Abstract
Aims
We aimed to assess the long‐term effect of a strategy of comprehensive vasodilation versus usual care on health‐related quality of life (HRQL) among patients with acute heart failure (AHF).
Methods and results
Health‐related quality of life was prospectively assessed by the generic 3‐levelled EQ‐5D and the disease‐specific Kansas City Cardiomyopathy Questionnaire (KCCQ) among adult AHF patients enrolled in an international, multicentre, randomised, open‐label blinded‐end‐point trial of a strategy that emphasized early intensive and sustained vasodilation using maximally tolerated doses of established oral and transdermal vasodilators according to systolic blood pressure. Changes in EQ‐5D and KCCQ from admission to 180 day follow‐up were individually compared between the intensive vasodilatation and the usual care group. Among 666 patients eligible for 180 day follow‐up, 284 (43%, median age 79 years, 35% women) and 198 (30%, median age 77 years, 35% women) had completed the EQ‐5D and KCCQ at baseline and follow‐up, respectively. There was a significant improvement in HRQL as quantified by both, EQ‐5D and KCCQ, from hospitalization to 180 day follow‐up, with no significant differences in the change of HRQL between both treatment strategies. For instance, 39 (26%) versus 33 (25%) patients had an improvement by at least one level in at least two categories in the EQ‐5D. Median increase in KCCQ overall summary score (KCCQ‐OSS) was 17.6 (IQR 2.0–42.6) in the intervention group versus 18.5 (IQR 3.9–39.3) in the usual care group (P < 0.001 vs. baseline, P = 0.945 between groups).
Conclusions
Among patients with AHF, long‐term HRQL quantified by EQ‐5D and KCCQ improved substantially, with overall no significant differences between a strategy of comprehensive vasodilation versus usual care.
Keywords: Acute heart failure, Health‐related quality of life, Sustained vasodilatation
Background
Patients requiring hospitalization for acute heart failure (AHF) continue to have unacceptable high rates of mortality and morbidity. 1 , 2 There is still no treatment for patients with AHF proven to be beneficial by a randomized control trial. Disappointingly, despite promising pilot data, also a recent randomized controlled multicentre study comparing an early and comprehensive strategy of aggressive vasodilation versus usual care reported a neutral effect on mortality and rehospitalization for AHF. 3 , 4
In addition to hard clinical endpoints such as death or rehospitalization, patient‐reported outcomes are increasingly recognized as providing important incremental insights regarding the medical value of novel therapies by displaying their clinical efficacy on the subjective well‐being. 5 , 6 Health‐related quality of life (HRQL) is of particular importance in chronic and progressive disorders such as heart failure (HF). 1 , 2 We therefore aimed to test the hypothesis that an early and comprehensive strategy of aggressive vasodilation would result in more pronounced improvements in HRQL quantified by using generic and disease‐specific questionnaires versus usual care in patients requiring hospitalization for AHF.
Methods
Study design and population
Goal Directed Afterload Reduction in Acute Congestive Cardiac Decompensation Study (GALACTIC) was a multicentre, randomized, open‐label blinded‐end‐point trial enrolling adult patients hospitalized due to AHF that emphasized early intensive and sustained vasodilation using maximally tolerated doses of established oral and transdermal vasodilators according to systolic blood pressure. 3 The final diagnosis was adjudicated by an independent cardiologist who had access to all patients' medical records. In situations of uncertainty about the diagnosis, cases were reviewed and adjudicated in conjunction with a second cardiologist. Patients who were concluded to not have AHF were excluded. Detailed methodology including patient population, randomization, and study procedures have been described before. 3 The study was carried out according to the principles of the Declaration of Helsinki and approved by the local ethics committees.
Assessment of health‐related quality of life
After providing written informed consent, patients received the generic EQ‐5D, as well as the disease‐specific Kansas City Cardiomyopathy Questionnaires (KCCQ), and were asked to complete the self‐administered forms without the help of external interviewers within the next 24 h. 7 , 8 For the 180 day follow‐up, HRQL questionnaires were send by mail. The primary outcomes for this analysis were the changes from baseline to 180 day follow‐up in the five dimensions of the 3‐levelled EQ‐5D (no problems/some problems/severe problems regarding mobility, self‐care, usual activity, pain/discomfort, anxiety/depression), and in the six domains of the KCCQ as well as on the composite KCCQ overall summary score (KCCQ‐OSS), all ranging from 0 to 100, with lower score indicating worse HRQL.
Statistical analysis
Continuous variables are presented as median with interquartile range and categorical variables by numbers and percentages. Differences in baseline characteristics of the study population were assessed using Mann–Whitney U or χ 2 test as appropriate. Changes in the percentages of patients reporting impairments according to the EQ‐5D were plotted with the use of alluvial diagrams. Differences in the changes of the KCCQ‐scores between both treatment groups were compared using empirical bootstrap with 1500 samples to estimate the 95% confidence interval of the point estimates. 9 Stuart–Maxwell and Wilcoxon signed‐rank tests were used to evaluate changes in HRQL from baseline to follow‐up for the ordinal EQ‐5D and the continuous KCCQ, respectively. All hypothesis testing was two‐tailed and P‐values of less than 0.05 were considered to indicate statistical significance. No adjustment for multiple testing was performed. Statistical analysis was operated using SPSS for Windows 26.0 (SPSS Inc., Chicago, IL) and R Statistical Software Version 4.0.0 (Vienna, Austria).
