Abstract
Aims
We tested the hypothesis that the effects of combined inspiratory muscle training and aerobic exercise training (IMT + AET) on muscle sympathetic nerve activity (MSNA) and forearm blood flow in patients with heart failure with reduced ejection fraction are more pronounced than the effects of AET alone.
Methods and results
Patients aged 30–70 years, New York Heart Association Functional Class II‐III, and left ventricular ejection fraction ≤40% were randomly assigned to four groups: IMT (n = 11), AET (n = 12), IMT + AET (n = 9), and non‐training (NT; n = 10). MSNA was recorded using microneurography. Forearm blood flow was measured by venous occlusion plethysmography and inspiratory muscle strength by maximal inspiratory pressure. IMT consisted of 30 min sessions, five times a week, for 4 months. Moderate AET consisted of 60 min sessions, three times a week for 4 months. AET (−10 ± 2 bursts/min, P = 0.03) and IMT + AET (−13 ± 4 bursts/min, P = 0.007) reduced MSNA. These responses in MSNA were not different between AET and IMT + AET groups. IMT (0.22 ± 0.08 mL/min/100 mL, P = 0.03), AET (0.27 ± 0.09 mL/min/100 mL, P = 0.01), and IMT + AET (0.35 ± 0.12 mL/min/100 mL, P = 0.008) increased forearm blood flow. No differences were found between groups. AET (3 ± 1 mL/kg/min, P = 0.006) and IMT + AET (4 ± 1 mL/kg/min, P = 0.001) increased peak oxygen consumption. These responses were similar between these groups. IMT (20 ± 3 cmH2O, P = 0.005) and IMT + AET (18 ± 3 cmH2O, P = 0.01) increased maximal inspiratory pressure. No significant changes were observed in the NT group.
Conclusions
IMT + AET causes no additive effects on neurovascular control in patients with heart failure with reduced ejection fraction compared with AET alone. These findings may be, in part, because few patients had inspiratory muscle weakness.
Keywords: Heart failure, Aerobic exercise training, Inspiratory muscle training, Muscle sympathetic nerve activity
Introduction
Despite advances in the treatment of heart failure, the prognosis of patients suffering from this syndrome remains poor. Fifty per cent of patients with heart failure with reduced ejection fraction (HFrEF) die during the first 5 years after the diagnosis of this syndrome. 1 Heart failure is characterized by sympathetic hyperactivation, peripheral vasoconstriction, and skeletal myopathy. 2 , 3 , 4 These physiological alterations contribute to exercise intolerance and dyspnoea, which adversely influences the quality of life in patients with HFrEF. 4
Exercise has been recommended for the treatment of patients with HFrEF. 5 Aerobic exercise training (AET) provokes a remarkable improvement in neurovascular control in these patients. Previous studies show that exercise training decreases muscle sympathetic nerve activity (MSNA) and peripheral vasoconstriction, 6 which contributes to the amelioration of skeletal myopathy. 2 Moreover, this nonpharmacological therapy improves functional capacity, quality of life, and prognosis in patients with heart failure. 7 , 8
More recently, inspiratory muscle training (IMT) emerged as a strategy for training in heart failure patients. This exercise paradigm reduces MSNA and increases muscle blood flow in patients with HFrEF. 9 , 10 , 11 Moreover, IMT seems to increase physical capacity in patients with cardiovascular disease. 9 , 12 Some investigators propose additional benefits of combined IMT + AET on exercise capacity, 13 , 14 but this training adaptation remains a controversial issue. 15 In the present study, we investigated the changes in MSNA, muscle blood flow, and functional capacity provoked by IMT, AET, and IMT + AET in patients with HFrEF.
Our hypothesis is that IMT + AET causes a more pronounced improvement in MSNA and muscle blood flow in patients with HFrEF than AET alone.
Methods
Study design
In this prospective, controlled trial, the patients were randomly divided into four groups: non‐training (NT, n = 10), IMT (n = 11), AET (n = 12), and combined IMT + AET (n = 9). Assessments were conducted at the beginning and after 4 months of interventions or clinical follow‐up. The study was approved by the Human Subject Protection Committee of the Clinical Hospital, School of Medicine, University of São Paulo (CAPPesq; 814/10). The trial is registered at www.ClinicalTrials.gov (NCT01747395). The investigation conforms to the principles outlined in the Declaration of Helsinki. All patients provided written informed consent to participate in the study.
It is noteworthy that eight patients in the NT, seven in the IMT, and seven in the AET groups were also involved in a previous study on skeletal muscle adaptation to IMT and AET. 16 The sample size calculation was based on a previous study about the effects of aerobic training on MSNA in patients with HFrEF. 6 The G‐Power 3.1.9.4 statistical program with a power of 90% and confidence interval of 95% (alpha of 5%) indicated that the sample size was five patients in each group. Because of dropouts observed in our previous studies, we made the decision to include more patients in each group.
Heart failure patients aged 30 to 70 years, New York Heart Association (NYHA) Functional Class II‐III, left ventricular ejection fraction (LVEF) ≤ 40%, peak oxygen uptake (VO2 peak) ≤ 20 mL/kg/min, body mass index ≤35 kg/m2, with optimized medication participated in the study. The exclusion criteria were myocardial infarction or heart surgery in the last 6 months, unstable angina, permanent pacemaker, cardiac resynchronization therapy or implantable cardiac defibrillator, atrial fibrillation, smoking, pregnancy, severe pulmonary disease, neurologic and orthopaedic diseases, neoplasia, chronic kidney disease with dialysis support, insulin‐dependent diabetes mellitus, and participation in a regular exercise programme. Changes in medication or hospitalization during the study were also exclusion criteria. The primary outcome was MSNA, and the secondary outcomes were muscle blood flow, physical capacity, respiratory muscle strength, and quality of life.
