-shaped structures (arrows) ceased rDNA transcription. Bars, 10 μm.
The phenotype of hNopp140N382 transfectants is reminiscent of the segregated nucleoli in actinomycin D-treated cells. Since actinomycin D is known as an inhibitor of rDNA transcription, we wonder whether hNopp140N382 exerts negative effects on rRNA synthesis. Cells transfected with G-hNopp140N382, a hNopp140N382 mutant fused N terminally with GFP, were subjected to the run-on assay. The GFP instead of FLAG tag was chosen in this study to avoid the double staining procedures due to the fact that anti-FLAG and anti-Br-UTP monoclonal antibodies belong to the same subclass (IgG1). GFP itself had no disturbing effect on rDNA transcription because the nucleolar uptake of Br-UTP occurred normally in the cell expressing GFP (Fig. 7C). Similar to the FLAG-tagged hNopp140N382, the GFP-tagged hNopp140N382 caused the formation of crescent-shaped structures in nucleoli at 60 h after transfection (Fig. 7E, GFP channel). Evidently, Br-UTP incorporation was abolished in these altered nucleoli (Fig. 7E, arrows), indicating that rDNA transcription was blocked after expression of hNopp140N382. In contrast, expression of GFP-tagged hNopp140C285 encompassing the C-terminal part of hNopp140 (amino acids 415 to 699) had no obvious effect on rDNA transcription at 60 h posttransfection (Fig. 7F, arrows). At an earlier time point (36 h) after transfection of G-hNopp140N382, the crescent-shaped structures were partially formed (Fig. 7D, arrows, GFP channel), whereas a few dot-like structures were still preserved in the residual part of the same nucleolus (Fig. 7D, arrowheads, GFP channel). This observation may represent an intermediate stage during the course of nucleolar alterations induced by hNopp140N382. Intriguingly, the labeling of Br-UTP was inhibited only at the site of crescent-shaped structures harboring large amounts of exogenous hNopp140N382 (Fig. 7D, arrows), while rDNA transcription remained active in the residual granules at the counterpart of the same nucleolus (Fig. 7D, arrowheads; note that green and red colors did not superimpose in the merged channel). These results demonstrated that the inhibition of rDNA transcription occurs progressively along with the expression of hNopp140N382. Because hNopp140N382 retained the abilities to localize to the nucleolus (Fig. 6A) and to interact with RNA polymerase I (Fig. 5), we conclude that mutant hNopp140N382 may exert negative effects on rDNA transcription through competitive binding of the functionally crippled mutant hNopp140 to RNA polymerase I.
DISCUSSION
In high eukaryotes, the nucleolus displays a concentric arrangement of three structural components: FC, DFC, and GC (50). The central element is the FC that is surrounded by a compact layer of the DFC. Evidence shows that rDNA transcription occurs largely at the boundary between the FC and the DFC (13, 20, 42, 58). The FC together with the surrounding DFC has been considered as a functional unit for rRNA synthesis and processing (47). Fibrillarin, a major constituent of the DFC, has been shown to colocalize with RNA polymerase I and nascent rRNA transcripts even after spreading out the nucleoli into separated spherical bodies (15). These observations suggest the existence of a close structural and functional relationship between FC and DFC. However, molecular mechanisms underlying these interactions remain largely unknown. Previously, Nopp140 homologous proteins have been found to localize mainly in the DFC (32, 39). Rat Nopp140 has been shown to associate with NAP57, a nucleolar protein present in the DFC (33). Here, we demonstrate that hNopp140 interacts and colocalizes with RNA polymerase I, resident in the FC. Therefore, Nopp140 interacts with both DFC and FC proteins. It is likely that a portion of Nopp140 is located in the vicinity of the FC where it can interact with RNA polymerase I. In this regard, Nopp140 may serve as a molecular link between FC and DFC in the nucleolus.
