Abstract
Aims
The Fontan operation has resulted in improved survival in patients with single‐ventricle congenital heart disease. As a result, there is a growing population of teenagers and adults with a Fontan circulation. Many co‐morbidities have been increasingly recognized in this population due to the unique features of the Fontan circulation. Standardization of how Fontan co‐morbid conditions are defined will help facilitate understanding, consistency and interpretability of research and clinical experience. Unifying common language usage in Fontan is a critical precursor step for data comparison of research findings and clinical outcomes and ultimately accelerating improvements in management for this growing group of patients. This manuscript aimed to create unified definitions for morbidities seen after the Fontan palliation.
Methods
In association of many congenital heart disease organizations, this work used Delphi methodology to reach a broad consensus among recognized experts regarding commonly used terms in Fontan care and research. Each definition underwent at least three rounds of revisions to reach a final definition through surveys sent to experts in the field of single‐ventricle care.
Results
The process of reaching a consensus on multiple morbidities associated with the Fontan procedure is summarized in this manuscript. The different versions that preceded reaching the consensus are also presented in the Supporting Information. Table 1 represents the final definitions according to the consensus.
Conclusions
We propose the use of these definitions for clinical care, future research studies, registry development and clinical trials.
Keywords: Fontan, Single ventricle, Congenital heart disease, Cirrhosis, Portal hypertension, Consensus
Introduction
Since its introduction in 1969, the Fontan procedure has transformed the lives of many patients with single‐ventricle physiology, offering them the potential for survival and improved quality of life well into adulthood. 1 The original procedure was described by Fontan in patients with tricuspid atresia. 2 Indications for the operation have expanded over time to include all other types of single ventricle, with incremental modifications and better outcomes. 2 The procedure connects the systemic veins directly to the branch pulmonary arteries. Thus, the systemic venous blood flows passively to the lungs without a sub‐pulmonary ventricle. 3 , 4 The procedure now is performed in almost all patients born with single‐ventricle congenital heart disease at an age between 2 and 5 years. Good Fontan candidates have good systolic ventricular function, atrioventricular valve function and sinus rhythm. Before the procedure, the patient undergoes a haemodynamic evaluation with cardiac catheterization to ensure low ventricular filling pressure, low pulmonary vascular resistance (PVR) and adequate pulmonary arteries. 3 , 4
It is estimated that there are 70 000 patients alive with a Fontan circulation worldwide. The circulation is characterized by an elevated central venous pressure due to connection with the pulmonary arteries and a low cardiac output. 3 , 4 , 5 As more patients with a Fontan circulation reach adulthood, a variety of cardiac and non‐cardiac complications involving almost every organ system are increasingly common, presenting significant management challenges. 3 , 4 Early recognition may be important to address potential haemodynamic causes and potentially prevent the progression of life‐threatening extra‐cardiac disease. 5 Many different terms have been used to describe disease states associated with the Fontan circulation, often without explicit definition that has created confusion and uncertainty. Standardization of how Fontan associated morbidities are defined will help facilitate understanding, consistency and interpretability of research and clinical experience. 6 , 7 Additionally, standardized definitions will allow direct comparison between clinical studies and aggregation of trial and registry datasets. 8 The present consensus statement proposes standardized definitions for a set of comorbid conditions commonly associated with the Fontan circulation. We present a brief summary of the available literature before each definition.
Methods and evidence review
In association with leading organizations and initiatives (Alliance of Adult Research in Congenital Cardiology [AARCC], the International Society for Adult Congenital Heart Disease [ISACHD], the National Paediatric Cardiology Quality Improvement Collaborative [NPC‐QIC], The Fontan Outcomes Network [FON], Fontan Outcomes Registry using CMR Examinations [FORCE] and the International Fontan Interest Group [IFIG]), we applied the University of California Los Angeles/RAND modified Delphi methodology to arrive at definitions for a set of predetermined common Fontan‐related diagnoses. 9 , 10
In summary, in order for a term to make it through this entire process, each definition underwent at least three rounds of revisions. The initial draft of this document was circulated to the above organizations for approval, and suggestions were incorporated in the document without changing the consensus definitions. The final definitions are summarized in Table 1 , and the percentage of agreement through the three stages of consensus is summarized in the supporting information table. The process is summarized in Figure 1 , and the Delphi methodology is summarized in Figure 2 and the Supporting Information.
Table 1.
Terms and definitions for patients with a Fontan circulation
| Term | Definition |
|---|---|
| Fontan circulatory failure | A broad, non‐specific term describing dysfunction of the Fontan circulation that affects a patient's ability to carry out daily life activities. Aetiology may include ventricular dysfunction, atrioventricular valve failure, increased pulmonary vascular resistance, recurrent arrhythmia, Fontan pathway obstruction, lymphatic insufficiency or end‐organ dysfunction. Symptoms may be related to heart failure, hypoxaemia or end‐organ dysfunction including liver disease, protein‐losing enteropathy and plastic bronchitis. Severe Fontan circulatory failure is defined as a composite of mortality, listing for heart transplantation, Fontan takedown or the decision to initiate palliative care due to lack of candidacy for heart transplantation |
| Fontan takedown | The deconstruction of one or more of the Fontan connections to partial cavo‐pulmonary anastomosis and/or an aorto‐pulmonary shunt due to Fontan circulatory failure |
| Systolic dysfunction | The presence of single‐ventricle ejection fraction < 50%. Severe single‐ventricle systolic dysfunction is defined as an ejection fraction < 30% |
| Diastolic dysfunction | A state of decreased single‐ventricle compliance leading to increased filling pressures. An end‐diastolic pressure or pulmonary capillary wedge pressure ≥12 mmHg at rest or ≥15 mmHg after rapid volume expansion is suggestive of diastolic dysfunction |
| Fontan pathway obstruction | An anatomical or functional narrowing detected by catheterization or cross‐sectional imaging anywhere in the cavo‐pulmonary pathways. This may or may not manifest with flow discrepancy on cardiac MRI or haemodynamic perturbation with a gradient as low as 1 mmHg at cardiac catheterization |
| Elevated pulmonary vascular resistance | Pulmonary vascular resistance (PVR) above 2 indexed Wood units (Wood units * m2 body surface area) in individuals with normal or near‐normal body surface area (BSA). In individuals with extremes of BSA, no data currently exist for the Fontan circulation, but the clinically applied cut‐off is >2.3 Wood units, a value that has been considered to indicate poor outcomes in the context of biventricular circulations |
| Sustained atrial or ventricular tachycardia | An atrial or ventricular rate of more than 100 beats per minute in adults, or two standard deviations above the mean in children, lasting for more than 30 s, or <30 s if associated with haemodynamic instability, or of any duration, if arrhythmia therapy is used. This definition excludes sinus tachycardia. Atrial and ventricular tachycardia should be categorized separately |
| Bradycardia | Heart rate below 50 beats per minute in adults or below the 5th percentile for age in children |
| Lymphatic insufficiency | Any abnormality in the form or function of the lymphatic system resulting in a clinical disease state. Depending on the location of the lymphatic vessel decompression, lymphatic insufficiency may manifest variably as protein‐losing enteropathy, plastic bronchitis, ascites or pleural effusion |
| Plastic bronchitis | A pulmonary lymphatic disorder characterized by the leakage of proteinaceous material into the airways, resulting in episodic expectoration or bronchoscopic visualization of bronchial shaped casts that can be associated with respiratory distress, wheezing or significant airway obstruction |
| Protein‐losing enteropathy | The state of increased enteric protein loss (as measured by faecal α1 antitrypsin [spot > 54 mg/dL, α1 antitrypsin clearance > 27 mL/24 h without diarrhoea and >56 mL/24 h with diarrhoea] or by nuclear scintigraphy using technetium‐99m‐labelled albumin). It can be subclinical or associated with (1) hypoalbuminaemia < 3.5 g/dL and total protein < 6 g/dL and (2) any of the following clinical symptoms: edema, abdominal distention or discomfort, diarrhoea or effusions (ascites, pleural or pericardial effusions) |
| Low exercise performance | Peak VO2 below 50% of the predicted value for the general non‐affected population of equivalent age, sex and body size on a symptom‐limited maximal exercise test. High performance is considered present when peak VO2 is above 80% of predicted values |
| Fontan‐associated liver disease (FALD) | The broad spectrum of liver disease and its consequences, attributable to Fontan haemodynamics. FALD includes varying degrees of hepatic fibrosis, compensated and decompensated cirrhosis, focal nodular hyperplasia, laboratory evidence of hepatic injury or impaired synthetic function and hepatocellular neoplastic lesions |
| Cirrhosis | A histologic diagnosis defined by bridging fibrosis and nodular regeneration. Although defined by histology, the diagnosis can be strongly suggested by radiological assessment (e.g. nodular liver contour, features of portal hypertension such as splenomegaly, collateral vessels, ascites and significantly elevated liver stiffness using ultrasound or magnetic resonance). Clinical features supporting the presence of cirrhosis include signs of (1) vasodilation, such as spider naevi and palmar erythema, and (2) portal hypertension, such varices bleeding, ascites or portosystemic shunting including encephalopathy. Laboratory findings often observed with cirrhosis include elevated liver enzymes, bilirubin and prothrombin time, with low platelet count and albumin, in the absence of protein‐losing enteropathy |
| Portal hypertension | Portal hypertension is intrinsic to the Fontan circulation, as portal pressure is inherently elevated due to the high suprahepatic central venous pressures. Though rare, in some cases, it can have an intrahepatic component. Portal hypertension can be clinically silent or present as any combination of splenomegaly, ascites, varices and thrombocytopenia |
VO2, oxygen consumption; MRI, magnetic resonance imaging.
