Abstract
Background
Cachexia is common in patients with chronic heart failure and is associated with poor prognosis. How best to measure body composition is not clear.
Methods and results
We characterized body composition in 120 patients with chronic heart failure: mean (SD) age 70 (10) years, left ventricular ejection fraction 44 (10) %, and median (Q1–Q3) N‐terminal pro B‐type natriuretic peptide 845 (355–1368) ng/L. We measured body composition using dual‐energy X‐ray absorptiometry (DEXA) and a multi‐frequency bioelectrical impedance analysis (BIA) device (Tanita BIA MC‐180MA). Mean (SD) fat mass (FM) was 27.2 (11.7) kg by BIA and 32.3 (12.2) kg by DEXA (mean difference −5.1 kg, 95% limits of agreement: −11.7, 1.5; 4% of values outside limit of agreement); mean (SD) lean mass (LM) was 56.6 (10.9) kg by BIA and 51.1 (9.9) kg by DEXA (mean difference 5.5 kg, 95% limits of agreement: −1.3, 12.3; 6% of values outside limit of agreement); and mean (SD) bone mass (BM) was 3.0 (0.5) kg by BIA and 2.8 (0.6) kg by DEXA (mean difference 0.2 kg, 95% limits of agreement: −0.5, 0.8; 5% of values outside limit of agreement). There was a close correlation between DEXA and BIA for both LM and FM (LM: r = 0.95, P < 0.001; FM: r = 0.96, P < 0.001) but less so for BM (r = 0.84, P < 0.001). Both DEXA and BIA body composition measurements correlated well with other measures of body size (body mass index, hip circumference, and waist circumference).
Conclusions
There are differences in the measurements of FM, LM, and BM between the two techniques, which should not be used interchangeably.
Keywords: Heart failure, Body composition, Dual‐energy X‐ray absorptiometry scan, Body composition analyser, Bioelectrical impedance analysis
Introduction
Cachexia is a common feature in the late stages of many chronic diseases but is under‐recognized. 1 Chronic heart failure (CHF) often leads to cachexia, with an estimated prevalence of between 5% and 15%. 1 Cachexia is an adverse prognostic indicator in patients with CHF. Patients with cachexia (defined as non‐oedematous and non‐intentional weight loss of more than 7.5% of previous weight over a period of at least 6 months) have a 50% mortality at 18 months. 2
Cachexia is characterized by loss of muscle with or without loss of fat mass. Defining cachexia is difficult, although consensus statements suggest features such as at least 5% loss of (oedema free) weight loss in 1 year or a body mass index (BMI) less than 20 kg/m2, accompanied by other symptoms or signs. 3 Weight loss of more than 6% within 9 to 12 months in patients with CHF is a strong predictor of adverse outcome. 4 Conversely, weight gain is associated with a better prognosis. 5
Detecting cachexia in patients with CHF can be particularly challenging because of changing amounts of extracellular fluid. A patient might be losing muscle and fat mass whilst their total weight is unchanged, or even increasing. Measuring just weight or BMI may miss a reduction in lean mass (LM) or fat mass (FM). 6 Techniques that measure body composition may thus help in detecting cachexia. We compared the segmental body composition of patients with CHF using two devices: dual‐energy X‐ray absorptiometry (DEXA), considered the gold standard for body composition analysis, 6 , 7 and a multi‐frequency bioelectrical impedance analysis (BIA) device.
Methods
We assessed body composition in 120 ambulatory patients with CHF enrolled in Studies Investigating Co‐morbidities Aggravating Heart Failure (SICA‐HF, ClinicalTrials.gov Identifier: NCT01872299) in Kingston upon Hull, UK. 8 SICA‐HF is an international observational study of the prevalence, incidence, and impact of key co‐morbidities in adults (≥18 years) with a clinical diagnosis of CHF and objective evidence of cardiac dysfunction as evidenced by either left ventricular ejection fraction (LVEF) ≤40%, left atrial dimension ≥4.0 cm, or plasma concentration of N‐terminal pro B‐type natriuretic peptide (NTproBNP) >400 ng/L. Patients with end‐stage renal failure or an alternative cause for raised NTproBNP were not enrolled in the study.
