Abstract
Aims
Right bundle branch block (RBBB) after heart transplantation (HTX) is a common finding, but its impact on post‐transplant survival remains uncertain. This study investigated the post‐transplant outcomes of patients with complete RBBB (cRBBB) ≤ 30 days after HTX.
Methods
This registry study analysed 639 patients receiving HTX at Heidelberg Heart Center between 1989 and 2019. Patients were stratified by diagnosis of cRBBB ≤ 30 days after HTX. Analysis included recipient and donor data, medication, echocardiographic features, graft rejections, atrial fibrillation, heart rates, permanent pacemaker implantation and mortality after HTX including causes of death.
Results
One hundred thirty‐nine patients showed cRBBB ≤ 30 days after HTX (21.8%), 20 patients with pre‐existing cRBBB in the donor heart (3.2%) and 119 patients with newly acquired cRBBB (18.6%). Patients with newly acquired cRBBB had a worse 1‐year post‐transplant survival (36.1%, P < 0.01) compared with patients with pre‐existing cRBBB (85.0%) or without cRBBB (86.4%), along with a higher percentage of death due to graft failure (P < 0.01). Multivariate analysis indicated cRBBB ≤ 30 days after HTX as significant risk factor for 1‐year mortality after HTX (HR: 2.20; 95% CI: 1.68–2.87; P < 0.01). Secondary outcomes showed a higher rate of an enlarged right atrium (P = 0.01), enlarged right ventricle (P < 0.01), reduced right ventricular function (P < 0.01), 30‐day atrial fibrillation (P < 0.01) and 1‐year permanent pacemaker implantation (P = 0.02) in patients with cRBBB after HTX.
Conclusions
Newly acquired cRBBB early after HTX is associated with increased post‐transplant mortality.
Keywords: Heart transplantation, Mortality, Right bundle branch block, Right heart strain, Survival
Introduction
Right bundle branch block (RBBB) is one of the most common electrocardiographic abnormalities in patients after heart transplantation (HTX). 1 , 2 , 3 , 4 , 5 , 6 Previous reported frequencies range from 19.6% to 69.4% depending on the definition of RBBB and the observed time interval after HTX. 7 , 8 On average, about half of all patients after HTX show incomplete (iRBBB) or complete RBBB (cRBBB) over time. 9 , 10 , 11 , 12 , 13
The cause of RBBB after HTX is still subject to controversy, but numerous reasons have been suggested including the posterior rotation of the implanted donor heart, surgical technique (biatrial or bicaval), prolonged ischaemic time, graft rejection, valvular regurgitation and right ventricular dysfunction. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 Moreover, chronic obstructive pulmonary disease (COPD) and increased pulmonary vascular resistance (PVR) that have been linked to right heart strain and increased mortality after HTX may provoke the manifestation of RBBB after HTX. 15 , 16
Results about the prognostic effect of RBBB after HTX are conflicting as several studies found an increased post‐transplant mortality in patients with RBBB after HTX, 3 , 4 , 9 , 11 whereas others could not confirm such effect. 1 , 5 , 6 , 10 , 13 , 14 Differences in sample size and study design may have contributed to inconsistencies because studies varied in definition of RBBB (iRBBB or cRBBB), length of follow‐up (30‐day, 1‐year or 5‐year) and analysed outcomes (mortality, graft rejection, surgical parameters or echocardiographic features). 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14
Time until diagnosis and duration of RBBB may also play a key role as the presence of cRBBB in the early stage after HTX may have another impact on post‐transplant mortality as the development of iRBBB several years after HTX. In addition, there might be an important difference between patients with newly acquired cRBBB after HTX and patients with cRBBB after HTX, which was already present in the donor heart before HTX. However, these questions have yet not been sufficiently answered in the literature. We therefore sought to investigate the post‐transplant outcomes of patients with cRBBB ≤ 30 days after HTX focusing on mortality with causes of death, echocardiographic features, graft rejections, bradycardia, permanent pacemaker (PPM) implantation and atrial fibrillation (AF) after HTX.
Patients and methods
Patients
We performed this study in accordance with the ethical standards of the Declaration of Helsinki. Approval was granted by the institutional review board (IRB) of Heidelberg University (ethical approval number: S‐286/2015, Version 1.2, 28‐07‐2020). Written informed consent was obtained from patients for inclusion in the Heidelberg HTX Registry allowing the clinical and scientific use of data. According to the ethical approval, no additional written informed consent was required for this observational study as only routine clinical data were analysed. 15 , 16 , 17 , 18 , 19
This study contained all adult patients (≥18 years) receiving HTX at Heidelberg Heart Center, Heidelberg, Germany, between 1989 and 2019. Patients with a second HTX were excluded. Donor hearts were routinely transported in an ice box (cold ischaemic storage), and Bretschneider solution was used as cardioplegic solution for myocardial protection. We initially stratified patients by diagnosis of cRBBB ≤ 30 days after HTX: patients with cRBBB ≤ 30 days after HTX (‘cRBBB group’) and patients without cRBBB ≤ 30 days after HTX (‘no cRBBB group’). Patients with cRBBB ≤ 30 days after HTX were further subdivided into patients with newly acquired cRBBB ≤ 30 days after HTX and patients with cRBBB ≤ 30 days after HTX, which was already present in the donor heart before HTX. Definition of cRBBB was based upon the following characteristic electrocardiographic (ECG) findings: (1) QRS duration ≥ 120 ms, (2) RSR' in leads V1 and/or V2, (3) S wave duration > 40 ms or greater than R wave duration in leads I and V6, (4) R wave peak time normal in leads V5 and V6 and (5) R wave peak time > 50 ms in lead V1. 20
Follow‐up
Follow‐up after HTX was performed in accordance with the standard of care at Heidelberg Heart Center. During the initial hospital stay, patients were continuously supervised by telemetry monitoring, and 12‐lead ECG was performed on a regular basis. Before discharge, 24‐h Holter was routinely performed. Diagnosis of cRBBB ≤ 30 days after HTX was based upon all available records pertaining to heart rhythm in the early post‐transplant period. 15 , 16 , 17 , 18 , 19
After discharge, patients were seen monthly during the first 6 months after HTX, then bimonthly until the end of the first year and thereafter usually three to four times per year (or if clinically indicated) at our HTX outpatient clinic. Routine follow‐up included medical history, physical examination, 12‐lead ECG, echocardiography, endomyocardial biopsy and blood tests including immunosuppressive drug monitoring. 15 , 16 , 17 , 18 , 19 , 21
Post‐transplant medication
Post‐transplant medication including immunosuppressive drug therapy was administered in accordance to centre standard. Patients initially received an anti‐thymocyte globulin‐based immunosuppression induction therapy after HTX. Cyclosporine A and azathioprine were used as the initial immunosuppressive drug therapy at the beginning of the study period. From 2001 onwards, azathioprine was subsequently substituted by mycophenolate mofetil, and cyclosporine A was consecutively replaced by tacrolimus from 2006 onwards. Steroids (prednisolone) were tapered incrementally during the first post‐transplant months and were finally discontinued 6 months after HTX (if clinically possible). 15 , 16 , 17 , 18 , 19
Statistical analysis
Data were analysed with SAS (Version 9.4, SAS Institute, Cary, NC, USA) and expressed as mean ± standard deviation (SD) or as count (n) with percentage (%). Difference of mean or hazard ratio (HR) with 95% confidence interval (CI) was used as measure of association. Student's t‐test/Mann–Whitney U test or analysis of variance (ANOVA)/Kruskal–Wallis test was used for continuous variables and chi‐squared‐test/Fisher's exact test for categorical variables, as appropriate. Kaplan–Meier estimator was employed to graphically display 1‐year post‐transplant survival. A P‐value of <0.05 was considered statistically significant. 15 , 16 , 17 , 18 , 19
Univariate analyses were performed to search for intergroup differences including recipient data, previous open‐heart surgery, principal diagnosis for HTX, donor data (including presence of cRBBB in the donor heart before HTX), transplant sex mismatch, preoperative data, perioperative data, post‐transplant medication, immunosuppressive drug therapy, post‐transplant echocardiographic features, graft rejections, post‐transplant AF, post‐transplant heart rates and PPM implantation after HTX. Causes of death within 1 year after HTX were grouped into the following categories: graft failure, acute rejection, infection/sepsis, malignancy and thromboembolic event/bleeding. Analysis of 1‐year mortality after HTX further included a multivariate analysis (Cox regression model) with the following eight clinically relevant parameters based on a predetermined model: cRBBB ≤ 30 days after HTX (in total), recipient age (decades), recipient preoperative PVR (Wood units), recipient COPD (in total), recipient severely reduced 30‐day right ventricular function (in total), recipient severely reduced 30‐day left ventricular function (in total), donor age (decades) and ischaemic time (hours). We did not include additional parameters in this multivariate analysis to avoid biased regression coefficients and to ensure a stable number of events (deceased patients) per analysed variable. In order to address clinically relevant questions with subgroup analyses, we decided to use the largest available number of patients for this study. Given the long study period of 30 years (1989–2019), we performed a sensitivity analysis to test the robustness of the study results and to investigate a possible era effect using a subgroup of patients with tacrolimus and mycophenolate mofetil as the immunosuppressive drug regimen was switched from 2006 onwards. 15 , 16 , 17 , 18 , 19
The primary outcome of this study was mortality after HTX. Secondary outcomes included post‐transplant echocardiographic features, graft rejections, post‐transplant AF, post‐transplant bradycardia and PPM implantation after HTX.