Results
Among 666 patients enrolled in 10 centres in five countries eligible for 180 day follow‐up (median age 78 years, 36% women), 284 (43%) and 198 (30%) had completed the EQ‐5D and KCCQ at baseline and follow‐up, respectively (Supporting Information, Figure S1 ). Patients who did fill out the HRQL questionnaires had comparable baseline characteristics to patients who did not (Supporting Information, Table S1 and S 2). Baseline characteristics including initial HRQL were comparable among patients randomized to comprehensive vasodilation versus usual care (Table 1 ). In‐hospital treatment differed significantly between both groups with substantially higher doses of nitrates, hydralazine, and ACEI/ARB/ARNIs and transiently resulted in significantly lower blood pressures in the comprehensive vasodilation group (Supporting Information, Figures S2 and S3 ).
Table 1.
Baseline characteristics of patients with (A) completed EQ‐5D questionnaire and (B) with completed KCCQ
| A | All patients with EQ‐5D | Standard care | Intensive vasodilatation | P‐value* |
|---|---|---|---|---|
| n = 284 | n = 134 | n = 150 | ||
| Age, years | 79 [71, 85] | 79 [71, 84] | 79 [71, 85] | 0.805 |
| Female gender | 100 (35) | 55 (41) | 45 (30) | 0.069 |
| BMI, kg/m2 | 27.1 [23.9, 30.5] | 27.2 [24.1, 30.8] | 27.0 [23.8, 30.4] | 0.729 |
| Diabetes | 85 (30) | 44 (33) | 41 (27) | 0.378 |
| Hypertension | 241 (85) | 111 (83) | 130 (87) | 0.463 |
| Coronary artery disease | 163 (57) | 77 (57) | 86 (57) | 1.000 |
| Stroke | 50 (18) | 25 (19) | 25 (17) | 0.777 |
| Atrial fibrillation | 145 (51) | 72 (54) | 73 (49) | 0.463 |
| Known heart failure | 189 (67) | 90 (67) | 99 (66) | 0.935 |
| NT‐proBNP, ng/L | 5479 [3042, 9307] | 5226 [3031, 9517] | 5724 [3106, 9134] | 0.806 |
| BUN, mmol/L | 8.5 [6.5, 12.4] | 8.2 [6.1, 12.5] | 8.6 [6.6, 12.3] | 0.674 |
| eGFR, mL/min/1.73 m2 | 51 [38, 71] | 51 [38.0, 70] | 51 [38, 72] | 0.924 |
| Sodium, mmol/L | 140 [137, 142] | 140 [137, 142] | 140 [137, 142] | 0.914 |
| ACEI/ARB/ARNI | 213 (75) | 103 (77) | 110 (73) | 0.583 |
| Beta‐blockers | 207 (73) | 101 (75) | 106 (71) | 0.449 |
| Diuretics | 212 (75) | 100 (75) | 112 (75) | 1.000 |
| EQ‐5D Mobility | 175 (62) | 81 (60) | 94 (63) | 0.794 |
| EQ‐5D Self‐Care | 86 (30) | 40 (30) | 46 (31) | 0.984 |
| EQ‐5D Usual Activity | 179 (63) | 85 (63) | 94 (63) | 0.992 |
| EQ‐5D Anxiety/Depression | 95 (33) | 48 (36) | 47 (31) | 0.5 |
| EQ‐5D Pain/Discomfort | 168 (59) | 76 (57) | 92 (61) | 0.503 |
| B | All patients with KCCQ | Standard care | Intensive vasodilatation | P‐value* |
|---|---|---|---|---|
| n = 198 | n = 94 | n = 104 | ||
| Age, years | 77 [70, 84] | 76 [68, 83] | 79 [72, 84] | 0.201 |
| Female gender | 69 (35) | 40 (43) | 29 (28) | 0.044 |
| BMI, kg/m2 | 27.8 [24.0, 30.9] | 27.8 [24.1, 31.6] | 27.4 [24.0, 30.5] | 0.440 |
| Diabetes | 62 (31) | 30 (32) | 32 (31) | 0.984 |
| Hypertension | 167 (84) | 79 (84) | 88 (85) | 1.000 |
| Coronary artery disease | 117 (59) | 56 (60) | 61 (59) | 1.000 |
| Stroke | 31 (16) | 15 (16) | 16 (15) | 1.000 |
| Atrial fibrillation | 100 (51) | 47 (50) | 53 (51) | 1.000 |
| Known heart failure | 126 (64) | 59 (63) | 67 (64) | 0.925 |
| NT‐proBNP, ng/L | 5298 [3081, 8244] | 4698 [2923, 8090] | 5783 [3512, 8338] | 0.351 |
| BUN, mmol/L | 8.5 [6.6, 12.4] | 8.6 [6.5, 12.6] | 8.5 [6.7, 12.0] | 0.818 |
| eGFR, mL/min/1.73 m2 | 54 [37, 73] | 54 [34, 70] | 53 [39, 75] | 0.441 |
| Sodium, mmol/L | 140 [137, 142] | 140 [137, 142] | 140 [137, 142] | 0.830 |
| ACEI/ARB/ARNI | 148 (75) | 73 (78) | 75 (72) | 0.464 |
| Beta‐blockers | 142 (72) | 69 (73) | 73 (70) | 0.732 |
| Diuretics | 150 (76) | 73 (78) | 77 (74) | 0.669 |
| KCCQ‐OSS | 43.5 [30.3, 62.8] | 42.6 [31.5, 57.9] | 43.8 [27.8, 64.9] | 0.897 |
ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor–neprilysin inhibitor; BMI, body mass index; BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate; KCCQ‐OSS, Kansas City Cardiomyopathy Questionnaire overall summary score; NT‐proBNP, N‐Terminal pro‐B‐type natriuretic peptide.
P‐value for comparison between two depicted groups.