Patients with an implanted cardioverter defibrillator or pacemaker were excluded as bioelectric impedance measurement is contraindicated in such patients. Patients who were unable to lie flat or who exceeded the 150 kg weight limit for the DEXA machine were also excluded.
For each patient, both body composition assessments were performed on the same day during a single scheduled visit, which also included a full cardiovascular clinical history and examination, blood tests (including full blood count, biochemical profile, and NTproBNP), an electrocardiogram, and an echocardiogram. Patients were clinically stable with no signs of cardiac decompensation.
We used the Tanita MC‐180 MA scales (Tanita Europe B. V, The Netherlands) to measure body weight and estimate body composition by BIA. The scales can detect percentage body fat between 1% and 75% in increments of 0.1% with a weight capacity of up to 200 kg. Patients stood bare foot on the scales with feet on metal plates and hands holding the handles. Weight and body composition [FM, LM, and bone mass (BM)] were determined.
We used the weight determined by the scales as an input to the DEXA scanner. Whole body composition was then measured using the DEXA scanner (LUNAR IDAX‐GE, UK) by experienced technical staff using the manufacturer's standard protocol. Patients lay supine for around 5 min. Body composition (FM, LM, and BM) was determined.
Patients wore light clothes for both investigations. All data were entered into a dedicated online database.
The investigation conformed to the principles outlined in the Declaration of Helsinki and was approved by relevant ethical bodies. All subjects gave their written informed consent.
Statistical analysis
Categorical data are presented as number and percentages; normally distributed continuous data as mean and standard deviation (SD); non‐normally distributed continuous variables as median and interquartile range.
Paired t tests were used to compare continuous variables between groups, and the χ 2 test was used for categorical variables. To compare measurements from DEXA and BIA, three methods of comparison were used: Pearson correlation coefficients, Pitman's test of difference in variance, and Bland–Altman plots. Pearson correlation coefficients were also used to determine the correlation between the measures of body composition (DEXA and BIA) and other measures of cachexia, echocardiographic findings, NTproBNP, and creatinine.
All analyses were performed using SPSS (v. 23) software. A two‐sided P value <0.05 was considered statistically significant.
Results
The characteristics of the patients are shown in Table 1 . The mean (SD) age of the population was 70 (10) years, and 24% were female. The mean (SD) for BMI was 30.2 (6.4) kg/m2, and LVEF was 44 ± 14%; and median (Q1–Q3) for NTproBNP 845 (355–1368) ng/L. The majority were classified as New York Heart Association Class I or II (87%).
Table 1.
Baseline characteristics
| Demographics | |
|---|---|
| Age (years) | 70 (10) |
| Male—no. (%) | 91 (76) |
| Female—no. (%) | 29 (24) |
| Weight (kg) | 86.8 (19.9) |
| Height (m) | 1.7 (0.1) |
| BMI (kg/m2) | 30.2 (6.4) |
| Waist circumference (cm) | 102.3 (16.3) |
| Hip circumference (cm) | 106.9 (14.0) |
| SBP (mm Hg) | 126 (23) |
| HR (beats/min) | 68 (13) |
| Medical history | |
|---|---|
| NYHA I—no. (%) | 34 (28) |
| NYHA II—no. (%) | 71 (59) |
| NYHA III—no. (%) | 15 (13) |
| Atrial fibrillation (%) | 37 (31) |
| Hypertension (%) | 63 (53) |
| Ischaemic heart disease (%) | 78 (65) |
| Diabetes (%) | 40 (33) |
| Previous cancer (%) | 16 (13) |
| Medication | |
|---|---|
| ACE‐I (%) | 86 (72) |
| ARB (%) | 27 (23) |
| Beta blocker (%) | 101 (84) |
| Loop diuretic (%) | 71 (59) |
| Blood results | |
|---|---|
| Urea (mmol/L) | 6.5 (5.3–9.5) |
| Creatinine (μmol/L) | 95 (80–118) |
| Bilirubin (μmol/L) | 16 (7) |
| Albumin (g/L) | 40 (3) |
| NTproBNP (ng/L) | 845 (355–1,368) |
| Echocardiography | |
|---|---|
| LVEDV (ml) | 139 (52) |
| LVEF (%) | 44 (14) |
| LAD (cm) | 4.0 (0.7) |
| TAPSE (mm) | 18 (5) |
ACE‐I, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; HR, heart rate; LAD, left atrial diameter; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; NTproBNP, N‐terminal pro B‐type natriuretic peptide; NYHA, New York Heart Association; SBP, systolic blood pressure; TAPSE, tricuspid annular plane systolic excursion.