Figure 1.
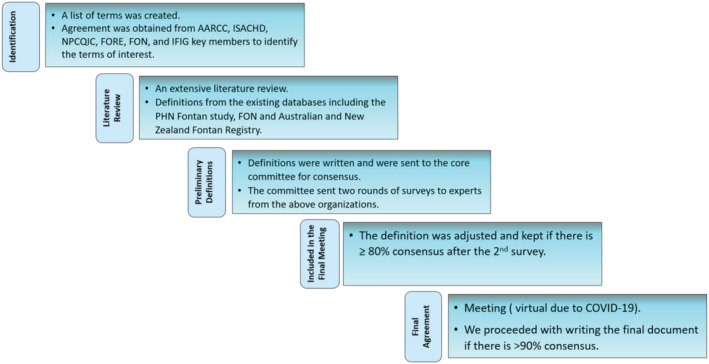
Steps in the process to reach consensus on standardized definitions.
Figure 2.
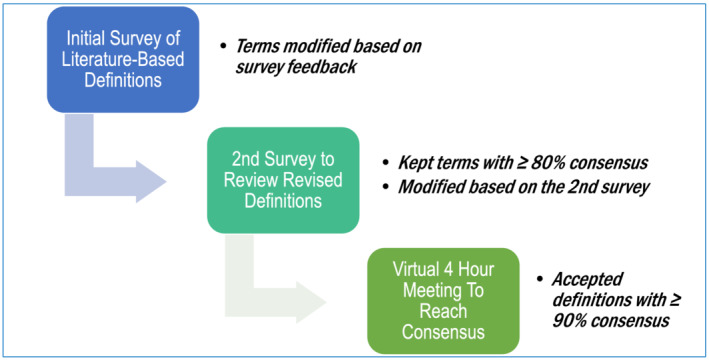
Steps in Delphi methodology.
Terms and definitions
Fontan circulatory failure
Fontan circulatory failure (FCF) is a broad, non‐specific term commonly used to indicate deteriorating clinical status. 11 , 12 Published studies have variably used FCF as a composite endpoint to include death, transplantation or listing for transplantation. 11 , 13 Additionally, the development of patient‐experienced exercise intolerance, inability to carry out daily life activities and Fontan takedown have sometimes been included in the definition of FCF. 2 , 14 , 15 , 16 Other variables included within the spectrum of FCF were heart failure symptoms and other co‐morbidities including arrhythmias, thromboembolism, protein‐losing enteropathy (PLE), plastic bronchitis (PB), oedema, persistent effusions and liver dysfunction. 15 , 17 , 18 The causes of FCF are similarly diverse and can include systolic or diastolic ventricular dysfunction, valve disease, elevated PVR, chronic arrhythmia, Fontan‐associated liver disease (FALD) or lymphatic insufficiency (PLE, ascites and PB). 14 , 19 As the aetiology of FCF is heterogeneous and ‘heart or pump failure’ is only seen in some of the cases, we avoided the term heart failure. This is similar to the term respiratory failure rather than lung failure used in pulmonary medicine as lung failure is a narrow term and capture only a portion of all cases. The consensus definition is below.
“A broad, non‐specific term describing dysfunction of the Fontan circulation that affects a patient's ability to carry out daily life activities. Etiology may include ventricular dysfunction, atrioventricular valve failure, increased pulmonary vascular resistance, recurrent arrhythmia, Fontan pathway obstruction, lymphatic insufficiency, or end‐organ dysfunction. Symptoms may be related to heart failure, Fontan pathway obstruction, hypoxemia or end‐organ dysfunction including liver disease, protein‐losing enteropathy, and plastic bronchitis. Severe Fontan circulatory failure is defined as a composite of mortality, listing for heart transplantation, Fontan takedown, or the decision to initiate palliative care due to lack of candidacy for heart transplantation.”
Fontan takedown
Fontan takedown to an intermediate palliative circulation is an important treatment option for patients with FCF. 16 The majority of Fontan takedown procedures are performed in the perioperative period after the Fontan operation to manage early FCF. Occasionally, Fontan takedown has been used as a strategy for late FCF years after the initial Fontan surgery. 16 , 20 In some cases, creating a fenestration or enlarging an existing fenestration may provide symptomatic improvement. In more severe cases, Fontan takedown usually includes reversion to a superior cavopulmonary connection, with or without an additional aorto‐pulmonary shunt. 20 , 21 This procedure carries a high perioperative morbidity and mortality as the haemodynamics and clinical state of the patient at the time of undergoing surgery are usually markedly deranged. 16 , 20 Despite these concerns, Fontan takedown can, in selected cases, effectively stabilize clinical status and act as a bridge to transplantation or provide time for rehabilitation and recovery prior to reattempting the Fontan procedure. 20 The consensus definition is below.
“The deconstruction of one or more of the Fontan connections to partial cavo‐pulmonary anastomosis and/or an aorto‐pulmonary shunt due to Fontan circulatory failure.”
Systolic ventricular dysfunction
A progressive decrease in ventricular systolic function was recognized as an important concern after the Fontan procedure. 19 , 22 The low ejection fraction can be related to altered preload without intrinsic ventricular dysfunction in some patients that may explain the lack of response to inotropes in such cases. Accurate evaluation of ventricular systolic function in patients with Fontan circulation by echocardiography remains challenging, and qualitative assessment is standard in many institutions given the heterogeneity of ventricular geometry in these patients. Contemporary techniques, including myocardial speckle tracking strain (deformation) imaging, may prove to be a helpful adjunct in evaluating myocardial function. 23 , 24 The complex three‐dimensional cardiac anatomy and the frequency of limited acoustic windows make cardiac magnetic resonance imaging (MRI) a gold standard to evaluate ventricular function in patients with a Fontan circulation. 24 , 25 Many adults with a Fontan circulation have a low resting cardiac index and reduced end‐systolic ventricular elastance. 26 Older age at the time of the evaluation and older age at Fontan seem to correspond to a higher risk for the development of systolic dysfunction in this patient population. 27 Once systolic dysfunction develops, and if severe enough, it may result in low cardiac output and symptoms of FCF. 14 The consensus definition is below.