Measures and procedures
Physical capacity
Physical capacity was evaluated by a maximal incremental cardiopulmonary exercise test on a cycle ergometer (Via Sprint 150P; Ergoline, Bitz, Germany). A ramp protocol with work rate increments of 5–15 W every minute until exhaustion was conducted as previously described. 16 Breath‐by‐breath analysis in a computerized system was used to assess the oxygen uptake (VO2) and carbon dioxide production (Vmax Encore 29 System; VIASYS Healthcare Inc, Yorba Linda, CA). Peak VO2 was defined as the maximum attained VO2 at the end of the exercise period in which the patient could no longer maintain the cycle ergometer at 60 rpm. Anaerobic threshold, respiratory compensation point, and VE/VCO2 slope were determined as previously described. 16 , 17 , 18 The cardiopulmonary exercise test was performed in a blinded manner.
Muscle sympathetic nerve activity
Muscle sympathetic nerve activity was directly recorded from the peroneal nerve (multiunit postganglionic) using the microneurography technique. 19 Signals were amplified by 50 000 to 100 000 and band‐passed filtered (700–2000 Hz). For recordings and analysis, nerve activity was rectified and integrated (time constant: 0.1 s) to obtain a mean voltage display (662C‐4 Nerve Traffic Analysis System; The University of Iowa, Iowa City, IA). MSNA was expressed as burst frequency (burst per min) and burst incidence (bursts per 100 heart beats). They were visually analysed by two investigators (C. E. N. and L. M. A. C.) in a blinded manner.
Forearm blood flow
Venous occlusion plethysmography was used to measure forearm blood flow (FBF) as previously described. 20 Briefly, the right arm was elevated above heart level to ensure adequate venous drainage. A mercury‐filled silastic tube attached to a low‐pressure transducer was placed around the forearm and connected to a plethysmograph (Hokanson‐AI‐6, USA). Sphygmomanometer cuffs were placed around the wrist and upper arm. At 20 s intervals, the upper arm cuff was inflated above venous pressure for 10 s. The increase in the tension in the silastic tube reflects the increase in the forearm volume and, consequently, the vasodilation. Forearm vascular conductance (FVC) was calculated by dividing FBF by mean arterial pressure times 100.
Blood pressure and heart rate
Blood pressure was noninvasively evaluated on a beat‐to‐beat basis by using a finger photoplethysmograph (Finometer Pro; Finapres Medical Systems, Amsterdam, the Netherlands). Heart rate was also recorded by Finometer Pro. 21
Respiratory muscle strength
Maximal inspiratory pressure (MIP) and maximal expiratory pressure (MEP) was assessed using a pressure transducer (MVD‐500 Globalmed, Porto Alegre, Brazil). Maximal static inspiratory and expiratory pressure was determined as previously described. 22
Quality of life
Quality of life was assessed using the Minnesota Living with Heart Failure Questionnaire. 23
Other measurements
Cardiac function was evaluated by left ventricular ejection fraction (LVEF) using two‐dimensional echocardiography, Simpson method (IE33; Philips Medical Systems, Andover, MA). 24 Plasma B‐type natriuretic peptide (BNP) levels were measured by venous blood sampling.
Inspiratory muscle training
Inspiratory muscle training consisted of 30 min session, five times a week, for 4 months. All patients exercised at 60% MIP using a resistive loading device (POWER breathe Plus®, POWER breathe International Limited, UK). Patients were instructed to maintain diaphragmatic breathing at 15 to 20 breaths/min. The intensity was assessed once a week. Four training sessions per week were performed at home, and one session was performed at the hospital under supervision. 16
Aerobic exercise training
Moderate AET consisted of 60 min session, three times a week, for 4 months under supervision. It included 5 min stretching exercises, 40 min of cycling, 10 min of local strengthening exercises, and 5 min of cool down. The cycling exercise was performed at anaerobic threshold up to 10% below the respiratory compensation point. 16
Combined inspiratory muscle training and aerobic exercise training
Combined inspiratory muscle training and aerobic exercise training was performed as previously described. 16 Briefly, AET was performed three times per week and IMT five times per week for 4 months. When both AET and IMT were scheduled for the same day, IMT was conducted at an alternative time.
Evaluation protocol
The evaluation protocol consisted of three visits within 1 week of initiating the intervention or clinical follow‐up and within 1 week of concluding the intervention or clinical follow‐up.
First visit
In the morning, after a light meal with no caffeine, cardiac function and physical capacity were evaluated.
Second visit
At least 2 h after a light meal without caffeine, in a quiet and acclimatized room (21–22°C), in a supine position, the participant's leg was positioned for microneurography recordings. A microelectrode was inserted into the peroneal nerve. Cuffs were placed for FBF, blood pressure, and heart rate evaluations. Measurements were conducted throughout the protocol.
Third visit
Quality of life questionnaire was obtained, and respiratory muscle strength was evaluated. Thereafter, a blood sample was obtained for BNP measures.
Statistical analysis
The data are presented as means ± SE. The Kolmogorov–Smirnov and Levene tests were used to assess normality of distribution and homogeneity. Baseline characteristics and absolute changes (Δ = post − pre) were tested by one‐way analysis of variance (ANOVA). In the case of significance, Tukey post hoc multiple comparisons were conducted. Nonparametric tests (Kruskal–Wallis test, Mann–Whitney U‐test, and Wilcoxon matched‐pairs test) were used when needed. A χ 2 test was used to assess categorical data differences. Probability values of P < 0.05 were considered statistically significant.
Results
From January 2012 to July 2016, 135 patients were screened to participate in the study. Eighty‐two patients did not meet the inclusion criteria. Fifty‐three patients were included in the study. Eleven patients did not complete the study: one death in the NT group; one death, two acute heart failures, one myocardial infarction, and one drop out in the IMT group; one acute heart failure, one diagnosis of neoplasia, and one development of atrial fibrillation in the AET group; and one insufficient medication intake, and one drop out in the IMT + AET group. No event was related to IMT or AET. The minimum attendance in the AET, IMT, and IMT+AET groups was 75%. Thus, 42 patients completed participation in the study: NT (n = 10), IMT (n = 11), AET (n = 12), and IMT + AET (n = 09) (Figure 1 ). MSNA recording was not possible in seven patients. Thus, the results of MSNA are representative of nine patients in NT; nine patients in IMT; nine patients in AET; and eight patients in IMT + AET.
