Abstract
Aims
While heart failure (HF) is a leading cause of death in adults with congenital heart disease (ACHD), few studies report contemporary outcomes after the first HF hospitalization. We examined outcomes of ACHD patients newly admitted for HF compared with ACHD patients without HF and the general HF population without ACHD.
Methods and results
Using population databases from a single‐payer health system from 1994 to 2018, ACHD patients newly admitted for HF were matched 1:1 to ACHD patients without HF (n = 4030 matched pairs). Similarly, ACHD patients newly admitted for HF were matched 1:1 to HF patients without ACHD (n = 4336 matched pairs). Patients with ACHD and HF (median age 68 years, 45% women) experienced higher mortality in short‐term [30 day adjusted hazard ratio (HR) 4.68, 95% confidence interval (CI) 4.06, 5.43, P < 0.001], near‐term (1 year HR 3.87, 95% CI 3.77, 4.92, P < 0.001), and long‐term (24 year HR 1.59, 95% CI 1.13, 2.36, P = 0.008) follow‐up. Patients with ACHD and HF had fewer baseline cardiovascular comorbidities than non‐ACHD HF but demonstrated higher 30 day (HR 1.56, 95% CI 1.41, 1.73, P < 0.001), 1 year (HR 1.30, 95% CI 1.20, 1.40, P < 0.001), and 24 year (HR 2.40, 95% CI 1.73, 3.38, P < 0.001) mortality. Those with ACHD and HF also exhibited higher cardiovascular readmission rates at 30 days with HRs 9.15 (95% CI; 8.00, 10.48, P < 0.001) vs. ACHD without HF, and 1.71 (95% CI; 1.54, 1.85, P < 0.001) vs. HF without ACHD, and the higher readmission risk extended to 10 year follow‐up.
Conclusions
Adults with congenital heart disease patients with new HF have high risks of death and cardiovascular hospitalization, and preventative strategies to improve outcomes are urgently needed.
Keywords: Heart failure, Adult congenital heart disease, Outcomes
Introduction
Adults with congenital heart disease (ACHD) are a growing population due to advances in medical and surgical therapies. 1 , 2 The majority of children with congenital heart disease now survive into adulthood; however, many are left with residual anatomical, physiological, and electrical abnormalities that increase their morbidity and mortality compared with the general population. 1 , 3 , 4 , 5
Heart failure (HF) is recognized as the leading cause of death, hospitalization, and reduced life expectancy in patients with ACHD. 4 , 6 , 7 Hospitalizations for HF have been identified to be prognostically important, with each hospital admission associated with worsening outcome. 8 In particular, the first hospitalization is a sentinel event that is important from the standpoint of preventive efforts to reduce the burden of HF. 9 However, few studies have reported the contemporary outcomes of ACHD patients after a first hospitalization with HF. 10 , 11 , 12 , 13 , 14 Such data are needed as it would improve allocation of resources and management of these patients.
Therefore, the aims of this study were to (i) characterize ACHD patients after a first admission with HF, (ii) compare the outcomes between ACHD patients after a first HF admission with ACHD patients without HF, and (iii) examine differences in outcomes in ACHD HF patients to the general HF population without ACHD.
Methods
Study design
We performed two retrospective cohort comparisons to determine outcomes in adult congenital heart disease (ACHD) patients after their first HF admission. One was between ACHD patients with a first admission for HF and ACHD patients with no HF admission within the previous 3 years. The second was between ACHD patients and non‐ACHD patients both with a first admission for HF.
Data sources
In Ontario (Canada), all residents are assigned a unique health insurance number to receive coverage for medically necessary services. All diagnoses and health services provided are systematically recorded until death. This study was conducted through linkages of multiple clinical and administrative databases housed at ICES (formerly the Institute for Clinical Evaluative Sciences), which holds multiple population‐based health databases of the Ontario population. ICES is a prescribed entity under Ontario's Personal Health Information Protection Act, which allows researchers to collect, link and analyse encoded population‐based administrative databases and clinical registries for conducting approved research studies, without the need to obtain individual patient consent, but under strict privacy and security policies, procedures, and practices (https://www.ices.on.ca/Data‐and‐Privacy). The use of data in this project was authorized under section 45 of Ontario's Personal Health Information Protection Act. This study complies with the Declaration of Helsinki.
Study population
We identified all ACHD patients in the population who were admitted to any hospital in Ontario from 1 April 1994 to 31 March 2018, via the Canadian Institute for Health Information Discharge Abstract Database (CIHI‐DAD), using International Classification of Diseases, ninth (ICD‐9) and tenth (ICD‐10) revisions, which have codes to designate congenital heart disease anomalies (Appendix 1). Using ICD‐9 code 428 and ICD‐10 code I50, we identified all ACHD and non‐ACHD patients who were hospitalized with HF and all readmissions after the index initial hospital admission (Appendix 2) using previously validated approaches. 15 We also excluded those less than 18 years of age, those ineligible for insurance coverage under the Ontario Health Insurance Plan, and non‐Ontario residents. Congenital heart disease severity was designated as per the American College of Cardiology/American Heart Association guidelines for the management of adults with congenital heart disease, which groups patients into three categories (simple, moderate, or great complexity), based on their unrepaired and repaired anatomy. 6 , 16 Patients for whom there was insufficient information were classified as undefined.
Adult congenital heart disease HF cases were ACHD patients who had a first hospitalization with the primary/most responsible diagnosis of HF at any time during the study period. These patients were then matched 1:1 to an ACHD control without HF. ACHD non‐HF controls had no HF admissions in the 3 years prior to the date of first HF hospitalization relative to the ACHD HF cases. Matching was performed using the following factors: age, sex, congenital heart disease complexity, and the admission date of the ACHD HF case hospitalization.
We also matched each ACHD HF patient 1:1 to a patient with HF but without ACHD (non‐ACHD HF control), matching on age, sex, and year of first HF hospitalization. For ACHD HF cases, the index date was the date of first HF hospitalization. For ACHD non‐HF controls, the index date was the corresponding date of first HF hospitalization for ACHD HF cases with whom they were matched. For non‐ACHD HF controls, the index date was the first HF hospitalization.
Comorbidities such as diabetes, hypertension, chronic obstructive pulmonary disease, and asthma were determined employing established and published, validated algorithms using the CIHI‐DAD and OHIP administrative databases. 17 , 18 , 19 , 20 , 21 Additional baseline patient characteristics were identified using administrative data sources, scanning for comorbidities by examining all hospital records in the 3 year period prior to the index date defined above. By scanning the 3 year period for all diagnostic codes in the CIHI‐DAD, we have previously reported that prior HF diagnoses can be identified, leaving only those with newly diagnosed HF after excluding those with pre‐existing disease. 9 Similarly, comorbid conditions can also be identified using this window of observation. 22 The Charlson comorbidity index was calculated excluding the HF diagnosis at the index admission. Cardiac procedures were determined from the CIHI‐DAD or the CIHI Same Day Surgery database using the Canadian Classification of Interventions coding system. Physician visits and procedures were also determined using the OHIP physician claims database. The Ontario Registered Persons Database was used to obtain sociodemographic information, including postal code, which was linked to Statistics Canada's 2006 and 2011 census data to obtain median neighbourhood income of subjects and served as a proxy for socioeconomic status.
Outcomes
We examined short‐term (30 days), near‐term (1 year), and long‐term (>20 years) occurrence of death from any cause. Mortality data were obtained from the Registered Persons Database of vital statistics. We also examined 1 year and long‐term (>20 years) HF hospitalizations and cardiovascular disease hospitalizations after the index date. Hospitalizations were identified using the CIHI‐DAD, and the primary reason for admission (cardiovascular disease or HF) was determined using the ICD‐9 and ICD‐10 coding systems, using diagnostic codes as previously published. 23
Statistical analysis
Continuous variables were summarized using median and interquartile range (IQR) or mean and standard deviation. The Kruskal–Wallis test was used to test comparison of medians and ANOVA for comparison of means. Categorical variables were reported as proportions and compared using the χ 2 test. The last follow‐up date for total mortality and hospitalization was 31 March 2019. Unadjusted event rates per 1000 person‐years for all‐cause mortality, HF hospitalization, and cardiovascular hospitalization were compared using Poisson regression. Admissions for elective procedures that lasted less than 48 h were excluded as an outcome event. In the primary analysis, all‐cause mortality, HF, and cardiovascular disease hospitalization events were analysed using multiple Cox regression models using cause‐specific hazards (that accounted for the competing risk of death in the latter two analyses), with the index date (as described above) as t = 0 time point. Hazard ratios (HR) were adjusted for ACHD complexity, income quintile, rural residence, Charlson comorbidity score category, history of myocardial infarction, renal disease, hypertension, atrial fibrillation, diabetes, chronic obstructive lung disease, asthma, implantable cardioverter defibrillator or pacemaker, previous valve procedure, coronary artery bypass grafting, or percutaneous coronary intervention, and previous electrical cardioversion using diagnostic and procedure codes as previously published by our group. 9 , 24 Analysis was performed stratified by CHD complexity and by dividing the accrual period into three equal time periods (1994–2001, 2002–2009, and 2010–2017). Kaplan–Meier curves were plotted for mortality outcomes and compared with the log‐rank statistic.