“The presence of single ventricle ejection fraction <50%. Severe single ventricle systolic dysfunction is defined as an ejection fraction <30%.”
Diastolic dysfunction
Whereas systolic function can be preserved late after the Fontan operation, diastolic dysfunction is relatively common and may play a substantial role in morbidity. 28 , 29 , 30 The chronic preload insufficiency may result in myocardial remodelling, increased ventricular stiffness, increased filling pressures and eventually a progressive decline in cardiac output. 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 Not surprisingly, compliance and relaxation abnormalities in the single ventricle have been documented after the Fontan operation. 29
It is likely that diastolic function abnormalities start as a secondary phenomenon due to altered preload and then progresses into more advance stage with elevated filling pressures. Why only some patients develop diastolic dysfunction and elevated end‐diastolic pressures is likely multifactorial, but a genetic component may be contributory. 34 Evidence of diastolic abnormality may be detected in up to 68% of patients with a Fontan circulation at a mean age of years using echocardiographic criteria normally applied to biventricular hearts. 35 , 36 It has yet to be confirmed, however, whether these Doppler patterns predict filling pressures of the systemic ventricle. Feature tracking deformation (strain) imaging may prove helpful in detecting diastolic dysfunction in this patient population. 37 , 38 At the moment, direct measurement of ventricular end‐diastolic pressure remains the gold standard for the evaluation of diastolic dysfunction. 39 , 40 Various studies have suggested >10 or 12 mmHg as defining an elevated end‐diastolic pressure, with the recent emergence of the potential role for provocative testing (fluid challenge) to help identify patients with occult diastolic dysfunction. 39 , 40 , 41 Data on the haemodynamic definition, however, are sparse, and further research is needed to provide more definitive guidance on specific cut‐offs and best options for provocative testing. The consensus definition is below.
“A state of decreased single ventricle compliance leading to increased filling pressures. An end‐diastolic pressure or pulmonary capillary wedge pressure ≥ 12 mm Hg at rest or ≥ 15 mmHg after rapid volume expansion is suggestive of diastolic dysfunction.”
Fontan pathway obstruction
An unobstructed Fontan pathway is essential for optimal Fontan haemodynamics. 42 , 43 Obstruction in the Fontan pathway can happen at any level from the vena cavae to the branch pulmonary arteries. 42 Some common locations include the inferior limb of the Fontan baffle, the lateral tunnel or extra‐cardiac conduit and the branch pulmonary arteries. The left pulmonary artery (LPA) can become compressed by the aortic root or ascending aorta, especially in patients with hypoplastic left heart syndrome (HLHS). 44 Right pulmonary artery (RPA) torsion may be associated with the use of an extra‐cardiac conduit for Fontan completion. 45 Fontan baffle obstruction and branch pulmonary artery stenosis or compression may result in elevated Fontan baffle pressures and are associated with worse exercise capacity. 46 Obstruction in the Fontan circuit may be associated with significant power loss and may expose the abdominal viscera, especially the liver, to a greater congestive burden. Previous elegant work using computational flow dynamics showed that power loss correlates with the minimum cross‐sectional area of the pulmonary arteries in Fontan circulations. 47 , 48 Stent implantation in the Fontan conduit and lateral tunnel baffle to eliminate obstruction has been reported to optimize haemodynamics with few side effects. 42 , 49 The consensus definition is below.
“An anatomical or functional narrowing detected by catheterization or cross‐sectional imaging anywhere in the cavo‐pulmonary pathways. This may or may not manifest with flow discrepancy on cardiac MRI or hemodynamic perturbation with a gradient as low as 1 mmHg at cardiac catheterization.”
Elevated pulmonary vascular resistance
Growth, development and regulation of the pulmonary vasculature are inherently abnormal in patients with single‐ventricle physiology. Reduced pulmonary blood flow can start in fetal life and continues after Glenn and Fontan palliation. 31 , 32 Pulsatile flow is often absent after completion of the superior cavopulmonary connection. 31 , 50 However, even before the loss of pulsatility, significant regional maldistribution of pulmonary blood flow may occur due to abnormal connections, ductal constriction, external compression or local alterations in small vessels, resulting in heterogeneous vascular resistance. 51 Further, the normal vasodilatory mechanisms may be suppressed due to the absence of pulsatile blood flow and the effect of gravity on venous return to the lungs, which may only become evident during exertion. 52 , 53 The resultant physiology, the relatively low flow and low pressure system, the presence of multiple sources of pulmonary blood flow and high prevalence of arteriovenous malformations together make it challenging to assess pulmonary resistance. Further larger‐scale data are needed to define expected findings in resting PVR and pressure–flow relationships in the pulmonary circulation in the absence of a right ventricle and to define the relationship between PVR and clinical outcomes. 50 , 51
The existing small retrospective and prospective published series have consistently reported worse outcomes when resting PVR exceeded 2 indexed Wood units. 54 , 55 There are currently no published data using non‐indexed PVR in patients with a Fontan circulation. The consensus definition is below.
“Pulmonary vascular resistance (PVR) above 2 indexed Wood units (Wood units * m2 body surface area) in individuals with normal or near normal body surface area (BSA). In individuals with extremes of BSA, no data currently exist for the Fontan circulation, but the clinically applied cut‐off is > 2.3WU, a value which has been considered to indicate poor outcomes in the context of biventricular circulations.”
Lymphatic insufficiency
The pathophysiology of PLE and PB is intricately related to lymphatic system abnormalities 56 , 57 (Figure 3 ). Elevated venous pressure at the microcirculation level results in increased lymphatic production. Chronic severe lymphatic overload may cause pathologic dilation and consequent dysfunction of the lymphangion, the functional unit of the lymphatic system. Proximal drainage from the lymphatic system into the venous circulation may become impaired due to high central venous pressures, and physical obstruction due to stenosis at the lympho‐venous connections causing exit block from the lymphatic circulation or from impaired contractile function of the lymphangion. 58 This leads to focal sites of abnormal lymphatic drainage and lymphatic proliferation due to increased lymphangiogenesis signalling. 59 Lympho‐venous channels abound in the lymphatic circulation, and anatomic variation of such lympho‐venous connections together with lymphatic rupture to lower pressure compartments determines specific clinical phenotypes of lymphatic insufficiency. Lymph effluent usually drains proximally at the level of the innominate vein into the central venous circulation. However, a considerable variation of these proximal connections has been documented, and prior cardiac surgical intervention has been associated with impaired or obstructed lymphatic drainage to the venous system. 56 , 60 , 61 Decompressing lymph drainage into the pleural cavity results in chylous pleural effusion, drainage into the peritoneal cavity causes ascites, into the bronchial tree (PB), into the bowels (PLE) and into a specific limb, lymphedema praecox. 62 Importantly, there are many causes of ascites or pleural effusion in the Fontan circulation, and the presence of these findings does not necessarily indicate that lymphatic insufficiency is the primary cause (Figure 4 ).
Figure 3.
