Abstract
Takotsubo cardiomyopathy (TCM), characterized by reversible ventricular dysfunction, has similar mortality to acute coronary syndrome. With the growing interest in the diagnosis of and interventions for TCM, many risk factors had been found to affect the prognosis of TCM patients, such as age, sex, and pre‐existing diseases. Because of the incomplete understanding of the pathophysiologic mechanism in TCM, evidence‐based medical therapy for this condition is lacking. Early intervention on risk factors may improve the outcomes of TCM. In this review, we sought to provide up‐to‐date evidence on risk factors and medical therapies that affect TCM outcome. We found that male sex, physical triggers, and certain comorbidities such as chronic kidney disease, malignant disease, higher body mass index, sepsis, chronic obstructive pulmonary disease, and anaemia were associated with poor TCM prognosis. In contrast, race, hyperlipidaemia, diabetes mellitus, and mood disorders were not clearly associated with TCM prognosis. We also reviewed the effect of medical therapies on TCM outcome, including angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers, β‐blockers, calcium channel blockers, and statins. The evidence that these medications confer a survival benefit on TCM patients is limited. Understanding these prognostic factors could help develop risk‐stratification tools for TCM and establish effective prevention and interventions for this not‐so‐benign condition. Further multicentre clinical studies with large samples and meta‐analyses of findings from previous studies are needed to address the inconsistent findings among the many potential risk factors for TCM.
Keywords: Risk factors, Treatment, Prognosis, Takotsubo cardiomyopathy
Introduction
Having been first introduced in Japan, Takotsubo cardiomyopathy (TCM) is characterized by temporary, reversible regional wall motion abnormalities that extend beyond a single coronary artery distribution without any evidence of acute coronary artery occlusion. 1 Takotsubo, which means ‘octopus trap’ in Japanese, is used to describe the classic morphology of the left ventricle in systole as seen on echocardiograms. 2 The true prevalence of the TCM remains unknown. Over the past few years, the number of reported cases has increased. Previous reports indicated that between 0.7% and 2.2% of acute coronary syndrome (ACS) cases were eventually discovered to be TCM. 3 , 4 , 5 , 6
Based on the initial presentation, TCM is classified into two clinical subtypes: primary and secondary. In primary TCM, TCM is the chief reason for the patient's acute presentation; this includes cases with clear triggers or any coexisting medical conditions that are risk factors for TCM. Secondary TCM is considered a complication of the patient's primary medical condition, such as medical, surgical, and psychiatric conditions. 7
Moreover, whereas TCM was once thought to be a reversible and benign condition, it is now recognized to have a mortality rate similar to that of ACS. 8 Major adverse cardiovascular events, such as cardiac arrhythmias, 9 cardiogenic shock, 8 and ventricular thrombus, 10 are also not uncommon in TCM patients. Rare conditions such as ventricular rupture 11 and ventricular septal defect 12 can occur, as well. The overall rarity of TCM had limited the study of TCM outcomes in the past. Recent advances in using administrative and registry databases have enabled us to uncover several prognostic factors in both short‐term and long‐term outcomes of TCM. A recent study has also identified distinct TCM clinical phenotypes according to their risk factors and found different in‐hospital outcomes among these phenotypes. 13 Understanding these risk factors could facilitate the development of risk‐stratification tools for TCM and effective interventions to improve TCM prognosis. In this review, we summarize the available evidence regarding risk factors and medical therapies and their effect on TCM prognosis.
Methods
We performed a systematic review of observational studies on the association between risk factors with TCM.
Search strategy
We searched the key words ‘apical ballooning syndrome,’ ‘broken heart syndrome,’ ‘stress cardiomyopathy,’ ‘takotsubo syndrome,’ ‘takotsubo cardiomyopathy,’ ‘takotsubo cardiomyopath*,’ ‘takotsubo,’ ‘ampulla cardiomyopathy,’ ‘neurogenic pulmonary edema,’ and ‘stress induced cardiomyopathy’ to identify articles from PubMed, Embase, and Cochrane databases from January 1, 2010 to February 28, 2020.
Inclusion and exclusion criteria
Studies were selected according to the following pre‐specified inclusion criteria (all of which had to be met for inclusion): (i) diagnosis of TCM on the basis of pre‐defined specific TCM diagnostic criteria or International Classification of Diseases (ICD) code; (ii) the end point of study was mortality, including short‐term and long‐term mortality; and (iii) the study sample comprised more than 100 patients. Exclusion criteria included duplicate reporting: if multiple articles reported the same number of patients and follow‐up time, the most recent article was selected. Single case reports and previous systematic reviews on TCM were also excluded.
Study selection
Two unblinded investigators (Lu and Li) independently screened the retrieved citations. Studies whose title or abstract indicated potential relevance to our study were fully reviewed, and their appropriateness for the study was judged independently by the two reviewers according to the previously described inclusion and exclusion criteria. Disagreements were resolved by consensus, with adjudication by a third party when needed.
Outcome measures
Two clinical outcomes were analysed: short‐term mortality and long‐term mortality (i.e. including both cardiac and non‐cardiac deaths). Data were extracted into standard spreadsheets and included date of study publication, sample size, diagnostic criteria, duration of follow‐up, analysis strategy, and clinical outcomes, as previously defined. Methodological study quality was assessed with the Quality in Prognostic Factor Studies tool. 14 The domains of patient selection, study attrition, measurement of prognostic factors, outcome measurement, study confounding, and statistical analysis and reporting were rated as having a low, moderate, or high risk of bias. Studies with five or six domains with a low risk of bias were classified as having a low overall risk of bias, and studies with two or more domains with a high risk of bias were classified as having a high overall risk of bias. All other studies were considered to have a moderate overall risk of bias. 15
Results
The process of study selection (Figure 1 ) identified 61 studies for review. Another two articles, which were published after we performed our search, were added at the suggestion of peer reviewers. Finally, we identified 63 observational studies. Quality assessment by the Quality in Prognostic Factor Studies checklist disclosed moderate bias in 42 studies, high bias in 20 studies, and low bias in 1 study.
Figure 1.
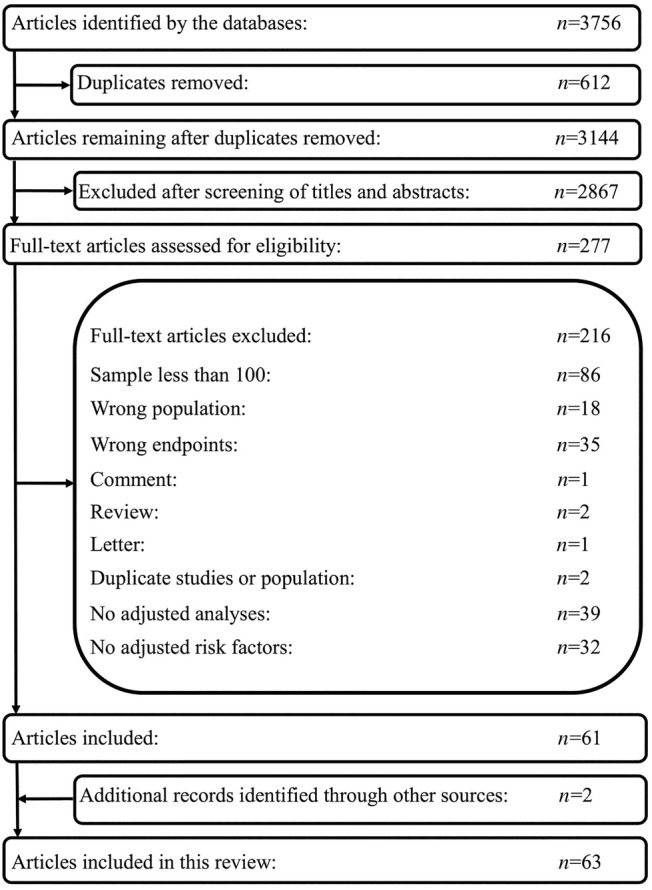
Flow chart of article selection process.
Factors affecting prognosis of Takotsubo cardiomyopathy
Sex
Female sex has been identified as a strong risk factor for TCM. 16 Previous studies have found that women comprise about 82.0% to 92.7% of the overall TCM patient population. 17 , 18 , 19 , 20 , 21 , 22 , 23 In a study of postmenopausal women who presented with ACS, approximately 5.9% of them were noted to have TCM. 16
Despite female sex being a strong risk factor for TCM, most of the studies we identified suggest that it is associated with favourable short‐term outcomes in TCM (Table 1 ). Some studies have found that male patients have poorer short‐term outcomes, such as higher rates of in‐hospital mortality 19 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 and complications. 18 , 20 , 32 One retrospective study of 14 639 patients found that female patients had a lower rate of in‐hospital mortality [odds ratio (OR) 0.521, 95% confidence interval (CI) 0.389–0.699]. 30 In another study that included 24 701 TCM patients, Brinjikji et al. found that male sex was associated with higher in‐hospital mortality (OR 2.07, 95% CI 1.71–2.49). 19 Similarly, a study of 39 662 TCM patients found that male patients had higher rates of in‐hospital mortality (OR 3.89, 95% CI 2.41–6.24) and in‐hospital complications, such as cardiogenic shock (OR 1.51, 95% CI 1.03–2.06), ventricular fibrillation (VF) or tachycardia (VT) (OR 1.52, 95% CI 1.07–2.16), and acute kidney injury (OR 1.93, 95% CI 1.44–2.59). 20 On the other hand, some studies have found no association between sex and TCM in‐hospital outcome. 8 , 23 , 33 , 34 , 35 , 36 , 37 , 38 , 39 A study of 1750 TCM patients found that female sex was not associated with in‐hospital outcomes, including catecholamine use, cardiogenic shock, ventilation use, cardiopulmonary resuscitation, and all‐cause mortality (P = 0.14). 8 Similarly, another study with relatively large sample size (705 male patients and 6805 female patients) did not find a sex difference in in‐hospital mortality in patients with TCM. 36
Table 1.
Clinical studies on the effect of sex in TCM patients
| Sex | Author (year) | Diagnostic criteria | Sample size | Type of study | Follow‐up | Mortality | Analysis strategy | Risk of bias |
|---|---|---|---|---|---|---|---|---|
| Male vs. Female | Brinjikji et al. (2012) | ICD‐9 | 24 701 | Retrospective | In‐hospital | OR 2.07 (95% CI 1.71–2.49), P < 0.0001 | Multivariable logistic regression | High |
| Isogai et al. (2014) | ICD‐10 | 3719 | Retrospective | In‐hospital | No difference | Multivariable logistic regression | High | |
| Lee et al. (2016) | Mayo criteria | 128 | Retrospective | In‐hospital | No difference | Multivariable logistic regression | Moderate | |
| Khera et al. (2016) | ICD‐9 | 11 193 | Retrospective | In‐hospital |
Primary diagnosis of TCM: OR 3.85 (95% CI 1.74–8.51), P = 0.0009 Secondary diagnosis of TCM: No difference |
Multivariable logistic regression | High | |
| Stiermaier et al. (2016) | Mayo criteria | 285 | Prospective | 6 months | No difference | Multivariate Cox regression | Moderate | |
| Stiermaier et al. (2016) | Mayo criteria | 286 | Prospective | Mean = 3.8 ± 2.5 years | HR 1.97 (95% CI 1.03–3.78), P = 0.04 | Multivariate Cox regression | Moderate | |
| Stiermaier et al. (2017) | Mayo criteria and ESC criteria (2016) | 387 | Prospective | Median = 2.9 years | HR 2.97 (95% CI 1.68–5.24), P < 0.001 | Multivariate Cox regression | Moderate | |
| El‐Battrawy et al. (2017) | Mayo criteria | 114 | Prospective | Mean = 1529 ± 1121 days | No difference | Multivariate Cox regression | Moderate | |
| Huseynov et al. (2017) | Mayo criteria | 114 | Prospective | Mean = 1591 ± 1079 days | HR 2.8 (95% CI 1.1–7.2), P = 0.02 | Multivariate Cox regression | Moderate | |
| Weidner et al. (2017) | Mayo criteria | 114 | Prospective | 5 years | HR 2.8 (95% CI 1.1–7.2), P = 0.02 | Multivariate Cox regression | Moderate | |
| Bill et al. (2017) | Mayo criteria | 114 | Prospective | 2 years | No difference | Multivariate Cox regression | Moderate | |
| Almendro‐Delia et al. (2018) | Modified Mayo criteria | 711 | Prospective | Median = 284 days | HR 1.93 (95% CI 1.46–2.55), P < 0.0001 | Multivariate Cox regression | Moderate | |
| Ansari et al. (2018) | Modified Mayo criteria | 114 | Prospective | 5 years | No difference | Multivariate Cox regression | Moderate | |
| Ghadri et al. (2018) | Modified Mayo criteria | 1613 | Prospective | 5 years | HR 1.75 (95% CI 1.13–2.70), P = 0.012 | Multivariate Cox regression | Moderate | |
| Stiermaier et al. (2018) | Mayo criteria and ESC criteria (2016) | 826 | Prospective | Median = 2.5 years | HR 1.65 (95% CI 1.09–2.51), P = 0.018 | Multivariate Cox regression | Moderate | |
| Stiermaier et al. (2018) | Mayo criteria and ESC criteria (2016) | 177 | Prospective | Median = 2.3 years | HR 5.52 (95% CI 1.74–17.47), P = 0.004 | Multivariate Cox regression | Moderate | |
| Giannakopoulos et al. (2019) | Mayo criteria | 138 | Prospective | 5 years | HR 2.7 (95% CI 1.1–6.5), P = 0.02 | Multivariate Cox regression | Moderate | |
| Hohneck et al. (2019) | Mayo criteria | 105 | Prospective | Mean = 1494 days | No difference | Multivariate Cox regression | Moderate | |
| Gietzen et al. (2019) | Mayo criteria | 138 | Prospective | 5 years | HR 2.7 (95% CI 1.1–6.5), P = 0.02 | Multivariate Cox regression | Moderate | |
| Lemor et al. (2019) | ICD‐9 | 39 662 | Retrospective | In‐hospital | OR 3.89 (95% CI 2.41–6.24), P < 0.001 | Multivariate regression | High | |
| Misumida et al. (2019) | ICD‐9 | 22 818 | Retrospective | In‐hospital | OR 3.80 (95% CI 1.94–7.46), P < 0.001 | Multivariable logistic regression | High | |
| Uribarri et al. (2019) | Modified Mayo criteria | 939 | Prospective | 5 years | No difference | Multivariate Cox regression | Moderate | |
| Alashi et al. (2020) | Modified Mayo criteria | 650 | Prospective | Median = 2.2 years | HR 1.75 (95% CI 1.06–2.89), P = 0.032 | Multivariate Cox regression | Moderate | |
| Arcari et al. (2020) | Mayo criteria and ESC criteria (2016) | 1071 | Prospective | Median = 576 days | OR 1.89 (95% CI 1.13–3.18), P = 0.015 | Multivariable logistic regression | Moderate | |
| Cammann et al. (2020) | Modified Mayo criteria | 2098 | Prospective | In‐hospital | OR 2.18 (95% CI 1.26–3.77), P = 0.005 | Multivariable logistic regression | Moderate | |
| Kimura et al. (2020) | Modified Mayo criteria | 421 | Retrospective | In‐hospital | No difference | Multivariable logistic regression | Moderate | |
| Malanchini et al. (2020) | ICD‐9 | 10 861 | Retrospective | In‐hospital | OR 2.32 (95% CI 1.65–3.27), P < 0.001 | Multivariable logistic regression | High | |
| Syed et al. (2020) | ICD‐9 and ICD‐10 | 260 144 | Retrospective | In‐hospital | OR 1.78 (95% CI 1.70–1.85), P < 0.01 | Stepwise logistic regression | High | |
| Female vs. Male | Kwon et al. (2013) | Modified Mayo criteria | 208 | Retrospective | In‐hospital | No difference | Multivariable logistic regression | Moderate |
| Krishnamoorthy et al. (2015) | ICD‐9 | 7510 | Retrospective | In‐hospital | No difference | Multivariable logistic regression | High | |
| Núñez‐Gil et al. (2016) | Modified Mayo criteria | 328 | Prospective | 18.1 ± 24.2 months | HR 0.258 (95% CI 0.080–0.832), P = 0.023 | Backwards Wald stepwise regression | Moderate | |
| Vallabhajosyula et al. (2018) | ICD‐9 | 2214 | Retrospective | In‐hospital | No difference | Multivariable logistic regression | High | |
| Cammann et al. (2019) | Modified Mayo criteria | 1604 | Prospective | 10 years | No difference | Multivariate Cox regression | Low | |
| Yerasi et al. (2019) | ICD‐9 | 12 255 | Retrospective | In‐hospital | OR 0.68 (95% CI 0.55–0.84), P < 0.001 | Multivariate regression | High | |
| Napierkowski et al. (2021) | ICD‐9 | 14 639 | Retrospective | In‐hospital | OR 0.521 (95% CI 0.389–0.699), P < 0.001 | Multivariable logistic regression | High | |
| Kato et al. (2021) | Modified Mayo criteria | 1670 | Prospective | 5 years | HR 0.56 (95% CI 0.37–0.85), P = 0.007 | Multivariate Cox regression | Moderate |
CI, confidence interval; ESC, European Society of Cardiology; HR, hazard ratio; ICD, International Classification of Diseases; NA, not applicable; OR, odds ratio; TCM, Takotsubo cardiomyopathy.
No consensus has been reached in terms of the long‐term effects of sex on TCM outcomes (Table 1 ). Some studies indicate that being male is associated with poorer outcomes. 21 , 22 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 Ghadri et al. found that the risk of all‐cause mortality of male patients was 1.75 times higher than that of female patients in a 5 year follow‐up study [hazard ratio (HR) 1.75, 95% CI 1.13–2.70] in a prospective study with a large sample (1450 female and 163 male). 42 Another retrospective study, which included 19 966 TCM patients, by Murugiah et al. noted that among patients with primary TCM, male patients had a higher 1 year mortality rate (12.0% vs. 6.6%, P < 0.05). Similarly, in the secondary TCM group, male patients also had a higher 1 year mortality rate than female patients (18.2% vs. 10.7%, P < 0.05). 22 Other studies found no association between sex and the long‐term outcomes of TCM. 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 One study by Uribarri et al. found that male sex was not associated with 5 year mortality in TCM patients (P = 0.076). 55 Another study that included 1604 TCM patients found that sex was not associated with 10 year mortality (P = 0.17). 56
Age
Although TCM occurs predominantly in postmenopausal women, younger individuals, even children, can also be affected by this condition. The mean age of study population ranges from 53 to 76 years. 22 , 27 , 61 , 62 , 63 However, whether the age gap translates into outcome differences is unclear.