Continuous variables are shown as mean (standard deviation), categorical variables as percentage. NTproBNP is shown as median (Q1–Q3).
Compared with DEXA, BIA gave a higher estimate of LM and BM but a lower estimate of FM (Bland–Altman plots: Figures 1, 2, 3, Table 2 ). There was a close correlation between LM (R = 0.95, P < 0.001) and FM (R = 0.96, P < 0.001) measured with the two devices (BIA and DEXA), although the correlation was less strong for BM (R = 0.84, P < 0.001). The Pitman's test of the difference in variance between DEXA and BIA was not significant for FM (−0.14, P = 0.14) but was significant for both LM (0.29, P ≤ 0.001) and BM (−0.21, P = 0.03).
Figure 1.
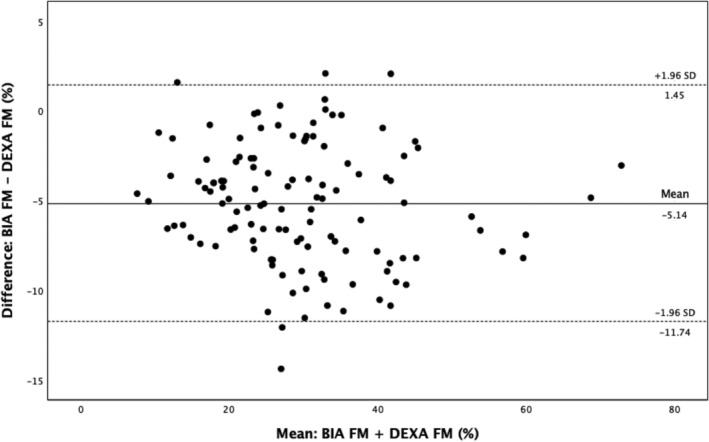
Bland–Altman analysis for FM measured by BIA and DEXA. BIA, bioelectrical impedance analysis; DEXA, dual‐energy X‐ray absorptiometry; FM, fat mass.
Figure 2.
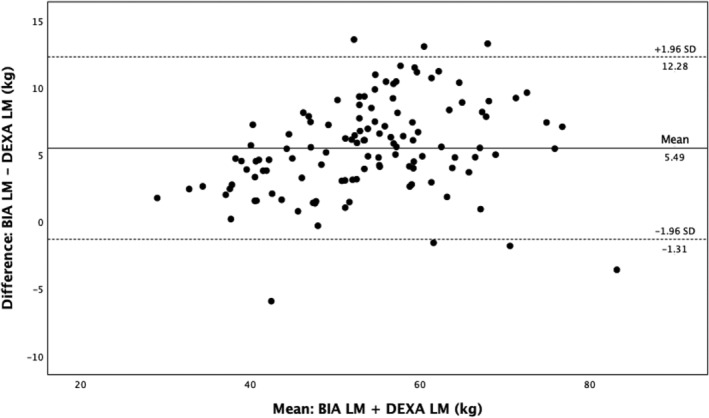
Bland–Altman analysis for LM measured by BIA and DEXA. BIA, bioelectrical impedance analysis; DEXA, dual‐energy X‐ray absorptiometry; LM, lean mass.
Figure 3.