The proportional hazard assumption was tested, using the Kolmogorov‐type supremum test from 1000 simulated patterns, to verify whether the HR varied by time. If the proportional hazards assumption was rejected (P < 0.05), time‐dependent HR were estimating using restricted cubic splines with three knots. For these estimated time‐dependent HR, 95% confidence intervals (CI) were obtained using the bootstrap approach, with 1000 bootstrap samples. The statistical significance of the effect was determined by using the equivalence between hypothesis testing and CI, whereby an association is significant at the alpha significance level if a 100‐alpha CI has an endpoint that is equal to the null value of the null hypothesis. Thus, using the 1000 bootstrap samples, we determined the confidence level of a CI whose endpoint was the null value. A two‐sided P value < 0.05 was deemed statistically significant. P values were not adjusted for multiple comparisons in these exploratory analyses. All analyses were conducted using SAS Enterprise Guide version 7.1 (SAS Institute, Cary, North Carolina).
Results
We identified 4450 ACHD cases with a first HF admission during our study period out of the 32 642 ACHD patients (Figure 1 ). After matching, we had 4030 ACHD HF cases and 4030 ACHD controls without HF. There were 294 798 adults without ACHD with a first HF admission during the study period. After matching, we had 4436 ACHD HF cases and 4436 non‐ACHD HF controls (Figure 2 ).
Figure 1.
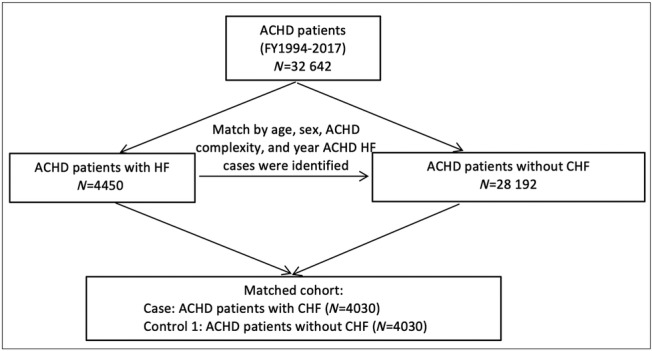
Flow chart demonstrating the creation of the matched adult congenital heart disease (ACHD) cohort with and without heart failure (HF).
Figure 2.
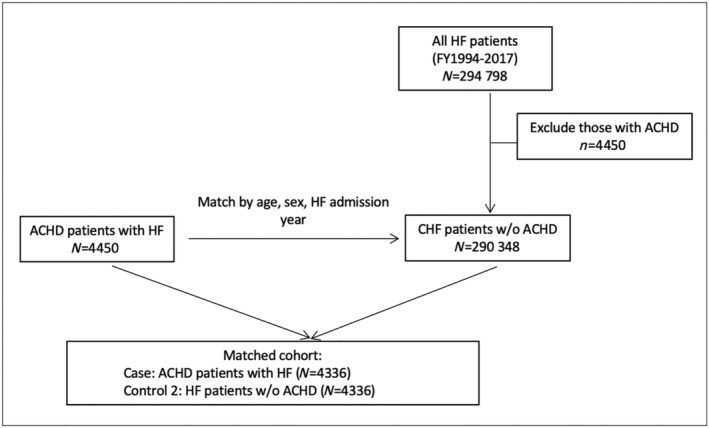
Flow chart demonstrating the creation of matched heart failure (HF) cohort with and without adult congenital heart disease (ACHD).
Congenital heart disease patients with and without heart failure
Baseline characteristics
Demographic and clinical characteristics of the ACHD HF cases and ACHD non‐HF controls are presented in Table 1 . The median age was 68 years (IQR 55–77 years), and 45% were female in the matched ACHD patients. Patients with simple, medium, and great complexity ACHD comprised 63.9%, 6.8%, and 1.3% of our matched cohort, respectively. Great complexity ACHD lesions were comprised mostly of patients with transposition of the great vessels, medium complexity were mainly patients with Tetralogy of Fallot, and simple ACHD were atrial septal defects and bicuspid aortic valves. ACHD HF cases were more likely in low‐income quintiles than ACHD non‐HF controls. They were also more likely than ACHD non‐HF controls to have a history of ischemic heart disease (38.3% vs. 8.1%), diabetes (34.3% vs. 17.7%), renal disease (11.8% vs. 1.1%), or COPD (32.1% vs. 16.7%), and a higher Charlson comorbidity score (1 [IQR 0–3] vs. 0 [IQR 0–0]) and all comparisons were statistically significant (all P < 0.001).
Table 1.
Baseline characteristics
| Characteristic | ACHD patients | HF patients | ||||
|---|---|---|---|---|---|---|
| With HF | Without HF | P value | With ACHD | Without ACHD | P value | |
| N | 4030 | 4030 | 4336 | 4336 | ||
| Demographics | ||||||
| Age, median (IQR) | 68 (55–77) | 68 (55–77) | 0.95 | 70 (58–79) | 70 (58–79) | 1 |
| Female | 1814 (45.0%) | 1814 (45.0%) | 1 | 1970 (45.4%) | 1970 (45.4%) | 1 |
| Rural | 664 (16.5%) | 606 (15.0%) | 0.08 | 724 (16.7%) | 688 (15.9%) | 0.30 |
| CHD complexity | ||||||
| High | 54 (1.3%) | 54 (1.3%) | 1 | 55 (1.3%) | NA | |
| Medium | 275 (6.8%) | 275 (6.8%) | 282 (6.5%) | NA | ||
| Simple | 2574 (63.9%) | 2574 (63.9%) | 2740 (63.2%) | NA | ||
| Undefined | 1127 (28.0%) | 1127 (28.0%) | 1259 (29.0%) | NA | ||
| Income quintile | ||||||
| 1 (lowest) | 966 (24.0%) | 761 (18.9%) | <0.001 | 1041 (24.0%) | 1121 (25.9%) | <0.001 |
| 2 | 837 (20.8%) | 777 (19.3%) | 912 (21.0%) | 1016 (23.4%) | ||
| 3 | 807 (20.0%) | 785 (19.5%) | 862 (19.9%) | 802 (18.5%) | ||
| 4 | 714 (17.7%) | 810 (20.1%) | 764 (17.6%) | 748 (17.3%) | ||
| 5 (highest) | 706 (17.5%) | 897 (22.3%) | 757 (17.5%) | 649 (15.0%) | ||
| Comorbidities | ||||||
| Asthma | 782 (19.4%) | 528 (13.1%) | <0.001 | 817 (18.8%) | 832 (19.2%) | 0.68 |
| COPD | 1293 (32.1%) | 673 (16.7%) | <0.001 | 1471 (33.9%) | 1611 (37.2%) | 0.002 |
| Renal disease | 475 (11.8%) | 44 (1.1%) | <0.001 | 537 (12.4%) | 607 (14.0%) | 0.03 |
| Diabetes mellitus | 1383 (34.3%) | 713 (17.7%) | <0.001 | 1500 (34.6%) | 1998 (46.1%) | <0.001 |
| Hypertension | 2791 (69.3%) | 2240 (55.6%) | <0.001 | 3116 (71.9%) | 3313 (76.4%) | <0.001 |
| Atrial fibrillation | 1085 (26.9%) | 209 (5.2%) | <0.001 | 1177 (27.1%) | 874 (20.2%) | <0.001 |
| Ischaemic heart disease | 1545 (38.3%) | 325 (8.1%) | <0.001 | 1702 (39.3%) | 1830 (42.2%) | 0.005 |
| Prior MI | 473 (11.7%) | 58 (1.4%) | <0.001 | 532 (12.3%) | 726 (16.7%) | <0.001 |
| Charlson score | 1 (0–3) | 0 (0–0) | <0.001 | 2 (0–3) | 2 (1–3) | <0.001 |
| 0 | 1160 (28.8%) | 3249 (80.6%) | <0.001 | 1219 (28.1%) | 870 (20.1%) | <0.001 |
| 1 | 884 (21.9%) | 385 (9.6%) | 947 (21.8%) | 913 (21.1%) | ||
| 2+ | 1986 (49.3%) | 396 (9.8%) | 2170 (50.0%) | 2553 (58.9%) | ||
| Physician visits within last year | ||||||
| Any | 3916 (97.2%) | 3620 (89.8%) | <0.001 | 4220 (97.3%) | 4148 (95.7%) | <0.001 |
| Family physician | 3910 (97.0%) | 3567 (88.5%) | <0.001 | 4217 (97.3%) | 4172 (96.2%) | 0.007 |
| Cardiologist | 2678 (66.5%) | 1530 (38.0%) | <0.001 | 2842 (65.5%) | 2193 (50.6%) | <0.001 |
| Cardiac tests or procedures within the last year | ||||||
| ECG | 3198 (79.4%) | 1986 (49.3%) | <0.001 | 3417 (78.8%) | 3103 (71.6%) | <0.001 |
| Echocardiogram | 2390 (59.3%) | 1286 (31.9%) | <0.001 | 2520 (58.1%) | 1740 (40.1%) | <0.001 |