Figure 1.
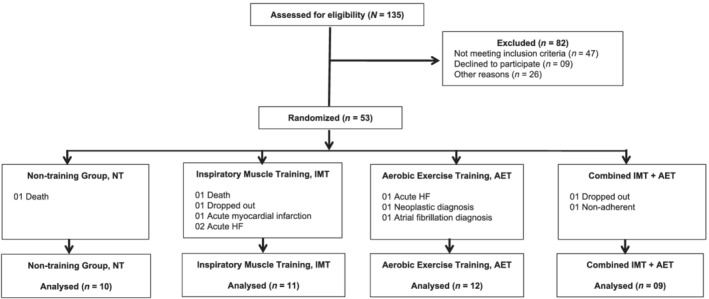
Schematic patient flowchart. NT, non‐training group; IMT, inspiratory muscle training group; AET, aerobic exercise training group; IMT + AET, combined inspiratory muscle training and aerobic exercise training group.
Baseline measures
Physical, clinical, functional capacity, neurovascular, and hemodynamic characteristics are shown in Table 1 . No differences in age, gender, weight, BMI, NYHA Functional Class, aetiology of heart failure, medication, co‐morbidity, LVEF, blood BNP concentration, peak VO2, VE/VCO2 slope, MIP, and incidence of inspiratory muscle weakness between groups were found. Baseline heart rate, blood pressure, MSNA, FBF, and FVC were also similar between groups. The quality of life was greater in the IMT + AET group compared with that in the NT, IMT, and AET groups.
Table 1.
Physical, clinical, and functional capacity in patients with heart failure with reduced ejection fraction assigned to inspiratory muscle training, aerobic exercise training, combined inspiratory muscle training and aerobic exercise training groups
| NT | IMT | AET | IMT + AET | P‐value | |
|---|---|---|---|---|---|
| (n = 10) | (n = 11) | (n = 12) | (n = 9) | ||
| Age, years | 57 ± 3 | 55 ± 3 | 57 ± 2 | 56 ± 3 | 0.94 |
| Gender, n | |||||
| Male | 6 | 3 | 7 | 7 | 0.14 |
| Female | 4 | 8 | 5 | 2 | |
| Weight, kg | 72 ± 4 | 77 ± 6 | 76 ± 6 | 80 ± 6 | 0.80 |
| BMI, kg/m2 | 27 ± 1 | 29 ± 2 | 28 ± 1 | 28 ± 1 | 0.80 |
| NYHA (II/III), n | 9/1 | 8/3 | 9/3 | 7/2 | 0.78 |
| Aetiology, n | |||||
| Ischemic | 6 | 1 | 4 | 6 | 0.20 |
| Hypertensive | 2 | 4 | 2 | 0 | |
| Idiopathic | 1 | 5 | 3 | 2 | |
| Chagasic | 1 | 0 | 2 | 0 | |
| Peripartum | 0 | 0 | 1 | 1 | |
| Alcoholic | 0 | 1 | 0 | 0 | |
| Medication, n | |||||
| Beta‐blocker | 10 | 11 | 12 | 9 | 1.00 |
| ACEI/ARB | 10 | 11 | 12 | 9 | 1.00 |
| Spironolactone | 10 | 10 | 12 | 8 | 0.50 |
| Diuretic | 9 | 11 | 12 | 7 | 0.17 |
| Statins | 5 | 6 | 5 | 6 | 0.72 |
| Hypoglycaemic drugs | 2 | 2 | 2 | 3 | 0.80 |
| Co‐morbidities, n | |||||
| Diabetes mellitus | 2 | 2 | 2 | 3 | 0.80 |
| Dyslipidaemia | 6 | 6 | 6 | 6 | 0.88 |
| Hypertension | 8 | 7 | 9 | 6 | 0.83 |
| LVEF, % | 25 ± 1 | 31 ± 2 | 26 ± 2 | 27 ± 1 | 0.23 |
| BNP, pg/mL | 197 ± 45 | 212 ± 116 | 265 ± 89 | 275 ± 90 | 0.91 |
| Peak VO2, mL/kg/min | 16 ± 1 | 16 ± 1 | 15 ± 1 | 17 ± 1 | 0.65 |
| VE/VCO2 slope | 37 ± 3 | 30 ± 2 | 34 ± 2 | 34 ± 2 | 0.10 |
| MIP, cmH2O | 85 ± 8 | 86 ± 9 | 87 ± 10 | 81 ± 8 | 0.97 |
| IMW, n | 2 | 3 | 3 | 3 | 0.93 |
| HR, b.p.m. | 68 ± 3 | 61 ± 2 | 65 ± 2 | 65 ± 3 | 0.40 |
| SBP, mmHg | 121 ± 4 | 121 ± 4 | 124 ± 5 | 116 ± 8 | 0.78 |
| DBP, mmHg | 66 ± 2 | 68 ± 4 | 69 ± 3 | 64 ± 4 | 0.72 |
| MBP, mmHg | 87 ± 2 | 86 ± 3 | 85 ± 3 | 85 ± 4 | 0.98 |
| MSNA, bursts/min | 44 ± 5 | 43 ± 3 | 46 ± 3 | 48 ± 4 | 0.80 |
| MSNA, HB | 64 ± 7 | 71 ± 6 | 72 ± 5 | 75 ± 6 | 0.62 |
| FBF, mL/min/100 mL | 2.05 ± 0.25 | 1.55 ± 0.16 | 1.51 ± 0.16 | 1.51 ± 0.17 | 0.17 |
| FVC, u.a. | 2.43 ± 0.30 | 1.82 ± 0.22 | 1.74 ± 0.21 | 1.88 ± 0.20 | 0.17 |
| Quality of life, u.a. | 55 ± 6 | 50 ± 6 | 56 ± 2 | 32 ± 4 a , b , c | 0.01 |
ACEI, angiotensin converter enzyme inhibitor; ARB, angiotensin receptor blockade; AET, aerobic exercise training group; BMI, body mass index; BNP, brain natriuretic peptide; DPB, diastolic blood pressure; FBF, forearm blood flow; FVC, forearm vascular conductance; HR, heart rate; IMT, inspiratory muscle training group; IMT + AET, combined inspiratory muscle training and aerobic exercise training group; IMW, inspiratory muscle weakness; LVEF, left ventricular ejection fraction; MIP, maximal inspiratory pressure; MSNA, muscle sympathetic nerve activity; NT, non‐training group; NYHA, New York Heart Association; SBP, systolic blood pressure; VE/VCO2, ventilation over carbon dioxide; VO2, oxygen uptake. Values are presented as mean ± standard error.
