Abstract
Aims
To systematically review randomized controlled trials assessing effects of sodium–glucose cotransporter 2 inhibitors (SGLT2is) on hospitalization for heart failure (HHF) and cardiac structure/function and explore randomized controlled trial (RCT)‐derived evidence for SGLT2i efficacy mechanisms in heart failure (HF).
Methods and results
Systematic searches of Medline and Embase were performed. In seven trials [3730–17 160 patients; low risk of bias (RoB)], SGLT2is significantly reduced the relative risk of HHF by 27–39% vs. placebo, including in two studies in patients with HF with reduced ejection fraction with or without type‐2 diabetes mellitus (T2DM). Improvements in conventional cardiovascular risk factors, including glycaemic levels, cannot account for these effects. Five trials (56–105 patients; low RoB) assessed the effects of 6–12 months of SGLT2i treatment on left ventricular structure/function; four reported significant improvements vs. placebo, and one did not. Five trials (low RoB) assessed SGLT2i treatment effects on serum N‐terminal pro B‐type natriuretic peptide levels; significant reductions vs. placebo were reported after 8–12 months (two studies; 3730–4744 patients) but not ≤12 weeks (three studies; 80–263 patients). Limited available RCT‐derived evidence suggests various possible cardioprotective SGLT2i mechanisms, including improved haemodynamics (natriuresis and reduced interstitial fluid without blood volume contraction/neurohormonal activation) and vascular function, enhanced erythropoiesis, reduced tissue sodium and epicardial fat/inflammation, decreased sympathetic tone, and beneficial changes in cellular energetics.
Conclusions
Sodium–glucose cotransporter 2 inhibitors reduce HHF regardless of T2DM status, and reversal of adverse left ventricular remodelling likely contributes to this efficacy. Hypothesis‐driven mechanistic trials remain sparse, although numerous trials are planned or ongoing.
Keywords: Systematic review, Randomized controlled trials, Sodium–glucose cotransporter 2 inhibitors, Heart failure, Cardiac structure/function, Mechanisms
Introduction
In the past 5 years, there has been a profound shift in the therapeutic focus of trials of sodium–glucose cotransporter 2 inhibitors (SGLT2is). Although initially explored and introduced as glucose‐lowering agents for patients with type 2 diabetes mellitus (T2DM), 1 clinical investigation of these molecules has evolved towards heart failure (HF) and chronic kidney disease (CKD) outcomes in patients with and without T2DM (Figure 1 ). This trend has been driven by the impressive cardiac and renal protective properties of SGLT2is, which are predominantly independent of their glucose‐lowering effects, thus diminishing hyperglycaemia as a prerequisite condition for their efficacy. Given the unexpected benefits of this drug class, it is not surprising that major event‐focused trials have been followed closely by studies exploring the mechanisms by which SGLT2is may exert their benefits (Figure 1 ).
Figure 1.
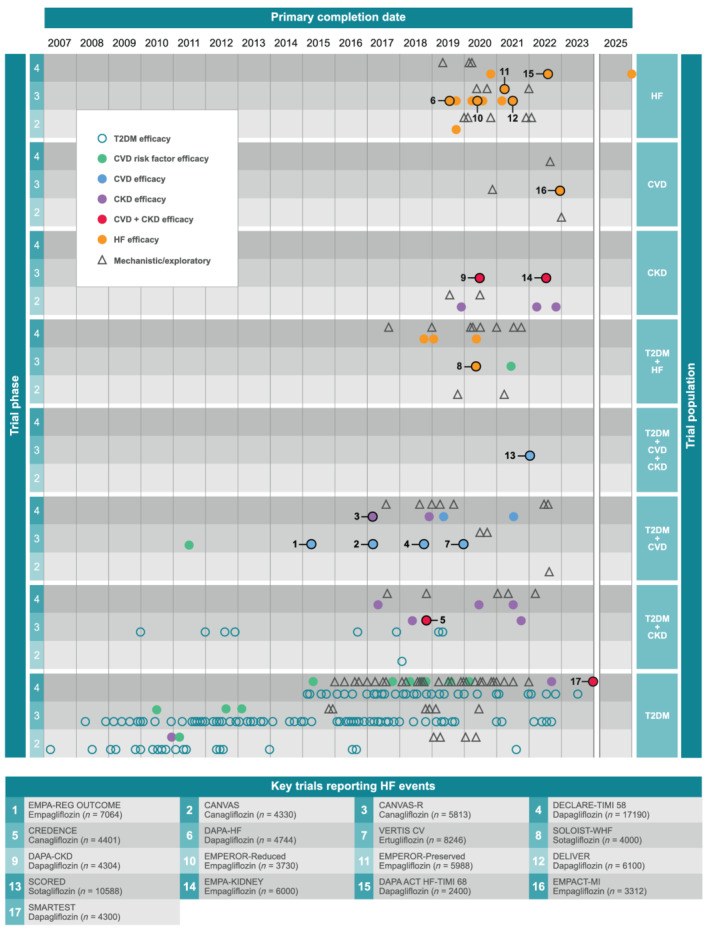
Evolution of SGLTi clinical trials over time. Only studies in patients with T2DM, HF, CVD, or CKD are included. We excluded phase 1 trials and studies assessing PK or safety only. Outcomes being assessed (KEY) by each study were broadly categorized according to the reported primary outcome for each trial. CVD trials include patients with or at risk of CVD. Major trials including assessments of HF‐event outcomes are numbered and described briefly in the appended table. CKD, chronic kidney disease; CVD, cardiovascular disease; HF, heart failure; T2DM, type 2 diabetes mellitus.
We aimed to systematically review randomized controlled trial (RCT) data assessing the effects of SGLT2is compared with placebo on hospitalization for HF (HHF), cardiac structure and cardiac function. We also aimed to review, in an exploratory manner, mechanistic evidence for how SGLT2is may exert their benefits, with a focus on RCT‐derived data. We also identify future trials that may help to address some of the remaining gaps in the clinical and mechanistic profiles of these drugs, and briefly discuss the implications of the current evidence for clinical practice.
Methods
Literature searches
This systematic review was performed in a Preferred Reporting Items for Systematic Reviews and Meta‐Analyses compliant manner (Supporting Information, Table S1 ) (Figure 2 ). 2 Searches were conducted using terms for ‘sodium‐glucose cotransporter 2 inhibitors’, ‘heart failure’, ‘cardiovascular disease’, ‘chronic kidney disease’, and ‘type 2 diabetes mellitus’ (Table S2 ). Database filters were applied to exclude congress abstracts, animal studies, reviews, editorials and letters, and to limit searches to studies reported in English in the past 10 years. Free‐text terms (e.g. real‐world, retrospective, and cross‐sectional) were used to minimize identification of non‐interventional studies by the targeted searches. Medline (via PubMed) and Embase (via Ovid) were searched on 16 August 2020, and results exported separately to EndNote. After removal of duplicates in EndNote, search results were combined and exported to Excel for screening. Titles and abstracts were screened by one author (MMB) and independently reviewed by a second author (RR).
Figure 2.
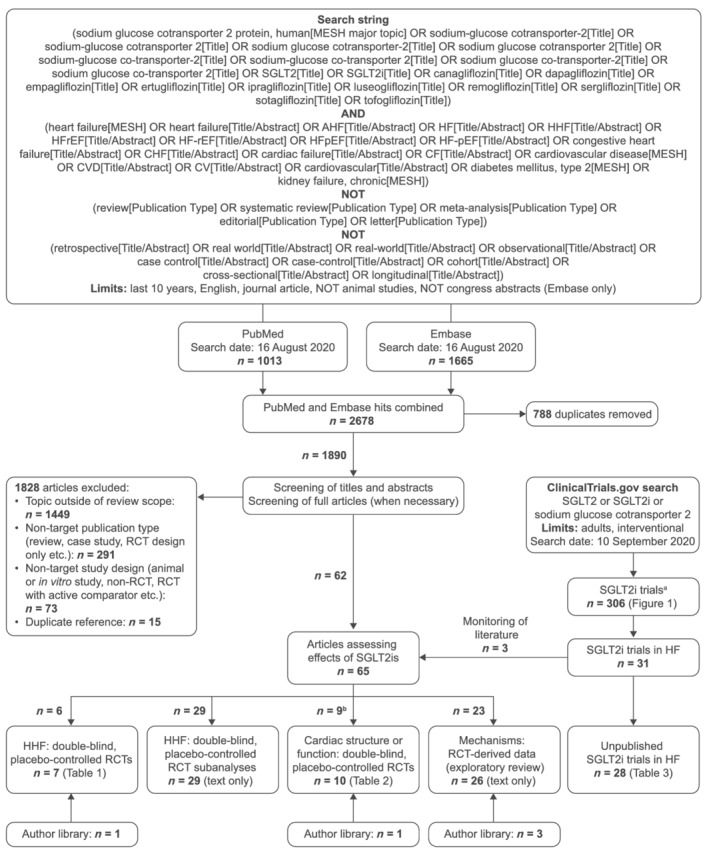
PRISMA flow diagram summarizing the search strategy. aPhase 1 trials and studies assessing PK and/or safety only were excluded. bIncludes two studies also included in Table 1 (i.e. HHF: double‐blind, placebo‐controlled RCTs). Hence, the total number of studies across categories adds up to 67 rather than 65. AHF, acute heart failure; CF, cardiac failure; CHF, congestive heart failure; CV, cardiovascular; CVD, cardiovascular disease; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HHF, hospitalization for heart failure; MESH, Medical Subject Headings; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta‐Analyses; RCT, randomized controlled trial; SGLT2, sodium–glucose cotransporter 2; SGLT2i, sodium–glucose cotransporter 2 inhibitor.
Study selection
We included RCT studies that assessed the effects of SGLT2is compared with placebo on HHF events (Table 1 ) and subanalyses of these trials (reported in the text only), as well as RCTs assessing effects of SGLT2is compared with placebo on cardiac structure and function as primary outcomes, or as secondary outcomes in large trials (>1000 patients) (Table 2 ). We also included RCTs assessing broader potential mechanisms of SGLT2i efficacy in HF (reported in the text only). Criteria for broader mechanistic studies were expanded to include mediation and in silico analyses incorporating RCT data; however, the wide range of potential mechanisms proposed for SGLT2is precluded an exhaustive account of the literature. In addition, mechanistic findings are supplemented in part by opinions held by the authors and wider supporting literature and should be considered more exploratory in nature.
Table 1.
Double‐blind, RCT data for effects of SGLT2is on HHF event rates
| Study and overall RoB assessment | Median follow‐up | Population and primary outcome(s) (met, Y/N) | Interventions (n) a | Baseline characteristics: SGLT2i [placebo] b | HHF events (%) and HHF rate c | HR (95% CI) |
|---|---|---|---|---|---|---|
|
Zinman et al. 3 EMPA‐REG OUTCOME (NCT01131676) RoB low |
3.1 years |
T2DM with CVD (N = 7020) MACE (Y; 14% reduction) |
Empagliflozin 10 mg or 25 mg (4687) or placebo (2333) |
Age: 63.1 [63.2] years Women: 29% [28%] BMI: 31 [31] kg/m2 SBP: 135 [136] mmHg HbA1c: 8.1% [8.1%] eGFR: 74.2 [73.8] mL/min/1.73 m2 |
Empagliflozin ▪ 126 (2.7) ▪ 9.4 per 1000 patient‐years Placebo ▪ 95 (4.1) ▪ 14.5 per 1000 patient‐years |
0.65 (0.50, 0.85); P = 0.002 |
|
Neal et al. 4 CANVAS and CANVAS‐Renal (NCT01032629 and NCT01989754) RoB low |
126 weeks |
T2DM with high CVD risk (66% established CVD) (N = 10 142) MACE (Y; 14% reduction) |
Canagliflozin 100 mg or 300 mg (5795) or placebo (4347) |
Age: 63.2 [63.4] years Women: 35% [37%] BMI: 32 [32] kg/m 2 SBP: 136 [137] mmHg HbA1c: 8.2% [8.2%] eGFR: 76.7 [76.2] mL/min/1.73 m2 A/C (median): 12.4 [12.1] |
Canagliflozin ▪ NR ▪ 5.5 (patients) per 1000 patient‐years Placebo ▪ NR ▪ 8.7 (patients) per 1000 patient‐years |
0.67 (0.52, 0.87); P = NR |
|
Perkovic et al. 5 CREDENCE NCT02065791 RoB low |
2.62 years |
T2DM with albuminuric CKD (N = 4401) ESRD, doubling of serum creatine, or death from renal or CV causes (Y; 30% reduction) |
Canagliflozin 100 mg (2202) or placebo (2199) |
Age: 62.9 [63.2] years Women: 35% [33%] BMI: 31 [31] kg/m2 SBP: 140 [140] mmHg HbA1c: 8.3% [8.3%] eGFR: 56.3 [56.0] mL/min/1.73 m2 A/C (median): 923 [931] |
Canagliflozin ▪ 89 (4.0) ▪ 15.7 per 1000 patient‐years Placebo ▪ 141 (6.4) ▪ 25.3 per 1000 patient‐years |
0.61 (0.47, 0.80); P < 0.001 |
|
Wiviott et al. 6 DECLARE‐TIMI 58 (NCT01730534) RoB low |
4.2 years |
T2DM with (41%), or at high risk of, atherosclerotic CVD (N = 17 160) CV death or HHF (Y; 17% reduction) MACE (N; 7% reduction) |
Dapagliflozin 10 mg (8582) or placebo (8578) |
Age: 63.9 [64.0] years Women: 37% [38%] BMI: 32 [32] kg/m2 SBP: 135 [135] mmHg HbA1c: 8.3% [8.3%] eGFR: 85.4 [85.1] mL/min/1.73 m2 |
Dapagliflozin ▪ 212 (2.5) ▪ 6.2 per 1000 patient‐years Placebo ▪ 286 (3.3) ▪ 8.5 per 1000 patient‐years |
0.73 (0.61, 0.88); P = NR |
|
Cannon et al. 7 VERTIS CV (NCT01986881) RoB low |
3.0 years |
T2DM with established atherosclerotic CVD (N = 8246) MACE (N; 3% reduction) |
Ertugliflozin 5 mg or 15 mg (5499) or placebo (2747) |
Age: 64.4 [64.4] years Women: 30% [31%] BMI: 32 [32] kg/m2 SBP: 134 [133] mmHg HbA1c: 8.2% [8.2%] eGFR: 76.1 [75.7] mL/min/1.73 m2 |
Ertugliflozin ▪ 139 (2.5) ▪ 7 per 1000 patient‐years Placebo ▪ 99 (3.6) ▪ 11 per 1000 patient‐years |
0.70 (0.54, 0.90); P = NR |
|
McMurray et al. 8 DAPA‐HF (NCT03036124) RoB low |
18.2 months |
HFrEF (NYHA class II–IV HF with LVEF ≤40%) (N = 4744) CV death or worsening HF (hospitalization or an urgent visit resulting in intravenous therapy for heart failure) (Y; 26% reduction) |
Dapagliflozin 10 mg (2373) or placebo (2371) |
Age: 66.2 [66.5] years Women: 24% [23%] BMI: 28 [28] kg/m2 SBP: 122 [122] mmHg eGFR: 66.0 [65.5] mL/min/1.73 m2 T2DM: 42% [42%] LVEF: 31.2% [30.9%] |
Dapagliflozin ▪ 231 (9.7) ▪ 69 per 1000 patient‐years Placebo ▪ 318 (13.4) ▪ 98 per 1000 patient‐years |
0.70 (0.59, 0.83); P = NR |
|
Packer et al. 9 EMPEROR‐Reduced (NCT03057977) RoB low |
16 months |
HFrEF (NYHA class II–IV HF with LVEF ≤ 40%) (N = 3730) CV death or HHF (Y; 25% reduction) |
Empagliflozin 10 mg (1863) or placebo (1867) |
Age: 67.2 [66.5] years Women: 24% [24%] BMI: 28 [28] kg/m2 SBP: 123 [121] mmHg eGFR: 61.8 [62.2] mL/min/1.73 m2 T2DM: 50% [50%] LVEF: 27.7% [27.2%] |
Empagliflozin ▪ 246 (13.2) ▪ 107 per 1000 patient‐years Placebo ▪ 342 (18.3) ▪ 155 per 1000 patient‐years |
0.69 (0.59, 0.81); P = NR |
A/C, albumin/creatinine; BMI, body mass index; CI, confidence interval; CKD, chronic kidney disease; CANVAS, Canagliflozin Cardiovascular Assessment Study; CREDENCE, Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation; CV, cardiovascular; CVD, cardiovascular disease; DAPA‐HF, Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure; DECLARE‐TIMI 58, Dapagliflozin Effect on Cardiovascular Events–Thrombolysis in Myocardial Infarction 58; eGFR, estimated glomerular filtration rate; EMPA‐REG OUTCOME, Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients; EMPEROR‐Reduced, Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Reduced Ejection Fraction; ESRD, end‐stage renal disease; HbA1c, glycated haemoglobin; HF, heart failure; HHF, hospitalization for heart failure; HR, hazard ratio; LVEF, left ventricular ejection fraction; MACE, major adverse cardiovascular events; NR, not reported; NYHA, New York Heart Association; RCT, randomized controlled trial; SBP, systolic blood pressure; SGLT2i, sodium–glucose cotransporter 2 inhibitor; T2DM, type 2 diabetes mellitus; VERTIS CV, Evaluation of Ertugliflozin Efficacy and Safety Cardiovascular Outcomes Trial; Y/N, yes/no.