| Holter | 677 (16.8%) | 359 (8.9%) | <0.001 | 700 (16.1%) | 493 (11.4%) | <0.001 |
| Stress test | 522 (13.0%) | 400 (9.9%) | <0.001 | 542 (12.5%) | 541 (12.5%) | 0.97 |
| Electrical cardioversion | 190 (4.7%) | 30 (0.7%) | <0.001 | 190 (4.4%) | 89 (2.1%) | <0.001 |
| Pacemaker | 113 (2.8%) | 31 (0.8%) | <0.001 | 122 (2.8%) | 85 (2.0%) | 0.009 |
| ICD | 56 (1.4%) | 7 (0.2%) | <0.001 | 55 (1.3%) | 37 (0.9%) | 0.06 |
| Pacemaker or ICD | 158 (3.9%) | 37 (0.9%) | <0.001 | 165 (3.8%) | 114 (2.6%) | 0.002 |
| Cardiac catheterization | 764 (19.0%) | 164 (4.1%) | <0.001 | 786 (18.1%) | 509 (11.7%) | <0.001 |
| PCI | 113 (2.8%) | 20 (0.5%) | <0.001 | 121 (2.8%) | 163 (3.8%) | 0.01 |
| CABG surgery | 193 (4.8%) | 41 (1.0%) | <0.001 | 199 (4.6%) | 116 (2.7%) | <0.001 |
| PCI or CABG surgery | 289 (7.2%) | 60 (1.5%) | <0.001 | 302 (7.0%) | 265 (6.1%) | 0.11 |
| Valve procedure | 299 (7.4%) | 96 (2.4%) | <0.001 | 304 (7.0%) | 46 (1.1%) | <0.001 |
CABG, coronary artery bypass graft; ICD, implantable cardioverter defibrillator; MI, myocardial infarction; NA, not applicable; PCI, percutaneous coronary intervention.
Resource utilization
Compared with ACHD non‐HF controls, in the year prior to their index HF admission, ACHD HF patients were significantly more likely to have visited family physicians (97.2% vs. 88.5%, P < 0.001) and cardiologists (66.5% vs. 38.0%, P < 0.001). ACHD HF patients were also more likely to undergo non‐invasive cardiac testing such as echocardiograms (59.3% vs. 31.9%, P < 0.001), Holter monitoring (16.8% vs. 8.9%, P < 0.001), stress testing (13.0% vs. 9.9%, P < 0.001) and undergo procedures such as electrical cardioversion (4.7% vs. 0.7%, P < 0.001), cardiac catheterization (19.0% vs. 4.1%, P < 0.001), and percutaneous or surgical coronary intervention (7.2% vs. 1.5%, P < 0.001).
Mortality of adult congenital heart disease patients with or without heart failure
All‐cause mortality was higher in ACHD HF cases (117 deaths per 1000 person‐years) compared with ACHD non‐HF controls (36 deaths per 1000 person‐years) during 37 243 person‐years of follow‐up examined (Table 2 ). Kaplan–Meier curves are shown in Supporting Information, Figure S1 . ACHD HF cases died at a younger age (75, IQR 64–83 years) than ACHD non‐HF controls (84, IQR 77–88 years, P < 0.001). Thirty day and 1 year mortality were also higher for ACHD HF cases after the first admission for HF compared with ACHD non‐HF controls and did not vary when the accrual time period was divided into three equal time periods (Appendix 3). During long‐term follow‐up, there was a violation of the proportional hazards assumption, indicating that the HR varied over time. Point estimates of the adjusted time‐dependent HR obtained using restricted cubic splines demonstrated that ACHD HF cases had higher HR for mortality compared with ACHD non‐HF patients that persisted over the duration of follow‐up (Table 3 ). Curves of these time‐dependent adjusted HR estimates demonstrated that the HR was highest at 30 days and decreased to plateau at around 10 years (Figure 3 ).
Table 2.
Crude event rates per 1000 patient‐years (95% confidence intervals)
| Variable | ACHD patients | HF patients | ||||
|---|---|---|---|---|---|---|
| With HF | Without HF | P value | With ACHD | Without ACHD | P value | |
| All‐cause death | ||||||
| # of events | 2660 | 1322 | 2970 | 3004 | ||
| Rate per 1000 person‐years | 117 (113, 122) | 36 (35, 37) | <0.001 | 130 (126, 135) | 132 (128, 137) | 0.485 |
| Heart failure hospitalization | ||||||
| # of events | 1847 | 57 | 1994 | 1734 | ||
| Rate per 1000 person‐years | 116 (111, 121) | 1.5 (1.2, 2.0) | <0.001 | 124 (119, 130) | 100 (95, 104) | <0.001 |
| Cardiovascular disease hospitalization | ||||||
| # of events | 2754 | 1710 | 2944 | 2559 | ||
| Rate per 1000 person‐years | 294 (285, 303) | 62 (59, 65) | <0.001 | 311 (302, 321) | 194 (187, 201) | <0.001 |
ACHD, adults with congenital heart disease; HF, heart failure.
Table 3.
Time‐dependent hazard ratios
| Variable | ACHD patients | HF patients | ||||
|---|---|---|---|---|---|---|
| With HF | Without HF | P value | With ACHD | Without ACHD | P value | |
| All‐cause death | ||||||
| 30 day | 4.68 (4.06, 5.43) | Reference | <0.001 | 1.56 (1.41, 1.73) | Reference | <0.001 |
| 1 year | 3.87 (3.77, 4.92) | Reference | <0.001 | 1.30 (1.20, 1.40) | Reference | <0.001 |
| 5 year | 1.78 (1.62, 1.74) | Reference | <0.001 | 0.76 (0.70, 0.83) | Reference | <0.001 |
| 10 year | 1.33 (1.20, 1.48) | Reference | <0.001 | 0.98 (0.90, 1.07) | Reference | 0.688 |
| 24 year | 1.59 (1.13, 2.36) | Reference | 0.008 | 2.40 (1.73, 3.38) | Reference | <0.001 |
| Heart failure hospitalization | ||||||
| 30 day | 141.9 (85.3, 263.6) | Reference | <0.001 | 1.49 (1.34, 1.66) | Reference | <0.001 |
| 1 year | 108.6 (72.0, 180.4) | Reference | <0.001 | 1.32 (1.22, 1.44) | Reference | <0.001 |
| 5 year | 38.2 (26.2, 58.1) | Reference | <0.001 | 1.19 (1.05, 1.36) | Reference | <0.001 |
| 10 year | 22.2 (16.3, 31.6) | Reference | <0.001 | 1.50 (1.28, 1.85) | Reference | <0.001 |
| 24 year | 9.28 (3.48, 28.72) | Reference | <0.001 | 2.87 (1.79, 5.43) | Reference | <0.001 |
| Cardiovascular disease hospitalization | ||||||
| 30 day | 9.15 (8.00, 10.48) | Reference | <0.001 | 1.71 (1.54, 1.85) | Reference | <0.001 |
| 1 year | 5.74 (5.21, 6.29) | Reference | <0.001 | 1.44 (1.21, 1.47) | Reference | <0.001 |
| 5 year | 1.70 (1.51, 1.89) | Reference | <0.001 | 1.49 (1.37, 1.66) | Reference | <0.001 |
| 10 year | 1.39 (1.19, 1.62) | Reference | <0.001 | 1.89 (1.58, 2.35) | Reference | <0.001 |
| 24 year | 0.88 (0.49, 1.53) | Reference | 0.648 | 3.69 (2.13, 6.98) | Reference | <0.001 |
ACHD, adults with congenital heart disease; HF, heart failure.