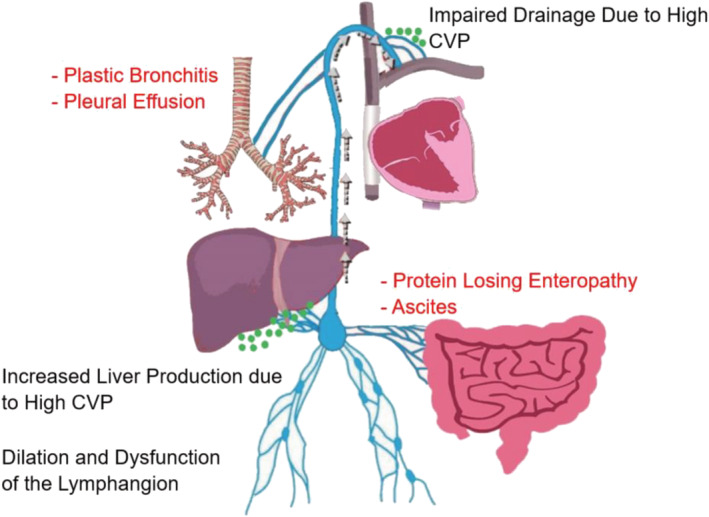
Lymphatic insufficiency in Fontan. The pathophysiology of the lymphatic insufficiency (black font) is increased central venous pressure (CVP), leading to increased lymphatic production from the liver and impaired lymphatic drainage. This leads to dilation and dysfunction of the lymphangion. Depending on the location for lymphatic decompression, a disease state may occur (red colour). The green dots represent the lymphatic drainage.
Figure 4.
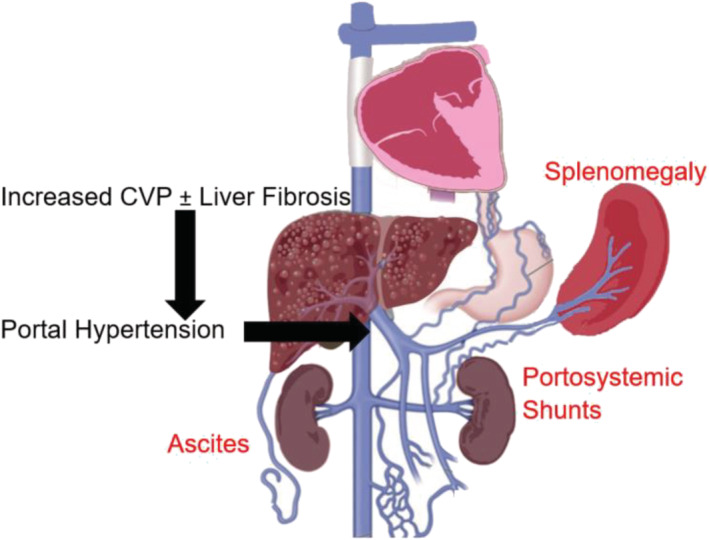
Portal hypertension in Fontan results from increased central venous pressure (CVP) with or without hepatic fibrosis (black font). Portal hypertension leads to splenomegaly, variceal ‘portosystemic shunts’ and ascites.
Imaging the lymphatic system remains challenging, but T2‐weighted MRI techniques are promising. 63 , 64 An improved understanding of lymphatic anatomy and the development of catheter‐based lymphatic interventions to embolize abnormal channels may result in significant improvement in the management of PB and some cases of PLE. 64 , 65 Additionally, diverting the lymphatic drainage of the thoracic duct to a lower pressure compartment (e.g. the systemic atrium) may alleviate symptoms of PLE and PB. 57 , 66 , 67 , 68 The consensus definition is below.
“Any abnormality in the form or function of the lymphatic system resulting in a clinical disease state. Depending on the location of the lymphatic vessel decompression, lymphatic insufficiency may manifest variably as protein‐losing enteropathy, plastic bronchitis, ascites or pleural effusion.”
Plastic bronchitis
PB is a serious complication of the Fontan circulation, occurring in 1%–4% of patients. 69 , 70 , 71 Risk factors include post‐operative prolonged pleural effusions or ascites. 72 The pathophysiology is thought to be related to abnormal lymphatic drainage around the airways. 61 The disease may manifest as episodic expectoration of bronchial‐shaped casts or visualization of these on bronchoscopy; this can cause wheezing and respiratory distress. Acute airway obstruction can be life threatening and may lead to suffocation, hypoxaemia and death. The consensus definition is below.
“A pulmonary lymphatic disorder characterized by the leakage of proteinaceous material into the airways, resulting in episodic expectoration or bronchoscopic visualization of bronchial shaped casts that can be associated with respiratory distress, wheezing or significant airway obstruction”
Protein‐losing enteropathy
PLE is a major cause of morbidity and mortality after the Fontan operation. 61 , 73 , 74 The prevalence is thought to be between 3% and 18% and can occur at any point after the Fontan operation. 61 , 75 The causes are poorly understood but likely include a combination of high venous pressure, abnormal mesenteric circulation, gut inflammation and abnormal lymphatic flow. 57 , 60 , 76 Though PLE is often progressive, some patients have a waxing‐and‐waning course. Many patients exhibit a preclinical phase with protein loss, but without any symptoms or hypoalbuminaemia. Remission can occur, with the only manifestation of prior PLE being either hypoalbuminaemia or abnormally high enteric protein loss. The consensus definition is below.
“The state of increased enteric protein loss [as measured by fecal α1 antitrypsin (spot > 54 mg/dl, α1 antitrypsin clearance > 27 ml/24 hours without diarrhea and > 56 ml/24 hours with diarrhea) or by nuclear scintigraphy using technetium‐99m labeled albumin]. It can be subclinical or associated with 1) hypoalbuminemia < 3.5 g/dl, and total protein < 6 g/dl and 2) any of the following clinical symptoms: edema, abdominal distention or discomfort, diarrhea, or effusions (ascites, pleural or pericardial effusions).”
Low exercise performance
Although Fontan completion may result in improved exercise capacity, as measured by peak oxygen consumption (VO2), patients with Fontan circulation generally have markedly reduced exercise capacity. 77 Most studies report a mean peak VO2 of 48%–65% of what would be predicted for age, sex and weight. 78 , 79 , 80 , 81 Decreased exercise capacity in patients with Fontan circulation is attributed to a blunted increase in stroke volume with exercise due to limited venous return, presumably due to a bottleneck in the pulmonary circulation. 82 Other factors include the inability to appropriately increase heart rate and systemic arterial desaturation. Further, the Fontan circulation is associated with decreased muscle mass, which may affect exercise capacity as it may decrease the ability to augment preload during exercise or to use oxygen during exercise. 83 , 84 Decreased exercise capacity is associated with increased mortality in patients with a Fontan circulation. 79 , 81 , 85 , 86 Extremely low peak VO2 in patients with a Fontan circulation (peak VO2 below 16.6 mL/kg/min) is an important mortality predictor. 85 , 87 Additionally, a decline in peak VO2 over time is also an important predictor of death and transplantation. 88 , 89 Peak heart rate and heart rate reserve may also provide prognostic information. 85 , 86 The consensus definition is below.
“Peak VO2 below 50% of the predicted value for the general non‐affected population of equivalent age, sex, and body size on a symptom‐limited maximal exercise test. High performance is considered present when peak VO2 is above 80% of predicted values.”
Fontan‐associated liver disease
FALD is a major non‐cardiac complication of the Fontan circulation, which may lead to substantial morbidity and early mortality. 4 The aetiology of FALD remains unclear, with elevated central venous pressure and possibly abnormal liver lymphatic drainage as important contributors. 90 Liver fibrosis is ubiquitous in adult patients with a Fontan circulation, and about two‐thirds have severe fibrosis. 91 Some risk factors recognized in previous studies included older age, longer time since the Fontan completion and perioperative insults. 4 , 12 , 91 , 92 . Weight control to prevent steatohepatitis, avoiding alcohol and hepatotoxic medication avoidance and vaccination against hepatitis may help slow progression of FALD. Recent expert guidance includes screening for liver disease every 3–5 years in childhood and every 1–2 years in adolescents using laboratory screening, 4 though there have been no empirical studies evaluating the benefit of any particular approach to liver evaluation. Abdominal ultrasound is reasonable at baseline and referral to hepatology should be considered in patients with abnormal screening results. 4 , 90 , 93 In addition, abdominal cross‐sectional imaging using MRI and computerized tomography (CT) scan is an important tool to screen for liver lesions. 94 The consensus definition is below.