All doses are once daily.
Presented as means unless otherwise specified.
Rate is based on number of events unless otherwise specified.
Table 2.
RCT data for effects of SGLT2is on cardiac structure, function, and biomarkers
| Study and overall RoB assessment | Follow‐up | Population | Interventions (n) a | Baseline characteristics: SGLT2i [placebo] b | Results c |
|---|---|---|---|---|---|
|
Brown et al. 10 Double‐blind RCT DAPA‐LVH (NCT02956811) RoB low |
12 months | T2DM, LVH and controlled blood pressure (N = 66) | Dapagliflozin 10 mg (32) or placebo (34) |
Age: 64 [67] years Women: 37% [47%] BMI: 32 [33] kg/m2 SBP: 130 [138] mmHg HbA1c: 62 [60] mmol/mol eGFR: 108 [97] mL/min/1.73 m2 Creatinine: 65 [71] μmol/L LVEF: 71% [73%] |
Primary outcome Mean (SD) change in LVM ▪ Dapagliflozin: −3.95 (4.85) g ▪ Placebo: −1.13 (4.55) g ▪ Difference: −2.82 (95% CI –5.13, −0.51); P = 0.018 |
|
Verma et al. 11 Double‐blind RCT EMPA‐HEART (NCT02998970) RoB low |
6 months | T2DM and CAD (N = 97) | Empagliflozin 10 mg (49) or placebo (48) |
Age (median): 64 [64] years Women: 10% [4%] BMI (median): 27 [27] kg/m2 SBP (median): 128 [134] mmHg HbA1c (median): 7.9% [7.9%] eGFR (median): 87 [88] mL/min/1.73 m2 Creatinine (median): 0.9 [0.9] mg/dL LVEF: 58% [56%] |
Primary outcome Mean (SD) change in LVMi ▪ Dapagliflozin: −2.6 (7.8) g/m2 ▪ Placebo: −0.01 (5.7) g/m2 ▪ Adjusted treatment effect: −3.4 (95% CI –5.9, −0.8); P = 0.01 |
|
Singh et al. 12 Double‐blind RCT REFORM (NCT02397421) RoB low d |
52 weeks | T2DM and HF (NYHA class I, II or III) with LVSD (N = 56) | Dapagliflozin 10 mg (28) or placebo (28) |
Age: 67 [67] years Women: 36% [32%] BMI: 33 [32] kg/m2 SBP: 135 [133] mmHg HbA1c: 63 [59] mmol/mol eGFR: 68 [76] mL/min/1.73 m2 Creatinine: 92 [84] μmol/L LVEF: 45% [47%] |
Primary outcome Mean (SD) change in LVESV ▪ Dapagliflozin: −8.9 (32.7) mL ▪ Placebo: −18.8 (51.0) mL ▪ Adjusted treatment effect: 4.8 (95% CI –13.3, 22.9); P = 0.59 |
|
Damman et al. 13 Double‐blind RCT EMPA‐RESPONSE (NCT03200860) RoB low |
30 days | Acute decompensated HF (N = 80) | Empagliflozin 10 mg (40) or placebo (39) |
Age: 79 [73] years Women: 40% [26%] Weight: 87 [83] kg SBP: 127 [121] mmHg eGFR: 55 [55] mL/min/1.73 m2 Creatinine: 114 [116] μmol/L T2DM: 38% [28%] LVEF: 36% [37%] |
Primary outcome Mean (SD) change in NT‐proBNP at 4 days ▪ Empagliflozin: −46 (32) pg/mL ▪ Placebo: −42 (31) pg/mL ▪ Difference: P = 0.63 Median (IQR) length of hospital stay ▪ Empagliflozin: 8 (6–10) days ▪ Placebo: 8 (6–9) days ▪ Difference: P = 0.58 Mean (SD) change in VAS dyspnoea score (AUC) at 4 days ▪ Empagliflozin: 1264 (1211) mm × h ▪ Placebo: 1650 (1240) mm × h ▪ Difference: P = 0.18 |
|
Jensen et al. 14 Double‐blind RCT EMPIRE HF (NCT03198585) RoB low |
12 weeks | HFrEF (NYHA class II or III HF with LVEF ≤ 40%) (N = 190) | Empagliflozin 10 mg. (95) or placebo (95) |
Age (median): 64 [63] years Women: 17% [13%] BMI (median): 29 [29] kg/m2 SBP (median): 117 [122]; mmHg HbA1c (median): 40 [39] mmol/mol eGFR: 73 [74] mL/min/1.73 m2 T2DM: 20% [15%] LVEF (median): 30% [30%] |
Primary outcome Median (IQR) NT‐proBNP at baseline vs. 12 weeks ▪ Empagliflozin: 582 (304–1020) vs. 478 (281–961) pg/mL ▪ Placebo: 605 (322–1070) vs. 520 (267–1075) pg/mL ▪ Adjusted change ratio: 0.98 (95% CI 0.82, 1.11); P = 0.7 |
|
Nassif et al. 15 Double‐blind RCT DEFINE‐HF (NCT02653482) RoB low |
12 weeks | HFrEF (NYHA class II or III HF with LVEF ≤ 40%) (N = 263) | Dapagliflozin 10 mg (131) or placebo (132) |
Age: 62 [60] years Women: 27% [26%] BMI (median): 31 [31] kg/m2 SBP: 122 [125] mmHg HbA1c: 7.0% [7.3%] eGFR: 67 [71] mL/min/1.73 m2 A/C (median): 19 [25] T2DM: 62% [64%] LVEF: 27% [26%] |
Primary outcomes Mean (95% CI) baseline‐adjusted change in NT‐proBNP at both Weeks 6 and 12 ▪ Dapagliflozin: 1133 (1036, 1238) pg/mL ▪ Placebo: 1191 (1089, 1304) pg/mL ▪ Adjusted change ratio: 0.95 (95% CI 0.84, 1.08); P = 0.43 Proportion of patients with a KCCQ increase of ≥5 points or a ≥20% reduction in NT‐proBNP at both week 6 and week 12 ▪ Dapagliflozin: 61.5% ▪ Placebo: 50.4% ▪ Adjusted OR: 1.8 (95% CI 1.03, 3.06); P = 0.039 |
|
Santos‐Gallego et al. 16 Double‐blind RCT EMPA‐TROPISM (NCT 03485222) RoB low |
6 months | Non‐diabetic with HFrEF (NYHA class II or III HF with LVEF < 50%) (N = 84) | Empagliflozin 10 mg (42) or placebo (42) |
Age: 64 [60] years Women: 36% [36%] BMI: 29 [30] HbA1c: 5.8% [5.8%] eGFR: 80 [83] mL/min/1.73 m2 Creatinine: 0.97 [0.95] mg/dL LVEF: 36% [37%] |
Primary outcome Mean (SD) change in LVEDV ▪ Empagliflozin: −25.1 (26) mL ▪ Placebo: −1.5 (25.4) mL ▪ Difference: P < 0.001 |
|
Lee et al. 17 Double‐blind RCT SUGAR‐DM‐HF (NCT03485092) RoB low |
9 months | T2DM or prediabetes and HFrEF (NYHA class II [77%] or III [23%] HF with LVEF ≤ 40%) (N = 105) | Empagliflozin 10 mg (52) or placebo (53) |
Age: 68 [69] years Women: 35% [19%] BMI: 31 [30] kg/m2 SBP: 126 [130] mmHg HbA1c: 7.5% [7.0%] eGFR (CKD‐EPI): 70 [65] mL/min Creatinine: 96 [104] μmol/L T2DM: 77% [79%] Prediabetes: 23% [21%] LVEF: 32% [33%] |
Primary outcome Mean (SD) change in LVESVi ▪ Empagliflozin: −7.9 (11.8) mL ▪ Placebo: −1.5 (11.3) mL ▪ Difference: −6.0 (95% CI − 10.8, −1.2); P = 0.015 |
|
McMurray et al. 8 Double‐blind RCT DAPA‐HF (NCT03036124) RoB low |
8 months |
HFrEF (NYHA class II or III HF with LVEF ≤ 40%) (N = 4744) |
Dapagliflozin 10 mg (2373) or placebo (2371) |
Age: 66 [67] years Women: 24% [23%] BMI: 28 [28] kg/m2 SBP: 122 [122] mmHg eGFR: 66 [66] mL/min/1.73 m2 T2DM: 42% [42%] LVEF: 31% [31%] |
Exploratory outcome Mean (SD) change in NT‐proBNP ▪ Dapagliflozin: −196 (2387) pg/mL ▪ Placebo: 101 (2944) pg/mL ▪ Difference: −303 (95% CI –457, −150); P < 0.001 |
|
Packer et al. 9 Double‐blind RCT EMPEROR‐Reduced (NCT03057977) RoB low |
52 weeks | HFrEF (NYHA class II–IV HF with LVEF ≤ 40%) (N = 3730) | Empagliflozin 10 mg (1863) or placebo (1867) |
Age: 67 [67] years Women: 24% [24%] BMI: 28 [28] kg/m2 SBP: 123 [121] mmHg eGFR: 62 [62] mL/min/1.73 m2 T2DM: 50% [50%] LVEF: 28% [27%] |
Exploratory outcome Median (IQR) change in NT‐proBNP ▪ Dapagliflozin: −244 (−890, 260) pg/mL ▪ Placebo: −141 (784, 585) pg/mL ▪ GMR: 0.87 (95% CI 0.82, 0.93); P = NR |
A/C, albumin/creatinine; AUC, area under curve; BMI, body mass index; CAD, coronary artery disease; CI, confidence interval; CKD‐EPI, Chronic Kidney Disease Epidemiology Collaboration; DAPA‐HF, Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure; DAPA‐LVH, Does Dapagliflozin Regress Left Ventricular Hypertrophy In Patients With Type 2 Diabetes; DEFINE‐HF, Dapagliflozin Effects on Biomarkers, Symptoms and Functional Status in Patients with HF with Reduced Ejection Fraction; EMPA‐HEART, Effects of Empagliflozin on Cardiac Structure in Patients with Type 2 Diabetes; EMPA‐RESPONSE, Effects of Empagliflozin on Clinical Outcomes in Patients With Acute Decompensated Heart Failure; EMPA‐TROPISM, Are the “Cardiac Benefits” of Empagliflozin Independent of Its Hypoglycemic Activity?; EMPEROR‐Reduced, Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Reduced Ejection Fraction; EMPIRE HF, Empagliflozin in Heart Failure Patients With Reduced Ejection Fraction; eGFR, estimated glomerular filtration rate; GMR, geometric mean ratio; HbA1c, glycated haemoglobin; HF, heart failure; HFrEF, heat failure with reduced ejection fraction; IQR, interquartile range; KCCQ, Kansas City Cardiomyopathy Questionnaire; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end‐systolic volume; LVESVi, left ventricular end‐systolic volume, indexed; LVH, left ventricular hypertrophy; LVM, left ventricular mass; LVMi, left ventricular mass, indexed; LVSD, left ventricular systolic dysfunction; NR, not reported; NT‐proBNP, N‐terminal pro B‐type natriuretic peptide; NYHA, New York Heart Association; RCT, randomized controlled trial; REFORM, Safety and Effectiveness of SGLT‐2 Inhibitors in Patients With Heart Failure and Diabetes; SBP, systolic blood pressure; SD, standard deviation; SGLT2i, sodium–glucose cotransporter 2; SUGAR‐DM‐HF, Studies of Empagliflozin and Its Cardiovascular, Renal and Metabolic Effects; T2DM, type 2 diabetes mellitus; VAS, visual analogue scale.