Results
Characteristics of RBBB after HTX
This study comprised a total of 639 patients including 139 patients with cRBBB ≤ 30 days after HTX (21.8%) and 500 patients without cRBBB ≤ 30 days after HTX (78.2%). Patients with cRBBB ≤ 30 days after HTX were further subdivided into 20 patients with pre‐existing cRBBB in the donor heart before HTX (3.2%) and 119 patients with newly acquired cRBBB after HTX (18.6%).
Mean duration of QRS interval in patients with cRBBB was 134.7 ± 12.5 ms, ranging from 120 to 181 ms. In patients with pre‐existing cRBBB in the donor heart before HTX, mean duration of QRS interval was 130.8 ± 8.8 ms, ranging from 120 to 149 ms. Patients with newly acquired cRBBB after HTX had a duration of QRS interval of 135.3 ± 13.0 ms, ranging from 120 to 181 ms.
Demographics and medication after HTX
Patients with cRBBB ≤ 30 days after HTX had a higher recipient age (53.8 ± 9.5 years vs. 51.6 ± 10.5 years; difference: 2.2 years, 95% CI: 0.4–4.0 years, P = 0.02), a higher percentage of recipient COPD (46 of 139 [33.1%] vs. 109 of 500 [21.8%]; difference: 11.3%, 95% CI: 2.7–19.9%; P = 0.01), a higher donor age (43.8 ± 13.2 years vs. 40.2 ± 13.4 years; difference: 3.6 years, 95% CI: 1.1–6.1 years, P = 0.01) and a longer ischaemic time (235.0 ± 70.7 min vs. 220.2 ± 67.4 min; difference: 14.8 min, 95% CI: 1.6–28.0 min, P = 0.03).
Analysis of preoperative right heart catheterization data showed a significantly higher right atrial pressure (11.8 ± 4.0 mmHg vs. 10.5 ± 4.0 mmHg; difference: 1.3 mmHg, 95% CI: 0.5–2.1 mmHg, P < 0.01), right ventricular pressure (15.7 ± 5.2 mmHg vs. 14.2 ± 4.9 mmHg; difference: 1.5 mmHg, 95% CI: 0.5–2.5 mmHg, P < 0.01), pulmonary arterial systolic pressure (45.9 ± 11.9 mmHg vs. 41.9 ± 12.4 mmHg; difference: 4.0 mmHg, 95% CI: 1.7–6.3 mmHg, P < 0.01), pulmonary arterial diastolic pressure (23.9 ± 7.4 mmHg vs. 21.3 ± 7.3 mmHg; difference: 2.6 mmHg, 95% CI: 1.2–4.0 mmHg, P < 0.01) or PVR (243.2 ± 116.2 mmHg vs. 198.1 ± 97.2 mmHg; difference: 45.1 mmHg, 95% CI: 23.8–66.4 mmHg, P < 0.01).
No statistically significant differences between both groups were found in regard to the remaining recipient data, previous open‐heart surgery, principal diagnosis for HTX, donor sex, donor body mass index, transplant sex mismatch, preoperative pulmonary artery pulsatility index or surgical HTX techniques (all P ≥ 0.05). Baseline characteristics are given in Table 1 .
Table 1.
Baseline characteristics
| Parameter | All (n = 639) | No cRBBB ≤ 30 days after HTX (n = 500) | cRBBB ≤ 30 days after HTX (n = 139) | Difference | 95% CI | P‐value |
|---|---|---|---|---|---|---|
| Recipient data | ||||||
| Age (years), mean ± SD | 52.1 ± 10.3 | 51.6 ± 10.5 | 53.8 ± 9.5 | 2.2 | 0.4–4.0 | 0.02* |
| Male sex, n (%) | 498 (77.9%) | 388 (77.6%) | 110 (79.1%) | 1.5% | −6.2%–9.2% | 0.70 |
| Body mass index (kg/m2), mean ± SD | 24.9 ± 4.0 | 24.9 ± 3.9 | 25.2 ± 4.3 | 0.3 | −0.5–1.1 | 0.45 |
| Arterial hypertension, n (%) | 350 (54.8%) | 269 (53.8%) | 81 (58.3%) | 4.5% | −4.8%–13.8% | 0.35 |
| Dyslipidaemia, n (%) | 406 (63.5%) | 323 (64.6%) | 83 (59.7%) | 4.9% | −4.3%–14.1% | 0.29 |
| Diabetes mellitus, n (%) | 215 (33.6%) | 169 (33.8%) | 46 (33.1%) | 0.7% | −8.2%–9.6% | 0.88 |
| COPD, n (%) | 155 (24.3%) | 109 (21.8%) | 46 (33.1%) | 11.3% | 2.7%–19.9% | 0.01* |
| Renal insufficiency a , n (%) | 368 (57.6%) | 287 (57.4%) | 81 (58.3%) | 0.9% | −8.4%–10.2% | 0.85 |
| eGFR (ml/min/1.73 m2), mean ± SD | 60.3 ± 21.7 | 60.7 ± 22.0 | 58.9 ± 20.5 | 1.8 | −2.1–5.7 | 0.37 |
| Previous open‐heart surgery | ||||||
| Overall open‐heart surgery, n (%) | 190 (29.7%) | 146 (29.2%) | 44 (31.7%) | 2.5% | −6.2%–11.2% | 0.58 |
| CABG surgery, n (%) | 78 (12.2%) | 59 (11.8%) | 19 (13.7%) | 1.9% | −4.5%–8.3% | 0.55 |
| Other surgery b , n (%) | 71 (11.1%) | 56 (11.2%) | 15 (10.8%) | 0.4% | −5.4%–6.2% | 0.89 |
| VAD surgery, n (%) | 55 (8.6%) | 40 (8.0%) | 15 (10.8%) | 2.8% | −2.9%–8.5% | 0.30 |
| Principal diagnosis for HTX | ||||||
| Ischaemic CMP, n (%) | 209 (32.7%) | 160 (32.0%) | 49 (35.2%) | 3.2% | −5.7%–12.1% | 0.47 |
| Non‐ischaemic CMP, n (%) | 339 (53.1%) | 269 (53.8%) | 70 (50.4%) | 3.4% | −6.0%–12.8% | 0.47 |
| Valvular heart disease, n (%) | 34 (5.3%) | 26 (5.2%) | 8 (5.8%) | 0.6% | −3.7%–4.9% | 0.80 |
| Cardiac amyloidosis, n (%) | 57 (8.9%) | 45 (9.0%) | 12 (8.6%) | 0.4% | −4.9%–5.7% | 0.89 |
| Donor data | ||||||
| Age (years), mean ± SD | 41.0 ± 13.4 | 40.2 ± 13.4 | 43.8 ± 13.2 | 3.6 | 1.1–6.1 | 0.01* |
| Male sex, n (%) | 278 (43.5%) | 221 (44.2%) | 57 (41.0%) | 3.2% | −6.1%–12.5% | 0.50 |
| Body mass index (kg/m2), mean ± SD | 24.8 ± 4.1 | 24.8 ± 4.1 | 24.8 ± 4.0 | 0.0 | −0.8–0.8 | 0.99 |
| Transplant sex mismatch | ||||||
| Mismatch, n (%) | 283 (44.3%) | 215 (43.0%) | 68 (48.9%) | 5.9% | −3.5%–15.3% | 0.21 |
| Donor (m) to recipient (f), n (%) | 31 (4.9%) | 24 (4.8%) | 7 (5.0%) | 0.2% | −3.9%–4.3% | 0.91 |
| Donor (f) to recipient (m), n (%) | 252 (39.4%) | 191 (38.2%) | 61 (43.9%) | 5.7% | −3.6%–15.0% | 0.23 |
| Preoperative data | ||||||
| RAP (mmHg), mean ± SD | 10.8 ± 4.1 | 10.5 ± 4.0 | 11.8 ± 4.0 | 1.3 | 0.5–2.1 | <0.01* |
| RVP (mmHg), mean ± SD | 14.5 ± 5.0 | 14.2 ± 4.9 | 15.7 ± 5.2 | 1.5 | 0.5–2.5 | <0.01* |