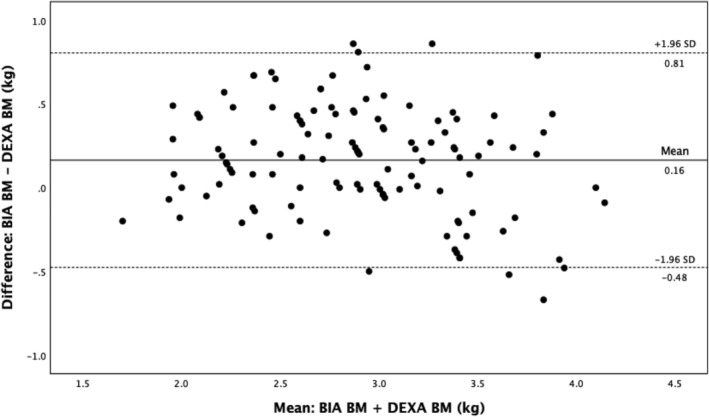
Bland–Altman analysis for BM measured by BIA and DEXA. BIA, bioelectrical impedance analysis; BM, bone mass; DEXA: dual‐energy X‐ray absorptiometry.
Table 2.
Body composition measured by BIA and DEXA
| DEXA kg (SD) | BIA kg (SD) | Mean difference (BIA − DEXA, kg) | 95% limits of agreement | % outside limits of agreement | |
|---|---|---|---|---|---|
| Mean LM | 51.1 (9.9) | 56.6 (10.9) | 5.5 | −1.3, 12.3 | 6 |
| Mean FM | 32.3 (12.2) | 27.2 (11.7) | −5.1 | −11.7, 1.5 | 4 |
| Mean BM | 2.8 (0.6) | 3.0 (0.5) | 0.2 | −0.5, 0.8 | 5 |
BIA, bioelectrical impedance analysis; BM, bone mass; DEXA, dual‐energy X‐ray absorptiometry; FM, fat mass; LM: lean mass.
Dual‐energy X‐ray absorptiometry measurements of FM and LM correlated well with other measures of body size (BMI, waist circumference, and hip circumference). Correlations between BM and measures of body size were weaker. There were at most only weak correlations between the DEXA measurements of FM, LM, and BM and measures of severity of heart failure, including echocardiographic severity of left ventricular dysfunction, NTproBNP, high sensitivity C reactive protein (hsCRP), creatinine, and age (Table 3 ).
Table 3.
Correlations between body composition and patient demographics, kidney function, NTproBNP, echocardiographic findings and measures of obesity
| Pearson correlation—R (P value) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (Years) | BMI (kg/m2) | Creatinine (mmol/L) | Log NTproBNP | hsCRP (mg/L) | LVEF (%) | LA volume | TAPSE (mm) | Hip circ (cm) | Waist circ (cm) | Waist–hip ratio | |
| DEXA FM (kg) | −0.35 (<0.001) | 0.91 (<0.001) | −0.15 (0.09) | −0.21 (0.02) | 0.16 (0.08) | 0.16 (0.09) | 0.01 (0.89) | 0.24 (0.01) | 0.82 (<0.001) | 0.76 (<0.001) | 0.20 (0.03) |
| DEXA LM (kg) | −0.23 (0.01) | 0.58 (<0.001) | 0.09 (0.33) | −0.14 (0.12) | 0.10 (0.27) | 0.14 (0.14) | 0.38 (<0.001) | 0.05 (0.63) | 0.58 (<0.001) | 0.74 (<0.001) | 0.51 (<0.001) |
| DEXA BM (kg) | −0.15 (0.11) | 0.37 (<0.001) | 0.07 (0.42) | −0.16 (0.08) | −0.01 (0.99) | 0.18 (0.05) | 0.26 (0.01) | 0.11 (0.23) | 0.38 (<0.001) | 0.58 (<0.001) | 0.50 (<0.001) |
| BIA FM (kg) | −0.36 (<0.001) | 0.91 (<0.001) | −0.15 (0.10) | −0.18 (0.53) | 0.193 (0.03) | 0.13 (0.17) | 0.06 (0.51) | 0.22 (0.02) | 0.81 (<0.001) | 0.72 (<0.001) | 0.14 (0.13) |
| BIA LM (kg) | −0.18 (0.05) | 0.58 (<0.001) | 0.07 (0.42) | −0.20 (0.03) | 0.03 (90.77) | 0.19 (0.04) | 0.27 (0.01) | 0.10 (0.28) | 0.60 (<0.001) | 0.77 (<0.001) | 0.54 (<0.001) |
| BIA BM (kg) | −0.16 (0.07) | 0.58 (<0.001) | 0.08 (0.39) | −0.20 (0.03) | 0.03 (0.72) | 0.19 (0.04) | 0.27 (0.01) | 0.10 (0.28) | 0.60 (<0.001) | 0.77 (<0.001) | 0.55 (<0.001) |
BIA, bioelectrical impedance analysis; BM, bone mass; BMI, body mass index; circ, circumference; DEXA, dual‐energy X‐ray absorptiometry; FM, fat mass; hsCRP, high sensitivity C reactive protein; LA, left atrial; LM, lean mass; LVEF, left ventricular ejection fraction; NTproBNP, N‐terminal pro B‐type natriuretic peptide; TAPSE, tricuspid annular plane systolic excursion.