The association between age and the short‐term outcomes in TCM is controversial (Table 2 ). Some studies have associated older age with a higher short‐term mortality rate. 22 , 24 , 28 , 30 , 33 , 34 , 35 , 36 , 37 , 44 , 64 , 65 , 66 , 67 , 68 For example, in one prospective study with 19 966 TCM patients (8068 patients with a primary diagnosis of TCM and 11 898 with a secondary diagnosis), patients were grouped by age into three categories: 65–74, 75–84, and ≥85 years. The patients 85 years or older had the highest overall in‐hospital mortality rate (primary TCM: 2.6% in patients aged more than 85 years vs. 1.5% in patients aged 75 to 84 vs. 0.74% in patients aged 65 to 74, P < 0.05; secondary TCM: 4.5% vs. 3.3% vs. 2.2%, P < 0.05) and the highest 30 day mortality rate (primary TCM, 4.9% vs. 2.7% vs. 1.7%, P < 0.05; secondary TCM 7.7% vs. 5.1% vs. 3.6%, P < 0.05). 22 Another study of 10 861 TCM patients found that greater age was independently associated with higher in‐hospital mortality (OR 1.05, 95% CI 1.04–1.07). In a subanalysis of sex in the same study, age predicted in‐hospital mortality in female patients (OR 1.06, 95% CI 1.04–1.08) but did not have the same predictive value among male patients (P = 0.08). 28
Table 2.
Clinical studies on the effects of age (years) in TCM patients
| Age | Author (year) | Diagnostic criteria | Sample size | Type of study | Follow‐up | Mortality | Analysis strategy | Risk of bias |
|---|---|---|---|---|---|---|---|---|
| Older vs. Younger | Brinjikji et al. (2012) | ICD‐9 | 24 701 | Retrospective | In‐hospital | 50–64 vs. <50: No difference; >64 vs. <50: No difference | Multivariable logistic regression | High |
| Kwon et al. (2013) | Modified Mayo criteria | 208 | Retrospective | In‐hospital | OR 1.063 (95% CI 1.007–1.123), P = 0.028 | Multivariable logistic regression | Moderate | |
| Isogai et al. (2014) | ICD‐10 | 3719 | Retrospective | In‐hospital | Per 10 year increase: OR 1.33 (95% CI 1.15–1.53), P < 0.001 | Multivariable logistic regression | High | |
| Krishnamoorthy et al. (2015) | ICD‐9 | 7510 | Retrospective | In‐hospital | OR 1.05 (95% CI 1.01–1.09), P = 0.02 | Multivariable logistic regression | High | |
| Nishida et al. (2015) | Mayo criteria | 251 | Prospective | Mean = 2.6 ± 2.8 years | HR 1.09 (95% CI 1.02–1.17), P < 0.01 | Multivariate Cox regression | Moderate | |
| Ghadri et al. (2016) | Modified Mayo criteria | 1750 | Prospective | 1 year | >70 vs. ≤70: No difference | Multivariate Cox regression | Moderate | |
| Girardey et al. (2016) | Madias criteria | 154 | Prospective | Median = 364 days |
Cardiac mortality: HR 1.11 (95% CI 1.03–1.2), P = 0.006 All‐cause mortality: No difference |
Multivariate Cox regression | Moderate | |
| Lee et al. (2016) | Mayo criteria | 128 | Retrospective | In‐hospital | No difference | Multivariable logistic regression | Moderate | |
| Khera et al. (2016) | ICD‐9 | 11 193 | Retrospective | In‐hospital | OR 1.04 (95% CI 1.01–1.08), P = 0.0081 | Multivariable logistic regression | High | |
| Núñez‐Gil et al. (2016) | Modified Mayo criteria | 328 | Prospective | Mean = 18.1 ± 24.2 months | Per 1 year increasing: No difference | Backwards Wald stepwise regression | Moderate | |
| Stiermaier et al. (2016) | Mayo criteria | 285 | Prospective | 6 months | No difference | Multivariate Cox regression | Moderate | |
| Stiermaier et al. (2016) | Mayo criteria | 286 | Prospective | Mean = 3.8 ± 2.5 years | >70 vs. ≤70: No difference | Multivariate Cox regression | Moderate | |
| Nayeri et al. (2017) | Mayo criteria | 306 | Prospective |
30 days 15 years |
No difference No difference |
Multivariate Cox regression | High | |
| Parodi et al. (2017) | In‐TAK criteria | 371 | Prospective | Mean = 26 ± 20 months | HR 1.11 (95% CI 1.06–1.16), P < 0.001 | Multivariate Cox regression | Moderate | |
| Stiermaier et al. (2017) | Mayo criteria and ESC criteria (2016) | 387 | Prospective | Median = 2.9 years | HR 1.04 (95% CI 1.01–1.07), P = 0.006 | Multivariate Cox regression | Moderate | |
| Almendro‐Delia et al. (2018) | Modified Mayo criteria | 711 | Prospective | Median = 284 days | Per 10 year increase: HR 2.61 (95% CI 1.18–3.42), P = 0.02 | Multivariate Cox regression | Moderate | |
| Ghadri et al. (2018) | Modified Mayo criteria | 1613 | Prospective | 5 years | >70 vs. ≤70: HR 1.50 (95% CI 1.08–2.09), P = 0.016 | Multivariate Cox regression | Moderate | |
| Kim et al. (2018) | Modified Mayo criteria | 257 | Prospective | Median = 5.8 ± 3.6 years | HR 1.057 (95% CI 1.035–1.080), P < 0.05 | Multivariate Cox regression | Moderate | |
| Stiermaier et al. (2018) | Mayo criteria and ESC criteria (2016) | 826 | Prospective | Median = 2.5 years | HR 1.05 (95% CI 1.03–1.07), P < 0.001 | Multivariate Cox regression | Moderate | |
| Stiermaier et al. (2018) | Mayo criteria and ESC criteria (2016) | 177 | Prospective | Median = 2.3 years | HR 1.10 (95% CI 1.04–1.18), P = 0.002 | Multivariate Cox regression | Moderate | |
| Vallabhajosyula et al. (2018) | ICD‐9 | 2214 | Retrospective | In‐hospital |
45–64 vs. 22–24: No difference 65–79 vs. 22–24: OR 1.8 (95% CI 1.1–3.3), P = 0.04; ≥80 vs. 22–24: OR 2.9 (95% CI 1.6–5.6), P = 0.001 |
Multivariable logistic regression | High | |
| Cammann et al. (2019) | Modified Mayo criteria | 1604 | Prospective | 10 years | >70 vs. ≤70: HR 1.6 (95% CI 1.16–2.20), P = 0.004 | Multivariate Cox regression | Low | |
| Jesel et al. (2019) | Madias criteria | 214 | Prospective | In‐hospital | Cardiovascular mortality: OR 1.072 (95% CI 1.023–1.123), P = 0.004 | Multivariate Cox regression | Moderate | |
| Misumida et al. (2019) | ICD‐9 | 22 818 | Retrospective | In‐hospital | Per 10 year increase: OR 1.46 (95% CI 1.10–1.95), P = 0.01 | Multivariable logistic regression | High | |
| Uribarri et al. (2019) | Modified Mayo criteria | 939 | Prospective | 5 years | >70 vs. ≤70: HR 2.894 (95% CI 1.657–5.054), P < 0.001 | Multivariate Cox regression | Moderate | |
| Yerasi et al. (2019) | ICD‐9 | 12 255 | Retrospective | In‐hospital |
50–64 vs. 18–49: OR 0.75 (95% CI 0.55–1.00), P = 0.05 65–79 vs. 18–49: No difference ≥80 vs. 18–49: No difference |
Multivariate regression | High | |
| Alashi et al. (2020) | Modified Mayo criteria | 650 | Prospective | Median = 2.2 years | Per each 10 years of age: HR 1.35 (95% CI 1.17–1.55), P < 0.001 | Multivariate Cox regression | Moderate | |
| El‐Battrawy et al. (2020) | ESC criteria (2016) | 906 | Prospective | Mean = 1038 ± 838 days | HR 1.05 (95% CI 1.03–1.07), P < 0.01 | Multivariate Cox regression | Moderate | |
| Lachmet‐Thébaud et al. (2020) | Madias criteria/In‐TAK criteria | 215 | Prospective | 1 year | Cardiac mortality: OR 1.09, (95% CI 1.02–1.16), P = 0.01 | Multivariate Cox regression | Moderate | |
| Malanchini et al. (2020) | ICD‐9 | 10 861 | Retrospective | In‐hospital | Per 1 year increasing: OR 1.05 (95% CI 1.04–1.07), P < 0.001 | Multivariate logistic regression | High | |
| Nayeri et al. (2020) | Mayo criteria | 538 | Prospective | 30 days | No difference | Multivariate logistic regression | Moderate | |
| Núñez‐Gil et al. (2020) | Modified Mayo criteria | 1097 | Prospective | Median = 27.5 months | HR 1.088 (95% CI 1.06–1.11), P < 0.001 | Multivariate Cox regression | Moderate | |
| Napierkowski et al. (2021) | ICD‐9 | 14 639 | Retrospective | In‐hospital | OR 1.023 (95% CI 1.013–1.035), P < 0.001 | Multivariable logistic regression | High | |
| Scudiero et al. (2020) | In‐TAk criteria | 561 | Prospective | Median = 29 months | HR 1.97 (95% CI 1.02–3.78), P = 0.042 | Multivariate Cox regression | Moderate | |
| Younger vs. Older | Cammann et al. (2020) | Modified Mayo criteria | 2098 | Prospective | In‐hospital | ≤50 vs. 51–74: No difference; ≥75 vs. 51–74: No difference | Multivariable logistic regression | Moderate |
CI, confidence interval; ESC, European Society of Cardiology; HR, hazard ratio; ICD, International Classification of Diseases; In‐TAK, International Takotsubo; NA, not applicable; OR, odds ratio; TCM, Takotsubo cardiomyopathy.
Other studies suggest that age is not associated with short‐term mortality in TCM patients (Table 2 ). 18 , 19 , 23 , 27 , 38 , 63 , 69 , 70 After a multivariable logistic regression analysis, Cammann et al. found no significant difference among different age groups in in‐hospital mortality (age 51–74 years old as reference, ≤50 years old, P = 0.14; ≥75 years old, P = 0.75) or 60 day mortality (P = 0.16). 27 In a univariate meta‐regression analysis of 54 studies with 4679 TCM patients, in‐hospital mortality in each study was not associated with age (P = 0.67, coefficient: 0.002, 95% CI 0.000–0.004), although the in‐hospital mortality rate had wide heterogeneity (I 2 = 78%). 63
Counterintuitively, some literature has shown that younger age is associated with more inpatient complications. 8 , 27 , 61 Compared with older groups of TCM patients (1194 patients 51–74 years old, 662 patients ≥ 75 years old), a younger group (242 patients ≤ 50 years old) more often had complications that required in‐hospital acute cardiac care interventions, such as invasive or non‐invasive ventilation (P < 0.001), catecholamine use (P < 0.001), cardiopulmonary resuscitation (P < 0.001), and cardiogenic shock (P = 0.004). 27 Another study that included 90 TCM patients found that being less than 55 years old was independently associated with ventricular arrhythmia (adjusted OR 9.5, 95% CI 1.7–52.6). 61 In addition, Templin et al. found that among TCM patients, older age (>70 years) was associated with fewer in‐hospital complications (OR 0.44, 95% CI 0.30–0.66)—including all‐cause mortality, cardiogenic shock, and the need for catecholamine administration, ventilation, and cardiopulmonary resuscitation—than younger age (≤70 years). 8
Results differ among outcome studies of the effect of age on the long‐term outcomes of TCM (Table 2 ). The majority of current studies suggest that older TCM patients have worse long‐term outcomes than younger patients. 22 , 26 , 27 , 41 , 42 , 44 , 47 , 48 , 49 , 55 , 56 , 62 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 Cammann et al. found that elderly patients with TCM (≥75 years old) had a higher long‐term (>60 days) mortality rate (P < 0.001) than younger (≤50 years old) and middle‐age patients (51 to 74 years old). 27 Similarly, another study found that older TCM patients had a higher 1 year mortality rate in both primary (12.5% in ≥85 years old vs. 7.0% in 75–84 vs. 5.3% in 65–74, P < 0.05) and secondary TCM patients (16.0% vs. 11.6% vs. 9.6%, P < 0.05), in a large sample of 19 966 TCM patients. 22 A prospective study of 1604 TCM patients found that being more than 70 years old is a predictor of worse long‐term outcomes (HR 1.6, 95% CI 1.16–2.20) during a 10 year follow‐up period. 56 However, some other studies suggest that age is not associated with long‐term outcomes. 40 , 46 , 52 , 53 , 69 , 79 , 80 , 81 One study that included 1750 TCM patients indicated that age > 70 years was not associated with 1 year mortality (P = 0.49). 69 Another study, by Núñez‐Gil et al., found that age was not associated with long‐term mortality (P = 0.085) or with complications such as all‐cause death, TCM recurrence, and readmission due to cardiovascular causes (P = 0.072) after a mean follow‐up time of 18.1 ± 24.2 months. 46
Race
When TCM was originally found in Japan, it was thought to occur predominantly in Asia. However, cases of TCM have been increasingly reported worldwide. Studies of the effect of race on TCM outcome have not yet produced definitive results. Some studies have found that race is associated with short‐term prognosis in TCM patients (Table 3 ). 22 , 82 , 83 One retrospective study that included 97 650 TCM patients showed that compared with White patients (89 624 patients), African American (AA) patients (8026 patients) had a lower incidence of inpatient complications, including cardiogenic shock (OR 0.58, 95% CI 0.46–0.75), the use of mechanical ventilation (OR 0.78, 95% CI 0.68–0.9), and the use of intra‐aortic balloon pump (OR 0.6, 95% CI 0.39–0.94), after adjustment for baseline characteristics. In the same study, mortality did not differ significantly between the White and AA patients (P = 0.21). 82 In a smaller study (205 TCM patients), Dias et al. had found that compared with White patients, AA patients had a longer hospital stay (15 days vs. 7 days, P < 0.05) and a higher incidence of acute respiratory failure requiring mechanical ventilation (44% vs. 20%, P < 0.05) during hospitalization, although inpatient mortality did not differ significantly between the two groups. 83 Murugiah et al. found a race‐based mortality difference in patients with secondary TCM (4.6% in the non‐White group vs. 2.8% in the White group, P < 0.05) but not in those with primary TCM. 22 A study of 24 701 TCM patients indicated that race was not associated with in‐hospital mortality after adjustment for age, Charlson comorbidity index (CCI), sex, and underlying critical illness. 19 The discrepancies among these studies may be due to the retrospective design of most of the studies. In addition, the variables adjusted to control bias were not the same in each study. Lastly, race was categorized differently in each study, which may have contributed to the different outcomes.
Table 3.
Clinical studies on the effects of race in TCM patients
| Race | Author (year) | Diagnostic criteria | Sample size | Type of study | Follow‐up | Mortality | Analysis strategy | Risk of bias |
|---|---|---|---|---|---|---|---|---|
| Black vs. White | Brinjikji et al. (2012) | ICD‐9 | 24 701 | Retrospective | In‐hospital | No difference | Multivariable logistic regression | High |
| Vallabhajosyula et al. (2018) | ICD‐9 | 2214 | Retrospective | In‐hospital | OR 1.6 (95% CI 1.1–2.4), P = 0.03 | Multivariable logistic regression | High | |
| Zaghlol et al. (2020) | ICD‐9 | 97 650 | Retrospective | In‐hospital | No difference | Multivariate regression | High | |
| Black vs. Hispanic | Brinjikji et al. (2012) | ICD‐9 | 24 701 | Retrospective | In‐hospital | No difference | Multivariable logistic regression | High |
| Hispanic vs. White | Vallabhajosyula et al. (2018) | ICD‐9 | 2214 | Retrospective | In‐hospital | No difference | Multivariable logistic regression | High |
| Asian vs. White | Brinjikji et al. (2012) | ICD‐9 | 24 701 | Retrospective | In‐hospital | No difference | Multivariable logistic regression | High |
| Vallabhajosyula et al. (2018) | ICD‐9 | 2214 | Retrospective | In‐hospital | No difference | Multivariable logistic regression | High | |
| Non‐White vs. White | Napierkowski et al. (2021) | ICD‐9 | 14 639 | Retrospective | In‐hospital | OR 1.412 (95% CI 1.071–1.861), P = 0.015 | Multivariable logistic regression | High |
AAs, African Americans; CI, confidence interval; HR, hazard ratio; ICD, International Classification of Diseases; NA, not applicable; OR, odds ratio; TCM, Takotsubo cardiomyopathy.
The long‐term outcome of TCM patients of different races also remains unclear (Table 3 ). One study that included 19 966 TCM patients found that the non‐White group had a higher 1 year mortality rate in both primary and secondary TCM (primary TCM: 8.9% in non‐Whites vs. 6.7% in Whites, P < 0.05; secondary TCM: 14.1% vs. 11.1%, P < 0.05). 22 In contrast, one study that included 205 TCM patients (152 White and 53 AA) found that the long‐term all‐cause mortality was not significantly different between the two groups (P = 0.40). 83 It is worth noting that the aforementioned studies compared the outcomes between races without adjusting for other variables.
Trigger events
A prospective study of 1750 TCM patients in the International Takotsubo Registry noted that about 36% of TCM cases were triggered by specific physical activities, 27.7% was triggered by emotion, 2.8% were triggered by both physical and emotional activities, and 28.5% of cases had no identifiable trigger. 8
Many studies show that physical triggers are associated with poor short‐term 8 , 70 , 80 , 84 , 85 and long‐term 41 , 42 , 49 , 55 , 62 , 75 , 86 , 87 outcomes in TCM (Table 4 ). One study divided TCM patients into three classes according to their triggers: emotional triggers as Class I (485 patients), physical triggers as Class II (532 patients with physical activities, medical conditions, or procedures as Class IIa, 98 patients with neurologic disorders as Class IIb), and no identifiable trigger as Class III (498 patients). The study showed that TCM patients in Classes II and III had higher rates of 30 day mortality (Class IIa, HR 5.3, 95% CI 2.05–13.7; Class IIb, HR 8.58, 95% CI 2.96–24.9; Class III, HR 2.81, 95% CI 1.01–7.81) and 5 year mortality (Class IIa, HR 3.78. 95% CI 2.21–6.44; Class IIb, HR 5.76, 95% CI 2.96–11.2; Class III: HR 2.14, 95% CI 1.20–3.82) than those with Class I triggers. 42 A study of 939 TCM patients from the Spanish National Registry on Takotsubo Syndrome used multiple Cox regression to show that patients with physical triggers (HR 3.073, 95% CI 1.758–4.302) and those with no identifiable trigger (HR 1.913, 95% CI 1.003–3.649) had a higher 5 year mortality rate than patients with emotional triggers group. 55
Table 4.