Figure 3.
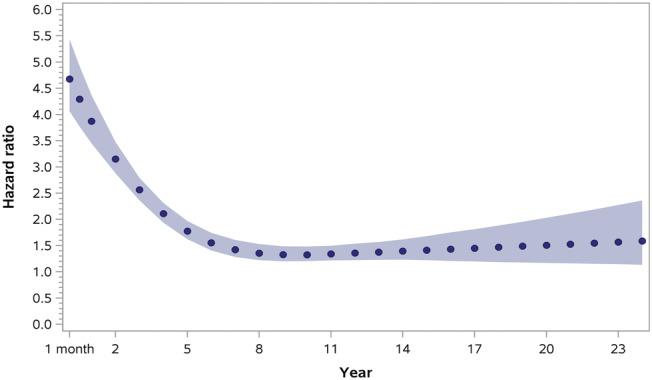
Time‐dependent adjusted hazard ratios for all‐cause mortality in the matched adult congenital heart disease (ACHD) cohort with and without heart failure (HF).
Hospitalizations among adult congenital heart disease patients with or without heart failure
Adults with congenital heart disease and HF were also more likely than ACHD non‐HF controls to be hospitalized with HF or for cardiovascular disease. The crude rate of hospitalization for HF was 116 per 1000 person‐years for ACHD HF cases vs. 1.5 per 1000 person‐years for ACHD non‐HF controls (Table 2 ). Rates of cardiovascular disease hospitalization were also higher in ACHD HF cases compared with ACHD non‐HF controls (294 vs. 62 per 1000 person‐years). Time‐dependent adjusted HRs for HF and cardiovascular disease hospitalization are presented in Table 3 and Figures S2 and S3 . ACHD HF patients had higher time‐dependent HR than ACHD non‐HF patients and the curves of the HR estimates for HF hospitalization after the date of first admission for HF for ACHD HF patients demonstrated that the HR was highest within the first 30 days and subsequently decreased over the study period.
Heart failure patients with and without congenital heart disease
Baseline characteristics
Demographic and clinical characteristics of the ACHD HF cases and non‐ACHD HF controls are presented in Table 1 . The median age was 70 years (IQR 58–79 years), and 45.4% were female in both groups. Patients with simple, medium, and great complexity ACHD comprised 63.2%, 6.5%, and 1.3% of the ACHD HF cohort, respectively. The ACHD diagnoses were similar to the aforementioned ACHD with and without HF cohort. Both groups were more likely to be derived from lower than higher income quintiles. Non‐ACHD HF comparators were more likely than ACHD HF cases to have a history of ischemic heart disease (42.2% vs. 39.3%), hypertension (76.4% vs. 71.9%), diabetes (46.1% vs. 34.6%), renal disease (14.0% vs. 12.4%), or COPD (37.2% vs. 33.9%), and a higher Charlson comorbidity score (2 [IQR 0–3] vs. 2 [IQR 1–3]), and all comparisons were statistically significant (P < 0.001).
Resource utilization
Compared with non‐ACHD HF comparators, in the year prior to their first HF admission, ACHD HF cases were significantly more likely to have visited family physicians (97.3% vs. 96.2%, P < 0.007) or cardiologists (65.5% vs. 50.6%, P < 0.001). ACHD HF cases were also more likely to undergo non‐invasive cardiac testing such as echocardiograms (58.1% vs. 40.1%, P < 0.001) and Holter monitoring (16.1% vs. 11.4%, P < 0.001), but not stress testing (12.5% vs. 12.5%, P = 0.97). ACHD HF cases were more likely to undergo invasive procedures such as electrical cardioversion (4.4% vs. 2.1%, P < 0.001), cardiac catheterization (18.1% vs. 11.7%, P < 0.001), and defibrillator or pacemaker implantation (3.8% vs. 2.6%, P < 0.002). ACHD HF cases were also more likely to have undergone valvular intervention (7.0% vs. 1.1%, P < 0.001) or coronary artery bypass surgery (7.2% vs. 1.5%, P < 0.001), whereas non‐ACHD HF comparators were more likely to have received percutaneous coronary intervention (3.8% vs. 2.8%, P = 0.011).
Mortality in heart failure patients with or without adult congenital heart disease
Crude mortality did not differ between ACHD HF and non‐ACHD HF during 22 854 person‐years of follow‐up (Table 2 ; Kaplan–Meier curves shown in Figure S4 ), but 30 day and 1 year mortality rates were higher for ACHD HF after the first HF hospital admission (Appendix 4). Graphs of the time‐dependent adjusted HR demonstrated a U‐shaped appearance with higher HR within the first few years after HF hospitalization in ACHD HF cases, which decreased and then subsequently increased with time (Figure 4 and Table 3). When stratified by ACHD disease complexity, medium and high complexity ACHD patients had higher time‐dependent HR for mortality at 30 days which decreased and then increased once again (Table 4).
Figure 4.
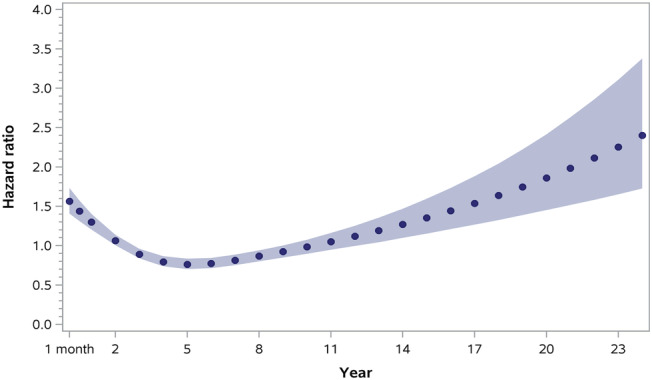
Time‐dependent adjusted hazard ratios for all‐cause mortality in matched heart failure (HF) cohort with and without adult congenital heart disease (ACHD).
Table 4.
Time‐dependent hazard ratios (95% CIs) for HF patients stratified by ACHD complexity
| Variable | ACHD simple | P value | ACHD medium | P value | ACHD great | P value | ACHD undefined | P value | Non‐ACHD HF |
|---|---|---|---|---|---|---|---|---|---|
| All‐cause death | |||||||||
| 30 day | 1.40 (1.24, 1.57) | <0.001 | 1.98 (1.49, 2.66) | <0.001 | 2.39 (0.88, 4.85) | 0.096 | 1.81 (1.59, 2.05) | <0.001 | Referent |
| 1 year | 1.15 (1.05, 1.26) | <0.001 | 1.55 (1.25, 1.91) | <0.001 | 2.10 (1.12, 3.51) | 0.036 | 1.55 (1.42, 1.70) | <0.001 | Referent |
| 5 year | 0.67 (0.61, 0.74) | <0.001 | 0.76 (0.55, 1.01) | 0.064 | 0.68 (0.09, 1.70) | 0.492 | 0.99 (0.87, 1.14) | 0.832 | Referent |
| 10 year | 0.90 (0.82, 0.99) | 0.036 | 1.00 (0.75, 1.29) | 0.920 | NR | n/a | 1.19 (1.04, 1.36) | 0.008 | Referent |
| 24 year | 2.50 (1.74, 3.68) | <0.001 | 2.71 (1.02, 6.81) | 0.048 | NR | n/a | 2.29 (1.37, 3.58) | <0.001 | Referent |
| HF hospitalization | |||||||||
| 30 day | 1.46 (1.30, 1.63) | <0.001 | 1.59 (1.16, 2.12) | 0.004 | 1.69 (0.65, 3.28) | 0.320 | 1.53 (1.31, 1.77) | <0.001 | Referent |
| 1 year | 1.22 (1.12, 1.33) | <0.001 | 1.57 (1.26, 1.91) | <0.001 | 2.12 (1.15, 3.39) | 0.016 | 1.50 (1.33, 1.69) | <0.001 | Referent |
| 5 year | 1.03 (0.90, 1.20) | 0.668 | 1.63 (1.18, 2.24) | 0.008 | 2.01 (0.53, 3.75) | 0.172 | 1.50 (1.26, 1.81) | <0.001 | Referent |
| 10 year | 1.41 (1.18, 1.76) | <0.001 | 1.87 (1.18, 2.97) | 0.008 | NR | n/a | 1.64 (1.27, 2.18) | <0.001 | Referent |
| 24 year | 3.44 (1.97, 6.84) | <0.001 | 2.86 (0.67, 11.73) | 0.152 | NR | n/a | 2.11 (0.88, 5.73) | 0.088 | Referent |
| CV hospitalization | |||||||||
| 30 day | 1.83 (1.65, 2.01) | <0.001 | 1.60 (1.25, 2.03) | <0.001 | 1.39 (0.59, 2.65) | 0.476 | 1.49 (1.30, 1.69) | <0.001 | Referent |
| 1 year | 1.38 (1.27, 1.50) | <0.001 | 1.40 (1.11, 1.70) | <0.001 | 1.75 (1.01, 2.73) | 0.040 | 1.55 (1.40, 1.74) | <0.001 | Referent |
| 5 year | 1.41 (1.27, 1.60) | <0.001 | 1.57 (1.18, 2.05) | 0.004 | 1.76 (0.84, 4.01) | 0.084 | 1.64 (1.43, 1.92) | <0.001 | Referent |
| 10 year | 1.94 (1.60, 2.49) | <0.001 | 2.15 (1.18, 3.93) | 0.004 | NR | n/a | 1.70 (1.22, 2.33) | 0.004 | Referent |
| 24 year | 4.80 (2.56, 9.80) | <0.001 | 5.20 (0.98, 32.55) | 0.064 | NR | n/a | 1.87 (0.67, 4.99) | 0.192 | Referent |
ACHD, adults with congenital heart disease; CV, cardiovascular; HF, heart failure; NR, not reportable due to small cells.