Greatly enlarged sphere-like structures in nucleoli were derived by ectopic expression of hNopp140 in excess amounts. RNA polymerase I and fibrillarin were mislocalized to fill up the entire sphere-like nucleolar area, whereas nucleolin was disseminated into the nucleoplasm. It is notable that only a particular subset of nucleolar proteins, mostly from FC and DFC, is retained in the hNopp140-derived sphere-like nucleoli. More importantly, gradually increased sizes of the hNopp140-containing nucleolar spheres, ranging from the functionally normal granules to the transcriptionally inactive bulky spheres, were correlated with the levels of ectopic expression of hNopp140. It seems that Nopp140 even in large excess tends to be incorporated into a spherical mass in the nucleolus. However, RNA polymerase I transcription occurs under conditions with balancing amounts of Nopp140 that may be important for maintaining the normal dotted granules, i.e., FC with the surrounding DFC. Interestingly, overexpression of an hNopp140 mutant that encompasses the central repeats (amino acids 83 to 605) produced a small number of spherical structures though mainly distributed in the nucleoplasm and cytoplasm (data not shown). Similar structures, termed R rings, derived by expression of the central repeated domain of rat Nopp140 (amino acids 59 to 585) have been reported (21). Likewise, these R rings attract nucleolar proteins of the FC and DFC including RNA polymerase I, UBF, fibrillarin, NAP57, and endogenous Nopp140, but exclude those proteins enriched in the GC, such as nucleolin and B23. Therefore, the central repeated domain of Nopp140 alone is possibly responsible for forming such sphere-like structures. Unlike the sphere-like nucleoli derived by overexpression of full-sized hNopp140, those spheres generated by the repeated domain of Nopp140 were only occasionally localized to the nucleolar regions (data not shown and reference 21). It seems that the N- and C-terminal domains of Nopp140 may contribute in some way to placing these sphere-like structures more effectively in nucleoli. Together, the previous observation and our results suggest that the repeated domain of Nopp140 may trigger the formation of a supracomplex harboring nucleolar proteins of the FC and DFC. Therefore, we speculate that Nopp140 may play a role in maintenance of the fundamental structure of the FC and DFC in the nucleolus.
The central repeated region of Nopp140 consists of acidic serine clusters alternating with basic stretches (8, 32, 39). A number of viral or cellular transcription factors for RNA polymerase II-dependent transcription, such as ICP4 (53), PC4/p15 (16, 26), and Sox-4 (60), carry similar acidic serine clusters. Some evidence suggests that these serine-rich regions are required for transcriptional activation possibly through interaction with basal transcription factors (24, 26, 53). Within the central repeated region, the domain on hNopp140 for interaction with the largest subunit of RNA polymerase I was mapped to amino acids 204 to 382. Accordingly, the central repeated region of Nopp140 may provide potentials for interactions with other nucleolar components essential for RNA polymerase I-dependent transcription. Casein kinase II has been shown to associate with RNA polymerase I holoenzyme (1, 19). Our results showed that both the largest subunit of RNA polymerase I and the α chain of casein kinase II were present in the immunoprecipitates of hNopp140. In this context, we reason that Nopp140 may complex with the RNA polymerase I holoenzyme.