versus NT.
versus IMT.
versus AET.
Effects of training
The changes in peak VO2 and peak workload obtained in the cardiopulmonary exercise test were greater in the AET and IMT + AET groups compared with those observed in the NT group (Figure 2A and 2B , respectively). The changes in peak ventilation obtained in the cardiopulmonary exercise test were also greater in the AET and IMT + AET groups than in the NT group (Table 2 ). The changes in peak ventilation were greater in the IMT + AET group compared with those found in the IMT group. VE/VCO2 slope and oxygen consumption efficiency slope (OUES) were not different between groups (Table 2 ). Likewise, LVEF and BNP were not different between groups (Table 2 ). Inspiratory muscle training increased MIP. IMT and IMT + AET elicited greater changes in MIP compared with that in AET (Figure 2C ). The interventions did not change maximal expiratory pressure. IMT, AET, and IMT + AET elicited greater changes in quality of life than those observed in the NT controls (Figure 2D ).
Figure 2.
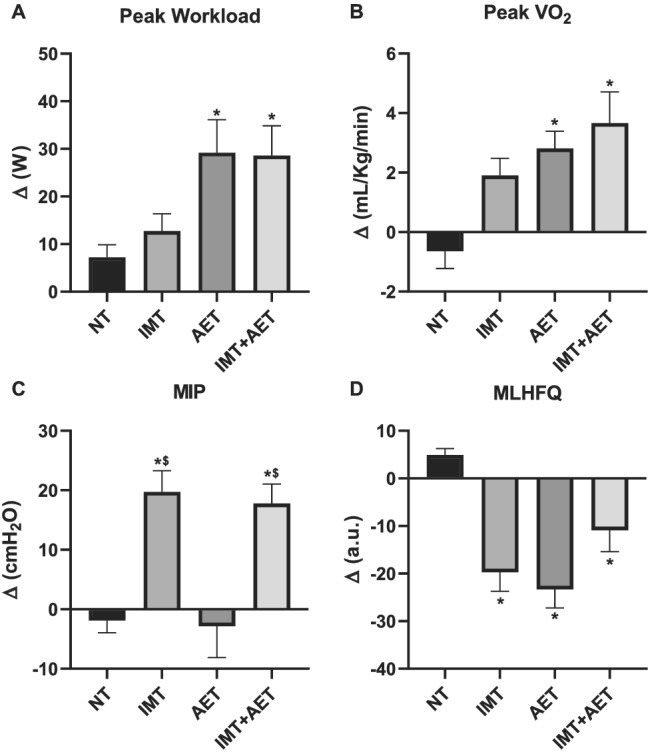
Effects of inspiratory muscle training (IMT), aerobic exercise training (AET), and combined inspiratory muscle training and aerobic exercise training (IMT+AET) on (A) peak workload, (B) peak oxygen consumption (VO2), (C) maximal inspiratory pressure (MIP), and (D) Minnesota Living with Heart Failure Questionnaire (MLHFQ) in patients with heart failure with reduced ejection fraction. NT, non‐trained. *vs. NT group, P < 0.05; $vs. AET group, P < 0.05.
Table 2.
Changes provoked by inspiratory muscle training, aerobic exercise training, and combined inspiratory muscle training and aerobic exercise training in patients with heart failure with reduced ejection fraction
| NT | IMT | AET | IMT+AET | P‐value | |
|---|---|---|---|---|---|
| (n = 10) | (n = 11) | (n = 12) | (n = 09) | ||
| LVEF, % | 1.60 ± 2.77 | 0.27 ± 1.86 | 2.75 ± 2.49 | 0.00 ± 1.60 | 0.80 |
| BNP, pg/mL | 58.80 ± 60.66 | −20.82 ± 36.39 | −2.92 ± 61.30 | −6.11 ± 52.63 | 0.74 |
| AT VO2, mL/kg/min | 0.04 ± 0.79 | −0.07 ± 0.56 | 1.21 ± 0.55 | 1.03 ± 0.67 | 0.35 |
| Peak VE, L/min | −4.69 ± 2.51 | 5.16 ± 2.13 | 7.95 ± 2.35 a | 16.59 ± 4.26 a , b | 0.002 |
| VE/VCO2 slope | −2.08 ± 2.43 | 0.80 ± 1.23 | −2.25 ± 1.55 | 2.09 ± 1.20 | 0.19 |
| OUES | −45.91 ± 66.60 | 46.87 ± 84.81 | 184.4 ± 76.33 | 21.87 ± 72.37 | 0.19 |
| MEP, cmH2O | −1.38 ± 4.72 | 3.00 ± 3.57 | 2.89 ± 4.25 | 2.66 ± 6.69 | 0.91 |
| HR, b.p.m. | −3.55 ± 2.96 | −1.77 ± 2.34 | −3.87 ± 1.67 | −2.60 ± 3.30 | 0.92 |
| SBP, mmHg | 2.56 ± 2.50 | 3.01 ± 3.89 | −4.10 ± 7.36 | 6.83 ± 4.80 | 0.52 |
| DPB, mmHg | 1.57 ± 2.06 | −1.57 ± 2.83 | −4.38 ± 2.29 | 1.26 ± 2.60 | 0.28 |
| MBP, mmHg | 1.22 ± 2.40 | −1.08 ± 3.50 | −2.98 ± 2.40 | 3.84 ± 3.25 | 0.40 |
AET, aerobic exercise training group; AT, anaerobic threshold; BNP, brain natriuretic peptide; DPB, diastolic blood pressure; HR, heart rate; IMT, inspiratory muscle training group; IMT + AET, combined inspiratory muscle training and aerobic exercise training group; LVEF, left ventricular ejection fraction; MBP, mean blood pressure; MEP, maximal expiratory pressure; NT, non‐training group; OUES, oxygen uptake efficiency slope; SBP, systolic blood pressure; VE, ventilation; VE/VCO2, ventilation over carbon dioxide; VO2, oxygen uptake. Values are presented as mean ± standard error.