Changes in health‐related quality of life
Among 284 patients eligible for analysis using EQ‐5D, the number of patients reporting any problems was highest regarding usual activities (at admission n = 179 [63%] vs. follow‐up n = 146 [51%]), mobility (n = 175 [62%] vs. n = 153 [54%]), and pain/discomfort (n = 168 [59%] vs. n = 164 [58%]). At 180 days, HRQL as assessed by EQ‐5D was improved in both groups, with two significant between‐group differences. While patients in the intensive vasodilatation group stated to have less pain/discomfort, they indicated to have more depression/anxiety at follow‐up than at admission, inversely to the patients in the usual care group (Tables 2 and 3 ; Figure 1 ). Summarized from baseline to follow‐up, 39 (26%) versus 33 (25%) patients had an improvement of at least one level in at least two categories, 27 (18%) versus 23 (17%) patients improved at least one level in one category, and 32 (21%) versus 40 (30%) patients had a constant level of impairment according to the EQ‐5D in the intensive vasodilatation versus usual care group (P‐values for comparison between groups 0.791, 0.854, and 0.100 respectively).
Table 2.
Summarized between‐group comparisons of change of HRQL from baseline to 180 day follow‐up in patients admitted due to AHF and randomized to usual care versus intensive vasodilatation
| (A) EQ‐5D | Overall | Standard care | Intensive vasodilatation | P‐value* |
|---|---|---|---|---|
| n = 284 | n = 134 | n = 150 | ||
| Improvement of at least one level in at least two categories | 72 (25) | 33 (25) | 39 (26) | 0.791 |
| Improvement of at least one level in one category | 50 (18) | 23 (17) | 27 (18) | 0.854 |
| Constant average level of impairment | 72 (25) | 40 (30) | 32 (21) | 0.100 |
| Deterioration of at least one level in at least one category | 90 (32) | 38 (28) | 52 (35) | 0.254 |
| (B) KCCQ | Overall | Standard care | Intensive vasodilatation | * |
|---|---|---|---|---|
| n = 198 | n = 94 | n = 104 | ||
| Symptom frequency | 30.2 [8.9, 50.0] | 29.2 [10.4, 47.4] | 33.3 [8.3, 50.5] | 0.801 |
| Symptom burden | 25.0 [0.0, 41.7] | 25.0 [8.3, 47.9] | 25.0 [0.0, 41.7] | 0.849 |
| Physical limitation | 10.0 [−4.2, 33.3] | 12.5 [−6.2, 33.3] | 9.2 [−4.2, 30.2] | 0.956 |
| Social limitation | 16.7 [0.0, 41.7] | 18.8 [0.0, 41.7] | 12.5 [−2.6, 42.2] | 0.419 |
| Self‐efficacy | 12.5 [0.0, 25.0] | 12.5 [0.0, 25.0] | 12.5 [0.0, 25.0] | 0.215 |
| Quality of life | 16.7 [8.3, 41.7] | 16.7 [8.3, 41.7] | 16.7 [8.3, 41.7] | 0.983 |
| KCCQ‐OSS | 18.0 [3.1, 39.3] | 17.6 [2.1, 41.0] | 18.5 [4.1, 38.8] | 0.943 |
AHF, acute heart failure; HRQL, health‐related quality of life; KCCQ‐OSS, Kansas City Cardiomyopathy Questionnaire overall summary score.
P‐value for comparison between two depicted groups.
Table 3.
Detailed between‐group comparisons of change of HRQL from baseline to 180 day follow‐up in patients admitted due to AHF and randomized to usual care versus intensive vasodilatation
| (A) EQ‐5D | All patients with EQ‐5D (n = 284) | Standard care (n = 134) | Intensive vasodilatation (n = 150) | P‐value* | ||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Follow‐up | Baseline | Follow‐up | Baseline | Follow‐up | |||
| MO | No problems | 109 (38) | 131 (46) | 53 (40) | 62 (46) | 56 (37) | 69 (46) | 0.360 |
| Some problems | 163 (57) | 148 (52) | 76 (57) | 70 (52) | 87 (58) | 78 (52) | ||
| Severe problems | 12 (4) | 5 (2) | 5 (4) | 2 (2) | 7 (5) | 3 (2) | ||
| SC | No problems | 198 (70) | 209 (74) | 94 (70) | 101 (75) | 104 (69) | 108 (72) | 0.443 |
| Some problems | 76 (27) | 65 (23) | 36 (27) | 28 (21) | 40 (27) | 37 (25) | ||
| Severe problems | 10 (4) | 10 (4) | 4 (3) | 5 (4) | 6 (4) | 5 (3) | ||
| UA | No problems | 105 (37) | 128 (49) | 49 (37) | 70 (52) | 56 (37) | 68 (45) | 0.435 |
| Some problems | 149 (53) | 126 (44) | 74 (55) | 57 (43) | 75 (50) | 69 (46) | ||