Clinical studies on the effects of triggers in TCM patients
| Triggers | Author (year) | Diagnostic criteria | Sample size | Type of study | Follow‐up | Mortality | Analysis strategy | Risk of bias |
|---|---|---|---|---|---|---|---|---|
| Physical triggers vs. Other triggers | Girardey et al. (2016) | Madias criteria | 154 | Prospective | Median = 364 days | No difference | Multivariate Cox regression | Moderate |
| Stiermaier et al. (2016) | Mayo criteria | 286 | Prospective | Mean = 3.8 ± 2.5 years | No difference | Multivariate Cox regression | Moderate | |
| Kato et al. (2017) | Modified Mayo criteria | 144 | Retrospective | In‐hospital | OR 5.03 (95% CI 1.01–39.8), P = 0.049 | Multivariable logistic regression | Moderate | |
| Kim et al. (2017) | Mayo criteria | 103 | Prospective | Median = 25.6 months | HR 3.77, (95% CI 1.02–13.9), P < 0.05 | Multivariate Cox regression | Moderate | |
| Nayeri et al. (2017) | Mayo criteria | 306 | Prospective |
30 days 15 years |
OR 7.55 (95% CI 2.21–25.71), P < 0.001 No difference |
Multivariate Cox regression | High | |
| Almendro‐Delia et al. (2018) | Modified Mayo criteria | 711 | Prospective | Median = 284 days | HR 1.43 (95% CI 1.20–3.80), P = 0.013 | Multivariate Cox regression | Moderate | |
| Ghadri et al. (2018) | Modified Mayo criteria | 1613 | Prospective | 5 years | Physical triggers vs. Emotional triggers: HR 3.78 (95% CI 2.21–6.44), P < 0.001 | Multivariable Cox regression | Moderate | |
| Kim et al. (2018) | Modified Mayo criteria | 257 | Prospective | Median = 5.8 ± 3.6 years | HR 1.723 (95% CI 1.126–2.638), P = 0.012 | Multivariate Cox regression | Moderate | |
| Cammann et al. (2019) | Modified Mayo criteria | 1604 | Prospective | 10 years | No difference | Multivariate Cox regression | Low | |
| Jesel et al. (2019) | Madias criteria | 214 | Prospective | In‐hospital | No difference | Multivariate Cox regression | Moderate | |
| Uribarri et al. (2019) | Modified Mayo criteria | 939 | Prospective | 5 years | Physical triggers vs. Emotional triggers: HR 3.073 (95% CI 1.758–4.302), P < 0.001 | Multivariable Cox regression | Moderate | |
| Alashi et al. (2020) | Modified Mayo criteria | 650 | Prospective | Median = 2.2 years | Physical triggers vs. No trigger: HR 2.64 (95% CI 1.63–4.20), P < 0.001 | Multivariate Cox regression | Moderate | |
| El‐Battrawy et al. (2020) | ESC criteria (2016) | 906 | Prospective | Mean = 1038 ± 838 days | HR 1.64 (95% CI 1.0–2.6), P = 0.04 | Multivariate Cox regression | Moderate | |
| Kimura et al. (2020) | Modified Mayo criteria | 421 | Retrospective | In‐hospital | Physical triggers vs. Emotional triggers: No difference | Multivariable logistic regression | Moderate | |
| Nayeri et al. (2020) | Mayo criteria | 538 | Prospective | 30 days | OR 3.93 (95% CI 1.71–9.05), P < 0.001 | Multivariate logistic regression | Moderate | |
| Stiermaier et al. (2020) | Mayo criteria and ESC criteria (2016) | 147 | Prospective | 3 years | HR 4.77 (95% CI 1.58–14.39), P = 0.006 | Multivariable Cox regression | Moderate | |
| Emotional triggers vs. Other triggers | Stiermaier et al. (2017) | Mayo criteria and ESC criteria (2016) | 387 | Prospective | Median = 2.9 years | HR 0.37 (95% CI 0.16–0.85), P = 0.020 | Multivariate Cox regression | Moderate |
| Stiermaier et al. (2018) | Mayo criteria and ESC criteria (2016) | 826 | Prospective | Median = 2.5 years | HR 0.41 (95% CI 0.25–0.67), P < 0.001 | Multivariate Cox regression | Moderate | |
| Cammann et al. (2019) | Modified Mayo criteria | 1604 | Prospective | 10 years | HR 0.56 (95% CI 0.33–0.96), P = 0.034 | Multivariate Cox regression | Low | |
| Alashi et al. (2020) | Modified Mayo criteria | 650 | Prospective | Median = 2.2 years | Emotional triggers vs. No trigger: No difference | Multivariate Cox regression | Moderate | |
| Cammann et al. (2020) | Modified Mayo criteria | 2098 | Retrospective | In‐hospital | OR 0.31 (95% CI 0.14–0.65), P = 0.002 | Multivariable logistic regression | Moderate | |
| Other triggers vs. Emotional triggers | Kimura et al. (2020) | Modified Mayo criteria | 421 | Retrospective | In‐hospital | No trigger vs. Emotional triggers: No difference | Multivariable logistic regression | Moderate |
| Ghadri et al. (2018) | Modified Mayo criteria | 1613 | Prospective | 5 years | Neurologic disorder triggers vs. Emotional triggers: HR 5.76 (95% CI 2.96–11.2), P < 0.001; No trigger vs. Emotional triggers: HR 2.14 (95% CI 1.20–3.82), P = 0.010 | Multivariable Cox regression | Moderate | |
| Uribarri et al. (2019) | Modified Mayo criteria | 939 | Prospective | 5 years | No trigger vs. Emotional triggers: HR 1.913 (95% CI 1.003–3.649), P = 0.049 | Multivariable Cox regression | Moderate |
CI, confidence interval; ESC, European Society of Cardiology; HR, hazard ratio; ICD, International Classification of Diseases; In‐TAK, International Takotsubo; OR: odds ratio; TCM, Takotsubo cardiomyopathy.
Emotional triggers, on the other hand, predict better short‐term 8 , 27 and long‐term 44 , 47 , 56 TCM outcomes (Table 4 ). Studies also associated emotional triggers with a lower in‐hospital complication rate. For example, one retrospective study that included 2098 TCM patients concluded that emotional triggers are associated with a lower in‐hospital mortality rate (OR 0.31, 95% CI 0.14–0.65). 27 Another study found that emotional triggers predicted a lower long‐term mortality rate during a median follow‐up of 2.5 years (HR 0.41, 95% CI 0.25–0.67). 44
Some retrospective studies found that different triggers do not affect short‐term 39 , 68 , 88 or long‐term 40 , 71 , 89 outcomes in TCM. One study that included 421 TCM patients found that different triggers were not associated with in‐hospital mortality in TCM patients (physical triggers vs. emotional triggers, P = 0.342; no triggers vs. emotional triggers, P = 0.937). 39 Another small retrospective study with 154 TCM patients found that physical and emotional triggers were not associated with in‐hospital complications (pulmonary oedema, cardiogenic shock, sustained VF or VT, complete atrioventricular block, thromboembolism, cardiac rupture, and cardiac death; P = 0.22 for physical triggers, P = 0.30 for emotional triggers, compared with no identifiable trigger). 88 In addition, one study that included 286 TCM patients found that different triggers were not associated with long‐term mortality during a mean follow‐up time of 3.8 ± 2.5 years. 40 In a study of 114 TCM patients, emotional triggers were not associated with complications (thromboembolic events, life‐threatening arrhythmias, all‐cause mortality, and rehospitalization) after a mean follow‐up of 1529 ± 1121 days (P = 0.15). 89
In a study of emotional triggers of TCM, compared with TCM triggered by negative emotions, TCM triggered by preceding pleasant emotional events did not show a more favourable outcome in terms of mortality, cardiogenic shock, ventricular or septal rupture, VT, ventricular thrombus, and new atrial fibrillation. 90
Effects of comorbidities
Takotsubo cardiomyopathy patients carry certain risk factors such as chronic kidney disease (CKD), mood disorders, and common cardiovascular comorbidities. 91 The effect of comorbidities on TCM outcome is discussed below. Studies are shown in Table 5 .
Table 5.
Clinical studies on the effects of comorbidities in TCM patients
| Comorbidities | Author (year) | Diagnostic criteria | Sample size | Type of study | Follow‐up | Mortality | Analysis strategy | Risk of bias |
|---|---|---|---|---|---|---|---|---|
| DM | DM vs. Non‐DM | |||||||
| Krishnamoorthy et al. (2015) | ICD‐9 | 7510 | Retrospective | In‐hospital | No difference | Multivariable logistic regression | High | |
| Khera et al. (2016) | ICD‐9 | 11 193 | Retrospective | In‐hospital | No difference | Multivariable logistic regression | High | |
| Stiermaier et al. (2016) | Mayo criteria | 286 | Prospective | Mean = 3.8 ± 2.5 years | HR 2.11 (95% CI 1.23–3.65), P < 0.01 | Multivariate Cox regression | Moderate | |
| Parodi et al. (2017) | In‐TAK criteria | 371 | Prospective | Mean = 26 ± 20 months | HR 2.86 (95% CI 1.29–6.35), P = 0.01 | Multivariate Cox regression | Moderate | |
| Almendro‐Delia et al. (2018) | Modified Mayo criteria | 711 | Prospective | Median = 284 days | HR 2.68 (95% CI 1.55–4.60), P < 0.0001 | Multivariate Cox regression | Moderate | |
| Ghadri et al. (2018) | Modified Mayo criteria | 1613 | Prospective | 5 years | No difference | Multivariable Cox regression | Moderate | |
| Stiermaier et al. (2018) | Mayo criteria and ESC criteria (2016) | 826 | Prospective | Median = 2.5 years | HR 1.66 (95% CI 1.16–2.39), P = 0.006 | Multivariate Cox regression | Moderate | |
| Uribarri et al. (2019) | Modified Mayo criteria | 939 | Prospective | 5 years | HR 2.750 (95% CI 1.758–4.302), P < 0.001 | Multivariable Cox regression | Moderate | |
| Yerasi et al. (2019) | ICD‐9 | 12 255 | Retrospective | In‐hospital | No difference | Multivariate regression | High | |
| Núñez‐Gil et al. (2020) | Modified Mayo criteria | 1097 | Prospective | Median = 27.5 months | HR 2.17 (95% CI 1.41–3.34), P < 0.001 | Multivariate Cox regression | Moderate | |
| Scudiero et al. (2020) | In‐TAK criteria | 561 | Prospective | Median = 29 months | HR 2.90 (95% CI 1.53–5.40), P = 0.001 | Multivariate Cox regression | Moderate | |
| Stiermaier et al. (2020) | Mayo criteria and ESC criteria (2016) | 147 | Prospective | 3 years | HR 6.15 (95% CI 2.18–17.32), P = 0.001 | Multivariable Cox regression | Moderate | |
| CKD | CKD vs. Non‐CKD | |||||||
| Isogai et al. (2014) | ICD‐10 | 3719 | Retrospective | In‐hospital | No difference | Multivariable logistic regression | High | |
| Khera et al. (2016) | ICD‐9 | 11 193 | Retrospective | In‐hospital | No difference | Multivariable logistic regression | High | |
| Weidner et al. (2017) | Mayo criteria | 114 | Prospective | 5 years | HR 3.1 (95% CI 1.4–7.0), P < 0.01 | Multivariate Cox regression | Moderate | |
| El‐Battrawy et al. (2017) | Mayo criteria | 114 | Prospective | Mean = 1529 ± 1121 days | HR 3.5 (95% CI 1.0–11.0), P = 0.03 | Multivariate Cox regression | Moderate | |
| Huseynov et al. (2017) | Mayo criteria | 114 | Prospective | Mean = 1591 ± 1079 days | HR 3.1 (95% CI 1.4–7.0), P < 0.01 | Multivariate Cox regression | Moderate | |
| El‐Battrawy et al. (2018) | Mayo criteria | 114 | Prospective | Mean = 3 years | HR 3.84 (95% CI 1.3–11.2), P = 0.01 | Multivariate Cox regression | Moderate | |
| Giannakopoulos et al. (2019) | Mayo criteria | 138 | Prospective | 5 years | HR 2.8 (95% CI 1.2–6.0), P = 0.01 | Multivariate Cox regression | Moderate | |
| Gietzen et al. (2019) | Mayo criteria | 138 | Prospective | 5 years | HR 2.8 (95% CI 1.2–6.0), P = 0.01 | Multivariate Cox regression | Moderate | |
| Yassin et al. (2019) | ICD‐9 | 2959 | Retrospective | In‐hospital | No difference | Multivariable logistic regression | High | |
| Syed et al. (2020) | ICD‐9 and ICD‐10 | 260 144 | Retrospective | In‐hospital | OR 1.2 (95% CI 1.16–1.30), P < 0.01 | Stepwise logistic regression | High | |
| Zalewska‐Adamiec et al. (2020) | Mayo criteria | 101 | Prospective | Mean = 7.2 years | OR 0.954 (95% CI 0.920–0.989), P = 0.01 | Multivariable logistic regression | Moderate | |
| Napierkowski et al. (2021) | ICD‐9 | 14 639 | Retrospective | In‐hospital | OR 1.756 (95% CI 1.266–2.436), P = 0.001 | Multivariable logistic regression | High | |
| Cancer | Cancer vs. Non‐cancer | |||||||
| Isogai et al. (2014) | ICD‐10 | 3719 | Retrospective | In‐hospital | OR 3.23 (95% CI 2.21–4.72), P < 0.001 | Multivariable logistic regression | High | |
| Krishnamoorthy et al. (2015) | ICD‐9 | 7510 | Retrospective | In‐hospital | OR 3.38 (95% CI 1.35–8.41), P < 0.01 | Multivariable logistic regression | High | |
| Lee et al. (2016) | Mayo criteria | 128 | Retrospective | In‐hospital | No difference | Multivariable logistic regression | Moderate | |
| Girardey et al. (2016) | Madias criteria | 154 | Prospective | Median = 364 days |
Cardiac mortality: HR 4.77 (95% CI 1.02–22.17), P = 0.046 All‐cause mortality: HR 2.62 (95% CI 1.26–5.44), P = 0.01 |
Multivariable Cox regression | Moderate | |
| Khera et al. (2016) | ICD‐9 | 11 193 | Retrospective | In‐hospital | No difference | Multivariable logistic regression | High | |
| Bill et al. (2017) | Mayo criteria | 114 | Prospective | 2 years | HR 2.76 (95% CI 0.8–9.0), P = 0.09 | Multivariate Cox regression | Moderate | |
| Almendro‐Delia et al. (2018) | Modified Mayo criteria | 711 | Prospective | Median = 284 days | HR 2.38 (95% CI 1.18–4.81), P = 0.016 | Multivariate Cox regression | Moderate | |
| El‐Battrawy et al. (2018) | Mayo criteria | 114 | Prospective | Mean = 3 years | No difference | Multivariate Cox regression | Moderate | |
| Ghadri et al. (2018) | Modified Mayo criteria | 1613 | Prospective | 5 years | HR 1.65 (95% CI 1.14–2.40), P = 0.009 | Multivariable Cox regression | Moderate | |
| Joy et al. (2018) | ICD‐9 | 122 855 | Retrospective | In‐hospital | Primary TCM: Solid cancer: OR 3.43 (95% CI 1.38–8.52), P = 0.008 Secondary TCM: Haematological cancer: OR 3.21 (95% CI 2.31–4.48), P < 0.001; Metastatic cancer: OR 1.99 (95% CI 1.46–2.71), P < 0.001 | Multivariable logistic regression | High | |
| Kim et al. (2018) | Modified Mayo criteria | 257 | Prospective | Mean = 5.8 ± 3.6 years | HR 1.857 (95% CI 1.214–2.841), P = 0.004 | Multivariable Cox regression | Moderate | |
| Stiermaier et al. (2018) | Mayo criteria and ESC criteria (2016) | 826 | Prospective | Median = 2.5 years | HR 2.12 (95% CI 1.44–3.12), P < 0.001 | Multivariate Cox regression | Moderate | |
| Giannakopoulos et al. (2019) | Mayo criteria | 138 | Prospective | 5 years | HR 3.6 (95% CI 1.4–9.3), P < 0.01 | Multivariate Cox regression | Moderate | |
| Cammann et al. (2019) | Modified Mayo criteria | 1604 | Prospective | 10 years | HR 1.84 (95% CI 1.29–2.61), P = 0.001 | Multivariate Cox regression | Low | |
| Gietzen et al. (2019) | Mayo criteria | 138 | Prospective | 5 years | HR 3.6 (95% CI 1.4–9.3), P < 0.01 | Multivariate Cox regression | Moderate | |
| Misumida et al. (2019) | ICD‐9 | 22 818 | Retrospective | In‐hospital | OR 5.36 (95% CI 1.32–21.80), P = 0.02 | Multivariable logistic regression | High | |
| Yerasi et al. (2019) | ICD‐9 | 12 255 | Retrospective | In‐hospital | OR 2.61 (95% CI 2.04–3.32), P < 0.001 | Multivariate regression | High | |
| El‐Battrawy et al. (2020) | ESC criteria (2016) | 906 | Prospective | Mean = 1038 ± 838 days | HR 1.99 (95% CI, 1.3–2.9), P < 0.01 | Multivariate Cox regression | Moderate | |
| Núñez‐Gil et al. (2020) | Modified Mayo criteria | 1097 | Prospective | Median = 27.5 months | HR 1.73 (95% CI 1.04–2.87), P = 0.03 | Multivariate Cox regression | Moderate | |
| Ding et al. (2020) | In‐TAK criteria | 1676 | Prospective | 6 months | HR, 1.96 (95% CI 1.32–2.87) P = 0.001 | Multivariate Cox regression | Moderate | |
| Stiermaier et al. (2020) | Mayo criteria and ESC criteria (2016) | 147 | Prospective | 3 years | HR 4.32 (95% CI 1.53–12.19), P = 0.006 | Multivariable Cox regression | Moderate | |
| Napierkowski et al. (2021) | ICD‐9 | 14 639 | Retrospective | In‐hospital | OR 2.16 (95% CI 1.398–3.337), P = 0.001 | Multivariable logistic regression | High | |
| Kato et al. (2021) | Modified Mayo criteria | 1670 | Prospective | 5 years | HR 2.24 (95% CI 1.57–3.21), P < 0.001 | Multivariate Cox regression | Moderate | |
| BMI | Lower BMI vs. Higher BMI | |||||||
| Desai et al. (2018) | ICD‐9 | 1140 | Retrospective | In‐hospital | Non‐obesity (BMI < 30) vs. Obesity (BMI > 30): No difference | Propensity score matching | High | |
| Zalewska‐Adamiec et al. (2020) | Mayo criteria | 101 | Prospective | Mean = 7.2 years | BMI < 20 vs. ≥20: OR 0.857 (95% CI 0.738–0.995), P = 0.042 | Multivariable logistic regression | Moderate | |
| Mood diseases | Mood diseases vs. Non‐mood diseases | |||||||
| Isogai et al. (2014) | ICD‐10 | 3719 | Retrospective | In‐hospital | OR 0.43 (95% CI 0.19–0.96), P = 0.039 | Multivariable logistic regression | High | |
| Krishnamoorthy et al. (2015) | ICD‐9 | 7510 | Retrospective | In‐hospital | Anxiety or depression: No difference | Multivariable logistic regression | High | |
| Khera et al. (2016) | ICD‐9 | 11 193 | Retrospective | In‐hospital | No difference | Multivariable logistic regression | High | |
| Nayeri et al. (2017) | Mayo criteria | 306 | Prospective |
30 days 15 years |
No difference No difference |
Multivariable logistic regression | High | |
| Kim et al. (2018) | Modified Mayo criteria | 257 | Prospective | Mean = 5.8 ± 3.6 years | Depression: HR 1.764 (95% CI 1.155–2.694), P = 0.009 | Multivariate Cox regression | Moderate | |
| Cammann et al. (2019) | Modified Mayo criteria | 1604 | Prospective | 10 years | HR 1.40 (95% CI 1.01–1.94), P = 0.041 | Multivariate Cox regression | Low | |
| Syed et al. (2020) | ICD‐9 and ICD‐10 | 260 144 | Retrospective | In‐hospital | Depression: OR 0.63 (95% CI 0.60–0.69), P < 0.01 | Stepwise logistic regression | High | |
| Napierkowski et al. (2021) | ICD‐9 | 14 639 | Retrospective | In‐hospital | Anxiety: OR 0.592 (95% CI 0.399–0.880), P = 0.01 | Multivariable logistic regression | High | |
| Sepsis | Sepsis vs. Non‐sepsis | |||||||
| Isogai et al. (2014) | ICD‐10 | 3719 | Retrospective | In‐hospital | OR 2.02 (95% CI 1.17–3.49), P = 0.011 | Multivariable logistic regression | High | |
| Krishnamoorthy et al. (2015) | ICD‐9 | 7510 | Retrospective | In‐hospital | No difference | Multivariable logistic regression | High | |
| Khera et al. (2016) | ICD‐9 | 11 193 | Retrospective | In‐hospital | OR 6.93 (95% CI 2.54–18.93), P = 0.0002 | Multivariable logistic regression | High | |
| Napierkowski et al. (2021) | ICD‐9 | 14 639 | Retrospective | In‐hospital | OR 4.278 (95% CI 3.047–6.007), P < 0.001 | Multivariable logistic regression | High | |
| TCM vs. Non‐TCM | ||||||||
| Vallabhajosyula et al. (2018) | ICD‐9 | 10 746 | Retrospective | In‐hospital | OR 0.58 (95% CI 0.51–0.65), P < 0.001 | Multivariable logistic regression | High | |
| COPD | COPD vs. Non‐COPD | |||||||
| Khera et al. (2016) | ICD‐9 | 11 193 | Retrospective | In‐hospital | No difference | Multivariable logistic regression | High | |
| Li et al. (2020) | ICD‐10 | 1748 | Retrospective | In‐hospital | OR 2.93 (95% CI 1.39–6.15), P = 0.005 | Multivariable logistic regression | High | |
| Napierkowski et al. (2021) | ICD‐9 | 14 639 | Retrospective | In‐hospital | OR 1.444 (95% CI 1.140–1.829), P = 0.002 | Multivariable logistic regression | High | |
| HLD | HLD vs. Non‐HLD | |||||||
| Khera et al. (2016) | ICD‐9 | 11 193 | Retrospective | In‐hospital | No difference | Multivariable logistic regression | High | |
| Kato et al. (2017) | Modified Mayo criteria | 144 | Retrospective | In‐hospital | No difference | Multivariable logistic regression | Moderate | |
| Li et al. (2021) | ICD‐10 | 2324 | Retrospective | In‐hospital | OR 0.46 (95% CI 0.24–0.89), P = 0.027 | Multivariable logistic regression | High | |
| Anaemia | Anaemia vs. Non‐Anaemia | |||||||
| Gaede et al. (2021) | Mayo criteria | 126 | Prospective | Median = 4.4 years | OR 3.93 (95% CI 1.02–2.08), P = 0.015 | Multivariable logistic regression | Moderate | |
| Lu et al. (2021) | ICD‐10 | 1731 | Retrospective | In‐hospital | No difference | Multivariable logistic regression | High | |
BMI, body mass index; CI, confidence interval; CKD, chronic kidney diseases; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; ESC, European Society of Cardiology; eGFR, estimated glomerular filtration rate; HLD, hyperlipidaemia; HR, hazard ratio; ICD, International Classification of Diseases; In‐TAK, International Takotsubo; NA, not applicable; OR, odds ratio; TCM, Takotsubo cardiomyopathy.