“The broad spectrum of liver disease and its consequences, attributable to Fontan hemodynamics. FALD includes varying degrees of hepatic fibrosis, compensated and decompensated cirrhosis, focal nodular hyperplasia, laboratory evidence of hepatic injury or impaired synthetic function, and hepatocellular neoplastic lesions.”
Please refer to the Supporting Information for the complete list of definitions and the steps taken to reach consensus on each definition. A complete list of references are also presented.
Conclusion
The above terms and definitions represent the consensus of a large panel of experts in single‐ventricle physiology and selected‐non‐cardiac specialties. We propose utilizing these definitions for clinical care, future research studies, registries and clinical trials. Unifying common language usage in Fontan is a critical precursor step for data aggregation, comparison of research findings and clinical outcomes and ultimately accelerating improvements in management for this growing group of patients.
Conflict of interest
None declared.
Funding
No authors report any funding related to this topic.
Tweet: #Fontan terminology is confusing and imprecisely defined. This consensus statement defines key morbidities to standardize the use of language in clinical care, research and registries. Twitter: @AlsaiedTarek @rahulrathodmd @sasha_opo @wernerbudts.
Supporting information
Supporting Information
Table S1. Oral medication at hospital discharge in patients without in‐hospital.
Acknowledgements
This work was funded by the Evan's Heart Fund.
Alsaied, T. , Rathod, R. H. , Aboulhosn, J. A. , Budts, W. , Anderson, J. B. , Baumgartner, H. , Brown, D. W. , Cordina, R. , D'udekem, Y. , Ginde, S. , Goldberg, D. J. , Goldstein, B. H. , Lubert, A. M. , Oechslin, E. , Opotowsky, A. R. , Rychik, J. , Schumacher, K. R. , Valente, A. M. , Wright, G. , and Veldtman, G. R. (2021) Reaching consensus for unified medical language in Fontan care. ESC Heart Failure, 8: 3894–3905. 10.1002/ehf2.13294.
Tarek Alsaied and Rahul H. Rathod contributed equally to this work and are considered first authors. Author names are presented in alphabetical order except for the first two authors and the last author.
References
- 1. Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax 1971; 26: 240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. d'Udekem Y, Iyengar AJ, Cochrane AD, Grigg LE, Ramsay JM, Wheaton GR, Penny DJ, Brizard CP. The Fontan procedure: contemporary techniques have improved long‐term outcomes. Circulation 2007; 116: I157–I164. [DOI] [PubMed] [Google Scholar]
- 3. Alsaied T, Bokma JP, Engel ME, Kuijpers JM, Hanke SP, Zuhlke L, Zhang B, Veldtman GR. Factors associated with long‐term mortality after Fontan procedures: a systematic review. Heart 2017; 103: 104–110. [DOI] [PubMed] [Google Scholar]
- 4. Daniels CJ, Bradley EA, Landzberg MJ, Aboulhosn J, Beekman RH 3rd, Book W, Gurvitz M, John A, John B, Marelli A, Marino BS, Minich LL, Poterucha JJ, Rand EB, Veldtman GR. Fontan‐associated liver disease: Proceedings from the American College of Cardiology stakeholders meeting, October 1 to 2, 2015, Washington DC. J Am Coll Cardiol 2017; 70: 3173–3194. [DOI] [PubMed] [Google Scholar]
- 5. Elder RW, McCabe NM, Hebson C, Veledar E, Romero R, Ford RM, Mahle WT, Kogon BE, Sahu A, Jokhadar M, McConnell ME, Book WM. Features of portal hypertension are associated with major adverse events in Fontan patients: the VAST study. Int J Cardiol 2013; 168: 3764–3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stout KK, Daniels CJ, Aboulhosn JA, Bozkurt B, Broberg CS, Colman JM, Crumb SR, Dearani JA, Fuller S, Gurvitz M, Khairy P, Landzberg MJ, Saidi A, Valente AM, Van Hare GF. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol 2019; 73: 1494–1563. [DOI] [PubMed] [Google Scholar]
- 7. Stout KK, Daniels CJ, Aboulhosn JA, Bozkurt B, Broberg CS, Colman JM, Crumb SR, Dearani JA, Fuller S, Gurvitz M, Khairy P, Landzberg MJ, Saidi A, Valente AM, Van Hare GF. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association task orce on clinical practice guidelines. J Am Coll Cardiol 2019; 73: e81–e192. [DOI] [PubMed] [Google Scholar]
- 8. Udink Ten Cate FE, Hannes T, Germund I, Khalil M, Huntgeburth M, Apitz C, Brockmeier K, Sreeram N. Towards a proposal for a universal diagnostic definition of protein‐losing enteropathy in Fontan patients: a systematic review. Heart 2016; 102: 1115–1119. [DOI] [PubMed] [Google Scholar]
- 9. Gurvitz M, Marelli A, Mangione‐Smith R, Jenkins K. Building quality indicators to improve care for adults with congenital heart disease. J Am Coll Cardiol 2013; 62: 2244–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fries JF. Methodology of validation of criteria for SLE. Scand J Rheumatol Suppl 1987; 65: 25–30. [DOI] [PubMed] [Google Scholar]
- 11. Deal BJ, Jacobs ML. Management of the failing Fontan circulation. Heart 2012; 98: 1098–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goldberg DJ, Shaddy RE, Ravishankar C, Rychik J. The failing Fontan: etiology, diagnosis and management. Expert Rev Cardiovasc Ther 2011; 9: 785–793. [DOI] [PubMed] [Google Scholar]
- 13. Egbe AC, Connolly HM, Miranda WR, Ammash NM, Hagler DJ, Veldtman GR, Borlaug BA. Hemodynamics of Fontan failure: the role of pulmonary vascular fisease. Circ Heart Fail 2017; 10: e004515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Book WM, Gerardin J, Saraf A, Valente AM, Rodriguez F 3rd. Clinical phenotypes of Fontan failure: implications for management. Congenit Heart Dis 2016; 11: 296–308. [DOI] [PubMed] [Google Scholar]
- 15. Hebson CL, McCabe NM, Elder RW, Mahle WT, McConnell M, Kogon BE, Veledar E, Jokhadar M, Vincent RN, Sahu A, Book WM. Hemodynamic phenotype of the failing Fontan in an adult population. Am J Cardiol 2013; 112: 1943–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Almond CS, Mayer JE Jr, Thiagarajan RR, Blume ED, del Nido PJ, McElhinney DB. Outcome after Fontan failure and takedown to an intermediate palliative circulation. Ann Thorac Surg 2007; 84: 880–887. [DOI] [PubMed] [Google Scholar]
- 17. De Rita F, Crossland D, Griselli M, Hasan A. Management of the failing Fontan. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2015; 18: 2–6. [DOI] [PubMed] [Google Scholar]