| PASP (mmHg), mean ± SD | 42.8 ± 12.4 | 41.9 ± 12.4 | 45.9 ± 11.9 | 4.0 | 1.7–6.3 | <0.01* |
| PADP (mmHg), mean ± SD | 21.9 ± 7.4 | 21.3 ± 7.3 | 23.9 ± 7.4 | 2.6 | 1.2–4.0 | <0.01* |
| PAPi (value), mean ± SD | 2.1 ± 0.9 | 2.1 ± 1.0 | 2.0 ± 0.8 | 0.1 | −0.1–0.3 | 0.16 |
| PVR (dyn·s·cm−5), mean ± SD | 207.9 ± 103.4 | 198.1 ± 97.2 | 243.2 ± 116.2 | 45.1 | 23.8–66.4 | <0.01* |
| Perioperative data | ||||||
| Ischaemic time (min), mean ± SD | 223.4 ± 68.4 | 220.2 ± 67.4 | 235.0 ± 70.7 | 14.8 | 1.6–28.0 | 0.03* |
| Biatrial HTX, n (%) | 164 (25.7%) | 131 (26.2%) | 33 (23.7%) | 2.5% | −5.5%–10.5% | 0.56 |
| Bicaval HTX, n (%) | 198 (31.0%) | 157 (31.4%) | 41 (29.5%) | 1.9% | −6.7%–10.5% | 0.67 |
| Total orthotopic HTX, n (%) | 277 (43.3%) | 212 (42.4%) | 65 (46.8%) | 4.4% | −5.0%–13.8% | 0.36 |
CABG, coronary artery bypass graft; CI, confidence interval; CMP, cardiomyopathy; COPD, chronic obstructive pulmonary disease; cRBBB, complete right bundle branch block; f, female; eGFR, estimated glomerular filtration rate; HTX, heart transplantation; m, male; n, number; PADP, pulmonary artery diastolic pressure; PAPi, pulmonary artery pulsatility index; PASP, pulmonary artery systolic pressure; PVR, pulmonary vascular resistance; RAP, right atrial pressure; RVP, right ventricular pressure; SD, standard deviation; VAD, ventricular assist device.
eGFR < 60 mL/min/1.73 m2.
Congenital, valvular or ventricular surgery.
Statistically significant at P < 0.05.
Analysis of the immunosuppressive drug therapy showed no statistically significant differences between both groups concerning the administration of cyclosporine A, tacrolimus, azathioprine or mycophenolate mofetil (all P ≥ 0.05). Patients with cRBBB ≤ 30 days after HTX had a lower percentage of angiotensin‐converting‐enzyme (ACE) inhibitors/angiotensin II receptor blockers (ARB) compared with patients without cRBBB ≤ 30 days after HTX (49 of 139 [35.3%] vs. 229 of 500 [45.8%]; difference: 10.5%, 95% CI: 1.5–19.5%; P = 0.03). There were no statistically significant differences between both groups in the administration of acetylsalicylic acid (ASA), beta blockers, ivabradine, calcium channel blockers or statins (all P ≥ 0.05). Medication after HTX is shown in Table 2 .
Table 2.
Medication after HTX
| Parameter | All (n = 639) | No cRBBB ≤ 30 days after HTX (n = 500) | cRBBB ≤ 30 days after HTX (n = 139) | Difference | 95% CI | P‐value |
|---|---|---|---|---|---|---|
| Immunosuppressive drug therapy | ||||||
| Cyclosporine A, n (%) | 347 (54.3%) | 273 (54.6%) | 74 (53.2%) | 1.4% | −8.0%–10.8% | 0.78 |
| Tacrolimus, n (%) | 292 (45.7%) | 227 (45.4%) | 65 (46.8%) | 1.4% | −8.0%–10.8% | 0.78 |
| Azathioprine, n (%) | 267 (41.8%) | 208 (41.6%) | 59 (42.4%) | 0.8% | −8.5%–10.1% | 0.86 |
| Mycophenolate mofetil, n (%) | 372 (58.2%) | 292 (58.4%) | 80 (57.6%) | 0.8% | −8.5%–10.1% | 0.86 |
| Steroids, n (%) | 639 (100.0%) | 500 (100.0%) | 139 (100.0%) | 0.0% | n.a. | n.a. |
| Concomitant medication | ||||||
| ASA, n (%) | 68 (10.6%) | 50 (10.0%) | 18 (12.9%) | 2.9% | −3.3%–9.1% | 0.32 |
| Beta blocker, n (%) | 114 (17.8%) | 93 (18.6%) | 21 (15.1%) | 3.5% | −3.4%–10.4% | 0.34 |
| Ivabradine, n (%) | 61 (9.5%) | 50 (10.0%) | 11 (7.9%) | 2.1% | −3.1%–7.3% | 0.46 |
| Calcium channel blocker, n (%) | 171 (26.8%) | 130 (26.0%) | 41 (29.5%) | 3.5% | −5.0%–12.0% | 0.41 |
| ACE inhibitor/ARB, n (%) | 278 (43.5%) | 229 (45.8%) | 49 (35.3%) | 10.5% | 1.5%–19.5% | 0.03* |
| Diuretic, n (%) | 639 (100.0%) | 500 (100.0%) | 139 (100.0%) | 0.0% | n.a. | n.a. |
| Statin, n (%) | 254 (39.7%) | 200 (40.0%) | 54 (38.8%) | 1.2% | −8.0%–10.4% | 0.81 |
| Gastric protection a , n (%) | 639 (100.0%) | 500 (100.0%) | 139 (100.0%) | 0.0% | n.a. | n.a. |
ACE inhibitor, angiotensin‐converting‐enzyme inhibitor; ARB, angiotensin II receptor blocker; ASA, acetylsalicylic acid; CI, confidence interval; cRBBB, complete right bundle branch block; HTX, heart transplantation; n, number; n.a., not applicable.
Gastric protection defined as proton pump inhibitor (PPI) or histamine receptor (H2) blocker.
Statistically significant at P < 0.05.
Primary outcome after HTX
In terms of the primary outcome of this study, patients with cRBBB ≤ 30 days after HTX had a significantly higher 30‐day (32.4% vs. 3.8%, difference: 28.6%, 95% CI: 20.6–36.6%, P < 0.01), intrahospital (43.9% vs. 7.2%, difference: 36.7%, 95% CI: 28.1–45.3%, P < 0.01), 31‐day‐to‐1‐year (24.4% vs. 9.8%, difference: 14.6%, 95% CI: 7.0–22.2%, P < 0.01), hospital discharge‐to‐1‐year (12.9% vs. 6.4%, difference: 6.5%, 95% CI: 0.5–12.5%, P = 0.01) and 1‐year all‐cause mortality after HTX (56.8% vs. 13.6%, difference: 43.2%, 95% CI: 34.4–52.0%, P < 0.01). Regarding the causes of death within 1 year after HTX, significantly more patients with cRBBB ≤ 30 days after HTX died from graft failure (32.4% vs. 2.0%, difference: 30.4%, 95% CI: 22.5–38.3%, P < 0.01) and infection/sepsis (21.6% vs. 9.0%, difference: 12.6%, 95% CI: 5.3–19.9%, P < 0.01). There were no statistically significant differences between groups concerning the causes of death within 1 year after HTX in terms of acute rejection, malignancy or thromboembolic event/bleeding. Causes of death within 1 year after HTX are provided in Table 3 .