Diabetes mellitus
The reported percentage of TCM patients with comorbid diabetes mellitus (DM) varies widely among studies (1.6–25.5%). 35 , 44 , 92 Although DM is a known risk factor in patients with cardiovascular disease, the association between DM and the outcomes of TCM is uncertain (Table 5 ). Many studies have found DM not to be associated with TCM outcome. 29 , 36 , 42 , 66 One study with 12 255 patients, for example, in‐hospital mortality did not differ between TCM patients with and without DM (P = 0.44), although patients with DM had a higher 90 day readmission rate (OR 1.36, 95% CI 1.27–1.47). 29 Some other studies found DM to be a predictor of mortality, especially long‐term mortality, in TCM patients. 40 , 41 , 44 , 55 , 74 , 77 , 78 , 88 For example, one retrospective study by Kato et al. found that TCM patients with a history of DM had more in‐hospital complications (pulmonary oedema, cardiogenic shock, sustained VT or VF, complete atrioventricular block, thromboembolism, cardiac rupture, and cardiac death) (OR 2.92, 95% CI 1.01–8.41). 88 Another study by Núñez‐Gil et al. of 1097 TCM patients found that DM was associated with long‐term mortality (median follow‐up = 27.5 months) (HR 2.17, 95% CI 1.41–3.34). 77
In contrast, Madias proposed the interesting hypothesis that DM has a protective effect on TCM patients. Because of diabetic autonomic neuropathy, TCM patients with DM may have a relatively lower level of catecholamine release than patients without DM, thus protecting individuals with DM from adverse events associated with TCM. 93 One single‐centre retrospective observational study of 114 TCM patients (88 without DM and 26 with DM) found that adverse events, including all‐cause mortality, TCM recurrence, thromboembolic events, life‐threatening arrhythmias, rehospitalization due to heart failure, and stroke, had a lower 1 year incidence in patients with DM than in patients without DM, although each adverse event rate was not significantly different between the two groups (P = 0.004). 94
Chronic kidney disease
Chronic kidney disease is a known independent risk factor for cardiovascular diseases such as heart failure 95 and myocardial infarction (MI). 96 Likewise, our review of the literature showed that CKD has different effects on in‐hospital outcomes. Several studies suggest that CKD is a predictor of worse short‐term outcomes in TCM patients. 30 , 31 , 32 , 97 In one small retrospective study, 61 TCM patients were separated into three groups: estimated glomerular filtration rate (eGFR) > 60 mL/[min 1.73 m2] (31 patients), eGFR 30–60 mL/[min 1.73 m2] (17 patients), and eGFR < 30 mL/[min 1.73 m2] (13 patients). In a multivariable Cox proportional hazard analysis, compared with the highest eGFR group, the lowest eGFR group (<30 mL/[min 1.73 m2]) had a higher incidence of overall inpatient complications (HR 2.84, 95% CI 1.20–6.69), while the middle eGFR group had no difference with the highest eGFR group (P = 0.184). 32 A large retrospective study with 2959 TCM patients also found that propensity‐matched patients with advanced CKD (1478 patients), defined as eGFR < 60 mL/[min 1.73 m2], were associated with a higher incidence of acute kidney injury and length of inpatient stay than non‐advanced CKD (eGFR > 60 mL/[min 1.73 m2]), although inpatient mortality was not different (P = 0.269). 98 In addition, several studies have found that CKD is not associated with inpatient mortality in TCM patients. 33 , 66
Regarding long‐term outcomes, CKD seems to have an adverse effect in TCM patients. Some studies with small cohorts have shown that CKD is a predictor of poor long‐term outcomes in TCM patients. 21 , 43 , 45 , 50 , 51 , 54 , 58 , 99 , 100 , 101 A study of 95 patients compared mortality between patients with an eGFR higher than 60 mL/[min 1.73 m2] (65 patients) with those who had eGFR < 60 mL/[min 1.73 m2] (30 patients). Patients in the lower eGFR group had a higher 3 year mortality rate (33.3% vs. 15.4%, P = 0.047) than patients in the higher eGFR group. In that study, elevated serum creatinine level was also an independent predictor of long‐term mortality (OR 13.813, 95% CI 1.8–105.9). 101 Another study compared TCM outcomes among three groups: eGFR > 60 mL/[min 1.73 m2] (45 patients), 60–30 mL/[min 1.73 m2] (43 patients), and <30 mL/[min 1.73 m2] (20 patients). Patients with eGFR < 30 mL/[min 1.73 m2] had more adverse events (all‐cause mortality, cardiovascular mortality, TCM recurrence, and hospitalization for any cardiovascular cause) than those who had eGFR > 60 mL/[min 1.73 m2] (HR 1.817, 95% CI 1.097–3.009). 100 On the other hand, only one small prospective study of 101 TCM patients found that patients with lower eGFR (<60 mL/[min 1.73 m2]) had a lower long‐term mortality rate (OR 0.954, 95% CI 0.920–0.989), after a mean follow‐up of 7.2 years. 102
Malignant disease
Malignant disease is found between 6.6% and 28.5% of TCM patients in different studies. 54 , 62 , 71 , 103 Malignant disease and associated chemotherapy have been reported to be associated with TCM under certain circumstances, 104 , 105 especially in cases of pheochromocytoma. 106
Past studies of malignant disease and TCM suggested that malignant disease is a predictor of higher inpatient adverse events. 24 , 29 , 30 , 33 , 36 , 103 In a study of 122 855 TCM patients, Joy et al. found that malignant disease was a predictor of inpatient mortality: solid‐tumour disease in primary TCM (OR 3.43, 95% CI 1.38–8.52), and haematological cancer (OR 3.21, 95% CI 2.31–4.48) and metastatic cancer (OR 1.99, 95% CI 1.46–2.71) in secondary TCM. 103 Another study of 14 639 TCM patients also showed comorbid malignant disease was associated with higher in‐hospital mortality (OR 2.160, 95% CI 1.398–3.337). 30
Malignant disease remains a predictor of poorer long‐term outcome in TCM patients. 26 , 41 , 42 , 43 , 44 , 45 , 51 , 54 , 56 , 60 , 62 , 71 , 75 , 77 , 86 , 107 , 108 , 109 The latest meta‐analysis of the association between malignant disease and TCM patients included 10 studies and 126 322 patients and suggested that malignant disease was associated with greater mortality in TCM patients (RR 2.23, 95% CI 1.64–3.03,), including both inpatient mortality (RR 2.26, 95% CI 1.34–3.82) and long‐term mortality (RR 2.04, 95% CI 1.63–2.55). 107 In one study of 1604 TCM patients, those with malignant disease had a higher 10 year mortality rate (HR 1.84, 95% CI 1.29–2.61). 56 In addition, compared with patients with MI, patients with TCM had higher incidence of new malignant disease over 4 year follow‐up (13 TCM patients had new cancer vs. 3 MI patients, P = 0.01), whereas TCM patients with malignant disease have greater mortality over 4 years of follow‐up than MI patients with malignant disease (P = 0.03). 43
In contrast, some studies found that malignant disease was not associated with the outcomes of TCM patients. 38 , 66 , 89 , 99 , 110 However, given the available data, we believe that malignant disease is associated with poor outcomes in TCM patients (Table 5 ).
Body mass index
As a common cardiovascular risk factor, the prevalence of body mass index (BMI) > 30 kg/m2 in TCM patients ranges from 8.5% to 12.7%. 36 , 111 , 112 However, the true association between BMI and TCM is still uncertain (Table 5 ).
Most current studies have associated higher BMI with a poor short‐term prognosis in TCM patients. 102 , 113 One 58‐patient retrospective study noted that a BMI less than 20 kg/m2 was associated with early recovery (less than 10 days) from left ventricular systolic dysfunction in TCM patients after multivariate logistic regression analysis (OR 0.11, 95% CI 0.01–0.55). 113 In addition, using the data from National Inpatient Sample (NIS) database, Desai et al. found that TCM patients with obesity (BMI > 30 kg/m2) had higher incidences of inpatient acute MI (9.0% vs. 7.4%, P = 0.025), cardiogenic shock (4.3% vs. 3.2%, P = 0.032), cardiac arrest (2.3% vs. 0.4%, P < 0.001), and respiratory failure (12.9% vs. 11.0%, P = 0.021). Overall inpatient mortality was not significantly different between two groups (P = 0.354) after propensity‐matching analysis in a 1:1 ratio (528 non‐obese and 612 obese patients). 111
However, another study supports a negative association between lower BMI and TCM patients' outcome. It categorized 80 TCM patients into three groups (6 patients with BMI < 18.5 kg/m2, 28 patients with 18.5 ≥ BMI < 25 kg/m2, and 46 patients with BMI ≥ 25 kg/m2) and found that the lowest‐BMI group had the highest 5 year mortality rate. In a multivariate analysis, higher BMI was associated with lower long‐term mortality (OR 0.768, 95% CI 0.599–0.985). 114
Mood disorders
There is a growing body of literature regarding the association between mental illness and TCM. 8 , 80 , 115 , 116 Compared with MI patients, TCM patients have a significantly higher prevalence of mood disorders. 8 , 115 Multiple case reports have described exacerbations of anxiety, depression, mania, and psychosis as potential triggers of TCM episodes, 117 , 118 and undergoing therapies such as serotonin‐norepinephrine reuptake inhibitors 119 and selective norepinephrine reuptake inhibitor 120 for mood disorders was also noted to be a risk factor for TCM.
According to the current literature, mood disorders have uncertain effects on the in‐hospital outcomes of TCM patients (Table 5 ). Some studies found that mood disorders predicted better short‐term outcomes in TCM patients. 30 , 31 , 33 One prospective study, registering 3719 TCM patients from Japan, found that psychiatric disorders were associated with lower inpatient mortality (OR 0.43, 95% CI 0.19–0.96). 33 Conversely, other studies have found that mood disorders were not associated with short‐term outcomes in TCM patients. 27 , 36 , 66 , 80 For example, one study of 7510 TCM patients found that mood disorders (anxiety or depression) were not associated with in‐hospital mortality in TCM patients. 36 Likewise, Nayeri et al. reported that pre‐existing psychiatric illness was not associated with 30 day mortality in TCM patients (P = 0.32). 80
The effect of mood disorders on the long‐term outcome of TCM remains unclear (Table 5 ). Using the data from the Mayo Clinic Takotsubo Syndrome Registry from 2002 to 2016, Kim et al. found that a history of depression was a predictor of long‐term mortality in TCM patients (HR 1.764, 95% CI 1.155–2.694). 62 On the other hand, the study from Nayeri et al. found that pre‐existing psychiatric illness was not associated with long‐term mortality (P = 0.621), but it was associated with a higher risk of recurrent TCM (OR 7.44, 95% CI 2.30–24.01) after adjustment for age, CCI score, and type of cardiomyopathy trigger. 80
Differing protocols to identify mood disorders in patients may account for the differing results found in each study. Different studies variously identified mood disorders by ICD codes, medications, or chart review. In addition, studies did not identify the severity of mood disorders, which may affect the outcome in TCM patients.
Sepsis
Sepsis has an incidence ranging from 2.8% to 7.1% for TCM patients 19 , 32 , 33 and is a potential trigger of TCM patients in many cases, as well. 121 , 122 TCM is estimated to be present in 0.15% of severe sepsis cases in the United States. 37 Our review of the literature revealed a dearth of studies that focus on the association between sepsis and the outcomes of TCM (Table 5 ).
Most studies we found support the conclusion that sepsis is associated with worse outcomes in TCM. 30 , 33 , 66 Napierkowski et al. studied 14 639 TCM patients and concluded that sepsis was a predictor of in‐hospital mortality (OR 4.278, 95% CI 3.047–6.007). 30 Several other investigators drew a similar conclusion. For example, Khera et al.'s multivariable logistic regression analysis showed that sepsis independently predicted in‐hospital mortality (OR 6.93, 95% CI 2.54–18.93) in TCM patients. 66 However, another study of 7510 TCM patients found that sepsis was not associated with in‐hospital outcomes in TCM patients (P = 0.67) after adjustment for demographics and risk factors. 36
In addition, in a study with approximately 7.1 million patients with sepsis, 10 746 of them were found to have TCM. The authors found that TCM was associated with lower in‐hospital mortality in sepsis patients (OR 0.58, 95% CI 0.51–0.65). 37
Chronic obstructive pulmonary disease
Approximately 10.1% to 18.7% TCM patients are diagnosed with chronic obstructive pulmonary disease (COPD). 123 , 124 , 125 COPD has also been reported as a trigger of TCM, especially in the acute exacerbation phase. 126
There are few studies on the association between COPD and outcomes in TCM patients (Table 5 ). One retrospective study by Khera et al. found that COPD was not associated with in‐hospital mortality in patients with TCM. 66 However, some other studies found that COPD predicted poor in‐hospital outcomes in TCM patients. In a study of 3139 TCM patients from the NIS database, Li et al. found that TCM patients with COPD had greater inpatient mortality (OR 2.93, 95% CI 1.39–6.15) and a higher risk of complications, including acute respiratory failure (OR 3.25, 95% CI 2.45–4.32) and cardiogenic shock (OR 1.76, 95% CI 1.1–2.81). The result was generated after patients were propensity‐matched in a 1:2 target ratio (678 patients in the COPD‐TCM group and 1070 in the non‐COPD‐TCM group) to avoid bias. 112 Another study of 14 639 TCM patients found that COPD was associated with greater in‐hospital mortality (OR 1.444, 95% CI 1.140–1.829). 30 In addition, a study by Kato et al. found that compared with those without lung disease, TCM patients with acute pulmonary triggers had greater 5 year mortality (HR 2.12, 95% CI 1.33–3.38), whereas chronic lung disease had no effect on mortality. 127
Hyperlipidaemia
Approximately 15.9% to 52% of TCM patients have comorbid hyperlipidaemia (HLD). 8 , 35 , 36 , 41 There is little evidence available regarding the association between TCM outcome and HLD (Table 5 ). One study of 11 193 TCM patients found that HLD was not associated with in‐hospital mortality in TCM patients (P = 0.1474). 66 One study of 144 TCM patients also found that HLD was not associated with in‐hospital mortality. 85 However, one recent retrospective study found that TCM patients with HLD had better in‐hospital outcomes than those without HLD. Those with comorbid HLD had less in‐hospital mortality (OR 0.46, 95% CI 0.24–0.89) and fewer severe in‐hospital complications such as cardiogenic shock (OR 0.59, 95% CI 0.39–0.88) and acute respiratory failure (OR 0.73, 95% CI 0.56–0.95). However, the rates of cardiac arrest, ventricular arrhythmia, and acute kidney injury did not differ significantly between the two groups (1162 patients with HLD and 1162 patients without HLD), the author attributed this result to statin use, the protection of low‐density lipoprotein cholesterol binding lipopolysaccharide in sepsis, and underlying malnutrition in patients without HLD. 128 More research is needed to study the association between TCM and HLD.