| Severe problems | 30 (11) | 20 (7) | 11 (8) | 7 (5) | 19 (13) | 13 (9) | ||
| PD | No | 116 (41) | 120 (42) | 58 (43) | 51 (38) | 58 (39) | 69 (46) | 0.019 |
| Some | 149 (53) | 151 (53) | 70 (52) | 76 (57) | 79 (53) | 75 (50) | ||
| Severe | 19 (7) | 13 (5) | 6 (5) | 7 (5) | 13 (9) | 6 (4) | ||
| AD | No | 189 (67) | 177 (62) | 86 (64) | 89 (66) | 103 (69) | 88 (59) | 0.039 |
| Some | 86 (30) | 98 (35) | 44 (33) | 41 31) | 42 (28) | 57 (38) | ||
| Severe | 9 (3) | 9 (3) | 4 (3) | 4 (3) | 5 (3) | 5(3) | ||
| (B) KCCQ | All patients with KCCQ (n = 198) | Standard care (n = 94) | Intensive vasodilatation (n = 104) | P‐value* | ||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Follow‐up | Baseline | Follow‐up | Baseline | Follow‐up | |||
| Symptom frequency | 37.5 [25.0, 59.9] | 78.5 [52.1, 91.7] | 35.4 [25.0, 57.8] | 74.0 [52.1, 91.7] | 39.6 [26.6, 60.4] | 79.2 [53.6, 94.8] | 0.459 | |
| Symptom burden | 50.0 [33.3, 66.7] | 83.3 [52.1, 91.7] | 50.0 [25.0, 58.3] | 83.3 [50.0, 100.0] | 50.0 [33.3, 75.0] | 83.3 [66.7, 91.7] | 0.382 | |
| Physical limitation | 54.2 [33.8, 68.2] | 70.8 [50.0, 87.5] | 54.2 [37.5, 66.7] | 70.8 [42.7, 87.5] | 54.2 [33.3, 75.0] | 70.8 [50.0, 87.5] | 0.457 | |
| Social limitation | 41.7 [25.0, 68.8] | 75.0 [37.5, 90.6] | 43.8 [25.0, 62.5] | 75.0 [43.8, 98.4] | 41.7 [18.8, 75.0] | 75.0 [31.2, 87.5] | 0.216 | |
| Self‐efficacy | 62.5 [50.0, 75.0] | 75.0 [62.5, 87.5] | 62.5 [50.0, 75.0] | 75.0 [62.5, 100.0] | 62.5 [43.8, 87.5] | 75.0 [62.5, 87.5] | 0.114 | |
| Quality of life | 41.7 [25.0, 58.3] | 75.0 [50.0, 90.6] | 41.7 [27.1, 58.3] | 75.0 [50.0, 91.7] | 41.7 [25.0, 60.4] | 75.0 [50.0, 84.4] | 0.429 | |
| KCCQ‐OSS | 43.5 [30.3, 62.8] | 71.8 [49.5, 87.0] | 42.6 [31.5, 57.9] | 70.3 [50.2, 89.5] | 43.8 [27.8, 64.9] | 72.8 [49.5, 84.1] | 0.378 | |
AHF, acute heart failure; AD = anxiety/depression; HRQL, health‐related quality of life; KCCQ‐OSS, Kansas City Cardiomyopathy Questionnaire overall summary score; MO, mobility; PD, pain/discomfort; SC, self‐care; UA, usual activities.
P‐value for comparison of change between standard care and intensive vasodilatation.
Figure 1.
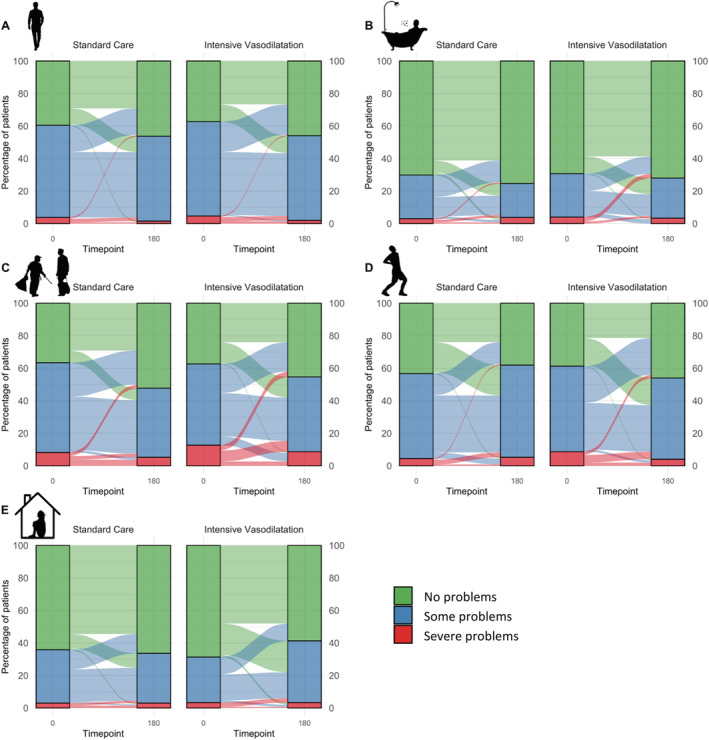
HRQL at baseline and 180 day follow‐up in patients assigned to usual care versus intensive vasodilatation according to the 3‐levelled EQ‐5D. Bars indicate percentage of randomized patients (n = 134 in standard care and n = 150 in the intensive vasodilatation) reporting no, some and severe impairments regarding (A) mobility, (B) self‐care, (C) usual activities, (D) pain/discomfort, and (E) anxiety/depression; flow indicates changes from admission to follow up.
In patients with available KCCQ‐scores (n = 198), symptom frequency (median score 37.5 [IQR 25.0–60.4]), quality of life (41.7 [IQR 25.0–60.4]), and social limitations (41.7 [IQR 23.4–68.8]) were the three domains with lowest scores at admission resulting in a baseline median KCCQ‐OSS of 43.5 (IQR 30.2–63.2). Median KCCQ‐OSS at baseline was comparable between patients who survived (41.2 [IQR 27.5–58.3], n = 365) and who died within the 180 day follow‐up (34.8 [27.5–58.3], n = 54, P = 0.076). From baseline to 180 day follow‐up, KCCQ‐scores increased significantly and to a similar extent in both treatment groups, e.g. median increase of KCCQ‐OSS was 17.6 (IQR 2.0–42.6) in the intervention group versus 18.5 (IQR 3.9–39.3) in the usual care group (both P < 0.001 vs. baseline, Supporting Information, Table S3 ; P = 0.943 between groups, Table 2 ). Highest increase was observed in Symptom Frequency (30.2 [IQR 8.9, 50.0]) and Symptom Burden (25.0 [IQR 0.0, 41.7]), resulting in median scores over 70 in all six domains regardless of treatment strategy. The comparison of the change in HRQL measured by KCCQ between the two groups is illustrated in Figure 2 .