Hospitalization among heart failure patients with adult congenital heart disease or without adult congenital heart disease
Adults with HF and congenital heart disease were also more likely to be readmitted with HF or for any cardiovascular reason during the study time period. The crude rate of post‐discharge readmission for HF in ACHD HF cases was 1.2 times that of ACHD non‐HF comparators (124 vs. 100 per 1000 person‐years) and 1.5 times for readmission for cardiovascular disease (311 vs. 194 per 1000 person‐years). Time‐dependent HR for HF and cardiovascular disease hospitalizations were higher for all ACHD HF patients at all follow‐up times (Table 3 , Figures S5 and S6 ) regardless of ACHD complexity (Table 4 ).
Discussion
This is the first study to demonstrate that ACHD patients with HF have higher rates of mortality and hospitalization, especially in the first 2 years after the index HF admission, when compared with non‐ACHD HF patients. Worse outcomes occurred despite the majority of ACHD patients having simple congenital lesions and fewer cardiovascular factors (diabetes, hypertension, and ischaemic heart disease) compared with the non‐ACHD HF population. This finding suggests that the mechanisms responsible for HF in ACHD patients may differ when compared with the general HF population and require further study. We also found that ACHD patients with HF had higher mortality compared with ACHD patients without HF and that their risk of dying or being hospitalized was significantly increased within the first year after their index HF admission. In addition, ACHD patients with HF had a higher burden of cardiovascular risk factors and other comorbidities, which may be potential targets for prevention in this population.
Previous published studies have focused on examining mortality between ACHD patients with and without HF. Overall, mortality in ACHD patients with HF compared with those without HF has not changed over the past decade as our results are similar to previous published studies. 25 , 26 Compared with ACHD patients without HF, we found that mortality in ACHD HF patients was highest in the first year after the new HF admission and then decreased somewhat but still remained elevated. This is consistent with the results of Wang et al., who studied ACHD patients over 40 years who had a new HF hospitalization and reported HR of 24.56 (95% CI, 14.35–89.60) at 1 month, 6.01 (95% CI, 4.02–10.72) at 1 year, and 1.65 (95% CI, 0.93–3.57) at 5 years. 26 The totality of studies clearly signify the importance of referring these patients to specialized HF and ACHD clinics, which have been demonstrated to be effective in reducing hospitalizations and mortality. 27 , 28 This referral appears especially crucial in the first 12 months after hospitalization for new HF. Published studies suggest that more than 80% of ACHD patients, including those with moderate or severe ACHD lesions, are not followed at specialized clinics despite demonstrated benefits and current guideline recommendations. 11 , 29 Thus, there is a need to improve long‐term strategies to appropriately direct the care of these patients as mortality and hospitalizations remain elevated during the study period and so continuous life‐long monitoring may be warranted.
Our ACHD HF population was predominantly patients with simple ACHD lesions. While there are reports of increased survival of medium and great complexity ACHD patients, the distribution of lesion complexity in our study was similar to historical papers examining ACHD patients in the population. 1 , 13 , 14 , 30 It could be that while more patients with medium or great complexity lesions are surviving into adulthood, their ACHD‐related complications still limit their lifespan. 5 The pattern of mortality, HF, and cardiovascular hospitalization in simple ACHD patients with HF warrants further study. These patients with corrected or haemodynamically insignificant mild ACHD may have inherent ventricular abnormalities, which combined with acquired heart disease, leads to the development of HF and increased risk of mortality. 31 Overall, the assumption that these simple ACHD patients can be managed similarly to non‐ACHD patients may not be valid.
We found that cardiovascular risk factors and a history of cardiovascular disease were significantly more frequent in ACHD patients with HF compared with ACHD patients without HF. Studies have shown that the prevalence of coronary artery disease in ACHD patients is similar to that found in the general population and that coronary artery disease is a known predictor of outcomes, especially in ACHD patients over the age of 60 years. 25 , 30 , 32 , 33 For ACHD patients with HF, coronary artery disease, systemic arterial hypertension, and chronic kidney disease are all predictors of 1 year HF hospitalization. 26 These findings indicate that primary cardiovascular prevention is important in these patients and that the physicians managing these ACHD patients should actively treat these conditions.
Finally, ACHD HF patients also required greater healthcare resource utilization, driven by both their congenital heart disease and acquired comorbidities. 30 Given the increasing numbers and ages of ACHD patients, the demand for healthcare will only increase. 4 , 10 , 33 , 34 Future studies are needed to develop specific pathways to optimize HF care in these patients such that comprehensive management is provided at every HF stage. 35 , 36
Limitations
There are inherent limitations due to the use of administrative data. The use of hospital discharge abstracts to identify incident HF has been used previously 37 ; however, it is possible that patients with HF that did not require hospitalization may be missed, leading to potential misclassification. However, given the adverse prognosis associated with HF, this would tend to attenuate the differences between the HF and non‐HF groups, which were clinically and statistically significant. Confirmation of the ACHD diagnoses with surgical claims records could not be performed because prior to 1991, a family‐based health card number system was used, making it difficult to link interventions to specific individuals within a family unit. Consequently, due to unspecified ICD coding, a significant portion of our ACHD population was categorized as undefined ACHD lesion complexity. Also, despite trends of increasing survival of patients with great complexity ACHD lesions, the majority of patients studied in our population had simple ACHD complexity lesions. Finally, we did not conduct sex‐specific analyses, in part due to the multiplicity of analyses required, and also the post‐hoc exploratory nature of such an endeavour. However, future studies that test sex‐specific hypotheses related to the interaction between ACHD and HF may be warranted.
Conclusions
In this large population‐based study, ACHD patients with HF were at higher risk of short‐term mortality and rehospitalization compared with ACHD patients without HF and the general HF population. These higher risks were present regardless of ACHD complexity severity and may be due to, at least in part, comorbidities such as hypertension and coronary artery disease. Our findings underscore the need for aggressive primary and secondary prevention in this population by all physicians who manage adults with congenital heart disease.
Conflict of interest
There are no conflicts of interest.
Funding
Funding for this study was provided by the Heart and Stroke Foundation of Canada and a foundation grant from the Canadian Institutes of Health Research (FDN 148446). Dr Tsang is supported by a Heart and Stroke Foundation of Canada National New Investigator Award. Dr Lee is the Ted Rogers Chair in Heart Function Outcomes, University Health Network, University of Toronto. Dr Austin is supported by a Mid‐Career Investigator award from the Heart and Stroke Foundation. The Institute for Clinical Evaluative Sciences (ICES) is supported in part by a grant from the Ontario Ministry of Health and Long‐Term Care. The opinions, results, and conclusions are those of the authors, and no endorsement by the Ministry of Health and Long‐Term Care or by the Institute for Clinical Evaluative Sciences is intended or should be inferred. Parts of this material are based on data and information compiled and provided by CIHI. However, the analyses, conclusions, opinions, and statements expressed herein are those of the author and not necessarily those of CIHI.