versus NT, P < 0.05.
versus IMT, P < 0.05.
Aerobic exercise training and IMT + AET similarly reduced MSNA. The comparisons between groups showed that the changes in MSNA burst frequency and burst incidence after IMT + AET and AET alone were greater than those observed in the NT group (Figure 3A and 3B , respectively). All interventions improved FBF and FVC. The comparisons between groups showed that the changes in FBF and FVC after IMT, AET, and IMT + AET were greater than those observed in the NT group (Figure 4A and 4B ). Heart rate, systolic, diastolic, and mean blood pressures did not change with IMT, AET, or IMT + AET (Table 2 ).
Figure 3.
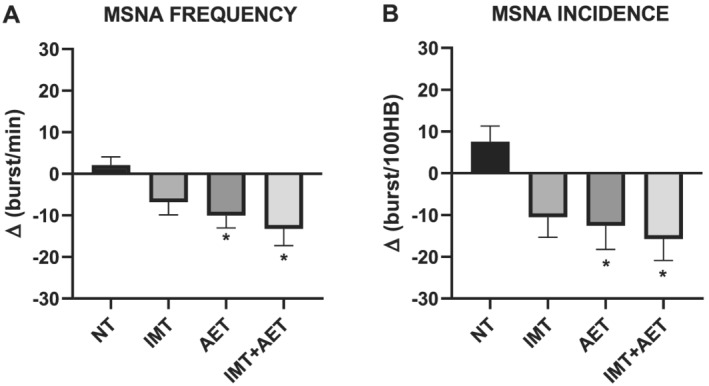
Effects of inspiratory muscle training (IMT), aerobic exercise training (AET), and combined inspiratory muscle training and aerobic exercise training (IMT + AET) on muscle sympathetic nerve activity (MSNA) burst frequency (A) and burst incidence (B) in patients with heart failure with reduced ejection fraction. NT, non‐trained. *vs. NT group, P < 0.05.
Figure 4.
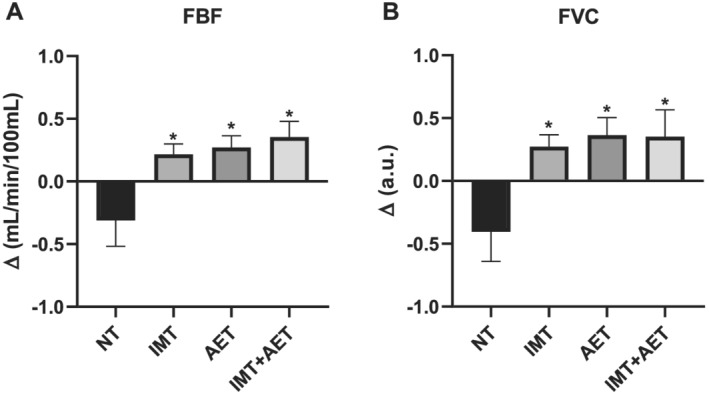
Effects of inspiratory muscle training (IMT), aerobic exercise training (AET), and combined inspiratory muscle training and aerobic exercise training (IMT + AET) on (A) forearm blood flow (FBF) and (B) forearm vascular conductance (FVC) in patients with heart failure with reduced ejection fraction. NT, non‐trained. *vs. NT group, P < 0.05.
Discussion
In the present study, we found that the magnitude of reduction in MSNA after 4 months of IMT + AET was similar to that observed after 4 months of AET alone. In addition, the increase in muscle blood flow was similar in patients who participated in IMT + AET and in patients who participated in AET alone.
Effects of combined inspiratory muscle training and aerobic exercise training
Because AET reduces MSNA, we raised the hypothesis that IMT + AET would elicit a greater decrease in MSNA than AET alone. Surprisingly, the changes in MSNA after IMT + AET were similar to those achieved by AET alone. There is no definitive explanation for these findings. One possibility is that the improvement in neurovascular control in exercise‐trained patients with HFrEF is fully achieved through AET. This interpretation seems to be unlikely because in a recent study, we found that high‐intensity interval training caused a more pronounced reduction in MSNA than moderate continuous aerobic exercise did. 25 IMT + AET is a very demanding exercise training protocol. Exercise sessions are scheduled every day and three times a week that include both AET and IMT sessions. This exercise paradigm may reduce motivation and interest in exercise sessions. Alternatively, the distribution of heart failure aetiology across groups may have influenced our findings. The rationale for this thought is that patients with heart failure associated with hypertension are those that mostly benefit from exercise training. 26 However, the distribution of aetiology was not significantly different between groups. 26 In line with MSNA responses, IMT + AET does not elicit additive effects on muscle blood flow. The changes in FBF and FVC were not different between IMT + AET and AET alone. As previously reported in a multicentre trial, 15 we found no additional improvement in physical capacity after IMT + AET compared with AET alone. Finally, IMT + AET did not change cardiac function (LVEF) and BNP levels.