All doses are once daily.
Presented as means unless otherwise specified.
Changes are at end of reported treatment period unless otherwise specified.
The REFORM trial was identified by RobotReviewer as having a high/unclear RoB in several domains, but this was largely driven by the separate publication of detailed methods, which reduced the level of detail reported in the primary paper. Assessment of the REFORM study design paper confirmed that the RoB was low for this trial across all domains. 18
Data extraction and risk of bias assessments
Extraction of data into tables was performed by a single author (MMB) and verified by a second author (RR). For trials assessing HHF, we extracted event rate data, hazard ratios, 95% confidence intervals and P values for the effects of SGLT2is compared with placebo (Table 1 ). For trials assessing cardiac structure and function, we extracted data for mean changes from baseline in primary outcomes, relevant summary statistics, and P values, for SGLT2is compared with placebo (Table 2 ). We also extracted information relating to study design (trial type, sample size, follow‐up period, etc.) and relevant baseline patient characteristics (age, sex, relevant comorbidities and laboratory measures, etc.). A semi‐automated risk of bias (RoB) assessment approach was employed, whereby studies were assessed at the study level across four categories (random sequence generation, allocation concealment, blinding of participants and personnel, and blinding of outcomes assessment) by a single author (RR) and the results independently verified using RobotReviewer (Tables 1 & 2 ). 19 RoB assessments were not performed for subanalyses of HHF‐event trial data, or studies assessing broader SGLT2i mechanisms (exploratory review), and data from these studies were synthesized directly from included papers into the review.
Searches of ongoing trials
We also searched ClinicalTrials.gov for ongoing or completed, but unpublished trials of SGLT2is in patients with HF. The publication status of trials identified by the ClinicalTrials.gov searches was monitored beyond the cut‐off date of the main literature search to allow up‐to‐date inclusion of data from large trials on effects of SGLT2is on HHF events, and trials on the effects of SGLT2is on cardiac structure and function in patients with HF (Figure 2 ).
Results
A Preferred Reporting Items for Systematic Reviews and Meta‐Analyses summary of the literature and ClinicalTrials.gov searches is presented in Figure 2 .
Randomized controlled trials evaluating effects on hospitalization for heart failure events
All studies assessing the impact of SGLT2is compared with placebo on HHF were deemed to have a low RoB (Table 1 ).
Type‐2 diabetes mellitus and high cardiovascular risk
The EMPA‐REG OUTCOME (empagliflozin) and CANVAS (canagliflozin) trials were conducted in patients with T2DM with high cardiovascular disease (CVD) risk, with or without underlying CVD (Table 1 ). 3 , 4 Both included major adverse CV events [MACE; cardiovascular (CV) death, non‐fatal myocardial infarction (MI) or non‐fatal stroke] as their primary endpoint and reported significant relative risk reductions of 14% for this outcome with SGLT2i treatment compared with placebo. 3 , 4 Substantial reductions in the risk of HHF were observed in both trials (35% and 33%, respectively).
Post hoc analyses of EMPA‐REG OUTCOME data indicate similar reductions in the risk of HHF with empagliflozin regardless of the following baseline characteristics: age; sex; ethnicity; body mass index (BMI); glycated haemoglobin (HbA1c), blood pressure, or uric acid levels; degree of kidney function; medication use; CVD or HF risk; presence of peripheral artery disease or atrial fibrillation; and history of coronary artery bypass grafting. 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 Post hoc analyses of CANVAS trial data reveal similar reductions in HHF risk with canagliflozin regardless of baseline age, sex, BMI, HbA1c or blood pressure levels, degree of kidney function or albuminuria, medication use, T2DM duration, or history of CVD. 33 , 34 , 35
The DECLARE‐TIMI 58 trial was the first to include a composite of CV death or HHF as a primary endpoint. In addition, the trial included patients with T2DM who had established atherosclerotic CVD (41%) or were at high risk of atherosclerotic CVD (59%) (Table 1 ). 6 A significant 17% reduction in the risk of the primary endpoint was reported for dapagliflozin compared with placebo, in addition to a 27% reduction in the risk of HHF. 6 Notably, the co‐primary endpoint of MACE was numerically less but not significantly improved with dapagliflozin in this trial (7% risk reduction vs. placebo). 6 The effects of dapagliflozin on HHF risk were not significantly affected by baseline age, T2DM duration, presence of HF with reduced ejection fraction (HFrEF), or history of MI, based on subsequent post hoc analyses. 36 , 37 , 38 , 39
Most recently, the VERTIS CV trial assessed MACE as its primary endpoint in patients with T2DM and established atherosclerotic CVD (Table 1 ). 7 The primary endpoint was not met based on a 3% reduction in the risk of MACE for ertugliflozin compared with placebo. Nevertheless, confidence intervals for this result still overlap with other studies. 7 Also, despite not meeting its primary endpoint, a 30% reduction in the risk of HHF was observed. No post hoc analyses of the VERTIS CV trial were identified at the time of our literature search.
Type‐2 diabetes mellitus with chronic kidney disease
The CREDENCE trial included patients with T2DM and albuminuric CKD, and reported a 30% reduction in the risk of its primary renal endpoint (end‐stage renal disease, doubling of serum creatine, or death from renal or CV causes) with canagliflozin compared with placebo (Table 1 ). 5 HHF risk was also significantly reduced by 39% with canagliflozin. 5 Post hoc analyses of CREDENCE data suggest there were no significant associations between baseline kidney function or history of CVD and the effects of canagliflozin on HHF risk. 40 , 41
Heart failure with reduced ejection fraction with or without type‐2 diabetes mellitus
Two trials of SGLT2is have now been completed in patients with HFrEF without the presence of T2DM as an inclusion criterion, both of which used a composite primary endpoint of CV death or worsening HF (Table 1 ). 8 , 9 In the DAPA‐HF trial, treatment with dapagliflozin significantly reduced the risk of the primary outcome by 26% compared with placebo, and the exploratory endpoint of HHF was reduced by 30%. 8 Nearly identical results were obtained with empagliflozin treatment in the EMPEROR‐Reduced trial, with significant risk reductions of 25% and 31% observed for the primary endpoint and HHF, respectively, compared with placebo. 9 Based on post hoc analyses, the effects of dapagliflozin on HF do not appear to be influenced by baseline age, presence of T2DM, left ventricular (LV) ejection fraction, Kansas City Cardiomyopathy Questionnaire score, or background HF medication or diuretic use. 42 , 43 , 44 , 45 , 46 , 47 , 48 No post hoc analyses of EMPEROR‐Reduced were identified at the time of our literature search.
Summary, implications, and future trials in heart failure
Sodium–glucose cotransporter 2 inhibitors exhibit remarkable consistency in terms of reducing HHF risk across trial populations (range: 27–39%; low RoB for all studies). 3 , 4 , 5 , 6 , 7 , 8 , 9 Crucially, with the results of the DAPA‐HF and EMPEROR‐Reduced trials, these benefits have been confirmed to extend to patients with established HFrEF regardless of T2DM status, 8 , 9 thus shifting the role of SGLT2is from prevention to active treatment of HFrEF.
A key question remaining is whether SGLT2is also benefit patients who have HF with preserved ejection (HFpEF), for which there are no treatment options strongly recommended in clinical guidelines. 49 It is likely that the EMPA‐REG OUTCOME trial included a significant proportion of patients with HFpEF. 3 Furthermore, results from the SOLOIST‐WHF trial investigating the dual SGLT1 and SGLT2 inhibitor sotagliflozin in patients with diabetes and recent worsening HF demonstrated a significant 33% reduction in the relative risk of CV death, HHF, and urgent visits for HF, which was maintained in the subgroup of patients with HFpEF. 50 These findings offer hope of similar results from the ongoing EMPEROR‐Preserved and DELIVER trials assessing the effects of empagliflozin and dapagliflozin, respectively, on CV death and HHF in patients with HFpEF (primary completion due first half of 2021) (Table 3 ).
Table 3.
Ongoing or unpublished (as of September 2020) RCTs in patients with HF
| Trial no. (name a ) | Population | Intervention (time period) | Comparator | Outcomes b | Study type | Status c | Primary completion c |
|---|---|---|---|---|---|---|---|
| NCT03977116 | HFrEF and T2DM (N = 100) | SGLT2i not specified (12 months) | Placebo | Primary: (1) All‐cause death. (2) CV death. (3) Ventricular arrhythmias recurrence. Secondary: Hospitalization for HF worsening | RCT, phase 4 | Complete | January 2019 |
| NCT03030235 (PRESERVED‐HF) | HFpEF (N = 320) | Dapagliflozin (12 weeks) | Placebo | Primary: (1) HF disease‐specific health status (KCCQ clinical summary score). Secondary: (1) HF health status (KCCQ overall summary score). (2) NT‐proBNP. (3) BNP. (4) 6MWT. (5) HbA1c | RCT, phase 4 | Recruiting | Estimated October 2020 |
| NCT03057951 (EMPEROR‐Preserved) | HFpEF (N = 5988) | Empagliflozin (38 months) | Placebo | Primary: Composite of time to first CV death or HHF. Secondary: (1) HHF. (2) eGFR. (3) Time to first chronic dialysis, renal transplant or sustained reduction of eGFR. (4) Time to first HHF event. (5) Time to CV death | RCT, phase 3 | Active, not recruiting | Estimated March 2021 |
| NCT03128528 (ELSI) | HFrEF (estimated N = 84) | Empagliflozin (14 weeks) | Placebo | Primary: Skin sodium content. Secondary: (1) Muscle sodium content. (2) Skin and muscle water content. (3) Sodium excretion. (4) 24 h urine sodium excretion. (5) Vascular stiffness (central systolic pressure) | RCT, phase 2 | Complete | January 2020 |
| NCT03030222 (EMBRACE‐HF) | HF (estimated N = 60) | Empagliflozin (12 weeks) | Placebo | Primary: Pulmonary artery diastolic pressure. Secondary: (1) Pulmonary artery diastolic pressure (interim time points). (2) Pulmonary artery systolic pressure. (3) Pulmonary artery systolic pressure (interim time points). (4) Mean pulmonary artery pressure. (5) Mean pulmonary artery pressure (interim time points) | RCT, phase 4 | Active, not recruiting | March 2020 |
| NCT03332212 (EMPA‐VISION) | HF (N = 72) | Empagliflozin (12 weeks) | Placebo | Primary: Creatine phosphate/adenosine triphosphate ratio | RCT, phase 3 | Complete | May 2020 |
| NCT03448419 (EMPERIAL‐Reduced) | HFrEF (N = 312) | Empagliflozin (12 weeks) | Placebo | Primary: Exercise capacity (6MWT); Secondary: (1) KCCQ total symptom score. (2) CHQ‐SAS dyspnoea score. (3) Exercise capacity (6MWT) (baseline to 6 weeks). (4) Clinical congestion score. (5) PGI‐S of HF symptoms | RCT, phase 3 | Complete | October 2019 |
| NCT03448406 (EMPERIAL‐Preserved) | HFpEF (N = 315) | Empagliflozin (12 weeks) | Placebo | Primary: Exercise capacity (6MWT); Secondary: (1) KCCQ total symptom score. (2) CHQ‐SAS dyspnoea score. (3) Exercise capacity (6MWT) (baseline to 6 weeks). (4) Clinical congestion score. (5) PGI‐S of HF symptoms | RCT, phase 3 | Complete | October 2019 |
| NCT03416270 (ERADICATE‐HF) | HF and T2DM (estimated N = 36) | Ertugliflozin (12 weeks) | Placebo | Primary: Proximal sodium reabsorption. Secondary: (1) GFR. (2) Effective renal plasma flow. (3) Systolic blood pressure. (4) Diastolic blood pressure. (5) Heart rate | RCT, Phase 2 | Not yet recruiting | Estimated March 2021 |
| NCT03554200 (EMPA Acute Heart Failure) | Acute HF (estimated N = 56) | Empagliflozin (30 days) | Placebo | Primary: Cardiac output. Secondary: (1) Haemodynamics (stroke volume). (2) Blood pressure. (3) QoL. (4) CV hospitalization or readmission for HF or renal failure. (5) CV death | RCT, phase 2 | Recruiting | Estimated October 2019 |
| NCT03619213 (DELIVER) | HFpEF (estimated N = 6100) | Dapagliflozin (39 months) | Placebo | Primary: Time to first of any component of the composite of CV death, HHF or urgent HF visit. Secondary: (1) Number of total (first and recurrent) HHF events and CV death. (2) KCCQ symptom score. (3) Time to CV death. (4) Time to all‐cause death | RCT, phase 3 | Recruiting | Estimated June 2021 |
| NCT03753087 | HFpEF and T2DM (estimated N = 100) | Empagliflozin (6 months) | SoC | Primary: 6MWT. Secondary: (1) LV mass index. (2) Left atrial volume index. (3) Left atrial stiffness. (4) Pulmonary artery systolic pressure. (5) e' velocity (echocardiography) | RCT, phase 4 | Recruiting | Estimated May 2020 |
| NCT03877224 (DETERMINE‐preserved) | HFpEF (N = 504) | Dapagliflozin (16 weeks) | Placebo | Primary: (1) KCCQ total score. (2) KCCQ physical limitation score. (3) 6MWT. Secondary: Physical activity (assessed using wearable activity monitor) | RCT, phase 3 | Complete | July 2020 |