Figure 2.
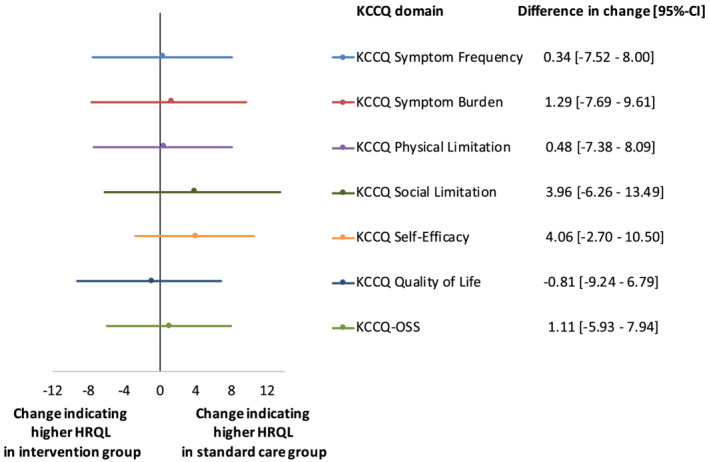
Differences in changes of HRQL between standard care and intensive vasodilatation group after 180 days of follow‐up. HRQL assessed by KCCQ. Differences in change displayed with bars intersecting vertical line representing no difference in change. Negative values indicating higher HRQL in intervention group and positive values higher change in usual care group. Calculations of change [95%‐confidence interval] was performed using empirical bootstrap method.
Discussion
This secondary analysis from the international multicentre study tested the hypothesis that an early and comprehensive strategy of aggressive vasodilation using commonly available drugs such as nitrates, hydralazine, and ACEI/ARB/ARNIs would result in more pronounced improvements in HRQL quantified using EQ‐5D and KCCQ versus usual care in patients admitted for AHF. We report three main findings: first, the disease‐specific KCCQ, which previously has been shown to be feasible even in the ED setting, documented very low scores indicating poor HRQL in symptom frequency, symptom burden, quality of life and social limitations at the time of admission. 10 With a 5‐point change in KCCQ widely considered to be the minimally noticeable clinical difference experienced by patients and a 20‐point change considered as a large substantial difference in HRQL, HRQL improved significantly and substantially from admission to the 180 day follow‐up in both randomization groups over all six domains. 11 , 12 Given the progressive natural history of HF and recent suggestions that even changes smaller than the traditional 5‐points may be clinically meaningful, this highlights the enormous medical value of current HF therapies and has not been shown in longitudinal data up to 180 days before. 1 , 2 , 13 Although sensitive to acute subjective changes of the health state, the KCCQ assessed early during hospital admission did not allow to predict 180 day mortality. Second, the measured improvements in HRQL from admission to 180 day follow‐up were smaller when using the generic EQ‐5D. After applying either of both strategies, about 50% of the patients continued to be impaired in mobility, usual activities, and/or had pain/discomfort. This might be explained by the EQ‐5D not being a HF specific, but a general assessment of the health state, which could be substantially affected by non‐cardiac comorbidities. It further highlights that as a single tool the EQ‐5D would not be well suited to monitor the HRQL in AHF patients. Third, a strategy of comprehensive vasodilation did not result in a statistically significant and/or clinically relevant more pronounced improvement in HRQL at 180 day follow‐up as quantified using EQ‐5D and KCCQ versus usual care. The neutral effect on HRQL at 180 days may at least partly be explained by three aspects: first, lack of effect of the early and comprehensive vasodilation strategy versus usual care on NT‐proBNP concentration quantifying intracardiac filling pressures at hospital discharge, second, smaller than expected difference in ACEI/ARB/ARNI doses at 180 day follow‐up between both groups, and third, confounding by medical and social factors occurring during 180 days and not differentially affected by both treatment strategies. 3
These findings corroborate and support the neutral findings of previous studies regarding intensive preload and afterload decrement as treatment strategy of patients with AHF. 14 , 15 , 16 Even when applying individualized and aggressive dosing strategies as in this trial short‐term vasodilatation may not influence the long‐term improvement of AHF patients' individually perceived physical and mental health significantly more than standard treatment according to current guidelines. 5 Nevertheless, whether specific phenotypes of AHF, e.g. patients with elevated systolic blood pressure at presentation, might benefit from early intensive vasodilatation remains unexplored. 16 Further, it has to be taken into account that important subjective changes in HRQL might occur shortly after presentation. A prior randomized controlled trial in patients presenting with AHF to the ED and comparable KCCQ‐OSS at baseline showed that HRQL increased not linearly, but had a more profound increase from baseline to 30 days and a plateau up to 90 days. 17 Although another former trial comparing comprehensive care bundle including the use of early intravenous nitrates versus usual care in older AHF patients presenting to the ED resulted in no significant difference in all‐cause mortality and rehospitalization at 30 days, the incremental effect of early comprehensive vasodilation on short‐term HRQL remains unknown and should be addressed in future studies. 18
Several limitations merit consideration. First, as in all studies using self‐administered HRQL‐questionnaires, selection bias might have occurred given the relatively low percentage of patients who had completed the questionnaires at both timepoints. While the help of an interviewer might have increased the number of patients completing the questionnaires, particular the more comprehensive KCCQ, it would have introduced the risk for patients to modify their answers to present themselves in a more favourable light. It is important to note that patients who did not fill‐out the HRQL questionnaires during admission had comparable baseline characteristics to patients who completed the questionnaires. Second, no specific sample size calculation was performed for the HRQL analyses. Due to the only moderate number of patients, this study likely was underpowered for some comparisons, EQ‐5D and KCCQ were analysed individually and results should be interpreted as exploratory. Third, these results cannot be generalized to patients with severe respiratory failure and/or hemodynamic instability requiring ICU‐admission, who were excluded from the GALACTIC study.