Table 3.
Causes of death within 1 year after HTX
| Parameter | All (n = 639) | No cRBBB ≤ 30 days after HTX (n = 500) | cRBBB ≤ 30 days after HTX (n = 139) | Difference | 95% CI | P‐value |
|---|---|---|---|---|---|---|
| Graft failure, n (%) | 55 (8.6%) | 10 (2.0%) | 45 (32.4%) | 30.4% | 22.5%–38.3% | <0.01* |
| Acute rejection, n (%) | 4 (0.6%) | 3 (0.6%) | 1 (0.7%) | 0.1% | −1.5%–1.7% | 0.87 |
| Infection/sepsis, n (%) | 75 (11.7%) | 45 (9.0%) | 30 (21.6%) | 12.6% | 5.3%–19.9% | <0.01* |
| Malignancy, n (%) | 3 (0.5%) | 2 (0.4%) | 1 (0.7%) | 0.3% | −1.2%–1.8% | 0.63 |
| Thromboembolic event/bleeding, n (%) | 10 (1.6%) | 8 (1.6%) | 2 (1.4%) | 0.2% | −2.1 – 2.5% | 0.89 |
| All causes, n (%) | 147 (23.0%) | 68 (13.6%) | 79 (56.8%) | 43.2% | 34.4%–52.0% | <0.01* |
CI, confidence interval; cRBBB, complete right bundle branch block; HTX, heart transplantation; n, number.
Statistically significant at P < 0.05.
Multivariate analysis revealed a more than twofold increased risk for 1‐year mortality after HTX in patients with cRBBB ≤ 30 days after HTX (HR: 2.20, CI: 1.68–2.87; P < 0.01). Furthermore, recipient age (HR: 1.20, CI: 1.08–1.33; P < 0.01), recipient preoperative PVR (HR: 1.13, CI: 1.04–1.22; P < 0.01), recipient COPD (HR: 2.92, CI: 2.33–3.66; P < 0.01), recipient severely reduced 30‐day RV function (HR: 9.38, CI: 5.76–15.28; P < 0.01) and recipient severely reduced 30‐day LV function (HR: 5.96, CI: 3.08–11.54; P < 0.01) were significant risk factors in the multivariate analysis for 1‐year mortality after HTX. The other two included variables (donor age and ischaemic time) showed no statistically significant effect on post‐transplant 1‐year mortality. Multivariate analysis for 1‐year mortality after HTX is given in Table 4 .
Table 4.
Multivariate analysis for 1‐year mortality after HTX
| Variable | Hazard ratio | 95% CI | P‐value |
|---|---|---|---|
| Complete RBBB ≤ 30 days after HTX (in total) | 2.20 | 1.68–2.87 | <0.01* |
| Recipient age (decades) | 1.20 | 1.08–1.33 | <0.01* |
| Recipient preoperative PVR (Wood units) | 1.13 | 1.04–1.22 | <0.01* |
| Recipient COPD (in total) | 2.92 | 2.33–3.66 | <0.01* |
| Recipient severely reduced 30‐day RV function (in total) | 9.38 | 5.76–15.28 | <0.01* |
| Recipient severely reduced 30‐day LV function (in total) | 5.96 | 3.08–11.54 | <0.01* |
| Donor age (decades) | 1.02 | 0.94–1.10 | 0.70 |
| Ischaemic time (hours) | 1.00 | 0.90–1.10 | 0.94 |
ACE inhibitor, angiotensin‐converting‐enzyme inhibitor; ARB, angiotensin II receptor blocker; CI, confidence interval; COPD, chronic obstructive pulmonary disease; HTX, heart transplantation; LV, left ventricle; PVR, pulmonary vascular resistance; RBBB, right bundle branch block; RV, right ventricle.
Statistically significant at P < 0.05.
Patients with cRBBB ≤ 30 days after HTX additionally displayed a worse 1‐year survival after HTX in the Kaplan–Meier survival analysis (43.2% vs. 86.4%, P < 0.01). Further stratification of the cRBBB group showed a significantly lower 1‐year post‐transplant survival in patients with newly acquired cRBBB ≤ 30 days after HTX (36.1%) in comparison with patients with pre‐existing cRBBB in the donor heart (85.0%) or patients without cRBBB ≤ 30 days after HTX (86.4%, P < 0.01). Kaplan–Meier estimators are shown in Figures 1 and 2 .
Figure 1.
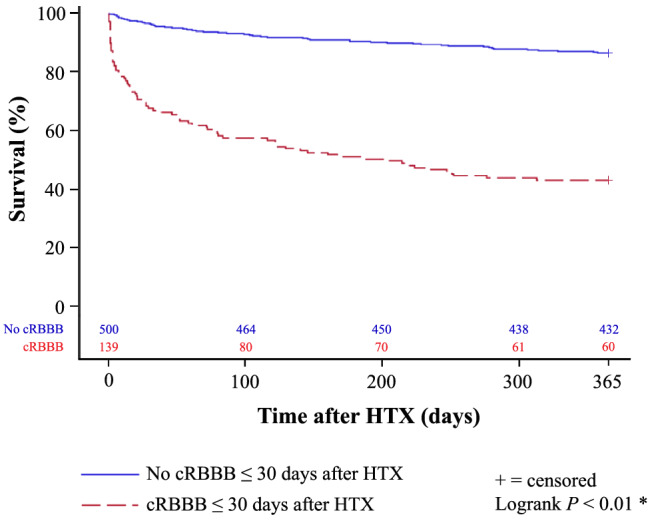
1‐year survival after HTX in patients with and without cRBBB ≤ 30 days after HTX (Kaplan–Meier estimator). Patients with cRBBB ≤ 30 days after HTX had a significantly worse 1‐year post‐transplant survival in the Kaplan–Meier survival analysis (43.2%) compared with patients without cRBBB ≤ 30 days after HTX (86.4%, P < 0.01). cRBBB, complete right bundle branch block; HTX, heart transplantation; *statistically significant (P < 0.05).
Figure 2.
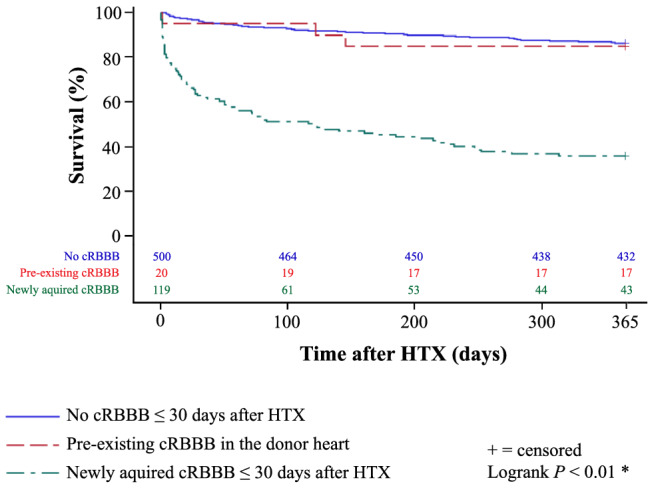
1‐year survival after HTX in patients with newly acquired cRBBB ≤ 30 days after HTX, with cRBBB ≤ 30 days after HTX which was already present in the donor heart before HTX, and without cRBBB ≤ 30 days after HTX (Kaplan–Meier estimator). Stratification of the cRBBB group showed a significantly lower 1‐year post‐transplant survival in patients with newly acquired cRBBB ≤ 30 days after HTX (36.1%) in comparison with patients with pre‐existing cRBBB in the donor heart (85.0%) or patients without cRBBB ≤ 30 days after HTX (86.4%, P < 0.01). cRBBB, complete right bundle branch block; HTX, heart transplantation; *statistically significant (P < 0.05).