| NCT03877237 (DETERMINE‐reduced) | HFrEF (N = 313) | Dapagliflozin (16 weeks) | Placebo | Primary: (1) KCCQ total score. (2) KCCQ physical limitation score. (3) 6MWT. Secondary: Physical activity (assessed using wearable activity monitor) | RCT, phase 3 | Complete | March 2020 |
| NCT03717194 (ERTU‐GLS) | HF and T2DM (estimated N = 120) | Ertugliflozin (24 weeks) | Placebo | Primary: Global longitudinal strain. Secondary: (1) E/e' ratio. (2) Ejection fraction. (3) LV mass. (4) HbA1c. (5) Body weight | RCT, phase 3 | Recruiting | Estimated September 2020 |
| NCT04049045 (EMPAG‐HF) | Acute HF (estimated N = 60) | Empagliflozin (5 days) | Placebo | Primary: Total urinary output. Secondary: (1) Renal function. (2) Net fluid output. (3) Worsening or persistent HF. (4) Intermediate care/intensive care and hospital length of stay. (5) Liver function | RCT, phase 2 | Recruiting | Estimated March 2021 |
| NCT04071626 (EMMED‐HF) | HFpEF and T2DM (estimated N = 52) | Ertugliflozin (12 weeks) | Placebo | Primary: Peak VO2. Secondary: (1) LV mass index. (2) Serum ketone bodies | RCT, phase 4 | Not yet recruiting | Estimated June 2021 |
| NCT04080518 (DAPA‐Shuttle1) | HF and T2DM (estimated N = 40) | Dapagliflozin (28 days) | Placebo | Primary: Urine osmolyte concentration. Secondary: (1) Plasma copeptin levels. (2) Tissue sodium content mobilization. (3) Muscle and liver lipid content | RCT, phase 4 | Recruiting | Estimated April 2020 |
| NCT04304560 | HFrEF and T2DM (estimated N = 60) | Dapagliflozin (3 months) | Placebo | Primary: (1) LV dimensions. (2) Systolic function. (3) Diastolic function. Secondary: (1) HbA1c. (2) Myeloperoxidase. (3) NT‐proBNP. (4) Galectin‐3 | RCT, phase 2 | Not yet recruiting | Estimated March 2021 |
| NCT04252287 (CHIEF‐HF) | HF (estimated N = 1900) | Canagliflozin (12 weeks) | Placebo | Primary: KCCQ total symptom score. Secondary: (1) Daily step count. (2) KCCQ individual domain scores | RCT, phase 3 | Recruiting | Estimated February 2021 |
| NCT04298229 (DICTATE‐AHF) | Acute HF and T2DM (estimated N = 240) | Dapagliflozin and protocolized diuretic therapy (5 days) | Protocolized diuretic therapy | Primary: Cumulative weight change. Secondary: (1) Worsening HF incidence. (2) Hospital readmission | RCT, phase 3 | Recruiting | Estimated May 2021 |
| NCT04385589 | Acute HFrEF and T2DM (estimated N = 100) | Dapagliflozin, insulin (if needed), diuretics and HF SoC (5 days) | Placebo, insulin, diuretics and HF SoC | Primary: (1) Body weight. (2) Diuresis effect. Secondary: (1) Renal function. (2) Patient‐reported dyspnoea | RCT, phase 4 | Recruiting | Estimated December 2020 |
| NCT04157751 (EMPULSE) | HF (estimated N = 500) | Empagliflozin (90 days) | Placebo | Primary: Composite of death, HF events, urgent HF visits, time to first HF event KCCQ total symptom score. Secondary: (1) KCCQ total symptom score ≥ 10‐point improvement. (2) KCCQ total symptom score. (3) NT‐proBNP. (4) Days alive and out of hospital to 30 days after initial hospital discharge. (5) Days alive and out of hospital to 90 days after randomization | RCT, phase 3 | Recruiting | Estimated June 2021 |
| NCT04363697 (DAPA ACT HF‐TIMI 68) | Acute HFrEF (estimated N = 2400) | Dapagliflozin (2 months) | Placebo | Primary: CV death or worsening HF. Secondary: (1) Composite of CV death, HF rehospitalization, urgent HF visit. (2) Composite of CV death or HF rehospitalization. (3) Composite of HF rehospitalization or urgent HF visit. (4) Hospital readmission. (5) CV death | RCT, phase 4 | Recruiting | Estimated July 2022 |
| NCT04475042 (STADIA‐HFpEF) | HFpEF (estimated N = 26) | Dapagliflozin (13 weeks) | Placebo | Primary: (1) LV e'. (2) E/e'/LV end‐diastolic volume index. Secondary: (1) KCCQ score. (2) 6MWT. (3) Left atrial volume. (4) Diastolic parameters. (5) Left atrial function | RCT, phase 2 | Recruiting | Estimated November 2021 |
| NCT04438213 | Acute HF (estimated N = 90) | Ertugliflozin (6 weeks) | Placebo or active control metolazone | Primary: (1) Natriuretic effect (urine sodium concentration). (2) Total body water at 7 days. (3) Total body water at 6 weeks | RCT, phase 2 | Not yet recruiting | Estimated January 2022 |
| NCT04249778 | HFrEF (estimated N = 392) | Dapagliflozin (26 weeks) | Placebo | Primary: Composite of HF hospital admissions, HF emergency department visits, HF urgent clinic visits or death after acute decompensated HF admission. Secondary: (1) KCCQ score. (2) CHQ‐SAS score. (3) NT‐proBNP. (4) 6MWT. (5) HbA1c | RCT, phase 4 | Recruiting | Estimated December 2025 |
| NCT04490681 (VERTICAL) | HF with non‐ischaemic cardiomyopathy (estimated N = 52) | Ertugliflozin (48 weeks) | Placebo | Primary: Extracellular volume change (cardiac MRI). Secondary: Cardiac MRI parameters (myocardial fibrotic change, ventricular remodelling, systolic function) | RCT, phase 3 | Not yet recruiting | Estimated December 2021 |
6MWT, 6 min walk test; BNP, B‐type natriuretic peptide; CHQ‐SAS, Chronic Heart Failure Questionnaire Self‐Administered Standardized; CV, cardiovascular; eGFR, estimated glomerular filtration rate; GFR, glomerular filtration rate; HbA1c, glycated haemoglobin; HF, heart failure; HHF, hospitalization for heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; KCCQ, Kansas City Cardiomyopathy Questionnaire; LV, left ventricular; MRI, magnetic resonance imaging; NT‐proBNP, N‐terminal pro B‐type natriuretic peptide; NYHA, New York Heart Association; PGI‐S, Patient Global Impression of Severity; QoL, quality of life; RCT, randomized controlled trial; SGLT2i, sodium–glucose cotransporter 2 inhibitor; SoC, standard of care; T2DM, type 2 diabetes mellitus; VO2, oxygen consumption.
If applicable.
If more than five secondary outcomes are listed on ClinicalTrials.gov, only the first five are shown here. Time points at which outcomes will be assessed are presented only if they differ from the time period stated for the intervention.
Status as of 10 September 2020.
Of note, both the DAPA‐HF and EMPEROR‐Reduced trials also reported significantly improved Kansas City Cardiomyopathy Questionnaire scores (indicating improved patient‐reported health status) with SGLT2i treatment compared with placebo in patients with HFrEF. 8 , 9 Several trials will soon provide further insights into the effects of SGLT2is on overall health status and quality of life in patients with HFrEF (DETERMINE‐Reduced, EMPERIAL‐Reduced, and CHIEF‐HF) and HFpEF (DETERMINE‐Preserved, EMPERIAL‐Preserved, and CHIEF‐HF) (Table 3 ), providing a more in‐depth picture of the benefits of these drugs beyond CV death and HHF.
Since the date of our literature search, results of the DAPA‐CKD study in patients with CKD with or without T2DM have also been reported. A significant 39% reduction in the relative risk of the composite endpoint of a sustained decline in estimated glomerular filtration rate of at least 50%, end‐stage renal disease, or death from renal or CV causes was observed with dapagliflozin compared with placebo in this study, which was similar in patients with or without T2DM. 51 In addition, there was a significant 29% reduction in the secondary composite endpoint of CV death or HHF (HHF not reported separately). 51 Primary completion of the EMPA‐KIDNEY trial, assessing empagliflozin in patients with CKD without T2DM, is due in 2022 (Figure 1 ).
Randomized controlled trials assessing effects on cardiac structure and function
Left ventricular structure and function
All studies assessing the impact of SGLT2is compared with placebo on LV structure and function were deemed to have a low RoB (Table 2 ).
The DAPA‐LVH study included 66 patients with T2DM, LV hypertrophy, and controlled blood pressure who were randomized to receive either dapagliflozin 10 mg or placebo daily for 12 months. 10 The primary outcome was the change in LV mass, an indicator of cardiac hypertrophy, assessed by cardiac magnetic resonance imaging. Dapagliflozin reduced LV mass significantly more than placebo in this trial (Table 2 ).
Cardiac hypertrophy was also assessed in the EMPA‐HEART study, in which 97 patients with T2DM and coronary artery disease (CAD) were randomized to receive empagliflozin 10 mg or placebo daily for 6 months. 11 This trial assessed the change in LV mass (indexed to body surface area) as its primary outcome, which was found to be significantly reduced with empagliflozin compared with placebo (Table 2 ).
In the REFORM trial, 56 patients with T2DM and HF with LV systolic dysfunction were randomized to receive dapagliflozin 10 mg or placebo daily for 52 weeks. 12 No significant differences were reported for the primary outcome of change in LV end‐systolic volume as assessed by cardiac magnetic resonance imaging (Table 2 ).
Change in LV end‐systolic volume (indexed to body surface area) was also assessed as the primary outcome in the SUGAR‐DM‐HF trial, which randomized 105 patients with HFrEF and T2DM or prediabetes to receive empagliflozin 10 mg or placebo daily for 9 months. 17 LV end‐systolic volume was reduced to a significantly greater extent with empagliflozin compared with placebo in this trial (Table 2 ).
Finally, in the EMPA‐TROPISM trial, change in LV end‐diastolic volume was assessed as the primary endpoint in 84 non‐diabetic patients with HFrEF who were randomized to receive empagliflozin 10 mg or placebo daily for 6 months. 16 This endpoint was significantly reduced in patients receiving empagliflozin compared with those who received placebo (Table 2 ).
N‐terminal pro B‐type natriuretic peptide as a marker of cardiac stress
Secretion of N‐terminal pro B‐type natriuretic peptide (NT‐proBNP) by cardiomyocytes in response to myocyte stretching is a marker of stress due to increased cardiac filling pressure. 52 All studies assessing the impact of SGLT2is compared with placebo on NT‐proBNP levels were deemed to have a low RoB (Table 2 ).
In the EMPA‐RESPONSE trial, 80 patients admitted for acute decompensated HF were randomized to receive empagliflozin 10 mg or placebo daily for 30 days. 13 A primary outcome of this study was the change in NT‐proBNP after 4 days of treatment, which was not found to be significantly different between treatment groups (Table 2 ).
Change in NT‐proBNP level was also assessed as a primary outcome with empagliflozin treatment in the EMPIRE HF study. 14 In this trial, 190 patients with HFrEF were randomized to receive empagliflozin 10 mg or placebo daily for 12 weeks. The change in mean NT‐proBNP level from baseline to 12 weeks was not significantly different between treatment groups (Table 2 ).
In the DEFINE‐HF trial, change in NT‐proBNP level was assessed as a primary outcome in 263 patients with HFrEF after randomization to 10 mg dapagliflozin or placebo daily for 12 weeks. 15 No significant difference between dapagliflozin and placebo was observed for this measure (Table 2 ).
In the DAPA‐HF and EMPEROR‐Reduced trials, changes in NT‐proBNP level were included as exploratory outcomes in patients with HFrEF. 8 , 9 Data from these trials indicated significantly reduced NT‐proBNP with SGLT2i treatment compared with placebo after 8–12 months (Table 2 ).
Summary, implications, and future trials in heart failure
Evidence from small, long‐term (6–12 month) trials in patients with T2DM, with concomitant LV hypertrophy or CAD, 10 , 11 and in patients with HFrEF with and without diabetes, 16 , 17 indicate that SGLT2i treatment may help to reverse adverse LV remodelling (low RoB for all studies). Indeed, to date, only the REFORM trial (low RoB) has included such a measure as its primary outcome and failed to detect a significant effect. 15 This may reflect differences in the population, such as inclusion of asymptomatic patients with New York Heart Association class I HF (vs. II–III in the other HF studies), or be due merely to chance, particularly given these are small trials. Several SGLT2i trials including measures of cardiac structure and function in patients with HF are nearing completion (EMBRACE‐HF, ERTU‐GLS, STADIA‐HFpEF, VERTICAL, EMMED‐HF, & NCT04304560) (Table 3 ).
None of the three trials assessing short‐term changes (4 days to 12 weeks) in NT‐proBNP levels in patients with HF detected a significant effect with SGLT2i treatment (low RoB for all studies). 13 , 14 , 15 However, larger, long‐term (8–12 month) trials have identified significant decreases in NT‐proBNP with SGLT2i treatment compared with placebo (low RoB for both studies). 8 , 9 In addition, changes in NT‐proBNP were assessed as secondary outcomes in both SUGAR‐DM‐HF and EMPA‐TROPISM and were significantly reduced with SGTL2i treatment compared with placebo. 16 , 17 Reduced NT‐proBNP may thus be a long‐term consequence of SGLT2i treatment, or larger sample sizes may be required to detect early changes. Several larger trials are assessing early NT‐proBNP changes as secondary outcomes in patients with HF (EMPULSE, NCT04249778, and PRESERVED‐HF) (Table 3 ).
Potential mechanisms of sodium–glucose cotransporter 2 inhibitor efficacy in heart failure
The following section is an exploratory, narrative synthesis of RCT‐derived data relating to mechanisms by which SGLT2is may improve HF outcomes. RoB assessments were not performed for studies in this section, and supplementary literature is cited in places for additional context.