Three classes of RNA polymerases, RNA polymerases I, II, and III, exist in eukaryotic cells and catalyze the synthesis of rRNA, mRNA, and 5S rRNA as well as tRNA, respectively. Several transcription regulators, such as nucleolin (14, 67), retinoblastoma protein (10, 64, 66), p53 (6, 9), and Staf (46), are known to modulate directly or indirectly the transcriptional activities of different classes of RNA polymerases. Rat Nopp140 has been shown to function as an RNA polymerase II transcription factor (36). Nevertheless, because of the major location in nucleoli, we believe that Nopp140 may play a role equivalent to that of the transcription factor RNA polymerase I. Previous evidence has suggested that the Nopp140 homologous proteins may be involved in rRNA biosynthesis. For example, SRP40, encoding a yeast homologue of Nopp140 (31), was initially identified as a weak multicopy suppressor of a temperature-sensitive mutation in the common AC40 subunit of RNA polymerase I and III (28). In addition, Cbf5p, the yeast homologue of the Nopp140-associated protein NAP57, is involved in rRNA biosynthesis and interacts genetically with the RNA polymerase I transcription factor RRN3 (7). In this report, the identification of an interaction between hNopp140 and RNA polymerase I unveils a linkage of Nopp140 with the rDNA transcription machinery. Furthermore, expression of a dominant negative mutant of hNopp140, hNopp140N382, resulted in abolishment of rDNA transcription. These results suggest that Nopp140 may play an important role in transcription catalyzed by RNA polymerase I. It would be interesting to further explore how Nopp140 participates in rDNA transcription, such as by examining whether Nopp140 has any effect on RNA polymerase I-dependent promoter activity.
Treatment with actinomycin D to block rDNA transcription (41) leads to the formation of crescent-shaped structures in nucleoli and the rearrangement of nucleolar proteins in a distinct pattern whereby the fibrillar components are segregated from the granular component (38, 43). Interestingly, cells expressing the hNopp140N382 mutant shut off rDNA transcription and displayed a phenotype similar to that of actinomycin D treatment. We have demonstrated that the mutant hNopp140N382 retains the abilities to interact with RNA polymerase I and to localize to the nucleolus. On the basis of dominant negative effects, we reason that the excess hNopp140N382 may impede rDNA transcription through interfering with the normal interaction between the endogenous hNopp140 and RNA polymerase I, resulting in consequences common to actinomycin D treatment. This notion is supported by observations that the hNopp140N382-transfected cells had no additional effects on the nucleolar changes when exposed to actinomycin D (0.08 μg/ml, a dose that selectively inhibits RNA polymerase I activity) (data not shown). Based on the negative effects of this hNopp140 mutant, the maintenance of an integral interaction between RNA polymerase I and the endogenous functionally competent Nopp140 appears to be essential for rDNA transcription.
The structural integrity of the nucleolus is believed to rely on transcription activities of the rRNA genes (47, 50). Evidence shows that the rDNA transcription machinery is attached to the nucleoskeleton (20, 65). Moreover, studies of yeast mutants defective in RNA polymerase I have implicated RNA polymerase I per se in maintenance of the intact nucleolar architecture as a structural element (37). These previous studies have indicated a crucial role for the rDNA transcription machinery in nucleolar organization. In the present report, we demonstrated an interaction between hNopp140 and RNA polymerase I and characterized roles for hNopp140 in relation to rRNA synthesis and maintenance of the nucleolar structure. Therefore, it would be intriguing to examine whether Nopp140 plays a role in organizing the nucleolus through its interaction with RNA polymerase I. Since the RNA polymerase I-interacting domain appears to be necessary for the ability of truncated hNopp140 to block rDNA transcription, we propose that the involvement of Nopp140 in rRNA synthesis may be due to its interaction with RNA polymerase I. However, it is still possible that alterations of the nucleolar architecture by excess hNopp140 or truncated hNopp140 also contribute to the impairment of rDNA transcription. Additional study is needed to clarify the cause-and-effect relationship of Nopp140 with regard to the RNA polymerase I-dependent transcription and the structural organization of the nucleolus.
ACKNOWLEDGMENTS
We are thankful to I. Grummt (German Cancer Research Center, Heidelburg, Germany) for anti-RPA194 antibody, to U. Scheer (University of Würzburg, Würzburg, Germany) for the S4 serum against fibrillarin, to S. J. Lo (National Yang-Ming University, Taipei, Taiwan) for pSV-NLS-LacZ, to J. Leszyk (University of Massachusetts) for mass spectrometry and protein sequencing, and to C.-H. Lin (National Yang-Ming University) for help with confocal microscopy and digital image processing.