Correlations were performed in 120 patients, but for LA volume, TAPSE, waist, and hip circumference n = 118.
Bioelectrical impedance analysis measurements of FM, LM, and BM correlated well with other measures of body size (BMI, waist circumference, and hip circumference). There were at most only weak correlations between the BIA measurements of FM, LM, and BM and measures of severity of heart failure, including echocardiographic severity of left ventricular dysfunction, NTproBNP, hsCRP, creatinine, and age (Table 3 ).
Discussion
We found similar measurements for body composition using DEXA and BIA in ambulatory patients with CHF, regardless of LVEF or sex. However, DEXA consistently gave higher values for FM and lower values for LM than BIA.
Cachexia is usually defined by crude measurements of weight or BMI. In patients with CHF, body weight may fluctuate due to differences in fluid retention. Hence, some method that measures body compartments independent of fluid retention might be helpful.
Bioelectrical impedance analysis uses the principle that conduction is directly proportional to the concentration of ions within a conductor. Conductivity in blood and urine is high, that of muscle is intermediate, and that of tissues such as bone, fat, or air is low. 9 , 10 In BIA, a low voltage is passed between surface electrodes on the feet and hands 9 , 11 to measure body water, which is then used to estimate fat‐free mass, subtracting the fat‐free mass value from weight estimates body fat.
Dual‐energy X‐ray absorptiometry, which is commonly used for bone mineral density measurements, uses the differential attenuation of the two X‐ray energies to determine body composition. 12 Subjects are scanned rectilinearly with the X‐ray source beneath a bed and a detector above the patient. 13 The radiation doses are small (5–7 μSv). 7 , 13 DEXA is a reference method for body composition analysis, being preferred to computed tomography (due to radiation exposure) and magnetic resonance imaging (due to cost). 13 , 14 , 15 Anker et al. first used DEXA scanning to show a significant reduction in lean, fat, and bone mass in 18 patients with heart failure with cachexia compared both with 36 patients with heart failure who were not cachectic and with healthy age‐matched controls. 16
Our results confirm, and expand, those reported by others who compared DEXA with BIA. Alves et al. compared two BIA devices (single‐frequency and multi‐frequency) to DEXA in 55 patients with stable heart failure and reduced ejection fraction (LVEF <45%). Compared with DEXA, the multi‐frequency device gave similar results for mean FM and fat‐free mass, but the single‐frequency device gave significantly higher results for FM and lower values for fat‐free mass. Both BIA methods had wide limits of agreement for FM and fat‐free mass compared with DEXA. 6
Oreopoulos et al. compared body composition in 140 patients with CHF using three devices: DEXA, BIA, and near infrared interactance (NIR). Compared with DEXA, BIA gave significantly higher values for LM and percentage fat in men and significantly higher values of LM in women. Compared with DEXA, NIR gave significantly higher values for LM in men. Both BIA and NIR had wide limits of agreement for body fat percentage and lean body mass compared with DEXA. 17
The devices used to assess body composition are thus not interchangeable. DEXA is the reference method for body composition analysis despite being a source of radiation. 15 , 17 BIA devices, which can assess body composition quickly and at the same time and place as an outpatient visit, are potentially much more convenient, but need further validation in the study of cachexia. 18
The identification of cachexia may improve with measurement of body composition, particularly in patients with CHF in whom changes in weight due to congestion may mask change in muscle and fat mass. The pathophysiology of cachexia is not well understood. There are no treatments proven to help in cardiac cachexia, 1 although the use of ACE inhibitors and beta blockers leads to an increase in body weight. 4 , 5 , 19 , 20
Conclusion
There are differences in the results for FM, LM, and BM between different methods for measuring body composition. Measures derived from DEXA and BIA should not be used interchangeably.