Conclusions
Among patients with AHF, long‐term HRQL quantified by EQ‐5D and KCCQ improved substantially, with overall no significant differences between a strategy of comprehensive vasodilation versus usual care.
Conflict of interest
Dr. Gualandro reported receiving personal fees from Servier. Dr. Walter has received research grants from the Swiss Heart Foundation (FF19097 and F18111) as well as the Swiss Academy of Medical Sciences and the Gottfried and Julia Bangerter‐Rhyner Foundation. Dr. Goudev reported receiving personal fees (speaking honoraria and advisory board membership) from Pfizer, Novartis, AstraZeneca, and Amge. Dr. Kozhuharov has received research grants from the Swiss National Science Foundation (P400PM‐194477), Gottfried und Julia Bangerter‐Rhyner‐Stiftung, and the European Society of Cardiology. Dr. Kobza received institutional grants from Abbott, Biosense‐Webster, Biotronik, Boston‐scientific, Medtronic and SIS‐Medical. Dr. Münzel reported being the principal investigator of the DZHK (German Center for Cardiovascular Research) Partner Site Rhine‐Main. Dr. Mueller has received research support from the Swiss National Science Foundation, the Swiss Heart Foundation, the KTI, the University Hospital Basel, Basel University, Abbott, Beckman Coulter, BRAHMS, Idorsia, LSI Medience, Norvartis, Ortho Clinical Diagnostics, Quidel, Roche, Siemens, and Singulex, as well as speaker/consulting honoraria from Acon, Amgen, Astra Zeneca, Bayer, BMS, Boehringer Ingelheim, Daiichi Sankyo, Idorsia, Osler, Novartis, Roche, and Sanofi. All other authors declare that they have no conflict of interest with this study.
Funding
This study was supported by the Swiss National Science Foundation, the Swiss Heart Foundation, the University of Basel, the University Hospital Basel, and the Stanley Thomas Johnson Foundation. The study funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Supporting information
Figure S1. Patient flow chart.
Figure S2. Medication doses in standard care versus early intensive and sustained vasodilatation group. Exemplarily for patients with completed EQ‐5D at baseline and follow‐up (n = 284); comparison assessed using Mann–Whitney‐U test. Bars indicate medians; box bottoms and tops, 25th and 75th percentiles; whiskers, upper and lower adjacent values; dots, outliers. ns: p‐value >0.05; *: p‐value < = 0.05; **: p‐value < = 0.01; ***: p‐value < = 0.001; ****: p‐value < = 0.0001
Furosemide‐equivalent dose on day 1 corresponds to intravenous furosemide application and on day 2 through 180‐day follow‐up corresponds to prescribed furosemide and/or torasemide dose × 4.
ACEI: angiotensin‐converting enzyme inhibitor; ARB: angiotensin receptor blocker; ARNI: angiotensin receptor–neprilysin inhibitor. Yellow boxes indicate early intensive and sustained vasodilation; blue boxes, usual care.
Figure S3. Systolic blood pressure in standard care versus early intensive and sustained vasodilatation group. Exemplarily for patients with completed EQ‐5D at baseline and follow‐up (n = 284); comparison assessed using Mann–Whitney‐U test. Bars indicate medians; box bottoms and tops, 25th and 75th percentiles; whiskers, upper and lower adjacent values; dots, outliers. ns: p‐value >0.05; *: p‐value < = 0.05; **: p‐value < = 0.01; ***: p‐value < = 0.001; ****: p‐value < = 0.0001
Table S1. Baseline characteristics of patients with and without completed EQ‐5D questionnaire/KCCQ at baseline. Including patients who died within 180‐day follow‐up. Comparisons assessed using Mann–Whitney U or Chi‐squared test as appropriate. Continuous variables represented as median [interquartile range] and categorical as number (percent). *p‐value for comparison between two depicted groups
Table S2. Baseline characteristics of patients with and without completed EQ‐5D questionnaire/KCCQ at baseline and 180‐day follow‐up. Comparisons assessed using Mann–Whitney U or Chi‐squared test as appropriate. Continuous variables represented as median [interquartile range] and categorical as number (percent). *p‐value for comparison between two depicted groups
ACEI: angiotensin converting enzyme inhibitor, ARB: angiotensin receptor blocker, ARNI: angiotensin receptor–neprilysin inhibitor, BMI: body mass index, BUN: blood urea nitrogen, eGFR: estimated glomerular filtration rate, KCCQ: Kansas City Cardiomyopathy Questionnaire, NT‐proBNP: N‐Terminal pro‐B‐type natriuretic peptide.
Table S3. Within‐group changes in HRQL from initial hospitalisation to 180‐day follow‐up in patients admitted due to AHF and randomised to usual care versus intensive vasodilatation. HRQL displayed by A number of patients reporting problems according to the EQ‐5D and B median score of six domains of the KCCQ. Comparison of changes in HRQL assessed using Stuart‐Maxwell and Wilcoxon signed‐rank test for categorical variables (number [percent]) and continuous variables (median [interquartile range]), respectively.
*p‐value for comparison between baseline and follow‐up; AHF: acute heart failure, AD: Anxiety/Depression, HRQL: health‐related quality of life, KCCQ‐OSS: Kansas City Cardiomyopathy Questionnaire overall summary score, MO: Mobility, PD: Pain/Discomfort, SC: Self‐Care, UA: Usual Activities.