Supporting information
Figure S1. Time‐dependent adjusted hazard ratios for 30‐day all‐cause mortality for the matched adult congenital heart disease (ACHD) cohort with and without heart failure (HF).
Figure S2. Time‐dependent adjusted hazard ratios for 1‐year all‐cause mortality in the matched adult congenital heart disease (ACHD) cohort with and without heart failure (HF).
Figure S3. Time‐dependent adjusted hazard ratios for 30‐day all‐cause mortality in the heart failure (HF) cohort with and without adult congenital heart disease (ACHD).
Figure S4. Time‐dependent adjusted hazard ratios for 1‐year all‐cause mortality in the heart failure (HF) cohort with and without adult congenital heart disease (ACHD).
Figure S5. Supporting Information.
Figure S6. Supporting Information.
ACHD complexity levels and associated ICD‐9 or ICD 10 code prefixes
| Diagnosis | ICD‐9 | ICD‐10 | Complexity |
|---|---|---|---|
| Univentricular heart hypoplastic left heart syndrome | 7453 7467 | Q204 Q206 Q224 Q226 Q234 | H |
| Truncus arteriosus | 7450 | Q200 Q214 | H |
| Transposition complex | 7451 | Q201 Q202 Q203 Q205 | H |
| Isomerism of atrial appendages | Q206 | H | |
| Hypoplastic right heart syndrome | Q226 | H | |
| Cor biloculare | 7457 | H | |
| Tetralogy of Fallot | 7452 | Q213 Q220 Q234 | M |
| Atrioventricular septal defect | 7456 | Q212 | M |
| Ebstein anomaly | 7462 | Q225 | M |
| Aortic coarctation | 7471 | Q251 | M |
| Anomalies of the pulmonary valve and pulmonary artery | 7473 7460 | Q220 Q221 Q222 Q223 Q255 Q256 Q257 | M |
| Congenital tricuspid valve disease | 7461 | Q224 | M |
| Congenital aortic stenosis and insufficiency subaortic stenosis | 7463 7464 | Q230 Q231 Q244 | S |
| Congenital mitral stenosis and insufficiency | 7465 7466 | Q232 Q233 | S |
| Ventricular septal defect | 7454 | Q210 | S |
| Atrial septal defect | 7455 | Q211 | S |
| Patent ductus arteriosus | 7470 | Q250 | S |
| Unspecified defect of septal closure | 7459 | Q219 | U |
| Anomalies of great veins | 7474 | Q26 | U |
| Other unspecified anomalies of the heart | 7468 7469 | Q24 Q208 Q209 | U |
| Other unspecified anomalies of circulation | 7479 | Q289 | U |
| Other unspecified anomalies of the aorta | 7472 | Q252 Q253 Q254 | U |
| Other congenital malformations of cardiac septa | Q218 | U | |
| Other congenital malformations of aortic and mitral valves | Q238 | U | |
| Other congenital malformations of tricuspid valve | Q228 | U | |
| Septal closure anom NEC | 7458 | U | |
| Congenital malformation of aortic and mitral valves, unspecified | Q239 | U | |
| Other congenital malformations of great arteries | Q258 | U | |
| Congenital malformation of tricuspid valve, unspecified | Q229 | U | |
| Congenital malformation of great arteries, unspecified | Q259 | U |
Diagnostic code prefixes for procedures, visits, and admissions
| Definition/code | |
|---|---|
| Physician care | |
| FP visit in past 1 year | OHIP spec = ‘00’ |
| Cardiologist visit in past 1 year | OHIP spec = ‘60’ |
| Any physician visit in past 1 year | OHIP fee code starts with ‘A’ |
| Diagnostic tests in past 1 year | |
| ECHO | OHIP fee code in: (‘G560’, ‘G561’, ‘G562’, ‘G566’, ‘G567’, ‘G568’, ‘G570’, ‘G571’, ‘G572’, ‘G574’, ‘G575’, ‘G577’, ‘G578’) |
| CIHI procedure code in: (‘0282’) ICD‐10 code in: (‘3IP30’) | |
| ECG | OHIP FEE CODE IN: (‘G310’, ‘G313’), no CIHI code needed |
| Stress test | OHIP FEE CODE IN: (‘G315’, ‘G319’, ‘G174’, ‘G111’, ‘G112’) |
| CIHI procedure code in: (‘0341’, ‘0342’, ‘0343’, ‘0344’) ICD‐10 code in: (‘2HZ08’) | |
| CATH | OHIP FEE CODE IN: (‘Z442’, ‘G297’) |
| CIHI procedure code in: (‘4892’, ‘4893’, ‘4894’, ‘4895’, ‘4896’, ‘4897’, ‘4898’, ‘4995’, ‘4996’, ‘4997’) ICD‐10 code in: (‘3IP10’) | |
| Holter | OHIP FEE CODE IN: (‘G311’, ‘G320’, ‘G65’, ‘G660’, ‘G661’, ‘G682’, ‘G683’, ‘G684’, ‘G685’, ‘G686’, ‘G687’, ‘G688’, ‘G689’, ‘G690’, ‘G692’, ‘G693’) |
| CIHI procedure code in: (‘0354’) ICD‐10 code in: (‘2HZ24JAKH’) | |
| PCI | OHIP FEE CODE IN: (‘Z434’, ‘G298’) |
| CIHI procedure code in: (‘4802’, ‘4803’) ICD‐10 code in: (‘1IJ50’, ‘1IJ57GQ’) | |
| CABG | OHIP FEE CODE IN: (‘R742’, ‘R743’) |
| CIHI procedure conde in: (‘481’) ICD‐10 code in: (‘1IJ76’) | |
| Valve | OHIP FEE CODE IN: (‘R736’, ‘R737’, ‘R738’, ‘R863’, ‘R876’, ‘R929’, ‘R730’, ‘R734’, ‘R735’, ‘R733’, ‘R773’, ‘R774’, ‘R724’, ‘R725’, ‘R772’, ‘R726’, ‘R727’, ‘R728’) |
| CIHI procedure code in: (‘4702’, ‘4703’, ‘4704’, ‘4705’, ‘4712’, ‘4713’, ‘4714’, ‘4715’, ‘4722’, ‘4723’, ‘4724’, ‘4725’, ‘4726’, ‘4727’, ‘4728’, ‘4729’, ‘4733’) ICD‐10 code in: (‘1HU’, ‘1HV’, ‘1HS’, ‘1HT’, ‘1HW’) | |
| Implantable defibrillator | OHIP FEE CODE IN: (‘R761’, ‘R753’) |
| CIHI procedure code in: (‘4974’) ICD‐10 code in: (‘1HZ53GRFS’, ‘1HZ53HAFS’, ‘1HZ53LAFS’, ‘1HZ53SYFS’) | |
| Pacemaker | OHIP FEE CODE IN: (‘R752’) |
| CIHI procedure code in: (‘4971’) ICD‐10 code in: (‘1HZ53GRNK’, ‘1HZ53GRNL’, ‘1HZ53GRNM’, ‘1HZ53GRNN’, ‘1HZ53GRFR’, ‘1HZ53LANK’, ‘1HZ53LANL’, ‘1HZ53LANM’, ‘1HZ53LANN’, ‘1HZ53QANK’, ‘1HZ53QANL’, ‘1HZ53QANM’, ‘1HZ53SYFR’) | |
| Electrical cardioversion | OHIP FEE CODE IN: (‘Z437’) |
| CIHI procedure code in: (‘1371’, ‘1372’) ICD‐10 code in: (‘1HZ09JA’) | |
| CHF and CVD | |
| CHF admission | admission to DAD with MRD as (ICD‐9428, ICD‐10 I50) |
| CVD admission | admission to DAD with |
| ICD‐9 (‘428’, ‘410’, ‘411’, ‘412’, ‘413’, ‘414’, ‘426’, ‘427’, ‘420’, ‘421’, ‘422’, ‘423’, ‘425’, ‘430’, ‘431’, ‘432’, ‘433’, ‘434’, ‘435’, ‘436’, ‘437’, ‘438’, ‘401’, ‘402’, ‘403’, ‘404’, ‘405’, ‘415’, ‘416’, ‘417’, ‘440’, ‘441’, ‘442’, ‘443’, ‘444’, ‘445’, ‘446’, ‘447’, ‘448’, ‘451’, ‘452’, ‘453’, ‘7854’, ‘390’, ‘391’, ‘392’, ‘393’, ‘394’, ‘395’, ‘396’, ‘397’, ‘398’, ‘7855’, ‘7802’, ‘7981’, ‘424’, ‘429’, ‘458’) | |
| ICD‐10 (‘I50’, ‘I20’, ‘I21’, ‘I22’, ‘I23’, ‘I24’, ‘I25’, ‘I44’, ‘I45’, ‘I46’, ‘I47’, ‘I48’, ‘I49’, ‘I30’, ‘I31’, ‘I32’, ‘I33’, ‘I40’, ‘I41’, ‘I42’, ‘I43’, ‘I514’, ‘I6’, ‘I10’, ‘I12’, ‘I13’, ‘I15’, ‘I26’, ‘I27’, ‘I28’, ‘I70’, ‘I71’, ‘I72’, ‘I73’, ‘I74’, ‘I77’, ‘I78’, ‘I79’, ‘I80’, ‘I81’, ‘I82’, ‘R02’, ‘I00’, ‘I01’, ‘I02’, ‘I05’, ‘I06’, ‘I07’, ‘I08’, ‘I09’, ‘R57’, ‘R55’, ‘R960’, ‘I34’, ‘I35’, ‘I36’, ‘I37’, ‘I38’, ‘I39’, ‘I51’, ‘I52’, ‘I95’, ‘I97’) | |
| The following ICES derived datasets were used to obtain comorbidity status | |
| ODD (to obtain DM status) | |
| ASTHMA | |
| HTN (hypertension) | |
| COPD | |
Mortality for ACHD patients with and without heart failure by accrual time