Conflict of interest
None declared.
Funding
The research leading to these results has received funding from the European Union Seventh Framework Programme (FP7/2007‐2013) (under grant agreement number 241558) (SICA‐HF).
Author contributions
P. S.: conceptualization, methodology, formal analysis, visualization, and writing—original draft; A. A.: formal analysis, visualization, writing—review and editing; V. B.: investigation, data curation, formal analysis; P. P.: investigation, data curation; A. B.: investigation; S. S.: investigation; J. G. F. C.: conceptualization, methodology, supervision; A. L. C.: conceptualization, methodology, funding acquisition, supervision, writing—review and editing.
Shah, P. , Abel, A. A. I. , Boyalla, V. , Pellicori, P. , Kallvikbacka‐Bennett, A. , Sze, S. , Cleland, J. G. F. , and Clark, A. L. (2021) A comparison of non‐invasive methods of measuring body composition in patients with heart failure: a report from SICA‐HF. ESC Heart Failure, 8: 3929–3934. 10.1002/ehf2.13402.
References
- 1. Anker SD, von Haehling S. Cachexia as a major underestimated and unmet medical need: facts and numbers. J Cachexia Sarcopenia Muscle 2010; 1: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anker SD, Ponikowski P, Varney S, Chua TP, Clark AL, Webb‐Peploe KM, Harrington D, Kox WJ, Poole‐Wilson PA, Coats AJ. Wasting as independent risk factor for mortality in chronic heart failure. Lancet 1997; 349: 1050–1053. [DOI] [PubMed] [Google Scholar]
- 3. Evans WJ, Morley JE, Argilés J, Bales C, Baracos V, Guttridge D, Jatoi A, Kalantar‐Zadeh K, Lochs H, Mantovani G, Marks D, Mitch WE, Muscaritoli M, Najand A, Ponikowski P, Rossi Fanelli F, Schambelan M, Schols A, Schuster M, Thomas D, Wolfe R, Anker SD. Cachexia: a new definition. Clin Nutr 2008; 27: 793–799. [DOI] [PubMed] [Google Scholar]
- 4. Anker SD, Negassa A, Coats AJ, Afzal R, Poole‐Wilson PA, Cohn JN, Yusuf S. Prognostic importance of weight loss in chronic heart failure and the effect of treatment with angiotensin‐converting‐enzyme inhibitors: an observational study. Lancet 2003; 361: 1077–1083. [DOI] [PubMed] [Google Scholar]
- 5. Sze S, Pellicori P, Kamzi S, Anton A, Clark AL. Effect of beta‐adrenergic blockade on weight changes in patients with chronic heart failure. Int J Cardiol 2018; 264: 104–112. [DOI] [PubMed] [Google Scholar]
- 6. Alves FD, Souza GC, Biolo A, Clausell N. Comparison of two bioelectrical impedance devices and dual‐energy X‐ray absorptiometry to evaluate body composition in heart failure. J Hum Nutr Diet 2014; 27: 632–638. [DOI] [PubMed] [Google Scholar]
- 7. Andreoli A, Scalzo G, Masala S, Tarantino U, Guglielmi G. Body composition assessment by dual‐energy X‐ray absorptiometry (DXA). Radiol Med 2009; 114: 286–300. [DOI] [PubMed] [Google Scholar]