Secondary outcomes after HTX
Assessment of echocardiographic features after HTX showed a lower percentage of normal sized right atrial (P = 0.01), right ventricular (P < 0.01) and left ventricular end‐diastolic diameter (P < 0.01) along with a higher rate of reduced right (P < 0.01) as well as left ventricular function (P < 0.01) in patients with cRBBB ≤ 30 days after HTX. Patients in the cRBBB group further had a higher rate of mitral and tricuspid regurgitation (both P < 0.01). Echocardiographic features after HTX are shown in Table 5.
Table 5.
Echocardiographic features after HTX
| Parameter | All (n = 639) | No cRBBB ≤ 30 days after HTX (n = 500) | cRBBB ≤ 30 days after HTX (n = 139) | Difference | 95% CI | P‐value |
|---|---|---|---|---|---|---|
| 30‐day end‐diastolic diameter | ||||||
| Normal RA (<35 mm), n (%) | 359 (56.2%) | 294 (58.8%) | 65 (46.8%) | 12.0% | 2.7%–21.3% | 0.01* |
| Normal RV (<30 mm), n (%) | 523 (81.8%) | 438 (87.6%) | 85 (61.2%) | 26.4% | 17.8%–35.0% | <0.01* |
| Normal LA (<40 mm), n (%) | 321 (50.2%) | 256 (51.2%) | 65 (46.8%) | 4.4% | −5.0%–13.8% | 0.35 |
| Normal LV (<55 mm), n (%) | 589 (92.2%) | 485 (97.0%) | 104 (74.8%) | 22.2% | 14.8%–29.6% | <0.01* |
| 30‐day RV function | ||||||
| Normal, n (%) | 515 (80.6%) | 441 (88.2%) | 74 (53.2%) | 35.0% | 26.2%–43.8% | <0.01* |
| Reduced, n (%) | 124 (19.4%) | 59 (11.8%) | 65 (46.8%) | 35.0% | 26.2%–43.8% | <0.01* |
| Mild, n (%) | 51 (8.0%) | 35 (7.0%) | 16 (11.5%) | 3.5% | ||
| Moderate, n (%) | 17 (2.7%) | 11 (2.2%) | 6 (4.3%) | 2.1% | ||
| Severe, n (%) | 56 (8.7%) | 13 (2.6%) | 43 (31.0%) | 28.4% | ||
| 30‐day LV function | ||||||
| Normal, n (%) | 579 (90.6%) | 477 (95.4%) | 102 (73.4%) | 22.0% | 14.4%–29.6% | 0.01* |
| Reduced, n (%) | 60 (9.4%) | 23 (4.6%) | 37 (26.6%) | 22.0% | 14.4%–29.6% | <0.01* |
| Mild, n (%) | 19 (3.0%) | 15 (3.0%) | 4 (2.9%) | 0.1% | ||
| Moderate, n (%) | 9 (1.4%) | 3 (0.6%) | 6 (4.3%) | 3.7% | ||
| Severe, n (%) | 32 (5.0%) | 5 (1.0%) | 27 (19.4%) | 18.4% | ||
| 30‐day tricuspid regurgitation | ||||||
| No, n (%) | 406 (63.5%) | 349 (69.8%) | 57 (41.0%) | 28.8% | 19.7%–37.9% | <0.01* |
| Yes, n (%) | 233 (36.5%) | 151 (30.2%) | 82 (59.0%) | 28.8% | 19.7%–37.9% | <0.01* |
| Mild, n (%) | 134 (21.0%) | 104 (20.8%) | 30 (21.6%) | 0.8% | ||
| Moderate, n (%) | 62 (9.7%) | 36 (7.2%) | 26 (18.7%) | 11.5% | ||
| Severe, n (%) | 37 (5.8%) | 11 (2.2%) | 26 (18.7%) | 16.5% | ||
| 30‐day mitral regurgitation | ||||||
| No, n (%) | 477 (74.6%) | 399 (79.8%) | 78 (56.1%) | 23.7% | 14.7%–32.7% | <0.01* |
| Yes, n (%) | 162 (25.4%) | 101 (20.2%) | 61 (43.9%) | 23.7% | 14.7%–32.7% | <0.01* |
| Mild, n (%) | 153 (23.9%) | 97 (19.4%) | 56 (40.3%) | 20.9% | ||
| Moderate, n (%) | 8 (1.3%) | 3 (0.6%) | 5 (3.6%) | 3.0% | ||
| Severe, n (%) | 1 (0.2%) | 1 (0.2%) | 0 (0.0%) | 0.2% |
CI, confidence interval; cRBBB, complete right bundle branch block; HTX, heart transplantation; LA, left atrium; LV, left ventricle; n, number; RA, right atrium; RV, right ventricle.
Statistically significant at P < 0.05.
Analysis of 30‐day graft rejection (P = 0.10), 30‐day bradycardia (P = 0.19) or 30‐day PPM implantation after HTX (P = 0.09) showed no statistically significant difference between groups. Patients with cRBBB ≤ 30 days after HTX had a higher rate of 30‐day post‐transplant AF (21.6% vs. 11.6%, P < 0.01) and a higher rate of 1‐year PPM implantation after HTX (4.3% vs. 1.2%, P = 0.02). Data regarding secondary outcomes after HTX are provided in Table 6 .
Table 6.
Secondary outcomes after HTX
| Parameter | All (n = 639) | No cRBBB ≤ 30 days after HTX (n = 500) | cRBBB ≤ 30 days after HTX (n = 139) | Difference | 95% CI | P‐value |
|---|---|---|---|---|---|---|
| 30‐day ≥ 1 graft rejection, n (%) | 118 (18.5%) | 99 (19.8%) | 19 (13.7%) | 6.1% | −0.6%–12.8% | 0.10 |
| 30‐day atrial fibrillation, n (%) | 88 (13.8%) | 58 (11.6%) | 30 (21.6%) | 10.0% | 2.6%–17.4% | < 0.01* |
| 30‐day bradycardia a , n (%) | 21 (3.3%) | 14 (2.8%) | 7 (5.0%) | 2.2% | −1.7%–6.1% | 0.19 |
| 30‐day PPM implantation, n (%) | 6 (0.9%) | 3 (0.6%) | 3 (2.2%) | 1.6% | −0.9%–4.1% | 0.09 |
| 1‐year PPM implantation, n (%) | 12 (1.9%) | 6 (1.2%) | 6 (4.3%) | 3.1% | 0.4%–6.6% | 0.02* |
CI, confidence interval; cRBBB, complete right bundle branch block; HTX, heart transplantation; n, number; PPM, permanent pacemaker.
Bradycardia defined as mean weekly heart rate < 60 beats per minute.
Statistically significant at P < 0.05.
Sensitivity analysis
A sensitivity analysis to test the robustness of the study results and to investigate a possible era effect was performed with a subgroup of patients receiving tacrolimus and mycophenolate mofetil (292 of 639 patients [45.7%]). This analysis showed comparable results regarding the primary outcome (mortality after HTX) and the secondary outcomes (post‐transplant echocardiographic features, graft rejections, post‐transplant AF, post‐transplant bradycardia and PPM implantation after HTX) supporting the robustness of the study results and minimizing the likelihood of an era effect.