| ACHD HF | ACHD without HF | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Accrual time | Number of people | 30 day event # | 30 day mortality | 1 year event # | 1 year mortality | 30 day event # | 30 day mortality | 1 year event # | 1 year mortality |
| 1994–2001 | 1089 | 67 | 6.15 (4.88–7.76) | 204 | 18.73 (16.55–21.2) | SC | SC | 21 | 1.93 (1.26–2.95) |
| 2002–2009 | 1251 | 82 | 6.55 (5.32–8.08) | 247 | 19.74 (17.66–22.08) | SC | SC | 21 | 1.68 (1.1–2.57) |
| 2010–2017 | 1690 | 107 | 6.33 (5.27–7.61) | 373 | 22.07 (20.18–24.14) | SC | SC | 50 | 2.96 (2.25–3.89) |
Crude mortality for HF patients with and without ACHD by accrual time
| ACHD HF | Non‐ACHD with HF | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Accrual time | Number of people | 30 day event # | 30 day mortality | 1 year event # | 1 year mortality | 30 day event # | 30 day mortality | 1 year event # | 1 year mortality |
| 1994–2001 | 1162 | 69 | 5.94 (4.72–7.46) | 235 | 20.22 (18.04–22.67) | 34 | 2.93 (2.1–4.07) | 188 | 16.18 (14.19–18.44) |
| 2002–2009 | 1346 | 87 | 6.46 (5.27–7.92) | 292 | 21.69 (19.6–24.01) | 36 | 2.67 (1.94–3.69) | 218 | 16.2 (14.34–18.29) |
| 2010–2017 | 1828 | 128 | 7 (5.92–8.28) | 445 | 24.34 (22.45–26.39) | 43 | 2.35 (1.75–3.16) | 294 | 16.08 (14.48–17.86) |
Small cells (SC): number between 1 and 5, suppressed to maintain anonymity.
Tsang, W. , Silversides, C. K. , Rashid, M. , Roche, S. L. , Alonso‐Gonzalez, R. , Austin, P. C. , and Lee, D. S. (2021) Outcomes and healthcare resource utilization in adult congenital heart disease patients with heart failure. ESC Heart Failure, 8: 4139–4151. 10.1002/ehf2.13529.
References
- 1. Marelli AJ, Mackie AS, Ionescu‐Ittu R, Rahme E, Pilote L. Congenital heart disease in the general population: changing prevalence and age distribution. Circulation 2007; 115: 163–172. [DOI] [PubMed] [Google Scholar]
- 2. Khairy P, Ionescu‐Ittu R, Mackie AS, Abrahamowicz M, Pilote L, Marelli AJ. Changing mortality in congenital heart disease. J Am Coll Cardiol 2010; 56: 1149–1157. [DOI] [PubMed] [Google Scholar]
- 3. Marelli AJ, Ionescu‐Ittu R, Mackie AS, Guo L, Dendukuri N, Kaouache M. Lifetime prevalence of congenital heart disease in the general population from 2000 to 2010. Circulation 2014; 130: 749–756. [DOI] [PubMed] [Google Scholar]
- 4. Verheugt CL, Uiterwaal CS, van der Velde ET, Meijboom FJ, Pieper PG, van Dijk AP, Vliegen HW, Grobbee DE, Mulder BJ. Mortality in adult congenital heart disease. Eur Heart J 2010; 31: 1220–1229. [DOI] [PubMed] [Google Scholar]
- 5. Greutmann M, Tobler D, Kovacs AH, Greutmann‐Yantiri M, Haile SR, Held L, Ivanov J, Williams WG, Oechslin EN, Silversides CK, Colman JM. Increasing mortality burden among adults with complex congenital heart disease. Congenit Heart Dis 2015; 10: 117–127. [DOI] [PubMed] [Google Scholar]
- 6. Stout KK, Broberg CS, Book WM, Cecchin F, Chen JM, Dimopoulos K, Everitt MD, Gatzoulis M, Harris L, Hsu DT, Kuvin JT, Law Y, Martin CM, Murphy AM, Ross HJ, Singh G, Spray TL, American Heart Association Council on Clinical Cardiology, Council on Functional Genomics and Translational Biology, and Council on Cardiovascular Radiology and Imaging . Chronic heart failure in congenital heart disease: a scientific statement from the American Heart Association. Circulation 2016; 133: 770–801. [DOI] [PubMed] [Google Scholar]
- 7. Diller GP, Kempny A, Alonso‐Gonzalez R, Swan L, Uebing A, Li W, Babu‐Narayan S, Wort SJ, Dimopoulos K, Gatzoulis MA. Survival prospects and circumstances of death in contemporary adult congenital heart disease patients under follow‐up at a large tertiary centre. Circulation 2015; 132: 2118–2125. [DOI] [PubMed] [Google Scholar]
- 8. Lee DS, Austin PC, Stukel TA, Alter DA, Chong A, Parker JD, Tu JV. “Dose‐dependent” impact of recurrent cardiac events on mortality in patients with heart failure. Am J Med 2009; 122: 162–169 e1. [DOI] [PubMed] [Google Scholar]
- 9. Anderson K, Ross HJ, Austin PC, Fang J, Lee DS. Health care use before first heart failure hospitalization: identifying opportunities to pre‐emptively diagnose impending decompensation. JACC Heart Fail 2020; 8: 1024–1034. [DOI] [PubMed] [Google Scholar]
- 10. Willems R, Werbrouck A, De Backer J, Annemans L. Real‐world healthcare utilization in adult congenital heart disease: a systematic review of trends and ratios. Cardiol Young 2019; 29: 553–563. [DOI] [PubMed] [Google Scholar]
- 11. Van De Bruaene A, Hickey EJ, Kovacs AH, Crean AM, Wald RM, Silversides CK, Redington AN, Ross HJ, Alba AC, Billia F, Nair K. Phenotype, management and predictors of outcome in a large cohort of adult congenital heart disease patients with heart failure. Int J Cardiol 2018; 252: 80–87. [DOI] [PubMed] [Google Scholar]
- 12. Moussa NB, Karsenty C, Pontnau F, Malekzadeh‐Milani S, Boudjemline Y, Legendre A, Bonnet D, Iserin L, Ladouceur M. Characteristics and outcomes of heart failure‐related hospitalization in adults with congenital heart disease. Arch Cardiovasc Dis 2017; 110: 283–291. [DOI] [PubMed] [Google Scholar]
- 13. Zomer AC, Vaartjes I, van der Velde ET, de Jong HMY, Konings TC, Wagenaar LJ, Heesen WF, Eerens F, Baur LHB, Grobbee DE, Mulder BJM. Heart failure admissions in adults with congenital heart disease; risk factors and prognosis. Int J Cardiol 2013; 168: 2487–2493. [DOI] [PubMed] [Google Scholar]
- 14. Rodriguez FH 3rd, Moodie DS, Parekh DR, Franklin WJ, Morales DL, Zafar F, Adams GJ, Friedman RA, Rossano JW. Outcomes of heart failure‐related hospitalization in adults with congenital heart disease in the United States. Congenit Heart Dis 2013; 8: 513–519. [DOI] [PubMed] [Google Scholar]
- 15. Schultz SE, Rothwell DM, Chen Z, Tu K. Identifying cases of congestive heart failure from administrative data: a validation study using primary care patient records. Chronic Dis Inj Can 2013; 33: 160–166. [PubMed] [Google Scholar]
- 16. Stout KK, Daniels CJ, Aboulhosn JA, Bozkurt B, Broberg CS, Colman JM, Crumb SR, Dearani JA, Fuller S, Gurvitz M, Khairy P, Landzberg MJ, Saidi A, Valente AM, van Hare GF. 2018 AHA/ACC Guideline for the management of adults with congenital heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019; 139: e637–e697. [DOI] [PubMed] [Google Scholar]
- 17. Juurlink D, Preyra C, Croxford R, Chong A, Austin P, Tu J, Laupacis A. Canadian Institute for Health Information Discharge Abstract Database: A Validation Study. Toronto: Institute for Clinical Evaluative Sciences; 2006. [Google Scholar]