Anaemia
A common chronic disease, anaemia, has been associated with poor outcome in TCM patients. About 12.7–22.82% of TCM patients have comorbid anaemia. 29 , 98 , 129 , 130
There are a few studies of the association between anaemia and the prognosis of TCM. One study of 4733 TCM patients found that anaemia was associated with more in‐hospital complications, such as cardiogenic shock (OR 3.14, 95% CI 2.12–4.64), ventricular arrhythmias (OR 1.88, 95% CI 1.20–2.95), acute kidney injury (OR 1.95, 95% CI 1.51–2.52), and acute respiratory failure (OR 1.93, 95% CI 1.49–2.50), although in‐hospital mortality was not different between patients with and without anaemia. 129 Another study with a small sample of 126 TCM patients associated anaemia with long‐term mortality (OR 3.93, 95% CI 1.02–2.08) during a median follow‐up time of 4.4 years. 131 Given the current evidence, we believe anaemia is associated with a poor prognosis in TCM patients. Future studies should focus on the optimal treatment for anaemia and the best threshold of transfusion in TCM patients.
Treatment
Evidence‐based medical therapy for TCM patients is lacking. TCM patients are routinely treated with supportive care, routine complication management, and conventional medical therapy, such as angiotensin‐converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), calcium channel blockers (CCBs), and β‐blockers. The evidence that using such medications translates into a survival benefit for TCM patients is limited.
Angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers
In addition to inhibiting the renin‐angiotensin‐aldosterone system and decreasing sympathetic system activation, ACEIs and ARBs also serve as direct and indirect antioxidants and can decrease nitrate tolerance and endothelial dysfunction, which in turn inhibits coronary artery spasm. 132 In theory, ACEIs/ARBs use may benefit TCM patients.
The association between ACEIs/ARBs use and the outcomes of TCM is not certain. Some studies have found that TCM patients who receive ACEIs/ARBs have better outcomes. 8 , 72 One study found that TCM patients who prescribed ACEIs/ARBs at discharge had less 1 year mortality than those without (33.6% vs. 36.6%, P = 0.001). 8 Another study of 326 TCM patients found those with ACEIs/ARBs had less long‐term cardiac mortality (46.7% vs. 70.7%, P = 0.048), although the all‐cause mortality difference was not statistically significant (P = 0.743). 72 Other studies, on the other hand, have found that ACEIs/ARBs use was not associated with in‐hospital outcomes 84 or long‐term outcomes 52 , 133 in TCM patients.
Some evidence suggests that ACEIs/ARBs use is beneficial for preventing TCM recurrence. A meta‐analysis of 31 studies and 1664 TCM patients showed that TCM patients receiving ACEIs/ARBs at discharge was correlated with a lower TCM recurrence rate (P = 0.016, r = −0.45) during long‐term follow‐up (mean 24.5 months). 134
β‐Blockers
In theory, given that the two most widely accepted mechanisms of TCM are sympathetic nervous system overactivation and catecholamine surge, β‐blockers may be able to prevent or reduce the severity of cardiac complications in TCM by counteracting the catecholamine effect. Although they are commonly prescribed for TCM patients during hospitalization and at discharge, the efficacy of β‐blockers against TCM remains uncertain.
Although β‐blockers could relieve ventricular discordance by blocking the adrenergic system in TCM patients with an elevated intraventricular pressure gradient, 135 they do not reduce the short‐term mortality rate in TCM patients according to our literature review (Table 6 ). 84 , 88 , 136 A retrospective outcome study found that those who received early β‐blocker administration (i.e. starting within the first 2 days of admission; 422 patients) had similar inpatient (P = 0.703), 15 day (P = 1.000), and 30 day mortality (P = 0.747) to those who did not receive any β‐blockers during hospitalization (1668 patients). 136 Another study by Kato et al. found that β‐blocker use before admission was associated with a higher rate of in‐hospital complications (pulmonary oedema, cardiogenic shock, sustained VT or VF, complete atrioventricular block, thromboembolism, cardiac rupture, and cardiac death) (OR 16.9, 95% CI 1.57–181.7). 88
Table 6.
Clinical studies on the effects of drugs in TCM patients
| Drug | Author (year) | Diagnostic criteria | Sample size | Type of study | Follow‐up | Mortality | Analysis strategy | Risk of bias |
|---|---|---|---|---|---|---|---|---|
| β‐blocker | β‐blocker vs. Non‐β‐blocker | |||||||
| Isogai et al. (2016) | ICD‐10 | 2110 | Retrospective | 15 days in‐hospital | No difference | Multivariable logistic regression | High | |
| 30 days in‐hospital | No difference | |||||||
| In‐hospital | No difference | |||||||
| Almendro‐Delia et al. (2018) | Modified Mayo criteria | 711 | Prospective | 1 year |
TCM with cardiogenic shock: HR 0.52 (95% CI 0.44–0.79), P = 0.011 TCM without cardiogenic shock: No difference |
Multivariate Cox regression | Moderate | |
CI, confidence interval; HR, hazard ratio; ICD, International Classification of Diseases; NA, not applicable; TCM, Takotsubo cardiomyopathy.
The effect of β‐blockers on the long‐term outcome of TCM is still uncertain (Table 6 ). Our literature review revealed that the majority of studies found β‐blocker administration not to be associated with long‐term TCM outcome. 8 , 52 , 133 , 134 , 137 For example, one meta‐analysis of 31 studies from Singh et al. found that discharging TCM patients with β‐blockers had no significant effect on the recurrence of TCM (P = 0.28) during an average of 24.5 months' follow‐up. 134 In addition, one study with a sample size of 711 found that TCM patients with cardiogenic shock who were prescribed β‐blockers at discharge had a lower 1 year mortality rate (HR 0.52, 95% CI 0.44–0.79) than patients who were not prescribed such medications. There was also no significant benefit of β‐blockers for TCM patients without cardiogenic shock (P = 0.853). 41
Calcium channel blockers
Calcium channel blockers are commonly used to treat cardiovascular diseases, especially hypertension and arrhythmia, and can be categorized as dihydropyridine CCBs (which have a greater effect on reducing systemic vascular pressure) and non‐dihydropyridine CCBs (which have a greater negative effect on cardiac conduction and contractility). Although the indications for the two types of CCBs differ, they both have counteractive effects on coronary vasospasm and microcirculatory dysfunction, which are thought to be the pathophysiologic causes of TCM. 138 , 139
Overall, the evidence of CCBs' effects on TCM outcome is limited. A retrospective study using multivariate logistic regression analysis found that TCM patients who were not taking CCBs at admission had a relatively delayed (more than 10 days) recovery from left ventricular systolic dysfunction to normal contraction compared with those taking CCBs (14 patients with dihydropyridine CCB and 2 patients with non‐dihydropyridine CCB) (OR 22.2, 95% CI 3.08–291.5). 113 The study was limited by its small sample size and its retrospective design.
Statins
Statins are one of the most frequently used medical therapies for primary and secondary prevention of cardiovascular disease. 140 The benefits of statins for the cardiovascular system have been widely accepted, 141 including not only cholesterol biosynthesis reduction 142 but also endothelial function improvement 143 and inflammation reduction. 144
However, our literature review did not find strong evidence of any short‐term or long‐term benefit of statins for TCM patients. 52 , 84 , 133 , 145 A retrospective study by Dias et al., involving 146 participants who received statins during hospitalization, found that statin use was not associated with major adverse cardiovascular events in TCM patients after multivariate analysis (P = 0.8). 84 A meta‐analysis of 511 TCM patients, including 203 patients prescribed statins at discharge, found that statin use was not associated with TCM reoccurrence during long‐term follow‐up (range, 24 to 30 months; P = 0.95). 145
Heterogeneity of studies
An increasing number of studies are examining the prognosis of TCM, especially with the rise of administrative databases and multicentre registries. However, these studies have substantial bias and heterogeneity. As Table 7 shows, administrative database and multicentre/single‐centre registry studies differ in several ways.
Table 7.
Differences between studies based on administrative databases and multicentre/single‐centre registries
| Variable | Administrative databases | Multicentre/single‐centre registries |
|---|---|---|
| Study type | Retrospective | Retrospective or prospective |
| Diagnosis criteria | ICD code | Mayo criteria, ESC criteria (2016), In‐TAK criteria, or Madias criteria |
| Sample size | Large (>3000) or moderate (1000–3000) | Moderate (1000–3000) or small (<1000) |
| Variables included | Less data: age, sex, race, and some comorbidities selected by ICD code | More data: age, sex, race, comorbidities, therapeutic schedule, laboratory, and imaging findings |
| Follow‐up time | In‐hospital | In‐hospital and long‐term |
ESC, European Society of Cardiology; ICD, International Classification of Diseases; In‐TAK, International Takotsubo.
First, the identification and diagnostic criteria of TCM differ significantly among these studies. ICD‐9 and ICD‐10 codes were widely used to identify TCM in studies based on administrative databases, while other specific diagnostic criteria, such as the Mayo and ESC criteria, were used in multicentre registries and single‐centre studies. Although previous studies showed that the discharge diagnosis of TCM by ICD code has a high accuracy and positive predictive value, 13 the validity of using the ICD code for TCM identification in public databases, such as the NIS, is lacking and infeasible because the data are deidentified, which compromises findings from these databases.
Second, many studies, both administrative database and multicentre/single‐centre registry studies, which aimed to study different prognostic factors with various follow‐up times in TCM patients, potentially have overlap among their patient samples. For example, many institutes and research groups that contributed to International Takotsubo Registry have also published prognostic studies based on their own patient cohorts. In addition, some studies used the same sample of TCM patients to study different factors, although the variables in the multivariable model and follow‐up times were different.
Third, the quality and sample size of these studies varied substantially. For example, studies based on administrative databases (e.g. the NIS database) usually have a large (over 3000) or moderate (1000 to 3000) sample of TCM patients but a high overall risk of bias. The limitation of database studies is the omission of certain potentially important variables, such as therapeutic schedules and laboratory and imaging findings, which may also be associated with the prognosis of TCM patients. In contrast, studies from multicentre or single‐centre registries have moderate or smaller samples (<1000), but they nonetheless have sufficient data to build the multivariable model with less bias. Thus, excluding studies from administrative databases means omitting a large number of TCM patients. Similarly, excluding studies from multicentre registries means excluding some high‐quality studies and reduces the credibility of the results.
Fourth, the follow‐up times were different between the two types of studies. Administrative databases often offer only in‐hospital information, so these data can only be used to examine associations between risk factors and short‐term outcomes in TCM patients. In contrast, many multicentre and single‐centre registries have both short‐term and long‐term follow‐up data. Therefore, the association between risk factors and TCM prognosis shown in the two types of studies may be different because of the different follow‐up times.
As a result, a statistical meta‐analysis would be challenging and inappropriate if it included all types of studies. Although one could use stricter inclusion criteria (e.g. by including only studies with specific TCM criteria) and exclusion criteria (e.g. excluding any potential duplicate studies) to synthesize the data, that would result in the exclusion of a significant portion of studies of several prognostic factors. So we describe the outcomes from different studies comprehensively to show the effects of as many risk factors as possible on patients with TCM.
Limitations
Our review also had limitations. First, the patient populations and potential predictors assessed were heterogeneous (e.g. the diagnostic criteria for TCM patients and the method of identifying each prognostic factor differed among these studies), which may have affected the interpretation and generalizability of the results. Second, because of the substantial heterogeneity among studies, we only reported findings from each study without performing meta‐analysis. Third, certain risk factors, such as COPD and anaemia, were examined in only a few studies, which could affect the certainty of the evidence.
Conclusion
Although they are widely recognized, the risk factors and treatments' effect on the outcome of TCM remain largely uncertain (Figure 2 ). The bulk of the evidence suggests that male sex, physical triggers, and certain comorbidities such as CKD, malignant disease, higher BMI, sepsis, COPD, and anaemia are associated with poor TCM prognosis. However, the association of race, DM, mood disorders, HLD, and medical therapies with TCM prognosis remains unclear. Further systemic review and meta‐analysis are needed to integrate findings from previous studies and to address the inconsistencies among many risk factors. Randomized controlled trials are warranted to determine whether these risk factors require early intervention and to identify the appropriate methods to intervene.
Figure 2.
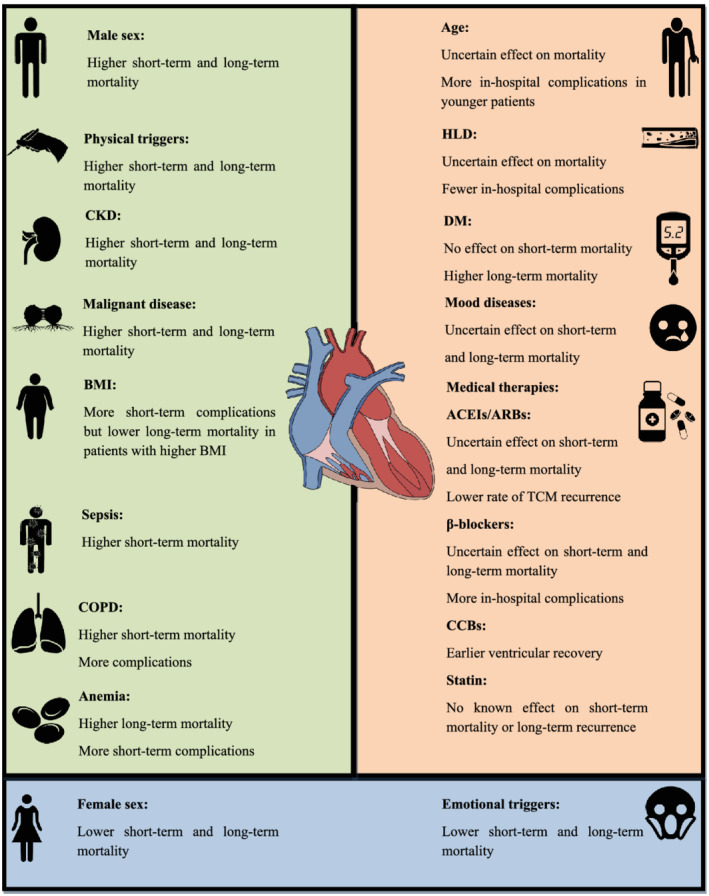
Prognostic factors of Takotsubo cardiomyopathy. ACEIs, angiotensin‐converting enzyme inhibitors; ARBs, angiotensin receptor blockers; BMI, body mass index; CCBs, calcium channel blockers; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus diabetes; HLD, hyperlipidaemia.
Conflict of interest
None declared.
Funding
This work was supported by Grant for 2019 Shantou Medical Science Talent Cultivation and Clinical Technology Promotion Project (190917105269872) and Grant for Key Disciplinary Project of Clinical Medicine under the Guangdong High‐level University Development Program (2020).
Acknowledgements
We acknowledge Stephen N. Palmer, PhD, ELS (Scientific Publications, Texas Heart Institute) and Alexander Yang, PhD (Center for Molecular Medicine and Genetics, Wayne State University School of Medicine), who contributed to the editing of the manuscript.
Lu, X. , Li, P. , Teng, C. , Cai, P. , Jin, L. , Li, C. , Liu, Q. , Pan, S. , Dixon, R. A. F. , and Wang, B. (2021) Prognostic factors of Takotsubo cardiomyopathy: a systematic review. ESC Heart Failure, 8: 3663–3689. 10.1002/ehf2.13531.
Xiaojia Lu and Pengyang Li contributed equally to the writing of this article and share primary authorship.