- 8. von Haehling S, Lainscak M, Doehner W, Ponikowski P, Rosano G, Jordan J, Rozentryt P, Rauchhaus M, Karpov R, Tkachuk V, Parfyonova Y, Zaritskey AY, Shlyakhto EV, Cleland JG, Anker SD. Diabetes mellitus, cachexia and obesity in heart failure: rationale and design of the Studies Investigating Co‐morbidities Aggravating Heart Failure (SICA‐HF). J Cachexia Sarcopenia Muscle 2010; 1: 187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. NIH . Bioelectrical impedance analysis in body composition measurement. NIH Technol Assess Statement 1994: 1–35. [Google Scholar]
- 10. Buchholz AC, Bartok C, Schoeller DA. The validity of bioelectrical impedance models in clinical populations. Nutr Clin Pract 2004; 19: 433–446. [DOI] [PubMed] [Google Scholar]
- 11. Segal KR, Gutin B, Presta E, Wang J, Van Itallie T. Estimation of human body composition by electrical impedance methods: a comparative study. J Appl Physiol 1985; 58: 1565–1571. [DOI] [PubMed] [Google Scholar]
- 12. Laskey MA. Dual‐energy X‐ray absorptiometry and body composition. Nutrition 1996; 12: 45–51. [DOI] [PubMed] [Google Scholar]
- 13. Chen Z, Wang Z, Lohman T, Heymsfield SB, Outwater E, Nicholas JS, Bassford T, LaCroix A, Sherrill D, Punyanitya M, Wu G, Going S. Dual‐energy X‐ray absorptiometry is a valid tool for assessing skeletal muscle mass in older women. J Nutr 2007; 137: 2775–2780. [DOI] [PubMed] [Google Scholar]
- 14. Plank LD. Dual‐energy X‐ray absorptiometry and body composition. Curr Opin Clin Nutr Metab Care 2005; 8: 305–309. [DOI] [PubMed] [Google Scholar]
- 15. Van Loan MD. Is dual‐energy X‐ray absorptiometry ready for prime time in the clinical evaluation of body composition? Am J Clin Nut 1998; 68: 1155–1156. [DOI] [PubMed] [Google Scholar]
- 16. Anker SD, Ponikowski PP, Clark AL, Leyva F, Rauchhaus M, Kemp M, Teixeira MM, Hellewell PG, Hooper J, Poole‐Wilson PA, Coats AJS. Cytokines and neurohormones relating to body composition alterations in the wasting syndrome of chronic heart failure. Eur Heart J 1999; 20: 683–693. [DOI] [PubMed] [Google Scholar]
- 17. Oreopoulos A, Kalantar‐Zadeh K, Mcalister FA, Ezekowitz JA, Fonarow GC, Johnson JA, Norris CM, Padwal RS. Comparison of direct body composition assessment methods in patients with chronic heart failure. J Cardiac Fail 2010; 16: 867–872. [DOI] [PubMed] [Google Scholar]
- 18. Kyle UG, Bosaeusb I, De Lorenzoc AD, Deurenbergd P, Eliae M, Gomez JM, Heitmann BL, Kent‐Smith L, Melchior J‐C, Pirlich M, Scharfetter H, Schols AMWJ, Pichard C, Composition of the ESPEN Working Group . Bioelectrical impedance analysis—part I: review of principles and methods. Clinl Nutr 2004; 23: 1226–1243. [DOI] [PubMed] [Google Scholar]
- 19. Lainscak M, Keber I, Anker SD. Body composition changes in patients with systolic heart failure treated with beta blockers: a pilot study. Int J Cardiol 2006; 106: 319–322. [DOI] [PubMed] [Google Scholar]
- 20. Clark AL, Coats AJS, Krum H, Katus HA, Mohacsi P, Salekin D, Schultz MK, Packer M, Anker SD. Effect of beta‐adrenergic blockade with carvedilol on cachexia in severe chronic heart failure: results from the COPERNICUS trial. J Cachexia Sarcopenia Muscle 2017; 8: 549–556c. [DOI] [PMC free article] [PubMed] [Google Scholar]