- 18. Lipscombe LL, Hwee J, Webster L, Shah BR, Booth GL, Tu K. Identifying diabetes cases from administrative data: a population‐based validation study. BMC Health Serv Res 2018; 18: 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gershon AS, Wang C, Guan J, Vasilevska‐Ristovska J, Cicutto L, To T. Identifying patients with physician‐diagnosed asthma in health administrative databases. Can Respir J 2009; 16: 183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gershon AS, Wang C, Guan J, Vasilevska‐Ristovska J, Cicutto L, To T. Identifying individuals with physician diagnosed COPD in health administrative databases. COPD 2009; 6: 388–394. [DOI] [PubMed] [Google Scholar]
- 21. Tu K, Campbell NR, Chen ZL, Cauch‐Dudek KJ, McAlister FA. Accuracy of administrative databases in identifying patients with hypertension. Open Med 2007; 1: e18–e26. [PMC free article] [PubMed] [Google Scholar]
- 22. Lee DS, Donovan L, Austin PC, Gong Y, Liu PP, Rouleau JL, Tu JV. Comparison of coding of heart failure and comorbidities in administrative and clinical data for use in outcomes research. Med Care 2005; 43: 182–188. [DOI] [PubMed] [Google Scholar]
- 23. Angaran P, Dorian P, Ha ACT, Thavendiranathan P, Tsang W, Leong‐Poi H, Woo A, Dias B, Wang X, Austin PC, Lee DS. Association of left ventricular ejection fraction with mortality and hospitalizations. J Am Soc Echocardiogr 2020; 33: 802–811 e6. [DOI] [PubMed] [Google Scholar]
- 24. Tam DY, Dharma C, Rocha R, Farkouh ME, Abdel‐Qadir H, Sun LY, Wijeysundera HC, Austin PC, Udell JA, Gaudino M, Fremes SE, Lee DS. Long‐term survival after surgical or percutaneous revascularization in patients with diabetes and multivessel coronary disease. J Am Coll Cardiol 2020; 76: 1153–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tutarel O, Kempny A, Alonso‐Gonzalez R, Jabbour R, Li W, Uebing A, Dimopoulos K, Swan L, Gatzoulis MA, Diller GP. Congenital heart disease beyond the age of 60: emergence of a new population with high resource utilization, high morbidity, and high mortality. Eur Heart J 2014; 35: 725–732. [DOI] [PubMed] [Google Scholar]
- 26. Wang F, Liu A, Brophy JM, Cohen S, Abrahamowicz M, Paradis G, Marelli A. Determinants of survival in older adults with congenital heart disease newly hospitalized for heart failure. Circ Heart Fail 2020; 13: e006490. [DOI] [PubMed] [Google Scholar]
- 27. Mylotte D, Pilote L, Ionescu‐Ittu R, Abrahamowicz M, Khairy P, Therrien J, Mackie AS, Marelli A. Specialized adult congenital heart disease care: the impact of policy on mortality. Circulation 2014; 129: 1804–1812. [DOI] [PubMed] [Google Scholar]
- 28. Kasper EK, Gerstenblith G, Hefter G, van Anden E, Brinker JA, Thiemann DR, Terrin M, Forman S, Gottlieb SH. A randomized trial of the efficacy of multidisciplinary care in heart failure outpatients at high risk of hospital readmission. J Am Coll Cardiol 2002; 39: 471–480. [DOI] [PubMed] [Google Scholar]
- 29. Beauchesne LM, Therrien J, Alvarez N, Bergin L, Burggraf G, Chetaille P, Gordon E, Kells CM, Kiess M, Mercier LA, Oechslin EN, Stein J, Tam JW, Taylor D, Williams A, Khairy P, Mackie AS, Silversides CK, Marelli AJ. Structure and process measures of quality of care in adult congenital heart disease patients: a pan‐Canadian study. Int J Cardiol 2012; 157: 70–74. [DOI] [PubMed] [Google Scholar]
- 30. Afilalo J, Therrien J, Pilote L, Ionescu‐Ittu R, Martucci G, Marelli AJ. Geriatric congenital heart disease: burden of disease and predictors of mortality. J Am Coll Cardiol 2011; 58: 1509–1515. [DOI] [PubMed] [Google Scholar]
- 31. Heiberg J, Ringgaard S, Schmidt MR, Redington A, Hjortdal VE. Structural and functional alterations of the right ventricle are common in adults operated for ventricular septal defect as toddlers. Eur Heart J Cardiovasc Imaging 2015; 16: 483–489. [DOI] [PubMed] [Google Scholar]
- 32. Giannakoulas G, Dimopoulos K, Engel R, Goktekin O, Kucukdurmaz Z, Vatankulu MA, Bedard E, Diller GP, Papaphylactou M, Francis DP, di Mario C, Gatzoulis MA. Burden of coronary artery disease in adults with congenital heart disease and its relation to congenital and traditional heart risk factors. Am J Cardiol 2009; 103: 1445–1450. [DOI] [PubMed] [Google Scholar]
- 33. Billett J, Cowie MR, Gatzoulis MA, Vonder Muhll IF, Majeed A. Comorbidity, healthcare utilisation and process of care measures in patients with congenital heart disease in the UK: cross‐sectional, population‐based study with case‐control analysis. Heart 2008; 94: 1194–1199. [DOI] [PubMed] [Google Scholar]
- 34. Seckeler MD, Thomas ID, Andrews J, Meziab O, Moe T, Heller E, Klewer SE. Higher cost of hospitalizations for non‐cardiac diagnoses in adults with congenital heart disease. Pediatr Cardiol 2018; 39: 437–444. [DOI] [PubMed] [Google Scholar]
- 35. Crossland DS, Van De Bruaene A, Silversides CK, Hickey EJ, Roche SL. Heart failure in adult congenital heart disease: from advanced therapies to end‐of‐life care. Can J Cardiol 2019; 35: 1723–1739. [DOI] [PubMed] [Google Scholar]
- 36. Steiner JM, Kirkpatrick JN, Heckbert SR, Sibley J, Fausto JA, Engelberg RA, Randall Curtis J. Hospital resource utilization and presence of advance directives at the end of life for adults with congenital heart disease. Congenit Heart Dis 2018; 13: 721–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Braga JR, Leong‐Poi H, Rac VE, Austin PC, Ross HJ, Lee DS. Trends in the use of cardiac imaging for patients with heart failure in Canada. JAMA Netw Open 2019; 2: e198766. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Time‐dependent adjusted hazard ratios for 30‐day all‐cause mortality for the matched adult congenital heart disease (ACHD) cohort with and without heart failure (HF).
Figure S2. Time‐dependent adjusted hazard ratios for 1‐year all‐cause mortality in the matched adult congenital heart disease (ACHD) cohort with and without heart failure (HF).
Figure S3. Time‐dependent adjusted hazard ratios for 30‐day all‐cause mortality in the heart failure (HF) cohort with and without adult congenital heart disease (ACHD).
Figure S4. Time‐dependent adjusted hazard ratios for 1‐year all‐cause mortality in the heart failure (HF) cohort with and without adult congenital heart disease (ACHD).
Figure S5. Supporting Information.
Figure S6. Supporting Information.