Effects of aerobic exercise training
Accumulated evidence shows that AET decreases MSNA in patients with HFrEF. Since the first report in 2003, 6 we know that moderate AET for 4 months provokes a remarkable reduction in MSNA. This response has been consistently replicated in different sets of heart failure patients. AET decreases MSNA regardless of age, gender, and aetiology. 26 , 27 , 28 In addition, AET increases muscle blood flow at rest and during exercise in patients with heart failure. 2 , 29 This neurovascular adaptation is confirmed in the present study. AET decreased MSNA and increased muscle blood flow. Skeletal muscle changes were not involved in our study. However, it is legitimate to suggest that the reduction in MSNA and the increase in muscle blood flow favour the amelioration in skeletal myopathy and, as a consequence, physical capacity. 2 , 16 In the present study as in others, 30 , 31 a 10% to 20% increase in peak VO2 is achieved through AET in patients with HFrEF.
Effects of inspiratory muscle training
It has been suggested that respiratory myopathy plays a role in the exercise intolerance in patients with HFrEF. 32 Despite the increased ventilatory work, the inspiratory muscle capacity may be decreased in patients with muscle inspiratory strength levels close to normal. 33 This pathophysiological characteristic is the rational for prescribing IMT for patients with HFrEF. In a previous study, some investigators reported that home‐based IMT provokes reduction in MSNA in patients with chronic heart failure. 11 In the present study, we found that IMT decreases MSNA, but this response was not significant. The differences in MSNA responses between studies may be due to inspiratory muscle weakness. In the present study, inspiratory muscle weakness was not an inclusion criterion. Further analysis, in which the patients from the IMT group were divided according to inspiratory muscle strength, revealed that the patients with inspiratory muscle weakness had a 26% reduction in MSNA versus 10% reduction in patients with preserved inspiratory muscle strength (data not shown). These findings lead us to speculate that the magnitude of improvement in sympathetic outflow after IMT depends on the baseline inspiratory muscle strength. Of course, we cannot exclude the possibility that a longer period of IMT is necessary to reduce MSNA in patients with HFrEF.
Previous studies demonstrated that IMT increases resting and exercise muscle blood flow in patients with heart failure. 10 The present study confirms this finding. IMT substantially increased forearm blood flow. However, it should be noted that this vascular response occurs regardless of the muscle involved in the exercise training, which confers to IMT a systemic training adaptation.
The effect of IMT on physical capacity in patients with HFrEF has been a matter of controversy. Some investigators report no changes in peak VO2 after IMT. 15 In contrast, others show that IMT increases peak VO2 in patients with HFrEF. 9 In the present study, we found a 12% increase in peak VO2, which is clinically relevant. 34 , 35 The amelioration in inspiratory muscle strength may have contributed to the increase in peak VO2 in our patients. In fact, the patients with inspiratory muscle weakness had a 20% improvement in peak VO2 versus 8% in the patients with preserved inspiratory muscle strength (data not shown).
Study limitations
We recognize limitations in our study. Because inspiratory muscle strength was not considered as an inclusion criterion, someone could argue that the lack of additional effect of IMT + AET on neurovascular control was due to the fact that few patients had inspiratory muscle weakness. In fact, further analysis showed that the changes in MSNA were more pronounced in patients with inspiratory muscle weakness than in patients without inspiratory muscle weakness. These findings strengthen the hypothesis that the effects of IMT + AET depend upon the baseline inspiratory muscle strength. Future studies should focus on this issue. The small number of patients who finished the study may lead to the interpretation that the sample size limited our study. However, it is important to note that this is a Herculean study. It involves complex measures and procedures. Supervised AET was conducted three times a week and IMT five times a week in very sick patients. As previously stated, from January 2012 to July 2016, 135 patients were screened. Fifty‐three patients were included in the study. Eleven did not finish the study for different reasons. More importantly, the sample size calculation indicated five patients in each group. Ten patients in the NT, 11 in the IMT, 12 in the AET, and 9 in the IMT + AET group finished the study. Thus, it is unlikely that sample size limited our findings. We found that IMT + AET does not have additive effects on neurovascular control in patients with HFrEF.
Clinical perspectives
Aerobic exercise training is a unique strategy in the treatment of patients with HFrEF. This exercise paradigm remarkably reduces sympathetic nerve activity and increases muscle blood flow. This is important information in clinical practice. Both MSNA and forearm blood flow are independent predictors of mortality in patients with HFrEF. 36 The present findings suggest that IMT + AET has no additional benefit in the neurovascular control in patients with HFrEF. Increase in exercise intensity seems to be a more suitable strategy in the amelioration of neurovascular control in patients with HFrEF. A recent study provides evidence that high‐intensity interval training provokes further improvement in MSNA and muscle blood flow in patients with HFrEF. 25
Conflict of interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
This study was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP #2015/22814‐5). LMAC was supported by FAPESP (#2013/15651‐7). C.E.N. was supported by Conselho Nacional de Pesquisa (CNPq, #303573/2015‐5).
Trevizan, P. F. , Antunes‐Correa, L. M. , Lobo, D. M. L. , Oliveira, P. A. , de Almeida, D. R. , Abduch, M. C. D. , Mathias Junior, W. , Hajjar, L. A. , Kalil Filho, R. , and Negrão, C. E. (2021) Effects of inspiratory muscle training combined with aerobic exercise training on neurovascular control in chronic heart failure patients. ESC Heart Failure, 8: 3845–3854. 10.1002/ehf2.13478.