Acknowledgements
We are indebted to the patients who participated in the study and to the emergency department staff as well as the laboratory technicians of all participating sites for their most valuable efforts. M. B., D. W., J. W., N. K., D. G, and C. M. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Belkin, M. , Wussler, D. , Gualandro, D. M. , Shrestha, S. , Strebel, I. , Goudev, A. , Maeder, M. T. , Walter, J. , Flores, D. , Kozhuharov, N. , Lopez‐Ayala, P. , Danier, I. , de Oliveira Junior, M. T. , Kobza, R. , Rickli, H. , Breidthardt, T. , Erne, P. , Münzel, T. , and Mueller, C. (2021) Effect of a strategy of comprehensive vasodilation versus usual care on health‐related quality of life among patients with acute heart failure. ESC Heart Failure, 8: 4218–4227. 10.1002/ehf2.13543.
References
- 1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola V‐P, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, ESC Scientific Document Group . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 2. Yancy CW, Mariell J, Biykem B, Javed B, Casey DE, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, JoAnn L, Masoudi FA, McBride PE, Peterson PN, Warner SL, Cheryl W. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017; 136: e137–e161. [DOI] [PubMed] [Google Scholar]
- 3. Kozhuharov N, Goudev A, Flores D, Maeder MT, Walter J, Shrestha S, Gualandro DM, de Oliveira Junior MT, Sabti Z, Müller B, Noveanu M, Socrates T, Ziller R, Bayés‐Genís A, Sionis A, Simon P, Michou E, Gujer S, Gori T, Wenzel P, Pfister O, Conen D, Kapos I, Kobza R, Rickli H, Breidthardt T, Münzel T, Erne P, Mueller C. Effect of a strategy of comprehensive vasodilation vs usual care on mortality and heart failure rehospitalization among patients with acute heart failure: the GALACTIC randomized clinical trial. JAMA 2019; 322: 2292–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Breidthardt T, Noveanu M, Potocki M, Reichlin T, Egli P, Hartwiger S, Socrates T, Gayat E, Christ M, Mebazaa A, Mueller C. Impact of a high‐dose nitrate strategy on cardiac stress in acute heart failure: a pilot study. J Intern Med 2010; 267: 322–330. [DOI] [PubMed] [Google Scholar]
- 5. Rumsfeld JS, Alexander KP, Goff DC, Graham MM, Ho PM, Masoudi FA, Moser DK, Roger VL, Slaughter MS, Smolderen KG, Spertus JA, Sullivan MD, Treat‐Jacobson D, Zerwic JJ. Cardiovascular health: the importance of measuring patient‐reported health status. Circulation 2013; 127: 2233–2249. [DOI] [PubMed] [Google Scholar]
- 6. Paitazoglou C, Bergmann MW, Özdemir R, Pfister R, Bartunek J, Kilic T, Lauten A, Schmeisser A, Zoghi M, Anker SD, Sievert H, Mahfoud F. One‐year results of the first‐in‐man study investigating the atrial flow regulator for left atrial shunting in symptomatic heart failure patients: the PRELIEVE study. Eur J Heart Fail 2021; 23: 800–810. [DOI] [PubMed] [Google Scholar]
- 7. EuroQol Group . EuroQol‐ a new facility for the measurement of health‐related quality of life. Health Policy 1990; 16: 199–208. [DOI] [PubMed] [Google Scholar]
- 8. Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol 2000; 35: 1245–1255. [DOI] [PubMed] [Google Scholar]
- 9. Botella J, Blázquez D, Suero M, Juola JF. Assessing individual change without knowing the test properties: item bootstrapping. Front Psychol 2018; 9: 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sauser K, Spertus JA, Pierchala L, Davis E, Pang PS. Quality of life assessment for acute heart failure patients from emergency department presentation through 30 days after discharge: a pilot study with the Kansas City Cardiomyopathy Questionnaire. J Card Fail 2014; 20: 18–22. [DOI] [PubMed] [Google Scholar]
- 11. Spertus J, Peterson E, Conard MW, Heidenreich PA, Krumholz HM, Jones P, McCullough PA, Pina I, Tooley J, Weintraub WS, Rumsfeld JS. Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J 2005; 150: 707–715. [DOI] [PubMed] [Google Scholar]
- 12. Pokharel Y, Khariton Y, Tang Y, Nassif ME, Chan PS, Arnold SV, Jones PG, Spertus JA. Association of serial Kansas City cardiomyopathy questionnaire assessments with death and hospitalization in patients with heart failure with preserved and reduced ejection fraction: a secondary analysis of 2 randomized clinical trials. JAMA Cardio 2017; 2: 1315–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Butler J, Khan MS, Mori C, Filippatos GS, Ponikowski P, Comin‐Colet J, Roubert B, Spertus JA, Anker SD. Minimal clinically important difference in quality of life scores for patients with heart failure and reduced ejection fraction. Eur J Heart Fail 2020; 22: 999–1005. [DOI] [PubMed] [Google Scholar]
- 14. O'Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, Heizer GM, Komajda M, Massie BM, McMurray JJV, Nieminen MS, Reist CJ, Rouleau JL, Swedberg K, Adams KF, Anker SD, Atar D, Battler A, Botero R, Bohidar NR, Butler J, Clausell N, Corbalán R, Costanzo MR, Dahlstrom U, Deckelbaum LI, Diaz R, Dunlap ME, Ezekowitz JA, Feldman D, Felker GM, Fonarow GC, Gennevois D, Gottlieb SS, Hill JA, Hollander JE, Howlett JG, Hudson MP, Kociol RD, Krum H, Laucevicius A, Levy WC, Méndez GF, Metra M, Mittal S, Oh B‐H, Pereira NL, Ponikowski P, Tang WHW, Wilson WH, Tanomsup S, Teerlink JR, Triposkiadis F, Troughton RW, Voors AA, Whellan DJ, Zannad F, Califf RM. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med 2011; 365: 32–43. [DOI] [PubMed] [Google Scholar]