This research was supported by grants NSC 86-2316-B010-002-BC, NSC 87-2314-B010-018, and NSC 88-2314-B010-037 from the National Science Council of the Republic of China.
REFERENCES
- 1.Albert A-C, Denton M, Kermekchiev M, Pikaard C S. Histone acetyltransferase and protein kinase activities copurify with a putative Xenopus RNA polymerase I holoenzyme self-sufficient for promoter-dependent transcription. Mol Cell Biol. 1999;19:796–806. doi: 10.1128/mcb.19.1.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allison L A, Moyle M, Shales M, Ingles C J. Extensive homology among the largest subunits of eukaryotic and prokaryotic RNA polymerases. Cell. 1985;42:599–610. doi: 10.1016/0092-8674(85)90117-5. [DOI] [PubMed] [Google Scholar]
- 3.Andrade L E C, Chan E K L, Raska I, Peebles C L, Roos G, Tan E M. Human autoantibody to a novel protein of the nuclear coiled body: immunological characterization and cDNA cloning of p80-coilin. J Exp Med. 1991;173:1407–1419. doi: 10.1084/jem.173.6.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benavente R, Reimer G, Rose K M, Hügle-Dörr B, Scheer U. Nucleolar changes after microinjection of antibodies to RNA polymerase I into the nucleus of mammalian cells. Chromosoma. 1988;97:115–123. doi: 10.1007/BF00327368. [DOI] [PubMed] [Google Scholar]
- 5.Biggs J, Searles L L, Greenleaf A L. Structure of the eukaryotic transcription apparatus: features of the gene for the largest subunit of Drosophila RNA polymerase II. Cell. 1985;42:611–621. doi: 10.1016/0092-8674(85)90118-7. [DOI] [PubMed] [Google Scholar]
- 6.Budde A, Grummt I. p53 represses ribosomal gene transcription. Oncogene. 1999;19:1119–1124. doi: 10.1038/sj.onc.1202402. [DOI] [PubMed] [Google Scholar]
- 7.Cadwell C, Yoon H-J, Zebarjadian Y, Carbon J. The yeast nucleolar protein Cbf5p is involved in rRNA biosynthesis and interacts genetically with the RNA polymerase I transcription factor RRN3. Mol Cell Biol. 1997;17:6175–6183. doi: 10.1128/mcb.17.10.6175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cairns C, McStay B. Identification and cDNA cloning of a Xenopus nucleolar phosphoprotein, xNopp180, that is the homolog of the rat nucleolar protein Nopp140. J Cell Sci. 1995;108:3339–3347. doi: 10.1242/jcs.108.10.3339. [DOI] [PubMed] [Google Scholar]
- 9.Cairns C A, White R J. p53 is a general repressor of RNA polymerase III transcription. EMBO J. 1998;17:3112–3123. doi: 10.1093/emboj/17.11.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cavanaugh A H, Hempel W M, Taylor L J, Rogalsky V, Todorov G, Rothblum L I. Activity of RNA polymerase I transcription factor UBF blocked by Rb gene product. Nature. 1995;374:177–180. doi: 10.1038/374177a0. [DOI] [PubMed] [Google Scholar]
- 11.Chen C-M, Chiang S-Y, Yeh N-H. Increased stability of nucleolin in proliferating cells by inhibition of its self-cleaving activity. J Biol Chem. 1991;266:7754–7758. [PubMed] [Google Scholar]
- 12.Chen H-K, Yeh N-H. The nucleolar phosphoprotein P130 is a GTPase/ATPase with intrinsic property to form large complexes triggered by F− and Mg2+ Biochem Biophys Res Commun. 1997;230:370–375. doi: 10.1006/bbrc.1996.5966. [DOI] [PubMed] [Google Scholar]
- 13.Dundr M, Raska I. Nonisotopic ultrastructural mapping of transcription sites within the nucleolus. Exp Cell Res. 1993;208:275–281. doi: 10.1006/excr.1993.1247. [DOI] [PubMed] [Google Scholar]