Discussion
Frequency and significance of RBBB after HTX
As the prognostic effect of RBBB after HTX is still subject to an ongoing debate, we performed this large study with 639 HTX recipients to elucidate the frequency and significance of early cRBBB after HTX. A total of 139 patients showed cRBBB ≤ 30 days after HTX (21.8%). This is in line with former studies describing a similar rate of early cRBBB after HTX. 1 , 10 Jessen et al. 10 reported a cRBBB rate of 17.3% (14 of 81) immediately after HTX, and Ferretto et al. 1 stated a cRBBB rate of 23.8% (57 of 240) on the first day after HTX. These data indicate that HTX recipients have a substantially higher percentage of cRBBB in comparison with the general population. 22 , 23 In the Copenhagen City Heart Study with 18 441 participants, the prevalence of cRBBB varied between 1.0% and 10.0%, depending on sex and increasing with age. 23 This difference is of clinical significance as the presence of cRBBB has been associated with increased mortality in the general population, in patients with myocardial infarction and in patients with congestive heart failure. 23 , 24 , 25 , 26
Several pathophysiological factors may contribute to the development of cRBBB after HTX. Positioning of the donor heart often results in a clockwise rotation on the long axis of the transplanted heart that may cause stretched‐induced cRBBB after HTX as the fibres of the right bundle branch are more susceptible to damage than the fibres of the left bundle branch. 1 , 7 , 8 , 13 , 27 , 28 , 29 In addition, the use of biatrial or bicaval HTX technique has been discussed to have an impact on the rate of cRBBB after HTX. Ferretto and colleagues 1 described a significantly lower incidence of cRBBB after HTX in patients with bicaval HTX (23.8%) in comparison with a control population with biatrial HTX (40.7%, P < 0.01) although both HTX techniques do not directly cause damage to the subhisian conduction system. 1 However, in this study, we could not detect a significant difference between patients with biatrial, bicaval or even total orthotopic HTX in regard to cRBBB ≤ 30 days after HTX. Another perioperative factor is prolonged ischaemic time, which has been linked to cRBBB after HTX. 3 , 6 , 12 We also found a longer ischaemic time in patients with early cRBBB after HTX (235.0 ± 70.7 min vs. 220.2 ± 67.4 min, P = 0.03) implicating ischaemia‐induced damage as a possible reason for cRBBB after HTX.
In addition to surgical parameters, right ventricular strain could be a cause for cRBBB after HTX. Gao and colleagues 2 found in a group of 50 HTX recipients an association between higher right heart and pulmonary pressures and patients with cRBBB after HTX. Similar findings were reported by Sandhu and colleagues 13 who found a higher rate of right ventricular dysfunction in patients with cRBBB after HTX (61.9% vs. 24.0%, P < 0.01). Assessment of preoperative right heart catheterization data in our study showed a significantly higher right atrial pressure, right ventricular pressure, pulmonary arterial systolic pressure, pulmonary arterial diastolic pressure and PVR in patients with early cRBBB after HTX.
Besides newly acquired cRBBB after HTX due to peri‐ and post‐transplant causes, a certain degree of cRBBB might have already been present in the donor heart before HTX. 29 , 30 , 31 In a large study of the California Transplant Donor Network, Khush et al. 31 reported that 1.8% (18 of 980) of potential donor hearts and 1.6% (9 of 560) of used donor hearts had cRBBB before HTX, which is comparable with our study with 3.2% (20 of 639) of used donor hearts showing cRBBB before HTX. These findings indicate that cRBBB after HTX is mostly a result of peri‐ and post‐transplant events.
Mortality and causes of death after HTX
Data regarding the association between RBBB and increased mortality after HTX are inconclusive as several studies found an increased post‐transplant mortality in patients with RBBB after HTX, 3 , 4 , 9 , 11 whereas others found no significant difference in mortality after HTX. 1 , 5 , 6 , 10 , 13 , 14 These divergent findings may result from differences in study design, sample size, definition of RBBB, onset of RBBB after HTX and follow‐up period. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14
We therefore decided to perform this large study with 639 HTX recipients specifically looking at 1‐year mortality of patients with cRBBB ≤ 30 days after HTX. In our study, patients with cRBBB ≤ 30 days after HTX had a significantly increased 1‐year all‐cause mortality after HTX (P < 0.01), along with a higher rate of death due to graft failure (P < 0.01) and infection/sepsis (P < 0.01). Multivariate analysis showed a more than twofold increased risk for 1‐year mortality after HTX in these patients (HR: 2.20, CI: 1.68–2.87; P < 0.01). Especially patients with newly acquired cRBBB ≤ 30 days after HTX had a significantly higher 1‐year post‐transplant mortality (63.9%) compared with patients with pre‐existing cRBBB in the donor heart (15.0%) or patients without cRBBB ≤ 30 days after HTX (13.6%, P < 0.01). Our data suggest that newly acquired cRBBB early after HTX is associated with increased post‐transplant mortality.
Right ventricular dysfunction and RBBB after HTX
Right ventricular dysfunction after HTX is associated with increased post‐transplant mortality and can be accompanied by derogations of the cardiac conduction system manifesting as RBBB, AF, bradycardia or even requirement for PPM implantation after HTX. 1 , 4 , 7 , 11 , 12 , 14 , 15 , 16 , 17 , 18 , 19 , 32 , 33 , 34 In our study, patients with cRBBB ≤ 30 days after HTX had a higher percentage of an enlarged right atrium (P = 0.01), an enlarged right ventricle (P < 0.01), tricuspid regurgitation (P < 0.01) and right ventricular dysfunction (P < 0.01), indicating the presence of severe right heart strain.
Graft rejection is a leading cause for right ventricular dysfunction and has been linked to the development of RBBB after HTX, but definition of graft rejection and temporal relationship varied between studies. 1 , 3 , 7 , 11 In contrast, we and other authors could not find a significant association between graft rejection and cRBBB after HTX. 2 , 8 , 10 , 13
Increased levels of cardiac biomarkers have been associated with graft rejection, right ventricular dysfunction, pulmonary hypertension and poor survival after HTX. 35 , 36 , 37 , 38 , 39 As several cardiac biomarkers such as cardiac troponin I (cTnI), cardiac troponin T (cTnT), high‐sensitivity cardiac troponin T (hsTnT), brain natriuretic peptide (BNP) or N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) are clinically used, comparison may be difficult. Furthermore, increased cardiac biomarkers have also been observed in HTX recipients without signs of cardiac graft impairment emphasizing the need for intraindividual assessment of differences as well as exclusion of non‐cardiac elevation of cardiac biomarker levels (reduced metabolic and renal clearance). 39
Bradycardia frequently occurs in the early stage after HTX and may require PPM implantation. 18 , 40 , 41 , 42 However, the impact of cRBBB early after HTX on the post‐transplant need for PPM implantation is uncertain. 1 , 4 , 8 , 43 Jones and colleagues 43 reported the presence of cRBBB early after HTX in 38.1% of patients who later received PPM implantation after HTX. Ferretto and colleagues 1 found a twofold increased rate of PPM implantation after HTX in patients with cRBBB early after HTX (10.5% vs. 5.5%) but could not reach statistical significance. In our study, we found a significantly higher rate of 1‐year PPM implantation in patients with cRBBB ≤ 30 days after HTX (4.3% vs. 1.2%, P = 0.02), implicating an association between cRBBB early after HTX and post‐transplant need for PPM implantation.
Cardiac arrhythmias after HTX have been linked to COPD and an increased PVR. 15 , 16 In the initial post‐transplant period, the right donor heart of HTX recipients with pre‐existing COPD is exposed to considerable physiological stress including pulmonary hypertension, hypoxaemia, hypercarbia and acidosis, which can provoke AF. 15 , 16 , 44 , 45 In our study, patients with cRBBB ≤ 30 days after HTX had a higher percentage of COPD (33.1% vs. 21.8%, P = 0.01), a twofold higher rate of early post‐transplant AF (21.6% vs. 11.6%, P < 0.01), and died significantly more often from infection/sepsis (21.6% vs. 9.0%, P < 0.01).
Our results indicate that cRBBB ≤ 30 days after HTX—especially newly acquired cRBBB—is associated with increased post‐transplant mortality, right ventricular dysfunction, need for PPM implantation and AF after HTX. These patients should hence be carefully monitored including 12‐lead ECG, echocardiography, myocardial biopsies and immunosuppressive drug levels.
Study limitations
Our findings are based on a single‐centre registry (Heidelberg HTX Registry). Interpretation of results should therefore be treated with caution as this study design carries certain limitations. Nevertheless, unlike many multicentre studies, our study provides an excellent granularity as patients received a standardized centre‐specific pre‐, peri‐ and post‐transplant treatment and follow‐up, reducing the likelihood of potential selection bias and confounders. Furthermore, this study included 639 patients analogous in sample size to multicentre studies. 15 , 16 , 17 , 18 , 19
This study included HTX recipients over a period of 30 years. A possible era effect due to changes in medical care might have affected our results. As the immunosuppressive drug regimen was changed from 2006 onwards, we performed a sensitivity analysis with patients who received tacrolimus and mycophenolate mofetil. This analysis showed similar findings supporting the robustness of our results. 15 , 16 , 17 , 18 , 19
Study results should be considered as hypothesis‐generating as several factors may affect post‐transplant mortality. Our data therefore can neither proof nor disproof a causal relationship between cRBBB ≤ 30 days after HTX and increased 1‐year mortality after HTX but merely indicate an association. In order to confirm our findings, further large multicentre trials are required to investigate the effects of cRBBB ≤ 30 days after HTX on post‐transplant outcomes.