- 18. Hebson C, Book W, Elder RW, Ford R, Jokhadar M, Kanter K, Kogon B, Kovacs AH, Levit RD, Lloyd M, Maher K, Reshamwala P, Rodriguez F, Romero R, Tejada T, Marie Valente A, Veldtman G, McConnell M. Frontiers in Fontan failure: a summary of conference proceedings. Congenit Heart Dis 2017; 12: 6–16. [DOI] [PubMed] [Google Scholar]
- 19. Alsaied T, Bokma J, Engel M, Kuijpers J, Hanke S, Zuhlke L, Zhang B, Veldtman G. Predicting long‐term mortality after Fontan procedures: a risk score based on 6707 patients from 28 studies. Submitted 2016. [DOI] [PubMed]
- 20. Trezzi M, Cetrano E, Giannico S, Iorio FS, Albanese SB, Carotti A. Long‐term outcomes after extracardiac Fontan takedown to an intermediate palliative circulation. Ann Thorac Surg 2018; 105: 599–605. [DOI] [PubMed] [Google Scholar]
- 21. Casella SL, Kaza A, Del Nido P, Lock JE, Marshall AC. Targeted increase in pulmonary blood flow in a bidirectional Glenn circulation. Semin Thorac Cardiovasc Surg 2018; 30: 182–188. [DOI] [PubMed] [Google Scholar]
- 22. Eicken A, Fratz S, Gutfried C, Balling G, Schwaiger M, Lange R, Busch R, Hess J, Stern H. Hearts late after Fontan operation have normal mass, normal volume, and reduced systolic function: a magnetic resonance imaging study. J Am Coll Cardiol 2003; 42: 1061–1065. [DOI] [PubMed] [Google Scholar]
- 23. Rathod RH, Prakash A, Kim YY, Germanakis IE, Powell AJ, Gauvreau K, Geva T. Cardiac magnetic resonance parameters predict transplantation‐free survival in patients with Fontan circulation. Circ Cardiovasc Imaging 2014; 7: 502–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ghelani SJ, Harrild DM, Gauvreau K, Geva T, Rathod RH. Comparison between echocardiography and cardiac magnetic resonance imaging in predicting transplant‐free survival after the Fontan operation. Am J Cardiol 2015; 116: 1132–1138. [DOI] [PubMed] [Google Scholar]
- 25. Bossers SS, Kapusta L, Kuipers IM, van Iperen G, Moelker A, Kroft LJ, Romeih S, de Rijke Y, Ten Harkel AD, Helbing WA. Ventricular function and cardiac reserve in contemporary Fontan patients. Int J Cardiol 2015; 196: 73–80. [DOI] [PubMed] [Google Scholar]
- 26. Saiki H, Eidem BW, Ohtani T, Grogan MA, Redfield MM. Ventricular‐arterial function and coupling in the adult Fontan circulation. J Am Heart Assoc 2016; 5: e003887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nakamura Y, Yagihara T, Kagisaki K, Hagino I, Kobayashi J. Ventricular performance in long‐term survivors after Fontan operation. Ann Thorac Surg 2011; 91: 172–180. [DOI] [PubMed] [Google Scholar]
- 28. Cheung YF, Penny DJ, Redington AN. Serial assessment of left ventricular diastolic function after Fontan procedure. Heart 2000; 83: 420–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hagler DJ, Seward JB, Tajik AJ, Ritter DG. Functional assessment of the Fontan operation: combined M‐mode, two‐dimensional and Doppler echocardiographic studies. J Am Coll Cardiol 1984; 4: 756–764. [DOI] [PubMed] [Google Scholar]
- 30. Gaynor JW, Bridges ND, Cohen MI, Mahle WT, Decampli WM, Steven JM, Nicolson SC, Spray TL. Predictors of outcome after the Fontan operation: is hypoplastic left heart syndrome still a risk factor? J Thorac Cardiovasc Surg 2002; 123: 237–245. [DOI] [PubMed] [Google Scholar]
- 31. Gewillig M, Brown SC. The Fontan circulation after 45 years: update in physiology. Heart 2016; 102: 1081–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gewillig M, Goldberg DJ. Failure of the fontan circulation. Heart Fail Clin 2014; 10: 105–116. [DOI] [PubMed] [Google Scholar]
- 33. Gewillig M, Daenen W, Aubert A, Van der Hauwaert L. Abolishment of chronic volume overload. Implications for diastolic function of the systemic ventricle immediately after Fontan repair. Circulation 1992; 86: II93–II99. [PubMed] [Google Scholar]
- 34. Liu X, Yagi H, Saeed S, Bais AS, Gabriel GC, Chen Z, Peterson KA, Li Y, Schwartz MC, Reynolds WT, Saydmohammed M, Gibbs B, Wu Y, Devine W, Chatterjee B, Klena NT, Kostka D, de Mesy Bentley KL, Ganapathiraju MK, Dexheimer P, Leatherbury L, Khalifa O, Bhagat A, Zahid M, Pu W, Watkins S, Grossfeld P, Murray SA, Porter GA Jr, Tsang M, Martin LJ, Benson DW, Aronow BJ, Lo CW. The complex genetics of hypoplastic left heart syndrome. Nat Genet 2017; 49: 1152–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Margossian R, Sleeper LA, Pearson GD, Barker PC, Mertens L, Quartermain MD, Su JT, Shirali G, Chen S, Colan SD. Assessment of Diastolic Function in Single‐Ventricle Patients After the Fontan Procedure. J Am Soc Echocardiogr 2016; 29: 1066–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF III, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Alexandru Popescu B, Waggoner AD, Houston T, Oslo N, Phoenix A, Nashville T, Hamilton OC, Uppsala S, Ghent LB, Cleveland O, Novara I, Rochester M, Bucharest R, St. Louis M. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2016; 17: 1321–1360. [DOI] [PubMed] [Google Scholar]
- 37. Michel M, Logoteta J, Entenmann A, Hansen JH, Voges I, Kramer HH, Petko C. Decline of Systolic and Diastolic 2D Strain Rate During Follow‐Up of HLHS Patients After Fontan Palliation. Pediatr Cardiol 2016; 37: 1250–1257. [DOI] [PubMed] [Google Scholar]
- 38. Grattan M, Mertens L. Mechanics of the Functionally Univentricular Heart‐How Little Do We Understand and Why Does It Matter? Can J Cardiol 2016; 321033 e11‐8: 1033.e11–1033.e18. [DOI] [PubMed] [Google Scholar]
- 39. Schwartz MC, Brock MA, Nykanen D, DeCampli W. Risk Factors for an Elevated Ventricular End‐Diastolic Pressure Prior to the Fontan Operation. Pediatr Cardiol 2018; 39: 315–323. [DOI] [PubMed] [Google Scholar]
- 40. Averin K, Hirsch R, Seckeler MD, Whiteside W, Beekman RH III, Goldstein BH. Diagnosis of occult diastolic dysfunction late after the Fontan procedure using a rapid volume expansion technique. Heart 2016; 102: 1109–1114. [DOI] [PubMed] [Google Scholar]
- 41. Cordina R, Ministeri M, Babu‐Narayan SV, Ladouceur M, Celermajer DS, Gatzoulis MA, Uebing A, Li W. Evaluation of the relationship between ventricular end‐diastolic pressure and echocardiographic measures of diastolic function in adults with a Fontan circulation. Int J Cardiol 2018; 259: 71–75. [DOI] [PubMed] [Google Scholar]
- 42. Udink ten Cate FEA, Trieschmann U, Germund I, Hannes T, Emmel M, Bennink G, Sreeram N. Stenting the Fontan pathway in paediatric patients with obstructed extracardiac conduits. Heart 2017; 103: 1111–1116. [DOI] [PubMed] [Google Scholar]