- 14.Egyhazi E, Pigon A, Chang J-H, Ghaffari S H, Dreesen T D, Wellman S E, Case S T, Olson M O. Effects of anti-C23 (nucleolin) antibody on transcription of ribosomal DNA in Chironomus salivary gland cells. Exp Cell Res. 1988;178:264–272. doi: 10.1016/0014-4827(88)90397-7. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Blanco M A, Miller D D, Sheetz M P. Nuclear spreads. I. Visualization of bipartite ribosomal RNA domains. J Cell Biol. 1995;128:15–27. doi: 10.1083/jcb.128.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ge H, Roeder R G. Purification, cloning, and characterization of a human coactivator, PC4, that mediates transcriptional activation of class II genes. Cell. 1994;78:513–523. doi: 10.1016/0092-8674(94)90428-6. [DOI] [PubMed] [Google Scholar]
- 17.Gharahdaghi F, Kirchner M, Fernandez J, Mische S M. Peptide-mass profiles of polyvinylidene difluoride-bound proteins by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry in the presence of nonionic detergents. Anal Biochem. 1996;233:94–99. doi: 10.1006/abio.1996.0012. [DOI] [PubMed] [Google Scholar]
- 18.Ginisty H, Sicard H, Roger B, Bouvet P. Structure and functions of nucleolin. J Cell Sci. 1999;112:761–772. doi: 10.1242/jcs.112.6.761. [DOI] [PubMed] [Google Scholar]
- 18a.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hannan R D, Hempel W M, Cavanaugh A, Arino T, Dimitrov S I, Moss T, Rothblum L. Affinity purification of mammalian RNA polymerase I. Identification of an associated kinase. J Biol Chem. 1998;273:1257–1267. doi: 10.1074/jbc.273.2.1257. [DOI] [PubMed] [Google Scholar]
- 20.Hozák P, Cook P R, Schöfer C, Mosgöller W, Wachtler F. Site of transcription of ribosomal RNA and intranucleolar structure in HeLa cells. J Cell Sci. 1994;107:639–648. doi: 10.1242/jcs.107.2.639. [DOI] [PubMed] [Google Scholar]
- 21.Isaac C, Yang Y, Meier U T. Nopp140 functions as a molecular link between the nucleolus and the coiled bodies. J Cell Biol. 1998;142:319–329. doi: 10.1083/jcb.142.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson D A, Hassan A B, Errington R J, Cook P R. Visualization of focal sites of transcription within human nuclei. EMBO J. 1993;12:1059–1065. doi: 10.1002/j.1460-2075.1993.tb05747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang W, Middleton K, Yoon H-J, Fouquet C, Carbon J. An essential yeast protein, CBF5p, binds in vitro to centromeres and microtubules. Mol Cell Biol. 1993;13:4884–4893. doi: 10.1128/mcb.13.8.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaiser K, Stelzer G, Meisterernst M. The coactivator p15 (PC4) initiates transcriptional activation during TFIIA-TFIID-promoter complex formation. EMBO J. 1995;14:3520–3527. doi: 10.1002/j.1460-2075.1995.tb07358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kass S, Tyc K, Steitz J A, Sollner-Webb B. The U3 small nucleolar ribonucleoprotein functions in the first step of preribosomal RNA processing. Cell. 1990;60:897–908. doi: 10.1016/0092-8674(90)90338-f. [DOI] [PubMed] [Google Scholar]
- 26.Kretzschmar M, Kaiser K, Lottspeich F, Meisterernst M. A novel mediator of class II gene transcription with homology to viral immediate-early transcriptional regulators. Cell. 1994;78:525–534. doi: 10.1016/0092-8674(94)90429-4. [DOI] [PubMed] [Google Scholar]