References
- 1. Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, van Wagner LB, Tsao CW. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics‐2020 update: a report from the American Heart Association. Circulation 2020; 141: e139–e596. [DOI] [PubMed] [Google Scholar]
- 2. Negrao CE, Middlekauff HR, Gomes‐Santos IL, Antunes‐Correa LM. Effects of exercise training on neurovascular control and skeletal myopathy in systolic heart failure. Am J Physiol Heart Circ Physiol 2015; 8: 792–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Coats AJ, Clark AL, Piepoli M, Volterrani M, Poole‐Wilson PA. Symptoms and quality of life in heart failure: the muscle hypothesis. Br Heart J 1994; 2: 36–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Clark AL, Poole‐Wilson PA, Coats AJ. Exercise limitation in chronic heart failure: central role of the periphery. J Am Coll Cardiol 1996; 5: 1092–1102. [DOI] [PubMed] [Google Scholar]
- 5. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL, American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines . 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013; 16: 240–327. [DOI] [PubMed] [Google Scholar]
- 6. Roveda F, Middlekauff HR, Rondon MU, Reis SF, Souza M, Nastari L, Barretto AC, Krieger EM, Negrão CE. The effects of exercise training on sympathetic neural activation in advanced heart failure: a randomized controlled trial. J Am Coll Cardiol 2003; 5: 854–860. [DOI] [PubMed] [Google Scholar]
- 7. Keteyian SJ, Leifer ES, Houston‐Miller N, Kraus WE, Brawner CA, O'Connor CM, Whellan DJ, Cooper LS, Fleg JL, Kitzman DW, Cohen‐Solal A, Blumenthal JA, Rendall DS, Piña IL, HF‐ACTION Investigators . Relation between volume of exercise and clinical outcomes in patients with heart failure. J Am Coll Cardiol 2012; 19: 1899–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Taylor RS, Walker S, Smart NA, Piepoli MF, Warren FC, Ciani O, Whellan D, O'Connor C, Keteyian SJ, Coats A, Davos CH, Dalal HM, Dracup K, Evangelista LS, Jolly K, Myers J, Nilsson BB, Passino C, Witham MD, Yeh GY, ExTraMATCH II Collaboration . Impact of exercise rehabilitation on exercise capacity and quality‐of‐life in heart failure: individual participant meta‐analysis. J Am Coll Cardiol 2019; 12: 1430–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dall'Ago P, Chiappa GR, Guths H, Stein R, Ribeiro JP. Inspiratory muscle training in patients with heart failure and inspiratory muscle weakness: a randomized trial. J Am Coll Cardiol 2006; 4: 757–763. [DOI] [PubMed] [Google Scholar]
- 10. Chiappa GR, Roseguini BT, Vieira PJ, Alves CN, Tavares A, Winkelmann ER, Ferlin EL, Stein R, Ribeiro JP. Inspiratory muscle training improves blood flow to resting and exercising limbs in patients with chronic heart failure. J Am Coll Cardiol 2008; 17: 1663–1671. [DOI] [PubMed] [Google Scholar]
- 11. Mello PR, Guerra GM, Borile S, Rondon MU, Alves MJ, Negrão CE, Dal Lago P, Mostarda C, Irigoyen MC, Consolim‐Colombo FM. Inspiratory muscle training reduces sympathetic nervous activity and improves inspiratory muscle weakness and quality of life in patients with chronic heart failure: a clinical trial. J Cardiopulm Rehabil Prev 2012; 5: 255–261. [DOI] [PubMed] [Google Scholar]
- 12. Ferreira JB, Plentz RD, Stein C, Casali KR, Arena R, Lago PD. Inspiratory muscle training reduces blood pressure and sympathetic activity in hypertensive patients: a randomized controlled trial. Int J Cardiol 2013; 1: 61–67. [DOI] [PubMed] [Google Scholar]
- 13. Winkelmann ER, Chiappa GR, Lima CO, Viecili PR, Stein R, Ribeiro JP. Addition of inspiratory muscle training to aerobic training improves cardiorespiratory responses to exercise in patients with heart failure and inspiratory muscle weakness. Am Heart J 2009; 5: 768.e1–768.e7. [DOI] [PubMed] [Google Scholar]
- 14. Neto MG, Martinez BP, Conceição CS, Silva PE, Carvalho VO. Combined exercise and inspiratory muscle training in patients with heart failure: a systematic review and meta‐analysis. J Cardiopulm Rehabil Prev 2016; 6: 395–401. [DOI] [PubMed] [Google Scholar]
- 15. Adamopoulos S, Schmid JP, Dendale P, Poerschke D, Hansen D, Dritsas A, Kouloubinis A, Alders T, Gkouziouta A, Reyckers I, Vartela V, Plessas N, Doulaptsis C, Saner H, Laoutaris ID. Combined aerobic/inspiratory muscle training vs. aerobic training in patients with chronic heart failure: The Vent‐HeFT trial: a European prospective multicentre randomized trial. Eur J Heart Fail 2014; 5: 574–582. [DOI] [PubMed] [Google Scholar]
- 16. Antunes‐Correa LM, Trevizan PF, Bacurau AVN, Ferreira‐Santos L, Gomes JLP, Urias U, Oliveira PA, Alves MJNN, de Almeida DR, Brum PC, Oliveira EM, Hajjar L, Kalil Filho R, Negrão CE. Effects of aerobic and inspiratory training on skeletal muscle microRNA‐1 and downstream‐associated pathways in patients with heart failure. J Cachexia Sarcopenia Muscle 2020; 1: 89–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Skinner JS, McLellan TM, McLellan TH. The transition from aerobic to anaerobic metabolism. Res Q Exerc Sport 1980; 1: 234–248. [DOI] [PubMed] [Google Scholar]