- 43. Alsaied T, Bokma JP, Engel ME, Kuijpers JM, Hanke SP, Zuhlke L, Zhang B, Veldtman GR. Predicting long‐term mortality after Fontan procedures: A risk score based on 6707 patients from 28 studies. Congenit Heart Dis 2017; 12: 393–398. [DOI] [PubMed] [Google Scholar]
- 44. Bichell DP, Lamberti JJ, Pelletier GJ, Hoecker C, Cocalis MW, Ing FF, Jensen RA. Late left pulmonary artery stenosis after the Norwood procedure is prevented by a modification in shunt construction. Ann Thorac Surg 2005; 79: 1656–1660 discussion 1660‐1. [DOI] [PubMed] [Google Scholar]
- 45. Konstantinov IE, Naimo PS, d'Udekem Y. Prevention of Right Pulmonary Artery Stenosis in Fontan Circulation: The Melbourne Modification of T‐Fontan Operation. Heart Lung Circ 2016; 25: 405–406. [DOI] [PubMed] [Google Scholar]
- 46. Zachary CH, Jacobs ML, Apostolopoulou S, Fogel MA. One‐lung Fontan operation: hemodynamics and surgical outcome. Ann Thorac Surg 1998; 65: 171–175. [DOI] [PubMed] [Google Scholar]
- 47. Dasi LP, Krishnankuttyrema R, Kitajima HD, Pekkan K, Sundareswaran KS, Fogel M, Sharma S, Whitehead K, Kanter K, Yoganathan AP. Fontan hemodynamics: importance of pulmonary artery diameter. J Thorac Cardiovasc Surg 2009; 137: 560–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tang E, Restrepo M, Haggerty CM, Mirabella L, Bethel J, Whitehead KK, Fogel MA, Yoganathan AP. Geometric characterization of patient‐specific total cavopulmonary connections and its relationship to hemodynamics. J Am Coll Cardiol Img 2014; 7: 215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mets JM, Bergersen L, Mayer JE Jr, Marshall AC, McElhinney DB. Outcomes of stent implantation for obstruction of intracardiac lateral tunnel Fontan pathways. Circ Cardiovasc Interv 2013; 6: 92–100. [DOI] [PubMed] [Google Scholar]
- 50. Ridderbos FJ, Wolff D, Timmer A, van Melle JP, Ebels T, Dickinson MG, Timens W, Berger RM. Adverse pulmonary vascular remodeling in the Fontan circulation. J. Heart Lung Transplant 2015; 34: 404–413. [DOI] [PubMed] [Google Scholar]
- 51. Gewillig M, Brown SC, Heying R, Eyskens B, Ganame J, Boshoff DE, Budts W, Gorenflo M. Volume load paradox while preparing for the Fontan: not too much for the ventricle, not too little for the lungs. Interact Cardiovasc Thorac Surg 2010; 10: 262–265. [DOI] [PubMed] [Google Scholar]
- 52. Levy M, Danel C, Laval AM, Leca F, Vouhe PR, Israel‐Biet D. Nitric oxide synthase expression by pulmonary arteries: a predictive marker of Fontan procedure outcome? J Thorac Cardiovasc Surg 2003; 125: 1083–1090. [DOI] [PubMed] [Google Scholar]
- 53. Yin Z, Wang Z, Zhu H, Zhang R, Wang H, Li X. Experimental study of effect of Fontan circuit on pulmonary microcirculation. Asian Cardiovasc Thorac Ann 2006; 14: 183–188. [DOI] [PubMed] [Google Scholar]
- 54. Egbe AC, Reddy YNV, Khan AR, Al‐Otaibi M, Akintoye E, Obokata M, Borlaug BA. Venous congestion and pulmonary vascular function in Fontan circulation: Implications for prognosis and treatment. Int J Cardiol 2018; 271: 312–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Miranda WR, Borlaug BA, Hagler DJ, Connolly HM, Egbe AC. Haemodynamic profiles in adult Fontan patients: associated haemodynamics and prognosis. Eur J Heart Fail 2019; 21: 803–809. [DOI] [PubMed] [Google Scholar]
- 56. Deal BJ, Mavroudis C, Backer CL. Arrhythmia management in the Fontan patient. Pediatr Cardiol 2007; 28: 448–456. [DOI] [PubMed] [Google Scholar]
- 57. Lasa JJ, Glatz AC, Daga A, Shah M. Prevalence of arrhythmias late after the Fontan operation. Am J Cardiol 2014; 113: 1184–1188. [DOI] [PubMed] [Google Scholar]
- 58. Weipert J, Noebauer C, Schreiber C, Kostolny M, Zrenner B, Wacker A, Hess J, Lange R. Occurrence and management of atrial arrhythmia after long‐term Fontan circulation. J Thorac Cardiovasc Surg 2004; 127: 457–464. [DOI] [PubMed] [Google Scholar]
- 59. Stephenson EA, Lu M, Berul CI, Etheridge SP, Idriss SF, Margossian R, Reed JH, Prakash A, Sleeper LA, Vetter VL, Blaufox AD, Pediatric Heart Network Investigators . Blaufox AD and Pediatric Heart Network I. Arrhythmias in a contemporary fontan cohort: prevalence and clinical associations in a multicenter cross‐sectional study. J Am Coll Cardiol 2010; 56: 890–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cecchin F, Johnsrude CL, Perry JC, Friedman RA. Effect of age and surgical technique on symptomatic arrhythmias after the Fontan procedure. Am J Cardiol 1995; 76: 386–391. [DOI] [PubMed] [Google Scholar]
- 61. Fishberger SB, Wernovsky G, Gentles TL, Gauvreau K, Burnetta J, Mayer JE Jr, Walsh EP. Factors that influence the development of atrial flutter after the Fontan operation. J Thorac Cardiovasc Surg 1997; 113: 80–86. [DOI] [PubMed] [Google Scholar]
- 62. Al‐Ghamdi AK, Abdelmalek SM, Bamaga MS, Azhar EI, Wakid MH, Alsaied Z. Bacterial contamination of Saudi “one” Riyal paper notes. Southeast Asian J Trop Med Public Health 2011; 42: 711–716. [PubMed] [Google Scholar]
- 63. Peters NS, Somerville J. Arrhythmias after the Fontan procedure. Br Heart J 1992; 68: 199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Carins TA, Shi WY, Iyengar AJ, Nisbet A, Forsdick V, Zannino D, Gentles T, Radford DJ, Justo R, Celermajer DS, Bullock A, Winlaw D, Wheaton G, Grigg L, d'Udekem Y. Long‐term outcomes after first‐onset arrhythmia in Fontan physiology. J Thorac Cardiovasc Surg 2016; 152: 1355–1363.e1. [DOI] [PubMed] [Google Scholar]
- 65. Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA, del Nido P, Fasules JW, Graham TP Jr, Hijazi ZM, Hunt SA, King ME, Landzberg MJ, Miner PD, Radford MJ, Walsh EP, Webb GD. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults With Congenital Heart Disease). Developed in Collaboration With the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2008; 52: e143–e263. [DOI] [PubMed] [Google Scholar]
- 66. Pundi KN, Pundi KN, Johnson JN, Dearani JA, Li Z, Driscoll DJ, Wackel PL, McLeod CJ, Cetta F, Cannon BC. Sudden cardiac death and late arrhythmias after the Fontan operation. Congenit Heart Dis 2017; 12: 17–23. [DOI] [PubMed] [Google Scholar]
- 67. Pundi KN, Johnson JN, Dearani JA, Pundi KN, Li Z, Hinck CA, Dahl SH, Cannon BC, O’Leary PW, Driscoll DJ, Cetta F. 40‐Year Follow‐Up After the Fontan Operation: Long‐Term Outcomes of 1,052 Patients. J Am Coll Cardiol 2015; 66: 1700–1710. [DOI] [PubMed] [Google Scholar]