Conclusion
To our knowledge, this is the largest study investigating the post‐transplant outcomes of patients with cRBBB ≤ 30 days after HTX, especially in regard to differences between patients with newly acquired cRBBB ≤ 30 days after HTX and patients with cRBBB ≤ 30 days after HTX, which was already present in the donor heart before HTX. A total of 139 patients showed cRBBB ≤ 30 days after HTX (21.8%), hereof 20 patients with pre‐existing cRBBB before HTX (3.2%) and 119 patients with newly acquired cRBBB after HTX (18.6%). Patients with newly acquired cRBBB after HTX had a worse 1‐year post‐transplant survival (36.1%, P < 0.01) compared with patients with pre‐existing cRBBB before HTX (85.0%) or without cRBBB after HTX (86.4%), along with a higher percentage of death due to graft failure (P < 0.01). Multivariate analysis indicated cRBBB ≤ 30 days after HTX as a significant risk factor for 1‐year mortality after HTX (HR: 2.20; 95% CI: 1.68–2.87; P < 0.01). Secondary outcomes showed a higher rate of an enlarged right atrium (P = 0.01), an enlarged right ventricle (P < 0.01), a reduced right ventricular function (P < 0.01), 30‐day AF (P < 0.01) and 1‐year PPM implantation (P = 0.02) in patients with cRBBB after early HTX.
Conflict of interest
None declared.
Funding
This work was supported by research grants from the German Society of Internal Medicine (Research Scholarship to AKR), the German Heart Foundation/German Foundation of Heart Research (Research Scholarship to RR), and the Faculty of Medicine, University of Heidelberg (Physician‐Scientist‐Program to RR).
Acknowledgements
We thank Viola Deneke and Berthold Klein for their assistance and advice.
Rahm, A.‐K. , Helmschrott, M. , Darche, F. F. , Thomas, D. , Bruckner, T. , Ehlermann, P. , Kreusser, M. M. , Warnecke, G. , Frey, N. , and Rivinius, R. (2021) Newly acquired complete right bundle branch block early after heart transplantation is associated with lower survival. ESC Heart Failure, 8: 3737–3747. 10.1002/ehf2.13494.
References
- 1. Ferretto S, Tafciu E, Giuliani I, Feltrin G, Bottio T, Gambino A, Fraiese A, Iliceto S, Gerosa G, Leoni L. Interventricular conduction disorders after orthotopic heart transplantation: risk factors and clinical relevance. Ann Noninvasive Electrocardiol 2017; 22: e12402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gao SZ, Hunt SA, Wiederhold V, Schroeder JS. Characteristics of serial electrocardiograms in heart transplant recipients. Am Heart J 1991; 122: 771–774. [DOI] [PubMed] [Google Scholar]
- 3. Leonelli FM, Pacifico A, Young JB. Frequency and significance of conduction defects early after orthotopic heart transplantation. Am J Cardiol 1994; 73: 175–179. [DOI] [PubMed] [Google Scholar]
- 4. Leonelli FM, Dunn JK, Young JB, Pacifico A. Natural history, determinants, and clinical relevance of conduction abnormalities following orthotopic heart transplantation. Am J Cardiol 1996; 77: 47–51. [DOI] [PubMed] [Google Scholar]
- 5. Marcus GM, Hoang KL, Hunt SA, Chun SH, Lee BK. Prevalence, patterns of development, and prognosis of right bundle branch block in heart transplant recipients. Am J Cardiol 2006; 98: 1288–1290. [DOI] [PubMed] [Google Scholar]
- 6. Yamamoto S, Bergsland J, Michalek SM. Evolution of right bundle branch block and other intraventricular conduction abnormalities in the transplanted human heart. Jpn Circ J 1990; 54: 1122–1129. [DOI] [PubMed] [Google Scholar]
- 7. Kim JH, Oh J, Kim MJ, Kim IC, Uhm JS, Pak HN, Kang SM. Association of newly developed right bundle branch block with graft rejection following heart transplantation. Yonsei Med J 2019; 60: 423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Golshayan D, Seydoux C, Berguer DG, Stumpe F, Hurni M, Ruchat P, Fischer A, Mueller X, Sadeghi H, von Segesser L, Goy JJ. Incidence and prognostic value of electrocardiographic abnormalities after heart transplantation. Clin Cardiol 1998; 21: 680–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Almenar L, Osa A, Arnau MA, Dolz LM, Rueda J, Palencia M. Right bundle branch block as a prognostic factor in heart transplantation. Transplant Proc 1999; 31: 2548–2549. [DOI] [PubMed] [Google Scholar]
- 10. Jessen ME, Olivari MT, Wait MA, Meyer DM, Yancey CW Jr, Ring WS. Frequency and significance of right bundle branch block after cardiac transplantation. Am J Cardiol 1994; 73: 1009–1111. [DOI] [PubMed] [Google Scholar]
- 11. Osa A, Almenar L, Arnau MA, Martínez‐Dolz L, Rueda J, Morillas P, Palencia M. Is the prognosis poorer in heart transplanted patients who develop a right bundle branch block? J Heart Lung Transplant 2000; 19: 207–214. [DOI] [PubMed] [Google Scholar]
- 12. Pickham D, Hickey K, Doering L, Chen B, Castillo C, Drew BJ. Electrocardiographic abnormalities in the first year after heart transplantation. J Electrocardiol 2014; 47: 135–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sandhu JS, Curtiss EI, Follansbee WP, Zerbe TR, Kormos RL. The scalar electrocardiogram of the orthotopic heart transplant recipient. Am Heart J 1990; 119: 917–923. [DOI] [PubMed] [Google Scholar]
- 14. Chang HY, Yin WH, Lo LW, Lin YJ, Chang SL, Hu YF, Feng AN, Chiang MC, Young MS, Chang CY, Chuang YC, Chong E, Chen SA, Wei J. The utilization of twelve‐lead electrocardiography for predicting sudden cardiac death after heart transplantation. Int J Cardiol 2013; 168: 2665–2672. [DOI] [PubMed] [Google Scholar]
- 15. Rivinius R, Helmschrott M, Ruhparwar A, Schmack B, Darche FF, Thomas D, Bruckner T, Katus HA, Ehlermann P, Doesch AO. Chronic obstructive pulmonary disease in patients after heart transplantation is associated with a prolonged hospital stay, early post‐transplant atrial fibrillation, and impaired post‐transplant survival. Clin Epidemiol 2018; 10: 1359–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rivinius R, Helmschrott M, Ruhparwar A, Schmack B, Darche FF, Thomas D, Bruckner T, Doesch AO, Katus HA, Ehlermann P. Elevated pre‐transplant pulmonary vascular resistance is associated with early post‐transplant atrial fibrillation and mortality. ESC Heart Fail 2020; 7: 176–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rivinius R, Helmschrott M, Ruhparwar A, Erbel C, Gleissner CA, Darche FF, Thomas D, Bruckner T, Katus HA, Doesch AO. The influence of surgical technique on early posttransplant atrial fibrillation – comparison of biatrial, bicaval, and total orthotopic heart transplantation. Ther Clin Risk Manag 2017; 13: 287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rivinius R, Helmschrott M, Rahm AK, Darche FF, Thomas D, Bruckner T, Doesch AO, Ehlermann P, Katus HA, Zitron E. Risk factors and survival of patients with permanent pacemaker implantation after heart transplantation. J Thorac Dis 2019; 11: 5440–5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rivinius R, Helmschrott M, Rahm AK, Darche FF, Thomas D, Bruckner T, Doesch AO, Ehlermann P, Katus HA, Zitron E. Combined amiodarone and digitalis therapy before heart transplantation is associated with increased post‐transplant mortality. ESC Heart Fail 2020; 7: 2082–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Surawicz B, Childers R, Deal BJ, Gettes LS, Bailey JJ, Gorgels A, Hancock EW, Josephson M, Kligfield P, Kors JA, Macfarlane P, Mason JW, Mirvis DM, Okin P, Pahlm O, Rautaharju PM, van Herpen G, Wagner GS, Wellens H, American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; American College of Cardiology Foundation; Heart Rhythm Society . AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part III: intraventricular conduction disturbances: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol 2009; 53: 976–981. [DOI] [PubMed] [Google Scholar]