Conventional cardiovascular disease risk factors
Reduced Hb1Ac levels with SGLT2i treatment do not account for their ability to reduce HHF events. First, changes in Hb1Ac to the degree observed with SGLT2i treatment in major event‐driven trials were relatively modest, 3 , 4 , 5 , 6 , 7 , 8 , 9 and changes of this scale do not lead to similar reductions in HHF with other drugs. Second, although the HbA1c‐reducing effects of SGLT2is decline as renal function declines, 53 reductions in HF events were consistent across different baseline estimated glomerular filtration rates. 30 , 34 , 40 , 53
Sodium–glucose cotransporter 2 inhibitors have also been consistently shown to reduce body weight and blood pressure in addition to HbA1c levels in key trials 3 , 4 , 5 , 6 , 7 , 8 , 9 ; however, these effects also cannot fully account for reduced HF events with SGLT2i treatment. In two post hoc analyses of EMPA‐REG OUTCOME data, simulation of the effects of empagliflozin compared with placebo on conventional CVD risk factors accounted for only 15% and 37% of the observed reduction in HHF risk observed in patients with T2DM and CVD. 54 , 55
A plethora of mechanisms outside of changes in conventional CVD risk factors have been proposed for how SGLT2is may improve HF outcomes (Figure 3 A ). However, not all have been the focus of RCT studies, which are reviewed in the following sections.
Figure 3.
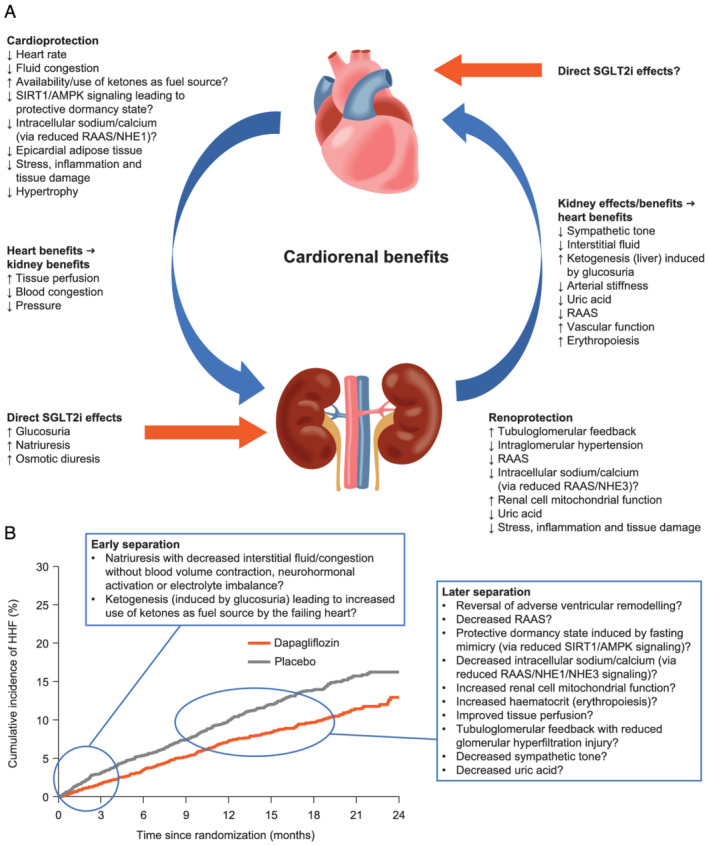
(A) Potential cardiorenal protective mechanisms that have been proposed to contribute to benefits observed with SGLT2is in patients with HF. (B) Potential mechanisms that may account for early vs. later benefits observed with SGLT2is in patients with HF (adapted from McMurray et al. 8 ). Copyright © (2019) Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society). AMPK, adenosine monophosphate‐activated protein kinase; HF, heart failure; HHF, hospitalization for heart failure; NHE, sodium‐hydrogen exchanger; RAAS, renin–angiotensin–aldosterone system; SGLT2i, sodium–glucose cotransporter 2 inhibitor; SIRT1, sirtuin 1.
Haemodynamic changes
In a pooled analysis (N = 4533) of double‐blind, RCT data in patients with inadequately controlled T2DM and blood pressure, the estimated plasma volume was significantly reduced with dapagliflozin 10 mg compared with placebo at 24 weeks. 56 Blood plasma volume was also significantly reduced with canagliflozin 300 mg daily compared with placebo at 1 week in a double‐blind RCT in 36 patients with inadequately controlled T2DM and hypertension controlled with renin–angiotensin–aldosterone system inhibitor therapy; however, in contrast with the previous study, this effect was largely attenuated at 12 weeks. 57
Taken together, the above evidence suggests that haemodynamic changes due to SGLT2i‐induced diuresis may contribute to their efficacy. However, this must be reconciled with a lack of evidence for reduced mortality in patients with HF treated with traditional diuretics. Modelling based on data from an open‐label, randomized study in healthy individuals predicted a two‐fold greater reduction in interstitial fluid (IF) volume than in blood volume for dapagliflozin. 58 In comparison, modelling data for bumetanide indicated a reduction in IF volume that was only 78% of that observed for blood volume. 58 These data suggest greater effects with SGLT2i treatment compared with diuretics on interstitial congestion. 58 A key prediction of this model is that larger reductions in IF volume relative to blood volume may more effectively relieve signs and symptoms of interstitial congestion, and provide relief of elevated cardiac filling pressures without the deleterious effects of excessive blood volume depletion, such as reduced arterial filling and neurohormonal activation. 58 This prediction appears to have been borne out in a subsequent double‐blind, crossover RCT in which 14 days of empagliflozin 10 mg daily did not cause neurohormonal activation, despite causing natriuresis and decreases in plasma blood volume. 59 Notably, several ongoing trials are assessing haemodynamic effects of SGLT2is in patients with HFrEF (ELSI, ERADICATE‐HF, DAPA‐Shuttle1, VERTICAL, and NCT04438213) (Table 3 ).
A recent double‐blind RCT (RECEDE‐CHF) investigating the effects of empagliflozin 25 mg once daily on top of loop diuretic (furosemide) treatment found that this combination significantly increased urine output compared with furosemide alone, without a significant increase in natriuresis after 6 weeks. 60 Although small (N = 23), this trial partly allays fears of an increased risk of volume depletion with combined SGLT2i and loop diuretic use. Indeed, there now appear to be numerous differences in the physiological effects of SGLT2is compared with diuretics (Table 4 ).
Table 4.
Physiological effects observed with SGLT2is and diuretics
| Physiological effect | SGLT2is | Diuretics |
|---|---|---|
| Sodium | ↔ | ↓ |
| Potassium | ↔ | ↓ |
| Magnesium | ↔ | ↓ |
| Uric acid | ↓ | ↑ |
| LDL cholesterol | ↔ | ↑ |
| Plasma glucose | ↓ | ↑ |
| Haematocrit | ↑ | ↔ |
| Heart rate | ↓ | ↑ |
| Systolic blood pressure | ↓ | ↓ |
| Intravascular volume | ↓ | ↓ |
| Interstitial volume | ↓ | ↔ |
| Myocardial infarction | ↔ | ↔ |
| Stroke | ↔ | ↓ |
| eGFR | ↓ then ↔ | ↓ |
| Intraglomerular pressure | ↓ | ↔ |
| Tubuloglomerular feedback | ↑ | ↔ |
| Renin/angiotensin II | ↓ | ↑ |
| Aldosterone | ↓ | ↑ |
| Sympathetic tone | ↓ | ↑ |
| Arginine vasopressin | ↔ | ↑ |
eGFR, estimated glomerular filtration rate; LDL, low‐density lipoprotein; SGLT2i, sodium–glucose cotransporter 2 inhibitor.
Tissue sodium content
The effects of dapagliflozin treatment compared with placebo on tissue sodium content at 6 weeks have been assessed in 59 patients with T2DM in a double‐blind, crossover RCT. 61 Tissue sodium content in the skin, but not muscle, was found to be significantly reduced with dapagliflozin compared with placebo, with no significant changes in water content observed in either tissue. 61 The ability of peripheral tissues to sequester sodium is a critical assumption of the haemodynamic model of differential volume regulation (IF vs. plasma volume) proposed above for SGLT2is. 58 In addition, increased tissue sodium content has been independently linked to LV hypertrophy in patients with CKD. 62 It has also been hypothesized that decreased renin–angiotensin–aldosterone system activation with SGLT2is may decrease sodium‐hydrogen exchanger 1 (NHE 1) activity in the heart, decreasing intracellular sodium and calcium to reduce cardiomyocyte death, as well as inhibiting NHE3 in the kidneys with potential renoprotective effects. 63 Indeed, SGLT2i‐driven inhibition of NHE3 sodium reabsorption (along with direct inhibition of sodium and glucose reabsorption through SGLT2 and osmotic diuresis coupled with peripheral sodium storage) was identified as a key mechanism required to explain benefits such as reduced glomerular pressure, and reduced blood and IF volume, in a model‐based analysis of clinical data. 64 Effects of dapagliflozin on tissue sodium are also being assessed in HF in the DAPA‐Shuttle1 and ELSI trials (Table 3 ).
Erythropoiesis
A mediation analysis found that increased haematocrit and haemoglobin levels, and reduced uric acid levels, were the most important mediators of reduced CV death with empagliflozin treatment in the EMPA‐REG OUTCOME trial. 65 Similarly, reduced uric acid levels, and increased erythrocyte and haemoglobin levels, were identified as important mediators of the effects of canagliflozin on HF events in the CANVAS trial programme, although haematocrit had a smaller mediating effect in this study compared with the EMPA‐REG OUTCOME trial. 65 , 66 Indeed, elevated haematocrit and/or haemoglobin levels have been consistently observed with SGLT2i treatment. 3 , 8 , 9
Although reductions in plasma volume may contribute to increased haematocrit and haemoglobin concentrations observed with SGLT2i treatment, it has been observed that the time course of changes in these variables does not align. 67 An alternative explanation may be that SGLT2i treatment increases production of red blood cells. This hypothesis was tested in 52 patients with T2DM randomized to dapagliflozin or placebo treatment for 12 weeks. Significant changes in markers indicative of enhanced erythropoiesis (increased erythroferrone, decreased hepcidin, and transiently increased erythropoietin) were observed in patients receiving dapagliflozin compared with placebo in this study. 68 The authors of this study hypothesize that erythropoiesis may be inhibited in a pro‐inflammatory state. 68 It has been argued separately that SGLT2is may prevent suppression of erythropoiesis through reduced renal metabolic ‘stress’ and inflammation as a result of diuresis without sympathetic activation. 68 Kidney stress may also be reduced via tubuloglomerular feedback due to SGLT2i‐induced natriuresis. 67
Epicardial fat
We identified one RCT assessing the effects of SGLT2is on epicardial adipose tissue (EAT) volume. 69 In this open‐label trial, 40 patients with T2DM and CAD were randomized to receive dapagliflozin or conventional treatment for 6 months. A significant reduction in EAT volume was observed with dapagliflozin compared with placebo, and the reduction was positively correlated with reductions in body weight and the pro‐inflammatory cytokine tumour necrosis factor‐α. 69 This is consistent with evidence that EAT expresses a pathogenic profile of adipocytokines in patients with CVD. 70 Accumulation and inflammation of EAT also stimulates secretion of leptin to promote fibrosis. 71 Reduction of EAT with SGLT2is may thus reduce cardiac inflammation and secretion of leptin to reduce adverse cardiac remodelling. 71
Inflammation
We identified a single, open‐label RCT that assessed the effects of SGLT2is on inflammation. 72 In this study, 102 patients with T2DM and insulin resistance were randomized to receive treatment with empagliflozin or placebo as add‐on therapy for 1 year. 72 Significant reductions in remnant‐like particle cholesterol and high‐sensitivity C‐reactive protein, as well as significantly reduced insulin resistance, were observed for empagliflozin compared with placebo in this trial. 72 Inflammation is a well‐known contributor to CVD, and patients with HF have elevated levels of pro‐inflammatory cytokines. 73 Although drugs that specifically target inflammation have not performed well in previous HF clinical trials, there is recent evidence that HF outcomes may be improved in patients with HF who have a cardio‐inflammatory phenotype. 73
Vascular function
In a double‐blind RCT conducted in 81 patients with T2DM and ischaemic heart disease, changes in flow‐mediated dilation and nitroglycerin‐mediated vasodilatation were not significantly different between dapagliflozin and placebo at 12 weeks, 74 although consistent trends were observed towards greater maintenance of these measures and reduced markers of endothelial dysfunction with dapagliflozin. 74 Secondary analysis of RCT data from 47 patients with T2DM and high CVD risk who were randomized to receive empagliflozin or placebo also found no significant difference in endothelial function (reactive hyperaemia index) between treatment groups. 75 However, arteriolar remodelling was significantly reduced, and retinal capillary flow was significantly improved, with 6 weeks of dapagliflozin treatment compared with placebo in 59 patients with T2DM in a double‐blind, crossover RCT. 76 In a subsequent double‐blind RCT conducted by the same research group, 6 weeks of treatment with empagliflozin was found to significantly improve various measures of aortic stiffness in 76 patients with T2DM compared with placebo. 77 These results are consistent with previous findings from a pooled analysis of data from double‐blind RCTs assessing canagliflozin 100 mg or 300 mg daily compared with placebo for 6 (N = 169) or 26 weeks (N = 2313) in patients with T2DM, which also identified significant reductions in markers of arterial stiffness. 78
Sympathetic nervous system activity
Overactivation of the sympathetic nervous system is a key characteristic of patients with HF and may contribute to poor outcomes through the modulation of factors such as increased vascular tone, contributing to macrovascular dysfunction and arterial stiffness, 79 and cardiac activity that may increase the risk of lethal ventricular arrhythmias and sudden cardiac death. 80 The sympathetic nervous system also provides a potential link between the renoprotective effects of SGLT2is and their CV benefits, because renal stress increases systemic sympathetic activity via the central nervous system. 81 Evidence suggesting that SGLT2is may suppress sympathetic activity comes from consistent decreases in resting heart rate based on pooled RCT data, 81 in contrast to diuretics, which increase heart rate. We identified two double‐blind RCTs (EMBODY and EMPA‐HEART) that assessed the impact of SGLT2i treatment on sympathetic cardiac activity. No significant differences in heart rate variability (HRV) measures, used to assess cardiac sympathetic activity, were observed in 105 patients with MI and T2DM treated with empagliflozin 10 mg daily for 24 weeks compared with those who received placebo. 82 HRV measures did, however, decrease significantly from baseline with empagliflozin, but not with placebo. 82 HRV measures were also not significantly altered after 6 months of treatment with empagliflozin 10 mg daily compared with placebo in 66 patients with T2DM and CAD in the EMPA‐HEART study, with the exception of a significant reduction in the standard deviation of normal to normal intervals. 83 The statistical significance of changes from baseline in HRV measures observed within treatment groups was not assessed in this study. 83 Further trials are needed to establish the relevance of this mechanism, particularly in patients with HF.