References
- 1. Isogai T, Yoshikawa T, Ueda T, Yamaguchi T, Imori Y, Maekawa Y, Sakata K, Murakami T, Mochizuki H, Arao K, Kimura A, Nagao K, Yamamoto T, Takayama M. Apical Takotsubo syndrome versus anterior acute myocardial infarction: findings from the Tokyo Cardiovascular Care Unit network registry. Eur Heart J Acute Cardiovasc Care 2019; 8: 86–95. [DOI] [PubMed] [Google Scholar]
- 2. Ono R, Falcão LM. Takotsubo cardiomyopathy systematic review: pathophysiologic process, clinical presentation and diagnostic approach to Takotsubo cardiomyopathy. Int J Cardiol 2016; 209: 196–205. [DOI] [PubMed] [Google Scholar]
- 3. Akashi YJ, Nakazawa K, Sakakibara M, Miyake F, Musha H, Sasaka K. 123I‐MIBG myocardial scintigraphy in patients with “takotsubo” cardiomyopathy. J Nucl Med 2004; 45: 1121–1127. [PubMed] [Google Scholar]
- 4. Bybee KA, Prasad A, Barsness GW, Lerman A, Jaffe AS, Murphy JG, Wright RS, Rihal CS. Clinical characteristics and thrombolysis in myocardial infarction frame counts in women with transient left ventricular apical ballooning syndrome. Am J Cardiol 2004; 94: 343–346. [DOI] [PubMed] [Google Scholar]
- 5. Ito K, Sugihara H, Katoh S, Azuma A, Nakagawa M. Assessment of Takotsubo (ampulla) cardiomyopathy using 99mTc‐tetrofosmin myocardial SPECT—comparison with acute coronary syndrome. Ann Nucl Med 2003; 17: 115–122. [DOI] [PubMed] [Google Scholar]
- 6. Pillière R, Mansencal N, Digne F, Lacombe P, Joseph T, Dubourg O. Prevalence of tako‐tsubo syndrome in a large urban agglomeration. Am J Cardiol 2006; 98: 662–665. [DOI] [PubMed] [Google Scholar]
- 7. Zhang L, Piña IL. Stress‐induced cardiomyopathy. Heart Fail Clin 2019; 15: 41–53. [DOI] [PubMed] [Google Scholar]
- 8. Templin C, Ghadri JR, Diekmann J, Napp LC, Bataiosu DR, Jaguszewski M, Cammann VL, Sarcon A, Geyer V, Neumann CA, Seifert B, Hellermann J, Schwyzer M, Eisenhardt K, Jenewein J, Franke J, Katus HA, Burgdorf C, Schunkert H, Moeller C, Thiele H, Bauersachs J, Tschöpe C, Schultheiss HP, Laney CA, Rajan L, Michels G, Pfister R, Ukena C, Böhm M, Erbel R, Cuneo A, Kuck KH, Jacobshagen C, Hasenfuss G, Karakas M, Koenig W, Rottbauer W, Said SM, Braun‐Dullaeus RC, Cuculi F, Banning A, Fischer TA, Vasankari T, Airaksinen KE, Fijalkowski M, Rynkiewicz A, Pawlak M, Opolski G, Dworakowski R, MacCarthy P, Kaiser C, Osswald S, Galiuto L, Crea F, Dichtl W, Franz WM, Empen K, Felix SB, Delmas C, Lairez O, Erne P, Bax JJ, Ford I, Ruschitzka F, Prasad A, Lüscher TF. Clinical features and outcomes of Takotsubo (stress) cardiomyopathy. N Engl J Med 2015; 373: 929–938. [DOI] [PubMed] [Google Scholar]
- 9. Stiermaier T, Eitel C, Denef S, Desch S, Schuler G, Thiele H, Eitel I. Prevalence and clinical significance of life‐threatening arrhythmias in Takotsubo cardiomyopathy. J Am Coll Cardiol 2015; 65: 2148–2150. [DOI] [PubMed] [Google Scholar]
- 10. Heckle MR, McCoy CW, Akinseye OA, Khouzam RN. Stress‐induced thrombus: prevalence of thromboembolic events and the role of anticoagulation in Takotsubo cardiomyopathy. Ann Transl Med 2018; 6: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jaguszewski M, Fijalkowski M, Nowak R, Czapiewski P, Ghadri J‐R, Templin C, Rynkiewicz A. Ventricular rupture in Takotsubo cardiomyopathy. Eur Heart J 2012; 33: 1027. [DOI] [PubMed] [Google Scholar]
- 12. Lu DY, Caplow J, Quatromoni N, Forde‐McLean R, Owens AT. Ventricular septal defect from Takotsubo syndrome. Case Rep Cardiol 2016; 2016: 2693062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li P, Dai Q, Cai P, Teng C, Pan S, Dixon RAF, Liu Q. Identifying different phenotypes in takotsubo cardiomyopathy by latent class analysis. ESC Heart Failure 2021; 8: 555–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med 2013; 158: 280–286. [DOI] [PubMed] [Google Scholar]
- 15. Granholm A, Zeng L, Dionne JC, Perner A, Marker S, Krag M, MacLaren R, Ye Z, Møller MH, Alhazzani W. Predictors of gastrointestinal bleeding in adult ICU patients: a systematic review and meta‐analysis. Intensive Care Med 2019; 45: 1347–1359. [DOI] [PubMed] [Google Scholar]
- 16. Sy F, Basraon J, Zheng H, Singh M, Richina J, Ambrose JA. Frequency of Takotsubo cardiomyopathy in postmenopausal women presenting with an acute coronary syndrome. Am J Cardiol 2013; 112: 479–482. [DOI] [PubMed] [Google Scholar]
- 17. Pérez‐Castellanos A, Martínez‐Sellés M, Mejía‐Rentería H, Andrés M, Sionis A, Almendro‐Delia M, Martín‐García A, Aguilera MC, Pereyra E, Linares Vicente JA, García de la Villa B, Núñez‐Gil IJ. Tako‐tsubo syndrome in men: rare, but with poor prognosis. Rev Esp Cardiol (Engl Ed) 2018; 71: 703–708. [DOI] [PubMed] [Google Scholar]
- 18. Murakami T, Yoshikawa T, Maekawa Y, Ueda T, Isogai T, Sakata K, Nagao K, Yamamoto T, Takayama M. Gender differences in patients with takotsubo cardiomyopathy: multi‐center registry from Tokyo CCU Network. PLoS ONE 2015; 10: e0136655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brinjikji W, El‐Sayed AM, Salka S. In‐hospital mortality among patients with takotsubo cardiomyopathy: a study of the National Inpatient Sample 2008 to 2009. Am Heart J 2012; 164: 215–221. [DOI] [PubMed] [Google Scholar]
- 20. Lemor A, Ramos‐Rodriguez AJ, De La Villa R, Hosseini Dehkordi SH, Vazquez de Lara F, Lee S, Rodriguez Rivera M, Casso Dominguez A, Argulian E. Impact of gender on in‐hospital outcomes in patients with Takotsubo syndrome: a nationwide analysis from 2006 to 2014. Clin Cardiol 2019; 42: 13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Weidner KJ, El‐Battrawy I, Behnes M, Schramm K, Fastner C, Kuschyk J, Hoffmann U, Ansari U, Borggrefe M, Akin I. Sex differences of in‐hospital outcome and long‐term mortality in patients with Takotsubo cardiomyopathy. Ther Clin Risk Manag 2017; 13: 863–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Murugiah K, Wang Y, Desai NR, Spatz ES, Nuti SV, Dreyer RP, Krumholz HM. Trends in short‐ and long‐term outcomes for takotsubo cardiomyopathy among medicare fee‐for‐service beneficiaries, 2007 to 2012. JACC Heart Fail 2016; 4: 197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Budnik M, Nowak R, Fijałkowski M, Kochanowski J, Nargiełło E, Piątkowski R, Peller M, Kucharz J, Jaguszewski M, Gruchała M, Opolski G. Sex‐dependent differences in clinical characteristics and in‐hospital outcomes in patients with takotsubo syndrome. Pol Arch Intern Med 2020; 130: 25–30. [DOI] [PubMed] [Google Scholar]
- 24. Misumida N, Ogunbayo GO, Kim SM, Abdel‐Latif A, Ziada KM, Sorrell VL. Clinical outcome of takotsubo cardiomyopathy diagnosed with or without coronary angiography. Angiology 2019; 70: 56–61. [DOI] [PubMed] [Google Scholar]
- 25. Santoro F, Núñez Gil IJ, Stiermaier T, El‐Battrawy I, Guerra F, Novo G, Guastafierro F, Tarantino N, Novo S, Mariano E, Romeo F, Romeo F, Capucci A, Bahlmann E, Zingaro M, Cannone M, Caldarola P, Marchetti MF, Montisci R, Meloni L, Thiele H, Di Biase M, Almendro‐Delia M, Sionis A, Akin I, Eitel I, Brunetti ND. Assessment of the German and Italian stress cardiomyopathy score for risk stratification for in‐hospital complications in patients with Takotsubo syndrome. JAMA Cardiol 2019; 4: 892–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Arcari L, Musumeci MB, Stiermaier T, El‐Battrawy I, Möller C, Guerra F, Novo G, Mariano E, Limite LR, Cacciotti L, Semeraro R, Volpe M, Romeo F, Caldarola P, Thiele H, Akin I, Brunetti ND, Eitel I, Santoro F. Incidence, determinants and prognostic relevance of dyspnea at admission in patients with Takotsubo syndrome: results from the international multicenter GEIST registry. Sci Rep 2020; 10: 13603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cammann VL, Szawan KA, Stähli BE, Kato K, Budnik M, Wischnewsky M, Dreiding S, Levinson RA, Di Vece D, Gili S, Citro R, Bossone E, Neuhaus M, Franke J, Meder B, Jaguszewski M, Noutsias M, Knorr M, Heiner S, D'Ascenzo F, Dichtl W, Burgdorf C, Kherad B, Tschöpe C, Sarcon A, Shinbane J, Rajan L, Michels G, Pfister R, Cuneo A, Jacobshagen C, Karakas M, Koenig W, Pott A, Meyer P, Roffi M, Banning A, Wolfrum M, Cuculi F, Kobza R, Fischer TA, Vasankari T, Airaksinen KEJ, Napp LC, Dworakowski R, MacCarthy P, Kaiser C, Osswald S, Galiuto L, Chan C, Bridgman P, Beug D, Delmas C, Lairez O, Gilyarova E, Shilova A, Gilyarov M, El‐Battrawy I, Akin I, Poledniková K, Toušek P, Winchester DE, Galuszka J, Ukena C, Poglajen G, Carrilho‐Ferreira P, Hauck C, Paolini C, Bilato C, Kobayashi Y, Shoji T, Ishibashi I, Takahara M, Himi T, Din J, Al‐Shammari A, Prasad A, Rihal CS, Liu K, Schulze PC, Bianco M, Jörg L, Rickli H, Pestana G, Nguyen TH, Böhm M, Maier LS, Pinto FJ, Widimský P, Felix SB, Braun‐Dullaeus RC, Rottbauer W, Hasenfuß G, Pieske BM, Schunkert H, Borggrefe M, Thiele H, Bauersachs J, Katus HA, Horowitz JD, Di Mario C, Münzel T, Crea F, Bax JJ, Lüscher TF, Ruschitzka F, Ghadri JR, Opolski G, Templin C. Age‐related variations in Takotsubo syndrome. J Am Coll Cardiol 2020; 75: 1869–1877. [DOI] [PubMed] [Google Scholar]
- 28. Malanchini G, Del Corral MP, De Filippo P, Ferrari P, Solomon A, Krepp J. Cardiac arrhythmias and In‐hospital mortality amongst patients with takotsubo cardiomyopathy: a retrospective study in an Italian population. Int J Cardiol Heart Vasc 2020; 31: 100608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yerasi C, Tripathi B, Banga S, McNown C, Jonnalagadda AK, Al‐Qaisi S, Miryala V, Nafisi S, Waksman R, Ben‐Dor I. Predictors of 90‐day readmission and in‐hospital mortality in takotsubo cardiomyopathy: an analysis of 28,079 index admissions. Cardiovasc Revasc Med 2019; 20: 973–979. [DOI] [PubMed] [Google Scholar]
- 30. Napierkowski S, Banerjee U, Anderson HV, Charitakis K, Madjid M, Smalling RW, Dhoble A. Trends and impact of the use of mechanical circulatory support for cardiogenic shock secondary to takotsubo cardiomyopathy. Am J Cardiol 2021; 139: 28–33. [DOI] [PubMed] [Google Scholar]
- 31. Syed M, Khan MZ, Osman M, Alharbi A, Khan MU, Munir MB, Balla S. Comparison of outcomes in patients with takotsubo syndrome with‐vs‐without cardiogenic shock. Am J Cardiol 2020; 136: 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ando K, Sukekawa H, Takahata A, Kobari Y, Tsuchiya H, Ishigaki D, Tamabuchi T, Koyama Y. Renal dysfunction indicative of outcomes in hospitalized patients with takotsubo syndrome. Eur Heart J Acute Cardiovasc Care 2018; 7: 723–731. [DOI] [PubMed] [Google Scholar]
- 33. Isogai T, Yasunaga H, Matsui H, Tanaka H, Ueda T, Horiguchi H, Fushimi K. Out‐of‐hospital versus in‐hospital Takotsubo cardiomyopathy: analysis of 3719 patients in the Diagnosis Procedure Combination database in Japan. Int J Cardiol 2014; 176: 413–417. [DOI] [PubMed] [Google Scholar]
- 34. Santoro F, Stiermaier T, Tarantino N, Guastafierro F, Graf T, Möller C, Di Martino LFM, Thiele H, Di Biase M, Eitel I, Brunetti ND. Impact of persistent ST elevation on outcome in patients with Takotsubo syndrome. Results from the GErman Italian STress Cardiomyopathy (GEIST) registry. Int J Cardiol 2018; 255: 140–144. [DOI] [PubMed] [Google Scholar]
- 35. Kwon SW, Kim BO, Kim MH, Lee SJ, Yoon JH, Chung H, Shim CY, Cho DK, Ryu SK, Yoon SJ, Yoon YW, Chang HJ, Rim SJ, Kwon HM, Jang Y, Hong BK. Diverse left ventricular morphology and predictors of short‐term outcome in patients with stress‐induced cardiomyopathy. Int J Cardiol 2013; 168: 331–337. [DOI] [PubMed] [Google Scholar]
- 36. Krishnamoorthy P, Garg J, Sharma A, Palaniswamy C, Shah N, Lanier G, Patel NC, Lavie CJ, Ahmad H. Gender differences and predictors of mortality in takotsubo cardiomyopathy: analysis from the National Inpatient Sample 2009‐2010 database. Cardiology 2015; 132: 131–136. [DOI] [PubMed] [Google Scholar]
- 37. Vallabhajosyula S, Deshmukh AJ, Kashani K, Prasad A, Sakhuja A. Tako‐Tsubo cardiomyopathy in severe sepsis: nationwide trends, predictors, and outcomes. J Am Heart Assoc 2018; 7: e009160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee JH, Uhm JS, Shin DG, Joung B, Pak HN, Ko YG, Hong GR, Lee MH. Clinical significance of changes in the corrected QT interval in stress‐induced cardiomyopathy. Korean J Intern Med 2016; 31: 507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kimura A, Yoshikawa T, Isogai T, Tanaka H, Ueda T, Yamaguchi T, Imori Y, Maekawa Y, Sakata K, Murakami T, Arao K, Nagao K, Yamamoto T, Takayama M. Impact of body temperature at admission on inhospital outcomes in patients with takotsubo syndrome: insights from the Tokyo Cardiovascular Care Unit Network Registry. Eur Heart J Acute Cardiovasc Care 2020; 9: 703–710. [DOI] [PubMed] [Google Scholar]
- 40. Stiermaier T, Moeller C, Oehler K, Desch S, Graf T, Eitel C, Vonthein R, Schuler G, Thiele H, Eitel I. Long‐term excess mortality in takotsubo cardiomyopathy: predictors, causes and clinical consequences. Eur J Heart Fail 2016; 18: 650–656. [DOI] [PubMed] [Google Scholar]
- 41. Almendro‐Delia M, Núñez‐Gil IJ, Lobo M, Andrés M, Vedia O, Sionis A, Martin‐García A, Cruz Aguilera M, Pereyra E, Martín de Miguel I, Linares Vicente JA, Corbí‐Pascual M, Bosch X, Fabregat Andrés O, Sánchez Grande Flecha A, Pérez‐Castellanos A, Pais JL, De Mora Martín M, Escudier Villa JM, Martín Asenjo R, Guillen Marzo M, Rueda Sobella F, Aceña Á, García Acuña JM, García‐Rubira JC. Short‐ and long‐term prognostic relevance of cardiogenic shock in takotsubo syndrome: results from the RETAKO registry. JACC Heart Fail 2018; 6: 928–936. [DOI] [PubMed] [Google Scholar]
- 42. Ghadri JR, Kato K, Cammann VL, Gili S, Jurisic S, Di Vece D, Candreva A, Ding KJ, Micek J, Szawan KA, Bacchi B, Bianchi R, Levinson RA, Wischnewsky M, Seifert B, Schlossbauer SA, Citro R, Bossone E, Münzel T, Knorr M, Heiner S, D'Ascenzo F, Franke J, Sarcon A, Napp LC, Jaguszewski M, Noutsias M, Katus HA, Burgdorf C, Schunkert H, Thiele H, Bauersachs J, Tschöpe C, Pieske BM, Rajan L, Michels G, Pfister R, Cuneo A, Jacobshagen C, Hasenfuß G, Karakas M, Koenig W, Rottbauer W, Said SM, Braun‐Dullaeus RC, Banning A, Cuculi F, Kobza R, Fischer TA, Vasankari T, Airaksinen KEJ, Opolski G, Dworakowski R, MacCarthy P, Kaiser C, Osswald S, Galiuto L, Crea F, Dichtl W, Empen K, Felix SB, Delmas C, Lairez O, El‐Battrawy I, Akin I, Borggrefe M, Horowitz J, Kozel M, Tousek P, Widimský P, Gilyarova E, Shilova A, Gilyarov M, Winchester DE, Ukena C, Bax JJ, Prasad A, Böhm M, Lüscher TF, Ruschitzka F, Templin C. Long‐term prognosis of patients with Takotsubo syndrome. J Am Coll Cardiol 2018; 72: 874–882. [DOI] [PubMed] [Google Scholar]
- 43. Sattler K, El‐Battrawy I, Gietzen T, Lang S, Zhou X, Borggrefe M, Akin I. Long term outcome of patients suffering from cancer and Takotsubo syndrome or myocardial infarction. Qjm 2018; 111: 473–481. [DOI] [PubMed] [Google Scholar]
- 44. Stiermaier T, Santoro F, El‐Battrawy I, Möller C, Graf T, Novo G, Santangelo A, Mariano E, Romeo F, Caldarola P, Fanelli M, Thiele H, Brunetti ND, Akin I, Eitel I. Prevalence and prognostic impact of diabetes in Takotsubo syndrome: insights from the international, multicenter GEIST registry. Diabetes Care 2018; 41: 1084–1088. [DOI] [PubMed] [Google Scholar]
- 45. Gietzen T, El‐Battrawy I, Lang S, Zhou XB, Ansari U, Behnes M, Borggrefe M, Akin I. Impact of T‐inversion on the outcome of Takotsubo syndrome as compared to acute coronary syndrome. Eur J Clin Invest 2019; 49: e13078. [DOI] [PubMed] [Google Scholar]