- 21. Stewart S, Winters GL, Fishbein MC, Tazelaar HD, Kobashigawa J, Abrams J, Andersen CB, Angelini A, Berry GJ, Burke MM, Demetris AJ, Hammond E, Itescu S, Marboe CC, McManus B, Reed EF, Reinsmoen NL, Rodriguez ER, Rose AG, Rose M, Suciu‐Focia N, Zeevi A, Billingham ME. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant 2005; 24: 1710–1720. [DOI] [PubMed] [Google Scholar]
- 22. Kreger BE, Anderson KM, Kannel WB. Prevalence of intraventricular block in the general population: the Framingham study. Am Heart J 1989; 117: 903–910. [DOI] [PubMed] [Google Scholar]
- 23. Bussink BE, Holst AG, Jespersen L, Deckers JW, Jensen GB, Prescott E. Right bundle branch block: prevalence, risk factors, and outcome in the general population: results from the Copenhagen City Heart study. Eur Heart J 2013; 34: 138–146. [DOI] [PubMed] [Google Scholar]
- 24. Go AS, Barron HV, Rundle AC, Ornato JP, Avins AL. Bundle‐branch block and in‐hospital mortality in acute myocardial infarction. National Registry of Myocardial Infarction 2 Investigators. Ann Intern Med 1998; 129: 690–697. [DOI] [PubMed] [Google Scholar]
- 25. Mueller C, Laule‐Kilian K, Klima T, Breidthardt T, Hochholzer W, Perruchoud AP, Christ M. Right bundle branch block and long‐term mortality in patients with acute congestive heart failure. J Intern Med 2006; 260: 421–428. [DOI] [PubMed] [Google Scholar]
- 26. Rasmussen PV, Skov MW, Ghouse J, Pietersen A, Hansen SM, Torp‐Pedersen C, Køber L, Haunsø S, Olesen MS, Svendsen JH, Melgaard J, Graff C, Holst AG, Nielsen JB. Clinical implications of electrocardiographic bundle branch block in primary care. Heart 2019; 105: 1160–1167. [DOI] [PubMed] [Google Scholar]
- 27. Alderman EL, Wexler L. Angiographic implications of cardiac transplantation. Am J Cardiol 1989; 64: 16E–21E. [DOI] [PubMed] [Google Scholar]
- 28. Desouza KA, Joseph SM, Cuculich PS, Ewald GA, Rudy Y. Noninvasive mapping of ventricular activation in patients with transplanted hearts. J Electrocardiol 2013; 46: 698–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Butman SM, Phibbs B, Wild J, Copeland JG. One heart, two bodies: insight from the transplanted heart and its new electrocardiogram. Am J Cardiol 1990; 66: 632–635. [DOI] [PubMed] [Google Scholar]
- 30. Villa AE, de Marchena EJ, Myerburg RJ, Castellanos A. Comparisons of paired orthotopic cardiac transplant donor and recipient electrocardiograms. Am Heart J 1994; 127: 70–74. [DOI] [PubMed] [Google Scholar]
- 31. Khush KK, Menza R, Nguyen J, Goldstein BA, Zaroff JG, Drew BJ. Electrocardiographic characteristics of potential organ donors and associations with cardiac allograft use. Circ Heart Fail 2012; 5: 475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Calzolari V, Angelini A, Basso C, Livi U, Rossi L, Thiene G. Histologic findings in the conduction system after cardiac transplantation and correlation with electrocardiographic findings. Am J Cardiol 1999; 84: 756–759. [DOI] [PubMed] [Google Scholar]
- 33. Foerster A. The conduction system in human cardiac allografts. A histological and immunopathological study. Pathol Res Pract 1992; 188: 783–790. [DOI] [PubMed] [Google Scholar]
- 34. Hickey KT, Sciacca RR, Chen B, Drew BJ, Pickham D, Carter EV, Castillo C, Doering LV. Electrocardiographic correlates of acute allograft rejection among heart transplant recipients. Am J Crit Care 2018; 27: 145–150. [DOI] [PubMed] [Google Scholar]
- 35. Avello N, Molina BD, Llorente E, Bernardo MJ, Prieto B, Alvarez FV. N‐terminal pro‐brain natriuretic peptide as a potential non‐invasive marker of cardiac transplantation rejection. Ann Clin Biochem 2007; 44: 182–188. [DOI] [PubMed] [Google Scholar]
- 36. Cuppoletti A, Roig E, Pérez‐Villa F, Marin JL, Orús J, Vallejos I, Rivera A, Botta C. Value of NT‐proBNP determinations in the follow‐up of heart transplantation. Transplant Proc 2005; 37: 4033–4035. [DOI] [PubMed] [Google Scholar]
- 37. De Santo LS, Torella M, Romano G, Maiello C, Buonocore M, Bancone C, Della Corte A, Galdieri N, Nappi G, Amarelli C. Perioperative myocardial injury after adult heart transplant: determinants and prognostic value. PLoS ONE 2015; 10: e0120813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Erbel C, Taskin R, Doesch A, Dengler TJ, Wangler S, Akhavanpoor M, Ruhparwar A, Giannitsis E, Katus HA, Gleissner CA. High‐sensitive troponin T measurements early after heart transplantation predict short‐ and long‐term survival. Transpl Int 2013; 26: 267–272. [DOI] [PubMed] [Google Scholar]
- 39. Talha S, Charloux A, Enache I, Piquard F, Geny B. Mechanisms involved in increased plasma brain natriuretic peptide after heart transplantation. Cardiovasc Res 2011; 89: 273–281. [DOI] [PubMed] [Google Scholar]
- 40. Jacquet L, Ziady G, Stein K, Griffith B, Armitage J, Hardesty R, Kormos R. Cardiac rhythm disturbances early after orthotopic heart transplantation: prevalence and clinical importance of the observed abnormalities. J Am Coll Cardiol 1990; 16: 832–837. [DOI] [PubMed] [Google Scholar]
- 41. Miyamoto Y, Curtiss EI, Kormos RL, Armitage JM, Hardesty RL, Griffith BP. Bradyarrhythmia after heart transplantation. Incidence, time course, and outcome. Circulation 1990; 82: IV313–IV317. [PubMed] [Google Scholar]
- 42. Thajudeen A, Stecker EC, Shehata M, Patel J, Wang X, McAnulty JH Jr, Kobashigawa J, Chugh SS. Arrhythmias after heart transplantation: mechanisms and management. J Am Heart Assoc 2012; 1: e001461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jones DG, Mortsell DH, Rajaruthnam D, Hamour I, Hussain W, Markides V, Banner NR, Wong T. Permanent pacemaker implantation early and late after heart transplantation: clinical indication, risk factors and prognostic implications. J Heart Lung Transplant 2011; 30: 1257–1265. [DOI] [PubMed] [Google Scholar]
- 44. Gasparova I, Kubatka P, Opatrilova R, Caprnda M, Filipova S, Rodrigo L, Malan L, Mozos I, Rabajdova M, Nosal V, Kobyliak N, Valentova V, Petrovic D, Adamek M, Kruzliak P. Perspectives and challenges of antioxidant therapy for atrial fibrillation. Naunyn Schmiedebergs Arch Pharmacol 2017; 390: 1–14. [DOI] [PubMed] [Google Scholar]
- 45. Korantzopoulos P, Kolettis TM, Galaris D, Goudevenos JA. The role of oxidative stress in the pathogenesis and perpetuation of atrial fibrillation. Int J Cardiol 2007; 115: 135–143. [DOI] [PubMed] [Google Scholar]