- 68. Williams RV, Travison T, Kaltman JR, Cecchin F, Colan SD, Idriss SF, Lu M, Margossian R, Reed JH, Silver ES, Stephenson EA, Vetter VL, Pediatric Heart Network Investigators . Comparison of Fontan Survivors with and without Pacemakers: A Report from the Pediatric Heart Network Fontan Cross‐sectional Study. Congenit Heart Dis 2013; 8: 32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gentles TL, Gauvreau K, Mayer JE Jr, Fishberger SB, Burnetta J, Colan SD, Newburger JW, Wernovsky G. Functional outcome after the Fontan operation: factors influencing late morbidity. J Thorac Cardiovasc Surg 1997; 114: 392–403 discussion 404‐5. [DOI] [PubMed] [Google Scholar]
- 70. Rychik J. The Relentless Effects of the Fontan Paradox. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 2016; 19: 37–43. [DOI] [PubMed] [Google Scholar]
- 71. Kreutzer C, Kreutzer G. The Lymphatic System: The Achilles Heel of the Fontan‐Kreutzer Circulation. World J Pediatr Congenit Heart Surg 2017; 8: 613–623. [DOI] [PubMed] [Google Scholar]
- 72. Mohanakumar S, Telinius N, Kelly B, Lauridsen H, Boedtkjer D, Pedersen M, de Leval M, Hjortdal V. Morphology and Function of the Lymphatic Vasculature in Patients With a Fontan Circulation. Circ Cardiovasc Imaging 2019; 12: e008074. [DOI] [PubMed] [Google Scholar]
- 73. Menon S, Chennapragada M, Ugaki S, Sholler GF, Ayer J, Winlaw DS. The Lymphatic Circulation in Adaptations to the Fontan Circulation. Pediatr Cardiol 2017; 38: 886–892. [DOI] [PubMed] [Google Scholar]
- 74. Rychik J. Protein‐losing enteropathy after Fontan operation. Congenit Heart Dis 2007; 2: 288–300. [DOI] [PubMed] [Google Scholar]
- 75. Rychik J, Goldberg D, Rand E, Semeao E, Russo P, Dori Y, Dodds K. End‐organ consequences of the Fontan operation: liver fibrosis, protein‐losing enteropathy and plastic bronchitis. Cardiol Young 2013; 23: 831–840. [DOI] [PubMed] [Google Scholar]
- 76. Dori Y, Itkin M. Etiology and new treatment options for patients with plastic bronchitis. J Thorac Cardiovasc Surg 2016; 152: e49–e50. [DOI] [PubMed] [Google Scholar]
- 77. Chavhan GB, Amaral JG, Temple M, Itkin M. MR Lymphangiography in Children: Technique and Potential Applications. Radiographics 2017; 37: 1775–1790. [DOI] [PubMed] [Google Scholar]
- 78. Geanacopoulos AT, Savla JJ, Pogoriler J, Piccione J, Phinizy P, DeWitt AG, Blinder JJ, Pinto E, Itkin M, Dori Y, Goldfarb SB. Bronchoscopic and histologic findings during lymphatic intervention for plastic bronchitis. Pediatr Pulmonol 2018; 53: 1574–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Dori Y, Keller MS, Rome JJ, Gillespie MJ, Glatz AC, Dodds K, Goldberg DJ, Goldfarb S, Rychik J, Itkin M. Percutaneous Lymphatic Embolization of Abnormal Pulmonary Lymphatic Flow as Treatment of Plastic Bronchitis in Patients With Congenital Heart Disease. Circulation 2016; 133: 1160–1170. [DOI] [PubMed] [Google Scholar]
- 80. James H, Witte MH, Bernas M, Barber B. Proposal for Prevention or Alleviation of Protein/Lymph‐Losing Enteropathy (PLE/LLE) After Fontan Circulation Treatment of Univentricular Hearts: Restoration of Lymph Balance With a "Lymphatic Right‐to‐Left Shunt". Lymphology 2016; 49: 114–127. [PubMed] [Google Scholar]
- 81. Antonio M, Gordo A, Pereira C, Pinto F, Fragata I, Fragata J. Thoracic Duct Decompression for Protein‐Losing Enteropathy in Failing Fontan Circulation. Ann Thorac Surg 2016; 101: 2370–2373. [DOI] [PubMed] [Google Scholar]
- 82. Hraska V. Decompression of thoracic duct: new approach for the treatment of failing Fontan. Ann Thorac Surg 2013; 96: 709–711. [DOI] [PubMed] [Google Scholar]
- 83. Singhi AK, Vinoth B, Kuruvilla S, Sivakumar K. Plastic bronchitis. Ann Pediatr Cardiol 2015; 8: 246–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Grutter G, Di Carlo D, Gandolfo F, Adorisio R, Alfieri S, Michielon G, Carotti A, Pongiglione G. Plastic bronchitis after extracardiac Fontan operation. Ann Thorac Surg 2012; 94: 860–864. [DOI] [PubMed] [Google Scholar]
- 85. Larue M, Gossett JG, Stewart RD, Backer CL, Mavroudis C, Jacobs ML. Plastic bronchitis in patients with fontan physiology: review of the literature and preliminary experience with fontan conversion and cardiac transplantation. World J Pediatr Congenit Heart Surg 2012; 3: 364–372. [DOI] [PubMed] [Google Scholar]
- 86. Schumacher KR, Singh TP, Kuebler J, Aprile K, O'Brien M, Blume ED. Risk factors and outcome of Fontan‐associated plastic bronchitis: a case‐control study. J Am Heart Assoc 2014; 3: e000865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Mertens L, Hagler DJ, Sauer U, Somerville J, Gewillig M. Protein‐losing enteropathy after the Fontan operation: an international multicenter study. PLE study group. J Thorac Cardiovasc Surg 1998; 115: 1063–1073. [DOI] [PubMed] [Google Scholar]
- 88. John AS, Johnson JA, Khan M, Driscoll DJ, Warnes CA, Cetta F. Clinical outcomes and improved survival in patients with protein‐losing enteropathy after the Fontan operation. J Am Coll Cardiol 2014; 64: 54–62. [DOI] [PubMed] [Google Scholar]
- 89. Meadows J, Jenkins K. Protein‐losing enteropathy: integrating a new disease paradigm into recommendations for prevention and treatment. Cardiol Young 2011; 21: 363–377. [DOI] [PubMed] [Google Scholar]
- 90. Itkin M, Piccoli DA, Nadolski G, Rychik J, DeWitt A, Pinto E, Rome J, Dori Y. Protein‐Losing Enteropathy in Patients With Congenital Heart Disease. J Am Coll Cardiol 2017; 69: 2929–2937. [DOI] [PubMed] [Google Scholar]
- 91. Nir A, Driscoll DJ, Mottram CD, Offord KP, Puga FJ, Schaff HV, Danielson GK. Cardiorespiratory response to exercise after the Fontan operation: a serial study. J Am Coll Cardiol 1993; 22: 216–220. [DOI] [PubMed] [Google Scholar]
- 92. Paridon SM, Mitchell PD, Colan SD, Williams RV, Blaufox A, Li JS, Margossian R, Mital S, Russell J, Rhodes J, Pediatric Heart Network Investigators . A cross‐sectional study of exercise performance during the first 2 decades of life after the Fontan operation. J Am Coll Cardiol 2008; 52: 99–107. [DOI] [PubMed] [Google Scholar]
- 93. Ohuchi H. Cardiopulmonary response to exercise in patients with the Fontan circulation. Cardiol Young 2005; 15: 39–44. [DOI] [PubMed] [Google Scholar]
- 94. Ohuchi H, Arakaki Y, Hiraumi Y, Tasato H, Kamiya T. Cardiorespiratory response during exercise in patients with cyanotic congenital heart disease with and without a Fontan operation and in patients with congestive heart failure. Int J Cardiol 1998; 66: 241–251. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Table S1. Oral medication at hospital discharge in patients without in‐hospital.