- 27.Lafontaine D, Bousquet-Antonelli C, Henry Y, Caizergues-Ferrer M, Tollervey D. The box H + ACA snoRNAs carry Cbf5p, the putative rRNA pseudouridine synthase. Genes Dev. 1998;12:527–537. doi: 10.1101/gad.12.4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lalo D, Carles C, Sentenac A, Thuriaux P. Interactions between three common subunits of yeast RNA polymerases I and III. Proc Natl Acad Sci USA. 1993;90:5524–5528. doi: 10.1073/pnas.90.12.5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li D, Meier U T, Dobrowolska G, Krebs E G. Specific interaction between casein kinase 2 and the nucleolar protein Nopp140. J Biol Chem. 1997;272:3773–3779. doi: 10.1074/jbc.272.6.3773. [DOI] [PubMed] [Google Scholar]
- 30.Lin C-G, Yang S-J, Hwang W-L, Su T-S, Lo S J. Demonstration of the presence of protease-cutting site in the spacer of hepatitis B viral Pol protein. J Virol Methods. 1995;51:61–73. doi: 10.1016/0166-0934(94)00118-z. [DOI] [PubMed] [Google Scholar]
- 31.Meier U T. Comparison of the rat nucleolar protein Nopp140 with its yeast homolog SRP40. Differential phosphorylation in vertebrates and yeast. J Biol Chem. 1996;271:19376–19384. [PubMed] [Google Scholar]
- 32.Meier U T, Blobel G. Nopp140 shuttles on tracks between nucleolus and cytoplasm. Cell. 1992;70:127–138. doi: 10.1016/0092-8674(92)90539-o. [DOI] [PubMed] [Google Scholar]
- 33.Meier U T, Blobel G. NAP57, a mammalian nucleolar protein with a putative homolog in yeast and bacteria. J Cell Biol. 1994;127:1505–1514. doi: 10.1083/jcb.127.6.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mélèse T, Xue Z. The nucleolus: an organelle formed by the act of building a ribosome. Curr Opin Cell Biol. 1995;7:319–324. doi: 10.1016/0955-0674(95)80085-9. [DOI] [PubMed] [Google Scholar]
- 35.Mémet S, Gouy M, Marck C, Sentenac A, Buhler J-M. RPA190, the gene coding for the largest subunit of yeast RNA polymerase A. J Biol Chem. 1988;263:2830–2839. [PubMed] [Google Scholar]
- 36.Miau L-H, Chang C-J, Tsai W-H, Lee S-C. Identification and characterization of a nucleolar phosphoprotein, Nopp140, as a transcription factor. Mol Cell Biol. 1997;17:230–239. doi: 10.1128/mcb.17.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oakes M, Nogi Y, Clark M W, Nomura M. Structural alterations of the nucleolus in mutants of Saccharomyces cerevisiae defective in RNA polymerase I. Mol Cell Biol. 1993;13:2441–2455. doi: 10.1128/mcb.13.4.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ochs R L, Lischwe M A, Spohn W H, Busch H. Fibrillarin: a new protein of the nucleolus identified by autoimmune sera. Biol Cell. 1985;54:123–133. doi: 10.1111/j.1768-322x.1985.tb00387.x. [DOI] [PubMed] [Google Scholar]
- 39.Pai C-Y, Chen H-K, Sheu H-L, Yeh N-H. Cell-cycle-dependent alterations of a highly phosphorylated nucleolar protein p130 are associated with nucleologenesis. J Cell Sci. 1995;108:1911–1920. doi: 10.1242/jcs.108.5.1911. [DOI] [PubMed] [Google Scholar]
- 40.Pai C-Y, Yeh N-H. Cell proliferation-dependent expression of two isoforms of the nucleolar phosphoprotein p130. Biochem Biophys Res Commun. 1996;221:581–587. doi: 10.1006/bbrc.1996.0639. [DOI] [PubMed] [Google Scholar]