- 15. Packer M, O'Connor C, McMurray JJV, Wittes J, Abraham WT, Anker SD, Dickstein K, Filippatos G, Holcomb R, Krum H, Maggioni AP, Mebazaa A, Peacock WF, Petrie MC, Ponikowski P, Ruschitzka F, van Veldhuisen DJ, Kowarski LS, Schactman M, Holzmeister J. Effect of ularitide on cardiovascular mortality in acute heart failure. N Engl J Med 2017; 376: 1956–1964. [DOI] [PubMed] [Google Scholar]
- 16. Chioncel O, Mebazaa A, Harjola VP, Coats AJ, Piepoli MF, Crespo‐Leiro MG, Laroche C, Seferovic PM, Anker SD, Ferrari R, Ruschitzka F, Lopez‐Fernandez S, Miani D, Filippatos G, Maggioni AP, ESC Heart Failure Long‐Term Registry Investigators . Clinical phenotypes and outcome of patients hospitalized for acute heart failure: the ESC Heart Failure Long‐Term Registry. Eur J Heart Fail 2017; 19: 1242–1254. [DOI] [PubMed] [Google Scholar]
- 17. Collins SP, Liu D, Jenkins CA, Storrow AB, Levy PD, Pang PS, Chang AM, Char D, Diercks DJ, Fermann GJ, Han JH, Hiestand B, Hogan C, Kampe CJ, Khan Y, Lee S, Lindenfeld J, Martindale J, McNaughton CD, Miller KF, Miller‐Reilly C, Moser K, Peacock WF, Robichaux C, Rothman R, Schrock J, Self WH, Singer AJ, Sterling SA, Ward MJ, Walsh C, Butler J. Effect of a self‐care intervention on 90‐day outcomes in patients with acute heart failure discharged from the emergency department: a randomized clinical trial. JAMA Cardiol 2021; 6: 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Freund Y, Cachanado M, Delannoy Q, Laribi S, Yordanov Y, Gorlicki J, Chouihed T, Féral‐Pierssens AL, Truchot J, Desmettre T, Occelli C, Bobbia X, Khellaf M, Ganansia O, Bokobza J, Balen F, Beaune S, Bloom B, Simon T, Mebazaa A. Effect of an emergency department care bundle on 30‐day hospital discharge and survival among elderly patients with acute heart failure: the ELISABETH randomized clinical trial. JAMA 2020; 324: 1948–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Patient flow chart.
Figure S2. Medication doses in standard care versus early intensive and sustained vasodilatation group. Exemplarily for patients with completed EQ‐5D at baseline and follow‐up (n = 284); comparison assessed using Mann–Whitney‐U test. Bars indicate medians; box bottoms and tops, 25th and 75th percentiles; whiskers, upper and lower adjacent values; dots, outliers. ns: p‐value >0.05; *: p‐value < = 0.05; **: p‐value < = 0.01; ***: p‐value < = 0.001; ****: p‐value < = 0.0001
Furosemide‐equivalent dose on day 1 corresponds to intravenous furosemide application and on day 2 through 180‐day follow‐up corresponds to prescribed furosemide and/or torasemide dose × 4.
ACEI: angiotensin‐converting enzyme inhibitor; ARB: angiotensin receptor blocker; ARNI: angiotensin receptor–neprilysin inhibitor. Yellow boxes indicate early intensive and sustained vasodilation; blue boxes, usual care.
Figure S3. Systolic blood pressure in standard care versus early intensive and sustained vasodilatation group. Exemplarily for patients with completed EQ‐5D at baseline and follow‐up (n = 284); comparison assessed using Mann–Whitney‐U test. Bars indicate medians; box bottoms and tops, 25th and 75th percentiles; whiskers, upper and lower adjacent values; dots, outliers. ns: p‐value >0.05; *: p‐value < = 0.05; **: p‐value < = 0.01; ***: p‐value < = 0.001; ****: p‐value < = 0.0001
Table S1. Baseline characteristics of patients with and without completed EQ‐5D questionnaire/KCCQ at baseline. Including patients who died within 180‐day follow‐up. Comparisons assessed using Mann–Whitney U or Chi‐squared test as appropriate. Continuous variables represented as median [interquartile range] and categorical as number (percent). *p‐value for comparison between two depicted groups
Table S2. Baseline characteristics of patients with and without completed EQ‐5D questionnaire/KCCQ at baseline and 180‐day follow‐up. Comparisons assessed using Mann–Whitney U or Chi‐squared test as appropriate. Continuous variables represented as median [interquartile range] and categorical as number (percent). *p‐value for comparison between two depicted groups
ACEI: angiotensin converting enzyme inhibitor, ARB: angiotensin receptor blocker, ARNI: angiotensin receptor–neprilysin inhibitor, BMI: body mass index, BUN: blood urea nitrogen, eGFR: estimated glomerular filtration rate, KCCQ: Kansas City Cardiomyopathy Questionnaire, NT‐proBNP: N‐Terminal pro‐B‐type natriuretic peptide.
Table S3. Within‐group changes in HRQL from initial hospitalisation to 180‐day follow‐up in patients admitted due to AHF and randomised to usual care versus intensive vasodilatation. HRQL displayed by A number of patients reporting problems according to the EQ‐5D and B median score of six domains of the KCCQ. Comparison of changes in HRQL assessed using Stuart‐Maxwell and Wilcoxon signed‐rank test for categorical variables (number [percent]) and continuous variables (median [interquartile range]), respectively.
*p‐value for comparison between baseline and follow‐up; AHF: acute heart failure, AD: Anxiety/Depression, HRQL: health‐related quality of life, KCCQ‐OSS: Kansas City Cardiomyopathy Questionnaire overall summary score, MO: Mobility, PD: Pain/Discomfort, SC: Self‐Care, UA: Usual Activities.