Cellular energetics
There has been substantial interest in the ‘ketone hypothesis’, which proposes that ketogenesis induced by SGLT2is may have favourable effects by providing an efficient fuel, in the form of ketone bodies, that can boost the function of the stressed heart in patients with HF. 84 More recently, the opposite of this hypothesis was proposed, in which SGLT2is are suggested to induce a protective ‘dormancy’ state, possibly through activation of adenosine monophosphate‐activated protein kinase/sirtuin 1 signalling. 85 , 86 At least two double‐blind RCTs in patients with T2DM that were identified by our searches support the notion of increased circulating ketones with SGLT2i use compared with placebo [dapagliflozin after 2 weeks (N = 18) 87 and empagliflozin after 4 weeks (N = 60) 88 ]. However, a study in a hypertensive HF rat model showed that empagliflozin was associated with reduced myocardial ketone body use, suggesting that increases in circulating ketone levels may be a secondary phenomenon. 89 The relative merits of the opposing ‘ketone’ and ‘dormancy state’ hypotheses, which have been the subject of intense debate, are better discussed in detail elsewhere. 86 Regardless, hypothesis‐driven RCTs supporting any firm conclusions are lacking, and given the potential importance of this mechanism, the number planned trials investigating aspects of myocardial energetics in patients with HF taking SGLT2is (EMPA‐VISION and EMMED‐HF) appears relatively small (Table 3 ).
Finally, in a study that analysed urine and plasma samples from a 6 ‐week, double‐blind, RCT of dapagliflozin in 31 patients with T2DM and elevated urine albumin levels, nine of 13 urine metabolites linked to mitochondrial metabolism were elevated compared with placebo, while none were elevated in plasma. These findings suggest that SGLT2is may improve renal cell mitochondrial function. 90
Summary, implications, and future trials in heart failure
It is helpful to consider proposed SGLT2i mechanisms in terms of those that may account for the early separation of SGLT2i and placebo HHF event curves at approximately 0–3 months, vs. further separation that seems to occur at later stages of treatment (Figure 3 B ). It is well recognized that interstitial congestion/fluid overload is associated with worse outcomes in patients with acute HF. 91 The early benefit of SGLT2i therapy may therefore relate to haemodynamic effects with natriuresis that is not accompanied by blood volume contraction, 58 neurohormonal activation, or electrolyte imbalance. 59 Ketogenesis, if found to be a clinically relevant mechanism of SGLT2i efficacy, could also have early benefits. The reversal of adverse ventricular remodelling, among other possible effects (Figure 3 B ), may help to account for long‐term SGLT2i benefits.
Although SGLT2is clearly have pleiotropic effects, not all mechanisms are likely to contribute equally to their success in HF. The consistency of the effects on HHF across trial populations and trial subgroups hints at a subset of key mechanisms that can operate across disease states, with disease‐state‐specific effects accounting for some of the variation in efficacy between populations.
Conclusions and future directions
Sodium–glucose cotransporter 2 inhibitors consistently reduce HHF rates in clinical trials, 3 , 4 , 5 , 6 , 7 , 8 , 9 including in patients with established HFrEF and CKD, regardless of T2DM status, 8 , 9 and they are likely to also be effective in patients with HFpEF. RCT data also support reversal of adverse LV remodelling as an effect of SGLT2i treatment that contributes to improved HF outcomes. However, the magnitude of this contribution is unknown, as is the contribution of other glucose‐independent SGLT2i mechanisms. Several intriguing and convincing hypotheses have been proposed, but hypothesis‐driven RCT data are sparse.
A limitation of this review is that our assessment of broader SGLT2i mechanisms was exploratory and may thus be more prone to bias. Conversely, a strength of this section, particularly in comparison with previous reviews, is that it focused primarily on systematically identified RCT data. Another limitation of this review is that ongoing SGLT2i trials registered in databases other than ClinicalTrials.gov may have been missed. We also summarized ongoing SGLT2i trials in patients with HF only, for practical reasons, and to maximize relevance. However, mechanistic insights will (and have) also come from ongoing studies in patients without HF, which are not captured here. Despite these limitations, we believe this review constitutes a rigorous and up‐to‐date assessment of the field.
A position paper from the European Society of Cardiology recently endorsed the use of dapagliflozin and empagliflozin in symptomatic patients with HFrEF already receiving guideline therapy, regardless of the presence of T2DM, based on their efficacy profile and data indicating no excess risk of renal adverse events, volume depletion, severe hypoglycaemia, fractures, amputations, or Fournier's gangrene. 92 Nevertheless, owing to their origins (Figure 1 ), the perception of SGLT2is as merely glucose‐lowering agents remains a communication challenge. At worse, this perception may lead to discontinuation in patients in whom negligible glycaemic effects are observed. Continued education around the glucose‐independent benefits of SGLT2is is thus of paramount importance to maximizing patient care, even if a sound mechanistic framework is currently lacking.
Upcoming trial results are likely to provide further clarity regarding the efficacy and mechanistic profiles of SGLT2is. Further research into SGLT2i mechanisms will offer new insights into the pathophysiological mechanisms contributing to disease progression in HF, and the nuanced functions of the kidney, potentially yielding novel drug targets for both CKD and HF.
Conflict of interest
AstraZeneca reviewed the draft manuscript for accuracy prior to submission, but did not contribute to the study concept, design, research or analysis, or to the drafting of the manuscript. MMB is an independent contractor for Oxford PharmaGenesis, Melbourne, Australia, which received an unrestricted grant for this study from AstraZeneca. RR has received honoraria and been on advisory boards or provided educational consultancy for AstraZeneca, Boehringer Ingelheim, Eli Lilly, Novo Nordisk, and Sanofi. JJA has received honoraria and travel support and been on advisory boards for AstraZeneca, Boehringer Ingelheim, and Eli Lilly. GFD has not received research funding support but provides educational and independent clinical consultancy to AstraZeneca and has been on Advisory Boards for AstraZeneca, Boehringer Ingelheim, Eli Lilly, Novartis, and Sanofi. NDC has had speaker engagements, Advisory Board roles, and received research funding from AstraZeneca, Boehringer Ingelheim, and Sanofi. AS has received honoraria, speaker fees, and consultancy fees; is a member of advisory boards; and has appeared on expert panels for Alphapharm, Amgen, Aspen, AstraZeneca, Bayer, Biotronik, Boehringer Ingelheim, Bristol Myers Squibb, CSL, Menarini, Merck Sharp and Dohm, Mylan, Novartis, Otsuka, Pfizer, Sanofi, Servier, and Vifor.
Funding
This work was supported by an unrestricted research grant from AstraZeneca.
Supporting information
Table S1. PRISMA Checklist.
Table S2. Search strategy to search Embase (via Ovid).
Rasalam, R. , Atherton, J. J. , Deed, G. , Molloy‐Bland, M. , Cohen, N. , and Sindone, A. (2021) Sodium‐glucose cotransporter 2 inhibitor effects on heart failure hospitalization and cardiac function: systematic review. ESC Heart Failure, 8: 4093–4118. 10.1002/ehf2.13483.
References
- 1. Chao EC, Henry RR. SGLT2 inhibition—a novel strategy for diabetes treatment. Nat Rev Drug Discov 2010; 9: 551–559. [DOI] [PubMed] [Google Scholar]
- 2. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ 2009; 339: b2700. [PMC free article] [PubMed] [Google Scholar]
- 3. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE, Investigators E‐RO. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 4. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR, Group CPC . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377: 644–657. [DOI] [PubMed] [Google Scholar]
- 5. Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW, Investigators CT. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019; 380: 2295–2306. [DOI] [PubMed] [Google Scholar]
- 6. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause‐Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS, Investigators D‐T. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019; 380: 347–357. [DOI] [PubMed] [Google Scholar]
- 7. Cannon CP, Pratley R, Dagogo‐Jack S, Mancuso J, Huyck S, Masiukiewicz U, Charbonnel B, Frederich R, Gallo S, Cosentino F, Shih WJ, Gantz I, Terra SG, Cherney DZI, McGuire DK, Investigators VC. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med 2020; 383: 1425–1435. [DOI] [PubMed] [Google Scholar]
- 8. McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Belohlavek J, Bohm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukat A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjostrand M, Langkilde AM, Committees D‐HT, Investigators . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019; 381: 1995–2008. [DOI] [PubMed] [Google Scholar]
- 9. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Bohm M, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner‐La Rocca HP, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde MF, Spinar J, Squire I, Taddei S, Wanner C, Zannad F, Investigators EM‐RT . Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020; 41: 3421–3432. [DOI] [PubMed] [Google Scholar]
- 10. Brown AJM, Gandy S, McCrimmon R, Houston JG, Struthers AD, Lang CC. A randomized controlled trial of dapagliflozin on left ventricular hypertrophy in people with type two diabetes: the DAPA‐LVH trial. Eur Heart J 2020; 41: 3421–3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Verma S, Mazer CD, Yan AT, Mason T, Garg V, Teoh H, Zuo F, Quan A, Farkouh ME, Fitchett DH, Goodman SG, Goldenberg RM, Al‐Omran M, Gilbert RE, Bhatt DL, Leiter LA, Jüni P, Zinman B, Connelly KA. Effect of empagliflozin on left ventricular mass in patients with type 2 diabetes mellitus and coronary artery disease: the EMPA‐HEART CardioLink‐6 randomized clinical trial. Circulation 2019; 140: 1693–1702. [DOI] [PubMed] [Google Scholar]
- 12. Singh JSS, Mordi IR, Vickneson K, Fathi A, Donnan PT, Mohan M, Choy AMJ, Gandy S, George J, Khan F, Pearson ER, Houston JG, Struthers AD, Lang CC. Dapagliflozin versus placebo on left ventricular remodeling in patients with diabetes and heart failure: the REFORM trial. Diabetes Care 2020; 43: 1356–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Damman K, Beusekamp JC, Boorsma EM, Swart HP, Smilde TDJ, Elvan A, van Eck JWM, Heerspink HJL, Voors AA. Randomized, double‐blind, placebo‐controlled, multicentre pilot study on the effects of empagliflozin on clinical outcomes in patients with acute decompensated heart failure (EMPA‐RESPONSE‐AHF). Eur J Heart Fail 2020; 22: 713–722. [DOI] [PubMed] [Google Scholar]
- 14. Jensen J, Omar M, Kistorp C, Poulsen MK, Tuxen C, Gustafsson I, Kober L, Gustafsson F, Faber J, Fosbol EL, Bruun NE, Brond JC, Forman JL, Videbaek L, Moller JE, Schou M. Twelve weeks of treatment with empagliflozin in patients with heart failure and reduced ejection fraction: a double‐blinded, randomized, and placebo‐controlled trial. Am Heart J 2020; 228: 47–56. [DOI] [PubMed] [Google Scholar]
- 15. Nassif ME, Windsor SL, Tang F, Khariton Y, Husain M, Inzucchi SE, McGuire DK, Pitt B, Scirica BM, Austin B, Drazner MH, Fong MW, Givertz MM, Gordon RA, Jermyn R, Katz SD, Lamba S, Lanfear DE, LaRue SJ, Lindenfeld J, Malone M, Margulies K, Mentz RJ, Mutharasan RK, Pursley M, Umpierrez G, Kosiborod M. Dapagliflozin effects on biomarkers, symptoms, and functional status in patients with heart failure with reduced ejection fraction: the DEFINE‐HF trial. Circulation 2019; 140: 1463–1476. [DOI] [PubMed] [Google Scholar]
- 16. Santos‐Gallego CG, Vargas‐Delgado AP, Requena JA, Garcia‐Ropero A, Mancini D, Pinney S, Macaluso F, Sartori S, Roque M, Sabatel‐Perez F, Cordero AR, Zafar MU, Fergus I, Atallah‐Lajam F, Contreras JP, Varley C, Moreno PR, Abascal VM, Lala A, Tamler R, Sanz J, Fuster V, Badimon JJ, investigators E‐T . Randomized trial of empagliflozin in non‐diabetic patients with heart failure and reduced ejection fraction. J Am Coll Cardiol 2021; 77: 243–255. [DOI] [PubMed] [Google Scholar]