- 46. Núñez‐Gil IJ, Almendro‐Delia M, Andrés M, Sionis A, Martin A, Bastante T, Córdoba‐Soriano JG, Linares JA, González Sucarrats S, Sánchez‐Grande‐Flecha A, Fabregat‐Andrés O, Pérez B, Escudier‐Villa JM, Martin‐Reyes R, Pérez‐Castellanos A, Rueda Sobella F, Cambeiro C, Piqueras‐Flores J, Vidal‐Perez R, Bodí V, García de la Villa B, Corbí‐Pascua M, Biagioni C, Mejía‐Rentería HD, Feltes G, Barrabés J. Secondary forms of Takotsubo cardiomyopathy: a whole different prognosis. Eur Heart J Acute Cardiovasc Care 2016; 5: 308–316. [DOI] [PubMed] [Google Scholar]
- 47. Stiermaier T, Santoro F, Eitel C, Graf T, Möller C, Tarantino N, Guastafierro F, Di Biase M, Thiele H, Brunetti ND, Eitel I. Prevalence and prognostic relevance of atrial fibrillation in patients with Takotsubo syndrome. Int J Cardiol 2017; 245: 156–161. [DOI] [PubMed] [Google Scholar]
- 48. Stiermaier T, Santoro F, Graf T, Guastafierro F, Tarantino N, De Gennaro L, Caldarola P, Di Biase M, Thiele H, Brunetti ND, Möller C, Eitel I. Prognostic value of N‐terminal pro‐B‐type natriuretic peptide in Takotsubo syndrome. Clin Res Cardiol 2018; 107: 597–606. [DOI] [PubMed] [Google Scholar]
- 49. Alashi A, Isaza N, Faulx J, Popovic ZB, Menon V, Ellis SG, Faulx M, Kapadia SR, Griffin BP, Desai MY. Characteristics and outcomes of patients with Takotsubo syndrome: incremental prognostic value of baseline left ventricular systolic function. J Am Heart Assoc 2020; 9: e016537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Huseynov A, El‐Battrawy I, Ansari U, Schramm K, Zhou X, Lang S, Borggrefe M, Akin I. Age related differences and outcome of patients with Takotsubo syndrome. J Geriatr Cardiol 2017; 14: 632–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Giannakopoulos K, El‐Battrawy I, Gietzen T, Ansari U, Borggrefe M, Akin I. Gender‐based comparison of takotsubo syndrome versus myocardial infarction. QJM 2019; 112: 355–362. [DOI] [PubMed] [Google Scholar]
- 52. Elesber AA, Prasad A, Lennon RJ, Wright RS, Lerman A, Rihal CS. Four‐year recurrence rate and prognosis of the apical ballooning syndrome. J Am Coll Cardiol 2007; 50: 448–452. [DOI] [PubMed] [Google Scholar]
- 53. Song BG, Hahn J‐Y, Cho SJ, Park YH, Choi SM, Park JH, Choi S‐H, Choi J‐H, Park SW, Lee SH, Gwon H‐C. Clinical characteristics, ballooning pattern, and long‐term prognosis of transient left ventricular ballooning syndrome. Heart Lung 2010; 39: 188–195. [DOI] [PubMed] [Google Scholar]
- 54. Sattler K, El‐Battrawy I, Lang S, Zhou X, Schramm K, Tülümen E, Kronbach F, Röger S, Behnes M, Kuschyk J, Borggrefe M, Akin I. Prevalence of cancer in Takotsubo cardiomyopathy: short and long‐term outcome. Int J Cardiol 2017; 238: 159–165. [DOI] [PubMed] [Google Scholar]
- 55. Uribarri A, Núñez‐Gil IJ, Conty DA, Vedia O, Almendro‐Delia M, Duran Cambra A, Martin‐Garcia AC, Barrionuevo‐Sánchez M, Martínez‐Sellés M, Raposeiras‐Roubín S, Guillén M, Garcia Acuña JM, Matute‐Blanco L, Linares Vicente JA, Sánchez Grande Flecha A, Andrés M, Pérez‐Castellanos A, Lopez‐Pais J. Short‐ and long‐term prognosis of patients with Takotsubo syndrome based on different triggers: importance of the physical nature. J Am Heart Assoc 2019; 8: e013701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cammann VL, Sarcon A, Ding KJ, Seifert B, Kato K, Di Vece D, Szawan KA, Gili S, Jurisic S, Bacchi B, Micek J, Frangieh AH, Napp LC, Jaguszewski M, Bossone E, Citro R, D'Ascenzo F, Franke J, Noutsias M, Knorr M, Heiner S, Burgdorf C, Koenig W, Thiele H, Tschöpe C, Rajan L, Michels G, Pfister R, Cuneo A, Jacobshagen C, Karakas M, Banning A, Cuculi F, Kobza R, Fischer TA, Vasankari T, Airaksinen KEJ, Dworakowski R, Kaiser C, Osswald S, Galiuto L, Dichtl W, Delmas C, Lairez O, Horowitz JD, Kozel M, Widimský P, Tousek P, Winchester DE, Gilyarova E, Shilova A, Gilyarov M, El‐Battrawy I, Akin I, Ukena C, Bauersachs J, Pieske BM, Hasenfuß G, Rottbauer W, Braun‐Dullaeus RC, Opolski G, MacCarthy P, Felix SB, Borggrefe M, Di Mario C, Crea F, Katus HA, Schunkert H, Münzel T, Böhm M, Bax JJ, Prasad A, Shinbane J, Lüscher TF, Ruschitzka F, Ghadri JR, Templin C. Clinical features and outcomes of patients with malignancy and takotsubo syndrome: observations from the International Takotsubo Registry. J Am Heart Assoc 2019; 8: e010881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ansari U, El‐Battrawy I, Fastner C, Behnes M, Sattler K, Huseynov A, Baumann S, Tülümen E, Borggrefe M, Akin I. Clinical outcomes associated with catecholamine use in patients diagnosed with Takotsubo cardiomyopathy. BMC Cardiovasc Disord 2018; 18: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. El‐Battrawy I, Lang S, Ansari U, Sattler K, Behnes M, Schramm K, Fastner C, Tülümen E, Zhou X, Hoffmann U, Borggrefe M, Akin I. Incidence and prognostic relevance of cardiopulmonary failure in takotsubo cardiomyopathy. Sci Rep 2017; 7: 14673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hohneck A, El‐Battrawy I, Lang S, Ansari U, Schramm K, Zhou X, Borggrefe M, Akin I. Protective effect of acquired long QT syndrome in Takotsubo syndrome. Intern Med J 2019; 49: 770–776. [DOI] [PubMed] [Google Scholar]
- 60. Bill V, El‐Battrawy I, Schramm K, Ansari U, Hoffmann U, Haghi D, Kuschyk J, Borggrefe M, Akin I. Coincidental coronary artery disease impairs outcome in patients with takotsubo cardiomyopathy. Qjm 2017; 110: 483–488. [DOI] [PubMed] [Google Scholar]
- 61. Auzel O, Mustafic H, Pillière R, El Mahmoud R, Dubourg O, Mansencal N. Incidence, characteristics, risk factors, and outcomes of Takotsubo cardiomyopathy with and without ventricular arrhythmia. Am J Cardiol 2016; 117: 1242–1247. [DOI] [PubMed] [Google Scholar]
- 62. Kim H, Senecal C, Lewis B, Prasad A, Rajiv G, Lerman LO, Lerman A. Natural history and predictors of mortality of patients with Takotsubo syndrome. Int J Cardiol 2018; 267: 22–27. [DOI] [PubMed] [Google Scholar]
- 63. Pelliccia F, Pasceri V, Patti G, Tanzilli G, Speciale G, Gaudio C, Camici PG. Long‐term prognosis and outcome predictors in Takotsubo syndrome: a systematic review and meta‐regression study. JACC Heart failure 2019; 7: 143–154. [DOI] [PubMed] [Google Scholar]
- 64. Citro R, Rigo F, Previtali M, Ciampi Q, Canterin FA, Provenza G, Giudice R, Patella MM, Vriz O, Mehta R, Baldi C, Mehta RH, Bossone E. Differences in clinical features and in‐hospital outcomes of older adults with tako‐tsubo cardiomyopathy. J Am Geriatr Soc 2012; 60: 93–98. [DOI] [PubMed] [Google Scholar]
- 65. Citro R, Rigo F, D'Andrea A, Ciampi Q, Parodi G, Provenza G, Piccolo R, Mirra M, Zito C, Giudice R, Patella MM, Antonini‐Canterin F, Bossone E, Piscione F, Salerno‐Uriarte J. Echocardiographic correlates of acute heart failure, cardiogenic shock, and in‐hospital mortality in tako‐tsubo cardiomyopathy. JACC Cardiovasc Imaging 2014; 7: 119–129. [DOI] [PubMed] [Google Scholar]
- 66. Khera R, Light‐McGroary K, Zahr F, Horwitz PA, Girotra S. Trends in hospitalization for takotsubo cardiomyopathy in the United States. Am Heart J 2016; 172: 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Nazir S, Ahuja KR, Soni RG, Raheja H, Saleem S, Hsiung I, Patel NJ, Eltahawy EA, Madias JE. Age‐related variations in takotsubo syndrome in the United States. Am J Cardiol 2020; 133: 168–170. [DOI] [PubMed] [Google Scholar]
- 68. Jesel L, Berthon C, Messas N, Lim HS, Girardey M, Marzak H, Marchandot B, Trinh A, Ohlmann P, Morel O. Atrial arrhythmias in Takotsubo cardiomyopathy: incidence, predictive factors, and prognosis. Europace 2019; 21: 298–305. [DOI] [PubMed] [Google Scholar]
- 69. Ghadri JR, Cammann VL, Napp LC, Jurisic S, Diekmann J, Bataiosu DR, Seifert B, Jaguszewski M, Sarcon A, Neumann CA, Geyer V, Prasad A, Bax JJ, Ruschitzka F, Lüscher TF, Templin C. Differences in the clinical profile and outcomes of typical and atypical takotsubo syndrome: data from the International Takotsubo Registry. JAMA Cardiol 2016; 1: 335–340. [DOI] [PubMed] [Google Scholar]
- 70. Nayeri A, Yuen A, Huang C, Cardoza K, Shamsa K, Ziaeian B, Wells QS, Fonarow G, Horwich T. Prognostic implications of pre‐existing medical comorbidity in Takotsubo cardiomyopathy. Heart Vessels 2020; 36: 492–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Girardey M, Jesel L, Campia U, Messas N, Hess S, Imperiale A, Blondet C, Trinh A, Ohlmann P, Morel O. Impact of malignancies in the early and late time course of Takotsubo cardiomyopathy. Circ J 2016; 80: 2192–2198. [DOI] [PubMed] [Google Scholar]
- 72. Citro R, Radano I, Parodi G, Di Vece D, Zito C, Novo G, Provenza G, Bellino M, Prota C, Silverio A, Antonini‐Canterin F, Rigo F, Vriz O, Galasso G, Bossone E, Salerno‐Uriarte J, Piscione F. Long‐term outcome in patients with Takotsubo syndrome presenting with severely reduced left ventricular ejection fraction. Eur J Heart Fail 2019; 21: 781–789. [DOI] [PubMed] [Google Scholar]
- 73. Nishida J, Kouzu H, Hashimoto A, Fujito T, Kawamukai M, Mochizuki A, Muranaka A, Kokubu N, Shimoshige S, Yuda S, Hase M, Tsuchihashi K, Miura T. “Ballooning” patterns in takotsubo cardiomyopathy reflect different clinical backgrounds and outcomes: a BOREAS‐TCM study. Heart Vessels 2015; 30: 789–797. [DOI] [PubMed] [Google Scholar]
- 74. Parodi G, Scudiero F, Citro R, Silverio A, Bellandi B, Zito C, Antonini‐Canterin F, Rigo F, Zocchi C, Bossone E, Salerno‐Uriarte J, Piscione F, Di Mario C, Armentano C, Astarita C, Coppola A, Ravera A, Prota C, Bottiglieri P, Bovelli D, Patella MM, Costantino MF, Gregorio G, Santoro M, Manganelli F, Rotondi F, Del Pace S, Pascotto M, Grolla E, Tagliamonte E, Bianchi A, Marinosci G, Pappalettera M, Pozzi A, Nardi F, Novo G, Bovenzi F. Risk stratification using the CHA2DS2‐VASc score in Takotsubo syndrome: data from the Takotsubo Italian network. J Am Heart Assoc 2017; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. El‐Battrawy I, Santoro F, Stiermaier T, Möller C, Guastafierro F, Novo G, Novo S, Santangelo A, Mariano E, Romeo F, Romeo F, Thiele H, Guerra F, Capucci A, Giannini I, Caldarola P, Brunetti ND, Eitel I, Akin I. Prevalence, management, and outcome of adverse rhythm disorders in takotsubo syndrome: insights from the international multicenter GEIST registry. Heart Fail Rev 2020; 25: 505–511. [DOI] [PubMed] [Google Scholar]
- 76. Lachmet‐Thébaud L, Marchandot B, Matsushita K, Dagrenat C, Peillex M, Sato C, Trimaille A, Reydel A, Trinh A, Ohlmann P, Jesel L, Morel O. Systemic inflammatory response syndrome is a major determinant of cardiovascular outcome in Takotsubo syndrome. Circ J 2020; 84: 592–600. [DOI] [PubMed] [Google Scholar]
- 77. Núñez‐Gil IJ, Vedia O, Almendro‐Delia M, Raposeiras‐Roubín S, Sionis A, Martin‐García AC, Martin‐García A, Andrés M, Blanco E, Martín‐de‐Miguel I, Uribarri A, Corbí‐Pascual M, Feltes G, Bosch X, Fabregat‐Andres O, López‐Pais J, Sánchez‐Grande‐Flecha A, Guillen‐Marzo M. Takotsubo syndrome and cancer, clinical and prognostic implications, insights of RETAKO. Med Clin (Barc) 2020; 155: 521–528. [DOI] [PubMed] [Google Scholar]
- 78. Scudiero F, Arcari L, Cacciotti L, De Vito E, Marcucci R, Passaseo I, Limite LR, Musumeci MB, Autore C, Citro R, Bossone E, Sanna GD, Bacchi B, Volpe M, Di Mario C, Parodi G. Prognostic relevance of GRACE risk score in Takotsubo syndrome. Eur Heart J Acute Cardiovasc Care 2020; 9: 721–728. [DOI] [PubMed] [Google Scholar]
- 79. Stiermaier T, Möller C, Graf T, Eitel C, Desch S, Thiele H, Eitel I. Prognostic usefulness of the ballooning pattern in patients with Takotsubo cardiomyopathy. Am J Cardiol 2016; 118: 1737–1741. [DOI] [PubMed] [Google Scholar]
- 80. Nayeri A, Rafla‐Yuan E, Farber‐Eger E, Blair M, Ziaeian B, Cadeiras M, McPherson JA, Wells QS. Pre‐existing psychiatric illness is associated with increased risk of recurrent Takotsubo cardiomyopathy. Psychosomatics 2017; 58: 527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Dastidar AG, Baritussio A, De Garate E, Drobni Z, Biglino G, Singhal P, Milano EG, Angelini GD, Dorman S, Strange J, Johnson T, Bucciarelli‐Ducci C. Prognostic role of CMR and conventional risk factors in myocardial infarction with nonobstructed coronary arteries. J Am Coll Cardiol Img 2019; 12: 1973–1982. [DOI] [PubMed] [Google Scholar]
- 82. Zaghlol R, Dey AK, Desale S, Barac A. Racial differences in takotsubo cardiomyopathy outcomes in a large nationwide sample. ESC Heart Fail 2020; 7: 1056–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Dias A, Franco E, Ross T, Hebert K. Role of race in Takotsubo syndrome presentation: short‐ and long‐term outcomes. JACC Heart failure 2019; 7: 444–445. [DOI] [PubMed] [Google Scholar]
- 84. Dias A, Franco E, Koshkelashvili N, Bhalla V, Pressman GS, Hebert K, Figueredo VM. Antiplatelet therapy in Takotsubo cardiomyopathy: does it improve cardiovascular outcomes during index event? Heart Vessels 2016; 31: 1285–1290. [DOI] [PubMed] [Google Scholar]
- 85. Kato K, Kitahara H, Saito Y, Fujimoto Y, Sakai Y, Ishibashi I, Himi T, Kobayashi Y. Impact of myocardial bridging on in‐hospital outcome in patients with takotsubo syndrome. J Cardiol 2017; 70: 615–619. [DOI] [PubMed] [Google Scholar]
- 86. Stiermaier T, Busch K, Lange T, Pätz T, Meusel M, Backhaus SJ, Frydrychowicz A, Barkhausen J, Gutberlet M, Thiele H, Schuster A, Eitel I. Prognostic value of different CMR‐based techniques to assess left ventricular myocardial strain in Takotsubo syndrome. J Clin Med 2020; 9: 3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kim HY, Doh JH, Jang SY, Kim EK, Hahn JY, Kim DK. Gender differences in clinical profiles of stress‐induced cardiomyopathy. J Cardiovasc Ultrasound 2017; 25: 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kato K, Sakai Y, Ishibashi I, Himi T, Fujimoto Y, Kobayashi Y. Predictors of in‐hospital cardiac complications in patients with Takotsubo syndrome. Heart Vessels 2018; 33: 1214–1219. [DOI] [PubMed] [Google Scholar]
- 89. El‐Battrawy I, Lang S, Ansari U, Behnes M, Hillenbrand D, Schramm K, Fastner C, Zhou X, Bill V, Hoffmann U, Papavassiliu T, Elmas E, Haghi D, Borggrefe M, Akin I. Impact of concomitant atrial fibrillation on the prognosis of Takotsubo cardiomyopathy. Europace 2017; 19: 1288–1292. [DOI] [PubMed] [Google Scholar]
- 90. Ghadri JR, Sarcon A, Diekmann J, Bataiosu DR, Cammann VL, Jurisic S, Napp LC, Jaguszewski M, Scherff F, Brugger P, Jäncke L, Seifert B, Bax JJ, Ruschitzka F, Lüscher TF, Templin C. Happy heart syndrome: role of positive emotional stress in takotsubo syndrome. Eur Heart J 2016; 37: 2823–2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Pelliccia F, Parodi G, Greco C, Antoniucci D, Brenner R, Bossone E, Cacciotti L, Capucci A, Citro R, Delmas C, Guerra F, Ionescu CN, Lairez O, Larrauri‐Reyes M, Lee PH, Mansencal N, Marazzi G, Mihos CG, Morel O, Nef HM, Nunez Gil IJ, Passaseo I, Pineda AM, Rosano G, Santana O, Schneck F, Song BG, Song J‐K, Teh AW, Ungprasert P, Valbusa A, Wahl A, Yoshida T, Gaudio C, Kaski JC. Comorbidities frequency in Takotsubo syndrome: an international collaborative systematic review including 1109 patients. Am J Med 2015; 128: 654.e11–654.e19. [DOI] [PubMed] [Google Scholar]