- 41.Perry R P, Kelley D E. Inhibition of RNA synthesis by actinomycin D: characteristic dose-response of different RNA species. J Cell Physiol. 1970;76:127–139. doi: 10.1002/jcp.1040760202. [DOI] [PubMed] [Google Scholar]
- 42.Puvion-Dutilleul F, Bachellerie J-P, Puvion E. Nucleolar organization of HeLa cells as studied by in situ hybridization. Chromosoma. 1991;100:395–409. doi: 10.1007/BF00337518. [DOI] [PubMed] [Google Scholar]
- 43.Reimer G, Rose K M, Scheer U, Tan E M. Autoantibody to RNA polymerase I in scleroderma sera. J Clin Investig. 1987;79:65–72. doi: 10.1172/JCI112809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robbins J, Dilworth S M, Laskey R A, Dingwall C. Two interdependent basic domains in nucleoplasmin nuclear targeting sequence: identification of a class of bipartite nuclear targeting sequence. Cell. 1991;64:615–623. doi: 10.1016/0092-8674(91)90245-t. [DOI] [PubMed] [Google Scholar]
- 45.Saez-Vasquez J, Pikaard C S. Extensive purification of a putative RNA polymerase I holoenzyme from plants that accurately initiates rRNA gene transcription in vitro. Proc Natl Acad Sci USA. 1997;94:11869–11874. doi: 10.1073/pnas.94.22.11869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schaub M, Myslinski E, Schuster C, Krol A, Carbon P. Staf, a promiscuous activator for enhanced transcription by RNA polymerases II and III. EMBO J. 1997;16:173–181. doi: 10.1093/emboj/16.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scheer U, Benavente R. Functional and dynamic aspects of the mammalian nucleolus. Bioessays. 1990;12:14–21. doi: 10.1002/bies.950120104. [DOI] [PubMed] [Google Scholar]
- 48.Scheer U, Rose K M. Localization of RNA polymerase I in interphase cells and mitotic chromosomes by light and electron microscopic immunocytochemistry. Proc Natl Acad Sci USA. 1984;81:1431–1435. doi: 10.1073/pnas.81.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scheer U, Thiry M, Goessens G. Structure, function and assembly of the nucleolus. Trends Cell Biol. 1993;3:236–241. doi: 10.1016/0962-8924(93)90123-i. [DOI] [PubMed] [Google Scholar]
- 50.Scheer U, Weisenberger D. The nucleolus. Curr Opin Cell Biol. 1994;6:354–359. doi: 10.1016/0955-0674(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 51.Seither P, Coy J F, Pouska A, Grummt I. Molecular cloning and characterization of the cDNA encoding the largest subunit of mouse RNA polymerase I. Mol Gen Genet. 1997;255:180–186. doi: 10.1007/s004380050487. [DOI] [PubMed] [Google Scholar]
- 52.Seither P, Iben S, Grummt I. Mammalian RNA polymerase I exists as a holoenzyme with associated basal transcription factors. J Mol Biol. 1998;275:43–53. doi: 10.1006/jmbi.1997.1434. [DOI] [PubMed] [Google Scholar]
- 53.Smith C A, Bates P, Rivera-Gonzalez R, Gu B, DeLuca N A. ICP4, the major transcriptional regulatory protein of herpes simplex virus type 1, forms a tripartite complex with TATA-binding protein and TFIIB. J Virol. 1993;67:4676–4687. doi: 10.1128/jvi.67.8.4676-4687.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith C M, Steitz J A. Sno storm in the nucleolus: new roles for myriad small RNPs. Cell. 1997;89:669–672. doi: 10.1016/s0092-8674(00)80247-0. [DOI] [PubMed] [Google Scholar]
- 55.Song C Z, Hanada K, Yano K, Maeda Y, Yamamoto K, Muramatsu M. High conservation of subunit composition of RNA polymerase I(A) between yeast and mouse and the molecular cloning of mouse RNA polymerase I 40-kDa subunit RPA40. J Biol Chem. 1994;269:26976–36981. [PubMed] [Google Scholar]