- 17. Lee MMY, Brooksbank KJM, Wetherall K, Mangion K, Roditi G, Campbell RT, Berry C, Chong V, Coyle L, Docherty KF, Dreisbach JG, Labinjoh C, Lang NN, Lennie V, McConnachie A, Murphy CL, Petrie CJ, Petrie JR, Speirits IA, Sourbron S, Welsh P, Woodward R, Radjenovic A, Mark PB, McMurray JJV, Jhund PS, Petrie MC, Sattar N. Effect of empagliflozin on left ventricular volumes in patients with type 2 diabetes, or prediabetes, and heart failure with reduced ejection fraction (SUGAR‐DM‐HF). Circulation 2021; 143: 516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Singh JS, Fathi A, Vickneson K, Mordi I, Mohan M, Houston JG, Pearson ER, Struthers AD, Lang CC. Research into the effect of SGLT2 inhibition on left ventricular remodelling in patients with heart failure and diabetes mellitus (REFORM) trial rationale and design. Cardiovasc Diabetol 2016; 15: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marshall IJ, Kuiper J, Wallace BC. RobotReviewer: evaluation of a system for automatically assessing bias in clinical trials. J Am Med Inform Assoc 2016; 23: 193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Verma S, Ji Q, Bhatt DL, Mazer CD, Al‐Omran M, Inzucchi SE, Wanner C, Ofstad AP, Zwiener I, George JT, Zinman B, Fitchett D. Association between uric acid levels and cardio‐renal outcomes and death in patients with type 2 diabetes: a subanalysis of EMPA‐REG OUTCOME. Diabetes Obes Metab 2020; 22: 1207–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bohm M, Slawik J, Brueckmann M, Mattheus M, George JT, Ofstad AP, Inzucchi SE, Fitchett D, Anker SD, Marx N, Wanner C, Zinman B, Verma S. Efficacy of empagliflozin on heart failure and renal outcomes in patients with atrial fibrillation: data from the EMPA‐REG OUTCOME trial. Eur J Heart Fail 2020; 22: 126–135. [DOI] [PubMed] [Google Scholar]
- 22. Kaku K, Lee J, Mattheus M, Kaspers S, George J, Woerle HJ, Investigators E‐RO. Empagliflozin and cardiovascular outcomes in Asian patients with type 2 diabetes and established cardiovascular disease—results From EMPA‐REG OUTCOME((R)). Circ J 2017; 81: 227–234. [DOI] [PubMed] [Google Scholar]
- 23. Inzucchi SE, Khunti K, Fitchett DH, Wanner C, Mattheus M, George JT, Ofstad AP, Zinman B. Cardiovascular benefit of empagliflozin across the spectrum of cardiovascular risk factor control in the EMPA‐REG OUTCOME trial. J Clin Endocrinol Metab 2020; 105: 3025–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fitchett D, Inzucchi SE, Cannon CP, McGuire DK, Scirica BM, Johansen OE, Sambevski S, Kaspers S, Pfarr E, George JT, Zinman B. Empagliflozin reduced mortality and hospitalization for heart failure across the spectrum of cardiovascular risk in the EMPA‐REG OUTCOME trial. Circulation 2019; 139: 1384–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Monteiro P, Bergenstal RM, Toural E, Inzucchi SE, Zinman B, Hantel S, Kis SG, Kaspers S, George JT, Fitchett D. Efficacy and safety of empagliflozin in older patients in the EMPA‐REG OUTCOME(R) trial. Age Ageing 2019; 48: 859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fitchett D, Butler J, van de Borne P, Zinman B, Lachin JM, Wanner C, Woerle HJ, Hantel S, George JT, Johansen OE, Inzucchi SE, investigators E‐ROt . Effects of empagliflozin on risk for cardiovascular death and heart failure hospitalization across the spectrum of heart failure risk in the EMPA‐REG OUTCOME(R) trial. Eur Heart J 2018; 39: 363–370. [DOI] [PubMed] [Google Scholar]
- 27. Wanner C, Inzucchi SE, Zinman B, Koitka‐Weber A, Mattheus M, George JT, von Eynatten M, Hauske SJ, Investigators E‐RO. Consistent effects of empagliflozin on cardiovascular and kidney outcomes irrespective of diabetic kidney disease categories: Insights from the EMPA‐REG OUTCOME trial. Diabetes Obes Metab 2020; 22: 2335–2347. [DOI] [PubMed] [Google Scholar]
- 28. Verma S, Mazer CD, Al‐Omran M, Inzucchi SE, Fitchett D, Hehnke U, George JT, Zinman B. Cardiovascular outcomes and safety of empagliflozin in patients with type 2 diabetes mellitus and peripheral artery disease: a subanalysis of EMPA‐REG OUTCOME. Circulation 2018; 137: 405–407. [DOI] [PubMed] [Google Scholar]
- 29. Verma S, Mazer CD, Fitchett D, Inzucchi SE, Pfarr E, George JT, Zinman B. Empagliflozin reduces cardiovascular events, mortality and renal events in participants with type 2 diabetes after coronary artery bypass graft surgery: subanalysis of the EMPA‐REG OUTCOME(R) randomised trial. Diabetologia 2018; 61: 1712–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fitchett D, Zinman B, Wanner C, Lachin JM, Hantel S, Salsali A, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE, investigators E‐ROt. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA‐REG OUTCOME(R) trial. Eur Heart J 2016;37:1526–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wanner C, Lachin JM, Inzucchi SE, Fitchett D, Mattheus M, George J, Woerle HJ, Broedl UC, von Eynatten M, Zinman B, Investigators E‐RO. Empagliflozin and clinical outcomes in patients with type 2 diabetes mellitus, established cardiovascular disease, and chronic kidney disease. Circulation 2018; 137: 119–129. [DOI] [PubMed] [Google Scholar]
- 32. Verma S, Sharma A, Zinman B, Ofstad AP, Fitchett D, Brueckmann M, Wanner C, Zwiener I, George JT, Inzucchi SE, Butler J, Mazer CD. Empagliflozin reduces the risk of mortality and hospitalization for heart failure across Thrombolysis In Myocardial Infarction Risk Score for Heart Failure in Diabetes categories: post hoc analysis of the EMPA‐REG OUTCOME trial. Diabetes Obes Metab 2020; 22: 1141–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Neuen BL, Ohkuma T, Neal B, Matthews DR, de Zeeuw D, Mahaffey KW, Fulcher G, Li Q, Jardine M, Oh R, Heerspink HL, Perkovic V. Effect of canagliflozin on renal and cardiovascular outcomes across different levels of albuminuria: data from the CANVAS program. J Am Soc Nephrol 2019; 30: 2229–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Radholm K, Figtree G, Perkovic V, Solomon SD, Mahaffey KW, de Zeeuw D, Fulcher G, Barrett TD, Shaw W, Desai M, Matthews DR, Neal B. Canagliflozin and heart failure in type 2 diabetes mellitus: results from the CANVAS program. Circulation 2018; 138: 458–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Neuen BL, Ohkuma T, Neal B, Matthews DR, de Zeeuw D, Mahaffey KW, Fulcher G, Desai M, Li Q, Deng H, Rosenthal N, Jardine MJ, Bakris G, Perkovic V. Cardiovascular and renal outcomes with canagliflozin according to baseline kidney function. Circulation 2018; 138: 1537–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Furtado RHM, Bonaca MP, Raz I, Zelniker TA, Mosenzon O, Cahn A, Kuder J, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Nicolau JC, Gause‐Nilsson IAM, Fredriksson M, Langkilde AM, Sabatine MS, Wiviott SD. Dapagliflozin and cardiovascular outcomes in patients with type 2 diabetes mellitus and previous myocardial infarction. Circulation 2019; 139: 2516–2527. [DOI] [PubMed] [Google Scholar]
- 37. Bajaj HS, Raz I, Mosenzon O, Murphy SA, Rozenberg A, Yanuv I, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Gause‐Nilsson IAM, Sabatine MS, Wiviott SD, Cahn A. Cardiovascular and renal benefits of dapagliflozin in patients with short and long‐standing type 2 diabetes: analysis from the DECLARE‐TIMI 58 trial. Diabetes Obes Metab 2020; 22: 1122–1131. [DOI] [PubMed] [Google Scholar]
- 38. Cahn A, Mosenzon O, Wiviott SD, Rozenberg A, Yanuv I, Goodrich EL, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Gause‐Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS, Raz I. Efficacy and safety of dapagliflozin in the elderly: analysis from the DECLARE‐TIMI 58 study. Diabetes Care 2020; 43: 468–475. [DOI] [PubMed] [Google Scholar]
- 39. Kato ET, Silverman MG, Mosenzon O, Zelniker TA, Cahn A, Furtado RHM, Kuder J, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Bonaca MP, Ruff CT, Desai AS, Goto S, Johansson PA, Gause‐Nilsson I, Johanson P, Langkilde AM, Raz I, Sabatine MS, Wiviott SD. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation 2019; 139: 2528–2536. [DOI] [PubMed] [Google Scholar]
- 40. Jardine MJ, Zhou Z, Mahaffey KW, Oshima M, Agarwal R, Bakris G, Bajaj HS, Bull S, Cannon CP, Charytan DM, de Zeeuw D, Di Tanna GL, Greene T, Heerspink HJL, Levin A, Neal B, Pollock C, Qiu R, Sun T, Wheeler DC, Zhang H, Zinman B, Rosenthal N, Perkovic V, Investigators CS. Renal, cardiovascular, and safety outcomes of canagliflozin by baseline kidney function: a secondary analysis of the CREDENCE randomized trial. J Am Soc Nephrol 2020; 31: 1128–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mahaffey KW, Jardine MJ, Bompoint S, Cannon CP, Neal B, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Capuano G, de Zeeuw D, Greene T, Levin A, Pollock C, Sun T, Wheeler DC, Yavin Y, Zhang H, Zinman B, Rosenthal N, Brenner BM, Perkovic V. Canagliflozin and cardiovascular and renal outcomes in type 2 diabetes mellitus and chronic kidney disease in primary and secondary cardiovascular prevention groups. Circulation 2019; 140: 739–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Martinez FA, Serenelli M, Nicolau JC, Petrie MC, Chiang CE, Tereshchenko S, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Ponikowski P, Sabatine MS, DeMets DL, Dutkiewicz‐Piasecka M, Bengtsson O, Sjostrand M, Langkilde AM, Jhund PS, McMurray JJV. Efficacy and safety of dapagliflozin in heart failure with reduced ejection fraction according to age: insights from DAPA‐HF. Circulation 2020; 141: 100–111. [DOI] [PubMed] [Google Scholar]
- 43. Docherty KF, Jhund PS, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, Ponikowski P, DeMets DL, Sabatine MS, Bengtsson O, Sjostrand M, Langkilde AM, Desai AS, Diez M, Howlett JG, Katova T, Ljungman CEA, O'Meara E, Petrie MC, Schou M, Verma S, Vinh PN, Solomon SD, McMurray JJV. Effects of dapagliflozin in DAPA‐HF according to background heart failure therapy. Eur Heart J 2020; 41: 2379–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jackson AM, Dewan P, Anand IS, Belohlavek J, Bengtsson O, de Boer RA, Bohm M, Boulton DW, Chopra VK, DeMets DL, Docherty KF, Dukat A, Greasley PJ, Howlett JG, Inzucchi SE, Katova T, Kober L, Kosiborod MN, Langkilde AM, Lindholm D, Ljungman CEA, Martinez FA, O'Meara E, Sabatine MS, Sjostrand M, Solomon SD, Tereshchenko S, Verma S, Jhund PS, McMurray JJV. Dapagliflozin and diuretic use in patients with heart failure and reduced ejection fraction in DAPA‐HF. Circulation 2020; 142: 1040–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dewan P, Solomon SD, Jhund PS, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, Ponikowski P, DeMets DL, Sabatine MS, Bengtsson O, Sjostrand M, Langkilde AM, Anand IS, Belohlavek J, Chopra VK, Dukat A, Kitakaze M, Merkely B, O'Meara E, Schou M, Vinh PN, McMurray JJV, Investigators D‐H. Committees. efficacy and safety of sodium‐glucose co‐transporter 2 inhibition according to left ventricular ejection fraction in DAPA‐HF. Eur J Heart Fail 2020; 22: 1247–1258. [DOI] [PubMed] [Google Scholar]
- 46. Solomon SD, Jhund PS, Claggett BL, Dewan P, Kober L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Inzucchi SE, Desai AS, Bengtsson O, Lindholm D, Sjostrand M, Langkilde AM, McMurray JJV. Effect of dapagliflozin in patients with HFrEF treated with sacubitril/valsartan: the DAPA‐HF trial. JACC Heart Fail 2020; 8: 811–818. [DOI] [PubMed] [Google Scholar]
- 47. Petrie MC, Verma S, Docherty KF, Inzucchi SE, Anand I, Belohlavek J, Bohm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukat A, Ge J, Howlett J, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Vinh PN, Schou M, Tereshchenko S, Kober L, Kosiborod MN, Langkilde AM, Martinez FA, Ponikowski P, Sabatine MS, Sjostrand M, Solomon SD, Johanson P, Greasley PJ, Boulton D, Bengtsson O, Jhund PS, McMmurray JJV. Effect of dapagliflozin on worsening heart failure and cardiovascular death in patients with heart failure with and without diabetes. JAMA 2020; 323: 1353–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kosiborod MN, Jhund PS, Docherty KF, Diez M, Petrie MC, Verma S, Nicolau JC, Merkely B, Kitakaze M, DeMets DL, Inzucchi SE, Kober L, Martinez FA, Ponikowski P, Sabatine MS, Solomon SD, Bengtsson O, Lindholm D, Niklasson A, Sjostrand M, Langkilde AM, McMurray JJV. Effects of dapagliflozin on symptoms, function, and quality of life in patients with heart failure and reduced ejection fraction: results from the DAPA‐HF trial. Circulation 2020; 141: 90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lam CSP, Chandramouli C, Ahooja V, Verma S. SGLT‐2 inhibitors in heart failure: current management, unmet needs, and therapeutic prospects. J Am Heart Assoc 2019; 8: e013389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, Lewis JB, Riddle MC, Voors AA, Metra M, Lund LH, Komajda M, Testani JM, Wilcox CS, Ponikowski P, Lopes RD, Verma S, Lapuerta P, Pitt B, Investigators S‐WT. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med 2021; 384: 117–128. [DOI] [PubMed] [Google Scholar]
- 51. Heerspink HJL, Stefansson BV, Correa‐Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE, McMurray JJV, Lindberg M, Rossing P, Sjostrom CD, Toto RD, Langkilde AM, Wheeler DC, Committees D‐CT, Investigators . Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020; 383: 1436–1446. [DOI] [PubMed] [Google Scholar]