- 92. Falola M, Fonbah W, McGwin G. Takotsubo cardiomyopathy versus ST‐elevation myocardial infarction in a large case‐control study: proposing a new mechanism. Int J Cardiol 2013; 167: 1079–1081. [DOI] [PubMed] [Google Scholar]
- 93. Madias JE. Low prevalence of diabetes mellitus in patients with Takotsubo syndrome: a plausible 'protective' effect with pathophysiologic connotations. Eur Heart J Acute Cardiovasc Care 2016; 5: 164–170. [DOI] [PubMed] [Google Scholar]
- 94. Bill V, El‐Battrawy I, Behnes M, Baumann S, Becher T, Elmas E, Hoffmann U, Haghi D, Fastner C, Kuschyk J, Papavassiliu T, Borggrefe M, Akin I. “Diabetes paradox” in Takotsubo cardiomyopathy. Int J Cardiol 2016; 224: 88–89. [DOI] [PubMed] [Google Scholar]
- 95. Bansal N, Zelnick L, Bhat Z, Dobre M, He J, Lash J, Jaar B, Mehta R, Raj D, Rincon‐Choles H, Saunders M, Schrauben S, Weir M, Wright J, Go AS. Burden and outcomes of heart failure hospitalizations in adults with chronic kidney disease. J Am Coll Cardiol 2019; 73: 2691–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Smilowitz NR, Gupta N, Guo Y, Mauricio R, Bangalore S. Management and outcomes of acute myocardial infarction in patients with chronic kidney disease. Int J Cardiol 2017; 227: 1–7. [DOI] [PubMed] [Google Scholar]
- 97. Bento D, Azevedo O, Santos R, Almeida A, Domingues K, Marmelo B, Reis L, Ruivo C, Guerreiro R, Lima R, Faria R, Marreiros A, Marques N. Short‐ and medium‐term prognosis of Takotsubo syndrome in a Portuguese population. Rev Port Cardiol 2019; 38: 349–357. [DOI] [PubMed] [Google Scholar]
- 98. Yassin AS, Adegbala O, Subahi A, Abubakar H, Akintoye E, Abdelrahamn M, Ahmed A, Agarwal A, Shokr M, Pahuja M, Elder M, Kaki A, Schreiber T, Mohamad T. Clinical impact of advanced chronic kidney disease on outcomes and in‐hospital complications of Takotsubo syndrome (broken‐heart‐syndrome): propensity‐matched national study. Int J Cardiol 2019; 277: 16–19. [DOI] [PubMed] [Google Scholar]
- 99. El‐Battrawy I, Lang S, Ansari U, Tülümen E, Schramm K, Fastner C, Zhou X, Hoffmann U, Borggrefe M, Akin I. Prevalence of malignant arrhythmia and sudden cardiac death in takotsubo syndrome and its management. Europace 2018; 20: 843–850. [DOI] [PubMed] [Google Scholar]
- 100. Santoro F, Ferraretti A, Ieva R, Musaico F, Fanelli M, Tarantino N, Scarcia M, Caldarola P, Di Biase M, Brunetti ND. Renal impairment and outcome in patients with takotsubo cardiomyopathy. Am J Emerg Med 2016; 34: 548–552. [DOI] [PubMed] [Google Scholar]
- 101. Zalewska‐Adamiec M, Małyszko J, Bachórzewska‐Gajewska H, Tomaszuk‐Kazberuk A, Kożuch M, Kralisz P, Dobrzycki S. Takotsubo syndrome and chronic kidney disease: a deadly duet in long‐term follow‐up. Pol Arch Intern Med 2018; 128: 518–523. [DOI] [PubMed] [Google Scholar]
- 102. Zalewska‐Adamiec M, Kuzma L, Dobrzycki S, Bachorzewska‐Gajewska H. The GRACE Scale in the prognosis of patients with Takotsubo syndrome. J Interv Cardiol 2020; 2020: 4340930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Joy PS, Guddati AK, Shapira I. Outcomes of Takotsubo cardiomyopathy in hospitalized cancer patients. J Cancer Res Clin Oncol 2018; 144: 1539–1545. [DOI] [PubMed] [Google Scholar]
- 104. Singh SB, Harle IA. Takotsubo cardiomyopathy secondary in part to cancer‐related pain crisis: a case report. J Pain Symptom Manage 2014; 48: 137–142. [DOI] [PubMed] [Google Scholar]
- 105. Coli S, Pigazzani F, Gaibazzi N. Midventricular Takotsubo cardiomyopathy after oxaliplatin infusion: an unreported side effect. J Cardiovasc Med (Hagerstown) 2015; 16: 646–649. [DOI] [PubMed] [Google Scholar]
- 106. Y‐Hassan S. Clinical features and outcome of pheochromocytoma‐induced Takotsubo syndrome: analysis of 80 published cases. Am J Cardiol 2016; 117: 1836–1844. [DOI] [PubMed] [Google Scholar]
- 107. Guo S, Xie B, Tse G, Roever L, Xia Y, Li G, Wang Y, Liu T. Malignancy predicts outcome of Takotsubo syndrome: a systematic review and meta‐analysis. Heart Fail Rev 2020; 25: 513–522. [DOI] [PubMed] [Google Scholar]
- 108. Möller C, Stiermaier T, Graf T, Eitel C, Thiele H, Burgdorf C, Eitel I. Prevalence and long‐term prognostic impact of malignancy in patients with Takotsubo syndrome. Eur J Heart Fail 2018; 20: 816–818. [DOI] [PubMed] [Google Scholar]
- 109. Ding KJ, Cammann VL, Szawan KA, Stähli BE, Wischnewsky M, Di Vece D, Citro R, Jaguszewski M, Seifert B, Sarcon A, Knorr M, Heiner S, Gili S, D'Ascenzo F, Neuhaus M, Napp LC, Franke J, Noutsias M, Burgdorf C, Koenig W, Kherad B, Rajan L, Michels G, Pfister R, Cuneo A, Jacobshagen C, Karakas M, Pott A, Meyer P, Arroja JD, Banning A, Cuculi F, Kobza R, Fischer TA, Vasankari T, Airaksinen KEJ, Paolini C, Bilato C, Carrilho‐Ferreira P, Opolski G, Dworakowski R, MacCarthy P, Kaiser C, Osswald S, Galiuto L, Dichtl W, Chan C, Bridgman P, Delmas C, Lairez O, El‐Battrawy I, Akin I, Gilyarova E, Shilova A, Gilyarov M, Kozel M, Tousek P, Widimský P, Winchester DE, Galuszka J, Ukena C, Horowitz JD, Di Mario C, Prasad A, Rihal CS, Pinto FJ, Crea F, Borggrefe M, Braun‐Dullaeus RC, Rottbauer W, Bauersachs J, Katus HA, Hasenfuß G, Tschöpe C, Pieske BM, Thiele H, Schunkert H, Böhm M, Felix SB, Münzel T, Bax JJ, Lüscher TF, Ruschitzka F, Ghadri JR, Bossone E, Templin C. Intraventricular thrombus formation and embolism in Takotsubo syndrome: insights from the International Takotsubo Registry. Arterioscler Thromb Vasc Biol 2020; 40: 279–287. [DOI] [PubMed] [Google Scholar]
- 110. Zaghlol R, Kashyap K, Al‐Shbool G, Basyal B, Desale S, Campia U, Barac A. Usefulness of malignancy as a predictor of worse in‐hospital outcomes in patients with Takotsubo cardiomyopathy. Am J Cardiol 2019; 123: 995–1001. [DOI] [PubMed] [Google Scholar]
- 111. Desai R, Singh S, Baikpour M, Goyal H, Dhoble A, Deshmukh A, Kumar G, Sachdeva R. Does obesity affect the outcomes in takotsubo cardiomyopathy? Analysis of the Nationwide Inpatient Sample database, 2010‐2014. Clin Cardiol 2018; 41: 1028–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Li P, Lu X, Teng C, Cai P, Kranis M, Dai Q, Wang B. The impact of COPD on in‐hospital outcomes in patients with Takotsubo cardiomyopathy. Int J Chron Obstruct Pulmon Dis 2020; 15: 2333–2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Shiomura R, Nakamura S, Takano H, Kato K, Inui K, Kubota Y, Komiyama H, Murai K, Asai K, Shimizu W. Impact of brain natriuretic peptide, calcium channel blockers, and body mass index on recovery time from left ventricular systolic dysfunction in patients with Takotsubo cardiomyopathy. Am J Cardiol 2015; 116: 515–519. [DOI] [PubMed] [Google Scholar]
- 114. Zalewska‐Adamiec M, Malyszko J, Bachórzewska‐Gajewska H, Tomaszuk‐Kazberuk A, Dobrzycki SJ. Takotsubo syndrome—fatal prognosis of patients with low body mass index in 5‐year follow‐up. Arch Med Sci 2020; 16: 282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. El‐Sayed AM, Brinjikji W, Salka S. Demographic and co‐morbid predictors of stress (takotsubo) cardiomyopathy. Am J Cardiol 2012; 110: 1368–1372. [DOI] [PubMed] [Google Scholar]
- 116. Nayeri A, Rafla‐Yuan E, Krishnan S, Ziaeian B, Cadeiras M, McPherson JA, Wells QS. Psychiatric illness in Takotsubo (stress) cardiomyopathy: a review. Psychosomatics 2018; 59: 220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Corrigan FE, Kimmel MC, Jayaram G. Four cases of takotsubo cardiomyopathy linked with exacerbations of psychiatric illness. Innov Clin Neurosci 2011; 8: 50–53. [PMC free article] [PubMed] [Google Scholar]
- 118. Maldonado JR, Pajouhi P, Witteles R. Broken heart syndrome (Takotsubo cardiomyopathy) triggered by acute mania: a review and case report. Psychosomatics 2013; 54: 74–79. [DOI] [PubMed] [Google Scholar]
- 119. Christoph M, Ebner B, Stolte D, Ibrahim K, Kolschmann S, Strasser RH, Schön S. Broken heart syndrome: Tako Tsubo cardiomyopathy associated with an overdose of the serotonin‐norepinephrine reuptake inhibitor Venlafaxine. Eur Neuropsychopharmacol 2010; 20: 594–597. [DOI] [PubMed] [Google Scholar]
- 120. Naguy A, Al‐Mutairi H, Al‐Tajali A. Atomoxetine‐related Takotsubo cardiomyopathy. J Psychiatr Pract 2016; 22: 232–233. [DOI] [PubMed] [Google Scholar]
- 121. Wang F, Wen W. Sepsis‐induced Takotsubo syndrome in young premenopausal women: two case reports. Medicine 2018; 97: e13718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Patel N, Shenoy A, Dous G, Kamran H, El‐Sherif N. Sepsis‐induced Takotsubo cardiomyopathy leading to Torsades de Pointes. Case Rep Cardiol 2016; 2016: 2384752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Tornvall P, Collste O, Ehrenborg E, Järnbert‐Petterson H. A case‐control study of risk markers and mortality in Takotsubo stress cardiomyopathy. J Am Coll Cardiol 2016; 67: 1931–1936. [DOI] [PubMed] [Google Scholar]
- 124. Zalewska‐Adamiec M, Bachorzewska‐Gajewska H, Tomaszuk‐Kazberuk A, Nowak K, Drozdowski P, Bychowski J, Krynicki R, Musial WJ, Dobrzycki S. Takotsubo cardiomyopathy: serious early complications and two‐year mortality—a 101 case study. Neth Heart J 2016; 24: 511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Hertting K, Krause K, Härle T, Boczor S, Reimers J, Kuck K‐H. Transient left ventricular apical ballooning in a community hospital in Germany. Int J Cardiol 2006; 112: 282–288. [DOI] [PubMed] [Google Scholar]
- 126. Rajwani A, Adam Z, Hall JA. Bronchogenic stress cardiomyopathy: a case series. Cardiology 2015; 130: 106–111. [DOI] [PubMed] [Google Scholar]
- 127. Kato K, Cammann VL, Napp LC, Szawan KA, Micek J, Dreiding S, Levinson RA, Petkova V, Würdinger M, Patrascu A, Sumalinog R, Gili S, Clarenbach CF, Kohler M, Wischnewsky M, Citro R, Vecchione C, Bossone E, Neuhaus M, Franke J, Meder B, Jaguszewski M, Noutsias M, Knorr M, Heiner S, D'Ascenzo F, Dichtl W, Burgdorf C, Kherad B, Tschöpe C, Sarcon A, Shinbane J, Rajan L, Michels G, Pfister R, Cuneo A, Jacobshagen C, Karakas M, Koenig W, Pott A, Meyer P, Roffi M, Banning A, Wolfrum M, Cuculi F, Kobza R, Fischer TA, Vasankari T, Airaksinen KEJ, Budnik M, Dworakowski R, MacCarthy P, Kaiser C, Osswald S, Galiuto L, Chan C, Bridgman P, Beug D, Delmas C, Lairez O, Gilyarova E, Shilova A, Gilyarov M, El‐Battrawy I, Akin I, Kozel M, Tousek P, Winchester DE, Galuszka J, Ukena C, Poglajen G, Carrilho‐Ferreira P, Hauck C, Paolini C, Bilato C, Sano M, Ishibashi I, Takahara M, Himi T, Kobayashi Y, Prasad A, Rihal CS, Liu K, Schulze PC, Bianco M, Jörg L, Rickli H, Pestana G, Nguyen TH, Böhm M, Maier LS, Pinto FJ, Widimský P, Felix SB, Opolski G, Braun‐Dullaeus RC, Rottbauer W, Hasenfuß G, Pieske BM, Schunkert H, Borggrefe M, Thiele H, Bauersachs J, Katus HA, Horowitz JD, Di Mario C, Münzel T, Crea F, Bax JJ, Lüscher TF, Ruschitzka F, Ghadri JR, Templin C. Prognostic impact of acute pulmonary triggers in patients with takotsubo syndrome: new insights from the International Takotsubo Registry. ESC Heart Fail 2021; 8: 1924–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Li P, Lu X, Teng C, Hadley M, Cai P, Dai Q, Wang B. The association between hyperlipidemia and in‐hospital outcomes in Takotsubo cardiomyopathy. Diabetes Metab Syndr Obes 2021; 14: 117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Lu X, Li P, Teng C, Cai P, Wang B. Anemia is associated with poor clinical outcomes in hospitalized patients with Takotsubo cardiomyopathy. Angiology 2021: 3319721999492. [DOI] [PubMed] [Google Scholar]
- 130. Yassin AS, Subahi A, Adegbala O, Abubakar H, Dawdy J, Mishra T, Akintoye E, Shereef H, Ghandour M, Alhusain R, Shokr M, Oviedo C, Afonso L. Clinical impact of atrial fibrillation on short‐term outcomes and in‐hospital mortality in patients with Takotsubo syndrome: a propensity‐matched national study. Cardiovasc Revasc Med 2020; 21: 522–526. [DOI] [PubMed] [Google Scholar]
- 131. Gaede L, Herchenbach A, Tröbs M, Marwan M, Achenbach S. Left ventricular contraction patterns in Takotsubo syndrome and their correlation with long‐term clinical outcome. Int J Cardiol Heart Vasc 2021; 32: 100708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Yasue H, Mizuno Y, Harada E. Coronary artery spasm—clinical features, pathogenesis and treatment. Proc Jpn Acad Ser B Phys Biol Sci 2019; 95: 53–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Gopalakrishnan M, Hassan A, Villines D, Nasr S, Chandrasekaran M, Klein LW. Predictors of short‐ and long‐term outcomes of Takotsubo cardiomyopathy. Am J Cardiol 2015; 116: 1586–1590. [DOI] [PubMed] [Google Scholar]
- 134. Singh K, Carson K, Usmani Z, Sawhney G, Shah R, Horowitz J. Systematic review and meta‐analysis of incidence and correlates of recurrence of takotsubo cardiomyopathy. Int J Cardiol 2014; 174: 696–701. [DOI] [PubMed] [Google Scholar]
- 135. Kyuma M, Tsuchihashi K, Shinshi Y, Hase M, Nakata T, Ooiwa H, Abiru M, Hikita N, Adachi T, Shoji T, Fujise Y, Shimamoto K. Effect of intravenous propranolol on left ventricular apical ballooning without coronary artery stenosis (ampulla cardiomyopathy): three cases. Circulation journal 2002; 66: 1181–1184. [DOI] [PubMed] [Google Scholar]
- 136. Isogai T, Matsui H, Tanaka H, Fushimi K, Yasunaga H. Early β‐blocker use and in‐hospital mortality in patients with Takotsubo cardiomyopathy. Heart 2016; 102: 1029–1035. [DOI] [PubMed] [Google Scholar]
- 137. Parodi G, Bellandi B, Del Pace S, Barchielli A, Zampini L, Velluzzi S, Carrabba N, Gensini GF, Antoniucci D. Natural history of tako‐tsubo cardiomyopathy. Chest 2011; 139: 887–892. [DOI] [PubMed] [Google Scholar]
- 138. Freedman SB. Calcium antagonist drugs in the treatment of coronary spasm, effort angina and hypertension. J Mol Cell Cardiol 1987; 19: 99–108. [DOI] [PubMed] [Google Scholar]
- 139. Brovkovych V, Kalinowski L, Muller‐Peddinghaus R, Malinski T. Synergistic antihypertensive effects of Nifedipine on endothelium: concurrent release of NO and scavenging of superoxide. Hypertension (Dallas, Tex : 1979) 2001; 37: 34–39. [DOI] [PubMed] [Google Scholar]
- 140. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U, Mihaylova B, Pedersen TR, Riccardi G, Richter DJ, Sabatine MS, Taskinen M‐R, Tokgozoglu L, Wiklund O. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020; 41: 111–188. [DOI] [PubMed] [Google Scholar]
- 141. Oesterle A, Laufs U, Liao JK. Pleiotropic effects of statins on the cardiovascular system. Circ Res 2017; 120: 229–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Istvan ES, Deisenhofer J. Structural mechanism for statin inhibition of HMG‐CoA reductase. Science (New York, NY) 2001; 292: 1160–1164. [DOI] [PubMed] [Google Scholar]
- 143. Förstermann U, Li H. Therapeutic effect of enhancing endothelial nitric oxide synthase (eNOS) expression and preventing eNOS uncoupling. Br J Pharmacol 2011; 164: 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Oduncu V, Tanalp AC, Erkol A, Sırma D, Dündar C, Akgün T, Türkyilmaz E, Kılıçgedik A, Gözübüyük G, Tigen K, Izgi A, Kirma C. Impact of chronic pre‐treatment of statins on the level of systemic inflammation and myocardial perfusion in patients undergoing primary angioplasty. Am J Cardiol 2011; 107: 179–185. [DOI] [PubMed] [Google Scholar]
- 145. Santoro F, Ieva R, Musaico F, Ferraretti A, Triggiani G, Tarantino N, Di Biase M, Brunetti ND. Lack of efficacy of drug therapy in preventing takotsubo cardiomyopathy recurrence: a meta‐analysis. Clin Cardiol 2014; 37: 434–439. [DOI] [PMC free article] [PubMed] [Google Scholar]