- 56.Suzuki N, Suzuki T, Uchida A, Thompson E A, Hosoya T. Effect of dexamethasone on nucleolar casein kinase II activity and phosphorylation of nucleolin in lymphosarcoma P1798 cells. J Steroid Biochem Mol Biol. 1992;42:305–312. doi: 10.1016/0960-0760(92)90133-4. [DOI] [PubMed] [Google Scholar]
- 57.Thiry M. Ultrastructural detection of DNA within the nucleolus by sensitive molecular immunocytochemistry. Exp Cell Res. 1992;200:135–144. doi: 10.1016/s0014-4827(05)80081-3. [DOI] [PubMed] [Google Scholar]
- 58.Thiry M, Goessens G. Distinguishing the sites of pre-rRNA synthesis and accumulation in Ehrlich tumor cell nucleoli. J Cell Sci. 1991;99:759–767. doi: 10.1242/jcs.99.4.759. [DOI] [PubMed] [Google Scholar]
- 59.Tollervey D, Lehtonen H, Jansen R, Kern H, Hurt E C. Temperature-sensitive mutations demonstrate roles for yeast fibrillarin in pre-rRNA processing, pre-rRNA methylation, and ribosome assembly. Cell. 1993;72:443–457. doi: 10.1016/0092-8674(93)90120-f. [DOI] [PubMed] [Google Scholar]
- 60.van de Wetering M, Oosterwegel M, van Norren K, Clevers H. Sox-4, an Sry-like HMG box protein, is a transcriptional activator in lymphocytes. EMBO J. 1993;12:3847–3854. doi: 10.1002/j.1460-2075.1993.tb06063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Voit R, Kuhn A, Sander E E, Grummt I. Activation of mammalian ribosomal gene transcription requires phosphorylation of the nucleolar transcription factor UBF. Nucleic Acids Res. 1995;23:2593–2599. doi: 10.1093/nar/23.14.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Voit R, Schnapp A, Kuhn A, Rosenbauer H, Hirschmann P, Stunnenberg H G, Grummt I. The nucleolar transcription factor mUBF is phosphorylated by casein kinase II in the C-terminal hyperacidic tail which is essential for transactivation. EMBO J. 1992;11:2211–2218. doi: 10.1002/j.1460-2075.1992.tb05280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wansink D G, Schul W, van der Kraan I, van Steensel B, van Driel R, de Jong L. Fluorescent labeling of nascent RNA reveals transcription by RNA polymerase II in domains scattered throughout the nucleus. J Cell Biol. 1993;122:283–293. doi: 10.1083/jcb.122.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weintraub S J, Chow K N B, Luo R X, Zhang S H, He S, Dean D C. Mechanism of active transcriptional repression by the retinoblastoma protein. Nature. 1995;375:812–815. doi: 10.1038/375812a0. [DOI] [PubMed] [Google Scholar]
- 65.Weipoltshammer K, Schofer C, Wachtler F, Hozák P. The transcription unit of ribosomal genes is attached to the nuclear skeleton. Exp Cell Res. 1996;227:374–379. doi: 10.1006/excr.1996.0287. [DOI] [PubMed] [Google Scholar]
- 66.White R J, Trouche D, Martin K, Jackson S P, Kouzarides T. Repression of RNA polymerase III transcription by the retinoblastoma protein. Nature. 1996;382:88–90. doi: 10.1038/382088a0. [DOI] [PubMed] [Google Scholar]
- 67.Yang T-H, Tsai W-H, Lee Y-M, Lei H-Y, Lai M-Y, Chen D-S, Yeh N-H, Lee S-C. Purification and characterization of nucleolin and its identification as a transcription repressor. Mol Cell Biol. 1994;14:6068–6074. doi: 10.1128/mcb.14.9.6068. [DOI] [PMC free article] [PubMed] [Google Scholar]