- 18. Chua TP, Ponikowski P, Harrington D, Anker SD, Webb‐Peploe K, Clark AL, Poole‐Wilson PA, Coats AJ. Clinical correlates and prognostic significance of the ventilatory response to exercise in chronic heart failure. J Am Coll Cardiol 1997; 7: 1585–1590. [DOI] [PubMed] [Google Scholar]
- 19. Fagius J, Wallin BG. Long‐term variability and reproducibility of resting human muscle nerve sympathetic activity at rest, as reassessed after a decade. Clin Auton Res 1993; 3: 201–205. [DOI] [PubMed] [Google Scholar]
- 20. Lobo DM, Trevizan PF, Toschi‐Dias E, Oliveira PA, Piveta RB, Almeida DR, Mady C, Bocchi EA, Lorenzi‐Filho G, Middlekauff HR, Negrão CE. Sleep‐disordered breathing exacerbates muscle vasoconstriction and sympathetic neural activation in patients with systolic heart failure. Circ Heart Fail 2016; 11: e003065. [DOI] [PubMed] [Google Scholar]
- 21. Guelen I, Westerhof BE, van der Sar GL, van Montfrans GA, Kiemeneij F, Wesseling KH, Bos WJ. Finometer, finger pressure measurements with the possibility to reconstruct brachial pressure. Blood Press Monit 2003; 1: 27–30. [DOI] [PubMed] [Google Scholar]
- 22. Neder JA, Andreoni S, Lerario MC, Nery LE. Reference values for lung function tests. II. Maximal respiratory pressures and voluntary ventilation. Braz J Med Biol Res 1999; 6: 719–727. [DOI] [PubMed] [Google Scholar]
- 23. Rector TS, Cohn JN. Assessment of patient outcome with the Minnesota Living with Heart Failure questionnaire: reliability and validity during a randomized, double‐blind, placebo‐controlled trial of pimobendan. Pimobendan Multicenter Research Group. Am Heart J 1992; 4: 1017–1025. [DOI] [PubMed] [Google Scholar]
- 24. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015; 3: 233–270. [DOI] [PubMed] [Google Scholar]
- 25. Sales ARK, Azevedo LF, Silva TOC, Rodrigues AG, Oliveira PA, Jordão CP, Andrade ACM, Urias U, Guimaraes GV, Bocchi EA, Alves MJNN, Hajjar LA, Filho RK, Grunewald ZI, Martinez‐Lemus LA, Padilla J, Negrão CE. High‐intensity interval training decreases muscle sympathetic nerve activity and improves peripheral vascular function in patients with heart failure with reduced ejection fraction. Circ Heart Fail 2020; 8: 007121. [DOI] [PubMed] [Google Scholar]
- 26. Antunes‐Correa LM, Ueno‐Pardi LM, Trevizan PF, Santos MR, da Silva CH, Franco FG, Alves MJ, Rondon MU, Negrao CE. The influence of aetiology on the benefits of exercise training in patients with heart failure. Eur J Prev Cardiol 2017; 4: 365–372. [DOI] [PubMed] [Google Scholar]
- 27. Antunes‐Correa LM, Melo RC, Nobre TS, Ueno LM, Franco FG, Braga AM, Rondon MU, Brum PC, Barretto AC, Middlekauff HR, Negrao CE. Impact of gender on benefits of exercise training on sympathetic nerve activity and muscle blood flow in heart failure. Eur J Heart Fail 2010; 1: 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Antunes‐Correa LM, Kanamura BY, Melo RC, Nobre TS, Ueno LM, Franco FG, Roveda F, Braga AM, Rondon MU, Brum PC, Barretto AC, Middlekauff HR, Negrao CE. Exercise training improves neurovascular control and functional capacity in heart failure patients regardless of age. Eur J Prev Cardiol 2012; 4: 822–829. [DOI] [PubMed] [Google Scholar]
- 29. Negrao CE, Middlekauff HR. Adaptations in autonomic function during exercise training in heart failure. Heart Fail Rev 2008; 1: 51–60. [DOI] [PubMed] [Google Scholar]
- 30. Ismail H, McFarlane JR, Nojoumian AH, Dieberg G, Smart NA. Clinical outcomes and cardiovascular responses to different exercise training intensities in patients with heart failure: a systematic review and meta‐analysis. JACC Heart Fail 2013; 6: 514–522. [DOI] [PubMed] [Google Scholar]
- 31. Ismail H, McFarlane JR, Dieberg G, Smart NA. Exercise training program characteristics and magnitude of change in functional capacity of heart failure patients. Int J Cardiol 2014; 1: 62–65. [DOI] [PubMed] [Google Scholar]
- 32. Laoutaris ID. The aerobic/resistance/inspiratory muscle training hypothesis in heart failure. Eur J Prev Cardiol 2018; 12: 1257–1262. [DOI] [PubMed] [Google Scholar]
- 33. Laoutaris ID, Adamopoulos S, Manginas A, Panagiotakos DB, Cokkinos DV, Dritsas A. Inspiratory work capacity is more severely depressed than inspiratory muscle strength in patients with heart failure: novel applications for inspiratory muscle training. Int J Cardiol 2016; 221: 622–626. [DOI] [PubMed] [Google Scholar]
- 34. Arena R, Myers J, Aslam SS, Varughese EB, Peberdy MA. Peak VO2 and VE/VCO2 slope in patients with heart failure: a prognostic comparison. Am Heart J 2004; 2: 354–360. [DOI] [PubMed] [Google Scholar]
- 35. Francis DP, Shamim W, Davies LC, Piepoli MF, Ponikowski P, Anker SD, Coats AJ. Cardiopulmonary exercise testing for prognosis in chronic heart failure: continuous and independent prognostic value from VE/VCO2 slope and peak VO2 . Eur Heart J 2000; 2: 154–161. [DOI] [PubMed] [Google Scholar]
- 36. Barretto AC, Santos AC, Munhoz R, Rondon MU, Franco FG, Trombetta IC, Roveda F, de Matos LN, Braga AM, Middlekauff HR, Negrão CE. Increased muscle sympathetic nerve activity predicts mortality in heart failure patients. Int J Cardiol 2009; 3: 302–307. [DOI] [PubMed] [Google Scholar]