- 52. Burnett JC Jr, Kao PC, Hu DC, Heser DW, Heublein D, Granger JP, Opgenorth TJ, Reeder GS. Atrial natriuretic peptide elevation in congestive heart failure in the human. Science 1986; 231: 1145–1147. [DOI] [PubMed] [Google Scholar]
- 53. Petrykiv S, Sjostrom CD, Greasley PJ, Xu J, Persson F, Heerspink HJL. Differential effects of dapagliflozin on cardiovascular risk factors at varying degrees of renal function. Clin J Am Soc Nephrol 2017; 12: 751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Coleman RL, Gray AM, Broedl Md UC, Fitchett D, George JT, Woerle HJ, Zinman B, Holman RR. Can the cardiovascular risk reductions observed with empagliflozin in the EMPA‐REG OUTCOME trial be explained by concomitant changes seen in conventional cardiovascular risk factor levels? Diabetes Obes Metab 2020; 22: 1151–1156. [DOI] [PubMed] [Google Scholar]
- 55. Kuo S, Ye W, Duong J, Herman WH. Are the favorable cardiovascular outcomes of empagliflozin treatment explained by its effects on multiple cardiometabolic risk factors? A simulation of the results of the EMPA‐REG OUTCOME trial. Diabetes Res Clin Pract 2018; 141: 181–189. [DOI] [PubMed] [Google Scholar]
- 56. Dekkers CCJ, Sjostrom CD, Greasley PJ, Cain V, Boulton DW, Heerspink HJL. Effects of the sodium‐glucose co‐transporter‐2 inhibitor dapagliflozin on estimated plasma volume in patients with type 2 diabetes. Diabetes Obes Metab 2019; 21: 2667–2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sha S, Polidori D, Heise T, Natarajan J, Farrell K, Wang SS, Sica D, Rothenberg P, Plum‐Morschel L. Effect of the sodium glucose co‐transporter 2 inhibitor canagliflozin on plasma volume in patients with type 2 diabetes mellitus. Diabetes Obes Metab 2014; 16: 1087–1095. [DOI] [PubMed] [Google Scholar]
- 58. Hallow KM, Helmlinger G, Greasley PJ, McMurray JJV, Boulton DW. Why do SGLT2 inhibitors reduce heart failure hospitalization? A differential volume regulation hypothesis. Diabetes Obes Metab 2018; 20: 479–487. [DOI] [PubMed] [Google Scholar]
- 59. Griffin M, Rao VS, Ivey‐Miranda J, Fleming J, Mahoney D, Maulion C, Suda N, Siwakoti K, Ahmad T, Jacoby D, Riello R, Bellumkonda L, Cox Z, Collins S, Jeon S, Turner JM, Wilson FP, Butler J, Inzucchi SE, Testani JM. Empagliflozin in heart failure: diuretic and cardiorenal effects. Circulation 2020; 142: 1028–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mordi NA, Mordi IR, Singh JS, McCrimmon RJ, Struthers AD, Lang CC. Renal and cardiovascular effects of SGLT2 inhibition in combination with loop diuretics in patients with type 2 diabetes and chronic heart failure: the RECEDE‐CHF trial. Circulation 2020; 142: 1713–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Karg MV, Bosch A, Kannenkeril D, Striepe K, Ott C, Schneider MP, Boemke‐Zelch F, Linz P, Nagel AM, Titze J, Uder M, Schmieder RE. SGLT‐2‐inhibition with dapagliflozin reduces tissue sodium content: a randomised controlled trial. Cardiovasc Diabetol 2018; 17: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Schneider MP, Raff U, Kopp C, Scheppach JB, Toncar S, Wanner C, Schlieper G, Saritas T, Floege J, Schmid M, Birukov A, Dahlmann A, Linz P, Janka R, Uder M, Schmieder RE, Titze JM, Eckardt KU. Skin sodium concentration correlates with left ventricular hypertrophy in CKD. J Am Soc Nephrol 2017; 28: 1867–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Packer M. Activation and inhibition of sodium‐hydrogen rxchanger is a mechanism that links the pathophysiology and treatment of diabetes mellitus with that of heart failure. Circulation 2017; 136: 1548–1559. [DOI] [PubMed] [Google Scholar]
- 64. Hallow KM, Greasley PJ, Helmlinger G, Chu L, Heerspink HJ, Boulton DW. Evaluation of renal and cardiovascular protection mechanisms of SGLT2 inhibitors: model‐based analysis of clinical data. Am J Physiol Renal Physiol 2018; 315: F1295–F1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Inzucchi SE, Zinman B, Fitchett D, Wanner C, Ferrannini E, Schumacher M, Schmoor C, Ohneberg K, Johansen OE, George JT, Hantel S, Bluhmki E, Lachin JM. How does empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA‐REG OUTCOME trial. Diabetes Care 2018; 41: 356–363. [DOI] [PubMed] [Google Scholar]
- 66. Li J, Woodward M, Perkovic V, Figtree GA, Heerspink HJL, Mahaffey KW, de Zeeuw D, Vercruysse F, Shaw W, Matthews DR, Neal B. Mediators of the effects of canagliflozin on heart failure in patients with type 2 diabetes. JACC Heart Fail 2020; 8: 57–66. [DOI] [PubMed] [Google Scholar]
- 67. Sano M, Goto S. Possible mechanism of hematocrit elevation by sodium glucose cotransporter 2 inhibitors and associated beneficial renal and cardiovascular effects. Circulation 2019; 139: 1985–1987. [DOI] [PubMed] [Google Scholar]
- 68. Ghanim H, Abuaysheh S, Hejna J, Green K, Batra M, Makdissi A, Chaudhuri A, Dandona P. Dapagliflozin suppresses hepcidin and increases erythropoiesis. J Clin Endocrinol Metab 2020; 105: e1056–e1063. [DOI] [PubMed] [Google Scholar]
- 69. Sato T, Aizawa Y, Yuasa S, Kishi S, Fuse K, Fujita S, Ikeda Y, Kitazawa H, Takahashi M, Sato M, Okabe M. The effect of dapagliflozin treatment on epicardial adipose tissue volume. Cardiovasc Diabetol 2018; 17: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Baker AR, Silva NF, Quinn DW, Harte AL, Pagano D, Bonser RS, Kumar S, McTernan PG. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc Diabetol 2006; 5: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lopaschuk GD, Verma S. Mechanisms of cardiovascular benefits of sodium glucose co‐transporter 2 (SGLT2) inhibitors: a state‐of‐the‐art review. JACC Basic Transl Sci 2020; 5: 632–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hattori S. Anti‐inflammatory effects of empagliflozin in patients with type 2 diabetes and insulin resistance. Diabetol Metab Syndr 2018; 10: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Adamo L, Rocha‐Resende C, Prabhu SD, Mann DL. Reappraising the role of inflammation in heart failure. Nat Rev Cardiol 2020; 17: 269–285. [DOI] [PubMed] [Google Scholar]
- 74. Zainordin NA, Hatta S, Mohamed Shah FZ, Rahman TA, Ismail N, Ismail Z, Abdul GR. Effects of dapagliflozin on endothelial dysfunction in type 2 diabetes with established ischemic heart disease (EDIFIED). J Endocr Soc 2020; 4: bvz017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tanaka A, Shimabukuro M, Machii N, Teragawa H, Okada Y, Shima KR, Takamura T, Taguchi I, Hisauchi I, Toyoda S, Matsuzawa Y, Tomiyama H, Yamaoka‐Tojo M, Ueda S, Higashi Y, Node K. Secondary analyses to assess the profound effects of empagliflozin on endothelial function in patients with type 2 diabetes and established cardiovascular diseases: the placebo‐controlled double‐blind randomized effect of empagliflozin on endothelial function in cardiovascular high risk diabetes mellitus: multi‐center placebo‐controlled double‐blind randomized trial. J Diabetes Investig 2020; 11: 1551–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ott C, Jumar A, Striepe K, Friedrich S, Karg MV, Bramlage P, Schmieder RE. A randomised study of the impact of the SGLT2 inhibitor dapagliflozin on microvascular and macrovascular circulation. Cardiovasc Diabetol 2017; 16: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Striepe K, Jumar A, Ott C, Karg MV, Schneider MP, Kannenkeril D, Schmieder RE. Effects of the selective sodium‐glucose cotransporter 2 inhibitor empagliflozin on vascular function and central hemodynamics in patients with type 2 diabetes mellitus. Circulation 2017; 136: 1167–1169. [DOI] [PubMed] [Google Scholar]
- 78. Pfeifer M, Townsend RR, Davies MJ, Vijapurkar U, Ren J. Effects of canagliflozin, a sodium glucose co‐transporter 2 inhibitor, on blood pressure and markers of arterial stiffness in patients with type 2 diabetes mellitus: a post hoc analysis. Cardiovasc Diabetol 2017; 16: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Holwerda SW, Luehrs RE, DuBose L, Collins MT, Wooldridge NA, Stroud AK, Fadel PJ, Abboud FM, Pierce GL. Elevated muscle sympathetic nerve activity contributes to central artery stiffness in young and middle‐age/older adults. Hypertension 2019; 73: 1025–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Verschure DO, van Eck‐Smit BL, Somsen GA, Knol RJ, Verberne HJ. Cardiac sympathetic activity in chronic heart failure: cardiac (123)I‐mIBG scintigraphy to improve patient selection for ICD implantation. Neth Heart J 2016; 24: 701–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sano M, Chen S, Imazeki H, Ochiai H, Seino Y. Changes in heart rate in patients with type 2 diabetes mellitus after treatment with luseogliflozin: subanalysis of placebo‐controlled, double‐blind clinical trials. J Diabetes Investig 2018; 9: 638–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Shimizu W, Kubota Y, Hoshika Y, Mozawa K, Tara S, Tokita Y, Yodogawa K, Iwasaki YK, Yamamoto T, Takano H, Tsukada Y, Asai K, Miyamoto M, Miyauchi Y, Kodani E, Ishikawa M, Maruyama M, Ogano M, Tanabe J, investigators Et . Effects of empagliflozin versus placebo on cardiac sympathetic activity in acute myocardial infarction patients with type 2 diabetes mellitus: the EMBODY trial. Cardiovasc Diabetol 2020; 19: 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Garg V, Verma S, Connelly KA, Yan AT, Sikand A, Garg A, Dorian P, Zuo F, Leiter LA, Zinman B, Juni P, Verma A, Teoh H, Quan A, Mazer CD, Ha ACT. Does empagliflozin modulate the autonomic nervous system among individuals with type 2 diabetes and coronary artery disease? The EMPA‐HEART CardioLink‐6 Holter analysis. Metabol Open 2020; 7: 100039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Mudaliar S, Alloju S, Henry RR. Can a shift in fuel energetics explain the beneficial cardiorenal outcomes in the EMPA‐REG OUTCOME study? A unifying hypothesis. Diabetes Care 2016; 39: 1115–1122. [DOI] [PubMed] [Google Scholar]
- 85. Avogaro A, Fadini GP, Del Prato S. Reinterpreting cardiorenal protection of renal sodium‐glucose cotransporter 2 inhibitors via cellular life history programming. Diabetes Care 2020; 43: 501–507. [DOI] [PubMed] [Google Scholar]
- 86. Packer M. SGLT2 inhibitors produce cardiorenal benefits by promoting adaptive cellular reprogramming to induce a state of fasting mimicry: a paradigm shift in understanding their mechanism of action. Diabetes Care 2020; 43: 508–511. [DOI] [PubMed] [Google Scholar]
- 87. Daniele G, Xiong J, Solis‐Herrera C, Merovci A, Eldor R, Tripathy D, DeFronzo RA, Norton L, Abdul‐Ghani M. Dapagliflozin enhances fat oxidation and ketone production in patients with type 2 diabetes. Diabetes Care 2016; 39: 2036–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Nishimura R, Tanaka Y, Koiwai K, Ishida K, Salsali A, Kaspers S, Kohler S, Lund SS. Effect of empagliflozin on free fatty acids and ketone bodies in Japanese patients with type 2 diabetes mellitus: a randomized controlled trial. Adv Ther 2019; 36: 2769–2782. [DOI] [PubMed] [Google Scholar]
- 89. Abdurrachim D, Teo XQ, Woo CC, Chan WX, Lalic J, Lam CSP, Lee PTH. Empagliflozin reduces myocardial ketone utilization while preserving glucose utilization in diabetic hypertensive heart disease: a hyperpolarized (13) C magnetic resonance spectroscopy study. Diabetes Obes Metab 2019; 21: 357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Mulder S, Heerspink HJL, Darshi M, Kim JJ, Laverman GD, Sharma K, Pena MJ. Effects of dapagliflozin on urinary metabolites in people with type 2 diabetes. Diabetes Obes Metab 2019; 21: 2422–2428. [DOI] [PubMed] [Google Scholar]
- 91. Metra M, Davison B, Bettari L, Sun H, Edwards C, Lazzarini V, Piovanelli B, Carubelli V, Bugatti S, Lombardi C, Cotter G, Dei CL. Is worsening renal function an ominous prognostic sign in patients with acute heart failure? The role of congestion and its interaction with renal function. Circ Heart Fail 2012; 5: 54–62. [DOI] [PubMed] [Google Scholar]
- 92. Seferovic PM, Fragasso G, Petrie M, Mullens W, Ferrari R, Thum T, Bauersachs J, Anker SD, Ray R, Cavusoglu Y, Polovina M, Metra M, Ambrosio G, Prasad K, Seferovic J, Jhund PS, Dattilo G, Celutkiene J, Piepoli M, Moura B, Chioncel O, Ben Gal T, Heymans S, Jaarsma T, Hill L, Lopatin Y, Lyon AR, Ponikowski P, Lainscak M, Jankowska E, Mueller C, Cosentino F, Lund LH, Filippatos GS, Ruschitzka F, Coats AJS, Rosano GMC. Heart Failure Association of the European Society of Cardiology update on sodium‐glucose co‐transporter 2 inhibitors in heart failure (an update on the sodium‐glucose co‐transporter 2 inhibitors in heart failure: beyond glycaemic control. A position paper of the Heart Failure Association of the European Society of Cardiology). Eur J Heart Fail 2020; 22: 1984–1986. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. PRISMA Checklist.
Table S2. Search strategy to search Embase (via Ovid).


